Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by memory loss, cognitive disorder, language dysfunction, and mental disability. The main neuropathological changes in AD mainly include amyloid plaque deposition, neurofibrillary tangles, synapse loss, and neuron reduction. However, the current anti-AD drugs do not demonstrate a favorable effect in altering the pathological course of AD. Moreover, long-term use of these drugs is usually accompanied with various side effects. Ginsenosides are the major active constituents of ginseng and have protective effects on AD through various mechanisms in both in vivo and in vitro studies. In this review, we focused on discussing the therapeutic potential effects and the mechanisms of pharmacological activities of ginsenosides in AD, to provide new insight for further research and clinical application of ginsenosides in the future. Recent studies on the pharmacological effects and mechanisms of ginsenosides were retrieved from Chinese National Knowledge Infrastructure, National Science and Technology Library, Wanfang Data, Elsevier, ScienceDirect, PubMed, SpringerLink, and the Web of Science database up to April 2023 using relevant keywords. Network pharmacology and bioinformatics analysis were used to predict the therapeutic effects and mechanisms of ginsenosides against AD. Ginsenosides presented a wide range of therapeutic and biological activities, including alleviating Aβ deposition, decreasing tau hyperphosphorylation, regulating the cholinergic system, resisting oxidative stress, modulating Ca2+ homeostasis, as well as anti-inflammation and anti-apoptosis in neurons, respectively. For further developing the therapeutic potential as well as clinical applications, the network pharmacology approach was combined with a summary of published studies.
Keywords: Panax ginseng, Ginsenosides, Alzheimer's disease, Neuroprotective effect, Pharmacological mechanisms, Network pharmacology
Graphical abstract
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by memory loss, cognitive disorder, language dysfunction, and mental disability [1,2]. The main neuropathological changes in AD mainly include amyloid plaque deposition, neurofibrillary tangles, synapse loss, and neuron reduction. The pathogenesis of AD has been attributed to intracellular neurofibrillary tangles in cortical areas of the brain caused by extracellular aggregates of amyloid β (Aβ) plaques and abnormally phosphorylated tau protein [3]. The epidemiological studies showed that the prevalence of AD has increased recently with an estimated morbidity of 50 million by 2020 and expected to reach 150 million by 2050 worldwide [[4], [5], [6]]. Although numbers of anti-AD drugs have been developed in the last decades, including anti-Aβ monoclonal antibodies, Aβ aggregation inhibitors, acetylcholinesterase (AChE) inhibitors, and N-methyl-d-aspartate (NMDA) receptor inhibitors, and these drugs can alleviate AD symptoms; however, none of them demonstrated a favorable effect in alleviating the pathological course of AD [6]. Moreover, long-term use of these drugs is often accompanied with various side effects, including nausea, vomiting, diarrhea, allergic reactions, loss of appetite, etc. [[7], [8], [9]].
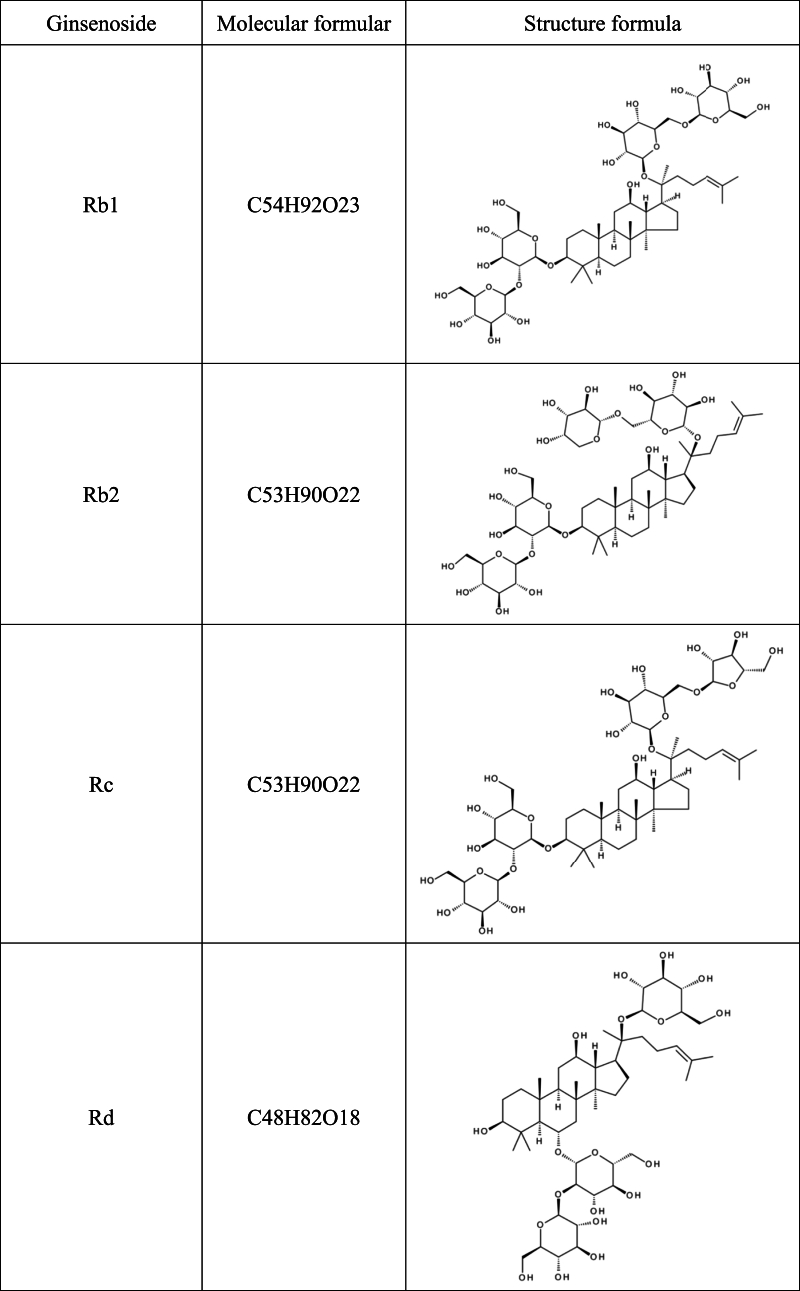
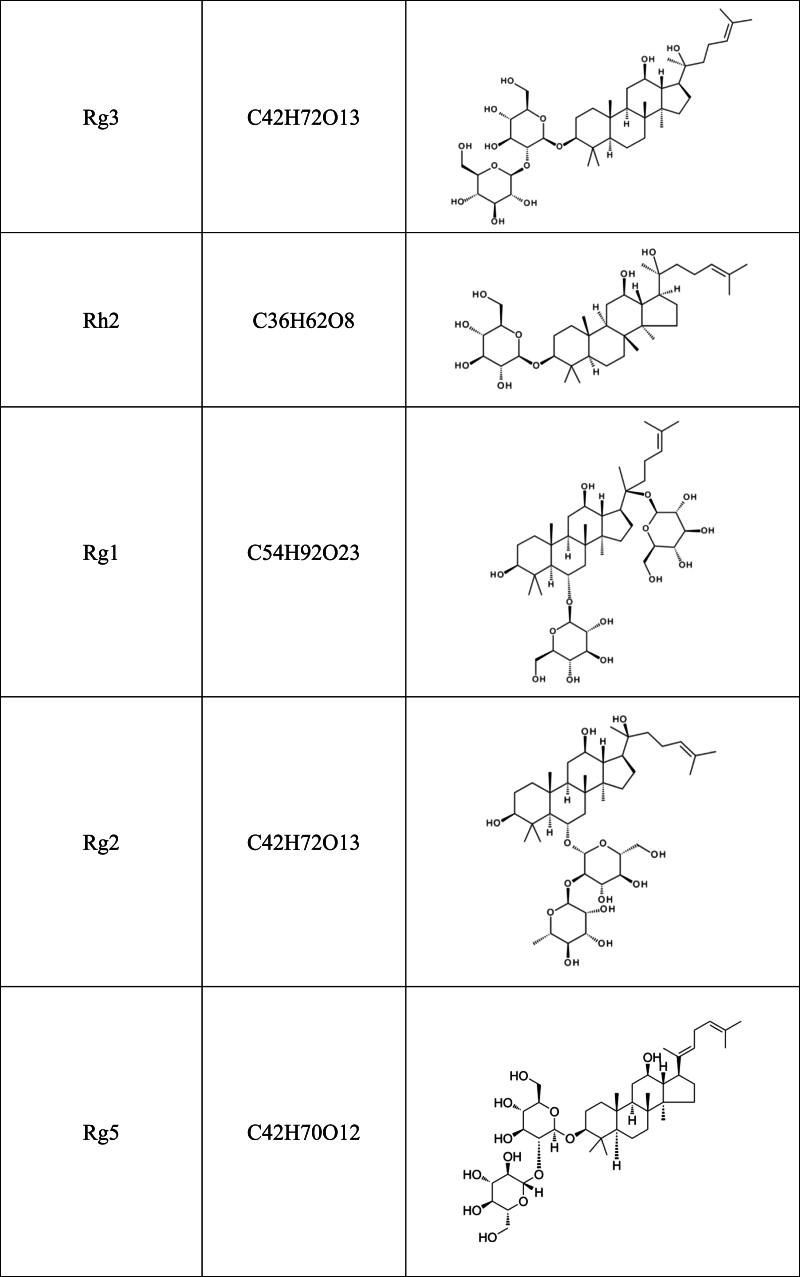
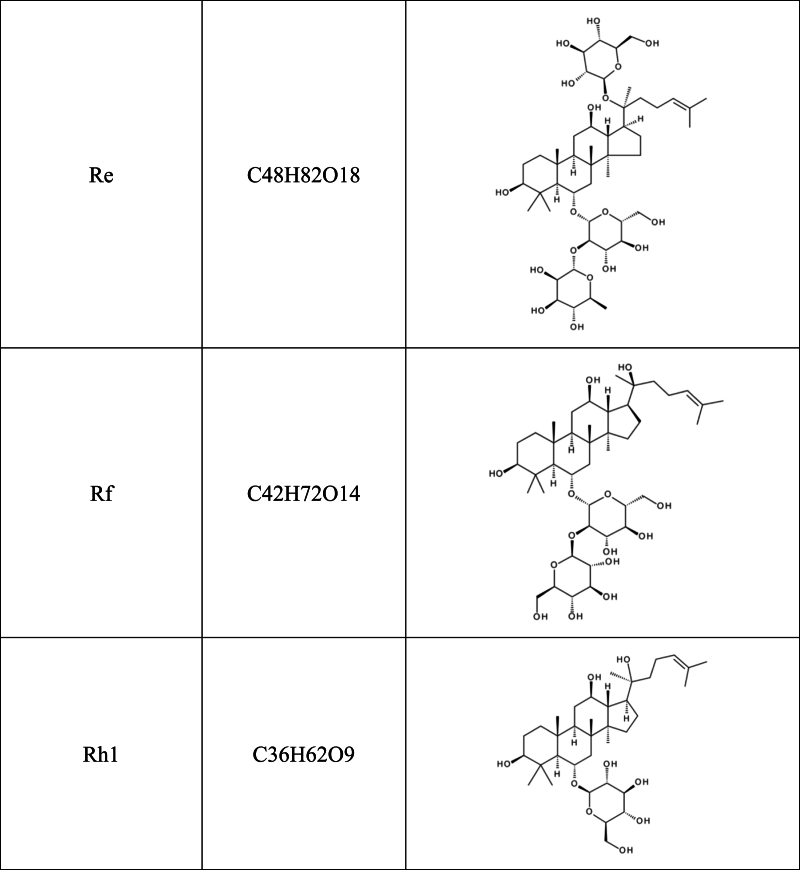
Recent studies have shown that herbal medicines and their active compounds can improve memory and cognitive function in AD patients with few adverse effects [10,11]. The Panax genus belongs to the Araliaceae family, which consists of at least nine species, including Panax ginseng, Panax quinquefolium, Panax notoginseng, and Panax japonicus. Panax genus has been considered one of the most important herbal medicines for thousands of years in China and east Asian, and its use as a nutritional supplement is rapidly increasing in North America and Europe. The bioactive components of Panax genus include saponins (ginsenosides), flavonoids, polysaccharides, volatile oils, polyenes, fatty acids, and polyacetylenic alcohols [[12], [13], [14]]. Among them, the major active constituents of Panax are ginsenosides, of which more than 40 have been identified so far [15]. Based on the carbon skeletons of the aglycones structure, ginsenosides were classified into protopanaxadiols (such as Rb1, Rb2, Rc, Rd, Rg3 and Rh2) and protopanaxatriols (such as Re, Rf, Rg1, Rg2 and Rh1). The steamed ginseng also has a unique saponin profile, such as Rg5, which is likely the result of heat transformation and deglycosylation [16](Table .1).
Table 1.
Chemical structural formula of ginsenoside.
Ginsenosides have been proven to be beneficial in the prevention and treatment of AD and other nervous system diseases [14,17,18]. Accumulating studies have suggested that ginsenosides have a protective effect on AD through various mechanisms both in vivo and in vitro [18,19]. In this review, we focus on discussing the therapeutic potential effects and the mechanisms of pharmacological activities of ginsenosides in AD, to provide new insight for further research and clinical application of ginsenosides in the future.
The following databases were consulted this review: Chinese National Knowledge Infrastructure, National Science and Technology Library, Wanfang Data, Elsevier, ScienceDirect, PubMed, SpringerLink, and the Web of Science database. More than 200 publications were consulted from the databases above from inception to April 2023. To retrieve the literatures, the following words or phrases were used alone or in various combinations in the titles and/or abstracts: “Ginseng”, “Ginsenosides”, “Renshen”, “Alzheimer”, “Alzheimer's disease”, “Anti-Alzheimer”, “Mechanism”, “Pharmacology” and “Neuroprotective effect”, “Panax ginseng”, “Neuroprotective effect”, and “Pharmacological mechanisms”, total 98 articles within two decades were used in this review.
2. Pharmacological mechanisms about anti-AD effects of ginsenosides
2.1. Ginsenosides inhibit Aβ deposition
The accumulation of Aβ is a pivotal contributor to the pathogenesis of AD [20]. Two main pathways involved in amyloid precursor protein (APP) metabolization, nonamyloidogenic and amyloidogenic. APP is cleaved by α-secretase via the nonamyloidogenic pathway, producing transmembrane fragment C83 and N-terminal fragment sAPPα. C83 is subsequently hydrolyzed by γ-secretase to generate the non-toxic fragment P3 and APP intracellular domain (AICD). In the amyloidogenic pathway, APP is cut by β-secretase to generate transmembrane fragment C99 and N-terminal fragment sAPPβ. C99 is then cleaved by γ-secretase to produce neurotoxic Aβ polypeptide fragments and AICD [21]. Aβ polypeptide fragments, as non-fibrillar oligomers, aggregate to amyloid fibrils in the cerebral cortex, inducing neurotoxicity and contributing to AD. Therefore, inhibiting β-secretase pathway and activating the nonamyloidogenic pathway are the potential targets for AD treatment.
A molecular docking study showed that ginsenosides Rb1, Rb2, F1, Rh1, Rh2 and compound K (CK) have high binding affinities for the beta-site amyloid precursor protein, which cleave the enzyme beta-secretase-1 (BACE1) and exhibits potential BACE1 inhibitory activity [22]. In APP/PS1 mice, 10 mg/kg intraperitoneal (i.p.) injection of ginsenoside Rg1 increased the expression of brain-derived neurotrophic factor (BDNF), a synaptic plasticity regulator in the central nervous system that improves memory and cognitive functions, and its receptor tyrosine kinase B (TrkB) in turn lowers the synthesis of Aβ1-42, improves synaptic plasticity and decreases neurofibrillary tangles [23,24]. In streptozotocin (STZ)-induced memory impaired rats, intracerebroventricular (i.c.v.) injection of Rg5 (5, 10, 20 mg/kg) alleviated Aβ deposition by enhancing the expressions of insulin-like growth factors 1 (IGF-1) and BDNF in the hippocampus and cerebral cortex [16]. Ginsenoside Rd (10 mg/kg for 2 moths) improved cognitive and memory function in ovariectomy (OVX) rats. In the same study, ginsenoside Rd increased the expression of α-secretase and the synthesis of soluble APPα (sAPPα) via the nonamyloidogenic pathway, which ameliorates the activity of amyloidogenic pathway and consequently decrease the Aβ level in neuronal cells from HT-22 mouse hippocampus [25]. Ginsenoside Rd was also found to activate the mitogen-activated protein kinase/phosphoinositide-3 kinase (MAPK/PI3K) pathway mediated by estrogen receptor (ER), increase the production of sAPPα through the nonamyloidogenic pathway, decrease extracellular Aβ level, and subsequently alleviate the impairment of cognitive and memory in ovariectomy rats [25]. In mouse neuroma cell N2a/APP695, ginsenoside Re (100 μM) inhibited the activity of BACE1 by activating peroxisome proliferator-activated receptor-γ (PPARγ) and reduced the generation of Aβ1-40 and Aβ1-42 [26]. In tg2576 AD model mice, ginsenoside Rh2 (10 mg/kg i.p. injection for 8 weeks) increased sAPPα levels and CTFα/β ratio, decreased Aβ1-40 and Aβ1-42 concentrations, and inhibited APP endocytosis by lowering levels of cholesterol and lipid raft, which resulted in a reduced number of senile plaques in brain tissue and improved cognitive functions [27].
2.2. Ginsenosides inhibit tau phosphorylation
Tau protein, a microtubule-associated protein expressed in neurons, plays an important role in AD pathogenesis [28]. Tau is switched from non-toxic to toxic when triggered by the upstream protein Aβ, and toxic tau protein in turn increases Aβ toxicity via a feedback loop [29,30]. The neurodegeneration in AD patients is positively associated with Aβ and tau protein [[31], [32], [33]]. The activation of p38 mitogen-activated protein kinase (p38 MAPKs) promotes tau phosphorylation, which impairs the ability of neurons and glial cells to eliminate Tau and Aβ aggregates [34]. P38 phosphorates tau and promotes the release of inflammatory cytokines in astrocytes and microglia, resulting in chronic neuroinflammation and neurodegeneration [35]. In Aβ1-40-induced SK-N-SH neuroblastoma cells, ginsenoside Rg1 (50, 100 and 150 μM) mitigated Aβ neurotoxicity to SK-N-SH neuroblastoma, decreased IL-1β release, and inhibited tau phosphorylation via inhibiting the activation of p38 MAPK [36].
Cyclin-dependent kinase 5 (CDK5) is a proline-directed serine/threonine kinase and can induce nerve cell death and neurodegeneration by altering mitochondrial morphology or transmembrane potential [37]. CDK5 becomes hyperactive when neurons are exposed to pathogenic stimuli, leading to aberrant hyperphosphorylation of CDK5 substrates, such as APP, tau, and neurofilament, and neurodegenerative disorders [38]. When intracellular Ca2+ levels rise, calpain cleaves CDK5 activators p35 and p39 into p25 and p29, and the binding of CDK5 and p25 causes aberrant hyperphosphorylation of several CDK5 substrates [[38], [39], [40], [41]]. Additionally, the increased Aβ level leads to hyperphosphorylation of tau by increasing the expression of p25 [42]. Inhibiting CDK5 decreases tau hyperphosphorylation and neurofibrillary tangles, thereby delaying the process of neurodegeneration [43]. Ginsenoside Rb1 (40 μM) down-regulated p25 expression and lowered CDK5 activity via the Ca2+/calpain/CDK5 signaling pathway in Aβ25-35-induced rat primary cortical neurons, efficiently maintaining intracellular Ca2+ homeostasis, inhibiting calpain activation and minimizing tau hyperphosphorylation [44].
Glycogen synthase kinase 3 (GSK-3) is another proline-directed serine/threonine kinase, its isoform, GSK-3β promotes Aβ formation and tau hyperphosphorylation through modulating the Wnt (Wingless/Integrated) and PI3K/AKT signaling pathways [45]. In Aβ1-40-injected SD rats APP transgenic mice, pretreated with ginsenoside Rd (30 mg/kg i.p. injection) not only reduced the expression of GSK-3β, but increased the activity of protein phosphatase 2A (PP2A), a key phosphatase involved in tau dephosphorylation [46]. In the aluminum-induced tau hyperphosphorylation ICR mice model, ginsenoside Rb1 (20 mg/kg orally administration) reduced the phosphorylation of GSK-3β and the level of PP2A by activating the PI3K/AKT pathway [47].
2.3. Ginsenosides reduce neuroinflammation
Neuroinflammation is an active immune response in the central nervous system mediated by astrocytes and microglia. Microglia act as macrophages that secrete cytokines and chemokines in response to stimuli phagocytosis and subsequently regulate the internalization and degradation of Aβ [48]. In Aβ1-42-induced rat primary microglial cells, ginsenoside Rg3 (25 μg/mL) increased microglial phagocytosis of Aβ by up-regulating the expression of macrophage scavenger receptor type A (MSR-A) that promoted Aβ uptake, internalization, and digestion [49].
Toll-like receptor 4 (TLR4) is a transmembrane protein that belongs to the toll-like receptor family and activates the innate immune system. Excessive activation of TLR4 triggers the generation of various inflammatory factors such as nuclear factor-κB (NF-κB), interferon beta (IFN-β) and tumor necrosis factor alpha (TNF-α) [50]. TLR4 is the primary receptor that specifically recognizes lipopolysaccharide (LPS). The combination of TLR4 and LPS activates NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways by regulating myeloid differentiation primary response protein 88 (Myd88), which increases pro-inflammatory factors secretion and triggers the infiltration of inflammatory cells into the brain [51,52]. In the Aβ 25-35-induced NG108-15 murine neuroglial cells, ginsenoside Rg1 (2, 4, 8, 16 and 32 μg/mL) reduced neuroinflammation by decreasing the level of NF-κB, TLR3 and TLR4, and lowering the production of TNF-α, IFN-β in cell supernatant [53]. In the APP/Tg mouse model, ginsenoside Rd (10 mg/kg i.p. injection for 6 months) decreased the expression of pro-inflammatory factors IL-1β, IL-6, and TNF-α, increased the expression of anti-inflammatory factor IL-10, and consequentially improved learning and memory function [54].
NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome contains NLRP3 core protein, apoptosis-associated speck-like protein (ASC) and pro-caspase-1, which are essential components of the innate immune system [55]. A decrease in Aβ deposition was observed in cases of insufficient NLRP3 expression mice [56]. Stimulating microglia cells to release IL-1 and inducing inflammatory response via activating the NLRP3 inflammasome contributes to Aβ clearance [57]. Therefore, decreasing NLRP3 inflammasome activation would be an effective therapeutic approach for reducing inflammatory injury in brain nerves [58]. Ginsenoside Rg3 (20 mM) was demonstrated to lower Nitric oxide (NO) generation and inhibiting the activation of NLRP3 inflammasome in murine macrophage Raw 264.7 cells by regulating the expression of inducible nitric oxide synthase (iNOS) [59]. CK (10 mg/kg orally administration for 10 weeks) inhibited the activation of NLRP3 inflammasome, declined the expression of cleaved caspase-1, and mature IL-1β in the hippocampus of diabetic model mice, improving memory and cognitive dysfunction of diabetic model mice [60].
2.4. Ginsenosides regulate the cholinergic nervous system
The cholinergic metabolism was closely associated with AD, indicated by decreased activity of Choline acetyl transferase (ChaT) and presynaptic cholinergic transmitter in the brain of AD patients [61,62]. Acetylcholine (Ach) is an important central neurotransmitter in neuromuscular junctions and associated with learning and memory behavior. Acetylcholinesterase (AChE) has a strong hydrolytic activity, which blocks signal transmission by hydrolyzing Ach. The disorder of synthesis, storage, and release of Ach increases AChE accumulation and combination of AChE with Presenilin-1 (PS1), which result in cognitive impairment [63,64].
Ginsenoside Rb1 (5 mg/kg i.p. injection for 4 days) was proved to enhance ACh release in the hippocampus of SD rats after anticholinergic drug treatment, and choline absorption was boosted by promoting ACh metabolism in the central nervous system, thus alleviating the memory loss induced by anticholinergic medication [65]. Rg5 (5, 10, 20 mg/kg i.c.v. injection for 28 days) was found to improve cognitive dysfunction in streptozotocin-induced memory impaired rats by reducing AChE activity [16]. In mouse neuroblastoma N2a cells, ginsenosides Rd and Re (5 μg/mL for 48 h) increased the expression of ChAT by up-regulating microtubule-associated protein (MAP2), nerve growth factor receptors p75, p21 and tropomyosin receptor kinase A (Trka),which resulted in an increase in the level of ACh and vesicular acetylcholine transporters for cholinergic neurotransmission [66]. In a high-fat diet induced C57BL/6 mouse model, ginsenoside Re (5, 10, 20 mg/kg orally administration for 4 weeks) increased ACh concentration in brain tissue by blocking the c-Jun NH2-terminal kinase (JNK) pathway, and decreased AChE activity, showing a beneficial effect in cholinergic neurons and mouse cognitive function [67].
2.5. Ginsenosides resist oxidative stress
Oxidative stress (OS) is one of the main causes of neurodegenerative disorders [[68], [69], [70]]. Aβ-40 and Aβ-42 induced Ca2⁺ signaling in astrocytes drives the oxidation process of decreased coenzyme II NADPH, resulting in excessive ROS and neurotoxicity [71]. On the other hand, excessive Aβ deposition aggravates OS and increases ROS production, which impairs permeability of the cell membrane, disrupts intracellular Ca2+ homeostasis, and causes the loss of mitochondrial function and neuronal cell death [[72], [73], [74], [75]].
In Aβ25-35-induced PC12 cells and primary cultured cortical neurons, ginsenosides Rb1 (1 mM for 24 h) and Rg1 (20 μM for 24 h) not only reduced excessive ROS and lipid peroxidation, but also lowered lactate dehydrogenase release, malondialdehyde (MDA) production, and superoxide dismutase (SOD) activity, subsequently enhancing cell survival [76,77]. Ginsenoside Rg2 (0.2 mmol/L for 24 h) demonstrated a neuroprotective effect in glutamate-induced PC12 cells by lowering excessive MDA and NO generation as well as intracellular free Ca2+ ions [78]. In d-galactosamine-induced rats, Rg3 (20 mg/kg/day i.p. injection for 60 days) increased the level of SOD and decreased the level of MDA, indicating that Rg3 alleviated the oxidative stress of AD rats by scavenging ROS. Rg3 improved the mitochondrial dysfunction of AD rats by regulating the abnormal energy metabolism and recovering the turbulent mitochondrial electron transport chain [79]. In Aβ-induced SH-SY5Y cells, Re (25 μM for 24 h) attenuated Aβ-induced ROS production and inhibited Aβ-triggered mitochondrial apoptosis by elevating the Bcl-2/Bax ratio, reducing the release of cytochrome c, and decreasing the caspase-3/9 ratio. Besides, Re activated Nrf2 (Nuclear factor erythroid 2–related factor 2), a regulator of cellular resistance to oxidants, indicated a beneficial effect on anti-oxidative and neuroprotection [80].
2.6. Ginsenosides regulate Ca2⁺ homeostasis
Metal ion homeostasis is critical for sustaining the function of the central nervous system. Cellular Ca2+ homeostasis regulates neural growth, differentiation, and synaptic plasticity, which are crucial for action potential and memory function. N-methyl-d-aspartate (NMDA) receptor locates in excitatory synapses and plays an important role in learning and memory. Aβ triggers NMDA-mediated Ca2+ influx and excitotoxicity in neurons, resulting in mitochondrial dysfunction, disruption of synaptic neurotransmission and cognitive impairment [81].
Ginsenoside Rg3 (10 μM for 2 days) regulates Ca2+ homeostasis by inhibiting NMDA receptors and shows a neuroprotective effect in rat primary hippocampal neurons [82]. In rat brain slices exposed to Aβ 25–35, ginsenoside Rg1 (20 μmol/L for 6 h) decreased hippocampal neuronal Ca2+ channel currents recorded by a whole-cell patch clamp by inhibiting the expression of mitogen-activated protein kinase (MAPK), thereby decreasing the neurotoxicity of Aβ induced by Ca2+ over influx [83]. What's more, ginsenoside Rg1 (1, 5 and 10 mg/kg orally administration for 8 weeks) improved diabetes-associated cognitive dysfunction by ameliorating neuronal Ca2+ overload and reducing Aβ generation in type 2 diabetic mice [84].
2.7. Ginsenosides inhibit cell apoptosis
Caspase-3 is a member of the caspase family and plays an essential role in cell apoptosis [85]. Caspase-3 immunoreactivity was increased remarkably in neurons and astrocytes of AD patients, as well as in neurofibrillary tangles and senile plaques [86]. Aβ accumulation was shown to activate caspase-3, trigger neuronal cell apoptosis, and increase neurofibrillary tangles, which are contributors to neuronal cell death and Aβ plaque formation in AD brain [87].
Ginsenoside Rg1 (12.5, 25, and 50 μmol/L for 24 h) was showed to attenuate isoflurane-induced caspase-3 activation in H4 human neuroglioma cells that express full-length APP (H4-APP cells) via inhibiting mitochondrial dysfunction, demonstrating a favor effect in neurotoxicity induced by APP [88]. In Aβ25-35 induced PC12 cells, ginsenoside Rg2 (20 mg/mL for 24 h) increased the level of B cell lymphoma protein 2 (Bcl-2) and down-regulated the level of Bcl-2 associated X protein (Bax) through the PI3K/AKT signaling pathway, thus inhibiting the activity of caspase-3 and decreasing the cells apoptosis [89]. Ginsenoside Rb1 (12.5, 25, and 50 mg/kg i.p. injection for 14 days) was proven to enhance cognitive and memory functions in Aβ1-40-induced rats by decreasing cleaved caspase-3 expression and increasing Bcl-2 expression in the hippocampus [90].
3. Network pharmacology of ginsenosides
Network pharmacology were used to explore the mechanisms of ginsenosides in AD. Combining traditional Chinese medicine (TCM) and network pharmacology should reveal the relationship between drugs, genes, and diseases. In this study, we identified and analyzed the core targets of the nine ginsenosides (Rb1, Rd, Re, Rg1, Rg2, Rg3, Rg5, Rh2 and CK) by using network pharmacology methods and found that their main pharmacological actions are anti-AD and neuroprotective effects.
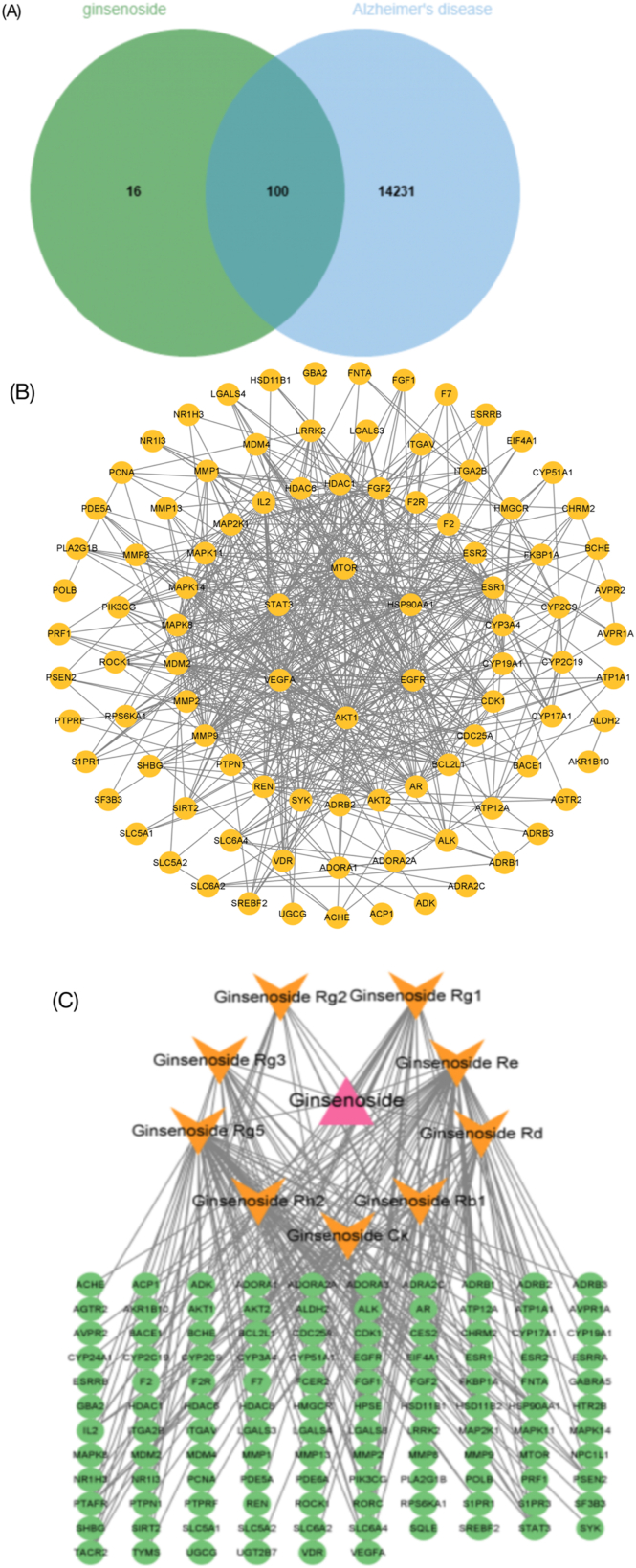
The target genes of nine ginsenosides were searched by the pharmacologic database and analysis platform of TCM System (TCMSP, https://tcmspw.com/tcmsp.php/). GeneCards human genome database and OMIM database (https://omim.org/) were used to identify the target genes of AD (Fig. 1A). Subsequently, a protein-protein interaction (PPI) network was produced for all target genes using STRING (https://cn.string-db.org/), and the PPI network cores were screened by Cytoscape software (Fig. 1B and C).
Fig. 1.
Network pharmacology revealed the targets characteristics of ginsenosides. (A) Venn diagram of targets in nine ginsenosides for AD. (B) The protein–protein interaction (PPI) network based on targets of nine ginsenosides for AD. (C) Nine ginsenosides-targets-AD network.
As shown in the core of the PPI network, AKT1, EGFR, VEGFA, MTOR HSP90AA1 and STAT3 possessed high scores. AKT serine/threonine kinase 1 (AKT1)-mediated signaling is crucial for the normal development and function of the nervous system. ROS-mediated oxidative modification of AKT1 contributes to synaptic dysfunction in AD, which is necessary for synaptic plasticity and maintenance [91]. Mammalian target of rapamycin (mTOR) signaling is critical to long lasting forms of synaptic plasticity, which is important to neurodegeneration [92]. Promoting AKT1-mTOR signaling at synapses could be a potential therapeutic strategy for AD. Vascular endothelial growth factors (VEGFs) are important in the growth and maintenance of both vascular and neural cells. VEGFA appears to protect against cognitive impairment in AD pathology, whose levels are reduced in both serum and CSF among AD cases [93]. Epidermal Growth Factor receptor (EGFR) regulates the morphology and function of astrocytes. Overexpressed EGFR is found in AD astrocytes [94]. Blocking EGFR activation in astrocytes could be beneficial for the treatment of neuroinflammation, such as AD. Heat shock protein 90 kDa alpha family class A member 1 (HSP90AA1) is decreased in AD and is negatively correlated with the activities of β-secretases [95]. Signal transducer and activator of transcription 3 (STAT3) is a transcriptional factor that involved in regulation of synaptic plasticity and cognition [96]. Abnormal activation of STAT3 in the hippocampus of AD mouse model can induce astrogliosis, which could be an important therapeutic target in AD [97].
4. Conclusion and perspectives
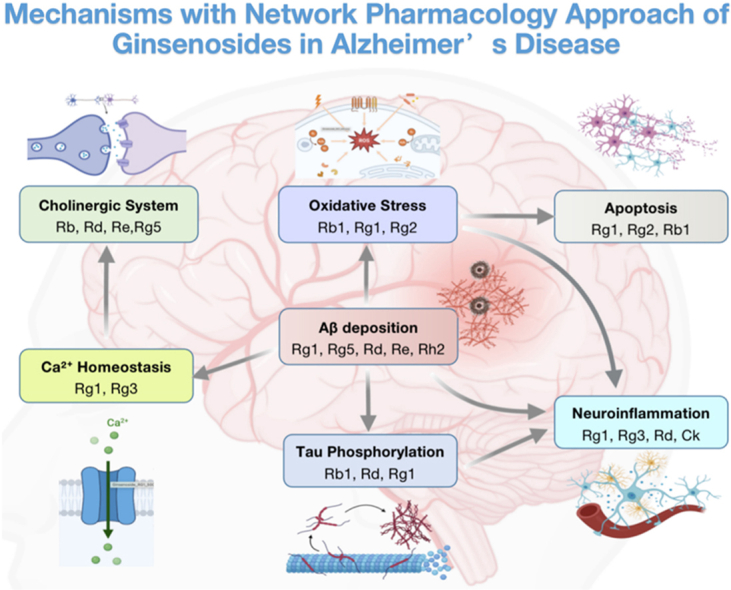
AD is a chronic neurodegenerative disorder, and a main cause of mortality and disability in the aging population. There are no ideal treatments for patients with AD to date, even though a lot of new drugs have been developed and applied in clinical practice recently. In this article, we reviewed studies on the neuroprotective effects of ginsenosides in different pathogenic processes of AD and have shown that ginsenosides, the main active component of ginseng, have a broad spectrum of pharmacological effects on the different AD pathogenic processes, including alleviation of Aβ deposition, tau hyperphosphorylation, neuroinflammation and neuron cell apoptosis, regulation of the cholinergic system, resistance to oxidative stress, and modulation of Ca2+ homeostasis (Table 2 and Fig. 2).
Table 2.
Neuroprotective mechanisms of ginsenosides in AD treatments.
| Ginsenoside | Subject | Dose | Duration | Targets | Actions | Reference |
|---|---|---|---|---|---|---|
| Rg1 | MaleAPP/PS1 mice | i.p. injection (10 mg/kg) | 30 days | BDNF, TrkB, BACE1, Aβ | Aβ deposition | [23,24] |
| SK-N-SH neuroblastoma cells | 50, 100 and 150 μM | 30 min | IL-1β, tau, p38 MAPK | tau phosphorylation | [36] | |
| NG108-15 mouse glial cells | 2, 4, 8, 16 and 32 μg/mL | 24 h | TLR3, TLR4, TNF-α, IFN-β | Neuroinflammation | [53] | |
| PC-12 cells | 20 μM | 24 h | ROS, MDA, LDH, SOD | Anti-oxidation | [77] | |
| T2DM mice | orally administration (1, 5 and 10 mg/kg) | 8 weeks | Ca2+, IP3, DAG, Aβ | Ca2+, IP3, DAG, Aβ | [84] | |
| SD rats | 20 μmol/L | 6 h | Ca2⁺, MAPK | Ca2⁺ homeostasis | [83] | |
| H4-APP cells | 12.5, 25, and 50 μmol/L | 24 h | Caspase-3 | Anti-apoptosis | [88] | |
| Rg2 | PC12 cells | 0.2 mmol/L | 24 h | MDA, NO | Anti-oxidation | [78] |
| PC12 cells | 20 mg/mL | 24 h | Caspase-3, Bcl2, BAX | Anti-apoptosis | [89] | |
| Rg3 | Rat primary microglial cells | 25 μg/mL | 8 h | Aβ, MSR-A | Neuroinflammation | [49] |
| D-Gal induced rat | i.p. injection (20 mg/kg/day) | 60 days | SOD, MDA | Anti-oxidation | [79] | |
| Murine macrophage cells | 20 mM | 16 h | NO, iNOS, NLRP3 | Neuroinflammation | [59] | |
| primary hippocampal neurons | 10 μM | 2 days | Ca2⁺, NMDAR | Ca2⁺ homeostasis | [82] | |
| Rg5 | STZ-induced rats | i.c.v. injection (5, 10 and 20 mg/kg) | 28 days | IGF-1, BDNF, Aβ | Aβ deposition | [16] |
| STZ-induced rats | i.c.v. injection (5, 10 and 20 mg/kg) | 28 days | AChE | Anticholinergic | [16] | |
| Rb1 | Rat primary cortical neurons | 40 μM | 24 h | p25, CDK5 | tau phosphorylation | [44] |
| Male SD rats | i.p. injection (5 mg/kg) | 4 days | ACh | Anticholinergic | [65] | |
| Female ICR mice | orally administation (20 mg/kg/day) | 4 months | GSK-3β, PP2A | tau phosphorylation | [47] | |
| PC-12 cells | 1 mM | 24 h | ROS, MDA, LDH, SOD | Anti-oxidation | [76] | |
| SD-rats | i.p. injection (12.5, 25, and 50 mg/kg) | 14 days | Caspase-3, Bcl2 | Anti-apoptosis | [90] | |
| Rd | OVX rats | i.p. injection (10 mg/kg) | 2 months | α-secretase, sAPPα, Aβ, MAPK/PI3K, ER | Aβ deposition | [25] |
| APP/Tg mouse | i.p. injection (10 mg/kg) | 6 months | IL-1β, IL-6, TNF-α, NF-κB, IL-10 | Neuroinflammation | [54] | |
| Male SD rats APP transgenic mice |
i.p. injection (30 mg/kg) i.p. injection (30 mg/kg) |
7 days 6 months |
GSK-3β, PP2A | tau phosphorylation | [46] | |
| N2a cells | 5 μg/mL | 48 h | ChAT, MAP2, p75, p21, Trka, VAChT, ACh | Anticholinergic | [66] | |
| Re | N2a/APP695 cells | 100 μM | 24 h | BACE1, PPARγ, Aβ | Aβ deposition | [26] |
| N2a cells | 5 μg/mL | 48 h | ChAT, MAP2, p75, p21, Trka, VAChT, ACh | Anticholinergic | [66] | |
| C57BL/6 mouse | orally administration (5, 10, 20 mg/kg) | 4 weeks | ACh, AChE, JNK | Anticholinergic | [67] | |
| SH-SY5Y cells | 25 uM | 24 h | Cytochrome c, caspase 3/9, Bal 2/BAX, Nrf2 | Anti-oxidation | [80] | |
| Rh2 | Tg2576 mice | i.p. injection (10 mg/kg) | 8 weeks | sAPPα, CTFα/β, APP, Aβ | Aβ deposition | [27] |
| CK | db/db mice | orally administration (10 mg/kg) | 10 weeks | NLRP3, caspase-1, IL-1β | Neuroinflammation | [60] |
Fig. 2.
Protective mechanisms of ginsenosides in AD. Ginsenosides presented a wide range of therapeutic and biological activities, including alleviating Aβ deposition, decreasing tau hyperphosphorylation, regulating the cholinergic system, resisting oxidative stress, modulating Ca2+ homeostasis, as well as anti-inflammation and anti-apoptosis in neurons, respectively.
Ginsenosides Rb1 and Rg1 are considered the main ginsenosides of ginseng, which exhibit neuroprotective properties in anti-oxidative stress and anti-apoptosis [88,90]. Rb1 and Rg1 were also found to protect against Aβ-induced neurotoxicity and decrease the level of Aβ deposition [76,77]. Nevertheless, there are somewhat differences between the ginsenoside Rb1 and Rg1: ginsenoside Rb1 is prone to inhibit tau phosphorylation and reinforce the cholinergic systems, while ginsenoside Rg1 decreases neuronal Ca2+ influx and reduces neuroinflammatory responses [36,44].
The specific mechanism of ginsenosides in the treatment of AD has been unable to be clearly elucidated so far. Recent research on AD with ginsenosides is basically based on in vitro and in vivo studies. There was a clinical study to investigate the effect of total ginsenosides extracted from ginseng, which showed that treatment with ginsenosides complex for 12 weeks significantly improved the Mini-Mental State Examination (MMSE) and Alzheimer's Disease Assessment Scale (ADAS) [98]. However, the clinical significance of this study was limited by its small sample size and open-label study design. Therefore, it is necessary to conduct clinical trials with a scientific design method to validate the effects of ginsenosides in the future.
Network pharmacology results revealed multiple potential therapeutic targets of ginsenosides in AD such as AKT1, mTOR, EGFR and STAT3. Previous studies already demonstrated that ginsenosides act on these targets and exert beneficial effects in various diseases. For instance, with regards to AKT and mTOR, one study showed that ginsenoside RK1 significantly alleviated radiation-induced intestinal epithelial cell apoptosis by suppressing the PI3K/AKT/mTOR pathway in the irradiated rats model [99]. In terms of targeting EGFR, ginsenoside Re and Rf inhibited the production of pro-inflammatory factors and the expression of important cytokines in periodontitis by inducing the expression of heme oxygenase 1 via EGFR signaling pathway in human periodontal ligament cells [100]. And ginsenoside Rg1 demonstrated an anti-fatigue effect by impacting the metabolism of taurine and mannose 6-phosphate through downregulating EGFR expression in chronic fatigue syndrome rat model [101]. As for targeting STAT3, protopanaxadiol could inhibit the phosphorylation of STAT3 and its translocation from the cytosol to the nucleus in hepatocellular carcinoma cell lines [102]. Ginsenoside F2 could suppress the Y705 phosphorylation of STAT3, decrease its nuclear translocation and inhibit the growth of human hepatocellular carcinoma Hep G2 cells [103]. Additionally, in human pancreatic cancer cell lines BxPC-3 and AsPC-1, ginsenoside Rg3 enhanced the efficacy of erlotinib to inhibit the proliferation of pancreatic cancer cells via induction of apoptosis and downregulation of the EGFR/PI3K/AKT pathway [104]. Therefore, future research should focus on the PPI networks of AKT1, EGFR, VEGFA, MTOR, HSP90AA1 and STAT3 to obtain more comprehensive understanding of ginsenosides in AD.
Ginsenosides can be transformed into smaller deglycosylated through bioconversion methods by intestinal bacteria. But oral administration always accompanied with low bioavailability which may be due to the poor solubility of deglycosylated metabolites of ginsenosides [[105], [106], [107]]. Different ginsenosides displayed various bioavailability in human when administrating orally. Previous pharmacokinetic study found that ginsenoside Rb1, Rb2, Rc, and Rd were identified in human plasma after oral administration, whereas ginsenosides Re, Rh1, and Rg1 could not be found after repeated oral administration [108]. Another study showed that ginsenoside Rg1, Re, Rb1, and Rd had high bioavailability in rats. The inconsistence results from previous studies may be due to the different techniques (steaming, drying, and extracting) used in processing ginseng. Currently, limited pharmacokinetic studies also restrict the development of drug containing ginsenosides. Besides, the existence of the blood-brain barrier (BBB) is another limitation for ginsenosides in AD treatment. However, the encapsulation and nanoparticle technology showed a considerable effect in vivo, which can be developed extensively in future clinical research [109,110].
In this review, we summarized and discussed the mechanisms of ginsenosides by using database and network pharmacology tools to build target-disease networks and to analyze the PPI core network and pathways. Further investigations on the interaction mechanism of ginsenosides in AD using molecular biological techniques such as proteomics, genomics, metabonomics, and bioinformatics analysis are needed to provide more therapeutic evidence of ginsenosides.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data are fully available without restriction.
CRediT authorship contribution statement
Shan He: Writing – review & editing, Writing – original draft, Validation, Conceptualization. Junhe Shi: Writing – review & editing, Validation. Hua Chai: Validation. Lina Ma: Validation. Hui Pei: Validation. Ping Zhang: Validation. Dazhuo Shi: Validation, Supervision. Hao Li: Writing – review & editing, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R&D Program of China(2022YFC3501400); National Natural Science Foundation of China (no. 82205233), and China Academy of Chinese Medical Sciences (no. CI2021A01401, CI2021A01403 and no. CI2021A01404).
Contributor Information
Dazhuo Shi, Email: shidztcm@163.com.
Hao Li, Email: xyhplihao1965@126.com.
References
- 1.C S.P. Alzheimer's disease and exercise: a literature review. Curr. Sports Med. Rep. 2017;16(1):19–22. doi: 10.1249/JSR.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 2.Burns A., Maurer K B.E. Alzheimer's disease. Lancet. 2002;360(9327):163–165. doi: 10.1016/S0140-6736(02)09420-5. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari S A.V., Kaushik A., Yndart A., Nair M. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols E.S.C., Vollset S., et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch C. World Alzheimer Report 2019: attitudes to dementia, a global survey. Alzheimer’s & Dementia J. 2020;16 [Google Scholar]
- 6.Cummings J L.G., Nahed P., et al. Alzheimer's disease drug development pipeline. Alzheimer's Dementia. 2022;8(1) doi: 10.1002/trc2.12295. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lythgoe Mp J.K., Prasad V. Regulatory decisions diverge over aducanumab for Alzheimer's disease. BMJ. 2022;376 doi: 10.1136/bmj-2021-069780. [DOI] [PubMed] [Google Scholar]
- 8.Cui D., et al. The combination of acetylcholinesterase inhibitor therapy and high‐frequency repetitive transcranial magnetic stimulation in Alzheimer's disease: a preliminary fMRI study. Alzheimer's Dementia. 2022;17(S4) [Google Scholar]
- 9.Rogers S.L., et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50(1):136. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z.K., Y H., Chen S.D. Traditional Chinese medicine: a promising candidate for the treatment of Alzheimer's disease. Transl. Neurodegener. 2013;2(1):6. doi: 10.1186/2047-9158-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M.R., Q Y., Hu B., An H.M. Signal pathways in the treatment of Alzheimer's disease with traditional Chinese medicine. Biomed. Pharmacother. 2022;152 doi: 10.1016/j.biopha.2022.113208. [DOI] [PubMed] [Google Scholar]
- 12.Ru W W.D., Xu Y., et al. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9(1):23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., P D., Xie J. Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin. Med. 2009;4:2. doi: 10.1186/1749-8546-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou M., et al. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B. 2021;11(7):1813–1834. doi: 10.1016/j.apsb.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lü J.M., Y Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009;7(3):293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu S., G J., Feng L., et al. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharm. 2014;19(2):317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Jia L Z.Y. Current evaluation of the millennium phytomedicine--ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr. Med. Chem. 2009;16(19):2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.J., J S., Kim S.Y., et al. Panax ginseng as an adjuvant treatment for Alzheimer's disease. J Ginseng Res. 2018;42(4):401–411. doi: 10.1016/j.jgr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira Zanuso B., d.O.D S.A., Miola V.F.B., Guissoni Campos L.M., Spilla C.S.G., Barbalho S.M. Panax ginseng and aging related disorders: a systematic review. Exp. Gerontol. 2022;161(11731) doi: 10.1016/j.exger.2022.111731. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y., et al. Presence of endogenous Aβ in plasma of patient with Alzheimer's disease differentiates Aβ oligomerization tendencies caused by synthetic Aβ. Alzheimer's Dementia. 2021;17(S5) [Google Scholar]
- 21.Stein Td A.N., DeCarli C., Chan S.L., Mattson M.P., Johnson J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J. Neurosci. 2004;24(35):7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpagam V., S N., Sathiyamoorthy S., et al. Identification of BACE1 inhibitors from Panax ginseng saponins-An Insilco approach. Comput. Biol. Med. 2013;43(8):1037–1044. doi: 10.1016/j.compbiomed.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Giuffrida Ml C.A., Rizzarelli E. A promising connection between BDNF and Alzheimer's disease. Aging. 2018;10(8):1791–1792. doi: 10.18632/aging.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F W.X., Li J., Niu Q. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer's disease model. Mol. Med. Rep. 2016;13(6):4909–4910. doi: 10.3892/mmr.2016.5103. [DOI] [PubMed] [Google Scholar]
- 25.Yan X., et al. 1879-0631. Ginsenoside Rd Promotes Non-amyloidogenic Pathway of Amyloid Precursor Protein Processing by Regulating Phosphorylation of Estrogen Receptor Alpha. (Electronic)) [DOI] [PubMed] [Google Scholar]
- 26.Cao G., et al. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016;793(793):101–108. doi: 10.1016/j.ejphar.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J L.W., Feng S.H., Wang M., He Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet. Mol. Res. 2014;13(2):3586–3598. doi: 10.4238/2014.May.9.2. [DOI] [PubMed] [Google Scholar]
- 28.Ballatore, C., J.Q. Lee Vm Fau - Trojanowski, and J.Q. Trojanowski, Tau-mediated Neurodegeneration in Alzheimer's Disease and Related Disorders. (1471-003X (Print)).. [DOI] [PubMed]
- 29.Morris M., M S., Vossel K., Mucke L. The many faces of tau. Neuron. 2011;70(3):410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom G.S. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 31.Ittner LM, K.Y., Delerue F, et al., Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 142(3): p. 387-397.. [DOI] [PubMed]
- 32.Götz J C.F., van Dorpe J., Nitsch R.M. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 33.Lewis J D.D., Lin W.L., et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X., R C., Boux H., Takeda A., Perry G., Smith M.A. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2000;59(10):880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- 35.D'Mello, S., When good kinases go rogue: GSK3, p38 MAPK and CDKs as therapeutic targets for Alzheimer's and huntington's disease. Int. J. Mol. Sci.. 22(11): p. 5911.. [DOI] [PMC free article] [PubMed]
- 36.Li W C.Y., Zhang L., Yin L., Li L. Ginsenoside Rg1 attenuates tau phosphorylation in SK-N-SH induced by Aβ-stimulated THP-1 supernatant and the involvement of p38 pathway activation. Life Sci. 2012;91:15–16. doi: 10.1016/j.lfs.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Cortés N., et al. CDK5: a unique CDK and its multiple roles in the nervous system. J Alzheimers Dis. 2019;68(3):843–855. doi: 10.3233/JAD-180792. [DOI] [PubMed] [Google Scholar]
- 38.Liu S.L., W C., Jiang T., Tan L., Xing A., Yu J.T. The role of Cdk5 in Alzheimer's disease. Mol. Neurobiol. 2016;53(7):4328–4342. doi: 10.1007/s12035-015-9369-x. [DOI] [PubMed] [Google Scholar]
- 39.Cárdenas-Aguayo Mdel C G.-V.L., DeRosa S., Meraz-Ríos M.A. The role of tau oligomers in the onset of Alzheimer's disease neuropathology. ACS Chem. Neurosci. 2014;5(12):1178–1191. doi: 10.1021/cn500148z. [DOI] [PubMed] [Google Scholar]
- 40.Guo, T., W. Noble, and D.P. Hanger, Roles of Tau Protein in Health and Disease. (1432-0533 (Electronic)).. [DOI] [PMC free article] [PubMed]
- 41.Serrano-Pozo A., F M., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pei Jj G.-I.I., Iqbal K., Bogdanovic N., Winblad B., Cowburn R.F. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Res. 1998;797(2):267–277. doi: 10.1016/s0006-8993(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 43.Pao Pc T.L. Three decades of Cdk5. J. Biomed. Sci. 2021;28(1):79. doi: 10.1186/s12929-021-00774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X., H T., Zhang J., Song J., Chen L., Zhu Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1's attenuation of beta-amyloid peptide25-35-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008;1200:99–106. doi: 10.1016/j.brainres.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 45.Jope R.S., J G. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Li L., L Z., Liu J., et al. Ginsenoside Rd attenuates beta-amyloid-induced tau phosphorylation by altering the functional balance of glycogen synthase kinase 3 beta and protein phosphatase 2A. Neurobiol. Dis. 2013;54:320–328. doi: 10.1016/j.nbd.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Zhao H.H., D J., Liu W.S., Liu H.L., Lai H., Lü Y.L. Involvement of GSK3 and PP2A in ginsenoside Rb1's attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013;241:228–234. doi: 10.1016/j.bbr.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 48.Lee, C.Y. and G.E. Landreth, The Role of Microglia in Amyloid Clearance from the AD Brain. (1435-1463 (Electronic)).. [DOI] [PMC free article] [PubMed]
- 49.Joo Ss L.D. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: enhancement of type a macrophage scavenger receptor expression. Arch Pharm. Res. (Seoul) 2005;28(10):1164–1169. doi: 10.1007/BF02972981. [DOI] [PubMed] [Google Scholar]
- 50.Vaure C L.Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014;5:316. doi: 10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y C.Y., Xu C., Zhang H., Lin C. TLR4 targeting as a promising therapeutic strategy for alzheimer disease treatment. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.602508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Bs L.Y., Gao X.Y., Zhai H.Q., Guo J.Y., Wang X.Y. Effects of ginsenoside Rg1 on the expression of toll-like receptor 3, 4 and their signalling transduction factors in the NG108-15 murine neuroglial cell line. Molecules. 2014;19(10):16925–16936. doi: 10.3390/molecules191016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J., Y X., Li L., et al. Ginsenoside Rd improves learning and memory ability in APP transgenic mice. J. Mol. Neurosci. 2015;57(4):522–528. doi: 10.1007/s12031-015-0632-4. [DOI] [PubMed] [Google Scholar]
- 55.Ising C V.C., Zhang S., et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575(7784):669–673. doi: 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heneka Mt K.M., Stutz A., et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webers, A., M.T. Heneka, andP.A. Gleeson, The Role of Innate Immune Responses and Neuroinflammation in Amyloid Accumulation and Progression of Alzheimer's Disease. (1440-1711 (Electronic)).. [DOI] [PubMed]
- 58.Leyns Ceg H.D. Glial contributions to neurodegeneration in tauopathies. Mol. Neurodegener. 2017;12(12):50. doi: 10.1186/s13024-017-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon Sj P.J., Choi S., et al. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem. Biophys. Res. Commun. 2015;463(4):1184–1189. doi: 10.1016/j.bbrc.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 60.Li Cw D.M., Gao Z.J., et al. Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food Funct. 2020;11(5):4416–4427. doi: 10.1039/c9fo02602a. [DOI] [PubMed] [Google Scholar]
- 61.Davies P., M A. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. 8000. [DOI] [PubMed] [Google Scholar]
- 62.Kar S I.A., Seto D., Auld D.S., Collier B., Quirion R. Amyloid beta-peptide inhibits high-affinity choline uptake and acetylcholine release in rat hippocampal slices. J. Neurochem. 1998;70(5):2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-Rodriguez Jj P.-H.M., Thyssen D., et al. Rapid β-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J. Neuropathol. Exp. Neurol. 2013;72(4):272–285. doi: 10.1097/NEN.0b013e318288a8dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campanari Ml G.-A.M., Belbin O., Galcerán J., Lleó A., Sáez-Valero J. Acetylcholinesterase modulates presenilin-1 levels and γ-secretase activity. J Alzheimers Dis. 2014;41(3):911–924. doi: 10.3233/JAD-140426. [DOI] [PubMed] [Google Scholar]
- 65.Benishin Cg L.R., Wang L.C., Liu H.J. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology. 1991;42(4):223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 66.Kim M.S., Y J., Kim H.J., et al. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol. Pharm. Bull. 2014;37(5):826–833. doi: 10.1248/bpb.b14-00011. [DOI] [PubMed] [Google Scholar]
- 67.Kim JM, P.C., Park SK, et al. , Ginsenoside Re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet-induced C57bl/6 mice. J. Agric. Food Chem.. 65(13): p. 2719-2729.. [DOI] [PubMed]
- 68.Hollis F K.A., Bagni C. Mitochondrial dysfunction in Autism Spectrum Disorder: clinical features and perspectives. Curr. Opin. Neurobiol. 2017;45:178–187. doi: 10.1016/j.conb.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Haider L., F M., Frischer J.M., et al. Oxidative damage in multiple sclerosis lesions. Brain Res. 2011;134(7):1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel Vp C.C. Nuclear transport, oxidative stress, and neurodegeneration. Int. J. Clin. Exp. Pathol. 2011;4(3):215–229. [PMC free article] [PubMed] [Google Scholar]
- 71.Angelova P.R., Abramov A.Y. Interaction of neurons and astrocytes underlies the mechanism of Aβ-induced neurotoxicity. Biochem. Soc. Trans. 2014;42(5):1286–1290. doi: 10.1042/BST20140153. [DOI] [PubMed] [Google Scholar]
- 72.Luo X., W G., Zheng J., Gendelman H.E., Ikezu T. C1q-calreticulin induced oxidative neurotoxicity: relevance for the neuropathogenesis of Alzheimer's disease. J. Neuroimmunol. 2003;135(1–2):62–71. doi: 10.1016/s0165-5728(02)00444-7. [DOI] [PubMed] [Google Scholar]
- 73.Noh, K.M. and J.Y. Koh, Induction and Activation by Zinc of NADPH Oxidase in Cultured Cortical Neurons and Astrocytes. (1529-2401 (Electronic)).. [DOI] [PMC free article] [PubMed]
- 74.Tu S., O S., Tu S., Lipton S.A., Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol. Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Llanos-González E., H C.Á., Pedrero-Prieto C.M., et al. Interplay between mitochondrial oxidative disorders and proteostasis in Alzheimer's disease. Front. Neurosci. 2020;13:1444. doi: 10.3389/fnins.2019.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie X., W H., Li C.L., et al. Ginsenoside Rb1 protects PC12 cells against β-amyloid-induced cell injury. Mol. Med. Rep. 2010;3(4):635–639. doi: 10.3892/mmr_00000308. [DOI] [PubMed] [Google Scholar]
- 77.Wu J., Y H., Zhao Q., Zhang X., Lou Y. Ginsenoside Rg1 exerts a protective effect against Aβ₂₅₋₃₅-induced toxicity in primary cultured rat cortical neurons through the NF-κB/NO pathway. Int. J. Mol. Med. 2016;37(3):781–788. doi: 10.3892/ijmm.2016.2485. [DOI] [PubMed] [Google Scholar]
- 78.Li N L.B., Dluzen D.E., Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007;111(3):458–463. doi: 10.1016/j.jep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y Y.X., Wang S., Song S. Ginsenoside Rg3 prevents cognitive impairment by improving mitochondrial dysfunction in the rat model of Alzheimer's disease. J. Agric. Food Chem. 2019;67(36):10048–10058. doi: 10.1021/acs.jafc.9b03793. [DOI] [PubMed] [Google Scholar]
- 80.Liu M., B X., Yu S., et al. Ginsenoside Re inhibits ROS/ASK-1 dependent mitochondrial apoptosis pathway and activation of nrf2-antioxidant response in beta-amyloid-challenged SH-SY5Y cells. Molecules. 2019;24:2687. doi: 10.3390/molecules24152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y L.P., Feng J., Wu M. Dysfunction of NMDA receptors in Alzheimer's disease. Neurol. Sci. 2016;37(7):1039–1047. doi: 10.1007/s10072-016-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S., R H. Ginsenosides inhibit NMDA receptor-mediated epileptic discharges in cultured hippocampal neurons. Arch Pharm. Res. (Seoul) 2004;27(5):524–530. doi: 10.1007/BF02980126. [DOI] [PubMed] [Google Scholar]
- 83.Quan Qk L.X., Yuan H.F., Wang Y., Liu W.L. Ginsenoside Rg1 inhibits high-voltage-activated calcium channel currents in hippocampal neurons of beta-amyloid peptide-exposed rat brain slices. Chin. J. Integr. Med. 2016 doi: 10.1007/s11655-015-2301-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 84.Dong X., K L., Huang L., et al. Ginsenoside Rg1 treatment protects against cognitive dysfunction via inhibiting PLC–CN–NFAT1 signaling in T2DM mice. Journal of Ginseng Research. 2022;47(3):458–468. doi: 10.1016/j.jgr.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stennicke Hr S.G. Biochemical characteristics of caspases-3, -6, -7, and -8. J. Biol. Chem. 1997;272(41):25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 86.Su Jh Z.M., Anderson A.J., Srinivasan A., Cotman C.W. Activated caspase-3 expression in Alzheimer's and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898(2):350–357. doi: 10.1016/s0006-8993(01)02018-2. [DOI] [PubMed] [Google Scholar]
- 87.Idan-Feldman A., O R., Gozes I. Tau and caspase 3 as targets for neuroprotection. Int. J. Alzheimer's Dis. 2012;2012 doi: 10.1155/2012/493670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miao Hh Z.Y., Ding G.N., Hong F.X., Xie Z.C., Tian M. Ginsenoside Rg1 attenuates isoflurane-induced caspase-3 activation via inhibiting mitochondrial dysfunction. Biomed. Environ. Sci. 2015;28(2):116–126. doi: 10.3967/bes2015.014. [DOI] [PubMed] [Google Scholar]
- 89.Cui J W.J., Zheng M., Gou D., Liu C., Zhou Y. Ginsenoside Rg2 protects PC12 cells against β-amyloid(25-35)-induced apoptosis via the phosphoinositide 3-kinase/Akt pathway. Chem. Biol. Interact. 2017;275:152–161. doi: 10.1016/j.cbi.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y L.Y., Yang W., et al. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer's disease induced by Aβ(1-40) Am J Transl Res. 2018;10(3):796–805. [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmad F., et al. Reactive oxygen species-mediated loss of synaptic Akt1 signaling leads to deficient activity-dependent protein translation early in Alzheimer's disease. Antioxidants Redox Signal. 2017;27(16):1269–1280. doi: 10.1089/ars.2016.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Querfurth H., Lee H.-K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegener. 2021;16(1):44. doi: 10.1186/s13024-021-00428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang H., M X., Xie L., Greenberg D.A., Jin K. Expression level of vascular endothelial growth factor in hippocampus is associated with cognitive impairment in patients with Alzheimer's disease. Neurobiol. Aging. 2013;34(5):1412–1415. doi: 10.1016/j.neurobiolaging.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Yj H.C., Shiao Y.J., Wang H.T., Lo Y.L., Lin A.M.Y. Anti-inflammatory effect of afatinib (an EGFR-TKI) on OGD-induced neuroinflammation. Sci. Rep. 2019;9(1):2516. doi: 10.1038/s41598-019-38676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qian Xh L.X., Chen S.D., Tang H.D. Integrating peripheral blood and brain transcriptomics to identify immunological features associated with Alzheimer's disease in mild cognitive impairment patients. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.986346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wan H.L., H X., Zhao Z.H., et al. STAT3 ameliorates cognitive deficits via regulation of NMDAR expression in an Alzheimer's disease animal model. Theranostics. 2021;11(11):5511–5524. doi: 10.7150/thno.56541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reichenbach N D.A., Plescher M., et al. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer's disease model. EMBO Mol. Med. 2019;11(2):e9665. doi: 10.15252/emmm.201809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee St C.K., Sim J.Y., Heo J.H., Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008;22(3):222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., et al. Ginsenoside Rk1 attenuates radiation-induced intestinal injury through the PI3K/AKT/mTOR pathway. Biochem. Biophys. Res. Commun. 2023;643:111–120. doi: 10.1016/j.bbrc.2022.12.072. [DOI] [PubMed] [Google Scholar]
- 100.Kim E.N., et al., Simultaneous quantitative analysis of ginsenosides isolated from the fruit of Panax ginseng C.A. Meyer and regulation of HO-1 expression through EGFR signaling has anti-inflammatory and osteogenic induction effects in HPDL cells, LID - 10.3390/molecules26072092 (2021)26(7):2092. [DOI] [PMC free article] [PubMed]
- 101.Lei, C., et al., Ginsenoside Rg1 Can Reverse Fatigue Behavior in CFS Rats by Regulating EGFR and Affecting Taurine and Mannose 6-phosphate Metabolism. (1663-9812 (Print)).. [DOI] [PMC free article] [PubMed]
- 102.Yang L., et al. Protopanaxadiol inhibits epithelial–mesenchymal transition of hepatocellular carcinoma by targeting STAT3 pathway. Cell Death Dis. 2019;10(9):630. doi: 10.1038/s41419-019-1733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan X., et al. A large-scale transcriptional analysis reveals herb-derived ginsenoside F2 suppressing hepatocellular carcinoma via inhibiting STAT3. Phytomedicine. 2023;120 doi: 10.1016/j.phymed.2023.155031. [DOI] [PubMed] [Google Scholar]
- 104.Jiang J., et al. 1950-6007. Ginsenoside Rg3 Enhances the Anti-proliferative Activity of Erlotinib in Pancreatic Cancer Cell Lines by Downregulation of EGFR/PI3K/Akt Signaling Pathway. (Electronic)) [DOI] [PubMed] [Google Scholar]
- 105.Leung, K.W. and A.S. Wong, Pharmacology of Ginsenosides: a Literature Review. (1749-8546 (Electronic)).. [DOI] [PMC free article] [PubMed]
- 106.Chen J., et al. Neuroprotective effects of red ginseng saponins in scopolamine-treated rats and activity screening based on pharmacokinetics. Molecules. 2019;24 doi: 10.3390/molecules24112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.St-Laurent T., Hammami R. The untapped potential of ginsenosides and American ginseng berry in promoting mental health via the gut–brain Axis. Nutrients. 2022;14 doi: 10.3390/nu14122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi M.-K., et al. Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. Journal of Ginseng Research. 2020;44(2):229–237. doi: 10.1016/j.jgr.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan D., et al. Functionalized curcumin/ginsenoside Rb1 dual-loaded liposomes: targeting the blood-brain barrier and improving pathological features associated in APP/PS-1 mice. J. Drug Deliv. Sci. Technol. 2023;86 [Google Scholar]
- 110.Shen, J., et al., Ginsenoside Rg1 Nanoparticle Penetrating the Blood-Brain Barrier to Improve the Cerebral Function of Diabetic Rats Complicated with Cerebral Infarction. (1178-2013 (Electronic)).. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available without restriction.