Abstract
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a treatable autoimmune disorder, for which different treatment options are available. Current first-line evidence-based therapies for CIDP include intravenous and subcutaneous immunoglobulins, corticosteroids and plasma exchanges. Despite lack of evidence, cyclophosphamide, rituximab and mycophenolate mofetil are commonly used in circumstances of refractoriness and, more debatably, of perceived overdependence on first-line therapies. Rituximab is currently the object of a randomized controlled trial for CIDP. Based on case series, and although rarely considered, haematopoietic autologous stem cell transplants may be effective in refractory disease, with low mortality and high remission rates. A new therapeutic option has appeared with efgartigimod, a neonatal Fc receptor blocker, recently shown to significantly lower relapse rate versus placebo, after withdrawal from previous immunotherapy. Other neonatal Fc receptor blockers, nipocalimab and batoclimab, are under study. The C1 complement-inhibitor SAR445088, acting in the proximal portion of the classical complement system, is currently the subject of a new study in treatment-responsive, refractory and treatment-naïve subjects. Finally, Bruton Tyrosine Kinase inhibitors, which exert anti-B cell effects, may represent another future research avenue. The widening of the therapeutic armamentarium enhances the need for improved evaluation of treatment effects and reliable biomarkers in CIDP.
Keywords: CIDP, immunoglobulins, corticosteroids, plasma exchange, efgartigimod
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is the most common chronic autoimmune peripheral nervous system disorder. The prevalence of CIDP has been reported to vary widely in different populations, with differences likely relating, in part, to use of different diagnostic criteria.1 An estimated worldwide prevalence of about 3 per 100,000 has been described.2 The incidence of CIDP is less than 1 per 100,000 per year. The latest European Academy of Neurology/Peripheral Nerve Society Guidelines published in 2021 have provided up-to-date directives and guidance on diagnosis and treatment of CIDP.3 Typical CIDP presents with symmetrical motor and sensory dysfunction of proximal and distal regions of the four limbs progressing over more than 8 weeks.3,4 CIDP may also present in variant forms, which can be focal, multifocal, distal, pure motor or motor-predominant, pure sensory or sensory-predominant. Electrophysiological support for the diagnosis allows further categorisation in what is either “CIDP” or “possible CIDP”. A number of supportive criteria may contribute to increasing the level of certainty of the diagnosis. These include cerebrospinal fluid (CSF) protein level, nerve ultrasonography, magnetic resonance (MR) neurography, nerve pathology as well as response to treatment.3 Of importance, the rate of misdiagnosis of CIDP is high, with overdiagnosis but also underdiagnosis being well-described, resulting from clinical and electrophysiological errors of interpretation.5–8 The implications on treatment are clear and may be considerable.
Evidence-based first-line treatments for CIDP include immunoglobulins, administered through intravenous (IVIg) or subcutaneous routes (SCIg), corticosteroids, given orally or intravenously, and plasma exchanges.3,9 Modalities of administration however vary, with the focus on using minimal effective doses and/or procedure frequencies. Although efficacious in the majority of subjects with CIDP, administered alone or in combination, other treatment avenues have been, are, and will be explored in future. Few are already frequently used in many centres. The justifications for this are multiple and include primarily refractoriness to first-line therapies, residual disability in ameliorated diseases, side-effects to available treatments, as well as, more debatably, their cost and availability, particularly when long-term therapy is required.10
Table 1 details the treatments for CIDP supported by randomized controlled trial (RCT) evidence. These, as well as available agents of unproven efficacy or under current investigation, are detailed in this review.
Table 1.
Randomized Controlled Trial Evidenced-Based Treatments for CIDP (Excluding Comparative Studies)
| Treatment | Trial(s) | Primary Endpoint | Overall Results |
|---|---|---|---|
| IVIg | - Vermeulen et al, 1993 - Hahn et al, 1996 - Thompson et al, 1996 - Mendell et al, 2001 - Hughes et al, 2008 |
MRS; Responder rate vs placebo NDS/CG/GS; cross-over vs placebo Timed 10 m walk/9-hole peg test/Hammersmith Disability Scale; cross-over vs placebo AMS; vs placebo INCAT; response-conditional cross-over vs placebo |
Favour IVIg over placebo |
| SCIg | - Markvardsen et al, 2013 - van Schaik et al, 2018 - ADVANCE CIDP-1, 2023 |
IKS in SCIg-treated vs placebo-treated, previous IVIg-responders INCAT-defined relapse rate in previous IVIg-responders; in 2 doses SCIg vs placebo INCAT-defined relapse rate in fSCIg-treated vs placebo-treated subjects, previously responsive to IVIg |
Favour SCIg over placebo |
| Corticosteroids | - Dyck et al, 1982 | NIS; unblinded; improvement comparing mean scores of treated vs no-treatment groups | Favour corticosteroids over no-treatment (not in ITT) |
| Plasma Exchange | - Dyck et al, 1986 - Hahn et al, 1996 |
NDS, proportion of improved subjects; parallel group vs sham PE NIS, mean group score change; cross-over design vs sham PE |
Favour plasma exchange vs sham plasma exchange |
| Efgartigimod alpha and hyaluronidase-qvfc (VYVGART Hytrulo) | - ADHERE, 2023 | INCAT-defined relapse rate vs placebo in previous responders | Favour VYVGART Hytrulo over placebo |
Abbreviations: AMS, average muscle strength; CG, clinical grade; fSCIg, facilitated SCIg; GS, grip strength; IKS, isokinetic strength; INCAT, inflammatory neuropathy cause and treatment; IVIg, intravenous immunoglobulin; ITT, intention to treat analysis; MRS, modified Rankin Scale; NIS, Neuropathy Impairment Scale; NDS, Neuropathy Disability Scale; SCIg, subcutaneous immunoglobulin.
Immunoglobulins for CIDP
The mechanisms through which immunoglobulins exert a therapeutic effect in CIDP are multiple and complex. They include inhibition of macrophage-induced demyelination, neutralization of pathogenic antibodies, anti-cytokine effects, inhibition of pathogenic antibody production and increase in catabolism, complement inhibition and regulatory T-cell effects.11,12
Five randomized controlled trials (RCTs) have demonstrated the efficacy of IVIg versus placebo, using parallel group or cross-over designs.13–18 All utilized one dose of IVIg of 2g/kg, administered over 2 or 5 days, with one long-term study, also using this dose initially but followed by a maintenance dose of 1g/kg every 3 weeks.18 All defined their primary outcome measure by improvement of disability using different scales. The efficacy of IVIg was demonstrated versus placebo in the short term, as early as within 6 weeks of initiation, as well as at 24 weeks. Of those five RCTs, one demonstrated long-term IVIg efficacy over 48 weeks.18
Three studies have been performed comparing IVIg to corticosteroids. These findings favored IVIg in one cross-over trial considering the primary disability outcome with, however, non-significant IVIg superiority.19 In another parallel group trial, a result significantly favoring IVIg was reported considering the chosen primary outcome of treatment discontinuation due to inefficacy or side-effects at 6 months.20 However, the proportion of responders at 2 weeks was similar in both groups. Although modified Rankin Scores were significantly improved in both groups at 6 months, analysis of inter-group comparison was impossible in view of treatment switch in non-responders. Hence, none of these 3 trials provided definite evidence for IVIg superiority vs corticosteroids on improving disability. An observer-blind trial compared plasma exchange to IVIg and found no difference in disability scores or weakness between the 2 treatments administered over 6 weeks.21
Quality of Life measures have also been shown to improve with IVIg, correlating with functional improvement.22 IVIg does not have demonstrated effects on stamina, fatigue, sensory symptoms, or pain. However, and in practice, these features are frequently considered as markers of treatment response by patients and occasionally also by physicians. This may therefore, as a result, influence and impact on therapeutic decisions, irrespective of true functional change.23
Although randomized controlled trials have used actual body weight to calculate dose administered, there are data supporting the use of ideal or dosing weight. Subsequent tailoring to individual needs is appropriate, although with variations in methodology, including switching to subcutaneous administration. In initial stages, IVIg is given as per routine practice, at a dose of 2 g/kg of ideal body weight, or what is known as “dosing weight” (which is calculated by adding 40% of the ideal body weight, except if the recorded weight is less or equal to the ideal body weight, in which case the dosing weight is considered equal to the recorded weight).24,25 This is because high doses calculated due to overweight/obese range BMIs do not produce better outcomes,26 while they may cause more side-effects27 and result in increased costs.24 Long-term dose requirements are not associated with body weight.26,28 Precise treatment modalities vary. In our unit, we administer two courses at 2 g/kg of dosing weight at a 4-week interval, after which we determine the ideal infusion frequency, by awaiting signs of deterioration before re-treating. Subsequently, after maintaining a dose of at 2g/kg per course until optimal benefit, corresponding ideally to recovery of pre-disease functional level, 15–25% dose reductions are performed every 2–3 courses, until, if this is achievable, complete wean off treatment.24 Of note, different studies have shown that up to 25–50% of patients requiring treatment for CIDP eventually go into remission, which obviously may go unnoticed if treatment is continued without alteration.24,29 It is debatable as to whether gradual weaning off immunoglobulins is preferable to abrupt interruption. Although the latter method has been advocated,30 this may on occasion, in our experience, result in major, not always subsequently fully reversible decline, with physical and psychological consequences. A recent Dutch study demonstrated that IVIg treatment withdrawal, performed progressively in steps of 25% dose reductions per course, was safe with regard to effective subsequent re-stabilization in case of relapse, bringing support to gradual IVIg wean.31 The above-mentioned IVIg dosing protocol that we use for CIDP furthermore allows substantial savings of 10–25%, of obvious importance considering cost and availability.24 There are otherwise reports of effective instant switching to administering 0.5g/kg every week, after a starting dose of 2 g/kg, with good results and tolerability. These are thought to be linked to lesser fluctuations of serum IgG levels.32 With this approach, dose reduction and treatment cessation may later be likewise considered.
Use of sub-cutaneous immunoglobulin (SCIg) has become widespread in recent years. A 12-week RCT of SCIg vs placebo was conducted between 2010 and 2011, in 30 Danish subjects with previously IVIg-responsive CIDP.33 Significant improvement in a number of measures including isokinetic strength, MRC scores and grip strength as well as disability was seen in the SCIg treated group compared to placebo, and SCIg was well-tolerated. Confirmation of these findings was subsequently brought by a large international RCT of 172 subjects from 69 centres, which demonstrated efficacy of 0.2 g/kg weekly SCIg versus placebo in preventing relapse in IVIg-responsive CIDP, with no additional benefit with a higher dose of 0.4 g/kg weekly.34 Otherwise, a 20-week randomized, single-blind, cross-over study demonstrated equivalence of SCIg and IVIg in treatment-naïve patients on motor performance although with earlier maximal improvement with IVIg.35 More recently, a large RCT of 132 subjects of hyaluronidase-facilitated SCIg 10% (ADVANCE-CIDP 1) has demonstrated its efficacy in a >20% relapse rate reduction versus placebo, in subjects with CIDP confirmed as previously IVIg-responsive.36 An IVIg-dependency test was however not used pre-inclusion, implying that a proportion of recruited participants may have been in remission. The main advantage of hyaluronidase-facilitated SCIg versus conventional SCIg is that it allows to help overcome the issue of the maximal volume that can be infused into the sub-cutaneous space by aiding dispersion of SCIg and its absorption into lymphatics.37 This enables reduction in frequency of infusions (which can be as infrequent as 4-weekly, instead of weekly), as well as infusion duration and number of needlesticks required. A continuation long-term 6-year follow-up study of safety profile is now ongoing (ADVANCE-CIDP 3).
The evidence for switching patients from IVIg to more convenient, home- and self-administered SCIg is hence today strong, and this is now available in most European and North American centres.
Plasma Exchange for CIDP
Plasma exchange is a therapeutic procedure utilized in antibody-mediated disease, which separates and then removes plasma from blood, in order to filter out pathogenic agents. Plasma exchange preferentially eliminates biologic substances of high molecular weight such as antibodies and antigen–antibody complexes.38
The evidence base for plasma exchange in CIDP comes from two RCTs, published in 198639 and 1996,40 which had included a total of just 52 participants. The first was a parallel group, and the second was a cross-over design. Both used the Neuropathy Impairment Score (NIS) as a secondary outcome and compared plasma exchange with sham plasma exchange, twice weekly for 3 weeks in the first, and 10 times over 4 weeks in the second. Both studies indicated improved short-term outcome with plasma exchanges, with the second indicating subsequent re-deterioration in the majority within the following 8 weeks.41 Short-term effects were also reported in earlier observational studies. This suggests concurrent therapies may be needed with plasma exchange and corticosteroids are often used in association, although whether they require systematic consideration is unproven. In our practice, we have however found that over 80% of plasma exchange-responders did not require additional therapies, with the rate and frequency of dependence being no different to those of subjects on long-term IVIg (unpublished data). Plasma exchanges are hence a proven and useful option in the treatment of CIDP and may be particularly helpful in case of refractoriness to corticosteroids and immunoglobulins, as well as high dependency on high doses of the former, which may frequently lead to intolerable side-effects. The lack of availability of plasma exchange may result in premature consideration of immunosuppressant therapy, which given the lack of evidence base, is unfortunate as well as inappropriate. More than unresponsiveness, a common anxiety for the treating neurologist can, in actual fact, be that of clear benefit with plasma exchanges justifying continuation, as long-term treatment is in practice may be laborious to arrange in many units. The limited access to plasma exchanges often results from the use of central lines, which themselves increase procedure risk.42 This may be greatly improved by use of peripheral line plasma exchange, shown to be safe and efficient,43 which opens up the possibility of out-patient procedures, instead of requiring in-patient admission.
In practice, plasma exchange is used in variable protocols in different units. This frequently mainly relates to local restrictions concerning availability as well as delivery of the procedure. In our service, we usually initially opt for four plasma exchanges over a couple of weeks, preferably through peripheral venous access. This is repeated at intervals of 2 to 4 weeks until optimal improvement is achieved. Subsequently, treatment frequency is reduced depending on individual requirements, with the ultimate objective being to stop treatment, if remission is achieved.
Corticosteroids for CIDP
Anti-inflammatory and immunosuppressive effects of corticosteroids are mediated by genomic effects that can result in increased production of anti-inflammatory proteins and reduced production of pro-inflammatory proteins.44 Corticosteroids also have rapid direct non-genomic mechanisms likely through heterogeneous receptors and pathways with similarly diverse impacts.45
Corticosteroids were the first described treatment, found to be dramatically effective for CIDP by Austin in 1958,46 in patients who relapsed after treatment withdrawal. Since then, corticosteroids have been extensively used worldwide to treat the disorder. Several retrospective studies have, in the last decades, demonstrated corticosteroid effectiveness,47–50 which have re-inforced the belief of their appropriateness, including as first-line therapy, particularly in view of the cost and availability of existing alternatives.
There is, however, very limited RCT-based evidence for corticosteroids for CIDP. A single unblinded RCT of only 35 subjects of oral prednisolone vs no treatment demonstrated non-significant superiority of corticosteroid therapy considering global group changes in Neuropathy Impairment Scores (NIS), with borderline significant superiority, but only considering numbers of improved subjects excluding patients having not completed the study, but not with an intention-to-treat analysis.51
A few studies have shown equivalence of corticosteroids to intravenous immunoglobulins, themselves of RCT-proven benefit in CIDP, as discussed elsewhere in this paper. One RCT, the PREDICT study, compared daily oral prednisolone with monthly pulse oral dexamethasone.52 The primary outcome was the proportion of patients achieving remission without treatment at 12 months. This study of 41 subjects showed no difference in the primary outcome or in any of multiple secondary outcomes, which included strength, sensory and quality of life measures. Importantly, however, monthly dexamethasone was significantly prompter in resulting in improvement (median time of 17 weeks vs 39 weeks). In terms of side-effect profile, sleeplessness, cushingoid facies, as well as marked weight gain (>3 kg) were found to be commoner with daily prednisolone.
Of note, corticosteroids may allow longer therapy-free remission or increased remission rates, than achieved with IVIg.53,54 This is an important consideration in therapeutic decision-making as it may justify using corticosteroids as a first-line treatment in subjects without contra-indications.
When using corticosteroids, it consequently appears that existing evidence from the PREDICT study supports pulse therapy, which offers greater speed of action as well as fewer side-effects. Oral dexamethasone furthermore requires no hospital visit. In our practice, based on above-mentioned trials, we routinely administer 4-weekly courses of 500 mg of intravenous methylprednisolone daily for four consecutive days20 or 40 mg of oral dexamethasone daily for 4 consecutive days.52 This is continued until optimal improvement occurs, not exceeding however a total of six courses. This may eventually be repeated after careful risk assessment. Gastric and bone protection is essential in patients on long-term steroid therapy.
A multicentre RCT of the association of IVIg and corticosteroids, the OPTIC study,55 based on the rationale of combining the advantages of the two treatments, in particular prompt effect for IVIg and increased chance/duration of remission for corticosteroids, was started but unfortunately recently suspended. Publication of further details is awaited.
Immunosuppression for CIDP
Only few RCTs were performed to evaluate the efficacy of immunosuppressant agents for CIDP, specifically with azathioprine,56 interferon β-1a,57 methotrexate58 and fingolimod,59 none of which demonstrated benefit. In spite of these trial results, azathioprine and methotrexate, probably as a result of their ongoing widespread use for other autoimmune disorders and overall perceived favourable side-effect profile, remain in use worldwide, particularly in lower-income countries.60 Cyclosporine and mycophenolate mofetil are also used in practice on a frequent basis, despite only limited data coming from case series or case reports.10 Other agents including interferon-α, alemtuzumab, natalizumab, etanercept, fludarabine and tacrolimus, have been described as occasionally effective, although they are not of common use in clinical practice.10
Cyclophosphamide has been described as effective in case series, most convincingly by Good et al, in a cohort of 15 subjects refractory to all 3 first-line therapies, where the drug was used monthly in association with high-dose corticosteroids.61 Amelioration was achieved within a mean of 4 months, complete remission in 11/15 (73.3%). Other reports of smaller series indicated subsequently similar results, in addition to, as detailed in recent systematic review and meta-analysis62, an unpublished conference abstract from China reporting an 88% responder-rate in 32 treated subjects. As a result, cyclophosphamide is today frequently utilized as first immunosuppressant in severe, refractory CIDP, in many centres.
Rituximab has been found to be effective in CIDP as reported by numerous case-series throughout the world, particularly in situations of refractoriness. Rituximab has shown efficacy in autoimmune neuropathy with paranodal antibodies,63 which are now no longer included in the CIDP spectrum, as per EAN/PNS Guidelines of 2021.3 An earlier report of 13 cases of CIDP from Italy, partly refractory and partly with high IVIg or plasma exchange requirements, described a responder rate of 70%, within a median of 2 months, lasting up to 1 year.64 Multiple other similarly retrospective reports from other countries documented similar rates.65 An ongoing Italian RCT (Eudra-CT: 2017–005034-36) aims to assess the efficacy of rituximab in allowing suspension of IVIg therapy without clinical worsening in CIDP.66
In practice, cyclophosphamide may be given through the intravenous route at a dose of 1 g/m2. Unless early and major improvement occurs, this is continued every month, for up to 6 months. Use of concurrent high-dose corticosteroids is routine in many units. However, it is noteworthy that the latest meta-analysis indicated associated corticosteroid therapy did not bring additional benefit.62 Repeating treatment with cyclophosphamide requires careful consideration of the side-effect risk versus the limited evidence base. Rituximab is given in CIDP in doses of 2 g in total over 2 weeks or 375 mg/m2 administered weekly for 4 weeks. Repeat treatment may be considered, but it is not always essential in patients for whom complete, or near-complete remission has occurred. Systematic further courses of treatment may not be required, expose to a greater side-effect risk and interestingly have only been used in one case out of every 12 published.65
Haematopoietic Autologous Stem Cell Transplant (ASCT) for CIDP
ASCT represents a further step in the immunosuppressive therapy available for CIDP. Small case series were reported as showing benefit of ASCT in patients with refractory CIDP, on disability level and electrophysiological parameters.67,68 A single-centre open-label large prospective study was reported in 2020 on 66 subjects with CIDP, of a mean age of 43 years, treated with ASCT.69 With regard to the widely perceived morbidity and mortality associated with ASCT, no treatment-related deaths occurred, there was a 5% rate of severe toxicity and no early opportunistic infections. The rate of treatment-free remission was of 80% at 6 months post-ASCT and remained stable at 5 years and the rate of assistance-free ambulation increased from 32% pre-ASCT to >80% after 1 year, remaining then stable up to 5 years. MRC scores, grip strength, INCAT scores and HR-QoL, all improved significantly. Of note, besides being of younger age by over 10 years compared to CIDP cohorts generally reported, subjects in this study had not, for their majority, received cyclophosphamide or rituximab. Two further case series of a total of 9 patients were published in 2021.70,71 A recent meta-analysis of a published series up to December 2022, evaluating 11 studies and 89 treated cases of a mean age of 42.1 years, found a response rate of 86%, remission rate of 85% and a rate of treatment-freedom post-ASCT of 81%.72 There were no ASCT-related deaths, although early neutropenic sepsis occurred in >30% of cases and long-term upper respiratory tract infections in >20%. Only 19/89 subjects had received cyclophosphamide as 2nd line treatment pre-ASCT, and only 18/89 had received rituximab, totalling less than 1 in 2 for these 2 agents. The results of this meta-analysis hence supported relative safety of ASCT, but specifically in younger patients, and also indirectly raised the question of its appropriateness when cyclophosphamide and rituximab have not been tried.
Potential Novel Treatments for CIDP
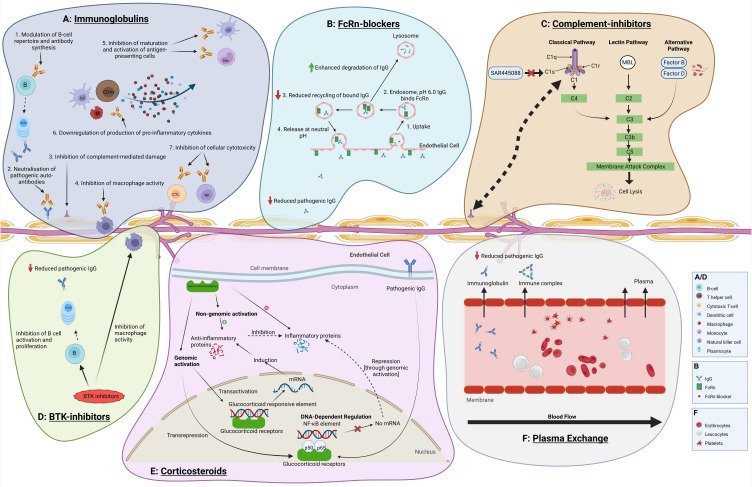
Figure 1 summarises the postulated mechanisms of action of current vs novel treatments for CIDP.
Figure 1.
Postulated mechanisms of action of treatments in CIDP: current vs novel. Created with Biorender.com. (A) Immunoglobulins are postulated to exert their action in multiple ways in CIDP: they modulate B-cell repertoire with impact upon antibody production, neutralise pathogenic antibodies, inhibit complement, suppress macrophage-mediated demyelination, downregulate production of inflammatory cytokines and inhibit antigen-presenting cells as well as cellular cytotoxicity. (B) FcRn-blockers exert their action in reducing binding of pathogenic antibodies to the FcRn. This reduces the protective effect of the FcRn on these antibodies from lysosomal degradation, and hence reducing auto-antibody serum life-span and pathogenic effects in CIDP. (C) Complement inhibitors selectively block downstream complement activation involved in the inflammatory processes causing demyelinating damage. (D) Corticosteroid act through genomic effects leading to increased production of anti-inflammatory proteins and reduced production of pro-inflammatory proteins, as well as rapid direct non-genomic anti-inflammatory effects, through heterogeneous receptors. (E) BTK-inhibitors inhibit B-cell activation and proliferation, and consequently pathogenic auto-antibody production, and reduce macrophage activity. (F) Plasma Exchange primarily removes pathogenic auto-antibodies from blood circulation.
FcRn Blockers
Monoclonal antibodies targeting the neonatal Fc receptor (FcRn) have been tried with success in other autoimmune disorders such as myasthenia gravis (MG).73 These monoclonal antibodies work by reducing binding of endogenous immunoglobulin, including pathogenic, to the FcRn. This reduced binding negates the protective effect of FcRn on endogenous immunoglobulin from lysosomal degradation, hence reducing their serum lifespan, with, consequently, favourable effects on the underlying auto-antibody-mediated disease.74
Efgartigimod, a humanized IgG1 Fc fragment blocking the FcRn, has recently been the subject of an RCT in CIDP, the ADHERE Study. The results of the study were just released by Argenx in July 2023.75 The ADHERE Study was a large multicentre, international two-staged trial of Efgartigimod alfa and hyaluronidase, to facilitate sub-cutaneous administration, in CIDP. Patients initially entered a run-in period during which previous treatment was stopped with mandatory clinical deterioration leading on to inclusion in stage A, during which the drug would be administered on an open-label basis. If amelioration occurred, patients then moved on to stage B, where they would be randomized to the drug or placebo for up to 48 weeks. The primary outcome was the proportion of relapses, which occurred in the two groups. The primary end-point was achieved with Efgartigimod alfa significantly reducing the risk of CIDP relapse in stage B by 61% (HR: 0.39; 95% CI: 0.25–0.61). Among other notable findings were a 78% improvement rate in subjects having received at least 4 injections of the active drug to reach full IgG-lowering effect, and meaningful improvements in stage A, reaching means of 7.7 points on the Inflammatory Rasch-Built Overall Disability Score (I-RODS) and of 12.3 KPa on grip strength, therefore approaching/reaching published minimal clinically important differences for these scales.76,77 However, also noteworthy was the high placebo response rate in stage B, of 46% at week 24 and 40% at week 48, despite the study's run-in period that ensured recruitment only of subjects with confirmed dependency on previous treatment. The full detailed results of the ADHERE Study are awaited.
Rozanolixizumab is a high affinity human anti-FcRn IgG4 monoclonal antibody, which has been the subject of an RCT for CIDP, which ended in March 2021. The results, still unpublished, appeared negative for the primary outcome, based on change from baseline to week 13 of the I-RODS score, with a lack of efficacy in 5/17 subjects in the treated group vs 4/17 in the placebo group.78 There was furthermore no inter-group difference in the change in I-RODS logit score.
Nipocalimab, a fully human anti-FcRn aglycosylated IgG1 monoclonal antibody, is currently under study in a multicentre RCT (arise Study), with comparable trial design to that of ADHERE.79 This RCT plans to enrol 300 participants and completion is expected in 2027.
Batoclimab, another fully human anti-FcRn monoclonal antibody, is also currently being evaluated in an RCT.80 The study plans to evaluate a dose of 340 mg SC weekly vs placebo, as well as comparing 2 doses of the drug (340 mg SC weekly vs 680 mg SC weekly). It consists of a 4-week screening phase, an up to 12-week washout phase, a 12-week randomized treatment phase and an up to 24-week randomized withdrawal phase. The primary outcome is the proportion of subjects remaining relapse-free at 36 weeks, comparing in the second trial phase, 340 mg of Batoclimab vs placebo.
Complement Pathway Inhibitors
SAR445088 is an anti-C1s humanized monoclonal antibody acting in the proximal portion of the classical complement system. In selectively inhibiting the C1-complex, SAR445088 suppresses downstream activation of complement that may prevent inflammatory processes implicated in causing CIDP. This selective inhibition may in addition offer a better safety profile than complement inhibitors targeting downstream components such as C5 inhibitors, especially with regard to infectious complications. This was suggested by the first study of SAR445088 in 93 healthy humans, which resulted in no serious infection, infection with encapsulated bacteria, or meningitis.81 An open-label, non-randomized Phase 2 study evaluating the efficacy, safety and tolerability of SAR445088 in 90 subjects with CIDP is currently ongoing, since 2021.82 The study aims to evaluate the drug in three different patient groups (i) with withdrawn but previously effective standard of care treatment, (ii) in patients refractory to standard of care treatment and (iii) in treatment-naïve patients. Data are to be analysed using Bayesian statistics with predefined efficacy criteria and placebo assumptions based on historical data derived from previously published RCTs.
Bruton Tyrosine Kinase Inhibitors
Bruton Tyrosine Kinase (BTK) plays an essential role in B-cell maturation, and its inhibition has therefore potential therapeutic effects in immune-mediated disease.83 BTK inhibitors have been tried with success in a number of autoimmune diseases, less so in some others.84 BTK inhibitors are currently used for chronic lymphocytic leukaemia and Waldenström’s macroglobulinemia.85 There is currently very limited knowledge and experience in the field of autoimmune neuropathy with only one case series describing the effective treatment of patients with anti-MAG (myelin associated glycoprotein) neuropathy with the BTK inhibitor, ibrutinib.86 Another BTK inhibitor, zanubrutinib, was recently reported as potentially effective in anti-MAG neuropathy.87 A Phase II single-arm open-label trial with zanubrutinib in combination with rituximab is currently ongoing for anti-MAG related polyneuropathy (MAGNAZ) in the Netherlands (NCT05939037).
Other BTK inhibitors are being studied in multiple sclerosis88,89 and NMO (neuromyelitis optica)-spectrum disorders.90 There are no current trials in CIDP, although this is a potentially important drug class for future consideration and availability of long-term data on haematological disorders may facilitate their application in the field of inflammatory neuropathy.91,92
Conclusions
CIDP is a disabling but treatable disorder, with a high response rate to available first-line treatments. The therapeutic management of CIDP is in practice highly dependent on accurately measuring outcome, timing and amplitude of improvement, all in relation to baseline function and gains that may be expected by each individual patient.76,93 There are no currently established biomarkers for CIDP, as opposed to autoimmune paranodopathy with detectable antibodies, and clinical assessment hence remains the only evaluation tool of treatment effects.
New drugs tried in CIDP are aimed at refractory patients, but also as an alternative for patients responsive to first-line agents, as well as those who are treatment-naïve. These different new drug classes are therefore being considered as a replacement for already available options for all patients with CIDP, without head-to-head comparisons of, for example, speed of action, amplitude of benefit and remission rate. Although understandable, primarily in view of the rarity of the disorder, such study designs will unfortunately not allow answering these important questions.
There also remains scope for optimized use of first-line treatments in CIDP, although this would require further comparative studies of existing drugs, which are unlikely to happen. Potential new treatments, starting with FcRn blockers in view of latest results with efgartigimod, today offer exciting additional hope for improved future management. CIDP is, however, a heterogeneous entity, and treatment responses vary widely from one patient to another and with substantial rates of treatment-free remission with existing therapies. A larger therapeutic armamentarium for patients with CIDP, resulting from recent and current studies, is highly desirable, but more research will be needed in future to determine the exact place and value of all available agents.
Acknowledgment
The figures in this manuscript were drawn with BioRender.com
Funding Statement
There is no funding to report.
Disclosure
YAR has received speaker/consultancy honoraria from Takeda, LFB, Polyneuron, Argenx, Janssen, Sanofi and Dianthus; educational support and research grants from LFB; educational support from CSL Behring, all outside the submitted work.
References
- 1.Rajabally YA, Simpson BS, Beri S, Bankart J, Gosalakkal JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: study of a UK population. Muscle and Nerve. 2009;39(4):432–438. doi: 10.1002/mus.21206 [DOI] [PubMed] [Google Scholar]
- 2.Broers MC, de Wilde M, Lingsma HF, van der Lei J, Verhamme KMC, Jacobs BC. Epidemiology of chronic inflammatory demyelinating polyradiculoneuropathy in The Netherlands. J Peripheral Nervous System. 2022;27(3):182–188. doi: 10.1111/jns.12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force-Second revision. Eur J Neurol. 2021;28(11):3556–3583. doi: 10.1111/ene.14959 [DOI] [PubMed] [Google Scholar]
- 4.Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. 2010;17(3):356–363. doi: 10.1111/j.1468-1331.2009.02930.x [DOI] [PubMed] [Google Scholar]
- 5.Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85(6):498–504. doi: 10.1212/WNL.0000000000001833 [DOI] [PubMed] [Google Scholar]
- 6.Allen JA, Ney J, Lewis RA. Electrodiagnostic errors contribute to chronic inflammatory demyelinating polyneuropathy misdiagnosis. Muscle and Nerve. 2018;57(4):542–549. doi: 10.1002/mus.25997 [DOI] [PubMed] [Google Scholar]
- 7.Broers MC, Bunschoten C, Drenthen J, et al. Misdiagnosis and diagnostic pitfalls of chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2021;28(6):2065–2073. doi: 10.1111/ene.14796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary UJ, Rajabally YA. Underdiagnosis and diagnostic delay in chronic inflammatory demyelinating polyneuropathy. J Neurol. 2021;268(4):1366–1373. doi: 10.1007/s00415-020-10287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;1:CD010369. doi: 10.1002/14651858.CD010369.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajabally YA. Unconventional treatments for chronic inflammatory demyelinating polyneuropathy. Neurodegenerative Dis Management. 2017;7(5):331–342. doi: 10.2217/nmt-2017-0017 [DOI] [PubMed] [Google Scholar]
- 11.Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther. 2004;102(3):177–193. doi: 10.1016/j.pharmthera.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Dalakas MC, Latov N, Kuitwaard K. Intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): mechanisms of action and clinical and genetic considerations. Expert Rev Neurother. 2022;22(11–12):953–962. doi: 10.1080/14737175.2022.2169134 [DOI] [PubMed] [Google Scholar]
- 13.Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN, Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;12:Cd001797. doi: 10.1002/14651858.CD001797.pub3 [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen M, van Doorn PA, Brand A, Strengers PF, Jennekens FG, Busch HF. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: a double blind, placebo controlled study. J Neurol Neurosurg. 1993;56(1):36–39. doi: 10.1136/jnnp.56.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain. 1996;119(Pt 4):1067–1077. doi: 10.1093/brain/119.4.1067 [DOI] [PubMed] [Google Scholar]
- 16.Thompson N, Choudhary P, Hughes RA, Quinlivan RM. A novel trial design to study the effect of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. 1996;243(3):280–285. doi: 10.1007/BF00868527 [DOI] [PubMed] [Google Scholar]
- 17.Mendell JR, Barohn RJ, Freimer ML, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001;56(4):445–449. doi: 10.1212/WNL.56.4.445 [DOI] [PubMed] [Google Scholar]
- 18.Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–144. doi: 10.1016/S1474-4422(07)70329-0 [DOI] [PubMed] [Google Scholar]
- 19.Hughes R, Bensa S, Willison H, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 2001;50(2):195–201. doi: 10.1002/ana.1088 [DOI] [PubMed] [Google Scholar]
- 20.Nobile-Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 2012;11(6):493–502. doi: 10.1016/S1474-4422(12)70093-5 [DOI] [PubMed] [Google Scholar]
- 21.Dyck PJ, Litchy WJ, Kratz KM, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 1994;36(6):838–845. doi: 10.1002/ana.410360607 [DOI] [PubMed] [Google Scholar]
- 22.Merkies IS, Bril V, Dalakas MC, et al. Health-related quality-of-life improvements in CIDP with immune globulin IV 10%: the ICE Study. Neurology. 2009;72(15):1337–1344. doi: 10.1212/WNL.0b013e3181a0fd80 [DOI] [PubMed] [Google Scholar]
- 23.Rajabally YA. Long-term immunoglobulin therapy for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle and Nerve. 2015;51(5):657–661. doi: 10.1002/mus.24554 [DOI] [PubMed] [Google Scholar]
- 24.Rajabally YA, Afzal S. Clinical and economic comparison of an individualised immunoglobulin protocol vs. standard dosing for chronic inflammatory demyelinating polyneuropathy. J Neurol. 2019;266(2):461–467. doi: 10.1007/s00415-018-9157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunn MP, Ellis L, Hadden RD, Rajabally YA, Winer JB, Reilly MM. A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. J Peripheral Nervous System. 2016;21(1):33–37. doi: 10.1111/jns.12158 [DOI] [PubMed] [Google Scholar]
- 26.Rajabally YA, Seow H, Wilson P. Dose of intravenous immunoglobulins in chronic inflammatory demyelinating polyneuropathy. J Peripheral Nervous System. 2006;11(4):325–329. doi: 10.1111/j.1529-8027.2006.00105.x [DOI] [PubMed] [Google Scholar]
- 27.Rajabally YA, Kearney DA. Thromboembolic complications of intravenous immunoglobulin therapy in patients with neuropathy: a two-year study. J Neurol Sci. 2011;308(1–2):124–127. doi: 10.1016/j.jns.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 28.Rajabally YA, Wong SL, Kearney DA. Immunoglobulin G level variations in treated chronic inflammatory demyelinating polyneuropathy: clues for future treatment regimens? J Neurol. 2013;260(8):2052–2056. doi: 10.1007/s00415-013-6938-7 [DOI] [PubMed] [Google Scholar]
- 29.Querol L, Rojas-Garcia R, Casasnovas C, et al. Long-term outcome in chronic inflammatory demyelinating polyneuropathy patients treated with intravenous immunoglobulin: a retrospective study. Muscle and Nerve. 2013;48(6):870–876. doi: 10.1002/mus.23843 [DOI] [PubMed] [Google Scholar]
- 30.Kapoor M, Compton L, Rossor A, et al. An approach to assessing immunoglobulin dependence in chronic inflammatory demyelinating inflammatory polyneuropathy. J Peripheral Nervous System. 2021;26(4):461–468. doi: 10.1111/jns.12470 [DOI] [PubMed] [Google Scholar]
- 31.Adrichem ME, Lucke IM, Vrancken A, et al. Withdrawal of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Brain. 2022;145(5):1641–1652. doi: 10.1093/brain/awac054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stino AM, Naddaf E, Dyck PJ, Dyck PJB. Chronic inflammatory demyelinating polyradiculoneuropathy-Diagnostic pitfalls and treatment approach. Muscle and Nerve. 2021;63(2):157–169. doi: 10.1002/mus.27046 [DOI] [PubMed] [Google Scholar]
- 33.Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2013;20(5):836–842. doi: 10.1111/ene.12080 [DOI] [PubMed] [Google Scholar]
- 34.van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Neurol. 2018;17(1):35–46. doi: 10.1016/S1474-4422(17)30378-2 [DOI] [PubMed] [Google Scholar]
- 35.Markvardsen LH, Sindrup SH, Christiansen I, Olsen NK, Jakobsen J, Andersen H. Subcutaneous immunoglobulin as first-line therapy in treatment-naive patients with chronic inflammatory demyelinating polyneuropathy: randomized controlled trial study. Eur J Neurol. 2017;24(2):412–418. doi: 10.1111/ene.13218 [DOI] [PubMed] [Google Scholar]
- 36.Bril V, Hadden RDM, Brannagan TH, et al. Hyaluronidase-facilitated subcutaneous immunoglobulin 10% as maintenance therapy for chronic inflammatory demyelinating polyradiculoneuropathy: the ADVANCE-CIDP 1 randomized controlled trial. J Peripheral Nervous System. 2023;28(3):436–449. doi: 10.1111/jns.12573 [DOI] [PubMed] [Google Scholar]
- 37.Jolles S. Hyaluronidase facilitated subcutaneous immunoglobulin in primary immunodeficiency. Immunotargets Ther. 2013;2:125–133. doi: 10.2147/ITT.S31136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams ME, Balogun RA. Principles of separation: indications and therapeutic targets for plasma exchange. Clin J Am Soc Nephrol. 2014;9(1):181–190. doi: 10.2215/CJN.04680513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyck PJ, Daube J, O’Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med. 1986;314(8):461–465. doi: 10.1056/NEJM198602203140801 [DOI] [PubMed] [Google Scholar]
- 40.Hahn AF, Bolton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham-controlled, cross-over study. Brain. 1996;119(Pt 4):1055–1066. doi: 10.1093/brain/119.4.1055 [DOI] [PubMed] [Google Scholar]
- 41.Mehndiratta MM, Hughes RA, Pritchard J. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2015;2015(8):Cd003906. doi: 10.1002/14651858.CD003906.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanhehco YC, Zantek ND, Alsammak M, et al. Vascular access practices for therapeutic apheresis: results of a survey. J Clin Apher. 2019;34(5):571–578. doi: 10.1002/jca.21726 [DOI] [PubMed] [Google Scholar]
- 43.Cardinale A, Pambrun E, Prelipcean C, Messikh Z, Moranne O. Feasibility, Efficacy, and Safety of Peripheral Venous Access for Chronic Double-Filtration Plasmapheresis with Regional Citrate Anticoagulation. Blood Purif. 2023;1–10. [DOI] [PubMed] [Google Scholar]
- 44.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–533. doi: 10.1038/ncprheum0898 [DOI] [PubMed] [Google Scholar]
- 45.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83(3):965–1016. doi: 10.1152/physrev.00003.2003 [DOI] [PubMed] [Google Scholar]
- 46.Austin JH. Recurrent polyneuropathies and their corticosteroid treatment; with five-year observations of a placebo-controlled case treated with corticotrophin, cortisone, and prednisone. Brain. 1958;81(2):157–192. doi: 10.1093/brain/81.2.157 [DOI] [PubMed] [Google Scholar]
- 47.Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Arch. Neurol. 1989;46(8):878–884. doi: 10.1001/archneur.1989.00520440064022 [DOI] [PubMed] [Google Scholar]
- 48.Cocito D, Paolasso I, Antonini G, et al. A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2010;17(2):289–294. doi: 10.1111/j.1468-1331.2009.02802.x [DOI] [PubMed] [Google Scholar]
- 49.Kuwabara S, Misawa S, Mori M, Tamura N, Kubota M, Hattori T. Long term prognosis of chronic inflammatory demyelinating polyneuropathy: a five year follow up of 38 cases. J Neurol Neurosurg. 2006;77(1):66–70. doi: 10.1136/jnnp.2005.065441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Börü ÜT, Erdoğan H, Alp R, et al. Treatment of chronic inflammatory demyelinating polyneuropathy with high dose intravenous methylprednisolone monthly for five years: 10-Year follow up. Clin Neurol Neurosurgery. 2014;118:89–93. doi: 10.1016/j.clineuro.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 51.Dyck PJ, O’Brien PC, Oviatt KF, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann. Neurol. 1982;11(2):136–141. doi: 10.1002/ana.410110205 [DOI] [PubMed] [Google Scholar]
- 52.van Schaik IN, Eftimov F, van Doorn PA, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9(3):245–253. doi: 10.1016/S1474-4422(10)70021-1 [DOI] [PubMed] [Google Scholar]
- 53.Nobile-Orazio E, Cocito D, Jann S, et al. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg. 2015;86(7):729–734. doi: 10.1136/jnnp-2013-307515 [DOI] [PubMed] [Google Scholar]
- 54.Rabin M, Mutlu G, Stojkovic T, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: search for factors associated with treatment dependence or successful withdrawal. J Neurol Neurosurg. 2014;85(8):901–906. doi: 10.1136/jnnp-2013-306105 [DOI] [PubMed] [Google Scholar]
- 55.Bus SRM, Zambreanu L, Abbas A, et al. Intravenous immunoglobulin and intravenous methylprednisolone as optimal induction treatment in chronic inflammatory demyelinating polyradiculoneuropathy: protocol of an international, randomised, double-blind, placebo-controlled trial (OPTIC). Trials. 2021;22(1):155. doi: 10.1186/s13063-021-05083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyck PJ, O’Brien P, Swanson C, Low P, Daube J. Combined azathioprine and prednisone in chronic inflammatory-demyelinating polyneuropathy. Neurology. 1985;35(8):1173–1176. doi: 10.1212/WNL.35.8.1173 [DOI] [PubMed] [Google Scholar]
- 57.Hadden RD, Sharrack B, Bensa S, Soudain SE, Hughes RA. Randomized trial of interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1999;53(1):57–61. doi: 10.1212/WNL.53.1.57 [DOI] [PubMed] [Google Scholar]
- 58.RMC Trial Group. Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurol. 2009;8(2):158–164. doi: 10.1016/S1474-4422(08)70299-0 [DOI] [PubMed] [Google Scholar]
- 59.Hughes R, Dalakas MC, Merkies I, et al. Oral fingolimod for chronic inflammatory demyelinating polyradiculoneuropathy (FORCIDP Trial): a double-blind, multicentre, randomised controlled trial. Lancet Neurol. 2018;17(8):689–698. doi: 10.1016/S1474-4422(18)30202-3 [DOI] [PubMed] [Google Scholar]
- 60.Mehreen S, Iftikhar S, Muhammad A, Aatif Siddique R, Shahid S. Efficacy of azathioprine and methotrexate in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). Pak J Pharm Sci. 2023;36(4):1361–1365. [PubMed] [Google Scholar]
- 61.Good JL, Chehrenama M, Mayer RF, Koski CL. Pulse cyclophosphamide therapy in chronic inflammatory demyelinating polyneuropathy. Neurology. 1998;51(6):1735–1738. doi: 10.1212/WNL.51.6.1735 [DOI] [PubMed] [Google Scholar]
- 62.Xiang Q, Cao Y, Song Z, et al. Cyclophosphamide for Treatment of Refractory Chronic Inflammatory Demyelinating Polyradiculoneuropathy: a Systematic Review and Meta-analysis. Clin Ther. 2022;44(8):1058–1070. doi: 10.1016/j.clinthera.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 63.Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology. 2014;82(10):879–886. doi: 10.1212/WNL.0000000000000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benedetti L, Briani C, Franciotta D, et al. Rituximab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a report of 13 cases and review of the literature. J Neurol Neurosurg. 2011;82(3):306–308. doi: 10.1136/jnnp.2009.188912 [DOI] [PubMed] [Google Scholar]
- 65.Chaganti S, Hannaford A, Vucic S. Rituximab in chronic immune mediated neuropathies: a systematic review. Neuromuscular Disorders. 2022;32(8):621–627. doi: 10.1016/j.nmd.2022.05.013 [DOI] [PubMed] [Google Scholar]
- 66.Briani C, Cocito D, Campagnolo M, Doneddu PE, Nobile-Orazio E. Update on therapy of chronic immune-mediated neuropathies. Neurol Sci. 2022;43(Suppl 2):605–614. doi: 10.1007/s10072-020-04998-y [DOI] [PubMed] [Google Scholar]
- 67.Mahdi-Rogers M, Kazmi M, Ferner R, et al. Autologous peripheral blood stem cell transplantation for chronic acquired demyelinating neuropathy. J Peripheral Nervous System. 2009;14(2):118–124. doi: 10.1111/j.1529-8027.2009.00221.x [DOI] [PubMed] [Google Scholar]
- 68.Press R, Askmark H, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation: a viable treatment option for CIDP. J Neurol Neurosurg. 2014;85(6):618–624. doi: 10.1136/jnnp-2013-306014 [DOI] [PubMed] [Google Scholar]
- 69.Burt RK, Balabanov R, Tavee J, et al. Hematopoietic stem cell transplantation for chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol. 2020;267(11):3378–3391. doi: 10.1007/s00415-020-10010-6 [DOI] [PubMed] [Google Scholar]
- 70.Masson-Roy J, Breiner A, Warman-Chardon J, et al. Autologous Hematopoietic Stem Cell Transplantation for Chronic Inflammatory Demyelinating Polyradiculoneuropathy. Can J Neurol Sci. 2021;48(6):760–766. doi: 10.1017/cjn.2021.30 [DOI] [PubMed] [Google Scholar]
- 71.Urbain F, Puyade M, Labeyrie C, et al. Hematopoietic stem cell transplantation in chronic inflammatory demyelinating polyneuropathy: French experience about four patients, under the behalf of French society for bone marrow transplantation. J Neurol. 2021;268(4):1536–1539. doi: 10.1007/s00415-021-10452-6 [DOI] [PubMed] [Google Scholar]
- 72.Zheng Y, Hu J, Sun C, Zhao C, Lin J. Efficacy of hematopoietic stem cell transplantation treatment in refractory chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta-analysis. Eur J Neurol. 2023;30(8):2570–2582. doi: 10.1111/ene.15857 [DOI] [PubMed] [Google Scholar]
- 73.Howard JF, Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526–536. doi: 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 74.Dalakas MC, Spaeth PJ. The importance of FcRn in neuro-immunotherapies: from IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors. Ther Adv Neurol Disord. 2021;14:1756286421997381. doi: 10.1177/1756286421997381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timmins P. argenx Reports Positive Topline Data from ADHERE Study of VYVGART Hytrulo in Patients with Chronic Inflammatory Demyelinating Polyneuropathy. Therapeutic Delivery. 2023;14(9):527.37786969 [Google Scholar]
- 76.Rajabally YA, Afzal S, Ghasemi M. Minimal important differences and self-identifying treatment response in chronic inflammatory demyelinating polyneuropathy. Muscle and Nerve. 2021;64(1):37–42. doi: 10.1002/mus.27250 [DOI] [PubMed] [Google Scholar]
- 77.Vanhoutte EK, Latov N, Deng C, et al. Vigorimeter grip strength in CIDP: a responsive tool that rapidly measures the effect of IVIG--the ICE study. Eur J Neurol. 2013;20(5):748–755. doi: 10.1111/j.1468-1331.2012.03851.x [DOI] [PubMed] [Google Scholar]
- 78.A Study to Assess the Efficacy, Safety and Tolerability of Rozanolixizumab in Subjects With Chronic Inflammatory Demyelinating Polyradiculoneuropathy (MyCIDPchoice); 2023. Available from: https://classic.clinicaltrials.gov/ct2/show/results/NCT03861481. Accessed February 9, 2024.
- 79.Efficacy and Safety Study of Nipocalimab for Adults With Chronic Inflammatory Demyelinating Polyneuropathy (CIDP); 2023. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05327114. Accessed February 9, 2024.
- 80.To Assess Efficacy and Safety of Batoclimab in Adult Participants With Active CIDP; 2023. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05581199. Accessed February 9, 2024.
- 81.Chow T, Shamszad P, Vinnard C, et al. First-in-human study with SAR445088: a novel selective classical complement pathway inhibitor. Clin Transl Sci. 2023;16(4):673–685. doi: 10.1111/cts.13481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Querol L, Lewis RA, Hartung HP, et al. An innovative phase 2 proof-of-concept trial design to evaluate SAR445088, a monoclonal antibody targeting complement C1s in chronic inflammatory demyelinating polyneuropathy. J Peripheral Nervous System. 2023;28(2):276–285. doi: 10.1111/jns.12551 [DOI] [PubMed] [Google Scholar]
- 83.Estupiñán HY, Berglöf A, Zain R, Smith CIE. Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects. Front Cell Dev Biol. 2021;9:630942. doi: 10.3389/fcell.2021.630942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ringheim GE, Wampole M, Oberoi K. Bruton’s Tyrosine Kinase (BTK) Inhibitors and Autoimmune Diseases: making Sense of BTK Inhibitor Specificity Profiles and Recent Clinical Trial Successes and Failures. Front Immunol. 2021;12:662223. doi: 10.3389/fimmu.2021.662223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarosiek S, Castillo JJ. Novel Agents in Waldenström Macroglobulinemia. Hematol Oncol Clin North Am. 2023;37(4):751–760. doi: 10.1016/j.hoc.2023.04.001 [DOI] [PubMed] [Google Scholar]
- 86.Castellani F, Visentin A, Campagnolo M, et al. The Bruton tyrosine kinase inhibitor ibrutinib improves anti-MAG antibody polyneuropathy. Neurol Neuroimmunol Neuroinflammation. 2020;7(4):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Min YG, Han HJ, Shin HY, et al. Therapeutic Outcomes and Electrophysiological Biomarkers in Anti-Myelin-Associated Glycoprotein Neuropathy: a Multicenter Cohort Study in South Korea. J Clin Neurol. 2024;20(1):50–58. doi: 10.3988/jcn.2023.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saberi D, Geladaris A, Dybowski S, Weber MS. Bruton’s tyrosine kinase as a promising therapeutic target for multiple sclerosis. Expert Opin Ther Targets. 2023;27(4–5):347–359. doi: 10.1080/14728222.2023.2218615 [DOI] [PubMed] [Google Scholar]
- 89.Krämer J, Bar-Or A, Turner TJ, Wiendl H. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat Rev Neurol. 2023;19(5):289–304. doi: 10.1038/s41582-023-00800-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furman MJ, Meuth SG, Albrecht P, et al. B cell targeted therapies in inflammatory autoimmune disease of the central nervous system. Front Immunol. 2023;14:1129906. doi: 10.3389/fimmu.2023.1129906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kohle F, Dalakas MC, Lehmann HC. Repurposing MS immunotherapies for CIDP and other autoimmune neuropathies: unfulfilled promise or efficient strategy? Ther Adv Neurol Disord. 2023;16:17562864221137129. doi: 10.1177/17562864221137129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Visentin A, Puthenparampil M, Briani C. Bruton tyrosine kinase inhibitors: can they be optimized for the treatment of neuroinflammatory disorders? Expert Opin Investig Drugs. 2023;32(12):1105–1111. doi: 10.1080/13543784.2023.2288076 [DOI] [PubMed] [Google Scholar]
- 93.Rajabally YA. Assessing the benefit of treatment in chronic inflammatory demyelinating polyneuropathy: the challenges of clinical practice. Neurodegenerative Dis Management. 2018;8(5):285–288. doi: 10.2217/nmt-2018-0027 [DOI] [PubMed] [Google Scholar]