Summary
Non-ribosomal peptide synthetases (NRPSs) assemble metabolites of medicinal and commercial value. Both serine and threonine figure prominently in these processes and separately can be converted to the additional NRPS building blocks 2,3-diaminopropionate (Dap) and 2,3-diaminobutyrate (Dab). Here we bring extensive bioinformatics, in vivo and in vitro experimentation to compose a unified view of the biosynthesis of these widely distributed non-canonical amino acids that both derive by pyridoxal-mediated β-elimination of the activated O-phosphorylated substrates followed by β-addition of an amine donor. By examining monobactam biosynthesis in Pseudomonas and in Burkholderia species where it is silent, we show that (2S,3R)-Dab synthesis depends on an l-threonine kinase (DabA), a β-replacement reaction with l-aspartate (DabB) and an argininosuccinate lyase-like protein (DabC). The growing clinical importance of monobactams to both withstand Ambler Class B metallo-β-lactamases and retain their antibiotic activity make reprogrammed precursor and NRPS synthesis of modified monobactams a feasible and attractive goal.
Subject areas: Chemistry
Graphical abstract

Highlights
-
•
DabABC operons in natural product and monobactam BGCs biosynthesize (2S,3R)-Dab
-
•
Presence of the dabABC operon differentiates C4-methylated monobactam BGCs
-
•
Dual functions of DabB and DabC give (2S,3R)-Dab or l-Dap with/without l-Thr kinase
-
•
Results enable the enzymatic production of further functionalized Dap and Dab analogs
Chemistry
Introduction
The rise of β-lactam resistant Gram-negative bacteria has been classified as one of the world’s most pressing clinical threats by both the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).1,2 One particularly alarming mediator of this resistance is the emergence of Ambler Class B metallo-β-lactamases (MBLs), which can inactivate most prescribed β-lactam antibiotics but for which no approved inhibitor currently exists. Only the monobactams with their unusual N-sulfamate possess an inherent stability to these enzymes.3 This class of molecules, which was established with the discovery of the natural product sulfazecin (1),4 has resulted in the FDA-approval of only one monobactam—aztreonam (2) (Figure 1B).5
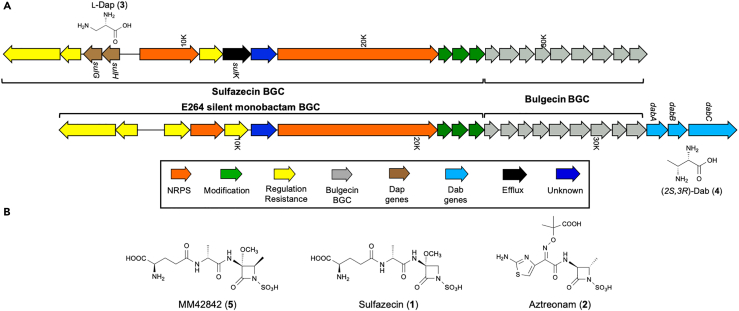
Figure 1.
Analysis of BGCs containing Dap and Dab operons
(A) sulfazecin-bulgecin and the cryptic monobactam in B. thailandensis E264. sulG-sulH and dabA-dabB-dabC are operons responsible for the biosynthesis of l-Dap (3) and (2S,3R)-Dab (4), respectively.
(B) Structures of MM42842, sulfazecin, and aztreonam.
Central to the biosynthesis of sulfazecin and other β-lactam antibiotics are members of the non-ribosomal peptide synthetase (NRPS) superfamily. These giant modular enzymes use the 20 common amino acids and select from among more than 500 specialized building blocks to assemble a vast array of secondary metabolites, many of which have important medical and commercial value. Biosynthetic innovations have evolved in core catalytic domains of these enzymes to modify certain amino acid substrates during peptide assembly to introduce new structural features in their products. Prominent among them are serine and threonine, which can be transformed to the α,β-dehydroamino acid residues dehydroalanine and dehydrobutyrine,6,7 respectively, and even create β-lactam rings embedded in their peptide products.8,9,10 Prior to and separate from the action of NRPSs, both serine and threonine can be further modified to produce the additional NRPS building blocks 2,3-diaminopropionate (Dap) and 2,3-diaminobutyrate (Dab), respectively. The biosynthesis of these widely-distributed non-canonical amino acids has been addressed previously but many questions remain unanswered.
In sulfazecin biosynthesis, the gene couple responsible for producing l-Dap (3), sulH and sulG, is highly homologous to the characterized sbnA, a PLP-dependent cysteine synthase, and sbnB, an NAD+ dependent dehydrogenase, genes from staphyloferrin biosynthesis as well as cmnB and cmnK from capreomycin biosynthesis, both of which have been previously characterized.11,12 l-Dap is biosynthesized from the primary metabolites O-phosphoserine (OPS, 6) and l-glutamate (l-Glu), which are condensed in a PLP-dependent reaction to generate N-(2-amino-2-carboxyethyl) glutamic acid (ACEGA, 7). This intermediate then undergoes an NAD+-dependent oxidation/hydrolysis to efficiently generate 2-oxoglutarate (2OG, 8) and the l-Dap (3) product.11
Aztreonam, and other synthetic monobactams that have been evaluated for clinical applications, differ importantly from naturally-occurring monobactams by the addition of a substituent at C-4 of the azetidinone ring, which has been demonstrated to improve both antimicrobial activity and stability to serine β-lactamases.13,14,15 In sulfazecin biosynthesis, the carbon framework of the β-lactam is derived from the non-canonical α,β-diamino acid l-Dap (3).16,17 We have recently demonstrated that the sulfazecin biosynthetic machinery in Pseudomonas acidophila can accept β-branched derivatives of l-Dap (3), resulting in the production of methyl and fluoromethyl C-4 substituted monobactams by chemical complementation.18 While in P. acidophila the syn-4-methyl-sulfazecin analogue arises from incorporation of the exogenously supplied amino acid (2S,3R)-diaminobutyrate (Dab, 4), this monobactam has also been isolated previously from Pseudomonas cocovenans and named MM42842 (5) (Figure 1B).19
Due to its altered antibacterial spectrum and β-lactamase-resistant properties, we were interested in investigating the biosynthetic origin of the C-4 β-methyl group in naturally occurring MM42842 (5) as a prelude to introducing additional modifications at this site. Based on the detection of the same product in an l-Dap-deficient strain supplemented with (2S,3R)-Dab (4), we hypothesized that MM42842 (5) biosynthesis natively incorporates this amino acid as a precursor by an NRPS. There are numerous examples of other natural products that contain 2,3-Dab diastereomers as building blocks including friulimicin, pacidamycin, napsamycin, and mureidomycins.20,21,22,23 In friulimicin biosynthesis, gene inactivation and substrate incorporation studies identified putative genes that were necessary for the production of (2S,3R)-Dab (4), implying that 2,3-Dab biosynthetic enzymes could be encoded in these biosynthetic gene clusters (BGCs),20,21 although the enzymes have yet been identified and characterized.
MM42842 remains the only naturally occurring C-4 substituted monobactam and its biosynthesis has not been uncovered. Herein, we conducted extensive bioinformatics analysis to identify new monobactam BGCs that harbor putative genes responsible for Dab biosynthesis. The in vivo and in vitro experimentation enabled us to present a unified view for the first time that a tri-enzyme cascade encoded by the dabABC operon in a silent MM42842 producer is required to synthesize (2S,3R)-Dab (4). By examining monobactams and their biosynthetic intermediates produced in Pseudomonas and in vitro biochemical reactions, we also identified an unexpected crossover activity of DabB and DabC to generate l-Dap directly from OPS (6) in the absence of DabA. Overall, the dabABC operon was found to be a genomic signature for monobactam biosynthetic BGCs that synthesize C-4 methylated β-lactam rings. On the other hand, simpler unmethylated monobactams such as sulfazecin are characterized by BGCs that do not contain dabABC-like genes, but instead encode SulG and SulH homologues that lead to the conversion of OPS (6) to l-Dap in a mechanistically distinct way.
Results and discussion
Bioinformatic analysis of sulfazecin homologous gene clusters
We initially hoped to identify homologous genes to friulimicin dabAB and pacidamycin pacQST within the MM42842 BGC. Unfortunately, the MM42842-producing P. cocovenenans strain has neither been sequenced nor is it accessible from any bacterial collection, leaving the identity of putative Dab biosynthetic genes in the MM42842 BGC unknown. Based on our hypothesis that activation of l-Thr as O-phospho-threonine (OPT, 9) is a requirement for activity of the PLP-dependent cysteine synthase homologue, we reasoned that the presence of an l-Thr kinase within a monobactam gene cluster would identify a producer of a C4-methyl monobactam such as MM42842 (5). To confirm our hypothesis, we searched a set of genomes of four known Dab and five known Dap producers with antiSMASH and indeed observed the presence of a putative l-Thr kinase consistently and exclusively in the Dab producers.24 We thus re-examined a set of previously identified monobactam gene clusters16 for an l-Thr kinase and several Burkholderia species were found that contained the anticipated kinase (Table 1).
Table 1.
BGCs containing l-Thr kinases genes and putative operons for biosynthesis of Dab or Dap
| Natural product | l-Threonine kinase | Dab or Dap Operons |
|---|---|---|
| Friulimicin | yes | DAB |
| Napsamycin | yes | DAB |
| Mureidomycin | yes | DAB |
| Pacidamycin | yes | DAB |
| Sulfazecin | No | DAP |
| Capreomycin | No | DAP |
| Staphyloferrin B | No | DAP |
| Viomycin | No | DAP |
| Edeine A | No | DAP |
We selected the Burkholderia thailandensis E264 monobactam BGC for further investigation. The overall gene organization and its encoded monobactam and bulgecin biosynthetic enzymes are very similar to those of the sulfazecin producer (Figure 1). Therefore, it seemed reasonable to predict the product from this BGC is similar to sulfazecin. However, sulK, encoding an RND efflux outer membrane lipoprotein, is absent from the E264 monobactam cluster. The most significant further difference is the absence of the Dap biosynthetic genes sulG and sulH and the addition of a 3-gene operon (dabABC) located at the end of E264 bulgecin BGC (Figure 1). This split monobactam–bulgecin–dabABC organization is also observed throughout all Burkholderia species harboring both monobactam BGCs and l-Thr kinase genes (Figure S1).
The dabABC operon encodes proteins sharing similarities to kinases (∼45/60, identity/similarity), PLP-dependent cysteine synthases (∼45/60), and lyase family protein/ATP-grasp domain-containing proteins (∼35/55), respectively. Similar operons are found in BGCs using 2,3-Dab as building blocks for other natural products such as friulimicin, napsamycin, and pacidamycin20,22 (Figure S2A). Interestingly, the Dap and Dab operons are organized differently across these BGCs, especially with the DabC homologous enzymes expressed either as N- and C-terminal individual proteins or the N-terminal domains fused to the PLP-dependent cysteine synthases (Figures S2A and S2B). In the case of friulimicin, gene inactivation, and genetic complementation showed that dabA and dabB, encoding a cysteine synthase and an arginosuccinate lyase homologous to the C-terminal E264 DabC, are essential for friulimicin biosynthesis (Figure S2A). Chemical complementation revealed that (2S,3R)-Dab (4), but not (2S,3S)-Dab (10), could restore friulimicin production, suggesting the former stereoisomer is the product synthesized by the Dab operon.20 Because of the high similarities to other Dab enzymes and tight association with monobactam BGCs, we proposed the dabABC operon is likely responsible for (2S,3R)-Dab (4) biosynthesis in B. thailandensis E264 even though the gene organization is different.
The monobactam gene cluster in B. thailandensis E264 is silent
Isolation of monobactam antibiotics have never been reported from any Burkholderia strains. Deletion of a global negative regulator scmR in B. thailandensis E264 resulted in increased levels of transcription of a monobactam-like BGC, but the β-lactam product of the cluster has never been described.25,26 We used three fermentation media with the WT E264 and the scmR deletion mutant of B. thailandensis ΔscmR to examine if any monobactam is produced by these strains. Fermentation supernatants and cell-free extracts were analyzed by a sensitive β-lactam-specific nitrocefin assay in which β-lactamase expression is induced by the presence of a β-lactam.27 However, no β-lactamase induction activity was detected by the assays over the entire course of the fermentations in all media tested, indicating that the monobactam BGC is silent in B. thailandensis (Figure S3A).
Analysis of the role of sulK in P. acidophila and B. thailandensis E264
A gene homologous to sulfazecin sulK is not present in the silent monobactam BGC of E264. Thus, we were interested in whether sulK is involved in sulfazecin (1) production in P. acidophila and the absence of such a gene caused silence in E264. During our previous search for the bulgecin BGC using transposon mutagenesis, we generated two bulgecin-deficient mutants in P. acidophila ΔsulG. The first mutant had Tn5 insertion in bulgecin biosynthetic gene bulE.28 To identify the second mutant, its genomic DNA (gDNA) was digested with restriction enzymes and probed with the kanamycin-resistance cassette from Tn5.16 DNA sequencing analysis of the positive 5.0-kb BamHI-digested fragment revealed that the Tn5 transposon had inserted into sulK of the sulfazecin BGC (Figures S4A and S4B). We grew the mutant in P. acidophila fermentation medium supplemented with 2 mM l-Dap (3) to chemically complement the sulG deficit. Both nitrocefin and bioassays showed that production of sulfazecin was still abolished in the ΔsulG/sulK::Tn5 mutant, indicating that sulK is in fact essential for the biosynthesis of both bulgecins and sulfazecin in P. acidophila (Figure S4C). Next, we constructed two expression vectors to test if expression of sulK in the B. thailandensis ΔscmR mutant could activate the silent BGC and result in production of monobactam. We observed that the concentration of resistance to chloramphenicol increased to 70 μg/mL in B. thailandensis ΔscmR (pSCrhaB2Plus/Cm) when expression was induced with rhamnose compared to <5 μg/mL in uninduced clones, indicating that the PrhaBAD promotor is functional in B. thailandensis. Then we attempted to activate the silent monobactam cluster by expression of sulK in ΔscmR. Five exconjugants of B. thailandensis ΔscmR (pSCrhaB2Plus/sulK) were tested and two were also supplemented with 2 mM l-Dap (3) to the medium. However, no detectable β-lactam metabolites were observed based on nitrocefin assays from strains where expression of sulK was induced. This result suggested that either sulK may not be essential for monobactam biosynthesis in B. thailandensis or additional factors are required to activate the silent BGC (Figure S3B).
A-domain substrate specificity of E264 A3
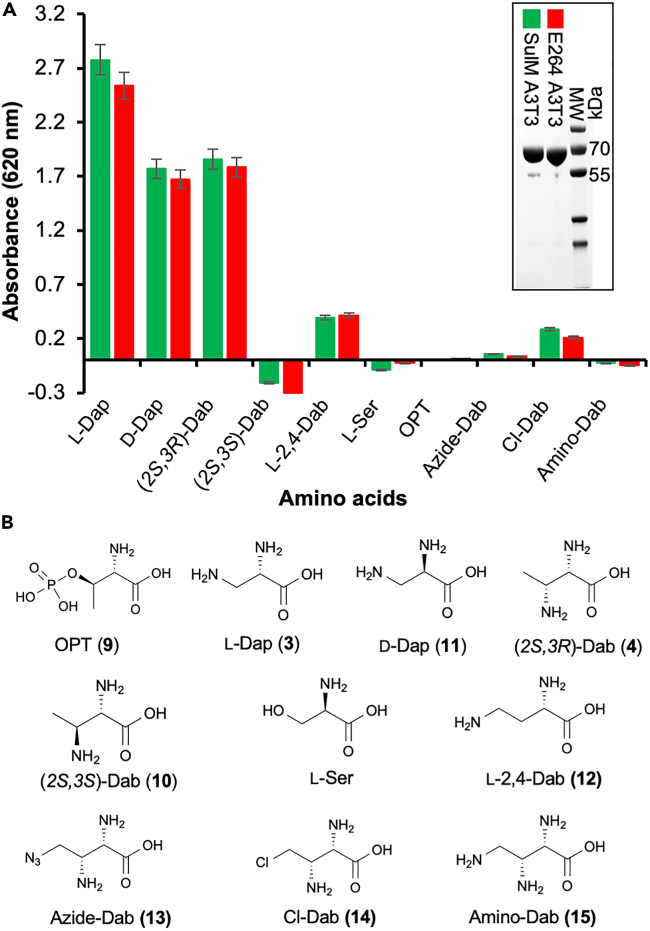
The large NRPS encoded in the E264 monobactam BGC showed ∼80% overall sequence similarity and share the same domain architecture as its counterpart SulM in the sulfazecin biosynthetic pathway.16 Although A-domain bioinformatic analysis29,30 failed to predict the amino acid activated by the last A-domain in the E264 large NRPS (E264 A3), the potential dab operon in its BGC and the results from previous chemical supplementation experiments in P. acidophila ΔsulG suggested that (2S,3R)-Dab (4) could be the native substrate of E264 A3.18 Therefore, the E264 A3T3 di-domain was expressed and used to analyze its substrate selectivity across several potential natural and synthetic amino acid substrates. As shown in Figure 2, colorimetric A-domain assays31 showed that E264 A3T3 displayed a similar substrate selectivity and activity compared to SulM A3T3. It clearly activated (2S,3R)-Dab (4), l-Dap (3) and d-Dap (11), while no activation of (2S,3S)-Dab (10), l-Ser and OPT (9) and only slight activity with l-2,4-Dab (12) and three synthetic l-Dap analogs (13–15) was seen.18 Interestingly, based on these endpoint assays E264 A3T3 actually seems to prefer l-Dap (3) over its proposed native substrate (2S,3R)-Dab (4), although E264 lacks the biosynthetic machinery (homologs to SulGH) to produce l-Dap (3) itself. To achieve a more detailed analysis, we applied the continuous 6-methyl-7-thioguanosine (MesG) hydroxylamine coupled assay32,33 to determine the kinetic parameters of E264 A3T3 for both l-Dap (3) and (2S,3R)-Dab (4) (Figure S5). In agreement with relative activities and previous kinetic investigations of SulM A3T3,18 the additional methyl-substituent in (2S,3R)-Dab (4) strongly increases KM by approximately a factor of 10, whereas kcat was only slightly diminished. Notably, E264 A3T3 exhibits an almost identical kcat/KM for l-Dap (3) (ca. 85 mM−1min−1) and the larger (2S,3R)-Dab (4) (5.4 mM−1min−1) compared to SulM A3T3 (Figure S5).
Figure 2.
Substrate specificity analysis of E264 A3T3 di-domain
(A) A-domain substrate specificity in vitro assays of SulM A3T3 (green bars) and E264 A3T3 (red bars). The purified proteins of SulM A3T3 and E264 A3T3 are also shown.
(B) Amino acid substrates used in A-domain assays.
Genetic complementation of P. acidophila ΔsulG mutant and in vivo functional characterization of the dabABC operon
The previous in vivo chemical supplementation and in vitro A-domain assays showed that the sulfazecin biosynthetic pathway can incorporate both l-Dap (3) and (2S,3R)-Dab (4) as building blocks to produce sulfazecin (1) and MM42842 (5), respectively.18 Next, we sought to see if the P. acidophila ΔsulG mutant could be genetically complemented by expression of the sulGH operon. To achieve this goal, we first established a functional gene expression system in P. acidophila. Whole-genome DNA sequencing has suggested that many species including P. acidophila and the isosulfazecin producer Pseudomonas mesoacidophila previously attributed to genus Pseudomonas should be re-classified as genus Burkholderia or Paraburkholderia.28,34 Thus, expression vectors and promoters developed for Pseudomonas may not actually be functional or genetically stable in these newly classified and untested species.35 We examined three expression vectors pSCrhaB2Plus (PrhaBAD), pJM101(Ptac), and pUCU20-ANT2-MCS (PantA), that were all originally developed for gene expression in P. aeruginosa.36,37,38 We found the PrhaBAD and Ptac promoters were either not induced or only at very low activity as the expression of the chloramphenicol-resistance reporter gene did not result in elevated resistance to the antibiotic in the P. acidophila ΔsulGH mutant (data not shown).
The expression vector pUCP20-ANT2-MCS contains an anthranilate-inducible PantA promoter that has been reported to be functional in a few Pseudomonas strains.39,38 We used a similar approach as earlier in B. thailandensis to identify if the vector is fully functional in P. acidophila and found resistance to high concentrations of chloramphenicol in P. acidophila ΔsulG (pUCP20-ANT2-MCS/Cm) achieved only when expression of the reporter gene was induced (Figure S6). The plasmid also appeared to be genetically stable in P. acidophila ΔsulG as it could be maintained for several generations without losing the kanamycin selection marker and the re-isolated plasmids from multiple transformants showed no deletions or rearrangement by restriction digestions (data not shown). Therefore, these results indicated that the pUCP20-ANT2-MCS is fully functional in P. acidophila.
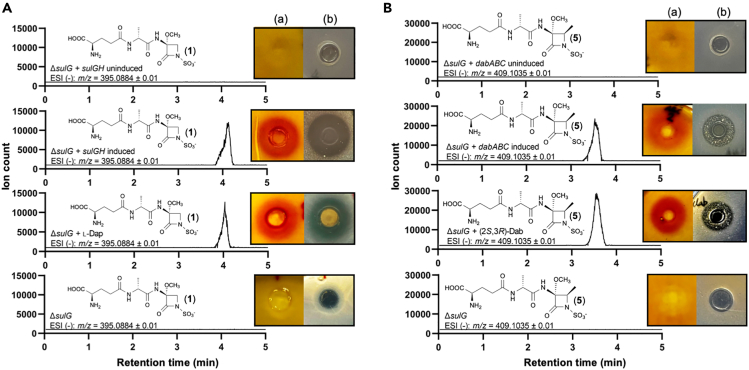
Then we tested if sulG could genetically complement the ΔsulG mutant and restore production of sulfazecin. Surprisingly, growth inhibition bioassay, nitrocefin assay, and Ultra Performance Liquid Chromatography – High Resolution Mass Spectrometry (UPLC-HRMS) did not show sulfazecin (1) production when expression of sulG was induced in ΔsulG (pUCP20-ANT2-MCS/sulG) cells. Sequence analysis revealed point mutations inside downstream sulH in the knockout mutant. These mutations appeared to be introduced by PCR during construction of the plasmid pEX18Km/FGmH16 and introduced into the genome by homologous recombination causing the loss of activity of its encoded protein. To confirm our assumption, we cloned the sulGH operon into pUCP20-ANT2-MCS. Upon induction of sulGH expression with anthranilate, we observed a sulfazecin (1) production in P. acidophila ΔsulG (pUCP20-ANT2-MCS/sulGH) comparable to WT by bioassay, nitrocefin assay, and UPLC-HRMS. Thus, the genetic complementation revealed both SulG and SulH are inactive in P. acidophila ΔsulG mutant. In addition, the overall sulfazecin (1) yields in the genetic complementation strain were comparable to those from the WT P. acidophila and P. acidophila ΔsulG supplemented with l-Dap (3), indicating that the PantA promoter is highly active and tightly controlled in P. acidophila, consistent with the results in other Pseudomonas strains (Figure 3A).38
Figure 3.
Nitrocefin assays, bioassays, and UPLC-HRMS assays of P. acidophila ΔsulG genetic and chemical complementation
(A) P. acidophila ΔsulG complemented by sulGH or l-Dap (3).
(B) P. acidophila ΔsulG complemented by dabABC or (2S,3R)-Dab (4). (a). Nitrocefin assay. (b). Bioassay on E. coli ESS.
Finally, to demonstrate if the dabABC operon in the silent in B. thailandensis E264 is responsible for the biosynthesis of (2S,3R)-Dab (4) in vivo, we constructed plasmid pUCP20-ANT2-MCS/dabABC, in which the expression of the dabABC operon was controlled by a single PantA promoter. Small but consistent inhibition zones on E. coli ESS and β-lactamase-induction activities on B. licheniformis were observed in cultures of P. acidophila ΔsulG (pUCP20-ANT2-MCS/dabABC) when induced. These activities were elevated after the supernatants of fermentation were concentrated by 7x before conducting the assays. In contrast, the ΔsulG mutant and uninduced cultures showed no antibacterial and β-lactamase-induction activities during the entire fermentation (Figure 3B). Therefore, β-lactam production was due to the induced expression of the dabABC operon. To further characterize the β-lactam product, the corresponding supernatants were partially purified before being analyzed by UPLC-HRMS. A mass corresponding to methyl-sulfazecin (MM42842, 5) was present in samples with expressed dabABC, but not in the mutant and uninduced controls. The mass and its retention time were identical to the MM42842 (5) produced from P. acidophila ΔsulG with added (2S,3R)-Dab (4) (Figure 3B). In conclusion, the in vivo genetic complementation clearly demonstrated that the dabABC operon encodes enzymes responsible for (2S,3R)-Dab (4) biosynthesis.
Functional characterization of DabA
Natural products friulimicin, napsamycin, and pacidamycin using either (2S,3R)-Dab (4) or (2S,3S)-Dab (10) as building blocks all harbor dabA homologs in their BGCs (Figure S2A; Table S1).20,21,40 DabA showed relatively high sequence similarities to kinases from various prokaryotic species (∼45/62). However, the two functionally characterized l-Thr kinases, PduX and BluE, in cobalamin biosynthesis, were not retrieved by BLASTP due to significant sequence differences among the members of this family of proteins.41,42 Despite the low sequence similarity, alignment of DabA with PduX and BluE revealed DabA contains all four conserved motifs in l-Thr kinases. Motifs I, II, and III are conserved in all members of the GHMP kinase family43 and probably involved in substrate (l-Thr) binding and interaction with the phosphates of ATP. The fourth conserved motif (Motif IV) is characteristic of PduX, BluE, and all other predicted but not experimentally verified l-Thr kinases, and is probably part of the active site or ATP-binding site.44 In addition, the five amino acid residues previously demonstrated to be critical for PduX activity by site-specific mutagenesis are also all conserved in DabA44 (Figure 4A).
Figure 4.
In vivo characterization of DabA and DabC
(A) Motifs I-III conserved in DabA and GHMP family kinases and Motif IV conserved in Pdux, BluE, and other l-Thr kinases. Identical and similar residues are highlighted in blue and yellow, respectively. Asterisks (∗) indicate residues demonstrated to be critical to enzyme activity.
(B) Nitrocefin assays, bioassays, and UPLC-HRMS assays in P. acidophila ΔsulG (pUCP20-ANT2-MCS/dabBC) showing sulfazecin (1) instead of MM42842 (5) was produced. (+) induced, (−) uninduced.
(C) Nitrocefin assays, bioassays, and UPLC-HRMS assays of MM42842 (5) produced in P. acidophila ΔsulG (pUCP20-ANT2-MCS/dabC) in the presence of 2 mM ACPAA (17), ΔsulG supplemented with ACPAA (17), or induced dabC in ΔsulG (pUCP20-ANT2-MCS/dabC) omitting of ACPAA (17). (a). Nitrocefin assay. (b). Bioassay on E. coli ESS.
In vitro characterization of DabA could not be carried out due to insolubility or insufficient quantities of soluble protein expressed in E. coli. PduX and BluE are involved in the biosynthetic salvage pathway of cobalamin.42 Searching the P. acidophila genome for l-Thr kinases (DabA, PduX, or BluE) and other key enzymes of the pathway did not identify homologous proteins, further suggesting the absence of a cobalamin pathway and l-Thr kinases in this strain. To determine if DabA is essential for production of (2S,3R)-Dab (4), plasmid pUCP20-ANT2-MCS/dabBC was constructed and transformed into the ΔsulG mutant to generate strain P. acidophila ΔsulG (pUCP20-ANT2-MCS/dabBC). Aliquots of the supernatants were withdrawn after expression of dabBC was induced and partially purified before analysis by UPLC-HRMS to determine if MM42842 (5) was produced. As shown in Figure 4B, no MM42842 (5) was detected in ΔsulG (pUCP20-ANT2-MCS/dabBC). The completely abolished production of MM42842 (5) associated with the absence of dabA revealed that DabA is essential for production of (2S,3R)-Dab (4). Therefore, combined with the bioinformatic analysis and DabB characterization (see below), this in vivo result strongly indicated that DabA is an l-Thr kinase that produces OPT (9) from l-Thr.
Functional characterization of DabB
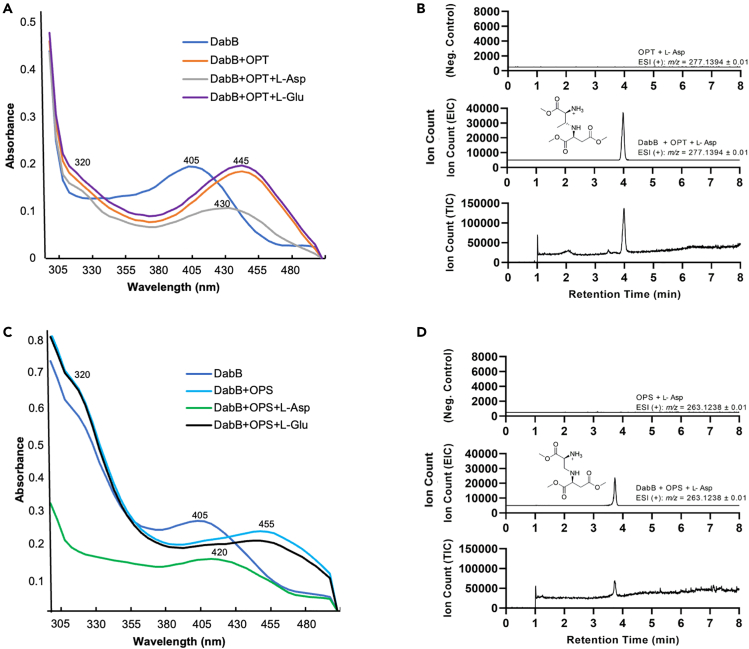
DabB and SulH are homologous to the class II PLP-dependent cysteine synthase enzymes and share ∼36% amino acid similarity. We thus hypothesized that DabB catalyzes a similar reaction as SulH and SbnA.11,16 Purified C-His6 DabB shows a maximal absorbance at 405 nm due to the Schiff base formed between PLP and a lysine residue (Lys40) at the active site. The λmax shifted to 320 and 445 nm when excess OPT (9) was mixed with the enzyme. OPS (6) also caused a spectral change to 320 and 455 nm when mixed with DabB. This absorption changes suggested that both OPT (11) and OPS (6) are used by DabB to form the analogous aminoacrylate intermediate coupled with phosphate release. l-Glu and l-Asp were then tested as coupling partners. We found the maximum absorbance moved to 430 nm when l-Asp, but not l-Glu, was added to DabB and OPT (9), indicating l-Asp is the exclusive amine donor to OPT (9)11 (Figure 5A). Similarly, l-Asp also induced a UV shift to 420 nm when mixed with DabB and OPS (6), but this shift was not observed when l-Glu was tested as amine donor, suggesting that l-Asp could be used with OPS (6) to form the addition product N-(2-amino-2-carboxylethyl) aspartic acid (ACEAA,16, Figures 5C; Figure 7B). This result supports the results that DabB and DabC can produce l-Dap (3) in vivo when OPT (9) is not available in cells (see below).
Figure 5.
In vitro characterization of DabB
(A) UV-vis spectra of DabB, complex of DabB with OPT (9), and DabB-OPT with l-Asp or l-Glu. The wavelength of maximum absorbance is labeled.
(B) UPLC-HRMS assays of ACPAA (17) trimethyl ester produced from OPT and l-Asp by DabB. (+): full reaction. (−): control reaction without DabB.
(C) UV-vis spectra of DabB, complex of DabB with OPS (6), and DabB-OPS with l-Asp or l-Glu. The wavelength of maximum absorbance is labeled.
(D) UPLC-HRMS assays of ACEAA (16) trimethyl ester produced from OPS and l-Asp by DabB. (+): full reaction. (−): control reaction without DabB.
Figure 7.
The proposed Dab and Dap biosynthetic pathways
(A) Proposed biosynthetic pathway to (2S,3R)-Dab (4) by DabA, DabB, and DabC.
(B) Proposed alternative biosynthetic pathway to l-Dap (3) by DabB and DabC.
The DabA and DabB analyses lead to the proposal that OPT (9) and l-Asp were likely the native substrates of DabB and form the product N-(1-amino-1-carboxy-2-propanyl) aspartic acid (ACPAA, 17) by linking the l-Thr β-carbon and l-Asp amine, while OPS (6) and l-Asp can be used as alternative substrates to produce ACEAA (16), similar as characterized in SulH and SbnA.11,16 In order to directly detect and analyze the DabB product using UPLC-HRMS, we conducted in vitro DabB reactions and derivatized the mixtures by esterification in methanol45 (Figure S8). As shown in Figures 5B and 5D, dominant mass ions at 277.14 and 263.124 were observed only in the full enzymatic reactions containing DabB, OPT (9) and l-Asp, and DabB, OPS (6), and l-Asp, respectively. They matched the predicted mass of ACPAA (17) and ACEAA (16) trimethyl esters and were not in the control reaction without DabB. No product was detected in reactions containing l-Glu as coupling amine donor, confirming that l-Glu is not used as substrate (data not shown). These data demonstrated that DabB can utilize either OPT (9) or OPS (6) as substrate to condense with l-Asp to produce ACPAA or ACEAA, respectively.
Functional characterization of DabC
The modeled structure of DabC using AlphaFold46 clearly showed two distinct domains (DabC-N and DabC-C) connected by a 16-aa linker (Figure S2B). The C-terminal domain showed structural similarity to known argininosuccinate lyases catalyzing the reversible breakdown of argininosuccinate to arginine and fumarate.47 Despite this coupled arrangement, DabC-N and DabC-C are also expressed as individual proteins or fused to DabB homologues in other Dab operons20,21,22,40 (Figure S2A), our in vivo genetic complementation data clearly showed the holo DabC functions to produce (2S,3R)-Dab (4) in concert with DabA and DabB.
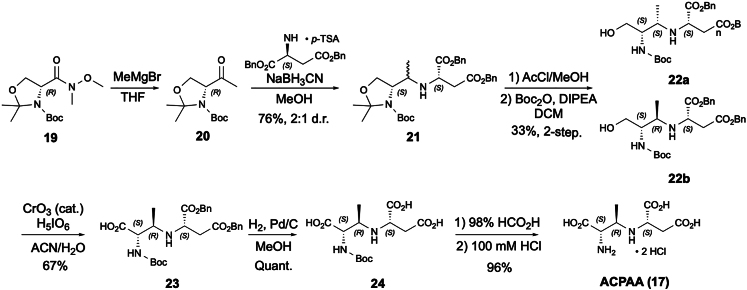
To provide enough substrate for in vitro characterization of DabC, its proposed substrate ACPAA (17) was chemically synthesized (Figure 6). To prepare ACPAA (17), we initially hoped to use an approach similar to that of Hsu et al. in their synthesis of the intermediate ACEGA (7) from l-Dap (3) biosynthesis.12 Using a cyclic sulfamidate (S1) derived from l-allo-Thr, we attempted to stereoselectively install the secondary amine by nucleophilic substitution with dibenzyl-l-aspartate (18) (Scheme S1). Although we previously found substitution with weakly basic, strong nucleophiles such as NaN3 to be facile with this substrate,18 the more basic, less nucleophilic amine of aspartate was not reactive. Only elimination byproduct was observed upon heating or extended reaction times. Reductive amination, on the other hand, is robust and selective48 and has been used to form N-alkyl bonds in α-amino acids.49,50
Figure 6.
Synthesis of ACPAA (17)
To avoid potential racemization at the α-stereocenter in a threonine-derived ketone, we elected to use a methyl ketone-homologue (20)51 to the well-known Garner aldehyde,52 which could be readily prepared from protected d-serine (19, Figure 6).53 Gratifyingly, reaction of 20 with dibenzyl-l-aspartate (18) and NaCNBH3 generated the product 20 in good yield, albeit as a poorly separable ∼2:1 mixture of diastereomers. Using the polar Felkin-Anh transition state model,54 we expected the major diastereomer to be the desired 3R-stereochemistry. While some resolution of the diastereomers could be achieved by silica gel chromatography at this stage, we hoped that a later intermediate would prove easier to resolve. Thus, the mixture of 21 was subjected to acidic methanolysis to remove the acetonide group, followed by Boc re-protection. While this reaction sequence gave only modest yields, the two diastereomers 22a and 22b could be separated and characterized.
Diastereomerically pure 22b was then transformed to the carboxylic acid 23 by a catalytic Jones oxidation procedure.55 Finally, hydrogenolysis of the esters and acidic N-deprotection, followed by anion exchange yielded ACPAA (17) as the dihydrochloride salt. 1H-NMR analysis of this product showed H2-H3 vicinal coupling constant of 3.2 Hz, in line with our expectations for the 2S,3R-stereochemistry.18
Attempts to biochemically characterize DabC failed because of our inability to obtain soluble protein in sufficient amount. With the synthesized ACPAA (17) in hand, we developed an in vivo method as an alternative approach to analyze the function of DabC. A dabC expression vector, pUCP20-ANT2-MCS/dabC, was constructed and delivered into the ΔsulG mutant. Nitrocefin assay showed the appearance of induced β-lactamase activity from P. acidophila ΔsulG (pUCP20-ANT2-MCS/dabC) when expression of dabC was induced and supplemented with ACPAA (17). UPLC-HRMS analysis of the partially purified fermentation supernatant revealed the active product as MM42842 (5) (Figure 4C). No MM42842 (5) was detected when dabC expression was induced but ACPAA (17) was not added to the fermentation medium. In another control fermentation, a trace amount of MM42842 (5) was detected by UPLC-HRMS when 2 mM ACPAA (17) was administered directly to the ΔsulG mutant (Figure 4C). The titer, however, was significantly less than that produced in the strain with induced dabC expression (<8% by ion count). The elimination of ACPAA (17) to yield (2S,3R)-Dab (4) and fumarate is a reversible reaction and, therefore, these species likely exist in equilibrium.47 We had also observed decomposition of ACPAA (17) into (2S,3R)-Dab (4) and fumarate in vitro and hypothesized the leaking production of MM42842 (5) is due to the instability of ACPAA (17) under the acidic fermentation conditions of P. acidophilia (pH 5.5–6.0). Collectively these data established that ACPAA (17) is the product of DabB and used by DabC to generate (2S,3R)-Dab (4) (Figure 4C).
DabB and DabC can produce l-Dap from OPS and l-Asp
Although UPLC-HRMS analysis revealed MM42842 (5) was not produced in P. acidophilia ΔsulG (pUCP20-ANT2-MCS/dabBC), the bioassays and nitrocefin assays clearly showed production of a β-lactam product in the strain when both dabB and dabC were expressed. We partially purified the active product and analyzed it by UPLC-HRMS. To our surprise, UPLC-HRMS data clearly showed production of sulfazecin (1) in the induced strain, but not when dabBC expression had not been induced (Figure 4B). This deduction is also in agreement with the DabB in vitro result showing the enzyme can use OPS (6) and l-Asp as alternate substrates to generate ACEAA (16). The data from dabBC expression in P. acidophila ΔsulG further indicated that ACEAA (16) produced by DabB can be cleaved by DabC to generate l-Dap (3). As OPT (9), OPS (6), and l-Asp all are available in ΔsulG (pUCP20-ANT2-MCS/dabABC) genetic complementation cells, we were therefore interested to see if both MM42842 (5) and sulfazecin (1) are produced in this strain. Indeed, sulfazecin (1) was produced as the minor product in the strain when the UPLC-HRMS data of earlier fermentation samples were re-examined. Neither MM42842 (5) nor sulfazecin (1) was produced when expression of dabABC was not induced (Figure S7). We reasoned that the higher MM42842 (5) production could be due to OPT (9) being a better substrate of DabB than OPS (6) or the larger OPT (9) precursor pool produced by overexpressed dabA under the strong PantA promoter in ΔsulG (pUCP20-ANT2-MCS/dabABC). In the event, this result indicated dual functions of DabB and DabC to produce (2S,3R)-Dab (4) from OPT and l-Asp and l-Dap (3) from OPS and l-Asp substrates.
Based on the in vivo and in vitro analyses of DabA, DabB, and DabC, the (2S,3R)-Dab (4) biosynthetic pathway and the proposed alternative functions of DabB, and DabC to produce l-Dap (3) are summarized in Figure 7. L-Dap (3) and 2,3-Dab are important non-canonical amino acids used as precursors to many natural products, some are of clinical importance. The absence of a threonine aldolase gene in dabABC operons in E264 silent monobactam BGCs is consistent with our chemical supplementation experiments and in vitro NRPS A-domain assays to demonstrate that (2S,3R)-Dab (4), not (2S,3S)-Dab (10) is the product of this three-gene operon. Our results clearly showed that the biosynthetic pathway begins similarly to l-Dap (3) by utilizing O-phosphorylated l-Ser or l-Thr, which are condensed with another amino acid as an amine donor in a PLP-dependent β-replacement reaction. However, rather than facilitating amine transfer by NAD+ dependent oxidation/hydrolysis, DabC acts as a lyase to cleave this intermediate to release the final diamine product and fumarate. Our data, therefore, provide an alternative mechanism for the biosynthesis of l-Dap, where DabC can eliminate fumarate (23) from ACEAA (16) that is produced by DabB from OPS (6) as an alternative substrate. A previous investigation found enzyme activities in cell-free-extracts that produce Dab from l-Thr in a mureidomycin producer. This study concluded that Dab was produced by a single PLP-dependent enzyme by a β-replacement reaction using ammonia as the nucleophile.56 In contrast, when searching the BGC of the mureidomycin in Streptomyces roseosporus, we found a threonine aldolase-containing dab-like operon that is highly similar to those in napsamycin and pacidamycin BGCs57 (Figure S2A), indicating that Dab biosynthesis in the mureidomycin pathway is functionally identical to (2S,3S)-Dab (10) in all these natural product producers.
Non-canonical amino acids can be incorporated into small molecules and proteins through non-ribosomal or semi-synthetic pathways and confer diversified architecture and bioactivity.58,59 However, many of these non-canonical amino acids must be synthesized by chemo- or semi-synthetic methods often in low yield and under harsh reaction conditions as they are not naturally produced. Our discovery will enable further production of these compounds by environmentally- and cost-friendly methods. It also provides an opportunity for the enzymatic production of further functionalized analogues that could be incorporated into modified monobactams with improved antibiotic activity and resistance to metallo-β-lactamases and other products. Finally, these discoveries will contribute to the discovery of new monobactam natural products by genome mining and guide their characterization.
Limitations of the study
While the synthetic role of the l-threonine kinase DabA was established in this study, its in vitro production and demonstration of catalytic activity could not be directly shown despite protracted effort. The varied fusions and structures of DabA, B and C may affect through association or complex formation overall synthetic ability, which will require further experimentation to assess.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER | |
|---|---|---|---|
| Biological and chemical reagents | |||
| Restriction enzymes | New England Biolabs | neb.com | |
| Modification enzymes | New England Biolabs | neb.com | |
| HiFi DNA assembly master mix | New England Biolabs | E2621S | |
| Phusion DNA polymerase | New England Biolabs | M0535 | |
| dNTP mix | Promega | U1515 | |
| [α-32P]-dCTP | Perkin-Elmer | NEG007X250UC | |
| Random DNA labeling system | Invitrogen | 18187-013 | |
| Ni-NTA agarose | McLab | NINTA-100 | |
| Amicon 3K ultra spin columns | Sigma-Aldrich | UFC500396 | |
| General chemicals and buffers | Thermo Scientific | fisherscientific.com | |
| Bacterial culture media | Boston Bioproduct | bostonbioproducts.com | |
| Antibiotics | Gold Biotechnology | goldbio.com | |
| l-Thr | Sigma-Aldrich | 1721646 | |
| l-Ser | Sigma-Aldrich | 1721404 | |
| OPT (9) | Sigma-Aldrich | 1727078 | |
| OPS (6) | Sigma-Aldrich | 1726826 | |
| l-Dap (3) | Matrix Scientific | #072732 | |
| D-Dap (11) | Chem-Impex | 05645 | |
| (2S,3R)-Dab (4) | Lichstrahl, M.S.et al.18 | N/A | |
| (2S,3S)-Dab (10) | Boc Sciences | B18BS09181 | |
| l-2,4-Dab (12) | Alfa Aesar | L10093 | |
| Azide-Dab (13) | Lichstrahl, M.S.et al.18 | N/A | |
| Cl-Dab (14) | Lichstrahl, M.S.et al.18 | N/A | |
| Amino-Dab (15) | Lichstrahl, M.S.et al.18 | N/A | |
| Nitrocefin | Toku-E | N005 | |
| Plasmids | |||
| pSCrhaB2plus | addgene | #164226 | |
| pSCrhaB2plus/Cm | This study | N/A | |
| pSCrhaB2plus/sulK | This study | N/A | |
| pACYC184 | NEB | #E4152 | |
| pRK2013 | Taton, A.et al.60 | N/A | |
| pJM101 | addgene | #74740 | |
| pGS9 | Selvaraj, and Iyer61, Shaw, and Berg62 | N/A | |
| pBluescript | Agilent Technologies | 212205 | |
| pCR-Blunt | Life Technologies | K270040 | |
| pCR-Blunt/dabB | This study | N/A | |
| pET29b | Millipore Sigma | 69872 | |
| pET29b/dabB | This study | N/A | |
| pET28b | Millipore Sigma | 69865 | |
| pET28b/E264A3T3-3 | This study | N/A | |
| pET28b/sulMA3T3 | This study | N/A | |
| pUCP20-ANT2-MCS | Hoffmann, L.38 | N/A | |
| pUCP20-ANT2-MCS/Cm | This study | N/A | |
| pUCP20-ANT2-MCS/sulG | This study | N/A | |
| pUCP20-ANT2-MCS/sulGH | This study | N/A | |
| pUCP20-ANT2-MCS/dabABC | This study | N/A | |
| pUCP20-ANT2-MCS/dabBC | This study | N/A | |
| pUCP20-ANT2-MCS/dabC | This study | N/A | |
| Bacterial strains | |||
| E. coli PR47 | Selvaraj, and Iyer61 Shaw, and Berg62 |
N/A | |
| E. coli DH5α | Thermo Fisher Scientific | 18265017 | |
| E. coli Rosetta2 (DE3) | Sigma-Aldrich | 70954 | |
| E. coli Rosetta2 (DE3) (pET29b/dabB) | This study | N/A | |
| E. coli Rosetta2 (DE3) (pET28b/sulMA3T3) | This study | N/A | |
| E. coli Rosetta2 (DE3) (pET28b/E264A3T3) | This study | N/A | |
| E. coli ESS | Aoki, H.63 | N/A | |
| E. coli HB101 (pRK2013) | ATCC | 37159 | |
| B. lichenifomis ATCC 14580 | ATCC | 14580 | |
| P. acidophila ATCC 31363 | ATCC | 31363 | |
| P. acidophila ΔsulG | This study | N/A | |
| P. acidophila ΔsulG/sulK::Tn5 | This study | N/A | |
| ΔsulG (pUCP20-ANT2-MCS/Cm) | This study | N/A | |
| ΔsulG (pUCP20-ANT2-MCS/sulG) | This study | N/A | |
| ΔsulG (pUCP20-ANT2-MCS/ sulGH) | This study | N/A | |
| ΔsulG (pUCP20-ANT2-MCS/ dabABC) | This study | N/A | |
| ΔsulG (pUCP20-ANT2-MCS/ dabBC) | This study | N/A | |
| ΔsulG (pUCP20-ANT2-MCS/dabC) | This study | N/A | |
| B. thailandensis E264 | ATCC | 700388 | |
| B. thailandensis ΔscmR | Guillouzer, L.S.et al.25 et al.26 | N/A | |
| E. coli DH5α (pSCrhaB2plus/Cm) | This study | N/A | |
| E. coli DH5α (pSCrhaB2plus/sulK) | This study | N/A | |
| Software and algorithms | |||
| antiSmash | Blin, K.24 | antismash.secondarymetabolites.org | |
| FramePlot 4.0beta | Ishikawa, J., and Hotta, K.64 | nocardia.nih.go.jp/fp4/ | |
| BLAST | Altschul, S.F.et al.65 | blast.ncbi.nlm.nih.gov/blast.cgi | |
| AlphaFold | Jumper, J.et al.46 | alphafold.ebi.ac.uk | |
| UPLC-HRMS data analysis | Waters | MassLynx | |
| Other | |||
| UPLC-HRMS | Waters | Acquity/Xevo-G2 | |
| PCR cycler | Bio-Rad | T100 Thermal Cycler | |
| Electroporator | Life-Technologies | Cell-Porator™ | |
| UV-VIS spectrophotometer | Varian | Cary 50 | |
| NMR | Bruker | UltraShield 300 or 400 MHz Avance | |
| Combiflash | Teledyne ISCO | EZ Prep | |
| Supelclean™ ENVI-Carb™ SPE Tube | Supelco Analytical | 57092 | |
| Primers | |||
| Primers | Sequences | Targets | |
|
dabB-F dabB-R |
5’-GGGCATATGACACA CGACACTGCATCGAACA-3’ 5’-GGGGGATCCCGCGTCGA GCGCGACGAGCTCCTG-3’ |
pET29b/dabB | |
|
Cm-pSCrha-F Cm-pSCrha-R |
5’-TGAAATTCAGCAGGATCACA-3’ 5’-TGCATGCCTGCAGGTCGACTTT ACGCCCCGCCCTGCCA-3’ |
pSCrhaB2plus/Cm | |
|
sulK-28b-F sulK-28b-R |
5’-GGGCATATGACACGCAGATC GGCAATGGCA-3’ 5’-GGGAAGCTTTTAGCAGCCG GATCTCAGTGGTGGTGG-3’ |
pET28b/sulK | |
|
E264A3T3-3-F E264A3T3-R |
5’-GGGCATATGGTGCTGCGC CAGGGTGAGCTGCGG-3’ 5’-GGGAAGCTTCAGATGCG CGGCGAGCGCCTC-3’ |
pET28b/E264A3T3 | |
|
pUCP20-Cm-F pUCP20-Cm-R |
5’-GGGCATATGGAGAAA AAAATCACTGG-3’ 5’-GGGAAGCTTTTACGCC CCGCCCTGCCAC-3’ |
pUCP20-ANT2-MCS/Cm | |
|
pUCP20-sulG-F pUCP20-sulG-R |
5’-AAACTAGTAGGAGATATAC ATATGAAATTCAACTCAATCGA AC-3’ 5’-TAAAACGACGGCCAGTGCCAC TAGACATACGCCAGAGTC-3’ |
pUCP20-ANT2-MCS/sulG | |
|
pUCP20-sulGH-F pUCP20-sulGH-R |
5’-AAACTAGTAGGAGATATACATAT GAAATTCAACTCAATCGA AC-3’ 5’-TAAAACGACGGCCAGTGCCAC TACATGGTCCACCGTTC-3’ |
pUCP20-ANT2-MCS/sulGH | |
|
pUCP20-dabABC-F pUCP20-dabABC-R |
5’-AAACTAGTAGGAGATATACATA TGAGCATCATCGAGAACAAC GAAGACC-3’ 5’-TAAAACGACGGCCAGTGCCAT CAGTCGCCCGCCAGCTG-3’ |
pUCP20-ANT2-MCS/dabABC | |
|
pUCP20-dabBC-F pUCP20-dabBC-R |
5’-AAACTAGTAGGAGATATACATATGA CACACGACACTGCAT CGAAC-3’ 5’-TAAAACGACGGCCAGTGCCATC AGTCGCCCGCCAGCTG-3’ |
pUCP20-ANT2-MCS/dabBC | |
|
pUCP20-dabC-F pUCP20-dabC-R |
5’-AAACTAGTAGGAGATATACATATGAC GGGTATCTTTGTCTTC ATCGAGAGC-3’ 5’-TAAAACGACGGCCAGTGCCATCAG TCGCCCGCCAGCTG-3’ |
pUCP20-ANT2-MCS/dabC | |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact: Prof. Craig A. Townsend (ctownsend@jhu.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
DabA, DabB, and DabC data have been deposited at GenBank and are publicly available as of the date of publication, under the accession number GenBank: BK065153 at NCBI’s GenBank database.
-
•
This paper does not report original codes.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
This research does not involve models or human/animal participants.
Method details
Construction of recombinant plasmids
All recombinant plasmids were confirmed by DNA sequence analysis (The Synthesis & Sequencing Facility at The Johns Hopkins University).
Construction of pSCrhaB2plus/Cm: The chloramphenicol-resistant gene was amplified from plasmid pACYC184 using primers Cm-pSCrha-F and Cm-pSCrha-R. The PCR product was assembled with NdeI/XbaI-digested pSCrhaB2plus using NEBuilder(R) HiFi DNA Assembly Master Mix (New England Biolabs) to give recombinant pSCrhaB2plus/Cm.
Construction of pSCrhaB2plus/sulK: sulK was amplified from genomic DNA of the WT P. acidophila using primers sulK-28b-F and sulK-28b-R. The PCR product was digested with NdeI and HindIII and ligated to NdeI/HindIII-digested pET28b to give recombinant plasmid pET28b/sulK. pET28b/sulK was digested with BlpI, blunt-ended with Klenow DNA polymerase, and digested with NdeI. The resulting sulK fragment was ligated into NdeI/XmnI-digested pSCrhaB2plu to generate pSCrhaB2plus/sulK.
Construction of pET29b/dabB: dabB was amplified from the genome of B. thailandensis E264 using primers dabB-F and dabB-R. The PCR product was ligated into plasmid pCR-Blunt and gave pCR-Blunt/dabB. dabB was excised with NdeI and BamHI and ligated into NdeI/BamHI-digested pET29b to give expression vector pET29b/dabB.
Construction of pET28b/E264A3T3-3: The fragment encoding A3T3 and upstream 16-a.a. linker region between the epimerization and adenylation domains was amplified using primers E264A3T3-3-F and E264A3T3-R. The PCR product was digested with NdeI/HindIII and ligated into pET28b digested with the same enzymes to generate expression vector pET28b/E264A3T3-3.
Construction of pUCP20-ANT2-MCS/Cm: The chloramphenicol-resistant gene was amplified from plasmid pACYC184 using primers pUCP20-Cm-F and pUCP20-Cm-R. The PCR product was digested with NdeI/HindIII and ligated into NdeI/HindIII-digested pUCP20-ANT2-MCS to give the expression vector pUCP20-ANT2-MCS/Cm.
Construction of pUCP20-ANT2-MCS-derived expression vectors: The biosynthetic genes sulG, sulGH, dabABC, dabBC, and dabC were amplified by PCR using primers listed in key resources table from the P. acidophila and B. thailandensis genomes. The PCR products were assembled with NdeI/HindIII-digested pUCP20-ANT2-MCS using NEBuilder(R) HiFi DNA Assembly Master Mix (New England Biolabs) to give recombinants pUCP20-ANT2-MCS/sulG, pUCP20-ANT2-MCS/sulGH, pUCP20-ANT2-MCS/dabABC, pUCP20-ANT2-MCS/dabBC, and pUCP20-ANT2-MCS/dabC, respectively.
Generation and identification of sulK transposon mutant in P. acidophila
A mutant library consisting of approximately 5,000 potentially independent Tn5 transposon insertional mutants of P. acidophila ΔsulG was constructed by biparental mating between P. acidophila ΔsulG and E. coli. A single colony of P. acidophila ΔsulG or E. coli PR47 was inoculated into 15 mL of sulfazecin seed medium or LB (Km) and cultures were grown at 30°C or 37°C for approximately 15 h to OD600 = 0.8 and 1.6, respectively, and 1 mL of PR47 and 2 mL of P. acidophila ΔsulG was harvested and washed with LB medium. Each cell type was resuspended in 0.5 mL LB medium, mixed in a 1:1 (donor/ recipient) ratio, and 200 μL of the mixed cells was plated on each King’s B agar plate (g/L: peptone 20; K2HPO4 1.5; MgSO4 1.5; glycerol 10; agar 20), followed by incubation at 30°C for 20 h. The resulting mated cells were harvested and resuspended in 10 mL of 10 mM NaPPi buffer (pH 7.0). 10 X serial dilutions were made from the cell suspension and 100 μL of each dilution was spread on YM agar (g/L: K2HPO4 0.5; MgSO4 0.2; NaCl 0.1; yeast extract 1.0; mannitol 10) containing 100 μg/mL carbenicillin (Car), 50 μg/mL Gentamicin (Gm) and 50 μg/mL Km. CarR/KmR/GmR exconjugants were obtained after growing at 30°C for 4-6 days.
To identify the sulK mutant, genomic DNA was digested with several restriction enzymes including BamHI, BstBI, DraIII, EcoRI, EcoRV, KpnI. The digested genomic DNAs were hybridized with an [α-32P]-dCTP-labeled kanamycin-resistant fragment isolated from Tn-5 by digestion of plasmid pGS9 with HindIII. The 5-kb positive fragment from the BamHI-digested genome was cloned into pBluescript to give plasmid pBluescript/Bam5.0. The plasmid was subjected to DNA sequence analysis to identify the location of Tn-5 insertion.
Protein expression and purification
Expression vectors pET29b/dab, pET28b/sulMA3T3 and pET28b/E264A3T3-3 were transformed into E. coli Rosetta2 (DE3) by electroporation and selected on LB (Cm, Km) plates, respectively. A single colony was inoculated into LB (Cm, Km) seed medium and seed cultures were grown at 37°C for 12-15 h before inoculating expression medium (LB+2% glycerol, or TB, Cm, Km) in either 2.8-L non-baffled flasks or in 5-L bioreactor. Cultures were grown at 37°C to OD600 = 0.6 - 0.7 and chilled immediately in an ice-water bath for 30 min and followed by induction with 0.5 or 1 mM IPTG. Expression was carried out at 20°C for 24 h. His-tagged proteins were purified with standard Ni-NTA affinity chromatography as described by the manufacturer (Gold Biotechnology, St. Louis, MO).
Transformation, gene expression and fermentation of B. thailandensis
B. thailandensis E264 and ΔscmR were grown on LB agar. The LB seed medium was inoculated from a freshly grown LB plates and grew at 37°C for 24 h before being transferred into sulfazecin fermentation medium, Medium 2 (g/L, glycerol 10, glucose 1, Na2S2O3 1, peptone 5, meat extract 5, NaCl 5, l-Glu 1, l-Ala 1, l-Thr 1, Cystine 0.2, l-Met 0.2, ZnSO4·7H2O 0.001, pH 7.0), or Medium 3 (g/L tryptone 5, peptone 5, meat extract 5, glucose 10, fructose 10, Na2S2O3 1, l-Thr 1, pH 7.0) at 1/20 ratio. Fermentation was carried out at 37°C for 96 h with rotation at 250 rpm.
pSCrhaB2plus expression vectors in E. coli DH5a were delivered into B. thailandensis ΔscmR through a triparental conjugation with HB101 (pKR2013) helper strain. 5 mL of each strain was grown for 15 h at 37°C with proper antibiotic selection. 150 μL of each culture was mixed and cells were collected by centrifugation at 13K rpm for 2 min. After 2X washing with 2 mL LB medium, cells were resuspended in 300 μL LB and spread on a nylon membrane sitting on LB agar plate. The plate was incubated at 37°C for 12 h and cells were scraped and washed 1X with LB medium before being resuspended in 300 μL LB. 100 μL cells were plated on LB containing 150 μg/mL trimethoprim (TMP), 60 μg/mL gentamicin (Gm) and incubated at 37°C for 2-3 d to select exconjugants. To test the genetic stability of exconjugants, plasmids were purified and ∼0.1-0.5 μg was used to transform E. coli DH5α. Plasmids purified from DH5α transformants were subjected to analysis by restriction digestions.
To test the PrhaBAD strength using a reporter gene in B. thailandensis ΔscmR (pSCrhaB2plus/Cm), a 5-mL culture containing 150 μg/mL TMP, 10 μg/mL Gm and 0.1% rhamnose was grown at 37°C for 24 h with 250 rpm rotation. 10 μL of culture was spotted on LB agar containing 150 μg/mL TMP and 70 μg/mL chloramphenicol (Cm) and incubated at 37 °C for 2–3 d. B. thailandensis ΔscmR (pSCrhaB2plus) was used as negative control. A similar protocol was used for expression of sulK in B. thailandensis ΔscmR (pSCrhaB2plus/sulK) but 0.2% rhamnose was used for induction. Supernatants of induced and uninduced ΔscmR (pSCrhaB2plus/sulK) were withdrawn every 24 h during fermentation and tested for production of MM42842 using the nitrocefin assay.
Transformation, gene expression and fermentation of P. acidophila
P. acidophila and its transformants were grown on sulfazecin seed agar (g/L: glucose 10, peptone 5, meat extract 5, NaCl 5, agar 20, pH 7.0) at 30°C. 50 μg/mL kanamycin (Km) was added if cells harboring pUCP20-ANT2-MCS derived plasmids. For growing in liquid medium, 50-mL sulfazecin seed medium was inoculated from a fresh plate and grown at 28°C for 60-72 h with 250 rpm rotation. 50 μg/mL Km was added if cells harboring pUCP20-ANT2-MCS derived plasmids. 2.5 mL seed culture was transferred to 50 mL sulfazecin fermentation medium (g/L: glycerol 30, glucose 1, Na2S2O3 1, peptone 5, meat extract 5, NaCl 5, cystine 0.2, pH 6.3). Fermentation was carried out at the same conditions as the seed culturing for 120 h. For chemical feeding with l-Dap (3) or (2S,3R)-Dab (4), the amino acid was added to 24-h fermentation cultures. The final concentration of each amino acid was 2 mM or 3 mM.
To transform the WT or its mutants, 1-2 μg of plasmid was mixed with 30 μl electroporation-competent cells. Electroporation was carried out at 1.9-2.0 kv using a BRL Cell-Porator. The transformed cells were immediately mixed with 2 mL of sulfazecin seed medium and incubated at 28°C for 8 h with rotation (250 rpm). Cells were collected by centrifugation at 17000 x g for 3 min and resuspended in 200 μL sulfazecin seed medium. 100 μL was plated on each sulfazecin seed agar plate containing 50 μg/mL Km. The plates were incubated at 30°C for 5 d until the resistant colonies appeared.
To induce gene expression in pUCP20-ANT2-MCS constructs for genetic complementation, the seed culture of transformants was transferred to 50 mL fermentation medium at a 1:20 ratio. To test the PantA strength using a reporter, expression of the chloramphenicol resistance gene was induced with 0, 0.3, 0.4, and 0.5 mM anthranilate at the beginning of fermentation, respectively. After 2 d, 100 μL of each culture was plated on sulfazecin seed agar containing 50 μg/mL Km and 80 μg/mL Cm. Plates were incubated at 30°C for 3-4 d.
For genetic complementation in ΔsulG with pUCU20-ANT2-MCS expression constructs, anthranilate was added to a concentration of 0.5 mM at the beginning of fermentation. More anthranilate was added after 24 h of fermentation to the final concentration of 1.0 mM. Production of monobactams was examined at 72 or 96 h of fermentation. For adminstration of ACPAA (17) to ΔsulG (pUCP20-ANT2-MCS/dabC), the compound was dissolved in 25 mM tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl, pH7.5), 50 mM NaCl, 5% glycerol and the pH was adjusted to 6.5 using 1 M NaOH. ACPAA (17) was added to the fermentation culture 24 h after expression of dabC was induced.
Detection of monobactam activities in P. acidophila
Fermentation broth (2 mL) was withdrawn and centrifuged for 3 min at 16000 x g. The antibacterial activity was assayed by using 250 μL supernatant against E. coli ESS seeded (0.4%) in nutrient agar. To analyze β-lactam products from fermentation, 250 μL supernatant was assayed on B. licheniformis ATCC 14580 mixed with 100 mL BA2 agar (g/L: BBL seed agar 30.5, NaCl 5). The plate was incubated for 5 h at 37°C and Induction of β-lactamase was visualized by overlaying with 1.5 mL of 40 μg/mL nitrocefin solution in phosphate buffer (pH 7.0).
A Supelclean™ ENVI-Carb™ SPE Tube (250 mg bed wt.) was conditioned with 2 mL of water and 2 mL of 50% acetonitrile, then equilibrated with 3 mL of water by gravity flow. A 500 μL supernatant was loaded on to the column, then washed with 2.5 mL of water by gravity flow. Products were eluted in 400 μL fractions of 50% acetonitrile by gravity flow, which were collected separately. Each elution fraction was directly analyzed (negative ion mode) by UPLC-HRMS as follows: ES- [binary gradient: water (solvent A), acetonitrile (solvent B), 0.3 mL/min]: 0-1 min isocratic 20% A; 1-7.5 min gradient 20% to 100% A; 7.5-8.4 min isocratic 100% A; 8.4-8.5 min gradient 100% to 20% A; 8.5-10 min isocratic 20% A. Waters ACQUITY UPLC BEH Amide Column, 130 Å, 1.7 μm, 2.1 mm × 100 mm. Monobactam products were detected between fractions two and four.
E264 and SulM A-domain pyrophosphate assay
A-domain activity was measured through colorimetric detection of released pyrophosphate using a modified in vitro assay. Each reaction was performed on a 100 μL scale containing 50 mM Tris-HCl, pH 8.0, 5 mM magnesium chloride (MgCl2), 5 mM adenosine 5’-triphosphate (ATP), 5 mM amino acid substrate, 40 mM hydroxylamine and purified A3T3 to a final concentration of 130 μg/mL (approx. 1.8 μM). Reactions were initiated by addition of enzyme and incubated for 70 min at 30°C. 1 mL Mo(VI) solution (20 mM sodium molybdate (NaMoO4) in 0.6 M hydrochloric acid and 60% acetonitrile) was added, vortexed briefly, and samples were incubated for 5 min at room temperature. 20 μL of bis(triphenylphosphoranylidene)ammonium chloride (BTPPACl, 50 mM in acetonitrile) was added, vortexed briefly, and samples were incubated for 5 min at room temperature. The mixture was then centrifuged for 20 min at 16,000 × g. After removal of the supernatant, the pellet was dissolved in 200 μL of acetonitrile. 20 μL ascorbic acid (0.5 M in 2 M hydrochloric acid and 60% acetonitrile) was added and the mixture was incubated at room temperature for 10 min. 200 μL acetonitrile was added and the absorbance at 620 nm was measured using a Varian Cary 50 UV-Vis spectrophotometer. Each amino acid substrate was blanked against an identical sample with no added enzyme.
Biochemical analysis of DabB
UV-vis spectral analyses of the DabB reactions were collected on a Varian Cary 50 UV-Visible spectrophotometer. The DabB-PLP spectra were measured at a concentration of 0.8 mg/mL in 50 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES, pH 7.5, 100 mM NaCl, 10% glycerol at room temperature. To measure the formation of the DabB aminoacrylate aldimine complex, OPT (9) or OPS (6) was mixed with DabB at final concentrations of 0.1 mM and 0.8 mg/mL and spectra were recorded immediately. To identify the amine donors and return of the resting state of DabB, l-Asp or l-Glu was added to DabB-OPT or DabB-OPS to a final concentration of 2 mM and 5 mM, respectively. The mixture was incubated at room temperature for 5 min before the spectra were recorded.
The in vitro assays for the formation of ACPAA by DabB were performed in 1-mL reactions containing the following components: 2 mg/mL purified DabB, 2 mg/mL OPT (9), and 6 mg/mL l-Asp in 25 mM Tris-HCl, pH 7.5, 50 mM NaCl, 10% glycerol. The negative control reaction had the same components but DabB. The reaction mixture was incubated at 30°C for 1 h and DabB was removed using Sigma-Aldrich Amicon 3K spin filters by centrifugation at 4000 x g for 30 min. The flow-through was lyophilized and resuspended in freshly made methanolic HCl. Esterification was performed at 37°C for 10 h and concentrated to dryness by rotary evaporation. The esterified ACPAA (17) was resuspended in 50% acetonitrile containing 0.1% formic acid for analysis by UPLC-HRMS.
The in vitro assays for the formation of ACEAA (16) by DabB were performed in 1-mL reactions containing the following components: 3 mg/mL purified DabB, 2 mg/mL OPS (9), and 6 mg/mL l-Asp in 25 mM Tris-HCl, pH 7.5, 50 mM NaCl, 10% glycerol. The negative control reaction had the same components but DabB. The reaction mixture was incubated at 30°C for 1 h and DabB was removed using Sigma-Aldrich Amicon 3K spin filters by centrifugation at 4000 x g for 30 min. The flow-through was lyophilized and resuspended in freshly made methanolic HCl. Esterification was performed at 37°C for 10 h and concentrated to dryness by rotary evaporation. The esterified ACEAA (16) was resuspended in 50% acetonitrile containing 0.1% formic acid for analysis by UPLC-HRMS.
Synthesis of ACPAA
tert-Butyl (R)-4-(methoxy(methyl)carbamoyl)-2,2-dimethyloxazolidine-3-carboxylate (19)
Compound 19 (Figure 6) was prepared according to a known procedure. Spectral data were consistent with literature values.
tert-Butyl (R)-4-acetyl-2,2-dimethyloxazolidine-3-carboxylate (20)
Compound 19 (Figure 6) (2.88 g, 10.0 mmol) was placed in a dry flask under Argon and dissolved in 30 mL of anhydrous tetrahydrofuran. The solution was cooled to -78°C and methylmagensium bromide (6.66mL, 20.0 mmol, 3.0 M in diethyl ether) was added dropwise. The reaction was stirred at this temperature 30 min, then allowed to warm to room temperature and stirred until complete consumption of the starting material was observed by thin layer chromatography. A saturated aqueous solution of ammonium chloride (30 mL) was added carefully and stirred at room temperature for 15 min. The mixture was extracted 3 × 30 mL of ethyl acetate, and the combined organics were washed with 30 mL of saturated brine, dried over anhydrous sodium sulfate and concentrated. Purification by flash chromatography (8/2 hexanes/ethyl acetate) yielded 20 (Figure 6) as a colorless oil (2.17 g, 90%). Spectral data were consistent with those observed in the literature.
Dibenzyl (1-((S)-3-(tert-butoxycarbonyl)-2,2-dimethyloxazolidin-4-yl)ethyl)-l-aspartate (21)
Compound 20 (1.03 g, 4.25 mmol) and dibenzyl-l-aspartate p-toluenesulfonate (2.48 g, 5.11 mmol) were dissolved in 20 mL of methanol. Sodium cyanoborohydride (402 mg, 6.38 mmol) was added and the reaction was stirred at room temperature for 18 h. A saturated aqueous solution of ammonium chloride (30 mL) was carefully added, and the mixture was stirred for 15 min before the methanol was removed by rotary evaporation. The aqueous phase was extracted 3 × 30 mL of ethyl acetate and the combined organics were washed with 30 mL of saturated brine, dried over anhydrous sodium sulfate and concentrated. Purification by flash chromatography (9/1 8/2 hexanes/ethyl acetate) afforded 21 (1.75 g, 76%) as a ∼ 2:1 mixture of diastereomers.
1H NMR (400 MHz; CDCl3): δ 7.42-7.30 (m, 10H), 5.22-5.03 (m, 4H), 4.07-3.66 (m, 4H), 3.24-3.01 (m, 1H), 2.86-2.60 (m, 2H), 1.64-1.40 (m, 15H), 1.02 (d, J = 6.7 Hz, 0.8H), 0.97 (d, J = 6.5 Hz, 2.2H) ; 13C NMR (100 MHz; CDCl3): δ 173.8, 170.7, 166.6, 153.3, 152.3, 135.8, 135.7, 129.0, 128.9, 128.7, 128.5, 128.4, 94.6, 94.1, 86.3, 80.2, 68.5, 67.1, 66.7, 63.9, 63.3, 62.2, 60.6, 60.2, 57.1, 55.9, 52.8, 52.7, 38.9, 28.6, 28.0, 19.2; HRMS (ESI) m/z: [M+H]+ calculated 541.2908, found 541.2916. Figures S9A and S9B.
Dibenzyl ((2S,3S)-3-((tert-butoxycarbonyl)amino)-4-hydroxybutan-2-yl)-l-aspartate and dibenzyl ((2S,3R)-3-((tert-butoxycarbonyl)amino)-4-hydroxybutan-2-yl)-l-aspartate (22a and 22b)
Acetyl chloride (6 mL) was added dropwise to 36 mL of methanol at 0°C and stirred for 15 min. Compound 21 (Figure 6) (1.75 g, 3.24 mmol) was dissolved in 15 mL of methanol and added to the above solution at 0°C, and the reaction was stirred at this temperature for 3 h. The solvent was removed by rotary evaporation and concentrated under high vacuum. The residue was re-dissolved in 50 mL of dichloromethane and cooled to 0°C. Di-tert-butyl dicarbonate (1.41 g, 6.48 mmol) and N,N-diisopropylethylamine (2.82 mL, 16.2 mmol) were added and the reaction was allowed to warm to room temperature and stirred for 18 h. A saturated aqueous solution of ammonium chloride (50 mL) was added and the layers were separated. The aqueous phase was extracted 2 × 30 mL of dichloromethane and the combined organics were dried over anhydrous sodium sulfate and concentrated. Purification by flash chromatography (55/45 50/50 hexanes/ethyl acetate) afforded 22a (first eluting) and 22b (second eluting) (534 mg, 33%), of which 50 mg were a 10:1 mixture of 22a/22b, 86 mg were a 1:1 mixture of 22a/22b, 112 mg were a 1:8 mixture of 22a/22b and 286 mg were pure 22b. A fraction of each diastereomer could be cleanly isolated to obtain characterization.
22a (Figure 6).
1H NMR (400 MHz; CDCl3): δ7.36-7.26 (m, 10H), 5.53 (d, J = 6.4 Hz), 5.15-5.05 (m, 4H), 3.88 (dd, J = 11.5, 3.4 Hz, 1H), 3.81 (dd, J = 8.3, 4.5 Hz, 1H), 3.53 (dd, J = 11.5, 3.4 Hz, 1H), 3.44-3.34 (m, 1H) 3.00-2.89 (m, 1H), 2.68 (ABX, JAB = 16.0 , JAX = 8.3, JBX = 4.5 Hz, 2H), 1.41 (s, 9H), 1.08 (d, J = 6.7 Hz); 13C NMR (100 MHz; CDCl3): δ 173.5, 170.8, 156.6, 135.5, 135.3, 128.8, 128.8, 128.7, 128.7, 128.6, 79.7, 67.6, 67.2, 63.0, 57.5, 55.4, 53.8, 38.5, 28.6, 18.1 HRMS (ESI) m/z: [M+H]+ calculated 501.2595, found 501.2602. Figures S9C and S9D.
22b (Figure 6)
1H NMR (400 MHz; CDCl3): δ 7.37-7.26 (m, 10H0, 5.23-5.15 (m, 1H), 5.15-5.03 (m, 4H), 3.77 (dd, J = 7.9, 4.7 Hz, 1H), 3.73-3.60 (m, 2H), 3.53-3.43 (m, 1H), 2.96-2.85 (m, 1H), 2.71 (ABX, JAB = 16.0 , JAX = 8.0, JBX = 4.7 Hz, 2H), 1.43 (s, 9H), 1.02 (d, J = 6.4 Hz) ; 13C NMR (100 MHz; CDCl3): δ 173.6, 170.6, 156.7, 135.5, 135.4, 128.8, 128.8, 128.8, 128.7, 128.7, 128.6, 79.7, 67.4, 67.0, 65.3, 55.5, 55.3, 53.9, 38.4, 28.6, 17.5 ; HRMS (ESI) m/z: [M+H]+ calculated 501.2595, found 501.2602. Figures S9E and S9F.
(2S,3R)-3-(((S)-1,4-Bis(benzyloxy)-1,4-dioxobutan-2-yl)amino)-2-((tert-butoxycarbonyl)amino)butanoic acid (23).
The procedure of Spangenberg et al. was used with minor modifications.55
A stock solution of Periodic acid/Chromium (VI) oxide was prepared by dissolving periodic acid (2.0 g, 8.77 mmol) and chromium (VI) oxide (4.0 mg, 0.04 mmol) in 19.8 mL of acetonitrile and 0.20 mL of water.
Compound 22b (Figure 6) (286 mg, 0.572 mmol) was dissolved in 5 mL of acetonitrile and cooled to 0°C, then 4.0 mL of the stock solution of periodic acid (1.66 mmol)/chromium (IV) oxide (0.008 mmol) was added dropwise. The reaction was stirred at this temperature for 1 h, then an additional 1.0 mL of the stock solution (0.44 mmol of periodic acid, 0.002 mmol chromium (IV) oxide) was added. After 30 min, the reaction was quenched by the addition of 20 mL of 100 mM potassium phosphate buffer pH 5.8. The mixture was extracted 3 × 20 mL of ethyl acetate, washed with 20 mL of saturated brine, dried over anhydrous sodium sulfate and concentrated. Purification by flash chromatography (1/1 hexanes/ethyl acetate 1% acetic acid) yielded 23 (Figure 6) (197 mg, 67%) as a colorless oil.
1H NMR (400 MHz; CDCl3): δ 7.38-7.26 (m, 10H), 5.54 (d, 4.8 Hz, 1H), 5.14 (ABq, J = 12.1 Hz, 2H), 5.06 (s, 2H), 4.17 (t, J = 4.4 Hz, 1H), 3.93 (t, J = 5.0 Hz, 1H), 3.45-3.36 (m, 1H), 2.92 (d, J = 5.0 Hz, 2H), 1.41 (s, 9H), 1.02 (d, J = 6.6 Hz) ; 13C NMR (100 MHz; CDCl3): δ 171.6, 171.4, 170.5, 155.6, 135.3, 135.0, 128.8, 128.7, 128.7, 80.3, 68.0, 67.2, 55.5, 54.6, 51.9, 35.7, 28.4, 14.4 ; HRMS (ESI) m/z: [M+H]+ calculated 515.2388, found 515.2393. Figures S9G and S9H.
(1S,2R)-1-Amino-1-carboxypropan-2-yl)-l-aspartic acid (ACPAA) dihydrochloride. (17)
Compound 23 (Figure 6) (35 mg, 0.068 mmol) was dissolved in 5 mL of methanol and 10% palladium on carbon (7 mg) was added. The reaction was placed in a pressure tube and shaken on a Parr apparatus under 40 psi of hydrogen gas for 18 h at room temperature. The solution was filtered over Celite and rinsed with methanol, then concentrated to obtain a white solid. The residue was dissolved in 4 mL of 98% formic acid and stirred at room temperature for 6 h. The reaction mixture was concentrated, then 3 mL of a 100 mM aqueous solution of hydrochloric acid was added and the solution was stirred at room temperature for 5 min. The water was removed under reduced pressure and this procedure was repeated three times. After the final repetition, the residue was dissolved in 3 mL of water, transferred to a tared vial, frozen and lyophilized to dryness to afford ACPAA (Figure 6) (20 mg, 96%) as an off-white, hygroscopic solid.
1H NMR (400 MHz; D2O): δ 4.54 – 4.51 (dd, J = 8, 3.9 Hz, 1H), 4.45 (d, J = 3.2, 1H), 4.00 – 3.94 (m, 1H), 3.24 – 3.18 (dd, J = 18.4, 3.9 Hz, 1H), 3.12 – 3.05 (dd, J = 18.4, 7.8 Hz, 1H), 1.42 (d, J = 6.7 Hz, 1H); 13C NMR (100 MHz; D2O): δ 173.3, 170.2, 169.0, 55.5, 53.2, 53.0, 33.6, 11.1; HRMS (ESI) m/z: [M+H]+ calculated 235.0925, found 235.0923. Figures S9I and S9J.
Quantification and statistical analysis
This study does not involve quantification and statistics.
Additional resources
This work is not part of a clinical trial.
Acknowledgments
This research was supported by NIH grant RO1 AI121072. We thank Professor T. Brüser, Leibniz University Hannover, for kindly providing expression vector pUCP20-ANT2-MCS. We thank Professor M. R. Seyedsayamdost, Princeton University, for generously providing B. thailandensis E264, B. thailandensis ΔscmR and helpful discussion. We are pleased to acknowledge the help and guidance of Drs. I. P. Mortimer (ESI-MS) and J. Catazaro (NMR), Department of Chemistry. L.K. acknowledges financial support from the Deutsche Forschungsgemeinschaft – 492438365.
Author contributions
R.F.L., M.S.L., T.A.Z., and C.A.T. conceived experiments and analyzed data. R.F.L., M.S.L., T.A.Z., L.K., and C.A.T. wrote the manuscript. R.F.L. carried out fermentation, vector construction, gene knockout, chemical and genetic complementation, protein expression and purification, and in vitro and in vivo product characterization and analyses. M.S.L. performed in vitro enzymatic analysis, derivatization of products, UPLC-HRMS analysis of in vivo and in vitro products, and synthesized ACPAA and Dabs. T.A.Z. carried out bioinformatic analysis, antiSMASH analysis, and DabB spectra and substrate analysis. L.K. carried out UPLC-HRMS analysis of in vivo and in vitro products, kinetic analysis of E264 A3T3 and synthesized ACPAA and Dabs.
Declaration of interests
The authors have no conflicts of interest.
Published: February 12, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109202.
Supplemental information
References
- 1.Centers for Disease Control and Prevention (U.S.) National Center for Emerging Zoonotic and Infectious Diseases (U.S.) National Center for HIV/AIDS, V.H., STD, and TB Prevention (U.S.) National Center for Immunization and Respiratory Diseases (U.S.) Antibiotic resistance threats in the United States, 2013. April 23, 2013. 2013. https://stacks.cdc.gov/view/cdc/20705
- 2.World Health Organization . European Centre for Disease Prevention and Control and World Health Organization; 2023. Antimicrobial resistance surveillance in Europe - 2021 data. [Google Scholar]
- 3.Hinchliffe P., Moreno D.M., Rossi M.A., Mojica M.F., Martinez V., Villamil V., Spellberg B., Drusano G.L., Banchio C., Mahler G., et al. 2-Mercaptomethyl Thiazolidines (MMTZs) Inhibit All Metallo-β-Lactamase Classes by Maintaining a Conserved Binding Mode. ACS Infect. Dis. 2021;7:2697–2706. doi: 10.1021/acsinfecdis.1c00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sykes R.B., Bonner D.P., Bush K., Georgopapadakou N.H., Wells J.S. Monobactams - Monocyclic beta-lactam antibiotics produced by bacteria. J. Antimicrob. Chemother. 1981;8:1–16. doi: 10.1093/jac/8.suppl_e.1. [DOI] [PubMed] [Google Scholar]
- 5.Sykes R.B., Bonner D.P., Bush K., Georgopapadakou N.H. Azthreonam (Sq 26,776), a synthetic monobactam specifically active against aerobic Gram-negative bacteria. Antimicrob. Agents Chemother. 1982;21:85–92. doi: 10.1128/Aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheadon M.J., Townsend C.A. Evolutionary and functional analysis of an NRPS condensation domain integrates β-lactam, ᴅ-amino acid, and dehydroamino acid synthesis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2026017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patteson J.B., Fortinez C.M., Putz A.T., Rodriguez-Rivas J., Bryant L.H., 3rd, Adhikari K., Weigt M., Schmeing T.M., Li B. Structure and function of a dehydrating condensation domain in nonribosomal peptide biosynthesis. J. Am. Chem. Soc. 2022;144:14057–14070. doi: 10.1021/jacs.1c13404. [DOI] [PubMed] [Google Scholar]
- 8.Gaudelli N.M., Long D.H., Townsend C.A. β-Lactam formation by a non-ribosomal peptide synthetase during antibiotic biosynthesis. Nature. 2015;520:383–387. doi: 10.1038/nature14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long D.H., Townsend C.A. Mechanism of integrated β-lactam formation by a nonribosomal peptide synthetase during antibiotic synthesis. Biochemistry-Us. 2018;57:3353–3358. doi: 10.1021/acs.biochem.8b00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long D.H., Townsend C.A. Acyl donor stringency and dehydroaminoacyl intermediates in β-Lactam formation by a non-ribosomal peptide synthetase. ACS Chem. Biol. 2021;16:806–812. doi: 10.1021/acschembio.1c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobylarz M.J., Grigg J.C., Takayama S.i.J., Rai D.K., Heinrichs D.E., Murphy M.E.P. Synthesis of L-2,3-diaminopropionic acid, a siderophore and antibiotic precursor. Chem. Biol. 2014;21:379–388. doi: 10.1016/j.chembiol.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Hsu S.H., Zhang S., Huang S.C., Wu T.K., Xu Z., Chang C.Y. Characterization of enzymes catalyzing the formation of the nonproteinogenic amino acid L-Dap in capreomycin biosynthesis. Biochemistry-Us. 2021;60:77–84. doi: 10.1021/acs.biochem.0c00808. [DOI] [PubMed] [Google Scholar]
- 13.Sykes R.B., Bonner D.P. Discovery and development of the monobactams. Rev. Infect. Dis. 1985;7:S579–S593. doi: 10.1093/clinids/7.supplement_4.s579. [DOI] [PubMed] [Google Scholar]
- 14.Carosso S., Liu R., Miller P.A., Hecker S.J., Glinka T., Miller M.J. Methodology for monobactam diversification: syntheses and studies of 4-thiomethyl substituted β-lactams with activity against Gram-negative bacteria, including carbapenemase producing Acinetobacter baumannii. J. Med. Chem. 2017;60:8933–8944. doi: 10.1021/acs.jmedchem.7b01164. [DOI] [PubMed] [Google Scholar]
- 15.Reck F., Bermingham A., Blais J., Capka V., Cariaga T., Casarez A., Colvin R., Dean C.R., Fekete A., Gong W., et al. Optimization of novel monobactams with activity against carbapenem-resistant Enterobacteriaceae – Identification of LYS228. Bioorg. Med. Chem. Lett. 2018;28:748–755. doi: 10.1016/j.bmcl.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Li R., Oliver R.A., Townsend C.A. Identification and characterization of the sulfazecin monobactam biosynthetic gene cluster. Cell Chem. Biol. 2017;24:24–34. doi: 10.1016/j.chembiol.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver R.A., Li R., Townsend C.A. Monobactam formation in sulfazecin by a nonribosomal peptide synthetase thioesterase. Nat. Chem. Biol. 2018;14:5–7. doi: 10.1038/Nchembio.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichstrahl M.S., Kahlert L., Li R., Zandi T.A., Yang J., Townsend C.A. Synthesis of functionalized 2,3-diaminopropionates and their potential for directed monobactam biosynthesis. Chem. Sci. 2023;14:3923–3931. doi: 10.1039/d2sc06893a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Box S.J., Brown A.G., Gilpin M.L., Gwynn M.N., Spear S.R. MM 42842, a new member of the monobactam family produced by Pseudomonas cocovenenans. II. Production, isolation and properties of MM 42842. J. Antibiot. 1988;41:7–12. doi: 10.7164/antibiotics.41.7. [DOI] [PubMed] [Google Scholar]
- 20.Müller C., Nolden S., Gebhardt P., Heinzelmann E., Lange C., Puk O., Welzel K., Wohlleben W., Schwartz D. Sequencing and analysis of the biosynthetic gene cluster of the lipopeptide antibiotic friulimicin in Actinoplanes friuliensis. Antimicrob. Agents Chemother. 2007;51:1028–1037. doi: 10.1128/AAC.00942-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Ostash B., Walsh C.T. Identification of the biosynthetic gene cluster for the pacidamycin group of peptidyl nucleoside antibiotics. Proc. Natl. Acad. Sci. USA. 2010;107:16828–16833. doi: 10.1073/pnas.1011557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaysser L., Tang X., Wemakor E., Sedding K., Hennig S., Siebenberg S., Gust B. Identification of a napsamycin biosynthesis gene cluster by genome mining. Chembiochem. 2011;12:477–487. doi: 10.1002/cbic.201000460. [DOI] [PubMed] [Google Scholar]
- 23.Jiang L., Wang L., Zhang J., Liu H., Hong B., Tan H., Niu G. Identification of novel mureidomycin analogues via rational activation of a cryptic gene cluster in Streptomyces roseosporus NRRL 15998. Sci. Rep. 2015;5:14111–14123. doi: 10.1038/srep14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blin K., Shaw S., Augustijn H.E., Reitz Z.L., Biermann F., Alanjary M., Fetter A., Terlouw B.R., Metcalf W.W., Helfrich E.J.N., et al. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023;51:W46–W50. doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillouzer L.S., Groleau M.C., Mauffrey F., Déziel E. ScmR, a global regulator of gene expression, quorum sensing, pH homeostasis, and virulence in Burkholderia thailandensis. J. Bacteriol. 2020;202 doi: 10.1128/JB.00776-19. e00776-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao D., Bushin L.B., Moon K., Wu Y., Seyedsayamdost M.R. Discovery of scmR as a global regulator of secondary metabolism and virulence in Burkholderia thailandensis E264. Proc. Natl. Acad. Sci. USA. 2017;114:E2920–E2928. doi: 10.1073/pnas.1619529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Callaghan C.H., Morris A., Kirby S.M., Shingler A.H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1972;1:283–288. doi: 10.1128/AAC.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsman M.E., Marous D.R., Li R., Oliver R.A., Byun B., Emrich S.J., Boggess B., Townsend C.A., Mobashery S. Whole-genome shotgun sequencing of two β-proteobacterial species in search of the bulgecin biosynthetic cluster. ACS Chem. Biol. 2017;12:2552–2557. doi: 10.1021/acschembio.7b00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Challis G.L., Ravel J., Townsend C.A. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 2000;7:211–224. doi: 10.1016/s1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 30.Stachelhaus T., Mootz H.D., Marahiel M.A. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 1999;6:493–505. doi: 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama C., Niikura H., Takakuwa M., Katano H., Hamano Y. Colorimetric detection of the adenylation activity in nonribosomal peptide synthetases. Methods Mol. Biol. 2016;1401:77–84. doi: 10.1007/978-1-4939-3375-4_5. [DOI] [PubMed] [Google Scholar]
- 32.Wilson D.J., Aldrich C.C. A continuous kinetic assay for adenylation enzyme activity and inhibition. Anal. Biochem. 2010;404:56–63. doi: 10.1016/j.ab.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duckworth B.P., Wilson D.J., Aldrich C.C. Measurement of nonribosomal peptide synthetase adenylation domain activity using a continuous hydroxylamine release assay. Methods Mol. Biol. 2016;1401:53–61. doi: 10.1007/978-1-4939-3375-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loveridge E.J., Jones C., Bull M.J., Moody S.C., Kahl M.W., Khan Z., Neilson L., Tomeva M., Adams S.E., Wood A.C., et al. Reclassification of the specialized metabolite producer Pseudomonas mesoacidophila ATCC 31433 as a member of the Burkholderia cepacia complex. J. Bacteriol. 2017;199:e00125-17–e00117. doi: 10.1128/JB.00125-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweizer H.P. Vectors to express foreign genes and techniques to monitor gene expression in Pseudomonads. Curr. Opin. Biotechnol. 2001;12:439–445. doi: 10.1016/s0958-1669(00)00242-1. [DOI] [PubMed] [Google Scholar]
- 36.Meisner J., Goldberg J.B. The Escherichia coli rhaSR-PrhaBAD inducible promoter system allows tightly controlled gene expression over a wide range in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2016;82:6715–6727. doi: 10.1128/AEM.02041-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogan A.M., Jeffers K.R., Palacios A., Cardona S.T. Improved dynamic range of a rhamnose-inducible promoter for gene expression in Burkholderia spp. Appl. Environ. Microbiol. 2021;87:e0064721. doi: 10.1128/AEM.00647-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann L., Sugue M.-F., Brüser T. A tunable anthranilate-inducible gene expression system for Pseudomonas species. Appl. Microbiol. Biotechnol. 2021;105:247–258. doi: 10.1007/s00253-020-11034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Retallack D., Schneider J.C., Chew L., Ramseier T., Allen J., Patkar A., Squires C., Talbot H., Mitchell J. Pseudomonas fluorescens – a robust expression platform for pharmaceutical protein production. Microb. Cell Factories. 2006;5:S28. doi: 10.1186/1475-2859-5-S1-S28. [DOI] [Google Scholar]
- 40.Rackham E.J., Grüschow S., Ragab A.E., Dickens S., Goss R.J.M. Pacidamycin biosynthesis: identification and heterologous expression of the first uridyl peptide antibiotic gene cluster. Chembiochem. 2010;11:1700–1709. doi: 10.1002/cbic.201000200. [DOI] [PubMed] [Google Scholar]
- 41.Fan C., Bobik T.A. The PduX enzyme of Salmonella enterica is an L-threonine kinase used for coenzyme B12 synthesis. J. Biol. Chem. 2008;283:11322–11329. doi: 10.1074/jbc.M800287200. [DOI] [PubMed] [Google Scholar]
- 42.Tavares N.K., VanDrisse C.M., Escalante-Semerena J.C. Rhodobacterales use a unique L-threonine kinase for the assembly of the nucleotide loop of coenzyme B12. Mol. Microbiol. 2018;110:239–261. doi: 10.1111/mmi.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreassi J.L., 2nd, Leyh T.S. Molecular functions of conserved aspects of the GHMP kinase family. Biochemistry-Us. 2004;43:14594–14601. doi: 10.1021/bi048963o. [DOI] [PubMed] [Google Scholar]
- 44.Fan C., Fromm H.J., Bobik T.A. Kinetic and functional analysis of L-threonine kinase, the PduX enzyme of Salmonella enterica. J. Biol. Chem. 2009;284:20240–20248. doi: 10.1074/jbc.M109.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma M., Kutz-Naber K.K., Li L. Methyl esterification assisted MALDI FTMS characterization of the orcokinin neuropeptide family. Anal. Chem. 2007;79:673–681. doi: 10.1021/ac061536r. [DOI] [PubMed] [Google Scholar]
- 46.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampaleanu L.M., Vallée F., Thompson G.D., Howell P.L. Three-dimensional structure of the argininosuccinate lyase frequently complementing allele Q286R. Biochemistry-Us. 2001;40:15570–15580. doi: 10.1021/bi011525m. [DOI] [PubMed] [Google Scholar]
- 48.Afanasyev O.I., Kuchuk E., Usanov D.L., Chusov D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019;119:11857–11911. doi: 10.1021/acs.chemrev.9b00383. [DOI] [PubMed] [Google Scholar]
- 49.Setoi H., Kayakiri H., Hashimoto M. Diastereoselective synthesis of an α, β-diaminocarboxylic acid: an efficient synthesis of FR900490, an immunomodulating peptide isolated from a fungus. Chem. Pharm. Bull. 1989;37:1126–1127. doi: 10.1248/cpb.37.1126. [DOI] [Google Scholar]
- 50.Ohfune Y., Tomita M., Nomoto K. Total synthesis of 2'-deoxymugineic acid, the metal chelator excreted from wheat root. J. Am. Chem. Soc. 1981;103:2409–2410. doi: 10.1021/ja00399a046. [DOI] [Google Scholar]
- 51.Ageno G., Banfi L., Giuseppe Guanti G.C., Manghisi E., Riva R., Rocca V. Enantiospecific and diastereoselective synthesis of 4,4-disubstituted-3-amino-2-azetidinones, starting from D-serine. Tetrahedron. 1995;51:8121–8134. doi: 10.1016/0040-4020(95)00427-A. [DOI] [Google Scholar]
- 52.Passiniemi M., Koskinen A.M. Garner’s aldehyde as a versatile intermediate in the synthesis of enantiopure natural products. Beilstein J. Org. Chem. 2013;9:2641–2659. doi: 10.3762/bjoc.9.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith M.W., Snyder S.A. A Concise total synthesis of (+)-scholarisine A empowered by a unique C–H arylation. J. Am. Chem. Soc. 2013;135:12964–12967. doi: 10.1021/ja406546k. [DOI] [PubMed] [Google Scholar]
- 54.Mengel A., Reiser O. Around and beyond Cram's rule. Chem. Rev. 1999;99:1191–1224. doi: 10.1021/cr980379w. [DOI] [PubMed] [Google Scholar]
- 55.Spangenberg T., Schoenfelder A., Breit B., Mann A. A new method for the synthesis of chiral beta-branched alpha-amino acids. Org. Lett. 2007;9:3881–3884. doi: 10.1021/ol071305m. [DOI] [PubMed] [Google Scholar]
- 56.Lam W.H., Rychli K., Bugg T.D.H. Identification of a novel beta-replacement reaction in the biosynthesis of 2,3-diaminobutyric acid in peptidylnucleoside mureidomycin. Org. Biomol. Chem. 2008;6:1912–1917. doi: 10.1039/b802585a. [DOI] [PubMed] [Google Scholar]
- 57.Liu N., Guan H., Niu G., Jiang L., Li Y., Zhang J., Li J., Tan H. Molecular mechanism of mureidomycin biosynthesis activated by introduction of an exogenous regulatory gene ssaA into Streptomyces roseosporus. Sci. China Life Sci. 2021;64:1949–1963. doi: 10.1007/s11427-020-1892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hickey J.L., Sindhikara D., Zultanski S.L., Schultz D.M. Beyond 20 in the 21st century: prospects and challenges of non-canonical amino acids in peptide drug discovery. ACS Med. Chem. Lett. 2023;14:557–565. doi: 10.1021/acsmedchemlett.3c00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou H., Li L., Zhang T., Shi M., Zhang N., Huang J., Xian M. Biosynthesis and biotechnological application of non-canonical amino acids: Complex and unclear. Biotechnol. Adv. 2018;36:1917–1927. doi: 10.1016/j.biotechadv.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Taton A., Lis E., Adin D.M., Dong G., Cookson S., Kay S.A., Golden S.S., Golden J.W. Gene transfer in Leptolyngbya sp. strain BL0902, a cyanobacterium suitable for production of biomass and bioproducts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selvaraj G., Iyer V.N. Transposon Tn5 specifies streptomycin resistance in Rhizobium spp. J. Bacteriol. 1984;158:580–589. doi: 10.1128/jb.158.2.580-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaw K.J., Berg C.M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979;92:741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aoki H., Sakai H., Kohsaka M., Konomi T., Hosoda J. Nocardicin A, a new monocyclic beta-lactam antibiotic. I. Discovery, isolation and characterization. J. Antibiot. (Tokyo) 1976;29:492–500. doi: 10.7164/antibiotics.29.492. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa J., Hotta K. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C conten. FEMS Microbiol. Lett. 1999;174:251–253. doi: 10.1111/j.1574-6968.1999.tb13576.x. [DOI] [PubMed] [Google Scholar]
- 65.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
DabA, DabB, and DabC data have been deposited at GenBank and are publicly available as of the date of publication, under the accession number GenBank: BK065153 at NCBI’s GenBank database.
-
•
This paper does not report original codes.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.