Abstract
Objectives:
We wished to assess time to protection from HIV-1 infection following oral tenofovir disoproxil and emtricitabine (TDF/FTC) as preexposure prophylaxis (PrEP), using ex-vivo rectal tissue infections and drug concentration measures in blood and rectal tissue.
Design/Methods:
Participants from the ANRS PREVENIR study (NCT03113123) were offered this sub-study after a 14-day wash-out. We used an ex-vivo model to evaluate rectal tissue HIV-1 susceptibility before and after PrEP, 2 h after two pills or 7 days of a daily pill of TDF/FTC. PrEP efficacy was expressed by the difference (after-before) of 14-day cumulative p24 antigen levels. TFV-DP and FTC-TP levels were measured in rectal tissue and PBMCs and correlated with HIV-1 infection.
Results:
Twelve and 11 men were analyzed in the 2 h–double dose and 7 days–single dose groups, respectively. Cumulative p24 differences after-before PrEP were -144 pg/ml/mg (IQR[−259;−108]) for the 2 h–double dose group (P = 0.0005) and -179 pg/ml/mg (IQR [−253;−86]) for the 7 days–single dose group (P = 0.001), with no differences between groups (P = 0.93). Rectal TFV-DP was below quantification after a double dose, but FTC-TP levels were similar to levels at 7 days. There was a significant correlation between rectal FTC-TP levels and p24 changes after a double dose (R = −0.84; P = 0.0001).
Conclusion:
Oral TDF/FTC provided similar protection against HIV-1 infection of rectal tissue 2 h after a double dose or 7 days of a daily dose. At 2 h, this protection seems driven by high FTC-TP concentrations in rectal tissue. This confirms the importance of combining TDF and FTC to achieve early protection.
Keywords: emtricitabine, HIV-1, preexposure prophylaxis, rectal tissue, tenofovir
Introduction
HIV is still an important concern for public health in the world according to numerous health organizations [1–3]. MSM are disproportionately affected by HIV infection [4–7]. Oral PrEP with a daily or on-demand fixed combination of tenofovir disoproxil (TDF) and emtricitabine (FTC) was demonstrated to be effective at reducing HIV-1 acquisition by up to 86% in MSM in double-blind randomized placebo control studies [8–10] and is now recommended in this population [11–14]. One of the challenges remaining in HIV prevention is to determine when each PrEP regimen starts to effectively protects against HIV acquisition. This is an area of ongoing controversy due to the lack of reliable clinical data.
In the macaques studies that informed the design of human PrEP trials, animals were treated preexposure 7–9 days with TDF/FTC for daily regimen and 2–24 h prior exposure in coitally dependent regimens [15–17]. In a post hoc analysis of the iPrEx trial, a randomized placebo controlled PrEP study in transgender women and MSM, a concentration of 16 fmol/106 cells of tenofovir diphosphate (TFV-DP), the active form of tenofovir, in the PBMCs was associated with a 90% reduction in HIV acquisition [18]. This 90% effective concentration (EC90) is obtained after the seventh daily dose of TDF/FTC in most of the population [18,19]. In a pharmacokinetics/pharmocodynamics (PK/PD) model in 47 healthy women using a combination of pharmacological dosages and ex-vivo infections of cells with HIV, authors reported that 81% of the population would obtain protection from a double dose of TDF/FTC in the colorectal tissue after 2 h [20].
Based on the available pharmacological and clinical data, it is recommended to take a single daily dose for at least 7 days or a double dose at least 2 h before exposure [11–14].
Determining an adequate PrEP dosing regimen and PrEP pharmacological concentration targets for efficacy is a challenge due to our inability to directly and reliably correlate ex-vivo efficacy and in-vivo efficacy in clinical trials or measure PK/PD in humans. Having a model to evaluate the time to protection of PrEP and explore PK/PD correlations in different dosing regimens would be helpful. We compared both validated dosing regimen of oral TDF/FTC in a PK/PD model of ex-vivo HIV-1 infection of rectal tissue to determine whether protection is similar at 2 h after a double dose and after 7 days of a daily dose and explore the PK/PD relationship after the preexposition dosing.
Materials and methods
Participants
The ANRS PREVENIR cohort study was a prospective observational cohort of HIV-negative adults at high-risk of HIV infection in Paris region taking a fixed-dose combination of TDF/FTC (245/200 mg) as daily (one pill per day) or on-demand PrEP (following the IPERGAY dosing recommendation). The PREVENIR cohort study design is described elsewhere [21]. A PK/PD sub-study was proposed to participants included and engaged in the study follow-up at the outpatient clinic of the Infectious Disease Department of Saint-Louis Hospital, Paris.
Eligible participants were 18-year-old or older, self-identifying MSM, who gave written consent to the sub-study and to a 14-day washout period without PrEP.
Main noninclusion criterion was contraindication to rectal biopsies (Supplemental Method).
Ethics and consent
Full inclusion and exclusion criteria for the ANRS PREVENIR trial are available at ClinicalTrials.gov NCT03113123 and EudraCT 2016A0157744. Prior to implementation, the sub-study protocol was reviewed and approved by the Institutional Review Board (Comité de Protection des Personnes) at Paris University IV and the French Drug Agency (Agence nationale de sécurité du medicament et des produits de santé). All participants provided written informed consent for the sub-study.
Study design
All the participants included in the study received their treatment assignment in a nonrandomized manner according to order of arrival into one of the two intervention groups “2 h – double dose” or “seven days – single dose.” In both groups, participants underwent two visits (V1 and V2). After a minimum of a 14-day wash-out period without any TDF/FTC intake, V1 was the baseline sampling visit in both groups, to assess tissue susceptibility to HIV in the ex-vivo model. V2 was the visit after exposure to TDF/FTC to assess the participant's decrease in tissue susceptibility to HIV. In the “2 h – double dose” group, V2 was 2 h after a double dose of TDF/FTC, and in the “seven days – single dose,” group V2 was the day after taking the seventh dose.
Sample collection and processing
At each visit, participants were sampled for blood, urine, and rectal tissue. Blood was drawn in EDTA tubes and then 25 μl were spotted on Whatman 903 filter paper (Dried Blood Spot, DBS). Plasma was collected after centrifugation. PBMCs were isolated from the buffy coat and frozen in fetal bovine serum with 4% DMSO at -80°C (for pharmacological dosages).
At each visit, 10 rectal biopsies were collected through a rigid anoscope, 8–12 cm from the anus. Six biopsies were placed immediately in complete medium (RPMI supplemented with 10% FCS, 1% Penstrep, 1% HEPES, 40 mg/l piperacillin-tazobactam, and 0.5 mg/l amphotericin) and were sent to the virology laboratory within 20 min to perform ex-vivo HIV-1 challenges. Two additional biopsies were put in polypropylene tubes and immediately frozen at −80°C until being analyzed for drug concentration assays. Two additional biopsies were frozen at −80°C for future studies.
Ex-vivo rectal challenge
We used an ex-vivo infection challenge model to evaluate rectal tissue susceptibility to HIV infection before and after taking PrEP, as previously described [22–26]. Rectal tissue explants were directly placed in a 105 TCID50/ml of HIVBaL-1 (a R5-tropic virus) solution for four hours at 37°C in 5% CO2. Explants were then washed and placed on a Gelfoam raft in culture media and incubated at 37°C in 5% CO2. Supernatants were collected entirely, stored at −80°C, and replaced with fresh media on Day (D)3, D7, and D10. On the final day of culture (D14), supernatants were collected and stored, and explants were discarded. Our model only evaluated the effects of oral PrEP.
Quantification of explant HIV infection
All supernatants were assessed for HIV levels using the Lenti-X p24 Rapid Titer ELISA kit (TAKARA Bio, EU, #632200). Limits of quantification were 20–200 pg/ml of p24.
Results are expressed at each collect day (D3, D7, and D10) as pg/ml and were standardized over explant weight and expressed in pg/ml/mg and cumulative p24 at D14 is expressed in pg/ml/mg.
Infection of rectal biopsies was defined by the moving average method; an explant was considered infected for a difference of mean of two consecutive measures of p24 antigen more than 5%.
Protection from infection was defined by a significant difference of the mean D14 cumulative p24 antigen value for each participant at V1 and V2 in the 2 h – double dose and 7 days – single-dose groups.
Pharmacological dosages
Liquid chromatography tandem mass spectrometry (LC–MS/MS) assays were used for the determination of TFV, FTC, and the active metabolite tenofovir diphosphate (TFV-DP) and emtricitabine triphosphate (FTC-TP) in the different matrices [27,28]. The measurements were developed and validated for each matrix according to the recommendations of the European Medicine Agency [27]. The method utilized a strong anion exchange isolation of mono-phosphate, di-phosphate, and tri-phosphate from intracellular matrix. The quantification of active intracellular phosphorylated fractions of drugs was done using a method developed and validated according to Bushman et al.[28]. TFV and FTC concentrations are presented in ng/ml for plasma and urine, TFV-DP and FTC-TP concentrations are presented in fmol and pmol per 3 mm punch for DBS, per mg of tissue for explants and per million cells for PBMC, respectively. Limits of quantification were 1 ng/ml for TFV and 10 ng/ml for FTC in plasma; 1 ng/ml in urine for TFV or FTC; 0.25 ng/sample in rectal explants for TFV or FTC; 17.4 fmol/0.4 ml of PBMC for TFV-DP and 0.202 pmol/0.4 ml of PBMC for FTC-TP in PBMCs and 10.445 fmol/sample for TFV-DP and 0.004 pmol/sample for FTC-TP in explants.
Statistical analysis
Results were summarized by numbers and percentages for categorical variables and by median and interquartile range or mean and standard deviation for continuous variables, when appropriate. All statistical tests were 2-sided with a significance level of 5%.
Drug levels of TFV-DP and FTC-TP in PBMC and rectal tissue biopsies were correlated to the fractional change in cumulative p24 level in rectal biopsies between V2 and V1 (=V2/V1), as previously reported with ex-vivo challenge models and oral TDF/FTC [24]. Spearman nonparametric correlation test was used to assess the association between drug concentrations and fractional change in cumulative p24. Participants for whom at least three quarters of the explants were not deemed susceptible to HIV at V1 before PrEP were excluded from the analysis.
Nonparametric paired Wilcoxon and Mann--Whitney tests were used to compare variations or differences V2-V1 of cumulative p24 within and between treatment groups, respectively. Fisher's exact test was used to compare the proportion of infected explants at visit V1 between treatment groups.
Risk reduction of infection between groups was compared using a generalized estimation equation model with a binomial distribution and a log link including the treatment group, visit and interaction between treatment group and visit. Analyses were performed using SAS software 9.4.
Results
Characteristics of participants
Screening of study participants took place between September 2019 and June 2021. A total of 25 participants were enrolled, and 23 participants were analyzed, excluding two individuals (one in each group) for insufficient infection of rectal tissue at V1. Characteristics of the 23 participants are summarized Table 1. The median participant age was 35 years (interquartile IQR [32;35]). All were self-identifying MSM. The median washout period of PrEP at V1 was 48 days (IQR [27;123]).
Table 1.
Participants characteristics before preexposure prophylaxis intake (V1) by study arm.
| Study arm | |||
| Participants characteristics (n = 23) | 2 h - double dose | 7 days - single dose | Total |
| Sex: Male, n (%) | 12 (100%) | 11 (100%) | 23 (100%) |
| Europe as place of birth, n (%) | 10 (83%) | 8 (73%) | 18 (78%) |
| Age, years, median [IQR] | 35 [33–44] | 45 [32–52] | 35 [32–49] |
| BMI, kg/m2, median [IQR] | 23.6 [21.4–25.7] | 23.4 [20.9–25.1] | 23.6 [21.4–25.1] |
| GFR at last visit before inclusion (ml/min), median [IQR] | 110 [101–131] | 106[91–115] | 107 [97–122] |
| Days since last intake of TDF/FTC (self-reported), median [IQR] | 31 [27–73] | 102 [42–141] | 48 [27–123] |
| Drug/metabolite in each matrix | |||
| TFV Plasma | |||
| Number >LLOQ/number tested | 1/12 | 2/11 | 3/23 |
| ng/ml, Median [IQR] | 2.2 | 2.8 [2.7–2.9] | 2.7[2.4–2.8] |
| TFV-DP DBS | |||
| Number >LLOQ/number tested | 6/10 | 3/11 | 11/21 |
| fmol/punch, Median [IQR] | 244 [173–275] | 358 [254–448] | 274 [230–379] |
| TFV-DP Rectum | |||
| Number >LLOQ/number tested | 0/11 | 0/11 | 0/22 |
| fmol/mg, Median [IQR] | – | – | – |
| TFV Rectum | |||
| Number >LLOQ/number tested | 1/10 | 0/11 | 1/21 |
| ng/g, Median [IQR] | 24.2 | – | 24.2 |
| TFV Urine | |||
| Number >LLOQ/number tested | 12/12 | 10/10 | 22/22 |
| ng/g, Median [IQR] | 24 [15–40] | 20 [17–45] | 22 [16–45] |
| FTC Plasma | |||
| Number >LLOQ/number tested | 0/12 | 0/11 | 0/23 |
| ng/ml, Median [IQR] | – | – | – |
| FTC-TP DBS | |||
| Number >LLOQ/number tested | 0/10 | 0/11 | 1/21 |
| pmol/punch, Median [IQR] | – | – | 0.15 |
| FTC-TP Rectum | |||
| Number >LLOQ/number tested | 0/11 | 0/11 | 0/22 |
| fmol/mg, Median [IQR] | – | – | – |
| FTC Rectum | |||
| Number >LLOQ/number tested | 0/10 | 0/11 | 0/21 |
| ng/g, Median [IQR] | – | – | – |
| FTC Urine | |||
| Number >LLOQ/number tested | 12/12 | 10/10 | 22/22 |
| ng/g, Median [IQR] | 31 [24–43] | 19 [9–49] | 28 [14–48] |
FTC plasma, 10 ng/ml; FTC Rectum, 0.25 ng/sample (approximatively 0.0125--0.025 ng/sample; FTC urine, 1ng/ml; FTC-TP Rectum, 0.004 pmol/sample (approximatively 2--4 fmol per mg); LLOQ, Lower limit of quantification; TFV plasma, 1 ng/ml; TFV Rectum, 0.25 ng/sample (approximatively 0.0125--0.025 ng/sample); TFV urine, 1 ng/ml; TFV-DP PBMC, 17.4 fmol/0.4 ml FTC-TP PBMC: 0.202 pmol/0.4 ml TFV-DP Rectum: 10.445 fmol/sample (approximatively 0.05--1.05 fmol per mg).
At V1, TFV was quantifiable in plasma in three of 23 cases (13%) and TFV-DP in DBS was quantifiable in 10 of 21 cases (48%) at a median level of 268 fmol/punch (IQR [228–305]). No participant had any level of FTC or FTC-TP detectable at V1. TFV and FTC were detectable at low levels in urine in all participants.
Ex-vivo rectal challenge
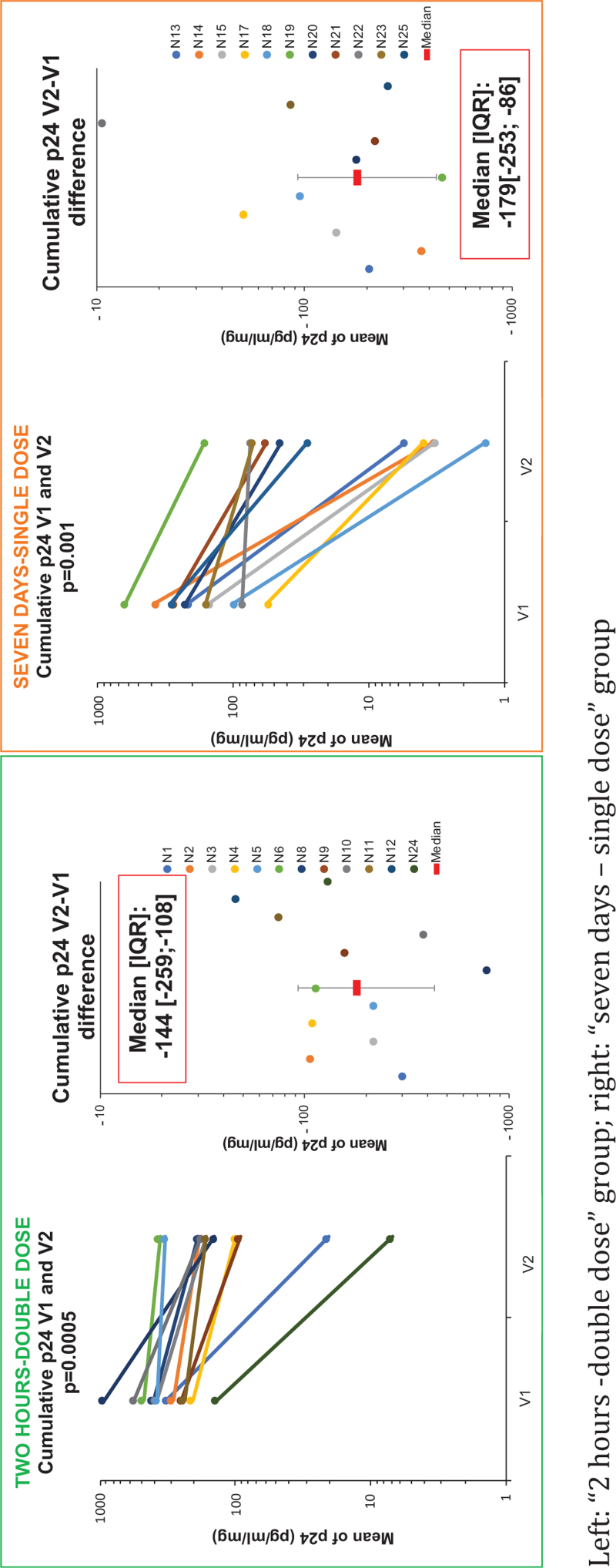
The primary endpoint was the difference of the mean D14 cumulative p24 value for each participant at V1 and V2 (Fig. 1, supplemental Table S1). Two hours after two pills, the median of mean D14 cumulative p24 difference (V2-V1) was -144 pg/ml/mg (IQR [−259;−108] (P = 0.0005) and -179 pg/ml/mg (IQR [−253;−86]) (P = 0.001) after 7 days of daily use of one pill. There was no statistical difference in the median cumulative p24 differences between treatment group (P = 0.93).
Fig. 1.
D14 cumulative p24 of ex-vivo rectal tissue HIV-1 infection before (V1) and after (V2) oral TDF/FTC PrEP for each participant.
Left: “2 h -double dose” group; right: “seven days – single dose” group.
Individual p24 kinetics were available in supplemental material (Figures S1 and S2). According to the moving average method, at V1, 80.6% (54/67) and 79.0% (49/62) of explants were infected in the 2 h – double dose and 7 days – single-dose groups, respectively (P = 0.82). At V2, 15.4% (10/65) and 6.2% (4/65) of explants were infected in the 2 h – double dose and 7 days – single-dose groups, respectively. This represented a risk reduction of ex-vivo HIV infection of 80.9 and 92.2% in the 2 h -- double dose and 7 days – single dose groups, respectively (P = 0.12). Mean of p24 at each day of culture for all participants in each group according to visit are represented in Supplemental (Figure S3).
Pharmacokinetics
We summarize median drug levels in all matrixes at V2 according to treatment group in Table 2. In the 7 days, single-dose group samples were obtained 2 h after the eighth dose in median.
Table 2.
Pharmacological dosages of TDF and FTC and their active forms in blood and rectum after preexposure prophylaxis intake (V2).
| Drug/metabolite | 2 h after two pills | Seven days of daily use of one pill | P value | ||
| Blood compartment | TFV | TFV Plasma | 0.046 | ||
| Number >LLOQ/number tested | 12/12 | 11/11 | |||
| Median [IQR] (ng/ml) | 321 [236–405] | 173 [50–320] | |||
| TFV-DP PBMC | 0.0002 | ||||
| Number >LLOQ/number tested | 10/11 | 9/9 | |||
| Median [IQR] (fmol/million) | 12.1 [5.3–18.5] | 100.9 [81.9–125.9] | |||
| FTC | FTC Plasma | 0.0004 | |||
| Number >LLOQ/number tested | 12/12 | 11/11 | |||
| Median [IQR] (ng/ml) | 2508 [2306–2807] | 849 [59–1591] | |||
| FTC-TP PBMC | 0.0004 | ||||
| Number >LLOQ/number tested | 11/11 | 9/9 | |||
| Median [IQR] (pmol/million) | 4.9 [3.4–9.7] | 22.8 [16.5–26.1] | |||
| Rectal compartment | TFV | TFV Rectum | <0.0001 | ||
| Number >LLOQ/number tested | 10/10 | 11/11 | |||
| Median [IQR] (ng/g) | 90 [45–142] | 3935 [3133–8854] | |||
| TFV-DP Rectum | 0.0002 | ||||
| Number >LLOQ/number tested | 0/11 | 9/11 | |||
| Median [IQR] (fmol/mg) | – | 7.0 [4.3–12.1] | |||
| FTC | FTC Rectum | 0.74 | |||
| Number >LLOQ/number tested | 11/11 | 11/11 | |||
| Median [IQR] (ng/g) | 4544 [3735–5036] | 4484 [2140–6538] | |||
| FTC-TP Rectum | 0.47 | ||||
| Number >LLOQ/number tested | 11/11 | 9/11 | |||
| Median [IQR] (fmol/mg) | 2.8 [2.1–3.3] | 5.2 [2.3–6.3] |
Values in bold indicate statistically significant P-values (P < 0.05).
FTC plasma, 10 ng/ml; FTC Rectum, 0.25 ng/sample (approximatively 0.0125--0.025 ng/sample); FTC-TP Rectum, 0.004 pmol/sample (approximatively 2--4 fmol per mg); LLOQs, TFV plasma: 1 ng/ml; TFV Rectum, 0.25 ng/sample (approximatively 0.0125 to 0.025 ng/sample); TFV-DP PBMC, 17.4 fmol/0.4 ml FTC-TP PBMC: 0.202 pmol/0.4 ml TFV-DP Rectum: 10.445 fmol/sample (approximatively 0.05--1.05 fmol per mg).
TFV and FTC in plasma were above the lower limit of quantification in all participants 2 h after taking two pills or after 7 days of a daily intake of one pill a day (Table 1). Median TFV levels reached 321 ng/ml (IQR [236–405]) in the 2 h after two pills regimen and lower at 173 ng/ml (IQR [50–320]) in the 7 days – single-dose group, with a significant difference between the groups (P = 0.046). Similarly, median FTC levels were 2508 ng/ml (IQR [2306–2807]) in the 2 h – double-dose group and lower at 849 ng/ml (IQR [59–1591]) in the 7 days – single-dose group, with a significant difference between the groups (P = 0.0004).
All but one participant showed displayed quantifiable levels of TFV-DP and FTC-TP in PBMCs in both groups. Median TFV-DP in PBMCs were lower (12.1 fmol/million; IQR [5.3–18.5]) in the 2 h – double-dose group compared to the 7 days – single-dose group (100.9 fmol/million; IQR [81.9–125.9]) (P = 0.0002). Four out of 11 (36.4%) participants at 2 h postdosing and nine out of nine (100%) after 7 days of daily intake presented TFV-DP concentrations in PBMCs equal or above the 16 fmol/106 cells cut-off associated with a 90% reduction in HIV-1 acquisition [18]. Median FTC-TP in PBMCs were lower (4.9 fmol/million (IQR [3.4–9.7]) in the 2-h double-dose group compared with the 7 days – single-dose group (22.8 fmol/million (IQR [16.5–26.1]) (P = 0.0004).
In rectal explants with a median weight of 5.1 mg (IQR [3.8–7.6]), TFV-DP were below the lower limit of quantification in all participants 2 h after intake of two pills but quantifiable for nine of 11 participants after the daily intake for 7 days of one pill. FTC-TP was detectable in the rectal tissue in nine of 11 participants in the 7 days – single-dose group and in all participants in the 2 h – double-dose group with no statistically significant difference noted (P = 0.47).
PK/PD relationship
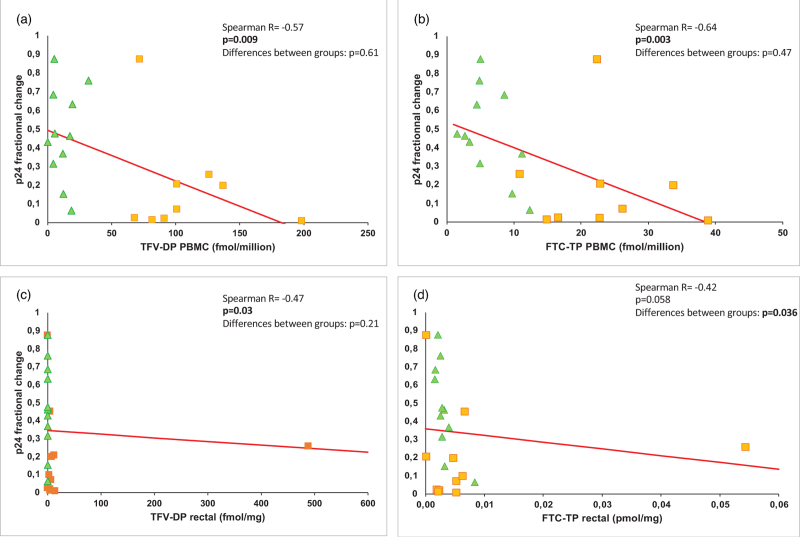
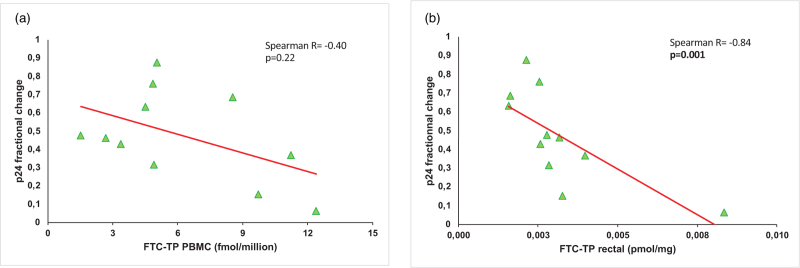
Ex-vivo infection results in rectal tissue were negatively correlated with TFV-DP and FTC-TP in PBMCs with a dose-effect response for all participants in both groups (Spearman R = −0.57; P = 0.009 and Spearman R = −0.64; P = 0.003, respectively) (Table S2, Fig. 2). There was also a PK/PD correlation for TFV-DP in rectal tissue for all participants (R = −0.47; P = 0.03) (Table S2). Rectal levels of FTC-TP showed a tendency to a correlation with a decrease of infectability (P = 0.058) (Table S2, Fig. 2). Although FTC-TP in the PBMC did not correlate with PrEP efficacy 2 h after a double dose, rectal FTC-TP concentration and p24 fractional change V2/V1 demonstrated an inverse correlation 2 h after a double dose (Spearman R = −0.84; P = 0.001) (Fig. 3).
Fig. 2.
Pharmacokinetic/pharmacodynamics correlation of TFV-DP and FTC-TP concentrations and ex-vivo rectal tissue efficacy in all participants.
(a) correlation with TFV-DP in PBMCs; (b) correlation with FTC-TP in PBMCs; (c) correlation with TFV-DP in rectal tissue; (d) correlation with FTC-TP in rectal tissue. Green triangles: 2 h – double-dose group; Orange squares: 7 days – single-dose group.
Fig. 3.
Pharmacokinetic/pharmacodynamics correlation of FTC-TP and ex-vivo rectal tissue efficacy in the 2 h - double-dose group.
(a) Correlation with FTC-TP in PBMCs; (b) correlation with FTC-TP in rectal tissue.
Discussion
We studied HIV-1 ex-vivo infectability of rectal tissue before and after taking oral TDF/FTC PrEP in 23 participants included in the ANRS PREVENIR prospective study. In our study, oral PrEP with TDF/FTC is similarly effective in significantly reducing ex-vivo HIV-1 infection of rectal tissue two hours after a double dose and 7 days after one pill a day. We observed mean cumulative p24 differences after PrEP exposure of −144 and −179 pg/ml/mg in the 2 h – double dose and 7 days – single-dose groups, respectively. Our ex-vivo model yield results of cumulative p24 levels before PrEP comparable to those reported in other ex-vivo studies with rectal explants at baseline [25,29,30].
Combining the results of both PrEP regimens, we saw an 86.5% reduction in explant infection without any significant difference between the dosing regimens. This result is in accordance with the main data of PREVENIR study showing no differences in efficacy between the two regimens with a similar incidence of 1.1 HIV infection IQR [0.2–3.2] per 1000 persons-year in both groups [21].
Before PrEP, TFV-DP in DBS was quantifiable in half of the cases at a level consistent with the use of one pill per week in the past 6--8 weeks [31]. Nondetectable FTC-TP concentrations in the DBS for all participants in this study and the low urine concentrations of TFV and FTC were consistent with an interruption of PrEP for more than 7 days [32,33].
Following oral intake of two pills of TDF/FTC, we report very low but quantifiable levels of TFV and no quantifiable level of TFV-DP in rectal tissue. This result could be due to the low mass of tissue analyzed but is consistent with a previous study describing TFV-DP concentration below the lower limit of quantification 30 min after intake and a Tmax of 24 h for oral TDF [22]. Conversely, we report similar levels of rectal FTC and FTC-TP with the loading doses of either two pills at 2 h or one pill per day for 7 days. This supports the concept that FTC-TP is the only active drug quantitatively present in the rectal tissue at the time of HIV exposure during an on-demand dosing regimen suggesting the importance of combining FTC to TDF with this dosing regimen. A similar result was noted in an IPERGAY substudy with FTC detected in rectal tissue much earlier than TFV, as soon as 30 min after intake [34]. These relatively quick levels of FTC compared with tenofovir might be attributed to differential drug transporter expression in the colon, with higher expression of P-gp, involved in TFV and TFV-DP efflux, as opposed to relatively low expression of MRP1, involved in FTC efflux [35,36]. Nonetheless, TFV was quantifiable as a prodrug in all rectal samples. Furthermore, intracellular phosphorylation of FTC exhibits faster kinetics than TFV, with a maximum concentration reached in 4 h (2−8 h) for FTC-TP and 24 h for TFV-DP, which could contribute to the observed results [22,37,38].
We observed PK/PD correlations with a dose-effect response for active forms of TDF and FTC in PBMC and their efficacy in the rectal tissue. We also observed a correlation for rectal TFV-DP and its efficacy; however, TFV-DP was not detectable in all rectal samples 2 h after a double dose of TDF/FTC making it unlikely to be effective in the rectal tissue 2 h after a loading dose of two pills. There was also a strong inverse correlation between drug level and p24 results after drug exposure for the rectal FTC-TP levels 2 h after a double dose of TDF/FTC. This result suggests that the main effect of PrEP in the rectal tissue closely after a double dose is due to the rapid distribution of the active form of FTC. This result is also consistent with a previous PK/PD model of EC90 determination in female genital and colorectal tissues, where most of the population could achieve expected early protection in the rectum with the combination TDF+FTC but not with TDF alone, suggesting a synergistic effect of both drugs [20]. In this model, using dosages of TFV-DP, FTC-TP and the ratio to their competing endogenous nucleotides and infections of TMZbl cells and CD4+ T cells, Cottrell et al.[20] estimated that 2 h after a double dose of TDF/FTC 81% of the population was protected at the colorectal level.
A question of interest in PrEP is whether the efficacy of PrEP happens at the mucosal or the systemic level. Two hours after a double dose, we observed a strong PK/PD correlation for FTC-TP levels in the rectal tissue and PrEP efficacy but not for FTC-TP levels in the PBMCs. This suggests that 2 h after a double dose of TDF/FTC the main effect of PrEP happens in the rectal tissue. Furthermore, although the “iPrEx EC90” level of TFV-DP in the PBMCs is usually admitted as a threshold for protection against HIV [39–41], in our study, most participants in the 2 h – double-dose group were below that threshold, but PrEP was still effective to reduce HIV ex-vivo infection of rectal tissue. The potential impact of FTC in the protection against HIV and the potential of different pharmacokinetics of the two drugs, especially in the first moments after intake, might have been overlooked in the past. In two other studies, demonstrating a linear PK/PD inverse correlation for HIV susceptibility with tissue TFV-DP in ex-vivo challenge model of rectal tissue, PK/PD correlation with tissue FTC-TP were not evaluated [23,24]. Our study originality comes from the examination of the very beginning of a dosing regimen of PrEP when protection is still uncertain. Studies of PK/PD in ex-vivo models in rectal tissue have mainly focused their attention to the steady state of PrEP dosing regimen [22–24].
Our study presents certain limitations. First, we only explored the preexposure dose of the dosing regimens of PrEP. The nature of the ex-vivo challenge prevents the exploration of postexposure doses. The importance of these postexposure doses was underlined in nonhuman primate models [16,42]. There is a noticeable intra and inter-individual variability of the model. Although ex-vivo efficacy of both dosing regimens seemed similar, our study was limited from a statistical standpoint due to the relatively small number of observations. We might have lacked power to demonstrate small differences between groups. We only tested the combination of TDF and FTC and not the effect of each drug alone. In a previous ex-vivo study, a linear PK/PD correlation has been shown with oral TDF alone [22]. We established the baseline tissue infectability values in people already taking PrEP which could lead to underestimating the infectability from residual levels of drugs. The wash-out period estimated from participants self-declarations should reduce this risk to negligeable and the pharmacological dosages at V1 confirmed that participants had stopped taking PrEP for at least a week [19,31,32].
In conclusion, this study supports the effectiveness of oral PrEP with TDF/FTC to protect rectal tissue from HIV-1 infection as soon as two hours after two pills. With other recommended regimens for PrEP (oral TAF/FTC and long-acting cabotegravir) data to determine the optimal timing of protection is limited [43–45]. Studies show low drug levels in the rectal tissue [45,46]. This warrants further exploration; the ex-vivo rectal challenge model is a promising translational model to study PrEP efficacy and evaluate new PrEP candidates or dosing regimens.
Acknowledgements
The authors wish to extend a special thanks to the participants who consented to the study, their dedication to clinical research is making science possible.
They convey their thanks to the ANRS both for funding the PREVENIR study and for a research grant for SC, this support was essential to our achievements.
They would like to express our gratitude towards The MaGee Women Institute for their technical support and their advice when setting up the experiments.
The authors express our most sincere appreciation to the clinical research team at Saint-Louis Hospital for being able to accommodate this study during the start of a pandemic.
They would also like to give thanks to the Institut Jean Bernard for giving us the opportunity to perform all the ex-vivo tests on site.
S.C. wrote the manuscript. J.M.M. and C.D. designed the study. S.C. and A.G. managed the samples and performed the ex-vivo infections. I.McG., A.S., and R.B. provided insight and training for the ex-vivo infections. L.G. performed the pharmacological measures. M.T. performed rectal biopsies. M.E.M. and L.A. performed the statistical analysis. S.C., A.A., J.M.M., and C.D. interpreted the results. All authors provided critical feedback on the manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.CDC. Let's stop HIV together. Cent. Dis. Control Prev. 2022. https://www.cdc.gov/stophivtogether/index.html [Accessed 3 October 2022]. [Google Scholar]
- 2. Santé Publique France. VIH/sida. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-sexuellement-transmissibles/vih-sida. [Accessed 3 October 2022] [Google Scholar]
- 3. World Health Organization. HIV. https://www.who.int/news-room/fact-sheets/detail/hiv-aids. [Accessed 3 October 2022] [Google Scholar]
- 4.Beyrer C, Sullivan P, Sanchez J, Baral SD, Collins C, Wirtz AL, et al. The increase in global HIV epidemics in MSM. AIDS Lond Engl 2013; 27:2665–2678. [DOI] [PubMed] [Google Scholar]
- 5.Martí-Pastor M, García de Olalla P, Barberá M-J, Manzardo C, Ocaña I, Knobel H, et al. Epidemiology of infections by HIV, Syphilis, Gonorrhea and Lymphogranuloma Venereum in Barcelona City: a population-based incidence study. BMC Public Health 2015; 15:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crepaz N, Hess KL, Purcell DW, Hall HI. Estimating national rates of HIV infection among men who have sex with men, persons who inject drugs and heterosexuals in the United States. AIDS Lond Engl 2019; 33:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stengaard AR, Combs L, Supervie V, Croxford S, Desai S, Sullivan AK, et al. HIV seroprevalence in five key populations in Europe: a systematic literature review, 2009 to 2019. Eurosurveillance 2021; 26:2100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet Lond Engl 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 11.WHO | Guideline on when to start antiretroviral therapy and on preexposure prophylaxis for HIV. WHO. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [Accessed 24 March 2021]. [Google Scholar]
- 12. Centers for Disease Control and Prevention: US Public Health Service: preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. 2021. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf. [Accessed 12 June 2022] [Google Scholar]
- 13.Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA 2020; 324:1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haute Autorité de Santé. Réponses rapides dans le cadre de la COVID-19 - Prophylaxie (PrEP) du VIH par ténofovir disoproxil / emtricitabine dans le cadre de l’urgence sanitaire. Available from: https://www.has-sante.fr/jcms/p_3262060/fr/reponses-rapides-dans-le-cadre-de-la-covid-19-prophylaxie-prep-du-vih-par-tenofovir-disoproxil-/-emtricitabine-dans-le-cadre-de-l-urgence-sanitaire. [Accessed 8 January 2024] [Google Scholar]
- 15.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, et al. Prevention of vaginal SHIV transmission in Macaques by a coitally-dependent Truvada regimen. PLoS One 2012; 7:e50632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Lerma JG, Cong M, Mitchell J, Youngpairoj AS, Zheng Q, Masciotra S, et al. Intermittent prophylaxis with oral Truvada protects macaques from rectal SHIV infection. Sci Transl Med 2010; 2:14ra4. [DOI] [PubMed] [Google Scholar]
- 17.García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong M, Masciotra S, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med 2008; 5:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and preexposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert SM, Glidden DV, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis 2015; 60:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 2016; 214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina J-M, Ghosn J, Assoumou L, Delaugerre C, Algarte-Genin M, Pialoux G, et al. Daily and on-demand HIV preexposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV 2022; 9:e554–e562. [DOI] [PubMed] [Google Scholar]
- 22.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% Gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012; 28:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGowan I, Wilkin T, Landovitz RJ, Wu C, Chen Y, Marzinke MA, et al. The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing preexposure prophylaxis regimens in MSM. AIDS Lond Engl 2019; 33:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan IM, Kunjara Na Ayudhya RP, Brand RM, Marzinke MA, Hendrix CW, Johnson S, et al. An open-label pharmacokinetic and pharmacodynamic assessment of tenofovir gel and oral emtricitabine/tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, et al. Long-acting rilpivirine as potential preexposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016; 3:e569–e578. [DOI] [PubMed] [Google Scholar]
- 26.McGowan IM, Chawki S, Hendrix CW, Anton PA, Marzinke MA, Brand RM, et al. A randomized, open label, crossover phase 1 safety and pharmacokinetic study of oral maraviroc and maraviroc 1% Gel (the CHARM-03 Study). AIDS Res Hum Retroviruses 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.guideline-bioanalytical-method-validation_en.pdf. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf [Accessed 16 October 2022]. [Google Scholar]
- 28.Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng J-H, Ray ML, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011; 56:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dezzutti CS, Park SY, Marks KM, Lawlor SE, Russo JR, Macio I, et al. Heterogeneity of HIV-1 replication in ectocervical and vaginal tissue ex vivo. AIDS Res Hum Retroviruses 2018; 34:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Khouja A, Shieh E, Fuchs EJ, Marzinke MA, Bakshi RP, Hummert P, et al. Examining the safety, pharmacokinetics, and pharmacodynamics of a rectally administered IQP-0528 gel for HIV pre-exposure prophylaxis: a first-in-human study. AIDS Res Hum Retroviruses 2021; 37:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldwirt L, Bauer R, Liegeon G, Charreau I, Delaugerre C, Cotte L, et al. Estimated pill intake with on-demand PrEP with oral TDF/FTC using TFV-DP concentration in dried blood spots in the ANRS IPERGAY trial. J Antimicrob Chemother 2021; 76:2675–2680. [DOI] [PubMed] [Google Scholar]
- 32.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2017; 62:e01710–e1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cressey TR, Siriprakaisil O, Kubiak RW, Klinbuayaem V, Sukrakanchana P, Quame-Amaglo J, et al. Plasma pharmacokinetics and urinary excretion of tenofovir following cessation in adults with controlled levels of adherence to tenofovir disoproxil fumarate. Int J Infect Dis 2020; 97:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonsart J, Saragosti S, Taouk M, Peytavin G, Bushman L, Charreau I, et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial. J Antimicrob Chemother 2017; 72:478–485. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann C, Gutmann H, Hruz P, Gutzwiller J-P, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos Biol Fate Chem 2005; 33:219–224. [DOI] [PubMed] [Google Scholar]
- 36.Kis O, Robillard K, Chan GNY, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci 2010; 31:22–35. [DOI] [PubMed] [Google Scholar]
- 37.Delaney WE, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother 2006; 50:2471–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng J-H, et al. Emtricitabine-triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother 2016; 60:6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of preexposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, et al. HIV preexposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2:e512–e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duwal S, Sunkara V, von Kleist M. Multiscale systems-pharmacology pipeline to assess the prophylactic efficacy of NRTIs against HIV-1. CPT Pharmacomet Syst Pharmacol 2016; 5:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson PL, Glidden DV, Bushman LR, Heneine W, García-Lerma JG. Tenofovir diphosphate concentrations and prophylactic effect in a macaque model of rectal simian HIV transmission. J Antimicrob Chemother 2014; 69:2470–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landovitz RJ, Li S, Grinsztejn B, Dawood H, Liu AY, Magnus M, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018; 15:e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarus G, Wangsaputra VK, Christianto, Louisa M, Soetikno V, Hamers RL. Safety and pharmacokinetic profiles of long-acting Injectable antiretroviral drugs for HIV-1 pre-exposure prophylaxis: a systematic review and meta-analysis of randomized trials. Front Pharmacol 2021; 12:664875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cottrell ML, Garrett KL, Prince HMA, Sykes C, Schauer A, Emerson CW, et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017; 72:1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 2014; 67:481–486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.