Abstract
Background
A probiotic mixture prevented epithelial barrier impairment in various experimental models. The objective was to evaluate its effects in patients suffering from IBS with diarrhea (IBS-D) with confirmed leaky gut.
Methods
IBS-D patients with increased intestinal permeability measured by radionuclide tracers were enrolled in this pilot, open-label, prospective, interventional, single-center, Phase IV study. Patients received two capsules of a multistrain probiotic a day for 30 days and were evaluated by repeated intestinal permeability tests, the Bristol Stool Scale, and patient-perceived quality of life and satisfaction.
Results
Of the 30 enrolled patients (mean age: 42.1 [SD: 13.1] years; female: 60%), 27 completed the study (full analysis set [FAS]), and 18 had no major protocol violation (per protocol set [PPS]). On D30, an improvement of intestinal permeability was observed in 81.5% of patients in FAS, normalization being observed in 37% of the participants (44% in PPS). The mean intestinal permeability was significantly decreased: baseline minus D30, 3.4 (95% CI: 1.7, 5.2); the IBS-QOL total score was significantly increased: D30 minus baseline, 8.0 (95% CI: 3.0, 12.9); and stool consistency was significantly improved. On D15 and D30, 96.3% of patients claimed that their IBS symptoms had been satisfactory alleviated, and a significant improvement was reported for the following VAS-IBS items: abdominal pain, diarrhea, and impact of gastrointestinal problems in daily life. Compliance and tolerance were satisfactory.
Conclusion
The multistrain probiotic tested may reduce intestinal permeability in a considerable proportion of patients and may improve abdominal pain, stool consistency, and quality of life. These results pave the way for larger, placebo-controlled clinical studies.
Keywords: Intestinal permeability, Irritable bowel syndrome, Leaky gut syndrome, Probiotics, Quality of life
Introduction
Irritable bowel syndrome (IBS) is a frequent functional gastrointestinal (GI) disorder with an estimated prevalence between 5 and 20%, depending on geographical region and assessment criteria [1]. A recent systematic review and meta-analysis reported that its global prevalence is of 9.2% using the Rome III criteria but 3.8% using the Rome IV criteria. The difference between Rome III and IV is thought to be due to the more restrictive criteria of the latter. One of the most common subtypes is IBS with diarrhea (IBS-D; 31.5%). IBS is associated with significantly reduced quality of life and increased healthcare costs [2].
Many drugs have been tested for the treatment of IBS-D, including antispasmodics, nonabsorbable antibiotics, and psychotropic agents. However, these do not always relieve symptoms, possibly because of the heterogeneous pathogenesis of the disease. Probiotics are also used in IBS, as they represent attractive approaches for IBS treatment given their safety and multiple mechanisms of action. These include the correction of dysbiosis, the production of antibacterial substances, the inhibition of pathogens and antagonism of toxins, and effects on visceral hypersensitivity [3, 4]. In fact, these agents have been used widely for decades and are listed as high and medium resources by the World Gastroenterology Organization [1]. Recent meta-analyses confirm their role in this disease but also note that their effects are highly strain-specific [5]. The British Society of Gastroenterology guideline coincides in stating that probiotics, as a group, may be an effective treatment for global symptoms and abdominal pain in IBS, but that it is not possible to recommend a specific species or strain [6]. A simplified algorithm for the management of IBS indicates that many studies suggest that probiotics may provide relief from overall or individual symptoms [1]. However, none of these studies mention the use of probiotics for leaky gut associated with IBS-D, which is a particularly important aspect in the efficacy of probiotics in IBS since a defective epithelial barrier function, which can be measured as increased gut permeability, has been implicated in the pathogenesis of IBS-D [7]. The effect of probiotics in regulating the expression of tight junction proteins has been studied as a possible favorable effect of probiotics on leaky gut syndrome [8]. Specifically, Nebot-Vivinus et al. [9] demonstrated that a multistrain probiotic (Lactibiane Tolérance®; PiLeJe Laboratoire, Paris, France; mixture of Bifidobacterium lactis LA 303, B. lactis LA 304, Lactobacillus acidophilus LA 201, Lactobacillus plantarum LA 301, and Lactobacillus salivarius LA 302) prevented epithelial barrier impairment in various models of epithelial barrier function both in vitro and in vivo. Indeed, this multistrain probiotic helped to prevent visceral hypersensitivity and repair disruption to the epithelial barrier that was induced by different inflammatory and stress conditions that mimic IBS. Additionally, the multistrain probiotic downregulated the response mediated through Toll-like receptor-4 in vitro and in vivo. The effects observed in these combined in vitro and in vivo experimental approaches logically led us to want to evaluate the impact of the multistrain probiotic on IBS symptoms in a clinical trial. As the results of the in vitro and in vivo tests obtained on intestinal permeability seemed particularly interesting and promising, we wished to evaluate the probiotic mixture in patients suffering from IBS-D diagnosed in accordance with Rome IV criteria and whose intestinal hyperpermeability had been confirmed beforehand (inclusion criterion), taking into account the fact that not all IBS-D patients have intestinal hyperpermeability [10, 11]. This pilot, open-label, prospective, interventional, single-center, Phase IV clinical study was thus a proof-of-concept study, aiming to evaluate the effects of supplementation with that multistrain probiotic in IBS-D patients with confirmed leaky gut.
Methods
Study Population
To be eligible for enrolment in this pilot, open-label, prospective, interventional, single-center, Phase IV study, subjects had to (i) give written informed consent; (ii) be male and/or female between 18 and 75 years of age; (iii) present, for the last 3 months and onset at least 6 months before the diagnosis, with recurrent abdominal pain, on average, at least one related to defecation, associated with a change in frequency of stool, associated with a change in form (appearance) of stool (Rome IV criteria) [12]; (iv) present diarrhea-predominant IBS (IBS-D) as defined by more than 25% of bowel movements with Bristol stool form type 6 or 7 and less than 25% of bowel movements with Bristol stool form type 1 or patient reports that abnormal bowel movements were usually diarrhea [12]; (v) have no evidence of organic disease at a colonoscopy performed 2 years within the enrollment; and (vi) have increased intestinal permeability measured by means of radioactive tracers. Particularly, to exclude the presence of other underlying organic diseases, enrolled subjects had to provide a colonoscopy performed 5 years within the enrollment showing no evidence of organic disease. Furthermore, other organic diseases, including celiac disease, were excluded during the screening visit based on medical history and common laboratory examinations usually prescribed according to clinical practice and included in the initial patient evaluation for patients with chronic diarrhea in the Digestive Disease Center of Fondazione Policlinico Universitario A. Gemelli IRCCS. These laboratory tests included total IgAs, anti-transglutaminase antibodies, cell blood count, C-reactive protein, C. difficile toxin, fecal culture tests, and research of fecal ova and parasites. Based on these evaluations, subjects were excluded from the study if they had or were suspected to have organic disease (such as celiac disease, inflammatory bowel disease, gastrointestinal neoplasia, unexplained anemia), a history of colonic or small bowel resection, or any major illness or evidence of an unstable clinical condition that could put him/her at risk when participating in the study. Subjects who used or had used probiotics, antibiotics, or investigational agents within 30 days before baseline, those who had repeated use of nonsteroidal anti-inflammatory drugs within 7 days from the enrollment (except for prophylactic use of a stable dose of aspirin up to 325 mg/day for cardiac disease), and those who used any product or ingredient that could affect intestinal permeability were also excluded. Finally, subjects with lactose intolerance and pregnant or breastfeeding women were excluded.
Study Procedures
Study participants with IBS-D and an increased intestinal permeability were screened in a 2-week period, and then, if eligibility was confirmed, they were enrolled consecutively and underwent a 30-day period. Baseline data were collected during the screening visit performed 7–14 days before enrollment (D7 or D14) and at the enrollment visit (D0). Data were then collected after 15 and 30 days of supplementation with the multistrain probiotic (D15 and D30).
At each visit, the physicians carried out a complete physical examination (including body temperature, weight, and vital signs), and the patients completed the Visual Analogue Scale for IBS (VAS-IBS) [13]. Patients also completed the IBS-quality of life questionnaire (IBS-QOL) at baseline and D30 [14, 15], and answered “yes” or “no” to a self-evaluation question assessing their treatment satisfaction (“Do you feel that your IBS symptoms have been satisfactorily alleviated by this treatment?”) on D15 and D30.
Intestinal permeability tests using radionuclide tracers (51Cr-ethylene-diamine-tetra-acetic acid (51Cr-EDTA) or 99mTc-diethylenetriaminepentaacetic acid according to their commercial availability) were performed at the screening visit and on D30 as previously described [16, 17, 18]. At the enrollment visit and D30, blood samples were taken for serum zonulin level measurement (ELISA; Cusabio Technology LLC, Huston, TX, USA) [19, 20, 21], and the Bristol Stool Scale was used to classify the form (appearance) of the feces [22].
Adverse events (AEs) and concomitant medications were recorded at all visits. IBS symptoms were recorded as AEs only if there was an increase in severity or frequency of the symptoms compared to baseline and if the increase was of a greater frequency and/or severity than the normal fluctuations in the disease for the patient.
Included patients took one capsule of the multistrain probiotic Lactibiane Tolérance® (PiLeJe Laboratoire) on an empty stomach twice a day (30 min before breakfast and 30 min before dinner) from D1 to D30. Lactibiane Tolérance® comprises a mixture of 5 viable lyophilized lactic acid bacterial strains (B. lactis LA 303, B. lactis LA 304, L. acidophilus LA 201, L. plantarum LA 301, and L. salivarius LA 302) at a total concentration of 1 × 1010 colony-forming units per capsule.
Statistical Analyses
As the study was a proof-of-concept study to evaluate the effect of the multistrain probiotic, no sample size calculation was performed. A sample size of 30 subjects was estimated to be sufficient to identify trends, which could be used to evaluate sample size for future controlled trials.
For the statistical analysis, patients were assigned before the database lock to each of the analysis sets according to specified definitions. Patients' exclusion from analysis was documented and justified in a Blind Data Review Report. Data were summarized by calculating means and SDs, or percentages.
The primary objective of this study was to demonstrate the effect of the multistrain probiotic in normalizing leaky gut in IBS-D patients. The primary end point was the proportion of patients with normal intestinal permeability after 30 days of treatment. Secondary end points were the proportion of patients answering “yes” to the self-evaluation question; mean levels of serum zonulin; and mean scores for the IBS-QOL, VAS-IBS, and the Bristol Stool Scale. Safety and tolerability were evaluated by the frequency of AEs, SAEs, and AEs leading to discontinuation of study treatment.
The main analysis (intention-to-treat analysis) was performed on the Full Analysis Set (FAS), which included all included patients who took at least one dose of the multistrain probiotic and have had at least one baseline and post-baseline value. A sensitive analysis was conducted on the per protocol set (PPS), which included all patients from the FAS who did not present at least one major deviation to the protocol liable to affect the efficacy of the multistrain probiotic. All analyses were conducted using SAS (SAS Institute, Cary, NC, USA).
Results
Study Follow-Up
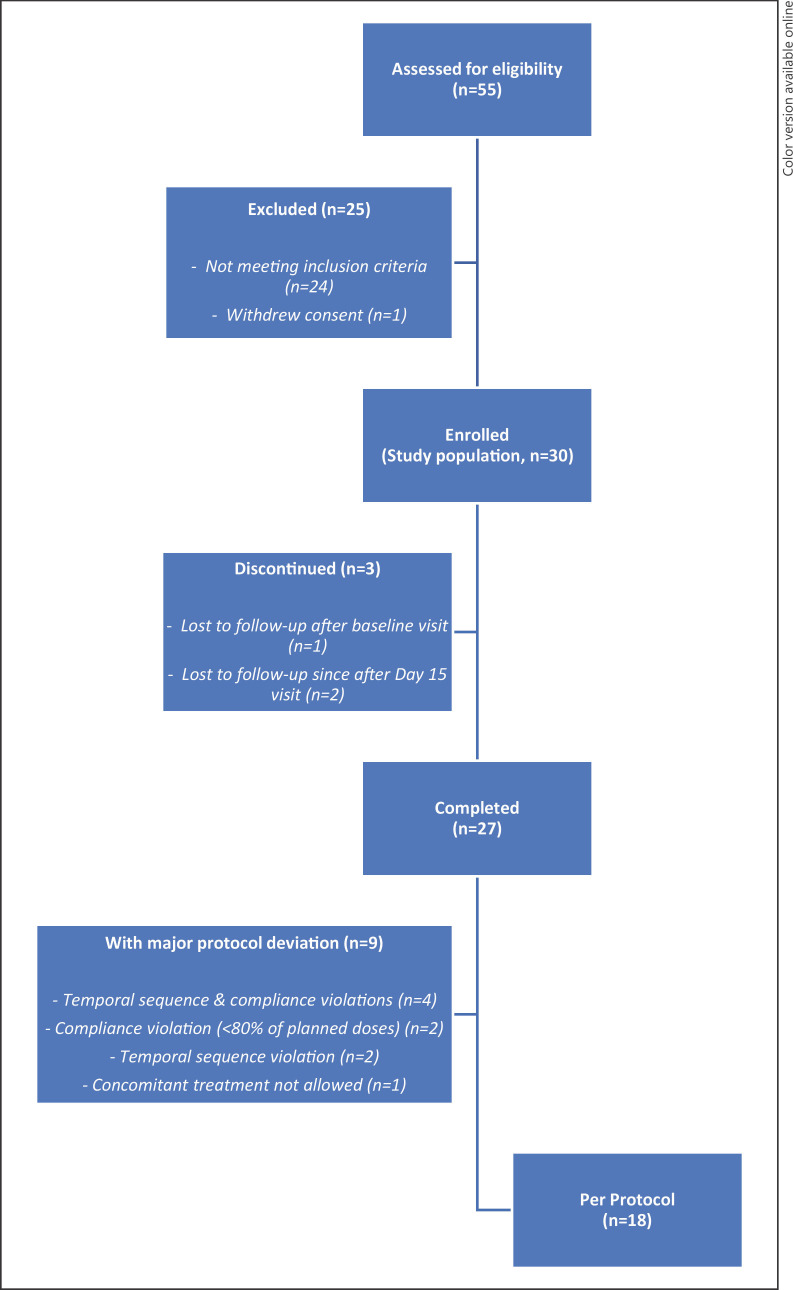
A total of 55 patients were assessed for eligibility (Fig. 1); 25 (45.5%) were excluded because they did not fulfill at least one inclusion criterion (n = 24) or as they withdrew their consent (n = 1). Consequently, 30 patients who met all inclusion criteria and none of the exclusion criteria were enrolled in the study and constituted the study population. Among them, 3 patients prematurely discontinued the study, one being lost to follow-up just after the baseline visit and two after D15 visit. Of the 27 patients who completed the study and took at least one dose of supplement, nine presented with at least one protocol violation. Most of these protocol violations were temporal sequence or compliance violations. The 9 patients with major protocol deviations were excluded from PPS.
Fig. 1.
Study flow diagram.
Baseline Characteristics and Treatment Compliance
Mean age ± standard deviation (SD) was 42.1 ± 13.1 years, ranging from 20 to 73 years (Table 1). 60% of patients were female; and 100% had an IBS diagnosis according to Rome IV criteria. Intestinal permeability assessed by means of radionuclide tracers was above the normal range in all patients, with a mean value of 8.0 ± 4.6 (n = 30).
Table 1.
Demographic and clinical characteristics at baseline (N = 30)
| Characteristics | Enrolled patients with available data | |
|---|---|---|
| Age (years) | Mean (SD) | 42.1 (13.1) |
| Min-max | 20–73 | |
|
| ||
| Gender | ||
| Female | n (%) | 18 (60.0) |
| Male | n (%) | 12 (40.0) |
|
| ||
| Weight, kg | Mean (SD) | 65.3 (15.3) |
| Min-max | 39–95 | |
|
| ||
| Height, cm | Mean (SD) | 168.6 (9.8) |
| Min-max | 152–199 | |
|
| ||
| SBP, mm Hg | Mean (SD) | 115.1 (12.4) |
| Min-max | 90–135 | |
|
| ||
| DBP, mm Hg | Mean (SD) | 66.4 (8.7) |
| Min-max | 50–85 | |
|
| ||
| Heart rate, bpm | Mean (SD) | 67.1 (9.2) |
| Min-max | 53–90 | |
|
| ||
| Respiratory rate | Mean (SD) | 13.1 (1.5) |
| Min-max | 11–17 | |
|
| ||
| IBS diagnosis (Rome IV) | ||
| Yes | n (%) | 30 (100.0) |
|
| ||
| Permeability tests (Radionuclide tracers)a | Mean (SD) Min-max | 8.0 (4.6) 3.1–21.4 |
|
| ||
| Bristol Stool scoreb (3 movements) | Mean (SD) | 5.6 (1.6) |
| Min-max | 2.0–7.0 | |
bpm, beats per minute; DBP, diastolic blood pressure; DTPA, diethylenetriaminepentaacetic acid; EDTA, ethylene-diamine-tetra-acetic acid; mm Hg, millimeter of mercury; SBP, systolic blood pressure; SD, standard deviation.
51Cr-EDTA or 99mTc-diethylenetriaminepentaacetic acid test performed at screening visit.
15 missing data.
Regarding concomitant diagnoses, no patient had concurrent disease: e.g., no patient had celiac disease, inflammatory bowel disease, gastrointestinal neoplasia, or lactose intolerance at baseline, and no patient reported unexplained anemia or history of colon or small bowel resection. In addition to IBS medications such as antispasmodic or antidiarrheic drugs, some patients took medication for anxiety or depression (n = 4), hypertension (n = 4), thyroid problems (n = 3), cardiac disorders (n = 2), vitamin D deficiency (n = 2), gastritis (n = 2), allergy, arthritis, epilepsy, hypercholesterolemia, hyperinsulinism, prostatic hypertrophy, urinary obstruction, or generalized pain (n = 1 for each). One patient took contraceptive and another hormonal therapy.
All enrolled patients took at least one dose of the multistrain probiotic. The 27 patients of the FAS took 88.2 ± 13.9% (mean ± SD; range: 48.3–111.3%), and the 18 patients of the PPS took 95.9 ± 5.6% (range: 84.8–111.3%) of the planned doses during the study period.
Intestinal Permeability Test with Radionuclide Tracers
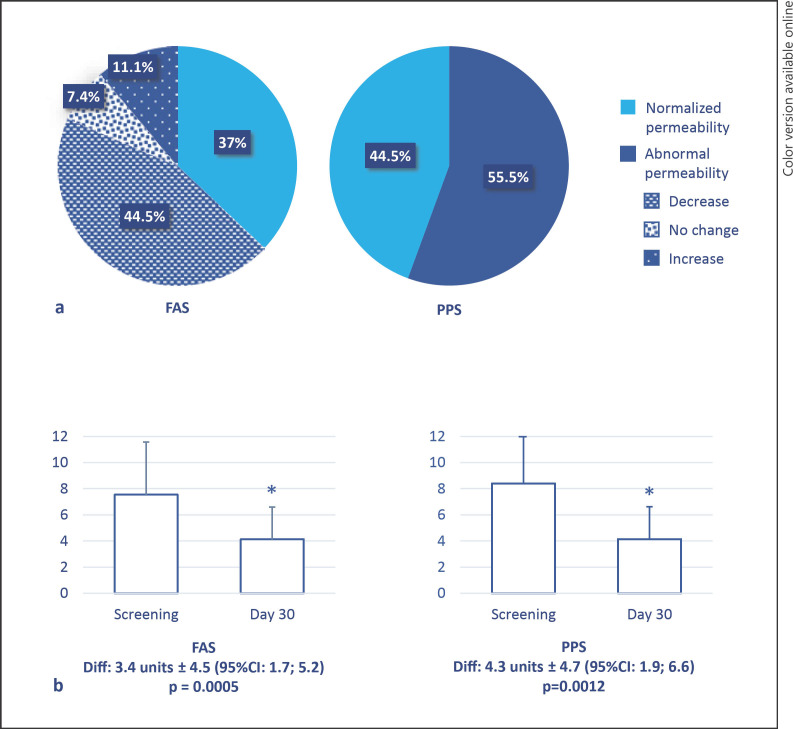
On D30 (n = 27), 10 patients (37%, 95% CI: 19%; 55%) had normalized intestinal permeability as determined using an intestinal permeability test with radionuclide tracers (measures were considered indicative of normal permeability when urinary excretion was less than 3% of the orally administered dose of tracer (Fig. 2). Of note, although intestinal permeability was not normalized, it was also improved in 12 of the 27 patients (44.5%), whereas intestinal permeability was unchanged in 2 patients (7.4%) and increased in 3 patients (11.1%). Therefore, a total of 22 patients (81.5%) showed improvement or normalization of intestinal permeability on D30. From screening to D30, the mean (±SD) intestinal permeability decreased from 7.5 (±4.0) to 4.1 (±2.5), leading to a statistically significant change of 3.4 units ± 4.5 (95% CI: 1.7; 5.2; p = 0.0005; n = 27). In the PPS (n = 18), 8 patients (44.5%, 95% CI: 21%; 67%) had normalized intestinal permeability, and the mean (±SD) intestinal permeability decreased from 8.4 (±4.4) at screening to 4.1 (±2.8) on D30, leading to a statistically significant change of 4.3 units ±4.7 (95% CI: 1.9; 6.6; p = 0.0012).
Fig. 2.
Change in intestinal permeability assessed with radionuclide tracers. a Proportion of patients with normalized and abnormal permeability in the FAS and PPS on day 30 compared to screening. Measures were expressed as percentages of the ingested dose of tracer and considered indicative of normal permeability when urinary excretion was less than 3% of the orally administered dose [37]. b Decrease in intestinal permeability between screening and day 30 in the FAS and PPS (mean ± SD; Diff, difference).
Serum Zonulin Concentration
Regarding serum zonulin concentration, the mean (±SD) concentration slightly decreased from 11.9 ng/mL (±3.9) at baseline to 11.1 ng/mL (±3.4) on D30, with a mean change of −0.6 ng/mL (95% CI: −1.9; 0.6; p = 0.3125). Similar result was observed in the PPS with decreased serum zonulin concentration on D30 (mean [±SD]: 10.4 [±3.1] on D30 versus 11.5 [±4.3] at baseline) but no statistically significant difference from baseline (mean change: −1.07, 95% CI: −2.5; 0.3; p = 0.1257).
Stool Appearance
From Baseline to D30, Bristol Stool Scale score decreased from 5.7 ± 0.8–4.7 ± 1.5 (Diff: 0.9 ± 1.6, 95% CI: 0.3; 1.6; p = 0.0057) in the FAS and from 5.8 ± 0.6–4.7 ± 1.6 (Diff: 1.1 ± 1.4; 95% CI: 0.4; 1.8; p = 0.0052) in the PPS, indicating improved stool consistency.
Patient-Reported Outcomes
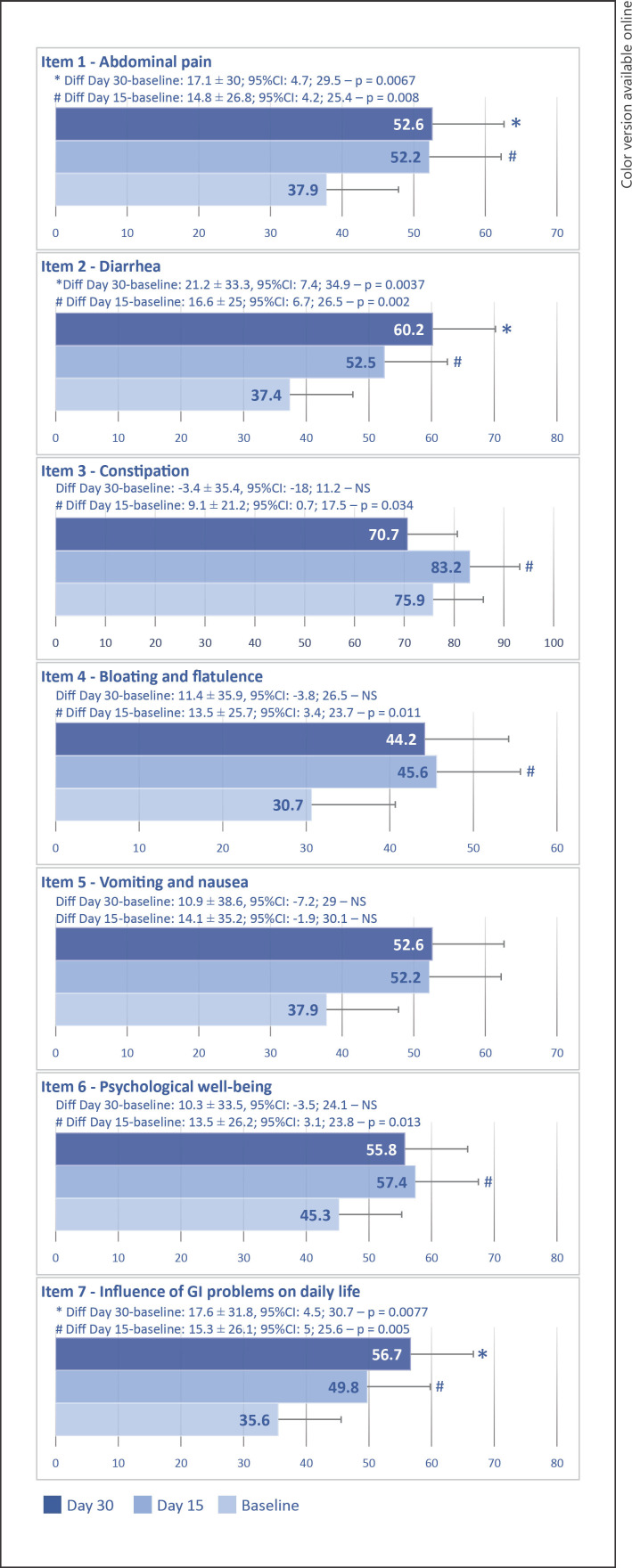
As compared with baseline, mean scores for the seven items of the VAS-IBS had increased on D15, statistical significance being observed for the following items: abdominal pain, diarrhea, constipation, bloating, flatulence, psychological well-being, and daily life (Fig. 3). On D30, changes from baseline in mean scores were statistically significant for the following items: abdominal pain, diarrhea, and daily life.
Fig. 3.
Changes in mean scores for the seven items of the VAS-IBS between baseline and days 15 and 30. Note: the question was “How have you been feeling during the past 2 weeks concerning…?” for item 1 to item 6 and “How much/little have your gastrointestinal problems influenced your daily life over the past 2 weeks?” for item 7.
On D15 and D30, respectively, 57.1% and 52.4% of patients reported having felt a defecation urgency during the last 2 weeks versus 71.4% at baseline. In addition, 71.4% of patients both on D15 and on D30 reported having felt that their bowel was not completely empty after visiting the bathroom during the last 2 weeks versus 81.0% at baseline.
Significant increase in the IBS-QOL total score indicating better health-related quality of life was found after 30 days of supplementation. Total score increased from 61.6 at baseline to 66.8 on D30, leading to a change of 8.0 points (95% CI: 3.0, 12.9). The increase was mainly due to increased scores for the following dimensions: dysphoria, interference with daily activities, food avoidance, and social reaction (Table 2).
Table 2.
Mean (SD) IBS-QOL scores at baseline and D30, with change from baseline following multistrain probiotic supplementation (N = 30)
| Scores | N | Mean | SD | 95% CI | |
|---|---|---|---|---|---|
| Total | Baseline | 22 | 61.6 | 19.6 | |
| (34 items) | D30 | 27 | 66.8 | 19.3 | |
| D30 - baseline | 21 | 8.0 | 10.9 | 3.0; 12.9 | |
|
| |||||
| Dysphoria | Baseline | 22 | 53.1 | 25.5 | |
| (9 items) | D30 | 27 | 62.0 | 25.2 | |
| D30 - baseline | 21 | 11.2 | 16.2 | 3.8; 18.5 | |
|
| |||||
| Interference with | Baseline | 22 | 57.8 | 26.2 | |
| daily activity | D30 | 27 | 61.6 | 25.6 | |
| (5 items) | D30 - baseline | 21 | 7.5 | 13.0 | 1.6; 13.4 |
|
| |||||
| Body image | Baseline | 26 | 67.3 | 18.7 | |
| (2 items) | D30 | 27 | 72.0 | 18.2 | |
| D30 - baseline | 25 | 3.8 | 16.6 | −3.1; 10.6 | |
|
| |||||
| Health worry | Baseline | 22 | 64.8 | 19.1 | |
| (2 items) | D30 | 27 | 68.8 | 20.0 | |
| D30 - baseline | 21 | 4.4 | 14.1 | −2.1; 10.8 | |
|
| |||||
| Food avoidance | Baseline | 26 | 35.3 | 25.1 | |
| (3 items) | D30 | 27 | 45.4 | 27.9 | |
| D30 - baseline | 25 | 10.7 | 21.2 | 1.9; 19.4 | |
|
| |||||
| Social reaction | Baseline | 22 | 73.0 | 21.3 | |
| (2 items) | D30 | 27 | 74.5 | 22.7 | |
| D30 - baseline | 21 | 6.3 | 12.2 | 0.7; 11.8 | |
|
| |||||
| Sexual concerns | Baseline | 26 | 85.1 | 14.6 | |
| (2 items) | D30 | 27 | 89.4 | 16.9 | |
| D30 - baseline | 25 | 4.5 | 13.0 | −0.9; 9.9 | |
|
| |||||
| Interpersonal relations | Baseline | 22 | 74.2 | 25.8 | |
| (9 items) | D30 | 27 | 78.1 | 20.4 | |
| D30 - baseline | 21 | 8.8 | 20.5 | −0.6; 18.1 | |
IBS-QOL, irritable bowel syndrome-quality of life questionnaire; CI, confidence interval; D, day; SD, standard deviation.
Finally, on D15 and D30, using the self-evaluation of symptom improvement, 96.3% of patients (26/27) claimed that their IBS symptoms have been satisfactorily alleviated using the multistrain probiotic supplementation. Similar results were observed in the PPS.
Safety and Tolerability
During the study, there were three AEs, all being GI disorders and all involving a single patient, in the same period. These AEs were of moderate severity and resolved within a week. These AEs (abdominal distension, abdominal pain, and defecation disorder) were consistent with usually reported symptoms in IBS patients. Relation with study treatment was considered unlikely by the investigator. No obvious changes were noted in weight or vital signs (blood pressure, and heart and respiratory rates). No patients were withdrawn from the study due to AEs, and no death was reported.
Discussion
Following 30-day supplementation with the multistrain probiotic evaluated, this pilot study found (1) an improvement of intestinal permeability measured with radionuclide tracers on D30 in 81.5% of patients, normalization being observed in 37% of the participants (44.5% of those who took the supplementation as per protocol); (2) improved stool consistency according to the Bristol Stool Scale score; (3) a significant improvement for the items 1, 2, and 7 of the VAS-IBS (i.e., abdominal pain, diarrhea, impact of gastrointestinal problems in daily life), which was significant from the first 15 days and then maintained over 30 days; (4) a significant improvement in health-related quality of life as assessed by the IBS-QOL total score; and (5) a large proportion of patients claiming that their symptoms had been alleviated (96.3%). The paucity of AEs and the absence of obvious changes in vital signs suggested that the multistrain probiotic was well tolerated.
Confounding factors were investigated, including the influence of lifestyle changes and weight. All the patients maintained the same weight with the exception of two who experienced 5.7% and 8.3% of weight loss (data not shown). In addition, a multivariate analysis (data not shown) revealed no impact of BMI on intestinal permeability outcomes. No other significant factors potentially explicative of the changes were identified in these analyses. It should also be noted that patients did not modify their concomitant treatments during the study.
Several randomized, placebo-controlled clinical trials evaluating the effect of different probiotics in IBS and IBS-D patients corroborate these data with a relief of symptoms, including abdominal pain and an amelioration of quality of life [4, 23, 24, 25]. Briefly, in a randomized placebo-controlled study including 400 adult patients with moderate to severe IBS-D, Ishaque et al. [4] showed that a multistrain probiotic formulation was well tolerated and associated with significant improvement in IBS-D symptoms and quality of life. Evaluating the effectiveness and safety of a multistrain probiotic preparation as compared with placebo in patients with IBS-D, Skrzydło-Radomanska et al. [23] found that the occurrence of AEs did not differ between the probiotic and placebo groups and that the probiotic significantly improved IBS symptoms and quality of life as compared with a placebo. In the study by Lewis et al. [24], 251 adults with either constipation, diarrhea (IBS-D), or mixed-pattern were randomized to receive one of the two probiotic strains studied or a placebo. Both strains were safe to consume, with no significant difference between the two probiotic groups and the placebo group, and may reduce IBS symptoms severity and improve the psychological well-being of IBS-affected individuals. In a randomized study involving 336 patients, Martoni et al. [25] evaluated the ability of two probiotics to improve abdominal pain and IBS symptomatology as compared with a placebo and found that both probiotics were well tolerated and improved abdominal pain and symptom severity scores with a corresponding normalization of bowel habits.
None of the abovementioned randomized, placebo-controlled clinical trials evaluated intestinal permeability. Intestinal permeability in patients with IBS after taking probiotics was measured in a few studies by using sugar tests [26, 27, 28]. In the randomized, single-blind placebo-controlled study by Zeng et al. [26], 30 patients with IBS-D were given either probiotic fermented milk (Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus acidophilus, and Bifidobacterium longum) or milk beverage containing no bacteria for 4 weeks. Small bowel permeability was measured as the ratio of lactulose and mannitol recovery, and colonic permeability was measured as the total mass of sucralose excretion. After supplementation with probiotics, small bowel permeability decreased significantly from 0.038 ± 0.024 at baseline to 0.023 ± 0.020 (p = 0.004). By contrast, colonic permeability improved neither in the probiotic group nor in the placebo group at week 4. In the Phase I, open-label trial by Boonma et al. [27], a 4 or 8-week supplementation with VSL#3 in 21 patients with IBS (not specifically IBS-D) led to a significant decrease in colonic permeability (per cent sucralose recovery) at week 8 (not significant after 4 weeks; data obtained in 5 patients). In a crossover double-blind randomized controlled study performed by Bonfrate et al. [28], in 25 patients with IBS supplemented with Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 with vitamin B6 for 30 days, a reduction of colonic permeability was observed in 5 patients who had increased permeability at baseline. One study measured serum zonulin levels in patients with IBS-D supplemented with probiotics [29]. In this recent study, Bifidobacterium longum ES1 (8 or 12 weeks at 1 × 109 colony-forming units/day) was evaluated in the treatment of 16 patients with IBS-D. Serum zonulin and cytokines were measured, and the clinical response was assessed by IBS-SSS. There was no significant reduction in serum zonulin levels in the overall study population. Zonulin levels diminished only in patients treated for 12 weeks (from 43.8 ± 6.8 ng/mL to 40.8 ± 5.0 ng/mL, p = 0.036), while interleukin (IL)-6, IL-8, IL-12p70, and tumor necrosis factor-α levels decreased, irrespective of treatment duration (p < 0.05). Clinical response was observed in 5/16 patients (31%): 4/8 (50%) were treated for 12 weeks and 1/8 (13%) were treated for 8 weeks. Abdominal pain improved only in patients treated for 12 weeks (5/8 vs. 0/8, p = 0.025), while stool consistency improved regardless of supplementation duration (p < 0.001). In another study, this is fecal zonulin levels that were measured in 10 patients with IBS-D supplemented with a synbiotic for 4 weeks [30]. They observed a reduction of fecal zonulin concentration (from 67 to 36 ng/mL; p = 0.035) along with an increased microbial diversity in gastric (p = 0.008) and duodenal (p = 0.025) mucosal specimens, reduction of CD4+ T cells (60 vs. 55%, p = 0.042) in the ascending colon, an increase in short-chain fatty acids (acetate 101 vs. 202 µmol/g; p = 0.007) and butyrate (27 vs. 40 µmol/g; p = 0.037) in fecal samples after treatment and a decrease of disease severity measured by IBS-SSS (237 vs. 54; p = 0.002). In our study, there was a tendency toward lower serum zonulin concentrations after than before supplementation. The lack of significance observed for serum zonulin concentration may be explained by the fact that baseline concentrations were within normal range (0.64–40 ng/mL according to the laboratory) and the low number of included patients. Zonulin is a protein that reversibly modulates intercellular tight junctions and is therefore a regulator of intestinal permeability. As such, zonulin is used as a marker of intestinal barrier impairment [31]. However, the use of serum zonulin as a marker is controversial, and the possibility that the method was not enough specific cannot be ruled out [31, 32]. First, commercial ELISAs have shortcomings that have been discussed in previous articles: measurements using these assays do not reflect actual zonulin levels but concentrations of other proteins [32, 33, 34]. Second, in several studies, zonulin failed to identify conditions where small intestinal permeability is a known characteristic [31, 33, 34]. In addition, in a recent study, it was shown that colonic permeability had no correlation with circulating zonulin levels; the authors concluded that they could not recognize the molecule as a circulating marker of colonic permeability [35].
The multistrain probiotic evaluated in the present study had already been characterized for its anti-inflammatory properties [36]. It was also investigated in vitro for its effects on the epithelial barrier function of confluent human colonic T84 cell monolayers in basal and inflammatory conditions and in mice for its impact on chronic stress-induced changes to intestinal permeability and visceral sensitivity (note that 51Cr-EDTA was used in this study) [9]. The present study completes and supports in humans (although the results will need to be confirmed in larger studies) what these preclinical studies had shown: the ability of the multistrain probiotic to reduce intestinal permeability and to alleviate IBS symptoms. The limitations of the clinical study are those of a pilot, open-label, single-center, proof-of-concept study: small sample size, no randomization, no comparison with a treatment or placebo arm. Therefore, the interpretation of these results must be tempered by recognition of these limitations. However, the results being promising of course, it would be worthwhile to continue the evaluation of this combination of bacterial strains in a larger population with a control group. It would also be interesting to evaluate its anti-inflammatory effects in humans and on the gut microbiota, as it has been reported that microbiota composition is strongly compromised in IBS. Furthermore, the interest of supplementation with this probiotic in other subpopulations of patients with IBS, IBS with constipation, or even IBS-D without leaky gut would deserve to be evaluated.
Conclusion
This study provides data suggesting that the multistrain probiotic tested may improve intestinal permeability in a considerable proportion of patients. Almost all patients (96.3%) claimed that their IBS symptoms were satisfactorily alleviated with the supplementation after 30 days. A significant improvement in stool consistency, abdominal pain, diarrhea, impact of gastrointestinal problems in daily life, and the total score of the IBS-QOL was reported. The multistrain probiotic was well tolerated. At this point, larger, placebo-controlled studies are needed to confirm this new information on the effect of the multistrain probiotic tested on leaky gut syndrome associated with IBS-D.
Statement of Ethics
This study, whose protocol and subject information form were approved on December 27, 2017 (Ethics Committee of Fondazione Policlinico A. Gemelli IRCCS; Protocol No. 5000/17-ID1801), was conducted in accordance with ICH GCP, all applicable subject privacy requirements, and the ethical principles that are outlined in the Declaration of Helsinki (2008). It took place in a single center in Italy (Fondazione Policlinico Universitario A. Gemelli, Roma). Written informed consent was obtained from participants. The first patient was enrolled on June 22, 2018, and the last visit of the last patient occurred on October 8, 2020. The study is registered on the ClinicalTrials.gov site under number NCT04898257. A TREND statement checklist is provided as supplementary data (see www.karger.com/doi/10.1159/000526712 for all online suppl. material).
Conflict of Interest Statement
Samira Ait Abdellah and Caroline Gal are employees of PiLeJe Laboratoire. Lucrezia Laterza is a lecturer for Janssen and a consultant for Actial Farmaceutica. Antonio Gasbarrini is an associate director of the journal. Other authors declare no conflict of interest.
Funding Sources
This study was sponsored by PiLeJe Laboratoire.
Author Contributions
Antonio Gasbarrini and Samira Ait Abdellah: conception and design of the study; Lucrezia Laterza, Venanzio Valenza, Carlo Romano Settanni, Marco Napoli, Elisa Schiavoni, Vincenzina Mora, and Valentina Petito: conduct of the study; Caroline Gal: follow-up of the study; All authors: manuscript writing, critical revision, and approval of the final version.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgment
The authors want to thank Claude Blondeau (PiLeJe Laboratoire), Matthieu Chanard, and Fabienne Péretz (Abelia Science) for their help in preparing this manuscript.
Funding Statement
This study was sponsored by PiLeJe Laboratoire.
References
- 1.Moayyedi P, Mearin F, Azpiroz F, Andresen V, Barbara G, Corsetti M, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J. 2017;5((6)):773–788. doi: 10.1177/2050640617731968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5((10)):908–917. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 3.Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46((Suppl l)):S52–S55. doi: 10.1097/MCG.0b013e318264e918. [DOI] [PubMed] [Google Scholar]
- 4.Ishaque SM, Khosruzzaman SM, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18((1)):71. doi: 10.1186/s12876-018-0788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley EMM, Fried M, Gwee K-A, Khalif I, Hungin P, Lindberg G, et al. Irritable bowel syndrome: a global perspective 2015. Available from: https://www.worldgastroenterology.org/UserFiles/file/guidelines/irritable-bowel-syndrome-english-2015.pdf. [DOI] [PubMed]
- 6.Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70((7)):1214–1240. doi: 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- 7.Gecse K, Roka R, Sera T, Rosztoczy A, Annahazi A, Izbeki F, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2012;85((1)):40–46. doi: 10.1159/000333083. [DOI] [PubMed] [Google Scholar]
- 8.La Fata G, Weber P, Mohajeri MH. Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob Proteins. 2018;10((1)):11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nebot-Vivinus M, Harkat C, Bzioueche H, Cartier C, Plichon-Dainese R, Moussa L, et al. Multispecies probiotic protects gut barrier function in experimental models. World J Gastroenterol. 2014;20((22)):6832–6843. doi: 10.3748/wjg.v20.i22.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586. doi: 10.1177/1756284821993586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146((1–2)):41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150((6)):1393–407.e5. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Bengtsson M, Ohlsson B, Ulander K. Development and psychometric testing of the visual analogue scale for irritable bowel syndrome (VAS-IBS) BMC Gastroenterol. 2007;7:16. doi: 10.1186/1471-230X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43((2)):400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 15.Andrae DA, Patrick DL, Drossman DA, Covington PS. Evaluation of the irritable bowel syndrome quality of life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual Life Outcomes. 2013;11:208. doi: 10.1186/1477-7525-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjarnason I, Peters TJ, Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet. 1983;1((8320)):323–325. doi: 10.1016/s0140-6736(83)91628-8. [DOI] [PubMed] [Google Scholar]
- 17.Resnick RH, Royal H, Marshall W, Barron R, Werth T. Intestinal permeability in gastrointestinal disorders. Use of oral [99mTc]DTPA. Dig Dis Sci. 1990;35((2)):205–211. doi: 10.1007/BF01536764. [DOI] [PubMed] [Google Scholar]
- 18.Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105((2)):323–327. doi: 10.1038/ajg.2009.558. [DOI] [PubMed] [Google Scholar]
- 19.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10:1096–10100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106((39)):16799–16804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh P, Silvester J, Chen X, Xu H, Sawhney V, Rangan V, et al. Serum zonulin is elevated in IBS and correlates with stool frequency in IBS-D. United European Gastroenterol J. 2019;7((5)):709–715. doi: 10.1177/2050640619826419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32((9)):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 23.Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierła JB, Kanarek E, et al. The effectiveness and safety of multi-strain probiotic preparation in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled study. Nutrients. 2021;13((3)):756. doi: 10.3390/nu13030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis ED, Antony JM, Crowley DC, Piano A, Bhardwaj R, Tompkins TA, et al. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in alleviating symptoms of Irritable Bowel Syndrome (IBS): a randomized, placebo-controlled study. Nutrients. 2020;12((4)):E1159. doi: 10.3390/nu12041159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 improve abdominal pain severity and symptomology in irritable bowel syndrome: randomized controlled trial. Nutrients. 2020;12((2)):E363. doi: 10.3390/nu12020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng J, Li Y-Q, Zuo X-L, Zhen Y-B, Yang J, Liu C-H. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28((8)):994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 27.Boonma P, Shapiro JM, Hollister EB, Badu S, Wu Q, Weidler EM, et al. Probiotic VSL#3 treatment reduces colonic permeability and abdominal pain symptoms in patients with irritable bowel syndrome. Front Pain Res. 2021;2:S230. doi: 10.3389/fpain.2021.691689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonfrate L, Di Palo DM, Celano G, Albert A, Vitellio P, De Angelis M, et al. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur J Clin Invest. 2020;50((3)):e13201. doi: 10.1111/eci.13201. [DOI] [PubMed] [Google Scholar]
- 29.Caviglia GP, Tucci A, Pellicano R, Fagoonee S, Rosso C, Abate ML, et al. Clinical response and changes of cytokines and zonulin levels in patients with diarrhoea-predominant irritable bowel syndrome treated with Bifidobacterium longum ES1 for 8 or 12 weeks: a preliminary report. J Clin Med. 2020;9:2353. doi: 10.3390/jcm9082353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser AM, Spindelboeck W, Halwachs B, Strohmaier H, Kump P, Gorkiewicz G, et al. Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur J Nutr. 2019;58((7)):2767–2778. doi: 10.1007/s00394-018-1826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talley NJ, Holtmann GJ, Jones M, Koloski NA, Walker MM, Burns G, et al. Zonulin in serum as a biomarker fails to identify the IBS, functional dyspepsia and non-coeliac wheat sensitivity. Gut. 2020;69((9)):1–3. doi: 10.1136/gutjnl-2019-318664. [DOI] [PubMed] [Google Scholar]
- 32.Massier L, Chakaroun R, Kovacs P, Heiker JT. Blurring the picture in leaky gut research: How shortcomings of zonulin as a biomarker mislead the field of intestinal permeability. Gut. 2021;70((9)):1801–182. doi: 10.1136/gutjnl-2020-323026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffler L, Crane A, Heyne H, Tönjes A, Schleinitz D, Ihling CH, et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol. 2018;9:189. doi: 10.3389/fendo.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ajamian M, Steer D, Rosella G, Gibson PR, D'Auria S. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLoS One. 2019;14((1)):e0210728. doi: 10.1371/journal.pone.0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meira de-Faria F, Bednarska O, Ström M, Söderholm JD, Walter SA, Keita ÅV. Colonic paracellular permeability and circulating zonulin-related proteins. Scand J Gastroenterol. 2021;56((4)):424–431. doi: 10.1080/00365521.2021.1879247. [DOI] [PubMed] [Google Scholar]
- 36.Drouault-Holowacz S, Foligne B, Dennin V, Goudercourt D, Terpend K, Burckel A, et al. Anti-inflammatory potential of the probiotic dietary supplement lactibiane tolerance: in vitro and in vivo considerations. Clin Nutr. 2006;25((6)):994–1003. doi: 10.1016/j.clnu.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Rossi M, Sganga G, Mazzone M, Valenza V, Guarneri S, Portale G, et al. Cardiopulmonary bypass in man: role of the intestine in a self-limiting inflammatory response with demonstrable bacterial translocation. Ann Thorac Surg. 2004;77((2)):612–618. doi: 10.1016/S0003-4975(03)01520-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.