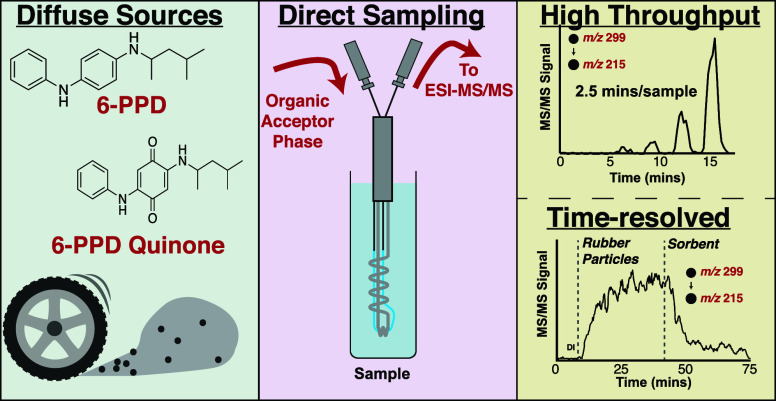
Abstract
The oxidative transformation product of a common tire preservative, identified as N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine quinone (6-PPDQ), has recently been found to contribute to “urban runoff mortality syndrome” in Coho salmon at nanogram per liter levels. Given the number of fish-bearing streams with multiple stormwater inputs, large-scale campaigns to identify 6-PPDQ sources and evaluate mitigation strategies will require sensitive, high-throughput analytical methods. We report the development and optimization of a direct sampling tandem mass spectrometry method for semiquantitative 6-PPDQ determinations using a thin polydimethylsiloxane membrane immersion probe. The method requires no sample cleanup steps or chromatographic separations, even in complex, heterogeneous samples. Quantitation is achieved by the method of standard additions, with a detection limit of 8 ng/L and a duty cycle of 15 min/sample. High-throughput screening provides semiquantitative concentrations with similar sensitivity and a full analytical duty cycle of 2.5 min/sample. Preliminary data and performance metrics are reported for 6-PPDQ present in representative environmental and stormwater samples. The method is readily adapted for real-time process monitoring, demonstrated by following the dissolution of 6-PPDQ from tire fragments and subsequent removal in response to added sorbents.
Introduction
N-(1,3-Dimethylbutyl)-N′-phenyl-p-phenylenediamine (6-PPD) is added to tire formulations (0.4–2% by mass) to mitigate oxidative damage to the tire.1 Tian et al. recently discovered that an ozonation product, N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine quinone (6-PPDQ), contributes to “urban runoff mortality syndrome” and is lethal to Coho salmon (Oncorhynchus kisutch) in the low microgram per liter range (LC50 = 0.8 μg/L). Given the continuous and widespread release of 6-PPDQ from tire wear, it represents a globally ubiquitous contaminant with many diffuse sources (roadways and stormwater drains). Identifying and prioritizing locations for targeted mitigation2−4 and evaluating treatment efficacy will require screening large sample sets that may be cost prohibitive. There is a clear and immediate need for rapid, sensitive, and cost-effective methods to measure 6-PPDQ.
Development of such methods is complicated by the fact that urban runoff/stormwater is a complex and highly variable sample matrix, presenting unique analytical challenges. In addition to salts and biogenic dissolved organic matter,5 numerous compounds deposited on and stored within anthropogenic surfaces (roofs, roads, etc.) are transported by rain events into streams and estuaries.6 Conventional analytical approaches such as LC-MS/MS provide several orthogonal identifiers for detected chemical species (retention time, m/z, and MSn product ions), yielding high analytical specificity. This approach was particularly powerful in the elegant nontargeted analysis that identified 6-PPDQ as the primary toxicant from tire-tread particles.1 However, this strategy is inherently time and cost intensive, requiring sample cleanup, preconcentration, and chromatographic steps. As communities re-evaluate the management of urban runoff, coupling conventional chromatographic workflows with targeted, direct analysis of 6-PPDQ (and structural analogues7) will be required to enable strategic allocation of resources and comprehensive monitoring of mitigation efforts.2,3 Direct mass spectrometry (MS) approaches are an excellent candidate method for this challenge, affording the sensitivity and selectivity of MS while obviating laborious sample cleanup and/or preconcentration steps. One such technique, condensed phase membrane introduction mass spectrometry (CP-MIMS),8 is a direct MS strategy that may provide rapid, in situ analysis of 6-PPDQ.
CP-MIMS typically employs a semipermeable polydimethylsiloxane (PDMS) capillary hollow fiber membrane (CHFM) to separate hydrophobic analytes from interfering matrix components (e.g., salts and particulate matter) directly in liquid/slurry samples.8,9 An organic acceptor continuously passed through the CHFM transports permeating analytes to the MS ion source.10,11 This approach has been paired with both electrospray ionization (ESI)10−14 and liquid electron ionization (LEI),15−19 enabling direct measurements of a wide array of analytes in diverse, complicated matrices, including PAHs in natural waters and sediments,17,18 naphthenic acids in constructed wetlands11 and oil-affected waters,14 pharmaceuticals in wastewater,20 and fatty acids in fish.13 The perm-selectivity of analytes with acid/base properties is pH-dependent and has been used to derive the pKa of trace organics in water.21 In addition, CP-MIMS can be used for real-time process monitoring and has been applied to monitor both remediation10,22 and organic synthesis.16 For further information about theoretical and operational considerations of CP-MIMS, see ref (8).
This work presents a direct membrane sampling strategy coupled to tandem MS, providing rapid 6-PPDQ analysis at nanogram per liter levels in complex samples. The method is used to measure fortified and real-world environmental samples by standard addition as well as in a high-throughput, direct calibration mode. The technique is also employed to monitor the release of 6-PPD and 6-PPDQ from tire and artificial turf particles in real time.
Materials and Methods
Standard Solutions
During method development and sample analysis, commercial standards of 6-PPDQ were unavailable to the authors. A sample of 6-PPDQ was therefore synthesized as an orange/brown solid (Figure S1). When a commercial analytical standard (>95%; 100 μg/mL in acetonitrile, ACP Chemicals, Montreal, QC) was subsequently obtained, the concentrations of the stock solutions from the synthesized 6-PPDQ were adjusted by 0.84 using the relative calibration slopes (Figure S2). All quantitative data presented here reflect this correction. 6-PPDQ and 6-PPD (>99.9%, Sigma-Aldrich, Oakville, ON) stock solutions were prepared in HPLC grade MeOH (Fisher Scientific, Ottawa, ON). Aqueous standard solutions were prepared gravimetrically in deionized water between 8.5 ng/L and 33.5 μg/L, containing <3% (v/v) methanol cosolvent. Further details are provided in the Supporting Information.
Environmental Samples
Surface water and stormwater samples were collected in amber glass bottles (40–1000 mL, PTFE-backed septa; Fisher Scientific) around Nanaimo, BC, in May and June 2021. Raw samples were stored at 4 °C and analyzed without further treatment within 1 week of sampling. The stability of 6-PPD and 6-PPDQ during storage is currently being investigated,23 so we caution the reader against overinterpreting quantitative results for environmental samples. Further details about environmental water samples are listed in Table S1.
Tire/Artificial Turf Particle Suspensions
Used tires were obtained from a local recycler (Nanaimo, BC). Small particles (<1 mm) were generated with hand tools. Artificial turf pellets (1–3 mm) were obtained from a landscaping pile (Nanaimo, BC) and measured without further treatment. Tire/artificial turf particles were suspended in a 36 mM dibasic potassium phosphate (>98%, Sigma-Aldrich) buffer (pH 7.11) solution at 0.5–10 g/L (50–1000 mg/100 mL), and desorption of 6-PPD and 6-PPDQ was monitored in real time. Activated carbon (Euroglas, Delft, The Netherlands) used in the sorption experiment was 100–200 mesh and added at a concentration of 1 g/L (100 mg/100 mL).
CP-MIMS Immersion Probe
A CP-MIMS immersion probe was constructed from a 7.6 cm length of dense PDMS hollow fiber membrane (inside diameter of 190 μm, outside diameter of 300 μm, Permselect, Medarray Inc., Ann Arbor, MI). The membrane was swelled with heptane (Sigma-Aldrich) and mounted on 31-gauge stainless steel capillaries (Microgroup, Medway, MA). A stainless steel wire support was epoxy potted into 1/4 in. stainless steel tubing alongside the capillary for improved probe ruggedness. The acceptor phase solution was a 15/85/0.03 (v/v/v) heptane/methanol/formic acid mixture (Optima 99.0%+, Fisher Scientific) and was pumped at a rate of 10 μL/min using a syringe pump (Chemyx Fusion 100, Stafford, TX) and a 10 mL gastight syringe (Hamilton, Reno, NV). The membrane probe was rinsed in a stirred solution of methanol for 1–3 min between samples and/or standards. To monitor carryover effects, deionized (DI) water blanks were interspersed every 5–10 samples. A delay time of ∼1.5 min was observed between the membrane probe and ion source. For standard addition experiments, samples were spiked with three consecutive aliquots of a methanolic stock of 6-PPDQ (measured gravimetrically), resulting in ∼100 ng/L additions. Freshly prepared aqueous 6-PPDQ standards were included throughout sample analysis to monitor instrument drift. Select samples were measured in triplicate to assess reproducibility (Table 1 and Table S4). No decline in membrane performance (sensitivity and response time) was observed, and a single membrane was used for weeks before being replaced.
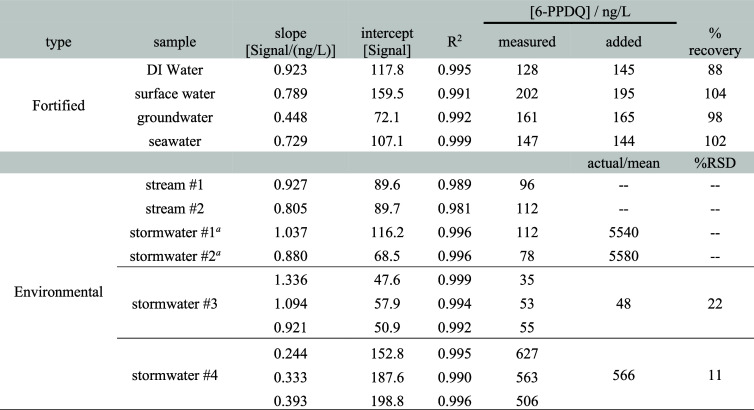
Table 1. Summary of Quantitative Results for CP-MIMS Standard Addition Analysis of 6-PPDQ in Fortified and Real Environmental Samples.
Stormwater samples 1 and 2 were diluted gravimetrically with DI water prior to analysis (49.8- and 71.6-fold, respectively). Concentrations in the original samples were 5.54 and 5.58 μg/L, respectively.
Mass Spectrometry Parameters
All quantitative work utilized an ESI triple quadrupole MS instrument (Q-Sight 220, PerkinElmer, Waltham, MA) with nebulization gas at 120 psi, an HSAID source at 320 °C, and a 4 kV capillary voltage. MS/MS transitions for 6-PPD and 6-PPDQ were developed using directly infused methanolic standards [∼500 μg/kg (see Table S2)] with 1000 ms dwell times. High-resolution, accurate mass (HRAM) analysis, using an Orbitrap Exploris 120 instrument (ThermoScientific, San Jose, CA), confirmed the identity of 6-PPDQ in the membrane permeate from an aqueous standard (Figure S3 and Table S3). For quantitative and time-resolved measurements, five-point boxcar smoothing was applied and the dominant m/z 299 → 215 MRM transition was employed. Qualifier ion transitions were monitored at m/z 299 → 243, m/z 299 → 256, and m/z 299 → 100 (Table S2) to confirm the presence of 6-PPDQ in all measured samples.
Results and Discussion
CP-MIMS is a simple, convenient, and fast analytical platform that involves immersion of a membrane probe directly in a sample. The sensitivity and selectivity can be tuned by adjustments made to the donor phase (pH), membrane (geometry), acceptor phase (co-solvents), ion source, and MS.7 The unusually low basicity of 6-PPDQ and the distinct fragmentation pathways reported1 suggested that this analyte was a good candidate for direct analysis by CP-MIMS. HRAM analysis of the membrane permeate in (+) ESI confirmed the presence of 6-PPDQ with observable parent ([M + H]+) and fragment ions agreeing (<3 ppm error) with reported values (Figure S3 and Table S3).
Method Development
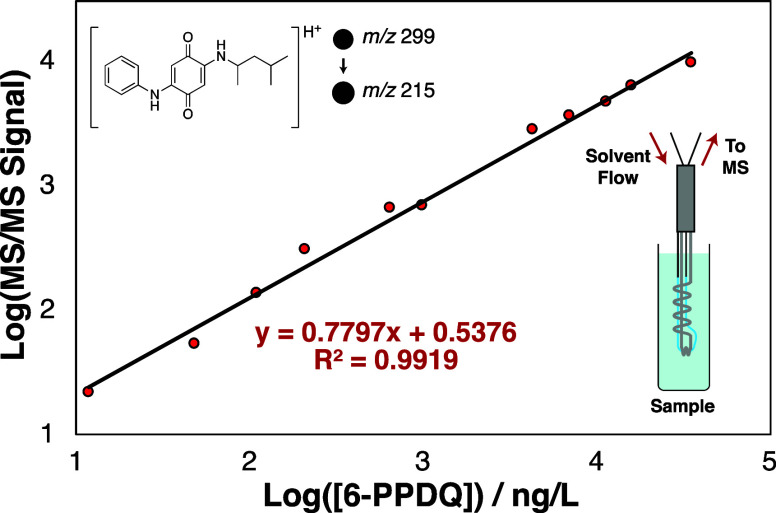
Method optimization focused on achieving rapid and sensitive 6-PPDQ analysis by (1) modifying the acceptor phase and (2) adjusting the membrane geometry. At the acceptor phase level, heptane was included to improve analyte permeation and reduce measurement response times.24 Additionally, volatile acids are known to enhance ionization efficiency in positive ESI. Direct infusion of 6-PPDQ in a 15/85 (v/v) heptane/methanol mixture at formic acid concentrations ranging from 0.006% to 0.32% (v/v) showed a maximum signal enhancement at 0.03% formic acid (Figure S4), which was 7.5 times greater than that of the same solvent system without formic acid. At the membrane level, a smaller capillary hollow fiber PDMS membrane (55 μm wall thickness) accommodates a slower acceptor phase flow (10 μL/min) compared to our standard CP-MIMS “J-probe” (170 μm wall thickness, 50–200 μL/min).10,11,14,19,22Figure S5 compares 6-PPDQ signal rise times in response to a step function increase in concentration using the two immersion probe designs. The thinner mini-probe yields a faster response time (t10–90% = 1 min) compared to that of the standard 170 μm thick CHFM (t10–90% = 8 min), achieving a full analytical duty cycle of 2.5 min/sample. Given the improved sensitivity afforded by inclusion of both heptane and formic acid in the acceptor, and the faster sample throughput with the thin membrane probe, this combination was used for all subsequent experiments. A log–log calibration curve (Figure 1) illustrates the 4 order of magnitude linear dynamic range between 8.4 and 33500 ng/L (R2 = 0.992). Linear calibration between 0 and 1000 ng/L was used for day-to-day calibrations and environmental samples (Figure S6; R2 = 0.995). On the basis of a signal-to-noise ratio of 3 for a 12 ng/L standard (Figure S7), the estimated limit of detection is 8 ng/L, 100-fold lower than the reported LC50 of 6-PPDQ for Coho salmon (800 ng/L).1 The intraday quantitation variability is <15% RSD (500 ng/L standard).
Figure 1.
Log–log calibration curve for aqueous 6-PPDQ standards using CP-MIMS with ESI in positive ion mode (m/z 299 → 215). The lower inset schematic shows the CP-MIMS direct immersion probe with the thin PDMS capillary hollow fiber membrane (blue) and stainless steel wire support (gray).
Without chromatographic separation, CP-MIMS is selective for 6-PPDQ on the basis of its PDMS permeability, its ability to form positive ions in ESI, and its efficient MRM transition at m/z 299 → 215. While compounds containing nitrogen are generally good proton acceptors and therefore well suited for (+) ESI, most are protonated at ambient pH in water and hence impermeable to PDMS. Figure S8 shows that 6-PPDQ remains in its neutral, membrane permeable (hydrophobic) form even under mildly acidic conditions while 6-PPD is permeable only at pH >7. While the ion at m/z 299 → 215 was employed for quantitation, MRM qualifier ions at m/z 243, 256, and 100 were also monitored.
Standard Addition Measurements
Given the complexity and variability of stormwaters, we approached quantitation using the method of standard additions.25 Standard addition curves (Figure S9) were generated with three spike additions to four constructed environmental water samples fortified with known quantities of 6-PPDQ. This approach requires ∼15 min/sample and gave excellent recoveries (88–104%) at a nominal concentration of 170 ng/L (Table 1). We applied the same approach to six environmental samples, including two from a small creek downstream from a highway overpass and four stormwaters (Table 1). Measured concentrations in stormwater samples ranged from 50 to 5500 ng/L, with two exhibiting 6-PPDQ concentrations well above the Coho LC50.1 Concentrations in stream samples were considerably lower (85–110 ng/L). Triplicate analyses of two stormwaters yielded RSD values of 22% and 11% for samples with concentrations of ∼50 and ∼600 ng/L, respectively. MRM qualifier ions were observed for all samples, confirming the presence of 6-PPDQ. However, given the complexity of stormwater-affected samples, we cannot rule out the possibility of interferents at this stage. Method selectivity will be improved in future work by coupling CP-MIMS directly with HRAM and/or field asymmetric ion mobility spectrometry (FAIMS) and verified by cross validation with LC-MS/MS.
High-Throughput Measurements
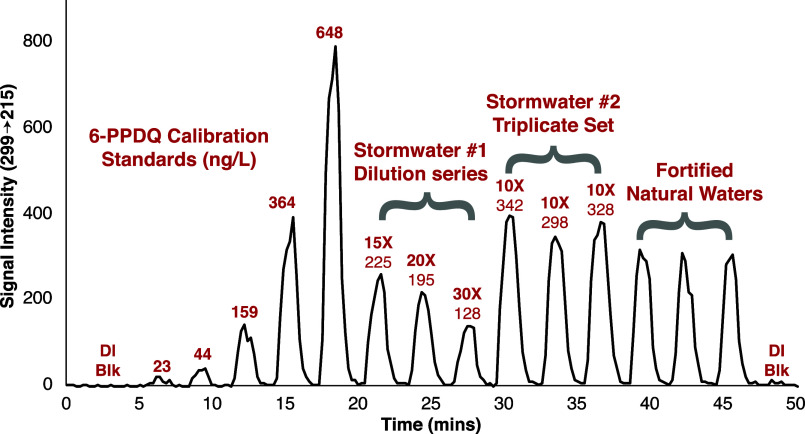
A method for high-throughput screening of 6-PPDQ in environmental samples was developed with a complete analytical duty cycle of 2.5 min/sample. This is accomplished by immersing the CP-MIMS probe directly in ambient samples for 90 s, followed by a 60 s methanol rinse, enabling 24 measurements per hour. While these timed exposures were performed manually, this approach can be interfaced with an autosampler.26Figure 2 gives a representative signal trace for 50 min of high-throughput screening, during which 16 measurements were performed. Table S4 summarizes performance metrics, including an RSD of <7% for triplicate analysis of stormwaters 1 and 2 and recoveries in fortified natural waters ranging from 112% to 147%. While the concentrations of 6-PPDQ for stormwaters 1 and 2 (Figure 2 and Table S4) are lower than those observed via the more quantitative standard addition method (Table 1), we are nonetheless encouraged by their relative agreement. Apparent differences may be due to matrix effects, which are not currently accounted for in rapid screening mode, and/or differences in sample hold time before analysis. Given that matrix effects are observed to influence the calibration slopes (Table 1 and Figure S9), we suggest that the rapid screening mode is semiquantitative and is currently best suited for samples with similar matrices. It can be used to quickly identify samples of interest for subsequent analysis by more quantitative methods. The use of isotopically labeled 6-PPDQ internal standards (unavailable during this study) will correct for matrix effects and instrument drift and is expected to improve the quantitation of the high-throughput method presented here.12
Figure 2.
Representative ion chronogram for the high-throughput measurement of 6-PPDQ by CP-MIMS. Sixteen analyses (seven calibrators and nine samples) were carried out over a 50 min window using 90 s sample exposure times with 60 s MeOH rinses. After dilution correction, the concentrations of 6-PPDQ in stormwaters 1 and 2 were 3.6 ± 0.3 and 3.2 ± 0.2 μg/L, respectively (Table S4). Dilution ratios and measured concentrations are shown with each sample peak. Fortified natural waters include a surface water, a seawater, and groundwater spiked with ∼200 ng/L 6-PPDQ.
Online Process Monitoring
CP-MIMS is readily adapted to provide real-time process and reaction monitoring, where the time-resolved MS signals follow dynamic changes in solution phase concentrations.8,16,27Figure 3A shows the desorption/dissolution of 6-PPDQ (black, left axis) from tire fragments suspended in an aqueous solution buffered at pH 7. After 30 min, the signal for 6-PPDQ appears to reach a steady state. An aliquot of this solution was collected, diluted in DI, and measured by CP-MIMS using standard additions to contain 4.2 μg/L 6-PPDQ and 0.2 μg/L 6-PPD. Figure 3A indicates that the 6-PPDQ is desorbing/dissolving faster than the parent 6-PPD (purple, right axis), which continues to increase in concentration, even after 30 min. We attribute this difference to the fact that 6-PPDQ is presumably enriched at the particle surface whereas 6-PPD is distributed throughout the tire particle. We have repeated this experiment with fragments obtained from tires produced in different years (2009, 2014, and 2016) and artificial turf pellets. The presence of 6-PPDQ in these suspensions was confirmed by qualifier fragment ions. The oldest tire released significantly less of each compound, whereas those from 2014 and 2016 exhibited similar levels (Table S5). A similar loading of the larger turf particles released negligible amounts of 6-PPD and 6-PPDQ. However, Figure 3B illustrates that when 10 g/L artificial turf particles were added, we observe 350 ng/L 6-PPDQ. The addition of an activated carbon sorbent (t = 40 min) yields a decrease in concentration of ∼90% over 10 min. Given the t10–90 rise time for 6-PPDQ with this membrane (Figure S5) is 5–30-fold faster than the adsorption/desorption processes observed in Figure 3, the changes in signal intensity reflect real-time changes in concentration within the sample. We propose therefore that CP-MIMS provides a powerful tool for the assessment of environmentally relevant processes that may characterize both sources and mitigation strategies.
Figure 3.
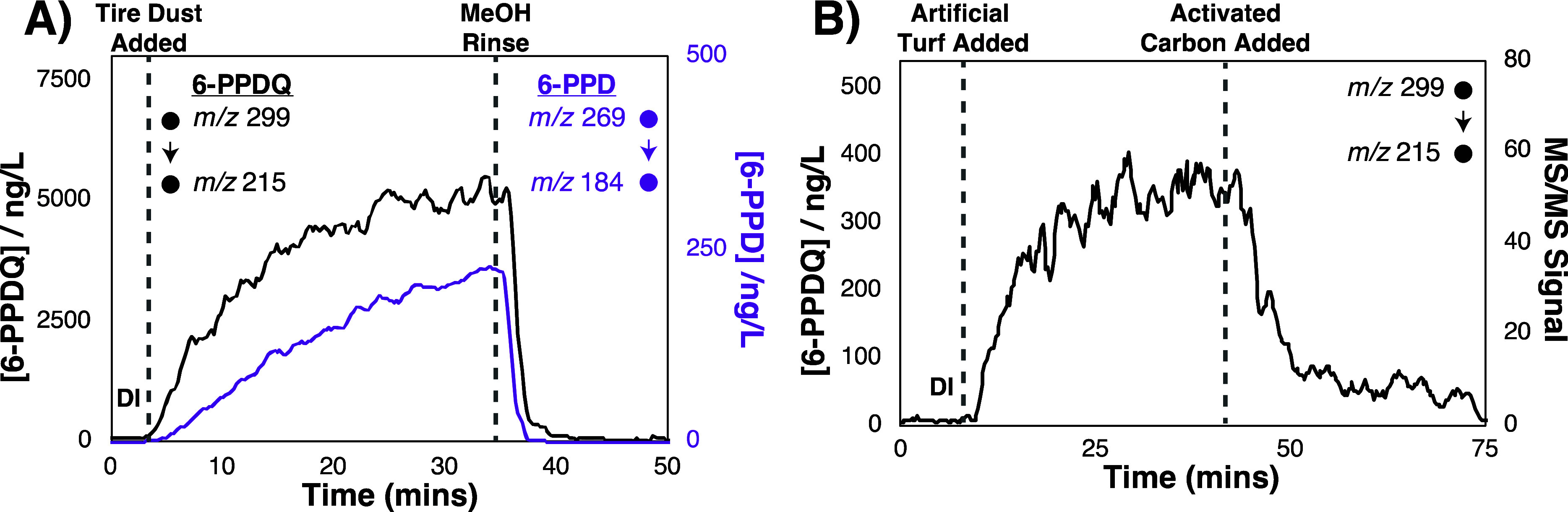
Time-resolved analysis of 6-PPD and 6-PPDQ in dynamic systems by CP-MIMS. (A) Desorption of 6-PPD (purple, right axis) and 6-PPDQ (black, left axis) from tire dust generated from a 2016 tire in a pH 7 buffered solution (0.5 g of tire dust/L). (B) Desorption of 6-PPDQ from a higher loading of artificial turf pellets (10 g/L), followed by sorption to activated carbon (1 g/L). The Y-axis concentrations are based on dilution of the equilibrated suspension (at t = 35 min in each run) and quantitation by standard additions.
In summary, we present preliminary results of an online membrane sampling method for the measurement of 6-PPDQ in the low nanogram per liter range. This approach provides a high-throughput platform and simple analytical workflow with an overall analysis time of 2.5 min, enabling semiquantitative measurement of 24 samples per hour. Analysis of 6-PPDQ by the method of standard additions (15 min/sample) yields improved quantitation with recoveries of 88–104% in fortified environmental samples. Natural water samples, including surface waters (n = 2) and stormwaters (n = 4), had 6-PPDQ concentrations ranging from 50 to 5500 ng/L. The use of CP-MIMS as a real-time monitoring strategy for dissolution and adsorption processes is also demonstrated. Given that environmental sources of 6-PPDQ are expected to be widespread, we believe that the direct MS method presented here will provide a powerful complement to conventional analytical approaches, providing a rapid, semiquantitative screening method for identifying hot spots and monitoring the efficacy of mitigation efforts. Ongoing method optimization and validation work includes the use of isotopically labeled standards, coupling to HRMS and FAIMS, and comparison to conventional LC-MS/MS methods.
Acknowledgments
The authors acknowledge Vancouver Island University and the University of Victoria for ongoing support of the Applied Environmental Research Laboratories and student researchers. This work was supported by infrastructure funded by the Canada Foundation for Innovation/British Columbia Knowledge Development Fund (32238 and 40274), operational support from the Natural Science and Engineering Research Council (NSERC) of Canada Discovery Grants RGPIN-2016-06454 (E.T.K.) and RGPIN-2021-02981 (C.G.G.), and NSERC graduate (J.M.) and undergraduate (A.J.) student scholarships. We thank the British Columbia Conservation Foundation (Thea Rodgers, Jamieson Atkinson) for assistance in collecting environmental samples.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.1c00794.
Details outlining 6-PPDQ synthesis, preparation of stock and standard solutions, water quality data, and additional data (PDF)
The authors declare no competing financial interest.
Notes
The synthesis was originally uploaded to a preprint server.28
Supplementary Material
References
- Tian Z.; Zhao H.; Peter K. T.; Gonzalez M.; Wetzel J.; Wu C.; Hu X.; Prat J.; Mudrock E.; Hettinger R.; Cortina A. E.; Biswas R. G.; Kock F. V. C.; Soong R.; Jenne A.; Du B.; Hou F.; He H.; Lundeen R.; Gilbreath A.; Sutton R.; Scholz N. L.; Davis J. W.; Dodd M. C.; Simpson A.; McIntyre J. K.; Kolodziej E. P. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. 10.1126/science.abd6951. [DOI] [PubMed] [Google Scholar]
- McIntyre J. K.; Davis J. W.; Hinman C.; Macneale K. H.; Anulacion B. F.; Scholz N. L.; Stark J. D. Soil bioretention protects juvenile salmon and their prey from the toxic impacts of urban stormwater runoff. Chemosphere 2015, 132, 213–9. 10.1016/j.chemosphere.2014.12.052. [DOI] [PubMed] [Google Scholar]
- Yang W.; Wang Z.; Hua P.; Zhang J.; Krebs P. Impact of green infrastructure on the mitigation of road-deposited sediment induced stormwater pollution. Sci. Total Environ. 2021, 770, 145294. 10.1016/j.scitotenv.2021.145294. [DOI] [PubMed] [Google Scholar]
- Werbowski L. M.; Gilbreath A. N.; Munno K.; Zhu X.; Grbic J.; Wu T.; Sutton R.; Sedlak M. D.; Deshpande A. D.; Rochman C. M. Urban Stormwater Runoff: A Major Pathway for Anthropogenic Particles, Black Rubbery Fragments, and Other Types of Microplastics to Urban Receiving Waters. ACS ES&T Water 2021, 1 (6), 1420–1428. 10.1021/acsestwater.1c00017. [DOI] [Google Scholar]
- Bressy A.; Gromaire M. C.; Lorgeoux C.; Saad M.; Leroy F.; Chebbo G. Towards the determination of an optimal scale for stormwater quality management: micropollutants in a small residential catchment. Water Res. 2012, 46 (20), 6799–810. 10.1016/j.watres.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Du B.; Lofton J. M.; Peter K. T.; Gipe A. D.; James C. A.; McIntyre J. K.; Scholz N. L.; Baker J. E.; Kolodziej E. P. Development of suspect and non-target screening methods for detection of organic contaminants in highway runoff and fish tissue with high-resolution time-of-flight mass spectrometry. Environ. Sci. Process Impacts 2017, 19 (9), 1185–1196. 10.1039/C7EM00243B. [DOI] [PubMed] [Google Scholar]
- Huang W.; Shi Y.; Huang J.; Deng C.; Tang S.; Liu X.; Chen D. Occurrence of Substituted p-Phenylenediamine Antioxidants in Dusts. Environ. Sci. Technol. Lett. 2021, 8 (5), 381–385. 10.1021/acs.estlett.1c00148. [DOI] [Google Scholar]
- Krogh E. T.; Gill C. G.. Condensed Phase Membrane Introduction Mass Spectrometry - Continuous, Direct and Online Measurements in Complex Samples. In Advances in the Use of Liquid Chromatography Mass Spectrometry (LC-MS) - Instrumentation Developments and Applications; Cappiello A., Palma P., Eds.; Elsevier: Amsterdam, 2018; Vol. 79, pp 173–203. [Google Scholar]
- Duncan K. D.; McCauley E. P.; Krogh E. T.; Gill C. G. Characterization of a condensed-phase membrane introduction mass spectrometry (CP-MIMS) interface using a methanol acceptor phase coupled with electrospray ionization for the continuous on-line quantitation of polar, low-volatility analytes at trace levels in complex aqueous samples. Rapid Commun. Mass Spectrom. 2011, 25 (9), 1141–51. 10.1002/rcm.4967. [DOI] [PubMed] [Google Scholar]
- Duncan K. D.; Letourneau D. R.; Vandergrift G. W.; Jobst K.; Reiner E.; Gill C. G.; Krogh E. T. A semi-quantitative approach for the rapid screening and mass profiling of naphthenic acids directly in contaminated aqueous samples. J. Mass Spectrom. 2016, 51 (1), 44–52. 10.1002/jms.3721. [DOI] [PubMed] [Google Scholar]
- Duncan K. D.; Richards L. C.; Monaghan J.; Simair M. C.; Ajaero C.; Peru K. M.; Friesen V.; McMartin D. W.; Headley J. V.; Gill C. G.; Krogh E. T. Direct analysis of naphthenic acids in constructed wetland samples by condensed phase membrane introduction mass spectrometry. Sci. Total Environ. 2020, 716, 137063. 10.1016/j.scitotenv.2020.137063. [DOI] [PubMed] [Google Scholar]
- Duncan K. D.; Vandergrift G. W.; Krogh E. T.; Gill C. G. Ionization suppression effects with condensed phase membrane introduction mass spectrometry: methods to increase the linear dynamic range and sensitivity. J. Mass Spectrom. 2015, 50 (3), 437–43. 10.1002/jms.3544. [DOI] [PubMed] [Google Scholar]
- Borden S. A.; Damer H. N.; Krogh E. T.; Gill C. G. Direct quantitation and characterization of fatty acids in salmon tissue by condensed phase membrane introduction mass spectrometry (CP-MIMS) using a modified donor phase. Anal. Bioanal. Chem. 2019, 411 (2), 291–303. 10.1007/s00216-018-1467-y. [DOI] [PubMed] [Google Scholar]
- Monaghan J.; Richards L. C.; Vandergrift G. W.; Hounjet L. J.; Stoyanov S. R.; Gill C. G.; Krogh E. T. Direct mass spectrometric analysis of naphthenic acids and polycyclic aromatic hydrocarbons in waters impacted by diluted bitumen and conventional crude oil. Sci. Total Environ. 2021, 765, 144206. 10.1016/j.scitotenv.2020.144206. [DOI] [PubMed] [Google Scholar]
- Termopoli V.; Famiglini G.; Palma P.; Cappiello A.; Vandergrift G. W.; Krogh E. T.; Gill C. G. Condensed Phase Membrane Introduction Mass Spectrometry with Direct Electron Ionization: On-line Measurement of PAHs in Complex Aqueous Samples. J. Am. Soc. Mass Spectrom. 2016, 27 (2), 301–8. 10.1007/s13361-015-1285-9. [DOI] [PubMed] [Google Scholar]
- Termopoli V.; Torrisi E.; Famiglini G.; Palma P.; Zappia G.; Cappiello A.; Vandergrift G. W.; Zvekic M.; Krogh E. T.; Gill C. G. Mass Spectrometry Based Approach for Organic Synthesis Monitoring. Anal. Chem. 2019, 91, 11916–11922. 10.1021/acs.analchem.9b02681. [DOI] [PubMed] [Google Scholar]
- Vandergrift G. W.; Monaghan J.; Krogh E. T.; Gill C. G. Direct Analysis of Polyaromatic Hydrocarbons in Soil and Aqueous Samples Using Condensed Phase Membrane Introduction Tandem Mass Spectrometry with Low-Energy Liquid Electron Ionization. Anal. Chem. 2019, 91 (2), 1587–1594. 10.1021/acs.analchem.8b04949. [DOI] [PubMed] [Google Scholar]
- Vandergrift G. W.; Krogh E. T.; Gill C. G. Direct, Isomer-Specific Quantitation of Polycyclic Aromatic Hydrocarbons in Soils Using Membrane Introduction Mass Spectrometry and Chemical Ionization. Anal. Chem. 2020, 92 (23), 15480–15488. 10.1021/acs.analchem.0c03259. [DOI] [PubMed] [Google Scholar]
- Vandergrift G. W.; Lattanzio-Battle W.; Krogh E. T.; Gill C. G. Condensed Phase Membrane Introduction Mass Spectrometry with In Situ Liquid Reagent Chemical Ionization in a Liquid Electron Ionization Source (CP-MIMS-LEI/CI). J. Am. Soc. Mass Spectrom. 2020, 31 (4), 908–916. 10.1021/jasms.9b00143. [DOI] [PubMed] [Google Scholar]
- Duncan K. D.; Volmer D. A.; Gill C. G.; Krogh E. T. Rapid Screening of Carboxylic Acids from Waste and Surface Waters by ESI-MS/MS Using Barium Ion Chemistry and On-Line Membrane Sampling. J. Am. Soc. Mass Spectrom. 2016, 27 (3), 443–50. 10.1007/s13361-015-1311-y. [DOI] [PubMed] [Google Scholar]
- Feehan J. F.; Monaghan J.; Gill C. G.; Krogh E. T. Direct measurement of acid dissociation constants of trace organic compounds at nanomolar levels in aqueous solution by condensed phase membrane introduction mass spectrometry. Environ. Toxicol. Chem. 2019, 38 (9), 1879–1889. 10.1002/etc.4519. [DOI] [PubMed] [Google Scholar]
- Willis M. D.; Duncan K. D.; Krogh E. T.; Gill C. G. Delicate polydimethylsiloxane hollow fibre membrane interfaces for condensed phase membrane introduction mass spectrometry (CP-MIMS). Rapid Commun. Mass Spectrom. 2014, 28 (7), 671–81. 10.1002/rcm.6828. [DOI] [PubMed] [Google Scholar]
- Hiki K.; Asahina K.; Kato K.; Yamagishi T.; Omagari R.; Iwasaki Y.; Watanabe H.; Yamamoto H. Acute Toxicity of a Tire Rubber-Derived Chemical, 6PPD Quinone, to Freshwater Fish and Crustacean Species. Environ. Sci. Technol. Lett. 2021, 8, 779–784. 10.1021/acs.estlett.1c00453. [DOI] [Google Scholar]
- Vandergrift G. W.; Krogh E. T.; Gill C. G. Polymer Inclusion Membranes with Condensed Phase Membrane Introduction Mass Spectrometry (CP-MIMS): Improved Analytical Response Time and Sensitivity. Anal. Chem. 2017, 89 (10), 5629–5636. 10.1021/acs.analchem.7b00908. [DOI] [PubMed] [Google Scholar]
- Harris D. C.; Lucy C. A.. Quantitative Chemical Analysis, 9th ed.; W. H. Freeman and Co.: New York, 2016. [Google Scholar]
- Duncan K. D.; Willis M. D.; Krogh E. T.; Gill C. G. A miniature condensed-phase membrane introduction mass spectrometry (CP-MIMS) probe for direct and on-line measurements of pharmaceuticals and contaminants in small, complex samples. Rapid Commun. Mass Spectrom. 2013, 27 (11), 1213–21. 10.1002/rcm.6560. [DOI] [PubMed] [Google Scholar]
- Letourneau D. R.; Gill C. G.; Krogh E. T. Photosensitized degradation kinetics of trace halogenated contaminants in natural waters using membrane introduction mass spectrometry as an in situ reaction monitor. Photochem. Photobiol. Sci. 2015, 14 (11), 2108–18. 10.1039/C5PP00286A. [DOI] [PubMed] [Google Scholar]
- Agua A.; Stanton R.; Pirrung M. Preparation of 2-((4-Methylpentan-2-Yl)amino)-5-(Phenylamino)cyclohexa-2,5-Diene-1,4-Dione (6PPD-Quinone), an Environmental Hazard for Salmon. ChemRxiv 2021, 10.26434/chemrxiv.13698985.v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.