Abstract
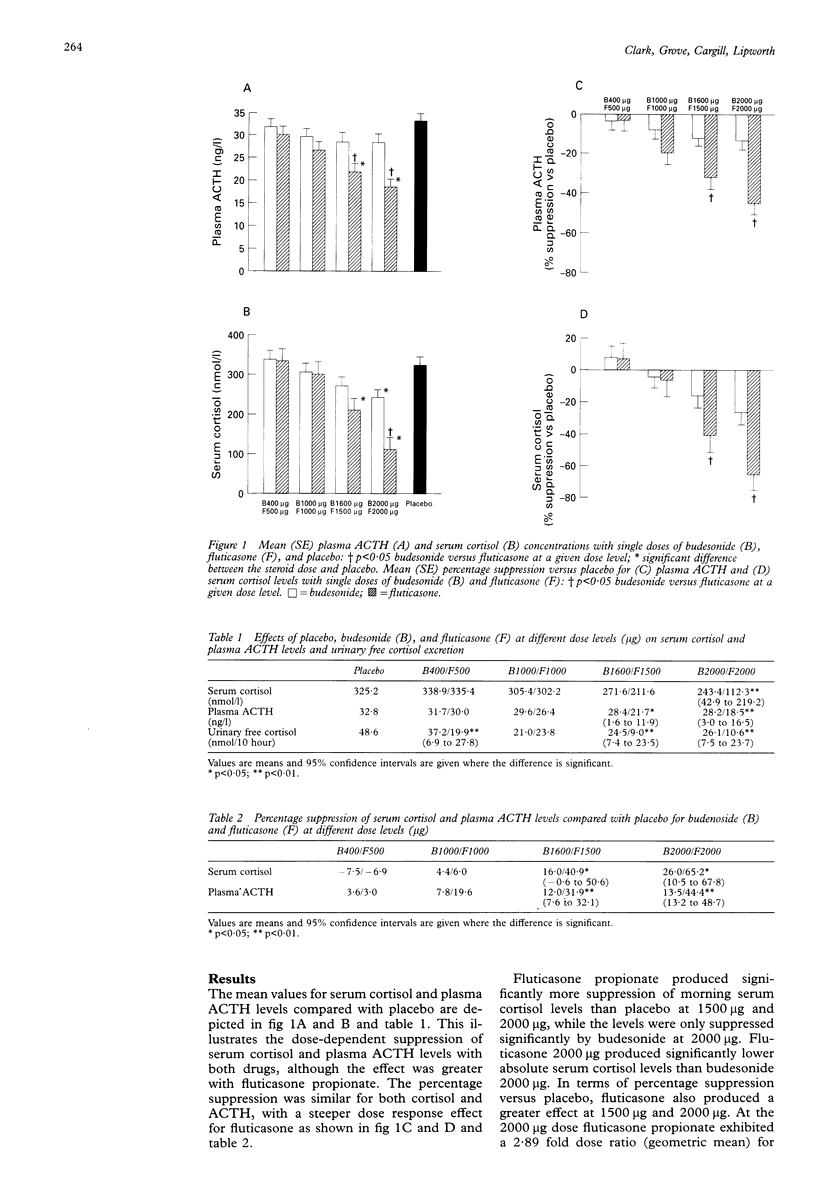
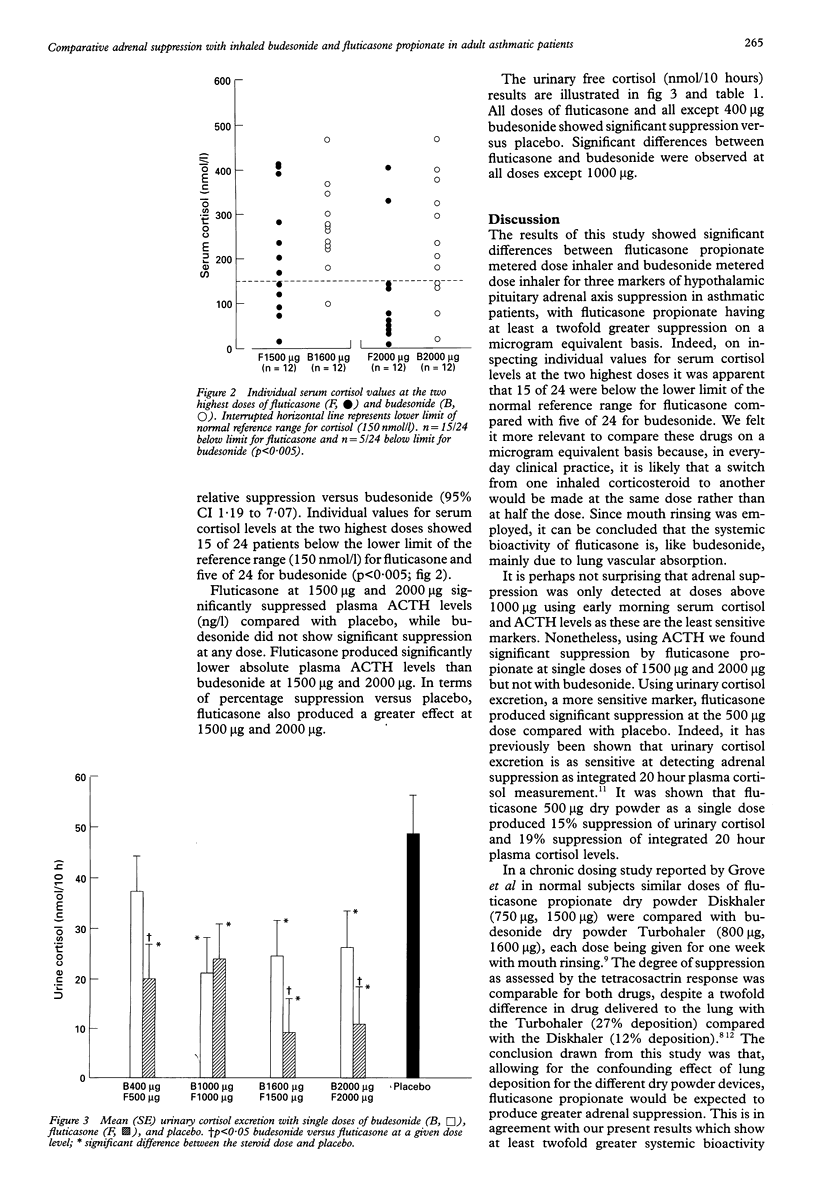
BACKGROUND: A study was performed to compare the adrenal suppression caused by inhaled fluticasone propionate and budesonide on a microgram equivalent basis, each given by metered dose inhaler to asthmatic patients. METHODS: Twelve asthmatic patients of mean age 29.9 years, with a forced expiratory volume in one second (FEV1) 92.9% predicted and forced expiratory flow 25-75% (FEF25-75) 69.5% predicted, on less than or equal to 400 micrograms/day inhaled corticosteroid, were studied in a double blind placebo controlled crossover design comparing single doses of inhaled budesonide 400, 1000, 1600, 2000 micrograms and fluticasone propionate 500, 1000, 1500, 2000 micrograms. Doses were administered at 22.00 hours by metered dose inhaler with mouth rinsing and measurements were made in the laboratory 10 hours later. RESULTS: Serum cortisol levels compared with placebo (mean 325.2 nmol/l) were suppressed by fluticasone at doses of 1500 micrograms (211.6 nmol/l) and 2000 micrograms (112.3 nmol/l) and by budesonide at 2000 micrograms (243.4 nmol/l). Fluticasone propionate 2000 micrograms produced lower absolute serum cortisol levels than budesonide 2000 micrograms (95% CI for difference 42.9 to 219.2). The dose ratio (geometric mean) for the relative potency was 2.89 fold (95% CI 1.19 to 7.07). In terms of percentage suppression versus placebo, fluticasone propionate also produced greater effects (means and 95% CI for difference): budesonide 1600 micrograms (16.0) versus fluticasone propionate 1500 micrograms (40.9) (95% CI -0.6 to 50.6), budesonide 2000 micrograms (26.0) versus fluticasone 2000 micrograms (65.2) (95% CI 10.5 to 67.8). Individual serum cortisol levels at the two highest doses showed 15 of 24 patients below the normal limit of the reference range (150 nmol/l) for fluticasone and five of 24 for budesonide. Fluticasone propionate also caused greater ACTH suppression than budesonide (as % versus placebo): budesonide 1600 micrograms (12.0) versus fluticasone propionate 1500 micrograms (31.9) (95% CI 7.6 to 32.1), budesonide 2000 micrograms (13.5) versus fluticasone propionate 2000 micrograms (44.4) (95% CI 13.2 to 48.7). For overnight 10 hour urinary cortisol (nmol/10 hours) there was a difference between the lowest doses of the two drugs: budesonide 400 micrograms (37.2) versus fluticasone propionate 500 micrograms (19.9) (95% CI 6.9 to 27.8). CONCLUSIONS: Like budesonide the systemic bioactivity of fluticasone propionate is mainly due to lung vascular absorption. Fluticasone propionate exhibited at least twofold greater adrenal suppression than budesonide on a microgram equivalent basis in asthmatic patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres J. G., Bateman E. D., Lundbäck B., Harris T. A. High dose fluticasone propionate, 1 mg daily, versus fluticasone propionate, 2 mg daily, or budesonide, 1.6 mg daily, in patients with chronic severe asthma. International Study Group. Eur Respir J. 1995 Apr;8(4):579–586. [PubMed] [Google Scholar]
- Bain B. M., Harrison G., Jenkins K. D., Pateman A. J., Shenoy E. V. A sensitive radioimmunoassay, incorporating solid-phase extraction, for fluticasone 17-propionate in plasma. J Pharm Biomed Anal. 1993 Jul;11(7):557–561. doi: 10.1016/0731-7085(93)80005-l. [DOI] [PubMed] [Google Scholar]
- Boe J., Bakke P., Rødølen T., Skovlund E., Gulsvik A. High-dose inhaled steroids in asthmatics: moderate efficacy gain and suppression of the hypothalamic-pituitary-adrenal (HPA) axis. Research Council of the Norwegian Thoracic Society. Eur Respir J. 1994 Dec;7(12):2179–2184. doi: 10.1183/09031936.94.07122179. [DOI] [PubMed] [Google Scholar]
- Borgström L., Bondesson E., Morén F., Trofast E., Newman S. P. Lung deposition of budesonide inhaled via Turbuhaler: a comparison with terbutaline sulphate in normal subjects. Eur Respir J. 1994 Jan;7(1):69–73. doi: 10.1183/09031936.94.07010069. [DOI] [PubMed] [Google Scholar]
- Fabbri L., Burge P. S., Croonenborgh L., Warlies F., Weeke B., Ciaccia A., Parker C. Comparison of fluticasone propionate with beclomethasone dipropionate in moderate to severe asthma treated for one year. International Study Group. Thorax. 1993 Aug;48(8):817–823. doi: 10.1136/thx.48.8.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahnén A., Eckernäs S. A., Brundin R. M., Ling-Andersson A. An assessment of the systemic activity of single doses of inhaled fluticasone propionate in healthy volunteers. Br J Clin Pharmacol. 1994 Dec;38(6):521–525. doi: 10.1111/j.1365-2125.1994.tb04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove A., Allam C., McFarlane L. C., McPhate G., Jackson C. M., Lipworth B. J. A comparison of the systemic bioactivity of inhaled budesonide and fluticasone propionate in normal subjects. Br J Clin Pharmacol. 1994 Dec;38(6):527–532. doi: 10.1111/j.1365-2125.1994.tb04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M. The human pharmacology of fluticasone propionate. Respir Med. 1990 Nov;84 (Suppl A):25–29. doi: 10.1016/s0954-6111(08)80004-2. [DOI] [PubMed] [Google Scholar]
- Högger P., Rohdewald P. Binding kinetics of fluticasone propionate to the human glucocorticoid receptor. Steroids. 1994 Oct;59(10):597–602. doi: 10.1016/0039-128x(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J. Clinical pharmacology of corticosteroids in bronchial asthma. Pharmacol Ther. 1993;58(2):173–209. doi: 10.1016/0163-7258(93)90049-j. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J. New perspectives on inhaled drug delivery and systemic bioactivity. Thorax. 1995 Feb;50(2):105–110. doi: 10.1136/thx.50.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchor R., Biddiscombe M. F., Mak V. H., Short M. D., Spiro S. G. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax. 1993 May;48(5):506–511. doi: 10.1136/thx.48.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipps G. H. Structure-activity relationships of topically active steroids: the selection of fluticasone propionate. Respir Med. 1990 Nov;84 (Suppl A):19–23. doi: 10.1016/s0954-6111(08)80003-0. [DOI] [PubMed] [Google Scholar]
- Ryrfeldt A., Andersson P., Edsbäcker S., Tönnesson M., Davies D., Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;122:86–95. [PubMed] [Google Scholar]


