Abstract
Background:
Alcohol exposure increases the risk of breast cancer. Alcohol consumption is a serious social and public health issue for adolescents. This study investigated the impact of adolescent alcohol consumption on mammary tumorigenesis and progression and compared it to that of adult alcohol exposure in animal models.
Methods:
Female adolescent (5 weeks) and adult (8 weeks) MMTV-Wnt1 mice were exposed to alcohol either chronically or acutely. For chronic alcohol exposure, animals were fed with a liquid diet containing 6.7% ethanol for 23 weeks. For acute exposure, animals were treated with alcohol (2.5 g/kg, 25% w/v) via intraperitoneal (IP) injection for 15 days.
Results:
In control animals, the tumor latency was 18.5–22 weeks. Both chronic and acute alcohol exposure in adolescent mice significantly shortened the tumor latency to 9.5 and 8.4 weeks, respectively. However, adult-initiated alcohol exposure had little effect on the tumor latency. Both adolescent- and adult-initiated alcohol exposure significantly increased lung metastasis. Adolescent-initiated alcohol exposure but not adult-initiated alcohol exposure increased breast cancer stem cell population. Adolescent-initiated alcohol exposure significantly altered the proliferation of mammary epithelial cells, ductal growth, and the formation of terminal end buds in the mammary glands. Adolescent-initiated alcohol exposure but not adult-initiated alcohol exposure increased the estradiol levels in the blood. Acute adolescent alcohol exposure also significantly increased progesterone levels. Furthermore, adolescent-initiated alcohol exposure activated PAK1 and p38γ MAPK which are critical regulators of mammary tumorigenesis and aggressiveness, respectively, while adult-initiated alcohol exposure only activated p38γ MAPK. In addition, both adolescent and adult alcohol significantly decreased the levels of a prognostic marker miR200b.
Conclusions:
Adolescent-initiated alcohol exposure enhanced both tumorigenesis and aggressiveness of mammary tumors, while adult-initiated alcohol exposure mainly promoted tumor metastasis. Thus, adolescent mice were more sensitive than adult mice in response to alcohol-induced tumor promotion.
Keywords: Adolescents, cancer stem cells, ethanol, mammary gland development, metastasis
Introduction
It has been well established that alcohol consumption is a risk factor for cancers (Chen et al., 2011; Mostofsky et al., 2016; Shield et al., 2016; Scheideler and Klein, 2018). Available evidence reveals a strong correlation between alcohol consumption and the risk of breast cancer, even at moderate levels of consumption. Due to the scale of alcohol consumption, the incidence of and mortality from alcohol exposure-associated breast cancer is large. In addition, alcohol consumption is associated with aggressive breast tumors (Vaeth and Satariano, 1998; Weiss et al., 1996). Alcohol increases breast cancer recurrence and there is an inverse association between alcohol consumption and prognosis (Boffetta et al, 2006; Shield et al., 2016; Simapivapan et al., 2016). Therefore, alcohol exposure may enhance both mammary carcinogenesis, and progression/aggressiveness of existing tumors.
Although breast cancer more likely occurs in older women, its diagnosis in young women is usually at advanced stages. Adolescents and young women have a higher rate than older women to present with aggressive subtypes and advanced diseases at diagnosis (Cathcart-Rake et al., 2021). Alcohol is the most widely used substance of abuse among America’s adolescents and young adults (Harding et al., 2016; Windle, 2016). Adolescent alcohol exposure correlates with higher lifetime risks for the development of alcohol dependence, behavioral disorders, and some chronic diseases (Crews et al., 2016; McKnight-Eily et al., 2017). For example, adolescent alcohol abuse profoundly impacts the brain structures and behavioral deficits (Crews et al., 2016). The adolescence is a crucial period of mammary gland development and therefore more sensitive to carcinogens (Aupperlee et al., 2013; 2015). However, the effects of adolescent alcohol consumption on breast cancer risk and progression have not been investigated.
In this study, we used an animal model, FVB MMTV-Wnt1 transgenic mice to study and compare the effects of adolescent alcohol on mammary tumorigenesis and metastasis to that of adult alcohol exposure. FVB MMTV-Wnt1 mice develop spontaneous mammary tumors at 3 to 6 months old age but have a low metastatic rate, offering a good model to examine the impact of alcohol on mammary tumor development. We initiated alcohol exposure at the age of 5 weeks old which modeled adolescent alcohol exposure or 8 weeks old which modeled adult alcohol exposure. We compared the effects of adolescent and adult alcohol exposure on the onset of mammary tumorigenesis and metastasis. We further investigated alcohol’s effects on breast cancer stem cells, the structure of mammary glands, estrogen/progesterone levels and some other prognosis markers. Our results indicated that alcohol exposure promoted mammary tumorigenesis and metastasis and adolescent mice are more sensitive to alcohol-induced tumor promotion.
Materials and Methods
Animals and treatment
FVB MMTV-Wnt1 [FVB.Cg-Tg (Wnt1)1Hev/J] mice were obtained from The Jackson Laboratories (Bar Harbor, ME), bred, and housed in a climate-controlled animal facility. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky and the University of Iowa. Only female mice were used for this study. For chronic alcohol exposure, mice at age of 5 weeks-old (adolescents) or 8 weeks old (adults) were assigned into control and alcohol exposure groups. Mice were exposed to alcohol by feeding with alcohol containing liquid diet (Bio-Serv, Flemington, NJ), while control mice were feed with isocaloric liquid diet in which maltose was used to substitute isocalorically for alcohol. The alcohol concentration in the diet increased as the following: week 1, 2% alcohol; week 2, 4% alcohol; weeks 3 and on: 6.7% alcohol. Diet was provided ad libitum for the experimental period. During the 23 weeks experimental period, body weights of mice were evaluated. No significant body weight difference was observed among these animals. For acute alcohol exposure, animals received an intraperitoneal (IP) injection of alcohol (2.5 g/kg, 25% w/v) for 15 days. The acute exposure regime was selected to model binge alcohol exposure and limit the alcohol exposure duration within the adolescent period. To monitor tumorigenesis, mice were examined one week after the initiation of chronic alcohol exposure or one week following acute alcohol exposure. Tumor development/growth was monitored weekly. Mice with tumors exceeding 20 mm maximum diameter were euthanized and evaluated for metastasis. Mammary tumor tissues or mammary glands were either immediately dissociated or fixed for the following procedures. To determine the blood alcohol concentrations (BACs), the blood was collected one week after feeding with 6.7% alcohol diet or 30 min after IP alcohol injection. The BACs were determined using Alcohol Analyser AM1 (Analox Instruments, MA). The mean BAC was 214.6 mg/dl for binge exposure, and 79.7 mg/dl for chronic alcohol exposure.
Analysis of tumor metastasis
When tumors reached 20 mm maximum diameter, mice were sacrificed, and lung tissues were removed and treated with 4% paraformaldehyde. The paraffin-embedded lung tissues were sectioned at a thickness of 5 μm. The Hematoxylin–Eosin (H&E)-stained sections were examined and photographed under a microscope.
Dissociation of mouse mammary tumor cells and determination of cancer stem cell (CSC) population
Dissociation of mouse mammary tumor cells was performed using reagents and procedures provided by STEMCELL Technologies Inc (Cambridge, MA). Briefly, resected mammary tumors were minced and incubated in collagenase/hyaluronidase-containing dissociation solution at 37°C for 4 hours. Pellets were washed with HBSS and Ammonium Chloride solution followed by incubations with Trypsin and then Dispase. Cells were washed by HBSS containing 2% FBS and then filtered through a 40 μm cell strainer. After centrifuging, the single-cell suspensions were collected for next experiments. The breast cancer stem cells were identified by aldehyde dehydrogenase (ALDH) activity as previously described (Xu et al., 2016a). Briefly, dissociated mouse mammary tumor cells (5×105 cells) were incubated with ALDEFLUOR assay buffer containing ALDH substrate for 45 min at 37°C. Some cells were stained under the same condition with a specific ALDH inhibitor as a negative control. Cells were sorted using flow cytometry and analyzed using WINMDI software. ALDEFLUOR-positive cells were considered as the population of CSCs. Data were presented relative to control groups.
Tumorsphere formation
Tumorsphere formation was determined as previously described (Xu et al., 2016a). Briefly, dissociated single mammary tumor cell suspension (1000 cells) from either control or alcohol-fed mice were plated on ultra-low attachment plates in full Essential 8™ basal medium without further alcohol exposure, and incubated at 37°C and 5% CO2 for 10 days. The ability of tumor cells to form spheres was determined manually and presented relative to control groups.
Three-dimensional cell culture assay
The assay was performed as previously described (Xu et al., 2016b). Briefly, 24-well cell culture plates were pre-coated with 150 μl Matrigel Matrix (BD Biosciences). Dissociated single mammary tumor cell suspension (1000 per well) were mixed with 200 μl ice-cold Matrigel and seeded in the pre-coated 24-well plates. After 30 min gelling, culture medium was added to the plates and the medium was changed every 2 to 3 days. The images of cells were captured by a Zeiss Axiovert 40C microscope. Number of scattering spheroids was counted.
Determination of plasma levels of estradiol and progesterone
The plasmas were collected and stored at −80°C. For the analysis of estradiol and progesterone levels, the samples were sent to Ligand Assay and Analysis Core Facility in University of Virginia Center for Research in Reproduction.
Determination of cell proliferation and morphology of mammary gland
Mammary gland whole mounts and immunohistochemical staining were performed according to a previously described method (Plante et al., 2011). Briefly, abdominal mammary tissues (glands #4) were excised from and spread directly on glass slides. Glands were fixed in Carnoy’s fixative (100% EtOH, chloroform, glacial acetic acid; 6:3:1) for 4 hours, then washed in 70% ethanol for 15 minutes followed by gradually dehydrating. Slides were rinsed in distilled water for 5 minutes then stained in carmine alum overnight at room temperature. Slides were kept in methyl salicylate. Mammary gland images were recorded using an Olympus BX51 microscope. The ductal growth was calculated as the distance from the lymph node to the farthest point of the longest duct and expressed relative to the distance to the farthest limit of the mammary fat pad. Terminal end buds (TEB) were counted manually and expressed relative to the controls. After taking pictures, the mammary glands were processed for immunohistochemical staining of Ki-67.
Measurement of miR-200b-3p
Mouse mammary tumor tissues were lysed by ultrasound, and RNA was extracted with Trizol kits. Real-time qPCR was performed to detect the relative miR-200b-3p level in tumor tissues. TaqMan Advanced miRNA cDNA Synthesis Kit (TaqMan) was used for reverse transcription, while TaqMan Universal Master Mix II (TaqMan) was used for qPCR. miRNA-200p-3p primer and reference primer Snord11 were synthesized by TaqMan Company.
Statistical analysis
Ehan-BreGslow-Wilcoxon test is used for analyzing differences in the tumor initiation curves among treatment groups. The prevalence of metastasis between was determined by the Fisher exact test. Data were analyzed using analysis of variance (ANOVA). A p-value less than 0.05 was considered statistically significant.
Results
Alcohol’s effects on mammary tumorigenesis and metastasis in adolescent and adult mice
We investigated and compared alcohol’s effect on tumor promotion in adolescent (5-weeks-old) and adult (8-weeks-old) FVB MMTV-Wnt1 mice. For adolescent alcohol exposure, both acute and chronic alcohol exposure significantly shortened the latency of tumorigenesis. Acute and chronic adolescent alcohol exposure significantly shortened the latency of tumorigenesis by 54.6% and 55.6%, respectively; it decreased from 18.5 weeks (control) to 8.4 weeks (acute alcohol exposure) and from 22 weeks (control) to 9.5 weeks (chronic alcohol exposure) (Fig. 1). Both adolescent and adult alcohol exposure significantly increased the percentage of mice developing tumors; for adolescent exposure, it increased from 50% in control to 79% in alcohol exposure group; for adult exposure, it increased from 53% in control to 80% in alcohol exposure group (Fig. 2a). Adult alcohol exposure did not significantly shorten the latency of tumorigenesis, although there was a trend of decrease (−11.9%) (Fig. 1).
Fig. 1.
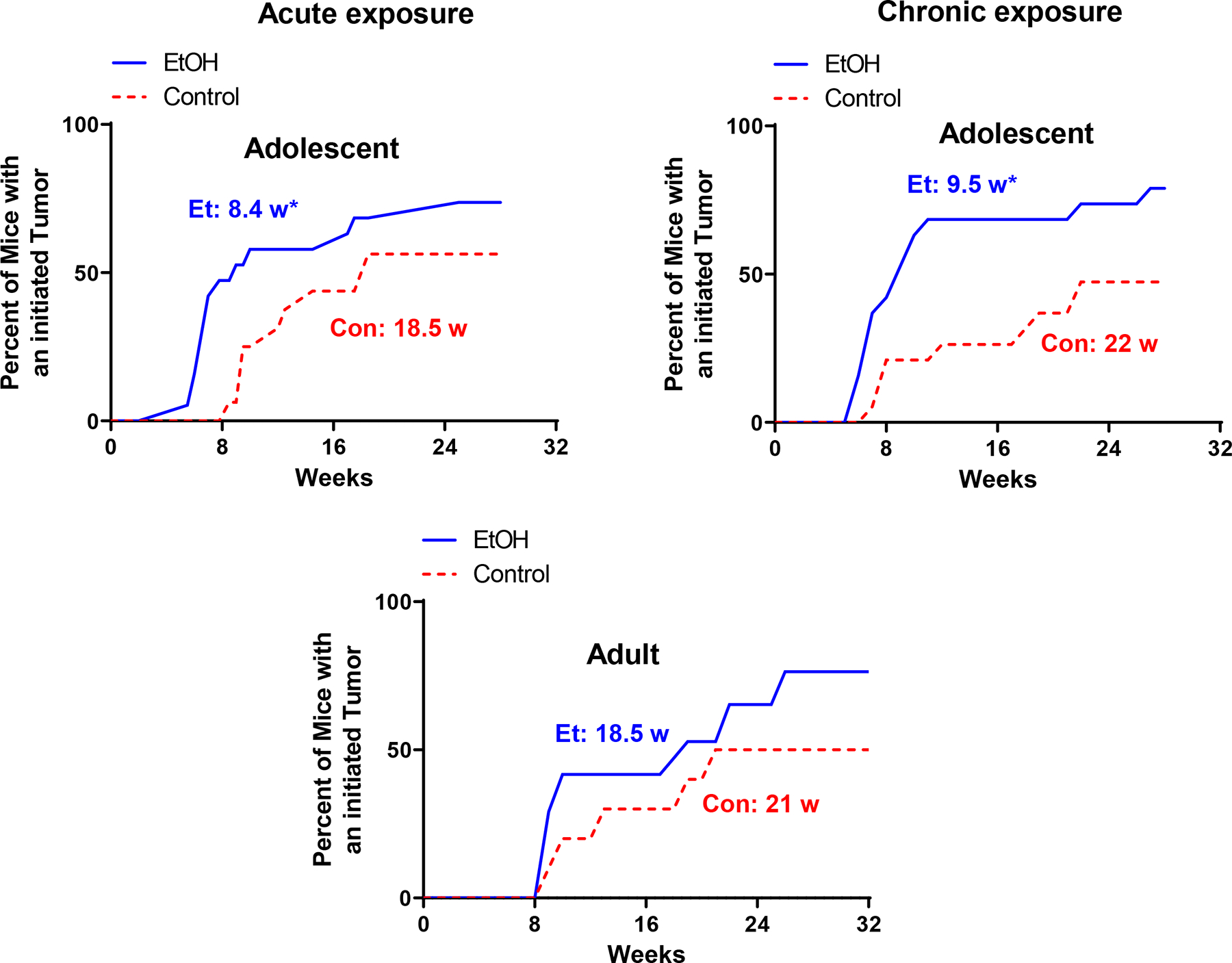
Alcohol’s effects on the latency of mammary tumorigenesis. Adolescent (5-weeks-old) and adult (8-weeks-old) MMTV-Wnt1 mice received short-term binge-like alcohol exposure (acute exposure) through IP injection or chronic exposure through liquid diet. The mice were monitored weekly for the growth of mammary tumors. For acute adolescent exposure, n = 13 for control and n = 14 for alcohol exposure group. For chronic adolescent alcohol exposure, n = 20 for control group and n = 19 for alcohol exposure group. For chronic adult alcohol exposure, n = 15 for both control and alcohol exposure group. *p< 0.05 as determined by the Fisher exact test.
Fig. 2.
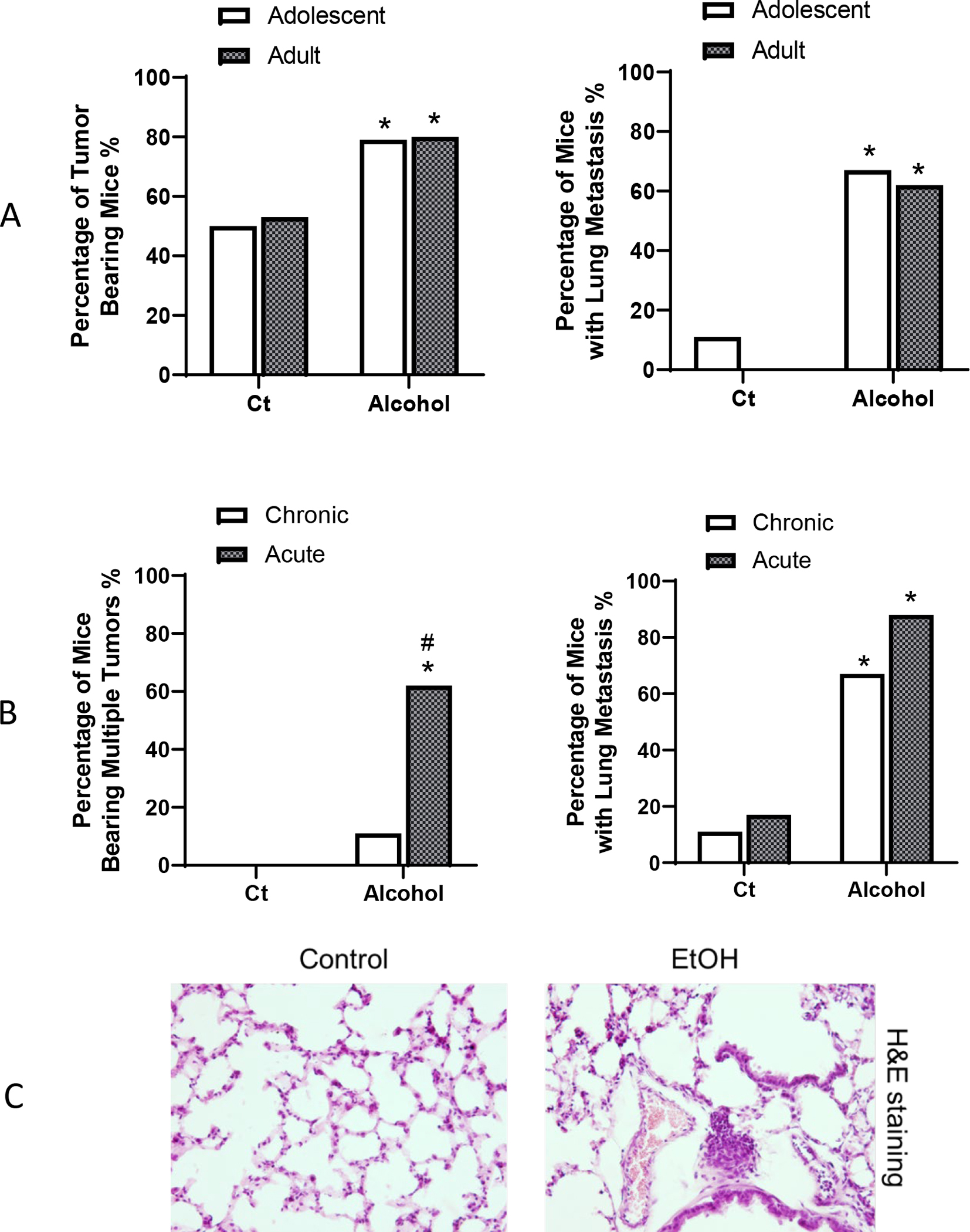
Alcohol’ effects on tumorigenesis and aggressiveness of breast cancer. A: Adolescent and adult FVB MMTV-Wnt1 mice received chronic alcohol exposure through liquid diet. When tumor size reached maximal diameter of 20 mm, the mice were euthanized, and the number of mice bearing mammary tumor and lung metastasis were determined. For adolescent alcohol exposure, n = 20 for control group and n= 19 for alcohol exposure group. For adult alcohol exposure, n = 15 for both control and alcohol-exposed group. *p< 0.05 as determined by the Fisher exact test. B. Adolescent FVB MMTV-Wnt1 mice received either acute alcohol exposure through IP injection or chronic alcohol exposure through liquid diet as described above. When tumors in mice reached 20 mm maximal diameter they were euthanized, number of mice bearing multiple tumors (more than 2 tumors/mouse) and lung metastasis were determined. For chronic exposure, n = 11 for control group and n = 14 for alcohol group. For acute exposure, n = 8 for both control and alcohol exposure group. * significant difference from controls, # significant difference from chronic exposure group, p< 0.05 as determined by the Fisher exact test. C. Representative images of lung metastasis. Adolescent FVB MMTV-Wnt1 mice received chronic exposure through liquid diet as described above. Mice were euthanized and lung tissues were obtained for Hematoxylin and Eosin (H&E) staining. Arrows indicate the metastases.
Both acute and chronic alcohol treatment increased the percentage of mice that developed multiple tumors in adolescent mice, and acute alcohol exposure-induced increase was significantly higher than that of chronic alcohol exposure (Fig. 2b). In addition, both acute and chronic alcohol exposure significantly enhanced tumor metastasis in the lung (Fig. 2b, 2c). We compared alcohol’s effects on tumor metastasis in adolescent and adult animals. Both adolescent and adult alcohol exposure similarly increased the lung metastasis (Fig. 2a).
Alcohol’s effects on mammary CSC population and tumorsphere formation
Breast cancer stem cells play an important role in chemoresistance, prognosis, and metastasis (He et al., 2021). We first examined alcohol’s effects on CSC population. Adolescent alcohol exposure significantly increased breast cancer stem cell population in mammary tumor tissues; however, adult alcohol exposure had little effect on CSC population (Fig. 3a). We then examined alcohol’s effects on the formation of tumorspheres, which is also an indication of cancer stem cells and tumor aggressiveness. Both adolescent and adult alcohol exposure increased the formation of tumorspheres, but the promoting effect of adolescent alcohol exposure was significantly stronger than that of adult alcohol exposure (Fig. 4a). Both adolescent and adult alcohol treatment increased the scattering of tumor cells in a 3-D cell culture assay, which is another indication of increased aggressiveness; the promoting effect adolescent alcohol exposure was significantly stronger than that of adult alcohol exposure (Fig. 4b). Similarly, acute alcohol exposure in adolescent mice also enhanced CSC population and tumorsphere formation (Fig. 3b and Fig. 4c).
Fig. 3.
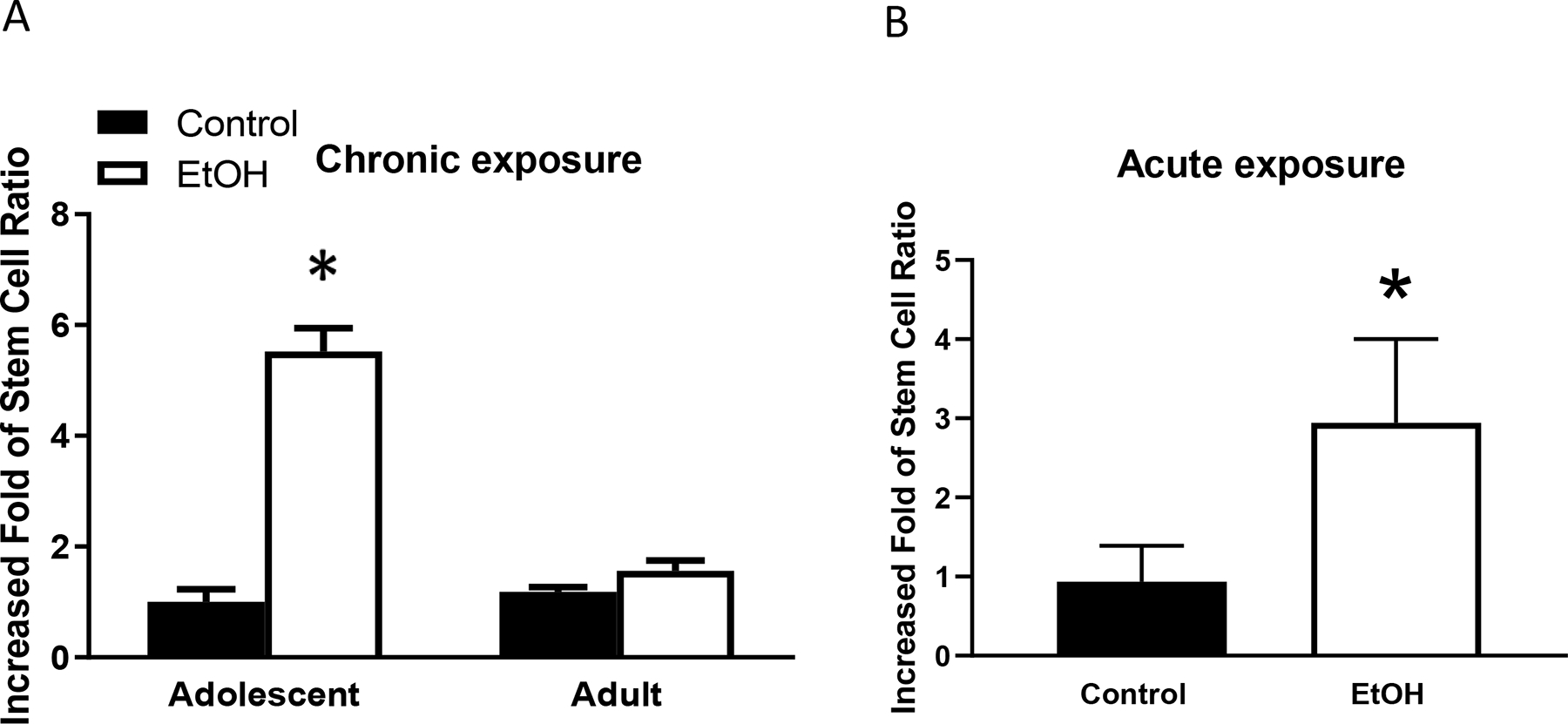
Alcohol’s effects on cancer stem cells (CSCs) in mammary tumors. Adolescent and adult MMTV-Wnt1 mice received chronic alcohol exposure through liquid diet or acute alcohol exposure through gavage as described above. When tumors in mice reached 20 mm maximal diameter, they were sacrificed, and mammary tumor cells were isolated for assaying CSC population. CSCs were determined by activity of aldehyde dehydrogenase (ALDH) using ALDEFLUOR kit. A. CSC population was determined following chronic alcohol exposure. n = 6 for each group. * significant difference from controls, p < 0.05. B. CSC population was determined following acute alcohol exposure in adolescent mice. n = 7 for each group. * significant difference from controls, p < 0.05.
Fig. 4.
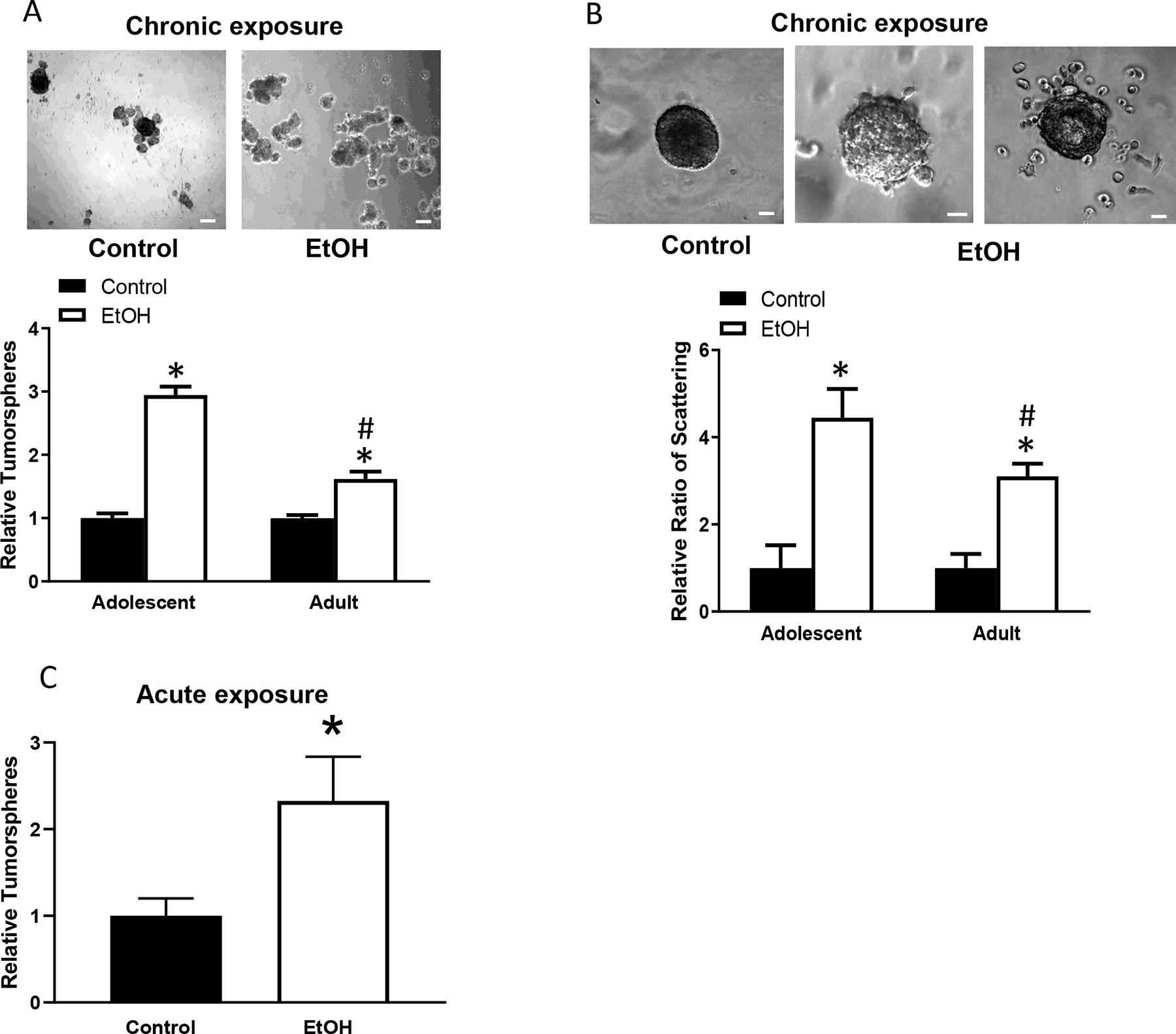
Alcohol’s effects on tumorsphere formation and cell scattering. Adolescent and adult MMTV-Wnt1 mice received chronic alcohol exposure through liquid diet or acute alcohol exposure through gavage as described above. Mammary tumor tissues were dissected and assayed for tumorsphere formation and cells scattering as described in the Materials and Methods. A. Tumorsphere formation was determined following chronic alcohol exposure. n = 6 for each group. * significant difference from controls; # significant difference from adolescent alcohol-exposed group, p < 0.05. B. Cell scatting was determined following chronic alcohol exposure. Number of spheroids/well or scattering spheroids was counted. n = 5 for each group, *denotes a significant difference from controls. # significant difference from adolescent alcohol-exposed group, p < 0.05. C. Tumorsphere formation was determined following acute alcohol exposure in adolescent mice. n = 7 for each group. * significant difference from controls. Bar = 50 μm
Alcohol’s effects on the proliferation and structure of mammary glands of adolescent mice
Since adolescent mice are still undergoing mammary gland development and appear more sensitive to alcohol-induced mammary tumor promotion, we investigated the impact of adolescent alcohol exposure on proliferation and structure of mammary glands. Chronic alcohol exposure (5 weeks of exposure) by liquid diet increased the proliferation of mammary epithelial cells as shown by an up-regulation of Ki67-positive cells in MMTV-Wnt1 mice (Fig. 5a). Chronic alcohol exposure decreased ductal growth but increased the amount of terminal end buds (TEBs)(Fig. 5b, 5c). Acute alcohol exposure did not significantly change the number of Ki67 positive cells and ductal growth (data not shown). Chronic adolescent alcohol exposure also inhibited ductal growth in wild type (WT) FVB mice (Fig. 5d). Although there was a trend of increase, alcohol did not significantly alter the number of TEBs in WT FVB mice (Fig. 5e).
Fig. 5.
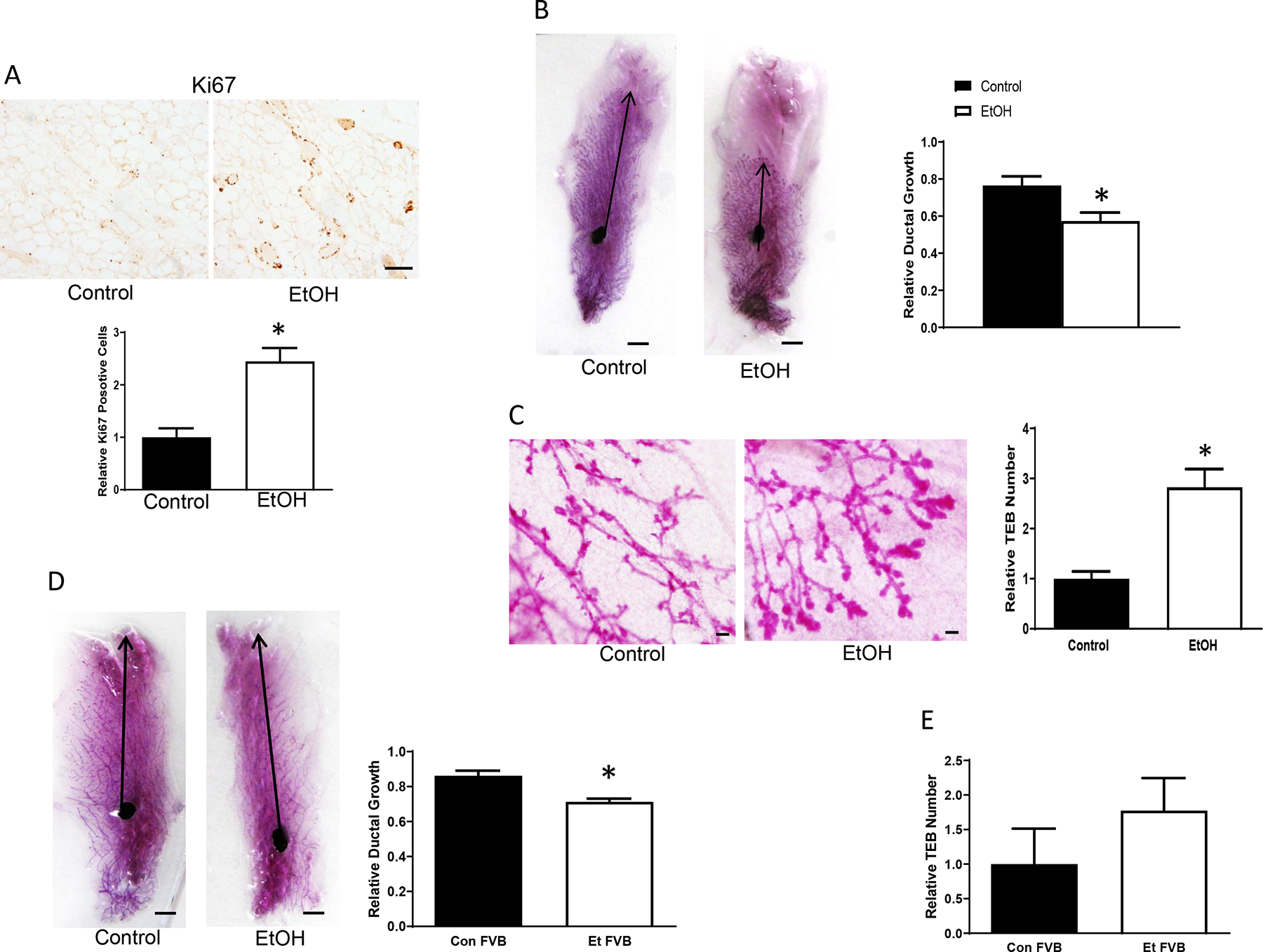
Alcohol’s effects on the development of mammary glands. Adolescent MMTV-Wnt1 mice received chronic alcohol exposure through liquid diet for 5 weeks as described above. A. Mammary glands were dissected and analyzed for Ki67 immunohistochemistry (IHC). Ki67-positive cells were counted and expressed relative to controls, n = 7 for each group. * significant difference from controls, p < 0.05. Bar = 25 μm. B. Mammary glands were collected for whole mount staining. Images were captured by a camera, and ductal growth was determined as the distance from the lymph node to the farthest point of the longest duct and expressed relative to the distance to the farthest limit of the mammary fat pad. n = 7 for each group. * significant difference from controls, p < 0.05. Bar = 5 mm. C: Terminal end buds (TEB) were counted and quantified relative to the controls, n = 7 for each group. * significant difference from controls, p < 0.05. Bar = 100 μm. D. Adolescent wild type (WT) FVB mice were exposed to alcohol through liquid diet for 5 weeks as described above. The ductal growth was determined as described above. n = 5 for each group. * significant difference from controls, p < 0.05. E. Adolescent WT FVB mice received chronic alcohol exposure through liquid diet for 5 weeks as described above, and relative TEB number was determined.
Alcohol’s effects on estradiol and progesterone levels
Since estrogen and progesterone play a critical role in mammary gland development and carcinogenesis (Aupperlee at al., 2013; Doan et al., 2017), we investigated alcohol’s effects on estradiol and progesterone levels in the plasma. Adolescent and adult MMTV-Wnt1 mice received chronic or acute alcohol exposure as described above. Chronic adolescent but not adult alcohol treatment increased the levels of estradiol in the plasma (Fig. 6a). However, acute adolescent alcohol exposure failed to significantly change plasma estradiol levels (data not shown). On the other hand, acute adolescent alcohol exposure significantly increased progesterone levels in the plasma (Fig. 6b) while chronic adolescent alcohol did not significantly alter progesterone levels in the plasma (data not shown).
Fig. 6.
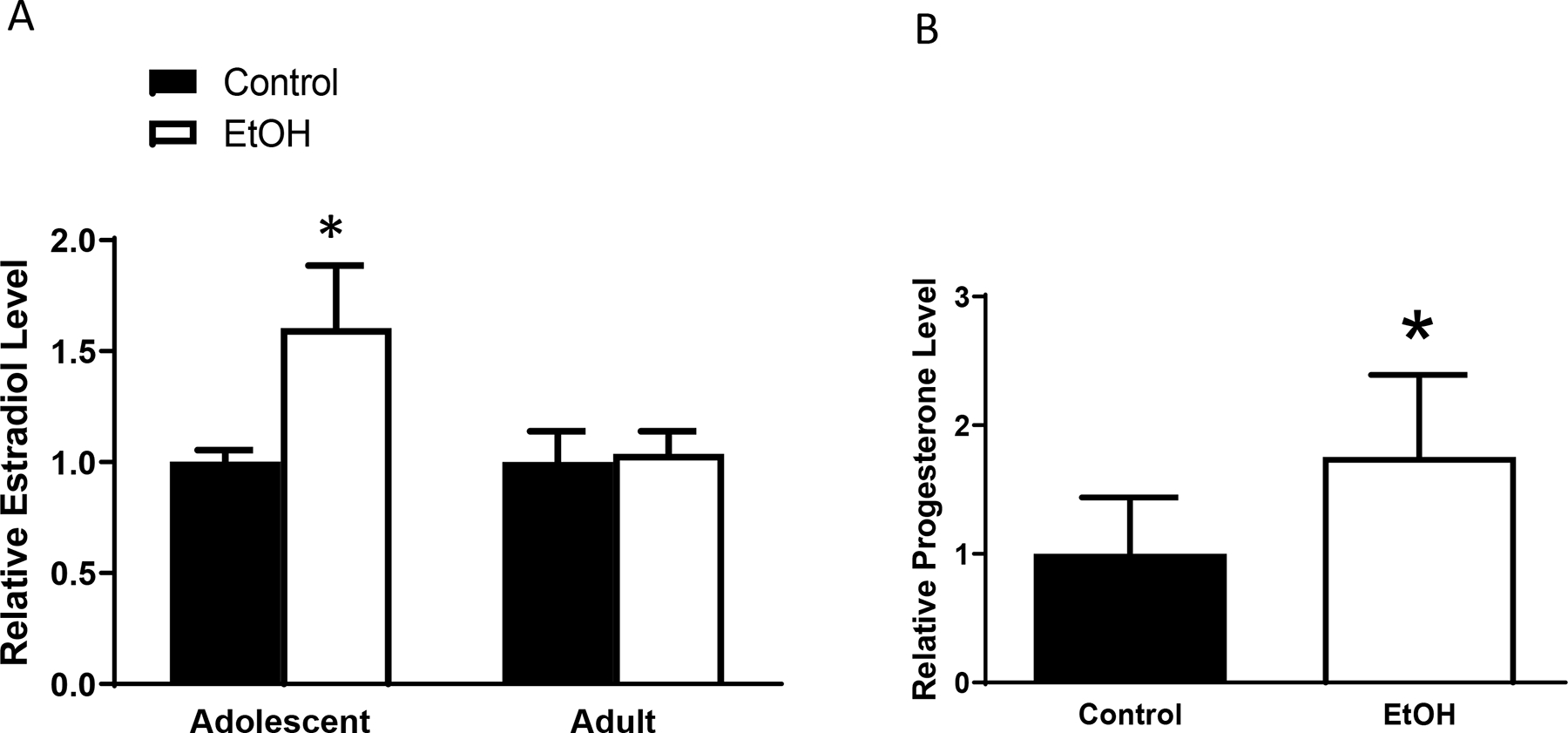
Alcohol’s effects estradiol and progesterone levels in the blood. Adolescent and adult MMTV-Wnt1 mice received either chronic alcohol exposure or acute alcohol exposure as described in the Materials and Methods. Mice were euthanized and estradiol and progesterone levels in the plasma were measured by ELISA. A: Estradiol levels in the plasma were determined following chronic alcohol exposure. n = 4 for each group. * significant difference from controls, p < 0.05. B: Progesterone levels in the plasma were determined following acute alcohol exposure. n = 6 for control group and 5 for alcohol-exposed group. * significant difference from controls, p < 0.05
Alcohol’s effects on intracellular signaling
p38γ MAPK activation is involved in the aggressiveness of mammary tumors (Xu et al., 2018). Chronic alcohol exposure increased the expression of both p38γ MAPK and phosphorylated p38γ MAPK in the mammary tissues of adolescent and adult MMTV-Wnt1 mice (Fig. 7). PAK1 activation is associated with mammary tumorigenesis (Shrestha et al., 2012; Zhan et al., 2017). Chronic alcohol exposure also enhanced the leve of PAK1 and phosphorylated PAK1 in the mammary tissues of adolescent mice. However, chronic alcohol treatment had little impact on the expression of PAK1 and phosphorylated PAK1 in the mammary tissues of adult MMTV-Wnt1 mice (Fig. 7).
Fig. 7.
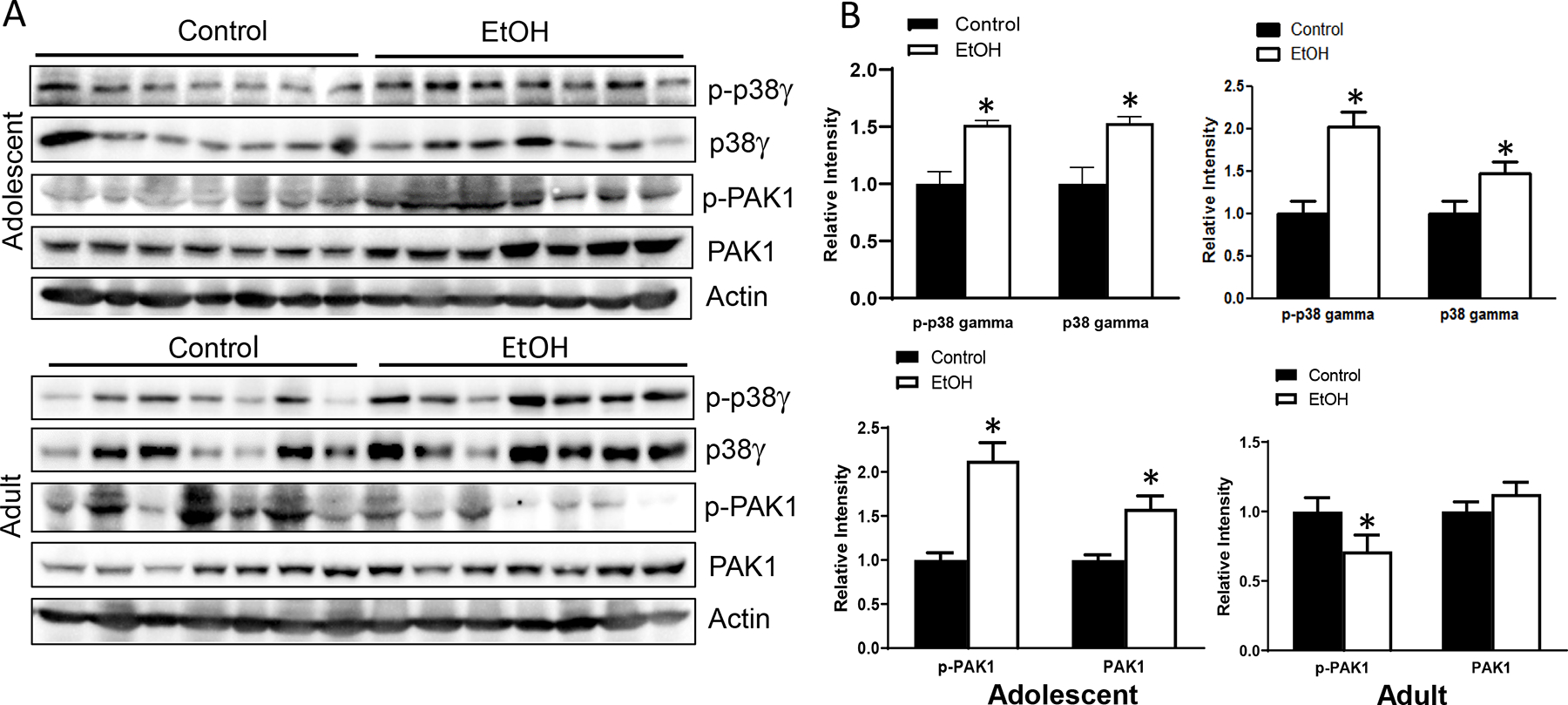
Alcohol’s effects on cell signaling pathways involved in mammary tumorigenesis and aggressiveness. Adolescent and adult MMTV-Wnt1 mice received alcohol exposure chronically through liquid diet as described above. When tumor size reached 20 mm, mice were sacrificed, tumor tissues were isolated, and protein was extracted. A. The expression of phosphorylated/total p38γ MAPK or p21 Activated Kinases 1 (PAK1) was examined in Western blots. B. The amount of p-p38γ MAPK, p38γ MAPK, p-PAK1 or PAK1 were determined, normalized to the actin. n = 7 for each group. * significant difference from controls, p < 0.05.
MicroRNA (miR)-200b-3p is a member of miR200b family and a tumor suppressor for mammary tumors; its levels inversely correlate to the prognosis of breast cancer (Amorim et al., 2019). The results shown in Fig. 8 indicated that alcohol exposure decreased the levels of miR200b.
Fig. 8.
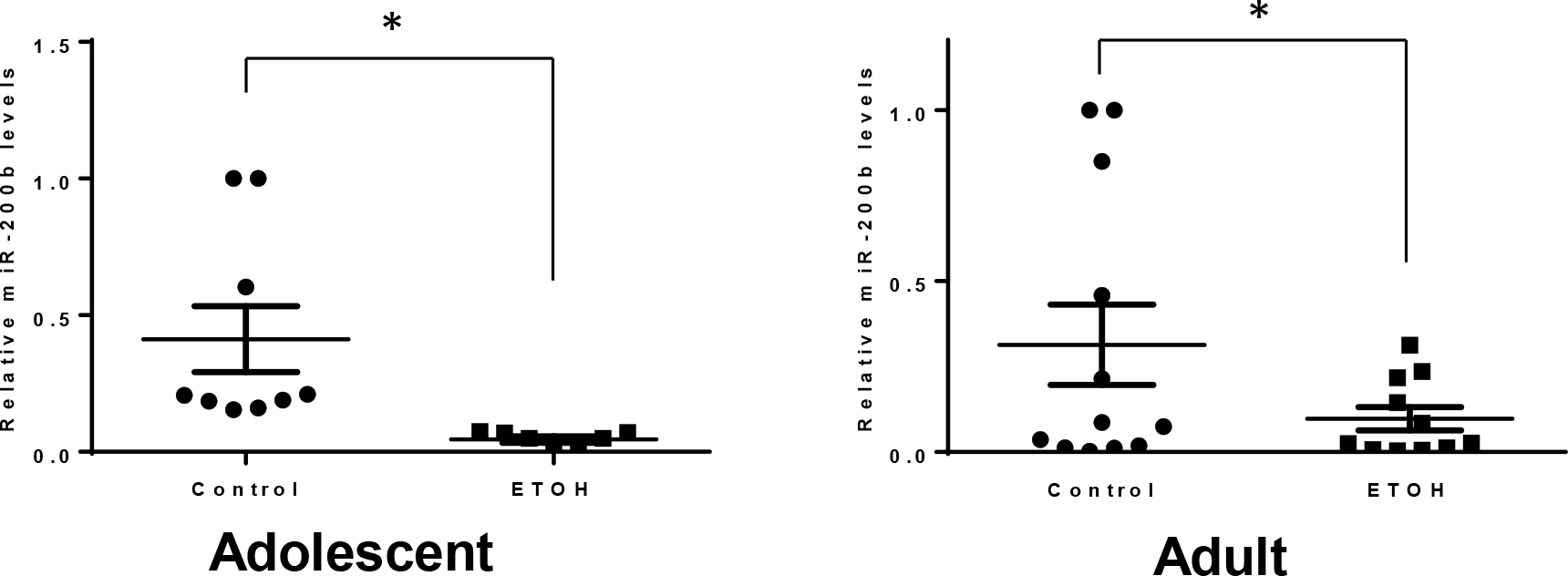
Alcohol’s effects on microRNA (miR)-200b-3p. Adolescent and adult MMTV-Wnt1 mice received chronic alcohol exposure as described above. As tumor size reached maximal diameter of 20 mm, the mice were sacrificed, and mammary tumor tissues were dissected for the analysis of miR-200b-3p expression. For adolescent alcohol exposure, n = 9 for both control and alcohol-exposed group. For adult alcohol exposure, n = 11 for both control and alcohol-exposed group * significant difference from controls, p < 0.05.
Discussion
We investigated the impact of adolescent-initiated alcohol exposure on breast tumorigenesis and progression in MMTV-Wnt1 mice and compared it to that of adult-initiated alcohol exposure. Adolescent-initiated alcohol exposure significantly shortened the latency of tumorigenesis and increased the percentage of mice developing multiple tumors as well as lung metastasis. Although adult-initiated alcohol exposure did not affect the tumor latency, it significantly increased the lung metastasis. Adolescent but not adult alcohol exposure significantly increased breast cancer stem cell population. Both adolescent and adult alcohol exposure promoted the tumorsphere formation and tumor cell scattering. Adolescent alcohol exposure also significantly altered the proliferation of mammary epithelial cells, ductal growth, and the number of terminal end buds in the mammary glands as well as estradiol/progesterone levels in the plasma. Furthermore, adolescent, and adult alcohol exposure appeared to differentially impact critical intracellular signaling regulators of mammary tumorigenesis and aggressiveness.
Alcohol exposure during mammary gland development
The development of mammary glands starts at the embryonic stage, and the expansion begins at adolescence or puberty. The development of mammary glands includes ductal elongation, cell proliferation, and bifurcation during adolescent, side branching during estrous cycles, and alveologenesis and lactogenesis during pregnancy and lactation (Russo and Russo, 2008; Li et al., 2012). At adolescence, rapid changes in the hormone levels drive increased proliferation and expanded ductal development. During this time, highly proliferative structures called end buds (EB) are developed and progress into the mammary fat pad, forming the extensive ductal network (Aupperlee et al., 2013).
The mammary gland is particularly susceptible to transformation during adolescent development (Jones et al., 2014). In adolescent rodents, terminal end buds (TEB) are responsible for ductal morphogenesis and likely to develop into malignant mammary tumors after exposure to carcinogens (Watson et al., 2008; Olson et al., 2010). It had been proposed disruption of adolescent development of mammary glands may increase breast cancer risk later in life (Ruder et al., 2008). The growth of TEB is greatly impacted by carcinogen exposure (Aupperlee et al., 2013). Therefore, the adolescent period is sensitivity to carcinogen exposure and subsequent mammary tumorigenesis (Aupperlee et al., 2013; 2015). Therefore, adolescent alcohol exposure-promoted tumorigenesis may result from its effects on mammary gland development.
Alcohol-induced alteration in estradiol and progesterone levels
During the adolescent, circulating ovarian hormones plays an important role in the growth of TEBs (Jones et al., 2014). Disruption of hormonal regulation during adolescent can create predispositions to abnormal mammary gland development and therefore increased breast cancer risk (Boyd et al., 2010). Estrogen is one of the most important hormones in mammary epithelial proliferation and differentiation during adolescent and progesterone is also involved in these processes (Brisken and O’Malley, 2010; Aupperlee et al., 2013). Both estrogen and progesterone are critically involved in the carcinogenesis of breast cancer (Doan et al., 2017). We demonstrate here that chronic adolescent alcohol exposure, but not adult alcohol exposure increases estradiol levels in the blood (Fig. 6a). Interestingly, it is acute adolescent alcohol exposure but not chronic alcohol exposure that significantly increased progesterone levels in the plasma (Fig. 6b). This suggests that chronic exposure regime may be functionally different from acute exposure regime. To draw a conclusion regarding the effect of alcohol exposure regimes on estradiol and progesterone, it is necessary to compare the effects of chronic or acute alcohol exposure during adolescent-and adult-restricted period.
Breast cancer stem cells (CSCs) and alcohol tumor promotion
CSCs is a subpopulation of cancer cells with self-renewal and differentiation capacity. Breast CSCs play an important role in tumor initiation, progression, metastasis, recurrence, and therapy resistance in breast cancer patients. It has been suggested that alcohol’s tumor promoting effects may be mediated by increasing the CSC population in cancers (Wang et al., 2017; Xu and Luo, 2017). In MMTV-Wnt1 mice, adolescent alcohol exposure significantly increases CSC population (Fig. 3), suggesting that activation of CSCs may underlie alcohol-enhanced aggressiveness of breast cancer. Although adult alcohol exposure does not increase CSC population, it promotes tumorsphere formation and cell scattering as well p38γ MAPK activation which may contributed to enhanced aggressiveness. Therefore, the mechanisms underlying adolescent and adult alcohol exposure-promoted aggressiveness of breast cancer are overlapping but have some differences.
Potential intracellular signaling involved in the effects of alcohol
The p21 Activated Kinases (PAKs) are serine threonine kinases and consist of 6 members, PAKs 1–6 (Rane and Minden, 2019). PAK1 and PAK4 are often associated with tumorigenesis including breast cancer development (Shrestha et al., 2012; Rane and Minden, 2019). PAK1 plays an important role in mammary tumorigenesis and acts as an oncogene in breast cancers (Shrestha et al., 2012; Zhan et al., 2017). We show that adolescent alcohol exposure, but not adult alcohol exposure activates PAK1, which is consistent with the finding that adolescent alcohol exposure promotes tumorigenesis.
The p38 MAPK family comprises four members, p38α, p38β, p38γ and p38δ, which have similar protein sequences (Cuenda and Rousseau, 2007). p38γ MAPK has unique functions and mediates the progression and aggressiveness of mammary tumors (Meng et al., 2011; Rosenthal et al 2011; Qi et al., 2015; Xu et al., 2016a; 2016b; 2018). We demonstrate that both adolescent and adult alcohol exposure activates p38γ MAPK, which is consistent with the results that alcohol exposure promotes tumor metastasis and increase the percentage of mice bearing multiple tumors.
Future studies
In this study, we establish a useful animal model (MMTV-Wnt1 mice) to study alcohol-promoted tumorigenesis and aggressiveness of breast cancer, especially for the impact of adolescent-initiated alcohol exposure on mammary tumor development. There are several remaining questions that warrant further investigation. Adolescent-initiated alcohol exposure enhanced both tumorigenesis and aggressiveness of mammary tumors, while adult-initiated alcohol exposure mainly promoted tumor metastasis. For adolescent- and adult-initiated alcohol exposure, there is an overlapping period from 8 weeks onward, despite the time of initial exposure was different. The similar effects, such as enhanced metastasis, caused by the two exposure regimes might be mediated by the overlapping periods of exposure. However, acute alcohol exposure which is initiated at adolescence (15 days of exposure starting at 5 weeks) significantly enhances tumorigenesis and tumor metastasis, suggesting that adolescent alcohol exposure is sufficient to promote mammary tumorigenesis and aggressiveness. A further study comparing the effects of chronic alcohol exposure during adolescent- and adult-restricted period may provide direct evidence.
It is noted that acute alcohol exposure more potently promotes tumorigenesis and progression while not affecting mammary gland structures and estradiol levels. The reason for that is currently unknown. One possibility is that acute alcohol exposure regime produces higher BACs than that of chronic alcohol exposure. It is also likely the mechanisms underlying acute alcohol exposure-induced tumor promotion may be different from that of chronic alcohol exposure. For example, acute but not chronic adolescent alcohol exposure increases progesterone levels in the blood. We will take advantage of this model system to explore these mechanisms. For example, amphiregulin and RANKL are targets of progesterone and involved in mammary gland development, (Aupperlee at al., 2013). We may investigate the effects of chronic and acute alcohol exposure regimes on amphiregulin and RANKL during adolescent- and adult-restricted period. In addition, we may employ RNA sequencing to screen differentially expressed genes in response to different alcohol exposure regimes.
The cellular and molecular mechanisms underlying alcohol-induced promotion of breast cancer are complex and it requires a systematic investigation. The current study provides insight into the potential involvement of some signaling pathways, hormonal regulation, and microRNAs. Further studies of blocking or enhancing these pathways are necessary to verify the involvement.
Funding:
National Institutes of Health (NIH) grants (AA017226, AA015407, and AA026344)
Abbreviations:
- ALDH
aldehyde dehydrogenase
- BAC
blood alcohol concentration
- EBs
end buds
- CSCs
cancer stems cells
- MMTV
mouse mammary tumor virus
- p38 MAPK
p38 mitogen-activated protein kinase
- PAKs
p21 Activated Kinases
- TEBs
terminal end buds
- Wnt1
Wingless/Integrated 1
Footnotes
Conflict of Interest
The authors declare no competing interests
References
- Amorim M, Lobo J, Fontes-Sousa M, Estevão-Pereira H, Salta S, Lopes P, Coimbra N, Antunes L, Palma de Sousa S, Henrique R, Jerónimo C (2019) Predictive and Prognostic Value of Selected MicroRNAs in Luminal Breast Cancer. Front Genet. 10:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperlee MD, Leipprandt JR, Bennett JM, Schwartz RC, Haslam SZ (2013). Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res. 15(3):R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperlee MD, Zhao Y, Tan YS, Zhu Y, Langohr IM, Kirk EL, Pirone JR, Troester MA, Schwartz RC, Haslam SZ (2015). Puberty-specific promotion of mammary tumorigenesis by a high animal fat diet. Breast Cancer Res. 17(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J (2006) The burn of cancer attributable to alcohol drinking. Int J Cancer 119:884–887 [DOI] [PubMed] [Google Scholar]
- Boyd AL, Salleh A, Humber B, Yee J, Tomes L, Kerr LR (2010). Neonatal experiences differentially influence mammary gland morphology, estrogen receptor {α} protein levels, and carcinogenesis in BALB/c mice. Cancer Prev Res (Phila). 3(11):1398–408. [DOI] [PubMed] [Google Scholar]
- Brisken C, O’Malley B (2010) Hormone action in the mammary gland. Cold Spring Harb Perspect Biol 2: a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart-Rake EJ, Ruddy KJ, Bleyer A, Johnson RH. (2021) Breast Cancer in Adolescent and Young Adult Women Under the Age of 40 Years. JCO Oncol Pract. 17(6):305–313 [DOI] [PubMed] [Google Scholar]
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC (2011) Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 306(17):1884–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL (2016). Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev. 68(4):1074–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Rousseau S. (2007) p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 1773(8):1358–75 [DOI] [PubMed] [Google Scholar]
- Doan TB, Graham JD, Clarke CL (2017). Emerging functional roles of nuclear receptors in breast cancer. J Mol Endocrinol. 58(3): R169–R190 [DOI] [PubMed] [Google Scholar]
- Harding FM, Hingson RW, Klitzner M, Mosher JF, Brown J, Vincent RM, Dahl E, Cannon CL (2016) Underage Drinking: A Review of Trends and Prevention Strategies. Am J Prev Med. 51(4 Suppl 2):S148–57. [DOI] [PubMed] [Google Scholar]
- He L, Wick N, Germans SK, Peng Y (2021) The Role of Breast Cancer Stem Cells in Chemoresistance and Metastasis in Triple-Negative Breast Cancer. Cancers (Basel). 13(24):6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Watson KL, Campbell CI, Moorehead RA (2014). IGF-IR mediated mammary tumorigenesis is enhanced during pubertal development. PLoS One. 2014 9(9):e108781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fu X, Ma G, Sun X, Dong X, Nagy T, Xing C, Li J, Dong JT (2012). Atbf1 regulates pubertal mammary gland development likely by inhibiting the pro-proliferative function of estrogen-ER signaling. PLoS One. 7(12):e51283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Zhang H, Liu G, Kreike B, Chen W, Sethi S, Miller FR, Wu G (2011). p38γ mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly (ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia. 13(5):472–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Henley SJ, Green PP, Odom EC, Hungerford DW (2017). Alcohol Screening and Brief Intervention: A Potential Role in Cancer Prevention for Young Adults. Am J Prev Med. 53(3S1):S55–S62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E, Mukamal KJ, Giovannucci EL, Stampfer MJ, Rimm EB (2016). Key Findings on Alcohol Consumption and a Variety of Health Outcomes From the Nurses’ Health Study. Am J Public Health. 106(9):1586–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante I, Stewart MK, Laird DW (2011) Evaluation of Mammary Gland Development and Function in Mouse Models J Vis Exp. (53):2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LK, Tan Y, Zhao Y, Aupperlee MD, Haslam SZ (2010). Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int J Obes (Lond). 34(9):1415–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Yin N, Ma S, Lepp A, Tang J, Jing W, Johnson B, Dwinell MB, Chitambar CR, Chen G. (2015) p38γ MAPK is a therapeutic target for triple-Negative breast cancer by stimulation of cancer stem-like cell expansion. Stem Cells. 33(9):2738–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal DT, Iyer H, Escudero S, Bao L, Wu Z, Ventura AC, Kleer CG, Arruda EM, Garikipati K, Merajver SD. (2011) p38γ promotes breast cancer cell motility and metastasis through regulation of RhoC GTPase, cytoskeletal architecture, and a novel leading edge behavior. Cancer Res. 71(20):6338–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ (2008): Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer 8:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Russo IH (2008). Breast development, hormones and cancer. Adv Exp Med Biol. 630:52–6 [DOI] [PubMed] [Google Scholar]
- Scheideler JK, Klein WMP (2018) Awareness of the Link between Alcohol Consumption and Cancer across the World: A Review. Cancer Epidemiol Biomarkers Prev.27(4):429–437 [DOI] [PubMed] [Google Scholar]
- Shield KD, Soerjomataram I, Rehm J (2016). Alcohol Use and Breast Cancer: A Critical Review. Alcohol Clin Exp Res. 40(6):1166–81 [DOI] [PubMed] [Google Scholar]
- Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, Wang S, Barretina J, Weir BA, Zhao JJ, Polyak K, Golub TR, Beroukhim R, Hahn WC (2012). PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene. 31(29):3397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simapivapan P, Boltong A, Hodge A (2016). To what extent is alcohol consumption associated with breast cancer recurrence and second primary breast cancer? A systematic review. Cancer Treat Rev. 50:155–167 [DOI] [PubMed] [Google Scholar]
- Vaeth PA, Satariano WA (1998) Alcohol consumption and breast cancer stage at diagnosis. Alcohol Clin Exp Res. 22:928–934 [PubMed] [Google Scholar]
- Wang Y, Xu M, Ke ZJ, Luo J. (2017) Cellular and molecular mechanisms underlying alcohol-induced aggressiveness of breast cancer. Pharmacol Res. 115:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Khaled WT (2008). Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development (Cambridge, England) 135: 995–1003. [DOI] [PubMed] [Google Scholar]
- Weiss HA, Brinton LA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB, Swanson CA (1996) Epidemiology of in situ and invasive breast cancer in women aged under 45. Br J Cancer 73:1298–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M (2016). Drinking Over the Lifespan: Focus on Early Adolescents and Youth. Alcohol Res. 38(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Ren Z, Wang X, Comer A, Frank JA, Ke ZJ, Huang Y, Zhang Z, Shi X, Wang S, Luo J (2016a) ErbB2 and p38γ MAPK mediate alcohol-induced increase in breast cancer stem cells and metastasis. Mol Cancer. 15(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang S, Ren Z, Frank JA, Yang XH, Zhang Z, Ke ZJ, Shi X, Luo J (2016b) Chronic ethanol exposure enhances the aggressiveness of breast cancer: the role of p38γ. Oncotarget. 7(3):3489–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Luo J. (2017) Alcohol and Cancer Stem Cells.Cancers (Basel). 9(11):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang S, Wang Y, Wu H, Frank JA, Zhang Z, Luo J (2018) Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochim Biophys Acta Mol Basis Dis. 1864(11):3605–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan MN, Yu XT, Tang J, Zhou CX, Wang CL, Yin QQ, Gong XF, He M, He JR, Chen GQ, Zhao Q (2017). MicroRNA-494 inhibits breast cancer progression by directly targeting PAK1. Cell Death Dis. 8(1):e2529. [DOI] [PMC free article] [PubMed] [Google Scholar]


