Abstract
Introduction
Lorlatinib was found to have improved efficacy versus crizotinib in the global phase 3 CROWN study (NCT03052608). Similar results were revealed for the Japanese population as for the overall population. We present results from the unplanned 3-year follow-up from the CROWN study in Japanese patients.
Methods
Patients were randomized to either lorlatinib 100 mg once daily (n = 25) or crizotinib 250 mg twice daily (n = 23). The primary end point was progression-free survival assessed by blinded independent central review. Secondary end points included objective and intracranial responses assessed by blinded independent central review and safety.
Results
At the data cutoff of September 20, 2021, median progression-free survival was not reached with lorlatinib and 11.1 months with crizotinib (hazard ratio = 0.36). Objective response rate was 72.0% with lorlatinib and 52.2% with crizotinib. For patients with baseline brain metastases, intracranial response rate was 100.0% versus 28.6% with lorlatinib versus crizotinib. Nine patients in the lorlatinib group received more than or equal to 1 subsequent anticancer systemic therapy, with ALK tyrosine kinase inhibitor as the most common first subsequent therapy. The safety profile was consistent with that reported previously, with no new safety signals.
Conclusions
This updated analysis in the Japanese population revealed prolonged benefits of lorlatinib over crizotinib in patients with treatment-naive advanced ALK-positive NSCLC with and those without brain metastases.
Keywords: Anaplastic lymphoma kinase, Japan, Lorlatinib, Non–small cell lung cancer
Introduction
ALK gene rearrangements occur in 4% to 5% of NSCLC cases, and this subset of tumors is sensitive to ALK tyrosine kinase inhibitors (TKIs).1, 2, 3, 4 Lorlatinib is a brain-penetrant, third-generation ALK TKI.5,6 Interim analysis from the phase 3 CROWN study (NCT03052608) revealed improved median progression-free survival (PFS) (not reached [NR] versus 9.3 mo) and intracranial response rate (82% versus 23%) compared with crizotinib in patients with treatment-naive advanced ALK-positive NSCLC.7 Subsequent analysis in the Japanese population of the CROWN study revealed similar results for lorlatinib versus crizotinib (median PFS: NR versus 11.1 mo; intracranial response rate: 100.0% versus 28.6%).8
Because the primary end point of median PFS along with median time to intracranial progression was NR with lorlatinib in the interim analysis, long-term follow-up is important to understand efficacy and safety of lorlatinib. In an unplanned 3-year follow-up CROWN study analysis, lorlatinib was found to have continued efficacy benefit over crizotinib in the overall population (median PFS: NR versus 9.3 mo; intracranial response rate: 83% versus 23%), including patients with and those without baseline brain metastases.9 Another concern is management of subsequent treatments after first-line treatment in patients with ALK-positive NSCLC. We aimed to provide results from this updated 3-year analysis for the Japanese population of the CROWN study, including subsequent treatments after first-line lorlatinib.
Materials and Methods
CROWN Study Design
The CROWN study (NCT03052608) is an ongoing global, randomized, phase 3 trial comparing lorlatinib with crizotinib in patients (N = 296) with treatment-naive advanced ALK-positive NSCLC.7,9 Full study design details were previously published along with global data from both the initial planned interim analysis and an unplanned 3-year follow-up analysis.7,9 Patients were randomized 1:1 to either lorlatinib 100 mg orally once daily or crizotinib 250 mg orally twice daily.
The protocol and amendments were approved by the institutional review board or independent ethics committee at each site and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the ethical principles of the Declaration of Helsinki, and all local laws. Written informed consent was obtained from all participants.
Japanese Subpopulation Analysis
Primary and secondary end points were analyzed in the Japanese subpopulation as previously published.8 The data cutoff for this updated analysis was September 20, 2021. Full analysis included all randomized patients. Safety analysis set included all patients who received more than or equal to one dose of the study treatment. Statistical analyses from the previous global and Japanese methods were used, including the Kaplan-Meier method for estimating time-to-event end points and Cox regression models for calculating hazard ratios (HRs).7,8
Results
Patients
In the Japanese subpopulation of the CROWN study, 25 and 23 patients were randomized to the lorlatinib and crizotinib groups, respectively. The safety analysis set for crizotinib included 22 patients because one randomized patient did not receive treatment. Patient demographics of the Japanese subgroup were previously described, with most baseline characteristics relatively balanced between the treatment groups.8 At the time of this analysis, treatment was ongoing in 14 patients (56.0%) in the lorlatinib group and one patient (4.5%) in the crizotinib group. Median follow-up for PFS (95% confidence interval [CI]) was 45.9 (34.9–49.5) months for lorlatinib and 38.6 (3.7–38.6) months for crizotinib.
Efficacy
At the time of this analysis, 25 of 48 patients had experienced disease progression by blinded independent central review (BICR) or died (11 [44.0%] with lorlatinib and 14 [60.9%] with crizotinib). Median PFS (95% CI) by BICR, which was the primary end point, was NR (11.3 mo–NR) with lorlatinib and 11.1 (5.4–14.8) months with crizotinib (HR = 0.36; 95% CI: 0.16–0.83) (Fig. 1A). PFS (95% CI) by BICR at 36 months was 54.2% (32.7%–71.4%) with lorlatinib and 7.8% (0.5%–29.1%) with crizotinib. Median PFS (95% CI) by investigator assessment was NR (16.4 mo–NR) with lorlatinib and 9.1 (5.4–11.1) months with crizotinib (HR = 0.25; 95% CI: 0.11–0.57); 36-month PFS (95% CI) was 62.5% (40.3%–78.4%) and 5.6% (0.4%–22.4%) with lorlatinib and crizotinib, respectively.
Figure 1.
Efficacy outcomes in the Japanese subpopulation (full analysis set). (A) Kaplan-Meier plot of PFS based on BICR assessment. (B) Kaplan-Meier plot of time to intracranial progression based on BICR assessment. BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; IC, intracranial; NR, not reached; PFS, progression-free survival; TTP, time to progression.
Confirmed objective response rate (95% CI) by BICR was 72.0% (50.6%–87.9%) with lorlatinib and 52.2% (30.6%–73.2%) with crizotinib, with an odds ratio of 2.345 (95% CI: 0.610–9.035) (Supplementary Table 1). For these patients, the median duration of response (95% CI) was NR (12.8 mo–NR) with lorlatinib and 9.4 (7.4–16.6) months with crizotinib. Response by BICR lasted more than or equal to 36 months in seven patients (38.9%) with lorlatinib and one patient (8.3%) with crizotinib. For the 10 patients with baseline brain metastases (lorlatinib, three; crizotinib, seven), the objective intracranial response rate (95% CI) was 100.0% (29.2%–100.0%) with lorlatinib and 28.6% (3.7%–71.0%) with crizotinib. Lorlatinib had intracranial activity in patients with (n = 3) and those without (n = 22) baseline brain metastases, with no new events observed in either subset (Fig. 1B). Crizotinib was less effective at preventing intracranial progression, with new events occurring in four patients (57.1%) with and three patients (18.8%) without baseline brain metastases. For these patients on crizotinib, the median time to intracranial progression (95% CI) was 11.1 months (3.5 mo–NR) and 27.4 months (16.6 mo–NR) for patients with and those without baseline metastases, respectively. In the full analysis set, the probability of intracranial PFS (95% CI) at 36 months was 100.0% (100.0%–100.0%) with lorlatinib and 24% (1.4%–61.9%) with crizotinib.
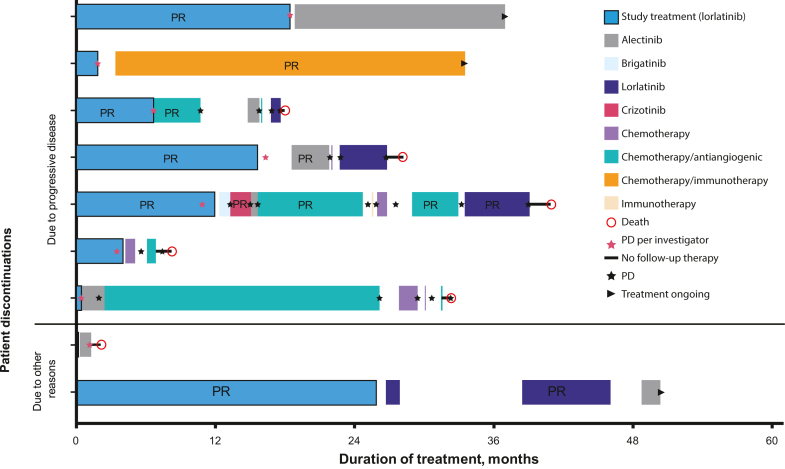
Nine patients (36.0%) in the lorlatinib group and 20 (87.0%) in the crizotinib group received more than or equal to one subsequent anticancer systemic therapy after discontinuation of the study treatment (Supplementary Table 2). Seven of the nine patients on lorlatinib discontinued study treatment due to progressive disease and two discontinued due to other reasons (Fig. 2). The most common first subsequent therapy after lorlatinib was an ALK TKI (n = 6). At the time of this analysis, three patients in the lorlatinib group were alive and receiving ongoing subsequent treatment.
Figure 2.
Subsequent anticancer systemic therapies in patients who discontinued lorlatinib. Patients discontinued study treatment due to progressive disease (n = 7) or other reasons (n = 2) and then received up to eight subsequent treatments. PD, progressive disease; PR, partial response.
Safety
Any-cause treatment-emergent adverse events (TEAEs) of any grade occurred in all patients in the safety analysis set. Serious TEAEs occurred in eight patients (32.0%) with lorlatinib and seven patients (31.8%) with crizotinib (Table 1). Grade 3 or 4 TEAEs occurred in 21 patients (84.0%) with lorlatinib and 16 patients (72.7%) with crizotinib. One patient died due to acute cardiac failure approximately two months after the last lorlatinib dose. Dose reduction or temporary discontinuation due to TEAEs occurred in 17 patients (68.0%) with lorlatinib and 17 patients (77.3%) with crizotinib (Table 1). TEAEs led to study treatment discontinuation in four patients (16.0%) with lorlatinib and six patients (27.3%) with crizotinib. Most frequent treatment-related AEs with lorlatinib were hypertriglyceridemia, hypercholesterolemia, cognitive effects, weight increased, and edema (Supplementary Fig. 1). Most frequent treatment-related AEs with crizotinib were nausea, diarrhea, vomiting, and vision disorders.
Table 1.
Summary of All Treatment-Emergent AEs
| Category, n (%) | Lorlatinib (n = 25) | Crizotinib (n = 22)a |
|---|---|---|
| Any AE | 25 (100.0) | 22 (100.0) |
| Serious AEs | 8 (32.0) | 7 (31.8) |
| Grade 3/4 AEs | 21 (84.0) | 16 (72.7) |
| Fatal AEs | 1 (4.0) | 0 |
| Discontinued from study due to AEs | 1 (4.0) | 0 |
| Discontinued study treatment due to AEs | 4 (16.0) | 6 (27.3) |
| Dose reduction or temporary discontinuation due to AEs | 17 (68.0) | 17 (77.3) |
AE, adverse event.
Safety end points were assessed in the safety analysis set, which included all patients who received more than or equal to one dose of the study treatment.
Discussion
At approximately 3 years of follow-up in the CROWN study, lorlatinib was found to have superior overall and intracranial efficacy over crizotinib in Japanese patients with treatment-naive advanced ALK-positive NSCLC. Efficacy data for lorlatinib versus crizotinib were consistent across the updated and interim analyses, revealing sustained efficacy in the Japanese population.8 In addition, the efficacy of lorlatinib in the updated analysis was comparable across the Japanese, Asian, and overall populations.9,10 Median PFS by BICR was NR with lorlatinib in the Japanese, Asian, and overall populations.9,10 Median PFS by BICR was 11.1 months with crizotinib in the Japanese and Asian populations and 9.3 months in the overall population.9,10 The proportion of patients with a confirmed objective response by BICR was consistently greater with lorlatinib than with crizotinib: Japanese (72% versus 52%), Asian (78% versus 57%), and overall (77% versus 59%) populations.9,10
The lorlatinib safety profile remained consistent with that in the previous data cutoff, with no new safety signals reported with longer exposure.8 Lorlatinib treatment was ongoing in 56.0% of patients at both data cutoffs. TEAEs resulted in one additional dose reduction or temporary discontinuation but no additional permanent discontinuations of lorlatinib treatment. In addition to remaining consistent over time, the safety profile was similar across populations, with a higher incidence of grade 3 or 4 AEs with lorlatinib than crizotinib in Japanese (84% versus 73%), Asian (80% versus 62%), and overall (76% versus 57%) populations.8, 9, 10
Patients with ALK-positive NSCLC have a high incidence of brain metastases (approximately 24%–35% at diagnosis; approximately 58% by 3 y post diagnosis), which are associated with high symptom burden, inferior quality of life, and poor prognosis.11, 12, 13 Lorlatinib was found to have intracranial activity in patients with and without baseline brain metastases, with no new events observed in either subset of patients. Crizotinib was less effective at preventing intracranial progression, with new events observed in 57.1% and 18.8% of patients with and without baseline brain metastases, respectively.
Although this study provides additional data supporting the first-line use of lorlatinib in Japanese patients, analysis is limited by low patient numbers. Nevertheless, the median duration of follow-up for PFS was longer in the Japanese population than in the overall population in the CROWN study (45.9 versus 36.7 mo),9 which may make the Japanese data indicative of the potential of lorlatinib, given the consistency with the outcomes in the overall population. Overall, these long-term efficacy and safety data for lorlatinib in the Japanese subpopulation of CROWN support its use in the first-line treatment of Japanese patients with treatment-naive advanced ALK-positive NSCLC with and without brain metastases.
CRediT Authorship Contribution Statement
Shunsuke Teraoka: Investigation, Writing—original draft, Writing—review and editing.
Hidetoshi Hayashi: Investigation, Writing—original draft, Writing—review and editing.
Yasushi Goto: Investigation, Writing—original draft, Writing—review and editing.
Makoto Nishio: Investigation, Writing—original draft, Writing—review and editing.
Shunichi Sugawara: Investigation, Writing—original draft, Writing—review and editing.
Takao Inoue: Investigation, Writing—original draft, Writing—review and editing.
Satoshi Oizumi: Investigation, Writing—original draft, Writing—review and editing.
Shigeyuki Toyoizumi: Conceptualization, Methodology, Writing—original draft, Writing—review and editing, Formal analysis.
Masakazu Matsumura: Conceptualization, Methodology, Writing—original draft, Writing—review and editing.
Rossella Messina: Conceptualization, Methodology, Writing—original draft, Writing—review and editing.
Terufumi Kato: Investigation, Writing—original draft, Writing—review and editing.
Disclosure
Dr. Teraoka has received honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Novartis Pharma K.K., Ono Pharmaceutical Co., Pfizer R&D Japan G.K., Taiho Pharmaceutical Co., and Thermo Fisher Scientific K.K.; and has participated on a data safety monitoring board or advisory board for Pfizer R&D Japan G.K. Dr. Hayashi has received contracted/support research grants from AstraZeneca K.K., Astellas Pharma, Merck Sharp & Dohme K.K., Ono Pharmaceutical Co., Nippon Boehringer Ingelheim Co., Novartis Pharma K.K., Pfizer Japan, Bristol Myers Squibb Company, Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Daiichi Sankyo Co., Merck Serono Co., Ltd./Merck Biopharma Co., Takeda Pharmaceutical Company Limited, Taiho Pharmaceutical Co., SymBio Pharmaceuticals Limited, AbbVie, inVentiv Health Japan, ICON Japan K.K., Gritstone bio, Parexel International, Kissei Pharmaceutical Co., EPS Corporation, Syneos Health, Pfizer R&D Japan G.K., A2 Healthcare Corporation, Quintiles/IQVIA Services JAPAN K.K., EP-CRSU Co., Linical Co., Eisai Co., CMIC Shift Zero K.K., Kyowa Hakko Kirin Co., Bayer Yakuhin, and Otsuka Pharmaceutical Co.; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca K.K., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Ono Pharmaceutical Co., and Pfizer Japan; and has received scholarship endowment from Bristol Myers Squibb Co.., Chugai Pharmaceutical Co., and Ono Pharmaceutical Co., Dr. Goto has received honoraria from Eli Lilly, Chugai, Taiho, Boehringer Ingelheim, Ono, Bristol Myers Squibb, Pfizer, Merck Sharp & Dohme, Novartis, Merck, and Thermo Fisher Scientific; has received grants from AbbVie, Eli Lilly, Pfizer, Bristol Myers Squibb, Ono, Novartis, Kyorin, Daiichi Sankyo, Novartis, and Preferred Network; has participated on a data safety monitoring board or advisory board for AstraZeneca, Chugai, Boehringer Ingelheim, Eli Lilly, Taiho, Pfizer, Novartis, Guardant Health, Illumina, Daiichi Sankyo, Ono, Bristol Myers Squibb, and Merck Sharp & Dohme; and has a leadership or fiduciary role in another board, society, committee, or advocacy group, paid or unpaid, for CancerNet Japan and JAMT. Dr. Nishio has received honoraria from Ono, Chugai, Taiho, Bristol Myers Squibb, Daiichi Sankyo, Lilly, AstraZeneca, Merck Sharp & Dohme, AbbVie, Takeda, Pfizer, Boehringer Ingelheim, Novartis, Nippon Kayaku, Merck, and Janssen. Dr. Sugawara has received honoraria from Pfizer, Chugai, Merck Sharp & Dohme K.K., AstraZeneca, Ono, Bristol Myers Squibb, Nippon Boehringer Ingelheim, Taiho, Eli Lilly, Novartis, Kyowa Kirin, Takeda, Nippon Kayaku, Merck, Amgen, AbbVie, Otsuka, Thermo Fisher Scientific, and Towa Pharmaceutical. Dr. Inoue has received honoraria from AstraZeneca, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Merck Sharp & Dohme, Ono, and Takeda. Dr. Oizumi has received honoraria from AstraZeneca, Merck Sharp & Dohme, and Eli Lilly; and has received grants for commissioned/joint research from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Chugai, Kissei, Ono, Pfizer, Merck Biopharma, Sanofi, Taiho, and Takeda. Dr. Kato has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eli Lilly, Merck Biopharma, Merck Sharp & Dohme, Novartis, Ono, Pfizer, and Roche; has received grants for commissioned/joint research from AbbVie, Amgen, AstraZeneca, Blueprint Medicines, Chugai, Eli Lilly, Haihe Biopharma, Merck Biopharma, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, and Takeda; has participated on a data safety monitoring board or advisory board for AbbVie, Amgen, AstraZeneca, BeiGene, Chugai, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Merck Biopharma, Merck Sharp & Dohme, Nippon Kayaku, Novartis, Ono, Pfizer, Taiho, and Takeda; and has other financial or nonfinancial interests with Eli Lilly (spouse). Drs. Toyoizumi, Matsumura, and Messina are employees of Pfizer.
Acknowledgments
The study was designed by the sponsor (Pfizer), study investigators, and members of the steering committee. Data were collected by investigators and analyzed by the sponsor. All authors, including those employed by the sponsor of the study, contributed to the interpretation of the data and the development, writing, and approval of the manuscript. Medical writing support was funded by the sponsor. All authors had full access to the raw data in the study, and the corresponding author had final responsibility for the decision to submit for publication. The authors thank the study participants. The authors acknowledge Hidenori Miura and Yukiyoshi Horiuchi of Pfizer R&D Japan for data collection and Taku Uryu of Pfizer R&D Japan for data analysis. Editorial and medical writing support was provided by Caitlin Cash, PhD, on behalf of Nucleus Global and was funded by Pfizer. This study was sponsored by Pfizer.
Ethics Statement
The CROWN trial was conducted in compliance with International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the ethical principles of the Declaration of Helsinki, and all local laws. The protocol and amendments were approved by the institutional review board or independent ethics committee at each study site. Written informed consent was obtained from all participants.
Data Sharing Statement
On request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Footnotes
Cite this article as: Teraoka S, Hayashi H, Goto Y, et al. Long-term efficacy and safety of lorlatinib in Japanese patients with ALK-positive advanced NSCLC—a brief report from the CROWN Study. JTO Clin Res Rep. 2024;5:100632.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100632.
Supplementary Data
References
- 1.Barlesi F., Mazieres J., Merlio J., et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 2.Solomon B., Varella-Garcia M., Camidge D.R. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009;4:1450–1454. doi: 10.1097/JTO.0b013e3181c4dedb. [DOI] [PubMed] [Google Scholar]
- 3.Goto Y., Yamamoto N., Masters E.T., et al. Treatment sequencing in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer in Japan: a real-world observational study. Adv Ther. 2020;37:3311–3323. doi: 10.1007/s12325-020-01392-0. [DOI] [PubMed] [Google Scholar]
- 4.Hida T., Nokihara H., Kondo M., et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 5.Johnson T.W., Richardson P.F., Bailey S., et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–4744. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 6.Shaw A.T., Felip E., Bauer T.M., et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw A.T., Bauer T.M., de Marinis F., et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi H., Teraoka S., Goto Y., et al. First-line lorlatinib versus crizotinib in ALK-positive NSCLC: Japanese subgroup analysis of CROWN. JTO Clin Res Rep. 2023;4 doi: 10.1016/j.jtocrr.2023.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon B.J., Bauer T.M., Mok T.S.K., et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023;11:354–366. doi: 10.1016/S2213-2600(22)00437-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q., Soo R.A., Chang G.C., et al. Asian subgroup analysis of the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive NSCLC. JTO Clin Res Rep. 2023;4 doi: 10.1016/j.jtocrr.2023.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerin A., Sasane M., Zhang J., et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ. 2015;18:312–322. doi: 10.3111/13696998.2014.1003644. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie C.S., Mustafa M.A., Richardson G.E., et al. Genomic alterations and the incidence of brain metastases in advanced and metastatic NSCLC: a systematic review and meta-analysis. J Thorac Oncol. 2023;18:1703–1713. doi: 10.1016/j.jtho.2023.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Rangachari D., Yamaguchi N., VanderLaan P.A., et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.