Abstract
Background
The efficacy and safety of opioid-free anaesthesia during bariatric surgery remain debated, particularly when administering multimodal analgesia. As multimodal analgesia has become the standard of care in many centres, we aimed to determine if such a strategy coupled with either dexmedetomidine (opioid-free anaesthesia) or remifentanil with a morphine transition (opioid-based anaesthesia), would reduce postoperative morphine requirements and opioid-related adverse events.
Methods
In this prospective double-blind study, 172 class III obese patients having laparoscopic gastric bypass surgery were randomly allocated to receive either sevoflurane–dexmedetomidine anaesthesia with a continuous infusion of lidocaine and ketamine (opioid-free group) or sevoflurane–remifentanil anaesthesia with a morphine transition (opioid-based group). Both groups received at anaesthesia induction a bolus of magnesium, lidocaine, ketamine, paracetamol, diclofenac, and dexamethasone. The primary outcome was 24-h postoperative morphine consumption. Secondary outcomes included postoperative quality of recovery (QoR40), incidence of hypoxaemia, bradycardia, and postoperative nausea and vomiting (PONV).
Results
Eighty-six patients were recruited in each group (predominantly women, 70% had obstructive sleep apnoea). There was no significant difference in postoperative morphine consumption (median [inter-quartile range]: 16 [13–26] vs 15 [10–24] mg, P=0.183). The QoR40 up to postoperative day 30 did not differ between groups, but PONV was less frequent in the opioid-free group (37% vs 59%, P=0.005). Hypoxaemia and bradycardia were not different between groups.
Conclusions
During bariatric surgery, a multimodal opioid-free anaesthesia technique did not decrease postoperative morphine consumption when compared with a multimodal opioid-based strategy. Quality of recovery did not differ between groups although the incidence of PONV was less in the opioid-free group.
Clinical trial registration
Keywords: dexmedetomidine, enhanced recovery after surgery, hypoxaemia, nausea, nociception, pain, remifentanil, vomiting
The systematic use of morphine or other opioids during surgery has been called into question in recent years. The liberal use of opioids can expose patients to side-effects which include excessive sedation, respiratory depression, postoperative nausea and vomiting (PONV), ileus, and urinary retention.1,2 It may also cause hyperalgesia, which has been closely linked to the amount of postoperative morphine consumed, and can increases the risk of chronic postsurgical pain.3 Obese patients are at particular risk of opioid-related adverse events, especially hypoxaemia, as they often suffer from obstructive sleep apnoea.4
Opioid-free anaesthesia (OFA) aims at replacing intraoperative opioids by combining non-opioid analgesics (e.g. dexmedetomidine, magnesium, lidocaine, dexamethasone, non-steroidal anti-inflammatory drugs [NSAIDs], and ketamine) to inhibit nociceptive input at different targets. Although several studies have reported that OFA may offer some benefits regarding postoperative comfort (e.g. decrease in pain and PONV), postoperative morphine consumption, or patient quality of recovery,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 doubt remains about its efficacy and safety.17 This is especially because of the lack of well conducted double-blinded, randomised controlled trials. Indeed, as most studies that show a positive effect of OFA do not blind the anaesthesiologist and nursing staff, these studies may have inadvertently created bias in favour of the interventional group. Furthermore, a recent large multicentre single-blinded study that compared dexmedetomidine with remifentanil during abdominal surgery was terminated early because of safety concerns.18 Opioid requirements and PONV were decreased, but the higher incidence of severe bradycardia and, paradoxically, postoperative hypoxaemia in the OFA group alarmed investigators.18
Multimodal analgesia in combination with opioids is increasingly being used in clinical care. This approach integrates many of the same drugs used during OFA (e.g. i.v. lidocaine, ketamine, NSAIDs, paracetamol, and dexamethasone), with the aim to decrease the need for opioids and reduce the incidence of opioid side-effects.19 Determining the difference between OFA and opioid-based anaesthesia (OBA) strategies thus requires further investigation with high-quality blinded studies of homogenous populations. This is especially the case for obese patients who are at high risk of opioid-related adverse events, especially hypoxaemia, as they often suffer from obstructive sleep apnoea.
This single-centre, prospective, randomised, double-blind trial tested the hypothesis that class III obese patients who undergo laparoscopic gastric bypass surgery using a multimodal OFA technique, when compared with a multimodal OBA technique, will consume less morphine after surgery.
Methods
Study design
This prospective, parallel-group, double-blind, randomised controlled superiority study was conducted at Chirec Delta Hospital (Brussels, Belgium). The study was approved by the Ethics committee of Erasme Hospital in Brussels on 10 August 2021 under the reference P2021/378/B4062021000196 and was registered on clinicaltrials.gov on 13 August 2021 under the number NCT05004519 before patient inclusion (principal investigator: MC). Written informed consent was obtained from all patients before surgery.
Patients
Inclusion criteria were severe obese class III patients (previously known as morbid obesity)20 (BMI >40 kg m−2 or >35 kg m−2 with at least one of the following comorbidities: diabetes mellitus, obstructive sleep apnoea syndrome, or arterial hypertension requiring triple therapy) aged ≥18 yr scheduled for elective laparoscopic gastric bypass surgery and who provided written informed consent.
Exclusion criteria were known allergies to any of the drugs used for anaesthesia or to any of their excipients; pregnancy or breastfeeding; atrioventricular, intraventricular, or sinoatrial block; and patients with a heart rate <50 beats min−1 at the preoperative consultation.
Randomisation, blinding, and data collection
An independent anaesthesiologist (AJ) who was not involved in patient management and who was not practicing at Delta Hospital randomly allocated patients (internet-based randomisation software: http://www.randomization.com, randomisation plan created on 1 October 2021, 09:23:42 UTC). Opaque envelopes labelled with the patient study number and containing the group allocation were then generated and provided to the research team by the same anaesthesiologist. The envelopes were kept in a locked box in the anaesthesiology department. On the morning of the surgery, the sealed opaque envelope containing the assigned patient number was opened by an independent anaesthesiologist who had no other role in the patients' perioperative management and who then prepared the blinded study solutions. Patients, surgeons, anaesthetists, post-anaesthesia care unit (PACU) nurses, and the statistician were all blinded to group allocation. Intraoperative data were collected by the principal investigator and postoperative data by nurses not involved in the trial and blinded to group allocation. All investigators remained blinded to the treatment allocation until the end of the study and the finalisation of the statistical analysis.
Study intervention
The independent anaesthesiologist not involved in any other aspect of the included patient's care prepared the different blinded solutions: a 100-ml infusion bag, a 1-ml syringe, and two supplementary 50 ml syringes for continuous drug infusion (syringe 1 and 2). Fig 1 shows the study design and medications received in each group.
Fig 1.
Study medications and timing. The study intervention consisted of four blinded interventions administered with a 100-ml infusion bag, a 1-ml syringe, and two 50-ml syringes. All medications were administered according to ideal bodyweight. OBA, opioid-based anaesthesia group; OFA, opioid-free anaesthesia group; PACU, post-anaesthesia care unit.
In the OFA group, the infusion bag of NaCl 0.9%, 100 ml contained dexmedetomidine 0.5 μg kg−1 and magnesium 40 mg kg−1. This infusion bag was administered during the 10 min preceding anaesthetic induction with propofol, ketamine, and lidocaine which both groups received. Syringe 1 contained dexmedetomidine 2 μg ml−1 and was infused at speeds of 0.2–0.4 ml kg−1 h−1, which corresponds to a dose of 0.4–0.8 μg kg−1 h−1. Syringe 2 contained lidocaine 2%, 49 ml and ketamine 50 mg, 1 ml. It was infused at 2 ml kg−1 h−1. The infusions in both syringes were started at induction of anaesthesia. Syringe 1 was stopped and a bolus of 1 ml of blinded NaCl 0.9% was administered upon completion of the surgical methylene blue test. The infusion in syringe 2 was continued until the end of the PACU stay at a reduced rate of 1 ml kg−1 h−1
In the OBA group, the infusion bag of NaCl 0.9%, 100 ml contained magnesium 40 mg kg−1. This infusion bag was administered during the 10 min preceding anaesthetic induction with propofol, ketamine, and lidocaine. Syringe 1 contained remifentanil 60 μg ml−1 and was infused at speeds of 0.2–0.4 ml kg−1 h−1, which corresponds to a dose of 0.2–0.4 μg kg−1 min−1. Syringe 2 contained NaCl 0.9%, 50 ml. It was infused at 2 ml kg−1 h−1. The infusions in both syringes were started at induction of anaesthesia. Syringe 1 was stopped and a bolus of 1 ml of blinded 10 mg morphine was administered upon completion of the surgical methylene blue test. The infusion in syringe 2 was continued until the end of the PACU stay at a reduced rate of 1 ml kg−1 h−1.
Anaesthetic management
The same surgeon and anaesthetist cared for all patients. Thromboprophylaxis was started the evening before surgery and consisted of a subcutaneous injection of low molecular weight heparin (enoxaparin 40 mg) which was continued for 10 days after surgery. Patients fasted 6 h for solids and 2 h for clear liquids before surgery. Apple juice (400 ml the evening before surgery and 2 h before surgery) was systematically given unless the patient was diabetic. No patient received anxiolytic drugs before surgery. All patients were warmed in the pre-induction room with a pulsed hot air cushion (3M™ Bair Hugger™, Saint Paul, MN, USA). Monitoring in the two groups was standardised and included a five-lead electrocardiogram, a noninvasive pulse oximetry which provided the pleth variability index (PVI, Masimo, Irvine, CA, USA), a noninvasive upper arm cuff recorded the arterial blood pressure every 5 min, a depth of anaesthesia monitoring system (Sed-Line, Masimo, Irvine, CA, USA), TOF-Scan (Idmed, Marseille, France), and the nociception level index (NOL) monitoring device (Medasense, Tel Aviv, Israel). NOL monitoring was blinded in both groups for an ancillary study. As a result, all patients were monitored with the NOL, but the data displayed were not available to the anaesthesiologist in charge of the patient. Data were collected at the end of the surgery for a potential secondary paper.
After the placement of one i.v. catheter (20 G in all patients), antibiotic prophylaxis was administered (cefazoline 2 g if patient's weight <120 kg or 3 g if >120 kg). After 5 min of pre-oxygenation to reach an expiratory fraction of oxygen of at least 90%, with the patient positioned in the reverse Trendelenburg position, anaesthesia was induced with propofol (2 mg kg−1), i.v. lidocaine (1.5 mg kg−1), i.v. ketamine (0.5 mg kg−1), and a neuromuscular blocking agent (rocuronium 1.2 mg kg−1) was given. All drug doses administered in mg kg−1 were calculated according to the patient's ideal body weight.21
Before surgical incision, all patients received paracetamol 15 mg kg−1, diclofenac 75 mg, dexamethasone 8 mg, and ondansetron 4 mg. Maintenance of anaesthesia was with sevoflurane in an O2/air mixture of 40%/60%, adapted to maintain the SedLine value between 35 and 50. Rocuronium boluses (10 mg) were given to maintain the post-tetanic count ≤1/10.
Fluid and vasopressor administration were also standardised in both groups. Fluids consisted of 1000 ml h−l baseline maintenance with a balanced crystalloid (Plasmalyte®, Baxter, Lessines, Belgium) and fluid challenges of 6 ml kg−1 over 10 min if PVI was >12% for more than 5 consecutive minutes during surgery. The mean arterial pressure was maintained at >65 mm Hg using ephedrine boluses. Ventilation settings included a tidal volume of 6–7 ml kg−1 of ideal body weight and a positive end-expiratory pressure value of 10 cm H2O. The ventilatory frequency was adjusted to maintain an end-tidal CO2 between 4.3 and 4.8 kPa. Recruitment manoeuvres were performed at least twice during surgery (after induction and before waking up the patient) but could be done at other times at the anaesthetist's discretion.
After completion of the surgical procedure, neuromuscular block was antagonised with sugammadex (4 mg kg−1). The tracheas of all patients were extubated in the operating room before transfer to the PACU. Oxygen was administered to the patient to ensure that the SpO2 was maintained >95%. The surgical technique was standardised and is detailed in Supplementary material 1.
Postoperative care
In both groups, postoperative analgesia consisted of paracetamol (1 g per 6 h), diclofenac (75 mg every 12 h for the first 2 postoperative days) and morphine titration in the PACU if visual analogue scale (VAS) was > 3 (2 mg per bolus). After PACU discharge (Aldrete score >9), morphine i.v. was self-administered by the patient using a patient-controlled analgesia pump (PCA) according to routine care (bolus of 1 mg with 5 min lock-out, and a maximum dose of 20 mg every 4 h for the first 24 h after surgery). PONV was assessed in the PACU (by the nurse), and 4 h and 24 h after surgery (by a member of the anaesthesia staff) and treated with alizapride if necessary. Patients received oxygen supplementation only when their SpO2 was <95%. Postoperative i.v. fluids consisted of a balanced crystalloid solution (Ringer's lactate solution) 1 L per 24 h. All other postoperative care was performed according to local practices. Sips of water were allowed, and patients were encouraged to get up and ambulate as soon as they returned to the ward. The preoperative, postoperative, and day +30 quality of recovery ‘Quality of Recovery-40 questionnaire’ (QOR 40) score was assessed by a data manager not involved in the study.
Outcomes
The primary outcome was total morphine consumption in the first 24 h after surgery. It was the sum of morphine titrated by the PACU nurses and the morphine administered by PCA at 24 h after surgery. Importantly, the 10 mg of blinded morphine given in the OBA group was not considered as it was given systematically during the intraoperative period to bridge analgesia upon remifentanil discontinuation.
Secondary outcomes included morphine consumption in the PACU and with the PCA, intensity of pain assessed by VAS at PACU arrival, 4 and 24 h after surgery, incidence of postoperative hypoxaemia (defined as an SpO2 <90% with 2 L min−1 of oxygen supplementation), bradycardia (defined as a heart rate <40 beats min−1) requiring treatment, PONV, antiemetic rescue treatment, and quality of recovery using the QoR40 scale. Exploratory outcomes included PACU and hospital length of stay, readmission after hospital discharge, and the incidence of any surgical complication at postoperative day 30.
Statistical methods
Local retrospective data analysis revealed that when OBA is applied (current institutional standard of care), patients consume 16.15 plus or minus 10.33 mg of morphine during the first 24 h after surgery. We calculated that 84 patients per group were required to have 80% power at a two-sided α level of 0.05 to demonstrate a 25% decrease in postoperative morphine consumption in the OFA group (absolute reduction of 4 mg). Considering potential dropout, we decided to include 172 patients.
For continuous variables, we tested the underlying assumptions of the t-test (homogeneity of the variances with the help of the Bartlett's homogeneity test and the normality of the residuals with the Shapiro–Wilk test). If both underlying assumptions were met, a t-test was performed on the data and the mean (standard deviation) are presented. Otherwise, the Wilcoxon signed-rank test was performed and the median and [25–75] percentiles are presented. Categorical variables were analysed with the χ2 test and counts and percentages for counts data are presented. We used linear mixed models to model the evolution of QoR40 over time.22 For all models, the following effects were tested: a group effect, a time effect, a time2 effect, a group × time interaction effect and a group × time2 interaction effect. We looked at the residuals of the model, and if these were not normally distributed, we used the bestNormalize R package to transform the outcome, and reported the results of this last linear mixed model. Maximum likelihood (ML) and multiple imputation (MI) use all available data in the study, produce unbiased estimates of the treatment effect, and correct P-values. We therefore presented the ML results, given the small number of missing values. All analyses were carried out by an independent statistician (JFF), who was blinded to group allocation. R software (R Core Team, 2021, Vienna, AU), version 4.2.0 was used for statistical analysis. For the primary outcome, a P-value <0.05 was considered as statistically significant. For all other outcomes, we did not apply corrections for multiple comparisons and the P-value should be considered as exploratory.
Results
A total of 183 patients were screened, 11 of whom declined to participate. A total of 172 class III obese patients were randomly allocated into two groups of 86 patients each. No patient was lost to follow-up and all patients were studied with an intention to treat approach (Fig 2). Patients were predominantly women and more than two-thirds of patients suffered from obstructive sleep apnoea. The patients' baseline characteristics were similar between groups (Table 1). Intraoperative data revealed that patients in the OFA group received a lower volume of fluid during surgery (1000 ml [1000–1300 ml] vs 1260 ml [1000–1350 ml]; P=0.031) (Table 2).
Fig 2.
Consort flowchart. OBA, opioid-based anaesthesia group; OFA, opioid-free anaesthesia group.
Table 1.
Baseline characteristics. Data are listed as number and (%) or mean (standard deviation) or median and [25th–75th] percentiles. ASA, American Society of Anesthesiology physical status; OBA, opioid-based anaesthesia; OFA, opioid-free anaesthesia.
| Variables | OFA group (N=86) | OBA group (N=86) |
|---|---|---|
| Age (yr) | 39 [32–48] | 37 [29–48] |
| Male (%) | 20 (23) | 19 (22) |
| Weight (kg) | 107 [99–120] | 110 [98–124] |
| Height (cm) | 165 [159–172] | 165 [160–175] |
| BMI (kg m−2) | 40 [36–42] | 40 [37–42] |
| ASA physical status 2/3 | 75 (87)/11 (13) | 81 (94)/5 (6) |
| Quality of recovery using the QoR-40 | 192 (6) | 193 (6) |
| Medications, n (%) | ||
| β-Blocker | 11 (13) | 10 (11) |
| Angiotensin-converting enzyme inhibitor | 7 (8) | 7 (8) |
| Angiotensin II receptor blockers | 5 (6) | 3 (3) |
| Aspirin | 2 (2) | 3 (3) |
| Calcium blocker | 6 (7) | 2 (2) |
| Hypoglycaemic agent, insulin, or both | 12 (14) | 10 (11) |
| Comorbidities, n (%) | ||
| Obstructive sleep apnoea syndrome | 59 (69) | 62 (71) |
| Arterial hypertension | 21 (24) | 17 (20) |
| Diabetes (any type) | 10 (12) | 7 (8) |
| Ischaemic heart disease | 1 (1) | 1 (1) |
Table 2.
Intraoperative data. Data are expressed as number and (%), or median and [25th–75th] percentiles. OBA, opioid-based anaesthesia; OFA, opioid-free anaesthesia; PVI, pleth variability index. ∗From stopping i.v. drugs and sevoflurane to extubation.
| Variables | OFA group (N=86) | OBA group (N=86) | P value |
|---|---|---|---|
| Anaesthesia duration (min) | 56 [50–63] | 59 [54–70] | 0.005 |
| Surgery duration (min) | 43 [38–50] | 43 [38–54] | 0.543 |
| Crystalloid bolus (ml) | 333 [300–391] | 350 [305–400] | 0.530 |
| Number of patients with a PVI >12% | 30 (35) | 45 (52) | 0.031 |
| Total crystalloid (ml) | 1000 [1000–1300] | 1260 [1000–1350] | 0.031 |
| Total ephedrine (mg) | 10 [6–12] | 10 [6–15] | 0.173 |
| Number of patients receiving ephedrine | 38 (44) | 37 (43) | 0.878 |
| Remifentanil (mg) | 0 | 0.65 [0.50–0.84] | |
| Dexmedetomidine (μg) | 20.4 [16.6–24.4] | 0 | |
| Time to wake up (min)∗ | 4 [3–5] | 8 [6–10] | <0.001 |
Bold character is significant results with a p value <0.05.
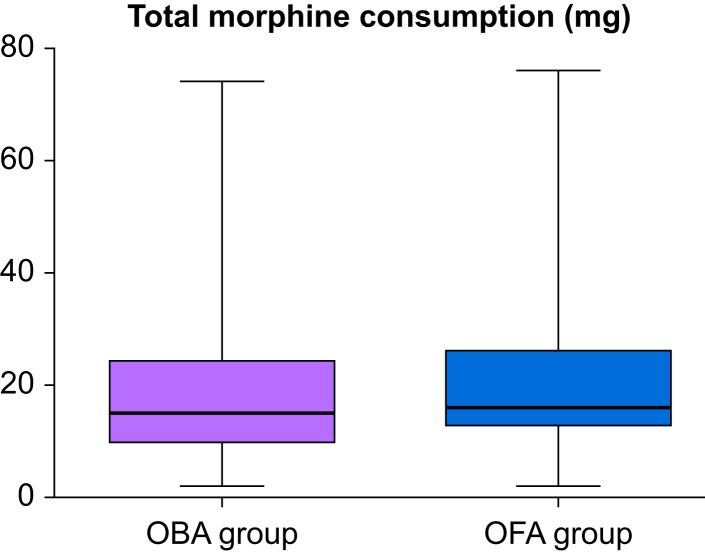
There was no difference in the primary outcome of total morphine consumption at 24 h after surgery median [inter-quartile range]: 16 mg [13–26 mg] vs 15 mg [10–24 mg], difference −2, 95% confidence interval [−5.0 to 1.0]; P=0.183 (Fig 3). Three patients in the OFA group and four patients in the OBA group did not receive morphine in the PACU. All patients requested morphine during their hospital stay. Moreover, 11 patients received >40 mg of morphine during the first 24 h after surgery (six in the OFA group and five in the OBA group). The VAS was higher at 4 h after surgery in the OFA group surgery (median [inter-quartile range]: 4 [3–5] vs 3 [2–4], P=0.015). There was no difference in the quality of recovery scores on postoperative days 1 and 30 (Fig 3). PONV and antiemetic administration, however, were less frequent in the OFA group (Table 3). There was no significant difference between groups in the incidence of bradycardia or postoperative hypoxaemia (Table 3). QoR40 did not differ between groups before surgery, at postoperative days 1 and 30 (Fig 4). Supplementary material 2 provides the QoR40 data and the fixed-effects of the mixed model.
Fig 3.
Primary outcome of total postoperative morphine consumption. Box plot presentation (median, percentiles 25–75 and min-max).OBA, opioid-based anaesthesia group; OFA, opioid-free anaesthesia group.
Table 3.
Outcome data. Data are expressed as number and (%), or median and [25th–75th] percentiles. ICU, intensive care unit; OBA, opioid-based anaesthesia; OFA, opioid-free anaesthesia; PACU, post-anaesthesia care unit; PCA, patient-controlled analgesia; PONV, postoperative nausea and vomiting. ∗From PACU arrival to up to 24 h post-surgery.
| Variables | OFA group (N=86) | OBA group (N=86) | P value |
|---|---|---|---|
| Primary outcome | |||
| Total postoperative morphine consumption (mg)∗ | 16 [13–26] | 15 [10–24] | 0.183 |
| Secondary outcomes | |||
| Total PACU morphine titration by ICU nurses (mg) | 8.0 [4.0–10.0] | 6.5 [4.0–10.0] | 0.703 |
| Total PCA morphine consumption (mg) | 9.5 [7.0–16.0] | 8.0 [4.0–14.0] | 0.087 |
| Visual analogue scale | |||
|
4 [3–5] | 4 [3–4] | 0.839 |
|
4 [3–5] | 3 [2–4] | 0.015 |
|
2 [1–3] | 2 [1–4] | 0.228 |
| Incidence of hypoxaemia, N (%) | 1 (1) | 7 (8) | 0.070 |
| PONV from PACU admission to 24 h post-surgery, N (%) | 32 (37) | 51 (59) | 0.005 |
|
30 (35) | 46 (53) | 0.021 |
|
9 (10) | 20 (23) | 0.041 |
|
7 (8) | 14 (16) | 0.162 |
| Patients requiring antiemetic treatment, N (%) | 32 (37) | 49 (57) | 0.015 |
| Bradycardia requiring atropine administration, N (%) | 2 (2) | 4 (5) | 0.681 |
| Exploratory outcomes | |||
| Any postoperative complications at day 30 | 0 (0) | 4 (5) | 0.120 |
| Readmission to the hospital after discharge, N (%) | 0 (0) | 4 (5) | 0.120 |
| Length of stay in the PACU (min) | 52 [35–75] | 58 [30–85] | 0.527 |
| Length of stay in the hospital (days) | 1 [1–1] | 1 [1–1] | 0.700 |
Bold character is significant results with a p value <0.05.
Fig 4.
Quality of recovery-40 questionnaire scores. Scores were measured before surgery, 1 day after surgery, and 30 days after surgery. OFA, opioid-free anaesthesia group; OBA, opioid-based anaesthesia group; QoR-40, Quality of recovery-40. ∗P<0.001 vs preop; †P<0.001 vs postoperative day 1.
Discussion
In this double-blind, single-centre, randomised controlled trial of obese class III patients undergoing gastric bypass surgery under general anaesthesia, OFA, when compared with OBA, was not associated with decreased postoperative morphine consumption. Visual analogue pain scores were even slightly higher at 4 h after surgery in patients who received OFA. However, patients in the OFA group had less PONV. Despite this, there was no difference in the quality of recovery scores on postoperative days 1 and 30. In addition, there was no significant difference in intraoperative bradycardia or postoperative hypoxaemia. It is important to underline that the studied population was homogeneous with respect to the type of surgery and the prevalence of grade III obesity, obstructive sleep apnoea, and female gender.
Several studies have investigated the impact of various OFA protocols on patients undergoing bariatric surgery.13,14,23, 24, 25 A recent meta-analysis demonstrated that these strategies, despite significant heterogeneity, were associated with decreased immediate postoperative pain.26 A pooled analysis also demonstrated decreased PACU morphine consumption and PONV. However, similarly to our results, 24 h postoperative morphine consumption was not different. Of note, most of these studies were only patient blinded and may thus suffer from performance bias, which was minimised in our study as patients, anaesthetists, nurses, surgeons, investigators, and the statistician were all blinded to group allocation. Furthermore, none of the studies included in this meta-analysis used a combination of remifentanil with a morphine bolus in the opioid group. Finally, in our study, perioperative multimodal analgesia was used in both the OFA and the OBA groups. Such a strategy may have provided additional analgesia in both groups that more radical approaches of pure opioid and opioid-free antinociception do not provide.
Beloeil and colleagues18 recently published the Postoperative and Opioid-free Anesthesia (POFA) multicentre randomised controlled trial that compared OFA with OBA during noncardiac surgery. In this study of a heterogeneous patient population, anaesthesiologists administered remifentanil antinociception coupled with a morphine transition of 0.05 mg kg−1 in the OBA group. In addition to ketamine and lidocaine boluses at induction, patients also had continuous doses of these agents during surgery in both groups. Patients in the OFA group received dexmedetomidine instead of remifentanil and had no morphine transition. OFA patients required less postoperative morphine and had less PONV. There was, however, a paradoxical and significantly higher incidence of hypoxaemia in the OFA group. Despite many patients in both groups suffering from obstructive sleep apnoea in our study, hypoxaemia occurred much less frequently than in the POFA study (i.e. 1% vs 8% and 8% vs 67% in the OFA and OBA groups, respectively). One reason for this difference between the two studies is the threshold defining hypoxaemia (i.e. an SpO2 of 95% in the POFA study vs 90% in our study). Other reasons may exist, such as the higher dose of dexmedetomidine used in the POFA study. Another observation that differs considerably between the POFA study and our study: the incidence of severe bradycardia. The POFA study was stopped prematurely because of an excessive incidence of bradycardia in the OFA group. Again, this adverse event was not reproduced in our study. The dose of dexmedetomidine probably plays a role, as the maximum infusion rate of dexmedetomidine in our study was set at 0.8 μg kg−1 h−1 while the mean infusion rate in the POFA study was 1.2 plus or minus 2 μg kg−1 h−1. Bradycardia is a side-effect observed with both remifentanil and dexmedetomidine and clinicians must administer these drugs in their therapeutic ranges to reduce their side-effects.27 Although our study does not demonstrate the superiority of the OFA technique for reducing postoperative morphine consumption, it suggests that dexmedetomidine antinociception may be safe when used in therapeutic doses during laparoscopic gastric bypass surgery in obese patients at high risk of obstructive apnoea.
The sophisticated blinding process and the homogeneity of the study population represent the main strength of our study. Some limitations must however be considered. Firstly, one may argue that giving morphine 10 mg at the end of the surgery in the OBA group and not considering it in the primary objective could create bias. However, our primary outcome was predefined as postoperative morphine consumption only from PACU admission to 24 h after surgery. As such, the morphine 10 mg transition given intraoperatively as our standard of care during remifentanil OBA in our institution was not added to the morphine consumed after surgery. Additionally, although this amount is greater than that patients received in the large POFA trial (0.05 mg kg−1),18 it is in our opinion more than reasonable for obese class III patients who have a median weight of >100 kg. Furthermore, a morphine 10 mg transition was our standard of care during remifentanil OBA and the patients in our centre who provided data for the sample size calculation required on average an additional morphine 16 mg after surgery. These elements helped us conclude that reducing the morphine transition dose would induce unnecessary pain to our patients. Despite this potential limitation, our study is to our knowledge the first double-blind, randomised, controlled trial that compares remifentanil with dexmedetomidine during multimodal antinociception for bariatric surgery. Secondly, the definition of a minimal clinically important difference in opioid consumption remains difficult to define, despite its frequent use as primary outcome in trials on postoperative pain management. Some may argue that the 25% morphine reduction (4 mg in absolute value) used to calculate our sample size may not be enough. Although it is true that the minimal clinically important difference of morphine milligram equivalents commonly recommended for postoperative pain control is an absolute reduction of i.v. morphine 10 mg in the 24 h after surgery,28 a recent paper on OFA used a 30% oral morphine equivalent reduction in their primary outcome in patients having hip surgery.29 This should correspond approximately to a reduction of i.v. morphine 4 mg in our study. Furthermore, Zhao and colleagues30 found, in 193 patients undergoing laparoscopic cholecystectomy, that every i.v. morphine 3–4 mg equivalent resulted in a ‘clinically meaningful event’ in one of 12 different opioid-related effects. Whereas it is true that this is not our study population, we may also presume that for obese patients, each morphine equivalent spared may be beneficial. Thirdly, both groups received multimodal analgesia that combined ketamine, lidocaine, magnesium, diclofenac, dexamethasone, and paracetamol boluses. This may limit the generalisation of our results. However, clinicians using intraoperative opioids adopt more and more frequently a multimodal analgesia strategy in order to reduce the amounts of opioids administered to their patients. Lastly, all patients received dexamethasone and ondansetron at anaesthesia induction and alizapride in the PACU if they had PONV. Although this is not based on the Apfel score, it was our local practice before the study and the results should not be biased by this practice as all patients received this treatment in this randomised, double-blind study. Also, a previous OFA trial focusing on PONV also used the same strategy of dexamethasone and ondansetron administered routinely after anaesthesia induction.14
In conclusion, during laparoscopic gastric bypass surgery a multimodal OFA technique did not decrease postoperative morphine consumption when compared with a multimodal OBA strategy. The quality of recovery up to postoperative day 30 did not differ between groups, although PONV was less frequent in the OFA group.
Authors’ contributions
Study design/conception: MC, KT, CEH, NG, JFF, JH, AJ, SC, YG, PVdL, ACD
Study conduct: MC, CEH, KT, NG, JH, ACD
Data analysis: AJ, SC, PVdL, YG, JFF
Writing paper: SC, AJ, PVdL, JFF
Editing paper: all authors
Revising paper: all authors
Declarations of interest
AJ is a consultant for Edwards Lifesciences, Irvine, CA, USA. SC has received honoraria for presentations from Medtronic, Medasense, and Med-Storm. All other authors declare that they have no conflicts of interest.
Funding
Departmental sources.
Acknowledgements
The authors thank the following persons for their help in the currect study: Maria De luliis, Cynthia Dessart, Severing Mohr, Celine Renard, Anais Tasiaux, Jean-Philippe Goreux.
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2024.100263.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Dinges H.C., Otto S., Stay D.K., et al. Side effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Analg. 2019;129:1153–1162. doi: 10.1213/ANE.0000000000003887. [DOI] [PubMed] [Google Scholar]
- 2.de Boer H.D., Detriche O., Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31:499–504. doi: 10.1016/j.bpa.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Lavand'homme P., Steyaert A. Opioid-free anesthesia opioid side effects: tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. 2017;31:487–498. doi: 10.1016/j.bpa.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Ingrande J., Lemmens H.J. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105(Suppl 1):i16–i23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]
- 5.Sin J.C.K., Tabah A., Mjj Campher, Laupland K.B., Eley V.A. The effect of dexmedetomidine on postanesthesia care unit discharge and recovery: a systematic review and meta-analysis. Anesth Analg. 2022;134:1229–1244. doi: 10.1213/ANE.0000000000005843. [DOI] [PubMed] [Google Scholar]
- 6.Miao M., Xu Y., Li B., Chang E., Zhang L., Zhang J. Intravenous administration of dexmedetomidine and quality of recovery after elective surgery in adult patients: a meta-analysis of randomized controlled trials. J Clin Anesth. 2020;65 doi: 10.1016/j.jclinane.2020.109849. [DOI] [PubMed] [Google Scholar]
- 7.Léger M., Perrault T., Pessiot-Royer S., et al. Opioid-free anesthesia protocol on the early quality of recovery after major surgery (SOFA trial): a randomized clinical trial. Anesthesiology Adv Access Published Nov. 2023;17 doi: 10.1097/ALN.0000000000004840. [DOI] [PubMed] [Google Scholar]
- 8.Hublet S., Galland M., Navez J., et al. Opioid-free versus opioid-based anesthesia in pancreatic surgery. BMC Anesthesiol. 2022;22:9. doi: 10.1186/s12871-021-01551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J.M., Tao Q.Y., He Y., Liu D., Niu J.Y., Zhang Y. Opioid-free anesthesia for pain relief after laparoscopic cholecystectomy: a prospective randomized controlled trial. J Pain Res. 2023;16:3625–3632. doi: 10.2147/JPR.S432601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Ma D., Lang B., et al. Effect of opioid-free anesthesia on the incidence of postoperative nausea and vomiting: a meta-analysis of randomized controlled studies. Medicine (Baltimore) 2023;102 doi: 10.1097/MD.0000000000035126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Cui Y., Cao L. The effect of opioid-free anaesthesia on the quality of recovery after endoscopic sinus surgery: a multicentre randomised controlled trial. Eur J Anaesthesiol. 2023;40:542–551. doi: 10.1097/EJA.0000000000001784. [DOI] [PubMed] [Google Scholar]
- 12.Cha N.H., Hu Y., Zhu G.H., Long X., Jiang J.J., Gong Y. Opioid-free anesthesia with lidocaine for improved postoperative recovery in hysteroscopy: a randomized controlled trial. BMC Anesthesiol. 2023;23:192. doi: 10.1186/s12871-023-02152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulbing S., Infanger L., Fleischmann E., Prager G., Hamp T. The performance of opioid-free anesthesia for bariatric surgery in clinical practice. Obes Surg. 2023;33:1687–1693. doi: 10.1007/s11695-023-06584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziemann-Gimmel P., Goldfarb A.A., Koppman J., Marema R.T. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 2014;112:906–911. doi: 10.1093/bja/aet551. [DOI] [PubMed] [Google Scholar]
- 15.Feng C.D., Xu Y., Chen S., et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. 2024;132:267–276. doi: 10.1016/j.bja.2023.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Hao C., Xu H., Du J., et al. Impact of opioid-free anesthesia on postoperative quality of recovery in patients after laparoscopic cholecystectomy – a randomized controlled trial. Drug Des Devel Ther. 2023;17:3539–3547. doi: 10.2147/DDDT.S439674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feenstra M.L., Jansen S., Eshuis W.J., van Berge Henegouwen M.I., Hollmann M.W., Hermanides J. Opioid-free anesthesia: a systematic review and meta-analysis. J Clin Anesth. 2023;90 doi: 10.1016/j.jclinane.2023.111215. [DOI] [PubMed] [Google Scholar]
- 18.Beloeil H., Garot M., Lebuffe G., et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. 2021;134:541–551. doi: 10.1097/ALN.0000000000003725. [DOI] [PubMed] [Google Scholar]
- 19.Ghai B., Jafra A., Bhatia N., Chanana N., Bansal D., Mehta V. Opioid sparing strategies for perioperative pain management other than regional anaesthesia: a narrative review. J Anaesthesiol Clin Pharmacol. 2022;38:3–10. doi: 10.4103/joacp.JOACP_362_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitahara C.M., Flint A.J., Berrington de Gonzalez A., et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 22.Geert Molenberghs G.V. Springer Series in Statistics (SSS) Verbeke & Molenberghs; 2001. Linear mixed models; p. 2000. [Google Scholar]
- 23.Berlier J., Carabalona J.F., Tête H., et al. Effects of opioid-free anesthesia on postoperative morphine consumption after bariatric surgery. J Clin Anesth. 2022;81 doi: 10.1016/j.jclinane.2022.110906. [DOI] [PubMed] [Google Scholar]
- 24.Torre A., Marengo M., Ledingham N.S., et al. Opioid-free anesthesia in bariatric surgery: a propensity score-matched analysis. Obes Surg. 2022;32:1673–1680. doi: 10.1007/s11695-022-06012-0. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed S.A., Abdelghany M.S., Afandy M.E. The effect of opioid-free anesthesia on the post-operative opioid consumption in laparoscopic bariatric surgeries: a randomized controlled double-blind study. J Opioid Manag. 2022;18:47–56. doi: 10.5055/jom.2022.0694. [DOI] [PubMed] [Google Scholar]
- 26.Hung K.C., Chiu C.C., Hsu C.W., et al. Impact of opioid-free anesthesia on analgesia and recovery following bariatric surgery: a meta-analysis of randomized controlled studies. Obes Surg. 2022;32:3113–3124. doi: 10.1007/s11695-022-06213-7. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu R., Turan A.M., Orhan-Sungur M., McGuire J., Radke O.C., Apfel C.C. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62:1266–1280. doi: 10.1111/j.1365-2044.2007.05221.x. [DOI] [PubMed] [Google Scholar]
- 28.Laigaard J., Pedersen C., Rønsbo T.N., Mathiesen O., Karlsen A.P.H. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: a systematic review. Br J Anaesth. 2021;126:1029–1037. doi: 10.1016/j.bja.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Chassery C., Atthar V., Marty P., et al. Opioid-free versus opioid-sparing anaesthesia in ambulatory total hip arthroplasty: a randomised controlled trial. Br J Anaesth. 2024;132:352–358. doi: 10.1016/j.bja.2023.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Zhao S.Z., Chung F., Hanna D.B., Raymundo A.L., Cheung R.Y., Chen C. Dose-response relationship between opioid use and adverse effects after ambulatory surgery. J Pain Symptom Manage. 2004;28:35–46. doi: 10.1016/j.jpainsymman.2003.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.