Abstract
Introduction
Low-frequency electromagnetic field (50 Hz) (EMF) can modify crucial neuronal processes. Existing data indicate that exposure to EMF may represent a mild stressor and contribute to disturbances of the hypothalamic-pituitary-adrenal (HPA) axis. The important regulatory pathways controlling HPA axis activity include two types of corticosteroid receptors: mineralocorticoid receptors (MRs) and glucocorticoid receptors. They are particularly abundant in the hippocampus, a key locus of HPA axis feedback control. The research aimed at determining whether (1) EMF exhibits hormesis, it means bidirectional action depending on EMF intensity (1 or 7 mT) and (2) repeated EMF exposure changes stress response to subsequent stress factors.
Methods
The exposure (7 days, 1 h/day) of adult rats to EMF (1 mT and 7 mT) was repeated 3 times. HPA axis hormones and their receptors were analysed after each following exposure. Moreover, the impact of EMF exposure on hormonal and behavioural responses to subsequent stress factor − open-field test was evaluated.
Results
Our data suggest that exposure to EMF can establish a new “set-point” for HPA axis activity. The direction and dynamics of this process depend on the intensity of EMF and the number of exposures. EMF of 1 mT induced an adaptive stress response, but 7 mT EMF caused sensitization. Consequently, EMF changed the vulnerability of the organism to a subsequent stress factor. We have also shown the increase in MR mRNA abundance in the hippocampus of 1 mT EMF-exposed rats, which can represent the possible neuroprotective response and suggest therapeutic properties of EMFs.
Keywords: Low-frequency electromagnetic field, Stress response, Hypothalamic-pituitary-adrenal axis, Open-field test, Mineralocorticoid receptor
Introduction
Exposure to electromagnetic field (EMF) has been suggested to adversely impact mammalian biology. EMF is pervasive in the contemporary environment, especially at extremely low frequencies (30–300 Hz) such as those emitted by electrical appliances and overhead power lines [1]. There is considerable interest in the health effects of EMF exposure (WHO 2007). Targets for EMF in the organism include the cell membrane (e.g., its permeability, inorganic ion transport, receptor function), second messenger synthesis, chromosome structural changes and chemical changes in DNA structure, gene expression, and protein synthesis [2, 3, 4, 5, 6, 7, 8, 9, 10].
Although some findings indicate the deteriorating effects of EMFs on stress responses (a detrimental increase in free radical levels and radical-evoked damages in macromolecules) [11], others failed to detect any effects [1, 12, 13, 14, 15, 16]. Indeed, some reports have shown that EMF of low intensity exerts a neuroprotective influence (the production of protective proteins [e.g., Hsp70 or BDNF] or an increase in the activity of antioxidant enzymes) [17, 18, 19, 20, 21].
No explanations have addressed the potential mechanisms for these contradictory results. We postulate that the direction and nature of EMF-induced changes depend on EMF intensity (magnetic flux density).
Many studies have suggested an association between chronic EMF exposure and psychiatric disorders [22, 23]. Existing data indicate that exposure to EMF may count as a mild stressor. This could lead to disturbances of neuroendocrine stress systems, notably of the hypothalamic-pituitary-adrenal (HPA) axis [13, 14, 24, 25, 26]. The HPA axis is regulated primarily through negative feedback mechanisms. Stress induces the hypothalamus (HYP) paraventricular nucleus to secrete corticotropin-releasing hormone (CRH) which stimulates anterior pituitary corticotrophs to secrete adrenocorticotrophic hormone (ACTH) through activation of CRH type 1 receptors (CRH-R1). Systemically released ACTH acts on the adrenal glands (AG, via melanocortin receptor 2 [MC2R]) to release glucocorticoids (corticosterone in rats) [27, 28]. Corticosterone initiates physiological and behavioural responses through widely expressed nuclear receptors of two types: mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs). Both MRs and GRs are particularly abundant in the hippocampus (HIP), where they underpin cognitive as well as neuroendocrine feedback effects [28, 29].
In some cases, high doses of chemical or environmental factors are detrimental to biological systems, whereas low doses induce endogenous survival systems, a phenomenon known as hormesis [30, 31, 32]. The hormetic dose-response relationship can occur after an initial homoeostasis disruption, which must then be re-established. It requires gene expression and protein synthesis that progresses over time; thus, the temporal feature of hormesis determines its final effect [30]. We hypothesized that initial exposure to EMF moves up or down the set-point of stress system activity; thus, each next EMF exposure would overlap the set-point status of stress response system activity established under the previous EMF exposure. Therefore, we explored whether the effect of EMF is hormetic and results in different activation of the stress system and the direction and dynamics of which depend on the intensity of the field.
Consequently, EMF can change the vulnerability of the organism to subsequent stress factors and thus to diseases, mainly related to the nervous system, in a two-way manner: compensatory or deleterious ones. The important mediators of this phenomenon can be corticosterone receptors, MRs and GRs, known to modulate the hippocampal function and determine the plasticity processes in this area [29, 33, 34, 35].
To explore this hypothesis, we determined the directions and dynamics of EMF-induced changes in stress system activity. Specifically, we examined (1) whether any adaptation of the HPA system occurs after repeated EMF applications, (2) if there is a relationship between these processes and the intensity of the EMF, and (3) whether EMF exposure changes the HPA axis response to a subsequent physiological stressor, and (4) what possible mechanisms might account for the impacts of repeated EMF exposure.
Materials and Methods
Animals
A total of 179 3-month-old male Wistar rats weighing 300–350 g were used. The number of animals was planned in accordance with 3R principles (Replacement, Reduction, and Refinement; EU Directive 2010/63/EU) [36]. Rats were housed in acrylic cages lined with wood shavings and kept on a 12:12 h day-night schedule (lights on at 7:00 a.m.) under standard laboratory conditions (temperature: 22 ± 2°C, humidity 55 ± 10%). Standard laboratory chow and water were available ad libitum. All procedures were approved by the Local Committee for Ethics in Animal Research in Bydgoszcz, Poland (decision number: 3/2018).
Experimental Design
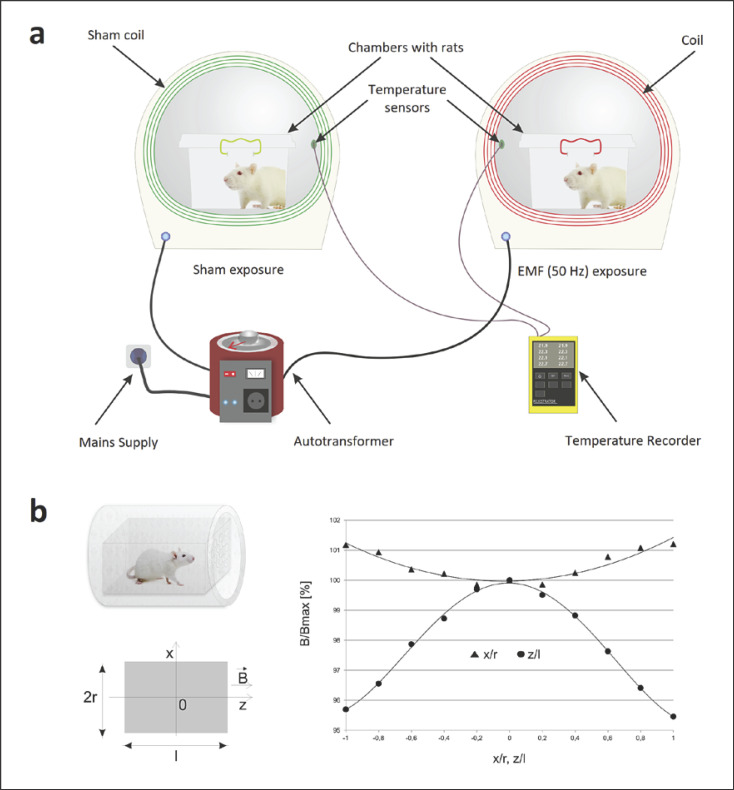
Experiments were conducted using the applicator (coil) used in magnetotherapy as a source of EMF (Elektronika i Elektromedycyna Sp. J., Otwock, Poland). The system for EMF exposure is shown in Figure 1. An EMF with the domination of magnetic component was generated by a 20-cm-diameter coil composed of 282 turns of copper wire. A detailed description of the apparatus has been published elsewhere [37, 38]. The coil and variable autotransformer power supply produced homogeneous, sine-wave alternating EMF at 50 Hz and with intensities 1 and 7 mT. The magnetic flux density was measured before each experiment using a Gauss metre (Model GM2; AlphaLab, Inc, USA). The non-homogeneity of the EMF within the area containing the animal's cage was approximately 10%. Control groups of animals were handled in an identical manner (in the sham exposure system with similar experimental procedures) to obtain the same experimental conditions, except for the presence of EMF (<10 µT). The temperature during experiments (for both control and EMF-exposed groups) was monitored using thermocouples mounted under each exposure system and was set to 26 ± 1°C. Experiments were conducted in an isolated room (with controlled light and temperature T = 23 ± 1°C). In our study, the EMF parameters were based on European Union Directive 2013/35/EU [39], which suggests that the limitation of EMF (50 Hz) exposure to 1 mT reduces the potential for damage, while exposure to EMF >6 mT causes measurable biological effects, e.g., DNA damage [8] or the generation of neuronal networks synchrony firing [40]. Such EMF parameters are commonly applied in magnetotherapy. Rats were repeatedly exposed for 1 h/day for 7 days to an EMF (50 Hz, 1 mT, or 7 mT) in a scheme corresponding to the extensive therapeutic application (frequency lower than 100 Hz, a magnetic flux density ranging from 0.1 mT to 20 mT, periods of exposure 30–60 min for 1–2 weeks) [41].
Fig. 1.
EMF exposure system. a The scheme of the EMF treatment. b The scheme of the coil with the plastic box containing the rat; the coordinates system; the distribution of the EMF along the main axis of the coil within the area of the animal's cage. B, magnetic flux density vector; B/Bmax, normalized magnetic flux density relative to the maximal value; z/l, normalized distance from the coil centre along z-axis; x/r, normalized distance from the solenoid centre along x-axis; l, coil length; r, coil radius.
After 7 days of habituation to the laboratory environment, each rat was placed in an opaque plastic box (12 cm × 20 cm × 14 cm) with a perforated plexiglass cover and with wood shaving bedding. Randomly assigned boxes were put into the centre of the EMF coil or sham exposure system (control). Animals were able to move freely within their chambers. After exposure to EMF or control conditions, the concentrations of HPA axis hormones and relative mRNA transcript abundance of their receptors were assessed − described in this research as “basal” (B) level. The second set of experiments evaluated open-field (OF) stress-induced levels of these parameters in animals previously exposed to EMF or control conditions. All procedures were carried out between 09:00 and 12:00 to avoid circadian fluctuations.
The rats were divided into the following groups.
EMF/B/1mT − animals exposed to EMF (50 Hz, 1 mT),
EMF/OF/1mT − animals exposed to EMF (50 Hz, 1 mT) and subsequently exposed to OF test,
EMF/B/7mT − animals exposed to EMF (50 Hz, 7 mT),
EMF/OF/7mT − animals exposed to EMF (50 Hz, 7 mT) and subsequently exposed to OF test,
C/B − control animals subjected to the same experimental procedure as the experimental groups 1, 3, without EMF exposure,
C/OF − control animals subjected to the same experimental procedure as the experimental groups 2, 4, without EMF exposure.
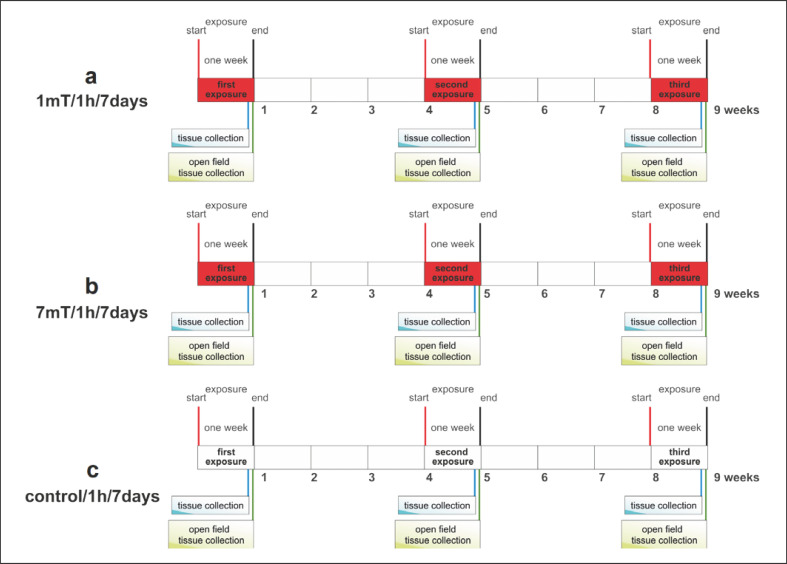
The experimental design is shown in Figure 2. Rats from groups EMF/B/1mT, EMF/OF/1mT, EMF/B/7mT, and EMF/OF/7mT were exposed for three periods (E1, E2, E3) of low (1 mT) or high (7 mT) EMF level every 3 weeks. We have chosen such a schedule as our previous experiments showed that the effect of a single exposure to EMF was still observed 3 weeks later in the case of EMF of 1 mT and even after 3 months from the end of the exposure to 7 mT [unpublished data].
Fig. 2.
Experimental design.
Each period included 7-day exposure, 1 h a day. After each period of exposure, a subset of rats from groups EMF/B/1mT and EMF/B/7mT was killed by decapitation to assess the effects of single or recurrent EMF exposure. Animals from groups EMF/OF/1mT and EMF/OF/7mT were tested in an OF apparatus, after E1, E2, or E3, and then decapitated to determine stress-induced changes in parameters as a consequence of prior exposure to EMF. Control rats (C/B and C/OF) were subjected to exactly the same experimental procedure but without EMF exposure. Body weights were monitored throughout the experimental period, but no changes were found.
The influence of EMF on concentrations of HPA axis hormones and receptors' relative mRNA transcript abundance in relevant brain structures, organs, and tissues was determined: CRH in the HYP, CRH-R1 and ACTH in the pituitary gland (PG), MC2R and CORT in the AG, ACTH and CORT in plasma as well as MRs and GRs in the HIP. To evaluate the direction and dynamics of the changes in the level of stress markers, they were analysed after each period (E1–E3) of 7-day exposure to control conditions or to EMF (1 mT and 7 mT) (“basal” level of parameters) and in animals from EMF-exposed groups and control group tested in OF apparatus (OF-induced level of parameters).
Sample Collection
Following decapitation, blood samples were collected via cardiac puncture in a solution of ethylenediaminetetraacetic acid disodium salt (Na2EDTA; Sigma-Aldrich, Germany). After centrifugation (20 min, 2,000 rpm), the resulting plasma was stored at −20°C until future ELISA analysis.
The organs (brains and AG) were quickly removed. From the brain, the following areas were dissected: the HYP, HIP, and PG. Each of the collected structures was weighed and immediately frozen in liquid nitrogen and stored at −80°C until further reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and ELISA analyses.
Determination of HPA Axis Hormone Concentrations: CORT, ACTH, CRH
Tissues were homogenized on ice in a pre-cooled PBS buffer containing a proteinase inhibitor cocktail (Roche Cat. No. 11836145001) (100 mg of tissue per millilitre). Rat Corticoliberin, Rat Corticotropin, and General Corticosterone ELISA kits were used to determine the concentrations of the hormones (EIAab Cat. No. E0835r, E0626r, E0540Ge, respectively). All procedures were conducted according to standard guidelines provided by the manufacturers. Each sample was measured in duplicate. Colourimetric changes in the assay were detected using a multi-mode microplate reader, Epoch 2 (BioTek Instruments, Inc., Winooski, UT, USA). The optical density was measured at 450 nm, setting the optical density of the blank well to zero. A standard curve was created by reducing the data using Gen5 Software, generating a four-parameter logistic curve-fit.
Assay of mRNA Encoding Receptors: GRs, MRs, MC2R, CRH-R1
RT-qPCR was performed to determine the relative mRNA transcript abundance of GRs, MRs, MC2R, CRH-R1 in collected organs. Total RNA was extracted using an HP RNA tissue kit RNA (Roche Cat. No. 11828665001), and concentration was measured by absorbance at 260 nm (A260/280) using a NanoDrop 2000 (Thermo Fisher). Approximately 1 μg of total RNA was used to generate complementary DNA (cDNA) by reverse transcription using EvoScript Universal cDNA Master (Roche Cat. No. 07 912 455 001) and random hexamer primers according to manufacturer's instructions. Next, 10 ng of cDNA was used to amplify the target gene by real-time qPCR using 0.2 µM specific primers (Sigma-Aldrich) and SYBR Green PCR Master Mix (Roche Cat. No. 12239264001) on LightCycler® 96 System (Roche) (online suppl. Table S1; see www.karger.com/doi/10.1159/000527878 for all online suppl. material). Initial denaturation was performed at 95°C for 10 min, followed by 50 cycles of 95°C for 15 s primer annealing (58–64°C). β-Actin and GAPDH were evaluated as housekeeping genes using the BestKeeper approach to determine the stability of gene transcript abundance under experimental conditions [42]. β-Actin was selected as the housekeeping gene for calculations (stability coefficient was 0.963 for β-actin vs. 2.0 for GAPDH). To determine the PCR efficiencies, standard curves for both target and control genes were obtained using a series of cDNA dilutions as a template. The relative gene transcript abundance was calculated according to the Pfaffl et al. [42] method using REST software. Results were reported as the fold change in gene transcript abundance.
OF Test
An OF test was used to evaluate the responsiveness of the animals to a stressful novel environment. The OF apparatus was a grey square box (100 cm × 100 cm), enclosed by walls (50 cm height) with a 100 W halogen bulb suspended 30 cm above the centre as the only source of light. To measure the thigmotaxis; the behaviour of locomotion close to the walls, avoiding the central area and the non-thigmotactic behaviour; locomotion in the central exposed area away from the walls, the arena for analysis was divided into zones (online suppl. Fig. S1): the border zone including corners, inner zone, and central zone. The following variables of behaviour were analysed (1) in the whole arena: latency to first movement (s); time of motor activity (s), distance moved (cm), and mean velocity − the distance moved per unit of time (cm/s); (2) in the border zone: total time spent in the zone (s), total time spent in corners (s); (3) in the central zone: total time spent in the zone (s), number of entries into the central zone. The OF apparatus was placed in a sound-isolated room with lighting conditions and environmental cues held constant throughout testing. Each trial lasted 5 min, and the box was cleaned with Mediseptol H Neutral (Alpinus Chemia, Poland). All behavioural sessions were recorded and analysed with the EthoVision 11 software (Noldus, Wageningen, The Netherlands).
Data Analysis
To analyse the effect of repeated exposure to EMF on stress responses, we applied 2-way general linear models (GLMs), separately for each hormone. To test the impact of EMF on behavioural stress responses, we used multivariate analysis of variance. The data were transformed when necessary to meet the GLM and multivariate analysis of variance assumptions (normality was tested with a Shapiro-Wilk test and homoscedasticity with a Levene test). Significant terms were further explored using the Bonferroni-corrected post hoc test adjusted to the design of particular experiments. Part of the data did not meet the criteria for parametric tests, even after transformations (natural logarithm, decimal logarithm, square root). For such data, we used a non-parametric analysis of variance (Kruskal-Wallis test with Bonferroni correction). Differences between compared data were considered significant when p < 0.05. The analyses were carried out using SPSS 25.0 package (IBM Inc.). The figures in the Results section present only significant effects of categorical factors displayed in the analysis (see online suppl. Tables S2–S5).
Results
EMF-Modified HPA Axis Hormone Concentrations and Receptor mRNA Abundance (“Basal”)
Overall, the data showed that EMF exposure activated HPA axis variables in a dose-related manner.
Hormones
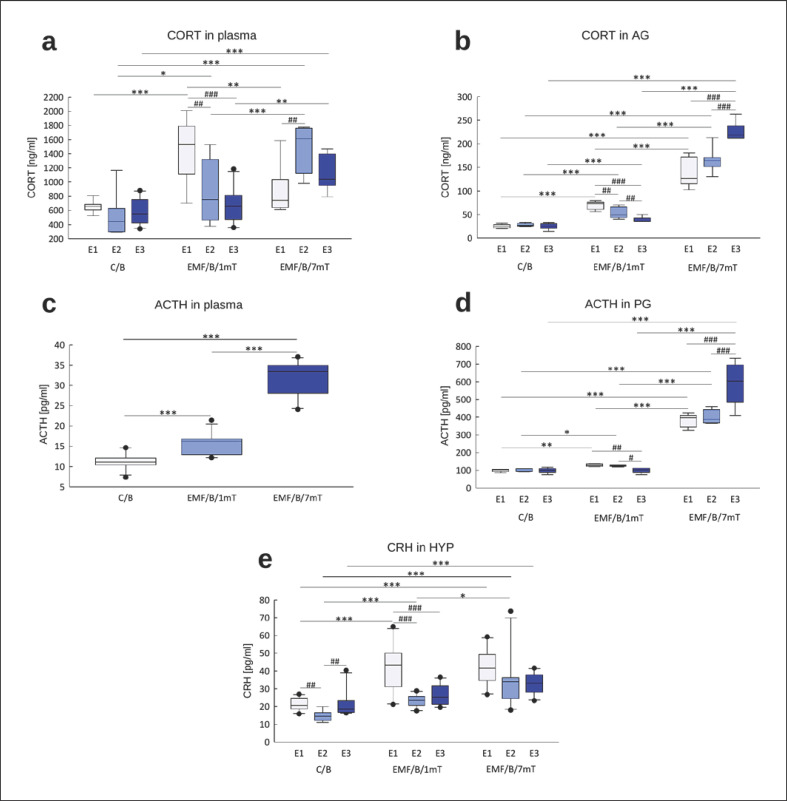
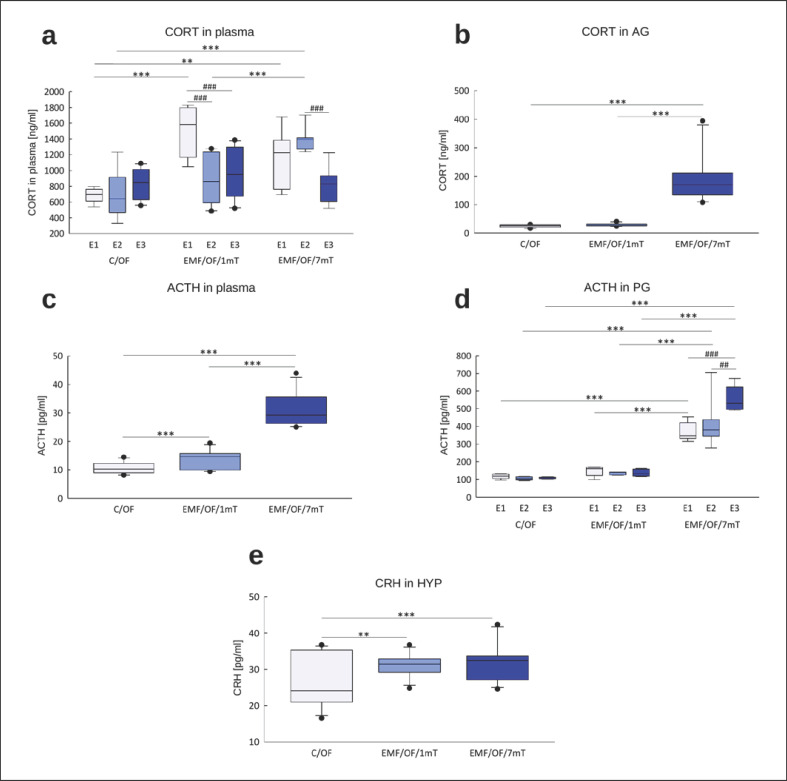
The profile of changes in “basal” plasma CORT concentrations (Fig. 3a) was similar to that of adrenal CORT concentrations (Fig. 3b). Under control circumstances, “basal” concentrations of corticosterone were low and were unaltered by single or repeated exposure to the apparatus, suggesting the set-up was not stressful per se. There was a significant effect of EMF dose, with the higher level of EMF producing greater elevation of CORT in adrenals and plasma (p < 0.001, online suppl. Table S2a, b). A single period (E1) of exposure to low 1 mT EMF significantly increased adrenal and plasma CORT concentrations (p < 0.001, Fig. 3a, b). Although there was no effect of the number of exposures (online suppl. Table S2a, b), a trend for lesser responses to repeated EMF exposure was found. Conversely, with the 7 mT EMF, there was no decline in CORT with repeated exposure.
Fig. 3.
“Basal” concentrations of HPA axis hormones: CORT in plasma (n = 77; 8–10 in each group for each exposure) (a), CORT in AG (n = 56; 5–7 in each group for each exposure) (b), ACTH in plasma (n = 86; 8–10 in each group for each exposure) (c), ACTH in PG (n = 45; 5 in each group for each exposure) (d), and CRH in the HYP (n = 89; 9–10 in each group for each exposure) (e) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Presented values are predicted by the GLM model for a significant intensity of the EMF (mT) × number of exposures (E1–E3) interaction (a, b, d, e) or significant effect of intensity of the EMF (mT) (c). Boxes indicate the lower quartile, median, and upper quartile, and whiskers indicate the range of changes (minimum to maximum). Statistically significant differences between animals from the same group are denoted #p < 0.05, ##p < 0.01, and ###p < 0.001 and these between experimental groups are denoted *p < 0.05, **p < 0.01, and ***p < 0.001. Note different scales on each plot.
As expected, ACTH concentrations in the pituitary and plasma parallelled the changes in CORT (online suppl. Table S2c, d). The higher EMF led to greater induction of ACTH (Fig. 3c, d). There was a significant effect of EMF intensity on ACTH concentration in plasma, with the higher level of EMF causing a greater elevation of hormone concentration (p < 0.001, Fig. 3c). In the EMF/B/1mT group, the ACTH concentrations in the pituitary (Fig. 3d) were significantly increased after E1 and E2. However, after the third exposure, there was attenuation of ACTH induction, and its concentration came back to the control value. In rats exposed to EMF of 7 mT, the concentration of ACTH in PG increased with each subsequent exposure.
CRH concentrations in the HYP increased with greater intensity of EMF and were further augmented with each subsequent exposure to EMF (online suppl. Table S2e; Fig. 3e). Single exposure (E1) to EMF of 1 mT significantly increased CRH compared to control (p < 0.001). After second exposure (E2), CRH was still elevated to receive the value not significantly different from control, perhaps suggesting habituation after E3. In the EMF/B/7mT group, a statistically significant increase in CRH concentration relative to the control group was noted after each subsequent exposure.
Receptors
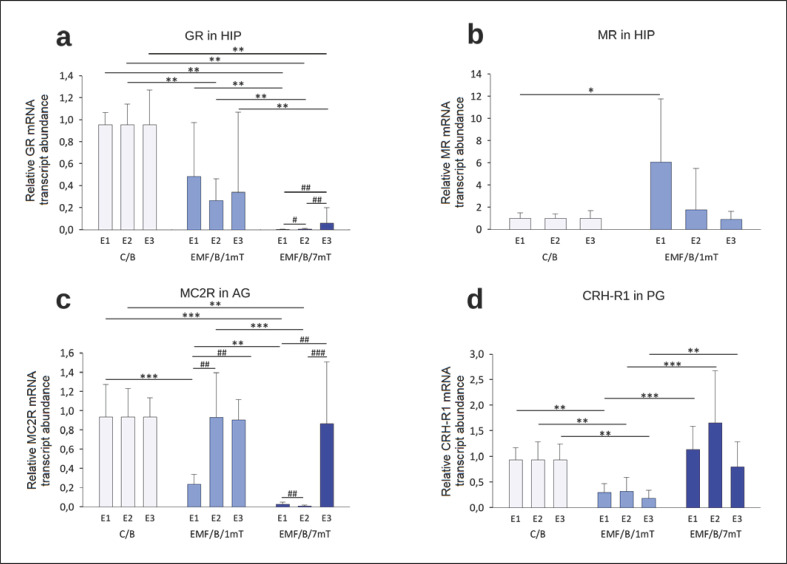
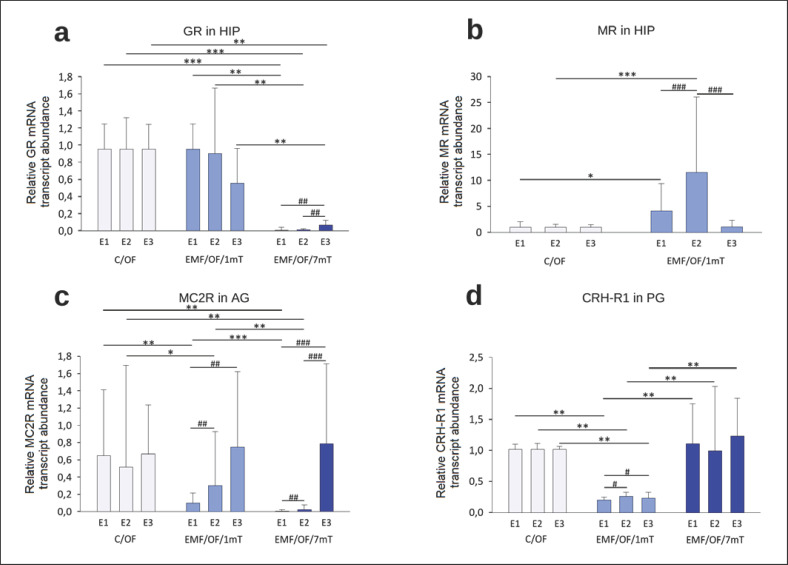
Figure 4 presents intergroup comparisons of the “basal” relative mRNA transcript abundance of (“mRNA”) encoding HPA axis receptors (expressed as fold change from an unrelated reference gene). Overall, the GR mRNA abundance in the HIP (Fig. 4a) of EMF-exposed groups was lower than control values, with a dose-response effect. However, the significant decrease of GR mRNA in the EMF/B/1mT group was observed only after E2 relative to the control group, while in the EMF/B/7mT group, GR mRNA was remarkably lower after all exposures to EMF in comparison to both: control and EMF/B/1mT groups. In animals from the EMF/B/1mT group, significantly higher hippocampal MR mRNA (Fig. 4b) was observed after E1; after E2, the receptor mRNA still tended to be elevated to receive the level not clearly different from the control value after E3. In rats exposed to EMF of 7 mT, the MR mRNA in the HIP was under the detectable threshold.
Fig. 4.
Receptor genes' “basal” relative mRNA transcript abundance (fold change vs. β-actin) GR (a) and MR (b) in the HIP, MC2R in AG (c), CRH-R1 in the PG (d) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Values are presented as mean ± SEM. Statistically significant differences between animals from the same group are denoted, #p < 0.05, ##p < 0.01, and ###p < 0.001 and these between experimental groups are denoted *p < 0.05, **p < 0.01, and ***p < 0.001 (n = 54; 6 in each group for each exposure). Note different scales on each plot.
The profile of changes of MC2R mRNA in AG (Fig. 4c) in EMF-exposed groups was different. In EMF/B/1mT, the lower of MC2R mRNA relative to that in the control group was noticed only after a single exposure (E1) to EMF (p < 0.001). The lower of MC2R mRNA was found in the EMF/B/7mT group in comparison to C/B and EMF/B/1mT after E1 and E2, which normalized to control level after E3.
Overall, CRH-R1 mRNA in the PG (Fig. 4d) in EMF/B/1mT was lower after each exposure to EMF in comparison to both: control and EMF/B/7mT groups. In rats exposed to EMF of 7 mT, the CRH-R1 mRNA was not significantly different relative to control group.
OF-Modified HPA Axis Hormone Concentrations and Their Receptors' Relative mRNA Transcript Abundance
Hormones
Generally, the direction of changes in hormone concentrations in animals exposed to EMF of 1 mT and 7 mT and then exposed to subsequent stress factor − OF test (EMF/OF/1mT and EMF/OF/7mT groups) − coincided with their “basal” concentrations observed only after exposure to EMF (EMF/B/1mT and EMF/B/7mT groups) (online suppl. Table S3; Fig. 5). CORT concentration in plasma in animals exposed to OF test was influenced by EMF intensity as well as by the number of exposures (online suppl. Table S3a; Fig. 5a). The concentration of CORT in EMF/OF/1mT was higher after E1 relative to the control group to reach the value typical for control animals already after E2. In EMF/OF/7mT, we observed a higher concentration of the hormone after E1, which was maintained after E2, and then after E3, a clear decrease in the CORT concentration was noticed. The adrenal concentration of CORT was enhanced only in animals exposed to EMF of 7 mT (Fig. 5b).
Fig. 5.
The OF-induced concentrations of HPA axis hormones: CORT in plasma (n = 78; 7–10 in each group for each exposure) (a), CORT in AG (n = 57; 5–7 in each group for each exposure) (b), ACTH in plasma (n = 90; 10 in each group for each exposure) (c), ACTH in the PG (n = 51; 5–7 in each group for each exposure) (d), and CRH in the HYP (n = 90; 10 in each group for each exposure) (e) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Presented values are predicted by the GLM model for a significant intensity of the EMF (mT) × number of exposures (E1–E3) interaction (a, d) or significant effect of intensity of the EMF (mT) (b, c, e). Boxes indicate the lower quartile, median, and upper quartile, and whiskers indicate the range of changes (minimum to maximum). Statistically significant differences between animals from the same group are denoted, ##p < 0.01 and ###p < 0.001 and these between experimental groups are denoted **p < 0.01 and ***p < 0.001. Note different scales on each plot.
In animals exposed to OF test, ACTH concentration in plasma and PG was influenced by EMF intensity (online suppl. Table S3c, d). The pituitary concentration of ACTH in EMF/OF/1mT did not differ from the value noticed in the control group, while in animals exposed to EMF of 7 mT, the concentration of ACTH was significantly enhanced in comparison to both control and EMF/OF/1mT groups at all steps of the experiment (Fig. 5d). In plasma, the hormone concentration increased with higher EMF intensity (Fig. 5c). CRH concentration in the HYP (Fig. 5e) was higher in both groups of rats exposed to EMF (1 and 7 mT) relative to control.
Receptors
The level and direction of changes in all receptors' relative mRNA transcript abundance (GRs, MRs, MC2R, and CRH-R1) in all groups after the OF test were not very different from their “basal” level (Fig. 6). There was an increase in MR mRNA in EMF/OF/1mT in comparison to the C/OF group, detected not only after E1 but also after E2 (Fig. 6b). Similarly, as in the EMF/B/7mT group, in the EMF/OF/7mT group, MR mRNA was not detectable.
Fig. 6.
Receptor genes' relative mRNA transcript abundance after OF test (fold change vs. β-actin) GR (a) and MR (b) in the HIP, MC2R in AG (c), CRH-R1 in the PG (d) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Values are presented as mean ± SEM. Statistically significant differences between animals from the same group are denoted #p < 0.05, ##p < 0.01, and ###p < 0.001 and these between experimental groups are denoted *p < 0.05, **p < 0.01, and ***p < 0.001 (n = 54; 6 in each group for each exposure). Note different scales on each plot.
Comparison between “Basal” and OF Test-Induced Concentrations of HPA Axis Hormones and Their Receptors' Relative mRNA Transcript Abundance
Hormones
We compared the “basal” concentrations of HPA hormones to the concentrations of these hormones after the OF test for each group: C/B versus C/OF; EMF/B/1mT versus EMF/OF/1mT; and EMF/B/7mT versus EMF/OF/7mT (online suppl. Table S4). In the control group, the increase in concentrations of CRH in the HYP, ACTH in the PG, and CORT in plasma after exposure to OF test was significant, which confirmed the stressogenic effect of this kind of stress. The exposure to subsequent stress factor profoundly decreased the ACTH in PG and CORT in AG in rats exposed to EMF of 1 mT. In animals exposed to EMF of 7 mT, the analysis did not reveal a significant effect of OF test on hormone concentrations.
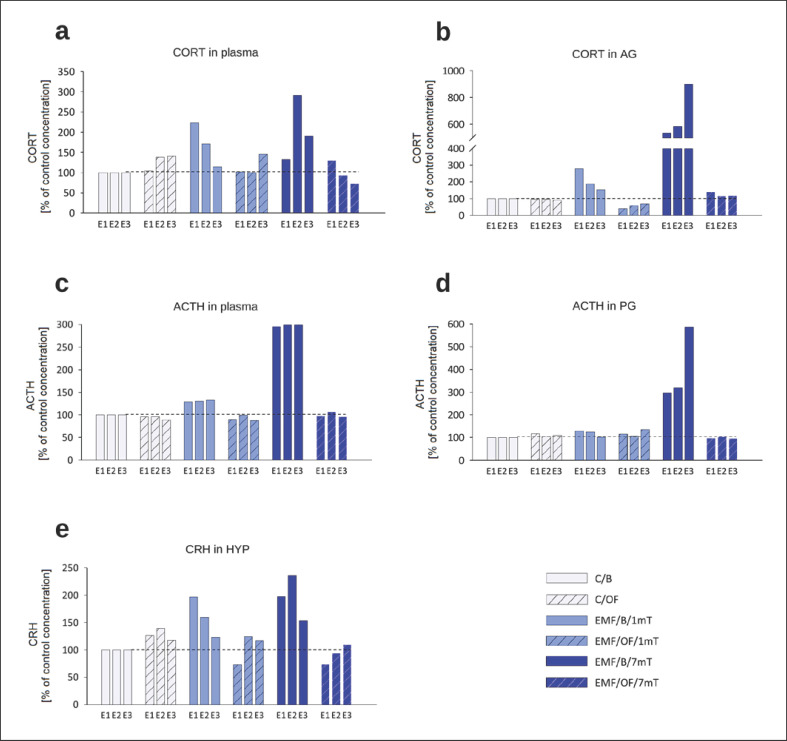
Interesting results have been received when we evaluated the percentage changes in the concentrations of hormones in comparison to their concentrations in the control (C/B) group separately after each exposure (set at 100% − reference value) (Fig. 7).
Fig. 7.
Percentage changes in the “basal” concentrations as well as OF-induced concentrations of hormones in each experimental group in relation to their level in control C/B group set at 100% after each subsequent exposure (E1–E3): CORT in plasma (a), CORT in AG (b), ACTH in plasma (c), ACTH in the PG (d) and CRH in the HYP (e) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Note different scales on each plot.
In the EMF/OF/1mT group, CORT concentrations in plasma (Fig. 7a) were lower than in EMF/B/1mT group after E1 and E2; however, after E3 when the “basal” concentration returned to a level close to the control one, we observed increased CORT concentrations after OF. In rats exposed to EMF of 7 mT and then to OF after E1, CORT was similar to the respective “basal” change ∼30% of the reference value. Then after E2 and E3, when the “basal” value increased to 292 and 190%, respectively, the OF-induced concentrations were decreased to 94 and 73% of C/B value, respectively. The “basal” concentration of CORT in AG (Fig. 7b) of animals exposed to EMF of 7 mT after each subsequent exposure was incredibly high (536, 585, 901%, respectively), while OF did not further increase CORT concentrations. In EMF/OF/1mT, similarly, the “basal” concentration of the hormone was higher than the reference value in the C/B group, (although the increase is not as high as in the EMF/B/7mT group), and again after OF test, the CORT concentration in AG was not further elevated.
It should be noticed the c.a. triple increase of the “basal” concentration of ACTH in plasma in EMF/B/7mT after each subsequent exposure in comparison to all other groups and the strikingly reduced concentrations of the hormone after OF test, which was similar to the control level (Fig. 7c). In the pituitary, the OF-induced changes in ACTH concentrations were similar (Fig. 7d). In both groups exposed to EMF (1 mT and 7 mT), OF test did not induce an increase in CRH concentrations (Fig. 7e).
Receptors
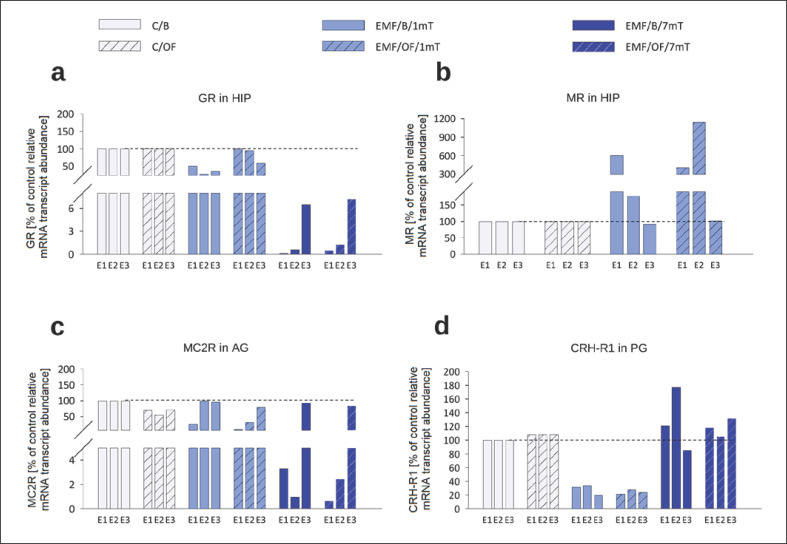
We evaluated the percentage changes in the relative mRNA transcript abundance of receptors (GR, MR, MC2R, and CRH-R1) after OF test in comparison to their mRNA transcript abundance in the control C/B group after each exposure (set at 100% − reference value) (Fig. 8). After the OF test, the hormone receptor mRNA did not differ from its “basal” abundance. The only important change concerned the MR and GR mRNA in the HIP in rats exposed to EMF of 1 mT. The OF-induced increase of MR mRNA was visible not only after the first exposure to EMF but also after the second one. We also noticed the restoration of GRs to the control level in this group.
Fig. 8.
Percentage changes in the “basal” as well as OF-induced relative mRNA transcript abundance of receptor genes in each experimental group in relation to their mRNA transcript abundance in control C/B group set at 100% after each subsequent exposure (E1–E3): GR (a) and MR in the HIP (b), MC2R in AG (c), CRH-R1 in the PG (d) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Note different scales on each plot.
Behavioural Analysis
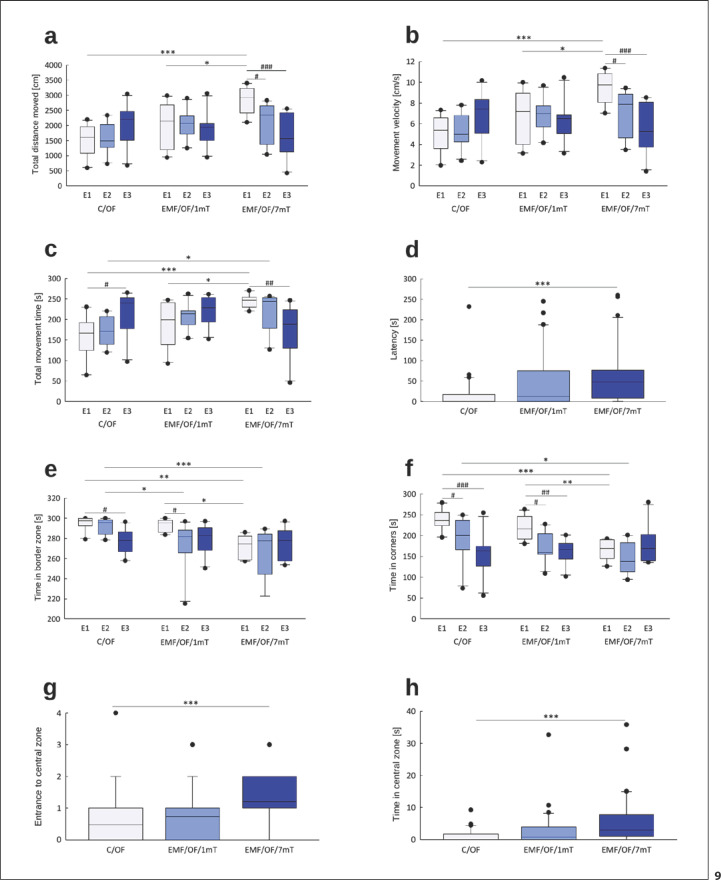
Overall, the activity level in rats exposed to EMF of 7 mT was higher relative to both control and animals exposed to EMF of 1 mT (online suppl. Table S5; Fig. 9).
Fig. 9.
Behavioural changes in OF test: total distance moved (cm) (a), movement velocity (cm/s) (b), total movement time (s) (c), latency (s) (d), time in border zone (s) (e), time in corners (s) (f), entrance to central zone (g), time in central zone (s) (h) in rats exposed once to three times (E1–E3) to EMF of 1 or 7 mT or control conditions. Presented values are predicted by the GLM model for a significant intensity of the EMF (mT) x number of exposures (E1–E3) interaction (a–c, e, f) or significant effect of intensity of the EMF (mT) (d, g, h). Boxes indicate the lower quartile, median, and upper quartile, and whiskers indicate the range of changes (minimum to maximum). Statistically significant differences between animals from the same group are denoted #p < 0.05, ##p < 0.01, and ###p < 0.001 and these between experimental groups are denoted *p < 0.05, **p < 0.01, and ***p < 0.001 (n = 90; 10 in each group for each exposure). Note different scales on each plot.
Whole Arena
The distance moved (Fig. 9a) by control animals was at a similar level after all exposures (E1–E3) with a slight increase after the last exposure. In the EMF/OF/1mT group, the distance moved during all subsequent tests did not differ from values noticed in C/OF group. The longest distance in OF was travelled by rats from EMF/OF/7mT after the first exposure (E1) to the EMF. After subsequent exposures to EMF (E2 and E3), the decrease in the value of this parameter was observed.
In all groups, the profile of changes in movement velocity (Fig. 9b) and total movement time (Fig. 9c) was similar to that observed in the case of distance moved as the parameters are related to each other. Rats exposed to EMF of 7 mT showed significantly shorter latency to start the movement after placement in OF than that noticed in control and exposed to 1 mT EMF rats (Fig. 9d).
Border Zone
In the control group, the time spent in the border zone (Fig. 9e) after E1 and E2 remained at a similar level and was significantly reduced after E3. In the EMF/OF/1mT group, the significant reduction in time spent in the border was observed already after E2. Rats exposed to EMF of 7 mT moved significantly less in the border zone already after E1; after E2, the value of the parameter was still decreased to receive the control value after E3.
The time spent in corners (Fig. 9f) did not differ between control and EMF/OF/1mT groups. However, in both groups, a significant gradual reduction in time spent in the corners after subsequent exposures was observed. Rats exposed to EMF of 7 mT spent significantly less time in the corners relative to other groups.
Central Zone
Rats exposed to EMF of 7 mT made more entries to the central zone (Fig. 9g) and spent a relatively greater proportion of time in the central zone of OF (Fig. 9h) than that noticed in control and exposed to 1 mT EMF rats.
Discussion
Our research has shown that the impact of repeated exposure to EMF on the HPA axis activity depends on the intensity of EMF. The increase of HPA axis hormone concentrations in rats exposed to EMF of 1 mT was maximal after the first exposure to EMF and was attenuated with each subsequent exposure. The initial increase in CRH induced the compensatory reaction − the reduction of relative mRNA transcript abundance of CRH-R1 receptors after each subsequent exposure to EMF. This may reflect an adaptive process aiming at diminishing the HPA axis activity in response to moderate stress [43]. The EMF-induced increase of ACTH release was accompanied by a parallel decline in MC2R mRNA transcript abundance only after the first exposure. Data suggest negative feedback on MC2R expression imposed by both its ligand (ACTH) and glucocorticoids [44]. The profile of corticosterone release from AG in the 1 mT EMF group appears to be a summated effect of several processes at higher levels of the HPA axis: the initial increase and then the decrease of hormone concentrations with each subsequent exposure. However, it should be stressed that the CORT concentrations were higher than that in control animals after each subsequent exposure. The results suggest that even a low intensity of EMF (1 mT) is a challenge, causing an HPA axis stress response, if relatively weak and temporary. Similarly, most other research also indicated that a low level of EMF (≤1 mT) causes an increase of HPA axis hormone concentrations [12, 13, 15].
In the group exposed to high EMF intensity (7 mT), the concentrations of HPA axis hormones were significantly higher than values recorded in both control and low-dose EMF (1 mT), and there was no apparent habituation with repeated exposure. Up-regulation of CRH predicts an enhanced capacity for stress excitation and is purported to reflect a “chronic stress-recruited” pathway [45], a phenomenon that can explain the high activity of the HPA axis in the 7 mT EMF-exposed group. The lack of down-regulation of CRH-R1 in this group can be the result of the stimulating role of a much higher concentration of CORT on the mRNA transcript abundance of the receptors in rats exposed to 7 mT than in 1 mT exposed ones. The ACTH concentration in the PG increased 4 times after first exposure and becomes even higher with each next exposure. Although we observed a decrease in relative MC2R mRNA transcript abundance after the 1st and 2nd exposure to EMF of 7 mT, the CORT release was not limited. The decrease in MC2R receptors may represent the adaptive response aimed to diminish the HPA axis activity; however, in such challenging stress situation, it seems that the regulatory mechanisms became insufficient or the HPA axis activity is regulated outside the main short-loop feedback. As a consequence of the cumulative effect of repeated severe stress, the glucocorticoid responses to a given stressor can be amplified [46]. A much stronger influence of the high-density EMF on the activation of stress systems was also demonstrated in other studies, e.g., increase in corticosterone secretion after chronic exposure to EMFs (3 mT) [14].
Apart from the negative feedback operating between components of the HPA axis, the important role in the regulation of HPA axis activity is played by GRs and MRs in the HIP. MRs play a crucial role at the onset of the stress reaction and increase neuronal excitability [35]. Finally, mineralocorticoid signalling is believed to play a major role in inhibiting HPA axis tone. GRs maintain the initiated stress response and then dampen neuron excitation, normalizing brain activity and promoting recovery [28].
MR-mediated corticosteroid negative feedback during stress may be an important mechanism that helps minimize the exposure of target tissue to corticosteroids, especially in the context of repeated stress [47, 48, 49]. Thus, increased MR mRNA might culminate in decreased HPA axis activity [50, 51] observed in our experiments with each subsequent exposure to 1 mT EMF. In contrast, hippocampal MR and GR down-regulation promotes CORT hypersecretion. The degree of such down-regulation varies with the nature and duration of the stressor applied, but in general, the stronger the stress, the more profound the MR and GR down-regulation [52, 53]. The increase in circulating CORT with each subsequent exposure to EMF of 7 mT may thus be related to loss of glucocorticoid feedback control of the HPA axis associated with decreased GR mRNA transcript abundance and even more with profound down-regulation of MR mRNA in the HIP. This phenomenon is also observed with other kinds of stress. In chronically stressed mice, the decrease in the relative mRNA transcript abundance of hippocampal MR and GR was accompanied with the increase of corticosterone concentration [54]. Also, changes in MR/GR balances have been found in patients with psychiatric disorders [55], emphasizing the importance of both CORT receptors, as particularly noted with high-intensity EMF exposure. Any implications for psychopathology are now important to determine.
Our results are consistent with the studies that confirmed the differences in the course of repeated exposure to some kind of stressor depending on stressor stimulus intensity [46, 56]. One weak/moderate challenge to the organism results in the inhibition of the HPA axis activity to the next second stimulus, as a consequence of feedback inhibition, characterized by a decreasing glucocorticoid response over time [57]. It was suggested that MR blockade impairs the ability to habituate to stress [48]. Our results showed that repeated exposure to EMF of 1 mT engenders a weak and temporary stress response. The decrease in CORT release with each exposure to 1 mT EMF may be partly explained by increased MR expression in the HIP. However, if stress stimuli are sufficiently intense, facilitation can override feedback inhibition, resulting in higher peak glucocorticoid concentrations to successive stimuli [56, 58]. The increasing activation of the HPA axis with each subsequent exposure to 7 mT EMF may reflect this phenomenon.
Our study is the first to show a hormetic, bidirectional effect of EMF (50 Hz) in vertebrates in vivo: adaptation at low EMF but sensitization at a higher magnetic flux density. A hormetic effect of radiofrequency EMF (1,800 MHz) on genomic DNA has been also reported in vitro in mouse embryonic fibroblasts [59]. In case of low-frequency EMF (50 Hz), the hormetic effect to our knowledge, has only been shown till now in Drosophila melanogaster [60]. Our results allowed us to conclude that repeated exposure to EMF of 1 mT shifts the set-point of stress systems activity, leading to adaptation to this kind of stress. Conversely, in animals exposed to EMF of 7 mT, the shift of the set-point of stress systems activity into augmented vulnerability was noticed. In consequence, the shifted set-point of HPA axis can influence the response to the subsequent heterotypic stress − the OF. It has been found that the novel stress-induced activation of the HPA axis after the repeated exposure to homotypic stress may cause increased glucocorticoid release, the shut-off of the stress response or in extreme cases hyporesponsiveness driven by adrenal exhaustion [61]. E.g., the strong social stress in mice finally caused them to cross the line from enhanced adrenal reactivity to adrenal exhaustion, a condition which was associated with substantial physical morbidity [62].
The profile and dynamics of OF-induced changes in hormone concentrations and their receptors' mRNA transcript abundance almost completely covered their basal levels − observed just after subsequent exposures to EMF or control conditions. However, if we evaluated the percentage changes in specific hormone concentrations, it became clear that in groups exposed to EMF, the release of hormones stimulated by another stress was diminished. This was most evident in the 7 mT EMF group, where OF-induced concentrations of all hormones of the HPA axis were severalfold lower than their respective “basal” concentrations. This may express a “ceiling effect” because “basal” CORT concentration was 5, 7, and 9 times higher than the value in control animals. Thus, it is likely that the further increase of CORT concentration in response to the next stressor was limited which reflects the adrenal exhaustion phenomenon. In animals exposed to 1 mT EMF, HPA hormonal responses to the OF challenge were attenuated. As levels of stress hormones were lower than after 7 mT EMF exposure, this is not likely to be adrenal exhaustion but is more probably an adaptation to heterotypic stressors. Interestingly, this coincided with the restoration of GR mRNA. Moreover, OF-induced increase in hippocampal MR mRNA appeared not only after the first exposure to EMF but was even higher after the second one. This suggests that previous EMF exposure acts as preconditioning which facilitates an adaptive response to subsequent stress events. A common observation in studies of hormesis is that exposure to low levels of one type of hormetic agent induces pathways responsible for brain plasticity to protect cells and organisms against subsequent heterotypic stress [33].
An association between EMF exposure and emotional behaviour has been indicated in many but not all studies. Behavioural effects of the EMF depend on the length, frequency, and intensity of exposure [15, 63]. Some reports noted reduced activity of animals after EMF exposure, and an anxiety-like behaviour, others observed anxiolytic effect or did not observe any changes. It is reasonable to speculate that EMF-induced behavioural changes may be attributed in part to the effect on HPA axis [33]. In our study, rats exposed to EMF of 7 mT showed less anxiety-related behaviour than that noticed in control and exposed to 1 mT EMF rats. This anxiolytic behaviour in the OF test may be due to substantive corticosterone release reduction. Moreover, the lower MR mRNA in the HIP, following 7 mT EMF exposure, could also underlie increased activity in OF. Indeed, MR antagonism decreases anxiety behaviour in rats [64].
We did not observe the difference in behaviour in OF tests in animals previously experienced with 1 mT EMF in comparison to control animals. We suggest that the stress system reaction evoked by the low-stress factor − 1 mT EMF − was not strong enough to change their behaviour. Similarly, mice prenatally exposed to EMF (50 Hz, 1 mT) did not show changes in anxiety-like behaviour [65].
The effects of exposure to EMF are particularly prevalent in the HIP [17, 19]. Corticosterone exerts a bidirectional, dose-dependent effect on hippocampal function. Whereas the highly elevated corticosterone concentrations potentiate excitotoxicity and disturb HPA axis function, low concentrations of corticosterone protect against excitotoxic neuronal damage and promote brain plasticity (hormesis-like effect) [29, 47]. High-density (8 mT) EMF causes memory impairment and cell death [8, 24]. On the other hand, low EMF (1 mT or less) stimulates hippocampal plasticity [17, 18, 19, 20]. Glucocorticoids impact the survival of hippocampal neurons and neuroplasticity via activation of MR [27, 29, 47]. The finding that EMF of 1 mT specifically induces MR mRNA in the HIP is in line with the previous findings that sub-lethal challenge can increase neuronal MR expression and may serve as a compensatory mechanism designed to induce neuronal plasticity. To summarize, the bidirectional effects of low- and high-density EMF exposure might be related to different MR mRNA abundance as they influence the HPA axis activity and also because of their neuroprotective value.
Conclusion
Our study suggests that the EMF intensity is the crucial factor determining the bidirectional influence of low-frequency EMF on the brain: positive − promoting brain plasticity that improves the neuroadaptation to subsequent stress exposures or negative − responsible for the disturbance of stress response and increasing sensitivity to subsequent stressors and perhaps the risk of stress-induced disorders. Thus, the proposed study is a pioneer because for the first time, we suggest such “hormetic mode of action” of EMF (50 Hz) in vertebrates (verified on rat model). The results can contribute to explaining the fundamental mechanisms of the bidirectional responses to EMF and provide a new view on possible therapeutic properties of the magnetic field as well as a new direction in the risk assessment of EMF exposure. We have shown for the first time the induction of hippocampal neuronal MR mRNA in response to EMF of 1 mT in rat brain. Although the consequences of increased MR expression under these circumstances are not precisely known, this phenomenon may represent an endogenous response to protect the brain from subsequent injury. It would be of interest to explore the long-lasting effects of EMF-induced MR changes. As the consequences of permanent changes in the stress response can be multidimensional, future research should further address the EMF-induced mechanisms, mainly related to their therapeutic properties. We are also convinced that the results of our research will help clarify the mechanisms of EMF impact on health − which is very important for humanity − living in more and more full of EMF environment.
Statement of Ethics
This study protocol was reviewed and approved by the Local Committee for Ethics in Animal Research in Bydgoszcz, Poland; approval number 3/2018.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by National Science Centre, Poland (Grant No. 2017/25/B/NZ7/00638) and project No. POWR.03.05.00-00-Z302/17 “Universitas Copernicana Thoruniensis In Futuro,” co-financed by the European Social Fund − the Operational Programme Knowledge Education Development. Module 5. Interdisciplinary PhD School “Academia Copernicana.”
Author Contributions
Justyna Rogalska conceived the project and got funding; Angelika Klimek, Hanna Kletkiewicz, and Joanna Wyszkowska prepared experimental protocols; Angelika Klimek, Hanna Kletkiewicz, Maciej Klimiuk, Agnieszka Siejka, Justyna Maliszewska, and Milena Jankowska conducted the experiments; Angelika Klimek and Hanna Kletkiewicz analysed the results; Angelika Klimek and Justyna Rogalska drafted the manuscript; Hanna Kletkiewicz, Justyna Maliszewska, Milena Jankowska, and Joanna Wyszkowska reviewed the manuscript; Angelika Klimek and Agnieszka Siejka prepared data visualization; Justyna Rogalska and Jonathan Seckl supervised.
Data Availability Statement
The data used to support the findings of this study are included in the article and its online supplementary material file. Further enquiries can be directed to the corresponding authors.
Supplementary Material
Supplementary data
Acknowledgments
We would like to thank Prof. Maria Stankiewicz (Nicolaus Copernicus University) for her valuable comments on the manuscript.
Funding Statement
This study was supported by National Science Centre, Poland (Grant No. 2017/25/B/NZ7/00638) and project No. POWR.03.05.00-00-Z302/17 “Universitas Copernicana Thoruniensis In Futuro,” co-financed by the European Social Fund − the Operational Programme Knowledge Education Development. Module 5. Interdisciplinary PhD School “Academia Copernicana.”
References
- 1.Touitou Y, Selmaoui B. The effects of extremely low-frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues Clin Neurosci. 2012;14((4)):381–399. doi: 10.31887/DCNS.2012.14.4/ytouitou. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson O. Disturbance of the immune system by electromagnetic fields: a potentially underlying cause for cellular damage and tissue repair reduction which could lead to disease and impairment. Pathophysiology. 2009;16((2–3)):157–177. doi: 10.1016/j.pathophys.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Corallo C, Battisti E, Albanese A, Vannoni D, Leoncini R, Landi G, et al. Proteomics of human primary osteoarthritic chondrocytes exposed to extremely low-frequency electromagnetic fields (ELF EMFs) and to therapeutic application of musically modulated electromagnetic fields (TAMMEF) Electromagn Biol Med. 2013;33((1)):3–10. doi: 10.3109/15368378.2013.782316. [DOI] [PubMed] [Google Scholar]
- 4.Li SS, Zhang ZY, Yang CJ, Lian HY, Cai P. Gene expression and reproductive abilities of male Drosophila melanogaster subjected to ELF-EMF exposure. Mutat Res Genet Toxicol Environ Mutagen. 2013;758((1–2)):95–103. doi: 10.1016/j.mrgentox.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Merla C, Liberti M, Consales C, Denzi A, Apollonio F, Marino C, et al. Evidences of plasma membrane-mediated ROS generation upon ELF exposure in neuroblastoma cells supported by a computational multiscale approach. Biochim Biophys Acta Biomembr. 2019;1861((8)):1446–1457. doi: 10.1016/j.bbamem.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari R, Lakshmi NK, Bhargava SC, Ahuja YR. Epinephrine, DNA integrity and oxidative stress in workers exposed to extremely low-frequency electromagnetic fields (ELF-EMFs) at 132 kV substations. Electromagn Biol Med. 2014;34((1)):56–62. doi: 10.3109/15368378.2013.869755. [DOI] [PubMed] [Google Scholar]
- 7.Duan W, Liu C, Zhang L, He M, Xu S, Chen C, et al. Comparison of the genotoxic effects induced by 50 Hz extremely low-frequency electromagnetic fields and 1800 MHz radiofrequency electromagnetic fields in GC-2 cells. Radiat Res. 2015;183((3)):305–314. doi: 10.1667/RR13851.1. [DOI] [PubMed] [Google Scholar]
- 8.Yin C, Luo X, Duan Y, Duan W, Zhang H, He Y, et al. Neuroprotective effects of lotus seedpod procyanidins on extremely low frequency electromagnetic field-induced neurotoxicity in primary cultured hippocampal neurons. Biomed Pharmacother. 2016;82:628–639. doi: 10.1016/j.biopha.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Ferroni L, Tocco I, De Pieri A, Menarin M, Fermi E, Piattelli A, et al. Pulsed magnetic therapy increases osteogenic differentiation of mesenchymal stem cells only if they are pre-committed. Life Sci. 2016;152:44–51. doi: 10.1016/j.lfs.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Haghighat N, Abdolmaleki P, Parnian J, Behmanesh M. The expression of pluripotency and neuronal differentiation markers under the influence of electromagnetic field and nitric oxide. Mol Cell Neurosci. 2017;85:19–28. doi: 10.1016/j.mcn.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Blank M, Goodman R. Electromagnetic fields stress living cells. Pathophysiology. 2009;16((2–3)):71–78. doi: 10.1016/j.pathophys.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Ebadi A, Sedghi H, Zare S, Hayatgeibi H, Alivandi S. Effects of 50 HZ magnetic field on some factors of immune system in the male Guinea pigs. Am J Immunol. 2005;1((1)):37–41. [Google Scholar]
- 13.Szemerszky R, Zelena D, Barna I, Bárdos G. Stress-related endocrinological and psychopathological effects of short- and long-term 50Hz electromagnetic field exposure in rats. Brain Res Bull. 2010;81((1)):92–99. doi: 10.1016/j.brainresbull.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Kitaoka K, Kitamura M, Aoi S, Shimizu N, Yoshizaki K. Chronic exposure to an extremely low-frequency magnetic field induces depression-like behavior and corticosterone secretion without enhancement of the hypothalamic-pituitary-adrenal axis in mice. Bioelectromagnetics. 2012;34((1)):43–51. doi: 10.1002/bem.21743. [DOI] [PubMed] [Google Scholar]
- 15.Mahdavi SM, Sahraei H, Yaghmaei P, Tavakoli H. Effects of electromagnetic radiation exposure on stress-related behaviors and stress hormones in male Wistar rats. Biomol Ther. 2014;22((6)):570–576. doi: 10.4062/biomolther.2014.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Sámano J, Flores-Poblano A, Verdugo-Díaz L, Juárez-Oropeza MA, Torres-Durán PV. Extremely low frequency electromagnetic field exposure and restraint stress induce changes on the brain lipid profile of Wistar rats. BMC Neurosci. 2018;19((1)):31. doi: 10.1186/s12868-018-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuccurazzu B, Leone L, Podda MV, Piacentini R, Riccardi E, Ripoli C, et al. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp Neurol. 2010;226((1)):173–182. doi: 10.1016/j.expneurol.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Tasset I, Medina FJ, Jimena I, Agüera E, Gascón F, Feijóo M, et al. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington's disease rat model: effects on neurotrophic factors and neuronal density. Neuroscience. 2012;209:54–63. doi: 10.1016/j.neuroscience.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Komaki A, Khalili A, Salehi I, Shahidi S, Sarihi A. Effects of exposure to an extremely low frequency electromagnetic field on hippocampal long-term potentiation in rat. Brain Res. 2014;1564:1–8. doi: 10.1016/j.brainres.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Sakhaie MH, Soleimani M, Pourheydar B, Majd Z, Atefimanesh P, Asl SS, et al. Effects of extremely low-frequency electromagnetic fields on neurogenesis and cognitive behavior in an experimental model of hippocampal injury. Behav Neurol. 2017;2017:9194261. doi: 10.1155/2017/9194261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cichoń N, Rzeźnicka P, Bijak M, Miller E, Miller S, Saluk J. Extremely low frequency electromagnetic field reduces oxidative stress during the rehabilitation of post-acute stroke patients. Adv Clin Exp Med. 2018;27((9)):1285–1293. doi: 10.17219/acem/73699. [DOI] [PubMed] [Google Scholar]
- 22.Bagheri Hosseinabadi M, Khanjani N, Ebrahimi MH, Haji B, Abdolahfard M. The effect of chronic exposure to extremely low-frequency electromagnetic fields on sleep quality, stress, depression and anxiety. Electromagn Biol Med. 2019;38((1)):96–101. doi: 10.1080/15368378.2018.1545665. [DOI] [PubMed] [Google Scholar]
- 23.Klimek A, Rogalska J. Extremely low-frequency magnetic field as a stress factor-really detrimental?-insight into literature from the last decade. Brain Sci. 2021;11((2)):174. doi: 10.3390/brainsci11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadidi M, Firoozabadi SM, Rashidy-Pour A, Sajadi AA, Sadeghi H, Taherian AA. Acute exposure to a 50 Hz magnetic field impairs consolidation of spatial memory in rats. Neurobiol Learn Mem. 2007;88((4)):387–392. doi: 10.1016/j.nlm.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Korpinar MA, Kalkan MT, Tuncel H. The 50 Hz (10 mT) sinusoidal magnetic field: effects on stress-related behavior of rats. Bratisl Lek Listy. 2012;113((09)):521–524. doi: 10.4149/bll_2012_117. [DOI] [PubMed] [Google Scholar]
- 26.de Kleijn S, Ferwerda G, Wiese M, Trentelman J, Cuppen J, Kozicz T, et al. A short-term extremely low frequency electromagnetic field exposure increases circulating leukocyte numbers and affects HPA-axis signaling in mice: ELF-EMF effect on stress regulation in mice. Bioelectromagnetics. 2016;37((7)):433–443. doi: 10.1002/bem.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krugers HJ, Karst H, Joels M. Interactions between noradrenaline and corticosteroids in the brain: from electrical activity to cognitive performance. Front Cell Neurosci. 2012;6:15. doi: 10.3389/fncel.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnett MG, Muglia LM, Laryea G, Muglia LJ. Genetic approaches to hypothalamic-pituitary-adrenal axis regulation. Neuropsychopharmacol. 2016;41((1)):245–260. doi: 10.1038/npp.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogalska J. Mineralocorticoid and glucocorticoid receptors in hippocampus: their impact on neurons survival and behavioral impairment after neonatal brain injury. Vitam Horm. 2010;82:391–419. doi: 10.1016/S0083-6729(10)82020-5. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese EJ. Overcompensation stimulation: a mechanism for hormetic effects. Crit Rev Toxicol. 2001;31((4–5)):425–470. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- 31.Mushak P. Temporal stability of chemical hormesis (CH): is CH just a temporary stop on the road to thresholds and toxic responses? Sci Total Environ. 2016;569–570:1446–1456. doi: 10.1016/j.scitotenv.2016.06.233. [DOI] [PubMed] [Google Scholar]
- 32.Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3((1)):13. doi: 10.1038/s41514-017-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cirulli F, Alleva E. The NGF saga: from animal models of psychosocial stress to stress-related psychopathology. Front Neuroendocrinol. 2009;30((3)):379–395. doi: 10.1016/j.yfrne.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Datson NA, van den Oever JM, Korobko OB, Magarinos AM, de Kloet ER, McEwen BS. Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology. 2013;154((9)):3261–3272. doi: 10.1210/en.2012-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koning A-SCAM, Buurstede JC, van Weert LTCM, Meijer OC. Glucocorticoid and mineralocorticoid receptors in the brain: a transcriptional perspective. J Endocr Soc. 2019;3((10)):1917–1930. doi: 10.1210/js.2019-00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes
- 37.Bieńkowski P, Wyszkowska J. Techniczne aspekty ekspozycji na pole magnetyczne ekstremalnie niskich częstotliwości (ELF) w badaniach biomedycznych [Technical aspects of exposure to magnetic fields of extremely low frequencies (ELF) in biomedical research] Med Pr. 2015;66((2)):185–197. doi: 10.13075/mp.5893.00164. [DOI] [PubMed] [Google Scholar]
- 38.Trawiński T, Szczygieł M, Wyszkowska J, Kluszczyński K. Analysis of magnetic field distribution and mechanical vibration of magnetic field exciter under different voltage supply. Inf Technol Biomed. 2010;69:613–622. [Google Scholar]
- 39.Directive 2013/35/EU of the European Parliament and of the Council of 26 June 2013 on the minimum health and safety requirements regarding the exposure of workers to the risks arising from physical agents (electromagnetic fields)
- 40.Calvo AC, Azanza MJ. Synaptic neurone activity under applied 50 Hz alternating magnetic fields. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124((1)):99–107. doi: 10.1016/s0742-8413(99)00059-6. [DOI] [PubMed] [Google Scholar]
- 41.Hug K, Röösli M. Therapeutic effects of whole-body devices applying pulsed electromagnetic fields (PEMF): a systematic literature review. Bioelectromagnetics. 2012;33((2)):95–105. doi: 10.1002/bem.20703. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30((9)):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochedalski T, Rabadan-Diehl C, Aguilera G. Interaction between glucocorticoids and corticotropin releasing hormone (CRH) in the regulation of the pituitary CRH receptor in vivo in the rat. J Neuroendocrinol. 1998;10((5)):363–369. doi: 10.1046/j.1365-2826.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 44.Agulleiro MJ, Sánchez E, Leal E, Cortés R, Fernández-Durán B, Guillot R, et al. Molecular characterization and functional regulation of melanocortin 2 receptor (MC2R) in the sea bass A putative role in the adaptation to stress. PLoS One. 2013;8((5)):e65450. doi: 10.1371/journal.pone.0065450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food. Proc Natl Acad Sci U S A. 2003;100((20)):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291((5)):E965–73. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 47.de Kloet ER, Vreugdenhil ER, Oitzl E, Joëls MS. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19((3)):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 48.Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation: mineralocorticoid receptor antagonists block habituation expression. J Neuroendocrinol. 2001;12((10)):1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- 49.Gądek-Michalska A, Spyrka J, Rachwalska P, Tadeusz J, Bugajski J. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol Rep. 2013;65((5)):1163–1175. doi: 10.1016/s1734-1140(13)71474-9. [DOI] [PubMed] [Google Scholar]
- 50.McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947((1)):41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- 51.Rogalska J, Kang P, Wotherspoon W, Macleod MR, Lai M. Effect of hyperthermia and anoxia on glucocorticoid and mineralocorticoid receptor expression in neonatal rat hippocampus. Neurosci Lett. 2009;450((2)):196–200. doi: 10.1016/j.neulet.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 52.Wang Q, Yu K, Wang J, Lin H, Wu Y, Wang W. Predator stress-induced persistent emotional arousal is associated with alterations of plasma corticosterone and hippocampal steroid receptors in rat. Behav Brain Res. 2012;230((1)):167–174. doi: 10.1016/j.bbr.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Zhang J, Sun H, Zhu H, Liu H, Yang Y. An enriched environment elevates corticosteroid receptor levels in the hippocampus and restores cognitive function in a rat model of chronic cerebral hypoperfusion. Pharmacol Biochem Behav. 2013;103((4)):693–700. doi: 10.1016/j.pbb.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 54.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav. 2008;53((2)):386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Von Werne Baes C, de Carvalho Tofoli SM, Martins CMS, Juruena MF. Assessment of the hypothalamic-pituitary-adrenal axis activity: glucocorticoid receptor and mineralocorticoid receptor function in depression with early life stress − a systematic review. Acta Neuropsychiatr. 2012;24((1)):4–15. doi: 10.1111/j.1601-5215.2011.00610.x. [DOI] [PubMed] [Google Scholar]
- 56.Rabasa C, Muñoz-Abellán C, Daviu N, Nadal R, Armario A. Repeated exposure to immobilization or two different footshock intensities reveals differential adaptation of the hypothalamic-pituitary-adrenal axis. Physiol Behav. 2011;103((2)):125–133. doi: 10.1016/j.physbeh.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65((5)):360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 58.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144((12)):5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 59.Sun C, Wei X, Fei Y, Su L, Zhao X, Chen G, et al. Mobile phone signal exposure triggers a hormesis-like effect in Atm+/+ and Atm-/- mouse embryonic fibroblasts. Sci Rep. 2016;6((1)):37423. doi: 10.1038/srep37423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graham JH, Fletcher D, Tigue J, McDonald M. Growth and developmental stability of Drosophila melanogaster in low frequency magnetic fields. Bioelectromagnetics. 2000;21((6)):465–472. [PubMed] [Google Scholar]
- 61.Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, et al. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148((2)):670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- 63.Janać B, Selaković V, Rauš S, Radenović L, Zrnić M, Prolić Z. Temporal patterns of extremely low frequency magnetic field-induced motor behavior changes in Mongolian gerbils of different age. Int J Radiat Biol. 2012;88((4)):359–366. doi: 10.3109/09553002.2012.652725. [DOI] [PubMed] [Google Scholar]
- 64.Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacol Biochem Behav. 1997;56((3)):507–513. doi: 10.1016/s0091-3057(96)00244-4. [DOI] [PubMed] [Google Scholar]
- 65.Alsaeed I, Al-Somali F, Sakhnini L, Aljarallah OS, Hamdan RMM, Bubishate SA, et al. Autism-relevant social abnormalities in mice exposed perinatally to extremely low frequency electromagnetic fields. Int J Dev Neurosci. 2014;37((1)):58–64. doi: 10.1016/j.ijdevneu.2014.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data used to support the findings of this study are included in the article and its online supplementary material file. Further enquiries can be directed to the corresponding authors.