Abstract
Ep-CAM, an epithelium-specific cell-cell adhesion molecule (CAM) not structurally related to the major families of CAMs, contains a cytoplasmic domain of 26 amino acids. The chemical disruption of the actin microfilaments, but not of the microtubuli or intermediate filaments, affected the localization of Ep-CAM at the cell-cell boundaries, suggesting that the molecule interacts with the actin-based cytoskeleton. Mutated forms of Ep-CAM were generated with the cytoplasmic domain truncated at various lengths. All of the mutants were transported to the cell surface in the transfectants; however, the mutant lacking the complete cytoplasmic domain was not able to localize to the cell-cell boundaries, in contrast to mutants with partial deletions. Both the disruption of the actin microfilaments and a complete truncation of the cytoplasmic tail strongly affected the ability of Ep-CAM to mediate aggregation of L cells. The capability of direct aggregation was reduced for the partially truncated mutants but remained cytochalasin D sensitive. The tail truncation did not affect the ability of the transfectants to adhere to solid-phase-adsorbed Ep-CAM, suggesting that the ability to form stable adhesions and not the ligand specificity of the molecule was affected by the truncation. The formation of intercellular adhesions mediated by Ep-CAM induced a redistribution to the cell-cell boundaries of α-actinin, but not of vinculin, talin, filamin, spectrin, or catenins. Coprecipitation demonstrated direct association of Ep-CAM with α-actinin. Binding of α-actinin to purified mutated and wild-type Ep-CAMs and to peptides representing different domains of the cytoplasmic tail of Ep-CAM demonstrates two binding sites for α-actinin at positions 289 to 296 and 304 to 314 of the amino acid sequence. The results demonstrate that the cytoplasmic domain of Ep-CAM regulates the adhesion function of the molecule through interaction with the actin cytoskeleton via α-actinin.
The epithelial cell adhesion molecule, Ep-CAM (also known as KS1/4 [33], EGP40 [42], or GA733-2 [45]), abundantly present in most epithelial tissues, functions as a homophilic Ca2+-independent cell-CAM (21–23). This molecule is not structurally related to any of the four major families of CAMs: the cadherins, integrins, immunoglobulin (Ig) family, and selectins (23). The extracellular domain of Ep-CAM consists of two epidermal growth factor-like domains, followed by a cysteine-poor region, a transmembrane domain, and a short cytoplasmic tail (26 amino acids). The exact contribution of Ep-CAM to the cellular interactions in epithelial tissues is unclear (23); however, the high evolutionary conservation—the murine and human proteins have 86% homology (2), and the sequences closely related to Ep-CAM can be found in the genomes of all mammals and birds (20)—suggests the functional importance of this protein.
In most adult epithelial tissues, enhanced expression of Ep-CAM is closely associated with either benign or malignant hyperproliferation. This is especially evident for squamous epithelia, which are Ep-CAM negative, and where Ep-CAM expression is related to early preneoplastic or dysplastic changes and carcinogenesis (14, 24, 35, 47). However, it is also true for other types of epithelium, such as transitional epithelium (urothelium), where the level of Ep-CAM expression correlates to the tumor grade (52), or simple epithelia, such as mammary gland, where enhanced levels of Ep-CAM in carcinomas are associated with a bad prognosis (46). In colon, where the normal level of Ep-CAM is relatively high, a further increase in Ep-CAM expression is observed in relation to polyp development (38). An interesting example of the relationship between Ep-CAM expression and proliferation in normal tissue is the hair follicle, where Ep-CAM is expressed only in the highly proliferative zone (47). These observations suggest that Ep-CAM may be an important adhesion receptor associated with a proliferative cell phenotype.
Recently we have demonstrated that the expression of ectopic Ep-CAM in Ep-CAM-negative epithelial cells, or in L cells transfected with E-cadherin, induced partial abrogation of the cadherin-mediated intercellular adhesions (25). However mutant Ep-CAM lacking the complete cytoplasmic domain had no effect on cadherins, which indicates the functional relevance of the cytoplasmic domain for the Ep-CAM.
Therefore, we investigated the role of the cytoplasmic domain in the formation of Ep-CAM-mediated intercellular adhesions. Association between CAMs and the cytoskeleton was demonstrated to be of importance for the formation of junctions and inside-out–outside-in signaling (1, 12, 30, 51). It was also demonstrated to define the binding specificity for adhesion receptors (7) and to control the delivery of the adhesion molecules to the appropriate domain of the cell membrane (27), as well to determine the half-life of the molecule (11, 27).
We report here that the cytoplasmic domain of Ep-CAM interacts with the actin cytoskeleton via a direct association with α-actinin. This connection is required for the formation and stabilization of Ep-CAM-mediated intercellular adhesions.
MATERIALS AND METHODS
Cell culture.
Human Ep-CAM-negative HBL-100 cells (clone HCA), Ep-CAM-positive RC-6 cells (both normal mammary epithelium-derived cell lines immortalized by simian virus 40 transformation), and murine fibroblast L cells (clone L929) were all cultured in Dulbecco’s modified minimal essential medium supplemented with 10% fetal calf serum, 100 U of penicillin per ml, and 100 U of streptomycin per ml. To disrupt the cytoskeleton, cells were treated for 2 h at 37°C by addition to the culture medium of either 10 μg of colchicine (Sigma Chemical Co., St. Louis, Mo.) per ml, 10 μg of cytochalasin D (Sigma Chemical Co.) per ml, or 10 mM acrylamide (Serva Feinbiochemica GmbH & Co., Heidelberg, Germany).
Antibodies.
The anti-Ep-CAM antibody 323/A3 (8), used in our previous studies (21, 22), was provided by Centocor, Inc. (Malvern, Pa.). Antibodies against talin (clone 8d4), vinculin (hVIN-1), spectrin (clone SB-SP1), filamin (clone FIL-2), and β-tubulin (clone TUB 2.1) were obtained from Sigma Chemical Co. Antibodies to α-catenin (clone 5) and β-catenin (clone 14) were obtained from Transduction Laboratories (Lexington, Ky.). Monoclonal antibody (MAb) to α-actinin CB-11 was obtained from ICN Biomedicals, Inc., (Costa Mesa, Calif.) and used for immunoblotting. A polyclonal rabbit antiserum to α-actinin was from Sigma Chemical Co. and was used for immunoprecipitation. Antibodies to E-cadherin (HECD-1) and keratin 18 (DC-10) were obtained from Thamer Diagnostica (Uithoorn, The Netherlands). Antibody to desmoplakins I and II 115F was kindly provided by D. Garrod (University of Manchester, Manchester, United Kingdom). A phalloidin-tetramethyl rhodamine isothiocyanate (TRITC) conjugate was from Sigma Chemical Co.
Construction of the mutated Ep-CAM cDNAs.
The cDNAs for Ep-CAM mutant forms missing, respectively, the terminal third of (Mu4), two-thirds of (Mu2), or complete (Mu1) cytoplasmic domain were prepared by PCR with the Pfu polymerase by proofreading (Stratagene, La Jolla, Calif.). The respective DNA fragments were amplified by using Ep-CAM cDNA (45) as a template with a general Ep-CAM sense primer, corresponding to the 5′ untranslated sequences of Ep-CAM mRNA (5′-TTT GCT AGC TTC TCG GCG CGC GCG CAG C-3′), and the mutant-specific reverse primers 5′-TTT AAG CTT TTA GGA AAT AAC CAG CAC-3′ for Mu1, 5′-TTT CTC GAG CTA CTT TGC CAT TCT CTT-3′ for Mu2, and 5′-TTT GCG GCC GCT CAC TCC TTT ATC TCA GC-3′ for Mu4. The general Ep-CAM sense primer was flanked by a 5′ NheI restriction site, and the mutant-specific reverse primers were flanked 3′ by HindIII, XhoI, and NotI restriction sites, respectively. The PCR products obtained were sequenced, and the sequence was controlled for any mistakes that could have occurred during the amplification. The mutant-specific PCR products, as well as the wild-type Ep-CAM cDNA, were subsequently cloned into the pMEP4 vector (Invitrogen BV, Leek, The Netherlands), containing the hygromycin resistance gene and the metallothionein promotor, which is inducible by divalent heavy metal ions (e.g., Zn2+ or Cd2+ ions). The vector also contains the OriP origin of replication and the EBNA-1 gene from the Epstein-Barr virus, which allows this vector to support self-replication in an episomal state in human and canine cells. In murine cells, the vector integrates into the chromosomal DNA.
Transfection of cells.
Cells were transfected with the DOTAP reagent (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer’s protocol. Stable clones of murine L cell transfectants were isolated as described previously (21); the transfectants of human HCA cells were selected and further cultured in the presence of 1 mg of hygromycin per ml in the culture medium.
Cell extraction with detergents.
Cells cultured on petri dishes were rinsed twice with ice-cold phosphate-buffered saline (PBS), and then the extraction buffer was added. The buffer contained 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 300 mM sucrose, complete protease inhibitor (Boehringer Mannheim), and one of the following detergents at various concentrations: Triton X-100 (0 to 1%), n-octyl-β-d-glucopyranoside (n-glucoside [0 to 100 mM]), CHAPS {3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate} (0 to 100 mM), or digitonin (0 to 0.5%). All detergents were from Boehringer Mannheim. Cells were incubated in extraction buffer at 4°C on a rotating platform for 15 min, scraped with a rubber policeman, collected, and spun down for 15 min at 15,000 × g. The supernatant (soluble fraction) was separated, and the collected pellet (insoluble fraction) was resuspended in buffer containing 1% Triton X-100–0.1% sodium dodecyl sulfate (SDS). The samples were used for gel electrophoresis. For immunofluorescent staining, the cells were incubated with the extraction buffer containing the designated detergent for 30 min on ice. The cells were rinsed with extraction buffer without the detergent, fixed with ice-cold methanol, air dried, and stained as described below.
Immunoblotting and immunoprecipitation.
Cells were extracted as described above. The lysates were cleared by centrifugation for 30 min at 15,000 × g and used for immunoprecipitation. Protein G-Sepharose beads (Pharmacia Biotech AB, Uppsala, Sweden) precoated with a specific antibody were incubated with cleared cell lysates for 2 to 16 h and washed five times in the same extraction buffer, and the immunoprecipitates were subjected to separation in a polyacrylamide gel.
Proteins were electrophoretically transferred from gels to Immobilon-P (Millipore, Bedford, Mass.) membrane. The Western blots were probed with mouse MAbs and were developed with the anti-mouse IgG Protoblot alkaline-phosphatase immunodetection system (Promega Biotec, Madison, Wis.) or the enhanced chemiluminescence detection system (Amersham Intl., Little Chalfont, United Kingdom).
Immunofluorescent staining.
Cells were grown on tissue culture plastic, fixed with 100% methanol (−20°C) for 15 min, and subsequently air dried. Alternatively, the cells were fixed in freshly prepared 4% paraformaldehyde in PBS–1 mM CaCl2–1 mM MgCl2 for 15 min on ice and permeabilized with 0.2% Nonidet P-40 (Sigma Chemical Co.). The fixed cells were blocked with 5% nonfat skim milk in PBS for 1 h at room temperature, washed, and incubated with a specific antibody. The cells were washed, and the bound MAb was detected with goat anti-mouse IgG–fluorescein isothiocyanate (FITC) or Texas red conjugate specific for the respective subclass of the MAb or with anti-rabbit IgG–FITC conjugate (Southern Biotechnology Associates, Inc., Birmingham, Ala.). After being washed in PBS and in distilled water, the preparations were embedded with Vectashield (Burlingame, Calif.) mounting reagent and were analyzed with the BRC-600 confocal fluorescent microscope (Bio-Rad Laboratories, Richmond, Calif.).
Flow cytometry.
Cells (106) were incubated for 1 h in the presence of the anti-Ep-CAM MAb 323/A3 (mouse IgG1), washed twice with cold PBS (by centrifugation and resuspension), and incubated with goat anti-mouse IgG1–FITC conjugate (Southern Biotechnology Associates, Inc.) for 1 h at 4°C. Cells were washed three times with cold PBS and analyzed by flow cytometry.
Cell aggregation assay.
Aggregation experiments were performed as described earlier (21). Briefly, cells were detached by treatment with 1 mM EDTA. Aggregation of cells was carried out in six-well plates (Nunc, Roskilde, Denmark). Single cells (5 × 105) resuspended in 2 ml of Ca2+-free HMCF (Hank’s solution containing 100 mM HEPES, 1% bovine serum albumin [BSA], and 100 μg of DNase I per ml) were placed in each well, and the plates were incubated on a rotating platform (100 rpm) at 37°C and 5% CO2. At distinct time points, 200-μl samples were analyzed with a CASY-1 cell counter (Scharfe System GmbH, Reutlingen, Germany) to determine the number of particles. At least 12 samples from two independent aggregation assays were measured. The degree of aggregation (D) was calculated as D = (N0 − Nt)/N0, where Nt is the number of remaining particles at time point t and N0 is the initial number of particles corresponding to the total number of cells (22). For prolonged aggregation (3), cells were detached with 0.05% trypsin and 1 mM EDTA, washed three times with PBS, resuspended in culture medium at a density of 0.5 × 106 cells/ml, and cultured overnight (16 h) on a rotating platform. Cell aggregates formed during the incubation period were gently dissociated by slow pipetting, and the cells were filtered through Mericloth to obtain monocellular suspensions and used for the aggregation assay as described above.
Cell adhesion to solid-phase-adsorbed Ep-CAM.
Adhesion assays were performed in 96-well tissue culture flat-bottom plates (Greiner B.V., Alphen a/d Rijn, The Netherlands) coated overnight with purified Ep-CAM (gift from D. Herlyn, Wistar Institute), produced in a baculovirus system (44) at a concentration of 10 μg of Ep-CAM protein in 50 μl of PBS per well. The coated wells were then blocked for 1 h with 1% BSA in PBS at 37°C and rinsed with PBS. Ten thousand cells labeled with 51Cr in 250 μl of PBS were added to each well. The cells were allowed to adhere to the well for 2 h, after which the wells were washed four times with PBS to remove the nonadherent cells. The adherent cells were lysed with 2% SDS–NaOH (1 M), and the released 51Cr was measured with a γ-counter.
α-Actinin binding assays.
To investigate the binding of labeled α-actinin to the wild-type and mutant Ep-CAMs, Mu1, -2, or -4 or wild-type Ep-CAMs from 1% Triton X-100 lysates of the transfected cell lines were preadsorbed to saturation on anti-mouse IgG Sac-Cel beads (IDS, Boldon, United Kingdom) coated with the 323/A3 MAb. After adsorption, the Sac-Cel beads were blocked with 1% BSA in PBS for 2 h and subsequently incubated in the presence of a 250,000-cpm/ml concentration of 125I-labeled chicken gizzard α-actinin in 1% BSA–PBS. After overnight incubation at 4°C, the beads were washed seven times. Bound 125I-α-actinin was measured with a gamma counter.
Peptides representing different fragments of the cytoplasmic tail of the Ep-CAM were cross-linked to the wells of 96-well Covalink plates (Nunc, Roskilde, Denmark). Cross-linking of the peptides to the NH2 groups of the plate was performed with 3-maleimidobenzoyl-N-hydroxy-succinimide ester (Boehringer Mannheim) via an extra cysteine residue added to the peptide’s NH2 terminus. The plates were washed and blocked with 1% BSA in PBS overnight. Chicken gizzard α-actinin (Sigma Chemical Co.) was iodinated with Iodogen beads (Pierce Chemical Co., Rockford, Ill.). A total of 100,000 cpm of 125I-α-actinin in 0.1% BSA–PBS was added per well to the peptide-coated 96-well plates. After overnight incubation at 4°C, the wells were washed five to seven times with 0.1% BSA–PBS. Bound 125I-α-actinin was measured with a gamma counter.
RESULTS
Cytochalasin D affects the subcellular localization of Ep-CAMs.
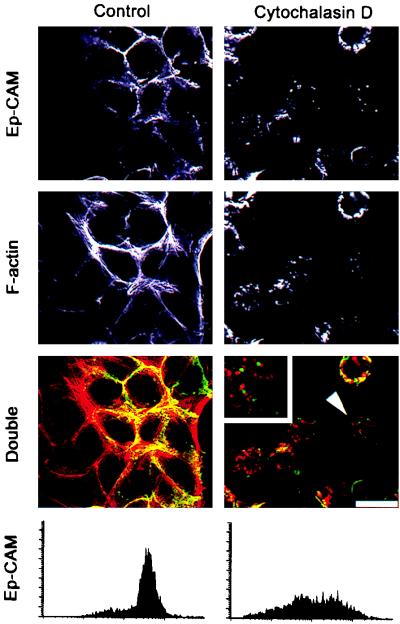
To investigate a possible interaction of Ep-CAM with the cytoskeleton, cells of a human mammary epithelial cell line, RC-6, which express endogenous Ep-CAM, were treated with agents that affect different types of cell cytoskeleton. Cytochalasin D (4), acrylamide (41), and colchicine (4) were used to disrupt, respectively, microfilaments (actin-containing cytoskeleton), intermediate filaments (cytokeratins), and microtubuli. The disruptive effect of the drugs was examined by immunostaining for the respective components of the different types of cytoskeleton: phalloidin-TRITC for actin microfilaments (Fig. 1), keratin 18 (not shown) for intermediate filaments, and tubulin (not shown) for microtubuli. After 2 h of culture of the cells in the presence of 10 μg of cytochalasin D per ml, a substantial portion of the cell surface Ep-CAM, previously localized at the cell-cell boundaries, was internalized (Fig. 1). The internalization was confirmed by immunostaining of living cells with anti-Ep-CAM MAb, which demonstrated a dramatic decrease in the number of Ep-CAMs at the cell surface (Fig. 1). As can be seen on double-immunofluorescent stainings, only Ep-CAM at the cell-cell boundaries colocalized with F-actin, in contrast to the intracellular Ep-CAM fraction. After the treatment with cytochalasin D, the depolymerized actin patches and intracellular Ep-CAM did not colocalize at large (Fig. 1), although some residual Ep-CAM at the areas of cell-cell contact still colocalized with actin.
FIG. 1.
Effect of cytochalasin D on the actin cytoskeleton and the subcellular localization of Ep-CAMs in human epithelial RC-6 cells. Control cells and cells treated for 2 h with 10 μg of cytochalasin D per ml were stained for either Ep-CAM with MAb 323/A3 (green fluorescence) or polymerized actin filaments with phalloidin-TRITC (red fluorescence). Note the substantial disappearance of the Ep-CAMs from the cell-cell boundaries after the treatment. Internalized Ep-CAM does not colocalize with actin patches in the treated cells in the cytochalasin D-treated, double-stained cells (note the cell marked by an arrowhead and presented at larger magnification in the upper left corner). The internalization of Ep-CAM was verified by staining with MAb 323/A3 of living RC-6 cells (flow cytometry histograms below). Bar, 25 μm.
Compared to the nontreated cells, no clear differences were seen with respect to Ep-CAM localization in cells treated with either acrylamide or colchicine (not shown). Stainings of the cells with antibodies against E-cadherin and desmoplakins I and II showed that the treatment with cytochalasin D also affected the adherens junctions, but not the desmosomes (not shown).
Detergent extractability of the Ep-CAMs.
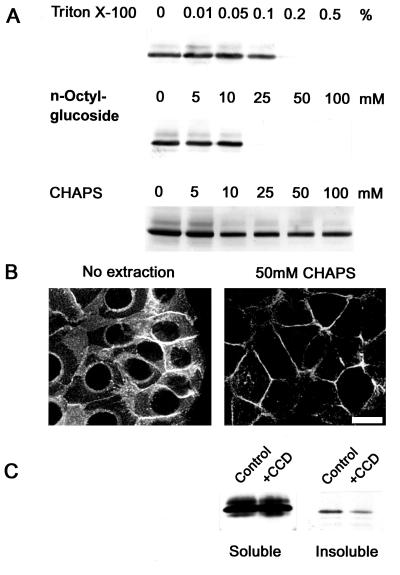
The relocation of the Ep-CAMs intracellularly induced by the treatment with cytochalasin D might indicate an interaction between Ep-CAM and the actin-based cytoskeleton. Therefore, we investigated whether an extraction of RC-6 cells with various detergents would allow us to discriminate between cytoskeleton-anchored and nonanchored fractions of the Ep-CAMs. Triton X-100 eluted no Ep-CAMs at concentrations below 0.1% and all Ep-CAMs at concentrations above 0.1% (Fig. 2A). Similarly n-octylglucoside eluted all Ep-CAMs at concentrations above 10 mM and no Ep-CAMs at lower concentrations (Fig. 2A). However, the zwitterionic detergent CHAPS did not elute any Ep-CAMs at concentrations of 0 to 10 mM, but did elute a discrete portion of them at concentrations above 10 mM. The remaining fraction of the Ep-CAMs could not be further extracted at CHAPS concentrations up to 100 mM (Fig. 2A). The residual cell-associated Ep-CAMs in detergent-extracted cells were localized at the cell-cell boundaries, presumably engaged in cell-cell adhesions, whereas the pool of the intracellular Ep-CAMs had been extracted (Fig. 2B). The size of the insoluble fraction of cellular Ep-CAM was 5 to 20%, as was estimated by titration of the total Ep-CAM along with insoluble Ep-CAM on Western blots, and varied between the individual experiments. Pretreatment of the cells with cytochalasin D decreased the fraction of the CHAPS-insoluble molecules approximately five times (Fig. 2C). This might be due to (i) dissociation of the Ep-CAM connections to the cytoskeleton or (ii) the increased intracellular pool of the Ep-CAMs that are detergent extractable.
FIG. 2.
Detergent-soluble and -insoluble fractions of cellular Ep-CAM. (A) Monolayers of RC-6 cells (106 cells/sample) were extracted with various concentrations of Triton X-100, n-octylglucoside, or CHAPS. The presence of Ep-CAM in detergent-insoluble pellets was detected by immunoblotting with MAb 323/A3. Note that CHAPS at concentrations above 10 mM discriminates between the detergent-soluble and -insoluble fractions of Ep-CAM. (B) The CHAPS-insoluble fraction of Ep-CAM represents the molecules that are localized at the cell-cell boundaries, as was revealed by immunofluorescent staining of the nonextracted and extracted (50 mM CHAPS) cells with MAb 323/A3. Note the disappearance of the intracellular fraction of Ep-CAM from the extracted cells. Bar, 20 μm. (C) Decrease of insoluble Ep-CAM in cells pretreated with cytochalasin D (+CCD [10 μg/ml, 2 h]) prior to extraction with 50 mM CHAPS.
To investigate whether these results indicate an association of Ep-CAM with the actin cytoskeleton, we generated a number of mutants with cytoplasmic domains truncated at various lengths.
Construction and expression of Ep-CAMs with a truncated cytoplasmic domain.
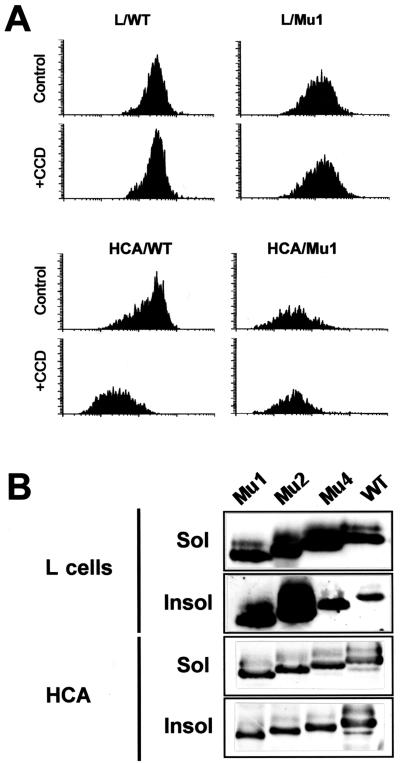
Three truncated forms of Ep-CAM were generated by PCR, as described in Materials and Methods. As shown in Fig. 3A, Mu1 has its cytoplasmic domain truncated up to amino acid 289, Mu2 has its truncated up to amino acid 296, and Mu4 has its truncated up to amino acid 304. The vectors with cDNAs for mutant or wild-type Ep-CAM, under the control of the inducible metallothionein promoter, were introduced by transfection into murine fibroblast L929 cells and into Ep-CAM-negative human epithelial HBL-100 (clone HCA) cells, respectively. As determined by SDS-polyacrylamide gel electrophoresis, the mobility of the mutant molecules expressed in the transfected cells differed from that of the wild-type Ep-CAMs to the expected degree (Fig. 3B). For all mutant forms, both the nonglycosylated and glycosylated forms were observed (not shown), and the differences in molecular weights between the two forms were similar for each mutant and the wild-type Ep-CAM. This suggests that the truncations in the cytoplasmic domain did not affect the glycosylation of the molecule. Molecules of similar molecular weights were found for each mutant form of Ep-CAM in both HCA and L929 transfectants (Fig. 4B).
FIG. 3.
Ep-CAM mutants with a deletion in the cytoplasmic domain. (A) Amino acid sequences of the intracellular domains for the wild-type (Wt) and mutant Ep-CAMs. (B) Expression of the wild-type and mutant Ep-CAMs in transfected human epithelial HCA cells, as detected by immunoblotting with MAb 323/A3 in lysates of individual cell lines transfected with the respective form of Ep-CAM.
FIG. 4.
Internalization and extractability of mutant and wild-type (WT) Ep-CAMs in transfected cells. (A) Decrease of surface wild-type Ep-CAM after cytochalasin D (+CCD) treatment as observed in HCA cells and not in L cells. The CCD treatment had no effect on the presence of the tailless Mu1 molecules at the cell surface in both cell lines. Cells were pretreated with 10 μg of cytochalasin D per ml for 2 h, detached, and stained with MAb 323/A3, and the immunofluorescence was analyzed by flow cytometry. The results are presented as flow cytometry histograms. (B) Extractability of mutant Ep-CAMs from HCA and L cells. The cells were lysed in 50 mM CHAPS, and the soluble (Sol) and insoluble (Insol) fractions were analyzed by immunoblotting with MAb 323/A3.
All mutants, similar to the wild-type Ep-CAMs, were transported to and expressed at the cell surface of the respective transfected cell lines, as was determined by flow cytometry. Upon induction of the transfected constructs by addition of heavy metal ions to the medium (1 to 10 μM CdCl2), enhanced levels of Ep-CAMs in cell lysates and at the cell surface were found for all mutants, as well as for wild-type Ep-CAM, as was determined by immunoblotting and flow cytometry, respectively (not shown). By modulating the expression levels of mutants in transfectants with different concentrations of CdCl2, it was possible to achieve approximately equal levels of Ep-CAMs at the surface of transfected cells.
The cytoplasmic domain is not required for the detergent insolubility of Ep-CAM.
We tested whether the deletions in the cytoplasmic domain would affect (i) their partial internalization upon depolymerization of the actin cytoskeleton and (ii) the detergent solubility of the Ep-CAMs. The treatment of L cell transfectants with cytochalasin D did not affect the presence of either wild-type Ep-CAMs or Mu1 molecules at the cell surface (Fig. 4A). To the contrary, in the HCA cells, the wild-type molecules were internalized upon this treatment, similar to what was observed for RC-6 cells. Mu1 Ep-CAMs did not internalize in relation to the disruption of the actin cytoskeleton (Fig. 4A). This indicates that internalization of the wild-type Ep-CAMs observed for epithelial cells most likely reflects some regulatory mechanism controlling the molecule’s presence at the cell surface, which is present in epithelial cells but absent in L cells.
Extraction of the wild-type and mutant forms with CHAPS from both L cell and HCA cell transfectants demonstrated that the presence of the cytoplasmic domain is not required for the detergent insolubility of the molecule (Fig. 4B). All mutant molecules had some nonextractable fraction in both cell lines, although the relative ratios between the soluble and insoluble forms varied for the different mutant forms. The low extractability was especially clear for Mu1 and Mu2 in L cells. Immunofluorescent staining of extracted cells showed that only the intracellular Ep-CAM was extractable, similar to the extraction observed for RC-6 cells (not shown). This agrees well with the low extractability for Mu1 and Mu2, since in cells transfected with these two mutants, the level of intracellular Ep-CAM was substantially lower than that for Mu4 and the wild-type molecules.
Despite the fact that in RC-6 cells the CHAPS extractability allowed discrimination between the fraction of Ep-CAMs involved in cell-cell adhesion and the intracellular fraction of Ep-CAM, the nonextractability does not reflect an interaction of the cytoplasmic domain of Ep-CAM with the cytoskeleton and most likely reflects an association of the Ep-CAM’s transmembrane domain with detergent-insoluble lipids, as was previously reported for the CD44 molecule (34).
The cytoplasmic domain is required for localization of Ep-CAM at the cell-cell boundaries.
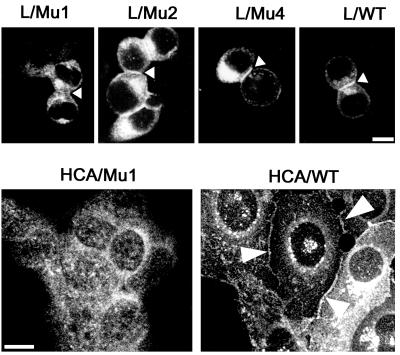
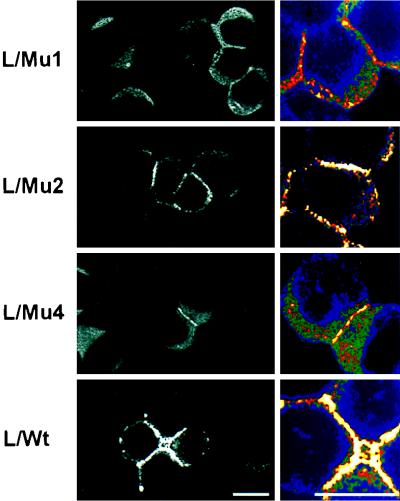
After transfection into L cells, the wild-type Ep-CAM localizes to the cell-cell boundaries (21). Apparently, the presence of the cytoplasmic tail is required for the intercellular localization of Ep-CAM, as was determined by immunofluorescent staining of L cell transfectants expressing different truncated forms of Ep-CAM (Fig. 5). The cells were seeded at high density to be forced into close proximity with each other, even if they did not interact well. Under these conditions, Mu2 and Mu4 molecules were capable of localizing at the cell-cell boundaries, similar to the wild-type molecules. In contrast, Mu1 molecules, which lack the entire cytoplasmic domain, were distributed all over the surface of L cell transfectants.
FIG. 5.
Subcellular localization of the wild-type and mutant Ep-CAMs in transfected fibroblast L cells and human epithelial HCA cells. The cells were fixed and stained with the anti-Ep-CAM MAb 323/A3, followed by a secondary FITC-labeled antimouse antibody. Note the absence of Mu1 molecules and the presence of Mu2, Mu4, and wild-type (WT) molecules at the cell-cell boundaries between L cell transfectants (marked by arrowheads). Similarly, in HCA cells, Mu1 molecules were located on the entire cell surface, in contrast to the wild-type Ep-CAM, which was present at the cell-cell boundaries (marked by arrowheads). Mu2 and Mu4 molecules were localized in HCA cells similar to the wild type (not shown). Bar, 10 μm.
In Ep-CAM-transfected cells that have other means of intercellular adhesion, such as epithelial HCA cells, in which the presence of N- and P-cadherin-mediated adherens junctions (25) contributes to the cell polarization, Mu1 Ep-CAMs were present all over the cell surface, in contrast to Mu2, and Mu4 molecules that were localized at cell-cell boundaries of the transfected HCA cells, similar to the wild-type Ep-CAM (Fig. 5).
In summary, the deletion of the cytoplasmic domain of Ep-CAM affected the ability of the molecule to localize into the cell-cell boundaries, which likely reflects the affected adhesion properties of the molecule.
Deletions in the cytoplasmic domain affect the ability of Ep-CAM to mediate cell adhesion.
Because HCA cells have their own means of intercellular interaction, and the disruption of the actin cytoskeleton affects the presence of Ep-CAM at the cell-cell boundaries, we used L cell transfectants to investigate the impact of the cytoplasmic domain and its possible association with actin cytoskeleton on the ability of Ep-CAM to direct cell aggregation.
L cell transfectants expressing approximately equal amounts of either mutant or wild-type Ep-CAM molecules (as estimated by flow cytometry) were tested in aggregation assays (Fig. 6A). The truncations affected the ability of all mutants to direct cell aggregation, compared to that of the wild-type molecule. However, both Mu2 and Mu4 demonstrated some, although rather little, ability to mediate cell aggregation within 2 h, in contrast to Mu1, which was not able to mediate cell aggregation at all (compared with aggregation of the parental cells). To evaluate more precisely the differences between the abilities of tested forms of Ep-CAM to mediate cell aggregation, we used the prolonged-cell-aggregation assay (3). After overnight culture in suspension, the cell aggregates were resuspended again as single-cell suspensions, and the cells were allowed to aggregate in either the presence or absence of cytochalasin D for 4 h. Treatment with cytochalasin D had previously been demonstrated to affect cell aggregation mediated by classic cadherins (18), which also depends on the interaction of cadherin molecules with the actin-based cytoskeleton. Cytochalasin D greatly affected not only aggregation of the wild-type Ep-CAM transfectants (Fig. 6B and C), but also that of the Mu2 and Mu4 transfectants, reducing the aggregation of the cells to the level of that of the Mu1 transfectants (Fig. 6B). Although in the latter experiments we used a clone that expressed twice as many Mu1 molecules at the cell surface as the clone with wild-type Ep-CAM, Mu1 was not able to mediate cell aggregation, and some background aggregation demonstrated by these cells was insensitive to cytochalasin D. A small difference in aggregation of Mu2 and Mu4 transfectants is likely related to the slight differences in the levels of expression between the two mutant forms. Both Mu2 and Mu4 transfectants formed multicellular aggregates in the absence of cytochalasin D that were substantially smaller than those formed by the wild-type Ep-CAM transfectants.
FIG. 6.
Effect of truncations in the cytoplasmic domain on cell adhesion properties of the Ep-CAM in L cells. (A) Aggregation in suspension (2 h in the absence of Ca2+) of the L929 transfectants, expressing various forms of Ep-CAM, is presented as the degree of aggregation. The relative levels of Ep-CAM expression at the surface of the transfectant cells used for the assay are presented (here and in panels B and D) above the bars as mean cell fluorescence, determined by flow cytometry with the 323/A3 MAb. LMC, mock transfectants of L cells. (B) After overnight culture in suspension, the cell aggregates were dispersed, and the cells were allowed to aggregate for 4 h in either the absence (solid bars) or presence (open bars) of 10 μg of cytochalasin D per ml in the aggregation media. For aggregation assays (A and B), the data presented were obtained from 12 independent measurements, and the standard deviation did not exceed 10%. (C) Micrographs of aggregates formed by L cells transfected with wild-type Ep-CAM in the presence and absence of cytochalasin D. (D) Adhesion of wild-type, Mu1, and blank vector (mock)-transfected L cells to the solid-phase-adsorbed purified Ep-CAM. The assay was performed as described in Materials and Methods, and the results are presented as percentages of cells attached to the substrate after a 2-h assay. The data presented were obtained from 12 independent measurements. The standard deviation did not exceed 10%.
Wild-type Ep-CAMs mediate homotypic binding (21). However, deletions in the cytoplasmic tail might change the ligand specificity of the adhesion molecule, as has been shown for PECAM (7), or might affect the avidity of the adhesion molecule to its ligand, as demonstrated for CD2 (13). To determine whether Mu1 Ep-CAM can still bind homotypically and have an avidity to a self-type ligand, we tested the adhesion of the Mu1 and wild-type Ep-CAM transfectants to purified Ep-CAM adsorbed on the surface of 96-well plates. As shown in Fig. 6D, both Mu1 and wild-type Ep-CAM transfectants adhered equally well to the solid-phase-adsorbed Ep-CAM.
Redistribution of α-actinin accompanies the formation of Ep-CAM-mediated adhesions.
The results presented above suggested that the deletion of the cytoplasmic domain did not affect the specificity of the Ep-CAM for its homotypic ligand but did affect the ability of the molecule to form stable intercellular adhesions. We investigated, therefore, whether the formation of cell-cell adhesions by the wild-type molecule is associated with a redistribution to the cell-cell boundaries of any molecules known to be associated with the cytoplasmic domain complexes of other adhesion molecules interacting with the actin-based cytoskeleton (1, 12, 51).
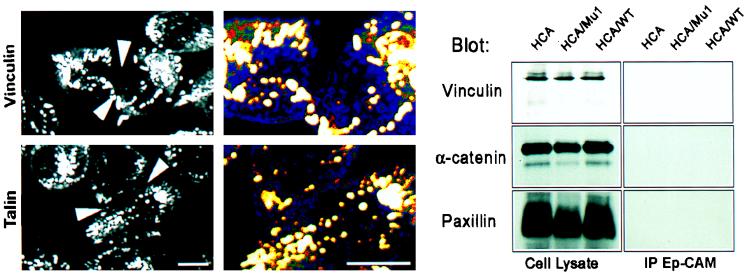
The parental L cells and transfectants for mutant and wild-type Ep-CAMs were grown in dense cultures, so that even poorly interacting cells could establish some intercellular contacts. Upon fixation, the cells were stained for α-actinin, talin, vinculin, filamin, spectrin, α-catenin, and β-catenin. When these experiments were performed with Mu1, -2, and -4 and wild-type Ep-CAM transfectants, all forms of Ep-CAM, except Mu1, induced the redistribution of α-actinin (Fig. 7), but not that of spectrin (not shown) or filamin (not shown), to the cell-cell boundaries. Accumulation of α-actinin along the intercellular boundaries, formed by Ep-CAM-mediated adhesions, was not accompanied by the accumulation of talin, vinculin, or catenins in these areas (see Fig. 10). At the same time, the transfectants showed the expected localization of vinculin and talin in focal adhesions. In control L cells transfected with E-cadherin, the expected presence of α- and β-catenins at the cell-cell boundaries was observed. L cells do not contain significant levels of either type of catenin; however, the catenins accumulate when these cells are transfected with E-cadherin cDNA. Transfection of L cells with Ep-CAM cDNA did not induce accumulation of α- and β-catenins in the transfectants, as was determined by immunoblotting (not shown). This makes the involvement of catenins in interconnections between Ep-CAM and the cytoskeleton highly unlikely.
FIG. 7.
Distribution of α-actinin in L cell transfectants expressing different forms of Ep-CAM, as detected by immunofluorescent staining of fixed cells with an α-actinin MAb, CB-11. Note the concentration of α-actinin along the areas of the intercellular contacts in Mu2, Mu4, and wild-type (WT) transfectants, in contrast to Mu1 transfectants. On the right are shown enlarged areas of cell-cell contact; the intensity of the fluorescent signal is presented as a pseudocolor increasing from blue to white. Note that the concentration of α-actinin at the cell-cell boundaries of Mu1 transfectants does not differ from the average density in cytoplasm, in contrast to all other cell types. Bar, 10 μm.
FIG. 10.
Vinculin, talin, and α-catenin are not involved in the Ep-CAM adhesion complex. (Left) Immunofluorescent staining of interacting wild-type Ep-CAM-transfected L (L/WT) cells with antibodies to vinculin and talin. Note the absence of both proteins in the areas of cell-cell contact, as marked by arrows, and their presence in cell-substrate adhesions. The marked areas are also presented at a larger magnification as pseudocolor pictures demonstrating that both molecules are present at the cell-cell boundaries at their average concentration in other areas not involved in adhesion. (Right) Immunoblotting of total lysates and immunoprecipitates (IP) from the n-octylglucoside lysates with anti-Ep-CAM MAb from HCA cell transfectants (similar to Fig. 9, the same precipitates are involved). Note the absence of all three molecules in immunoprecipitates. Bar, 10 μm.
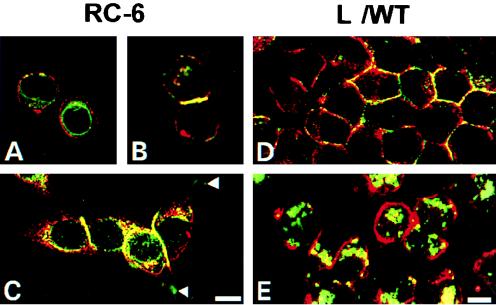
Double-immunofluorescent stainings for Ep-CAM and α-actinin have shown that both in RC-6 cells and in L cell transfectants, only the fraction of Ep-CAM involved in cell-cell adhesion (which is present at the cell-cell boundaries) colocalized with α-actinin (Fig. 8). Intracellular Ep-CAM does not colocalize with α-actinin (Fig. 8C and D) and neither does Ep-CAM that was present at the cell surface of single cells (Fig. 8A). Colocalization of Ep-CAM and α-actinin was observed only when two single cells established a contact (Fig. 8B). In well-spread RC-6 cells, Ep-CAM colocalized with α-actinin only at the lateral domains of cells engaged in intercellular contact, but not in other sites where α-actinin was abundantly present, such as areas of cell-substrate adhesions (Fig. 8C). Treatment with cytochalasin D of L/WT cells did not affect the presence of Ep-CAM at the cell surface; however, it did not colocalize with α-actinin any longer, with the majority of the latter molecules being relocated from the juxtamebrane space into cytoplasm (Fig. 8E). Taking into account the fact that cytochalasin D affects the ability of Ep-CAM to mediate cell aggregation, these observations strongly suggest that α-actinin may interact with Ep-CAMs and that this interaction is directly related to the formation of Ep-CAM-mediated cell-cell adhesions.
FIG. 8.
Colocalization of Ep-CAM and α-actinin in RC-6 (A, B, and C) and L (D and E) cells. Double-immunofluorescence staining was performed with fixed RC-6 and L cells with MAb 323/A3 for Ep-CAM (red fluorescence) and MAb CB-11 for α-actinin (green fluorescence). The L cells shown in panel E were cultured for 2 h in the presence of 10 μg of cytochalasin D per ml prior to fixation. Note the absence of colocalization between the two molecules in single cells and also in the areas of adhesion plaques (arrows), as well as in L cells treated with cytochalasin D. Bars, 10 μm (A, B, and C) and 5 μm (D and E).
Ep-CAM is directly associated with α-actinin.
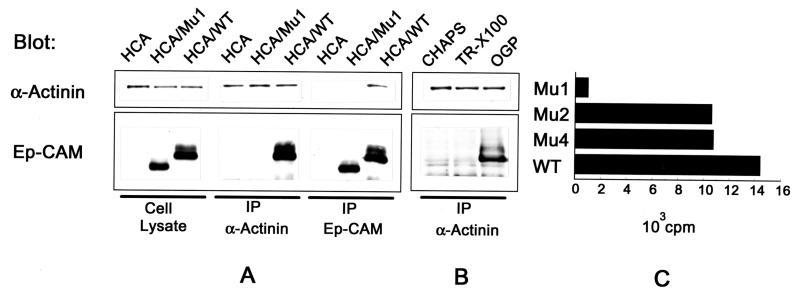
To investigate whether Ep-CAM directly interacts with α-actinin, immunoprecipitations were performed from lysates of HCA cells expressing either wild-type Ep-CAM or Mu1. As shown in Fig. 9A, anti-α-actinin antibody coprecipitated the wild-type, but not the Mu1 Ep-CAMs. The reverse precipitation with an anti-Ep-CAM MAb (reactive with an epitope in the extracellular domain of the molecule) showed that α-actinin was coprecipitated only with molecules that contained the cytoplasmic domain (Fig. 9A). The coprecipitation of Ep-CAM with α-actinin was observed only in the lysates prepared with 50 mM n-octylglucoside as a solubilizing detergent, but not with Triton X-100 at concentrations above 0.1%, although both detergents were shown to elute all cellular Ep-CAMs (Fig. 9B). Apparently, Triton X-100 seems to disrupt the binding of Ep-CAM to α-actinin, whereas n-octylglucoside elutes all Ep-CAMs without disrupting the complex of α-actinin and Ep-CAM. When the CHAPS-extractable molecules were immunoprecipitated, no coprecipitation of α-actinin was observed (Fig. 9B). Although the immunoprecipitations from HCA cells are more demonstrative (due to approximately 10 times higher levels of Ep-CAM per total cell protein in the transfectants), coprecipitations of Ep-CAM and α-actinin were also demonstrated for L cell transfectants and RC-6 cells (not shown).
FIG. 9.
Interaction of Ep-CAM and α-actinin. (A) Detection of Ep-CAM and α-actinin by immunoblotting with the respective antibodies (323/A3 and CB-11) in cell lysates and immunoprecipitates (IP) obtained with antibodies to Ep-CAM (MAb 323/A3) and α-actinin (polyclonal serum) from 50 mM n-octylglucoside extracts of the parental HCA cells, Mu1, or wild-type (WT) Ep-CAM transfectants. (B) Detection of Ep-CAM and α-actinin in immunoprecipitates with anti-α-actinin antibody from lysates of HCA/WT transfectants prepared with either CHAPS (50 mM), Triton X-100 (0.2%), or n-octylglucoside (OGP [50 mM]). Note that Ep-CAM is coprecipitated only from the lysates obtained with n-octylglucoside. (C) Binding of α-actinin to a solid-phase immobilized wild-type and mutant Ep-CAM molecules. Mu1, -2, and -4 and wild-type Ep-CAM from 1% Triton X-100 (TR-X100) lysates of the respective HCA cell transfectants were immobilized on Sac-Cel beads precoated with anti-Ep-CAM MAb. Binding of 125I-α-actinin to the immobilized wild-type or mutant Ep-CAM molecules is presented after subtraction of the background binding of α-actinin to the beads precoated with nontransfected HCA cell lysate.
When the precipitation was performed with an anti-Ep-CAM MAb, no vinculin, talin, or α- or β-catenin was detected in precipitates on immunoblots performed with the respective antibodies (Fig. 10).
To confirm the coimmunoprecipitation results and to analyze the binding of α-actinin to Mu2 and Mu4 of Ep-CAM, we investigated the binding of α-actinin to solid-phase-adsorbed mutant and wild-type Ep-CAMs. The wild-type and mutant Ep-CAMs from 1% Triton X-100 lysates of the respective HCA transfectants were adsorbed at approximately equimolar amounts to Sac-Cel beads coated with the anti-Ep-CAM MAb, and the beads were used in 125I-α-actinin binding assays. As is shown in Fig. 9C, the beads with immobilized Mu2, Mu4, and wild-type Ep-CAMs were capable of binding 125I-α-actinin, in contrast to Mu1. However, the binding of 125I-α-actinin to Mu2 and Mu4 Ep-CAMs was reduced compared to the binding of 125I-α-actinin to wild-type Ep-CAMs.
Two binding sites for α-actinin are present in the cytoplasmic domain of Ep-CAM.
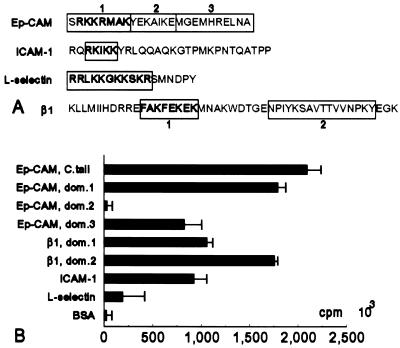
Because Mu1 Ep-CAMs did not bind α-actinin, and Mu2 and Mu4 Ep-CAM molecules did, but showed a reduced binding capacity compared to the wild-type Ep-CAM, it appears that the domain 1 (Fig. 10A) of the cytoplasmic domain is involved in α-actinin binding, while domain 2 has no impact on binding, and the remaining part of the cytoplasmic domain (domain 3), which is present only in the wild-type Ep-CAM molecule, has some additional effect on α-actinin binding (Fig. 11A). To confirm this assumption, peptides representing the three domains of the Ep-CAM cytoplasmic tail were covalently bound to a 96-well plate, and, subsequently, 125I-α-actinin was added to the wells. Figure 11B shows that the domain 1 and domain 3 peptides were both capable of binding α-actinin, in contrast to the domain 2 peptide, which did not bind α-actinin. The peptide corresponding to the complete Ep-CAM cytoplasmic domain demonstrated increased α-actinin binding compared to either domain 1 or domain 3, which suggests simultaneous involvement of both domains 1 and 3 in α-actinin binding to the cytoplasmic tail of Ep-CAM. Under the conditions tested, the control peptides representing α-actinin binding sequences from the cytoplasmic domains of β1 integrin, intercellular adhesion molecule 1 (ICAM-1), and L-selectin (Fig. 11A) showed binding activity similar to or lower than (for L-selectin) that of the peptides (domains 1 and 3) from Ep-CAM (Fig. 11B).
FIG. 11.
Binding of α-actinin to peptides representing the fragments of cytoplasmic domains of various CAMs. (A) Sequences of the cytoplasmic domains of Ep-CAM, ICAM-1, L-selectin, and β1 integrin. Boxes indicate various peptides used for the assay. Numbers indicate the respective domains of the cytoplasmic tail of Ep-CAM or β1 integrin. Boldface letters indicate the previously identified α-actinin binding sequences within the cytoplasmic domains of ICAM-1, L-selectin, and β1 integrin molecules and the sequence of a similar amino acid composition in the cytoplasmic domain of Ep-CAM. (B) Binding of purified 125I-labelled α-actinin to various cytoplasmic domain peptides (tested in parallel assays [n = 10]; the error bars represent ± 2ς). The whole cytoplasmic domain of Ep-CAM (C. tail) or its separate fragments (domains [dom.] 1, 2, and 3) were used. Peptide sequences used for other molecules are indicated in panel A by the fragments in boxes.
DISCUSSION
Interaction with the cytoskeleton is involved in a number of functions of adhesion molecules, such as the ability to form junctions and the mediation of outside-inside and inside-outside signaling (6, 9, 15, 16, 37, 40, 48, 49). Ep-CAM is a new type of adhesion molecule not structurally related to cadherins, integrins, Igs, or selectins (21, 22), and as was discussed in the introduction, it seems to be an adhesion receptor associated with a highly proliferative state of epithelial cells. Previous results suggest that one of the possible functions for Ep-CAM in epithelial cells is the regulation of cadherin-mediated adhesions and that the cytoplasmic domain of the molecule is essential for this effect (25).
Here we have demonstrated that the cytoplasmic domain is required for the adhesion function of the Ep-CAM, since the cytoplasmic truncation affects the ability of the molecule to form stable adhesions (Fig. 5 and 6), although it does not seem to affect the homotypic interaction between Ep-CAMs. The cytoplasmic domain of Ep-CAM mediates interactions of the molecule with the actin cytoskeleton, and stable Ep-CAM-mediated intercellular adhesions may be formed only with the availability of F-actin for the anchorage of the Ep-CAMs (Fig. 6 and 8). Ep-CAM is anchored to the actin cytoskeleton via α-actinin, with the latter molecule bound directly to the cytoplasmic domain of the Ep-CAM (Fig. 7 to 9). The results of assays of α-actinin binding to Ep-CAMs with their cytoplasmic domains truncated at different lengths and to peptides representing various fragments of the Ep-CAM cytoplasmic domain indicated the presence of two independent α-actinin binding sites within the cytoplasmic tail of Ep-CAM, localized at positions 289 to 296 and 304 to 314 of the amino acid sequence of the Ep-CAM, respectively (Fig. 9C and 11).
Many adhesion molecules interact with the actin cytoskeleton—some directly like the α2 integrin (17), and others indirectly, such as the β1, β2, and β3 integrins, ICAMs, L-selectin, and cadherins (12). Ep-CAM interacts with the actin cytoskeleton via α-actinin, a molecule mediating binding to cytoplasmic domain of a number of other molecules: β1, β2, and β3 integrins (28, 32), ICAM-1 (4), and L-selectin (31). Comparison of the motifs that are present in the cytoplasmic domains of molecules interacting with α-actinin, including Ep-CAM, reveals no clear similarities, except for the arginine- and lysine-rich consensus that can be found in the first Ep-CAM α-actinin binding site and the α-actinin binding sites of ICAM-1 and L-selectin (Fig. 11A). Most likely, the overall charge of the binding site is crucial for the binding of α-actinin, as was suggested for the cytoplasmic domains of β1 integrin (29, 36) and ICAM-1 (4). At the same time, scrambled peptides of the α-actinin binding sequences from the cytoplasmic domains of β1 integrin and ICAM-1 revealed that at least some sequence specificity should be present for α-actinin binding, since not all scrambled peptides were capable of binding α-actinin (4, 29). For β1 integrin, also containing two sites for α-actinin binding, it is still questionable whether both of them are engaged in α-actinin binding in the cytoplasmic complex of integrins (29), although it is seems that both are required for the positioning of the β1 integrin into cell-matrix adhesion contacts (36). Only the first α-actinin binding site, FAKFEKEKMN, may actually be bound to α-actinin (29, 36); alternatively, the folding of the cytoplasmic domain in the native integrin may bring the two segments together, in such a way that they both form a three-dimensional α-actinin binding site (29). In the case of Mu2 Ep-CAM, containing only one α-actinin binding domain, the molecule could localize into cell-cell boundaries and form cell-cell connections, but has a reduced ability to mediate cell aggregation, demonstrating the requirement of both binding sites for the fully functional Ep-CAM.
The first α-actinin binding site in the Ep-CAM cytoplasmic tail is located in immediate proximity to the transmembrane domain, similar to ICAM-1 and L-selectin molecules, and in contrast to the β1 integrin cytoplasmic domain, where the first site is 11 amino acids away from the end of the transmembrane domain. However, the two binding sites in both the Ep-CAM and β1 integrin cytoplasmic domains are approximately the same distance from each other (10 amino acids for Ep-CAM and 9 amino acids for β1 integrin), which supports the suggestion that the two sites form a conformational structure that effectively binds α-actinin.
Different molecules can be additionally associated with the complex formed by α-actinin and the cytoplasmic tails of various CAMs, such as vinculin, which is present within the complex between β1 integrin and α-actinin (28) and which associates with α-actinin in the complex formed by L-selectin (31). Talin is also present in both complexes; however, neither talin nor vinculin was found in the cytoskeleton anchorage complexes of ICAM-1 and β2 integrin (4, 32). Ep-CAM seems to interact with α-actinin directly, and we did not observe talin, vinculin, or catenins in this complex. It is interesting that the fraction of the Ep-CAMs that is detected intracellularly, as we reported earlier (22), seems not to be associated with α-actinin (Fig. 8 and 9). Only the molecules that are present at the cell-cell boundaries and that are presumably engaged in contact with their counterpart on the other cell colocalize with α-actinin. We also observed that in single cells, Ep-CAMs that are at the cell surface do not colocalize with α-actinin, whereas in interacting epithelial cells they do, and we can hardly find areas where the surface Ep-CAM is not colocalized with α-actinin. It is highly suggestive that in interacting epithelial cells, all Ep-CAMs either are anchored via α-actinin to the actin cytoskeleton, being engaged in cell-cell adhesion, or are otherwise eliminated from the cell surface. For epithelial cells, there clearly exists a mechanism controlling the presence of Ep-CAM at the cell surface (Fig. 1 and 4). When the cytoskeleton connections are disrupted (i.e., by treatment with cytochalasin D), the Ep-CAMs can no longer participate in cell-cell contact, which results in the internalization of Ep-CAM. The internalization of many type I transmembrane proteins is controlled by the cytoplasmic domain, and indeed an internalization-related motif, YEKA (19, 39), is present in the Ep-CAM at position 297 to 300 (Fig. 11A). The absence of this motif in Mu1 and Mu2 peptides may, in particular, explain their low extractability with CHAPS. What mechanism is behind the control of Ep-CAM’s presence at the cell surface remains to be investigated, but it is plausible that one of the mechanisms is the availability of α-actinin to participate in the molecule’s connection to the cytoskeleton.
Since transfectants expressing Mu1 Ep-CAM bind to solid-phase-adsorbed Ep-CAM as well as those expressing wild-type Ep-CAM, the deletion of the cytoplasmic domain does not seem to affect the homotypic binding properties of the molecule, but instead affects the ability of the molecules to form stable adhesions. When one of the interacting molecules is stabilized, as in the case of solid-phase-adsorbed Ep-CAM, the cells expressing Mu1 Ep-CAMs were able to form relatively stable contacts. In this respect, Ep-CAM resembles E-cadherin (26), C-CAM (5), and myelin protein 0 (50), all of which are homotypic adhesion molecules that require a cytoplasmic domain to mediate cell-cell adhesions. In contrast, a glycophosphatidylinositol-linked mutant of ICAM-1 is fully functional (4). It’s plausible that the importance of the cytoplasmic domain for many homophilic adhesion molecules is caused by the necessity of their oligomerization when forming adhesions.
The involvement of α-actinin in interactions with the cytoskeleton of several major types of adhesion molecules involved in cell-cell and cell-substrate interactions of epithelial cells suggests that this protein may be one of the central molecules regulating the coordinated expression of different types of adhesion molecules. It is known that the amount of cellular α-actinin may be crucial for defining the cell phenotype, since a twofold increase in cellular α-actinin suppresses the tumorigenicity of simian virus 40-transformed 3T3 cells (10). Up-regulation of Ep-CAM was observed in actively proliferating cell populations (24) and in some carcinoma cell lines may be up to 109 Ep-CAMs per cell (unpublished observation). Therefore, it is conceivable that overexpression of Ep-CAM may cause a major redistribution of cellular α-actinin and, as a consequence, may affect other types of adhesion systems that employ α-actinin in their connections to the cytoskeleton. The abrogation of adherens junctions mediated by classical cadherins upon expression of ectopic Ep-CAM in transfected cells was indeed observed (25). The binding of Ep-CAM to the actin cytoskeleton mediated by α-actinin may contribute to the cross talk between various cell-cell and cell-matrix adhesion systems.
ACKNOWLEDGMENTS
This research was supported by the Dutch Cancer Foundation (grant RUL 95-1107) and a research grant from Centocor, Inc., Malvern, Pa.
REFERENCES
- 1.Ben-Ze’ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- 2.Bergsagel P L, Victor-Kobrin C, Timblin C R, Trepel J, Kuehl W L. A murine cDNA encodes a pan-epithelial glycoprotein that is also expressed on plasma cells. J Immunol. 1992;148:590–596. [PubMed] [Google Scholar]
- 3.Berndorff D, Gessner R, Kreft B, Schnoy N, Lajous-Petter A-M, Loch N, Reutter W, Hortsch M, Tauber R. Liver-intestine cadherin: molecular cloning and characterization of a novel Ca2+-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol. 1994;125:1353–1369. doi: 10.1083/jcb.125.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpén O, Pallai P, Staunton D E, Springer T A. Association of intercellular adhesion molecule-1 (I-CAM1) with actin-containing cytoskeleton and α-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung P H, Culic O, Qui Y, Early K, Thompson N, Hixson D C, Lin S H. The cytoplasmic domain of C-CAM is required for C-CAM mediated adhesion function: studies of a C-CAM transcript containing an unspliced intron. Biochem J. 1993;295:427–435. doi: 10.1042/bj2950427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 7.DeLisser H M, Chilkotowsky J, Yan H-C, Daise M L, Buck C A, Albelda S M. Deletions in the cytoplasmic domain of platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J Cell Biol. 1994;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards D P, Grzyb K T, Dressler L G, Mansel R E, Zava D T, Sledge G W, Jr, McGuire W L. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986;46:1306–1317. [PubMed] [Google Scholar]
- 9.Fagotto F, Funayama N, Glück U, Gumbiner B M. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glück U, Kwiatkowski D J, Ben-Ze’ev A. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with α-actinin cDNA. Proc Natl Acad Sci USA. 1993;90:382–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green S A, Setiadi H, McEver R P, Kelly R B. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumbiner B M. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron. 1993;11:551–564. doi: 10.1016/0896-6273(93)90068-3. [DOI] [PubMed] [Google Scholar]
- 13.Hahn W C, Bierer B E. Separable portion of the CD2 cytoplasmic domain involved in signalling and ligand avidity regulation. J Exp Med. 1993;178:1831–1836. doi: 10.1084/jem.178.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.High A S, Robinson P A, Klein C E. Increased expression of a 38 kd cell-surface glycoprotein MH99 (KS1/4) in oral mucosal dysplasias. J Oral Pathol Med. 1996;25:10–13. doi: 10.1111/j.1600-0714.1996.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 15.Hynes R O. Integrins: versatility, modulation, and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 16.Juliano R L, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieffer J D, Plopper G, Ingber D E, Hartwig J H, Kupper T S. Direct binding of F actin to the cytoplasmic domain of the α2 integrin chain in vitro. Biochem Biophys Res Commun. 1995;217:466–474. doi: 10.1006/bbrc.1995.2799. [DOI] [PubMed] [Google Scholar]
- 18.Kreft B, Berndorff D, Böttinger A, Finnemann S, Wedlich D, Hortsch M, Tauber R, Gessner R. Ll-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions. J Cell Biol. 1997;136:1109–1121. doi: 10.1083/jcb.136.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ktistakis N T, Thomas D, Roth M G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990;111:1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnenbach A J, Seng B A, Wu S, Robbins S, Scollon M, Pyrc J J, Druck T, Huebner K. Retroposition in a family of carcinoma-associated antigen genes. Mol Cell Biol. 1993;13:1507–1515. doi: 10.1128/mcb.13.3.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litvinov S V, Velders M P, Bakker H A M, Fleuren G J, Warnaar S O. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvinov S V, Bakker H A M, Gourevitch M M, Velders M P, Warnaar S O. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhesion Commun. 1994;2:417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- 23.Litvinov S V. Ep-CAM: a homophilic cell-cell adhesion molecule with EGF-like domains. Trends Glycosci Glycotechnol. 1995;7:375–384. [Google Scholar]
- 24.Litvinov S V, van Driel W, van Rhijn C M, Bakker H A M, van Krieken H, Fleuren G J, Warnaar S O. Expression of Ep-CAM in cervical squamous epithelia correlates with an increased proliferation and the disappearance of markers for terminal differentiation. Am J Pathol. 1996;148:865–875. [PMC free article] [PubMed] [Google Scholar]
- 25.Litvinov S V, Balzar M, Winter M J, Bakker H A M, Briaire-de Bruijn I H, Prins F, Fleuren G J, Warnaar S O. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J Cell Biol. 1997;139:1337–1348. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–3694. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neame S J, Isacke C M. The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J Cell Biol. 1993;121:1299–1310. doi: 10.1083/jcb.121.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otey C A, Pavalko F M, Burridge K. An interaction between α-actinin and the β1 integrin subunit in vitro. J Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otey C A, Vasquez G B, Burridge K, Erickson B W. Mapping of the α-actinin binding site within the β1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- 30.Pasqualini R, Hemler M E. Contrasting roles for integrin β1 and β5 cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol. 1994;125:447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavalko F M, Walker D M, Graham L, Goheen M, Doerschuk C M, Kansas G S. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via α-actinin: receptor positioning in microvilli does not require interaction with α-actinin. J Cell Biol. 1995;129:1155–1164. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavalko F M, LaRoche S M. Activation of human neutrophils induces an interaction between the integrin β2-subunit (CD18) and the actin binding protein α-actinin. J Immunol. 1993;151:3795–3807. [PubMed] [Google Scholar]
- 33.Perez M S, Walker L E. Isolation and characterization of a cDNA encoding the KS1/4 epithelial carcinoma marker. J Haematol. 1989;142:3662–3667. [PubMed] [Google Scholar]
- 34.Perschl A, Lesley J, English N, Hyman R, Trowbridge I S. Transmembrane domain of CD44 is required for its detergent insolubility in fibroblasts. J Cell Sci. 1995;108:1033–1041. doi: 10.1242/jcs.108.3.1033. [DOI] [PubMed] [Google Scholar]
- 35.Quack J J, van Dongen G, Brakkee J G, Hayashida D J, Balm A J, Snow G B, Meijer C J. Production of a monoclonal antibody (K931) to a squamous cell carcinoma associated antigen identified as the 17-1A antigen. Hybridoma. 1990;9:377–387. doi: 10.1089/hyb.1990.9.377. [DOI] [PubMed] [Google Scholar]
- 36.Reszka A A, Hayashi Y, Horwitz A F. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosales C, O’Brien V, Kornberg L, Juliano R. Signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1995;1242:77–98. doi: 10.1016/0304-419x(95)00005-z. [DOI] [PubMed] [Google Scholar]
- 38.Salem R R, Wolf B C, Sears H F, Lavin P T, Ravikumar T S, DeCoste D, D’Emilia J C, Herlin M, Scholm M. Expression of colorectal carcinoma-associated antigens in colonic polyps. J Surg Res. 1993;55:249–255. doi: 10.1006/jsre.1993.1136. [DOI] [PubMed] [Google Scholar]
- 39.Sandoval I V, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz M A. Transmembrane signalling by integrins. Trends Cell Biol. 1992;2:304–308. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- 41.Seely K A, Aggeler J. Modulation of milk protein synthesis through alteration of the cytoskeleton in mouse epithelial cells cultured on a reconstituted basement membrane. J Cell Physiol. 1991;146:117–130. doi: 10.1002/jcp.1041460116. [DOI] [PubMed] [Google Scholar]
- 42.Simon B, Podolsky K, Moldenhauer G, Isselbacher K J, Gattoni-Cellini S, Brand S J. Epithelial glycoprotein is a member of a family of epithelial cell surface antigens homologous to nidogen, a matrix adhesion protein. Proc Natl Acad Sci USA. 1990;87:2755–2759. doi: 10.1073/pnas.87.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinardi L, Einheber S, Cullen T, Milner T A, Giancotti F G. A recombinant tail-less integrin β4 subunit disrupts hemidesmosomes, but does not suppress α6β4-mediated cell adhesions to laminins. J Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strassburg C P, Kasai Y, Seng B A, Miniou P, Zaloudik J, Herlyn D, Koprowski H, Linnenbach A J. Baculovirus recombinant expressing a secreted form of a transmembrane carcinoma-associated antigen. Cancer Res. 1992;52:815–821. [PubMed] [Google Scholar]
- 45.Szala S, Froehlich M, Scollon M, Kasai Y, Steplewski Z, Koprowski H, Linnenbach A J. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci USA. 1990;87:3542–3546. doi: 10.1073/pnas.87.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tandon A K, Clark G M, Chamness G C, McGuire W L. Association of the 323/A3 surface glycoprotein with tumor characteristics and behaviour in human breast cancer. Cancer Res. 1990;50:3317–3321. [PubMed] [Google Scholar]
- 47.Tsubura A, Senzaki H, Sasaki M, Hilgers J, Morii S. Immunohistochemical demonstration of breast-derived and/or carcinoma-associated glycoproteins in normal skin appendages and their tumors. J Cutan Pathol. 1992;19:73–79. doi: 10.1111/j.1600-0560.1992.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 48.Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 49.Williams M J, Hughes P E, O’Toole T E, Ginsberg M H. The inner world of cell adhesion: integrin cytoplasmic domains. Trends Cell Biol. 1994;4:109–112. doi: 10.1016/0962-8924(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 50.Wong M-H, Filbin M T. The cytoplasmic domain of the myelin Po protein influences the adhesive interactions of its extracellular domain. J Cell Biol. 1994;126:1089–1097. doi: 10.1083/jcb.126.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada K M, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- 52.Zorzos J, Zizi A, Bakiras A, Pectasidis D, Skarlos D V, Zorzos H, Elemenoglou J, Likourinas M. Expression of a cell surface antigen recognized by the monoclonal antibody AUA1 in bladder carcinoma: an immunohistochemical study. Eur Urol. 1995;28:251–254. doi: 10.1159/000475060. [DOI] [PubMed] [Google Scholar]