Abstract
Fisheries and aquaculture output have exploded due to an alarming increase in consumption due to the global understanding of the nutritional advantages of fish. Inadvertently, the methods produce a massive amount of fish waste, posing a serious environmental threat. Recycling this waste has now become a major point of controversy that must be resolved. It is critical to emphasize the utility of discarded marine by-products for the creation of high-value commodities such as marine collagen (MC), which can be considered a sustainable solution. Because of its biocompatibility, biodegradability, safety, minimal immunogenicity, and low production costs, MC has various benefits over terrestrial collagen. Many academics have recently become interested in the use of MC as a scaffold. This review focuses on the intriguing contribution of MC in the production of MC-based scaffolds.
Key words: Marine collagen, rapid prototyping, scaffold, tilapia, tissue engineering
INTRODUCTION
Fish is an important part of a well-balanced diet because it is high in protein and vitamins. Multiple associations’ dietary scientific guideline recommends eating fish twice a week to minimize the risk of stroke, depression, neurodegenerative diseases, and various other chronic diseases.[1] Because of a large increase in total output and consumption, the aquaculture sector grew greatly in 2018.
According to statistics from “The State of World Fisheries and Aquaculture 2020 (SOFIA-2020),” roughly 88% of overall fish production in 2018 was used for direct human consumption.[2] However, 35% of the global harvest of production was lost or thrown away.[2] Total fish production is predicted to increase from 179 million to 204 million tonnes by 2030, i.e., an estimated increase of 32% (26 million tonnes).[2]
Over the last two decades, rapid population growth and excessive use of nonrenewable resources have had a severe impact on the environment, prompting the development of sustainable methods.[3,4] Sustainable Development Goal (SDG) 14– ”Conserve and sustainably use the oceans, seas, and marine resources for sustainable development”– has been a major contributor for attaining all of the SDGs.[2] The proportion of fish stocks that are within biologically viable levels is a significant indication for determining the desired success. Unfortunately, from 90% in 1974 to 65.8% in 2017, the fraction of biologically sustainable fish stocks has fallen.[2]
To overcome present environmental challenges, it is vital to place a greater emphasis on better fish waste management methods.[5] As a result, the researchers have shifted their focus to develop a circular economy, which is defined by the European Commission as “the production of renewable biological resources and the conversion of these resources and waste streams into value-added products, such as food, feed, bio-based products, and bioenergy.”[3] In this environment conscious age, growing awareness and interest in the applicability of discarded marine by-products is a fantastic long-term strategy which in turn is a benefit to the socioeconomic sector. Valorization solutions for discarding fish by-products could contribute to economic growth in the long run.[3]
Various value-added items wasted in the form of fish skin, scales, bones, and fins can be used to extract important and valuable products of high economic worth through efficient fish waste management. Collagen is the most intriguing molecule recovered so far from the many amazing potentials of fish by-products.
COLLAGEN STRUCTURE
Collagen is derived from the Greek which means “glue maker.”[6] It is the most structurally and functionally distinctive component of biological tissues extracellular matrix (ECM). In 1955, Rich and Crick[7] postulated the “triple helical structure” of collagen.[6] It is made up of three 1400 amino acid long “helicoidal” chains. There are 44 genes present on the 17th pair of the chromosome, which codes for the collagen protein. The strong structure is made up of a three-amino-acid sequence called Gly-X-Y, with glycine (Gly) in the middle and proline and hydroxyproline (Hyp) occupying the X and Y positions, respectively.[8] Water molecules add to the structure’s strength. Water bridges prevent the “imino-poor region” from unraveling. Furthermore, water molecules that form a single hydrogen bond to the backbone destabilize the triple helix, as they merely provide thermal energy to the structure.[9]
Collagen biosynthesis follows the central dogma by Rich and Crick, 1958.[6] The transcription of the genetic information in the nucleus, formation of messenger RNA (mRNA), and transportation of the mRNA to the endoplasmic reticulum for the synthesis of proteins. Finally, in the posttranslational modification Hyp and hydroxylysine (Hyl), polypeptide chains are formed. The hydroxylation of proline and lysine is prime in the helix stability and cross-linkage. However, it ceases once the triple helix is formed.[7] Although the cross-linking is an extracellular event, it is modified by intracellular posttranslational processing.[8]
The cross-linking of proline and lysine hydroxylation at the time of chain elongation is an enzyme-dependent process. Moreover, the extent of hydroxylation varies for different types of collagen. Similarly, lysine hydroxylation governs the nature of the cross-link formed, and as a consequence, the biomechanical properties of the tissue. In addition, the varied rates of periodontal ligament (PDL) destruction among individuals speculate distinctive lysine/Hyl cross-linking variations in collagen. It highlights the complexity of collagen formation and the importance of posttranslational modifications in the functional integrity of the tissue.[9] Yamada et al. concluded that the hydroxylation degree of proline in fish collagens is 35%–48%, which is similar to mammalian collagens.[10]
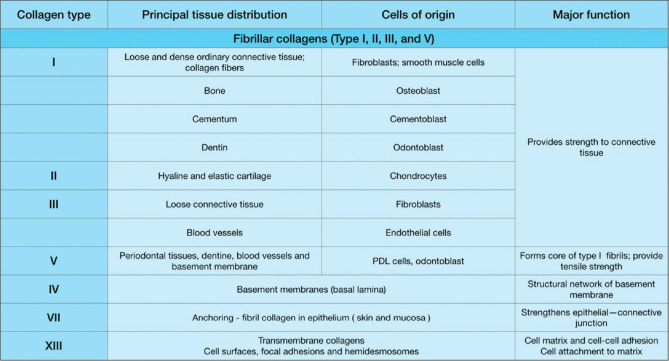
Collagen is divided into two categories[9,10,11] [Figure 1]. fibrillar collagens, as they form fibrils (collagen types I, II, III, and V), and nonfibrillar collagens (collagen types IV, VII, VII, IX, X, and XIII), which are further divided into subfamilies: Type IV (basement membrane) and Type VII (anchoring fibril) are network forming, while Type XIII is a plasma membrane collagen. The only type I, II, and III of the almost 20 forms of collagen found in humans are used in biomedicine. Type I is the most prevalent protein in the body, and it gives bones, tendons, and skins their mechanical strength. Collagen’s most attractive trait is that it self-aggregates and cross-links to build an elaborate three-dimensional (3D) architecture of highly structured woven fiber networks. These networks are capable of resisting tensile stress in several directions while also promoting cell development.[3]
Figure 1.
Different types of collagen, their functions, distribution, and structure. PDL – Periodontal ligament
FISH WASTE BOOM OR BOON?
Collagen is primarily obtained from the skin of porcine and bovine animals. However, the use of mammalian-derived collagens was prohibited due to health and religious concerns. This, stimulated an urgent necessity to search for collagen sources beyond the available options, which are terrestrial mammals.[11] Marine organisms are a rich source of biologically active compounds that are structurally and functionally novel. It has several advantages over mammalian collagen, including the fact that it is safe and poses no risk of disease transmission. It is water-soluble, easily extracted, abundant, and has higher bioavailability when compared to porcine and bovine collagen.[11] As a result, marine collagens (MC) are an excellent replacement for mammalian collagens. Table 1 highlights the differences between mammalian and MC.
Table 1.
Difference between mammalian and marine collagen
| Mammalian | Marine |
|---|---|
| Expensive | Cheap (comparatively) |
| Low bioavailability | More bioavailable can be obtained from fish-by-products |
| Less absorption capability | Higher absorption capability (up to 1.5 times more efficiently into the body) |
| High thermal stability | Low thermal stability |
| High-molecular-weight and particle size | Low-molecular-weight and small particle size so more rapid bloodstream circulation |
| Soluble in the organic solvent | Soluble in water |
| Risk of disease transmission | No risk reported |
Among the various MCs, the collagen from tilapia species is considered the most suitable candidate for replacing mammalian collagen because of its higher thermal stability.[12] One of the most widely grown tilapia species is Oreochromis, which is native to Africa and the Middle East. Tilapia can thrive in a wide range of salinities, from freshwater to seawater, and can tolerate acidic (pH 5) and alkaline (pH 9) environments, as well as low oxygen (2 mg/l) and high ammonia (50 mg/l). Furthermore, it is one of the most important fish groups grown and sold. As a result, it accounts for approximately 60%–70% of industrial by-products (skin, scales, and bone), which are a rich source of collagens and other bioactive molecules.[13]
As a result, the focus of this review is on the applicability of MC in tissue engineering (TE) and its enigmatic role in scaffold fabrication. The first section discusses MC isolation, characteristics, and functional properties. The second section defines scaffold key factors as well as the relatively recent use of marine-based collagen scaffolds in dentistry and regenerative medicine. Finally, it describes the instrumental futuristic role of MC in periodontal tissue engineering work.
COLLAGEN EXTRACTED FROM THE MARINES
Extraction of marine collagen
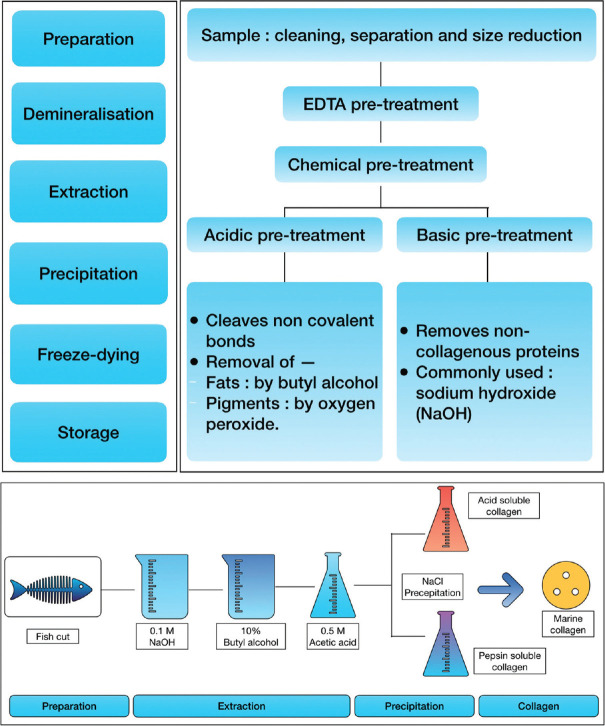
Sponge, jellyfish, squid, and fish can all be used to extract MC. The extraction methodology includes steps such as preparation, demineralization, extraction, precipitation, freeze drying, and recovery [Figure 2].[14] The preparation consists of washing and cleaning to ensure complete impurity removal, followed by separating the parts and cutting them to facilitate maximum extraction. The strong cross-links in the collagen triple helix structure makes it insoluble in cold water. As a result, for efficient and smooth extraction of MC, a mild chemical pretreatment with dilute acids and bases is required.
Figure 2.
Steps involved in the extraction of marine collagen and preparation and isolation of tilapia skin collagen. EDTA – Ethylenediaminetetraacetic acid, NaCl – Sodium Chloride
Noncovalent bonds are cleaved during acidic pretreatment and noncollagenous proteins are removed during alkaline pretreatment. Demineralization is used to extract collagen more efficiently from mineral-rich bone, cartilage, and scales. Demineralization is done effectively using citric acid, HCl and ethylenediaminetetraacetic acid, achieving efficiencies >90%. The extracted solubilized collagen is centrifuged. The obtained collagen is later salted out by adding sodium chloride to it. Finally, the precipitate is dialyzed and freeze-dried.[11,13]
Freeze drying is one of the many extraction techniques. The extraction and purification techniques are mainly influenced by several procedures, such as acid extraction, enzymatic hydrolysis, and fermentation. However, attention in choosing the best extraction technique is done by focusing on the final yield of collagen obtained. It was demonstrated in a study that collagen peptide extracted from Tilapia fish skin by freeze drying showed enhanced levels of osteocalcin secretion capacity in human PDL fibroblasts.[15]
Characteristics and functional properties of extracted collagen
Compared to porcine and bovine collagen, collagen obtained from fish has important characteristic properties such as efficient adsorption up to 1.5-fold into the body, higher bioavailability, biodegradability, biocompatibility, and nonimmunogenicity.[12] Tilapia piscidin, an antibacterial, increases cell proliferation by activating epidermal growth factor, transforming growth factor, and vascular endothelial growth factor, increases the functional properties.
Ullah et al. found that scaffolds made of chitosan, collagen extracted from tilapia scale and glycerine helped human fibroblast and keratinocyte cell adhesion and spreading. Furthermore, with the gradual increase in tilapia collagen and glycerine, the proliferation rate of human fibroblasts gets affected, but human keratinocytes remained unchanged.[16]
The composition of amino acids in MC is comparable to that of presently used mammalian collagen. Glycine makes up more than 30% of all amino acids. In comparison, MC contains serine and threonine residues, especially in cold water species. Collagen from warm-water fishes such as tilapia, on the other hand, has a similar amino acid composition to mammalian collagen. The hydroxyl groups of Hyp and Hyl are important in helix stability because they form intramolecular hydrogen bonds.[12]
The Hyp content of collagen influences properties such as rigidity, temperature (Td) stability, and denaturation Td. The amount of hydroxylation of proline and lysine has a direct effect on collagen’s thermal stability. As a result, the higher the amino acid content, the greater the thermal stability of collagen. Because of its lower amino acid content, MC denaturation Td is less than mammalian collagen. As a result, variations in Hyp content may influence the Td of collagens from different species. Compared with warm-water fish, collagen from cold-water fish has fewer Hyp contents hence less thermal stability.[11]
The most commonly used collagen extraction methods are “acetic acid” and “pepsin hydrolysis.” The Pepsin hydrolysis method produces more collagen than the acetic acid method. As a result of the cleavage of cross-linked molecules in the “telopeptide” region during the process, an unstable collagen protein structure is formed, increasing collagen solubility and contributing to the final high collagen yield.[12] Furthermore, enzyme treatment can reduce the antigenicity of telopeptides. The yield can be calculated using the following equation:[12]
Yield (%) = (Weight of lyophilized collagen) (Weight of initial dry fish by-product)-1 × 100
Other factors such as Td change, time, and solvent concentration all have an impact on collagen yield.[12] As Td rises, viscosity decreases, increasing the mass transfer rate. A Td range of 4°C–10°C is usually considered for collagen extraction from fish sources because pepsin cleaves the collagen triple helix cross-links at this Td. Nonetheless, Tds above the denaturation point cause the isolated proteins to thermally degrade.
Denaturation Td is defined as “the point at which the collagen triple helix structure is deformed to a random spiral structure.” In an acidic solution, the Td of most MC is <30°C. A low Td is thus considered undesirable because denaturation will alter the structure of collagen; thus, a high Td is required for biomaterial applications. In general, marine fish scales should be kept between 26°C and 29°C and marine fish skin should be kept between 28°C and 30°C.[12,17] The collagen recovery rate increases as the extraction time increases. Excessive extraction times, on the other hand, cause chain degradation and breakage.
In one of the studies, Alfaro et al. found that with the gradual increase in time from 3 to 15 hours the yield also increased ie; it resulted in a 1.72 percent increase in yield, indicating a positive relationship between temperature and time for extracting collagen from Wami tilapia.[18] Because of its low cost and good performance in breaking down collagen cross-links, 0.5 M of AcOH is commonly used for collagen extraction. As with higher molarities, it may cause peptide degradation, lowering final product yield and purity.[12]
Tilapia collagens have higher thermal stability, proline and Hyp amino acid compositions, biocompatibility, biodegradability, immunogenicity, and hemostasis properties comparable to mammalian collagen. As a result, it is suitable for use as biomaterials in TE applications.
TISSUE ENGINEERING
The tooth’s unique structure, which includes both hard and soft tissues, as well as the periodontium’s hierarchical structure, makes a successful regeneration a significant foreseeable challenge. Periodontal homeostasis is challenged several times throughout life, by various genetic and environmental factors. However, over the last decade, new concepts of reparative paradigm and biologics have emerged in dental tissue engineering.[16] Many significant advances have been made in the field of periodontal tissue engineering. The 21st century appears to be a period in history when human genetics and clinical dentistry, molecular biology, bioengineering, and bioinformatics come together.[16,17,18] Tissue engineering, a filtered bio-inspired by-product, is an opportunity at this convergence. The regeneration of the attachment apparatus is the holy grail of periodontal therapy. The tissue engineering approach, as first proposed by Langer and Vacanti,[19] combines three key elements:
Conductive scaffolds
Signalling molecules
Stem/progenitor cells.
Scaffold
To achieve tissue regeneration, the use of tissue engineering may provide a more notable level of manipulation of the components related to the wound healing process. In this case, a biomaterial (scaffold) serves as a framework for the cells that will shape the new tissues, as well as biological signaling molecules (signals) that train these cells to shape the desired tissue type. Another important requirement for tissue design is the creation of new vascular support and adaptive mechanical environments for developing tissues.[20,21]
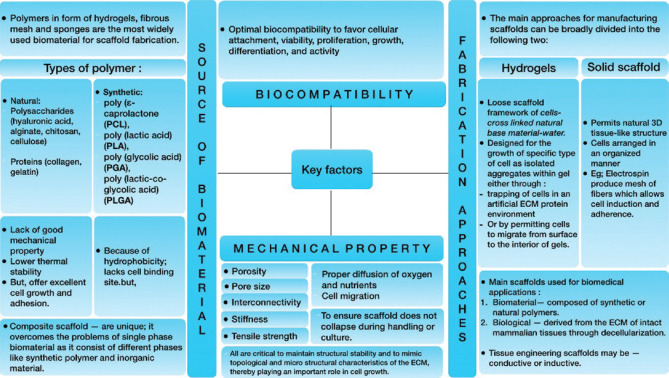
A scaffold is made by selecting an appropriate biomaterial, i.e., a substance engineered to interact with the biological systems to carry out functions like improving cell support while also mimicking in vivo cell physiology and morphology.[22] As a result, the scaffold should ideally provide a supporting 3D structure with various biological, mechanical, and chemical cues necessary to respond to external stimuli.[21,23] Figure 3 depicts the key considerations for scaffold fabrication.[11]
Figure 3.
Key factors in scaffold fabrication. ECM – Extracellular matrix
Signaling molecules
Among all the varied bioactive proteins (signaling molecules), the most attention was received to– growth factors that are mitogenic (cell proliferation) and chemotactic (cell recruiting) agents and morphogens which function by altering the cellular phenotype (osteoinduction).[24] Since the late 1990s, the focus has been mainly on two approaches for the preparation that contains biological mediators for the enhancement of cells near the periodontal wound. The first is widely used and accepted, such as enamel matrix derivative and autologous platelet-rich plasma preparation. The second utilizes recombinant growth factors such as recombinant human basic fibroblast growth factor and recombinant human platelet-derived growth factor-BB. These biological mediators primarily enhance periodontal and bone regeneration. All are commercially available as Emdogain (Straumann)– enamel matrix derivative, GEM 21S (Lynch biologics)– PDGF-BB, Infuse bone graft (Medtronic)– BMP-2, Osigraft (Stryker Biotech)– BMP-7.[25]
Stem cells
Stem cell are clonogenic, relatively undifferentiated cell that is capable of self-renewal and multi-lineage differentiation.[26] The widely known source of stem cells is bone marrow containing hematopoietic and nonhematopoietic cells, known as mesenchymal stem cells. It was first identified in 1966 that mesenchymal cells (MSCs) harbor a unique population of multipotent cells, characteristics of differentiating into multiple tissue-specific lineages.[26] In tissue engineering-based approaches, the expanding knowledge and extensive information about the cells with the periodontal-regenerative phenotype and MSC, it has become possible to culture cells.
Of particular interest is the capacity of progenitor cell populations derived from PDL. Evidence supports that PDL stem cells (PDLSCs) are capable to differentiate into either cementoblasts, osteoblasts, or collagen-forming cells.[27,28] These cells are capable to form clonogenic colonies that possess properties of postnatal stem cells and are self-renewable. When transplanted into immunocompromised rodents, PDLSCs show the capacity to generate a cementum PDL-like structure and contribute to periodontal tissue repair.[29]
With the development of recombinant growth factors and morphogens and the use of synthetic scaffolds, the level of success has improved.[30] As tissue engineering approaches are likely to improve clinical results, clinicians need to understand the biology and clinical parameters/limitations of these techniques and venture into more biocompatible methods.[31]
TILAPIA FISH SCAFFOLD IN TISSUE ENGINEERING
Many researchers have been drawn to fish collagen-based scaffolds, for which several studies have demonstrated their utility in regenerative medicine. For the first time, Liu and Sun proposed hydrolyzed tilapia fish collagen (HFC) as a promising bioactive ingredient for periodontal tissue regeneration.[32] The study looked at human PDL cells (hPDLCs) cultured with HFC irrespective of any inducing agents and discovered that tilapia collagen promotes hPDLC viability. There was also a surge in the production of alkaline phosphatase and osteocalcin via the Extracellular signal-regulated kinase (ERK) signaling pathways, indicating osteogenic differentiation of hPDLCs. As a result, authors believe that HFC has good osteoinductive properties and hence can be a good candidate for alveolar bone regeneration.
Zhou et al. created a composite scaffold comprising of biomimetic electrospun nanofiber membrane made of collagen (Col) extracted from tilapia fish with with bioactive glass (BG) and chitosan (CS) to evaluate its (Col/BG/CS) biological effects on hPDLCs, its mechanical properties and antibacterial activity (Streptococcus mutans).[33] Its periodontal tissue regeneration effects were also validated in dogs by treating class II furcation defects and promoting bone regeneration. As a result, the tilapia collagen scaffold has shown clinical potential as a guided tissue or bone regeneration membrane for inducing periodontal tissue regeneration.
Collagen is a promising biomaterial, according to the findings of various studies, because: (1) it improves cell-to-cell attachment, (2) promotes the viability of cells, (3) up-regulates gene expression of proteins in hPDLCs and (4) accelerates matrix mineralization.[34] Researchers have also successfully developed a human skin substitute for the treatment of skin wounds. Similarly, an oral mucosa substitute is required due to the restricted feasibility of donor soft tissues. Terada et al.[35] created a composite scaffold from tilapia scale collagen and chitosan equivalent to oral mucosa on which primary oral keratinocytes were cultured. It produced a stratified multilayered epithelial layer with superficial keratinization [Table 2].
Table 2.
Tilapia fish collagen-based scaffold for tissue engineering application
| Form of marine collagen | Origin | Exaction technique | Biological assessment | Outcomes | Reference |
|---|---|---|---|---|---|
| Collagen peptide | Tilapia scales | Enzymatic hydrolysis | Human periodontal ligament cells | Promoted cell viability Up-regulated the expression of osteogenic markers and the production of osteogenic-related proteins | Liu and sun[32] |
| Scaffold | Tilapia fish collagen | Electrospinning | Human periodontal ligament cells A dog class II furcation defect model in vivo | Enhanced cell viability and osteogenic gene expression Promoted the expression of RUNX-2 and OPN protein promoted bone regeneration | Zhou et al.[33] |
| Scaffold | Tilapia fish scale collagen | Freeze-drying/dehyrothermal cross-linked | Primary oral keratinocytes | Produced multilayered, polarized, stratified epithelial layer with superficial keratinization | Terada et al.[35] |
RUNX-1 - Runt-related transcription factor -1, OPN - osteopontin proteins
PerioCol-GTR (Eucare pharmaceuticals limited, Chennai) is a commercially available MC. It is dispatched as pale white color material predominantly containing intact Type I collagen membrane of fish origin. It is resorbable, nontoxic, nonimmunogenic, and biocompatible. Its predictable resorption time is 6–8 weeks. It is indicated in the management of recession, intra-bony defects, furcation defects, and bone augmentation procedures. The mode of action is based on Melcher’s hypothesis, wherein the membrane acts as a barrier membrane and prevents the migration of epithelial cells into the defects. It also serves as an excellent scaffold for migrating periodontal cells. KolSpon® Tape (Universe Surgical Equipment Co, Chennai) is Sterile Type 1 acellular collagen tape of fish origin for wound healing.
CURRENT TREATMENT MODALITIES AND LIMITATIONS
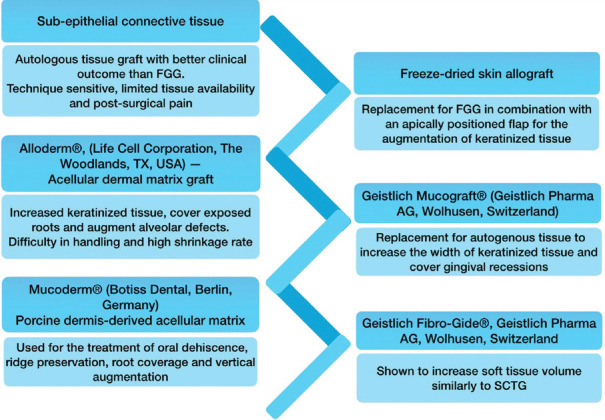
Soft tissue augmentation is typically used to replace tissue loss either on the natural tooth or around an exposed implant. The treatment chosen should address both functional and aesthetic concerns. Several techniques are considered depending on the need, with autologous tissue grafts being the most reliable.[36,37] However, it has several limitations, including postoperative pain and discomfort, technique sensitivity, limited tissue availability, and reliance on palate anatomical position. As a result, several substitutes have been sought to reduce postsurgical morbidity[38] [Figure 4].
Figure 4.
The current treatment modalities with their limitations. FGG – Free gingival graft, SCTG – Subepithelial connective tissue graft
The alternative biological scaffolds mentioned above are promising biomaterials because they reduce morbidity, are cost-effective, and significantly reduce surgical time. However, outbreaks like bovine spongiform encephalopathy in recent years have raised an alarming issue concerning health and questioned the use of collagen-derived products from terrestrial animals.[2] Furthermore, due to religious beliefs, Hinduism forbids the use of bovine collagen, while Islamic and Jewish cultures forbid the use of porcine collagen. As a result, MC is an excellent replacement for mammalian collagen.[2] Furthermore, these biological scaffolds must be tailored to the defect in order to achieve a “perfect-fit patient-tailored graft” with an accurate inner architecture of the defect and an outer shape that mimics soft tissue, resulting in a harmonious result and aesthetically pleasing tissue restoration. This paves the way for 3D printing.
RAPID PROTOTYPING AND TISSUE ENGINEERING - AN EMERGING TREATMENT APPROACH
Tissue engineering is no longer limited to cell culture but has enormous potential in the creation of 3D structures capable of cell adhesion and differentiation. It is the most common method to build a scaffold.[39] Previously, reproducing a tissue relied heavily on scaffold fabrication techniques that were completely blind to tissue architecture and complexity.[40] Today, using advanced techniques such as “Rapid prototyping (RP),” it appears possible to create a variety of geometries that can perfectly fit any tissue defect while also precisely mimicking inner tissue architecture.
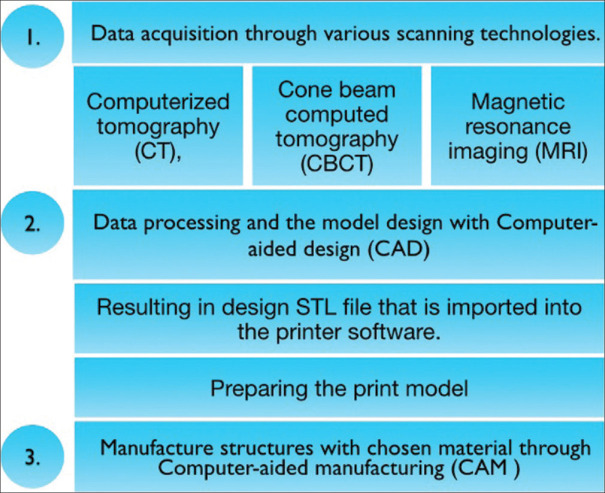
RP is based on an additive process that builds complex anatomy by adding layers upon layers.[41] RP is regarded as an important tissue engineering technology because it has enabled the creation of a custom-designed geometrical biomimetic scaffold. It successfully replaced manual handling via three processing steps, and this novel method is referred to as “digital workflow.” Figure 5 depicts the novel 3D printing process. The data from cone beam computed tomography (CBCT)/computed tomography (CT)/magnetic resonance imaging – or any imaging technology – are transferred into computer-aided design (CAD) software to create a customized CAD model. The CAD data are converted to a Standard Triangle Language or Standard Tessellation Language (STL) file format before being transferred to an RP system for scaffold fabrication. Thus, CAD data are now converted into a sequence of cross-sectional layers that will form a solid model layer by layer from bottom to top.
Figure 5.
Steps of 3D printing. 3D – Three-dimensional, STL – Standard Triangle Language or Standard Tessellation Language
There are over 20 commercially available RP systems:[41,42,43]
Liquid-based — stereolithography and two-photon polymerization fall into this category
Solid-based — fused deposition modeling comes in this category
Powder-based — Selective laser sintering and 3D printing fall in this category.
“Bioprinting” is the 3D printing technology that includes cells, and the hydrogels — the residence of cells, have been called “bio-inks.”
FUTURE PERSPECTIVE: A CONCEPTUALIZED 3D-BIOMIMETIC COMPOSITE SCAFFOLD
3D scaffolds have solved many problems that conventional approaches had. As conventional scaffold designs do not compartmentalize different tissues, the spatial arrangement of PDL fibers is difficult. To address the same limitation, combining CAD and computer-aided manufacturing with 3D bioprinting appears to be a valuable alternative approach. Tissue engineering has enabled the combination of specific cells with these 3D scaffolds that mimic native ECM cues and interact with native cells and growth factors. The interaction between cells and scaffold material is critical for smooth cellular adhesion and proliferation. Among the various materials available, tilapia fish collagen may be a promising biomimetic scaffold. As a result, an ideal scaffold should translate functionally as well as topographically because it serves as a temporary “engineered replacement” of the native ECM, which later resorbs and is replaced by new natural tissue.[14] A customized anatomically designed composite scaffold of tilapia fish collagen/bioactive glass/chitosan specific for the defect would facilitate regeneration and its architecture would direct the integration of PDL and mineralized tissue structures allowing for the replication of such functional interface that naturally mimics tooth anatomy.
LIMITATIONS
The topic discussing tilapia fish waste as a boon is fairly new in the field of dentistry. Very limited studies have been conducted in humans to evaluate the efficacy of MC products based on tilapia skin. In addition, a better understanding of the physicochemical properties of fish collagen is necessary. To evaluate the therapeutic potential of MC-based scaffolds, a comprehensive and complete investigation is paramount. As no single material can fulfill the required properties of biomaterial. It is advised to use a combinatorial approach by fabricating a composite biomaterial scaffold. However, a better understanding is necessary concerning the pros and cons of various biomaterials. In addition, any tissue-engineered scaffold for periodontia should address the following key points:
Resorption time of the membrane;
Adaptability into the defect with more controlled and predictable geometry for the alignment of connective tissue fiber;
Focus on molecular biology to optimize cementum regeneration;
and, lastly, economically acceptable.
CONCLUSION
Fish waste is not only a major environmental problem but also a huge economic loss. For this reason, a sustainable approach towards fish management is the need of the hour. With better fish-waste management two of these major issues can be resolved while adding value to it as well. The Waste Framework Directive[44] aims to prevent the generation of waste as much as possible and to use the waste generated as a resource for reusing, recycling, and recovery of waste produced. In this way, with the use of fish by-products, one could contribute to the development and increase of economic growth.[45] The review comprehensively illustrates the utilization of membranes obtained from tilapia fish scales, highlighting how this waste could become an enormous resource for the production of value-added products (e.g., collagen), with potential applications in the biomedical field.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Raji CA, Erickson KI, Lopez OL, Kuller LH, Gach HM, Thompson PM, et al. Regular fish consumption and age-related brain gray matter loss. Am J Prev Med. 2014;47:444–51. doi: 10.1016/j.amepre.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rome: The State of World Fisheries and Aquaculture c2020 –. [cited 2023 Apr 12]. Available from:https://www.fao.org/documents/card/en/c/ca9229en . Internet.
- 3.Coppola D, Lauritano C, Palma Esposito F, Riccio G, Rizzo C, de Pascale D. Fish waste: From problem to valuable resource. Mar Drugs. 2021;19:116. doi: 10.3390/md19020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew PT. Fish Waste Utilization in India. In: Meenakumari B, Boopendranath MR, Edwin L, Sankar TV, Gopal N, Ninan G, editors. Coastal Fishery Resources of India: Conservation and Sustainable Utilisation. 1st ed. Cochin: Society of Fisheries Technologists (India); 2010. pp. 463–79. [Google Scholar]
- 5.Arvanitoyannis IS, Kassaveti A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int J Food Sci Technol. 2008;43:726–45. [Google Scholar]
- 6.Rich A, Crick FH. The molecular structure of collagen. J Mol Biol. 1961;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- 7.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 8.Kivirikko KI. Posttranslational processing of collagens. In: Bittar EE, Bittar N, editors. Principles of Medical Biology. 3rd ed. Brazil: Elsevier Science &Technology; 1996. pp. 233–54. [Google Scholar]
- 9.Owczarzy A, Kurasiński R, Kulig K, Rogóż W, Szkudlarek A, Maciążek-Jurczyk M. Collagen-structure, properties and application. Eng Biomater. 2020;156:17–23. [Google Scholar]
- 10.Yamada S, Yamamoto K, Ikeda T, Yanagiguchi K, Hayashi Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed Res Int. 2014;2014:302932. doi: 10.1155/2014/302932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim YS, Ok YJ, Hwang SY, Kwak JY, Yoon S. Marine Collagen as A promising biomaterial for biomedical applications. Mar Drugs. 2019;17:467. doi: 10.3390/md17080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zain NM, Saidin S, Sosiawan A. Properties of Tilapia Collagen as a Biomaterial for Tissue Engineering: A Review. IOP Conference Series: Materials Science and Engineering. 2020;932 [Google Scholar]
- 13.Valenzuela-Rojo DR, López-Cervantes J, Sánchez-Machado DI. Tilapia (Oreochromis aureus) Collagen for Medical Biomaterials. Seaweed Biomaterials. BoD (Books on Demand) 2018:47–66. [Google Scholar]
- 14.Jafari H, Lista A, Siekapen MM, Ghaffari-Bohlouli P, Nie L, Alimoradi H, et al. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers (Basel) 2020;12:2230. doi: 10.3390/polym12102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elango J, Selvaganapathy PR, Lazzari G, Bao B, Wenhui W. Biomimetic collagen-sodium alginate-titanium oxide (TiO (2)) 3D matrix supports differentiated periodontal ligament fibroblasts growth for periodontal tissue regeneration. Int J Biol Macromol. 2020;163:9–18. doi: 10.1016/j.ijbiomac.2020.06.173. [DOI] [PubMed] [Google Scholar]
- 16.Ullah S, Zainol I, Chowdhury SR, Fauzi MB. Development of various composition multicomponent chitosan/fish collagen/glycerin 3D porous scaffolds: Effect on morphology, mechanical strength, biostability and cytocompatibility. Int J Biol Macromol. 2018;111:158–168. doi: 10.1016/j.ijbiomac.2017.12.136. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura H, Yunoki S, Kondo E, Ikoma T, Tanaka J, Yasuda K. In vivo biological responses and bioresorption of tilapia scale collagen as a potential biomaterial. J Biomater Sci Polym Ed. 2009;20:1353–68. doi: 10.1163/092050609X12457418396658. [DOI] [PubMed] [Google Scholar]
- 18.da Trindade Alfaro A, Fonseca GG, Balbinot E, de Souza NE, Prentice C. Yield, viscosity, and gel strength of wami tilapia (Oreochromis urolepis hornorum) skin gelatin: Optimization of the extraction process. Food Sci Biotechnol. 2014;23:765–73. [Google Scholar]
- 19.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 20.Izumi Y, Aoki A, Yamada Y, Kobayashi H, Iwata T, Akizuki T, et al. Current and Future Periodontal Tissue Engineering. Periodontology 2000. 2000;56:166–87. doi: 10.1111/j.1600-0757.2010.00366.x. [DOI] [PubMed] [Google Scholar]
- 21.Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 22.Srisuwan T, Tilkorn DJ, Wilson JL, Morrison WA, Messer HM, Thompson EW, et al. Molecular aspects of tissue engineering in the dental field. Periodontol 2000. 2006;41:88–108. doi: 10.1111/j.1600-0757.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 23.Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S. Tissue engineered constructs for periodontal regeneration: Current status and future perspectives. Adv Healthc Mater. 2018;7:e1800457. doi: 10.1002/adhm.201800457. [DOI] [PubMed] [Google Scholar]
- 24.Kao RT, Murakami S, Beirne OR. The use of biologic mediators and tissue engineering in dentistry. Periodontol 2000. 2009;50:127–53. doi: 10.1111/j.1600-0757.2008.00287.x. [DOI] [PubMed] [Google Scholar]
- 25.Mullin DR. Tissue engineering: Applications in oral and maxillofacial surgery and periodontics, second edition. J Prosthodont. 2008;17:602–3. [Google Scholar]
- 26.Owen M, Friedenstein AJ. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang QZ, Nguyen AL, Yu WH, Le AD. Human oral mucosa and gingiva: A unique reservoir for mesenchymal stem cells. J Dent Res. 2012;91:1011–8. doi: 10.1177/0022034512461016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCulloch CA, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res. 1991;26:144–54. doi: 10.1111/j.1600-0765.1991.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 29.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 30.Bartold PM, Narayanan AS. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000. 2006;40:29–49. doi: 10.1111/j.1600-0757.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- 31.Slavkin HC, Bartold PM. Challenges and potential in tissue engineering. Periodontol 2000. 2006;41:9–15. doi: 10.1111/j.1600-0757.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Sun J. Hydrolyzed tilapia fish collagen induces osteogenic differentiation of human periodontal ligament cells. Biomed Mater. 2015;10:065020. doi: 10.1088/1748-6041/10/6/065020. [DOI] [PubMed] [Google Scholar]
- 33.Zhou T, Liu X, Sui B, Liu C, Mo X, Sun J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed Mater. 2017;12:055004. doi: 10.1088/1748-605X/aa7b55. [DOI] [PubMed] [Google Scholar]
- 34.Subhan F, Hussain Z, Tauseef I, Shehzad A, Wahid F. A review on recent advances and applications of fish collagen. Crit Rev Food Sci Nutr. 2021;61:1027–37. doi: 10.1080/10408398.2020.1751585. [DOI] [PubMed] [Google Scholar]
- 35.Terada M, Izumi K, Ohnuki H, Saito T, Kato H, Yamamoto M, et al. Construction and characterization of a tissue-engineered oral mucosa equivalent based on a chitosan-fish scale collagen composite. J Biomed Mater Res B Appl Biomater. 2012;100:1792–802. doi: 10.1002/jbm.b.32746. [DOI] [PubMed] [Google Scholar]
- 36.Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T, Giannobile WV. Regenerative medicine for periodontal and peri-implant diseases. J Dent Res. 2016;95:255–66. doi: 10.1177/0022034515618887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nesic D, Schaefer BM, Sun Y, Saulacic N, Sailer I. 3D printing approach in dentistry: The future for personalized oral soft tissue regeneration. J Clin Med. 2020;9:2238. doi: 10.3390/jcm9072238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol 2000. 2009;51:208–19. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 40.Habib AA, Sheikh NA. 3D printing review in numerous applications for dentistry. J Inst Eng India Ser C. 2022;103:991–1000. [Google Scholar]
- 41.Peltola SM, Melchels FP, Grijpma DW, Kellomäki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40:268–80. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 42.Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, et al. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 2014;20:533–42. doi: 10.1089/ten.tec.2013.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L, Fang Y, Liao Y, Chen G, Gao C, Zhu P. 3D printing and digital processing techniques in dentistry: A review of literature. Adv Eng Mater. 2019;21:1801013. [Google Scholar]
- 44.D’Adamo I. Adopting a circular economy: Current practices and future perspectives. Soc Sci. 2019;8:1–5. [Google Scholar]
- 45.Geissdoefer M, Savaget P, Bocken NMP, Hultink EJ. The circular economy –a new sustainability paradigm?'. J Clean Prod. 2017;143:757–68. [Google Scholar]