Abstract
We assessed trends in behavioral risk for HIV infection among men who have sex with men (MSM). Seattle MSM participated in random digit dial telephone surveys in 2003 (n = 400) and 2006 (n = 400). Fourteen percent in 2003 and 9% in 2006 reported unprotected anal intercourse with a partner of different or unknown HIV status (non-concordant UAI; odds ratio [OR] = 0.7; 95% confidence interval [CI]: 0.5, 1.2). Compared to participants in 2003, participants in 2006 met a greater proportion of their anal sex partners through the Internet (OR = 2.0; 95% CI: 1.2, 3.1). Although the proportion of anal sex partnerships formed online increased between 2003 and 2006, Internet partnerships were not more risky than those initiated elsewhere. While the emergence of the Internet as a venue through which men meet partners demonstrates that sexual risk among MSM remains highly dynamic, our findings suggest that sexual risk behavior among MSM is currently stable.
Keywords: Men who have sex with men, HIV, Behavioral surveillance, Random digit dial
Introduction
Since the mid-1990s, scientists have documented substantial increases in the rates of bacterial sexually transmitted infections (STI), sexual risk behavior, and HIV diagnoses among men who have sex with men (MSM) [1–4]. Some investigators have hypothesized that the rise of methamphetamine use [5], the expansion of the Internet as a venue to meet sex partners [6, 7], and the advent of effective antiretroviral therapy for HIV [2], all trends coincident with increases in sexual risk and STI and HIV diagnoses, may have played a causal role in the behavior change of MSM. Thus, behavioral surveillance has become an important component of HIV prevention activities [8], and monitoring behavioral surrogates of HIV transmission may facilitate the timely assessment of a population’s HIV risk [9].
Although researchers and public health officials have developed an array of behavioral surveillance programs [2, 8, 10–18], few population-based data are available to assess trends in risk among MSM, the group at highest risk for HIV infection in the US and many other nations [3, 19]. We present an analysis of serial random digit dial surveys conducted among MSM in Seattle, Washington in 2003 and 2006. These surveys were conducted as a form of population-based behavioral surveillance designed to assess trends in the population’s level of HIV-related sexual risk.
Methods
RDD Procedure
We conducted two RDD telephone household surveys of MSM in Seattle, Washington (population 592,800) in 2003 and 2006. The sampling universe included all households in the 3 zip (postal) codes in King County with high estimated concentrations of MSM. We described methods for identifying these zip codes previously [20], and identical methods used in 2003 and 2006 identified the same sampling frame.
From February 3 to May 18, 2003 and from August 15 to December 11, 2006, Gilmore Research Group (Seattle, WA) used RDD to sample households with the selected telephone prefixes and conducted anonymous computer-assisted telephone interviews. Interviewers called each sampled number at least eight times on different days and at different times before stopping contact attempts. On contacting a household, interviewers determined if the household was in a targeted zip code and whether more than one man at least 18 years of age resided in the household. If these criteria were met, the interviewer randomly selected one man for participation in the survey. After an introduction, the interviewer asked whether the respondent had ever had sex with a man since age 14; those responding affirmatively were eligible to participate. We chose this behavioral eligibility criterion to be consistent with other population-based samples of MSM, to capture men who may be less sexually active, and to sample men who may not identify as gay, homosexual, or bisexual [17]. The University of Washington Institutional Review Board approved all procedures.
In 2003, interviewers attempted 17,105 calls and screened 5,077 households. In 2006, interviewers attempted 35,519 calls and screened 4,808 households. The response rate, defined as the number of completed interviews divided by the estimated number of all telephone numbers dialed that belonged to households with at least one MSM, was 46% in 2003 and 22% in 2006 [21]. This rate is based on the assumption that telephone numbers not contacted or screened for eligibility had a similar proportion of eligible households as those contacted and screened. Eight and ten percent of households screened in the target zip codes had at least one MSM in 2003 and 2006, respectively. The completion (cooperation) rate, defined as the number of identified MSM who completed the survey, was 97% (400/412) in 2003 and in 87% (400/459) in 2006.
Study Instrument and Outcomes
Survey questions covered respondents’ STI/HIV testing and history, substance use, sexual behavior, and socio-demographics, and included detailed partner-by-partner sexual behavior questions concerning the participants’ three most recent anal sex partners in the prior 12 months. We defined sexually active respondents as those who reported one or more male sex partner in the prior 12 months. Seventy-eight percent (311/400) in 2003 and 70% (280/400) in 2006 reported sex with another man in the preceding 12 months.
Using the partner-by-partner information, we adopted the sexual risk hierarchy used in two population-based surveys in California [22, 23]. This variable has six categories of risk related to sexual activity in the prior 12 months indicating the respondent’s highest level of sexual risk: (1) no male sex partners; (2) no male anal sex partners; (3) only protected anal intercourse; (4) unprotected anal intercourse (UAI) with partners of the same perceived HIV status (concordant UAI); (5) UAI with a partner of unknown or different HIV status where the HIV-negative partner is the insertive partner and the receptive partner is either HIV-positive or HIV-unknown, or where the HIV positive partner is the receptive partner and the insertive partner is either HIV-negative or HIV-unknown (non-concordant UAI, risk to the insertive partner); and (6) UAI with a partner of unknown or different HIV status where the HIV-negative partner is the receptive partner and the insertive partner is HIV-positive or HIV-unknown, or where the HIV-positive partner is the insertive partner and the receptive partner is HIV-negative or HIV-unknown (non-concordant UAI, risk to the receptive partner).
STD Clinic Behavioral Data Collection
While our RDD sampling procedures were designed to provide a representative sample of Seattle MSM, we only had two time points. Therefore, to contextualize our data temporally, we also present sentinel sexual behavior data collected at the Public Health—Seattle & King County (PHSKC) STD clinic. During new problem visits, clinicians ask patients if they have sex with men, women, or both in the prior year, and record the number of clients’ male and female sex partners in the prior 2 months. We defined MSM as men who reported a male sex partner in the past 12 months. We calculated the proportion of men reporting two or more male sex partners in the prior 2 months for 1993–2007. This number was chosen to define a population of men having multiple sex partners. For men with more than one clinic visit in a calendar year, we used data only from the first new problem visit in each year. PHSKC has monitored this metric as part of routine STD surveillance since 1993 and does not have other sentinel sources of behavioral surveillance on MSM for a similarly long time period [24].
Statistical Methods
First, we compared the 2003 and 2006 populations. We used chi-squared and Kruskal–Wallis tests to compare socio-demographic characteristics; quantile regression to compare the median number of male sex partners and the median number of male anal sex partners in the prior year; [25] and, logistic regression to compare self-reported HIV status, HIV testing in the prior year, participants’ highest level of sexual risk behavior in the prior year, and substance use in the prior 6 months. We adjusted quantile and logistic regression models for socio-demographic variables that differed statistically (P < 0.05) between the 2003 and 2006 samples.
Second, we used partner-by-partner data to evaluate differences in locations where men met anal sex partners between 2003 and 2006. We used generalized estimating equations (GEE) to compare the context of anal sex partnership formation accounting for multiple partners reported by a single participant [26]. We adjusted these analyses for socio-demographic characteristics that differed statistically between the 2003 and 2006 samples and for the total number of anal sex partners about whom the participant provided information (one, two, or three).
Third, we combined the 2003 and 2006 data to explore individual-level and partnership-level correlates of non-concordant UAI, again using GEE. In our multivariable model, we included predictors that were significant (P ≤ 0.1) on univariable analyses and adjusted for RDD year. All GEE models used exchangeable working correlation matrices and robust standard error estimation, except for those among the smaller sample of HIV-positive participants, which used jackknife standard error estimates [27].
Finally, using data on MSM STD clinic attendees, we plotted the proportion of men reporting two or more male sex partners in the prior 2 months. Since this metric is part of routine behavioral surveillance, we knew the shape of the plot prior to statistical testing. Therefore, we performed a post-hoc analysis [28] using GEE to evaluate trends in male sex partners between 1993–1999 and 2000–2006, where the outcome was a binary variable indicating number of sex partners in the prior 2 months (none or one, two or more) and the predictor was calendar year.
Results
Study Population
We observed substantial differences in the socio-demographic characteristics of the 2003 and 2006 samples (Table 1). Compared to men in the 2003 sample, men participating in the 2006 RDD survey were older, lived in Seattle longer, reported greater annual incomes, and were more likely to have health insurance. The populations did not differ statistically with respect to race/ethnicity, education, or sexual orientation. Therefore, in comparisons of the 2003 and 2006 samples, we adjusted all medians and odds ratios (ORs) for age, years in Seattle, income, and health insurance.
Table 1.
Socio-demographic characteristics of MSM participating in the 2003 and 2006 random digit dial studies, Seattle, Washington
| Characteristic | 2003 RDD (n = 400) | 2006 RDD (n = 400) | χ2 | P value |
|---|---|---|---|---|
| Age, median (range) | 38 (19–90) | 44 (20–83) | 50.5 | <0.01 |
| Years living in Seattle, median (range) | 12 (0–90) | 16 (0–75) | 25.9 | <0.01 |
| Race/ethnicity, n (%) | 8.16 | 0.32 | ||
| Native American, Alaska Native | 4 (1.0) | 2 (0.5) | ||
| Asian, Asian American, Native Hawaiian, Pacific Islander | 8 (2.0) | 8 (2.0) | ||
| Hispanic or Latino | 16 (4.0) | 9 (2.3) | ||
| White | 341 (85.3) | 348 (87.0) | ||
| Other, multiracial | 18 (4.5) | 19 (4.8) | ||
| Missing | 1 (0.3) | 6 (1.5) | ||
| Annual household income, n (%) | 15.7 | 0.01 | ||
| <$15,000 | 42 (10.5) | 35 (8.8) | ||
| $15,000–$29,999 | 92 (23.0) | 66 (16.5) | ||
| $30,000–$49,999 | 118 (29.5) | 99 (24.8) | ||
| $50,000–$74,999 | 61 (15.3) | 72 (18.0) | ||
| >$75,000 | 72 (18.0) | 110 (27.5) | ||
| Missing | 15 (3.8) | 18 (4.5) | ||
| Education, n (%) | 9.19 | 0.06 | ||
| ≤High school | 75 (9.9) | 55 (10.9) | ||
| Technical or vocational training, some 4-year college | 99 (24.8) | 77 (19.3) | ||
| ≥College | 261 (65.3) | 279 (69.8) | ||
| Sexual orientation, n (%) | 5.55 | 0.35 | ||
| Gay, homosexual | 331 (82.8) | 314 (78.5) | ||
| Straight, heterosexual | 29 (7.3) | 40 (10.0) | ||
| Bisexual | 30 (7.5) | 38 (9.5) | ||
| Unknown/other | 10 (2.5) | 8 (2.0) | ||
| Health insurance, n (%) | 5.92 | 0.02 | ||
| Yes | 318 (79.5) | 344 (86.0) | ||
| No | 82 (20.5) | 56 (14.0) | ||
| Living with domestic partner, n (%) | 1.07 | 0.59 | ||
| Yes | 96 (24.0) | 93 (23.2) | ||
| No | 303 (75.8) | 307 (76.8) | ||
| Unknown | 1 (0.2) | 0 |
HIV Status
Comparing the 2003 and 2006 populations, 13% of men in each year reported being HIV-positive (χ2(1) = 0.33, P = 0.57), 77 and 79% reported being HIV-negative (χ2(1) = 1.12, P = 0.29), and 10 and 8% reported having never tested or having tested but having never learned their test results (χ2(1) = 0.67, P = 0.41). The participants aware of their HIV status comprise the sample for all subsequent analyses (359 in 2003 and 367 in 2006).
HIV Testing
In 2003, sexually active HIV-negative MSM reported having tested a median of 8 months prior to the interview (interquartile range [IQR]: 3–18 months). In 2006, this interval increased to 12 months (IQR: 12–24; F(1,473) =18.72; P < 0.01). In 2003, 74% of 242 sexually active HIV-negative MSM reported having tested for HIV in the prior 12 months compared with 60% of 231 sexually active men in 2006 (OR = 0.6; 95% CI: 0.4, 0.99; χ2(1) = 0.67; P = 0.04).
Sexual Risk Behavior
The median number of male sex partners in the prior year was 2 (IQR: 1–6) in 2003 and 1 (IQR: 0–4) in 2006 (F(1,684) = 4.92; P = 0.03). In 2003, the median number of male anal sex partners in the prior year was 1 (IQR: 1–3) and 1 (IQR: 1–2) in 2006 (F(1,548) = 0.01; P = 0.98).
Combining the two survey populations, 27% of HIV-positive men and 9% of HIV-negative men reported non-concordant UAI in the prior 12 months. Fewer HIV-negative and HIV-positive men reported non-concordant UAI in 2006 than in 2003, but this difference was not statistically significant (Table 2). This difference in non-concordant UAI reflected the decline in the report of non-concordant UAI with risk to the receptive partner from 2003 to 2006, a difference of borderline statistical significance in HIV-positive men. Compared to HIV-positive respondents in 2003, HIV-positive men in 2006 were significantly more likely to report not having had sex in the prior 12 months; they were also less likely to report protected anal sex and twice as likely to report concordant UAI as their riskiest behaviors, though these differences were not statistically significant. Among HIV-negative respondents, we detected no statistically significant changes in risk behavior from 2003 to 2006 according to the six-level risk variable. Reclassification of a random 13% of men who reported being HIV-negative but not having tested in the prior 2 years as HIV-positive changed the proportion of men reporting non-concordant UAI by less than 1%.
Table 2.
Highest level of sexual risk among MSM of known HIV status participating in the 2003 and 2006 random digit dial studies, Seattle, Washington
| 2003 RDD | 2006 RDD | Adjusted OR (95% CI)a | χ2 | P-value | |
|---|---|---|---|---|---|
| No sex, n (%) | |||||
| HIV-positive men | 9/53 (17) | 14/50 (28) | 4.2 (1.1, 15.8) | 4.62 | 0.03 |
| HIV-negative men | 64/306 (20.9) | 86/317 (27.1) | 1.2 (0.8, 1.8) | 0.75 | 0.39 |
| All men | 73/359 (20.3) | 100/367 (27.3) | 1.3 (0.9, 2.0) | 2.03 | 0.15 |
| No anal sex, n (%) | |||||
| HIV-positive men | 7/53 (13) | 4/50 (8) | 0.6 (0.1, 2.2) | 0.73 | 0.39 |
| HIV-negative men | 53/306 (17.3) | 59/317 (18.6) | 0.9 (0.6, 1.3) | 0.46 | 0.50 |
| All men | 60/359 (16.7) | 63/367 (17.2) | 0.8 (0.5, 1.3) | 0.84 | 0.36 |
| Only protected anal sex, n (%) | |||||
| HIV-positive men | 11/53 (21) | 6/50 (12) | 0.3 (0.1, 1.1) | 3.20 | 0.07 |
| HIV-negative men | 75/306 (24.5) | 74/317 (23.3) | 1.0 (0.7, 1.5) | 0.01 | 0.93 |
| All men | 86/359 (24.0) | 80/367 (21.8) | 0.9 (0.6, 1.3) | 0.20 | 0.66 |
| Concordant unprotected anal sex, n (%) | |||||
| HIV-positive men | 8/53 (15) | 15/50 (30) | 2.1 (0.8, 5.9) | 2.14 | 0.14 |
| HIV-negative men | 79/306 (25.8) | 74/317 (23.3) | 1.1 (0.8, 1.7) | 0.29 | 0.59 |
| All men | 87/359 (24.2) | 89/367 (24.3) | 1.2 (0.8, 1.8) | 1.01 | 0.32 |
| Non-concordant unprotected anal sex, risk to insertive partner, n (%) | |||||
| HIV-positive men | 7/53 (13) | 8/50 (16) | 1.5 (0.4, 5.0) | 0.39 | 0.53 |
| HIV-negative men | 13/306 (4.3) | 11/317 (3.5) | 1.0 (0.4, 2.3) | 0.01 | 0.91 |
| All men | 20/359 (5.6) | 19/367 (5.2) | 1.1 (0.6, 2.2) | 0.14 | 0.71 |
| Non-concordant unprotected anal sex, risk to receptive partner, n (%) | |||||
| HIV-positive men | 9/53 (17) | 3/50 (6) | 0.2 (0.1, 1.1) | 3.24 | 0.07 |
| HIV-negative men | 21/306 (6.9) | 11/317 (3.5) | 0.6 (0.3, 1.3) | 1.58 | 0.20 |
| All men | 30/359 (8.4) | 14/367 (3.8) | 0.5 (0.3, 1.0) | 3.66 | 0.06 |
| Any non-concordant unprotected anal sex, n (%)b | |||||
| HIV-positive men | 17/53 (32) | 11/50 (22) | 0.7 (0.3, 2.0) | 0.39 | 0.53 |
| HIV-negative men | 34/306 (11.1) | 22/317 (6.9) | 0.7 (0.4, 1.3) | 1.17 | 0.28 |
| All men | 50/359 (13.9) | 33/367 (9.0) | 0.7 (0.5, 1.2) | 1.41 | 0.23 |
| Missing, n (%) | |||||
| HIV-positive men | 1/53 (2) | 0/50 (0) | Not estimable | – | – |
| HIV-negative men | 1/306 (0.3) | 2/317 (0.6) | 2.4 (0.2, 29.2) | 0.50 | 0.48 |
| All men | 2/359 (0.6) | 2/367 (0.5) | 1.1 (0.1, 8.2) | 0.01 | 0.94 |
RDD random digit dial, OR odds ratio, CI confidence interval
Adjusted for health insurance status, income, age, and years living in Seattle
Includes men who report either non-concordant unprotected anal sex, risk to the insertive partner or non-concordant unprotected anal sex, risk to the receptive psartner, and one man who reported non-concordant unprotected anal sex but did not indicate whether he was the receptive or insertive partner
Among sexually active men, the proportion of men reporting methamphetamine use in the prior 6 months was 4% in 2003 and 6% in 2006 (χ2(1) = 0.76; P = 0.38), the proportion of men reporting sildenafil use in the prior 6 months was 15% in 2003 and 25% in 2006 (χ2(1) = 0.73; P = 0.39), and the proportion of men reporting inhaled nitrites in the prior 6 months was 16% in 2003 and 20% in 2006 (χ2(1) = 0.11; P = 0.74).
Partnership-Level Analyses: Contexts of Anal Sex Partnership Formation
For the partner-by-partner module of the survey, the 430 participants who reported anal sex in the prior year provided information about 794 anal sex partnerships. Only 5 (1%) men provided information on fewer partners than the survey requested. The number of partners about whom participants provided information declined from 439 in 2003 to 355 in 2006; this difference was of borderline statistical significance (χ2(1) = 5.31; P = 0.07).
The proportion of anal sex partnerships initiated through the Internet increased, while the proportion of anal sex partnerships initiated at a park, on the street, or at a beach declined (Table 3). Compared to 2003, HIV-negative participants reported fewer anal sex partnerships initiated at bathhouses or sex clubs in 2006. The proportion of anal sex partnerships formed at a bar or dance club, in a social public setting, or through friends remained stable.
Table 3.
Context of anal sex partnership formation in the prior 12 months among sexually active MSM participating in the 2003 and 2006 random digit dial studies, Seattle, Washington
| 2003 RDD % of partnerships | 2006 RDD % of partnerships | Adjusted OR (95% CI)a | χ2 | P value | |
|---|---|---|---|---|---|
| Bar or club | |||||
| HIV-positive men | 15/89 (17) | 12/62 (19) | 0.7 (0.2, 3.1) | 0.20 | 0.66 |
| HIV-negative men | 88/350 (25.4) | 73/293 (25.6) | 1.1 (0.7, 1.6) | 0.09 | 0.76 |
| All men | 104/439 (23.7) | 87/355 (24.5) | 1.1 (0.7, 1.6) | 0.11 | 0.74 |
| Internet | |||||
| HIV-positive men | 24/89 (27) | 15/62 (42) | 2.7 (0.8, 9.4) | 2.51 | 0.12 |
| HIV-negative men | 58/350 (16.6) | 77/293 (26.6) | 1.8 (1.1, 3.1) | 5.13 | 0.02 |
| All men | 82/439 (18.7) | 104/355 (29.3) | 2.0 (1.2, 3.1) | 8.32 | <0.01 |
| Bathhouse/sex club | |||||
| HIV-positive men | 4/89 (5) | 5/62 (8) | 2.0 (0.2, 20.2) | 0.38 | 0.54 |
| HIV-negative men | 23/350 (6.6) | 9/293 (3.1) | 0.3 (0.1, 0.9) | 3.96 | 0.05 |
| All men | 27/439 (6.2) | 14/355 (3.9) | 0.6 (0.3, 1.4) | 1.35 | 0.25 |
| Park/beach/street | |||||
| HIV-positive men | 13/89 (15) | 6/62 (10) | 0.9 (0.2, 4,0) | 0.03 | 0.86 |
| HIV-negative men | 31/350 (8.9) | 8/293 (2.7) | 0.3 (0.1, 0.7) | 6.59 | 0.01 |
| All men | 44/439 (10.0) | 14/355 (3.9) | 0.4 (0.2, 0.9) | 5.44 | 0.02 |
| Social public settingb | |||||
| HIV-positive men | 13/89 (15) | 4/62 (6) | 0.6 (0.1, 4.5) | 0.28 | 0.60 |
| HIV-negative men | 52/350 (15.0) | 35/293 (12.0) | 0.8 (0.5, 1.3) | 0.77 | 0.38 |
| All men | 65/439 (15.4) | 44/355 (11.9) | 0.7 (0.4, 1.1) | 2.28 | 0.13 |
| Through a friend | |||||
| HIV-positive men | 14/89 (16) | 5/62 (8) | 0.4 (0.1, 2.0) | 1.32 | 0.26 |
| HIV-negative men | 52/350 (14.9) | 31/293 (10.6) | 0.8 (0.4, 1.4) | 0.72 | 0.40 |
| All men | 66/439 (15.0) | 36/355 (10.1) | 0.7 (0.4, 1.1) | 2.29 | 0.13 |
| Otherc | |||||
| HIV-positive men | 4/89 (5) | 3/62 (5) | 1.4 (0.1, 26.5) | 0.04 | 0.84 |
| HIV-negative men | 39/350 (11.1) | 50/293 (17.1) | 1.5 (0.8, 2.6) | 1.86 | 0.17 |
| All men | 43/439 (9.8) | 53/355 (14.9) | 1.4 (0.8, 2.5) | 1.82 | 0.18 |
RDD random digit dial, OR odds ratio, CI confidence interval
Adjusted for health insurance status, income, age, years living in Seattle, and total number of partners about whom the participant provided information
Social public settings include work, community activities, hobbies, sports, gym, and parties at friends’ houses
Other includes telephone sex lines, dating/personal ads, adult video or book stores, and other unspecified venues
Partnership-Level Analyses: Correlatesof Non-concordant Unprotected Anal Intercourse
The 361 HIV-negative men who had anal sex in the prior year provided information about partner HIV status and condom use for 630 (98%) of the 643 anal sex partnerships about which they provided some information. The partnerships of older men, men earning less than $30,000 annually, and men who use methamphetamine were more likely to involve non-concordant UAI than partnerships of younger men, men earning $30,000 or more annually, and men who did not report methamphetamine use, respectively (Table 4). Non-concordant UAI partnerships were less likely to be formed online than at a bar or dance club. A smaller proportion of anal sex partnerships formed via the Internet involved non-concordant UAI than partnerships formed through friends, bathhouses or sex clubs, or in public settings, though these differences were not statistically significant. Additionally, anal sex partnerships in which the HIV-negative participant misrepresented his HIV status or did not disclose his HIV status were more likely to include non-concordant UAI than anal sex partnerships in which the participant disclosed being HIV-negative.
Table 4.
Multivariable individual and partnership-level correlates of non-concordant unprotected anal intercourse among MSM reporting anal sex partnerships in the prior 12 months, Seattle, Washington, 2003–2006
| % Of partnerships without non-concordant UAI | % Of partnerships with non-concordant UAI | Multivariable OR (95% CI)a | |
|---|---|---|---|
| HIV-negative men (n = 630 anal sex partnerships of 361 men) b | |||
| Age | |||
| 19–29 | 115/120 (95.8) | 5/120 (4.2) | REF |
| 30–39 | 216/238 (90.3) | 22/238 (9.2) | 4.6 (1.2, 17.5) |
| 40–49 | 163/176 (92.6) | 13/176 (7.4) | 5.2 (1.2, 21.4) |
| ≥50 | 81/94 (86) | 13/94 (14) | 8.6 (1.6, 46.2) |
| Annual income | |||
| <$30,000 | 124/150 (82.7) | 26/150 (17.3) | REF |
| ≥$30,000 | 434/461 (94.1) | 27/461 (5.9) | 0.3 (0.1, 0.6) |
| Methamphetamine use, prior 6 months | |||
| No | 558/605 (92.2) | 47/605 (7.8) | REF |
| Yes | 19/25 (76) | 6/25 (24) | 5.0 (1.4, 17.7) |
| Participant’s disclosure of HIV status | |||
| Disclosed as HIV-negative | 477/497 (96.0) | 20/497 (4.0) | REF |
| Disclosed as HIV-positive or unknown | 5/7 (71) | 2/7 (29) | 7.1 (1.3, 37.5) |
| Did not disclose | 95/126 (75.4) | 31/126 (24.6) | 6.5 (3.1, 13.6) |
| Meeting placec | |||
| Online | 127/135 (94) | 8/135 (6) | REF |
| Bar/dance club | 144/161 (89) | 17/162 (11) | 2.3 (1.1, 5.0) |
| Through a friend | 76/83 (92) | 7/83 (8) | 3.2 (0.9, 10.6) |
| Bathhouse/sex club | 23/30 (77) | 7/30 (23) | 2.3 (0.9, 6.4) |
| Social public setting | 81/85 (95) | 4/85 (5) | 1.7 (0.5, 5.6) |
| Park/beach/street | 34/39 (87) | 5/39 (13) | 1.9 (0.4, 8.1) |
| Other/unknown | 92/97 (95) | 5/97 (5) | 0.9 (0.3, 3.0) |
| HIV-positive men (n = 151 anal sex partnerships of 69 men) d | |||
| Participant’s disclosure of HIV status | |||
| Disclosed as HIV-positive | 101/113 (89) | 12/113 (11) | REF |
| Disclosed as HIV-negative or unknown | 3/4 (75) | 1/4 (25) | REF |
| Did not disclose | 17/34 (50) | 17/34 (50) | 6.8 (1.6, 28.6) |
CI confidence interval, OR odds ratio, RDD random digit dial, REF reference group, UAI unprotected anal intercourse
Adjusted for RDD year. Only significant associations (P < 0.05) are shown in the table
For HIV-negative men, model also includes individual-level binary variables indicating non-white race/ethnicity (OR = 0.4; 95% CI: 0.1, 1.4) and Viagra use in the prior 6 months (OR = 2.1; 95% CI: 0.7, 5.9). Model χ2(14) = 45.94; P < 0.001
Social public settings include work, community activities, hobbies, sports, gym, and parties at friends’ houses. Other includes telephone sex lines, dating/personal ads, adult video or bookstores, and other unspecified venues
For HIV-positive men, model also includes individual-level binary variables indicating annual income C$30,000 (OR = 4.3; 95% CI: 0.8,23.3); not being a lifelong Seattle resident (OR = 1.2; 95% CI: 0.1, 13.6); ≥6 male sex partners (OR = 0.3; 95% CI: 0.1, 2.2); ≥3 male anal sex partners (OR = 6.1; 95% CI: 0.6, 61.4); and, a partnership-level variable indicating a one-time partnership (OR = 1.1; 95% CI: 0.4, 3.1). Model F(7, 69) = 1.66; P = 0.13. Note that we present the F-statistic because this model used jackknife standard error estimation for small sample sizes as reported in the “Methods” section
The 69 HIV-positive men reporting anal sex in the prior year provided information on a total of 151 anal sex partners. Anal sex partnerships in which the participant did not disclose his HIV status were more likely to involve non-concordant UAI than partnerships in which the participant did disclose his HIV status or disclosed his HIV status as negative or unknown (Table 4).
Behavioral Trends in MSM STD Clinic Attendees
According to data from our STD clinic, the proportion of MSM reporting two or more sex partners in the prior 2 months increased from 24% in 1993 to 34% in 1999 (χ2(1) = 24.55, P < 0.01), after which it has remained stable at 35–38% (χ2(1) = 0.08, P = 0.50; Fig. 1).
Fig. 1.
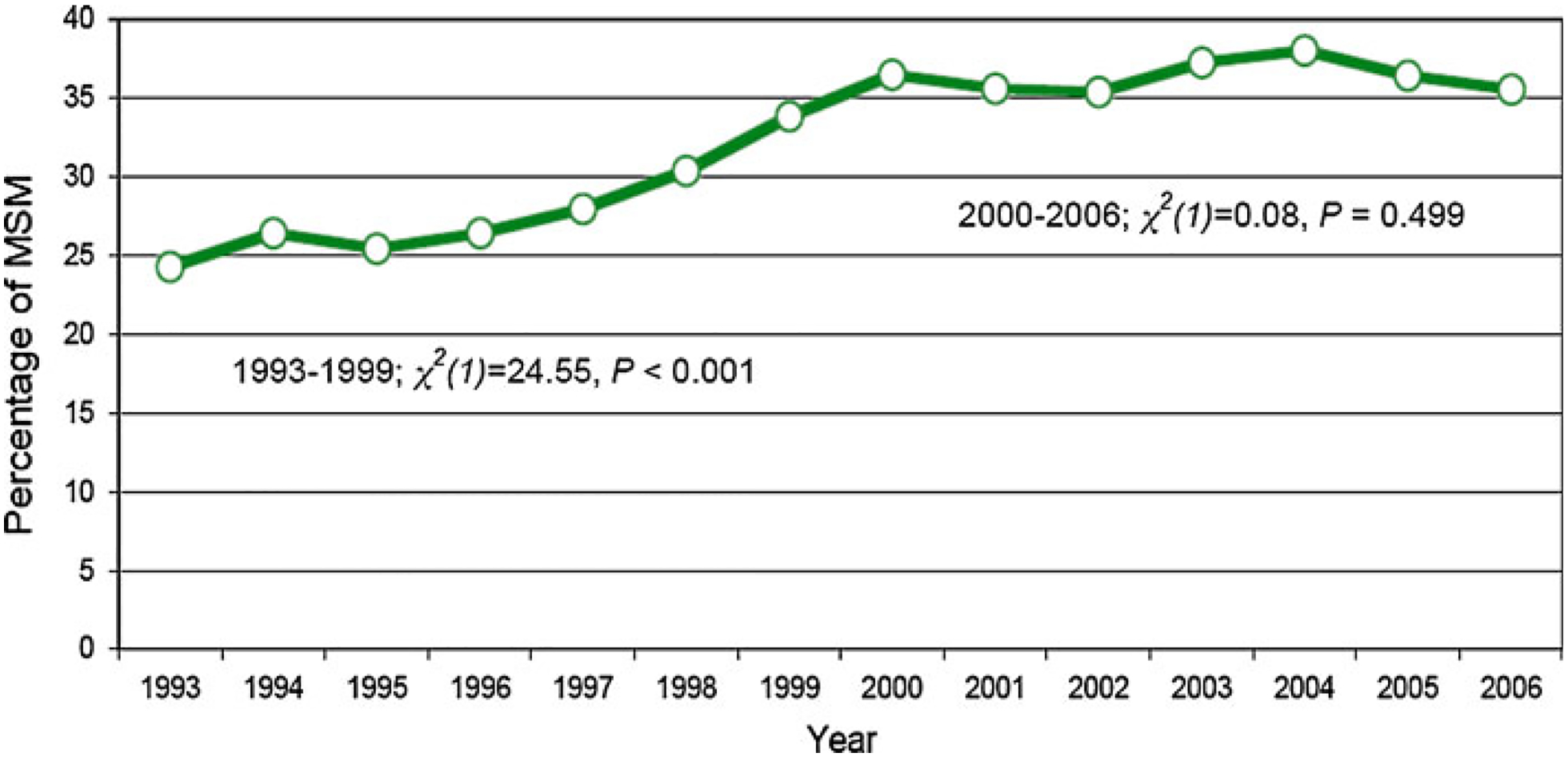
Percentage of men who have sex with men (MSM) STD clinic attendees who report two or more sex partners in the prior 2 months, Seattle and King County, Washington, 1993–2006
Discussion
Using data from our two RDD studies of MSM in Seattle, WA, we found that a minority of men engaged in behaviors likely to lead to HIV transmission. Despite a significant change in the context of partnership formation characterized by an increase in the proportion of anal sex partnerships initiated via the Internet, the population’s sexual risk was stable between 2003 and 2006.
Most serial cross-sectional studies of MSM have reported that sexual risk behavior among MSM increased in the mid-1990s and early 2000s [1, 2, 4], and we observed a similar trend among MSM in our STD clinic. The findings we present here, as well as behavioral surveillance data collected in London [29], suggest that sexual risk behavior among MSM stabilized in the early and mid-2000s at a level higher than in the mid-1990s. Across our RDD samples, the absolute level of risk expressed as the proportion of population who reported practicing non-concordant UAI—11% overall, 27% in HIV-positive MSM, and 9% in HIV-negative MSM—is very similar to other studies conducted in the Western U.S. In particular, our 2003 estimate of the proportion of MSM reporting non-concordant UAI in the prior year (14%) is similar to estimates of non-concordant UAI in a 2002 San Francisco RDD (15%) [22] and a 2003 statewide RDD of MSM living in California (11%) [23], but lower than that reported in a 2003 behavioral surveillance survey in London gyms (22%) [10]. We are not aware of other population-based estimates of non-concordant UAI with which to compare out 2006 findings. Of note, our 2006 estimate of non-concordant UAI among HIV-positive men (22%) is comparable to that reported in a 2005–2006 survey of randomly selected men attending an HIV clinic in Seattle (27%) [30], suggesting that estimates of this metric from a more readily accessible population may be roughly comparable.
While sexual risk, as measured by number of male sex partners and non-concordant UAI, appears to have declined or stabilized in recent years, sexual behavior among MSM remains dynamic. We found that more MSM are engaging in concordant UAI as their highest risk sexual behavior. Although not statistically significant, the large increase in concordant UAI among HIV-positive men suggests that the practice of choosing sex partners and practices based on HIV status is increasing (i.e., serosorting). We recently observed a similar trend in data from our STD clinic [31], and Elford and colleagues have observed increases in concordant UAI among HIV-positive men in London [29]. In the absence of other population-level changes in sexual behavior, increased concordant UAI among HIV-positive MSM should lead to a reduction in HIV transmission; however, it may increase rates of superinfection and transmission of drug-resistant HIV [32]. Our findings highlight the need to more clearly define the risks and benefits, both at the individual and population levels, of trends in concordant UAI that have been documented in several geographic areas [22, 29, 33].
Our study also supports prior reports documenting the expanding role of the Internet in sexual partnership formation among MSM [6, 29, 34, 35]. The increase in the proportion of anal sex partnerships initiated online was not accompanied by a concurrent increase in the total number of partnerships formed. Among HIV-negative men, we found that Internet anal sex partnerships were less likely to involve non-concordant UAI than partnerships formed in some other settings, a difference that was significant when comparing Internet partnerships to those initiated in bars or dance clubs. These findings are consistent with some reports [36–38], but not others [39]. In general, studies that have examined both the individual-level and partnership-level impact of Internet sex-seeking find that while MSM who use the Internet to find sex partners are more likely to engage in sexual risk behavior, Internet partnerships are not more likely to include non-concordant UAI than partnerships formed through other means [36, 37]. Our findings are consistent with this work and suggest that while the Internet has clearly changed the landscape of risk, its impact on the magnitude of risk in the population is uncertain.
Our results have implications for behavioral surveillance more generally. Researchers and public health officials have employed an array of approaches to behavioral surveillance, including sentinel surveillance; RDD studies; and, venue-based sampling [2, 8, 10–18]. Each approach has its advantages and disadvantages. Sentinel surveillance is relatively simple and inexpensive, and provides public health agencies with timely access to serial cross-sectional data from what are often comparable samples over time [1, 2, 10, 31, 40–42]. However, the extent to which any sentinel population is representative of the “target” population of MSM is uncertain [35, 43, 44]. Venue-based sampling yields diverse samples of MSM in a relatively short period of time [45–47]. However, this approach is typically resource intensive and generates high-risk samples whose behaviors may not be representative of the general population of MSM [8, 48, 49]. Also, it remains uncertain whether serially recruited samples are comparable.
RDD sampling is relatively inexpensive and is meant to enroll representative and reproducible populations. However, our experience calls into question whether RDD sampling can indeed accomplish these goals. The 2006 response rate was 22%, down from 46% in 2003. In part, the older, wealthier, less mobile population recruited in 2006 may reflect real changes in the men living in the three sampled zip codes; between 2003 and 2006, the median price of homes sold in the sampling frame increased by $100,000–$200,000 [50]. However, the differences in the 2003 and 2006 populations may reflect problems with RDD sampling methodology [51]. San Francisco RDD surveys in 1997 and 2002 were also demographically dissimilar [22]. While these studies did not report response rates, other RDD surveillance projects, such as the Behavioral Risk Factor Surveillance System [52] and the National Immunization Survey [53] have experienced marked declines in response rates over the past 10–15 years. These declines are contemporaneous with the widespread use of answering machines [54], and, more recently, an increase in the proportion of US adults who use only wireless telephones [55]. Wireless-only adults are more likely to be male, less than 30 years of age, Hispanic, uninsured, to live below the federal poverty line and with unrelated roommates, and to rent their home [55, 56]. As a consequence, RDD samples may be systematically biased. Although we controlled for differences in our 2003 and 2006 samples to mitigate potential confounding, adjustment does not eliminate selection bias and confounding due to unmeasured variables. The deterioration of RDD sampling seen in our study is clearly widespread [51–53, 57]. Unfortunately, at present, what alternative approaches might be best employed to sample a representative population of MSM remains uncertain. Evaluating new sampling approaches is a critical research question.
We cannot be certain of the extent to which our low 2006 response rate biases our findings, as we did not sample non-respondents [57, 58]. While many of our findings on absolute risk levels of and trends in sexual risk are comparable with data from other cities, particularly those in the Western US, they may not generalize to other populations. Specifically, our study included few non-white MSM, limiting the generalizability of our findings to populations of racial and ethnic minorities who are most severely affected by the HIV epidemic. There is a small probability (<0.016) that we sampled the same man in 2003 and 2006; because men participated anonymously, we could not know who participated twice and account for the non-independence of the samples.
In summary, in a population-based study, we found that a minority of MSM engage in behaviors that may result in HIV transmission, and that non-concordant UAI and number of anal sex partners among MSM in Seattle were stable between 2003 and 2006, despite a dramatic increase in the proportion of anal sex partnerships initiated through the Internet and a possible increase in concordant UAI. These observations are consistent with sentinel surveillance data and suggest that behavioral risk among MSM has plateaued following a period of increasing sexual risk in the mid-1990s through the early 2000s. Our data also show that the current level of sexual risk is much higher than it was in the mid-1990s and recent CDC data indicate that the number of HIV cases among MSM has increased since that time [3]. Clearly, HIV prevention among MSM is not working well enough. This reality signals the need to develop strategies that engage MSM as partners in employing traditional HIV prevention practices more effectively, in researching the contexts in which sexual risk and HIV transmission occurs, and in developing innovative, effective, multi-level HIV prevention programs. Our work suggests that Internet-based cohort studies and Internet-based outreach, education, and counseling interventions may be key to reaching large numbers of MSM to broaden the public health impact of HIV prevention interventions. Such programs, employed in concert with prevention through more traditional venues, would seek to increase the frequency of HIV testing (especially HIV RNA testing), broaden access to antiretroviral treatment (particularly among men with CD4 counts greater than 350 cells/mm3), reduce substance use and sexual risk facilitated by substance use, and promote open communication about HIV in sexual partnerships.
Acknowledgments
Devon Brewer provided valuable feedback during the preparation of the manuscript. The authors thank JoElla Weybright, Laurie Burke, and others at Gilmore Research Group for their efforts in collecting the data and help in refining the sampling design. The 2003 RDD was supported by a Comprehensive STD Prevention System Syphilis Elimination grant from the Centers for Disease Control and Prevention and the 2006 RDD was supported by a grant to MRG (NIH K23 AI01846). TWM was supported by NIH T32 AI07140.
Contributor Information
Timothy W. Menza, Center for AIDS and STD, University of Washington, 325 Ninth Ave, Box 359931, Seattle, WA 98104, USA Department of Epidemiology, University of Washington, Seattle, WA, USA; Public Health-Seattle & King County, Seattle, WA, USA.
Roxanne P. Kerani, Public Health-Seattle & King County, Seattle, WA, USA
H. Hunter Handsfield, Department of Medicine, University of Washington, Seattle, WA, USA; Battelle Research Institute, Seattle, WA, USA.
Matthew R. Golden, Center for AIDS and STD, University of Washington, 325 Ninth Ave, Box 359931, Seattle, WA 98104, USA Department of Epidemiology, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA; Public Health-Seattle & King County, Seattle, WA, USA.
References
- 1.Chen SY, Gibson S, Katz MH, et al. Continuing increases in sexual risk behavior and sexually transmitted diseases among men who have sex with men: San Francisco, Calif, 1999–2001, USA. Am J Public Health. 2002;92(9):1387–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Katz MH, Schwarcz SK, Kellogg TA, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;92(3):388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton KA, Imrie J. Increasing rates of sexually transmitted diseases in homosexual men in Western Europe and the United States: why? Infect Dis Clin North Am. 2005;19(2):311–31. [DOI] [PubMed] [Google Scholar]
- 5.Mansergh G, Purcell DW, Stall R, et al. CDC consultation on methamphetamine use and sexual risk behavior for HIV/STD infection: summary and suggestions. Public Health Rep. 2006;121(2):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarlane M, Bull SS, Rietmeijer CA. The Internet as a newly emerging risk environment for sexually transmitted diseases. JAMA. 2000;284(4):443–6. [DOI] [PubMed] [Google Scholar]
- 7.Klausner JD, Wolf W, Fischer-Ponce L, et al. Tracing a syphilis outbreak through cyberspace. JAMA. 2000;284(4):447–9. [DOI] [PubMed] [Google Scholar]
- 8.MacKellar DA, Gallagher KM, Finlayson T, et al. Surveillance of HIV risk and prevention behaviors of men who have sex with men–a national application of venue-based, time–space sampling. Public Health Rep. 2007;122(Suppl 1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansky A, Sullivan PS, Gallagher KM, et al. HIV behavioral surveillance in the US: a conceptual framework. Public Health Rep. 2007;122(Suppl 1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elford J, Bolding G, Davis M, et al. Trends in sexual behaviour among London homosexual men 1998–2003: implications for HIV prevention and sexual health promotion. Sex Transm Infect. 2004;80(6):451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart GJ, Williamson LM. Increase in HIV sexual risk behaviour in homosexual men in Scotland, 1996–2002: prevention failure? Sex Transm Infect. 2005;81(5):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson LM, Dodds JP, Mercey DE, et al. Increases in HIV-related sexual risk behavior among community samples of gay men in London and Glasgow: how do they compare? J Acquir Immune Defic Syndr. 2006;42(2):238–41. [DOI] [PubMed] [Google Scholar]
- 13.Hickson FC, Reid DS, Davies PM, et al. No aggregate change in homosexual HIV risk behaviour among gay men attending the Gay Pride festivals, United Kingdom, 1993–1995. AIDS. 1996;10(7):771–4. [DOI] [PubMed] [Google Scholar]
- 14.Nardone A, Mercey D, Johnson AM, et al. Developing surveillance for HIV transmission and risk behaviours among high-risk groups in a central London health district. J Public Health Med. 1999;21(2):208–14. [DOI] [PubMed] [Google Scholar]
- 15.Elford J, Bolding G, Davis M, et al. Web-based behavioral surveillance among men who have sex with men: a comparison of online and offline samples in London, UK. J Acquir Immune Defic Syndr. 2004;35(4):421–6. [DOI] [PubMed] [Google Scholar]
- 16.Wiessing LG, Houweling H, Sandfort TG, et al. Reaching homosexual men for HIV surveillance through a gay magazine. Eur J Epidemiol. 1999;15(5):429–37. [DOI] [PubMed] [Google Scholar]
- 17.Catania JA, Osmond D, Stall RD, et al. The continuing HIV epidemic among men who have sex with men. Am J Public Health. 2001;91(6):907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster RD, Darrow WW, Paul JP, et al. HIV infection and associated risks among young men who have sex with men in a Florida resort community. J Acquir Immune Defic Syndr. 2003;33(2):223–31. [DOI] [PubMed] [Google Scholar]
- 19.Baral S, Sifakis F, Cleghorn F, et al. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brewer DD, Golden MR, Handsfield HH. Unsafe sexual behavior and correlates of risk in a probability sample of men who have sex with men in the era of highly active antiretroviral therapy. Sex Transm Dis. 2006;33(4):250–5.21. [DOI] [PubMed] [Google Scholar]
- 21.American Association for Public Opinion Research. Standard definitions: final dispositions of case codes and outcome rates for surveys. Lenexa, Kansas: AAPOR; 2006. [Google Scholar]
- 22.Osmond DH, Pollack LM, Paul JP, et al. Changes in prevalence of HIV infection and sexual risk behavior in men who have sex with men in San Francisco: 1997–2002. Am J Public Health. 2007;97(9):1677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Q, Osmond DH, Tholandi M, et al. HIV prevalence and sexual risk behaviors among men who have sex with men: results from a statewide population-based survey in California. J Acquir Immune Defic Syndr. 2006;41(2):238–45. [DOI] [PubMed] [Google Scholar]
- 24.Public Health—Seattle & King County. Public Health—Seattle & King County STD Data and Statistical Resources. Available at: http://www.kingcounty.gov/healthservices/health/communicable/std/statistics.aspx. Accessed 10 Oct 2008.
- 25.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62(5): 511—517, e511. [DOI] [PubMed] [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 27.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57(1): 126–34. [DOI] [PubMed] [Google Scholar]
- 28.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elford J, Bolding G, Sherr L, et al. High-risk sexual behaviour among London gay men: no longer increasing. AIDS. 2005; 19(18):2171–4. [DOI] [PubMed] [Google Scholar]
- 30.Golden MR, Wood RW, Buskin SE, et al. Ongoing risk behavior among persons with HIV in medical care. AIDS Behav. 2007;11(5):726–35. [DOI] [PubMed] [Google Scholar]
- 31.Golden MR, Stekler J, Hughes JP, et al. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008;49(2):212–8. [DOI] [PubMed] [Google Scholar]
- 32.Smith DM, Richman DD, Little SJ. HIV superinfection. J Infect Dis. 2005;192(3):438–44. [DOI] [PubMed] [Google Scholar]
- 33.Mao L, Crawford JM, Hospers HJ, et al. “Serosorting” in casual anal sex of HIV-negative gay men is noteworthy and is increasing in Sydney, Australia. AIDS. 2006;20(8):1204–6. [DOI] [PubMed] [Google Scholar]
- 34.Bolding G, Davis M, Hart G, et al. Where young MSM meet their first sexual partner: the role of the Internet. AIDS Behav. 2007;11(4):522–6. [DOI] [PubMed] [Google Scholar]
- 35.Evans AR, Wiggins RD, Mercer CH, et al. Men who have sex with men in Great Britain: comparison of a self-selected internet sample with a national probability sample. Sex Transm Infect. 2007;83(3):200–5. (discussion 205). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolding G, Davis M, Hart G, et al. Gay men who look for sex on the Internet: is there more HIV/STI risk with online partners? AIDS. 2005;19(9):961–8. [DOI] [PubMed] [Google Scholar]
- 37.Horvath KJ, Rosser BR, Remafedi G. Sexual risk taking among young internet-using men who have sex with men. Am J Public Health. 2008;98(6):1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiasson MA, Hirshfield S, Remien RH, et al. A comparison of on-line and off-line sexual risk in men who have sex with men: an event-based on-line survey. J Acquir Immune Defic Syndr. 2007;44(2):235–43. [DOI] [PubMed] [Google Scholar]
- 39.Berry M, Raymond HF, Kellogg T, et al. The Internet, HIV serosorting and transmission risk among men who have sex with men, San Francisco. AIDS. 2008;22(6):787–9. [DOI] [PubMed] [Google Scholar]
- 40.Rietmeijer CA, Patnaik JL, Judson FN, et al. Increases in gonorrhea and sexual risk behaviors among men who have sex with men: a 12-year trend analysis at the Denver Metro Health Clinic. Sex Transm Dis. 2003;30(7):562–7. [DOI] [PubMed] [Google Scholar]
- 41.Truong HM, Kellogg T, Klausner JD, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Infect. 2006;82(6):461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Bij AK, Kolader ME, de Vries HJ, et al. Condom use rather than serosorting explains differences in HIV incidence among men who have sex with men. J Acquir Immune Defic Syndr. 2007;45(5):574–80. [DOI] [PubMed] [Google Scholar]
- 43.Schwarcz S, Spindler H, Scheer S, et al. Assessing representativeness of sampling methods for reaching men who have sex with men: a direct comparison of results obtained from convenience and probability samples. AIDS Behav. 2007;11(4):596–602. [DOI] [PubMed] [Google Scholar]
- 44.Dodds JP, Mercer CH, Mercey DE, et al. Men who have sex with men: a comparison of a probability sample survey and a community based study. Sex Transm Infect. 2006;82(1):86–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valleroy LA, MacKellar DA, Karon JM, et al. HIV prevalence and associated risks in young men who have sex with men. Young Men’s Survey Study Group. JAMA. 2000;284(2):198–204. [DOI] [PubMed] [Google Scholar]
- 46.Stueve A, O’Donnell LN, Duran R, et al. Time–space sampling in minority communities: results with young Latino men who have sex with men. Am J Public Health. 2001;91(6):922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi KH, McFarland W, Neilands TB, et al. An opportunity for prevention: prevalence, incidence, and sexual risk for HIV among young Asian and Pacific Islander men who have sex with men, San Francisco. Sex Transm Dis. 2004;31(8):475–80. [DOI] [PubMed] [Google Scholar]
- 48.Xia Q, Tholandi M, Osmond DH, et al. The effect of venue sampling on estimates of HIV prevalence and sexual risk behaviors in men who have sex with men. Sex Transm Dis. 2006;33(9):545–50. [DOI] [PubMed] [Google Scholar]
- 49.Pollack LM, Osmond DH, Paul JP, et al. Evaluation of the center for disease control and prevention’s HIV behavioral surveillance of men who have sex with men: sampling issues. Sex Transm Dis. 2005;32(9):581–9.50. [DOI] [PubMed] [Google Scholar]
- 50.City-data.com. Available at: http://www.city-data.com. Accessed 9 Sep 2008.
- 51.Kempf AM, Remington PL. New challenges for telephone survey research in the twenty-first century. Annu Rev Public Health. 2007;28:113–26. [DOI] [PubMed] [Google Scholar]
- 52.CDC. BRFSS Annual Survey Data: Summary Data Quality Reports. Available at: http://www.cdc.gov/brfss/technical_infodata/quality.htm. Accessed 9 Sep 2008.
- 53.CDC. Trends in the National Immunization Survey (NIS) repsonse rates and vaccination coverage rates. Available at: http://www.cdc.gov/nis/notice.htm. Accessed 23 Sep 2008.
- 54.Oldendick RW. The answering machine generation: who are they and what problem do they pose for survey research? Public OpinQ. 1994;58:264–73.55. [Google Scholar]
- 55.Blumberg SJ, Luke JV. Wireless substitution: early release of estimates based on data from the National Health Interview Survey, July–December 2006. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/nhis.htm. Accessed 9 Sep 2008. [Google Scholar]
- 56.Blumberg SJ, Luke JV, Cynamon ML. Telephone coverage and health survey estimates: evaluating the need for concern about wireless substitution. Am J Public Health. 2006;96(5):926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White E, Armstrong B, Saracci R. Principles of exposure measurement in epidemiology: collecting, evaluating and improving measures of disease risk factors. 2nd ed. New York: Oxford University Press; 2008. [Google Scholar]
- 58.Austin MA, Criqui MH, Barrett-Connor E, et al. The effect of response bias on the odds ratio. Am J Epidemiol. 1981;114(1): 137–43. [DOI] [PubMed] [Google Scholar]


