Abstract
Background
Gamification has been used successfully to promote various desired health behaviors. Previous studies have used gamification to achieve desired health behaviors or facilitate their learning about health.
Objective
In this scoping review, we aimed to describe digital gamified tools that have been implemented or evaluated across various populations to encourage vaccination, as well as any reported effects of identified tools.
Methods
We searched Medline, Embase, CINAHL, the Web of Science Core Collection, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, Academic Search Premier, PsycInfo, Global Health, and ERIC for peer-reviewed papers describing digital gamified tools with or without evaluations. We also conducted web searches with Google to identify digital gamified tools lacking associated publications. We consulted 12 experts in the field of gamification and health behavior to identify any papers or tools we might have missed. We extracted data about the target population of the tools, the interventions themselves (eg, type of digital gamified tool platform, type of disease/vaccine, type and design of study), and any effects of evaluated tools, and we synthesized data narratively.
Results
Of 1402 records, we included 28 (2%) peer-reviewed papers and 10 digital gamified tools lacking associated publications. The experts added 1 digital gamified tool that met the inclusion criteria. Our final data set therefore included 28 peer-reviewed papers and 11 digital gamified tools. Of the 28 peer-reviewed papers, 7 (25%) explained the development of the tool, 16 (57%) described evaluation, and 2 (7%) reported both development and evaluation of the tool. The 28 peer-reviewed papers reported on 25 different tools. Of these 25 digital gamified tools, 11 (44%) were web-based tools, 8 (32%) mobile (native mobile or mobile-enabled web) apps, and 6 (24%) virtual reality tools. Overall, tools that were evaluated showed increases in knowledge and intentions to receive vaccines, mixed effects on attitudes, and positive effects on beliefs. We did not observe discernible advantages of one type of digital gamified tool (web based, mobile, virtual reality) over the others. However, a few studies were randomized controlled trials, and publication bias may have led to such positive effects having a higher likelihood of appearing in the peer-reviewed literature.
Conclusions
Digital gamified tools appear to have potential for improving vaccine uptake by fostering positive beliefs and increasing vaccine-related knowledge and intentions. Encouraging comparative studies of different features or different types of digital gamified tools could advance the field by identifying features or types of tools that yield more positive effects across populations and contexts. Further work in this area should seek to inform the implementation of gamification for vaccine acceptance and promote effective health communication, thus yielding meaningful health and social impacts.
Keywords: digital gamified tools, digital game, vaccination, gamification, vaccine uptake, scoping review, review method, vaccine, gamified, COVID-19, COVID, SARS-CoV-2, health behaviour, health behavior, health promotion, behavior change, behaviour change
Introduction
Vaccination is one of the most cost-effective methods of preventing the spread of vaccine-preventable diseases. If vaccination coverage falls below the thresholds that are safe for the prevention of epidemic transmission, the incidence of vaccine-preventable diseases increases [1,2]. For example, measles returned over the past 2 decades, and the incidence of measles in the European Union increased in 2017-2018 [3].
In 2019, prior to the COVID-19 pandemic, the World Health Organization identified vaccine hesitancy (ie, the reluctance or refusal to be vaccinated despite the availability of vaccination services) as 1 of the top 10 threats to worldwide health [4]. Vaccine hesitancy is one of the several reasons some people are un- or undervaccinated [5-9]. Interventions addressing vaccine hesitancy are therefore necessary to promote vaccine acceptance and uptake. As the contributors of vaccine acceptance are diverse, no single intervention will solve this issue [10]. Multicomponent interventions tailored to local barriers to vaccine acceptance and uptake are known to be the most effective [11,12]. Misinformation and conspiracy theories spread online, where extensive antivaccine content is shared [13-15], potentially negatively influencing views about vaccines [16,17]. Efforts have been made to counter vaccine misinformation and mistrust by targeting various groups, such as parents, non–health care workers [18,19], and adolescents [20], and delivering information about the risks and benefits of different types of vaccines, for instance, human papillomavirus (HPV) vaccination [21] and measles, mumps, and rubella vaccines [22,23]. Along with traditional communication strategies, the use of other strategies to inform and educate about immunizations, for example, with digital gamified tools, may help encourage vaccine uptake.
Gamification is defined as the use of game design elements in nongame contexts [24]. It includes several aspects and features, such as fun interfaces, immediate success or feedback, reward systems (levels, point scores, badges), challenges and competitions, team playing, avatars, and quizzes. Previous studies have used gamification to achieve desired health behaviors [25-27] or facilitate their learning about health [28]. Gamification draws on elements from serious games, meaning fully developed digital games used to train and educate players [29,30]. For example, a serious game “Land of Secret Gardens” facilitates conversations about HPV with preteens. In the game, preteens need to protect their bodies with a “potion,” which offers a metaphor for the HPV vaccine [31]. However, serious games and digital gamified tools are distinct but related concepts. Serious games use gaming as a central and primary medium [32]. In contrast, digital gamified tools (eg, apps) or gamified interventions are not complete game experiences but have gaming features, such as rewards systems, scoring of points, or engaging users in different challenges [33]. In this study, we defined digital gamified tools as digital apps with the aforementioned gaming features. Our definition includes serious games that meet the criteria, that is, they must include such gaming features. This scoping review provides insight into the reported effects of digital gamified tools to increase vaccine uptake. Our review built upon existing reviews in the field by including a comprehensive search of both published literature and online tools, as well as an examination of both the characteristics and the reported effects of these digital tools. This review was distinct in that it focused specifically on digital gamified tools and their effects, rather than simply the effectiveness of gamification in general. In doing so, this review aimed to fill a gap in the literature by providing evidence-based answers to the question of whether gamification “works” to increase vaccine uptake. Therefore, the objectives of this scoping review [34] were, first, to review digital gamified tools that have been implemented or evaluated across various populations to encourage vaccine uptake and, second, to describe any reported effects of the identified tools in terms of influence on users’ knowledge or behavior toward vaccination. Our research questions can therefore be summarized as follows:
What digital gamified tools intended to encourage vaccination exist and have been described in the literature?
Do these tools demonstrate any effects on knowledge, attitudes, beliefs, and behaviors about vaccination?
Methods
Search Strategy
For peer-reviewed papers, we searched Medline (Ovid), Embase (Ovid), CINAHL (EBSCO), the Web of Science Core Collection, the Cochrane Database of Systematic Reviews (Ovid), the Cochrane Central Register of Controlled Trials (Ovid), Academic Search Premier (EBSCO), PsycInfo (Ovid), Global Health (Ovid), and ERIC (Ovid) with no language or date restrictions. The proposed search terms were, for example, “vaccine,” “vaccination,” “immunization,” “video games,” “gamification,” “application,” and “virtual reality” (see Multimedia Appendix 1 for the full search strategy). The search was conducted on January 26 and 27, 2022.
We also conducted an online Google search on May 5, 2022, for any digital tools with gamified features that deliver informative or educative messages on vaccination. The search terms we used were “vaccination,” “immunization,” “electronic game,” “computer game,” “mobile game,” “interactive game,” and “digital game” (see Multimedia Appendix 1 for the full search strategy). We reviewed the first 30 results for each search, as it is rare for users to click past the third page of 10 search results per page, and therefore, researchers analyzing medical content available on the web often use 30 as a threshold [35-37]. On May 6, 2022, we conducted the same searches in private browsing mode to ascertain whether our results had been affected by a “filter bubble” [38], that is, the way Google search results are adapted to one’s previous browsing activity. Our search strategy was constructed and reviewed by 2 librarians. Following the librarians’ advice, we expanded our search strategy to include ERIC and Global Health databases.
Study Selection and Screening Process
We used PICO (Population, Intervention, Comparison, and Outcome) to structure study inclusion and exclusion criteria (Table 1). Our population of interest was the general public or any subgroup, including health care professionals and students. We sought studies describing tools with gamification techniques or gamified elements, including gamified web-based quizzes to deliver informative or educative messages on vaccination. Posters, preprints, editorials, conference proceedings, news bulletins, and paper-based or board games were excluded. Our comparator was any control, including offering no education on vaccination or comparing participants before and after an intervention. Our outcomes of interest included common outcomes associated with vaccine uptake, namely knowledge (comprehension, understanding), attitudes (for or against vaccination), beliefs (perceived benefits, perceived risks), and behaviors toward vaccines (vaccination intention [ie, intention to get vaccinated or not get vaccinated] and vaccine uptake [ie, receiving or not receiving a vaccine]). We excluded papers that did not present the description or evaluation of a concrete digital gamified tool.
Table 1.
Inclusion and exclusion criteria.
| Component | Inclusion criteria | Exclusion criteria | Question related to the criteria |
| Type of report |
|
|
Has the study or research described the development of the tool and evaluated it? |
| Population |
|
N/Aa | Who is the audience for whom the key message was intended? |
| Intervention |
|
|
Does the study or tool aim to deliver an informative or educative message on vaccination? |
| Comparator |
|
N/A | N/A |
| Outcome |
|
|
Has the study or tool been evaluated for the outcomes that encourage vaccine uptake? |
aN/A: not applicable.
For Google-searched digital gamified tools, our inclusion and exclusion criteria used the same specifications regarding population and intervention. We did not apply comparison and outcome criteria to web-based tools because we did not expect these to report evaluation studies.
We reported this review according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (see the PRISMA checklist in Multimedia Appendix 2) [39]. We registered our protocol on the Open Science Framework [40].
Expert Consultations
After extracting information from peer-reviewed papers and tools identified via a Google search, we contacted experts in the field of digital gamified tools (eg, developers and researchers working on the topic in Canada and worldwide who were already known to the research team) to complement our online searches and ensure completeness. Specifically, we sent emails to 12 experts about the results of our searches and asked them to alert us to any games or papers we might have missed.
Data Charting
We developed a form in Microsoft Excel to guide the charting of data. We pretested and reviewed the form with team members to ensure we were accurately and adequately capturing relevant data. Data charting occurred independently with verification. Specifically, a reviewer (author HH) identified and screened all studies and digital gamified tools for their eligibility. Screening results were verified by a second reviewer (author DG). The data charting was then performed by a reviewer (HH) and again verified by a second reviewer (DG). Any conflicts throughout screening or data charting were resolved by a third reviewer (author ED). From the included papers, we charted data about (1) the type and design of study (developmental or evaluation study, user testing, randomized controlled trial, etc), (2) the vaccine(s) addressed (COVID-19, HPV, etc), (3) the purpose of the study or intervention, (4) the digital gamified tool platform (web based, native mobile app, mobile-enabled web app, virtual reality), and (5) the characteristics of study participants. For the evaluated interventions, we charted data about preselected outcomes that are widely used to predict health-related behaviors and to assess outcomes in studies of interventions about vaccination and immunization [11-14]. Specifically, we extracted data about the tools’ effects on knowledge, attitudes, beliefs (perceived benefits, perceived risks), and behavioral intentions. Emotional, cultural, and social factors can also influence a decision about vaccination [29,30]. Therefore, we also extracted data about other outcomes that the studies may have evaluated. Because we sought to understand all possible effects, we did not prespecify any of these as a primary outcome. We organized the extracted data in tables and synthesized them descriptively.
Quality Assessment
To assess the quality of the studies that evaluated their interventions, we used the Mixed Methods Appraisal Tool (MMAT) developed by Pluye et al [41]. Two reviewers independently conducted the quality assessment, resolving disagreements through discussion until reaching a consensus. A third and a fourth reviewer (authors HH and HW) intervened to settle any remaining conflicts.
Data Synthesis
We summarized data using a narrative approach involving framework and content analysis. We classified each digital gamified tool platform using the 4 types of digital gamified tools: web-based tool, native mobile app, mobile-enabled web app, virtual reality tool. For the type of digital gamified tool, we classified web-based tools that explicitly noted their suitability for mobile use (eg, by smartphone or tablet) as mobile-enabled apps. We classified web-based tools without such an explicit statement as web based only, even though they may be functional on mobile devices. For the type and design of study, we grouped randomized designs together, including traditional randomized controlled trials with only 2 study arms and factorial designs with more than 2 study arms. Although these methods are not exactly the same, they all use randomization to minimize potential biases and are therefore functionally equivalent for our purposes of understanding what kinds of evaluations have been undertaken [42]. We summarized the main characteristics of tools, including PICO elements, in a tabular display. We used the PRISMA 2020 flowchart to describe the process of study selection [43].
Results
Papers Identified and Scope of Literature
We identified a total of 2082 records through database searches. After removing duplicates, we screened 1402 (67.3%) database records. Through Google searches, we identified 10 digital gamified tools and 2 papers. In a private browsing mode search, there was no change in search results. Of the 12 experts contacted, 2 (17%) responded and suggested 2 papers and 2 links, of which 1 (50%) digital gamified tool met the inclusion criteria and was included in our review. Through these methods, our final data set included 28 (2%) peer-reviewed papers and 11 digital gamified tools. Figure 1 shows our PRISMA diagram.
Figure 1.
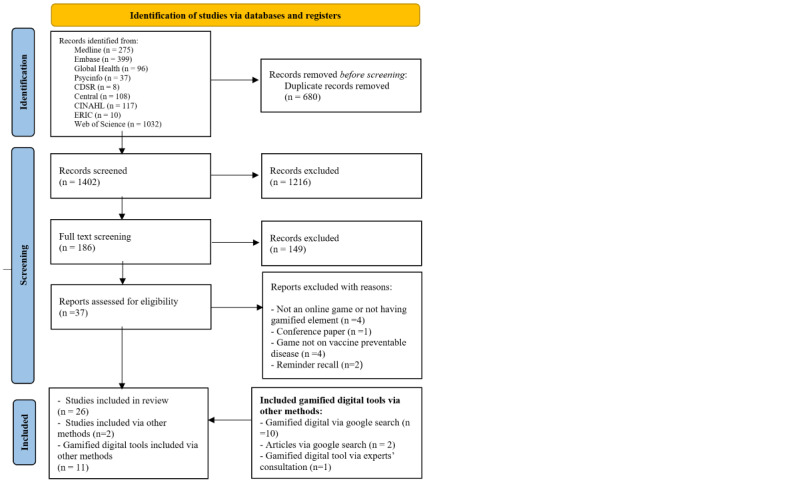
PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Of the 28 peer-reviewed papers, 7 (25%) explained the development of the tool, 16 (57%) described evaluation, and 2 (7%) reported both development and evaluation of the tool (Table 2). To report our results, we grouped studies together that reported the same tool, meaning 28 peer-reviewed papers reporting on 25 different tools. Of these 25 digital gamified tools, 11 (44%) were web-based tools, 7 (28%) mobile (native mobile or mobile-enabled web) apps, 6 (24%) virtual reality tools, and 1 (4%) offered in both mobile and web-based versions (for details, see Table 2). The most common single vaccines addressed in the tools were influenza (n=6, 24%, tools) and HPV (n=6, 24%, tools). Other tools addressed COVID-19 (n=2, 8%); measles, mumps, influenza, and smallpox (n=2, 8%); a hypothetical disease (n=2, 8%); other vaccine-preventable diseases (n=6, 24%); and the role vaccines play in preventing the spread of disease with no particular vaccine specified (n=1, 4%). Of the 10 digital gamified tools identified via a Google search and 1 suggested by the expert (a total of 11 digital gamified tools; see Table 3), the largest group (n=5, 45%) addressed COVID-19, and the rest were about other vaccine-preventable diseases. The 11 gamified elements identified in the Google search and expert feedback identified 6 types of gamified elements: reward points, serious games, physical trading cards, certificates, role-playing, and quizzes (see Table 3). The most common type was reward points, which appeared in 5 (45%) cases. Two cases used serious games, one case used physical trading cards and reward points, one case used certificates, one case used role-playing, and one case used quizzes. Additional characteristics of the studies included (eg, country of origin, sample size, participant characteristics) are detailed in Multimedia Appendix 3 [31,44-70]. The expanded versions of Table 2 [31,44-70] and Table 3 [71-81] are provided in Multimedia Appendix 4.
Table 2.
General information about the studies.
| Type of study and author(s) | Type of digital gamified tool platform | Type of disease/vaccine | Type and design of study (development or evaluation, iterative design, randomized controlled trial, etc) | |||
| Evaluation studies | ||||||
|
|
Betsch and Böhm [44] | Web-based tool | Hypothetical | Evaluation: online experiment | ||
|
|
Carolan et al [45] | Web-based tool | Measles, mumps, influenza, and smallpox | Evaluation: pre-post study | ||
|
|
Cates et al [31] | Web-based tool | HPVa | Evaluation: pilot randomized controlled trial | ||
|
|
Dale et al [46] | Native mobile app | Influenza | Evaluation: nonrandomized trial | ||
|
|
Darville et al [47] | Web-based tool | HPV | Evaluation: randomized controlled trial | ||
|
|
Eley et al [48], McNulty et al [49] | Web-based tool | Bacteria, vaccine-preventable disease | Evaluation: quantitative followed by qualitative research design | ||
|
|
Fadda et al [50], Fadda et al [51] | Native mobile app | MMR vaccines | Evaluation: mixed methods research design | ||
|
|
Ibuka et al [52] | Web-based tool | Hypothetical disease | Evaluation: experimental design | ||
|
|
Kaufman and Flanagan [53] | Web-based tool | Not reported | Evaluation: experimental design | ||
|
|
Lee et al [54] | Native mobile app | Influenza | Evaluation: randomized controlled trial | ||
|
|
Mitchell et al [55], Laplana [56] | Web-based tool | Influenza | Evaluation: pre-post study | ||
|
|
Mottelson et al [57] | Virtual reality tool | COVID-19 | Evaluation: randomized controlled trial (2×2 factorial design) | ||
|
|
Nowak et al [58] | Virtual reality tool | Influenza | Evaluation: one-way between-subjects design with random assignment | ||
|
|
Real et al [59] | Virtual reality tool | Influenza | Evaluation: quasi- randomized controlled trialb | ||
|
|
Woodall et al [60] | Mobile-enabled web app | HPV | Evaluation: clinic-cluster randomized trial | ||
|
|
Vandeweerdt et al [61] | Virtual reality tool | COVID-19 | Evaluation: randomized controlled trial | ||
| Development studies | ||||||
|
|
Amresh et al [62] | Web-based tool | HPV | Development: iterative design | ||
|
|
Bertozzi et al [63] (data extracted for the game related to vaccines) | Web-based tool | Influenza | Development: iterative design | ||
|
|
Carolan et al [64] | Web-based tool | Measles, mumps, influenza, and smallpox | Development: iterative design | ||
|
|
de Araujo Lima et al [66] | Native mobile app | Vaccine-preventable diseases | Development: heuristic evaluation by users, content evaluation by experts | ||
|
|
Kafai et al [65] | Virtual reality | Dragon swooping cough virus to reflect real-life features of infectious viruses, such as Ebola. | Development: user feedback via surveys (asking users questions) and log files (observing user behaviors) | ||
|
|
Real et al [67] | Native mobile app | HPV | Development: usability testing | ||
|
|
Streuli et al [68] | Virtual reality | Pediatric vaccines | Development: Community-based participatory research and co-design | ||
| Development and evaluation studies | ||||||
|
|
Davies et al [69] | Mobile or web app (multiple formats available) | Hepatitis B | Development and evaluation: Participatory Action Research | ||
|
|
Ruiz-López et al [70] | Native mobile app | HPV | Development and evaluation: Iterative design and evaluation via questionnaire | ||
aHPV: human papillomavirus.
bAllocation to a study arm was performed according to work schedules, which are often arbitrary. We therefore considered this quasi-randomization.
Table 3.
Tools from Google search and expert suggestions.
| Digital gamified tool | Type of disease/vaccine | Type of digital gamified tool platform | Gamification elements (eg, rewards, role-playing, leaderboard, serious game) |
| Antidote COVID-19 [71] | COVID-19 | Native mobile app | Reward points |
| The Vaccination Game [72] | H11N7 and influenza | Web-based tool | Serious game |
| Help take down COVID-zilla! [73] | COVID-19 | Web-based tool | Role-playing |
| Just the Vax! [74] | Vaccine-preventable disease | Web-based tool | Reward points |
| COVID Invaders [75] | COVID-19 | Web-based tool | Reward points |
| Vax Pack Hero [76] | Vaccine-preventable disease | Web-based tool | Reward points and physical trading cards |
| Flu's Clues [77] | Influenza | Web-based tool | Certificate of completion for solving the influenza mystery |
| Virus Fighter [78] | COVID-19, influenza, Ebola, measles | Web-based tool | Serious game |
| Immunization411: for preteens and teens’ online training [79] | Tdap meningococcal vaccine, varicella, HPVa, influenza | Web-based tool | Reward points |
| COVID Chronicles [80] | COVID-19 | Web-based tool | Reward points |
| I Boostb [81] | Vaccine-preventable disease | Web-based tool | Quiz |
aHPV: human papillomavirus.
bSuggested by an expert.
The studies were conducted in 26 different countries, with the majority of studies coming from the United States (n=13, 46%, studies) and the United Kingdom (n=5, 18%, studies). Study populations included students at various levels (elementary school to college, specialty programs, eg, nursing and pediatric residency), parents of vaccine-eligible children, adults from the general population, members of particular sociocultural communities (eg, immigrants, Indigenous peoples), and convenience samples, such as players of a game, attendees of a conference, and employees of an organization. Sample sizes ranged from 8 to 50,286. Whenever papers reported study participant characteristics such as age, sex, gender, ethnocultural identity, or socioeconomic levels, we extracted summary data, as shown in Multimedia Appendix 3.
Reported Effects of Evaluated Interventions
In total, 18 (64%) of 28 studies evaluated at least 1 of our outcomes of interest, while 11 (39%) studies reported the effects of the evaluated interventions on more than 1 outcome of interest. Summarized outcomes and their MMAT quality assessments are shown in Table 4. Multimedia Appendix 5 provides full details.
Table 4.
Outcomes of evaluation studies included.
| Type of digital gamified tool platform and study | Knowledge (comprehension/understanding, etc) | Attitudes (for/against vaccination, etc) | Beliefs (risk perceptions, etc) | Behavioral intentions (getting vaccinated or not, etc) | Others (eg, emotions) | MMATa quality score | |||||||
| Web-based tool | |||||||||||||
|
|
Betsch and Böhm [44] | —b | Negative vaccine attitudes with compulsory vaccination | — | Decreased vaccine uptake with compulsory vaccination | Increased level of anger with compulsory vaccination | 60% quality criteria met | ||||||
|
|
Carolan et al [45] | — | No significant effect on attitudes towards vaccination | — | — | Increased confidence in information needs | 80% quality criteria met | ||||||
|
|
Cates et al [31] | Increase in knowledge about immunization | — | — | Positive increase in intentions to vaccinate | Increase in vaccination self-efficacy, decisional balance towards vaccination | 100% quality criteria met | ||||||
|
|
Darville et al [47] | — | — | Positive effects on beliefs towards vaccination | Increase in intentions to vaccinate | — | 60% quality criteria met | ||||||
|
|
Eley et al [48], McNulty et al [49] | Improvements in knowledge about immunization | — | — | — | — | 100% quality criteria met | ||||||
|
|
Ibuka et al [52] | — | — | — | — | Free riding in vaccination decisions decreases vaccine acceptance | 80% quality criteria met | ||||||
|
|
Kaufman and Flanagan [53] | The digital version of the game was less effective at facilitating learning | The digital version of the game was less effective at attitude change | — | — | The digital version of the game was perceived to be complicated to use | 20% quality criteria met | ||||||
|
|
Mitchell et al [55], Laplana [56] | Increase in knowledge | Positive increase in attitudes for vaccination | — | Increase in vaccine uptake after accessing the game | — | 80% quality criteria met (Mitchell et al [55]) | ||||||
| Mobile app | |||||||||||||
|
|
Dale et al [46] | — | — | — | Positive increase in intentions to vaccinate | — | 80% quality criteria met | ||||||
|
|
Fadda et al [50], Fadda et al [51] | Improvements in knowledge about immunization | — | — | Increase in intentions to vaccinate | Increase in psychological empowerment and confidence in the decision | 80% quality criteria met (Fadda et al [50], Fadda et al [51]) | ||||||
|
|
Lee et al [54] | — | — | — | Increase in intentions to vaccinate | — | 80% quality criteria met | ||||||
|
|
Woodall et al [60] | — | — | Increase in beliefs towards vaccination | Increase in intentions to vaccinate | Increase in vaccine confidence | 40% quality criteria met | ||||||
|
|
Ruiz-López et al [70] | Increase in knowledge after playing the game | — | — | — | — | 100% quality criteria met | ||||||
| Virtual reality tool | |||||||||||||
|
|
Mottelson et al [57] | — | — | — | Increase in vaccination intention when both the personal and collective benefit of COVID-19 vaccination was communicated | Increase in COVID-19 empathy, vaccination recommendation, and vaccination readiness | 80% quality criteria met | ||||||
|
|
Nowak et al [58] | — | — | Positive effects on beliefs towards vaccination | Increase in intentions to vaccinate | — | 100% quality criteria met | ||||||
|
|
Real et al [59] | — | Increase in attitudes in favour of vaccination | — | — | — | 60% quality criteria met | ||||||
|
|
Vandeweerdt et al [61] | — | — | — | Increase in intentions to vaccinate | Virtual reality intervention increases a sense of collective responsibility | 100% quality criteria met | ||||||
| Mobile or web app (multiple formats available) | |||||||||||||
|
|
Davies et al [69] | Increase in knowledge about immunization | — | — | — | — | 80% quality criteria met | ||||||
aMMAT: Mixed Methods Appraisal Tool.
bNot reported.
Effects on Knowledge (Includes Comprehension/Understanding, etc)
Overall, the 28 included studies suggested that digital gamified tools may positively influence knowledge. Of 7 (25%) studies that assessed knowledge, 6 (86%) showed an increase in knowledge about immunization in general [31,48,51,55,69,70]. All these 6 (86%) studies were of high quality (≥80%). One study of low quality (≤25%) reported that a digital game is less effective at increasing knowledge compared to its original board game format [53]. When considering only the high-quality (≥80%) studies, we observed that digital gamified tools are associated with increased knowledge.
Effects on Attitudes (for or Against Vaccination)
Overall, digital gamified tools appeared to have mixed effects on attitudes toward vaccination. Of 5 (18%) of 28 studies that assessed attitudes, 2 (40%), one of high quality (≥80%) and the other of medium quality (60%), showed an increase in positive attitudes toward vaccination [55,59]. In addition, 2 (40%) studies, one of high quality (≥80%) and the other of low quality (20%), reported no or less effect on attitudes toward vaccination [45,53], and 1 (20%) study comparing voluntary and compulsory vaccines in a game context showed negative attitudes regarding compulsory vaccination [44]. When considering only the high-quality (≥80%) studies, we observed inconsistent effects on attitudes.
Effects on Beliefs (Perceived Benefits, Perceived Risks)
Overall, digital gamified tools demonstrated positive effects on beliefs toward vaccination. In total, 3 (11%) of 28 studies, 1 (33%) of high quality (100%) and 2 (67%) of medium quality (60% and 40%), evaluated the effects of digital gamified tools on beliefs toward vaccination. All 3 (100%) studies showed positive effects on beliefs toward vaccination [47,58,60]. When considering only the high-quality (≥80%) studies, we observed that digital gamified tools are associated with positive beliefs about vaccines.
Effects on Behavioral Intentions
Overall, the 28 included studies suggested that digital gamified tools may positively influence intentions to receive vaccines. In total, 11 (39%) studies evaluated the effects of digital gamified tools on behavioral intentions with regard to vaccines. Of these 11 studies, 1 (9%) of medium quality (60%) showed a decrease in vaccination intention when compulsory vaccination was introduced within a game context [44], whereas 10 (91%) studies, 3 (30%) of medium quality (60% and 40%) and 7 (70%) of high quality (≥75%), showed increased intentions to vaccinate [31,46,47,51,54,55,57,58,60,61]. When considering only the high-quality (≥80%) studies, digital gamified tools appeared to be consistently associated with increased vaccination intention.
Other Outcomes
In total, 9 (32%) of 28 studies have also evaluated the effects of digital gamified tools on other outcomes. Of these, 4 (44%) studies reported an increase in confidence in vaccines (medium quality=40%) [60], confidence in information needs (high quality=80%) [45], decisional balance in support of vaccination (high quality=100%) [31], and confidence in vaccine decisions (high quality=80%) [50]. In addition, 1 (11%) study of high quality (80%) reported an increase in empathy toward those vulnerable to COVID-19 and vaccination recommendations [57], and 2 (22%) studies of high quality (100% and 80%) reported an increase in vaccination self-efficacy and readiness [31,57]. An increase in psychological empowerment (high quality=80%) [51] and in emotions such as anger toward compulsory vaccination (medium quality=60%) [44] was also reported by 2 (22%) studies. One study of high quality (80%) reported that the concept of free riding decreases vaccine acceptance [52], whereas another study of high quality (100%) reported that virtual reality intervention increases collective responsibility [61]. When considering only the high-quality (≥80%) studies, we observed a variety of positive effects associated with digital gamified tools, including confidence in vaccines, confidence in decisions about vaccines, empathy toward vulnerable people, collective responsibility, psychological empowerment, and vaccination self-efficacy and readiness.
Effects of the Platform (Web Based, Mobile, Virtual Reality)
The study designs of the 28 included papers did not permit us to formally compare the effects of different platforms in a robust way. Upon inspection, there did not appear to be a strong effect of the platform. In other words, we did not observe evidence in favor of web-based, mobile, or virtual reality apps over the other 2 types of apps.
Discussion
Principal Findings
The broad objective of this scoping review was to map the state of the science regarding digital gamified tools and their effects. In other words, we wished to answer a common question at the intersection of public health and digital health: does gamification encourage vaccination and influence knowledge, attitudes, beliefs, and behaviors related to vaccination? By mapping both published literature and tools currently available online, we observed 2 principal findings.
First, our results suggest that gamification can increase predictors of vaccine uptake, such as knowledge, attitudes, beliefs, behaviors, and vaccination intention. This finding is similar to the findings of a previous review by Montagni et al [82] suggesting that gamification can contribute to changed behaviors and improved knowledge of vaccination. Similarly, other reviews have suggested the potential benefits of gamification for non–vaccination-related behavior change, such as a systematic review suggesting that gamification interventions could be a feasible way to improve health-related outcomes among cancer survivors [83] and another review suggesting their effectiveness in improving physical activity [84]. Such previous work became even more relevant during the COVID-19 pandemic, as many jurisdictions sought to optimize vaccine uptake in the context of an “infodemic” (ie, overabundance of information, true, false, and misleading, about the pandemic and recommended preventive behaviors) [85]. Half of the digital gamified tools identified in our web search addressed COVID-19, suggesting an active interest in using a gamified approach in the pandemic context. Recent research by Plechatá et al [86] published after our data extraction steps were complete suggested that explaining the concept of herd immunity with gamification has a positive impact on the COVID-19 vaccination intention.
Second, our review suggests that although gamification has the potential to enhance the impact of education strategies, gamified tools alone may not wholly address gaps in vaccine acceptance and uptake. Although some of the identified tools did increase vaccination, the increases did not fully close gaps between previous and desired vaccine uptake. This finding aligns with those of Tozzi et al [87], which suggested that promising results could be achieved by combining gamification with educative and informative tools to improve immunization programs. This finding also aligns with previous reviews suggesting the use of digital gamified interventions as a public health tool of interest in enhancing vaccine uptake [82,88]. Further research published by Real et al [89] after our systematic search similarly observed that integrating gamification, such as virtual reality, in training modules enhances uptake of the HPV vaccine. Integrating gamified features may work because they make digital tools acceptable and more fun to use and may reduce the chances of people feeling pushed toward vaccination. In parallel, gamification may be a promising strategy for increasing knowledge, skills, and confidence among health professionals engaging in discussions about vaccines with their patients [90,91].
In addition to these findings drawn directly from our review of the included tools, we offer a broader observation based on the contents of this scoping review, along with the larger landscape of vaccine acceptance research: context is key. Although an engaging approach may work for some groups or in some situations, it may be less well accepted among other groups and in other situations. For instance, a casual and approachable style of communication will work for the younger audience to convey vaccine information but might be deemed insufficient to health care professionals in a more formal setting, such as hospitals. A good understanding of the factors associated with low vaccine acceptance at the local level is needed prior to developing gamified tools [92]. Future research in this area should consider possible contextual factors, such as local culture, social and demographic characteristics of users, and different influences on vaccine hesitancy and acceptance in different regions. To help better match games to the context(s) in which they will be played, when developing games, developers and researchers may wish to consider involving potential players from different contexts early and often. This aligns with previous work [93,94] suggesting that involving users earlier in developing tools may help in designing interventions suitable for a targeted context. One of the examples in our review was an intervention by Cates et al [31] designed to explain HPV vaccines to teenagers using a “secret garden” theme. Involving potential game players early in the development of the game may have contributed toward its positive effects on vaccination intention.
The implications of this research extend beyond the immediate reported effects of gamified tools and delve into the strategic dimensions of public health policy and communication efforts. Considering the insights gleaned from the findings, this study supports a comprehensive and well-informed approach to integrating gamification into strategies for promoting vaccination. As gamification continues to demonstrate its potential in enhancing vaccine uptake, it is crucial to navigate this terrain thoughtfully, considering the various factors that influence its impact. This includes not only the technological and behavioral aspects but also the larger sociocultural context in which vaccination decisions are made. Therefore, our study emphasizes the importance of a comprehensive approach that fosters a mutually beneficial relationship between technological innovation, evidence-based strategies, and an intricate understanding of local contexts. This approach has the potential to make gamification a sustainable and adaptable tool in the arsenal of public health interventions, rather than just a passing trend.
The review does not find a clear advantage for any platform in terms of reported effects. It was challenging to measure the impact of the platforms on behavioral outcomes and calls for more focused research to better understand the specific elements within each platform that drive behavior change. In essence, our study suggests that the reported effects of an app may not be solely determined by its platform but rather by the strategic incorporation of mechanics and elements that facilitate the desired behavior change.
Gamification can influence knowledge, attitudes, and beliefs about vaccines, which can affect vaccine uptake. This is consistent with theories of change proposing that cognitive changes can lead to behavioral outcomes. Although our study mainly examines the immediate effects of gamification on these cognitive aspects, it also offers some implications for using gamification as a potentially viable strategy to improve vaccine acceptance.
Strengths and Limitations
Our study has 5 main limitations. First, because we aimed to capture all relevant evidence and examples, as is typical in a scoping review, we included a broad range of study designs and did not draw conclusions about the relative advantages or disadvantages of different game platforms and features. Given the rapid growth within this field of research, it would be difficult to truly prioritize evidence according to quality criteria at this point. In the future, it may be possible to conduct a systematic review and meta-analysis, restricting included studies to randomized experiments or randomized controlled trials. Such future work may include approaches such as a network meta-analysis to allow for comparison of the effects of different game types or game features. Based on the existing literature, it is difficult to conclude whether certain games are more or less likely to achieve their aims. Second, our results may be influenced by publication bias. It is possible that groups that have developed digital gamified tools that showed disappointing results simply did not publish their studies. This bias could lead to an overestimation of the reported effects of these tools. This highlights the importance of further research to fully understand the real impact of these tools and thus accurately inform policy decisions about the development and use of these tools. Third, and related to the previous 2 points, the rapid growth in this area may mean that we missed more recent evidence in literature published after January 2022 and web searches after May 2022. Fourth, the majority of digital gamified tools on vaccination represented in publications and online were developed in high-income countries. This finding aligns with the findings of previous work by Ohannessian et al [88], who also reported a predominance of high-income countries. This may reflect more widespread internet access and resources for developing digital gamified tools in high-income countries. It may also reflect publication bias in the scientific literature (ie, there may be fewer papers written about digital gamified tools in lower-income countries) and online (ie, tools developed and published in lower-income countries may not be ranked highly by search engines and therefore may not have appeared in our web searches). Tools developed in lower-income countries may also take different forms; for example, they may be text message–based interventions (with or without gamification) rather than web-based tools and therefore would be less likely to be identified in web searches. Analog games from high-income countries were similarly excluded from the scope of our study [95]. Nondigital games, such as board and card games, have demonstrated positive impacts on educational knowledge, cognitive function, and social interactions [96,97]. Such games can support diverse learning across subjects and settings, fostering interactions that develop skills, such as computational thinking and teamwork, and have positive impacts on academic achievement and vocabulary acquisition compared to digital games [97-99]. We restricted our scoping review to digital gamified tools because the review was intended to provide an evidence base for digital game development. Although nondigital games are also potentially useful interventions, the implementation and distribution of such interventions is more challenging, especially in a geographically dispersed country, such as Canada. Fifth, and finally, as we used Google and private browsing in Google, there may be a possibility that different search engines would provide different results.
This study also has 2 main strengths. First, by systematically examining the current literature and currently available tools online, we were able to offer an updated overview of the potential effects of including gamification in digital tools about vaccination. Second, by conducting a scoping review to broadly map the literature, future work can more easily identify and select key outcomes for systematic reviews and meta-analyses in this domain.
Conclusion
Digital gamified tools have the potential to improve vaccine uptake by increasing knowledge and promoting positive attitudes, beliefs, behaviors, and vaccination intention. Further evaluations of these innovative digital tools, including head-to-head comparisons of different features and different platforms, will add more knowledge about what works and what does not in order to achieve public health goals more efficiently. In the wider context of health policy, digital gamified tools may be useful components of multifaceted strategies to improve vaccination rates throughout society.
Acknowledgments
This work was supported by a research grant from the Canadian Institutes of Health Research (GA3177725). The authors gratefully acknowledge the assistance of Frédéric Bergeron (librarian) for reviewing the search strategy. We appreciate the assistance of Charles Racine and Créscence Joëlle Mefou Tasong in conducting the quality appraisal of the studies that evaluated their interventions.
Abbreviations
- HPV
human papillomavirus
- MMAT
Mixed Methods Appraisal Tool
- PICO
Population, Intervention, Comparison, and Outcome
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Search strategy.
PRISMA-ScR checklist.
Characteristics of the studies included in the review.
Expanded version of Table 2 (general information about the studies) and Table 3 (tools from Google search and expert suggestions).
MMAT quality assessment. MMAT: Mixed Methods Appraisal Tool.
Footnotes
Authors' Contributions: All authors provided substantial contributions to this paper’s conception and edits and approved the final version of the manuscript.
Conflicts of Interest: None declared.
References
- 1.Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009 May 07;360(19):1981–1988. doi: 10.1056/nejmsa0806477. [DOI] [PubMed] [Google Scholar]
- 2.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016 Mar 15;315(11):1149–1158. doi: 10.1001/jama.2016.1353. https://europepmc.org/abstract/MED/26978210 .2503179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plans-Rubió P. Low percentages of measles vaccination coverage with two doses of vaccine and low herd immunity levels explain measles incidence and persistence of measles in the European Union in 2017-2018. Eur J Clin Microbiol Infect Dis. 2019 Sep 10;38(9):1719–1729. doi: 10.1007/s10096-019-03604-0.10.1007/s10096-019-03604-0 [DOI] [PubMed] [Google Scholar]
- 4.Ten health issues WHO will tackle this year. World Health Organization. [2021-10-04]. http://tinyurl.com/39cd7tuf .
- 5.Attwell K, Betsch C, Dubé E, Sivelä J, Gagneur A, Suggs LS, Picot V, Thomson A. Increasing vaccine acceptance using evidence-based approaches and policies: insights from research on behavioural and social determinants presented at the 7th Annual Vaccine Acceptance Meeting. Int J Infect Dis. 2021 Apr;105:188–193. doi: 10.1016/j.ijid.2021.02.007. https://linkinghub.elsevier.com/retrieve/pii/S1201-9712(21)00093-X .S1201-9712(21)00093-X [DOI] [PubMed] [Google Scholar]
- 6.MacDonald NE, Dube E, Comeau JL. Have vaccine hesitancy models oversimplified a complex problem to our detriment? The Adapted Royal Society of Canada vaccine uptake framework. Vaccine. 2022 Jun 23;40(29):3927–3930. doi: 10.1016/j.vaccine.2022.05.052. https://europepmc.org/abstract/MED/35637069 .S0264-410X(22)00669-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubé Eve, Gagnon D, MacDonald N, Bocquier A, Peretti-Watel P, Verger P. Underlying factors impacting vaccine hesitancy in high income countries: a review of qualitative studies. Expert Rev Vaccines. 2018 Nov;17(11):989–1004. doi: 10.1080/14760584.2018.1541406. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald NE, Comeau J, Dubé. Graham J, Greenwood M, Harmon S, McElhaney J, Meghan McMurtry C, Middleton A, Steenbeek A, Taddio A. Royal society of Canada COVID-19 report: enhancing COVID-19 vaccine acceptance in Canada. FACETS. 2021 Jan 01;6(1):1184–1246. doi: 10.1139/facets-2021-0037. [DOI] [Google Scholar]
- 9.Dolu İ, Turhan Z, Yalnız Dilcen H. COVID-19 vaccine acceptance is associated with vaccine hesitancy, perceived risk and previous vaccination experiences. Disaster Med Public Health Prep. 2021 Dec 23;17:e97. doi: 10.1017/dmp.2021.370. https://europepmc.org/abstract/MED/34937599 .S1935789321003700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behavioural and social drivers of vaccination: tools and practical guidance for achieving high uptake. World Health Organization. [2023-01-10]. http://tinyurl.com/3uteanyc .
- 11.Dubé E, Gagnon D, MacDonald NE, SAGE Working Group on Vaccine Hesitancy Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015 Aug 14;33(34):4191–4203. doi: 10.1016/j.vaccine.2015.04.041. https://linkinghub.elsevier.com/retrieve/pii/S0264-410X(15)00505-8 .S0264-410X(15)00505-8 [DOI] [PubMed] [Google Scholar]
- 12.Omer SB, Benjamin RM, Brewer NT, Buttenheim AM, Callaghan T, Caplan A, Carpiano RM, Clinton C, DiResta R, Elharake JA, Flowers LC, Galvani AP, Lakshmanan R, Maldonado YA, McFadden SM, Mello MM, Opel DJ, Reiss DR, Salmon DA, Schwartz JL, Sharfstein JM, Hotez PJ. Promoting COVID-19 vaccine acceptance: recommendations from the Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA. Lancet. 2021 Dec 11;398(10317):2186–2192. doi: 10.1016/S0140-6736(21)02507-1. https://europepmc.org/abstract/MED/34793741 .S0140-6736(21)02507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills M, Rahal C, Brazel D, Yan J, Gieysztor S. COVID-19 vaccine deployment: behaviour, ethics, misinformation and policy strategies. The Royal Society; the British Academy. [2024-02-10]. http://tinyurl.com/2bc87r5f .
- 14.Oehler RL. On measles, vaccination, social media activism and how to win back our role as our patients’ best advocates. Clin Infect Dis. 2020 Jan 02;70(2):338–340. doi: 10.1093/cid/ciz656.5532609 [DOI] [PubMed] [Google Scholar]
- 15.Tasnim S, Hossain M, Mazumder H. Impact of rumors or misinformation on coronavirus disease (COVID-19) in social media. SocArXiv preprint posted online 2020. doi: 10.31235/osf.io/uf3zn. https://osf.io/preprints/socarxiv/uf3zn/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witteman HO, Zikmund-Fisher BJ. The defining characteristics of Web 2.0 and their potential influence in the online vaccination debate. Vaccine. 2012 May 28;30(25):3734–3740. doi: 10.1016/j.vaccine.2011.12.039.S0264-410X(11)01962-1 [DOI] [PubMed] [Google Scholar]
- 17.Haase N, Schmid P, Betsch C. Impact of disease risk on the narrative bias in vaccination risk perceptions. Psychol Health. 2020 Mar;35(3):346–365. doi: 10.1080/08870446.2019.1630561. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman J, Ryan R, Walsh L, Horey D, Leask J, Robinson P, Hill S. Face-to-face interventions for informing or educating parents about early childhood vaccination. Cochrane Database Syst Rev. 2018 May 08;5(5):CD010038. doi: 10.1002/14651858.CD010038.pub3. https://europepmc.org/abstract/MED/29736980 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lendacki FR, Forst LS, Mehta SD, Kerins JL. COVID-19 vaccination requirements, encouragement and hesitancy among non-health care, non-congregate workers in Chicago: results from the WEVax survey. BMC Public Health. 2023 May 25;23(1):951. doi: 10.1186/s12889-023-15781-x. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-023-15781-x .10.1186/s12889-023-15781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden SD, Moracco KE, Feld AL, Turner KL, DeFrank JT, Brewer NT. Process evaluation of an intervention to increase provision of adolescent vaccines at school health centers. Health Educ Behav. 2014 Dec;41(6):625–632. doi: 10.1177/1090198114531773.1090198114531773 [DOI] [PubMed] [Google Scholar]
- 21.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011 Mar 21;29(14):2537–2541. doi: 10.1016/j.vaccine.2011.01.065.S0264-410X(11)00125-3 [DOI] [PubMed] [Google Scholar]
- 22.Shourie S, Jackson C, Cheater F, Bekker H, Edlin R, Tubeuf S, Harrison W, McAleese E, Schweiger M, Bleasby B, Hammond L. A cluster randomised controlled trial of a web based decision aid to support parents' decisions about their child's measles mumps and rubella (MMR) vaccination. Vaccine. 2013 Dec 05;31(50):6003–6010. doi: 10.1016/j.vaccine.2013.10.025. https://linkinghub.elsevier.com/retrieve/pii/S0264-410X(13)01401-1 .S0264-410X(13)01401-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andriani Y, Rusmil K, Akbar I. Measles-rubella immunization health education using animated videos and text messages via WhatsApp. Midwifery. 2020. [2024-02-10]. https://www.academia.edu/download/84881544/52501-208272-1-PB.pdf .
- 24.Groh F. Gamificationtate of the art definition and utilization. RTMI’12: 4th Seminar on Research Trends in Media Informatics; February 14, 2012; Ulm, Baden-Württemberg. 2012. pp. 39–46. [Google Scholar]
- 25.Lister C, West JH, Cannon B, Sax T, Brodegard D. Just a fad? Gamification in health and fitness apps. JMIR Serious Games. 2014 Aug 04;2(2):e9. doi: 10.2196/games.3413. https://games.jmir.org/2014/2/e9/ v2i2e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson D, Deterding S, Kuhn K, Staneva A, Stoyanov S, Hides L. Gamification for health and wellbeing: a systematic review of the literature. Internet Interv. 2016 Nov;6:89–106. doi: 10.1016/j.invent.2016.10.002. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(16)30038-0 .S2214-7829(16)30038-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sardi L, Idri A, Fernández-Alemán JL. A systematic review of gamification in e-Health. J Biomed Inform. 2017 Jul;71:31–48. doi: 10.1016/j.jbi.2017.05.011. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(17)30106-5 .S1532-0464(17)30106-5 [DOI] [PubMed] [Google Scholar]
- 28.Cugelman B. Gamification: what it is and why it matters to digital health behavior change developers. JMIR Serious Games. 2013 Dec 12;1(1):e3. doi: 10.2196/games.3139. https://games.jmir.org/2013/1/e3/ v1i1e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawyer B, Rejeski D. Serious Games: Improving Public Policy through Game-Based Learning and Simulation. Washington, DC: Woodrow Wilson International Center for Scholars; 2002. [Google Scholar]
- 30.Alvarez J, Djaouti D. An introduction to serious game - definitions and concepts. In: Fauquet-Alekhine P, Soler L, editors. Serious Games & Simulation for Risks Management. Paris: LARSEN Science; 2011. pp. 11–15. [Google Scholar]
- 31.Cates JR, Fuemmeler BF, Stockton LL, Diehl SJ, Crandell JL, Coyne-Beasley T. Evaluation of a serious video game to facilitate conversations about human papillomavirus vaccination for preteens: pilot randomized controlled trial. JMIR Serious Games. 2020 Dec 03;8(4):e16883. doi: 10.2196/16883. https://games.jmir.org/2020/4/e16883/ v8i4e16883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Carvalho CV, Coelho A. Game-based learning, gamification in education and serious games. Computers. 2022 Mar 04;11(3):36. doi: 10.3390/computers11030036. [DOI] [Google Scholar]
- 33.Fleming TM, Bavin L, Stasiak K, Hermansson-Webb E, Merry SN, Cheek C, Lucassen M, Lau HM, Pollmuller B, Hetrick S. Serious games and gamification for mental health: current status and promising directions. Front Psychiatry. 2016;7:215. doi: 10.3389/fpsyt.2016.00215. https://europepmc.org/abstract/MED/28119636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020 Oct;18(10):2119–2126. doi: 10.11124/JBIES-20-00167.02174543-202010000-00004 [DOI] [PubMed] [Google Scholar]
- 35.Hargrave DR, Hargrave UA, Bouffet E. Quality of health information on the internet in pediatric neuro-oncology. Neuro Oncol. 2006 Apr;8(2):175–182. doi: 10.1215/15228517-2005-008. https://europepmc.org/abstract/MED/16533758 .15228517-2005-008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrescu P. Google organic click-through rates in 2014. MOZ Blog. 2014. [2024-02-10]. https://moz.com/blog/google-organic-click-through-rates-in-2014 .
- 37.van der Marel S, Duijvestein M, Hardwick JC, van den Brink GR, Veenendaal R, Hommes DW, Fidder HH. Quality of web-based information on inflammatory bowel diseases. Inflamm Bowel Dis. 2009 Dec;15(12):1891–1896. doi: 10.1002/ibd.20976. [DOI] [PubMed] [Google Scholar]
- 38.Resnick P, Garrett R, Kriplean T, Munson S, Stroud N. Bursting your (filter) bubble: strategies for promoting diverse exposure. CSCW '13: Computer Supported Cooperative Work; February 23-27, 2013; San Antonio, TX. 2013. [DOI] [Google Scholar]
- 39.PRISMA for scoping reviews. PRISMA. [2024-02-10]. http://tinyurl.com/23j8b9aw .
- 40.Gamification tool- enviromental scan. OSF Registries. [2024-02-09]. https://osf.io/nv8af .
- 41.Pluye P, Gagnon M, Griffiths F, Johnson-Lafleur J. A scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in mixed studies reviews. Int J Nurs Stud. 2009 Apr;46(4):529–546. doi: 10.1016/j.ijnurstu.2009.01.009.S0020-7489(09)00014-5 [DOI] [PubMed] [Google Scholar]
- 42.Montgomery AA, Peters TJ, Little P. Design, analysis and presentation of factorial randomised controlled trials. BMC Med Res Methodol. 2003 Nov 24;3:26. doi: 10.1186/1471-2288-3-26. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-3-26 .1471-2288-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=33782057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betsch C, Böhm R. Detrimental effects of introducing partial compulsory vaccination: experimental evidence. Eur J Public Health. 2016 Jun;26(3):378–381. doi: 10.1093/eurpub/ckv154.ckv154 [DOI] [PubMed] [Google Scholar]
- 45.Carolan K, Verran J, Crossley M, Redfern J, Whitton N, Amos M. Impact of educational interventions on adolescent attitudes and knowledge regarding vaccination: a pilot study. PLoS One. 2018;13(1):e0190984. doi: 10.1371/journal.pone.0190984. https://dx.plos.org/10.1371/journal.pone.0190984 .PONE-D-17-28113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dale LP, White L, Mitchell M, Faulkner G. Smartphone app uses loyalty point incentives and push notifications to encourage influenza vaccine uptake. Vaccine. 2019 Jul 26;37(32):4594–4600. doi: 10.1016/j.vaccine.2018.04.018.S0264-410X(18)30487-0 [DOI] [PubMed] [Google Scholar]
- 47.Darville G, Anderson–Lewis C, Stellefson M, Lee Y, MacInnes J, Pigg R, Gilbert J, Thomas S. Customization of avatars in a HPV digital gaming intervention for college-age males: an experimental study. Simul Gaming. 2018 Oct 17;49(5):515–537. doi: 10.1177/1046878118799472. [DOI] [Google Scholar]
- 48.Eley CV, Young VL, Hayes CV, Verlander NQ, McNulty CAM. Young people’s knowledge of antibiotics and vaccinations and increasing this knowledge through gaming: mixed-methods study using e-Bug. JMIR Serious Games. 2019 Feb 01;7(1):e10915. doi: 10.2196/10915. https://games.jmir.org/2019/1/e10915/ v7i1e10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNulty CAM, Lecky DM, Farrell D, Kostkova P, Adriaenssens N, Koprivová Herotová T, Holt J, Touboul P, Merakou K, Koncan R, Olczak-Pienkowska A, Avô AB, Campos J, e-Bug Working Group Overview of e-Bug: an antibiotic and hygiene educational resource for schools. J Antimicrob Chemother. 2011 Jun;66(Suppl 5):v3–12. doi: 10.1093/jac/dkr119. http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=21680584 .dkr119 [DOI] [PubMed] [Google Scholar]
- 50.Fadda M, Galimberti E, Fiordelli M, Romanò L, Zanetti A, Schulz PJ. Effectiveness of a smartphone app to increase parents' knowledge and empowerment in the MMR vaccination decision: a randomized controlled trial. Hum Vaccin Immunother. 2017 Nov 02;13(11):2512–2521. doi: 10.1080/21645515.2017.1360456. https://europepmc.org/abstract/MED/29125783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fadda M, Galimberti E, Fiordelli M, Schulz PJ. Evaluation of a mobile phone–based intervention to increase parents’ knowledge about the measles-mumps-rubella vaccination and their psychological empowerment: mixed-method approach. JMIR Mhealth Uhealth. 2018 Mar 07;6(3):e59. doi: 10.2196/mhealth.8263. https://mhealth.jmir.org/2018/3/e59/ v6i3e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibuka Y, Li M, Vietri J, Chapman GB, Galvani AP. Free-riding behavior in vaccination decisions: an experimental study. PLoS One. 2014;9(1):e87164. doi: 10.1371/journal.pone.0087164. https://dx.plos.org/10.1371/journal.pone.0087164 .PONE-D-13-37383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaufman. Flanagan Lost in translation: comparing the impact of an analog and digital version of a public health game on players? perceptions, attitudes, and cognitions. Int J Gaming Comput Mediat Simul. 2013 Jul;5(3):1–9. doi: 10.4018/jgcms.2013070101. https://www.igi-global.com/article/lost-in-translation/93025 . [DOI] [Google Scholar]
- 54.Lee W, Stück D, Konty K, Rivers C, Brown CR, Zbikowski SM, Foschini L. Large-scale influenza vaccination promotion on a mobile app platform: a randomized controlled trial. Vaccine. 2020 Apr 16;38(18):3508–3514. doi: 10.1016/j.vaccine.2019.11.053.S0264-410X(19)31595-6 [DOI] [PubMed] [Google Scholar]
- 55.Mitchell G, Leonard L, Carter G, Santin O, Brown Wilson C. Evaluation of a 'serious game' on nursing student knowledge and uptake of influenza vaccination. PLoS One. 2021 Jan 14;16(1):e0245389. doi: 10.1371/journal.pone.0245389. https://dx.plos.org/10.1371/journal.pone.0245389 .PONE-D-20-24096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laplana JP. BJN Awards 2019: innovation runner up: creating a staff flu vaccination game. Br J Nurs. 2019 Aug 08;28(15):1032–1034. doi: 10.12968/bjon.2019.28.15.1032. [DOI] [PubMed] [Google Scholar]
- 57.Mottelson A, Vandeweerdt C, Atchapero M, Luong T, Holz C, Böhm R, Makransky G. A self-administered virtual reality intervention increases COVID-19 vaccination intention. Vaccine. 2021 Nov 05;39(46):6746–6753. doi: 10.1016/j.vaccine.2021.10.004. https://linkinghub.elsevier.com/retrieve/pii/S0264-410X(21)01311-6 .S0264-410X(21)01311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nowak GJ, Evans NJ, Wojdynski BW, Ahn SJG, Len-Rios ME, Carera K, Hale S, McFalls D. Using immersive virtual reality to improve the beliefs and intentions of influenza vaccine avoidant 18-to-49-year-olds: considerations, effects, and lessons learned. Vaccine. 2020 Jan 29;38(5):1225–1233. doi: 10.1016/j.vaccine.2019.11.009.S0264-410X(19)31516-6 [DOI] [PubMed] [Google Scholar]
- 59.Real FJ, DeBlasio D, Beck AF, Ollberding NJ, Davis D, Cruse B, Samaan Z, McLinden D, Klein MD. A virtual reality curriculum for pediatric residents decreases rates of influenza vaccine refusal. Acad Pediatr. 2017;17(4):431–435. doi: 10.1016/j.acap.2017.01.010.S1876-2859(17)30012-8 [DOI] [PubMed] [Google Scholar]
- 60.Woodall WG, Zimet G, Kong A, Buller D, Reither J, Chilton L, Myers V, Starling R. Vacteens.org: a mobile web app to improve HPV vaccine uptake. Front Digit Health. 2021;3:693688. doi: 10.3389/fdgth.2021.693688. https://europepmc.org/abstract/MED/34713171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandeweerdt C, Luong T, Atchapero M, Mottelson A, Holz C, Makransky G, Böhm R. Virtual reality reduces COVID-19 vaccine hesitancy in the wild: a randomized trial. Sci Rep. 2022 Mar 17;12(1):4593. doi: 10.1038/s41598-022-08120-4. doi: 10.1038/s41598-022-08120-4.10.1038/s41598-022-08120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amresh A, Chia-Chen A, Baron C. A game based intervention to promote HPV vaccination among adolescents. SeGAH 2019: IEEE 7th International Conference on Serious Games and Applications for Health; August 5-7, 2019; Kyoto, Japan. 2019. [DOI] [Google Scholar]
- 63.Bertozzi E, Krilov L, Walker D. Successful game development partnerships between academics and physicians: two case studies. IJGCMS. 2013 Jul;5(3):97. doi: 10.4018/jgcms.2013070107. [DOI] [Google Scholar]
- 64.Carolan K, Verran J, Amos M, Crossley M, Redfern J, Whitton N, Louttit D. SimFection: a digital resource for vaccination education. J Biol Educ. 2018 May 24;53(2):225–234. doi: 10.1080/00219266.2018.1469534. [DOI] [Google Scholar]
- 65.Kafai Y, Fields D, Giang M, Fefferman N, Sun J, Kunka D, Wong J. Designing for massive engagement in a tween community: participation, prevention, and philanthropy in a virtual epidemic. IDC '17: 2017 Conference on Interaction Design and Children; June 27-30, 2017; Stanford, CA. 2017. [DOI] [Google Scholar]
- 66.de Araujo Lima ID, de Leon CGRMP, Ribeiro LM, da Silva ICR, Vilela DM, Fonseca LMM, de Góes F dos SN, Funghetto SS. A serious game (Immunitates) about immunization: development and validation study. JMIR Serious Games. 2022 Feb 18;10(1):e30738. doi: 10.2196/30738. https://games.jmir.org/2022/1/e30738/ v10i1e30738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Real FJ, Rosen BL, Bishop JM, McDonald S, DeBlasio D, Kreps GL, Klein M, Kahn JA. Usability evaluation of the novel smartphone application, HPV Vaccine: Same Way, Same Day, among pediatric residents. Acad Pediatr. 2021;21(4):742–749. doi: 10.1016/j.acap.2020.11.023.S1876-2859(20)30633-1 [DOI] [PubMed] [Google Scholar]
- 68.Streuli S, Ibrahim N, Mohamed A, Sharma M, Esmailian M, Sezan I, Farrell C, Sawyer M, Meyer D, El-Maleh K, Thamman R, Marchetti A, Lincoln A, Courchesne E, Sahid A, Bhavnani SP. Development of a culturally and linguistically sensitive virtual reality educational platform to improve vaccine acceptance within a refugee population: the SHIFA community engagement-public health innovation programme. BMJ Open. 2021 Sep 14;11(9):e051184. doi: 10.1136/bmjopen-2021-051184. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=34521673 .bmjopen-2021-051184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davies J, Bukulatjpi S, Sharma S, Caldwell L, Johnston V, Davis JS. Development of a culturally appropriate bilingual electronic app about hepatitis B for indigenous Australians: towards shared understandings. JMIR Res Protoc. 2015 Jun 10;4(2):e70. doi: 10.2196/resprot.4216. https://www.researchprotocols.org/2015/2/e70/ v4i2e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz-López Tomás, Sen S, Jakobsen E, Tropé Ameli, Castle PE, Hansen BT, Nygård Mari. FightHPV: design and evaluation of a mobile game to raise awareness about human papillomavirus and nudge people to take action against cervical cancer. JMIR Serious Games. 2019 Apr 08;7(2):e8540. doi: 10.2196/games.8540. https://games.jmir.org/2019/2/e8540/ v7i2e8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.New mobile game in collaboration with WHO raises COVID-19 vaccine awareness. Eurogamer. [2024-02-09]. http://tinyurl.com/4p5snk46 .
- 72.The vaccination game. mrcwimm. [2024-02-09]. https://mrcwimm.itch.io/the-vaccination-game .
- 73.Help take down COVID-zilla!: protect your friends and family. Alberta Health Services. [2024-02-09]. http://tinyurl.com/yp82u75j .
- 74.Just the vax! Vaccine Education Center. [2024-02-09]. https://media.chop.edu/data/files/vaccine-trivia-game/index.html .
- 75.COVID invaders. Get One Desk. [2024-02-09]. https://www.getonedesk.com/covid-invaders .
- 76.Vax pack hero. Vax Pack Hero. [2024-02-09]. https://vaxpackhero.com/
- 77.Flu's clues: virtual flu game. Families Fighting Flu. [2024-02-09]. https://www.familiesfightingflu.org/virtual-flu-game/
- 78.Virus fighter. Virus Fighter. [2024-02-09]. https://www.virusfighter.org/
- 79.Immunization411: for preteens and teens’ online training. Missouri Department of Health and Senior Services. [2024-02-09]. http://tinyurl.com/553ztd28 .
- 80.COVID chronicles. COVID Chronicles. [2024-02-09]. http://tinyurl.com/yc73frrh .
- 81.I boost. Public Health Association of BC. [2024-02-09]. https://iboostimmunity.com/
- 82.Montagni I, Mabchour I, Tzourio C. Digital gamification to enhance vaccine knowledge and uptake: scoping review. JMIR Serious Games. 2020 May 18;8(2):e16983. doi: 10.2196/16983. https://games.jmir.org/2020/2/e16983/ v8i2e16983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ning Y, Jia Z, Zhu R, Ding Y, Wang Q, Han S. Effect and feasibility of gamification interventions for improving physical activity and health-related outcomes in cancer survivors: an early systematic review and meta-analysis. Support Care Cancer. 2022 Dec 30;31(1):92. doi: 10.1007/s00520-022-07550-0.10.1007/s00520-022-07550-0 [DOI] [PubMed] [Google Scholar]
- 84.Mazeas A, Duclos M, Pereira B, Chalabaev A. Evaluating the effectiveness of gamification on physical activity: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2022 Jan 04;24(1):e26779. doi: 10.2196/26779. https://www.jmir.org/2022/1/e26779/ v24i1e26779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Infodemic. World Health Organization. [2023-01-24]. https://www.who.int/health-topics/infodemic .
- 86.Plechatá A, Vandeweerdt C, Atchapero M, Luong T, Holz C, Betsch C, Dietermann B, Schultka Y, Böhm R, Makransky G. Experiencing herd immunity in virtual reality increases COVID-19 vaccination intention: evidence from a large-scale field intervention study. Comput Hum Behav. 2023 Feb;139:107533. doi: 10.1016/j.chb.2022.107533. https://linkinghub.elsevier.com/retrieve/pii/S0747-5632(22)00353-3 .S0747-5632(22)00353-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tozzi AE, Gesualdo F, D'Ambrosio A, Pandolfi E, Agricola E, Lopalco P. Can digital tools be used for improving immunization programs? Front Public Health. 2016;4:36. doi: 10.3389/fpubh.2016.00036. https://europepmc.org/abstract/MED/27014673 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ohannessian R, Yaghobian S, Verger P, Vanhems P. A systematic review of serious video games used for vaccination. Vaccine. 2016 Aug 31;34(38):4478–4483. doi: 10.1016/j.vaccine.2016.07.048.S0264-410X(16)30646-6 [DOI] [PubMed] [Google Scholar]
- 89.Real FJ, Ollberding NJ, Meisman AR, DeBlasio DJ, Pero MB, Davis D, Cruse B, Klein MD, Kahn JA, Rosen BL. Impact of a virtual reality curriculum on human papillomavirus vaccination: a pilot trial. Am J Prev Med. 2022 Nov;63(5):865–873. doi: 10.1016/j.amepre.2022.05.003. https://europepmc.org/abstract/MED/35778065 .S0749-3797(22)00293-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richman AR, Torres E, Wu Q, Eldridge D, Lawson L. The evaluation of a digital health intervention to improve human papillomavirus vaccine recommendation practices of medical students. J Cancer Educ. 2023 Aug 17;38(4):1208–1214. doi: 10.1007/s13187-022-02250-z.10.1007/s13187-022-02250-z [DOI] [PubMed] [Google Scholar]
- 91.Raikhel AV, Blau K, Alberty K, Cornia P, Rodriguez RA, Steinberg KP, Wu C. Vax the Max, a gamification intervention for COVID-19 vaccination task engagement in the inpatient setting. Am J Med Qual. 2023;38(1):47–56. doi: 10.1097/JMQ.0000000000000094. https://europepmc.org/abstract/MED/36472420 .00008488-202301000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017 Dec;18(3):149–207. doi: 10.1177/1529100618760521. [DOI] [PubMed] [Google Scholar]
- 93.Vaisson G, Provencher T, Dugas M, Trottier M, Chipenda Dansokho S, Colquhoun H, Fagerlin A, Giguere AMC, Hakim H, Haslett L, Hoffman AS, Ivers NM, Julien A, Légaré F, Renaud J, Stacey D, Volk RJ, Witteman HO. User involvement in the design and development of patient decision aids and other personal health tools: a systematic review. Med Decis Making. 2021 Apr;41(3):261–274. doi: 10.1177/0272989X20984134. [DOI] [PubMed] [Google Scholar]
- 94.Hakim H, Bettinger JA, Chambers CT, Driedger SM, Dubé E, Gavaruzzi T, Giguere AMC, Kavanagh É, Leask J, MacDonald SE, Orji R, Parent E, Paquette J, Roberge J, Sander B, Scherer AM, Tremblay-Breault M, Wilson K, Reinharz D, Witteman HO. A web application about herd immunity using personalized avatars: development study. J Med Internet Res. 2020 Oct 30;22(10):e20113. doi: 10.2196/20113. https://www.jmir.org/2020/10/e20113/ v22i10e20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Epstein D. Game design and behaviour change using Vaxcards: a collectable card game to increase vaccine confidence. Monash University. 2022. [2024-02-10]. http://tinyurl.com/bdhxbbfj .
- 96.Noda S, Shirotsuki K, Nakao M. The effectiveness of intervention with board games: a systematic review. Biopsychosoc Med. 2019;13:22. doi: 10.1186/s13030-019-0164-1. https://bpsmedicine.biomedcentral.com/articles/10.1186/s13030-019-0164-1 .164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bayeck R. Examining board gameplay and learning: a multidisciplinary review of recent research. Simul Gaming. 2020 Apr 16;51(4):411–431. doi: 10.1177/1046878119901286. [DOI] [Google Scholar]
- 98.Talan T, Doğan Y, Batdı V. Efficiency of digital and non-digital educational games: a comparative meta-analysis and a meta-thematic analysis. J Res Technol Educ. 2020 Apr 20;52(4):474–514. doi: 10.1080/15391523.2020.1743798. [DOI] [Google Scholar]
- 99.Naderi S, Moafian F. The victory of a non-digital game over a digital one in vocabulary learning. Comput Educ Open. 2023 Dec;4:100135. doi: 10.1016/j.caeo.2023.100135. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.
PRISMA-ScR checklist.
Characteristics of the studies included in the review.
Expanded version of Table 2 (general information about the studies) and Table 3 (tools from Google search and expert suggestions).
MMAT quality assessment. MMAT: Mixed Methods Appraisal Tool.


