Abstract
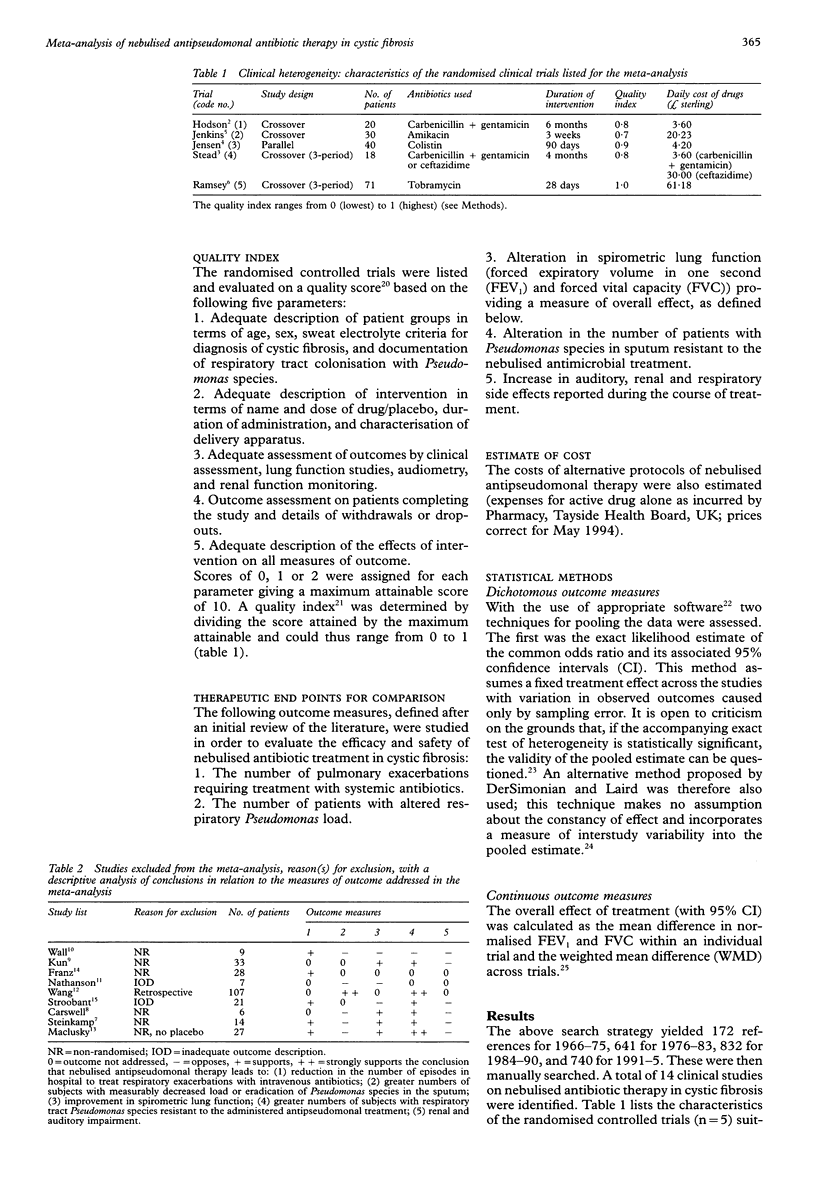
BACKGROUND: To establish the benefits and risks of nebulised antipseudomonal therapy in cystic fibrosis the results of relevant randomised controlled trials were combined. METHODS: The therapeutic end points compared were (a) number of pulmonary exacerbations requiring treatment with systemic antibiotics, (b) measurable alteration in respiratory tract pseudomonal load, (c) alteration in lung function on spirometric assessment, (d) development of resistance in respiratory tract Pseudomonas strains to the nebulised antipseudomonal used in each randomised controlled trial, and (e) renal and auditory impairment. RESULTS: Five studies were suitable for meta-analysis, eight others could not be included because of inadequate outcome description or the lack of appropriate randomisation. Meta-analysis shows benefit for nebulised antipseudomonal antibiotic therapy with no demonstrable adverse effect other than a possible increase in in vitro antibiotic resistance of Pseudomonas aeruginosa of the respiratory tract. CONCLUSIONS: Although inferences drawn from individual randomised controlled trials concerning the benefits and risks of this form of therapy are conflicting, pooled effect size establishes benefit with nebulised antipseudomonal antibiotic therapy and emphasises its relevance to the integration of information in other areas of controversy relating to the treatment of this disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg C. B. A measure to aid in the interpretation of published clinical trials. Stat Med. 1985 Jan-Mar;4(1):1–9. doi: 10.1002/sim.4780040103. [DOI] [PubMed] [Google Scholar]
- Carswell F., Ward C., Cook D. A., Speller D. C. A controlled trial of nebulized aminoglycoside and oral flucloxacillin versus placebo in the outpatient management of children with cystic fibrosis. Br J Dis Chest. 1987 Oct;81(4):356–360. doi: 10.1016/0007-0971(87)90184-7. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dickersin K., Scherer R., Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994 Nov 12;309(6964):1286–1291. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson M. E., Penketh A. R., Batten J. C. Aerosol carbenicillin and gentamicin treatment of Pseudomonas aeruginosa infection in patients with cystic fibrosis. Lancet. 1981 Nov 21;2(8256):1137–1139. doi: 10.1016/s0140-6736(81)90588-2. [DOI] [PubMed] [Google Scholar]
- Jensen T., Pedersen S. S., Garne S., Heilmann C., Høiby N., Koch C. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother. 1987 Jun;19(6):831–838. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- Kun P., Landau L. I., Phelan P. D. Nebulized gentamicin in children and adolescents with cystic fibrosis. Aust Paediatr J. 1984 Mar;20(1):43–45. doi: 10.1111/j.1440-1754.1984.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Lefebvre C. The Cochrane Collaboration: the role of the UK Cochrane Centre in identifying the evidence. Health Libr Rev. 1994 Dec;11(4):235–242. doi: 10.1046/j.1365-2532.1994.1140235.x. [DOI] [PubMed] [Google Scholar]
- MacLusky I. B., Gold R., Corey M., Levison H. Long-term effects of inhaled tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Pediatr Pulmonol. 1989;7(1):42–48. doi: 10.1002/ppul.1950070110. [DOI] [PubMed] [Google Scholar]
- MacLusky I., Levison H., Gold R., McLaughlin F. J. Inhaled antibiotics in cystic fibrosis: is there a therapeutic effect? J Pediatr. 1986 May;108(5 Pt 2):861–865. doi: 10.1016/s0022-3476(86)80758-2. [DOI] [PubMed] [Google Scholar]
- McElvaney N. G., Hubbard R. C., Birrer P., Chernick M. S., Caplan D. B., Frank M. M., Crystal R. G. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet. 1991 Feb 16;337(8738):392–394. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- McElvaney N. G., Nakamura H., Birrer P., Hébert C. A., Wong W. L., Alphonso M., Baker J. B., Catalano M. A., Crystal R. G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992 Oct;90(4):1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S. When will nebulized chemotherapy come of age? Respir Med. 1994 Apr;88(4):245–247. doi: 10.1016/0954-6111(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Pitcher-Wilmott R. W., Levinsky R. J., Gordon I., Turner M. W., Matthew D. J. Pseudomonas infection, allergy, and cystic fibrosis. Arch Dis Child. 1982 Aug;57(8):582–586. doi: 10.1136/adc.57.8.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey B. W., Boat T. F. Outcome measures for clinical trials in cystic fibrosis. Summary of a Cystic Fibrosis Foundation consensus conference. J Pediatr. 1994 Feb;124(2):177–192. doi: 10.1016/s0022-3476(94)70301-9. [DOI] [PubMed] [Google Scholar]
- Ramsey B. W., Dorkin H. L., Eisenberg J. D., Gibson R. L., Harwood I. R., Kravitz R. M., Schidlow D. V., Wilmott R. W., Astley S. J., McBurnie M. A. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993 Jun 17;328(24):1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. M., Vertrees J. E., Carr J., Cipolle R. J., Uden D. L., Giebink G. S., Canafax D. M. Clinical efficacy of antimicrobial drugs for acute otitis media: metaanalysis of 5400 children from thirty-three randomized trials. J Pediatr. 1994 Mar;124(3):355–367. doi: 10.1016/s0022-3476(94)70356-6. [DOI] [PubMed] [Google Scholar]
- Shak S., Capon D. J., Hellmiss R., Marsters S. A., Baker C. L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkamp G., Tümmler B., Gappa M., Albus A., Potel J., Döring G., von der Hardt H. Long-term tobramycin aerosol therapy in cystic fibrosis. Pediatr Pulmonol. 1989;6(2):91–98. doi: 10.1002/ppul.1950060207. [DOI] [PubMed] [Google Scholar]
- Wall M. A., Terry A. B., Eisenberg J., McNamara M., Cohen R. Inhaled antibiotics in cystic fibrosis. Lancet. 1983 Jun 11;1(8337):1325–1325. doi: 10.1016/s0140-6736(83)92428-5. [DOI] [PubMed] [Google Scholar]


