Abstract
PURPOSE
Bosutinib is approved for adults with chronic myeloid leukemia (CML): 400 mg once daily in newly diagnosed (ND); 500 mg once daily in resistant/intolerant (R/I) patients. Bosutinib has a different tolerability profile than other tyrosine kinase inhibitors (TKIs) and potentially less impact on growth (preclinical data). The primary objective of this first-in-child trial was to determine the recommended phase II dose (RP2D) for pediatric R/I and ND patients.
PATIENTS AND METHODS
In the phase I part of this international, open-label trial (ClinicalTrials.gov identifier: NCT04258943), children age 1-18 years with R/I (per European LeukemiaNet 2013) Ph+ CML were enrolled using a 6 + 4 design, testing 300, 350, and 400 mg/m2 once daily with food. The RP2D was the dose resulting in 0/6 or 1/10 dose-limiting toxicities (DLTs) during the first cycle and achieving adult target AUC levels for the respective indication. As ND participants were only enrolled in phase II, the ND RP2D was selected based on data from R/I patients.
RESULTS
Thirty patients were enrolled; 27 were evaluable for DLT: six at 300 mg/m2, 11 at 350 mg/m2 (one DLT), and 10 at 400 mg/m2 (one DLT). The mean AUCs at 300 mg/m2, 350 mg/m2, and 400 mg/m2 were 2.20 μg h/mL, 2.52 μg h/mL, and 2.66 μg h/mL, respectively. The most common adverse event was diarrhea (93%; ≥grade 3: 11%). Seven patients stopped because of intolerance and eight because of insufficient response. Complete cytogenetic and major molecular response to bosutinib appeared comparable with other published phase I/II trials with second-generation TKIs in children.
CONCLUSION
Bosutinib was safe and effective. The pediatric RP2D was 400 mg/m2 once daily (max 600 mg/d) with food in R/I patients and 300 mg/m2 once daily (max 500 mg/d) with food in ND patients, which achieved targeted exposures as per adult experience.
INTRODUCTION
Chronic myeloid leukemia (CML) is a rare disease in children, accounting for 3% of all pediatric leukemias.1,2 CML is caused by the t(9;22)(q34;q11.2) translocation, resulting in the BCR::ABL1 fusion oncogene (Ph+).3
CONTEXT
Key Objectives
To define the recommended phase II dose (RP2D) of bosutinib in resistant/intolerant (R/I) and newly diagnosed (ND) pediatric patients with Ph+ chronic myeloid leukemia (CML), on the basis of tolerability and pharmacokinetic (PK) targets (derived from adults), and to assess the preliminary antileukemic activity in R/I patients.
Knowledge Generated
The RP2D in R/I pediatric patients was established at 400 mg/m2 once daily. The dose for ND patients was based on the safety and PK data in R/I children and target exposure as derived from adults, and established as 300 mg/m2 once daily. Response to bosutinib in R/I patients appeared comparable with other second-generation tyrosine kinase inhibitors (TKIs) in children.
Relevance (C.F. Craddock)
-
The development of TKIs with a favorable toxicity profile and high levels of clinical activity in children with Ph+ CML remains an area of unmet need. The presented data define a well-tolerated dosing schedule with promising evidence of clinical activity for the second-generation TKI bosutinib and justify ongoing prospective trials of this novel agent.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
The introduction of tyrosine kinase inhibitors (TKIs) targeting the BCR::ABL1 protein, such as imatinib, has drastically improved the prognosis of Ph+ CML.3 With imatinib, more than 90% of children achieve complete hematologic response (CHR) and around 60% achieve complete cytogenetic response (CCyR) after 1 year of treatment.4-7 However, in the long term, approximately 30% of children have an unsatisfactory response or intolerance to imatinib.7 Dasatinib, a second-generation TKI approved for this pediatric indication, led to 82% CCyR rate in imatinib resistant/intolerant (R/I) patients with chronic phase (CP) Ph+ CML.8-11 In addition, nilotinib was also approved for pediatric patients and led to around 80% CCyR rate in resistant patients, but it requires twice daily dosing under fasting conditions.12,13 Side effects of imatinib and dasatinib mostly consist of musculoskeletal pain, asthenia, and skin rash.1,7,10,11 Nilotinib is frequently associated with increased bilirubin, nausea, and vomiting.2,3,12
Bosutinib is a dual Src and Bcr-Abl inhibitor, approved for adults at the recommended dose of 400 mg (max 600 mg) orally once daily for newly diagnosed (ND) CP Ph+ CML and 500 mg (max 600 mg) once daily for patients previously treated with one or more TKIs.14 Treatment with bosutinib in adults is mostly associated with GI toxicities, rash, and increased transaminases (BYOND study).15 GI toxicity (mainly diarrhea) may lead to dose reduction during treatment.2,3,15,16 Animal models showed that bosutinib does not cross the blood-brain barrier, differently from dasatinib.14
Of particular relevance for children, there is evidence that long-term exposure to imatinib results in growth impairment.1,17 Impaired growth may be related to off-target binding, such as inhibition of c-KIT and PDGF-R, and/or the development of an acquired growth hormone deficiency.18-21 Preclinical data indicated that this toxicity may not be observed or be less prominent with bosutinib.22
We report the results of the phase I part of the ITCC-054/COG AAML1921 trial, which aimed to identify a recommended phase II dose (RP2D) for R/I and ND pediatric patients with Ph+ CML.
PATIENTS AND METHODS
Study Design
ITCC-054/COG AAML1921 (ClinicalTrials.gov identifier: NCT04258943) is a phase I/II multicenter, single-arm, open-label study conducted in the context of a pediatric investigation plan and a pediatric written request. The study was conducted under the International Ethical Guidelines for Biomedical Research Involving Human Subjects, ICH Guidelines for Good Clinical Practice, and the Declaration of Helsinki, and approved by the institutional review board or ethics committee in all participating centers. The study is sponsored by the Erasmus Medical Center in Europe and the Children's Oncology Group (COG) in the United States, and funded by Pfizer Inc. It is open in 21 sites of the Innovative Therapies for Children with Cancer Consortium based in Europe, and 45 COG sites.
The age of eligible patients ranged from 1 year to less than 18 years at enrollment, had a diagnosis of Ph+ CML (either in chronic, acute phase, or blast crisis), were resistant or intolerant to at least one previous TKI (per protocol definition according to 2013 European LeukemiaNet criteria), and did not suffer from major organ toxicities.23 Main exclusion criteria consisted of known T315I or V299L BCR::ABL1 mutations and extramedullary disease only (Data Supplement, Table S1 [online only]). Patients and/or parents provided written informed consent and were enrolled between November 2016 and August 2022.
A modified rule-based design (6 + 4), following the principles of the rolling six design, was chosen to allow a better characterization of the pharmacokinetic (PK) parameters defining the RP2D on the basis of a simulation study showing that six to 10 patients are needed to demonstrate that target exposure in children is in the adult range.24,25 We defined the RP2D as the dose resulting in 0/6 or 1/10 dose-limiting toxicities (DLTs, definition in Table 1; patients without DLT had to receive ≥75% of the planned dose in cycle 1 to be evaluable), and resulting in a geometric mean area under the concentration-time curve at steady state (AUCss) of 3.15 ng·µr/mL (±20%) for R/I patients and 2.27 μg h/mL (±20%) for ND patients. Target AUCs for both ND and R/I patients were based on a population PK analysis pooling data (n = 1,401) from adults treated with bosutinib and are equivalent to the adult exposure achieved at 400 and 500 mg/d, respectively.26 The PK sampling schema is provided in the Data Supplement (Table S2). The RP2D for ND patients was extrapolated from PK and safety data obtained in R/I subjects, and target exposure was based on adult data. The Protocol was amended to add a new cohort of ND patients in CP in the phase II part of the study, after approval of bosutinib for this indication in adults (Data Supplement, Table S3).
TABLE 1.
Definition of Dose-Limiting Toxicity
| Nonhematologic AEsa | Hematologic AEsa |
|---|---|
| Any grade ≥3 toxicity, despite optimal treatment | Grade 4 neutropenia or thrombocytopenia and lasting ≥7 days (not explained by persistent leukemia) |
| Any grade ≥2 toxicity requiring discontinuation/interruption for ≥7 days | |
| Clinically significant laboratory abnormality grade ≥3 and lasting ≥7 days despite optimal treatment |
Abbreviations: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events.
AEs were graded based on the CTCAE version 4.03 and assessed only during the first cycle of treatment (28 days).
Study Treatment
A treatment cycle was defined as 28 days, regardless of missed doses. Available formulations included tablets (dissolved for nasogastric administration if needed) and capsules (which could be opened and sprinkled on food), which could be used in combination. The bioequivalence of these formulations was established based on data from the trials ClinicalTrials.gov identifiers: NCT04549480, NCT05032690, and NCT04916769 provided by Pfizer Inc.
Dose levels were amended in protocol version 4 as the exposure observed in the first patients treated at 300 mg/m2 were insufficient to yield the target AUC for R/I patients. Consequently, we tested daily doses of 350 mg/m2 and 400 mg/m2 (dose schema; Data Supplement, Table S4). Maximum daily dose was capped at 600 mg in R/I patients as per adult label. Body surface area (BSA) was calculated using the Mosteller formula.27 Moderate or strong CYP3A inducers and inhibitors and proton pump inhibitors were prohibited.
End Points and Assessment
The primary objective was to determine the RP2D of bosutinib for R/I pediatric patients with Ph+ CML. Secondary objectives included overall safety and preliminary antileukemic activity.
The primary end points were the incidence of DLTs and PK parameters. Secondary end points included estimations of toxicity and efficacy outcomes. As a post hoc analysis, the cumulative incidence of treatment discontinuation because of unsatisfactory response and intolerance was added. A full list of end points and definitions of efficacy, safety assessments, and mutation analysis can be found in the Data Supplement (Tables S5-S7).
Statistical Analysis
Cumulative incidence of response was obtained using one minus the Kaplan-Meier (KM) estimate. Event-free survival (EFS) and overall survival (OS) were estimated using the KM method (definition in the Data Supplement, Table S6). The cumulative incidence of treatment discontinuation was estimated using a competing risk setting (insufficient response v intolerance). Statistical methodology details are provided in the Data Supplement.
RESULTS
Patients
At the data cutoff of September 19, 2022, 30 patients were screened, 29 enrolled (one screen failure), and 28 treated (one patient did not start the treatment because of low absolute neutrophil count; safety and efficacy analysis set); 27 were evaluable for DLT (one patient was not evaluable because of withdrawal of consent after <21 days of treatment in cycle 1, in the absence of a DLT; Data Supplement, Fig S1). Baseline demographics are summarized in Table 2. All 28 treated patients were in CP at the time of enrollment.
TABLE 2.
Patient Characteristics (N = 28, all patients receiving at least one dose of bosutinib)
| Characteristic | Total No. of Patients |
|---|---|
| Sex, No. (%) | |
| Male | 16 (57.1) |
| Female | 12 (42.9) |
| Age at enrollment, years | |
| Median (range) | 12 (1-17) |
| >1 to ≤6, No. (%) | 6 (21.4) |
| >6 to ≤12, No. (%) | 10 (35.7) |
| >12, No. (%) | 12 (42.9) |
| Reason for enrollment, No. (%) | |
| Resistant | 23 (82.1) |
| Intolerant | 5 (17.9) |
| Time from diagnosis, months | |
| Median (range) | 17 (3-82) |
| Characteristic | Total No. of Patients, (%) | |
|---|---|---|
| Previous Lines of Treatment (TKIs) | Resistanta | Intoleranta |
| One | 13 (46.4) | 3 (10.7) |
| Two | 8 (28.6) | 1 (3.6) |
| Three | 2 (7.1) | 1 (3.6) |
| Characteristic | Total No. of Patients, (%) | |
|---|---|---|
| Last TKI Received | Resistanta | Intoleranta |
| Imatinib | 10 (35.5) | 3 (10.7) |
| Dasatinib | 12 (42.9) | 1 (3.6) |
| Nilotinib | 1 (3.6) | 1 (3.6) |
| Characteristic | Total No. of Patients, (%) | |
|---|---|---|
| Response Status at Enrollmentb | Resistant | Intolerant |
| CCyR | 14 (50) | 2 (7.1) |
| Partial CyR | 3 (10.7) | 2 (7.1) |
| Minor CyR | 1 (3.6) | 0 |
| Minimal CyR | 2 (7.1) | 0 |
| No CyR | 1 (3.6) | 0 |
| NA | 2 (7.1) | 1 (3.6) |
| MMR/≥MR3 | 1c (3.6) | 3 (10.7) |
| MR2 | 9 (32.1) | 1 (3.6) |
| MR1 | 7 (25) | 0 |
| No MR | 5 (17.8) | 0 |
| NA | 1 (3.6) | 1 (3.6) |
Abbreviations: CCyR, complete cytogenetic response; CyR, cytogenetic response; ELN, European LeukemiaNet; MMR, major molecular response; MR, molecular response; NA, not available; PB, peripheral blood; TKI, tyrosine kinase inhibitor.
Resistance has been defined either suboptimal/warning or failure response on the basis of ELN 2013 criteria for all patients depending on whether they received only one or more than one line of treatment with TKIs (see Appendix 3 and 4 of the Protocol). Intolerance was based on the treating physician's judgment.
Results based on central laboratory analysis at the time of screening. Molecular response was based on PB analysis and on bone marrow when PB was NA.
One patient was included as resistant with MR2 molecular response on the basis of local peripheral blood results. The central laboratory confirmation later showed MR3 in PB (MR2 on the basis of bone marrow analysis), but because the patient was already enrolled, treatment was continued.
Overall, 490 bosutinib 28-day cycles (median cycles per patient, 15; range, 1-66) were administered. Eleven patients (39%) were still on treatment at the time of data cutoff: seven stopped because of intolerance, eight because of insufficient response, and two completed study treatment and transitioned to adult care (after 24 and 19 months of study treatment).
Safety
Six patients were enrolled at 300 mg/m2 once daily, without DLTs. The dose was escalated to 350 mg/m2 once daily, and 11 patients were enrolled (two patients consented simultaneously). One DLT occurred (grade 3 nausea/vomiting and diarrhea). This patient continued at 250 mg/m2 once daily and discontinued the treatment after seven cycles because of increased transaminase levels. The dose was further escalated to 400 mg/m2 once daily, and 11 patients were treated, as one subject was replaced (not evaluable for DLTs). One patient experienced a DLT (grade 3 transaminase increase, grade 2 bilirubin increase, and grade 3 rash with treatment interruption >7 days), which resolved completely, and continued the treatment at the reduced dose of 300 mg/m2 once daily.
The most common adverse events (AEs) were diarrhea (93%, n = 26), abdominal pain (71%, n = 20), vomiting (68%, n = 19), nausea (61%, n = 12), and maculopapular rash or other skin disorders (39%, n = 11; and 43%, n = 12, respectively). AEs assessed as (possibly, probably or definitely) related to bosutinib are reported in Table 3.
TABLE 3.
Most Frequent Adverse Events (frequency >3)
| Adverse Event Term | Gr 1-2, No. (%) | Gr ≥3, No. (%) | Gr 1-2 Related to Bosutinib,a No. (%) | Gr ≥3 Related to Bosutinib,a No. (%) |
|---|---|---|---|---|
| Diarrhea | 23 (82) | 3 (11) | 20 (71) | 2 (7) |
| Abdominal pain | 19 (68) | 1 (4) | 15 (54) | 1 (4) |
| Vomiting | 16 (57) | 3 (11) | 12 (43) | 3 (11) |
| Nausea | 17 (61) | 0 | 15 (54) | 0 |
| Fever | 11 (39) | 1 (4) | 5 (18) | 0 |
| Skin and subcutaneous tissue disorders | 11 (39) | 1 (4) | 6 (21) | 1 (4) |
| Rash maculopapular | 8 (29) | 3 (11) | 4 (14) | 3 (11) |
| Headache | 9 (32) | 1 (4) | 5 (18) | 0 |
| ALT increased | 4 (14) | 5 (18) | 4 (14) | 5 (18) |
| Fatigue | 7 (25) | 1 (4) | 6 (21) | 1 (4) |
| Pain in extremity | 7 (25) | 1 (4) | 4 (14) | 0 |
| Constipation | 7 (25) | 0 | 2 (7) | 0 |
| GI disorders | 7 (25) | 0 | 4 (14) | 0 |
| Anorexia | 6 (21) | 0 | 5 (18) | 0 |
| Creatinine increased | 6 (21) | 0 | 5 (18) | 0 |
| Infections and infestations | 5 (18) | 1 (3) | 0 | 0 |
| Metabolism and nutrition disorders | 6 (21) | 0 | 4 (14) | 0 |
| Stomach pain | 6 (21) | 0 | 5 (18) | 0 |
| AST increased | 3 (11) | 2 (7) | 3 (11) | 2 (7) |
| Rhinitis infective | 5 (18) | 0 | 0 | 0 |
| Cough | 4 (14) | 0 | 1 (3) | 0 |
| CPK increased | 4 (14) | 0 | 3 (11) | 0 |
| Flatulence | 4 (14) | 0 | 2 (7) | 0 |
| General disorders—other | 4 (14) | 0 | 2 (7) | 0 |
| Platelet count decreased | 3 (11) | 1 (4) | 2 (7) | 1 (4) |
| Rash acneiform | 4 (14) | 0 | 4 (14) | 0 |
Abbreviations: CPK, creatine phosphokinase; Gr, grade.
Possibly, probably, and definitely related to bosutinib on the basis of the treating physician's judgment.
Importantly, some patients suffered of persistent low-grade gastrointestinal toxicity, mostly diarrhea, protracted for over a year.
Among grade 3 and 4 AEs, the most common were transaminase elevation (18%, n = 5), maculopapular skin rash (11%, n = 3), vomiting (11%, n = 3), and diarrhea (11%, n = 3). No grade 5 AEs occurred. A full list of AEs (Table S8), laboratory and hematologic abnormalities (Table S9), and AEs by age class and dose level (Tables S10A and S10B) are provided in the Data Supplement. No patient developed a clinically significant prolonged QTc (Data Supplement, Table S11). Neither cases of arrhythmia nor abnormalities in cardiac function were registered at the echocardiograms performed every 12 months. Eleven patients had their dose level reduced because of AEs, and seven stopped the treatment because of intolerance (protracted diarrhea, nausea/vomiting, neutropenia, and rash), of whom two were already intolerant to imatinib or dasatinib.
Pharmacokinetics
In total, 386 samples from 27 patients were available for PK analysis. The geometric mean AUCss at 300 mg/m2, 350 mg/m2, and 400 mg/m2 were 2.20 x 103 ng·hr/ml (range, 1.54-3.10), 2.52 x 103 ng·hr/ml (range, 1.85-4.62), and 2.66 x 103 ng·hr/ml (range, 1.47-3.92), respectively. The geometric mean peak plasma concentrations at 300 mg/m2, 350 mg/m2, and 400 mg/m2 were 188.5 ng/mL (range, 110-262), 221.2 ng/mL (range, 121-528), and 198.1 ng/mL (range, 111-297), respectively, and it was generally reached approximately 3 hours after the administration across all dose levels. The geometric mean trough concentrations at 300 mg/m2, 350 mg/m2, and 400 mg/m2 were 46.58 ng/mL (range, 20.9‐103), 46.33 ng/mL (range, 29.8-124), and 48.88 ng/mL (range, 11.7-137), respectively. In 10 patients (33%), the capped dose of 600 mg/d was administered, five of which at the highest dose level. The target steady-state exposure for ND patients (2.37% x 103 ng·hr/ml ± 20%) was achieved at 300 mg/m2/d, while for R/I patients, the target exposure (3.15% x 103 ng·hr/ml ± 20%) was achieved at 400 mg/m2/d.
Efficacy
The median follow-up was 23.8 months (range, 1.8-61.5). At the data cutoff date, the cumulative proportions of CHR, major cytogenetic response (MCyR), CCyR, and major molecular response (MMR) by the end of treatment, as best response, were 100% (95% CI, 87.7 to 100), 96.4% (95% CI, 81.7 to 99.9), 92.9% (95% CI, 76.5 to 99.1), and 46.4% (95% CI, 27.5 to 66.1), respectively (Fig 1A). All patients entering the study not in CHR (n = 6), achieved CHR by month 4. Considering only those patients who achieved MCyR, CCyR, and/or MMR for the first time on study, the cumulative incidence of MCyR was 71.4% (95% CI, 17.9 to 93.6) at 6 months (no patient at risk after month 9), while for CCyR was 83.3% (95% CI, 40.5 to 96.4) at 6 months and was maintained at 12 months. The cumulative incidence of MMR was 26.1% (95% CI, 10.3 to 45.2) at 6 months and increased to 39.1% (95% CI, 19.4 to 58.5) at 12 months (Fig 1B). In patients without baseline response (screening), the median time to respond was 3 months for MCyR and CCyR and 28 months for MMR. Among the patients achieving MMR while on study (n = 10), five achieved MR4/MR4.5 (Data Supplement, Table S6). All patients who achieved or entered the study in MCyR, CCyR, and/or MMR maintained the response except three patients, who lost CCyR after four, 15, and 31 cycles, respectively. Baseline response for resistant versus intolerant patients is reported in Table 2. In a post hoc analysis, no statistically significant differences in the cumulative incidence of MMR, CCyR, or CHR were observed across the dose levels, previous lines of therapy, or age groups (Data Supplement, Figs S2 and S3 and Table S12), but the study was also not powered to detect such differences. Notably, MMR was reached only by one of five children at risk in the class age >1 years ≤6 years, with a cumulative incidence of MMR of 20% in this age group (P = .08).
FIG 1.
(A) Proportions of patients in CHR, MCyR, CCyR, and MMR at baseline (screening), and 3, 6, 9, and 12 months. (B) Cumulative incidence of first-time achieving CHR, MCyR, CCyR, and MMR on treatment at 3, 6, 9, and 12 months (patients with response at screening excluded). CCyR, complete cytogenetic response; CHR, complete hematologic response; MCyR, major cytogenetic response; MMR, major molecular response.
The OS was 100% (95% CI, not available) at 1 and 2 years, and 85.7% (95% CI, 63.3 to 100) at 3 years. One patient died due to meningitis after hematopoietic stem-cell transplantation (HSCT) 15 months after the last dose of bosutinib. EFS rates at 1, 2, and 3 years were 96.3% (95% CI, 89.4 to 100), 91.92% (95% CI, 81.7 to 100), and 70.0% (95% CI, 47.0 to 100), respectively (Fig 2).
FIG 2.
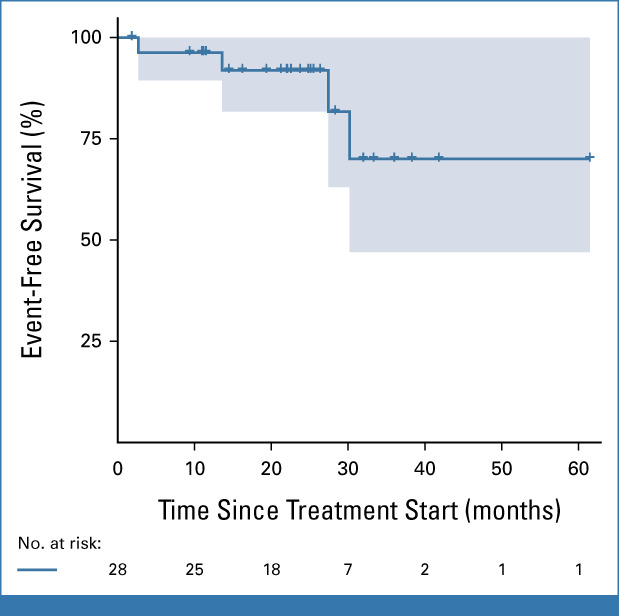
Event-free survival (Kaplan-Meier method). Events were defined as either (1) death due to any cause; (2) transformation to accelerated phase or blast crisis at any time; (3) loss of CHR (as defined in the Data Supplement); (4) loss of CCyR (as defined in the Data Supplement); (5) loss of MMR (as defined in the Data Supplement); and (6) for patients not achieving a CHR: doubling of WBC at least 1 month apart with the second value >20 × 109/L and maintained in subsequent assessments for at least 2 weeks. Only one patient died, two lost CCyR, and one lost both CCyR and MMR (counted as one event at the time of loss of CCyR). Crosses represent censored patients. CCyR, complete cytogenetic response; CHR, complete hematologic response; MMR, major molecular response.
At the time of data cutoff, eight patients stopped the treatment because of insufficient response per investigator judgment, of whom two were treated at 300 mg/m2, three at 350 mg/m2, and three at 400 mg/m2 once daily. As a post hoc analysis, the cumulative incidence of treatment discontinuation is shown in Figure 3. Three of the patients who did not obtain sufficient response underwent HSCT. We did not record emerging mutations of T315I or V299L in BCR::ABL1, or any other mutations in exons 5 and 6 in ABL1, in patients achieving the end of treatment.
FIG 3.
Cumulative incidence of treatment discontinuation. (A) Cumulative incidence of treatment discontinuation as per investigator judgment in a competing risk setting. Discontinuation because of insufficient response is displayed in red and included loss of CCyR and/or MMR or failure to achieve CCyR and/or MMR. Discontinuation because of toxicities is displayed in blue. (B) Cumulative incidence of treatment discontinuation because of all causes. Crosses represent censored patients. CCyR, complete cytogenetic response; MMR, major molecular response.
DISCUSSION
In this first-in-child dose-finding study, bosutinib showed a tolerability profile consistent with data known in adults.15 Only two DLTs occurred (at 350 and 400 mg/m2). The RP2D for R/I patients was established at 400 mg/m2 once daily (max 600 mg/d). The RP2D for ND patients was extrapolated from safety and exposure data in R/I pediatric patients on the basis of the AUC of the recommended dose for ND adult patients and was established at 300 mg/m2 once daily (max 500 mg/d).
The most common AEs were GI toxicities, with almost all patients experiencing at least mild (grade 1-2) events, while grade 3 or higher AEs occurred in approximately 10% of the patients. This frequency is higher when compared with imatinib and dasatinib (2%-5% grade 3-4 GI toxicities).28,29 A small proportion of patients have persistent GI complaints, mainly diarrhea, which may affect compliance and quality of life. A real-world strategy to prevent early discontinuation in adult patients consists of starting with a lower dose (200-300 mg/d) of bosutinib followed by gradually increasing the dose, but this was not tested in this first-in-child dose-finding study.16 Although musculoskeletal pain is commonly reported in patients treated with imatinib (40%-50%), our study confirms that these events were less common with bosutinib (approximately 10%-15%).28-30 Another frequent adverse event was skin rash, which occurred in 40% (n = 11) of the subjects, similarly to published data for other TKIs used in children.7,11,12 As observed in adult patients, the impact of bosutinib on cardiac function was negligible; however, longer follow-up may be needed to better assess cardiac side effects.30
It remains to be established whether bosutinib might show a less toxic profile on longitudinal growth as demonstrated in murine models.20,22 All TKIs approved in children for Ph+ CML show a negative impact on height, especially when started before puberty.18,21,31 The potential benefit of bosutinib will be better evaluable in our phase II cohort in ND patients, as the enrollment of pretreated subjects precludes a firm assessment in the R/I cohort.
In terms of PK, the AUC increased almost linearly with each dose level, even if 50% of the patients treated at 400 mg/m2/d received the maximum dose of 600 mg/d. This might suggest that the solubility and saturation in the gastrointestinal tract were not saturated in the investigated dose range. Such phenomena were observed in adults receiving 600 mg/d (selected as maximum daily dose in our R/I cohort).30,32 A higher BSA-adjusted dose was necessary in younger children to achieve the target exposure as defined in adult studies, whereas in older children, the dose was capped as in adults if the BSA-adjusted dose was higher than 600 mg. These differences in PKs might be influenced by a different absorption of the drug in younger children, who generally have a higher gastric pH compared with adults and less water in the gastrointestinal tract.33 In addition, although bosutinib was instructed to be administered after a meal, food intake was not standardized. Bosutinib is likely classifiable as a biopharmaceutics classification system class IV drug, characterized by low permeability and low solubility, the latter being pH-dependent and increased by food intake, especially when rich in fat.14
In adults, higher bosutinib concentrations have been associated with higher probability of response, likely reaching the plateau of exposure efficacy at recommended doses in adults.14 In adults resistant to imatinib, it was suggested that bosutinib doses ≥350 mg/d were associated with an increased rate of MCyR.25,30 In our study, we did not identify a clear dose-efficacy relationship, which might be due to the limited sample size or that participants are at or near the exposure-efficacy plateau.
In terms of preliminary efficacy, the cumulative incidence of CCyR and MMR appears comparable with the other published pediatric phase I/II trials with second-generation TKIs.11,12 The main reasons to discontinue treatment in this study were equally attributable to intolerance and loss of response/insufficient response.
Currently, a dose-finding trial of asciminib (targeting the ABL myristoyl pocket STAMP) in children is ongoing (ClinicalTrials.gov identifier: NCT04925479).34 In adults, it showed a higher MMR rate and a lower treatment discontinuation rate because of toxicities compared with bosutinib.35 Ponatinib is the other TKI under investigation in children (ClinicalTrials.gov identifier: NCT03934372); available data are mostly based on case reports.36,37 In adults, it proved effective, particularly in patients with T315I-mutated CML, but at the expense of more frequent cardiovascular events.38,39
Since Ph+ CML is a very rare disease in children, one of the main limitations of this trial was the slow enrollment rate. Six years were needed to complete the phase I part, despite adding additional centers in the United States since 2019 and finally recruiting in over 60 centers globally. The number of TKIs now approved for children further reduces the number of eligible patients for dose-finding trials. To resolve this problem, it might be crucial to limit the number of dose levels tested, and use PK modeling to define the starting dose and implement extrapolation from adult data where feasible.40
In conclusion, the phase I portion of this study indicates that bosutinib is safe and effective in the R/I pediatric population. The phase II part of the trial, enrolling ND and R/I patients, is ongoing.
ACKNOWLEDGMENT
The authors would like to thank the ITCC consortium and the COG study group for providing the infrastructure and the collaborative environment to run early clinical trials in pediatric oncology, and Pfizer Inc for providing funding and a clinical research collaboration to implement the study. Sponsorship for the study was provided by Erasmus MC/Sophia Children's Hospital, Department of Pediatrics, Rotterdam, the Netherlands. The authors thank all patients and their families for participating in the trial.
Erica Brivio
Research Funding: Pfizer (Inst)
Edoardo Pennesi
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
Vincent H.J. van der Velden
Research Funding: BD Biosciences (Inst), Pfizer (Inst), Janssen (Inst), Novartis/Navigate BioPharma (Inst), Agilent (Inst)
Patents, Royalties, Other Intellectual Property: Patent EuroFlow MRD antibody tubes. No financial relationship (Inst)
Chad Hudson
Employment: Hematologics
Karey Kowalski
Employment: Pfizer, CVS
Stock and Other Ownership Interests: Pfizer
Patents, Royalties, Other Intellectual Property: Author for dose regimen patent for oncology compound (Inst)
Travel, Accommodations, Expenses: Pfizer
Luke Kuttschreuter
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Pfizer
Carmelo Rizzari
Honoraria: Servier, Jazz Pharmaceuticals, Clinigen Group, Amgen, SERB
Consulting or Advisory Role: Servier, Jazz Pharmaceuticals, Clinigen Group, SERB
Jessica Pollard
Consulting or Advisory Role: Syndax
Research Funding: AbbVie (Inst), Servier (Inst)
Matthew Kutny
Research Funding: Jazz Pharmaceuticals (Inst), Incyte (Inst), Merck (Inst), Novartis (Inst)
Yves Bertrand
Travel, Accommodations, Expenses: Servier
Jean-Pierre Bourquin
Travel, Accommodations, Expenses: Servier, Amgen, Jazz Pharmaceuticals
Jayashree Motwani
Honoraria: Roche
Consulting or Advisory Role: Sobi
Speakers' Bureau: Roche
Travel, Accommodations, Expenses: Roche
Inge M. van der Sluis
Consulting or Advisory Role: Clinigen Group (Inst), Jazz Pharmaceuticals (Inst)
Speakers' Bureau: Servier (Inst)
Research Funding: Servier (Inst), Amgen (Inst)
Franco Locatelli
Honoraria: Miltenyi Biotec, Bluebird Bio, Sobi, Amgen, Novartis
Consulting or Advisory Role: Amgen, Novartis, Pfizer
Michael E. Roth
Research Funding: Eisai, Pfizer
Nobuko Hijiya
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Stemline Therapeutics, Incyte, Novartis
Consulting or Advisory Role: Novartis, Incyte, Stemline Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst)
Christian M. Zwaan
Consulting or Advisory Role: Takeda (Inst), Pfizer (Inst), AbbVie (Inst), Jazz Pharmaceuticals (Inst), Incyte (Inst), Novartis (Inst), Kura Oncology (Inst), Gilead Sciences (Inst), Johnson&Johnson, Sanofi, Syndax, Bristol Meyers Squibb, Roche, Daiichi Sankyo, Servier, Astra Zeneca
Research Funding: Takeda (Inst), AbbVie/Genentech (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst), Kura Oncology (Inst), Daiichi Sankyo (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 63rd Annual Meeting of the American Society of Hematology, Orlando, FL, December 11-14, 2021.
SUPPORT
Supported by Erasmus MC Sophia Children's Hospital, Rotterdam, the Netherlands, and financially supported by Pfizer Inc.
CLINICAL TRIAL INFORMATION
E.B. and E.P. contributed equally to this work. N.H. and C.M.Z. jointly supervised the work.
AUTHOR CONTRIBUTIONS
Conception and design: Erica Brivio, Marieke E. Willemse, Alwin D.R. Huitema, Luke Kuttschreuter, Jessica Pollard, Inge M. van der Sluis, Michael E. Roth, Christian M. Zwaan
Administrative support: Marieke E. Willemse
Provision of study materials or patients: Erica Brivio, Marieke E. Willemse, Francisco J. Bautista Sirvent, Carmelo Rizzari, Jessica Pollard, Matthew Kutny, Sara Zarnegar-Lumley, Michele Redell, Stacy Cooper, Arnaud Petit, Julie Krystal, Jean-Pierre Bourquin, Jayashree Motwani, Nobuko Hijiya, Christian M. Zwaan
Collection and assembly of data: Erica Brivio, Edoardo Pennesi, Marieke E. Willemse, Vincent H.J. van der Velden, Berna H. Beverloo, Monique L. den Boer, Lukas A.J. Rammeloo, Chad Hudson, Nyla Heerema, Karey Kowalski, Luke Kuttschreuter, Francisco J. Bautista Sirvent, Andrew Bukowinski, Carmelo Rizzari, Jessica Pollard, Laura Murillo-Sanjuán, Matthew Kutny, Sara Zarnegar-Lumley, Michele Redell, Stacy Cooper, Yves Bertrand, Arnaud Petit, Julie Krystal, Markus Metzler, Jean-Pierre Bourquin, Jayashree Motwani, Inge M. van der Sluis, Franco Locatelli, Nobuko Hijiya, Christian M. Zwaan
Data analysis and interpretation: Erica Brivio, Edoardo Pennesi, Alwin D.R. Huitema, Yilin Jiang, Harm D.R. van Tinteren, Vincent H.J. van der Velden, Berna H. Beverloo, Karey Kowalski, Huadong Zhao, Luke Kuttschreuter, Francisco J. Bautista Sirvent, Andrew Bukowinski, Jessica Pollard, Stacy Cooper, Donna Lancaster, Inge M. van der Sluis, Franco Locatelli, Michael E. Roth, Nobuko Hijiya, Christian M. Zwaan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Bosutinib in Resistant and Intolerant Pediatric Patients With Chronic Phase Chronic Myeloid Leukemia: Results From the Phase I Part of Study ITCC054/COG AAML1921
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Erica Brivio
Research Funding: Pfizer (Inst)
Edoardo Pennesi
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
Vincent H.J. van der Velden
Research Funding: BD Biosciences (Inst), Pfizer (Inst), Janssen (Inst), Novartis/Navigate BioPharma (Inst), Agilent (Inst)
Patents, Royalties, Other Intellectual Property: Patent EuroFlow MRD antibody tubes. No financial relationship (Inst)
Chad Hudson
Employment: Hematologics
Karey Kowalski
Employment: Pfizer, CVS
Stock and Other Ownership Interests: Pfizer
Patents, Royalties, Other Intellectual Property: Author for dose regimen patent for oncology compound (Inst)
Travel, Accommodations, Expenses: Pfizer
Luke Kuttschreuter
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Pfizer
Carmelo Rizzari
Honoraria: Servier, Jazz Pharmaceuticals, Clinigen Group, Amgen, SERB
Consulting or Advisory Role: Servier, Jazz Pharmaceuticals, Clinigen Group, SERB
Jessica Pollard
Consulting or Advisory Role: Syndax
Research Funding: AbbVie (Inst), Servier (Inst)
Matthew Kutny
Research Funding: Jazz Pharmaceuticals (Inst), Incyte (Inst), Merck (Inst), Novartis (Inst)
Yves Bertrand
Travel, Accommodations, Expenses: Servier
Jean-Pierre Bourquin
Travel, Accommodations, Expenses: Servier, Amgen, Jazz Pharmaceuticals
Jayashree Motwani
Honoraria: Roche
Consulting or Advisory Role: Sobi
Speakers' Bureau: Roche
Travel, Accommodations, Expenses: Roche
Inge M. van der Sluis
Consulting or Advisory Role: Clinigen Group (Inst), Jazz Pharmaceuticals (Inst)
Speakers' Bureau: Servier (Inst)
Research Funding: Servier (Inst), Amgen (Inst)
Franco Locatelli
Honoraria: Miltenyi Biotec, Bluebird Bio, Sobi, Amgen, Novartis
Consulting or Advisory Role: Amgen, Novartis, Pfizer
Michael E. Roth
Research Funding: Eisai, Pfizer
Nobuko Hijiya
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Stemline Therapeutics, Incyte, Novartis
Consulting or Advisory Role: Novartis, Incyte, Stemline Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst)
Christian M. Zwaan
Consulting or Advisory Role: Takeda (Inst), Pfizer (Inst), AbbVie (Inst), Jazz Pharmaceuticals (Inst), Incyte (Inst), Novartis (Inst), Kura Oncology (Inst), Gilead Sciences (Inst), Johnson&Johnson, Sanofi, Syndax, Bristol Meyers Squibb, Roche, Daiichi Sankyo, Servier, Astra Zeneca
Research Funding: Takeda (Inst), AbbVie/Genentech (Inst), Pfizer (Inst), Jazz Pharmaceuticals (Inst), Kura Oncology (Inst), Daiichi Sankyo (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Suttorp M, Millot F: Treatment of pediatric chronic myeloid leukemia in the year 2010: Use of tyrosine kinase inhibitors and stem-cell transplantation. Hematol Am Soc Hematol Educ Progr 2010:368-376, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Ford M, Mauro M, Aftandilian C, et al. : Management of chronic myeloid leukemia in children and young adults. Curr Hematol Malig Rep 17:121-126, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hijiya N, Suttorp M: How I treat chronic myeloid leukemia in children and adolescents. Blood 133:2374-2384, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Champagne MA, Capdeville R, Krailo M, et al. : Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: Results from a Children’s Oncology Group phase 1 study. Blood 104:2655-2660, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Millot F, Guilhot J, Nelken B, et al. : Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia 20:187-192, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Millot F, Baruchel A, Guilhot J, et al. : Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: Results of the French national phase IV trial. J Clin Oncol 29:2827-2832, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Suttorp M, Schulze P, Glauche I, et al. : Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: Results from a phase III trial. Leukemia 32:1657-1669, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Tokarski JS, Newitt JA, Chang CYJ, et al. : The structure of dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res 66:5790-5797, 2006 [DOI] [PubMed] [Google Scholar]
- 9.de la Fuente J, Baruchel A, Biondi A, et al. : Managing children with chronic myeloid leukaemia (CML): Recommendations for the management of CML in children and young people up to the age of 18 years. Br J Haematol 167:33-47, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Zwaan CM, Rizzari C, Mechinaud F, et al. : Dasatinib in children and adolescents with relapsed or refractory leukemia: Results of the CA180-018 phase I dose-escalation study of the Innovative Therapies for Children With Cancer Consortium. J Clin Oncol 31:2460-2468, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Gore L, Kearns PR, de Martino ML, et al. : Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: Results from a phase II trial. J Clin Oncol 36:1330-1338, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijiya N, Maschan A, Rizzari C, et al. : Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome–positive chronic myeloid leukemia. Blood 134:2036-2045, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hijiya N, Zwaan CM, Rizzari C, et al. : Pharmacokinetics of nilotinib in pediatric patients with Philadelphia chromosome–positive chronic myeloid leukemia or acute lymphoblastic leukemia. Clin Cancer Res 26:812-820, 2020 [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency : Bosulif. https://www.ema.europa.eu/en/medicines/human/EPAR/bosulif
- 15.Hochhaus A, Gambacorti-Passerini C, Abboud C, et al. : Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: Primary results of the phase 4 BYOND study. Leukemia 34:2125-2137, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes JE, Apperley JF, Deangelo DJ, et al. : Management of adverse events associated with bosutinib treatment of chronic-phase chronic myeloid leukemia: Expert panel review. J Hematol Oncol 11:143, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samis J, Lee P, Zimmerman D, et al. : Recognizing endocrinopathies associated with tyrosine kinase inhibitor therapy in children with chronic myelogenous leukemia. Pediatr Blood Cancer 63:1332-1338, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Patterson B, Samis J, Gore L, et al. : PF416 growth rate and endocrine effects of dasatinib therapy observed in a retrospective analysis of a phase 2 clinical trial for pediatric patients with chronic myeloid leukemia in chronic phase (cml-cp). HemaSphere 3:161, 2019 [Google Scholar]
- 19.Narayanan KR, Bansal D, Walia R, et al. : Growth failure in children with chronic myeloid leukemia receiving imatinib is due to disruption of GH/IGF-1 axis. Pediatr Blood Cancer 60:1148-1153, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Ulmer A, Tabea Tauer J, Glauche I, et al. : TK inhibitor treatment disrupts growth hormone axis: Clinical observations in children with CML and experimental data from a juvenile animal model. Klinische Padiatrie 225:120-126, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Bansal D, Shava U, Varma N, et al. : Imatinib has adverse effect on growth in children with chronic myeloid leukemia. Pediatr Blood Cancer 59:481-484, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Tauer JT, Hofbauer LC, Jung R, et al. : Micro-osmotic pumps for continuous release of the tyrosine kinase inhibitor bosutinib in juvenile rats and its impact on bone growth. Med Sci Monit Basic Res 19:274-278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baccarani M, Deininger MW, Rosti G, et al. : European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood 122:872-884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skolnik JM, Barrett JS, Jayaraman B, et al. : Shortening the timeline of pediatric phase I trials: The rolling six design. J Clin Oncol 26:190-195, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Janssen JM, Zwaan CM, Schellens JHM, et al. : Clinical trial simulations in paediatric oncology: A feasibility study from the innovative Therapies for Children with Cancer Consortium. Eur J Cancer 85:78-85, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Garrett M, Knight B, Cortes JE, et al. : Population modeling of bosutinib exposure-response in patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer Med 12:17981-17992, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Edelbi R, Lindemalm S, Eksborg S: Estimation of body surface area in various childhood ages—Validation of the Mosteller formula. Acta Paediatr 101:540-544, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Bristol-Myers Squibb : SPRYCEL (dasatinib) tablet for oral use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf
- 29.Novartis : GLEEVEC (imatinib mesylate) tablets for oral use [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021588s035lbl.pdf
- 30.Cortes JE, Kantarjian HM, Brümmendorf TH, et al. : Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome–positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 118:4567-4576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hijiya N, Maschan A, Rizzari C, et al. : A phase 2 study of nilotinib in pediatric patients with CML: Long-term update on growth retardation and safety. Blood Adv 5:2925-2934, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbas R, Hsyu PH: Clinical pharmacokinetics and pharmacodynamics of bosutinib. Clin Pharmacokinet 55:1191-1204, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Reinoso-Barbero F, Calvo C, Ruza F, et al. : Reference values of gastric intramucosal pH in children. Paediatr Anaesth 8:135-138, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Hijiya N, Kapoor S, Descamps L, et al. : Trial in progress: A multicenter, open-label, phase ib/II study to determine the dose and safety of asciminib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with ≥1 prior tyrosine kinase inhibitor. Blood 138, 2021. (suppl 1; abstr 2561) [Google Scholar]
- 35.Réa D, Mauro MJ, Boquimpani C, et al. : A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood 138:2031-2041, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossoff J, Huynh V, Rau RE, et al. : Experience with ponatinib in paediatric patients with leukaemia. Br J Haematol 189:363-368, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Millot F, Suttorp M, Versluys AB, et al. : Ponatinib in childhood Philadelphia chromosome-positive leukaemias: An international registry of childhood chronic myeloid leukaemia study. Eur J Cancer 136:107-112, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. : Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: Final 5-year results of the phase 2 PACE trial. Blood 132:393-404, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortes J, Apperley J, Lomaia E, et al. : Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: A randomized, open-label phase 2 clinical trial. Blood 138:2042-2050, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ursino M, Zohar S, Lentz F, et al. : Dose-finding methods for Phase I clinical trials using pharmacokinetics in small populations. Biom J 59:804-825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]




