Abstract

Ionic memristor devices are crucial for efficient artificial neural network computations in neuromorphic hardware. They excel in multi-bit implementation but face challenges like device reliability and sneak currents in crossbar array architecture (CAA). Interface-type ionic memristors offer low variation, self-rectification, and no forming process, making them suitable for CAA. However, they suffer from slow weight updates and poor retention and endurance. To address these issues, the study demonstrated an alkali ion self-rectifying memristor with an alkali metal reservoir formed by a bottom electrode design. By adopting Li metal as the adhesion layer of the bottom electrode, an alkali ion reservoir was formed at the bottom of the memristor layer by diffusion occurring during the atomic layer deposition process for the Na:TiO2 memristor layer. In addition, Al dopant was used to improve the retention characteristics by suppressing the diffusion of alkali cations. In the memristor device with optimized Al doping, retention characteristics of more than 20 h at 125 °C, endurance characteristics of more than 5.5 × 105, and high linearity/symmetry of weight update characteristics were achieved. In reliability tests on 100 randomly selected devices from a 32 × 32 CAA device, device-to-device and cycle-to-cycle variations showed low variation values within 81% and 8%, respectively.
Keywords: alkali cation, reservoir layer, artificial neural networks, synaptic response, adhesion layer
Introduction
The resistive switching (RS) characteristics resulting from the state change of materials, including ionic memristor, ferroelectric tunnel junction (FTJ), phase-change memory, and magnetic tunnel junction (MTJ), have been considered promising candidates as next-generation nonvolatile memories (NVMs).1−10 They have in common the characteristic to express nonvolatile multi-bit (or analog) resistance states, depending on the magnitude, duration time, and number of programming pulses, used in a two-terminal structure, enabling them to be utilized as synapse devices in neuromorphic hardware. The matrix-vector multiplier using memristors with a crossbar-array architecture (CAA) is the foremost scheme for chip implementation (analog accelerator) of artificial neural networks (ANNs).11−14 Among the promising memristors, the ionic memristor is particularly suited for neuromorphic and analog computation in terms of switching speed, simple structure (metal/insulator/metal), availability of various materials (transition metal oxides, TMOs), and multi-bit capability (larger on/off ratio).13−16 However, long-standing issues such as the sneak current issue in CAA and reliability issues (such as device-to-device and cycle-to-cycle variation) caused by the RS mechanism “defect control” have hindered neuromorphic hardware implementation.17−19
The RS characteristics of ionic memristors observed in TMOs are classified into filament-type and interface-type.6,20 In the case of filament-type RS, it is mainly observed in TMO thin films with high resistance. When a high voltage is applied with a compliance current, a conductive path (called filament) consisting of a large amount of oxygen vacancy inside of the oxide film can be formed by soft breakdown, and then a part of the conductive filament is reversibly broken/recovered by an appropriate programming voltage to implement the RS characteristics.6,19,20 On the other hand, the interface-type RS is a characteristic observed in relatively lower resistance TMO films, which induces a change in the interface resistance between the electrode and the oxide by redistribution of oxygen vacancies in the TMO film when the programming voltage is applied.6,19,20 Therefore, a filament-type memristor can be considered a small memristor in an insulating thin film and has a parallel structure of a capacitor and a memristor. Filament-type memristors have the disadvantage of requiring an essential filament-forming process, but they generally exhibit faster switching speeds and stable retention and endurance characteristics compared to interface-type memristors.17−20 And, a hallmark of the RS behavior of filament-type memristors is the abrupt resistance change characteristic during the break/recovery of local conducting filaments. This characteristic is due to the fact that the resistance change behavior occurs within a very small filament, resulting in a large amount of current passing through the narrow filament and a fast switching speed. In the past, filament-type memristors have been mainstream for NVM (resistive RAM) applications that require only 1-bit implementation,21−23 but recently, interface-type memristor devices that exhibit gradual switching characteristics have attracted attention in neuromorphic hardware applications that require multi-bit implementation.1,10,11,13,14 Since filament-type memristors have difficulty controlling filament formation and ensuring uniformity, the device-to-device and cycle-to-cycle variation is larger than that of interface-type memristors. In addition, because they exhibit an Ohmic conduction regime in the low-resistance state (LRS), a cell selector such as a transistor or ovonic threshold switching (OTS) device is essential to avoid errors caused by the sneak current in the CAA.24−26 Filament-type memristors, which typically demand operating currents of the mA range for programming, require a relatively large channel of transistors for a cell selector, which is disadvantageous in terms of integration. In addition, when OTS and memristors are stacked in a series structure, the compatibility issue in the operating voltage and current of the OTS and memristor devices still hinders reliability. Abrupt overvoltage or overcurrent delivered to the memristor layer during switching of the OTS element causes damage such as hard breakdown or irreversible RS. On the other hand, interface-type memristors utilize interface resistance, so self-rectifying RS can be implemented by asymmetric interface configuration, which can implement selector-less CAA. However, the interface-type memristor is relatively inferior to the filament-type memristor in terms of weight update (switching) speed, retention, and endurance (deterioration by oxygen vacancy clustering), and these properties should be improved.27−31 One of the solutions for this is to use alkali metal cations with high mobility in solids, such as Li and Na, instead of oxygen anion.10,32 Highly mobile alkali cations are expected to offer improvement in switching speed, and simultaneity, it is disadvantageous for retention characteristics. Therefore, in order to improve the retention characteristics, we need to consider introducing a diffusion barrier to prevent the redistribution of alkali ions in the oxide matrix by the program voltage from returning to equilibrium or a reservoir layer that can safely store alkali cations.
The introduction of alkali metals in the fabrication process for on-chip analog accelerators may pose compatibility issues with CMOS processes. The CAA is formed in the back end of line (BEOL) process. At this stage, the insertion of an alkali metal layer on top of the completed CMOS devices in the front end of line (FEOL) could lead to diffusion concerns into the transistor (selector) components beneath each synapse cell. However, unlike in active arrays, the passive array with self-rectifying memristors eliminates the need for transistors (selectors) beneath each synapse cell, thereby mitigating potential issues with CMOS devices by diffusion. Additionally, introducing a diffusion barrier can minimize the spread of alkali ions. In the case of cross-contamination issues in the process, another consideration could be combining the drive chip and the synapse array devices, manufactured separately, through chip bonding. We expect such methods to circumvent compatibility issues with the CMOS process.
In general, the redox phenomenon of the electrodes at the interface of the memristor oxide layer and the electrodes can also contribute and help to induce RS,33−35 but in order to avoid the redox effect with the electrodes and consider only the ion migration effect inside of the TMO thin film (or to ensure reliability), novel metals such as Au and Pt (not easily redoxable) are used. However, novel metals require an adhesion layer due to their weak adhesion to the oxide (insulating substrate), and the adhesion layer diffuses during the device fabrication process, causing the oxide layer to be unintentionally doped with the adhesion layer material.36 In this study, we propose a strategy for fabricating an alkali ion-based memristor device by utilizing the doping effect of the adhesion layer. Since lithium is rapidly oxidized and forms stable LixO when exposed to the atmosphere, a high-temperature process is required to dope the TMO (memristor) film. However, when the Li metal is used as an adhesion layer of the BE, it can be safely protected from oxidation by the Au (BE) layer until the oxide film (memristor layer) is deposited, and at the same time, it can be effectively doped locally by diffusion during the memristor deposition process (supplied to the oxide layer). In this case, the asymmetric interface of the TE and BE is formed, which facilitates the implementation of a self-rectifying memristor with high selectivity, and the diffused Li ions form a Li reservoir layer at the bottom interface, which can be expected to improve the endurance characteristics similar to previous reports such as bilayer oxide memristor devices (active layer/oxygen reservoir layer).37,38 We have previously reported highly reliable memristor characteristics with in-situ ALD-grown Na:TiO2 memristors using NaOH aqueous solution as a homemade reactant.10 In this study, we adopted the Na:TiO2 as the memristor layer to maximize the self-rectifying characteristics. (See Figure S1). In addition, we performed a systematic experiment on the memristor characteristics according to the amount of Al doping to suppress the diffusion of alkali cations in the TiO2 matrix (for the retention characteristic). As a result, with the increase of Al doping amount, the retention characteristics were improved, but simultaneously, the operating current and switching speed decreased. We also observed that Al doping changes the linearity and symmetry of the long-term potentiation/depression (LTP/LTD) curve under fixed weight update pulse conditions. Although there was a trade-off between switching speed and retention characteristics, the controllability of retention characteristics, switching speed, and linearity and symmetry of weight update by the Al doping can be useful control factors to implement device characteristics required for the neuromorphic hardware. As a result, retention characteristics were improved up to 20 h at 125 °C and +1 V reading conditions, and no degradation was observed in the 550,000 cycles endurance test.
Results and Discussion
Figure 1a represents the atomic layer deposition (ALD) supercycle configured to grow Al,Na:TiO2 in this experiment. The Ti cycles in a supercycle consisted of n cycles of [titanium isopropoxide (TTIP) pulse 1s → Ar purge 5s → reactant pulse 1s → Ar purge 5s] for Na:TiO2 and 1 cycle of [trimethylaluminum (TMA) pulse 1s → Ar purge 5s → reactant pulse 1s → Ar purge 5s] for Al doping. Al cycles were organized in the ratio of 1:0, 83:1, 41:1, and 27:1, and the numbers of Al cycles were 0, 3, 6, and 9 within a total of 250 cycles to complete four different Al,Na:TiO2 memristors of equal thickness. (Hereafter, the four samples are referred to as Al 0, Al 3, Al 6, and Al 9.) Aqueous NaOH solution was used as a homemade reactant in all the processes to induce in-situ Na doping during the film growth process.10Figure 1b shows a schematic (left) and a scanning transmission electron microscope (STEM) image (right) of the completed device structure, which is fabricated as a Au(80 nm)/Li(5 nm)/Al,Na:TiO2 (18 nm)/Au(40 nm)/Li(5 nm) structure on a SiO2 (300 nm)/p-Si substrate. Here, the Li adhesion layer underneath the BE is diffused out to the top of the BE and doped on the TiO2 bottom interface in a 250 °C ALD process of 2 h. In the bright field STEM image in Figure 1b (light elements are shown as bright, and heavy elements are shown as dark), the Li adhesion layer between the Au BE and the SiO2 substrate is identified. In addition, a large amount of bright-colored excess Li or Na elements was also identified at the top and bottom interfaces of the TiO2 layer. In the memristor device, these two regions serve as an alkali metal reservoir, and the TiO2 layer acts as a diffusion barrier, which provides stable storage for Li ions redistributed by the program voltage (accumulated at the interface) to improve the retention characteristics and endurance characteristics. Figure 1c is a schematic illustration of the doping process of the Li adhesion layer in the Al,Na:TiO2 growth process and the defect species that can be induced. As shown in the figure, the three doped elements, Li, Al, and Na, can occupy the Ti site (substitutional defects: Li’’’Ti, Al’Ti, Na’’’Ti) or interstitial sites (interstitial defects: Na•i, Li•i, Al•••i) in the TiO2 matrix. Broken charge neutrality by those defects can also induce oxygen vacancies (V••O) and Ti defect occupying interstitial sites (Ti••••i) to compensate for charge valence.39 In the case of substitutional defects (Li’’’Ti, Al’Ti, Na’’’Ti), they form a negative charge defect that can accommodate (or trap) electrons in TiO2, as marked by the blue dashed line in Figure 1c, which acts as a p-type defect and shifts the Fermi level to the valence band maximum (VBM) in the band gap.40−45 On the other hand, the positive charge defect species, Li, Al, Na, and Ti occupying interstitial sites and the O vacancy (marked by green dashed line) such as Na•i, Li•i, Al•••i, Ti••••i, and V••O, can donate electrons as an n-type defect and shift the Fermi level to the conduction band minimum (CBM).40−45 This study considers the simplified eight types of defects listed above.
Figure 1.

(a) Schematic representation of the Al,Na:TiO2in-situ ALD process. One supercycle consists of nTi cycles composed of TTIP and homemade reactant and 1Al cycle composed of TMA and homemade reactant. The amount of Al doping was controlled by adjusting the nTi:1Al ratio. (b) Schematic diagram (left) and cross-sectional bright field STEM image (right) of the self-rectifying alkali-ion memristor device. The device with Au (80 nm)/Li (5 nm)/Al,Na:TiO2 (18 nm)/Au (40 nm)/Li (5 nm) structure was fabricated on SiO2 (300 nm)/p-Si substrate. The Li adhesion layer and Li Na reservoir are marked in the device schematic and bright field STEM image as black and red arrows, respectively. (c) Schematic representation of in-situ ALD growth (left) and schematic band diagram (right) of Al,Na:TiO2. From the Li adhesion layer, Li diffuses over the bottom electrode and dopes into the bottom of the Al,Na:TiO2 film. The eight defect species that can be considered in this process are indicated by dotted boxes, p-type defects (Li’’’Ti, Al’Ti, Na’’’Ti), and n-type defects (Na•i, Li•i, Al•••i, Ti••••i, V••O) indicated by blue and green dotted boxes, respectively. The p-type and n-type defects form energy levels at the bottom and top within the TiO2 bandgap, respectively, and shift the Fermi level in the CBM or VBM direction. The concentration of each defect species affects the direction of the Fermi level shift depending on the dominant species.
Figures 2a–d show the dynamic secondary ion mass spectrometry (SIMS) analysis results of Al 0, Al 3, Al 6, and Al 9 thin films measured without TE. First, as shown in the figure, the Li and Na cation concentration is high at the bottom interface between the TiO2 layer and the Au BE (marked by yellow boxes). This results from the Li adhesion layer under the BE being doped into the TiO2 film (bottom interface region) by diffusion during the 250 °C ALD process for TiO2 growth.36 Interestingly, in all films, the Ti and O concentrations at the bottom interface are relatively higher and lower, respectively, than inside the film. And on the other hand, in the case of the top interface, the Ti:O ratio is lower than the film inside. Therefore, while the concentration of Ti increases from the surface to the bottom interface, the concentration of O decreases. First, the high Ti concentration and low oxygen concentration at the bottom interface (indicated by the red arrows in the figure) can be interpreted as the result of Li in the adhesion layer diffusing into the bottom interface and occupying Ti sites (Li’’’Ti), leading to n-type defects Ti••••i and V••O.43 In addition, it was also observed that Na ions were concentrated together with Li at the bottom interface. As shown in Figure 2e, at the initial stage of in-situ Na:TiO2 ALD growth using NaOH aqueous solution, the diffused Li results in the presence of three surface species (which can be considered), Ti-OH, Li-OH, and O-Li. In this case, Na doping with desolvation of Na-H2O cluster is expected to be relatively promoted by the Li-OH and O-Li surface, which has a larger partial charge than Ti-OH. The electronegativities of Li, Ti, and O are 0.98, 1.54, and 3.44, respectively. The desolvation of Na ions is a dominant factor for Na doping (to form chemical bonds on the surface), because Na ions in the reactant are introduced into the chamber in a solvated form by water molecules. Therefore, Li-OH and Li-O with a large partial charge on the surface are expected to promote Na doping. As shown in Figures 2a–d, both the top and bottom interfaces are rich in Na and Li, but the concentration distribution of Ti and oxygen vacancies is different at the top and bottom interfaces. The difference in Ti:O ratio at the top and bottom interfaces can be understood that the bottom interface is due to the participation of Li and Na in the ALD growth, while the Na- and Li-rich at the top interface is caused by the exposure of the TiO2 thin film to the atmosphere after the process, rather than during the ALD process. Various molecules are known to be adsorbed on the (n-type) surface by the excess electrons in TiO2.46−50 In this study, we can expect oxygen absorption when taken out from a chamber of an Ar environment and exposed to the atmosphere after the film deposition is completed. From the excess oxygen detected on the surface, we can consider the adsorbed molecule species such as superoxo (O2–), peroxo (O22–), OOH, etc., or diffusion into the TiO2 surface and the resulting formation of a negatively charged surface.46−50 And these excess oxygens on the surface could lead to the diffusion of Na and Li cations to the surface. Figure S2a shows the XPS measurement results of Li 1s, and Li was detected on the surface of all thin film samples, in agreement with the SIMS analysis results. The compositional analysis of Ti, O, and Li based on the XPS results of the Al 0 sample showed that the at% of each element was 26.70 at%, 62.03 at%, and 11.27 at%, respectively. (Na was excluded from the quantitative analysis because it overlapped with the Ti Auger peak) Assuming the TiO2 composition of the thin film, this resulted in an approximately 1:1 excess of O and Li. Consequently, alkali cation reservoirs were already formed at both the top and bottom interfaces before the top electrode deposition. Due to the compositional gradient of Ti and O, the top interface formed a robust and high Schottky barrier with relatively excess oxygen, while the Schottky barrier at the bottom interface has a lower height and smaller depletion width by the excess Ti and O vacancies. In the case of Al, it was found that the amount of Al doping gradually increased with the increase of Al cycles in Al 0, Al 3, Al 6, and Al 9 films (Figure S2b shows the results of Al 2p measurements, which are in agreement with the SIMS results), and the amount of Al doping did not affect the distribution of Li, Na, Ti, and O in the film. Since Al was introduced as a single cycle, it can be confirmed that the AlO layer is not formed in a laminate structure but is diffused and uniformly distributed in the film.
Figure 2.
Dynamic SIMS depth profile results of (a) Al 0, (b) Al 3, (c) Al 6, and (d) Al 9/Au (40 nm)/Li (5 nm) samples. The yellow box in the figure indicates the bottom interfacial region of the Al,Na:TiO2 layer with the Au BE. (e) Schematic representation of three simplified surface species Ti-OH, Li-OH, and O-Li considering results by diffused Li. (f) O 1s and (g) Ti 2p XPS results of Al 0, Al 3, Al 6, and Al 9 samples. The dotted line and red arrow in the figures indicate the binding energy of the main peak in the Al 0 sample and the direction of the peak shift, respectively. (h) Schematic representation of the native oxygen vacancy present in TiO2 and (i) the passivation effect and additional oxygen vacancies created by Al doping (top) and the resulting band diagram (bottom). (Defects caused by alkali ions are excluded.)
Figures 2f and g are the O 1s and Ti 2p XPS measurement results. Table 1 summarizes the atomic percent of the four elements (Li, Al, Ti, O) and the ratio of oxygen vacancy peak intensity obtained from the XPS results of each sample. (Na is excluded due to overlap with Na 1s and Ti Auger peaks.) First, it is noted that the oxygen vacancy peak (labeled as Ov) exhibits a slight increase with the rise in aluminum (Al) doping, a consequence of the Schottky defect. While it varies depending on doping concentration and environment, it is generally reported that the Al’Ti defect, where the Al3+ substitutes the Ti4+ accompanied by an oxygen vacancy, is more dominant than the Al••••i defect that occupies the interstitial site.41,43,45 On the one hand, despite the increase in the n-type Ov defect, both the O 1s and Ti 2p XPS peaks, as illustrated in Figures 2f and g, undergo a red shift of approximately 0.10 eV, indicated by red arrows. This shift is attributed to the Al doping effect, which, akin to p-type doping, moves the Fermi level toward the conduction band minimum (CBM). Figures 2f and i provide simplified representations of the defect types and band diagrams before and after Al doping. The native defect Ov present in undoped TiO2 generates a shallow trap state in the bandgap and two electrons, as shown in Figure 2f. In thermal equilibrium, these two electrons are unable to function as carriers. However, when a strong electric field is applied to the 18-nm-thick film, mechanisms like trap-to-trap hopping or the Poole–Frenkel effect can mobilize them, allowing their participation in electrical conduction. When Ti neighboring an oxygen vacancy is substituted by Al, it can passivate the oxygen vacancies as the green dotted box. While Al doping creates new oxygen vacancies due to Schottky defects as the orange dotted box, it can also create a deep trap level in the bandgap that can accommodate the electron as the blue dotted box. Thus, Al doping acts as a p-type dopant by passivating oxygen vacancies and trapping electrons. This reduces the number of electrons available for transport in the film, increasing the resistivity, and shifts the Fermi level, increasing the band offset. Therefore, the red shift observed in the XPS results is a reflection of the above case.
Table 1. Atomic Percent of Al 0, Al 3, Al 6, and Al 9 Films as Derived from XPS Data, along with the Intensity Ratios of the Oxygen Vacancy Peaks Determined through O 1s Peak Fitting.
| Li 1s (at %) | Al 2p (at %) | Ti 2P (at %) | O 1s (at %) | Ov/(OL+Ov) (peak area) | |
|---|---|---|---|---|---|
| Al 0 | 11.44 | 1.14 | 26.27 | 61.15 | 0.126 |
| Al 3 | 11.12 | 1.35 | 26.34 | 61.19 | 0.140 |
| Al 6 | 11.63 | 1.65 | 25.73 | 60.99 | 0.145 |
| Al 9 | 10.81 | 1.76 | 26.20 | 61.23 | 0.151 |
Author: Figure S3a shows the X-ray diffraction (XRD) results of Al 0, Al 3, Al 6, and Al 9 thin films, where diffraction peaks of TiO2 films were not observed. In a previous study using Pt (BE)/Ti (adhesion), an anatase phase was observed in the same ALD process.10 However, in this study using Au (BE)/Li (adhesion) as the BE, the crystallinity was very weak, suggesting that the diffusion of Li hindered the crystallization of TiO2. From the Raman spectra in Figure S3b, very faint peaks at 140, 240, 340, and 432 cm–1 (indicated by red arrows) were observed in the Al 0 sample. Assuming the rutile phase, the peak observed at 140 cm–1 is the B1g peak, while the other three peaks (240, 340, and 432 cm–1) are observed in lithiated TiO2 (LixTiO2, 0.3 < x < 0.5) (or lithium titanate) and are related to the vibrations of TiO6, LiO6, and LiO4 (F2g, F2g, Eg), respectively.51,52 The Al 0 Raman spectrum reveals that Li is doped in the TiO2 layer and has a weak crystalline or weak ordered structure. These soft peaks were not observed in Al-doped thin films, which is interpreted as a result of Al doping increasing the structural disorder.53Figure S3c shows the fast-Fourier-transform (FFT) pattern and high-resolution transmission electron microscopy (HRTEM) image of the TiO2 layer (the center of the film) of the Al 0 sample, where a rutile phase was observed. In Al 3, Al 6, and Al 9 films, the same low crystallinity rutile phase was observed as in Al 0, but the change in crystallinity with Al doping was difficult to determine from the FFT pattern. In the case of diffraction spots of the (130) planes of the rutile (indicated by the yellow solid circle), diffraction spots with larger d-spacing were observed in the 90° rotation direction (indicated by the red circle). Their d-spacing was 1.45 and 1.52 Å, respectively, showing an increase in d-spacing of about 4.83%. (This change was also observed in the (210) plane.) This is interpreted as a result of Li cations occupying the interstitial sites of the rutile, causing lattice expansion and distortion (a and b lattice parameter changes).
Figure 3 shows the RS characteristics of Al 0, Al 3, Al 6, and Al 9 memristor devices measured for 10 cycles each (arrows and numbers indicate the sweep sequence), which shows the gradual resistance change characteristics without any forming process. First, the Al 0 device shows gradual resistance change characteristics with self-rectifying characteristics. This is due to the asymmetric top and bottom interfaces caused by the composition gradient of Ti and O, as shown in the SIMS analysis. In the case of the bottom interface, since Li is supplied during the thin film deposition process, it forms a Li–O bond (or Na–O) and occupies a Ti site, as shown in Figure 2. On the other hand, in the case of the top interface, it can be expected that the alkali cations have occupied the interstitial site by the electrochemical migration after thin film deposition and formed an alkali metal reservoir layer. Considering this pristine state, the migration of the alkali cations in the bottom reservoir is relatively difficult compared to the alkali cations of the top interface. Therefore, in the pristine state, the device starts with a high-resistance state (HRS). Figure 3e is a schematic illustration of the RS mechanism, where the numbers in the figure correspond to the states of the sweep sequence number shown in the I–V curve. Both the top and bottom interfaces participate in the RS event, representing the process of changing the resistance of the top and bottom interfaces by migration of alkali ions (this has been confirmed by dynamic SIMS and Schottky barrier measurement in our previous work).1,10,54 As shown 1 → 2 in Figure 3e, the alkali cation migrates to the bottom interface under positive voltage, reducing the Schottky barrier height and the depletion width of the bottom interface. In this case, the device switches to a low resistance state (LRS), and then when a negative voltage is applied (Sequence #3), the device exhibits HRS with an increased Schottky barrier of the top interface (reverse biased) by the reduced alkali cations, and the alkali cation moves back to the top interface, inducing LRS switching (Sequence #4).10 The top and bottom interfaces of the pristine state are in an equilibrium state with respect to chemical composition and charge valence. As described above, the redistribution of the alkali cation by the programming voltage breaks the charge valence at the top and bottom interfaces, and a force is exerted to return to equilibrium. This impairs retention in the interface-type memristor. In the device of this study, abundant negative charge defect species at the bottom interface, as shown in Figures 1 and 2, could make it easy to hold cations Li+ and Na+. (Role of alkali cation reservoir) And the middle of TiO2 layer acts as a diffusion barrier, Al dopant in TiO2 acts to increase the diffusion barrier, which can improve the retention characteristics. Figures 3a–d show that the device loses its self-rectifying characteristic (asymmetry of the DC curve) with an increase of Al dopant. This behavior is caused by the formation of the n-type defect states V••O accompanied by the p-type defect Ai’i, as shown in the band diagram on the right side of each DC curve, which facilitates the electron injection from the top electrode to the conduction band of TiO2. On the other hand, the current level at a positive voltage decreased with the increase of Al dopant, which means an increase of interface resistance at the bottom interface. As confirmed by the XPS data, the Fermi level shifted (redshift) toward the VBM with the increase of Al doping, which acts as a factor in increasing the Schottky barrier height at the bottom interface. In addition, the p-type defect Ai’i serves to trap the n-type carriers and reduce the carrier concentration in TiO2. We cannot exclude the structural factor that the increase in structural disorder caused by Al doping (as shown in Figure S3) could also contribute to the increase in resistance. As a result, Al doping increases the resistance of the memristor and weakens its self-rectifying characteristics. The selectivity values using 1/3 biasing scheme “I@Vop/I@–1/3 Vop” for each device Al 0, 3, 6, 9, extracted from self-rectifying asymmetric curves, were 2 × 104, 1 × 104, 2 × 103, and 3 × 101, respectively. Here, the measured current value of the noise level at –1/3Vop was used for the I@–1/3Vop, and it is expected that a higher selectivity will be observed in actual large-scale arrays. Low operating current is advantageous in terms of power consumption, but on the other hand, it also reduces the sensing margin, which is a trade-off dilemma for memristors that need to implement multi-bit. This is an important issue for neuromorphic hardware, which emphasizes energy-efficient computing. Consequently, the capacity to regulate the operating current by means of aluminum (Al) doping allows for the optimization of the aforementioned trade-off factors. Figure 3f presents the results of a three-dimensional graph depicting the depth profiles of O, Ti, Li, and Na from the surface to the bottom electrode interface for the Al 0 sample, based on SIMS data. As illustrated, O and Ti exhibit compositional gradients, which contribute to the self-rectifying characteristics. The distribution of alkali ions shows higher concentrations at the top and bottom interface regions of the thin film compared to the middle, indicating stability at these locations in the pristine state and suggesting the middle region acts as a barrier. The anticipated band diagram under this condition is represented on the left in Figure 3g, corresponding to the initial state or HRS@positive voltage. Upon application of a positive programming voltage, Na and Li dopants migrate toward the bottom interface, as depicted in the band diagram on the right in Figure 3g, resulting in a reduction in both the height of the Schottky barrier and the depletion width at the bottom interface which induce LRS@positive voltage.
Figure 3.

Resistive switching properties (10 cycles of DC sweep curves) of (a) Al 0, (b) Al 3, (c) Al 6, and (d) Al 9 devices fabricated by 5 um × 5 um crosspoint structure and schematic band diagram (right) of each devices. Above and below the band schematic are labeled n-type defects (red) and p-type defects (blue) species, respectively. The n-type (red) and p-type (blue) defect species are labeled above and below the band diagram. (e) A schematic representation of the RS mechanism of the self-rectifying alkai-ion memristor. Here, both the top and bottom interfaces change their interface resistance by the redistribution of alkali ions. (f) Three-dimensional compositional distribution graph for elements O, Ti, Li, and Na from the surface to the bottom interface, constructed based on SIMS data of the Al 0 sample. (g) Expected band diagrams for each state: HRS@positive voltage (left); LRS@positive voltage (right).
Figure 4 shows the retention characteristics of Al 0, Al 3, Al 6, and Al 9 memristor devices, which were measured with a read voltage of +1 V at 125 °C and read interval of 2 s for 20 h under relatively harsh conditions (to accelerate degradation) after entirely switching to HRS and LRS using DC sweep. As shown in the figure, an improvement in retention characteristics was clearly observed with increasing Al doping level, but at the same time, the on/off ratio with operating current decreased. In general, it is common for the resistance of LRS to increase gradually with time, but in the case of the LRS curve of Al 0, it was observed that the resistance decreased from 100 s to 2,000 s and then increased continuously. It is expected that highly mobile alkali cations, which are responsible for the resistance change in this study, exhibit unstable behavior in the 125 °C environment. Interestingly, the retention properties became increasingly stable as the amount of Al dopant increased. This is understood to be a consequence of Al doping hindering the diffusion of redistributed alkali ions back to their equilibrium state, as by the programming voltage. This phenomenon occurs in the same context as the observed decrease in the on/off ratio. Several factors influencing the diffusion properties of Li in solids are notable, including defect type, defect concentration, migration barrier, and structural symmetry. As shown in the XPS results, Al doping increases the oxygen vacancy and simultaneously passivates the oxygen vacancy, which would act as a factor to facilitate the diffusion of alkali ions from the defect perspective (lowering the migration barrier). On the contrary, an increase in structural disorder observed in Figure S3b acts as a factor hindering the migration of alkali ions. Therefore, based on the analytical data obtained in this study, it is reasonable to understand that the stability of retention properties with Al doping observed in Figure 4 is due to an increase in structural disorder. For more details, We measured Warburg impedance to investigate the Li (or Na) diffusion coefficient in Al 0, Al 3, Al 6, and Al 9 devices, and the measured Warburg plots (ω–1/2 vs ZIm) are given in Figure 4e. Due to the small electrode area (5 μm × 5 μm) and thin film thickness (18 nm), diffusional impedance was observed,55 and the Warburg coefficient (AW) was extracted in the high-frequency region. Utilizing the fitted slopes in conjunction with the Warburg coefficient–diffusivity relationship (see Eq. S1 in the Supporting Information for details), we calculated the Li (or Na) diffusion coefficients for the Al 0, Al 3, Al 6, and Al 9 devices, which are presented in Table 2. The derived values fell within the acceptable range (from 10–6 to 10–15 cm2/s in Rutile TiO2) found in the literature.56−58 (Note that this analysis focuses on the relative values between devices rather than the absolute values.) As shown in Table 2, the measured diffusion coefficient decreased with increased aluminum dopant. In devices from Al 0 (undoped) to Al 3 (3 cycles doping), there is a significant decrease in the diffusion coefficient, followed by relatively smaller reductions for Al 6 and Al 9. This trend is analogous to the changes in structural disorder observed in Raman spectra. In TiO2, the migration of Li ions is known to be strongly dependent on the density (Ti–O distance) and the crystal structure (symmetry, migration path, and the size of the migration channel). Therefore, the TiO2 rutile phase is recognized for having highly anisotropic Li ion migration paths. In the [110] directions, the zigzag path results in low mobility (∼10–15 cm2/s), while along the c-axis, the straight migration path leads to significantly higher Li ion mobility (∼10–6 cm2/s).56 Therefore, we infer that the structural disorder by Al doping reduces the diffusivity of Li (or Na) ions and contributes to improved retention characteristics. On the other hand, such a reduction in diffusivity adversely affects the weight update speed. To compare the weight update speeds, we measured the conductance change of Al 0, Al 3, Al 6, and Al 9 devices according to pulse width, as presented in Figure 4f. The amplitude was fixed at +5 V, and five different pulse widths (5 μs, 10 μs, 20 μs, 30 μs, 50 μs) were used for weight updates. The pulse width-dependent average change in conductance was obtained by utilizing the slope of the conductance vs pulse number plot (potentiation). As shown in Figure 4f, although the average conductance change rate of each device increases with the pulse width, it decreases significantly with the increase in Al doping amount. Therefore, while a decrease in diffusivity is effective in improving retention characteristics, it also reduces the weight update speed. However, this feature could be advantageous for implementing multi-bit operations if appropriately used. In this study, we cannot completely rule out the influence of oxygen ion migration in the observed resistance change characteristics. However, considering the measured diffusion coefficient values, these are approximately 10–1000 times higher than the range reported in the literature for oxygen ion mobility in Rutile TiO2 at room temperature.59−63 Consequently, under the applied RS voltage, the redistribution of defects within the thin film is likely dominated by the migration of Li (or Na) rather than oxygen migration. As previously mentioned, in filament-type devices, current flows through a very narrow conducting path, leading to Joule heating, which thermally assists the migration of oxygen (both current-driven and electric field-driven), making it relatively easier.64,65 (Ion migration strongly depends on temperature.) However, thermal assistance is significantly less for the interface-type devices in our study, considering the current passing through the entire interface during switching (below 10 μA). Therefore, it can be anticipated that the migration of Li, which has higher mobility, becomes the dominant factor in the resistive switching characteristics rather than the less mobile oxygen ions.
Figure 4.
Retention properties of (a) Al 0, (b) Al 3, (c) Al 6, and (d) Al 9 devices, measured by +1 V every 2 s at 125 °C, after fully switching to LRS and HRS using ±3 V DC sweep. (e) Warburg plots (ω–1/2 vs Zim) measured at room temperature for Al 0, Al 3, Al 6, and Al 9. The AW values marked on each plot are the Warburg coefficients obtained from the slopes. (f) Pulse width-dependent average change in conductance for Al 0, Al 3, Al 6, and Al 9 at 5 μs, 10 μs, 20 μs, 30 μs, and 50 μs. The average change in conductance was measured by employing pulses of varying widths (5 μs, 10 μs, 20 μs, 30 μs, and 50 μs) over 100 weight update cycles. For each cycle, conductance was measured using a read voltage of +1 V for potentiation, and the slope of 100 cycles of potentiation was used to determine the average conductance change.
Table 2. Diffusion Coefficients at Room Temperature Derived from Warburg Impedance Measurements for Al 0, Al 3, Al 6, and Al 9 Devices.
| Al 0 | Al 3 | Al 6 | Al 9 | |
|---|---|---|---|---|
| DLi (or Na) (cm2/s) | 1.68 × 10–12 | 8.96 × 10–13 | 7.65 × 10–13 | 6.96 × 10–13 |
Figures 5a–d show the results of weight update characteristics using 256 + (−) 5 V, 5 us pulses for long-term potentiation (LTP) (long-term depression, LTD) curves using a read voltage of +1 V for Al 0, Al 3, Al 6, and Al 9 memristor devices. The left of each figure represents the LTP/LTD curves repeated for 6 cycles in a single device (cycle-to-cycle variation), while the right shows the results of a polar plot (pulse number vs conductance) for the LTP/LTD curve obtained from one cycle in 20 different devices (device-to-device variation). As the Al dopant increases, the resistance change rate decreases for a constant programming pulse size, i.e., the switching speed decreases. The resistance change rate means the amount of ions that are redistributed, which depends on the magnitude of the write voltage, the amount of current, and the diffusivity of the ions that cause the resistance change. Therefore, Al dopant decreases the weight update rate because it decreases the amount of current passing through the memristor and the diffusivity of Li+ and Na+ cations. And it also affects the linearity and symmetry of the LTP/LTD curve, as shown in the figure, which means that Al dopant can control the linearity and symmetry of the LTP/LTD curve. In the polar plot, the blue and red curves represent LTP and LTD, respectively. (The device-to-device variation illustrated by violin plots is depicted in Figure S4.) The symmetry between each semicircle indicates the symmetry of the weight update, and the size distribution of each semicircle demonstrates the device-to-device variation. As the doping amount of Aluminum increases, the available conductance range decreases, but it is also possible to reduce the cycle-to-cycle and device-to-device variations. Considering the stochastic weight update characteristics due to ion migration, it can be anticipated that a higher switching ratio may also lead to a large fluctuation in weight update, which degrades reliability. Consequently, Al dopant loses in switching speed but gains in linearity, symmetry, retention, and reliability. Therefore, Al doping can be used as a control factor in the design of memristor devices.
Figure 5.
Six cycles of LTP/LTD curves obtained from 1 device (left) and polar plots (pulse number vs conductance) for the LTP/LTD curves obtained from 20 different devices (right) of (a) Al 0, (b) Al 3, (c) Al 6, and (d) Al 9 devices. 256 weight updates were performed using ±5 V amplitude and 5 μs width of pulse train and read using +1 V at each step. Simulation results of 28 × 28 (e) digit and (f) fashion MNIST classification of Al 0, Al 3, Al 6, and Al 9 devices derived based on the long-term potentiation and depression curves obtained in (a)–(d).
Figures 5e and f show the results of 28 × 28 (pixel) digit MNIST and 28 × 28 (pixel) fashion MNIST classification simulations based on the linearity and symmetry of Al 0, Al 3, Al 6, and Al 9 memristor devices. In 28 × 28 pixel handwritten digit MNIST and fashion MNIST image recognition, 28 × 28 pre-neurons corresponding to the 28 × 28 pixel images act as the input layer of our multilayer perceptron. The fully connected 300 hidden neurons in a single hidden layer and 10 output neurons correspond to 10 handwritten digits and clothing data sets. Each neural network was trained for up to 40 epochs, with each epoch training and testing 10,000 images at random. During the forward propagation stage, the activation of each neuron is propagated to the next neurons via synaptic weights and transferred through a nonlinear function. The sigmoid function serves as the mathematical activation unit in a neural network. Python was used to implement the pattern recognition processing described above. Recognition rates for 10 handwritten digits and 10 types of clothing images are shown for training based on 60,000 training data sets and 10,000 test data sets, respectively. The nonlinearity and asymmetry of the LTP/LTD and the accuracy (%) obtained by each device for Digit and Fashion MNIST are summarized in Table S1. As is well-known, the linearity and symmetry of the weight update are the most important factors determining the accuracy of neuromorphic hardware.13 As a result, the Al 6 device with the highest symmetry and linearity has the highest recognition rate.
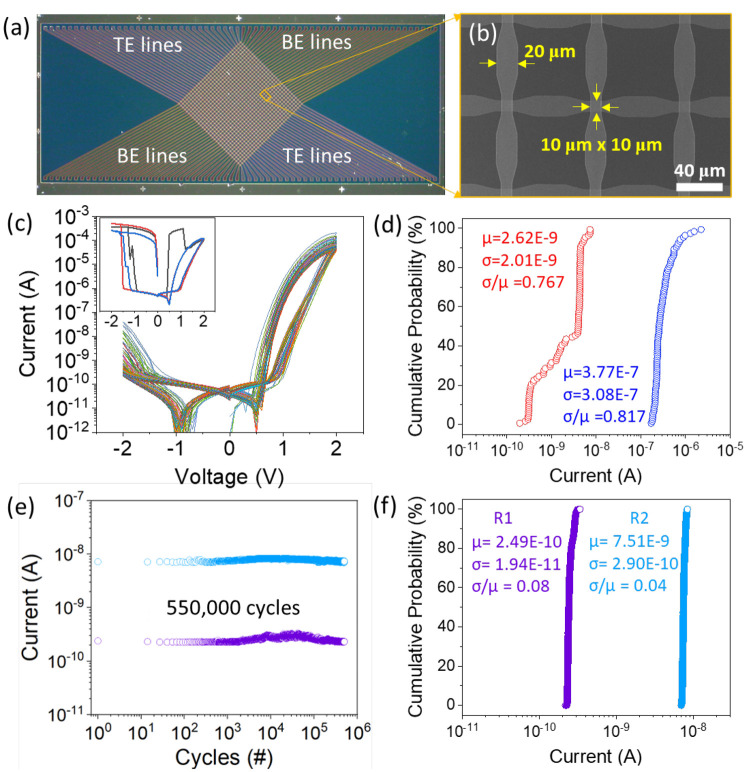
Figures 6a and b show an optical microscope image of a 32 × 32 crossbar array device fabricated based on an Al 6 device and a field emission scanning electron microscope (FE-SEM) image of the three cells enlarged, respectively. As shown in the SEM image, the area where the TEs and BEs are crossed is 10 μm × 10 μm, which is four times larger than the unit device in Figure 4 (5 μm × 5 μm). The thickness was reduced by 30% compared to the unit device through thickness optimization, and the DC sweep range was limited to ±2 V to avoid hard breakdown. The measured DC switching curves of 100 devices randomly selected from 1,024 devices in a 32 × 32 array are shown in Figure 6c. First, the figure shows that the stable RS characteristics were obtained without interference by sneak current from the self-rectifying characteristic (high selectivity). The base current was about 1.5 orders of magnitude higher than that of the unit device, which was confirmed by leakage within the switch matrix device. Figure 6c inset shows the I–V curves of the three devices that failed by hard breakdown out of 100 devices. Therefore, 97% of the 100 devices worked well without failure, and to check the device-to-device variation, the cumulative probability of each current level of HRS and LRS under the +1 V read condition is shown for the 97 working devices. The relative standard deviation for each resistance state (HRS and LRS) of the 97 devices in the crossbar arrays (not unit devices) was measured to be 76.7% and 81.7%, respectively. This variation is expected to be due to the uniformity issue of the TEs and BEs deposited by the thermal evaporator (compared to the ALD process of the memristor layer). Figure 6e shows the endurance characteristics measured under the conditions of programming pulse width 100 μs, ±5 V, DC read +1 V. A longer pulse width was used compared to the LTP/LTD measurement condition in Figure 5 to better distinguish between the two resistance states of R1 and R2. As a result, degradation was not observed up to 550,000 cycles. The cumulative probability obtained from the measured endurance data is shown in Figure 6f to confirm the cycle-to-cycle variation. The relative standard deviations for the R1 and R2 resistance states were measured to be 8% and 4%, respectively, indicating a very low cycle-to-cycle variation. In order to compare the performance of the self-rectifying alkali ion memristor devices proposed in this study, we listed the device structures with device performance indicators such as endurance, retention, switching speed, and test scale characteristics from previous reports of oxygen ion-based interface-type memristor devices in Table S2. The comparison table shows that the self-rectifying alkali-ion memristor of the Au/Li/Al,Na:TiO2/Au/Li device proposed in this study exhibits relatively improved RS characteristics.
Figure 6.
(a) Optical microscope image of a 32 × 32 crossbar array device fabricated using Al 6. (b) SEM image of three magnified device cells in a 32 × 32 crossbar array device. (c) ±2 V DC sweep curves measured on randomly 100 selected devices. The inset is the DC curves of the three devices that experienced hard breakdown among the 100 devices. (d) A cumulative probability plot of HRS and LRS extracted at read voltage +1 V from 97 devices, excluding the 3 failed devices. (e) Endurance data measured 5.5 × 105 times using switching condition pulse amplitude ±5 V, width 100 μs, and read condition +1 V (DC). (f) Cumulative probability plot extracted from endurance data for cycle-to-cycle variation.
Conclusions
Memristor devices are essential circuit elements for the implementation of neuromorphic hardware, but reliability issues due to sneak current and large cycle-to-cycle and device-to-device variations in the crossbar array structure have been long-standing obstacles. In this study, we developed an alkali ion-based self-rectifying memristor device and the fabrication method using the diffusion and doping effect of the adhesion layer. The device exhibited relatively high reliability in terms of cycle-to-cycle and device-to-device variation and sneak current issues. As the name “interface type” implies, the resistance change event occurs at the interface, and the control of the interface is very important. When we consider that the ALD process is generally performed at 150 °C ∼ 300 °C except for amorphous material deposition, interdiffusion occurring at the interface with the BE is a very important factor that should be considered for interface memristors. We derived the formation of alkali ion reservoir layers at the top and bottom interfaces by using the diffusion effect of an adhesion layer and obtained a self-rectifying memristive characteristic by the electrochemical migration of alkali cations. Since the alkali cations have high mobility in the solid, it exhibits faster switching speed and higher reliability than the oxygen anion-based interface type memristor.
Experimental Methods
Sample Preparation
The 5 nm Li metal (adhesion layer) and 40 nm thick Au metal (BE) were deposited on 2 μm thick patterned photoresist (for the BE)/ SiO2(300 nm)/ Si substrate using a thermal evaporator (in situ). The base pressure in the thermal evaporator chamber was less than 5.0 × 10–7 Torr, and the deposition rate was 1 Å/s for both Li and Au on a thickness monitor and controlled the thickness. After the deposition process, the substrate was taken out from the thermal evaporator chamber, and the lift-off process was performed using acetone to complete the BE. And Al, Na:TiO2 thin films (18 nm thick) were grown on the completed substrate having Au (40 nm)/Li (5 nm) BEs using an ALD process (IC-100 ITECHU, Inc. KR). NaOH dissolved in deionized water at concentrations of 30 wt % was used as the reactant. And TTIP (Ezchem Co., Ltd., Korea) and TMA (Ezchem Co., Ltd., Korea) were used as the titanium and aluminum precursors, respectively. The substrate temperature was held at 250 °C during the ALD growth, and the base pressure of the chamber was maintained at 0.375 Torr. The ALD process was performed as shown in Figure 1a (growth rate: 0.72 Å/cycle), while Ar was used as a carrier gas. Single devices of the cross-point structure were fabricated using top and bottom electrodes with a 5 μm line width to measure the electrical properties. For a 32 × 32 CAA memristor device, 13 nm thick Al 6 film was deposited on line patterned 32 bottom electrodes with a 10 μm line width. Finally, a 32 × 32 CAA was completed via patterning the top electrode Au (80 nm)/Li (5 nm) by using the lift-off method.
Characterizations and Measurements
The thickness of the grown films was measured using atomic force microscopy (AFM, XE-70, Park Systems Co.), and the crystallinity was investigated using the X-ray diffractometer (D8 Advance, Bruker)). Raman spectroscopy (inVia Raman microscopes, Renishaw) was performed to observe structural changes as a function of Al dopant. A cross-sectional STEM sample was prepared using a focused ion beam (FIB, FEI Helios Nano Lab 450 HP), and STEM images were obtained using a JEOL ARM 200F instrument at an accelerating voltage of 200 kV. Dynamic SIMS analysis for the depth profile of the Al,Na:TiO2 (18 nm)/ Au (40 nm)/ Li (5 nm)/ SiO2 (300 nm)/ p-Si structure (Al 0, 3, 6, 9 samples) was performed using IMS-7f auto magnetic sector SIMS (CAMECA). The depth profile data was acquired from the area of 150 μm × 150 μm. Electrical measurements of single memristor devices and memristor devices in 32 × 32 CAA were performed using a source meter (Keithley 2634B), arbitrary function generator (Tektronix AFG31152), pulse function arbitrary noise generator (Keysight 81150A), mixed domain oscilloscope (Tektronix MDO34 3-BW-350), customized switch matrix (multichannel device characterization system, SNM), and 64 pin multicontact probe card (MS-TECH) as shown in Figure S5. Impedance spectroscopy measurements for extracting the Warburg coefficient were conducted using an LCR meter (Wayne Kerr 4100), scanning the range from 1 MHz to 20 Hz, with a +1V amplitude.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Grant Nos. NRF-2022R1C1C1006337 and NRF- 2022M3F3A2A01044952). This work was partly supported by the GRRC program of Gyeonggi province (GRRCKYUNGHEE2023-B02), Sub-nm process development for functional thin film microstructure modulation to demonstrate next-generation semiconductor devices.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.3c11325.
Comparative data of DC sweep curves for undoped TiO2 and Na:TiO2 thin films; results of Li 1s, Al 2p XPS measurements for Al 0, Al 3, Al 6, and Al 9 films; XRD and Raman measurement results for Al 0, Al 3, Al 6, and Al 9 films, and FFT pattern and HRTEM image of the Al 0 film; Warburg coefficient–diffusivity relationship; device-to-device variation illustrated by violin plots; the device setup for the electrical characteristic analysis of a 32 × 32 crossbar array device; the nonlinearity and asymmetry parameters extracted from LTP/LTD curves; comparison table of the device performance with previous results (PDF)
Author Contributions
† B.-M.L. and Y.-M.L. contributed equally to this work. H.-S.L. conceived the idea and designed the experiments. B.-M.L. and Y.-M.L. conducted materials growth, device fabrication, spectroscopy measurements, and I–V measurements. M.K. and S.J.K. performed ANN simulations. S.K. conducted SEM and (S)TEM analysis. J.J.Y. and H.-S.L. supervised the research process. The manuscript was written with the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sangwan V. K.; Lee H.-S.; Bergeron H.; Balla I.; Beck M. E.; Chen K.-S.; Hersam M. C. Multi-terminal memtransistors from polycrystalline monolayer molybdenum disulfide. Nature 2018, 554, 500–504. 10.1038/nature25747. [DOI] [PubMed] [Google Scholar]
- Auciello O.; Scott J. F.; Ramesh R. The Physics of Ferroelectric Memories. Phys. Today 1998, 51, 22–27. 10.1063/1.882324. [DOI] [Google Scholar]
- Mathews S.; Ramesh R.; Venkatesan T. Ferroelectric Field Effect Transistor Based on Epitaxial Perovskite Heterostructures. Science 1997, 276, 238–240. 10.1126/science.276.5310.238. [DOI] [PubMed] [Google Scholar]
- Lee J.; Choi S.; Lee C.; Kang Y.; Kim D. GeSbTe deposition for the PRAM application. Appl. Surf. Sci. 2007, 253, 3969–3976. 10.1016/j.apsusc.2006.08.044. [DOI] [Google Scholar]
- Li Z.; Zhang S. Domain-wall dynamics driven by adiabatic spin-transfer torques. Phys. Rev. B 2004, 70, 024417. 10.1103/PhysRevB.70.024417. [DOI] [PubMed] [Google Scholar]
- Sawa A. Resistive switching in transition metal oxides. Mater. Today 2008, 11, 28–36. 10.1016/S1369-7021(08)70119-6. [DOI] [Google Scholar]
- Lee H.-S.; Choi S. G.; Park H.-H.; Rozenberg M. J. A new route to the Mott-Hubbard metal-insulator transition: Strong correlations effects in Pr0.7Ca0.3MnO3. Sci. Rep. 2013, 3, 1704. 10.1038/srep01704. [DOI] [Google Scholar]
- Lee H.-S.; Park H.-H. Tunneling Electroresistance Effect with Diode Characteristic for Cross-Point Memory. ACS Appl. Mater. Interfaces 2016, 8, 15476–15481. 10.1021/acsami.6b03780. [DOI] [PubMed] [Google Scholar]
- Waser R.; Dittmann R.; Staikov G.; Szot K. Redox-Based Resistive Switching Memories - Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. 10.1002/adma.200900375. [DOI] [PubMed] [Google Scholar]
- Kim S.-E.; Lee J.-G.; Ling L.; Liu S. E.; Lim H.-K.; Sangwan V. K.; Hersam M. C.; Lee H.-S. Sodium-Doped Titania Self-Rectifying Memristors for Crossbar Array Neuromorphic Architectures. Adv. Mater. 2022, 34, 2106913. 10.1002/adma.202106913. [DOI] [PubMed] [Google Scholar]
- Yang J. J.; Strukov D. B.; Stewart D. R. Memristive devices for computing. Nat. Nanotechnol. 2013, 8, 13–24. 10.1038/nnano.2012.240. [DOI] [PubMed] [Google Scholar]
- Lee H.-S.; Sangwan V. K.; Rojas W. A. G.; Bergeron H.; Jeong H. Y.; Yuan J.; Su K.; Hersam M. C. Dual-Gated MoS2Memtransistor Crossbar Array. Adv. Funct. Mater. 2020, 30, 2003683. 10.1002/adfm.202003683. [DOI] [Google Scholar]
- Kang S.-H.; Yang S.; Lee D.; Kim S.; Suh J.; Lee H.-S. Investigating Series and Parallel Oxide Memtransistors for Tunable Weight Update Properties. ACS Appl. Electron. Mater. 2023, 5, 3232–3240. 10.1021/acsaelm.3c00325. [DOI] [Google Scholar]
- Jeong D. S.; Kim K. M.; Kim S.; Choi B. J.; Hwang C. S. Memristors for Energy-Efficient New Computing Paradigms. Adv. Electron.Mater. 2016, 2, 1600090. 10.1002/aelm.201600090. [DOI] [Google Scholar]
- Yuan J.; Liu S. E.; Shylendra A.; Rojas W. A. G.; Guo S.; Bergeron H.; Li S.; Lee H.-S.; Nasrin S.; Sangwan V. K.; Trivedi A. R.; Hersam M. C. Reconfigurable MoS2Memtransistors for Continuous Learning in Spiking Neural Networks. Nano Lett. 2021, 21, 6432–6440. 10.1021/acs.nanolett.1c00982. [DOI] [PubMed] [Google Scholar]
- Jeong D. S.; Hwang C. S. Nonvolatile Memory Materials for Neuromorphic Intelligent Machines. Adv. Mater. 2018, 30, 1704729. 10.1002/adma.201704729. [DOI] [PubMed] [Google Scholar]
- Strukov D. B.; Snider G. S.; Stewart D. R.; Williams R. S. The missing memristor found. Nature 2008, 453, 80–83. 10.1038/nature06932. [DOI] [PubMed] [Google Scholar]
- Valov I.; Linn E.; Tappertzhofen S.; Schmelzer S.; van den Hurk J.; Lentz F.; Waser R. Nanobatteries in redox-based resistive switches require extension of memristor theory. Nat. Commun. 2013, 4, 1771. 10.1038/ncomms2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser R.; Dittmann R.; Staikov G.; Szot K. Redox-Based Resistive Switching Memories - Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. 10.1002/adma.200900375. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Lee S.; Noh T. W. Resistive switching phenomena: A review of statistical physics approaches. Appl. Phys. Rev. 2015, 2, 031303. 10.1063/1.4929512. [DOI] [Google Scholar]
- Jeong D. S.; Thomas R.; Katiyar R. S.; Scott J. F.; Kohlstedt H.; Petraru A.; Hwang C. S. Emerging memories: resistive switching mechanisms and current status. Rep. Prog. Phys. 2012, 75, 076502. 10.1088/0034-4885/75/7/076502. [DOI] [PubMed] [Google Scholar]
- Choi B. J.; Jeong D. S.; Kim S. K.; Rohde C.; Choi S.; Oh J. H.; Kim H. J.; Hwang C. S.; Szot K.; Waser R.; Reichenberg B.; Tiedke S. Resistive switching mechanism of TiO2 thin films grown by atomic-layer deposition. J. Appl. Phys. 2005, 98, 033715. 10.1063/1.2001146. [DOI] [Google Scholar]
- Kwon D.-H.; Kim K. M.; Jang J. H.; Jeon J. M.; Lee M. H.; Kim G. H.; Li X.-S.; Park G.-S.; Lee B.; Han S.; Kim M.; Hwang C. S. Atomic structure of conducting nanofilaments in TiO2 resistive switching memory. Nat. Nanotechnol. 2010, 5, 148–153. 10.1038/nnano.2009.456. [DOI] [PubMed] [Google Scholar]
- Shi L.; Zheng G.; Tian B.; Dkhil B.; Duan C. Research progress on solutions to the sneak path issue in memristor crossbar arrays. Nanoscale Adv. 2020, 2, 1811–1827. 10.1039/D0NA00100G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q.; Yang J. J. Memristive crossbar arrays for brain-inspired computing. Nat. Mater. 2019, 18, 309–323. 10.1038/s41563-019-0291-x. [DOI] [PubMed] [Google Scholar]
- Li C.; Hu M.; Li Y.; Jiang H.; Ge N.; Montgomery E.; Zhang J.; Song W.; Dávila N.; Graves C. E.; Li Z.; Strachan J. P.; Lin P.; Wang Z.; Barnell M.; Wu Q.; Williams R. S.; Yang J. J.; Xia Q. Analogue signal and image processing with large memristor crossbars. Nat. Electron. 2018, 1, 52–59. 10.1038/s41928-017-0002-z. [DOI] [Google Scholar]
- Li Q.; Yu S.; Yang P.; Wang Q.; Liu S. A large memory window and low power consumption self-rectifying memristor for electronic synapse. Electron. Lett. 2023, 59, e12717 10.1049/ell2.12717. [DOI] [Google Scholar]
- Jeon K.; Kim J.; Ryu J. J.; Yoo S.-J.; Song C.; Yang M. K.; Jeong D. S.; Kim G. H. Self-rectifying resistive memory in passive crossbar arrays. Nat. Commun. 2021, 12, 2968. 10.1038/s41467-021-23180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Long S.; Liu Q.; Lv H.; Liu M. Resistive Switching Performance Improvement via Modulating Nanoscale Conductive Filament, Involving the Application of Two-Dimensional Layered Materials. Small 2017, 13, 1604306. 10.1002/smll.201604306. [DOI] [PubMed] [Google Scholar]
- Kim K. M.; Jeong D. S.; Hwang C. S. Nanofilamentary resistive switching in binary oxide system; a review on the present status and outlook. Nanotechnology 2011, 22, 254002. 10.1088/0957-4484/22/25/254002. [DOI] [PubMed] [Google Scholar]
- Schmitt R.; Spring J.; Korobko R.; Rupp J. L. M. Design of Oxygen Vacancy Configuration for Memristive Systems. ACS Nano 2017, 11, 8881–8891. 10.1021/acsnano.7b03116. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosillo J. C.; Balaish M.; Hood Z. D.; Nadkarni N.; Fraggedakis D.; Kim K. J.; Mullin K. M.; Pfenninger R.; Bazant M. Z.; Rupp J. L. M. Lithium-Battery Anode Gains Additional Functionality for Neuromorphic Computing through Metal-Insulator Phase Separation. Adv. Mater. 2020, 32, 1907465. 10.1002/adma.201907465. [DOI] [PubMed] [Google Scholar]
- Li S.-L.; Shang D. S.; Li J.; Gang J. L.; Zheng D. N. Resistive switching properties in oxygen-deficient Pr0.7Ca0.3MnO3 junctions with active Al top electrodes. J. Appl. Phys. 2009, 105, 033710. 10.1063/1.3073987. [DOI] [Google Scholar]
- Lee H.-S.; Park H.-H.; Rozenberg M. J. Manganite-based memristive heterojunction with tunable non-linear I-V characteristics. Nanoscale 2015, 7, 6444–6450. 10.1039/C5NR00861A. [DOI] [PubMed] [Google Scholar]
- Asanuma S.; Akoh H.; Yamada H.; Sawa A. Relationship between resistive switching characteristics and band diagrams of Ti/Pr1-xCaxMnO3 junctions. Phys. Rev. B 2009, 80, 235113. 10.1103/PhysRevB.80.235113. [DOI] [Google Scholar]
- Yang J. J.; Strachan J. P.; Xia Q.; Ohlberg D. A. A.; Kuekes P. J.; Kelley R. D.; Stickle W. F.; Stewart D. R.; Medeiros-Ribeiro G.; Williams R. S. Diffusion of Adhesion Layer Metals Controls Nanoscale Memristive Switching. Adv. Mater. 2010, 22, 4034–4038. 10.1002/adma.201000663. [DOI] [PubMed] [Google Scholar]
- Lee M.-J.; Lee C. B.; Lee D.; Lee S. R.; Chang M.; Hur J. H.; Kim Y.-B.; Kim C.-J.; Seo D. H.; Seo S.; Chung U.-I.; Yoo I.-K.; Kim K. A fast, high-endurance and scalable non-volatile memory device made from asymmetric Ta2O5-x/TaO2-x bilayer structures. Nat. Mater. 2011, 10, 625–630. 10.1038/nmat3070. [DOI] [PubMed] [Google Scholar]
- Liu J.; Yang H.; Ji Y.; Ma Z.; Chen K.; Zhang X.; Zhang H.; Sun Y.; Huang X.; Oda S. An electronic synaptic device based on HfO2TiOx bilayer structure memristor with self-compliance and deep-RESET characteristics. Nanotechnology 2018, 29, 415205. 10.1088/1361-6528/aad64d. [DOI] [PubMed] [Google Scholar]
- Alvarez Roca R.; Guerrero F.; Eiras J. A.; Guerra J.D.S. Structural and electrical properties of Li-doped TiO2 rutile ceramics. Ceram. Int. 2015, 41, 6281–6285. 10.1016/j.ceramint.2015.01.052. [DOI] [Google Scholar]
- Stashans A.; Lunell S.; Bergström R.; Hagfeldt A.; Lindquist S.-E. Theoretical study of lithium intercalation in rutile and anatase. Phys. Rev. B 1996, 53, 159–170. 10.1103/PhysRevB.53.159. [DOI] [PubMed] [Google Scholar]
- Gesenhues U.; Rentschler T. Crystal Growth and Defect Structure of Al3+-Doped Rutile. J. Solid State Chem. 1999, 143, 210–218. 10.1006/jssc.1998.8088. [DOI] [Google Scholar]
- Yong Z.; Qi Y.-Y.; Hu Y.-H.; Pei L. Defect-induced ferromagnetism in rutile TiO2: A first-principles study. Chin. Phys. B 2013, 22, 127101. 10.1088/1674-1056/22/12/127101. [DOI] [Google Scholar]
- Roose B.; Pathak S.; Steiner U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. 10.1039/C5CS00352K. [DOI] [PubMed] [Google Scholar]
- Liu D.; Li S.; Zhang P.; Wang Y.; Zhang R.; Sarvari H.; Wang F.; Wu J.; Wang Z.; Chen Z. D. Efficient planar heterojunction perovskite solar cells with Li-doped compact TiO2 layer. Nano Energy 2017, 31, 462–468. 10.1016/j.nanoen.2016.11.028. [DOI] [Google Scholar]
- Liu Y.; Sang B.; Hossain M. A.; Gao K.; Cheng H.; Song X.; Zhong S.; Shi L.; Shen W.; Hoex B.; Huang Z. A novel passivating electron contact for high-performance silicon solar cells by ALD Al-doped TiO2. Sol Energy 2021, 228, 531–539. 10.1016/j.solener.2021.09.083. [DOI] [Google Scholar]
- Tian H.; Xu B.; Fan J.; Xu H. Intrinsic Role of Excess Electrons in Surface Reactions on Rutile TiO2 (110): Using Water and Oxygen as Probes. J. Phys. Chem. C 2018, 122, 8270–8276. 10.1021/acs.jpcc.7b12451. [DOI] [Google Scholar]
- Xiong F.; Yin L.-L.; Li F.; Wu Z.; Wang Z.; Sun G.; Xu H.; Chai P.; Gong X.-Q.; Huang W. Anatase TiO2(001)-(1 × 4) Surface Is Intrinsically More Photocatalytically Active than the Rutile TiO2(110)-(1 × 1) Surface. J. Phys. Chem. C 2019, 123, 24558–24565. 10.1021/acs.jpcc.9b06319. [DOI] [Google Scholar]
- Setvín M.; Aschauer U.; Scheiber P.; Li Y.-F.; Hou W.; Schmid M.; Selloni A.; Diebold U. Reaction of O2 with subsurface oxygen vacancies on TiO2 anatase (101). Science 2013, 341, 988–991. 10.1126/science.1239879. [DOI] [PubMed] [Google Scholar]
- Setvin M.; Hulva; Parkinson G. S.; Schmid M.; Diebold U. Electron transfer between anatase TiO2 and an O2 molecule directly observed by atomic force microscopy. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E2556–E2562. 10.1073/pnas.1618723114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi Y.; Nosaka Y. OH Radical Formation at Distinct Faces of Rutile TiO2 Crystal in the Procedure of Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 23832–23839. 10.1021/jp408244h. [DOI] [Google Scholar]
- Bhatia A.; Hallot M.; Leviel C.; Roussel P.; Pereira-Ramos J.-P.; Lethien C.; Baddour-Hadjean R. Electrochemical Lithium Insertion into TiO2 Anatase ALD Thin Films for Li-Ion Microbatteries: An Atomic-Scale Picture Provided by Raman Spectroscopy. Adv. Mater. Interfaces 2023, 10, 2202141. 10.1002/admi.202202141. [DOI] [Google Scholar]
- Kim S.-M.; Kim S.; Ling L.; Liu S. E.; Jin S.; Jung Y. M.; Kim M.; Park H.-H.; Sangwan V. K.; Hersam M. C.; Lee H.-S. Linear and Symmetric Li-Based Composite Memristors for Efficient Supervised Learning. ACS Appl. Mater. Interfaces 2022, 14, 5673–5681. 10.1021/acsami.1c24562. [DOI] [PubMed] [Google Scholar]
- Islam M. N.; Podder J.; Hossain K. S.; Sagadevan S. Band gap tuning of p-type al-doped tio2 thin films for gas sensing applications. Thin Solid Films 2020, 714, 138382. 10.1016/j.tsf.2020.138382. [DOI] [Google Scholar]
- Yang J. J.; Borghetti J.; Murphy D.; Stewart D. R.; Williams R. S. A Family of Electronically Reconfigurable Nanodevices. Adv. Mater. 2009, 21, 3754–3758. 10.1002/adma.200900822. [DOI] [Google Scholar]
- Ho C.; Raistrick I. D.; Huggins R. A. Application of A-C Techniques to the Study of Lithium Diffusion in Tungsten Trioxide Thin Films. J. Electrochem. Soc. 1980, 127, 343–350. 10.1149/1.2129668. [DOI] [Google Scholar]
- Johnson O. W. One-Dimensional Diffusion of Li in Rutile. Phys. Rev. 1964, 136, A284–A290. 10.1103/PhysRev.136.A284. [DOI] [Google Scholar]
- Bach S.; Pereira-Ramos J. P.; Willman P. Investigation of lithium diffusion in nano-sized rutile TiO2 by impedance spectroscopy. Electrochim. Acta 2010, 55, 4952–4959. 10.1016/j.electacta.2010.03.101. [DOI] [Google Scholar]
- Arrouvel C.; Peixoto T. C.; Valerio M. E. G.; Parker S. C. Lithium migration at low concentration in TiO2 polymorphs. Comput. Theor. Chem. 2015, 1072, 43–51. 10.1016/j.comptc.2015.09.002. [DOI] [Google Scholar]
- Gruenwald T. B.; Gordon G. Oxygen diffusion in single crystals of titanium dioxide. J. Inorg. Nucl. Chem. 1971, 33, 1151–1155. 10.1016/0022-1902(71)80184-7. [DOI] [Google Scholar]
- Millot F.; Picard C. Oxygen self-diffusion in non-stoichiometric rutile TiO2-x at high temperature. Solid State Ion. 1988, 28–30, 1344–1348. 10.1016/0167-2738(88)90384-0. [DOI] [Google Scholar]
- Marmitt G. G.; Nandi S. K.; Venkatachalam D. K.; Elliman R. G.; Vos M.; Grande P. L. Oxygen diffusion in TiO2 films studied by electron and ion Rutherford backscattering. Thin Solid Films 2017, 629, 97–102. 10.1016/j.tsf.2017.03.024. [DOI] [Google Scholar]
- Van Orman J. A.; Crispin K. L. Diffusion in Oxides. Rev. Mineral. Geochem. 2010, 72, 757–825. 10.2138/rmg.2010.72.17. [DOI] [Google Scholar]
- Singh J.; Raj B. Temperature dependent analytical modeling and simulations of nanoscale memristor. Eng. Sci. Technol. Int. J. 2018, 21, 862–868. 10.1016/j.jestch.2018.07.016. [DOI] [Google Scholar]
- Strachan J. P.; Pickett M. D.; Yang J. J.; Aloni S.; Kilcoyne A. L. D.; Medeiros-Ribeiro G.; Williams R. S. Direct Identification of the Conducting Channels in a Functioning Memristive Device. Adv. Mater. 2010, 22, 3573–3577. 10.1002/adma.201000186. [DOI] [PubMed] [Google Scholar]
- Strachan J. P.; Strukov D. B.; Borghetti J.; Yang J. J.; Medeiros-Ribeiro G.; Williams R. S. The switching location of a bipolar memristor: chemical, thermal and structural mapping. Nanotechnology 2011, 22, 254015. 10.1088/0957-4484/22/25/254015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.