Abstract
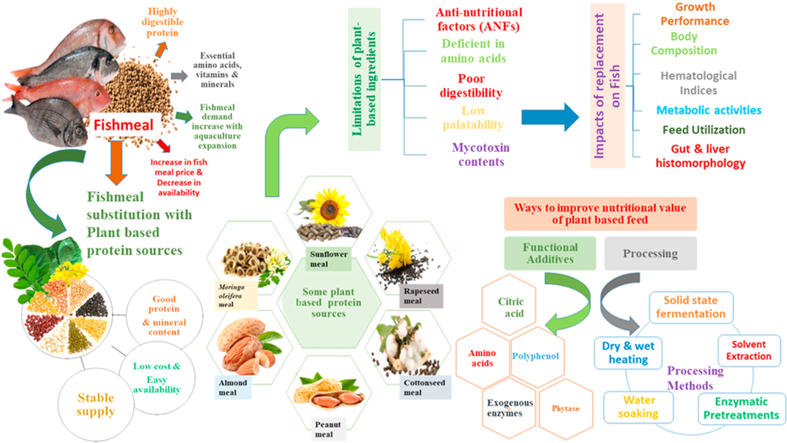
High protein content, excellent amino acid profile, absence of anti-nutritional factors (ANFs), high digestibility and good palatability of fishmeal (FM), make it a major source of protein in aquaculture. Naturally derived FM is at risk due to an increase in its demand, unsustainable practices, and price. Thus, there is an urgent need to find affordable and suitable protein sources to replace FM. Plant protein sources are suitable due to their widespread availability and low cost. However, they contained certain ANFs, deficiency of some amino acids, low nutrient bioavailability and poor digestibility due to presence of starch and fiber. These unfavourable characteristics make them less suitable for feed as compared to FM. Thus, these potential challenges and limitations associated with various plant proteins have to be overcome by using different methods, i.e. enzymatic pretreatments, solvent extraction, heat treatments and fermentation, that are discussed briefly in this review. This review assessed the impacts of plant products on growth performance, body composition, flesh quality, changes in metabolic activities and immune response of fishes. To minimize the negative effects and to enhance nutritional value of plant products, beneficial functional additives such as citric acid, phytase and probiotics could be incorporated into the plant-based FM. Interestingly, these additives improve growth of fishes by increasing digestibility and nutrient utilization of plant based feeds. Overall, this review demonstrated that the substitution of fishmeal by plant protein sources is a plausible, technically-viable and practical option for sustainable aquaculture feed production.
Keywords: Fishmeal substitution, Plant products, Anti-nutritional factors, Physiological impacts, Sustainability
Graphical abstract
1. Introduction
Fish is a primary affordable protein source for over 950 million humans, providing about 16% of animal protein [1]. The high-quality and readily digestible fish protein with its unique content of eight essential amino acids (EAAs) is considered superior to other protein sources such as beef, milk, and eggs. Moreover, it is the principal source of omega-3 fatty acids, many vitamins, i.e. vitamin A, and minerals that are not available in many terrestrial protein sources. Fish and fish items form an essential part of the global food supply, meeting nutritional demands in both developed and developing countries [2].
With the rapid increase in world population and their demand for seafood as well, aquaculture is the best way to produce animal protein. Aquaculture can solve the problem of food shortage. Basically, it is the production of aquatic animals (fishes, crustaceans and mollusks) as well as aquatic plants under controlled conditions [3]. Aquaculture plays a crucial role in improving food security and generating home for fish farmers, particularly in developing regions. Furthermore, this industry is producing aquatic food at 7.5% growth rate per year since 1970 [4].
Fishmeal (FM) is used to feed aquatic animals. Nutrient rich FM is the best source of high quality protein with balanced amino acid content, omega-3 polyunsaturated fatty acids, minerals, vitamins (biotin, choline, vitamin A, D, E, and B12) as well as trace elements (iodine and selenium). It is highly digestible and palatable feed for farm animals. Commercial FM is mostly prepared from small, wild-caught sea fishes having high oil and bone percentage that are not appropriate for human use. A small proportion is also prepared from the byproducts of many seafood items made for human consumption. Major fishes used in a FM are shads, smelts, anchovies, sardines, menhaden, and herrings. High-quality FM usually has crude protein 60–72% and lipids are only 6–10% or 4–20% by weight as most oil is extracted during FM processing [5].
Aquafeed is a critical factor influencing the production of the aquaculture industry that affects its production. Exponential development of aquaculture to provide cheap protein to world's ever growing population has resulted an increase in demand for FM. Extensive overfishing for FM production led to a decline in marine species that further limits FM production [6]. Moreover, FM production has decreased due to restrictions on fishing. Due to all these factors, FM price has increased about 300% in recent years [7].
Expensive feed accounts for more than 50% of aquaculture production [8]. Overuse of FM also pollutes water owing to its high phosphorus content [9]. As FM is a limited world resource so its use as a single protein source for fish production is not viable. Therefore, now it is imperative to look for alternative protein sources to substitute FM for aquaculture sustainability. The current review highlights the significance of the use of different plant protein sources to replace FM. This review also focuses on the effects of using these products on different fish species. Moreover, the strategies to minimize the negative effects of plant based sources have also been discussed.
2. Fishmeal substitution with plant products
Plant protein sources are the best to substitute FM due to their ideal protein content, low cost, and stable supply from a large range of resources and are suitable feed for aquatic animals. Amino acid profile, energy density, fiber, and nutrient absorption are major factors for the substitution with plant based protein sources in aquafeed. However, plant proteins have some disadvantages over the FM that are a high proportion of cellulose [10], imbalance amino acid profile, and absence of some EAAs i.e. lysine, tryptophan, and methionine, and low palatability. Plants also have anti-nutritional factors (ANFs) i.e. phytic acid and inhibitors [11].
The use of alternative plant products will reduce feed cost and dependence on FM improving economic benefits. It is estimated that the aquaculture sector consumed 3.72 million tonnes of fishmeal (60.8 percent of global fishmeal production) in 2008. However, it is estimated that the aquaculture feed sector consumed about 6.8 million tonnes of soybean meal (25.1 percent of total compound aquafeeds by weight) in 2008. Other plant proteins being increasingly used include corn products, pulses, oilseed meals and protein from other cereals products. The total use of FM by the aquaculture sector is expected to decrease due to plant products [12]. These steps are pivotal for the sustainability of aquaculture [13]. This review explains some of the effective plant products such as soybean meal, black cumin seed meal, canola meal, lupin meal, rapeseed meal, rice, guar meal, almond meal and Moringa oleifera meal that has been utilized in fish feed. Conventional and non-conventional plant protein sources have been chosen for the novelty and uniqueness in research. Moreover, this review demonstrates the effects of substitution of plant ingredients with FM on different fish species.
3. Candidate plant products used in fish feed
3.1. Soybean meal
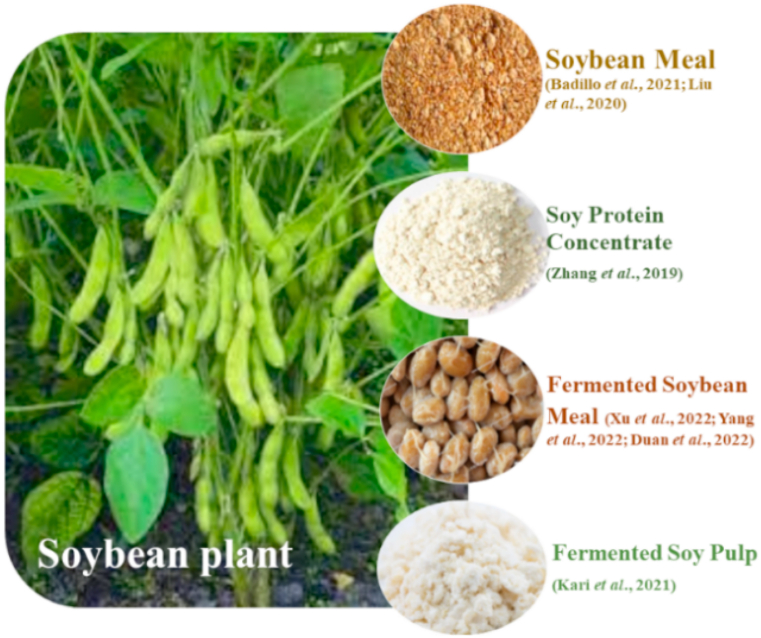
Soybean meal (SBM) is first largest protein source having balanced amino acid profile with a low price and consistent supply making it an ideal to replace FM [14] (Fig. 1). Soybeans have about 41% protein and 20% ether extract based on dry matter [15]. However, SBM has low palatability, poor EAA content, and several ANFs i.e. phytates, tannins, trypsin inhibitors, and oligosaccharides [16]. Many studies have shown that SBM inclusion in high proportion in aquafeed affects the growth process, digestion, absorption, anti-oxidative capacity as well as causes damage to the gut of aquatic animals [17]. These negative effects can be minimized by fermentation of SBM to enhance probiotics and increase palatability. It also activates some components improving fish intestinal microbiota, increasing digestion and nutrient absorption [18] (Table 1).
Fig. 1.
Different forms of soybeans used as fishmeal substitute.
Table 1.
Soybean forms as substitute for fishmeal in diet of different fish species.
| Fish Species |
Soybean Forms | Tested inclusion levels |
Duration | Optimum inclusion Level |
Effects | References |
|---|---|---|---|---|---|---|
| Pacific fat sleeper (Dormitator latifron) | Soybean meal (SBM) | 0, 40, 70, & 100% | 60 days | 100% | No significant difference in WG, SGR, SR up to 100% ↑ADC, Crude protein & lipid up to 100 % ↓Feeding cost |
[155] |
| African catfish (Clarias gariepinus) | Fermented soy pulp (FSP) | 0, 25, 50, 75 & 100 % | 70 days | 50% | ↑ WG, SGR, RBC, LYM, LAB, TB up to 50% ↑ALB, GLOB, TP ↑FCR = ↑ FSP |
[156] |
| Crussian carp (Carassius auratus gibelio♀) × Common carp (Cyprinus carpio♂) | SBM | 0, 20, 40, 60, 80 & 100 % |
60 days | <38.52–41.81% | ↑WG, SGR up to optimum level ↓HF, HMV, Digestive enzyme activity = ↑SBM ↑Fusobacteria, Proteobacteria & Actinobacteria ↓Firmicutes and Bacteroidetes |
[157] |
| Obscure puffer (Takifugu obscurus) | Dehulled & defatted SBM + lysine, methionine, & taurine |
0, 15, 30, 45, 60 & 75% |
56 days | 40% | ↓ RBC, hemoglobin, methemoglobin, whole body lipid content ∞ ↑ SBM ↑ ALT & AST activity ↓GSH-Px & CAT activity |
[26] |
| Amberjack (Seriola dumerili) | SBM + inosine | 0, 25, 50 & 75% with or without inosine at 0.6% | 56 days | 50–75% | Inosine improve SBM utilization, innate immune responses, gut morphology & stress resistance against low salinity exposure TSP, ACH50, LA, BA, PA ↑ with inosine |
[25] |
| Rice field eel (Monopterus albus) | Soy protein concentrate (SPC) | 0, 15, 30, 45, 60 & 75% | 56 days | 26% | WG & FI ↓, FCR↑ over optimum level Improvement in T-AOC, SLP, digestive enzyme activity & alteration in growth-related genes expression pattern in skeletal muscle |
[158] |
| Crucian Carp (C. auratus) | Fermented soybean meal (FSM) | 0, 20, 40, 60 & 80% | 56 days | 40% | Serum T-AOC, POD & IgM ↑ upto 40% Significant differences in the midgut & hindgut microbiota |
[23] |
| Largemouth bass (Micropterus salmoides) | FSM | Control, 75, 150, 225 & 300 g/kg | 56 days | 150 g/kg | ↓WG, T-AOC, SOD, lipid & protein retention over optimum level ↑ FCR Gut Cetobacterium↓ & Mycoplasma↑ over 150 g/kg ↑ resistance against bacterial infection |
[24] |
| Hybrid Snakehead (Channa argus × Ch. Maculate) | FSM | 0, 72, 144, 216 & 288 g/kg | 60 days | 50 g/kg of FM replaced by FSM | WG & SGR ↓, FCR↑ ADCDM, ADCCP, PER, TC, intestinal muscle thickness & villus height decrease |
[159] |
| Japanese seabass (Lateolabrax japonicas) | Conventional SBM + functional additives (FAS) | Control, 30% SBM without and with 0.0665% FAS | 105 days | – | Growth, immunity, T-AOC & disease resistance ↓ at 50% FM replacement FAS ↑ antioxidant capacity & regulate gut microbiota |
[27] |
| Turbot (Scophthalmus maximus) | Extruded full-fat soybean (EFS) | 0, 10, 20, & 30% | 60 days | 20% | Serum LDL & TC, ADCCP, ADCDM, & gut probiotics reduced over 20% Water ammonia & nitrogen discharge, TSS, & fine solid accumulation increased over 20% |
[160] |
| South American Catfish (Rhamdia quelen) | FSM | 0, 7, 14, 21 and 28% | 56 days | 21% | ↓ Vibrionaceae intestinal pathogenic bacteria up to 20 % No significant impact on gut lactic & heterotrophic bacteria concentrations, gut morphology & enzymatic activities |
[22] |
| Turbot (S. maximus L.) | SBM or L. acidophilus-fermented SBM (LASM) | Control, SBM, LASBM |
56 days | 45% SFM + 1 × 108 CFU g−1 (L. acidophilus) | SBM = ↓growth, ↓ activities of intestinal digestive & immune-related enzymes LASM = ↓ SM-induced negative effects on growth performance, morphology & microbiota, immune-related & digestive enzymes |
[13] |
| Rainbow trout (Oncorhynchus mykiss) | Enzymatically hydrolyzed SM (ESM) + amino acid methionine & lysine (Met + Lys) + bile acid | Control, 45% ESM, E + A = ESM+0.76% (Met + Lys), E + B = ESM 0.02% bile acid, & E + AB = ESM + 0.76% (Met + Lys)+ 0.02% bile acid |
56 days | – | ESM = ↓growth performance, ↑ liver health problem, ↓ lipid & SFA & ↑ protein load in muscle E + A = ↑growth performance, pepsin activity & fillet protein content E + B = ↓ growth & protease activity, ↑ amylase activity, ↑ SFA, MUFA & PUFA, ↑ lipid utilization E + AB = ↑ liver health |
[161] |
Abbreviations: ACH50: Alternative complement pathways, ADC: Apparent digestibility coefficient, ADCCP: Apparent digestibility coefficient of crude protein, ADCDM: Apparent digestibility coefficient of dry matter, ALB: Albumin, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, BA: Bactericidal activity, CAT: Catalase, FCR: Food conversion ratio, GLOB: Globulin, GSH-Px: Glutathione, HF: Height of fold (hindgut), HMV: Height of microvillus (hindgut), IgM: Immunoglobulin M,LA: Lysozyme activity, LAB: Lactic bacteria, LDL: Low density lipoprotein, LYM: Lymphocytosis, MUFA: Monounsaturated fatty acids, PA: Peroxidase activity, PER: Protein efficiency ratio, POD: Peroxidase, PUFA: Polyunsaturated fatty acids, RBC: Red Blood Cell, SFA: Saturated fatty acids, SGR: Specific growth rate, SLP: Serum lipid profile, SOD: Superoxide dismutase, T-AOC: Total antioxidant capacity, TB: Total bacteria, TC: Total cholesterol, TP: Total protein, TSP: Total serum protein, TSS: Total suspended solid, WG: Weight gain.
Fermented soybean meal (FSM) is produced by introducing certain microorganisms into SBM for a fixed time period for fermentation by which ANFs are broken down and specific bioactive substances such as short peptides, probiotics, flavonoids, and organic acids are produced that improve the growth and health of aquatic animals [19]. Mostly, the solid state fermentation process is utilized to produce FSM that includes the grinding, steam sterilizing and adding of microorganisms for fermentation for a fixed period. Fungi and bacteria that are mostly used are Lactobacillus, Aspergillus, Bacillus, Saccharomyces, etc. Fermentation process, strains composition, and conditions show great impact on the FSM quality [20,21]. According to de [[22], [23], [24]], FSM significantly (p<0.05) improved the growth, intestinal morphology and increased disease resistance of south American catfish, crucian carp and largemouth bass, respectively.
Another way to improve effects of SBM is inclusion of functional additives such as lysine, methionine, taurine and inosine either separately or in combination [25,26]. A blend of antioxidants including ethoxyquin, a 1:1 standardized amalgam of carvacrol and thymol along with chelated trace elements, i.e., Mintrex® Cu, Zn and Mn, with conventional SBM when fed to Japanese seabass significantly improved the health and disease resistance as well as mitigate the adverse effects of SBM [27].
3.2. Cottonseed meal
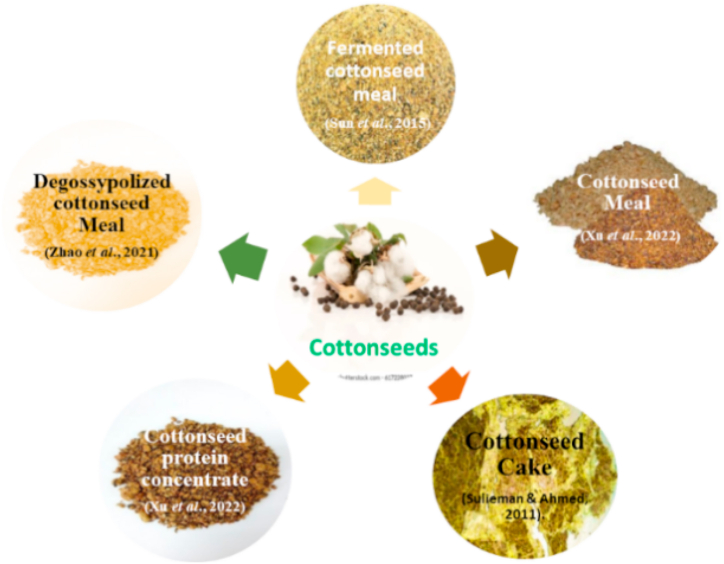
Cottonseed meal (CSM) is another plant protein source for fish feed due to its good protein content, high yield and low price (Fig. 2). Cottonseeds have about 51.20% crude protein, 9.3% ash, 1.6% ether extract, 7.02% fiber and 2.7 kcal/kg mass energy. Chemically active cyclopropenoid fatty acids, sterculic acid, and malvic acid are also found. But it is lysine deficient [28]. In 2013, the estimated cottonseed yield was 45.5 million tons in the world [2]. ANFs such as gossypol are found in cottonseeds that bound with lysine making its availability low to fish. Gossypol has negative impacts on the aquatic animals affecting their growth, fertility, and reproduction. Therefore, cottonseeds are converted to cottonseed protein concentrate through a large variety of processing methods i.e. dehulling, delinting, solvent extraction, and oil extraction at low temperature. These processes remove nonstarch polysaccharides, aflatoxins, and ANFs i.e. gossypol and protein denaturation is also greatly decreased [29,30] (Table 2). The study of [31] exhibited that cotton seed protein concentrate can replace 45% of FM without compromising health, growth and antioxidant status of largemouth bass. Similarly [32], revealed 10–50% replacement of FM in rainbow trout improved the growth, hematology, antioxidant parameters, and body composition by using concentrated dephenolization cottonseed protein.
Fig. 2.
Different forms of cottonseeds that are suitable to replace fishmeal.
Table 2.
Cottonseed forms as substitute for fishmeal in diet of different fish species.
| Fish Species | Cottonseed Forms |
Tested Inclusion levels |
Duration | Optimum inclusion Level |
Effects | References |
|---|---|---|---|---|---|---|
| Largemouth bass (M. salmoides) | Cottonseed protein concentrate (CPC) | 0, 15, 30, 45 & 70% |
84 days | 45% or 385.0 g/kg | WG↓, FCR↑, flesh composition & texture↓ over 45% ↓Serum glucose, TGC, TP at 70% Lower serum cholesterol content in all CPC levels |
[31] |
| Rainbow trout (O. mykiss) | Concentrated dephenolization cottonseed protein | 0, 10, 20, 30, 40 & 50 % |
56 days | 10–50% | At 10–50%, no negative impacts on growth performance, body composition, gut histomorphology, antioxidant & hematological indices ↑AOC at 30–50% |
[32] |
| Silver sillago (Sillago sihama Forsskál) | Low-gossypol cottonseed meal (LCSM) + AA (methionine & lysine) | 0,16, 32, 48 & 64 % |
56 days | 16% | WG& SGR ↑ up to 16% ↑expression of intestinal t TNF-α, NF-κB & IL-1β but ↓ZO-1, TGF-β3, IL-10 expression LCSM↑ = intestinal amylase activity↑ but intestinal trypsin activity ↓ & morphological damage to mid gut occur |
[30] |
| Nile tilapia (Oreochromis niloticus) | CSM + exogenous protease | FM: CSM = 2:1, 1:1, 1:2, All three diets without or with 2500 U protease per kg diet |
84 days | First & second group with protease | Protease improve growth performance, nutrient assimilation, & hematological indices Change in gene expression of GH & IGF-I Improve physiological indices |
[162] |
| Southern flounder (Paralichthys lethostigma) | Genetically improved (GI) (glandless) & Genetically modified low-gossypol (GMO) CSM |
0%, 50, 75 & 100% FM protein replace by GI or GMO-CSM protein, 100% R-CSM | 56 days | 75% GI or GMO-CSM | No effect on entire body omega-3 PUFAs, or liver gossypol up to 75% No adverse effect on body composition & growth performance up to 75% |
[163] |
| Rohu (Labeo rohita) | Acidified phytase + CSM | 0, 25, 50 & 75% with citric acid 0 & 2.5% & phytase 0 & 750 FTU/kg |
84 days | 50% with CA; 2.5%, PHY; 750 FTU/kg |
Highest WG, SGR, FCR, crude protein & fat at optimum level Improvement in growth & body composition |
[164] |
| Golden Pompano (Trachinotus ovatus) | CSM | 0,20,40 & 60% | 42 days | 20% | Up to 20% no impact on muscle proximate composition & free EAA concentration Over 20% impaired glucose metabolism & muscle nutritive metabolism disorders |
[165] |
| Turbot (S. maximus L.) | Low-gossypol CSM |
0, 15, 25, 35 & 45% |
56 days | 35% | At 45% FER↓, PER↓, plasma glucose & cholesterol ↓, intestinal villi height ↓ ADCs of dry matter, crude protein & lysine ↓ |
[166] |
| Snubnose pompano (T. ovatus) | CSM + lysine & methionine |
0, 25, 50, 75 & 100 % |
70 days | 100% | No negative impact on growth, metabolism, and general health at total replacement | [33] |
| Largemouth bass (M. salmoides) | CPC + yeast based paraprobiotic | Control, 23.5% CPC & 23.5% CPC +800 mg/kg yeast-based paraprobiotic |
65 days | 40% with yeast-based paraprobiotic | Improved growth performance Regulation in intestinal barrier & peptide transport related functions MsYF improve intestinal health by altering intestinal permeability, microbiota & inflammatory environment | [34] |
| Pearl gentian groupers (Epinephelus fuscoguttatus♀ × E. lanceolatus♂) | CPC | 0 (FM, control), 10, 30 & 50 % | 56 days | 50% | No significant impact on WG, SGR, FCR, SR At 50% body lipid content ↑ Changes in genes related to hepatic lipid metabolism & enzyme activity |
[167] |
| Black sea bream (Acanthopagrus schlegelii) | Fermented cottonseed meal (FCM) | 0, 80, 160 & 240 g kg−1 |
56 days | 16% | ↓ SGR, WG, PPV, PER, & hepatosomatic index ↑ FCR, ADCDM ↓ADCs of dietary protein and lipid |
[168] |
Abbreviations: AA: Amino acids, ADC: Apparent digestibility coefficient, AOC: Antioxidant capacity, EAA: Essential amino acid, FA: Fatty acid, FCR: Feed conversion ratio, FER: Feed efficiency ratio, GH: Growth hormone, IGF-I: Insulin like growth factor I, IL-10: Interleukin 10, IL-1β: Interleukin one beta, MsYF: Multi-strain yeast fractions, NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, PER: Protein efficiency ratio, PPV: Protein productive value, PUFAs: Polyunsaturated fatty acids, SGR: Specific growth rate, SR: Survival rate, TGC: Triglycerides, TGF-β3: Transforming growth factor beta-3, TNF-α: Tumor necrosis factor-α, TP: Total protein, WG: Weight gain, ZO-1: Tight junction proteins ZO-1.
Functional feed additives i.e. methionine, lysine and yeast based paraprobiotics are also largely supplemented with CSM to boost health and growth of fish [33,34]. Moreover, microbial fermentation of CSM also reduces concentrations of liver and dietary gossypol [35]. CSM fermentation with fungal mixture of Saccharomyces cerevisiae and Candida tropicalis for 48 h has improved its protein content from 20 to 33.5% [36] while fermentation with yeast has increased protein content from 8.745 to 12.67% [37].
3.3. Corn gluten meal
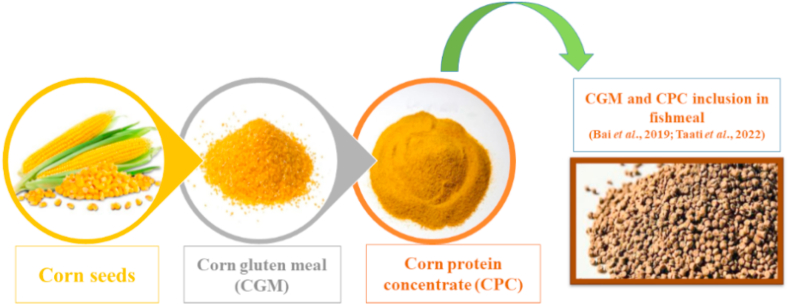
Corn gluten meal (CGM) is obtained as a byproduct of wet grinding process for separation of protein, gums, fiber and starch parts from corn [38] (Fig. 3). It is beneficial plant protein source due to local availability, high protein content that is 60–70% of dry matter, low fiber content, and specifically due to low cost [39]. Moreover, it has few ANFs as compared to other plant based sources of protein [40]. In a study [40], demonstrated that 10% FM can be replaced by CGM in sea bass. The deficiency of EAAs i.e. lysine and arginine [41], and non-soluble carbohydrates i.e. raw starch up to 20% limit its inclusion in fish feed [42]. These non-soluble carbohydrates are reduced by enzymatic treatment and converted to corn protein concentrate (CPC) with high protein parts. Currently, there are two commercial types of CPC used in fish feed that are Lysto™ and Empyreal®75. The Empyreal®75 has higher methionine content (1.8 %) while lower in lysine (1.14 %) with crude protein 78%. Lysto™ has methionine 1.6%, lysine 6.2% with crude protein 77% [43] (Table 3).
Fig. 3.
Different forms of corn seeds used to replace fishmeal.
Table 3.
Corn products as substitute for fishmeal in diet of different fish species.
| Fish Species |
Corn Forms |
Tested Inclusion Levels |
Duration | Optimum inclusion level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Spotted rose snapper (Lutjanus guttatus) | CGM + crystalline Arginine & Lysine | 0, 20, 40, 60, 80, or 100% | 70 days | 60% | ↑Protein retention, ↓ lipid retention & ↓trypsin activity at 60 % Over 60% hypocholesterolemia & hypertriglyceridemia with negative effects on growth & FCR |
[40] |
| Turbot (S. maximus) | CGM | 0, 21.2, 31.8, & 42.6 % CGM to replace 0, 33, 50, & 67 FM protein |
56 days | - | Negative impacts on growth & gut health by inducing enteritis ↓ intestinal immunity & AOC, ↓nutrient digestibility & feed utilization |
[169] |
| Ussuri catfish (Pseudobagrus ussuriensis) | CGM | 0, 10, 20, 30, 40, 50 & 60 % |
56 days | 40% | WG, SGR, FI, PER, ADCs ↓ over 40% but ADC of phosphorus ↑ ↑nitrogen excretion but ↓ phosphorus excretion Pepsin & serum lysozyme activity ↓ but ALT & AST activity ↑ |
[170] |
| Asian seabass (Lates calcarifer) | CGM | 0, 5, 10, 15, & 20% |
45 days | 10% | Best FCR & ADCs up to 10% Crude lipid & gross energy highest at 20% |
[171] |
| Siberian sturgeon (Acipenser baerii) | Corn protein concentrate (CPC) | 0, 15, 30, & 45 % |
56 days | 15% | TL, FW, SGR, PER increase up to 15% FCR↓, SR = 100% |
[172] |
| Rainbow trout (O. mykiss) | CPC | 0, 30, 60, 90 & 120 g/kg CPC |
56 days | 81.0–82.2 g/kg | ↑ Redness & yellowness of fillet ↑ ALT & LDH level at 120 g/kg ↑serum lysozyme activity ↓Proline & Threonine ∞↑ CPC |
[173] |
Abbreviations: ADC: Apparent digestibility coefficient, ALT: Alanine aminotransferase, AOC: Antioxidant capacity, AST: Aspartate aminotransferase, CPC: Corn protein concentrate, FCR: Feed conversion ratio, FW: Final weight, LDH: Lactate dehydrogenase, PER: Protein efficiency ratio, SGR: Specific growth rate, SR: Survival rate, TL: Total length, WG: Weight gain.
3.4. Rapeseed meal
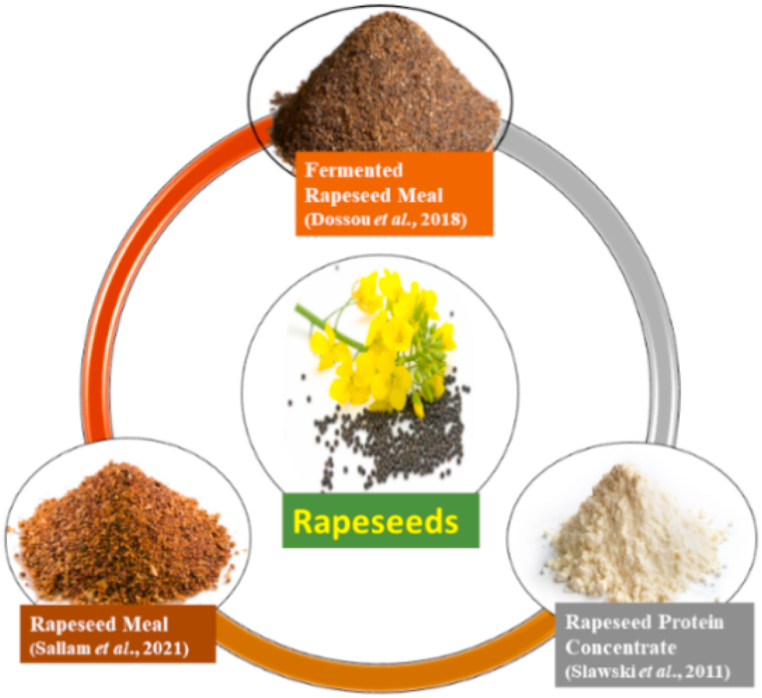
Rapeseed meal (RSM) has good protein content from 32 to 45% of dry matter, comparatively balanced amino acid content, low cost, and constant supply (Fig. 4). It also provides vitamins, minerals, and other microelements. It has lower lysine value than SBM but rich in sulfur amino acids [44]. Besides, RSM has a large number of ANFs i.e. tannis, phytic acid, sinnapine, erucic acid, glucosinolates, and indigestible carbohydrates. These factors show adverse effects on fish growth and health status [45,46] due to low palatability [47], poor digestibility and decrease in feed utilization [48]. In recent years, RSM yield has increased greatly becoming the second most important and commonly used protein source after SBM [49]. During microbial and enzymatic denaturation of rapeseed protein, certain functional bioactive peptides are released that perform specific biological activities including antihypertensive, immunomodulatory and antimicrobial [50,51]. Moreover, some degradation products from rapeseeds i.e. sinigrin and phenolic components, are observed having main antioxidant role with positive impacts in foods [52,53] (Table 4). Rapeseed protein concentrate (RPC) is also used in fish feed that is obtained after washing of RSM with aqueous ethanol [54]. For example [55], found that RSM, when fed to Nile tilapia and mango tilapia, showed improved weight gain and specific growth rate at 10% inclusion level while RPC replaces 33% of FM in Common carp.
Fig. 4.
Different forms of rapeseeds used to replace fishmeal.
Table 4.
Rapeseed meal as substitute for fishmeal in diet of different fish species.
| Fish Species | Rapeseed forms | Tested Inclusion levels |
Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Major carp (Catla catla) | RSM with Probiotic | 34% RSM with 0, 1, 2, 3, 4 & 5 g/kg of probiotics | 70 days | 34% RSM with 2 g/kg of probiotics | High carcass composition, RBCs, WBCs, Hb & PLT at optimum level while low above optimum level Good immunological indices with 0, 1, 3 & 5 g/kg of probiotics |
[174] |
| Nile tilapia (O. niloticus) & Mango tilapia (Sarotherodon galilaeus) | RSM | 0, 10, 20, & 30% | 84 days | 10% | ↑ FW, WG, SGR, WGR up to 10% ↑Mucosal & intestinal villi length and goblet cell number at 30% ↑AST & ALT with ↑RSM |
[55] |
| Red Sea Bream (Pagrus major) | Aspergillus oryzae fermented RSM (RM-Koji) | 0, 25, 50, 75 & 100% |
56 days | 50% | ↑growth, nutrient assimilation & boosted immune responses & anti-oxidative impacts up to 50% | [175] |
| Asian red-tailed catfish (Hemibagrus wyckioides) | RSM | 0, 11.2, 22.4, 33.6, & 44.8 % |
56 days | 11.2% | No negative impact on growth & health up to 11.2% ↓FGR, antioxidant capacity & digestive enzymes activities ↑Plasma aspartate aminotransferase & hepatic γ-glutamyl transferase activities |
[103] |
| Ussuri catfish (P. ussuriensis) | RSM | 0, 10, 20, 30, 40 & 50% |
56 days | 17% | ↓FW, SGR, FI, FER, PER, ADCs = ↑RSM Hepatic AST, ALT & IGF-I gene expression level ↓ over 17% but muscles IGF-I gene expression level ↑ at 50% |
[176] |
| Common Carp (C. carpio L). | Rapeseed Protein Concentrate (RPC) | 0%, 33%, 66%, or 100% | 56 days | 33% | ↓FI, FER, SGR over 33% | [177] |
| Rainbow trout (O. mykiss Walbaum) |
RPC | 0, 66 & 100% | 84 days | – | No significant effect on growth performance, FI, FER, intestinal morphology & blood parameters | [178] |
| Rainbow trout (O. mykiss) | Rapeseed protein isolate | 0, 33, 66 & 100% | 56 days | 66% | No significant effect on FCR, health parameters & body composition except ash content↓ ↓FI& FGR at total replacement |
[179] |
Abbreviations: AKP: Alkaline phosphatase, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, FA: Fatty acid, FCR: Feed conversion ratio, FER: Feed efficiency ratio, FGR: Fish growth response, FI: Feed intake, FW: Final weight, Hb: Hemoglobin, IGF-I: Insulin-like growth factor I, PLT: Platelets, RBC: Red blood cell, RSH: Rapeseed meal high, RSL: Rapeseed meal low, SGR: Specific growth rate, TG: Triglycerides, TP: Total protein, WBC: White blood cell, WG: Weight gain, WGR: Weight gain rate.
3.5. Canola meal
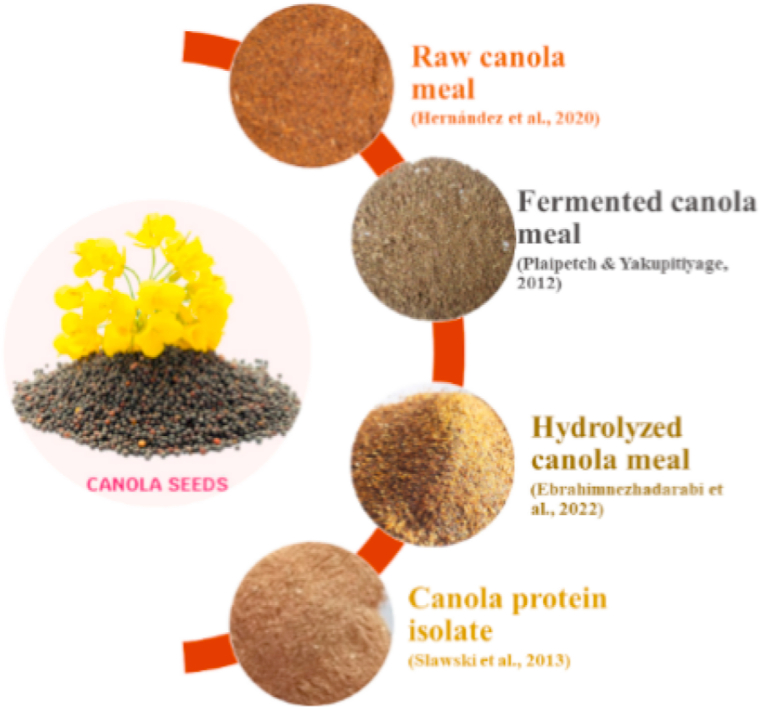
Canola meal (CM) is the solid matter left after the processing of canola seeds to extract oil [56] (Fig. 5). By weight up to 67%, CM is obtained from seeds after oil extraction [57]. It is most important source of plant protein as it has a minimum of 40% protein (dry matter) with easy availability and low cost. However, like other plant-based sources, it also contains ANFs i.e. non digestible oligosaccharides, glucosinolates and phytic acid, that limit its use in fish feed [48,58]. These factors have adverse impacts on fishes i.e. increase in gut viscosity and decrease in nutrient assimilation. High fiber content dilutes the density of nutrients [59], and phytates that are negatively charged make bonds with minerals decreasing their bioavailability [60]. The glucosinolates and their degraded products result in iodine deficiency, low feed intake, and increase in size of the thyroid gland, liver, and kidney [61].
Fig. 5.
Different byproducts of canola seeds used as fishmeal replacers.
Different techniques are used to lower or vanish ANFs that include alkaline and acid treatments, thermal extrusion [62], enzymatic process [63], microbial fermentation [64], and tail end dehulling [65]. All these methods are observed to decrease ANFs, but the benefits are restricted due to net protein loss, high processing cost, and/or partial reduction of ANFs. CM nutritional composition is greatly enhanced by fermentation using microbes to decrease ANFs [64] (Table 5) but due to high simple carbohydrate levels in CM, fermentation effect is limited to complex sugars. The removal of simple sugars by washing CM prior to fermentation increase microbial effect on complex carbohydrates i.e. fiber. These processes increase overall digestibility in fish species [66]. According to Refs. [67,68], CM when supplemented with phytase, citric acid and polyphenols, improved the nutrient digestibility, hematology and growth performance of C. mrigala and C. carpio, respectively.
Table 5.
Canola meal as substitute for fishmeal in diet of different fish species.
| Fish Species | Canola forms | Tested Inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Common carp (C. carpio) | Polyphenols + Canola meal (CM) |
55% CM with 0, 100,200, 300, 400, 500 & 600 mg/kg |
70 days | 400 mg/kg of polyphenol with 55 % CM | Highest minerals absorption, best hematological parameters as well as proximate composition at optimum level | [180] |
| Mrigal carp (Cirrhinus mrigala) | CM + phytase & citric acid (CA) | 0, 25, 50& 75 % without or with 750 FTU kg−1 & CA 2.5% |
90 days | CM 50% with phytase 750 FTU kg-1 & CA 2.5% |
Maximum WG & lowest FCR as well as best nutrient digestibility values at optimum level Dose dependent decrease in fish gut pH by CA |
[67] |
| Nile tilapia (O. niloticus) | Processed canola meal (PCM) | 0, 12.5, 25, 37.5 & 50% |
36 days | 25% | No effect on digestive enzymes, liver antioxidative status & mucosal immunity Liver SOD, GPx & CAT genes expression ↑ = PCM↑ Growth performance & intestine & liver tissue histoarchitecture ↓ over 25% |
[181] |
| Blunt snout bream (Megalobrama amblycephala) | CM | 208.2, 279.4, 350.5, 421.4, & 492.8 g/kg |
112 days | 50% | SGR, FER, PER ↑ but FI ↓ up to 50% PepT1 gene expression in gut ↑ then ↓ IGF-1 expression ↑ with ↑ CM Expression of TOR↓, AKT,4E-BP2 & S6K1 expressions ↑with CM ↑ |
[182] |
| Asian Sea Bass (L. Calcarifer) | Yeast fermented CM |
25, 50, 75 & 100% |
60 days | 50% | ↓ FW, PER, & nutrient digestibility while ↑ FCR over 50% ↓ Body crude protein, ash, Ca, Mg, P & their utilization with ↑ replacement |
[183] |
| Beluga (Huso huso) | Hydrolyzed canola protein | 0, 300, 400 & 500 mg of protein |
500 mg | WG, SGR, PER & expression of genes highest up to 500 mg but lowest protein amount | [184] | |
| Spotted rose snapper (L. guttatus) | CM | 0, 150, 300, & 450 g/kg of CM protein |
70 days | 300 g/kg | ↓SGR above optimum level ↑ CM protein = ↓FGR, ↓PER, ↓ ADC No effect on SR, FI & hematological parameters |
[185] |
| Rainbow trout (O. mykiss W.) | Canola protein isolate | 0, 25, 50%, 75 & 100% |
70 days | 75% | No negative impact on FGR, FI, FCR & palatability up to 100% ↑ WG, FCR, PER up to 75% No effect on SR & whole body composition |
[186] |
Abbreviations: 4E-BP2: Eukaryotic initiation factor 4E binding protein 2, ADC: Apparent digestibility coefficient, AKT: Protein kinase B, CAT: Catalase, FCR: Feed conversion ratio, FER: Feed efficiency ratio, FGR: Fish growth response, FI: Feed intake, FW: Final weight, GPx: Glutathione peroxidase, IGF-I: Insulin like growth factor I, PepT1: Peptide transporter 1, PER: Protein efficiency ratio, S6K1: Ribosomal protein S6 kinase 1, SGR: Specific growth rate, SOD: Superoxide dismutase, SR: Survival rate, TOR: Target of rapamycin, WG: Weight gain.
3.6. Peanut meal
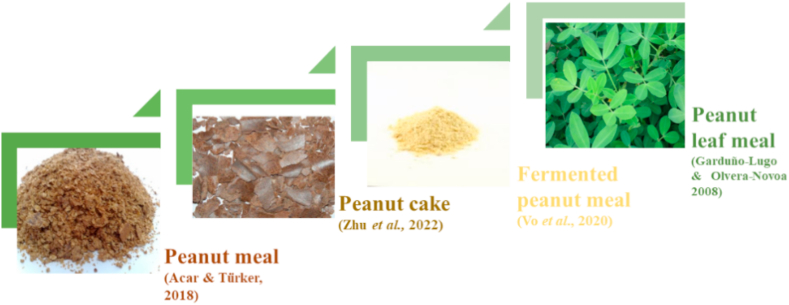
The peanut belonging to the legume family has high oil and protein content with high palatability as compared to other plant protein sources [69,70] (Fig. 6). The extracted peanut oil is used by humans [71] and left over peanut meal (PM) is rich in protein (55.94 %) and amino acid arginine (6.15%). It also has ANFs, i.e. trypsin, tannins, and amylase inhibitors, that show adverse impacts on fish health. Various methods are used to lower or eliminate these ANFs that are roasting and germination and a combination of both to enhance the peanut quality [72] (Table 6).
Fig. 6.
Different peanut products to replace fishmeal.
Table 6.
Peanut products as substitute for fishmeal in diet of different fish species.
| Fish Species |
Peanut Products | Tested Inclusion levels |
Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Hybrid Grouper (E. fuscoguttatus ♀ × E. lanceolatus ♂) |
Peanut cake (PNK) | 0, 6.6, 13.2, 19.8, 26.4, 33.0 & 39.6% of PNK by replacing 0, 11, 22, 33, 44, 55 & 66% of FM |
56 days | 15% | FCR & SGR ↑ over 33% TP first ↓ then ↑, TC ↓ Fat deposition in liver ↑ Harmful bacteria of gut ↑ over 44% WGR ↓ over 22% |
[75] |
| Hybrid Grouper (E. fuscoguttatus ♀ × E. lanceolatus ♂) |
Peanut meal (PM) | 0, 10, 20, 30, 40 & 50 % |
70 days | 50% | At higher levels ↑ in innate immune-related enzyme activity & gene expression, but ↓ antimicrobial peptide gene expression ↑ Gut pathogenic bacteria & ↓ Gut beneficial bacteria |
[70] |
| Barramundi (L. calcarifer) | Germinated, fermented & untreated PM | 15%, 30% & 60% GMP, FMP, UMP | 56 days | 60% FPM |
At 60% GPM & UPM, ↑ liver lipid droplets & myodigeneration but ↓ acidic mucins in distal gut Stress increase plasma cortisol |
[187] |
| Rainbow Trout (O. mykiss) | PM | 0, 10, 20 & 30% | 60 days | 10% | No negative impacts on growth performance, feed utilization, hematological & serum biochemical parameters up to 10% | [188] |
| Nile tilapia (O. niloticus L.) | Peanut leaf meal | 0, 10, 20 & 30% | 126 days | 20% | ↓Growth performance & ↑ lipid content over 20% No effect on SR |
[189] |
Abbreviations: FCR: Feed conversion ratio, SGR: Specific growth rate, SR: Survival rate, TC: Total cholesterol, TP: Total protein, WGR: Weight gain rate.
Peanut cake, obtained from oil extraction process by squeezing oil [73], has great nutritional value specifically rich protein content in range of 410–450 g/kg (dry matter) but imbalance amino acid content. In comparison with PM, peanut cake contains slightly lower protein and higher lipid quantity, while other nutritional factors are same [74]. [70,75] investigated that 50% PM and 15% peanut cake, respectively, could be the optimum FM replacement levels when fed to hybrid grouper.
3.7. Guar meal
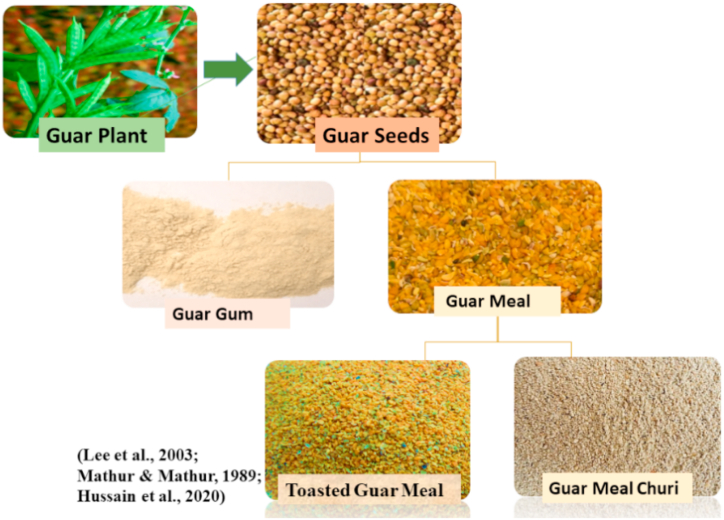
Guar, Cyamopsis tetragonoloba, from family Fabaceae, is a leguminous plant commonly called cluster bean (Fig. 7). It is low emission summer crop and used in feed of farm animals [76]. It occurs commonly in the East and South East of Pakistan and North and North West of India [77]. Guar meal is comparatively cheap high protein product gained after guar gum extraction that is valuable for producers of livestock [78]. Major ANF present is Galactomannan which is d-mannose polymer linked to d-galactose via α-1, 4 linkages attached to alternate β-1, 6 mannose units. This meal contains glactomannan 75–85%, moisture 8–14%, protein 5–6%, fiber 2–3% and ash 0.5–1%. The lowest crude protein present is 50% in guar meal in comparison with SBM which has 48% crude protein [79]. Guar meal contains tryptophan 0.68%, methionine 0.73%, cystine 0.79%, meth + cystine 1.51%, threonine 1.94%, isoleucine 2.31%, valine 2.35% lysine 3.22%, arginine 3.62%, and leucine 3.7% [80,81]. Guar meal is also free from aflatoxin, Salmonella, and E. coli as well as a good binding agent in feed formation [82]. It contains about 1.3 g per kg of saponin content [83]. Gum presence and ANFs limit its use in fish feed as an alternate plant protein source. Guar gum is largely utilized as thickner, stabilizer, and emulsifier in oil and food industries. After the gum extraction, guar seeds are toasted at high temperature to eliminate natural inhibitor trypsin that increases its digestibility and nutritional value [79] (Table 7). Similarly [84], found that when toasted guar meal was added to L. rohita, it substituted FM by 60%.
Fig. 7.
Different Guar products used for fishmeal replacement.
Table 7.
Guar Meal as substitute for fishmeal in different fish species.
| Fish species | Guar forms | Tested Inclusion levels | Duration | Optimum inclusion level | Effects | Reference |
|---|---|---|---|---|---|---|
| Rohu (L. rohita) | Toasted Guar Meal | 0, 30, 60 & 90% |
60 days | 60% | Lowest crude protein & highest moisture, fat & gross energy at 90% while highest crude protein & lowest moisture, fat & energy at 30% Gut digestive enzyme activity ↓ |
[84] |
| Nile tilapia (O. niloticus) | Guar Sprout Meal | 0, 25, 50, 75 & 100% |
60 days | 25–50% | ↓GR, SGR, FCR, PER & feed utilization over 50% SR = 100% |
[190] |
| Asian Catfish, (Pangasianodon hypophthalmus) | Guar meal | 0, 5, 10, 15 & 20% guar meal to replace SBM at 0, 25, 50,75 & 100% | 45 days | 10% | FCR & SR ↓ over 10% Adverse impacts on growth performance & feed utilization over 10% |
[191] |
| Rohu (L. rohita) | Enzyme treated guar meal |
0, 25 & 50 % guar meal Pre-treated with Protease & multienzymes separately | 60 days | 25% | ↑Growth with protease & multienzymes treated feed ↑ Gut digestive enzyme activities ↓carcass moisture & ash levels |
[192] |
| Common Carp (C. carpio L.) | Guar meal | 0, 25, 50, 75 & 100% |
84 days | 50% | ↓ADC of protein, energy & lipid over 50% Significant differences in FW, SGR, FCR, FER, PER & FI |
[193] |
| Mrigal carp (C. mrigala) | Guar meal with phytase enzyme & citric acid (CA) |
Phytase 0, 500 & 1000 FTU kg-1 & 0, 2.5 & 5% CA with 56% guar meal in each dose | 90 days | 2.5% CA & 1000 FTU kg-1 with 56 % guar meal |
Maximum growth performance, minerals & nutrient digestibility at optimum level ↓ Feed cost & nutrient's discharge via feces |
[194] |
Abbreviations: FCR: Feed conversion ratio, FER: Feed efficiency ratio FGR: Fish growth response, FI: Feed intake, FW: Final weight, PER: Protein efficiency ratio, SBM: Soybean meal, SGR: Specific growth rate, SR: Survival rate, growth rate, WG: Weight gain.
3.8. Sunflower meal
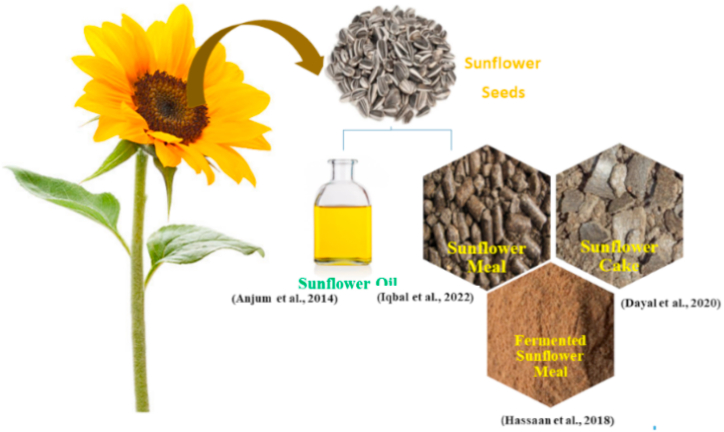
Sunflower commonly called “Sooraj Mukhi” is fourth largest crop cultivated globally and contributes up to 30% of gross domestic production of cooking oil [85] (Fig. 8). Sunflower meal (SFM) is cheap protein and energy source. It comprises of oil extracted kernels that are rich in protein and sunflower hulls that have high fiber content about 180–230 g per kg [86]. It has 36–40% of crude protein with high tryptophan and methionine content [87]. In comparison with SBM, SFM contains lower lysine and sulfur amino acids but has other amino acids specifically aspartic and glutamic acid [88]. Main factors that restrict SFM use in fish feed are arginase and protease inhibitors [89], high fiber content and high phenolic compounds mainly caffeic and chlorogenic acids that also reduce solubility of protein [90]. ANFs can be inactivated by exogenous enzymes that improve digestion and absorption of nutrients of plant based protein for aquatic animals [91] (Table 8) [92,93]. found that 50% and 25% inclusion levels were optimum when SFM and fermented SFM were substituted, respectively.
Fig. 8.
Sunflower seed byproducts as substitute of fishmeal.
Table 8.
Sunflower Meal as substitute for fishmeal in diet of different fish species.
| Fish species | Sunflower seed forms | Tested Inclusion levels |
Duration | Optimum inclusion level | Effects | Reference |
|---|---|---|---|---|---|---|
| Nile tilapia (O. niloticus) | Sunflower meal (SFM) | 0, 25, 50, 75 & 100% |
90 days | 50% | ↑Growth performance & feed utilization but body indices ↓ up to 50% ↑whole-body protein, serum total protein &albumin up to 50% but whole body lipid, SGPT & SGOT activity increase over 50%, intestinal histology also effected by SFM |
[92] |
| Nile tilapia (O. niloticus) | Fermented SFM with Saccharomyces cerevisiae & Bacillus subtilis |
(0%), YFSFM (25, 50,75%) & BFSFM (25,50, 75%) |
84 days | 25% | Serum P & Ca, cholesterol, TGC & HDL lowest at 75% LDL & blood parameters not much effected ALT & AST values lowest at 25% Best growth performance & digestibility at 25% |
[93] |
| Nile tilapia (O. niloticus) | SFM with exogenous xylanase | FM: SFM = (2:1, 1:1 & 1:2), each diet without or with 0.5 g/kg of exogenous xylanase | 84 days | 270g/kg with 0.5 g/kg of exogenous xylanase | Exogenous xylanase improved growth, digestive enzymes, nutrient digestibility, histological morphometric of liver & intestine and nutrient retention | [195] |
| Common carp (C. carpio) | SFM | 0, 25, 50, 75, & 100% |
70 days | 75% | Highest WG at 25 % while lowest at 100% Significant decrease in TGC at 100% No adverse effects on growth, body composition, or hematological & plasma biochemical indices at 75% |
[196] |
| Rohu (L. rohita) | SFM with Se Nanoparticles | 50% SFM with 0, 0.5, 1, 1.5, 2, 2.5 & 3 mg/kg NPs |
90 days | 50% SFM with 1 mg/kg Se NPs |
Best hematological parameters, nutrient digestibility, & growth performance at optimum level but highest SGR at 2 mg/kg NPs | [197] |
| Mrigal carp (C. mrigala) | SFM with Se Nanoparticles | 50 % SFM with Se NPs0, 0.5, 1, 1.5, 2, 2.5 & 3 mg/kg | 90 days | 50 % SFM with Se NPs 1.5 mg/kg |
Best body composition & increase in mineral absorption at optimum level Maximum Fe, Mn & Cr absorption at 2 mg/kg Highest absorption of Mg & Zn at 1 mg/kg |
[198] |
Abbreviations: ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, Ca: Calcium, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, P: Phosphorus, SGOT: Glutamic-oxalacetic transaminase, SGPT: Serum glutamic-pyruvic transaminase, SGR: Specific growth rate, TGC: Triglycerides, WG: Weight gain.
3.9. Moringa oleifera meal
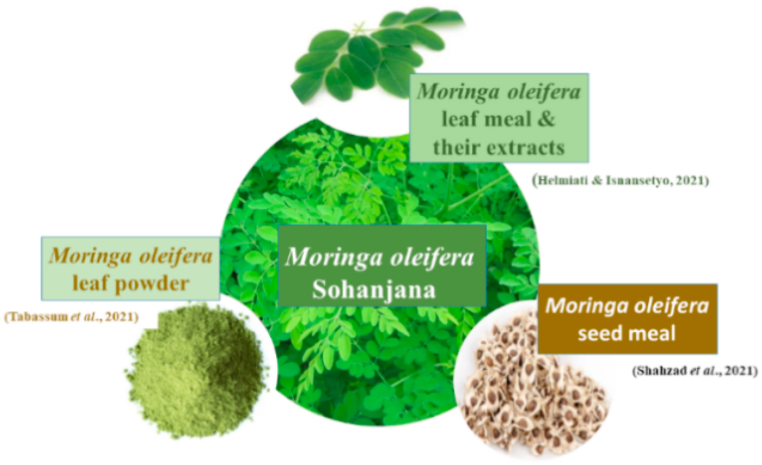
Moringa oleifera, from family Moringaceae is a slender tree having softwood [94] (Fig. 9). It is largely found in Southern Punjab, commonly known as “drumstick or sohanjana” in Pakistan [95]. It is deciduous tree and ranges in size from 10 to 12 m [96]. In Pakistan, moringa leaves are utilized in tea, pods as vegetable, and roots to prepare pickle [97]. Typically, it is a multipurpose plant having several use in livestock, agriculture, pharmaceutics, human and other biological systems [98]. The pods and leaves have high mineral content like zinc, calcium, phosphorous, magnesium, manganese in small quantities, and are efficient source of vitamins (specifically C, B), proteins, amino acids, betacarotene, flavinoids and several phenolics [99]. Moringa fat free kernel and raw kernel meals contain 61.4% and 36.7% crude protein, respectively [60] while in leaves crude protein is 23–30% and crude fiber is less than 5.9% [100], resulting its good palatability for livestock and fish [101]. There are ten EAAs present in M. oleifera including lysine, histidine, leucine, isoleucine, valine, methionine, phenylalanine, tyrosine, tryptophan, and threonine [102]. ANFs present are tannins, saponins, lignins, phytates and oxalates. Tannins react with amylase and trypsin enzymes resulting non digestible compounds that decrease palatability and feed intake in fish [101]. These ANFs also give bitter taste resulting in low acceptability to aquatic animals [60]. The nutritional value of M. oleifera can be enhanced by fermentation process [103], phytase pretreatments [104], and by introducing organic acids i.e. citric acid to plant parts [68] (Table 9). For instance Refs. [68,105,106], exhibited that M. oleifera leaf meal (MOLM) replaced 10% FM, M. oleifera seed meal (MOSM) substituted 36% with 950 FTU per kg phytase and MOSM replaced 35% with 3% citric acid, respectively.
Fig. 9.
Different forms of Moringa oleifera used to substitute fishmeal.
Table 9.
Moringa oleifera as substitute for fishmeal in diet of different fish species.
| Fish Species |
Moringa oleifera forms | Tested Inclusion Doses |
Duration | Best Inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Mrigal carp (C. mrigala) | Moringa oleifera leaf meal (MOLM) | 0, 10, 20, 30, 40 & 50% |
90 days | 10% | Maximum growth performance & nutrient digestibility at 10% RBCs, WBCs, Hb ↓ = ↑ MOLM |
[105] |
| Common carp (C. carpio) |
Moringa oleifera seed meal (MOSM) + MOLM with phytase pretreatment |
36% moringa + phytase levels 0, 500, 650, 800, 950, 1100 & 1250 FTU kg−1 |
70 days | 36% with 950 FTU per kg | ↑ WG, SGR, FCR, nutrient digestibility & mineral absorption up to optimum level ↓ Discharge of minerals & nutrients in water bodies |
[199] |
| Mrigal carp (C. mrigala) | Citric Acid Acidified MOSM |
35 % MOSM with 0, 1, 2, 3, 4 & 5% CA levels |
90 days | 35% with 3% CA | Improved mineral absorption, carcass composition & hematological parameters up to optimum level, Maximum crude protein & fat content up to 3% | [68] |
| Nile tilapia (O. niloticus) | MOSM + MOLM with phytase | 36% moringa with PHY levels 0, 500, 650, 800, 950, 1100 & 1250 FTU per kg |
70 days | 36% with 950 FTU per kg | Maximum values of RBCs, PLT & Hb at optimum level Enhanced hemato-immunological parameters, body composition, fish health & growth gene expressions |
[106] |
| Gibel carp (C. auratus gibelio) | Fermented Moringa oleifera leaf meal (FMOLM) | a basal diet with 10% FM & others 20, 40, 60 | 50 days | 40% | ↑ growth, antioxidant & immune response & regulate expression of immune-related genes & ↑ disease resistance against A. hydrophila via TLR2 pathway up to 40% | [200] |
| Red tilapia (Oreochromis sp.) | FMOLM | 0, 10, 20 & 30 % |
60 days | 20% | At 20%, hematocrit & leukocrit ↑, phagocytic activity & phagocytic index ↑ but suppression in monocyte Increase in lymphocytes & total plasma protein |
[201] |
| Catfish (C. gariepinus) | MOLM | 0, 0.5, 1.0 & 1.5% |
56 days | 1.5% | Best hematological parameters at 0 & 1.5% hematological performance increase by MOLM | [202] |
| Rainbow trout (O. mykiss) | MOLM | 0, 10, 20, 30 & 40% |
90 days | 20% | ↑ WG & SGR but ↓ FCR up to 20% ↑ Blood Proteins & SOD, CAT, & GPx activity ↓AST & ALT activity ↑ IL-6, IL-8 & IL-10 expression compared to β-actin gene |
[203] |
Abbreviations: ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, CAT: Catalase, FCR: Feed conversion ratio, GPx: Glutathione peroxidase, Hb: Hemoglobin, IL-10: Interleukin −10, IL-6: Interleukin −6, IL-8: Interleukin −8, PLT: Platelets, RBC: Red blood cell, SGR: Specific growth rate, SOD: Superoxide dismutase, TLR2: Toll-like receptors 2, WBC: White blood cell, WG: Weight gain.
3.10. Almond meal
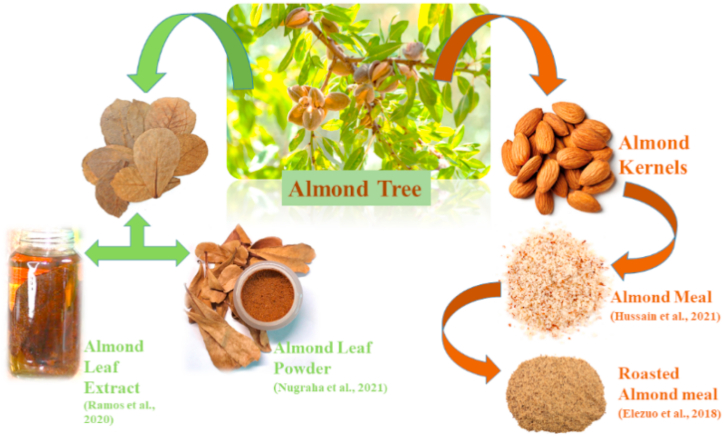
Terminalia catappa, from family Combretacea, is commonly known as wild almond, sea almond, Indian almond or tropical almond (Fig. 10). The annual fruit production is about 700,000 tons worldwide [107]. It is mostly found in Taiwan, the Malay Peninsula, tropical Asia and India. Almond nut or seed, Prunus amygdalus, is categorized into two groups: (1) Bitter almond “Prunus amygdalus amara” (2) Sweet almond “Prunus amygdalus dulcis” [108]. The sweet almonds provide edible nuts [107]. Almond kernels have brown skin layer covering with an intermediate shell and an outer hull. They have 24.5% crude protein, 6% ash, 36% crude protein and rich in vitamin E as well as phenolic compounds. Almond meal also contains considerable oil quantity because extraction of oil is done by cold pressing method. Owing to unique antioxidant effects, almond meal boosts immune system, making it effective substitute for FM [109]. Available research data has shown that almond meal is under study as a possible alternative of FM in diet of different fish species (Table 10). Up to 40% almond meal based diet significantly improved the growth performance, nutrient absorption and hematology of L. rohita [68].
Fig. 10.
Different almond meals used to replace fishmeal.
Table 10.
Almond meal as substitute for fishmeal in diet of different fish species.
| Fish species | Almond forms | Tested inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Nile tilapia (O. niloticus) | Almond leaf meal | 0, 25, 50, 75 & 100% | 70 days | 25–100% | ↑ WG, SGR, FCR ↑ survival against Salmonella typhi |
[204] |
| Rohu (L. rohita) | Almond meal | 0, 20, 40, 60, 80 & 100% | 70 days | 40% | Significant ↑ in FGR, nutrient digestibility & hematology up to 40% Hb & RBCs ↓ over 40% |
[205] |
| Snakehead (Ch. striata) | Almond leaf powder | 0, 0.25, 0.50 & 0.75 g L-1 | 40 days | 0.50 g/L | ↑ survival, SGR, FCR, PER Normal hematological & physiochemical parameters up to 0.5 g/L | [206] |
| Leaf fish (Monocirrhus polyacanthu | Almond leaf extract | 0.25, 0.50, 0.75, 1.00 & 1.25 g/L | 15 days | 0.75 g/L | ↓Mortality & ↑ growth performance ↑WG, SGR, length, survival up to 0.75 g/L ↑water quality parameters value |
[114] |
| African catfish (C. gariepinus) | Roasted tropical almond meal | 0, 25, 50, 75 & 100% | 105 days | 75% | ↑WG, ↑SGR, ↓ FCR at 75% ↓WG, ↓SGR & ↑FCR at 100% Economic depression over 75% |
[109] |
| Cardinal tetra (Paracheirodon axelrodi) | Tropical Almond leaves |
0, 0.5, 1.5 & 2.5 g/L | _ | 0.5–1.5 g/L | ↑WG, length, survival (100%) | [115] |
Abbreviations: FCR: Feed conversion ratio, FGR: Fish growth response, Hb: Hemoglobin, PER: Protein efficiency ratio, RBCs: Red blood cells, SGR: Specific growth rate, WG: Weight gain.
Terminalia catappa leaf extract (TCE) has been utilized in culture of aquatic animals by farmers as an eco-friendly substitute due to high levels of tannins and phenolic compounds [110]. Although it controls fungal, parasitic and bacterial infections [[111], [112], [113]], it also works as a behavioral stimulator for spawning substrate and reproduction [114]. Improvement in growth of fish, survival rate as well as water quality parameters have been noticed by TCE use for Leaf fish, Monocirrhus polyacanthus, & Cardinal tetra, Paracheirodon axelrodi, [114,115].
3.11. Black cumin seed meal
Nigella sativa, commonly called black cumin seed is from family Ranunculacea and is a medicinal herb with remedial characteristics (Fig. 11). It has been utilized for therapeutics purposes for over 2000 years. N. sativa is indigenous to Southern Europe, Southwest Asia and North Africa and is cultured in different regions of world including Middle Eastern Mediterranean region, Saudi Arabia, Turkey, Pakistan, India, and South Europe [116,117]. Therapeutic effects of black seeds have been recorded as anticancer, antihistaminic, antidiabetic, antioxidant, antiviral, antifungal, antiprotozoal, antibacterial, anticholesterol, anti-inflammatory and immonomodulator [118]. Black seed comprise of crude protein 20.8%, lipids 34.8%, ash 3.7%, carbohydrate 33.7% and 7.0% moisture [119]. Black seeds and oil have been utilized commonly for treating many health conditions belonging to immune, respiratory and cardiovascular system, digestive tract, liver and kidney functions [117]. Most of its pharmacological characters are because of thymoquinone (18.4–24.0%) that is main bioactive element of its volatile oil (0.4–2.5% of seed oil) [117,120]. Black cumin seed cake (BCSC) is a byproduct of agro-industry obtained after oil extraction from seeds and is nutritionally valuable for animals. It has crude protein 27.5% and crude oil 14.8% [120]. As a whole, owing to local availability, low price, and effectiveness, the use of N. sativa and its oil in aquaculture has been urged as a feed ingredient for better fish health, making them more resistant to bacterial infections such as Burkholderia cepacia [121] and Aeromonas hydrophila [122] (Table 11).
Fig. 11.
Different Black cumin seed forms used to replace fishmeal.
Table 11.
Black cumin seeds as substitute for fishmeal in diet of different fish species.
| Fish species | Black Seed forms | Tested Inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Nile tilapia (O. niloticus) | Black cumin powder against Burkholderia cepacia | 30 g/kg of NS-incorporated diet | 45 days | _ | Protective effects on immune response, antioxidant capacity, hepato-renal function, modulation of gene expression, antibacterial activity against Burkholderia cepacia | [121] |
| Tilapia (O. niloticus) | Black cumin flour against Aeromonas hydrophila | 0, 20, 35, 50 and 65 g/Kg | 90 days | 50 g/kg | Boosted the immune system | [122] |
| Mirror carp (C. carpio var. specularis) | Black cumin seed cake (BCSC) | SBM replaced by 0%, 25% & 50% of BCSC | 63 days | < 25% | ↑BCSC level = ↓Growth & feed intake No significant impact on serum total protein, globulin, ALT, AST & glucose ↓ Cholesterol & triglyceride at > 25% No significant impact on palmitic acid, stearic acid, linolenic acid, linoleic acid, SFA, MUFA & PUFA of muscle |
[207] |
| Common carp (C. carpio) | Black Seed | Control, 0.25, 0.5 & 1% | 60 days | _ | Black seed remove negative effects of glyphosate exposure Stable levels of biochemical serum parameters & cholesterol, higher levels of immune defences & antioxidant enzymes, lower lipid peroxidation, metabolic enzymes & cortisol levels than control fish |
[208] |
| Nile tilapia (O. niloticus) | Black seed meal (BSM) | 0, 25, 50, 75 & 100% of BSM alternative to SBM | 60 days | 50% | ↓Growth performance & feed utilization above 50% No impact on carcass crude protein ↑Ash content = ↑ BSM Feed costs decrease upto 50% |
[209] |
| Rainbow trout (O. mykiss) | Methanolic extract of black cumin | 0, 0.1 & 0.5 g/kg | 30 days | _ | Stimulate some innate humoral immune responses, but ineffective for cytokine-related gene trancriptions, ↓FCR, ↑Respiratory burst activity | [210] |
| Rainbow Trout (O. mykiss, Walbaum) | Black Cumin Seed Powder | 0, 0.5, 1, 2.5, 5, 10 & 20 g/kg | 60 days | _ | Positive differences in plasma protein, MCH, MCHC, RDW_SD, PLT and MPV at all levels | [211] |
| African catfish (C. gariepinus) | Black seed | Control, 4-nonylphenol (NP)-treated, 1, 2.5 & 5% black seed treated groups | 21 days | _ | Black seed with 4-NP significantly decrease nephrotoxic effect of 4-NP & sustain normal kidney function & structure | [212] |
Abbreviations: SBM: Soybean meal, ALT = Alanine aminotransferase, AST = aspartate aminotransferase, SFA=Saturated fatty acids, MUFA = Monounsaturated fatty acids, PUFA= Polyunsaturated fatty acids, FCR= Feed conversion ratio, MCH = Mean cell hemoglobin, MCHC = Mean cell hemoglobin concentration, RDW-SD= Red cell distribution width, PLT= Platelet, MPV = Mean platelet volume.
3.12. Lupin meal
Lupins plants from genus lupinus are found worldwide having many species but mostly cultivated species are only four including narrow-leaf lupin (L. angustifolius), white lupin (L. albus), pearl lupin (L. mutabilis) and yellow lupin (L. luteus) [123] (Fig. 12). Lupin seed meals (LSMs) are included as supplement globally in livestock feed formulation due to its good nutritious value and potential to grow on infertile lands [124]. LSM has good protein content, high dietry fiber and locally available at low price [125]. Comparatively, they have low ANFs including tannis, phytic acid, oligosaccharides and alkaloids than present in other plant based ingredients. Owing to their availability and suitability, LSMs are routinely utilized in aquafeed [126]. Pretreatment of lupin with Lactobacilli enhanced its nutritional value as well as micronizatin process increased starch digestibility and destroyed the ANFs [127,128]. The advantageous influence of lupin in comparison with FM has been prescribed in diets of different fish species (Table 12). Lupin meal can be used in rainbow trout up to 30% without adverse effects on growth performance, hematological and serum biochemical markers [129].
Fig. 12.
Mostly cultivated lupin species.
Table 12.
Lupin meal as substitute for fishmeal in diet of different fish species.
| Fish Species |
Lupin forms | Tested Inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Rainbow trout (O. mykiss) | Lupin meal | 0, 15, 30, 45, & 60% | 60 days | 30% | Best growth performance upto 30 % ↓ Heamatocrit & MCV at 60% ↓TP, TGC, cholesterol, ALP, LDH in all treated groups |
[129] |
| Cobia (Rachycentron canadum) | Lupin kernel meal (LKM) | 0, 200, 400, 600 & 800 g/kg of FM replaced by LKM | 56 days | 200 g/kg of FM replaced by LKM | ↓FBW, SGR, FI & ADCs = ↑LKM ↓Haematological indices & histopathological changes ↑ AST & ALT |
[213] |
| Nile tilapia (O. niloticus) | Different UK lupin meal cultivars | Yellow lupin, blue lupin, fermented yellow lupin & fermented blue lupin | 49 days | Dehulled (kernel meal) Lupinus luteus cv. Pootalong | Significant increase in WG, SGR, FCR, PER and K with fermented meals Improved enterocyte height |
[214] |
| Barramundi (L. calcarifer) | Fermented lupin | 0, 30, 45, 60 & 75% | 61 days | _ | Fermentation decresed anti-nutrients & upgraded amino acid profile of lupin ↑ in final weight & length of Barramundi |
[128] |
| Gilthead sea bream (Sparus aurata L.) | Micronized lupin seed meal (MLSM) | 10, 20 & 30% of raw lupin & 20 & 30% MLSM |
84 days | MLSM | MLSM promote significantly higher growth rates than raw lupin seeds | [127] |
MCV = Mean cellular volume, TP = total protein, TGC = triglyceride, ALP = alkaline phosphatase, LDH = lactate dehydrogenase, FBW = final body weight, SGR = specific growth rate, FI = feed intake, ADCs = Apparent digestibility coefficients, ALT = Alanine aminotransferase, AST = aspartate aminotransferase, WG = weight gain, SGR = specific growth rate, FCR = feed conversion ratio, PER = protein efficiency ratio K = condition factor.
3.13. Rice (rice protein concentrate and rice distiller's grains)
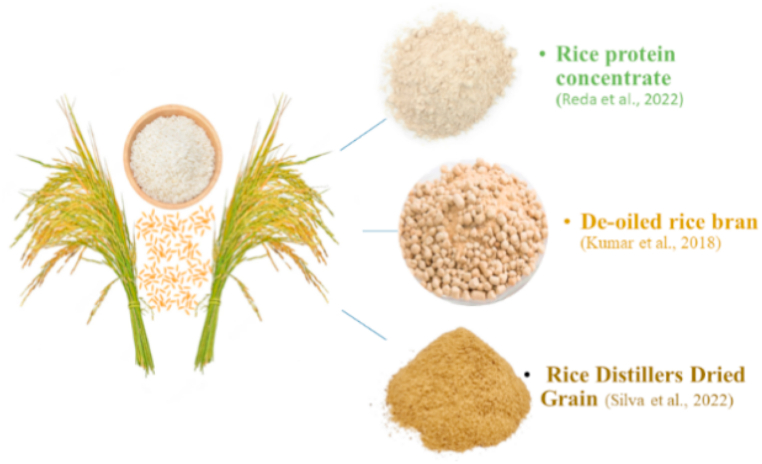
Rice is an important food crop globally. Rice protein concentrate (RPC), a byproduct of rice processing, has great nutritional value as it contains about 66–70% protein and 10–11% lipid, becoming an ideal replacer of FM (Fig. 13). But, it is deficient in lysine that limits its use in aquafeeds. Recently, RPC has become available at commercial level for use in fish and animal feed [130,131]. RPC can replace up to 25% of the FM protein in the diet of O. niloticus, enhancing the antioxidant capacity, immunocompetence, and disease resistance [132]. Enzymatic rice protein is a treated product of RPC via action of polysaccharides and proteases have many monosaccharides and short peptides which improve digestion and growth [133,134]. This processing also reduces adverse effects of rice protein due to disulfide bonds i.e. reduction in growth as well as immune response of fishes [135,136]. Fermented rice protein (FRP) is obtained by microbial fermentation has high acid, good solubility, low viscosity and heat stability [137]. Rice or rice bran fermentation by different types of bacteria is reported for use as food for animals. However, only few studies on use of FRP in aquatic animals feed are present. Another byproduct is rice distillers dried grain (rice DDG), obtained from rice grains during ethanol production, have 70.4% crude protein (about 3.6% lysine), 2.9% crude fiber and 9.7% lipid [138], also utilized in aquafeed. De-oiled rice bran (DORB) comprise of 6–10% of rice by weight with greater nutritional value than many other agri-products. It is free of fat, also called rice polish being good substitute for FM [139] (Table 13). DORB has crude lipid 0.33%, crude protein 15.30%, crude fiber 14.45%, nitrogen free extract 63.88% and ash 6.01% [140].
Fig. 13.
Rice byproducts used to replace fishmeal.
Table 13.
Rice as substitute for fishmeal in diet of different fish species.
| Fish species | Rice forms | Tested Inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Nile tilapia (O. niloticus) | Rice protein concentrate | 0, 25, 50 & 75% | 5 months | 25% | ↓Antioxidant capacity & immune indices over 25 % ↑mRNA levels of intestinal cytokines at 25% ↓ Gut microbial diversity ↑ Immunocompetence & disease resistance |
[132] |
| Channel catfish (Ictalurus punctatus) | Enzymatic rice protein | 0, 2.5, 5.0 & 7.5% | 56 days | 2.5 % | Improved intestinal morphology & digestion at 2.5% Upset gut microbiota equilibrium, results in damage to gut mucosa & inflammation at 7.5% |
[215] |
| Hybrid groupers (E. fuscoguttatus ♀× E. lanceolatus♂) | Fermented rice protein (FRP) | 10, 30 & 50 % | 56 days | 10% | ↑Activities of intestinal digestive enzymes & brush border enzymes by FRP ↓Expression of immune-related genes & relative abundance of Enterococcus & Bacteroides |
[216] |
| Silver catfish (Rhamdia quelen) | Rice ethanol distillery residue | 0, 25 & 50% | 60 days | _ | Reduced final growth, deposition of body protein & increased free liver amino acids | [217] |
| Red Seabream (P. major) | Rice Distillers Dried Grain | 0, 5, 10, 15, 20 & 25% | 70 days | 25% | No negative effects on overall body performance | [218] |
| Rohu (L. rohita) | De-oiled rice bran | 0, 20, 30, 40, 50 & 60% | 60 days | 40% | Significant effect on digestive, metabolic enzyme activities & haematological parameters High oxidative stress activity at 60% increased metabolic activity & immune responses upto 40% |
[219] |
3.14. Brewer's spent grains and spent yeast
The breweries produce large volumes of byproducts including spent grains, spent yeast and spent hops, that are potentially used as aquafeed. The recent exponential rise in number of global small brewing industries produce huge amount of byproducts which are available at low or no price. Brewers' spent grains (BSG) are the biggest byproduct of beer brewing process accounts for 70–85% of total byproducts, almost 1/3 of total malt weight from production of beer, and 40 million MT of BSG are generated worldwide annually. BSG is a lignocellulosic residue that mainly comprise of fiber (30–50%), protein (19–30%) and also lipids, vitamins and minerals [[141], [142], [143], [144]]. Brewers’ spent yeast (BSY) is the second main byproduct from brewing process and also a source of protein (40%–50% DW) minerals and vitamins [145], and several other bioactive elements including mannan oligosaccharides, b-glucans, and nucleic acids [146]. Many studies have shown that BSG and BSY can successfully substitute a part of dietary fishmeal in different fish species (Table 14), such as in Oncorhynchus mykiss where, 15% BSG + BSY and 20% yeast can replace FM.
Table 14.
Brewery byproducts as substitute for fishmeal in diet of different fish species.
| Fish species | Brewery byproducts | Tested Inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Rainbow trout (O. mykiss) | Dry & hydrolyzed spent yeast (DSY & HSY) & spent grains (DSG & HSG) | 10 & 20% of DSY, HSY & 7.5% & 15% of DSG & HSG to the basal mixture | 60 days | 20% yeast & 15% spent grain | ↑ final weight, protein digestibility & food conversion, No change in muscle nutritional composition | [220] |
| European seabass (Dicentrarchus labrax) | Fermented brewer's spent grain (BSG) | Control, 0.4 & 0.8% BSG-extract pretreated + direct inclusion | 66 days | Pretreated 0.4% BSG-extract | ↑ Dietary reducing sugars content, antioxidant activity & cellulase and xylanase activities No effect on growth performance & whole-body composition ↑Feed efficiency & protein utilization |
[221] |
| Rainbow trout (O. mykiss) & Gilthead seabream (S. aurata) |
Spent yeast & Spent grain | Dry & hydrolyzed spent yeast & spent grains | 30 days | 20–30% brewers' spent yeast & spent grain | Good protein, lipid & amino acid digestibility | [222] |
| Striped catfish, (Pangasianodon hypophthalmus) | Brewer's spent grains (BSG) | SBM replaced by 0, 25, 50, 75 or 100% of BSG | 50% | Improvement in growth performance, nutrient utilization and feed conversion upto 50% | [223] | |
| Nile Tilapia, (O. niloticus) | Brewer's waste | 25, 50, 75 & 100% | 70 days | 50% | ↓ Feed intake & utilization with increase level no adverse effect on growth and feed utilization upto 50% | [224] |
| Thai Panga (P. hypophthalmus × Pangasius bocourti) |
Brewer's yeast (Saccharomyces cerevisiae) | 30, 45, 60 or 75% | 9 months | 45% | Improved growth performance & immune response No effect on feed efficiency, blood hematology & meat quality ↑ lysozyme activity & total immunoglobulin (Ig) |
[225] |
3.15. Distiller's grains or distiller's dried grains with solubles
Distiller's grains (DG) or distiller's dried grains with solubles (DDGS) are the byproducts of the distillation process of cereals for production of ethanol. These are alternate protein sources. Starch rich cereals including corn, cassava, rice, millet, wheat, barley and sweet potato are sources of DDGS. These are used potentially for ethanol production [147]. DDGS are rich in protein, fat and phosphorus with low cost and high yield. They contain 30–35% crude protein, 10% crude fat and about 11% crude fiber. It might be utilized as superior source of protein for production and breeding [148]. DDGS are nutritionally valuable due to B vitamins, an unknown growth promotor factor as well as saccharides obtained during the fermentation [149]. Excluding fiber, other ANFs are absent in DDGS which interrupt the health and growth of fishes, as present in other plant protein sources, e.g., SBM, CSM, CM or SFM [150,151]. Further processing increases protein content in DDGS producing higher protein (∼40%) distillers dried grains (HP-DDGS). Annually over 40 million MT DDGS are obtained from the process of distillation in the United States alone and are a promising source for aquafeeds [152]. DDGSs have yeast as well that is a potential source of nucleotides and betaglucans, that may boost immune system in fish. Attempts have been done to utilize DDGS in aquaculture sector and its propriety has been checked in both in salmonids i.e. O. mykiss and omnivorous species i.e. tilapia with positive effects [153,154] (Table 15). However, more research studies are needed to find the optimum proportions of DDGS for aquafeed.
Table 15.
Distiller's grains as substitute for fishmeal in diet of different fish species.
| Fish species | Distiller's grains | Tested Inclusion levels | Duration | Optimum inclusion Level |
Effects | Reference |
|---|---|---|---|---|---|---|
| Hybrid grouper (E. fuscoguttatus ♀ × E. lanceolatus ♂) | Corn distillers dried grains with solubles (DDGS) | 6.67, 13.33, 20, 26.67 & 33.33% | 56 days | 7.80% | ↑WGR & SGR upto 13.33% ↓ Amylase activity in gut, ↓mRNA levels of anti-inflammatory factors tgf-β1 & il-10, ↑mRNA levels of pro-inflammatory factors il-1β & tnf-α, microvilli damage = ↑DDGS |
[226] |
| Grass Carp (Ctenopharyngodon idellus) | Dried Distillers Grains with Solubles (DDGS) | Native DDGS20 & DDGS30, US-imported DDGS20 & DDGS30 | 60 days | US-imported DDGS30 | US-imported DDGS30 = ↑ growth via regulating genes involved in myogenesis & hypertrophy ↑Raw muscle collagen but negatively influence antioxidant capacity & cooked muscle texture |
[227] |
| Yellow Perch (Perca flavescens) | High-protein distillers dried grains (HP-DDG) | 25, 50, 75 & 100% | 105 days | 50% | ↓Crude protein content & amino acids = ↑HP-DDG = ↓ growth | [228] |
| Common Carp (C. carpio) | Distillers dried grains with solubles (DDGS) | 0, 25, 35% | 49 days | _ | No effects on growth parameters, flesh quality & blood biochemical profile, ↓ in liver tissue, CAT, GSH, MDA & carbonylated protein Variation in microbial density |
[229] |
| Striped catfish (P. hypophthalmus) | High protein distillers dried grains | 0, 25, 50 & 75% | 98 days | 25% | ↓SGR, ↑FCR, ↓whole-body protein content & plasma proteins, ↑lipids | [230] |
| Turbot (S. maximus) | Corn distillers dried grains with solubles | 10, 17.5, or 25% | 84 days | _ | ↓Growth performance & impaired overall nitrogen & energy metabolism | [231] |
| Minor carp (Labeo bata) | Distiller's grain soluble | Brewery waste (test diet) &SBM (reference diet) | 60 days | _ | No effect on survival, growth parameters & biochemical composition of whole body tissue by brewery waste ↓ Feed cost |
[232] |
WGR = weight gain rate, SGR = specific growth rate, CAT = catalase, GSH = glutathione, MDA = malondialdehyde, SBM = soybean meal.
4. Conclusion
Natural conventional as well as non-conventional plant products are receiving more attention as promising substitutes due to their cheap availability in large quantities and predictable supply all year round but they are mostly replaced partially at specific levels in aquafeed. Above their optimum level of replacement, they exert negative effects on fish growth performance, body composition, metabolic activities and other biological parameters due to their ANFs. These factors may reduce by different methods such as heating, washing, solvent extraction, fermentation and enzymatic pretreatments. Also, incorporation of enzymes or bacteria into the plant proteins may increase feed utilization as these bacteria and enzymes stabilize the ANFs in the intestine of fish. Plant use in fish feed also establishes a good relationship between aquaculture and agriculture and can provide augmented production of aquaculture in a cost effective, eco-friendly and sustainable way. In the future, competition will increase for natural resources due to climate change, population growth and bio-economy development. In this case, aquaculture will play a key role in fulfilling the global demands for protein, elaborating innovating technology and exploring the use of alternative sustainable feed ingredients.
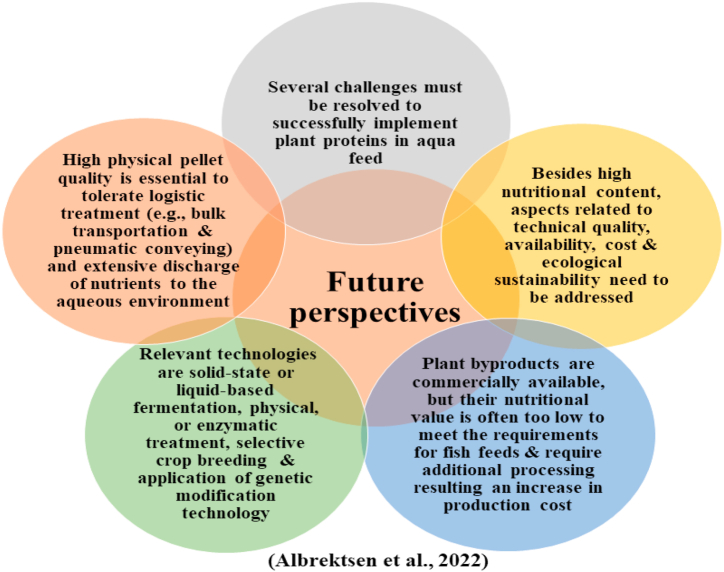
5. Future recommendations
There is an urgent need to find compatible and efficient techniques by which these negative factors are removed from plant products and improving their digestibility and palatability as FM. Moreover, alternative plant protein sources and specific methods and techniques to optimize the nutritional composition of plant feeds should be determined. Additionally, research should also look at potential nutritional issues in fishes caused by consuming more plant proteins. Furthermore, by collating all the available knowledge, establishing a coordinated research effort through strategic planning is necessary to improve the nutritional content of plant feed ingredients in anticipation of increased usage in aquaculture. While plant components might lower feed prices, relevant economic strategy and incentive are needed to encourage feed millers to produce feed at lower costs. These measures will ultimately minimize the cost and dependence on naturally-sourced aquatic feed while increasing sustainability and resilience in aquacultural production (Fig. 14).
Fig. 14.
Future perspectives of using plant-based protein sources.
Authors declaration statement
According to all authors, there is no conflict of interest with the publication of this paper.
Role of funding source
The authors are grateful to the HEC Pakistan for funding Projects No. 20–4892/NRPU/R&D/HEC/14/1145 and 5649/Punjab/NRPU/R&D/HEC/2016 at the Department of Zoology, Government College University Faisalabad, Pakistan, for the provision of facilities. We thank the Cynthia and George Mitchell Foundation, University of California, Santa Cruz, United States, for partially supporting the work. We also thank the University of California Santa Cruz, Dean of Social Sciences and Executive Vice Chancellor.
Ethical statement
No ethical approval is required for this research.
Data availability statement
Not applicable.
CRediT authorship contribution statement
Syed Makhdoom Hussain: Writing – original draft, Supervision, Investigation, Conceptualization. Aumme Adeeba Bano: Writing – original draft. Shafaqat Ali: Writing – review & editing, Software, Data curation. Muhammad Rizwan: Writing – review & editing, Software, Data curation. Muhammad Adrees: Writing – review & editing, Software, Data curation. Ameer Fawad Zahoor: Writing – review & editing, Software, Data curation. Pallab K. Sarker: Writing – review & editing. Majid Hussain: Writing – review & editing. Muhammad Zubair-ul-Hassan Arsalan: Writing – review & editing. Jean Wan Hong Yong: Funding acquisition. Adan Naeem: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Syed Makhdoom Hussain, Email: drmakhdoomhussain@gcuf.edu.pk.
Jean Wan Hong Yong, Email: jean.yong@slu.se.
References
- 1.Pradeepkiran J.A. Aquaculture role in global food security with nutritional value: a review. Translational Animal Science. 2019;3(2):903–910. doi: 10.1093/tas/txz012. Pradeepkiran, J. A. (2019). Aquaculture role in global food security with nutritional value: a review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FAO “Food and Agriculture Organization” . vol. 223. FAO; Rome, Italy: 2014. (The State of World Fisheries and Aquaculture). [Google Scholar]
- 3.FAO “Food and Agriculture Organization” . Food and Agriculture Organization of the United Nations; Rome Italy: 2018. The state of world fisheries and aquaculture. Meeting the Sustainable Development Goals. [Google Scholar]
- 4.Fiorella K.J., Okronipa H., Baker K., Heilpern S. Contemporary aquaculture: implications for human nutrition. Curr. Opin. Biotechnol. 2021;70:83–90. doi: 10.1016/j.copbio.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Cho J.H., Kim I.H. Fishmeal–nutritive value. J. Anim. Physiol. Anim. Nutr. 2011;95(6):685–692. doi: 10.1111/j.1439-0396.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S., Smith A.D., Knudsen E.E. Ending overfishing while catching more fish. Fish Fish. 2015;16(4):716–722. doi: 10.1111/faf.12077. [DOI] [Google Scholar]
- 7.Beal C.M., Gerber L.N., Thongrod S., Phromkunthong W., Kiron V., Granados J., Huntley M.E. Marine microalgae commercial production improves sustainability of global fisheries and aquaculture. Sci. Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-33504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye G., Dong X., Yang Q., Chi S., Liu H., Zhang H., Zhang S. Low-gossypol cottonseed protein concentrate used as a replacement for fishmeal for juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂): effects on growth performance, immune responses, and intestinal microbiota. Aquaculture. 2020;524 doi: 10.1016/j.aquaculture.2020.735309. [DOI] [Google Scholar]
- 9.Song S.K., Beck B.R., Kim D., Park J., Kim J., Kim H.D., Ringø E. Prebiotics as immune stimulants in aquaculture: a review. Fish Shellfish Immunol. 2014;40(1):40–48. doi: 10.1016/j.fsi.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Jannathulla R., Rajaram V., Kalanjiam R., Ambasankar K., Muralidhar M., Dayal J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquacultre Research. 2019;50(12):3493–3506. doi: 10.1111/are.14324. [DOI] [Google Scholar]
- 11.Liu T., Han T., Wang J., Liu T., Bian P., Wang Y., Cai X. Effects of replacing fishmeal with soybean meal on growth performance, feed utilization and physiological status of juvenile red lip mullet Liza haematocheila. Aquaculture Reports. 2021;20 doi: 10.1016/j.aqrep.2021.100756. [DOI] [Google Scholar]
- 12.FAO . vol. 2011. 2011. (Food and Agriculture Organization of the United Nations). Rome. [Google Scholar]
- 13.Li C., Tian Y., Wang L., Zhang B., Ma Q. Effects of replacing fishmeal by raw or Lactobacillus acidophilus-fermented soybean meal on growth, intestinal digestive and immune-related enzyme activities, morphology, and microbiota in turbot (Scophthalmus maximus L.) Aquacult. Nutr. 2022;2022(6) doi: 10.1155/2022/2643235. [DOI] [Google Scholar]
- 14.Zhou Q.C., Mai K.S., Tan B.P., Liu Y.J. Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentron canadum) Aquacult. Nutr. 2005;11(3):175–182. doi: 10.1111/j.1365-2095.2005.00335.x. [DOI] [Google Scholar]
- 15.Potter L.M., Potchanakorn M. World Soybean Res Conf. III: Proceedings. CRC Press; 2022, February. Digestibility of the carbohydrate fraction of soybean meal by poultry; pp. 218–224. [Google Scholar]
- 16.Urán P.A., Aydin R., Schrama J.W., Verreth J.A.J., Rombout J.H.W.M. Soybean meal-induced uptake block in the distal enterocytes of Atlantic salmon (Salmo salar L.) Etiology of Soybean-Induced Enteritis in Fish. 2008;25 [Google Scholar]
- 17.Lin Y.H., Cheng M.Y. Effects of dietary organic acid supplementation on the growth, nutrient digestibility and intestinal histology of the giant grouper Epinephelus lanceolatus fed a diet with soybean meal. Aquaculture. 2017;469:106–111. doi: 10.1016/j.aquaculture.2016.11.032. [DOI] [Google Scholar]
- 18.Ringø E., Sperstad S., Myklebust R., Refstie S., Krogdahl Å. Characterization of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.): the effect of fishmeal, standard soybean meal and a bioprocessed soybean meal. Aquaculture. 2006;261(3):829–841. doi: 10.1016/j.aquaculture.2006.06.030. [DOI] [Google Scholar]
- 19.Mukherjee R., Chakraborty R., Dutta A. Role of fermentation in improving nutritional quality of soybean meal—a review. Asian-Australas. J. Anim. Sci. 2016;29(11):1523. doi: 10.5713/ajas.15.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H., Qu Y., Li J., Liu X., Wu R., Wu J. Improvement of the protein quality and degradation of allergens in soybean meal by combination fermentation and enzymatic hydrolysis. Lwt. 2020;128 doi: 10.1016/j.lwt.2020.109442. [DOI] [Google Scholar]
- 21.Yasar S., Tosun R., Sonmez Z. Fungal fermentation inducing improved nutritional qualities associated with altered secondary protein structure of soybean meal determined by FTIR spectroscopy. Measurement. 2020;161 doi: 10.1016/j.measurement.2020.107895. [DOI] [Google Scholar]
- 22.de Oliveira N.S., Ha N., da Cunha L., Cipriani L.A., Neto A.T., Skoronski E., Perez Fabregat T.E.H. Fermentation of soybean meal with Lactobacillus acidophilus Allows greater inclusion of vegetable protein in the diet and can reduce Vibrionacea in the intestine of the south American catfish (Rhamdia quelen) Animals. 2022;12(6):690. doi: 10.3390/ani12060690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q., Yang Z., Chen S., Zhu W., Xiao S., Liu J., Lan S. Effects of replacing dietary FishMeal by soybean meal Co-fermented using Bacillus subtilis and Enterococcus faecium on serum antioxidant indices and gut microbiota of crucian carp Carassius auratus. Fishes. 2022;7(2):54. doi: 10.3390/fishes7020054. [DOI] [Google Scholar]
- 24.Yang H., Bian Y., Huang L., Lan Q., Ma L., Li X., Leng X. Effects of replacing fishmeal with fermented soybean meal on the growth performance, intestinal microbiota, morphology and disease resistance of largemouth bass (Micropterus salmoides) Aquaculture Reports. 2022;22 doi: 10.1016/j.aqrep.2021.100954. [DOI] [Google Scholar]
- 25.Hossain S., Koshio S., Ishikawa M., Yokoyama S., Sony N.M., Islam J., Fujieda T. Substitution of dietary fishmeal by soybean meal with inosine administration influences growth, digestibility, immunity, stress resistance and gut morphology of juvenile amberjack Seriola dumerili. Aquacult. 2018;488:174–188. doi: 10.1016/j.aquaculture.2018.01.037. [DOI] [Google Scholar]
- 26.Ye H., Xu M., Liu Q., Sun Z., Zou C., Chen L., Ye C. Effects of replacing fishmeal with soybean meal on growth performance, feed utilization and physiological status of juvenile obscure puffer, Takifugu obscurus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;216:75–81. doi: 10.1016/j.cbpc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Mai K., Ai Q. Conventional soybean meal as fishmeal alternative in diets of Japanese seabass (Lateolabrax japonicus): effects of functional additives on growth, immunity, antioxidant capacity and disease resistance. Antioxidants. 2022;11(5):951. doi: 10.3390/antiox11050951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dharmakar P., Aanand S., Kumar J.S.S., Ande M.P., Pereira P.P.J.J. Fermented cottonseed meal as an alternative for groundnut oil cake in aquafeed. International Journal of Fisheries and Aquatic Studies. 2022;10(1):151–154. doi: 10.22271/fish.2022.v10.i1b.2642. [DOI] [Google Scholar]
- 29.Wan M., Yin P., Fang W., Xie S., Chen S.J., Tian L.X., Niu J. The effect of replacement of fishmeal by concentrated dephenolization cottonseed protein on the growth, body composition, haemolymph indexes and haematological enzyme activities of the Pacific white shrimp (Litopenaeus vannamei) Aquacult. Nutr. 2018;24(6):1845–1854. doi: 10.1111/anu.12823. [DOI] [Google Scholar]
- 30.Liu H., Dong X., Tan B., Du T., Zhang S., Yang Y., Liu H. Effects of fishmeal replacement by low‐gossypol cottonseed meal on growth performance, digestive enzyme activity, intestine histology and inflammatory gene expression of silver sillago (Sillago sihama Forsskál) (1775) Aquacult. Nutr. 2020;26(5):1724–1735. doi: 10.1111/anu.13123. [DOI] [Google Scholar]
- 31.Xu X., Yang H., Zhang C., Bian Y., Yao W., Xu Z., Leng X. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides) Aquaculture. 2022;548 doi: 10.1016/j.aquaculture.2021.737551. [DOI] [Google Scholar]
- 32.Zhao W., Liu Z.L., Niu J. Growth performance, intestinal histomorphology, body composition, hematological and antioxidant parameters of Oncorhynchus mykiss were not detrimentally affected by replacement of fishmeal with concentrated dephenolization cottonseed protein. Aquaculture Reports. 2021;19 doi: 10.1016/j.aqrep.2020.100557. [DOI] [Google Scholar]
- 33.Prabu D.L., Vijayagopal P., Ebeneezar S., Kalidas C., Rameshkumar P., Varghese E., Muniswaran B.R. Enzymological, histological, and serum biomarker responses of snubnose pompano on complete replacement of fishmeal using cottonseed meal supplemented with lysine and methionine in the diet. Fish Physiol. Biochem. 2022:1–20. doi: 10.21203/rs.3.rs-1127485/v1. [DOI] [PubMed] [Google Scholar]
- 34.Xie X., Wang J., Guan Y., Xing S., Liang X., Xue M., Leclercq E. Cottonseed protein concentrate as fishmeal alternative for largemouth bass (Micropterus salmoides) supplemented a yeast-based paraprobiotic: effects on growth performance, gut health and microbiome. Aquaculture. 2022;551 doi: 10.1016/j.aquaculture.2022.737898. [DOI] [Google Scholar]
- 35.Lim S.J., Lee K.J. A microbial fermentation of soybean and cottonseed meal increases antioxidant activity and gossypol detoxification in diets for Nile tilapia, Oreochromis niloticus. J. World Aquacult. Soc. 2011;42(4):494–503. doi: 10.1111/j.1749-7345.2011.00491.x. [DOI] [Google Scholar]
- 36.Mageshwaran V., Parvez N. Gossypol detoxification and lysine enrichment in cottonseed cake by solid state fermentation. J. Pure Appl. Microbiol. 2016;10(2):1333–1339. [Google Scholar]
- 37.Dharmakar P., Aanand S., Stephen Sampath Kumar J. Assessment of solid-state fermented meals of cottonseed, sunflower seed and dried brewery waste on the growth and survival of Rohu (Labeo rohita) in nursery phase. TNJFU. 2021;2021:8–194. [Google Scholar]
- 38.Wang Y.H., Yuan Y., Yang X.Q., Wang J.M., Guo J., Lin Y. Comparison of the colloidal stability, bioaccessibility and antioxidant activity of corn protein hydrolysate and sodium caseinate stabilized curcumin nanoparticles. J. Food Sci. Technol. 2016;53(7):2923–2932. doi: 10.1007/s13197-016-2257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glencross B. In: Aquafeed Formulation. first ed. Nates Sergio., editor. Academic Press; Cambridge. UK: 2016. Understanding the nutritional and biological constraints of ingredients to optimize their application in aquaculture feeds; pp. 33–73. [Google Scholar]
- 40.Hernández C., Lizárraga-Velázquez C.E., Contreras-Rojas D., Sánchez-Gutiérrez E.Y., Martínez-Montaño E., Ibarra-Castro L., Peña-Marín E.S. Fishmeal replacement by corn gluten in feeds for juvenile spotted rose snapper (Lutjanus guttatus): effect on growth performance, feed efficiency, hematological parameters, protease activity, body composition, and nutrient digestibility. Aquaculture. 2021;531 doi: 10.1016/j.aquaculture.2020.735896. [DOI] [Google Scholar]
- 41.NRC . National Academies Press; Washington D.C: 2011. Nutrient Requirements of Fish and Shrimp. [Google Scholar]
- 42.Corradini E., Marconcini J.M., Agnelli J.A., Mattoso L.H. Thermoplastic blends of corn gluten meal/starch (CGM/starch) and corn gluten meal/polyvinyl alcohol and corn gluten meal/poly (hydroxybutyrate-cohydroxyvalerate) (CGM/PHB-V) Carbohydr. Polym. 2011;83:959–965. doi: 10.1016/j.carbpol.2010.09.004. [DOI] [Google Scholar]
- 43.Nguyen L., Davis D.A. Comparison of crystalline lysine and intact lysine used as a supplement in practical diets of channel catfish (Ictalurus punctatus) and Nile tilapia (Oreochromis niloticus) Aquaculture. 2016;464:331–339. doi: 10.1016/j.aquaculture.2016.07.005. [DOI] [Google Scholar]
- 44.Drew M.D., Ogunkoya A.E., Janz D.M., Van Kessel A.G. Dietary influence of replacing fishmeal and oil with canola protein concentrate and vegetable oils on growth performance, fatty acid composition and organochlorine residues in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2007;67:260–268. doi: 10.1016/j.aquaculture.2007.01.002. [DOI] [Google Scholar]
- 45.Olukosi O.A., Kasprzak M.M., Kightley S., Carre P., Wiseman J., Houdijk J.G.M. Investigations of the nutritive value of meals of double-low rapeseed and its influence on growth performance of broiler chickens. Poultry Sci. 2017;96:3338–3350. doi: 10.3382/ps/pex157. [DOI] [PubMed] [Google Scholar]
- 46.Adem H.N., Tressel R.P., Pudel F., Slawski H., Schulz C. Rapeseed use in aquaculture. OCL. 2014;21(1):D105. doi: 10.1051/ocl/2013041. [DOI] [Google Scholar]
- 47.Rao S.B.N., Prasad K.S., Rajendran D. Publisher: Satish Serial Publishing House; Delhi, India: 2013. Recent advances in amelioration of anti-nutritional factors in livestock feed stuffs. Animal Nutrition & Reproductive Physiology (Recent Concepts) pp. 655–678. [Google Scholar]
- 48.Wu J., Muir A. Comparative structural, emulsifying, and biological properties of 2 major canola proteins, cruciferin and napin. J. Food Sci. 2008;73:210–216. doi: 10.1111/j.1750-3841.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 49.USDA Oilseeds: world markets and trade. 2017. https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf Retrieved from.
- 50.He R., Girgih A.T., Malomo S.A., Ju X., Aluko R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the mem‐ brane ultrafiltration fractions. J. Funct.Foods. 2013;5:219–227. doi: 10.1016/j.jff.2012.10.008. [DOI] [Google Scholar]
- 51.Akbari A., Wu J. An integrated method of isolating napin and cruciferin from defatted canola meal. LWT--Food Sci. Technol. 2015;64(1):308–315. doi: 10.1016/j.lwt.2015.05.046. [DOI] [Google Scholar]
- 52.Alashi A.M., Blanchard C.L., Mailer R.J., Agboola S.O., Mawson A.J., He R., Aluko R.E. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014;146:500–506. doi: 10.1016/j.foodchem.2013.09.081. [DOI] [PubMed] [Google Scholar]
- 53.Mazumder A., Dwivedi A., Plessis J.D. Sinigrin and its therapeutic benefits. Molecules. 2016;21:1–11. doi: 10.3390/molecules21040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanasundara J.P., McIntosh T.C., Perera S.P., Withana-Gamage T.S., Mitra P. Canola/rapeseed protein-functionality and nutrition. OCl. 2016;23(4):D407. doi: 10.1051/ocl/2016028. [DOI] [Google Scholar]
- 55.Sallam E.A., Matter A.F., Mohammed L.S., Azam A.E., Shehab A., Mohamed Soliman M. Replacing fishmeal with rapeseed meal: potential impact on the growth performance, profitability measures, serum biomarkers, antioxidant status, intestinal morphometric analysis, and water quality of Oreochromis niloticus and Sarotherodon galilaeus fingerlings. Vet. Res. Commun. 2021;45(4):223–241. doi: 10.1007/s11259-021-09803-5. [DOI] [PubMed] [Google Scholar]
- 56.Olukomaiya O.O., Fernando W.C., Mereddy R., Li X., Sultanbawa Y. Solid-state fermentation of canola meal with Aspergillus sojae, Aspergillus ficuum and their co-cultures: effects on physicochemical, microbiological and functional properties. Lwt. 2020;127 doi: 10.1016/j.lwt.2020.109362. [DOI] [Google Scholar]
- 57.Östbring K., Malmqvist E., Nilsson K., Rosenlind I., Rayner M. The effects of oil extraction methods on recovery yield and emulsifying properties of proteins from rapeseed meal and press cake. Foods. 2020;9(1):19. doi: 10.3390/foods9010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshie-Stark Y., Wada Y., Wäsche A. Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem. 2008;107(1):32–39. doi: 10.1016/j.foodchem.2007.07.061. [DOI] [Google Scholar]
- 59.Booth M.A., Allan G.L., Frances J., Parkinson S. Replacement of fish meal in diets for Australian silver perch, Bidyanus bidyanus: IV. Effects of dehulling and protein concentration on digestibility of grain legumes. Aquaculture. 2001;196(1–2):67–85. doi: 10.1016/S0044-8486(00)00578-0. [DOI] [Google Scholar]
- 60.Francis G., Makkar H.P., Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199(3–4):197–227. doi: 10.1016/S0044-8486(01)00526-9. [DOI] [Google Scholar]
- 61.Tripathi M., Mishra A. Glucosinolates in animal nutrition: a review. Anim. Feed Sci. Technol. 2007;132(1–2):1–27. doi: 10.1016/j.anifeedsci.2006.03.003. [DOI] [Google Scholar]
- 62.Croat J., Karki B., Berhow M., Iten L., Muthukumarappan K., Gibbons W. Utilizing pretreatment and fungal incubation to enhance the nutritional value of canola meal. J. Appl. Microbiol. 2017;123(2):362–371. doi: 10.1111/jam.13507. [DOI] [PubMed] [Google Scholar]
- 63.Hussain S.M., Afzal M., Nasir S., Javid A., Makhdoom S.M., Jabeen F., Shah S.Z.H. Efficacy of phytase supplementation in improving mineral digestibility in Labeo rohita fingerlings fed on canola meal-based diets. Iran. J. Fish. Sci. 2016;15(2):645–661. doi: 10.22092/IJFS.2018.114560. [DOI] [Google Scholar]
- 64.Alhomodi A.F., Zavadil A., Berhow M., Gibbons W.R., Karki B. Application of cocultures of fungal mycelium during solid‐state fermentation of canola meal for potential feed application. J. Am. Oil Chem. Soc. 2021;98(5):509–517. doi: 10.1002/aocs.12479. [DOI] [Google Scholar]
- 65.Hansen J.Ø., Skrede A., Mydland L.T., Øverland M. Fractionation of rapeseed meal by milling, sieving and air classification—effect on crude protein, amino acids and fiber content and digestibility. Anim. Feed Sci. Technol. 2017;230:143–153. doi: 10.1016/j.anifeedsci.2017.05.007. [DOI] [Google Scholar]
- 66.Kasiga T., White B.M., Gibbons W.R., Brown M.L. Amino acid availability of processed Brassica carinata meals in hybrid striped bass Morone chrysops♀ x M. saxatilis♂. Aquaculture Reports. 2021;19 doi: 10.1016/j.aqrep.2021.100597. [DOI] [Google Scholar]
- 67.Arsalan M., Hussain S.M., Ali S., Ahmad B., Sharif A. Use of phytase and citric acid supplementation on growth performance and nutrient digestibility of Cirrhinus mrigala fingerlings fed on canola meal based diet. Braz. J. Biol. 2021;83 doi: 10.1590/1519-6984.246568. [DOI] [PubMed] [Google Scholar]
- 68.Hussain M., Hussain S.M., Iqbal R., Shahzad M.M., Shah S.Z.H., Akram A.M., Arsalan Muhammad Zubair ul Hassan. Effect of citric acid acidified Moringa oleifera seed meal based diet on minerals absorption, carcass composition and hematological indices of Cirrhinus mrigala fingerlings. Pakistan J. Zool. 2021;54(4):1737–1745. doi: 10.17582/journal.pjz/20190531050527. [DOI] [Google Scholar]
- 69.Jiang X., Ding H., Liu Q., Wei Y., Zhang Y., Wang Y., Hu Y. Effects of peanut meal extracts fermented by Bacillus natto on the growth performance, learning and memory skills and gut microbiota modulation in mice. Br. J. Nutr. 2020;123(4):383–393. doi: 10.1017/S0007114519002988. [DOI] [PubMed] [Google Scholar]
- 70.Ye G., Dong X., Yang Q., Chi S., Liu H., Zhang H., Zhang S. Dietary replacement of fishmeal with peanut meal in juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂): growth performance, immune response, and intestinal microbiota. Aquaculture Reports. 2020;17 doi: 10.1016/j.aqrep.2020.100327. [DOI] [Google Scholar]
- 71.Zhao X., Chen J., Du F. Potential use of peanut by-products in food processing: a review. J. Food Sci. Technol. 2012;49(5):521–529. doi: 10.1007/s13197-011-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ejigui J., Savoie L., Marin J., Desrosiers T. Influence of traditional processing methods on the nutritional composition and antinutritional factors of red peanuts (Arachis hypogea) and small red kidney beans (Phaseolus vulgaris) J. Biol. Sci. 2005;5:597–605. doi: 10.3923/jbs.2005.597.605. [DOI] [Google Scholar]
- 73.Neto S.G., Oliveira R.L., de Lima F.H.S., de Medeiros A.N., Bezerra L.R., Viégas J., de Freitas Neto M.D. Milk production, intake, digestion, blood parameters, and ingestive behavior of cows supplemented with by-products from the biodiesel industry. Trop. Anim. Health Prod. 2015;47:191–200. doi: 10.1007/s11250-014-0706-2. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y.L., Gao J.L., Y.X M., Zhang X.L. Quality comparison of alcohol leached peanut protein concentrate obtained from different peanut meal. Grain Oil Food Technology. 2013;21(3):57–61. [Google Scholar]
- 75.Zhu Z., Yi Y., Zhang X., Lin Y., Chi S., Yang Q., Tan B. The growth performance, antioxidant capacity, liver fat metabolism and intestinal flora composition responsiveness to fishmeal replacement by peanut cake for juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂) Aquaculture Reports. 2022;23 doi: 10.1016/j.aqrep.2022.101060. [DOI] [Google Scholar]
- 76.Gresta F., Cristaudo A., Trostle C., Anastasi U., Guarnaccia S., Catara P., Onofri A. Germination of guar (Cyamopsis tetragonoloba (L.) Taub.) genotypes with reduced temperature requirements. Aust. J. Crop. Sci. 2018;12(6):954–960. doi: 10.21475/ajcs.18.12.06.PNE1049. [DOI] [Google Scholar]
- 77.Hussain M., Rehman A.U., Khalid M.F. Feeding value of guar meal and the application of enzymes in improving nutritive value for broilers. World Poultry Sci. J. 2012;68(2):253–268. doi: 10.1017/S0043933912000311. [DOI] [Google Scholar]
- 78.Lee J.T., Bailey C.A., Cartwright A.L. Guar meal germ and hull fractions differently affect growth performance and intestinal viscosity of broiler chickens. Poultry Sci. 2003;82:1589–1595. doi: 10.1093/ps/82.10.1589. [DOI] [PubMed] [Google Scholar]
- 79.Mathur O.P., Mathur C.S. Feeding of protected protein and urea supplementation for enhanced growth and feed utilization in Magra lambs. Indian J. Anim. Nutr. 1989;6(3):274–278. [Google Scholar]
- 80.Heo P.S., Lee S.W., Kim D.H., Lee G.Y., Kim K.H., Kim Y.Y. Various levels of guar meal supplementation on growth performance and meat quality in growing-finishing pigs. J. Anim. Sci. 2009;87(2):144. [Google Scholar]
- 81.Rajamohamed K.M. Ph.D. Dissertation, Central Institute of Brackish water Aquaculture, University of Madras; Chennai, Tamil Nadu, India: 2012. Role of exogenous enzymes in maximizing the use of novel and conventional alternate plant protein sources in the diet of tiger shrimp, Penaeus monodon. [Google Scholar]
- 82.Lund P., Weisbjerg M.R., Hvelplund T. Profile of digested feed amino acids from untreated and expander treated feeds estimated using in situ methods in dairy cows. Livest. Sci. 2008;114(1):108–116. doi: 10.1016/j.livsci.2007.04.012. [DOI] [Google Scholar]
- 83.Singh K., Khetrapaul N. Development of potato and guar bean vegetable and raita using fresh guar (Cyamopsis tetragonoloba) pods. Journal of Food Legumes. 2011;24(3):235–238. [Google Scholar]
- 84.Barlaya G., Ananda Kumar B.S., Huchchappa R.C., Basumatary P., Kannur H. Effect of fish meal replacement with toasted guar meal on growth, food conversion, digestive enzyme activity and final carcass composition of rohu, Labeo rohita. Aquacult. Res. 2021;52(11):5551–5557. doi: 10.1111/are.15430. [DOI] [Google Scholar]
- 85.Anjum M.A., Hussain Z., Khan S.H., Ahmad N., Amer M.Y., Iftikhar N. Assessment of poultry feed ingredients used in commercial compound feed. Pakistan Journal of Life and Social Sciences. 2014;12(2):69–73. [Google Scholar]
- 86.Laudadio V., Bastoni E., Introna M., Tufarelli V. Production of low-fibre sunflower (Helianthus annuss L.) meal by micronization and air-classification processes. CyTA - J. Food. 2013;11(4):398–403. doi: 10.1080/19476337.2013.781681. [DOI] [Google Scholar]
- 87.Mushtaq A., Roobab U., Denoya G.I., Inam‐Ur‐Raheem M., Gullón B., Lorenzo J.M., Aadil R.M. Advances in green processing of seed oils using ultrasound‐assisted extraction: a review. J. Food Process. Preserv. 2020;44(10) doi: 10.1111/jfpp.14740. [DOI] [Google Scholar]
- 88.Shchekoldina T., Aider M. Production of low chlorogenic and caffeic acid containing sunflower meal protein isolate and its use in functional wheat bread making. J. Food Sci. Technol. 2014;51:2331–2343. doi: 10.1007/s13197-012-0780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tacon A.G.J., Webster J.L., Martinez C.A. Use of solvent extracted sunflower seed meal in complete diets for fingerling rainbow trout (Salmo gairdneri Richardson) Aquaculture. 1984;43(4):381–389. doi: 10.1016/0044-8486(84)90246-1. [DOI] [Google Scholar]
- 90.González‐Pérez S., Vereijken J.M. Sunflower proteins: overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007;87(12):2173–2191. doi: 10.1002/jsfa.2971. [DOI] [Google Scholar]
- 91.Soltan M. Effect of dietary fishmeal replacement by poultry by‐ product meal with different grain source and enzyme supplementation on performance, feces recovery, body composition and nutrient balance of Nile tilapia. Pakistan J. Nutr. 2009;8(4):395–407. [Google Scholar]
- 92.Iqbal M., Yaqub A., Ayub M. Effects of partial and full dietary substitution of fish meal and soybean meal by sunflower meal on growth performance, feed consumption, body indices, serum chemistry and intestine morphology of Oreochromis niloticus. Turk. J. Fish. Aquat. Sci. 2022;22(10) 10.4194/TRJFAS20784. [Google Scholar]
- 93.Hassaan M.S., Soltan M.A., Mohammady E.Y., Elashry M.A., El-Haroun E.R., Davies S.J. Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture. 2018;495:592–601. doi: 10.1016/j.aquaculture.2018.06.018. [DOI] [Google Scholar]
- 94.Padayachee B., Baijnath H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. South African Journal of Biotechnology. 2020;129:304–316. doi: 10.1016/j.sajb.2019.08.021. [DOI] [Google Scholar]
- 95.Hassan S.K.U., Khalique A., Pasha T.N., Sahota A.W. Effect of dried Moringa oleifera leaves on growth performance and immune response of broilers. JAPS: Journal of Animal and Plant Sciences28. 2018;(6):1579–1583. [Google Scholar]
- 96.Shahzad U., Khan M.A., Jaskani M.J., Khan I.A., Korban S.S. Genetic diversity and population structure of Moringa oleifera. Conserv. Genet. 2013;14:1161–1172. [Google Scholar]
- 97.Anwar F., Bhanger M. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem. 2003;51:6558–6563. doi: 10.1021/jf0209894. [DOI] [PubMed] [Google Scholar]
- 98.Falowo A.B., Mukumbo F.E., Idamokoro E.M., Lorenzo J.M., Afolayan A.J., Muchenje V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: a review. Food Res. Int. 2018;106:317–334. doi: 10.1016/j.foodres.2017.12.079. [DOI] [PubMed] [Google Scholar]
- 99.Majhi S. Department of Pharmaceutical Technology Jadavpur University; Kolkata. 700032 India: 2013. Nutritional Value of Moringa Oleifera as a Dietary Supplement. Thesis, Degree of Master of Pharmacy. [Google Scholar]
- 100.Wu D., Cai Z., Wei Y., Zhang C., Liang G., Guo Q. Research advances in moringa as a new plant protein feed. Chin. J. Animal Nutrition. 2013;25:503–511. [Google Scholar]
- 101.Su B., Chen X. Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 2020;7:53. doi: 10.3389/fvets.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sánchez-Machado D.I., Núñez-Gastélum J.A., Reyes-Moreno C., Ramírez-Wong B., López-Cervantes J. Nutritional quality of edible parts of Moringa oleifera. Food Anal. Methods. 2010;3:175–180. doi: 10.1007/s12161-009-9106-z. [DOI] [Google Scholar]
- 103.Zhang X., Wang H., Zhang J., Lin B., Chen L., Wang Q., Deng J. Assessment of rapeseed meal as fishmeal alternative in diets for juvenile Asian red-tailed catfish (Hemibagrus wyckioides) Aquaculture Reports. 2020;18 doi: 10.1016/j.aqrep.2020.100497. [DOI] [Google Scholar]
- 104.Shahzad M.M., Hussain S.M., Akram A.M., Javid A., Hussain M., Hussain Shah S.Z., Chaudhary A. Improvement in nutrient digestibility and growth performance of Catla catla fingerlings using phytase in Moringa oleifera leaf meal based diet. Pakistan J. Zool. 2020;52(1):157–168. doi: 10.17582/journal.pjz/2020.52.1.157.168. [DOI] [Google Scholar]
- 105.Tabassum S., Hussain S.M., Ali S., Arsalan M., Ahmad B., Asrar M., Sharif A. Partial replacement of fishmeal with Moringa oleifera leaf meal in practical diets of Cirrhinus mrigala fingerlings. Brazilian Journal of Biotechnology. 2021;83 doi: 10.1590/1519-6984.246333. [DOI] [PubMed] [Google Scholar]
- 106.Shahzad M.M., Rafique T., Hussain S.M., Hussain Z., Zahoor M.Y., Hussain M., Bashir S. Effect of phytase supplemented Moringa by-products based diets on the performance of Oreochromis niloticus fingerlings. Journal of Animal and Plant Sciences. 2021;31(1):228–295. doi: 10.36899/JAPS.2021.1.0216. [DOI] [Google Scholar]
- 107.Ezeokonkwo C.A., Dodson W.L. The potential of Terminalia catappa (Tropical almond) seed as a source of dietary protein. J. Food Qual. 2004;27(3):207–219. doi: 10.1111/j.1745-4557.2004.tb00650.x. [DOI] [Google Scholar]
- 108.Monaghan E.K. Florida State University; USA: 2008. Chemical composition and protein antigenicity-almond (Prunus dulcis) and Macadamia nut (Macadamia integrifolia) Seeds. PhD Thesis. [Google Scholar]
- 109.Elezuo K.O., Falaye A.E., Omoike A., Ajani E.K., Obute C.U. Growth performance of Clarias gariepinus juveniles fed graded levels of roasted tropical almond (Terminalia catappa linnaeus) kernel meal based diets. Journal of Biololgy and Agriculture Healthcare. 2018;8(22) [Google Scholar]
- 110.Annegowda H.V., Nee C.W., Mordi M.N., Ramanathan S., Mansor S.M. Evaluation of phenolic content and antioxidant property of hydrolysed extracts of Terminalia catappa L. leaf. Asian J. Plant Sci. 2010;9(8):479. doi: 10.3923/ajps.2010.479.485. [DOI] [Google Scholar]
- 111.Chitmanat C., Tongdonmuan K., Nunsong W. The use of crude extracts from traditional medicinal plants to eliminate Trichodina sp. in tilapia (Oreochromis niloticus) fingerlings. Songklanakarin J. Sci. Technol. 2005;27:359–364. [Google Scholar]
- 112.Neelavathi P., Venkatalakshmi P., Brindha P. Antibacterial activities of aqueous and ethanolic extracts of Terminalia catappa leaves and bark against some pathogenic bacteria. Int. J. Pharm. Pharmaceut. Sci. 2013;5(1):114–120. [Google Scholar]
- 113.Terças A.G., Monteiro A.D.S., Moffa E.B., Santos J.R.D., Sousa E.M.D., Pinto A.R., Monteiro C.D.A. Phytochemical characterization of Terminalia catappa Linn. Extracts and their antifungal activities against Candida spp. Front. Microbiol. 2017;8:595. doi: 10.3389/fmicb.2017.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramos F.M., Abe H.A., Couto M.V.S.D., Paixão P.E.G., Martins M.L., Carneiro P.C.F.…Fujimoto R.Y. Terminalia catappa improves growth performance and survival of the Amazon leaf fish (Monocirrhus polyacanthus) larvae submitted to handling stress. Aquacult. Res. 2020;51(11):4805–4808. doi: 10.1111/are.14783. [DOI] [Google Scholar]
- 115.Nurhidayat N., Wardin L., Sitorus E. The survival and growth performance of juvenile cardinal tetra (Paracheirodon axelrodi) with application of tropical almond (Terminalia catappa) leaves. Nusantara Bioscience. 2016;8(1) doi: 10.13057/nusbiosci/n080101. [DOI] [Google Scholar]
- 116.Ziaee T., Moharreri N., Hosseinzadeh H. Review of pharmacological and toxicological effects of Nigella sativa and its active constituents. Journal of Medicinal Plants. 2012;11(42):16–42. [Google Scholar]
- 117.Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Altinterim B. 2010. Çörekotu (Nigella Sativa, L.) Yağının Gökkuşağı Alabalığı (Oncorhynchus mykiss, Walbaum, 1792)’nın Immün Sistemine Etkisinin Araştırılması/A study on the effect of black seeds (Nigella sativa) oil on immune system of rainbow trout (Oncorhynchus mykiss) [Google Scholar]
- 119.Atta M.B. Some characteristics of nigella (Nigella sativa L.) seed cultivated in Egypt and its lipid profile. Food Chem. 2003;83(1):63–68. doi: 10.1016/S0308-8146(03)00038-4. [DOI] [Google Scholar]
- 120.Dey B.K., Hossain M.M.M., Alam M.E. Effect of black cumin seed oil on growth, innate immunity and resistance against Pseudomonas fluorescens infection in Nile tilapia Oreochromis niloticus. Aquacult. Int. 2020;28(4):1485–1499. doi: 10.1007/s10499-020-00539-8. [DOI] [Google Scholar]
- 121.Mahboub H.H., Elsheshtawy H.M., Sheraiba N.I., Fahmy E.M., Mohamed E.A., Abdelnaeim N.S., Ahmed S.A. Dietary black cumin (Nigella sativa) improved hemato-biochemical, oxidative stress, gene expression, and immunological response of Nile tilapia (Oreochromis niloticus) infected by Burkholderia cepacia. Aquaculture Reports. 2022;22 doi: 10.1016/j.aqrep.2021.100943. [DOI] [Google Scholar]
- 122.Silviana N.R., Pamungkas W., Grandiosa R. Utilizing of black cumin (Nigella sativa) flour to increase the immunity system of tilapia (Oreochromis niloticus) against Aeromonas hydrophila bacteria attack. Jurnal Akuakultur Indonesia. 2022;21(2):161–177. doi: 10.19027/jai.21.2.161-177. [DOI] [Google Scholar]
- 123.Abraham E.M., Ganopoulos I., Madesis P., Mavromatis A., Mylona P., Nianiou-Obeidat I., Vlachostergios D. The use of lupin as a source of protein in animal feeding: genomic tools and breeding approaches. Int. J. Mol. Sci. 2019;20(4):851. doi: 10.3390/ijms20040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kohajdova Z., KaroVičoVá J., Schmidt Š. Lupin composition and possible use in bakery-a review. Czech J. Food Sci. 2011;29(3):203–211. doi: 10.17221/252/2009-cjfs. [DOI] [Google Scholar]
- 125.Pham H., Fotedar R., Nguyen C., Siddik M. Feed utilisation efficiency of lupin inclusion in cobia: role of dietary organic selenium supplementation. Mod. Appl. Sci. 2016;10(10):180–192. doi: 10.5539/mas.v10n10p180. [DOI] [Google Scholar]
- 126.Glencross B., Rutherford N., Hawkins W. A comparison of the growth performance of rainbow trout (Oncorhynchus mykiss) when fed soybean, narrow‐leaf or yellow lupin meals in extruded diets. Aquacult. Nutr. 2011;17(2):e317–e325. doi: 10.1111/j.1365-2095.2010.00765.x. [DOI] [Google Scholar]
- 127.Pereira T.G., Oliva‐Teles A. Evaluation of micronized lupin seed meal as an alternative protein source in diets for gilthead sea bream Sparus aurata L. juveniles. Aquacult. Res. 2004;35(9):828–835. https://doi:10.1111/j.1365-2109.2004.01073.x [Google Scholar]
- 128.Van Vo B., Bui D.P., Nguyen H.Q., Fotedar R. Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture. 2015;444:62–69. doi: 10.1016/j.aquaculture.2015.03.019. [DOI] [Google Scholar]
- 129.Acar Ü., Kesbiç O.S., Yılmaz S., Karabayır A. Growth performance, haematological and serum biochemical profiles in rainbow trout (Oncorhynchus mykiss) fed diets with varying levels of lupin (Lupinus albus) meal. Aquacult. Res. 2018;49(7):2579–2586. doi: 10.1111/are.13724. [DOI] [Google Scholar]
- 130.Palmegiano G.B., Daprà F., Forneris G., Gai F., Gasco L., Guo K., Zoccarato I. Rice protein concentrate meal as a potential ingredient in practical diets for rainbow trout (Oncorhynchus mykiss) Aquaculture. 2006;258(1–4):357–367. doi: 10.1016/j.aquaculture.2006.04.011. [DOI] [Google Scholar]
- 131.Güroy D., Şahin İ., Güroy B., Merrifield D.L., Bulut M., Tekinay A.A. Replacement of fishmeal with rice protein concentrate in practical diets for European sea bass Dicentrarchus labrax reared at winter temperatures. Aquacult. Res. 2013;44(3):462–471. doi: 10.1111/j.1365-2109.2011.03053.x. [DOI] [Google Scholar]
- 132.Reda R.M., Maricchiolo G., Quero G.M., Basili M., Aarestrup F.M., Pansera L., Rahman A.N.A. Rice protein concentrate as a fishmeal substitute in Oreochromis niloticus: effects on immune response, intestinal cytokines, Aeromonas veronii resistance, and gut microbiota composition. Fish Shellfish Immunol. 2022 doi: 10.1016/j.fsi.2022.05.048. [DOI] [PubMed] [Google Scholar]
- 133.Tavano O.L., Berenguer‐Murcia A., Secundo F., Fernandez‐Lafuente R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018;17(2):412–436. doi: 10.1111/1541-4337.12326. [DOI] [PubMed] [Google Scholar]
- 134.Toldrá F., Gallego M., Reig M., Aristoy M.C., Mora L. Recent progress in enzymatic release of peptides in foods of animal origin and assessment of bioactivity. J. Agric. Food Chem. 2020;68(46):12842–12855. doi: 10.1021/acs.jafc.9b08297. 10.1021/acs. jafc.9b08297. [DOI] [PubMed] [Google Scholar]
- 135.Amagliani L., O'Regan J., Kelly A.L., O'Mahony J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017;59:18–26. doi: 10.1016/j.jfca.2016.12.026. [DOI] [Google Scholar]
- 136.Abasubong K.P., Liu W.B., Adjoumani Y.J.J., Xia S.L., Xu C., Li X.F. Xylooligosaccharides benefits the growth, digestive functions and TOR signaling in Megalobrama amblycephala fed diets with fish meal replaced by rice protein concentrate. Aquaculture. 2019;500:417–428. doi: 10.1016/j.aquaculture.2018.10.048. [DOI] [Google Scholar]
- 137.Yu Z. Hunan Agricultural University; Changsha, China: 2013. Fermentation optimization of rice protein and its replacement effect of fish mean on feeding in cultivation of Carassius auratus. Master Thesis. [Google Scholar]
- 138.Taranu I., Nguyen T.T., Pham K.D., Gras M.A., Pistol G.C., Marin D.E., Chu-Ky S. Rice and cassava distillers dried grains in Vietnam: nutritional values and effects of their dietary inclusion on blood chemical parameters and immune responses of growing pigs. Waste and Biomass Valorization. 2019;10(11):3373–3382. doi: 10.1007/s12649-018-0341-7. [DOI] [Google Scholar]
- 139.Limbu S.M., Shoko A.P., Lamtane H.A., Kishe-Machumu M.A., Joram M.C., Mbonde A.S., Mgaya Y.D. Supplemental effects of mixed ingredients and rice bran on the growth performance, survival and yield of Nile tilapia. Oreochromis niloticus reared in fertilized earthen ponds. Springer Plus. 2016;5(1):1–13. doi: 10.1186/s40064-015-1643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar S., Sahu N.P., Gupta S., Deo A.D., Shamna N., Ranjan A. Inclusion level of deoiled rice bran (DORB) in the diet of Labeo rohita (Hamilton, 1882) fingerlings: effect on growth and gene expression of IGF-I and IGF-II. Aquaculture. 2017;481:211–217. doi: 10.1016/j.aquaculture.2017.08.025. [DOI] [Google Scholar]
- 141.Mussatto S.I. Brewer's spent grain: valuable feedstock for industrial applications. J. Sci. Food Agric. 2014;94:1264–1275. doi: 10.1002/jsfa.6486. [DOI] [PubMed] [Google Scholar]
- 142.Mullen A.M., Álvarez C., Pojić M., Hadnadev T.D., Papageorgiou M. Food Waste Recovery. Academic Press; 2015. Classification and target compounds; pp. 25–57. [DOI] [Google Scholar]
- 143.Lynch K.M., Steffen E.J., Arendt E.K. Brewers' spent grain: a review with an emphasis on food and health. J. Inst. Brew. 2016;122(4):553–568. doi: 10.1002/jib.363. [DOI] [Google Scholar]
- 144.Bjerregaard M.F., Charalampidis A., Frøding R., Shetty R., Pastell H., Jacobsen C., Hobley T.J. Processing of brewing by-products to give food ingredient streams. Eur. Food Res. Technol. 2019;245(3):545–558. doi: 10.1007/s00217-018-03224-6. [DOI] [Google Scholar]
- 145.Ovie S.O., Eze S.S. Utilization of Saccharomyces cerevisiae in the partial replacement of fishmeal in Clarias gariepinus diets. Int. J. Appl. Agric. Res. 2014;2:83–88. [Google Scholar]
- 146.Ferreira I.M.P.L.V.O., Pinho O., Vieira E., Tavarela J.G. Brewer's Saccharomyces yeast biomass: characteristics and potential applications. Trends Food Sci. Technol. 2010;21(2):77–84. doi: 10.1016/j.tifs.2009.10.008. [DOI] [Google Scholar]
- 147.Liu K., Han J. Changes in mineral concentrations and phosphorus profile during dry-grind processing of corn into ethanol. Bioresour. Technol. 2011;102(3):3110–3118. doi: 10.1016/j.biortech.2010.10.070. [DOI] [PubMed] [Google Scholar]
- 148.Yan F. Northwest Agriculture and Forestry University of Science and Technology; 2019. Nutrient content of corn DDGS and evaluation of biological potency of broilers. (Master) [Google Scholar]
- 149.Han J., Liu K. Changes in composition and amino acid profile during dry grind ethanol processing from corn and estimation of yeast contribution toward DDGS proteins. J. Agric. Food Chem. 2010;58(6):3430–3437. doi: 10.1021/jf9034833. [DOI] [PubMed] [Google Scholar]
- 150.Kluth H., Rodehutscord M. Effect of the duration of prefeeding on amino acid digestibility of wheat distillers dried grains with solubles in broiler chicken. Poultry Sci. 2010;89(4):681–687. doi: 10.3382/ps.2009-00409. [DOI] [PubMed] [Google Scholar]
- 151.Oliva-Teles A., Enes P., Peres H. 2015. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. feed and feeding practices in aquaculture; pp. 203–233. [DOI] [Google Scholar]
- 152.Lim C., Li E., Klesius P.H. Distiller's dried grains with solubles as an alternative protein source in diets of tilapia. Rev. Aquacult. 2011;3(4):172–178. doi: 10.1111/j.1753-5131.2011.01054.x. [DOI] [Google Scholar]
- 153.Øverland M., Krogdahl Å., Shurson G., Skrede A., Denstadli V. Evaluation of distiller's dried grains with solubles (DDGS) and high protein distiller's dried grains (HPDDG) in diets for rainbow trout (Oncorhynchus mykiss) Aquaculture. 2013;416–417:201–208. doi: 10.1016/j.aquaculture.2013.09.016. [DOI] [Google Scholar]
- 154.Suprayudi M.A., Yaniharto D., Priyoutomo N., Kurnianto A., Ekasari J., Jusadi D., Haga Y. Evaluation of practical diets containing high levels of corn distillers dried grains with soluble on red tilapia floating net cage production performance. Pakistan J. Nutr. 2015;14(10):708. [Google Scholar]
- 155.Badillo-Zapata D., Musin G.E., Palma-Cancino D.J., Guerrero-Galván S.R., Chong-Carrillo O., Vega-Villasante F. Total or partial replacement of fishmeal with soybean meal in the diet of the Pacific fat sleeper Dormitator latifrons juveniles. Latin American Journal of Aquatic Research. 2021;49(1):40–47. doi: 10.3856/vol49-issue1-fulltext-2564. [DOI] [Google Scholar]
- 156.Kari Z.A., Kabir M.A., Mat K., Rusli N.D., Razab M.K.A.A., Ariff N.S.N.A., Wei L.S. The possibility of replacing fishmeal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus) Aquaculture Reports. 2021;21 doi: 10.1016/j.aqrep.2021.100815. [DOI] [Google Scholar]
- 157.Liu X., Han B., Xu J., Zhu J., Hu J., Wan W., Miao S. Replacement of fishmeal with soybean meal affects the growth performance, digestive enzymes, intestinal microbiota and immunity of Carassius auratus gibelio♀× Cyprinus carpio♂. Aquaculture Reports. 2020;18 doi: 10.1016/j.aqrep.2020.100472. [DOI] [Google Scholar]
- 158.Zhang J., Zhong L., Peng M., Chu W., Liu Z., Dai Z., Hu Y. Replacement of fishmeal with soy protein concentrate in diet of juvenile rice field eel Monopterus albus. Aquaculture Reports. 2019;15 doi: 10.1016/j.aqrep.2019.100235. [DOI] [Google Scholar]
- 159.Duan Z., Zhang C., Huang L., Lan Q., Hu J., Li X., Leng X. An evaluation of replacing fishmeal with fermented soybean meal in diet of hybrid snakehead (Channa argus× Channa maculata): growth, nutrient utilization, serum biochemical indices, intestinal histology, and microbial community. Aquacult. Nutr. 2022;2022(2):1–13. doi: 10.1155/2022/2964779. [DOI] [Google Scholar]
- 160.Zhang Y., Yang P., Sun H., Hou Y., Zhang Y., Liu H. Evaluation of extruded full-fat soybean as the substitution for fishmeal in diets for juvenile Scophthalmus maximus based on growth performance, intestinal microbiota, and aquaculture water quality. Aquaculture. 2023 doi: 10.1016/j.aquaculture.2022.738734. [DOI] [Google Scholar]
- 161.Hang Y., Fu Y., Jin C., Hua X. Effects of supplemental amino acids and bile acid in a completely replaced fishmeal by enzymatically hydrolysed soybean meal diet on growth performance, liver health and fillet quality of rainbow trout (Oncorhynchus mykiss) Aquacult. Res. 2022;53(9):3297–3308. doi: 10.1111/are.15837. [DOI] [Google Scholar]
- 162.Hassaan M.S., El-Sayed A.I.M., Soltan M.A., Iraqi M.M., Goda A.M., Davies S.J., Ramadan H.A. Partial dietary fishmeal replacement with cotton seed meal and supplementation with exogenous protease alters growth, feed performance, hematological indices and associated gene expression markers (GH, IGF-I) for Nile tilapia, Oreochromis niloticus. Aquaculture. 2019;503:282–292. doi: 10.1016/j.aquaculture.2019.01.009. [DOI] [Google Scholar]
- 163.Alam M.S., Watanabe W.O., Carroll P.M., Gabel J.E., Corum M.A., Seaton P., Dowd M.K. Evaluation of genetically-improved (glandless) and genetically-modified low-gossypol cottonseed meal as alternative protein sources in the diet of juvenile southern flounder Paralichthys lethostigma reared in a recirculating aquaculture system. Aquaculture. 2018;489:36–45. doi: 10.1016/j.aquaculture.2018.02.006. [DOI] [Google Scholar]
- 164.Ahmad B., Hussain S.M., Ali S., Arsalan M., Tabassum S., Sharif A. Efficacy of acidified phytase supplemented cottonseed meal based diets on growth performance and proximate composition of Labeo rohita fingerlings. Braz. J. Biol. 2021;83 doi: 10.1590/1519-6984.24779. [DOI] [PubMed] [Google Scholar]
- 165.Qin Y., He C., Geng H., Wang W., Yang P., Mai K., Song F. Muscle nutritive metabolism changes after dietary fishmeal replaced by cottonseed meal in golden pompano (Trachinotus ovatus) Metabolites. 2022;12(7):576. doi: 10.3390/metabo12070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang X., Zhou H., Liu C., Mai K., He G., Wang X. Fishmeal substitution with low-gossypol cottonseed meal in the diet for juvenile turbot (Scophthalmus maximus L.): effects on growth, nutrients utilization and haematological responses. Aquaculture Reports. 2022;24 doi: 10.1016/j.aqrep.2022.101149. [DOI] [Google Scholar]
- 167.He Y., Guo X., Tan B., Dong X., Yang Q., Liu H., Chi S. Replacing fishmeal with cottonseed protein concentrate in feed for pearl gentian groupers (Epinephelus fuscoguttatus♀× E. lanceolatus♂): effects on growth and expressions of key genes involved in appetite and hepatic glucose and lipid metabolism. Aquaculture Reports. 2021;20 doi: 10.1016/j.aqrep.2021.100710. [DOI] [Google Scholar]
- 168.Sun H., Tang J.W., Yao X.H., Wu Y.F., Wang X., Liu Y., Lou B. Partial substitution of fishmeal with fermented cottonseed meal in juvenile black sea bream (Acanthopagrus schlegelii) diets. Aquaculture. 2015;446:30–36. doi: 10.1016/j.aquaculture.2015.04.020. [DOI] [Google Scholar]
- 169.Bai N., Gu M., Liu M., Jia Q., Pan S., Zhang Z. Corn gluten meal induces enteritis and decreases intestinal immunity and antioxidant capacity in turbot (Scophthalmus maximus) at high supplementation levels. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bu X., Lian X., Zhang Y., Chen F., Tang B., Ge X., Yang Y. Effects of replacing fishmeal with corn gluten meal on growth, feed utilization, nitrogen and phosphorus excretion and IGF‐I gene expression of juvenile Pseudobagrus ussuriensis. Aquacult. Res. 2018;49(2):977–987. doi: 10.1111/are.13545. [DOI] [Google Scholar]
- 171.Nandakumar S., Ambasankar K., Ali S.S.R., Syamadayal J., Vasagam K. Replacement of fishmeal with corn gluten meal in feeds for Asian seabass (Lates calcarifer) Aquacult. Int. 2017;25(4):1495–1505. [Google Scholar]
- 172.Taati R., Pajand Z., Mostafavi H. Replacement of fish meal with corn protein concentrate and its effect on growth, survival, and body composition of siberian sturgeon (Acipenser baerii) Journal of Veterinary Research. 2022;77(1):11–18. doi: 10.22059/JVR.2021.317813.3130. [DOI] [Google Scholar]
- 173.Hosseini Shekarabi S.P., Shamsaie Mehrgan M., Banavreh A., Foroudi F. Partial replacement of fishmeal with corn protein concentrate in diets for rainbow trout (Oncorhynchus mykiss): effects on growth performance, physiometabolic responses, and fillet quality. Aquacult. Res. 2021;52(1):249–259. doi: 10.1111/are.14887. [DOI] [Google Scholar]
- 174.Shahzad M.M., Rashid H., Hussain S.M., Bashir S., Khalid F., Nisar A. Improvement in body composition and blood parameters of Catla catla fingerlings by supplementing rapeseed meal based diet with probiotics. Pakistan J. Zool. 2022:1–9. doi: 10.17582/journal.pjz/20211011061010. [DOI] [Google Scholar]
- 175.Dossou S., Koshio S., Ishikawa M., Yokoyama S., Dawood M.A., El Basuini M.F., Zaineldin A.I. Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-Koji) Fish Shellfish Immunol. 2018;75:253–262. doi: 10.1016/j.fsi.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 176.Bu X.Y., Wang Y.Y., Chen F.Y., Tang B.B., Luo C.Z., Wang Y., Yang Y.H. An evaluation of replacing fishmeal with rapeseed meal in the diet of Pseudobagrus ussuriensis: growth, feed utilization, nonspecific immunity, and growth‐related gene expression. J. World Aquacult. Soc. 2018;49(6):1068–1080. doi: 10.1111/jwas.12470. [DOI] [Google Scholar]
- 177.Slawski H., Adem H., Tressel R.P., Wysujack K., Koops U., Schulz C. Replacement of fishmeal by rapeseed protein concentrate in diets for common carp (Cyprinus carpio L.) Isr. J. Aquac. Bamidgeh. 2011;17(6):605–612. http://hdl.handle.net/10524/36285 [Google Scholar]
- 178.Slawski H., Adem H., Tressel R.P., Wysujack K., Koops U., Kotzamanis Y., Schulz C. Total fishmeal replacement with rapeseed protein concentrate in diets fed to rainbow trout (Oncorhynchus mykiss Walbaum) Aquacult. Int. 2012;20(3):443–453. doi: 10.1007/s10499-011-9476-2. [DOI] [Google Scholar]
- 179.Kaiser F., Harloff H.J., Tressel R.P., Kock T., Schulz C. Effects of highly purified rapeseed protein isolate as fishmeal alternative on nutrient digestibility and growth performance in diets fed to rainbow trout (Oncorhynchus mykiss) Aquacult. Nutr. 2021;27(5):1352–1362. doi: 10.1111/anu.13273. [DOI] [Google Scholar]
- 180.Hussain S.M., Gohar H., Asrar M., Shahzad M.M., Rasul A., Hussain M., Umair M. Effects of polyphenols supplemented canola meal based diet on proximate composition, minerals absorption and hematology of Cyprinus carpio fingerlings. Pakistan J. Zool. 2020;54(3):1071–1079. doi: 10.17582/journal.pjz/20190214170258. [DOI] [Google Scholar]
- 181.Mohammadi M., Imani A., Farhangi M., Gharaei A., Hafezieh M. Replacement of fishmeal with processed canola meal in diets for juvenile Nile tilapia (Oreochromis niloticus): growth performance, mucosal innate immunity, hepatic oxidative status, liver and intestine histology. Aquaculture. 2020;518 doi: 10.1016/j.aquaculture.2019.734824. [DOI] [Google Scholar]
- 182.Zhou Q.L., Habte‐Tsion H.M., Ge X., Xie J., Ren M., Liu B., Pan L. Graded replacing fishmeal with canola meal in diets affects growth and target of rapamycin pathway gene expression of juvenile blunt snout bream, Megalobrama amblycephala. Aquacult. Nutr. 2018;24(1):300–309. doi: 10.1111/anu.12560. [DOI] [Google Scholar]
- 183.Plaipetch P., Yakupitiyage A. Use of yeast-fermented canola meal to replace fishmeal in the diet of Asian sea bass Lates calcarifer (Bloch, 1790) J. Aquacult. Res. Dev. 2012;3(2):1–5. doi: 10.4172/2155-9546.1000125. [DOI] [Google Scholar]
- 184.Ebrahimnezhadarabi M., Hosseinifard S.M., Changizi R., Vatandoust S., Ghobadi S. The effects of hydrolyzed canola meal protein on growth, body composition and expression of growth genes and appetite of beluga fish (Huso huso) Applied Biology. 2022;35(1):20–35. doi: 10.22051/JAB.2021.34652.1404. [DOI] [Google Scholar]
- 185.Hernández C., Olmeda-Guerrero L., Chávez-Sánchez M.C., Ibarra-Castro L., Gaxiola-Cortez G., Martínez-Cárdenas L. Nutritional evaluation of canola meal as fishmeal replacement for juvenile spotted rose snapper (Lutjanus guttatus): effects on growth performance, hematological parameters, body composition, and nutrient digestibility. Anim. Feed Sci. Technol. 2020;269 doi: 10.1016/j.anifeedsci.2020.114683. [DOI] [Google Scholar]
- 186.Slawski H., Nagel F., Wysujack K., Balke D.T., Franz P., Schulz C. Total fishmeal replacement with canola protein isolate in diets fed to rainbow trout (Oncorhynchus mykiss W.) Aquacult. Nutr. 2013;19(4):535–542. doi: 10.1111/anu.12005. [DOI] [Google Scholar]
- 187.Vo B.V., Siddik M.A., Chaklader M.R., Fotedar R., Nahar A., Foysal M.J., Nguyen H.Q. Growth and health of juvenile barramundi (Lates calcarifer) challenged with DO hypoxia after feeding various inclusions of germinated, fermented and untreated peanut meals. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0232278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Acar Ü., Türker A. The effects of using peanut meal in rainbow trout (Oncorhynchus mykiss) diets on the growth performance and some blood parameters. Aquactic Studies. 2018;18(2):5–13. doi: 10.4194/2618-6381-v18202. [DOI] [Google Scholar]
- 189.Garduño‐Lugo M., Olvera‐Novoa M.Á. Potential of the use of peanut (Arachis hypogaea) leaf meal as a partial replacement for fishmeal in diets for Nile tilapia (Oreochromis niloticus L.) Aquacult. Res. 2008;39(12):1299–1306. doi: 10.1111/j.1365-2109.2008.01995.x. [DOI] [Google Scholar]
- 190.Priyadarshini N., Kaippilly D., Pradhan C., Saroglia M. Effect of guar sprout meal on growth, nutrient utilization and hematological characteristics of genetically improved farmed tilapia (Gift) fingerlings. International Journal of Current Microbiology and Applied Sciences. 2020;9(7):3587–3599. doi: 10.20546/ijcmas.2020.907.419. [DOI] [Google Scholar]
- 191.Kalanjiam M.A.R., Malaikozhundan B., Girija V., Gobi N., Vaseeharan B. Effect of guar (Cyamopsis tetragonolobus) meal based diets on growth performance and feed utilization in asian catfish, Pangasianodon hypophthalmus fingerlings. J. Fish. Soc. Taiwan. 2014;41(2):135–144. [Google Scholar]
- 192.Chovatiya S. Sardar Patel University. Ph.D. thesis; 2011. An Integrated Approach for Feed Development for Indian Major Carps (IMCs) to Enhance Their Production by Using Probiotics, Enzymes and Different Agrobased Unconventional Feed. [Google Scholar]
- 193.El-Saidy D.M.S.D., Gaber M.M., Abd-El-Shafy A.S.A. Evaluation of cluster bean meal Cyamposis tetragonoloba as a dietary protein source for common carp Cyprinus carpio L. J. World Aquacult. Soc. 2005;36(3):311–319. doi: 10.1111/j.1749-7345.2005.tb00335.x. [DOI] [Google Scholar]
- 194.Hussain S.M., Ahmad N., Shahzad M.M., Javid A., Aslam N., Hussain M., Riaz D. Efficacy of phytase enzyme and citric acid on growth performance, nutrients and mineral digestibility of Cirrhinus mrigala fingerlings fed guar meal-based diet. Iran. J. Fish. Sci. 2020;19(3):1573–1588. doi: 10.22092/IJFS.2018.117462. [DOI] [Google Scholar]
- 195.Hassaan M.S., Mohammady E.Y., Soaudy M.R., Abdel Rahman A.A. Exogenous xylanase improves growth, protein digestibility and digestive enzymes activities in Nile tilapia, Oreochromis niloticus, fed different ratios of fishmeal to sunflower meal. Aquacult. Nutr. 2019;25(4):841–853. doi: 10.1111/anu.12903. [DOI] [Google Scholar]
- 196.Rahmdel K.J., Noveirian H.A., Falahatkar B., Lashkan A.B. Effects of replacing fishmeal with sunflower meal on growth performance, body composition, hematological and biochemical indices of common carp (Cyprinus carpio) fingerlings. Fishries and Aquatic Life. 2018;26(2):121–129. doi: 10.2478/aopf-2018-0013. [DOI] [Google Scholar]
- 197.Ahmad N., Hussain S.M., Azam S.M., Shahzad M.M., Noureen A., Yaqoob R., Ahmad S. Effects of Se nanoparticles supplementation on growth performance, hematological parameters and nutrient digestibility of Labeo rohita fingerling fed sunflower meal based diet. Braz. J. Biol. 2022;84 doi: 10.1590/1519-6984.253555. [DOI] [PubMed] [Google Scholar]
- 198.Ahmad N., Hussain S.M., Rasul A., Shahzad M.M., Javid A., Azmat H., Ahmad B. Effect of Nano-se particles supplemented sunflower meal based diets on mineral absorption and carcass composition of Cirrhinus mrigala fingerlings. Pakistan J. Zool. 2021;54(3):1103–1113. doi: 10.17582/journal.pjz/20190421160425. [DOI] [Google Scholar]
- 199.Shahzad M.M., Bashir S., Hussain S.M., Javid A., Hussain M., Ahmed N., Khalid F. Effectiveness of phytase pre-treatment on growth performance, nutrient digestibility and mineral status of common carp (Cyprinus carpio) juveniles fed Moringa by-product based diet. Saudi J. Biol. Sci. 2021;28(3):1944–1953. doi: 10.1016/j.sjbs.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang X., Sun Z., Cai J., Wang J., Wang G., Zhu Z., Cao F. Effects of dietary fishmeal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III) Fish Shellfish Immunol. 2020;102:430–439. doi: 10.1016/j.fsi.2020.04.051. [DOI] [PubMed] [Google Scholar]
- 201.Helmiati S., Isnansetyo A. vol. 919. IOP Publishing; 2021. The replacement of fishmeal with fermented Moringa leaves meal and its effect on the immune response of red tilapia (Oreochromis sp.) (IOP Conf. Series: Earth and Environmental Science). 1. [DOI] [Google Scholar]
- 202.Eyiwunmi F.A., Augustine O., Ovie W.K. The hematological parameters of catfish (Clarias gariepinus) fed fish feeds with replaced premix using moringa leaf meal (MLM) Madridge Journal of Aquaculture Research and Development. 2018;2(1):35–39. doi: 10.18689/mjard-1000107. [DOI] [Google Scholar]
- 203.Labh S.N. Expression of immune genes and stress enzyme profiles of rainbow trout (Oncorhynchus mykiss) fed moringa oleifera leaf meal (MLM) International Journal of Biologiclal Innovation. 2020;2(2):155–164. doi: 10.46505/IJBI.2020.2212. [DOI] [Google Scholar]
- 204.Adebayo A.N., Omosowone O.O. Efficacy of Terminalia catappa leaf as an alternative to synthetic antibiotics in the diet of Oreochromis niloticus challenged with Salmonella typhi. Asian Journal of Fisheries and Aquatic Research. 2022:34–42. doi: 10.9734/ajfar%2F2022%2Fv16i430379. [DOI] [Google Scholar]
- 205.Hussain S.M., Nisar S., Jamil M., Bashir F., Arslan M.Z., Tabassum S., Sharif A. Effects of almond meal (Terminalia catappa) based diets on nutrient utilization, growth and hematology of Labeo rohita fingerlings. JAPS: Journal of Animal and Plant Sciences. 2021;31(6):1828–1835. doi: 10.36899/JAPS.2021.6.0387. [DOI] [Google Scholar]
- 206.Nugraha F.P., Rahardjo S., Saputra A. Survival and growth performance of snakehead juvenile (Channa striata) with various dosages of Terminalia catappa leaf powder. Aquaculture, Aquarium, Conservation & Legislation. 2021;14(2):762–773. [Google Scholar]
- 207.Aydın B. A preliminary assessment of the effects of dietary black cumin seed cake on growth performance, serum biochemical parameters and fatty acid composition of mirror carp (Cyprinus carpio var. specularis) fingerlings. Aquaculture Reports. 2021;21 doi: 10.1016/j.aqrep.2021.100847. [DOI] [Google Scholar]
- 208.Yousefi M., Adineh H., Reverter M., Hamidi M.K., Vatnikov Y.A., Kulikov E.V., Van Doan H. Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish Shellfish Immunol. 2021;109:12–19. doi: 10.1016/j.fsi.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 209.Ahmed H.A., Abusina G., Elnady A., Abdelrahman H., Abedo A.E. Evaluation of substituting black seed meal (Nigella sativa L.) as protein source in Nile tilapia diets. Biosci. Res. 2018;15(2):1191–1198. [Google Scholar]
- 210.Altunoglu Y.C., Bilen S., Ulu F., Biswas G. Immune responses to methanolic extract of black cumin (Nigella sativa) in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2017;67:103–109. doi: 10.1016/j.fsi.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 211.Bektaş Z.H., Savaşer S., Akçimen U., Ceylan M., Yener O., Bulut C. Using of black cumin seed powder (Nigella sativa) as immunostimulant and growth promoter in rainbow trout, Oncorhynchus mykiss (Walbaum) Turk. J. Fish. Aquat. Sci. 2019;19(12):987–999. doi: 10.4194/1303-2712-v19_12_01. [DOI] [Google Scholar]
- 212.Kotb A.M., Abd-Elkareem M., Abou Khalil N.S., Sayed A.E.D.H. Protective effect of Nigella sativa on 4-nonylphenol-induced nephrotoxicity in Clarias gariepinus (Burchell, 1822) Sci. Total Environ. 2018;619:692–699. doi: 10.1016/j.scitotenv.2017.11.131. [DOI] [PubMed] [Google Scholar]
- 213.Pham H.D., Siddik M.A., Fotedar R., Chaklader M.R., Foysal M.J., Nguyen C.M., Munilkumar S. Substituting fishmeal with lupin Lupinus angustifolius kernel meal in the diets of cobia Rachycentron canadum: effects on growth performance, nutrient utilization, haemato-physiological response, and intestinal health. Anim. Feed Sci. Technol. 2020;267 doi: 10.1016/j.anifeedsci.2020.114556. [DOI] [Google Scholar]
- 214.Bowyer P.H., El-Haroun E.R., Salim H.S., Davies S.J. Benefits of a commercial solid-state fermentation (SSF) product on growth performance, feed efficiency and gut morphology of juvenile Nile tilapia (Oreochromis niloticus) fed different UK lupin meal cultivars. Aquaculture. 2020;523 [Google Scholar]
- 215.Zhu B.P., Zhou J., Wang Z., Hu Y., Cai M., Yang L., Hu Y. Interactions between intestinal morphology, digestion, inflammatory responses, and gut microbiota of juvenile channel catfish elicited by dietary enzymatic rice protein. Fish Shellfish Immunol. 2022;127:155–165. doi: 10.1016/j.fsi.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 216.He Y., Guo X., Tan B., Dong X., Yang Q., Liu H., Chi S. Replacing fishmeal with fermented rice protein in diets for hybrid groupers (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂): effects on growth, digestive and absorption capacities, inflammatory-related gene expression, and intestinal microbiota. Aquaculture Reports. 2021;19 doi: 10.1016/j.aqrep.2021.100603. [DOI] [Google Scholar]
- 217.Silva V.C.D., Adorian T.J., Goulart F.R., Lovatto N.D.M., Bender A.B.B., Speroni C.S., Silva L.P.D. Rice ethanol distillery residue as a protein source in the diet of silver catfish (Rhamdia quelen) J. Appl. Aquacult. 2022;34(1):97–111. doi: 10.1080/10454438.2020.1822255. [DOI] [Google Scholar]
- 218.Choi J., Rahman M.M., Lee S.M. Rice distillers dried grain is a promising ingredient as a partial replacement of plant origin sources in the diet for juvenile red seabream (Pagrus major) Asian-Australas. J. Anim. Sci. 2014;27(12):1736–1743. doi: 10.5713/ajas.2014.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kumar S., Sahu N.P., Shamna N., Ranjan A. Feeding higher level of de-oiled rice bran causes stress to Labeo rohita fingerlings. Aquaculture. 2018;484:184–190. doi: 10.1016/j.aquaculture.2017.11.029. [DOI] [Google Scholar]
- 220.Estevez A., Padrell L., Iñarra B., Orive M., San Martin D. Brewery by-products (yeast and spent grain) as protein sources in rainbow trout (Oncorhynchus mykiss) feeds. Front. Mar. Sci. 2022 doi: 10.3389/fmars.2022.862020. [DOI] [Google Scholar]
- 221.Fernandes H., Castro C., Salgado J.M., Filipe D., Moyano F., Ferreira P., Peres H. Application of fermented brewer's spent grain extract in plant-based diets for European seabass juveniles. Aquaculture. 2022;552 doi: 10.1016/j.aquaculture.2022.738013. [DOI] [Google Scholar]
- 222.Nazzaro J., San Martin D., Perez-Vendrell A.M., Padrell L., Iñarra B., Orive M., Estévez A. Apparent digestibility coefficients of brewer's by-products used in feeds for rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata) Aquaculture. 2021;530 doi: 10.1016/j.aquaculture.2020.735796. [DOI] [Google Scholar]
- 223.Jayant M., Hassan M.A., Srivastava P.P., Meena D.K., Kumar P., Kumar A., Wagde M.S. Brewer's spent grains (BSGs) as feedstuff for striped catfish, Pangasianodon hypophthalmus fingerlings: an approach to transform waste into wealth. J. Clean. Prod. 2018;199:716–722. doi: 10.1016/j.jclepro.2018.07.213. [DOI] [Google Scholar]
- 224.Zerai D.B., Fitzsimmons K.M., Collier R.J., Duff G.C. Evaluation of brewer's waste as partial replacement of fishmeal protein in Nile tilapia, Oreochromis niloticus, diets. J. World Aquacult. Soc. 2008;39(4):556–564. doi: 10.1111/j.1749-7345.2008.00186.x. [DOI] [Google Scholar]
- 225.Pongpet J., Ponchunchoovong S., Payooha K. Partial replacement of fishmeal by brewer's yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus× Pangasius bocourti) Aquacult. Nutr. 2016;22(3):575–585. doi: 10.1111/anu.12280. [DOI] [Google Scholar]
- 226.Zhu Z., Kou S., Zhang X., Lin Y., Chi S., Yang Q., Tan B. Evaluation of corn distillers dried grains with solubles (DDGS) replacement for fishmeal in the diet for juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂) Aquaculture Reports. 2022;25 doi: 10.1016/j.aqrep.2022.101224. [DOI] [Google Scholar]
- 227.Abouel Azm F.R., Kong F., Wang X., Zhu W., Yu H., Long X., Tan Q. The interaction of dried distillers grains with solubles (DDGS) type and level on growth performance, health, texture, and muscle-related gene expression in grass carp (Ctenopharyngodon idellus) Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.832651. 10.3389%2Ffnut.2022.832651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Von Eschen A.J., Brown M.L., Rosentrater K. Effect of increasing dietary high protein distillers dried grains on yellow perch Perca flavescens performance. J. Appl. Aquacult. 2022;34(3):702–714. doi: 10.1080/10454438.2021.1885558. [DOI] [Google Scholar]
- 229.Barbacariu C.A., Rimbu C.M., Dirvariu L., Burducea M., Boiangiu R.S., Todirascu-Ciornea E., Dumitru G. Evaluation of DDGS as a low-cost feed ingredient for common carp (Cyprinus carpio Linneus) cultivated in a semi-intensive system. Life. 2022;12(10):1609. doi: 10.3390/life12101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Allam B.W., Khalil H.S., Mansour A.T., Srour T.M., Omar E.A., Nour A.A.M. Impact of substitution of fish meal by high protein distillers dried grains on growth performance, plasma protein and economic benefit of striped catfish (Pangasianodon hypophthalmus) Aquaculture. 2020;517 doi: 10.1016/j.aquaculture.2019.734792. [DOI] [Google Scholar]
- 231.Diógenes A.F., Castro C., Miranda A.C., Oliva-Teles A., Peres H. Dietary replacement of fishmeal by corn distillers dried grains with solubles (DDGS) in diets for turbot (Scophthalmus maximus, Linneaus, 1758) Juveniles. Aquaculture. 2018;492:113–122. doi: 10.1016/j.aquaculture.2018.04.005. [DOI] [Google Scholar]
- 232.Hassan M.A., Aftabuddin M., Meena D.K., Mishal P., Gupta S.D. Effective utilization of distiller's grain soluble—an agro-industrial waste in the feed of cage-reared minor carp Labeo bata in a tropical reservoir. Environ. Sci. Pollut. Control Ser. 2016;23(16):16090–16095. doi: 10.1007/s11356-016-6732-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.