Abstract
Aims/Introduction
To evaluate the relative contributions of the area under the C‐peptide curve (AUCC) in diabetic retinopathy (DR) during an oral glucose tolerance test and C‐peptide release test in patients with type 2 diabetes.
Materials and Methods
We retrospectively analyzed the data of 969 patients. Their general characteristics were retrieved. A series of parameters for assessing pancreatic β‐cells function, such as the AUCC for six time periods: 0–60 min (AUCC0–60), 0–120 min (AUCC0–120), 0–180 min (AUCC0–180), 60–120 min (AUCC60–120), 60–180 min (AUCC60–180) and 120–180 min (AUCC120–180); the area under the glucose‐time curve for six time periods: 0–60 min (AUCG0–60), 0–120 min (AUCG0–120), 0–180 min (AUCG0–180), 60–120 min (AUCG60–120), 60–180 min (AUCG60–180) and 120–180 min (AUCG120–180) and their related indexes, were calculated through 0–180 min oral glucose tolerance test and C‐peptide release test. We used univariate analysis to examine the potential factors affecting DR. Spearman's correlation was used to analyze the correlation between AUCC‐related indexes and DR. The logistic regression model was used to investigate AUCC and its related indexes’ contribution to incidence DR. A smooth curve fitting model was used to determine the correlation, non‐linear relationship, and threshold effect between AUCC and DR.
Results
Of the 969 patients with type 2 diabetes, 469 (48.40%) and 500 (51.60%) were classified as the DR group and non‐DR group. Compared with the non‐DR group, the DR patients had lower AUCC and AUCC/AUCG. Spearman's correlation analysis showed that AUCC‐related indexes were all negatively correlated with DR. The logistic regression analysis determined that there were associations between AUCC and DR in the adjusted models. The odds ratio values of AUCC0–60, AUCC0–120, AUCC0–180, AUCC0–60/AUCG0–60, AUCC0–120/AUCG0–120, AUCC0–180/AUCG0–180, AUCC60–120, AUCC60–180, AUCC120–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180 and AUCC120–180/AUCG120–180 were 0.817 (0.750, 0.890), 0.925 (0.895, 0.955), 0.951 (0.932, 0.970), 0.143 (0.060, 0.340), 0.194 (0.093, 0.406), 0.223 (0.116, 0.427), 0.886 (0.842, 0.933), 0.939 (0.915, 0.963), 0.887 (0.846, 0.930), 0.253 (0.133, 0.479), 0.282 (0.160, 0.497) and 0.355 (0.220, 0.573), respectively. AUCC showed a non‐linear relationship with DR, with an inflection point. The inflection points of AUCC180/AUCG180, AUCC60–120, AUCC60–180, AUCC120–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180, AUCC120–180/AUCG120–180 and DR were 17.51, 0.542, 6.6, 15.7, 8.23, 0.534, 0.593 and 0.808 (P < 0.0001). When the indexes related to the AUCC were less than the inflection point value, they were significantly negatively associated with DR.
Conclusions
The indexes related to the AUCC for six time periods during an oral glucose tolerance test and C‐peptide release test was closely associated with the incidence to DR in patients with type 2 diabetes. AUCC has the added advantage of being a cheap and convenient risk assessment over traditional ophthalmic screening.
Keywords: Area under the C‐peptide curve, Beta‐cell function, Diabetic retinopathy
The indexes related to the area under the C‐peptide curve for six time periods during an oral glucose tolerance test and C‐peptide release test might be independent predictors of the incidence of diabetic retinopathy in patients with type 2 diabetes. The area under the C‐peptide curve has the added advantage of being a cheap and convenient risk assessment over traditional ophthalmic screening.
INTRODUCTION
Diabetes is a common chronic non‐communicable disease with an estimated global prevalence of 9.3% in 2019 1 , reaching 700 million people worldwide by 2045 2 . Diabetic retinopathy (DR) is the most common microvascular complication of diabetes, with approximately one‐third of people with diabetes suffering from DR 3 . DR is the leading cause of vision loss in people with diabetes, and its early stages are asymptomatic. Early screening with simple and cost‐effective means is essential to identify patients who need treatment or are at risk of treatment, and to reduce national healthcare costs for controlling the disease 4 .
Pancreatic β‐cells dysfunction is closely associated with poor glycemic control. Glycemic variability and poorer glycemic control caused by β‐cell dysfunction might lead to an increased risk of diabetic complications 5 . Large glycemic variability is positively associated with microvascular and macrovascular complications, and mortality 6 , 7 . Intensive blood glucose control can restore part of pancreatic β‐cells. The UK Prospective Diabetes Study reported that intensive glycemic control with sulfonylureas or insulin significantly reduced the risk of microvascular complications, but not macrovascular complications. Therefore, the relative roles of pancreatic β‐cells dysfunction on DR in patients with type 2 diabetes constitute a highly interesting topic 8 .
The oral glucose tolerance test (OGTT) is a screening tool for identifying diabetes. There is a growing interest in finding novel biomarkers from the OGTT that can reflect pancreatic β‐cells function. Such emerging risk indicators include OGTT product of C‐peptide and glucose, ratio of C‐peptide to glucose, area of C‐peptide under the insulin release curve (AUCC), and the correlation ratio 9 , 10 . AUCC and its ratio to glycemic area were associated with pancreatic β‐cells function and risk for diabetes 11 . The correlation indexes of AUCC during a 2‐h OGTT to the development of DR has not yet been studied.
The aim of the present study was to analyze whether the AUCC can predict the occurrence of DR by OGTT combined with the C‐peptide release test.
METHODS
Participants
All participants in the present study were from the Department of Endocrinology, Harbin Medical University, Harbin, China. Type 2 diabetes patients with or without DR were eligible for this study. The exclusion criteria are as follows: (1) positive autoimmune antibodies to diabetes or other specific types of diabetes; (2) hospitalized patients mainly diagnosed with acute diabetic complications, such as diabetic ketoacidosis and diabetic hypertonic coma; (3) active or chronic inflammation, active infection or autoimmune disease; (4) hematological diseases, recent blood transfusion before enrollment or malignant tumors; (5) acute or chronic kidney/liver disease; (6) other eye diseases affecting the diagnosis of DR were excluded, such as macular degeneration, retinal vein obstruction and glaucoma; and (7) incomplete basic information, such as medication history and past disease history. Based on inclusion and exclusion criteria, 969 diabetes patients were enrolled from January 2016 to August 2022 and assigned to either the DR group (n = 469) or type 2 diabetes group (n = 500; Figure 1). All patients gave written informed consent to the use of their clinical data in this study.
Figure 1.
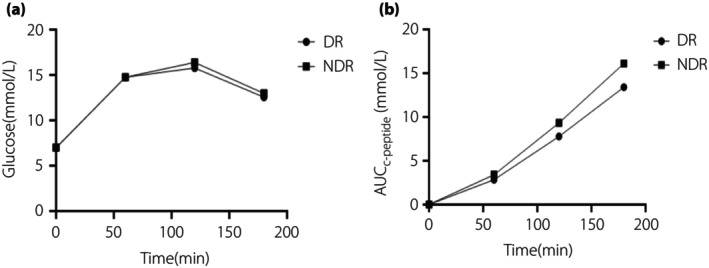
Functional curve of islets of diabetic retinopathy (DR) and non‐diabetic retinopathy (NDR). (a) Glucose‐time curve of the DR group and NDR group, the x‐axis is time and the y‐axis is glucose value. (b) The area under the C‐peptide curve time curve of the DR group and NDR group, the x‐axis is time and the y‐axis is the area under the C‐peptide curve value.
Study definition and diagnosis of DR
The diagnosis of type 2 diabetes conforms to the criteria published by the American Diabetes Association 12 . A professional ophthalmologist performed an eye examination and assessment on the International Clinical Diabetic Retinopathy Disease Severity Scale. If the Early Treatment Diabetic Retinopathy Study identified any characteristic lesions, a diagnosis of DR was made. A history of smoking and drinking referred to people who have not quit or abstained from alcohol, respectively, from the past to the present.
Sociodemographic characteristics
Sociodemographic characteristics, including sex, age, smoking history, alcohol history, diabetes duration, insulin, body mass index (BMI; BMI = height [m] / weight [kg]2), systolic blood pressure and diastolic blood pressure (DBP) were collected retrospectively. Classification was carried out according to: 0 = age ≤44 years; 1 = age 45–59 years; 2 = age ≥60 years; 0 = diabetes duration <5 years; 1 = diabetes duration 5–14 years; 2 = diabetes duration 15–24 years; 3 = diabetes duration ≥25 years; 0 = BMI <18.5; 1 = BMI 18.5–24.0; 2 = BMI 24.0–28.0; 3 = BMI ≥28.0; 0 = systolic blood pressure ≤140 mmHg; 1 = systolic blood pressure >140 mmHg; 0 = DBP ≤90 mmHg; and 1 = DBP >90 mmHg.
Peripheral blood parameters
After 3‐h OGTT (75 g) and 12‐h overnight fasting, participants were evaluated at 6:00 am in the Department of Endocrinology, The First Affiliated Hospital of Harbin Medical University. Blood samples were collected at 0, 60, 120 and 180 min to measure blood glucose (GC0, GC60, GC120, GC180) and C‐peptide levels after glucose intake (C‐P0, C‐P60, C‐P120, C‐P180). After overnight fasting, glycated hemoglobin (HbA1c), total cholesterol, triglycerides, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), neutrophil, lymphocyte, monocyte, creatinine and uric Acid were evaluated. The levels of fasting glucose, total cholesterol, triglycerides, uric acid and creatinine were measured by enzymatic methods. The content of fasting C‐peptide was determined by radioimmunoassay. HbA1c was determined by ion exchange high‐performance liquid chromatography. Homogeneous assays were used to determine LDL‐C and HDL‐C levels. White blood cell counts and differentials were detected by automated hematology analyzer (Sysmex XT‐2000i,SYSMEX, Japan).
Analysis and calculation
GraphPad Prism9.0 (San Diego, CA, USA) was used to calculate the area under the OGTT curve (AUC) of glucose (AUCG) and C‐peptide. AUCG, AUCC and AUCC/AUCG are OGTT and C‐peptide release test derived pancreatic β‐cells function indices.
Statistical analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA), EmpowerStats (http://www.empowerstats.com, X&Y Solutions; Boston, MA, USA) and GraphPad Prism 9.0 were used for data analysis and chart building. Normally distributed continuous variables are expressed as the mean ± standard deviation. Categorical variables are expressed in frequency and percentage terms. Baseline characteristics were compared between DR and non‐DR participants. Variables conforming to the normal distribution were compared between groups through analysis of the independent samples t‐test. Variables not conforming to the normal distribution were compared between groups using a non‐parametric test. The differences between classification variables groups were analyzed using the χ2‐test. The relationships between DR and AUCC‐related indexes were quantified with Spearman's correlation analysis. Spearman's correlation analysis also examined the relationship between the AUCC and its related indexes for six time periods and other variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using a binary logistic regression to assess the association of AUCC‐related indexes with DR. To exclude the influence of other variables, we adjusted the original model. Model 1 was adjusted according to sex and age. In model 2, sex, age, smoking history, drinking history, diabetes course, insulin use, and HbA1c were adjusted. In model 3, DBP and HDL‐C were further adjusted. The generalized additive model in EmpowerStats was used for smooth curve fitting of AUCC‐related indexes to DR incidence. AUCC‐related indexes are continuous variables, and DR incidence is a classified variable. Whether there was a non‐linear relationship between them was tested and whether there was a threshold effect was determined. According to the predetermined interval, the inflection point is selected, and the inflection point with the maximum likelihood of the model is selected. P < 0.05 was considered statistically significant.
RESULTS
Relationship between sociodemographic characteristics and DR in type 2 diabetes patients
The study included 969 patients, including 469 DR patients (men, n = 303; women, n = 166) and 500 non‐DR patients (men, n = 296; women, n = 204). Except for LDL‐C, the remaining variables were not normally distributed. Univariate analysis of sociodemographic characteristics in patients with type 2 diabetes showed that smoking history (P < 0.001) and DBP (P = 0.029) were associated with DR (Table 1).
Table 1.
Univariate analysis of sociodemographic characteristics and diabetic retinopathy in type 2 diabetes patients
| DR n = 469 | NDR n = 500 | χ2/t/Z | P‐value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 303 (64.6) | 296 (59.2) | 2.996 | 0.083 |
| Female | 166 (35.4) | 204 (40.8) | ||
| Age (years) | ||||
| ≤44 | 61 (13.0) | 66 (13.2) | −1.263 | 0.207 |
| 45–59 | 224 (47.8) | 226 (45.2) | ||
| ≥60 | 184 (39.2) | 208 (41.6) | ||
| Smoking history | ||||
| Yes | 175 (37.3) | 133 (26.6) | 12.811 | <0.001 |
| No | 294 (62.7) | 367 (73.4) | ||
| Alcohol history | ||||
| Yes | 148 (31.6) | 142 (28.4) | 1.150 | 0.284 |
| No | 321 (68.4) | 358 (71.6) | ||
| Diabetes duration (years) | ||||
| <5 | 128 (27.3) | 131 (26.2) | −0.943 | 0.346 |
| 5–14 | 194 (41.3) | 192 (38.4) | ||
| 15–24 | 128 (27.3) | 146 (29.2) | ||
| ≥25 | 19 (4.1) | 31 (6.2) | ||
| Insulin | ||||
| Yes | 280 (59.7) | 306 (61.2) | 0.227 | 0.633 |
| No | 189 (40.3) | 194 (38.8) | ||
| BMI | ||||
| <18.5 | 19 (4.1) | 6 (1.2) | −1.319 | 0.187 |
| 18.5–24 | 135 (28.8) | 166 (33.2) | ||
| 24–28 | 209 (44.6) | 236 (47.2) | ||
| ≥28 | 106 (22.5) | 92 (18.4) | ||
| SBP | ||||
| ≤140 | 227 (48.4) | 242 (48.4) | −0.037 | 0.971 |
| >140 | 242 (51.6) | 258 (51.6) | ||
| DBP | ||||
| ≤90 | 385 (82.1) | 414 (82.8) | −2.181 | 0.029 |
| >90 | 84 (17.9) | 86 (17.2) | ||
BMI, body mass index; DBP, diastolic blood pressure; DR, diabetic retinopathy; NDR, non‐diabetic retinopathy; SBP, systolic blood pressure.
Relationship between blood parameters and DR in patients with type 2 diabetes
Univariate analysis of blood parameters in patients with type 2 diabetes showed that HbA1c (P = 0.020), HDL‐C (P = 0.008), LDL‐C (P = 0.001), neutrophil (P = 0.011), AUCC0–60 (P < 0.001), AUCC0–120 (P < 0.001), AUCC0–180 (P < 0.001), AUCC0–60/AUCG0–60 (P < 0.001), AUCC0–120/AUCG0–120 (P < 0.001), AUCC0–180/AUCG0–180 (P < 0.001), AUCC60–120 (P < 0.001), AUCC60–180 (P < 0.001), AUCC120–180 (P < 0.001), AUCC60–120/AUCG60–120 (P < 0.001), AUCC60–180/AUCG60–180 (P < 0.001) and AUCC120–180/AUCG120–180 (P < 0.001) were associated with DR (Table 2, Figure 1).
Table 2.
Univariate analysis of blood parameters and diabetic retinopathy in type 2 diabetes patients
| DR | NDR | t/Z | P‐value | |
|---|---|---|---|---|
| HbA1c (%) | 8.89 ± 1.89 | 8.71 ± 2.06 | −2.331 | 0.020 |
| TC (mmol/L) | 4.79 ± 1.32 | 4.74 ± 1.18 | −0.320 | 0.749 |
| TG (mmol/L) | 2.28 ± 1.77 | 2.11 ± 1.63 | −1.291 | 0.197 |
| HDL‐C (mmol/L) | 1.10 ± 0.25 | 1.07 ± 0.28 | −2.648 | 0.008 |
| LDL‐C (mmol/L) | 2.70 ± 1.13 | 2.92 ± 0.92 | −3.327 | 0.001 |
| Neutrophil (×109/L) | 3.96 ± 1.33 | 3.78 ± 1.36 | −2.534 | 0.011 |
| Lymphocyte (×109/L) | 2.10 ± 0.74 | 2.10 ± 0.68 | −0.269 | 0.788 |
| Monocyte (×109/L) | 0.43 ± 0.16 | 0.45 ± 0.18 | −1.355 | 0.175 |
| eGFR | 101.22 ± 21.41 | 100.68 ± 20.11 | −1.332 | 0.183 |
| Uric acid (μmol/L) | 344.18 ± 90.81 | 343.89 ± 92.78 | −0.130 | 0.896 |
| GC0 | 6.96 ± 1.55 | 7.00 ± 1.61 | −0.362 | 0.717 |
| GC60 | 14.75 ± 3.34 | 14.76 ± 3.10 | −0.257 | 0.797 |
| GC120 | 15.77 ± 3.75 | 16.40 ± 9.63 | −1.075 | 0.283 |
| GC180 | 12.55 ± 4.16 | 12.98 ± 3.95 | −1.843 | 0.065 |
| C‐P0 | 1.78 ± 1.14 | 1.74 ± 1.18 | −0.925 | 0.355 |
| C‐P60 | 4.45 ± 2.77 | 4.41 ± 2.74 | −0.274 | 0.784 |
| C‐P120 | 6.36 ± 3.47 | 6.30 ± 3.60 | −0.589 | 0.556 |
| C‐P180 | 6.00 ± 3.24 | 6.00 ± 3.26 | −0.154 | 0.877 |
| AUCG0–60 | 10.84 ± 2.30 | 10.87 ± 1.94 | −0.521 | 0.602 |
| AUCG0–120 | 26.19 ± 5.43 | 26.31 ± 6.72 | −0.094 | 0.925 |
| AUCG0–180 | 40.71 ± 8.57 | 40.68 ± 12.10 | −0.577 | 0.564 |
| AUCC0–60 | 2.84 ± 1.75 | 3.41 ± 2.00 | −4.890 | <0.001 |
| AUCC0–120 | 7.78 ± 4.62 | 9.33 ± 5.16 | −5.153 | <0.001 |
| AUCC0–180 | 13.41 ± 7.65 | 16.11 ± 8.44 | −5.536 | <0.001 |
| AUCC0–60/AUCG0–60 | 0.27 ± 0.17 | 0.32 ± 0.20 | −4.809 | <0.001 |
| AUCC0–120/AUCG0–120 | 0.31 ± 0.20 | 0.38 ± 0.23 | −4.971 | <0.001 |
| AUCC0–180/AUCG0–180 | 0.35 ± 0.24 | 0.43 ± 0.27 | −5.312 | <0.001 |
| AUCC60–120 | 4.94 ± 2.94 | 5.91 ± 3.23 | −5.231 | <0.001 |
| AUCC60–180 | 10.56 ± 6.04 | 12.69 ± 6.60 | −5.608 | <0.001 |
| AUCC120–180 | 5.62 ± 3.21 | 6.78 ± 3.49 | −5.759 | <0.001 |
| AUCC60–120/AUCG60–120 | 0.34 ± 0.24 | 0.42 ± 0.27 | −5.044 | <0.001 |
| AUCC60–180/AUCG60–180 | 0.38 ± 0.27 | 0.47 ± 0.30 | −5.398 | <0.001 |
| AUCC120–180/AUCG120–180 | 0.43 ± 0.33 | 0.54 ± 0.36 | −5.543 | <0.001 |
AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180, the area under the C‐peptide curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180, 120–180 min; AUCG0–60, AUCG0–120, AUCG0–180, AUCG60–120, AUCG60–180 and AUCG120–180, the area under the glucose‐time curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180 and 120–180 min; C‐P0, C‐P60, C‐P120, C‐P180, 0, 60, 120 and 180 min C‐peptide after the oral glucose tolerance test; GC0, GC60, GC120, GC180, 0, 60, 120 and 180 min blood glucose after the oral glucose tolerance test.
DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NDR, non‐diabetic retinopathy; TC, total cholesterol; TG, triglycerides.
Correlation analysis of AUCC ‐related indexes and DR in patients with type 2 diabetes
The correlation between AUCC‐related indexes and DR of patients with type 2 diabetes was analyzed using Spearman's correlation. The results showed that AUCC0–60 (r = −0.157, P < 0.001), AUCC0–120 (r = −0.166, P < 0.001), AUCC0–180 (r = −0.178, P < 0.001), AUCC0–60/AUCG0–60 (r = −0.155, P < 0.001), AUCC0–120/AUCG0–120 (r = −0.160, P < 0.001), AUCC0–180/AUCG0–180 (r = −0.171, P < 0.001), AUCC60–120 (r = −0.168, P < 0.001), AUCC60–180 (r = −0.180, P < 0.001), AUCC120–180 (r = −0.185, P < 0.001), AUCC60–120/AUCG60–120 (r = −0.162, P < 0.001), AUCC60–180/AUCG60–180 (r = −0.173, P < 0.001) and AUCC120–180/AUCG120–180 (r = −0.178, P < 0.001) were inversely associated with DR (Table 3).
Table 3.
Correlation analysis of area under the C‐peptide curve‐related indexes and diabetic retinopathy in type 2 diabetes patients
| r | P | |
|---|---|---|
| AUCC0–60 | −0.157 | <0.001 |
| AUCC0–120 | −0.166 | <0.001 |
| AUCC0–180 | −0.178 | <0.001 |
| AUCC0–60/AUCG0–60 | −0.155 | <0.001 |
| AUCC0–120/AUCG0–120 | −0.160 | <0.001 |
| AUCC0–180/AUCG0–180 | −0.171 | <0.001 |
| AUCC60–120 | −0.168 | <0.001 |
| AUCC60–180 | −0.180 | <0.001 |
| AUCC120–180 | −0.185 | <0.001 |
| AUCC60–120/AUCG60–120 | −0.162 | <0.001 |
| AUCC60–180/AUCG60–180 | −0.173 | <0.001 |
| AUCC120–180/AUCG120–180 | −0.178 | <0.001 |
C‐P0, C‐P60, C‐P120, C‐P180, 0, 60, 120 and 180 min C‐peptide after the oral glucose tolerance test; GC0, GC60, GC120, GC180, 0, 60, 120 and 180 min blood glucose after the oral glucose tolerance test; AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180, AUCC120–180, the area under the C‐peptide curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180, 120–180 min; AUCG0–60, AUCG0–120, AUCG0–180, AUCG60–120, AUCG60–180, AUCG120–180, the area under the glucose‐time curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180, 120–180 min.
Relationship between AUCC indicators and prevalence of DR
Using binary logistic regression models, the AUCC – AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180 – and its ratio to the corresponding glucose curve – AUCC0–60/AUCG0–60, AUCC0–120/AUCG0–120, AUCC0–180/AUCG0–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180 and AUCC120–180/AUCG120–180 – were all associated with the risk of DR (P < 0.001). After adjusting for age, sex, duration of diabetes, hypertension, smoking, insulin use and HbA1c, the above variables were still independently associated with the risk of DR (P < 0.001; Table 4). The results of the adjusted model 3 showed that after further adjusting for diastolic blood pressure, HDL‐C, LDL‐C and neutrophil, AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180, AUCC120–180, AUCC0–60/AUCG0–60, AUCC0–120/AUCG0–120, AUCC0–180/AUCG0–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180 and AUCC120–180/AUCG120–180, a 1‐unit increase, patients' risk of developing DR decreased by 0.842‐, 0.933‐, 0.956‐, 0.185‐, 0.225‐, 0.248‐, 0.896‐, 0.944‐, 0.896‐, 0.277‐, 0.302‐ and 0.373‐fold (Table 4).
Table 4.
Binary logistic regression analysis
| Non‐adjusted | Adjust I | Adjust II | Adjust III | |
|---|---|---|---|---|
| AUCC0–60 | 0.848 (0.792, 0.909)* | 0.849 (0.792, 0.910)* | 0.854 (0.791, 0.921)* | 0.817 (0.750, 0.890)* |
| AUCC0–120 | 0.936 (0.912, 0.961)* | 0.936 (0.912, 0.962)* | 0.936 (0.909, 0.964)* | 0.925 (0.895, 0.955)* |
| AUCC0–180 | 0.958 (0.943, 0.973)* | 0.958 (0.943, 0.974)* | 0.958 (0.941, 0.975)* | 0.951 (0.932, 0.970)* |
| AUCC0–60/AUCG0–60 | 0.197 (0.098, 0.396)* | 0.196 (0.097, 0.395)* | 0.202 (0.093, 0.437)* | 0.143 (0.060, 0.340)* |
| AUCC0–120/AUCG0–120 | 0.239 (0.132, 0.434)* | 0.237 (0.130, 0.431)* | 0.233 (0.119, 0.456)* | 0.194 (0.093, 0.406)* |
| AUCC0–180/AUCG0–180 | 0.268 (0.159, 0.453)* | 0.266 (0.157, 0.450)* | 0.257 (0.142, 0.465)* | 0.223 (0.116, 0.427)* |
| AUCC60–120 | 0.901 (0.863, 0.941)* | 0.902 (0.864, 0.941)* | 0.902 (0.860, 0.947)* | 0.886 (0.842, 0.933)* |
| AUCC60–180 | 0.947 (0.927, 0.967)* | 0.947 (0.928, 0.968)* | 0.947 (0.925, 0.970)* | 0.939 (0.915, 0.963)* |
| AUCC120–180 | 0.901 (0.866, 0.937)* | 0.901 (0.866, 0.938)* | 0.902 (0.863, 0.943)* | 0.887 (0.846, 0.930)* |
| AUCC60–120/AUCG60–120 | 0.297 (0.174, 0.508)* | 0.294 (0.172, 0.505)* | 0.298 (0.164, 0.543)* | 0.253 (0.133, 0.479)* |
| AUCC60–180/AUCG60–180 | 0.330 (0.205, 0.529)* | 0.327 (0.203, 0.527)* | 0.326 (0.192, 0.556)* | 0.282 (0.160, 0.497)* |
| AUCC120–180/AUCG120–180 | 0.402 (0.269, 0.599)* | 0.399 (0.267, 0.596)* | 0.401 (0.256, 0.627)* | 0.355 (0.220, 0.573)* |
Adjust I model adjusted for: sex and age. Adjust II model adjusted for: sex, age, smoking history, history of alcohol consumption, diabetes course, insulin use and glycated hemoglobin. Adjust III model adjusted for: sex, age, smoking history, history of alcohol consumption, diabetes course, insulin use, body mass index; glycated hemoglobin, diastolic blood pressure; high‐density lipoprotein; low‐density lipoprotein and neutrophils. *: P<0.001. AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180, the area under the C‐peptide curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180 and 120–180 min; AUCG0–60, AUCG0–120, AUCG0–180, AUCG60–120, AUCG60–180 and AUCG120–180, the area under the glucose‐time curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180 and 120–180 min.
Smooth curve fitting
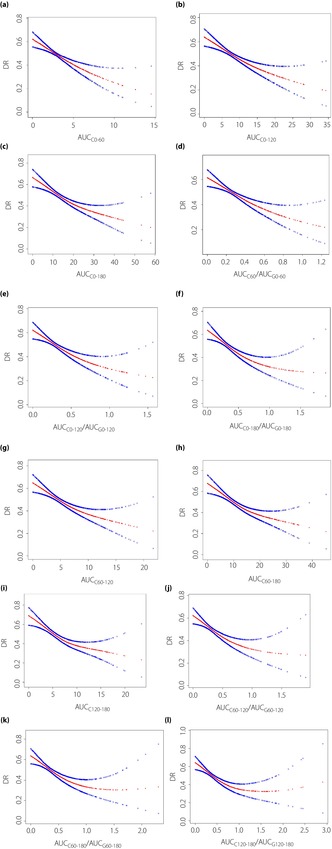
Smooth curve fitting was used to assess whether there was a dose‐dependent effect between the AUCC‐related indexes of islet function and DR in type 2 diabetes patients. After adjusting for risk factors, non‐linear relationships were found between AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180, its ratio to the corresponding glucose curve: AUCC0–180/AUCG0–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180, AUCC120–180/AUCG120–180 and DR (Figure 2, Table 5). The inflection points were 17.51, 0.542, 6.6, 15.7, 8.23, 0.534, 0.593 and 0.808, respectively, and the risk of DR decreased with the increase of the ratio when the indexes related to the area under the islet function curve was less than the inflection point value (P < 0.0001). When it was greater than the inflection point value, there was no significant correlation with DR (P > 0.05).
Figure 2.
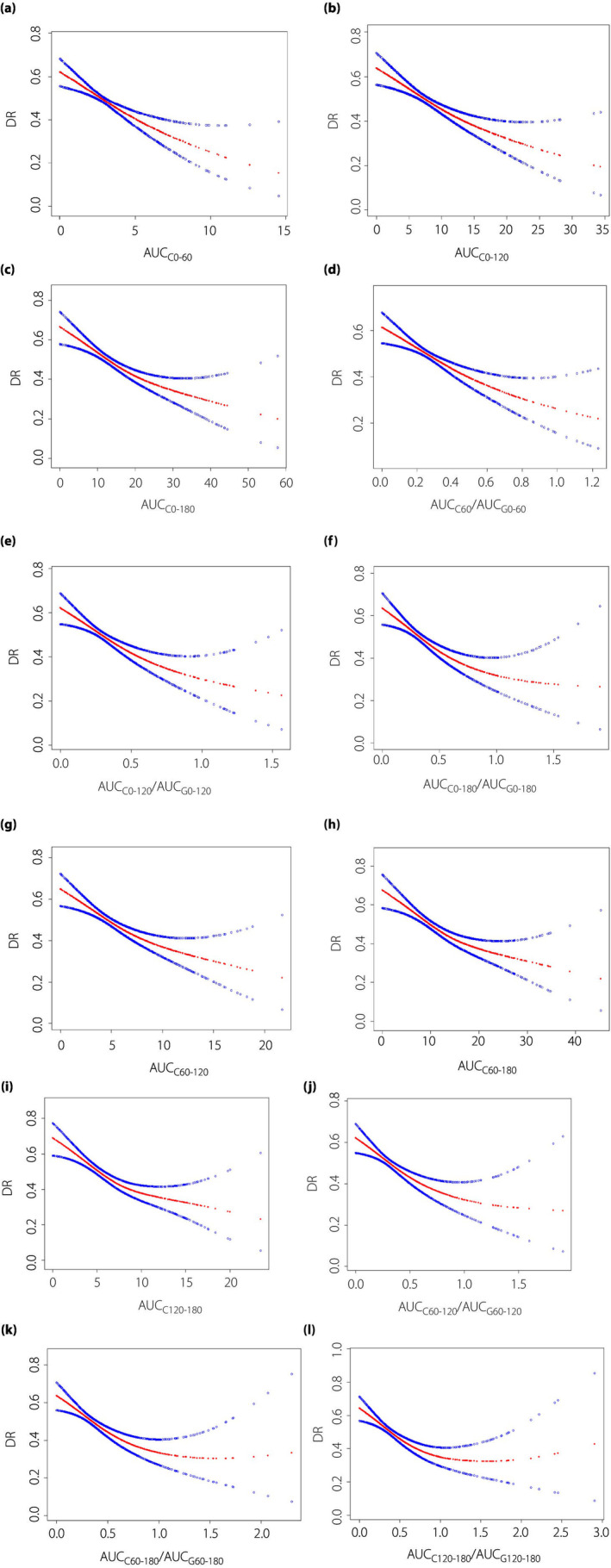
The non‐linear relationship between the incidence of diabetic retinopathy and area under the C‐peptide curve (AUCC)‐related indexes: (a) AUCC0–60, (b) AUCC0–120, (c) AUCC0–180, (d) AUCC0–60/AUCG0–60, (e) AUCC0–120/AUCG0–120, (f) AUCC0–180/AUCG0–180, (g) AUCC60–120, (h) AUCC60–180, (i) AUCC120–180, (j) AUCC60–120/AUCG60–120, (k) AUCC60–180/AUCG60–180 and (l) AUCC120–180/AUCG120–180 in type 2 diabetes patients. The x‐axis is AUCC‐related indexes and y‐axis is the incidence of diabetic retinopathy. The red lines are smooth curve fit, and the blue lines on both sides show the 95% confidence intervals. A non‐linear relationship between them was detected after adjusting for sex, age, smoking history, history of alcohol consumption, diabetes course, insulin use and glycated hemoglobin. Abbreviations: AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180, AUCC120–180: the area under the C‐peptide curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180, 120–180 min; AUCG0–60, AUCG0–120, AUCG0–180, AUCG60–120, AUCG60–180, AUCG120–180: the area under the glucose‐time curve for sic time periods: 0–60, 0–120, 0–180, 60–120, 60–180, 120–180 min.
Table 5.
Results of the two‐piecewise linear regression model
| Model I | Model II | P | |||||
|---|---|---|---|---|---|---|---|
| One‐line effect | Fold point (K) | <K segment effect 1 | >K‐segment effect 2 | The difference between 2 and 1 | P‐value at the fold point | ||
| AUCC0–60 | 0.847 (0.789, 0.909) <0.0001 | 4.36 | 0.788 (0.700, 0.888) <0.0001 | 0.938 (0.809, 1.088) 0.3996 | 1.190 (0.947, 1.495) 0.1347 | −0.418 (−0.668, −0.168) | 0.14 |
| AUCC0–120 | 0.936 (0.911, 0.962) <0.0001 | 10.46 | 0.903 (0.859, 0.950) <0.0001 | 0.973 (0.924, 1.024) 0.2963 | 1.077 (0.987, 1.175) 0.0952 | −0.350 (−0.583, −0.117) | 0.098 |
| AUCC0–180 | 0.958 (0.943, 0.974) <0.0001 | 17.51 | 0.933 (0.904, 0.962) <0.0001 | 0.986 (0.956, 1.017) 0.3667 | 1.057 (1.002, 1.115) 0.0412 | −0.375 (−0.606, −0.145) | 0.043 |
| AUCC0–60/AUCG0–60 | 0.194 (0.094, 0.398) <0.0001 | 0.419 | 0.087 (0.025, 0.298) 0.0001 | 0.565 (0.130, 2.449) 0.4454 | 6.498 (0.648, 65.146) 0.1115 | −0.429 (−0.684, −0.174) | 0.116 |
| AUCC0–120/AUCG0–120 | 0.243 (0.132, 0.449) <0.0001 | 0.494 | 0.115 (0.041, 0.319) <0.0001 | 0.689 (0.199, 2.380) 0.5558 | 6.015 (0.876, 41.325) 0.0680 | −0.463 (−0.717, −0.208) | 0.072 |
| AUCC0–180/AUCG0–180 | 0.274 (0.160, 0.470) <0.0001 | 0.542 | 0.120 (0.047, 0.306) <0.0001 | 0.757 (0.269, 2.127) 0.5969 | 6.314 (1.177, 33.879) 0.0316 | −0.480 (−0.732, −0.229) | 0.034 |
| AUCC60–120 | 0.901 (0.863, 0.941) <0.0001 | 6.6 | 0.843 (0.778, 0.913) <0.0001 | 0.968 (0.892, 1.049) 0.4266 | 1.148 (1.000, 1.318) 0.0494 | −0.373 (−0.605, −0.141) | 0.051 |
| AUCC60–180 | 0.947 (0.927, 0.967) <0.0001 | 15.7 | 0.919 (0.888, 0.951) <0.0001 | 0.994 (0.950, 1.040) 0.7898 | 1.082 (1.011, 1.158) 0.0230 | −0.501 (−0.744, −0.258) | 0.025 |
| AUCC120–180 | 0.901 (0.866, 0.937) <0.0001 | 8.23 | 0.842 (0.788, 0.900) <0.0001 | 0.994 (0.914, 1.082) 0.8959 | 1.181 (1.038, 1.343) 0.0113 | −0.522 (−0.767, −0.278) | 0.012 |
| AUCC60–120/AUCG60–120 | 0.297 (0.174, 0.508) <0.0001 | 0.534 | 0.134 (0.053, 0.342) <0.0001 | 0.761 (0.280, 2.070) 0.5921 | 5.663 (1.097, 29.241) 0.0384 | −0.462 (−0.713, −0.211) | 0.041 |
| AUCC60–180/AUCG60–180 | 0.330 (0.205, 0.529) <0.0001 | 0.593 | 0.138 (0.059, 0.323) <0.0001 | 0.854 (0.364, 2.003) 0.7172 | 6.182 (1.458, 26.216) 0.0135 | −0.500 (−0.752, −0.249) | 0.015 |
| AUCC120–180/AUCG120–180 | 0.402 (0.269, 0.599) <0.0001 | 0.808 | 0.190 (0.102, 0.356) <0.0001 | 1.270 (0.578, 2.789) 0.5518 | 6.678 (2.015, 22.133) 0.0019 | −0.695 (−0.979, −0.411) | 0.002 |
AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180, the area under the C‐peptide curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180 and 120–180 min; AUCG0–60, AUCG0–120, AUCG0–180, AUCG60–120, AUCG60–180 and AUCG120–180, the area under the glucose‐time curve for six time periods: 0–60, 0–120, 0–180, 60–120, 60–180 and 120–180 min.
DISCUSSION
DR is a common microvascular complication of type 2 diabetes with a high prevalence. Early diagnosis of DR is vital to reduce the risk of disease development and save considerable disease management costs 3 , 13 . Studies have shown that the secretory function of pancreatic β‐cells is closely related to the occurrence and development of DR 14 , 15 . Novel biomarkers based on OGTT and C‐peptide release test, such as AUCC, have been widely recognized for assessing islet function 16 . However, no studies have evaluated the relationship between AUCC and the development of DR. In the present study, we found that the AUCC related to the secretory function of pancreatic β‐cells in response to stimulation is an independent risk factor for DR, and there is a non‐linear correlation between them and DR, with an inflection point in type 2 diabetes.
OGTT is the gold standard for diabetes diagnosis. It has been widely used both in clinical practice and in research settings. Meanwhile, the OGTT combined with C‐peptide release test is the most frequently used method for assessing the secretory function of pancreatic β‐cells. As C‐peptide secretion reflects pancreatic β‐cells function, it has emerged as a reliable, simple and inexpensive clinical marker. Impaired pancreatic β‐cells function is recognized as a cornerstone of diabetes pathophysiology. The use of C‐peptide instead of insulin as a biomarker for assessing pancreatic β‐cells might better predict the progression toward type 2 diabetes, because it is not affected by the impaired hepatic clearance of insulin 17 . New biomarkers of pancreatic β‐cells function, such as AUCC and AUCG, have been increasingly studied. In patients with type 1 diabetes, mixed‐meal tolerance test AUCC is used as the gold standard to show pancreatic β‐cells secreting capacity 18 , and AUCC/AUCG is a previously described and validated OGTT‐derived pancreatic β‐cells function index 19 . Mari et al. 20 showed that the high human regular U‐500 insulin treatment group showed a 34.0% increase in AUCC/AUCG and a significant improvement in pancreatic β‐cells function after 24 weeks of treatment.
The relationship between pancreatic β‐cells function and diabetes complications is contradictory based on indicators related to pancreatic β‐cells secretory function in OGTT and C‐peptide release experiments. Shin et al. 21 found that the lower the AUCC level in type 2 diabetes patients, the more albuminuria, and the greater the likelihood of progression to diabetic nephropathy. In a study of 456 patients with type 2 diabetes, AUCC/AUCG was not an independent risk factor for diabetic peripheral neuropathy 22 . Lingshu et al. 23 found that compared with patients with simple diabetes, diabetes patients with coronary heart disease had a lower AUCC. However, on average, there was no significant difference in hydrops below the C‐peptide release curve between diabetes patients with and without a history of myocardial infarction. Currently, few studies have evaluated the relationship between indicators related to secretion function of pancreatic β‐cells and DR based on OGTT and C‐peptide release tests.
The present study found that smoking status, DBP, HbA1c and HDL‐C were all related to DR. The UK Prospective Diabetes Study concluded that HbA1c and smoking status were independent risk factors for retinopathy; in normotensive patients, the risk of retinopathy progression and vision loss associated with strict blood pressure control was reduced 24 . The NO BLIND multicenter, observational study results found that high HDL‐C was a risk factor for DR in type 2 diabetes 25 . In the present study, smoking status, DBP, HbA1c and HDL‐C were also associated with islet function. Nicotine triggers pancreatic β‐cells senescence, mainly through a series of mechanisms involving Ca2+ influx and reactive oxygen species generation, which related to the damage of pancreatic β‐cells and impaired insulin secretion 26 . Elevated blood pressure promoted islet blood supply, resulting in an increased number and volume of islet cells, and consequent enhancement of glucose‐stimulated insulin secretion 27 . There is a negative correlation between HbA1c and pancreatic β‐cells function. As HDL‐C improves regulation of pancreatic β‐cells insulin secretion, HDL‐C‐elevating therapies could, thus, possibly improve glucose‐stimulated insulin secretion 28 .
We found that the pancreatic β‐cells secretion function‐related indices based on OGTT and C‐peptide release in DR patients – AUCC0–60, AUCC0–120, AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180 –and its ratio to the corresponding glucose curve – AUCC0–60/AUCG0–60, AUCC0–120/AUCG0–120, AUCC0–180/AUCG0–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180 and AUCC120–180/AUCG120–180 – were lower than diabetes patients without DR. Meanwhile, these indexes were related to DR. The adjusted ORs were 0.854, 0.936, 0.958, 0.202, 0.233, 0.257, 0.902, 0.947, 0.902, 0.298, 0.326 and 0.401, respectively. After futher adjusting BMI, DBP, HDL‐C, LDL‐C and neutrophil, AUCC‐related indexes were still independent risk factors for DR.
Our research shows that pancreatic β‐cells function plays an important role in the incidence of DR. Combined with the clinical convenience and practicability of the above research results, we believe that AUCC0–120/AUCG0–120 is the most effective index to predict DR. The present study also found a non‐linear relationship between AUCC0–180, AUCC60–120, AUCC60–180 and AUCC120–180 and its ratio to the corresponding glucose curve – AUCC0–180/AUCG0–180, AUCC60–120/AUCG60–120, AUCC60–180/AUCG60–180, AUCC120–180/AUCG120–180 and DR – with the inflection points of 17.51, 0.542, 6.6, 15.7, 8.23, 0.534, 0.593 and 0.808, and the risk of DR decreased with the increase of the ratio when the index related to the area under the islet function curve was smaller than the value of the inflection point (P < 0.0001).
The present study is the first to find an inflection point between the indicators related to pancreatic β‐cells secretory function and DR based on OGTT and C‐peptide release test. Previous research found that the form of the relationship between C‐peptide and the incidence of retinopathy was approximately linear near to the lower limit of detection of the assay (0.003 nmol/L), with no evidence of a threshold effect 29 . Suzuki et al. 30 found that the incidence of proliferative DR in type 2 diabetes patients with a known duration of diabetes of >10 years was highest in the group with the lowest pancreatic β‐cells insulin secretory capacity. Ahlquist et al. 31 identified that the risk of DR is highest in the insulin‐deficient group of patients compared with the most insulin‐resistant group. Chowdhury et al. 32 found that lower fasting and postprandial pancreatic β‐cells responsiveness and function at diagnosis showed a significant negative contribution to the development of DR by 5 years. When AUCC‐related indexes of type 2 diabetes patients are less than the inflection point, the fundus examination should be closely monitored to reduce the risk of DR.
To prevent the development of DR, it is necessary to evaluate pancreatic β‐cell function in patients with type 2 diabetes by OGTT. The decrease in secretion function of pancreatic β‐cells is followed by large fluctuations in blood glucose, resulting in large blood glucose variability. At the same time, glucose variability can cause oxidative stress in pancreatic β‐cells 33 , which is associated with overproduction of reactive oxygen species, oxidative damage to DNA and depletion of superoxide dismutase activity 34 , leading to progressive deterioration of pancreatic β‐cells 35 . Thus, a vicious cycle is formed between decreased pancreatic β‐cell function and blood glucose variability. Blood glucose fluctuations caused by poor pancreatic β‐cell function might lead to increased oxidative stress, inflammation, dysfunction and altered gene expression in endothelial cells 6 , 36 , thus increasing the risk of DR. Intensive hypoglycemia can improve pancreatic β‐cell function and, thus, reduce the risk of DR. The UK Prospective Diabetes Study found that intensive blood glucose control with sulfonylurea or insulin can significantly reduce the risk of microvascular complications in patients with type 2 diabetes, and the risk of retinopathy can be reduced by 21% 8 . The Epidemiology of Diabetes Interventions and Complications study also showed a continued reduction in the risk of progressive retinopathy after the trial ended 37 .
The present study had several limitations. First, this was a cross‐sectional study and, therefore, no causal inferences can be drawn; further studies need to be prospective. Second, this was a single‐center study, and was carried out in a northeastern Chinese population, so the findings should be carefully extrapolated to other populations with different genetic backgrounds.
In conclusion, the present study shows for the first time that OGTT islet function‐related markers in diabetes patients are closely associated with DR. OGTT islet function‐related markers are independent risk factors for DR, especially when they do not exceed the inflection point, and a decrease in islet function‐related markers predicts an increase in the incidence of DR in diabetes patients.
In conclusion, the present study showed that the AUCC, which responds to pancreatic β‐cell secretory function, was negatively correlated with DR. A non‐linear relationship between AUCC and DR was observed in type 2 diabetes. In patients with type 2 diabetes, it is necessary to evaluate pancreatic β‐cell function based on AUCC as early as possible to intervene and reduce blood glucose exposure, and prevent the occurrence and development of DR at an early stage.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: This study was performed inaccordance with the principles of the Declaration of Helsinkiand approved by the institutional ethics review board of The First Affiliated Hospital of Harbin Medical University.
Informed consent: Informed consent was not required for the study.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
REFERENCES
- 1. Gerstein Hertzel C, Naveed S, Julio R, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021; 385: 896–907. [DOI] [PubMed] [Google Scholar]
- 2. Motala Ayesha A, Claude MJ, Kaushik R, et al. Type 2 diabetes mellitus in sub‐Saharan Africa: Challenges and opportunities. Nat Rev Endocrinol 2022; 18: 219–229. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation . IDF Diabetes Atlas, 9th edn. Brussels: International Diabetes Federation, 2019. [Google Scholar]
- 4. Rhys W, Suvi K, Belma M, et al. Global and regional estimates and projections of diabetes‐related health expenditure: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract 2020; 162: 108072. [DOI] [PubMed] [Google Scholar]
- 5. Catherine G, Shing KC, Saadia A, et al. Long‐term glycemic variability and risk of adverse outcomes: A systematic review and meta‐analysis. Diabetes Care 2015; 38: 2354–2369. [DOI] [PubMed] [Google Scholar]
- 6. Ourania P, Marios M, Alexandros T, et al. Short‐term glycemic variability and its association with macrovascular and microvascular complications in patients with diabetes. J Diabetes Sci Technol, 2022: P1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bao S, Zhiying L, Jiecan Z. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol 2021; 20: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 9. Vollenbrock Charlotte E, Dick M, Pim D, et al. Fasting and meal‐stimulated serum C‐peptide in long‐standing type 1 diabetes mellitus. Diabet Med 2023; 40: e15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andreas F, Martin H, Andreas P, et al. Considering insulin secretory capacity as measured by a fasting C‐peptide/glucose ratio in selecting glucose‐lowering medications. Exp Clin Endocrinol Diabetes 2022; 130: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ismail Heba M, Ping X, Libman Ingrid M, et al. The shape of the glucose concentration curve during an oral glucose tolerance test predicts risk for type 1 diabetes. Diabetologia 2018; 61: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of medical Care in Diabetes‐2021. Diabetes Care 2021; 44(Suppl 1): S15–S33. [DOI] [PubMed] [Google Scholar]
- 13. Deponti GJ, Romero BJP, Lucena Moises M, et al. Efficacy of smartphone‐based retinal photography by undergraduate students in screening and early diagnosing diabetic retinopathy. Int J Retina Vitreous 2022; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharmistha RC, Thomas Rebecca L, Dunseath Gareth J, et al. Incidence of diabetic retinopathy in newly diagnosed subjects with type 2 diabetes mellitus over 5 years: Contribution of Β‐cell function. J Diabetes Complications 2022; 36: 108028. [DOI] [PubMed] [Google Scholar]
- 15. Sharmistha RC, Thomas Rebecca L, Dunseath Gareth J, et al. Diabetic retinopathy in newly diagnosed subjects with type 2 diabetes mellitus: Contribution of β‐cell function. J Clin Endocrinol Metab 2016; 101: 572–580. [DOI] [PubMed] [Google Scholar]
- 16. Ismail Heba M, Carmella E‐M, DiMeglio LA, et al. Associations of HbA1c with the timing of C‐peptide responses during the oral glucose tolerance test at the diagnosis of type 1 diabetes. Pediatr Diabetes 2019; 20: 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ernesto M, Bolli Geremia B, Frier Brian M, et al. C‐peptide determination in the diagnosis of type of diabetes and its management: A clinical perspective. Diabetes Obes Metab 2022; 24: 1912–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer Jerry P, Alexander FG, Greenbaum Carla J, et al. C‐peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta‐cell function: Report of an ADA workshop, 21–22 October 2001. Diabetes 2004; 53: 250–264. [DOI] [PubMed] [Google Scholar]
- 19. Andrea T, Alexandra K‐W, Giovanni P. Insulinogenic indices from insulin and C‐peptide: Comparison of beta‐cell function from OGTT and IVGTT. Diabetes Res Clin Pract 2006; 72: 298–301. [DOI] [PubMed] [Google Scholar]
- 20. Andrea M, Julio R, Ma X, et al. Optimized human regular U‐500 insulin treatment improves β‐cell function in severely insulin‐resistant patients with long‐standing type 2 diabetes and high insulin requirements. Endocr Pract 2015; 21: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 21. Shin SJ, Lee YJ, Hsaio PJ, et al. Relationships between beta‐cell function and diabetic duration and albuminuria in type 2 diabetes mellitus. Pancreas 1997; 14: 192–198. [DOI] [PubMed] [Google Scholar]
- 22. Qianna Z, Ning Y, Xiangjun C, et al. Total body adiposity, triglycerides, and leg fat are independent risk factors for diabetic peripheral neuropathy in Chinese patients with type 2 diabetes mellitus. Endocr Pract 2019; 25: 270–278. [DOI] [PubMed] [Google Scholar]
- 23. Lingshu W, Peng L, Aixia M, et al. C‐peptide is independently associated with an increased risk of coronary artery disease in T2DM subjects: A cross‐sectional study. PloS One 2015; 10: e0127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: Risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001; 44: 156–163. [DOI] [PubMed] [Google Scholar]
- 25. Carlo SF, Clara PP, Aldo G, et al. (eds). High HDL cholesterol: A risk factor for diabetic retinopathy? Findings from NO BLIND study. Diabetes Res Clin Pract 2019; 150: 236–244. [DOI] [PubMed] [Google Scholar]
- 26. Lingli S, Xiaohua W, Tianye G, et al. Nicotine triggers islet β cell senescence to facilitate the progression of type 2 diabetes. Toxicology 2020; 441: 152502. [DOI] [PubMed] [Google Scholar]
- 27. Paulina N, Irena K. Changes in the pancreas caused by different types of hypertension. Acta Biochim Pol 2017; 64: 591–595. [DOI] [PubMed] [Google Scholar]
- 28. Drew Brian G, Kerry‐Anne R, Duffy Stephen J, et al. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol 2012; 8: 237–245. [DOI] [PubMed] [Google Scholar]
- 29. Ook CJ, Hyeok CD, Jin CD, et al. Relationship between serum C‐peptide level and diabetic retinopathy according to estimated glomerular filtration rate in patients with type 2 diabetes. J Diabetes Complications 2015; 29: 350–355. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki K, Watanabe K, Motegi T, et al. High prevalence of proliferative retinopathy in diabetic patients with low pancreatic B‐cell capacity. Diabetes Res Clin Pract 1989; 6: 45–52. [DOI] [PubMed] [Google Scholar]
- 31. Emma A, Petter S, Annemari K, et al. Novel subgroups of adult‐onset diabetes and their association with outcomes: A data‐driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6: 361–369. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz Stanley S, Solomon E, Corkey Barbara E, et al. The time is right for a new classification system for diabetes: Rationale and implications of the β‐cell‐centric classification schema. Diabetes Care 2016; 39: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhi‐Qiang H, Hong‐Liang L, Ling G, et al. Involvement of chronic stresses in rat islet and INS‐1 cell glucotoxicity induced by intermittent high glucose. Mol Cell Endocrinol 2008; 291: 71–78. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Huijun Y, Ye H, et al. Panax Quinquefolius saponin of stem and leaf attenuates intermittent high glucose‐induced oxidative stress injury in cultured human umbilical vein endothelial cells via PI3K/Akt/GSK‐3 β pathway. Evid Based Complement Alternat Med 2013; 2013: 196283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Z, Haichen S, Shuang L, et al. Glycemic variability is associated with vascular calcification by the markers of endoplasmic reticulum stress‐related apoptosis, Wnt1, galectin‐3 and BMP‐2. Diabetol Metab Syndr 2019; 11: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michael Y, Steven J, Craig Maria E, et al. Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. J Clin Endocrinol Metab 2022; 107: e2221–e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group , Lachin John M, Saul G, et al. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000; 342: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]


