Abstract
Background
Therapeutic drug monitoring provides important guidance for treatment of patients with inflammatory bowel disease (IBD) and could help to early identify treatment failure. This study aimed to validate a finger prick–based capillary blood sampling technique to measure biological trough levels and C-reactive protein (CRP) and evaluate patient performance and -support.
Methods
In this prospective cohort study, patients with IBD receiving infliximab (IFX) or vedolizumab (VEDO) therapy performed finger prick–based capillary blood sampling at home. Additionally, blood was collected through routinely performed in-hospital venepuncture prior to biological infusion. IFX, VEDO, and CRP concentrations were measured by enzyme-linked immunosorbent assay. The concordance between methods was statistically evaluated and a survey was conducted to assess practicality and patient support.
Results
In total, 81 patients (46 IFX, 35 VEDO) were enrolled. Mean differences between both methods were 0.42 (95% confidence interval, -1.74 to 2.58) μg/mL for IFX and 0.72 (95% confidence interval, -5.50 to 6.94) μg/mL for VEDO. Passing-Bablok regressions demonstrated no evidence for systematic or proportional biases. Venous and capillary IFX (ρ = 0.96, P < .001) and VEDO (ρ = 0.97, P < .001) levels strongly correlated and showed high intermethod agreement (Cohen’s kappa: IFX = 0.82; VEDO = 0.94). Similarly, venous and capillary CRP levels were strongly correlated (ρ = 0.99, P < .001). Most patients (>95%) were able to successfully perform the self-sampling at home without prior instructions.
Conclusions
This study clinically validated a finger prick–based capillary blood self-sampling technique allowing concomitant home monitoring of biological levels and CRP for patients with IBD, who reported substantial support, tolerability, and practicality.
Keywords: inflammatory bowel disease, therapeutic drug monitoring, home sampling, infliximab, vedolizumab
Key Messages.
What is already known?
Therapeutic drug monitoring of biologics and C-reactive protein from a patient’s home setting might optimize treatment outcomes and relieves pressure on healthcare systems.
What is new here?
A finger prick–based capillary blood self-sampling technique, carried out by a patient individually at their home, is validated. Patient survey show a high level of support for home monitoring using this finger prick–based sampling technique.
How can this study help patient care?
When utilizing this self-sampling technique, patients with inflammatory bowel disease can be readily monitored within their home setting, allowing for clinicians to closely monitor their patients and make timely treatment decisions.
Introduction
Over the past 2 decades, biologicals have become the mainstay of treatment for patients with inflammatory bowel diseases (IBDs). In particular, tumor necrosis factor α (TNF-α) inhibitors (eg, infliximab [IFX], adalimumab), anti-integrin inhibitors (eg, vedolizumab [VEDO]), and interleukin-12p40/23 inhibitors (eg, ustekinumab) have been well established in the therapeutic armamentarium for IBD. These therapeutic agents have shown to effectively induce and maintain clinical remission while also drastically decreasing the need for hospitalization and surgery in patients with IBD.1,2 Management of IBD using biological therapies remains challenging because treatment is accompanied by lack and/or loss of clinical, biochemical, and endoscopic responses. Importantly, approximately 30% to 40% of patients with Crohn’s disease (CD) and ulcerative colitis (UC) achieve remission after biological therapy. Eventually, about 50% of patients who initially responded to treatment will lose response over time.2 Nonresponse and loss of response to biological therapy are largely attributed to pharmacokinetic (PK) or pharmacodynamic (PD) factors. For instance, inadequate drug exposure and formation of antidrug antibodies (ADAs) are associated with biological treatment failure.3-6 However, ineffective use of biological therapy has a negative impact on disease burden and healthcare costs.7 Thus, improvements in monitoring of PK/PD domains are of pivotal importance, and increasing efforts are being made to optimize treatment efficacy through therapeutic drug monitoring (TDM) of biological therapy.
TDM aims to identify treatment failure, including over- and undertreatment, by monitoring biological trough levels and ADA detection, which may promptly allow for dose adjustment or switches to alternative treatment regimens.5,6 Likewise, monitoring of inflammatory biomarkers such as C-reactive protein (CRP) allows to identify acute inflammatory events, and may predict clinical response after treatment initiation, and high levels often precede disease flares.8,9 With regard to the concurrent monitoring of CRP and biological drug concentrations, studies have reported correlations between CRP concentrations and biological therapy response. For example, reduced CRP levels from initiation of anti-TNF therapy at baseline compared with low levels of CRP at weeks 12 to 14 were associated with a maintained response to anti-TNF therapy, while elevated concentrations of CRP in patients with CD predicted nonresponse to anti-TNF therapy treatment.10,11 TDM and CRP monitoring at home are increasingly advocated, as they reduces travel costs and save time for patients, relieve pressure on healthcare systems by reducing dependence on healthcare providers, and may significantly reduce disease burden.12 Home monitoring can be achieved by leveraging capillary blood sampling techniques through a finger prick, which is less invasive compared with in-hospital venepuncture and can be easily performed by patients themselves at home. Using MiniCollect tubes, a “wet” sample can be obtained, and sample centrifugation directly yields serum,13-16 creating a patient-friendly method to facilitate TDM of biologicals and CRP monitoring at home.
In this study, we aimed to clinically validate a capillary blood sampling technique17 individually performed by patients with IBD at home using a finger-prick test. This test would enable simultaneous monitoring of biological trough levels (TDM), here IFX and VEDO, and systemic inflammation (CRP) at home, for patients with IBD receiving biological therapy.
Methods
Study Design and Study Population
In this prospective observational cohort study, patients ≥18 years of age with an established diagnosis of IBD were included between May 2022 and October 2022 at the outpatient IBD clinic of the University Medical Centre Groningen (UMCG). Patients were diagnosed with either CD or UC and received induction or maintenance therapy with either IFX or VEDO according to standard treatment guidelines. Exclusion criteria were the presence of visual, physical, or mental impairment rendering patients unable to use a capillary sampling device, the inability to read or understand Dutch language, the presence of severe Raynaud’s phenomenon or digital ischemia, or unwillingness to participate in the study. This study was approved by the Institutional Review Board (in Dutch: “Medisch Ethische Toetsingscommissie”) of the UMCG (no. 2021/710) and was performed in accordance with the principles of the Declaration of Helsinki (2013). All patients provided written informed consent for their participation in the study.
Data Collection
All included patients received a package at their homes, sent by the investigators, which contained all items and instructions necessary to perform the finger prick at home. (Supplementary Figure S1) Patients were requested to perform the finger prick with the capillary sampling device at home on the same morning that they visited the hospital to receive their biological infusion. After obtaining the sample, the patient sent it by post directly to Sanquin Diagnostics Laboratories. The finger prick was performed using a contact-activated lancet (BD Microtainer; 2.0 × 1.5 mm). Capillary blood was collected within a MiniCollect tube (Greiner BioOne), which was sent using a CoverMed medical envelope with a rigid safety bag (DaklaPack). During the hospital visit on the same day, a venous blood sample was obtained according to standard procedures, before patients received their biological infusion. Afterward, patients were requested to complete a survey with questions to gain insight into their experience of using the capillary sampling device (Supplementary Table S2).
Sample Preparation and Measurements of IFX and VEDO Trough Levels and CRP Concentrations
Self-obtained capillary samples collected in MiniCollect tubes were sent by mail from a patient’s home using a medical envelop. Once samples arrived at Sanquin Diagnostics Laboratory, samples were spun down at 3000 rpm for 10 minutes at room temperature on a Hettich Rotixa 50RS rotor in order to collect the serum. After preparing the samples in the laboratory, samples directly proceeded to analysis, meaning that there was no delay between sample preparation and sample analysis. At room temperature and at a temperature of 4 °C, samples are analyzable up to 21 days. At increased temperatures of 37 °C, samples remain stable up to 14 days after collection. Preparation of venous samples was comparable to capillary samples. However, the collection of spun-down serum occurred at the clinical laboratory of the UMCG and was subsequently stored in -80 °C freezers and was sent to Sanquin Diagnostics Laboratory for analysis in 1 to 3 weekly batches. All biological trough levels were measured using enzyme-linked immunosorbent assays (ELISAs) (Sanquin Diagnostics Laboratories). The IFX ELISAs were performed as described previously.18 Briefly, 2 mg/L monoclonal anti-TNF-7 antibodies in phosphate-buffered saline were coated on Maxisorp ELISA plates. After washing with phosphate-buffered saline/0.02% Tween, recombinant TNF-α (0.01 mg/L) (Active Bioscience) was diluted in a high-performance ELISA (HPE) buffer (Sanquin) and added and incubated for 1 hour at room temperature. Subsequently, the plates were washed again and incubated for 1 hour with patient (venous or capillary) serum, serially diluted in HPE buffer. Next, the plates were washed and incubated for 1 hour with biotinylated IFX-specific rabbit anti-idiotype antibodies (0.25 mg/L in HPE buffer). After washing, streptavidin-poly-horseradish peroxidase (1/25 000, in HPE buffer) was added for 1 hour at 37 °C. Finally, the ELISA was developed with 100 mg/L tetramethylbenzidine in 0.11 M sodium acetate (pH 5.5) containing 0.003% (v/v) H2O2, and the reaction was stopped with 2 M H2SO4. Absorption was measured at 450 nm. Likewise, VEDO ELISAs were performed as previously described.16,19 Polyclonal anti-VEDO antibodies were coated to the plate, VEDO was captured, and subsequently detected with biotinylated F(ab’)2 fragments of the polyclonal anti-VEDO antibody in combination with streptavidin–horseradish peroxidase. The lower limits of quantification for IFX and VEDO were 0.03 μg/mL and 0.01 μg/mL, respectively. CRP levels in venous samples were determined by nephelometry using the Siemens Dimension Vista clinical analyzer. CRP concentrations in capillary samples were determined using an ELISA-based assay, as described previously.20 Briefly, polyclonal rabbit anti-CRP (KH61) was coated to the plates to capture CRP. After washing, biotinylated anti-rCRP was added and plates were developed with streptavidin coupled to monomeric peroxidase.
Study Outcomes and Definitions
The primary study outcome was the agreement in trough levels of IFX and VEDO, and CRP concentrations between capillary samples and venous samples (according to standard procedures). Secondary outcome parameters included practicality of the technique, reflected by the fraction of patients unable to provide a capillary sample, and patients’ experience and support of the capillary sampling technique, as measured through a patient experience survey specifically designed for this study by the investigators.
Sample Size Calculation
Previous studies have been conducted using dried blood spot technology to compare biological trough levels between capillary and venous samples.14,18,21 The most recent study investigated IFX trough levels in pediatric patients with IBD, data of which were used as input for a sample size calculation.21 In that study, a mean difference in IFX levels between venous and capillary samples of -0.14 μg/mL was reported, with lower and upper limits of agreement of -1.39 and 1.12 μg/mL, respectively. Thus, the calculated SD of these differences was 0.64. Considering that the limits of agreement did not exceed 2.0 μg/mL and that this difference was deemed relevant for clinical decision making, a sample size of 32 patients per type of biologic would be required to reliably perform comparison of both methods (while allowing a maximum difference between both methods of 2.0 μg/mL, assuming a type I error rate of 0.05, and type II error rate of 0.20). This sample size calculation was based on a previously described method used to calculate sample sizes for assessing agreement between 2 clinical measurement methods (using Bland-Altman analysis).22
Statistical Analysis
Demographic and clinical characteristics of the study population were presented as mean ± SD, median (interquartile range [IQR]), or proportion and percentage. Assessment of normality was performed visually using normal probability (Q-Q) plots and histograms, and statistically using Shapiro-Wilk tests. Correlations between venous and capillary measurements were calculated using Spearman’s rank correlation coefficients. Bland-Altman plots were established to quantify the degree of agreement between capillary and venous blood test results, visualizing the differences of the 2 measurements on the vertical axis, and the average of the 2 measurements on the horizontal axis. Three horizontal reference lines were superimposed on the plots: 1 line designating the average difference between both measurements and 2 lines designating the upper and lower limits of agreements set at ±1.96 SD of measurement differences (corresponding to 95% confidence intervals [CIs]). Both methods were considered to be in agreement when the difference was small, that is when most data points (>80%) did not exceed the 95% limits of agreement.22,23 To ascertain this, 95% CIs were added to these upper and lower limits of agreement, to be 95% certain that both tests did not substantially disagree. To quantify the consistency (interrater reliability) of both tests, Cohen’s kappa was calculated, after having divided IFX trough levels into low (0-5 μg/mL), adequate (5-10 μg/mL), and high (>10 μg/mL) levels and VEDO trough levels into insufficient (<12 μg/mL) and sufficient (>12 μg/mL) levels, which represent desirable therapeutic ranges for IFX and VEDO, respectively.24,25 CRP was classified as normal (≤5 mg/L) and elevated (>5 mg/L) levels for calculation of Cohen’s kappa. In addition to Bland-Altman analysis, Passing-Bablok linear regression was performed to calculate the slope and intercept of the linear regression line between the 2 tests. Here, the intercept represents the systematic bias between the 2 tests, in which no bias is assumed when zero (0) is present within the 95% CI of the intercept. The slope represents the proportional bias between the 2 tests, and no bias is assumed to be present here when one (1) is within the 95% CI of the slope. Statistical analyses and data visualization were performed using the Python programming language (v.3.9.0; Python Software Foundation; https://python.org) using the pandas (v.1.4.2), numpy (v.1.21.0), matplotlib (v.3.5.1), and seaborn (v.0.12.1) packages.
Results
Characteristics of the Study Population
A total of 81 patients with IBD were included and completed the full study program, consisting of 46 (56.8%) patients with CD, 33 (40.7%) patients with UC, and 2 (2.5%) patients with indeterminate IBD (IBD unclassified). Patients were treated with either IFX (n = 46, 56.8%) or VEDO (n = 35, 43.2%). Mean age of the study population was 42.7 ± 13.6 years, and sex distributions were fairly equal (male/female: 45.7%/54.3%). Most patients (n = 57, 70.4%) were biological-naive, while almost one-third (n = 24, 29.6%) received prior biological therapy. Demographic and clinical characteristics of the study population at time of sampling are presented in Table 1. For more extensive characteristics including concomitant medication and information regarding the schedules and dosages that patients were receiving at the time of sampling, please refer to Supplementary Table S1.
Table 1.
Demographic and clinical characteristics of the study population.
| Variable | Total (N = 81) | IFX (n = 46) | VEDO (n = 35) |
|---|---|---|---|
| Age, y | 42.7 ± 13.6 | 40.2 ± 12.1 | 45.9 ± 14.8 |
| Sex | |||
| Female | 44 (54.3) | 29 (63.0) | 15 (42.9) |
| Male | 37 (45.7) | 17 (37.0) | 20 (57.1) |
| BMI, kg/m2 | 26.9 ± 7.3 | 27.4 ± 8.2 | 26.2 ± 5.9 |
| IBD diagnosis | |||
| CD | 46 (56.8) | 39 (84.8) | 7 (20.0) |
| UC | 33 (40.7) | 5 (10.9) | 28 (80.0) |
| IBD-U | 2 (2.5) | 2 (4.3) | 0 (0.0) |
| Disease activity | |||
| HBI | 4 (1-6.3) | 4 (1-6) | 4 (1-7) |
| SCCAI | 3 (1-5) | 4 (2-6) | 3 (1-l5) |
| CRP, mg/L | 1.7 (0.8-3.7) | 1.5 (0.7-2.5) | 2.6 (1.1-5.0) |
| Biological trough levels | |||
| Drug level in venous sample, μg/mL | — | 6.5 (3.6-9.1) | 16 (10-24) |
| Antidrug antibodies | |||
| Detectable in venous sample | 4 (4.9) | 1 (2.2) | 3 (3.7) |
Values are mean ± SD, n (%),or median (interquartile range). P values ≤ .05 were considered statistically significant.
Abbreviations: BMI, body mass index; CD, Crohn’s disease; CRP, C-reactive protein; HBI, Harvey-Bradshaw Index; IBD, inflammatory bowel disease; IBD-U, inflammatory bowel disease unclassified; IFX, infliximab; SCCAI, Simple Clinical Colitis Activity Index; UC, ulcerative colitis; VEDO, vedolizumab.
Venous and Capillary Concentrations of IFX and VEDO Demonstrate Excellent Agreement
In total, 75 (92.6%) patients provided both a venous (following an in-hospital venepuncture) and capillary (following the at-home-performed finger prick) blood sample, of which data were available to compare biological trough levels between both measurement methods. The average transit time from self-sampling by the patient until arrival of capillary sampling at the diagnostic laboratory by post was 24 to 48 hours. During the study period, a minimum temperature of 1.7 °C and a maximum temperature of 35.5 °C were reported by the Royal Netherlands Meteorological Institute, the Dutch national weather service. Median blood volume of the obtained samples available for analysis was 50 μL. For the remaining 6 (7.4%) patients, no paired data were available due to insufficient capillary sample volume (n = 2), loss of MiniCollect tubes during the mailing process (n = 3), or an omitted venepuncture (n = 1). Of the 75 patients with paired data, 41 patients were treated with IFX and 34 patients were treated with VEDO. Comparative analysis between venous and capillary trough levels was performed using Bland-Altman plots (Figure 1).
Figure 1.
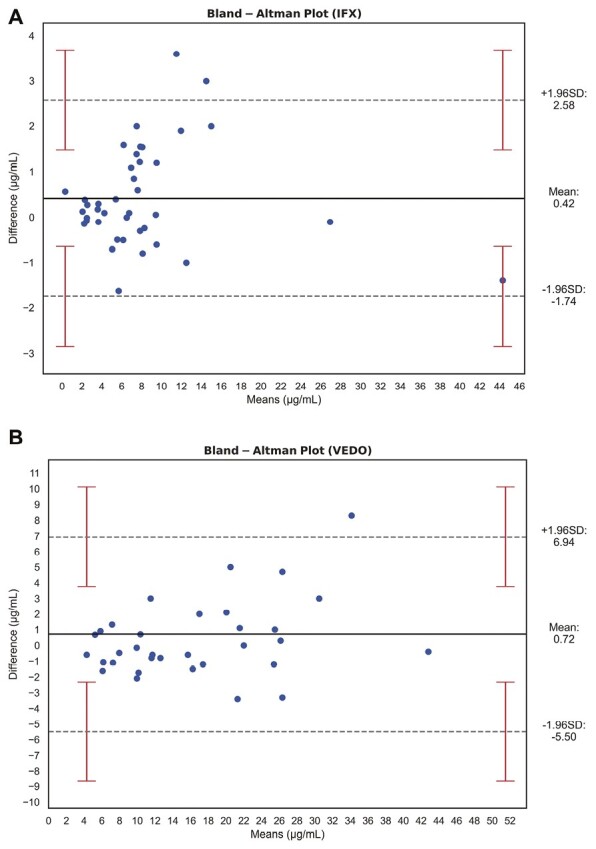
Bland-Altman plots showing the comparison of venous and capillary measurements of (A) infliximab (IFX) trough levels (μg/mL) and (B) vedolizumab (VEDO) trough levels (μg/mL). The black bold lines indicate the mean difference, the gray dashed lines indicate 95% limits of agreement, and the red error bars show the 95% confidence interval of the limits of agreement. Venous and capillary concentrations of IFX and VEDO showed excellent agreement.
For IFX, the mean difference between both methods was 0.42 μg/mL, with corresponding 95% limits of agreement of -1.74 (95% CI, -2.84 to -0.64) and 2.58 (95% CI, 1.48 to 3.68) μg/mL. All but 2 outliers (which were still within the 95% CI of the upper limit of agreement) were within the 95% limits of agreement (Figure 1A), indicating unbiased agreement between the 2 methods. For VEDO, a similar pattern was observed (Figure 1B), with a mean difference of 0.72 μg/mL, with corresponding limits of agreement of -5.50 (95% CI, -2.33 to -8.67) and 6.94 (95% CI, 3.77 to 10.11) μg/mL. These findings were sustained by the presence of very strong and statistically significant correlations between venous and capillary IFX trough levels (ρ = 0.96, P < .001) and venous and capillary VEDO trough levels (ρ = 0.97, P < .001) (Supplementary Figure S2). When IFX trough levels were categorized as low (0-5 μg/mL), adequate (5-10 μg/mL), or high (>10 μg/mL), the value for weighted Cohen’s kappa was 0.82 (95% CI, 0.64 to 0.96; P < .001), showing excellent intermethod agreement. When VEDO trough levels were categorized as insufficient (<12 μg/mL) and sufficient (>12 μg/mL), Cohen’s kappa was 0.94 (95% CI, 0.82 to 1.00; P < .001), also demonstrating excellent agreement. To better evaluate the potential existence of systematic or proportional bias between both methods, Passing-Bablok regression was performed (Figure 2). For IFX trough levels, neither systematic nor proportional bias was supported because zero (0) and one (1) were enclosed within the 95% CI of the calculated intercept (-0.12 [95% CI, -0.66 to 0.32]) and slope (1.08 [95% CI, 0.97 to 1.21]), respectively (Figure 2A). Likewise, for VEDO trough levels, no systematic (intercept -1.23 [95% CI, -2.41 to 0.12]) or proportional bias (slope 1.06 [95% CI, 0.97 to 1.17]) could be demonstrated.
Figure 2.
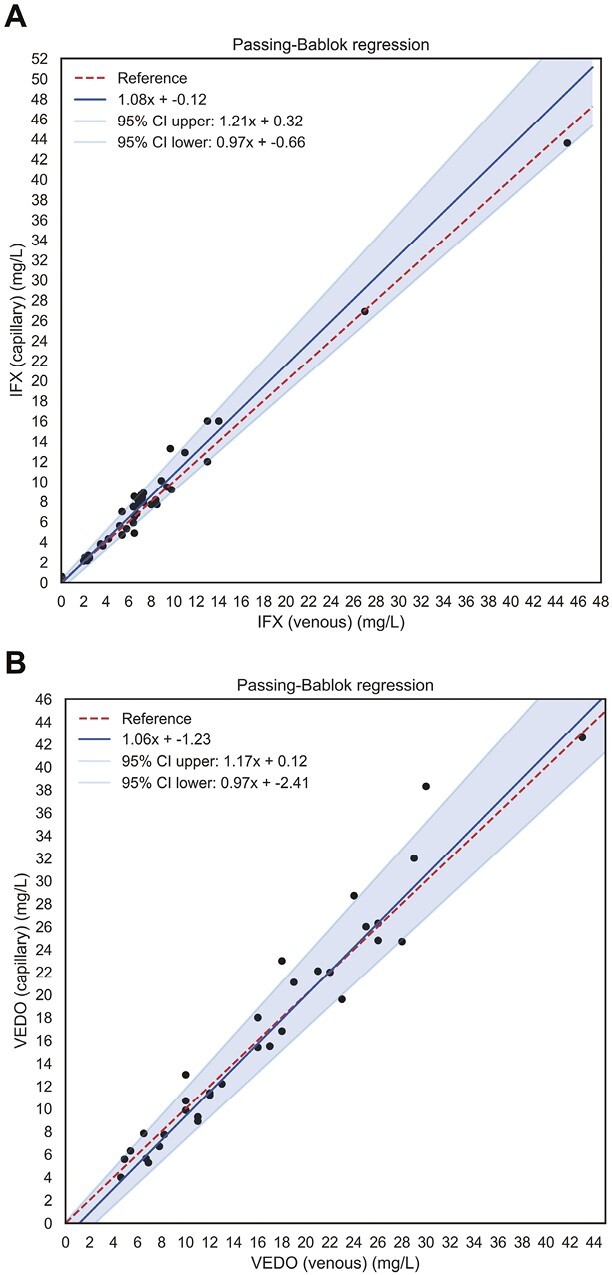
Passing-Bablok regression lines showing the associations between venous and capillary concentrations (μg/mL) of (A) IFX and (B) VEDO. The solid blue lines are the regression lines, with the surrounding blue shades representing 95% confidence intervals. The red dashed lines represent the reference lines (lines of identity). Venous and capillary concentrations of IFX and VEDO were highly correlated in the absence of systematic or proportional bias. CI, confidence interval; IFX, infliximab; VEDO, vedolizumab.
Presence of ADAs Against IFX and VEDO
Four (4.9%) patients presented with detectable ADAs in venous samples. One patient treated with IFX presented with a positive ADA titer (310 AE/mL), and 3 VEDO-treated patients presented with ADA concentrations between 4 and 10 AE/mL, a range in which ADAs against VEDO are deemed detectable but not quantifiable. No statistically relevant comparisons could be made between capillary and venous samples for these low numbers of patients with ADAs.
CRP Measurements in Capillary Samples
In total, samples of 71 (87.7%) patients could be analyzed for CRP concentrations. Venous and capillary CRP concentrations significantly correlated with each other (ρ = 0.99, P < .001) (Supplementary Figure S3). When Passing-Bablok regression was performed, no systematic (intercept 0.01 [95% CI, -0.07 to 0.05]), albeit with a slight proportional increase in CRP levels (slope 1.11 [95% CI, 1.05 to 1.17]), could be demonstrated in favor of the capillary samples (Figure 3). When CRP concentrations were categorized as low (<5 μg/mL) and elevated (>5 μg/mL), Cohen’s kappa was 0.91 (95% CI, 0.84 to 0.98; P < .001), demonstrating excellent agreement.
Figure 3.
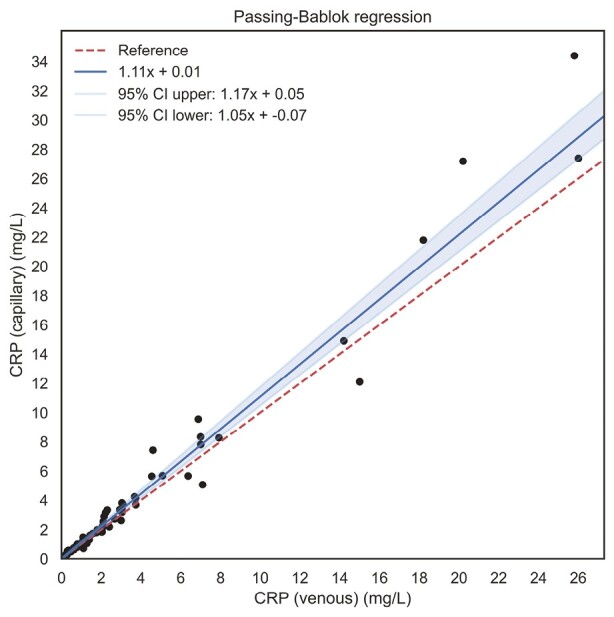
Passing-Bablok regression lines showing the associations between venous and capillary concentrations (μg/mL) of C-reactive protein (CRP). Venous and capillary concentrations of CRP show no systematic bias (as determined by Passing-Bablok regression), albeit a slight proportional bias toward higher levels in capillary samples. CI, confidence interval.
Patients Report a High Degree of Support and Tolerability to the Capillary Finger Prick Home Sampling Device
All patients completed a questionnaire asking questions about their experience and practicality of performing the finger prick–based capillary sampling device in the home setting. Almost all patients (97.5%) were able to successfully perform the finger prick by collecting sufficient blood in the MiniCollect tube. All patients (100%) found the written instructions sufficiently clear. In total, 59 (72.8%) patients performed the finger prick independently, while 22 (27.2%) patients asked their partner, sibling, or parents for help. Most patients (87.7%) were willing to periodically perform the finger pick in the future, if such a clinical practice would be implemented. Finally, patients rated several aspects related to their experience with the finger prick device on a 10-point visual analog scale (VAS) (Figure 4). Median VAS scores for pain and tension were very low (median 2 [IQR, 1-8] and 1 [IQR, 1-10], respectively). Most patients felt sufficiently competent to perform the finger prick (self-efficacy: median 10 [IQR, 4-10]; performance: median 10 [IQR, 5-10]), read and understand the instructions beforehand (median 10 [IQR, 7-10]), and send the sample by mail to the laboratory (median 10 [IQR, 4-10]). Most patients assessed the practice of massaging blood from finger (median 8 [IQR, 1-10]) and catching blood drops in the MiniCollect tube (median 8 [IQR, 1-10]) as very straightforward, yet a smaller fraction of patients reported some slight difficulties with these aspects. Supplementary Table S2 provides a complete overview of the results of the patient experience survey.
Figure 4.
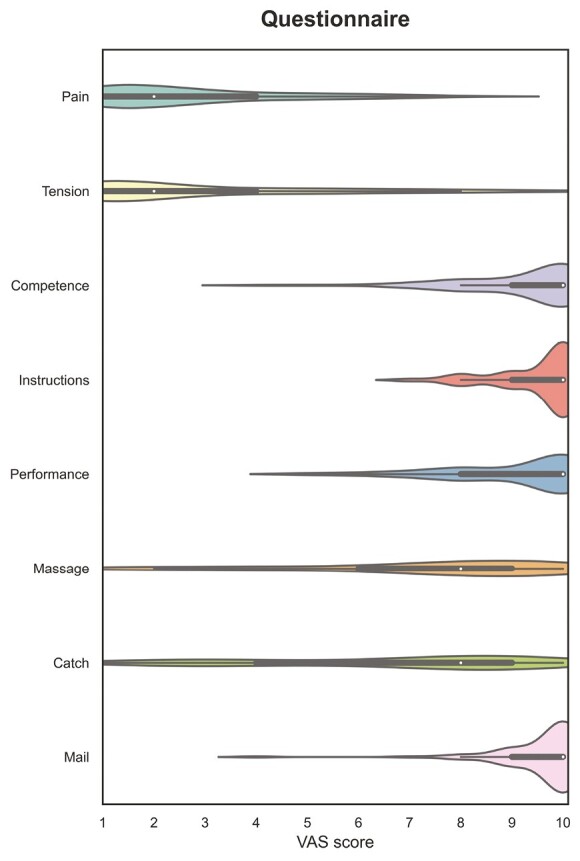
Violin plots detailing questionnaire items related to patients’ experience and tolerability regarding the finger prick capillary sampling device. The white dots indicate the median visual analog scale (VAS) scores given by patients, while the bars indicate the total range of reported values.
Discussion
In this prospective cohort study, we demonstrated that a finger prick–based capillary blood sampling test can be effectively used at home by patients with IBD to reliably measure IFX and VEDO trough levels as well as CRP concentrations. For both IFX and VEDO, venous and capillary blood concentrations exhibited excellent concordance in the absence of any systematic or proportional bias between both measurements. Furthermore, we report a high degree of patient satisfaction and tolerability for using the “wet” capillary self-sampling technique at home. In clinical practice, TDM for patients with IBD receiving IFX and VEDO treatment is impeded by the significant delay (up to 2-3 weeks) that may occur between blood sample withdrawal and the clinical availability of test results. This complicates routine management because this delays clinical decision making upon biological trough levels. As such, finger-prick testing using a capillary blood sampling device would be an easy, tolerable, and robust method for at-home monitoring of biological treatment and systemic inflammation (CRP) for patients with IBD.
Over the past 2 decades, biological therapy has gained a predominant place within the treatment of IBD, showing significant improvements in reducing clinical symptoms, the induction and maintenance of endoscopic and histological remission, and reductions in hospitalization rates and the need for surgery.26 Until the date, however, about one-third of patients treated with induction therapy exhibited primary nonresponse, and one-third experienced loss of response over time.27,28 Obviously, sufficient drug exposure is critical to achieve therapeutic efficacy (ie, reaching and maintaining drug levels within the therapeutic range). Insufficient drug exposure and the development of ADAs are associated with (PD) nonresponse to biologicals.29 Following the principles of TDM, drug levels, ADAs, and inflammatory biomarkers such as CRP could be monitored on a regular basis. Desirable therapeutic drug level ranges are estimated to lie within the range of 5 to 10 μg/mL for IFX24 and above 12 μg/mL for VEDO,25 which have been associated with mucosal healing.30,31 However, due to high interindividual PK differences, it is difficult to precisely determine clinically relevant cutoffs. Furthermore, we lack evidence to provide clear therapeutic windows for both drugs.32 Follow-up studies are needed to better characterize interindividual PK differences, optimal therapeutic ranges, and their clinical benefits. Additionally, TDM may aid in increasing cost-effectiveness of biological therapy by early discontinuation of futile therapy and enabling personalized dose titrations based on PK factors.33,34 As such, TDM offers guidance in making therapeutic decisions, although we should not solely rely on it when performing dose adjustments or making switches to alternative treatments.
This study shows that a “wet” finger prick–based capillary blood self-sampling technique using MiniCollect tubes is able to reliably detect biological trough levels and CRP concentrations, which are currently routinely measured in standard clinical practice through venous sampling. Compared with other sampling techniques, such as dried blood spot or volumetric absorptive microsampling, “wet” samples are potentially less labor-intensive and easier to implement in practice.13-15 Most (>95%) patients who participated in this study experienced the finger prick as a straightforward, easy-to-follow, accessible, and feasible test, reflecting a high degree of patient support, tolerability, and practicality, without the need for prior training. This patient support for home monitoring would allow for reactive and proactive TDM, without the need for patients to frequently visit the hospital. IBD can have a major impact on quality of life35; thus, offering options such as home-based blood tests could contribute to reducing disease burden for patients while simultaneously allowing treating physicians to closely monitor their patients and make timely treatment decisions. As such, implementation of finger prick–based capillary blood self-sampling in clinical practice could make TDM more widely available and more accessible for patients, thereby also increasing their contribution to shared therapeutic decision making with their treating physicians. For example, drug levels measured at home using finger prick–based capillary self-sampling would allow real-time dose adjustments instead of waiting for the next upcoming hospital visit for drug level measurements. Finally, other laboratory tests in addition to TDM could be integrated in this test method, and residual blood could be stored for retrospectively adding laboratory measurements.
For future implementation of capillary home-based sampling, it is important to acknowledge the differences in trough levels reached when administering intravenous or subcutaneous therapy. In the present study, we included patients with IBD on intravenous therapy. A study investigating real-world data of patients from IBD who switched from an intravenous to a 14-day treatment cycle of subcutaneously injected IFX therapy reported median serum IFX trough levels obtained during subcutaneous therapy of 11.0 (IQR, 7.5-15.1) μg/mL.36 Likewise, a study investigating real-world data of patients from IBD who switched from intravenous to subcutaneous VEDO therapy reported median serum VEDO trough levels during subcutaneous therapy of 19.0 (IQR, 13.0–23.0) μg/mL, compared with median VEDO trough levels of 8.1 (IQR, 5.2–14) μg/mL of patients on intravenous therapy.37 The trough concentrations from subcutaneous therapy as reported by these studies are still within the reliable concentration ranges (roughly < 20 μg/mL for IFX and < 35 μg/mL for VEDO) as we could detect in the present study, suggesting that the validity of the capillary blood–based sampling method could be comparably useful for measuring concentrations from subcutaneously administered biologicals.
In a different cohort of patients with IBD from the United Kingdom, Chee et al38 investigated a capillary sampling technique similar to the self-sampling technique used in our cohort. Capillary adalimumab, IFX, VEDO, and ustekinumab drug concentrations, as well as anti-adalimumab and anti-IFX antibody concentrations, proved equivalent to measurements in blood obtained through venepuncture.38 Our study confirmed the reliability of TDM of biological therapy trough home-based capillary self-sampling. Similar to our study, this study also reported a high degree of patient satisfaction with a finger prick–based self-sampling device, which was even preferred over conventional venepuncture. Approximately three-quarters of patients successfully performed the remote capillary testing. In our study, almost all patients (>95%) were fully competent in performing the blood collection. In addition, we obtained detailed information about potential barriers that patients report with using the finger prick–based capillary self-sampling device. For instance, some patients experienced difficulties in obtaining sufficient blood drops from the finger or had difficulties collecting the drops in the tube. About one quarter of patients also requested help from another individual (ie, a relative) to perform the finger prick–based blood withdrawal. Still, a major advantage of the finger prick–based capillary self-sampling device is that it could also potentially be used by patients administering subcutaneous injections with biological such as adalimumab or ustekinumab, which may likewise aid in therapeutic guidance and reduce the frequency of required hospital visits.
Some limitations of this study warrant recognition. First, analysis of ADAs is an important domain of TDM, yet ADAs could not be adequately analyzed in our cohort due to small sample numbers with detectable ADAs. However, the aforementioned study conducted by Chee et al38 investigated a capillary technique similar to the self-sampling technique used in our cohort and found sufficient agreement between venous and capillary samples regarding ADA titers to IFX and adalimumab. This finding appears very hopeful for the use of at-home capillary self-sampling for both drug concentrations and ADA detection. In addition, this study was of cross-sectional nature, whereas longitudinal measurements would allow to investigate the capacity of the capillary blood sampling test in capturing the dynamics of trough levels and CRP across various ranges and different clinical conditions. Capillary samples were found to be stable at outside temperatures ranging from 1.7 to 37 °C; however, additional temperature stability tests to establish optimized shipping conditions need to be conducted.
Discussion
This study clinically validated the use of a finger prick–based capillary blood sampling device that allows for simultaneous monitoring of biological trough levels and CRP at home. This study showed strong correlations between venous and capillary measurements for IFX, VEDO, and CRP levels, and all values obtained fell within acceptable limits of agreement, proving this finger prick–based capillary blood sampling test to be useful for TDM at home. Future studies are warranted to further implement the finger prick–based capillary blood sampling test in relation to clinically relevant outcomes such as the number of required hospital visits, biological response rates, and cost-effectiveness.
Supplementary Material
Acknowledgments
The authors thank all participating patients and the infusion center of the University Medical Centre Groningen for the practical assistance during the study.
Contributor Information
Antonius T Otten, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Groningen, the Netherlands.
Hedwig H van der Meulen, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Groningen, the Netherlands.
Maurice Steenhuis, Biologics Laboratory, Sanquin Diagnostic Services, Amsterdam, the Netherlands.
Floris C Loeff, Biologics Laboratory, Sanquin Diagnostic Services, Amsterdam, the Netherlands.
Daan J Touw, Department of Pharmaceutical Analysis, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, the Netherlands.
Jos G W Kosterink, Department of PharmacoTherapy, Epidemiology and Economy, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, the Netherlands.
Henderik W Frijlink, Department of Pharmaceutical Technology and Biopharmacy, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, the Netherlands.
Theo Rispens, Department of Immunopathology, Sanquin Research, Amsterdam, the Netherlands.
Gerard Dijkstra, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Groningen, the Netherlands.
Marijn C Visschedijk, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Groningen, the Netherlands.
Arno R Bourgonje, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Groningen, the Netherlands.
Author Contribution
A.T.O., F.C.L., M.S., G.D., M.C.V., and A.R.B. conceptualized and initiated the study. ATO, H.H.v.d.M., and A.R.B. gathered and prepared the data. A.T.O., H.H.v.d.M., M.S., and A.R.B. analyzed the data. A.R.B. performed data visualization. A.T.O., H.H.v.d.M., and A.R.B. wrote the first draft of the manuscript. G.D., M.C.V., and A.R.B. supervised the study. All authors contributed to manuscript revision, read and approved the final version of the manuscript to be submitted for publication.
Funding
No external funders were involved in this study and thus funders had no role in the design of the study, collection, analysis, or interpretation of data or in writing the manuscript.
Conflicts of Interest
G.D. has received a research grant from Royal DSM; and received speaker fees from Pfizer, AbbVie, and Janssen Pharmaceuticals, outside the submitted work. A.R.B. has received a research grant from Janssen Pharmaceuticals, outside the submitted work. D.J.T. has received research grants from Chiesi; served on the advisory board of Pure-IMS; and served as vice-chair of the Medical Advisory Board of Sanquin, unrelated to the submitted work. All other authors have no conflicts of interest to declare.
Data Availability
The dataset(s) used for the current study are available from the corresponding author upon reasonable request.
References
- 1. Katsanos KH, Papamichael K, Feuerstein JD, et al. Biological therapies in inflammatory bowel disease: beyond anti-TNF therapies. Clin Immunol. 2018;206:9-14. [DOI] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Lémann M.. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33(8):870-879. [DOI] [PubMed] [Google Scholar]
- 3. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182-197. [DOI] [PubMed] [Google Scholar]
- 4. Papamichael K, Cheifetz AS.. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol. 2016;7(4):289-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655-1668.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nanda KS, Cheifetz AS, Moss AC.. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): A meta-analysis. Am J Gastroenterol. 2013;108(1):40-47; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Valk ME, Mangen M-JJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63(1):72-79. [DOI] [PubMed] [Google Scholar]
- 8. Solem CA, Loftus EV Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707-712. [DOI] [PubMed] [Google Scholar]
- 9. Chen P, Zhou G, Lin J, et al. Serum biomarkers for inflammatory bowel disease. Front Med (Lausanne). 2020;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reinisch W, Wang Y, Oddens BJ, Link R.. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: a post-hoc analysis from ACCENTI. Aliment Pharmacol Ther. 2012;35(5):568-576. [DOI] [PubMed] [Google Scholar]
- 11. Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. 2014;8(2):129-136. [DOI] [PubMed] [Google Scholar]
- 12. Overton PM, Shalet N, Somers F, Allen JA.. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer Adherence. 2021;15:811-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Denniff P, Spooner N.. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal Chem. 2014;86(16):8489-8495. [DOI] [PubMed] [Google Scholar]
- 14. Berends SE, Bloem K, de Vries A, et al. Monitoring of adalimumab concentrations at home in patients with inflammatory bowel disease using dried blood samples. Ther Drug Monit. 2020;l42(2):289-294. [DOI] [PubMed] [Google Scholar]
- 15. Wilhelm AJ, den Burger JCG, Swart EL.. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet. 2014;53(11):961-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloem K, Schaap T, Boshuizen R, et al. Capillary blood microsampling to determine serum biopharmaceutical concentration: Mitra® microsampler vs dried blood spot. Bioanalysis. 2018;10(11):815-823. [DOI] [PubMed] [Google Scholar]
- 17. Toorop AA, Steenhuis M, Loeff FC, et al. Fingerprick blood samples to measure serum natalizumab concentrations. Mult Scler. 2022;29(3):457-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vande Casteele N, Buurman DJ, Sturkenboom MG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36(8):765-771. [DOI] [PubMed] [Google Scholar]
- 19. Löwenberg M, Vermeire S, Mostafavi N, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology. 2019;157(4):997-1006.e6. [DOI] [PubMed] [Google Scholar]
- 20. Padilla ND, Bleeker WK, Lubbers Y, et al. Rat C-reactive protein activates the autologous complement system. Immunology. 2003;109(4):564-571. doi: 10.1046/j.1365-2567.2003.01681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zijlstra M, Jongsma MME, de Vries A, Schaap T, Bloem K, de Ridder L.. Infliximab level between venous and capillary blood using novel device strongly correlate in paediatric inflammatory bowel disease patients. J Pediatr Gastroenterol Nutr. 2021;72(1):56-60. [DOI] [PubMed] [Google Scholar]
- 22. Lu MJ, Zhong WH, Liu YX, et al. Sample size for assessing agreement between two methods of measurement by Bland-Altman method. Int J Biostat. 2016;12(2):20150039. [DOI] [PubMed] [Google Scholar]
- 23. Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310. [PubMed] [Google Scholar]
- 24. van Hoeve K, Dreesen E, Hoffman I, et al. Higher infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J Crohns Colitis. 2018;12(11):1316-1325. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Dulai PS, Vande Casteele N, et al. Systematic review with meta-analysis: association between vedolizumab trough concentration and clinical outcomes in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2019;50(8):848-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Billiet T, Rutgeerts P, Ferrante M, Van Assche G, Vermeire S.. Targeting TNF-α for the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2014;14(1):75-101. [DOI] [PubMed] [Google Scholar]
- 27. Ding NS, Hart A, De Cruz P.. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43(1):30-51. [DOI] [PubMed] [Google Scholar]
- 28. Peyrin-Biroulet L, Danese S, Argollo M, et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(5):838-846.e2. [DOI] [PubMed] [Google Scholar]
- 29. Papamichael K, Cheifetz AS.. Therapeutic drug monitoring in inflammatory bowel disease: for every patient and every drug? Curr Opin Gastroenterol. 2019;35(4):302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14(4):550-557.e2. [DOI] [PubMed] [Google Scholar]
- 31. Yacoub M, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47(7):906-912. [DOI] [PubMed] [Google Scholar]
- 32. Tamilarasan AG, Cunningham G, Irving PM, Samaan MA.. Recent advances in monoclonal antibody therapy in IBD: practical issues. Frontline Gastroenterol. 2019;10(4):409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martelli L, Olivera P, Roblin X, Attar A, Peyrin-Biroulet L.. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J Gastroenterol. 2017;52(1):19-25. [DOI] [PubMed] [Google Scholar]
- 34. Papamichael K, Cheifetz AS.. Optimizing therapeutic drug monitoring in inflammatory bowel disease: a focus on therapeutic monoclonal antibodies. Expert Opin Drug Metab Toxicol. 2021;17(12):1423-1431. [DOI] [PubMed] [Google Scholar]
- 35. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A.. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part I. Inflamm Bowel Dis. 2018;24(4):742-751. [DOI] [PubMed] [Google Scholar]
- 36. Roblin X, Veyrard P, Bastide L, et al. Subcutaneous injection of infliximab CT-P13 results in stable drug levels within 14-day treatment cycle in Crohn’s disease. Aliment Pharmacol Ther. 2022;56(1):77-83. doi: 10.1111/apt.16852 [DOI] [PubMed] [Google Scholar]
- 37. Bergqvist V, Holmgren J, Klintman D, Marsal J.. Real-world data on switching from intravenous to subcutaneous vedolizumab treatment in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55(11):1389-1401. doi: 10.1111/apt.16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chee D, Nice R, Hamilton B, et al. Patient-led remote IntraCapillary pharmacoKinetic sampling (fingerPRICKS) for therapeutic drug monitoring in patients with inflammatory bowel disease. J Crohns Colitis. 2022;16(2):190-198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset(s) used for the current study are available from the corresponding author upon reasonable request.


