Abstract
Alcohol exposure has detrimental effects on both the developing and mature brain. Endoplasmic reticulum (ER) stress is one of the mechanisms that contributes to alcohol-induced neuronal damages. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER stress responsive protein and is neuroprotective in multiple neuronal injury and neurodegenerative disease models. MANF deficiency has been shown to exacerbate alcohol-induced ER stress and neurodegeneration. However, it is unknown whether MANF supplement is sufficient to protect against alcohol neurotoxicity. Alcohol alters MANF expression in the brain, but the mechanisms underlying alcohol modulation of MANF expression remain unclear. This study was designed to determine how alcohol alters MANF expression in neuronal cells and whether exogeneous MANF can alleviate alcohol neurotoxicity. We showed that alcohol increased MANF transcription and secretion without affecting MANF mRNA stability and protein degradation. ER stress was necessary for alcohol-induced MANF upregulation, as pharmacological inhibition of ER stress by 4-PBA diminished alcohol-induced MANF expression. In addition, the presence of ER stress response element II (ERSE-II) was required for alcohol-stimulated MANF transcription. Mutations or deletion of this sequence abolished alcohol-regulated transcriptional activity. We generated MANF knockout (KO) neuronal cells using CRISPR/Cas9. MANF KO cells exhibited increased unfolded protein response (UPR) and were more susceptible to alcohol-induced cell death. On the other hand, MANF upregulation by addition of recombinant MANF protein or adenovirus gene transduction, protected neuronal cells against alcohol-induced cell death. Further studies using early postnatal mouse pups demonstrated that enhanced MANF expression in the brain by intracerebroventricular (ICV) injection of MANF adeno-associated viruses ameliorated alcohol-induced cell death. Thus, alcohol increased MANF expression through inducing ER stress, which could be a protective response. Exogenous MANF was able to protect against alcohol-induced neurodegeneration.
Keywords: MANF, alcohol neurotoxicity, neurodegeneration, endoplasmic reticulum stress
Graphical abstract

(By Figdraw)
Alcohol triggers endoplasmic reticulum (ER) stress and neurodegeneration. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER stress responsive protein that is neuroprotective in various neurodegenerative disease models. In this study, we demonstrated that alcohol upregulated MANF expression through ER stress and the activation of ER stress response element II (ERSE-II). MANF deficient neuronal cells exhibited increased unfolded protein response (UPR) and were more susceptible to alcohol-induced neuronal damage. Exogenous MANF supplementation protected neurons against alcohol neurotoxicity in both neuronal cell culture and postnatal mouse brains. These results suggest that alcohol-induced MANF upregulation could be a protective response against alcohol neurotoxicity.
Introduction
Alcohol is a commonly consumed substance that can pose detrimental effects on the structure, physiology, and function of the central nervous system (CNS) [1, 2]. Immature neurons are especially vulnerable to alcohol neurotoxicity. The exposure of developing fetus to alcohol during pregnancy can result in a condition known as fetal alcohol spectrum disorder (FASD). FASD manifests a wide variety of alcohol-related physiological and cognitive impairments in children [3]. FASD is a highly prevalent, yet preventable cause of mental disabilities worldwide. Numerous clinical and animal studies have shown that even acute and moderate alcohol exposure during development can induce profound damages to the developing brain with long-term effects that can impair neuronal function later in life [4–6]. The neurobehavioral impairments in individuals with FASD arise from the structural and functional changes in the CNS, with neurodegeneration being the most profound characteristic [7, 8]. Chronic heavy alcohol intake in adult also results in structural and functional alterations in the brain [9, 10]. Brain imaging revealed smaller frontal cortical and white matter regions with larger ventricles in the alcoholics brain, which was associated with functional deficits in human alcoholics [11].
Alcohol-induced neurodegeneration may be mediated by multiple cellular and molecular mechanisms, including neuroinflammation [12], oxidative stress [13], autophagy [14], disruption of neurotrophic factor functions [15], and dysregulation of microRNA [16]. Recently, the involvement of endoplasmic reticulum (ER) stress has been suggested as a contributing factor in the process of alcohol-induced neurodegeneration [17, 18]. The ER is an organelle regulating protein synthesis and folding, posttranslational modification and secretion, and lipid metabolism. ER stress can be induced by various pathological insults that affect proper protein folding and disturb the homeostasis of the ER, including glucose deprivation, abnormal ER Ca2+ levels, inflammation, and toxic chemical exposure. ER stress can be resolved by the activation of a highly conserved adaptive response called unfolded protein response (UPR). UPR is regulated by three ER-transmembrane proteins: protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6). It alleviates ER stress by suppressing the rate of protein synthesis, increasing the ER protein folding capacity, and eliminating aberrant proteins through ER-associated protein degradation (ERAD) [19]. If the UPR is insufficient to overcome ER stress, prolonged UPR activation can ultimately result in apoptosis [20, 21]. In neurons, the maintenance of ER homeostasis is essential for normal neuronal functions as the dysregulation of UPR often lead to neurodegeneration. The aggregation of misfolded/unfolded proteins is a common pathogenesis observed in many neurodegenerative diseases, including Parkinson’s disease [22], Alzheimer’s disease [23], and stroke [24]. We and other investigators have demonstrated that alcohol exposure can cause ER stress and cell death in the developing, mature, and aged CNS [25–31]. Pharmacologically inhibiting ER stress has the potential to protect alcohol-sensitive immature neurons from the detrimental effects of alcohol-induced neurodegeneration [32].
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER-stress inducible protein that was originally identified as a secreted trophic factor to promote dopaminergic neuron survival in cell cultures [33]. It is widely expressed in the developing and mature brain [34, 35]. MANF is involved in multiple neurodevelopment processes, including neurogenesis [36], neurite outgrowth [37, 38], and neuron migration [39]. Increasing evidence suggest that MANF not only regulates normal neurodevelopment and survival [40, 41], but also protects neurons from pathological conditions such as brain ischemia, Alzheimer’s disease, Parkinson’s disease, traumatic brain injury, and retinal degeneration [33, 40–48].
Previously, we have demonstrated that deficiency of neuronal MANF exacerbated alcohol-induced neurodegeneration [49], and alcohol exposure caused ER stress and increased MANF expression in the developing brain [26, 32, 49]. In this study, we investigated the mechanisms underlying alcohol-induced upregulation of MANF expression and sought to determine whether exogenous MANF supplementation is sufficient to protect neurons against alcohol neurotoxicity.
Materials and Methods
Reagents and antibodies
The following reagents and materials were used: MEM (11095–080), high glucose DMEM (10569–010), and antibiotic-antimycotic (15240112) were from Life Technologies (Carlsbad, CA). FBS (35–010-CV) was from Corning (Glendale, AZ). MTT (M5655), DAPI (D9542), tunicamycin (T7765), and fibronectin (F3667) were from Sigma-Aldrich (St. Louis, MO). Ethanol 200 Proof (64-17-5) was from Decon Labs (King of Prussia, PA). Paraformaldehyde (PFA) (15714) was from Electron Microscopy Sciences (Hatfield, PA). Recombinant human MANF (3748-MN-050) was from R&D systems (Minneapolis, MN). Recombinant human MANF HA-tagged MANF adenovirus (mouse) (279970540200) and CMV Null control adenovirus (000047A) were from Applied Biological Materials (Richmond, BC, Canada). VECTASHIELD mounting medium (H-1400) was from Vector Laboratories (Burlingame, CA). Actinomycin D (GR300–0005) was purchased from Enzo Lifesciences (Farmingdale, NY). Cycloheximide (10187) was purchased from VWR (Radnor, PA). Trizol Reagent (15596026) and Lipofectamine 2000 transfection reagent (11668027) were purchased from Invitrogen (Waltham, MA). DNase I recombinant, RNase-free (04716728001) was from Roche (South San Francisco CA). RNase inhibitor (N8080119), High-Capacity cDNA Reverse Transcription Kit (4368814), and TaqMan™ fast universal PCR master mix (4352042) were from Applied Biosystems (Waltham, MA). Taqman gene expression assay for Manf (Mm00512511) and 18 s rRNA (Mm03928990) were purchased from Thermo Fisher Scientific (Rockford, IL). DC protein assay kit (5000112) was from Bio-Rad Laboratories (Hercules, CA). Sodium phenylbutyrate (4-PBA) (SML0309) was from MilliporeSigma (Burlington, MA). Cytiva Amersham™ ECL™ Prime Western Blotting Detection Reagent (45-002-401) was purchased from GE Healthcare Life Sciences (Piscataway, NJ). Luciferase Assay System (E1500) was purchased from Promega (Madison, WI). Control CRISPR/Cas9 plasmid (sc-418922), mouse ARP double nickase plasmid (sc-428989-NIC), UltraCruz transfection reagent (sc-395739), and plasmid transfection medium (sc-108062) were purchased from Santa Cruz Biotechnology (Dallas, TX). Fisherbrand™ Superfrost™ Plus Microscope Slides (12-550-15) and Cytiva Vivaspin™ 6 Sample Concentrators (45-001-571) were purchased from Fisher Scientific (Hampton, NH).
The following antibodies were used: ATF6 antibody (LS-B2516, 1:1000) was purchased from Life Span Biosciences (Seattle, WA). Cleaved caspase-3 (9661, 1:1000), phosphorylated-eIF2α (3398, 1:1000), eIF2α (9722, 1:1000), ATF4 (11815, 1:1000), IRE1α (3294, 1:1000), phosphorylated-PERK (3179, 1:1000), PERK (3192, 1:1000), HA-Tag (3724, 1:1000), and β-Actin (3700, 1:10000) antibodies were from Cell Signaling Technology (Danvers, MA). GRP78 antibody (NBP1–06274, 1:2000) was from Novus Biologicals (Littleton, CO). XBP1s antibody (658802, 1:1000) was from BioLegend (San Diego, CA). Phosphorylated-IRE1α (ab48187, 1:1000), PDIA1 (ab2792, 1:100) and MANF (ab67271 and ab67203, 1:1000) antibodies were from Abcam (Cambridge, MA). CHOP antibody (MA1–250, 1:1000) was from Thermo Fisher Scientific (Rockford, IL). Horseradish peroxidase (HRP)-conjugated anti-rabbit (NA934V, 1:200) and anti-mouse (NA931V, 1:200) secondary antibodies were from GE Healthcare Life Sciences (Piscataway, NJ). Alexa-594 conjugated anti-rabbit antibody (A-11012, 1:200) was purchased from Invitrogen (Waltham, MA).
Cell culture and treatments
Mouse neuronal cell line N2a (ATCC®CCL-131, RRID:CVCL_0470) and human neuronal cell line SH-SY5Y (ATCC®CRL-2266, RRID:CVCL_0019) were purchased from ATCC (Manassas, VA). These cell lines are not listed as a commonly misidentified cell line by the International Cell Line Authentication Committee. N2a and SH-SY5Y cells were maintained in MEM and DMEM, respectively, supplemented with 10% FBS and 1% antibiotic-antimycotic at 37°C in 5% CO2 in a humidified incubator. The culture medium was changed every 2 days, and cells were subcultured after reaching 90% confluence. A maximum of 5 cell passages were used. No further authentication was performed in the laboratory. For alcohol treatment, N2a and SH-SY5Y cells were starved in serum free medium overnight and then incubated with complete medium containing 0.4% weight/volume (w/v) ethanol, unless noted otherwise. Cells were put into a sealed container with 5% CO2 to maintain proper ethanol concentrations in the medium. To decide the appropriate concentration of recombinant human MANF (rhMANF) treatment, rhMANF was added into the medium at various concentrations of 5, 10, 20, and 50 ng/ml two hours prior to alcohol treatment. 20 ng/ml rhMANF were chose for subsequent experiments as it generated the best outcome. For ER stress induction, cells were incubated with 5 μg/ml tunicamycin or 1.5 μM thapsigargin for 24 hours.
RNA extraction and cDNA synthesis
Cells were dissociated from the plates by trypsin. After PBS wash, total RNA was extracted from cells using Trizol reagent. One μg of total RNA was reverse transcribed into cDNA using SuperScriptTM III reverse transcriptase and random primers according to the manufacturer’s instruction (Invitrogen).
Quantitative real-time PCR (qPCR)
qPCR was performed on the QuantStudio 3 Real-Time PCR Systems using the TaqMan™ fast universal PCR master mix. Taqman gene expression assay for Manf (Mm00512511) and 18 s rRNA (Mm03928990) were purchased from Thermo Fisher Scientific. All reactions were performed in triplicates. The relative Manf expression was normalized to the housekeeping 18 s rRNA using cycle time (Ct) values. The relative differences among different treatments were calculated as relative fold changes normalized to control using 2^−ΔΔCt method.
Protein extraction and immunoblotting
Protein was extracted from cells or postnatal mice brains as previously described [36]. Briefly, samples were lysed on ice for 15 minutes in RIPA buffer (150 mM NaCl, 1 mM EGTA, 50 mM Tris-HCl pH 7.5, 0.5% NP-40, 0.25% SDS, and freshly added protease inhibitors). Protein concentration was determined using the DC protein assay according to the manufacture’s instruction. To enrich MANF protein from the culture medium, 3 ml of conditioned medium from each treatment was centrifuged at 4,000 × g for 90 minutes at 25 °C using the Cytiva Vivaspin™ 6 Sample Concentrators (45-001-571).
For immunoblotting, 20–30 μg protein samples from cell lysates or 20 μl enriched culture medium were separated on 12% or 15% polyacrylamide gels by electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked in 5% BSA and incubated with primary antibodies at 4°C overnight. Membranes were then washed with TBST and incubated with horseradish peroxidase conjugated secondary antibodies. Blots were developed using the Cytiva Amersham™ ECL™ Prime Western Blotting Detection Reagent on Chemi™Doc imaging system (Bio- Rad Laboratories, Hercules, CA). Band intensity was quantified using Image Lab software (Bio-Rad Laboratories). β-actin was used as housekeeping control for proteins from cell lysates and ponceau staining image was used as loading control for proteins from culture media.
Immunocytochemistry and immunofluorescence
Cells were seeded in 24-well plates onto sterile coverslips coated with 10μg/ml fibronectin. Following treatment, the cells were fixed using 4% PFA and made permeable using 0.25% Triton X-100 in PBS. Subsequently, the cells were blocked in PBS with 1% BSA and 2% goat serum for 30 minutes and incubated with primary antibodies for 1 hour at room temperature and Alexa fluor dyes-conjugated secondary antibodies for 30 minutes in the dark. DAPI was used to counterstain the nuclei.
For immunofluorescence labeling, postnatal mouse brains were removed, post-fixed in 4% PFA for 48 hours at 4 C°, cryopreserved in 30% sucrose in PBS, frozen and sectioned sagittally at a thickness of 10 μm using a cryostat. Brain sections were mounted onto superfrost/plus slides. Slides were incubated with primary antibodies at 4 C° overnight and Alexa fluor-conjugated secondary antibodies for 1 hour at room temperature in the dark. DAPI was used to counterstain the nuclei.
Images were obtained using the IX83 inverted fluorescent microscope (Olympus). Fluorescent intensity and the number of positive cells were analyzed using the Olympus cellSens Dimention software.
mRNA stability assay
To determine MANF mRNA stability, N2a cells were cultured in 6-well plates and starved in serum free medium overnight. Cells were then treated for 0, 0.5, 1, 3, 6, and 8 hours with 10 μg/ml actinomycin D with or without 0.4% (w/v) alcohol in complete media. Total RNA was extracted, and reverse transcribed into cDNA for subsequent qPCR analysis on MANF transcripts.
Cycloheximide (CHX)-chase assay
N2a cells were cultured in 6-well plates and starved in serum free medium overnight. Cells were then treated for 0, 0.5, 1, 3, and 6 hours with 100 μg/ml CHX with or without 0.4% (w/v) alcohol in complete media. Protein was obtained from the cell lysate and subsequently utilized for immunoblotting analysis. The level of MANF was normalized to actin and the ratio of each time points to 0 hour was calculated.
Pharmacological inhibition of ER stress by 4-PBA
Sodium phenylbutyrate (4-PBA) inhibits ER stress by acting as a chemical chaperone that facilitate protein folding, thus prevents their aggregation. N2a cells were preincubated with 10 mM of 4-PBA (SML0309, MilliporeSigma) for 2 hours then treated with alcohol as described above.
Luciferase activity assay
Luciferase reporter constructs with wild type or mutant mouse MANF promoter regions were described previously [50] and generously provided by Dr. Oh-Hashi from Gifu University, Japan. N2a cells were cultured in 6-well plates and transfected with 1 μg/well reporter constructs using Lipofectamine 2000. Forty-eight hours after the transfection, cells were treated with 0.4% (w/v) ethanol or 5 μg/ml tunicamycin for 6 hours. Luciferase activities were detected using the Luciferase Assay System (Promega) according to the manufacture’s instruction. Relative MANF promoter activities were normalized to cells transfected with pGL3-Basic empty vector.
Generation of MANF knockout (KO) N2a cells using CRISPR/Cas9
MANF KO N2a cells were generated and described previously [37]. Briefly, N2a cells were seeded onto 6-well plate and transfected with 1 μg of either control CRISPR/Cas9 or mouse ARP double nickase plasmids (Santa Cruz Biotechnology). Forty-eight hours after the transfection, cells were selected for GFP by flow cytometry and selected by 1 μg/ml puromycin for 5 days. Single cells were dispensed into 96-well plates by cell sorting using BD FACSAria II Cell Sorter (BD Biosciences, Franklin Lakes, NJ), and allowed to expand in complete media for 2 weeks. Protein was extracted from each single cell colony and immunoblotting for MANF was performed to confirm MANF knock out. We generated 16 MANF KO colonies in N2a cells and 2–3 colonies were used for experiments in this study to confirm data reproducibility.
Cell viability assay
MTT assay was used to determine cell viability. Cells were seeded in 96-well plates and starved in serum free medium overnight and then incubated with complete media with or without alcohol. Five-hundred μg/ml MTT was added to each well and incubated at 37°C for 2 hours. After the incubation, culture medium in each well was replaced with 100 μl DMSO. Results were detected using the SpectraMax iD3 Multi-Mode Microplate Reader (Molecular Devices, San Jose,CA) at the wavelength of 570 nm. The percentage of cell survival after alcohol exposure was calculated by normalizing alcohol- treated groups to no alcohol control groups at each time points.
Adenovirus transduction
Crude viral stock of HA-tagged mouse MANF adenovirus (MANF-AD) and CMV-Null control adenovirus (Ctrl-AD) were produced at a titer ranging from 1×107- 1×108 pfu/ml by the manufacture (Applied Biological Materials). They were amplified in HEK293 cells as described previously [37]. To obtain MANF overexpression, N2a cells were incubated with 120 μl per well MANF-AD in 6-well plate for 2 hours. Cells underwent subsequent alcohol treatment, MTT analysis, and immunoblot at desired time points as described above. Although Enzyme-linked immunosorbent assay (ELISA) was used in previous reports to determine MANF secretion [51], the current study aimed to detect both endogenous MANF and HA-tagged exogenous MANF and therefore employed immunoblotting approach. To enrich MANF in the culture media, 3 ml of conditioned medium from each treatment was centrifuged at 4,000 × g for 90 minutes at 25 °C using the Cytiva Vivaspin™ 6 Sample Concentrators (45-001-571). The enriched culture media were subject to immunoblot as described in the previous section.
Neonatal intracerebroventricular (ICV) injection of adeno-associated virus (AAV)
All experimental procedures were performed following regulations for the Care and Use of Laboratory Animals set forth by the National Institutes of Health (NIH) Guide and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa (#3042295). pAAV-CMV>mManf[NM_029103.4](ns):T2A:EGFP:WPRE vector (MANF-AAV) and pAAV-CMV>EGFP:WPRE vector (control-AAV) were generated and packaged into AAV-PHP.eB virus at a minimum titer of 1013 viral particles (vp)/ml by VectorBuilder (Chicago, IL). Viruses were diluted with sterile PBS and mixed with 0.04% trypan blue. For free-hand ICV delivery, postnatal day 0 (PD0) neonatal C57BL/6 mice were cryo-anesthetized and injected with control- or MANF-AAV at a dose of 1010 vp/cerebral hemisphere for both hemispheres as previously described [52]. Injections were performed using a 10 μl Hamilton 701 Cemented Needle Special (SN) Syringe with a 10 millimeter 33-gauge 30° bevel needle (Hamilton Company, Reno, NV). The skull was pierced at 2/5 distance from the lambda to the eye with 3 mm in depth. After injection, pups were placed on a heating pad to recover body heat. Brains of injected mice were collected and cryosectioned on PD3, 7, and 14 for the assessment of GFP expression. Widespread GFP expression was observed in the postnatal brain at all time points, indicative of successful AAV expression.
Animals and alcohol exposure
All experimental procedures were performed following regulations for the Care and Use of Laboratory Animals set forth by the National Institutes of Health (NIH) Guide and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa (#3042295). Four pregnant C57BL/6 mouse dams (RRID:MGI:2159769) were purchased from The Jackson Laboratory. Dams were single housed with ad libitum access to chow and water on a 14-hour light/10-hour dark cycle. They gave birth to a total of 21 pups. Pups were numbered from 1 to 21 and then divided randomly into 4 groups using the RAND formula in Excel: control-AAV+PBS (n=5), control-AAV+EtOH (n=5), MANF-AAV+PBS (n=5), MANF-AAV+EtOH (n=6). On PD0, pups were injected with control- or MANF-AAV as described above. On PD7, they were subcutaneously injected with either PBS control or ethanol (2.5 g/kg, 20% solution in PBS) twice with a 2-hour interval as previously described [49]. The first and second injection were performed at 9 and 11 am, respectively. After 8 hours following the initial injection, pups were cryo-anesthetized and decapitated to collect the brain. Neuronal apoptosis was assessed by immunofluorescence labeling and immunoblotting analysis for cleaved-caspase 3. Briefly, freshly dissected postnatal mouse brains were cut in half saggitally with one half frozen on dry ice for protein extraction and immunoblot as described above. The other half was fixed in 4% PFA and used for fluorescence labeling for cleaved-caspase 3 as describe above. Fluorescence images were obtained using the IX83 inverted fluorescent microscope (Olympus). Two sections were examined for each brain. The total number of cleaved-caspase 3 positive cells in the cerebral cortex and cerebellum was counted using the Olympus cellSens Dimention software. The timeline for the experimental procedure is shown in Figure 7A. Investigators were blinded for experimental groups when assessing the immunofluorescence labeling.
Figure 7. Effects of MANF on alcohol-induced cell death in the developing mouse cerebral cortex.
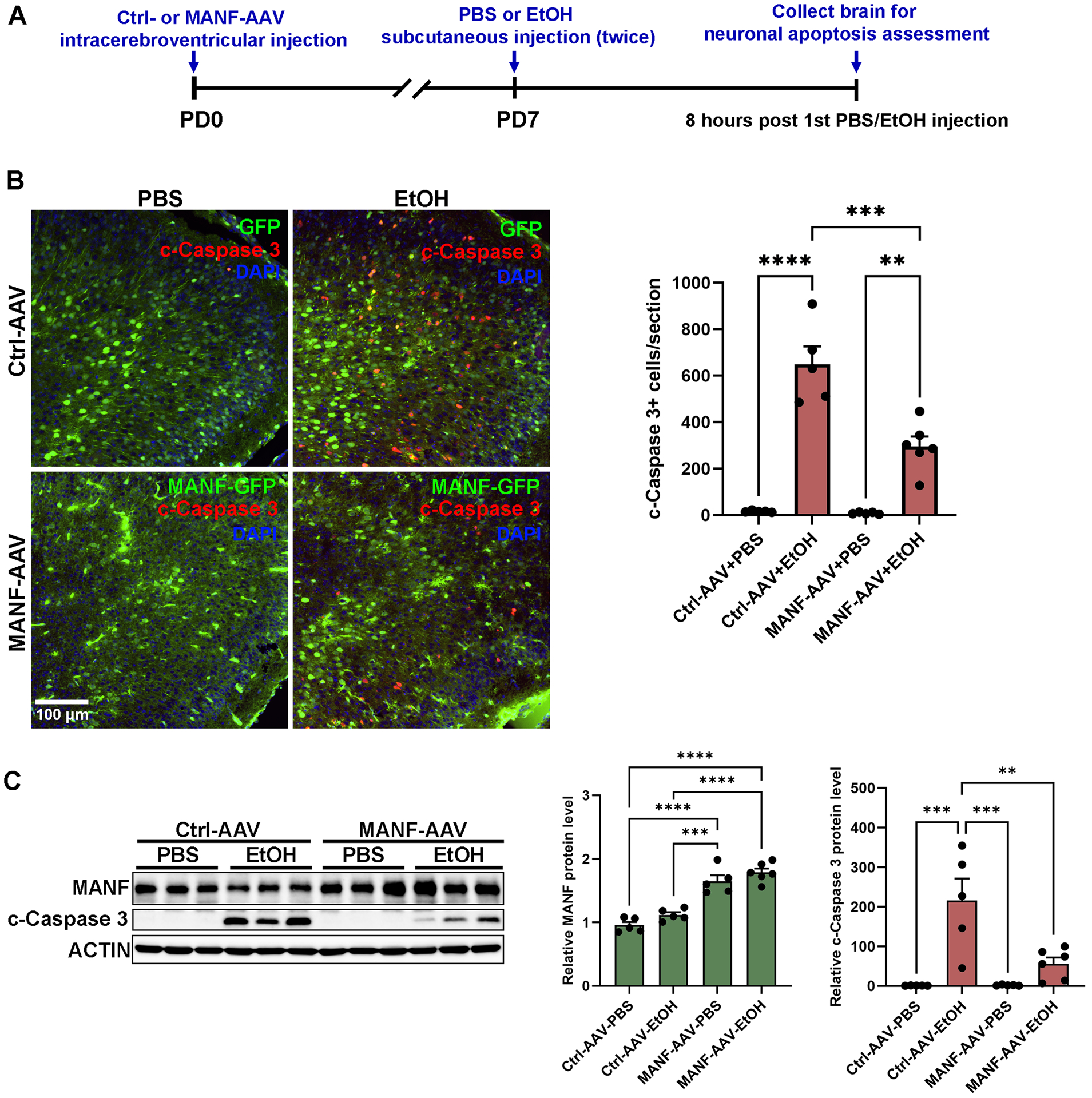
A: Time-line diagram showing the experimental procedure. Newborn pups were ICV-injected with either control- or MANF-AAV. The pups were then injected with PBS control or alcohol subcutaneously at PD7. Eight hours after alcohol treatment, the pups were sacrificed, and the cerebral cortices were dissected. B: Representative fluorescent images of GFP (green) and cleaved-caspase 3 (red) in the PD7 cerebral cortex. The number of cleaved-caspase 3 positive cells in the cerebral cortex was quantified. The data was expressed as mean ± SEM. n = 5–6 animals per group. One-way ANOVA followed by Tukey post hoc test, F (3, 17) = 45.37, p < 0.0001. ** p < 0.01, *** p < 0.001, **** p < 0.0001. C: Representative immunoblots of MANF and cleaved-caspase 3 in the PD7 cerebral cortex. The data was expressed as mean ± SEM. n = 5–6 animals per group. One-way ANOVA followed by Tukey post hoc test, MANF: F (3, 17) = 40.23, p < 0.0001, cleaved-caspase 3: F (3, 17) = 12.69, p = 0.0001. ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Statistical analysis
Three to five independent cell culture preparations were used for all in vitro experiments and 5–6 animals per group were used for in vivo experiments. Sample size was not determined by statistical method as the number of independent experiments we used was sufficient to detect significant changes as reported previously [36]. No exclusion criteria were pre-determined. Data of all animals in the experiments were included for analysis.
All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad software, La Jolla, CA). Test for outlier were not performed. The data were displayed as mean ± SEM. Data were tested for normal distribution using the Shapiro-Wilk normality test. Two tailed Student’s unpaired t-test was used for comparison between two groups that were normally distributed. One-way analysis of variance (ANOVA) was used to compare more than two groups of data with one variable. Two-way ANOVA was used for comparison of more than two groups with two variables. ANOVA tests were followed by Tukey post hoc test. p values of < 0.05 were considered significant.
Results
Alcohol increases MANF transcription and secretion without altering the stability of MANF mRNA and protein
To examine the effect of alcohol exposure on MANF expression and secretion, N2a and SH-SY5Y cells were treated with 0.4% (w/v) ethanol for various length of time, then total RNA and protein were extracted from cell lysates or conditioned culture media. Quantitative real-time PCR analysis revealed an upregulation of MANF transcripts that peaked at 6 hours after alcohol treatment in both N2a and SH-SY5Y cells (Fig. 1A, 1B). A corresponding increase in MANF protein level was observed after 12 hours of alcohol treatment (Fig. 1C, 1D). Increased MANF immunofluorescent signal was also detected in N2a cells 12 hours following alcohol exposure, which was co-localized with the MANF interacting protein PDIA1 in the ER (Fig. 1E) [53]. Although MANF localized in the ER, its secretion can be induced in pathological conditions [54]. Next, we detected the amount of secreted MANF in the conditioned media and found that it was elevated by alcohol treatment in a time dependent manner for both N2a and SH-SY5Y cells (Fig. 1C, 1D).
Figure 1. Effects of alcohol on MANF expression and secretion in neuronal cells.
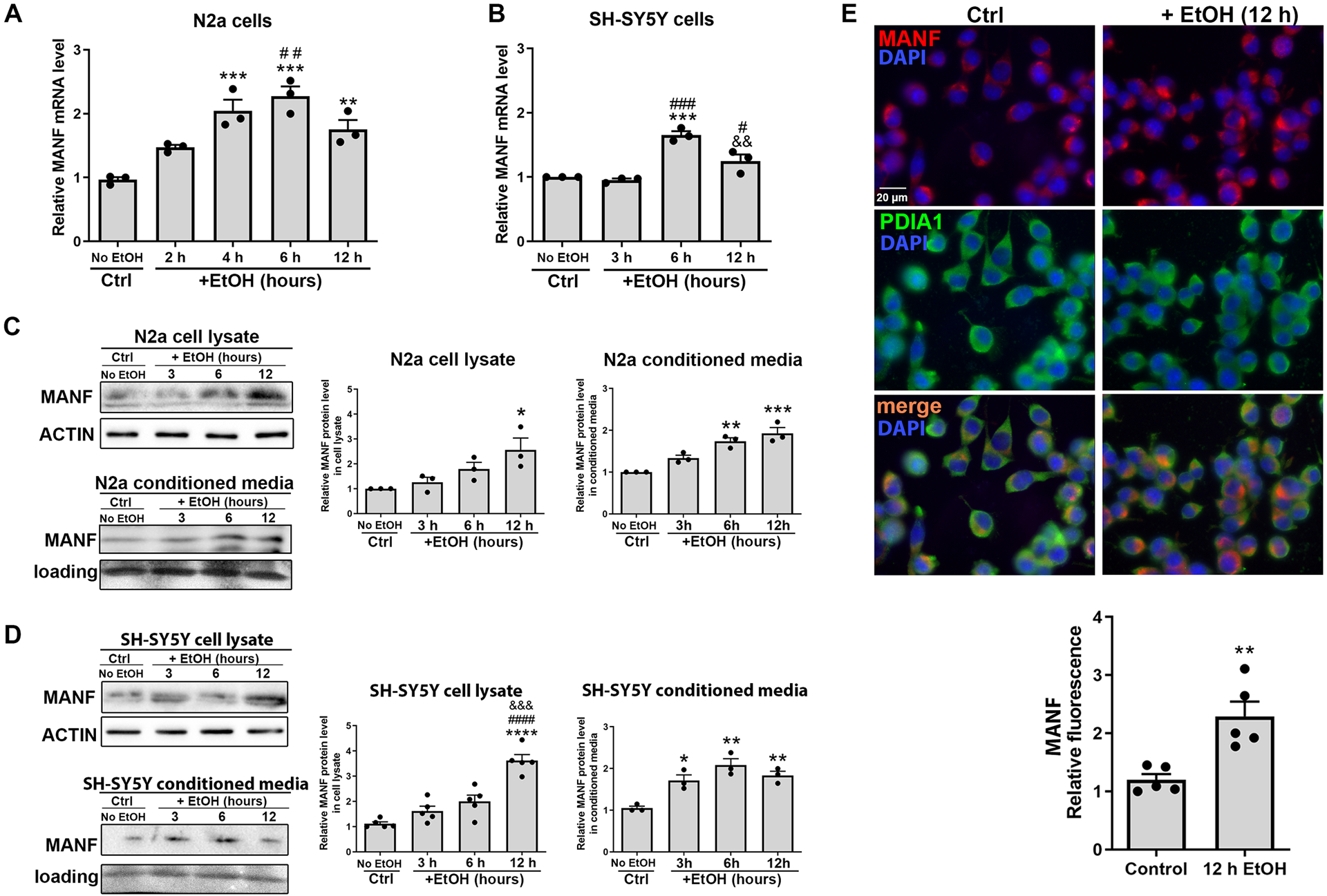
A-B: The levels of MANF mRNA in control and alcohol-treated (0.4% w/v) N2a and SH-SY5Y cells were examined by real-time PCR. The data was expressed as mean ± SEM from 3 independent cell culture preparations. One-way ANOVA followed by Tukey post hoc test, N2a cells: F (4, 10) = 16.97, p = 0.0002, SH-SY5Y cells: F (3, 8) = 29.18, p = 0.0001. ** p < 0.01 or *** p < 0.001 when compared to control; # p < 0.05 or ## p < 0.01 or ### p < 0.001 when compared to 2 h alcohol-treated group (N2a cells) or 3 h alcohol-treated group (SH-SY5Y cells); && p < 0.01 when compared to 6 h alcohol-treated groups. C-D: Representative immunoblots showing MANF protein levels in cell lysates and conditioned media in control and alcohol-treated cells. The data was expressed as mean ± SEM from 3–5 independent cell culture preparations. One-way ANOVA followed by Tukey post hoc test, N2a cell lysate: F (3, 8) = 5.636, p = 0.0226, SH-SY5Y cell lysate: F (3, 16) = 29.69, p < 0.0001, N2a conditioned media: F (3, 8) = 22.37, p = 0.0003, SH-SY5Y conditioned media: F (3, 8) = 14.33, p = 0.0014. * p < 0.05 or ** p <0.01 or *** p <0.001 or **** p < 0.0001 when compared to control; #### p < 0.0001 when compared to 3 h alcohol-treated group; &&& p < 0.001 when compared to 6 h alcohol-treated group. E: Representative fluorescent images of MANF (red) and PDIA1 (green) in N2a cells after 12 hours of alcohol treatment. The data was expressed as mean ± SEM from 5 independent cell culture preparations. t-test, t = 4.017, df = 8, p = 0.0039. ** p < 0.01.
Alcohol-induced upregulation of MANF could be mediated by either increased transcription/translation or enhanced mRNA/protein stability. We then examined the effect of alcohol exposure on MANF mRNA stability. N2a cells were treated with actinomycin D, which inhibits new RNA synthesis so that the degradation of pre-existing MANF mRNA could be examined. The level of MANF mRNA in N2a cells was measured at various time points by qPCR following alcohol exposure. The result showed that the rate of MANF mRNA degradation was not affected by alcohol (Fig. 2A).
Figure 2. Effects of alcohol on the stability of MANF mRNA and protein.
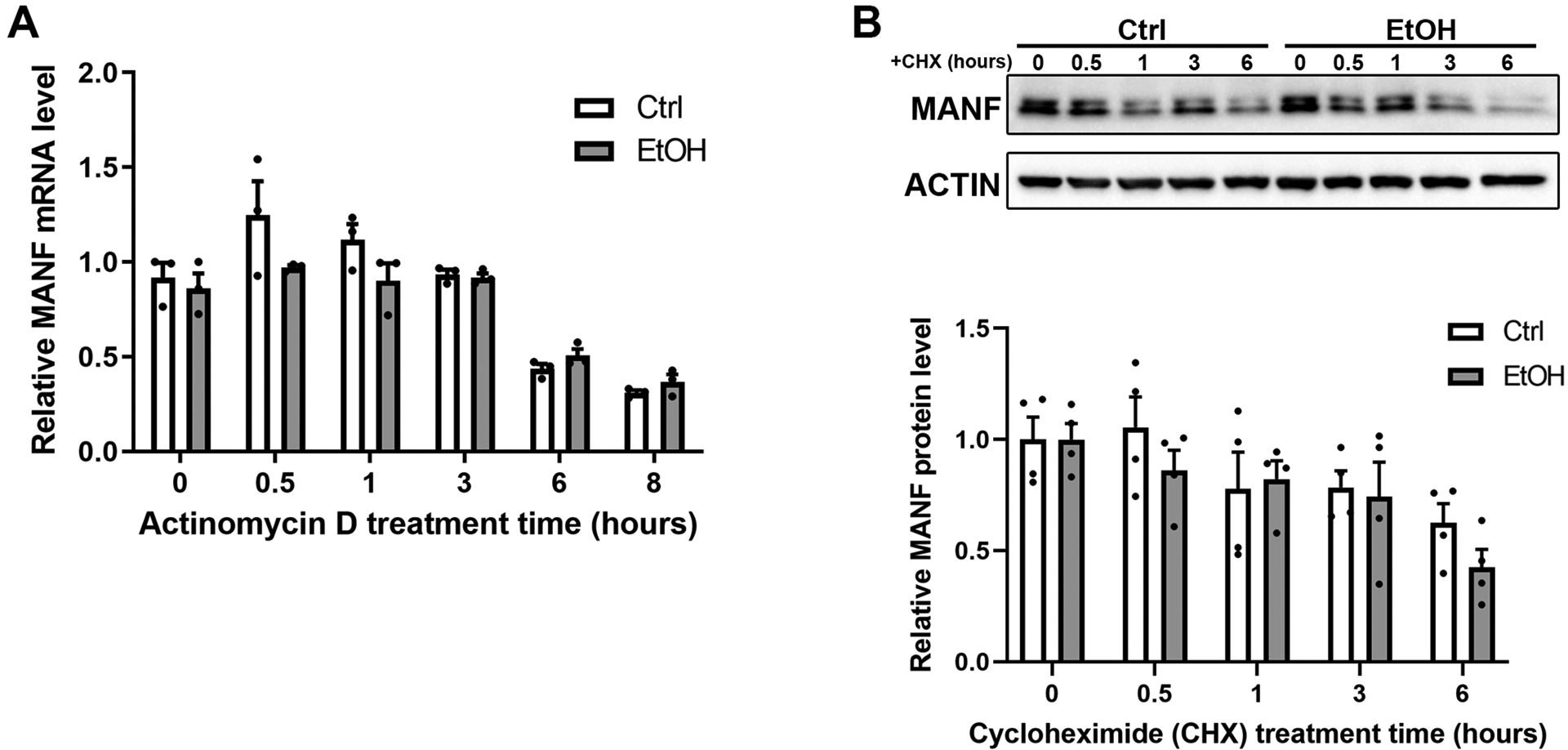
A: The levels of MANF mRNA in control and alcohol-treated (0.4% w/v) N2a cells were examined by real-time PCR after new RNA synthesize was inhibited by actinomycin D (10 μg/ml). The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test, Time: F (1.595, 6.380) = 33.38, p = 0.0005, EtOH: F (1, 4) = 4.997, p = 0.0891, Interaction: F (5, 20) = 1.750, p = 0.1693. No statistical significance was detected between control and EtOH groups. B: Representative immunoblots showing MANF protein levels in control and alcohol-treated (0.4% w/v) N2a cells after new protein synthesis was blocked by CHX (100 μg/ml). The data was expressed as mean ± SEM from 4 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test, Time: F (4, 30) = 5.883, p = 0.0013, EtOH: F (1, 30) = 1.268, p = 0.2691, Interaction: F (4, 30) = 0.5218, p = 0.7204. No statistical significance was detected between control and EtOH groups.
Similarly, we also examined whether alcohol affected the stability of MANF protein. CHX inhibits new protein synthesis by blocking ribosomal protein translation. N2a cells were treated with 100 μg/ml of CHX for various timepoints and then MANF protein half-life was detected by immunoblotting. As shown in Fig. 2B, MANF protein level was degraded to about 75% after 3 hours, and to about 60% after 6 hours. Alcohol did not significantly affect the rate of MANF protein degradation (Fig. 2B).
Alcohol-induced MANF expression is dependent on elevated ER stress
Studies have shown that alcohol can induce ER stress in both the developing and mature mouse brain [25, 49]. We examined the protein levels of unfolded protein response (UPR) genes in alcohol treated N2a cells and confirmed that all UPR genes were upregulated (Fig. 3A and 3B), indicating that alcohol indeed triggered ER stress in N2a cells. Notably, while all the UPR genes were upregulated by 12 hours of alcohol treatment, some UPR genes were elevated as soon as 3 hours post alcohol treatment, such as p-PERK and XBP1s. MANF is known as a protein that responds to ER stress. To determine whether alcohol-induced MANF expression is dependent on ER stress activation, N2a cells were treated with alcohol with or without pre-incubation of an ER stress inhibitor 4-PBA. The level of MANF transcripts were examined after 6 hours of alcohol treatment and MANF protein levels were examined after 12 hours of treatment. While alcohol alone upregulated MANF expression as expected, pre-incubation of 4-PBA significantly diminished alcohol-induced MANF upregulation (Fig. 4A and 4B). MANF transcription is regulated by several transcription factors in the UPR pathways, such as the binding of ATF6 and XBP1s with the MANF promoter region with ER stress response element II (ERSE-II) [50, 55–57]. To further examine if ERSE-II transactivation is required for alcohol-induced MANF transcription, MANF promoter luciferase reporter constructs with wild type or mutant/deleted ERSE-II motifs were transfected into N2a cells. The consensus sequence of the ERSE-II motif and the mutated/deleted sequences were shown in Fig. 4C. Transfected cells were incubated with alcohol for 6 hours. Tunicamycin was used as a positive control as high level of MANF expression was able to be induced by tunicamycin in N2a cells (Fig. S1). Luciferase activity was significantly elevated by both alcohol and tunicamycin treatment in cells expressing wild type MANF promoter (Fig. 4D), and as expected, the luciferase activity was higher in tunicamycin-treated group than in alcohol-treated group. However, mutation or deletion of the ATF6/XBP1s binding site in the ERSE-II region of MANF promoter abolished the luciferase activity in both tunicamycin- and alcohol-treated groups (Fig. 4D). These data indicated that ER stress and ERSE-II were required for alcohol-induced MANF upregulation.
Figure 3. Effects of alcohol on ER stress.
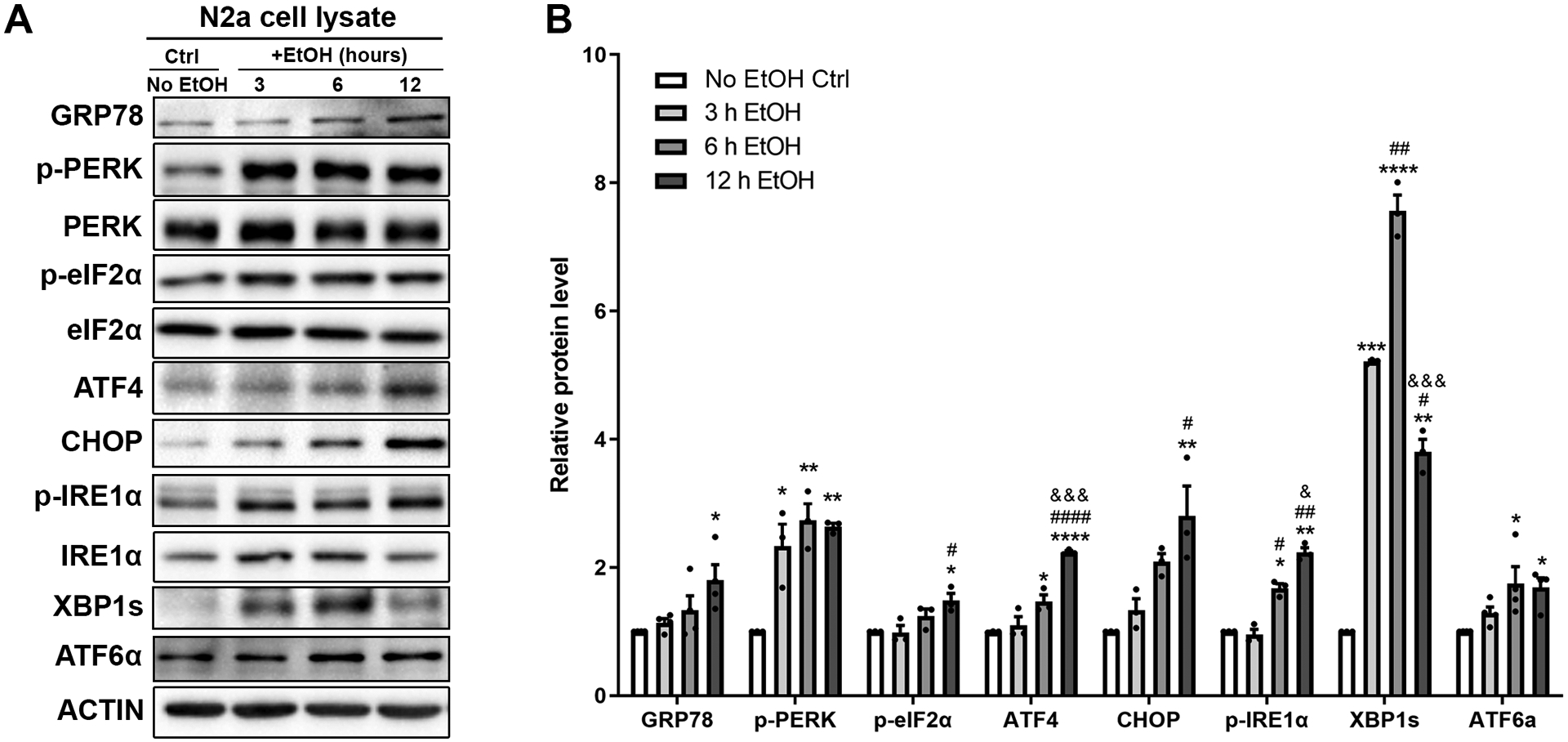
A-B: Representative immunoblots showing the expression of UPR proteins in control and alcohol-treated (0.4% w/v) N2a cell lysates. The data was expressed as mean ± SEM from 3–4 independent cell culture preparations. One-way ANOVA followed by Tukey post hoc test, GRP78: F (3, 12) = 4.254, p = 0.0290, p-PERK: F (3, 8) = 13.75, p = 0.0016, p-eIF2α: F (3, 8) = 11.63, p = 0.0027, ATF4: F (3, 8) = 106.5, p < 0.0001, CHOP: F (3, 8) = 9.668, p = 0.0049, p-IRE1α: F (3, 8) = 84.94, p < 0.0001, XBP1s: F (3, 8) = 312.0, p < 0.0001, ATF6α: F (3, 12) = 7.390, p = 0.0046. * p < 0.05 or ** p < 0.01 or *** p < 0.001 or **** p < 0.0001 when compared to control; # p < 0.05 or ## p < 0.01 or #### p < 0.0001 when compared to 3 h alcohol-treated group; & p < 0.05 or &&& p < 0.001 when compared to 6 h alcohol-treated group.
Figure 4. Effects of alcohol and 4-PBA on MANF expression and transcription activity.
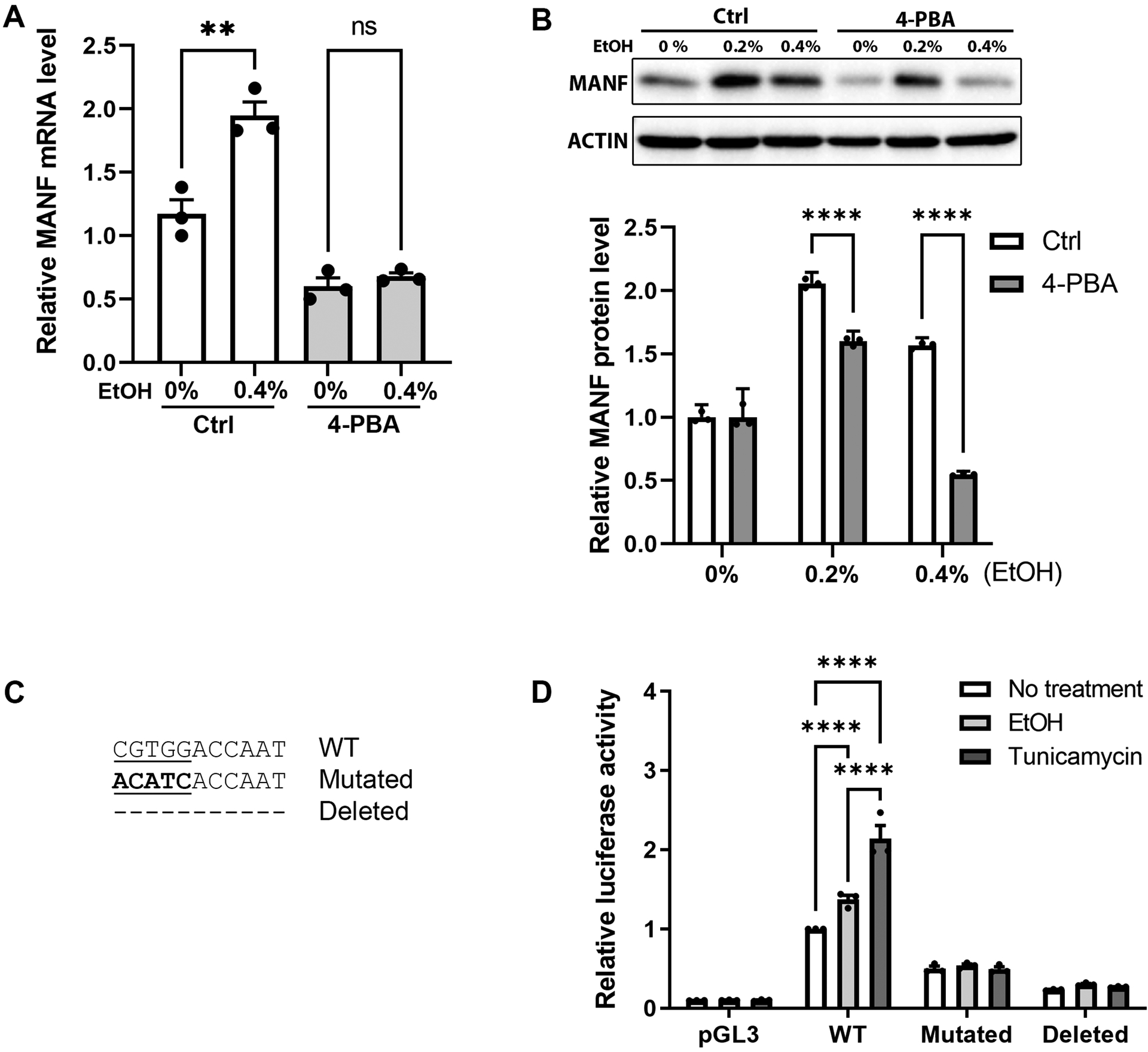
A: Real-time PCR result showing the levels of MANF mRNA in control and alcohol-treated (0.4% w/v) N2a cells with or without the pretreatment of ER stress inhibitor 4-PBA. Cells were incubated with 0.4% (w/v) ethanol for 6 hours with or without 2 hours pretreatment of 10 mM 4-PBA. The data was expressed as mean ± SEM from 3 independent cell culture preparations. One-way ANOVA followed by Tukey post hoc test, F (3, 8) = 52.15, p < 0.0001. ** p < 0.01, ns not statistically significant. B: Representative immunoblots of MANF expression in control and alcohol-treated N2a cells with or without the pretreatment of ER stress inhibitor 4-PBA. Cells were incubated with 0.2% or 0.4% (w/v) ethanol for 12 hours with or without 2 hours pretreatment of 10 mM 4-PBA. The data was expressed as mean ± SEM from 3 independent cell culture preparations. t-test, 0%: t = 4.444e-006, df = 4, p = 0.999997, 0.2%: t = 16.48, df = 4, p = 0.000079, 0.4%: t = 64.48, df = 4, p <0.000001. **** p < 0.0001. C: The consensus sequence of ERSE-II in MANF promoter is shown and ATF6/XBP1s binding site is underlined. Mutated sequence is bolded. Dashed line represents the deletion of the entire ERSE-II sequence. D: N2a cells were transfected with luciferase reporter constructs with MANF promotor regions containing wild type (WT), mutated, or deleted ERSE-II sequence. After the transfection, cells were treated with ethanol (0.4% w/v) or tunicamycin (5 μg/ml) for 6 hours. Luciferase activity was measured and normalized to cells that were transfected with WT construct and with no treatment. The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test, Mutations: F (3, 24) = 445.6, p < 0.0001, Treatment: F (2, 24) = 32.67, p < 0.0001, Interaction: F (6, 24) = 31.84, p < 0.0001. **** p < 0.0001.
MANF-deficient neuronal cells are more susceptible to alcohol-induced cell death
To investigate the effects of MANF deficiency on neuronal cell death induced by alcohol, MANF knockout (KO) N2a cells were generated using CRISPR/Cas9 (Fig. 5A) [37]. The basal expression level of UPR genes GRP78 and XBP1s were increased in MANF KO cells (Fig. 5B), suggesting a predisposition of ER stress in MANF deficient cells. Control and MANF KO cells were exposed to alcohol and the amount of cleaved-caspase 3 was examined at different time points. While in control cells cleaved-caspase 3 was induced by 48 hours of alcohol exposure, it was detected in MANF KO cells as early as 24 hours post alcohol treatment (Fig. 5C) and its expression level was much higher in MANF KO cells than in control cells. To further assess cell viability, MTT assay was perform. As shown in Fig. 5D, alcohol exposure caused more reduction of cell viability over time in MANF KO cells than control cells, indicating MANF-deficient neuronal cells are more susceptible to alcohol-induced cell death (Fig. 5D). To further examine whether the increased susceptibility to alcohol neurotoxicity was specific to MANF knockout, cells were incubated with 20 ng/ml recombinant human MANF (rhMANF) 2 hours prior to and during alcohol treatment. MTT assay revealed that the addition of rhMANF was able to rescue the decrease in cell survival induced by alcohol in MANF knockout (KO) cells (Fig. 5D).
Figure 5. Effects of MANF deficiency on alcohol-induced cell death.
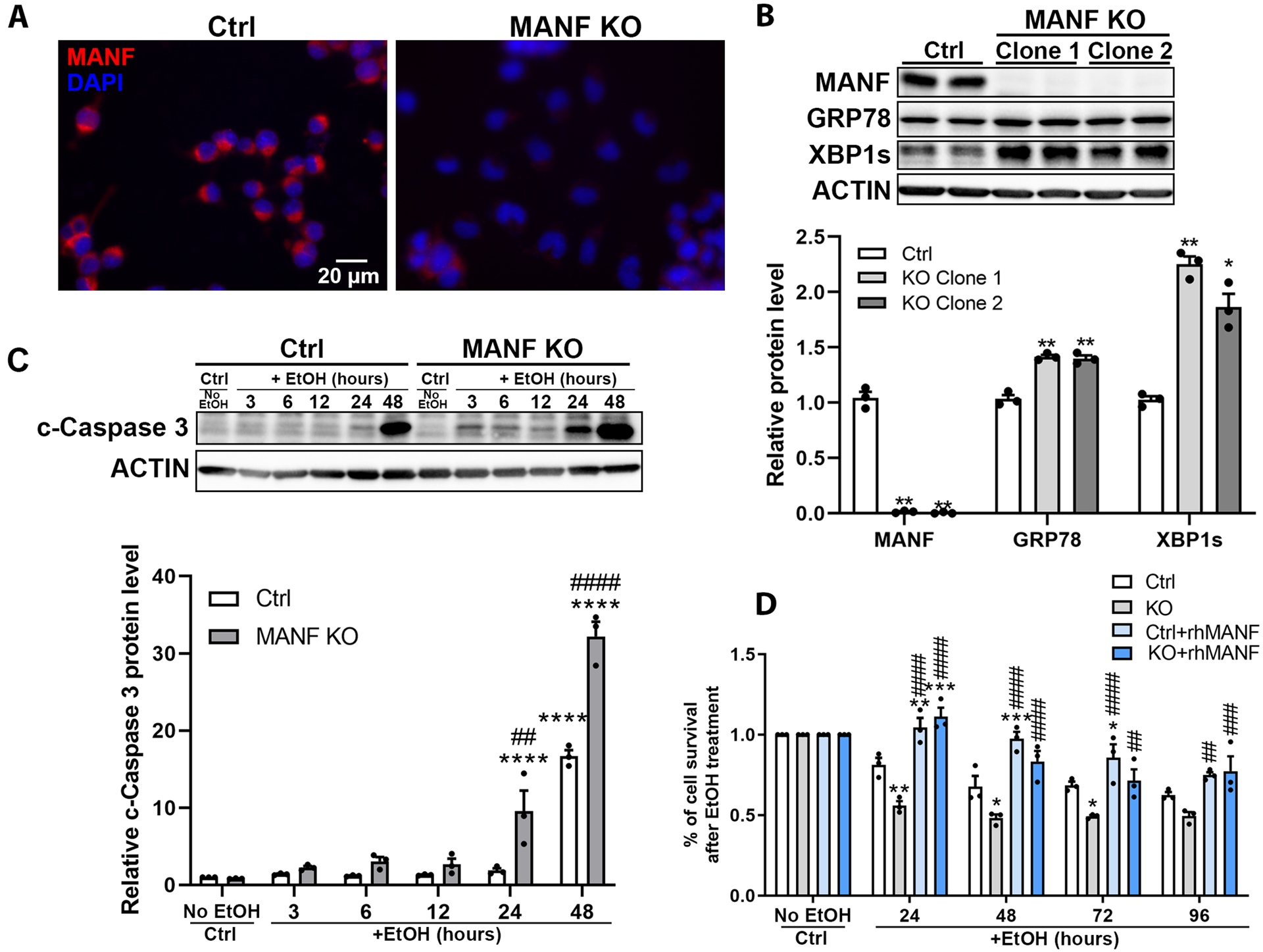
A: Representative fluorescent images confirming MANF knockout (KO) in N2a cells. B: Representative immunoblots of MANF, GRP78, and XBP1s expression in control and MANF KO N2a cells. The data was expressed as mean ± SEM from 3 independent cell culture preparations. One-way ANOVA followed by Tukey post hoc test, MANF: F (2, 6) = 361.0, p < 0.0001, GRP78: F (2, 6) = 60.17, p = 0.0001, XBP1s: F (2, 6) = 58.41, p = 0.0001. * p < 0.05 or ** p < 0.01 when compared to control cells. C: Representative immunoblots of cleaved-caspase 3 expression in control and alcohol-treated (0.4% w/v) control and MANF KO N2a cells. The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test, Time: F (5, 24) = 161.4, p < 0.0001, KO: F (1, 24) = 59.67, p < 0.0001, Interaction: F (1, 24) = 59.67, p < 0.0001. **** p < 0.0001 when compared to all earlier time points. ## p < 0.01 or #### p < 0.0001 when compared between control and MANF KO cells within each time points. D: N2a cells were incubated with or without 20 ng/ml rhMANF. Cell viability was tested by MTT assay at various time points after alcohol treatment (0.4% w/v). Percentage of cell survival was calculated by normalizing the alcohol treated group by no alcohol control group at each time points. The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test, Time: F (4, 40) = 38.64, p < 0.0001, KO: F (3, 40) = 48.86, p < 0.0001, Interaction: F (12, 40) = 4.994, p < 0.0001. * p < 0.05, ** p < 0.01, *** p < 0.001 when compared to control; ## p < 0.01, ### p <0.01, #### p < 0.0001 when compared to KO at each time points.
Exogenous MANF protects neuronal cells against alcohol-induced cell death
Overexpression of MANF can alleviate cellular damages induced by ER stress in multiple disease models [46, 58–61]. However, little is known on whether MANF can mitigate alcohol-induced ER stress and cell death in neuronal cells. To determine whether MANF overexpression is protective on alcohol neurotoxicity, N2a cells were transduced with control- or MANF-adenovirus (MANF-AD). MANF-AD transduction resulted in strong intracellular MANF overexpression, which can be further augmented by ER stress inducers tunicamycin and thapsigargin (Fig. 6A and 6B). MTT assay demonstrated that alleviation of alcohol-induced cell death was observed in MANF-AD transduced N2a cells (Fig. 6E). Notably, MANF-AD transduced exogenous MANF can be secreted into culture media and the secretion was enhanced by thapsigargin treatment, which was consistent with previous reports showing MANF was induced by thapsigargin [62] (Fig. 6C and 6D). As a result, the protective effect of MANF against alcohol may be due to the action of both intracellular and extracellular MANF.
Figure 6. Effects of exogenous MANF on alcohol-induced cell death.
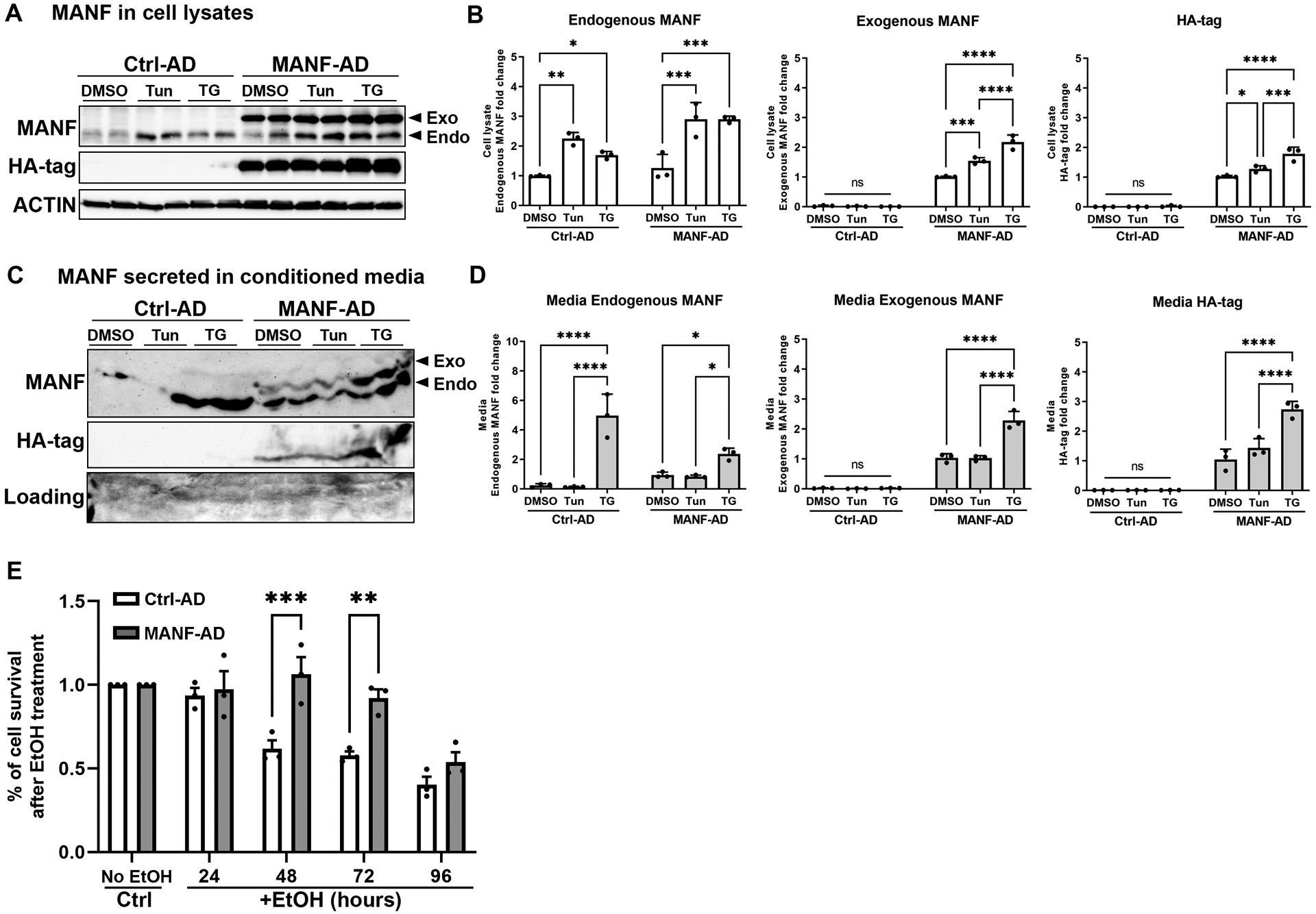
A: N2a Cells were transduced with Ctrl-AD or MANF-AD for 4 hours, then treated with DMSO or tunicamycin (5 μg/ml) or thapsigargin (1.5 μM) for 24 hours. Representative immunoblots of MANF and HA-tag expression in N2a cell lysates. Exogenous (exo) and endogenous (endo) MANF were distinguished by the shift of migration on SDS-PAGE gel electrophoresis. Exogenous MANF migrated slower than endogenous MANF because of the HA tag. Tun: tunicamycin; TG: thapsigargin. B: Quantification of the cell lysates immunoblots. The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test. Endogenous MANF: Treatment F (2, 12) = 35.41, p < 0.0001, Ctrl- vs MANF-AD F (2, 12) = 22.67, p = 0.0005, Interaction F (2, 12) = 3.33, p = 0.0707. Exogenous MANF: Treatment F (2, 12) = 41.68, p < 0.0001, Ctrl- vs MANF-AD F (2, 12) = 933.4, p < 0.0001, Interaction F (2, 12) = 45.01, p < 0.0001. Ha-tag: Treatment F (2, 12) = 22.21, p < 0.0001, Ctrl- vs MANF-AD F (2, 12) = 769.4, p < 0.0001, Interaction F (2, 12) = 20.1, p = 0.0001. * p<0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns not statistically significant. C: Representative immunoblots of MANF and HA-tag expression in conditioned culture media. D: Quantification of the conditioned media immunoblots. The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test. Endogenous MANF: Treatment F (2, 12) = 49.6, p < 0.0001, Ctrl- vs MANF-AD F (2, 12) = 1.788, p = 0.206, Interaction F (2, 12) = 13.82, p = 0.00008. Exogenous MANF: Treatment F (2, 12) = 39.97, p < 0.0001, Ctrl- vs MANF-AD F (2, 12) = 459.5, p < 0.0001, Interaction F (2, 12) = 38.93, p < 0.0001. Ha-tag: Treatment F (2, 12) = 23.82, p < 0.0001, Ctrl- vs MANF-AD F (2, 12) = 272.1, p < 0.0001, Interaction F (2, 12) = 23.66, p = 0.0001. * p<0.05, **** p < 0.0001, ns not statistically significant. E: The viability of cells transduced with Ctrl-AD and MANF-AD was tested by MTT assay at various time points following alcohol exposure. Percentage of cell survival was calculated by normalizing the alcohol treated group by no alcohol control group at each time points. The data was expressed as mean ± SEM from 3 independent cell culture preparations. Two-way ANOVA followed by Tukey post hoc test, Time: F (4, 20) = 24.70, p < 0.0001, MANF: F (1, 20) = 25.93, p < 0.0001, Interaction: F (4, 20) = 5.276, p = 0.0046. ** p < 0.01, *** p < 0.001.
To determine if the protective effect of MANF observed in cell cultures can be replicated in vivo, we enhanced MANF expression in the developing brain through the intracerebroventricular (ICV) injection of MANF-AAV to the mouse brain. Control- or MANF-AAV were administered to neonatal mouse pups on postnatal day 0 (PD0) via bilateral ICV injection (Fig. 7A). Widespread GFP expression was observed in the postnatal brain on PD3, 7, and 14, indicative of successful AAV expression (Fig. S2 and data not shown). By PD7, GFP expression was detected in multiple brain regions, including the olfactory bulb, cerebral cortex, hippocampus, striatum, thalamus, and pontine nuclei (Fig. S2). Immunoblot of cerebral cortex lysates indicated a ~1.5-fold increase of MANF protein expression when compared to its endogenous level (Fig. 7C). Control- or MANF-AAV injected pups were exposed to alcohol on PD7, and then apoptosis was assessed by immunofluorescence labeling and immunoblotting for cleaved-caspase 3. Alcohol exposure led to widespread neuronal apoptosis throughout the developing brain with some areas, such as the cortex, superior and inferior colliculus, and cerebellum showing more susceptibility (data not shown). Alcohol exposure caused increased numbers of cleaved-caspase 3+ cells in control-AAV injected cerebral cortex (Fig. 7B). Consistently, immunoblotting also showed an upregulation of cleaved-caspase 3 expression (Fig. 7C). Applaudably, MANF overexpression significantly alleviated alcohol-induced neuronal apoptosis in the cerebral cortex as indicative by reduced cleaved-caspase 3 expression (Fig. 7B and 7C). Notably, alcohol exposure induced a similar elevation in the number of cleaved-caspase 3+ cells in the cerebellum of both control- and MANF-AAV injected animals, where exogenous MANF/GFP expression was not detected (Fig. S2H and H’, Fig. S3). Taken together, these results suggested that MANF was neuroprotective and exogenous MANF was sufficient to ameliorate alcohol-induced neuronal apoptosis.
Discussion
In this study, we provided evidence that alcohol induces the expression of MANF in neuronal cells. MANF expression was reported to be induced by ER stress inducers in different types of tissues and cells [44, 57, 63]. Animal studies also indicated that MANF expression was induced in pathological conditions where ER stress was activated. For example, MANF expression was induced by ischemia-related ER stress in neurons, glial cells, and cardiac tissues [64–67]. MANF upregulation was implicated to attenuate ER stress-related cellular damages by modulating the stress via unknown mechanisms, probably through facilitating protein folding and regulating immune response [45, 68–70].
Alcohol exposure has been reported to induce MANF expression in multiple tissues including postnatal mouse brain, adult mouse liver and mouse pancreatic acinar cells [26, 32, 49, 71, 72]. However, the molecular mechanism of how alcohol stimulates MANF expression and whether enhanced MANF expression alleviates alcohol neurotoxicity are unclear. Numerous studies have shown that alcohol interferes with protein homeostasis in the ER, causing ER stress and triggering unfolded protein response (UPR) in the pancreas, liver, heart, and brain (reviewed in [73–75]). Our results support that alcohol-induced MANF expression in neuronal cells is mediated by ER stress, because pharmacological inhibition of UPR activation by 4-PBA diminished alcohol-induced MANF expression.
We demonstrated that alcohol increased MANF transcription without affecting MANF mRNA stability and protein degradation. The expression of ER molecular chaperones, such as GRP78 and GRP94, are often regulated by the ERSE located in the promoter region [76]. Under ER stress conditions, ATF6 and/or XBP1s recognize and bind to CCACG and form complex with the general transcription factor NF-Y that binds to the CCAAT region, resulting in the transcriptional upregulation of ER chaperones. ERSE-II with the consensus sequence of ATTGG-N-CCACG is another ERSE-like cis-acting element first identified in the human homocysteine-induced endoplasmic reticulum protein (HERP) gene [55]. It also possesses the ATF6/XBP1s binding sequence of CCACG and the NF-Y binding sequence of CCAAT, although they are located at the opposite strands [56]. ERSE-II is present in both human and mouse MANF promoter region, while an additional canonical ERSE was identified in the human MANF promoter [57, 77]. Mizobuchi et al. demonstrated that MANF expression in mouse tissues and cell cultures were induced by ER stress via ERSE-II [57]. Oh-Hashi et al. further showed that ATF6α, but not ATF6β nor XBP1s, strongly induced MANF promoter activity via ERSE-II in N2a mouse neuronal cells [50]. XBP1s was reported to mainly bind to the canonical ERSE promoter region and promote MANF transcription in human tissues [77]. We showed that the presence of ERSE-II was necessary for alcohol-induced MANF transcription in mouse neuronal cells. ATF6α and XBP1s were induced by alcohol in neuronal cells. Mutations or deletion of the ATF6/XBP1s binding sequence in the ERSE-II abolished alcohol-induced MANF luciferase reporter activity. Taken together, these results suggest alcohol may enhance MANF transcription through promoting ATF6/ XBP1s binding to ERSE-II. This conclusion is supported by the evidence that cells lacking ATF6 or XBP1s exhibited a decrease in MANF expression [78]. In addition, a mouse model of spinocerebellar ataxia demonstrated a decline in MANF expression, which was attributed to reduced association of XBP1s with the MANF promoter [79].
We also detected increased MANF secretion after alcohol exposure. Although MANF is located in the ER, its extracellular secretion has been reported to be protective against many ER stress-associated diseases, including kidney diseases, skeletal diseases, diabetes, cardiac ischemia, stroke, and Parkinson’s disease in rodent models and human patients [42, 54, 64, 67, 80–82]. The mechanism of MANF secretion is not fully understood. Based on the available information, MANF secretion is regulated in conjugation by interaction of MANF with KDEL receptors and calcium-dependent binding of MANF to GRP78 [62, 83, 84]. ER retention is largely relied on Lys-Asp-Glu-Leu (KDEL), a canonical C-terminal ER-retention sequence that are possessed by many soluble ER resident proteins such as GRP78, GRP94, and protein disulfide isomerases (PDIs) [85]. MANF possessed a C-terminal Arg-Thr-Asp-Leu (RTDL) sequence that resembles the function of the canonical KDEL but with a weaker affinity to the KDEL receptor, which renders its secretion under stress conditions [67, 84, 86, 87]. Increased MANF secretion was observed with deletion of the RTDL sequence [83]. In addition, it was reported that the secretion of MANF was partially regulated by the calcium-dependent interaction of MANF with the ER molecular chaperone GRP78 [62]. GRP78 is a major ER chaperone molecule critical for protein folding and the activation of the UPR sensors. In unstressed condition, GRP78 anchors to the luminal domain of the ER stress sensors, thereby preserving their inactive state. Upon ER stress, GRP78 dissociates with the sensors and governs the activation of UPR [19]. Similar to the binding of GRP78 to UPR sensors, direct physical interaction of MANF and GRP78 was detected in a manner that is dependent on calcium levels [62]. Depletion of ER calcium by chemical calcium depletion compounds triggered the dissociation of the MANF-GRP78 complex, facilitating the subsequent secretion of MANF [62]. Alcohol has long been known to disrupt neuron calcium homeostasis and calcium responses by increasing cytoplasmic free calcium level, altering voltage- and ligand-gated calcium channels, and depleting intracellular calcium pool in the ER and mitochondrial [88–90]. Whether the increased MANF secretion in response to alcohol is due to the calcium-dependent MANF-GRP78 interaction requires further investigations.
The most devastating outcome of alcohol exposure is neurodegeneration observed in individuals with excessive alcohol consumption or prenatal alcohol exposure [6, 9]. It is usually associated with long-lasting cognitive and behavioral abnormalities throughout an individual’s life in alcoholics and children with FASD. Alcohol triggered widespread apoptotic neuronal death in a third trimester equivalent alcohol exposure mouse model [7]. Evidence have suggested that ER stress is one of the contributing factors for alcohol-induced neurodegeneration. A study using cultured SH-SY5Y cells and primary cerebellar granule neurons showed that alcohol significantly potentiated tunicamycin- and thapsigargin-induced expression of UPR genes, indicating that alcohol was synergistic with chemical ER inducers in enhancing ER stress [91]. Activation of the UPR was observed in the developing mouse brain using a third-trimester equivalent alcohol exposure model [26]. When alcohol induced UPR activation was blocked pharmacologically, neuronal apoptosis was significantly reduced [32]. MANF has been implicated to attenuate ER stress-related cellular damages in multiple diseases. We have previously shown that MANF deficiency rendered neurons more susceptible to alcohol induced ER stress and neuronal apoptosis in the postnatal mouse brain in a neuron specific MANF knockout model [49].
We showed that exogenous MANF was able to protect neuronal cells against alcohol neurotoxicity, which was indicated by in vitro studies showing that the treatment of rhMANF or MANF overexpression by adenovirus offered protection to alcohol-induced reduction of viability in N2a cells. Similar protection was observed in vivo studies showing that enhanced MANF by ICV-injection of MANF-AAV significantly decreased the number of alcohol-induced apoptotic neurons in the developing cortex. These observations were in line with previous studies that MANF overexpression protect neurons from ER stress-induced cell death in various disease models. For example, MANF overexpression reduced cerebral ischemia-induced neurodegeneration and promoted functional recovery after stroke [64, 92]. In Parkinson’s disease models, intrastriatal injection of MANF protected and reinstated the functionality of dopaminergic neurons [42, 93]. Addition of MANF also facilitated retinal neurons recovery in various eye injury models [45, 46, 48, 94]. The function of MANF in ER stress modulation is still unclear and it is unknown whether intracellular and extracellular MANF acts via similar or different molecular mechanisms. Intracellular overexpression of MANF reduced UPR gene p-eIF2α, ATF4, CHOP, XBP1s, GRP78 and ATF6α in a 6-hydroxydopamine (6-OHDA)-induced PD model in SH-SY5Y cells [95]. However, the same study showed that extracellular addition of MANF failed to alter any of the UPR genes, but instead it may protect cells against 6-OHDA-induced toxicity via the PI3K/AKT/mTOR pathway [95]. Furthermore, results were also controversial for the effect of MANF on UPR genes expression. For example, extracellular addition of MANF was reported to attenuated cerebral ischemia-induced ER stress by inhibiting the expression of GRP78, p-IRE1, and XBP1s [96]. However, in an earlier study, extracellular MANF was shown to elevate GRP78 expression in 6-OHDA-treated SH-SY5Y cells [97]. As a result, further investigations are needed to understand the mechanisms by which MANF modulates ER stress and UPR.
In summary, we demonstrated that alcohol increased MANF transcription and secretion in neuronal cells. The elevation of UPR and the presence of ERSE-II were necessary for alcohol-induced MANF expression. MANF deficient neuronal cells exhibited increased GRP78 and XBP1s and were more vulnerable to alcohol-induced cell death. Enhanced MANF expression alleviated alcohol-induced cell death in cultured neuronal cells and in the postnatal mouse brain. Taken together, MANF may be beneficial for the therapy/treatment of alcohol neurotoxicity.
Supplementary Material
Acknowledgements:
This work was supported by the National Institutes of Health (NIH) grants AA017226 and AA015407. It was also supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development [Biomedical Laboratory Research and Development: Merit Review (BX001721)]. We thank Dr. Oh-Hashi from Gifu University for generously providing us the luciferase reporter constructs. The graphical abstract figure was made using the free online tool Figdraw developed by the Home for Researchers.
Abbreviations
- AAV
adeno-associated virus
- AKT
protein kinase B
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- CHX
cycloheximide
- CHOP
CCAAT-enhancer-binding proteins homologous protein
- CNS
central nervous system
- CRISPR
clustered regularly interspaced short palindromic repeats
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- ERAD
ER-associated protein degradation
- ERSE-II
ER stress response element II
- FASD
fetal alcohol spectrum disorder
- GRP78
glucose-regulating protein 78
- GRP94
glucose-regulated protein 94
- HERP
homocysteine-induced endoplasmic reticulum protein
- HRP
horseradish peroxidase
- ICV
intracerebroventricular
- IRE1α
inositol-requiring enzyme 1α
- KO
knockout
- KDEL
lysine-aspartic acid-glutamic acid-leucine
- mTOR
mammalian target of rapamycin
- MANF
mesencephalic astrocyte-derived neurotrophic factor
- rhMANF
recombinant human mesencephalic astrocyte-derived neurotrophic factor
- PD
postnatal day
- PDIs
protein disulfide isomerases
- PERK
protein kinase R-like ER kinase
- PI3K
phosphoinositide 3-kinases
- RRID
Research Resource Identifier
- RTDL
arginine-threonine-aspartic acid-leucine
- TG
thapsigargin
- Tun
tunicamycin
- UPR
unfolded protein response
- vp
viral particles
- WT
wild type
- XBP1s
spliced form of X-box binding protein 1
- 4-PBA
sodium phenylbutyrate
- 6-OHDA
6-hydroxydopamine
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Patel F and Mandal P. Effect of Alcohol on Brain Development. 2018.
- 2.Squeglia LM, et al. , Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol Clin Exp Res, 2014. 38(10): p. 2509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson SN, Bernes GA, and Doyle LR, Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated With Prenatal Alcohol Exposure. Alcohol Clin Exp Res, 2019. 43(6): p. 1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gursky ZH, Spillman EC, and Klintsova AY, Single-day Postnatal Alcohol Exposure Induces Apoptotic Cell Death and Causes long-term Neuron Loss in Rodent Thalamic Nucleus Reuniens. Neuroscience, 2020. 435: p. 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikonomidou C, et al. , Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science, 2000. 287(5455): p. 1056–60. [DOI] [PubMed] [Google Scholar]
- 6.Jarmasz JS, et al. , Human Brain Abnormalities Associated With Prenatal Alcohol Exposure and Fetal Alcohol Spectrum Disorder. J Neuropathol Exp Neurol, 2017. 76(9): p. 813–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olney JW, et al. , Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res, 2002. 133(2): p. 115–26. [DOI] [PubMed] [Google Scholar]
- 8.Nunez CC, Roussotte F, and Sowell ER, Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Res Health, 2011. 34(1): p. 121–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Crews FT and Nixon K, Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol, 2009. 44(2): p. 115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfonso-Loeches S and Guerri C, Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci, 2011. 48(1): p. 19–47. [DOI] [PubMed] [Google Scholar]
- 11.Harper C and Matsumoto I, Ethanol and brain damage. Curr Opin Pharmacol, 2005. 5(1): p. 73–8. [DOI] [PubMed] [Google Scholar]
- 12.Crews FT, et al. , The role of neuroimmune signaling in alcoholism. Neuropharmacology, 2017. 122: p. 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández JA, López-Sánchez RC, and Rendón-Ramírez A, Lipids and Oxidative Stress Associated with Ethanol-Induced Neurological Damage. Oxid Med Cell Longev, 2016. 2016: p. 1543809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J, Autophagy and ethanol neurotoxicity. Autophagy, 2014. 10(12): p. 2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boschen KE and Klintsova AY, Neurotrophins in the Brain: Interaction With Alcohol Exposure During Development. Vitam Horm, 2017. 104: p. 197–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sushma, et al. , Alcohol induced impairment/abnormalities in brain: Role of MicroRNAs. Neurotoxicology, 2021. 87: p. 11–23. [DOI] [PubMed] [Google Scholar]
- 17.Ji C, Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int, 2012. 2012: p. 216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang F and Luo J, Endoplasmic Reticulum Stress and Ethanol Neurotoxicity. Biomolecules, 2015. 5(4): p. 2538–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter P and Ron D, The unfolded protein response: from stress pathway to homeostatic regulation. Science, 2011. 334(6059): p. 1081–6. [DOI] [PubMed] [Google Scholar]
- 20.Rasheva VI and Domingos PM, Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis, 2009. 14(8): p. 996–1007. [DOI] [PubMed] [Google Scholar]
- 21.Hetz C and Saxena S, ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol, 2017. 13(8): p. 477–491. [DOI] [PubMed] [Google Scholar]
- 22.Costa CAD, et al. , The Endoplasmic Reticulum Stress/Unfolded Protein Response and Their Contributions to Parkinson’s Disease Physiopathology. Cells, 2020. 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerakis Y and Hetz C, Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. Febs j, 2018. 285(6): p. 995–1011. [DOI] [PubMed] [Google Scholar]
- 24.Xin Q, et al. , Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int, 2014. 68: p. 18–27. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, et al. , Binge ethanol exposure induces endoplasmic reticulum stress in the brain of adult mice. Toxicol Appl Pharmacol, 2018. 356: p. 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke Z, et al. , Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin Exp Res, 2011. 35(9): p. 1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, et al. , Chronic Voluntary Alcohol Drinking Causes Anxiety-like Behavior, Thiamine Deficiency, and Brain Damage of Female Crossed High Alcohol Preferring Mice. Frontiers in Pharmacology, 2021. 12(258). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, et al. , Effects of Chronic Voluntary Alcohol Drinking on Thiamine Concentrations, Endoplasmic Reticulum Stress, and Oxidative Stress in the Brain of Crossed High Alcohol Preferring Mice. Neurotox Res, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George AK, et al. , Exercise Mitigates Alcohol Induced Endoplasmic Reticulum Stress Mediated Cognitive Impairment through ATF6-Herp Signaling. Sci Rep, 2018. 8(1): p. 5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dlugos CA, ATF6 and caspase 12 expression in Purkinje neurons in acute slices from adult, ethanol-fed rats. Brain Res, 2014. 1577: p. 11–20. [DOI] [PubMed] [Google Scholar]
- 31.Dlugos CA, Smooth endoplasmic reticulum dilation and degeneration in Purkinje neuron dendrites of aging ethanol-fed female rats. Cerebellum, 2006. 5(2): p. 155–62. [DOI] [PubMed] [Google Scholar]
- 32.Li H, et al. , 4-Phenylbutyric Acid Protects Against Ethanol-Induced Damage in the Developing Mouse Brain. Alcohol Clin Exp Res, 2019. 43(1): p. 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrova P, et al. , MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci, 2003. 20(2): p. 173–88. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, et al. , Spatiotemporal expression of MANF in the developing rat brain. PLoS One, 2014. 9(2): p. e90433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindholm P, et al. , MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci, 2008. 39(3): p. 356–71. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, et al. , Deficiency of Mesencephalic Astrocyte-derived Neurotrophic Factor Affects Neurogenesis in Mouse Brain. Brain Res Bull, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen W, et al. , Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Regulates Neurite Outgrowth Through the Activation of Akt/mTOR and Erk/mTOR Signaling Pathways. Frontiers in Molecular Neuroscience, 2020. 13(188). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng K-Y, et al. , MANF Is Essential for Neurite Extension and Neuronal Migration in the Developing Cortex. eneuro, 2017. 4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng KY, et al. , MANF Promotes Differentiation and Migration of Neural Progenitor Cells with Potential Neural Regenerative Effects in Stroke. Mol Ther, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palgi M, et al. , Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc Natl Acad Sci U S A, 2009. 106(7): p. 2429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YC, et al. , MANF regulates dopaminergic neuron development in larval zebrafish. Dev Biol, 2012. 370(2): p. 237–49. [DOI] [PubMed] [Google Scholar]
- 42.Voutilainen MH, et al. , Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci, 2009. 29(30): p. 9651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Airavaara M, et al. , Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol, 2010. 225(1): p. 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apostolou A, et al. , Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res, 2008. 314(13): p. 2454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neves J, et al. , Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science, 2016. 353(6294): p. aaf3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao FJ, et al. , Identification of Mesencephalic Astrocyte-Derived Neurotrophic Factor as a Novel Neuroprotective Factor for Retinal Ganglion Cells. Front Mol Neurosci, 2017. 10: p. 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko JA, et al. , Functional analysis of mesencephalic astrocyte-derived neurotrophic factor in retinal ganglion cells under oxidative stress. Cell Biochem Funct, 2020. [DOI] [PubMed] [Google Scholar]
- 48.Lu J, et al. , Photoreceptor Protection by Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF). eNeuro, 2018. 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, et al. , MANF is neuroprotective against ethanol-induced neurodegeneration through ameliorating ER stress. Neurobiol Dis, 2021. 148: p. 105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh-Hashi K, Hirata Y, and Kiuchi K, Transcriptional regulation of mouse mesencephalic astrocyte-derived neurotrophic factor in Neuro2a cells. Cell Mol Biol Lett, 2013. 18(3): p. 398–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang JX, et al. , Mesencephalic astrocyte-derived neurotrophic factor (MANF) prevents the neuroinflammation induced dopaminergic neurodegeneration. Exp Gerontol, 2023. 171: p. 112037. [DOI] [PubMed] [Google Scholar]
- 52.Au - Kim J-Y, et al. , Intracerebroventricular Viral Injection of the Neonatal Mouse Brain for Persistent and Widespread Neuronal Transduction. JoVE, 2014(91): p. e51863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eesmaa A, et al. , The cytoprotective protein MANF promotes neuronal survival independently from its role as a GRP78 cofactor. J Biol Chem, 2021: p. 100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim Y, et al. , Mesencephalic Astrocyte-Derived Neurotrophic Factor as a Urine Biomarker for Endoplasmic Reticulum Stress-Related Kidney Diseases. J Am Soc Nephrol, 2016. 27(10): p. 2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokame K, Kato H, and Miyata T, Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem, 2001. 276(12): p. 9199–205. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto K, et al. , Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem, 2004. 136(3): p. 343–50. [DOI] [PubMed] [Google Scholar]
- 57.Mizobuchi N, et al. , ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct, 2007. 32(1): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 58.Bell PA, et al. , Mesencephalic astrocyte-derived neurotropic factor is an important factor in chondrocyte ER homeostasis. Cell Stress Chaperones, 2019. 24(1): p. 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hakonen E, et al. , MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hellman M, et al. , Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem, 2011. 286(4): p. 2675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu S, et al. , Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Abeta toxicity via attenuating Abeta-induced endoplasmic reticulum stress. J Neuroinflammation, 2019. 16(1): p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glembotski CC, et al. , Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem, 2012. 287(31): p. 25893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, et al. , Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol, 2015. 283(3): p. 157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Airavaara M, et al. , Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J Comp Neurol, 2009. 515(1): p. 116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu YQ, et al. , Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J Cereb Blood Flow Metab, 2010. 30(1): p. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen Y, et al. , Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J Neuroinflammation, 2012. 9: p. 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tadimalla A, et al. , Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res, 2008. 103(11): p. 1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arrieta A, et al. , Mesencephalic astrocyte-derived neurotrophic factor is an ER-resident chaperone that protects against reductive stress in the heart. J Biol Chem, 2020. 295(22): p. 7566–7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan Y, et al. , MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat Commun, 2019. 10(1): p. 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sousa-Victor P, et al. , MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab, 2019. 1(2): p. 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu H, et al. , MANF protects pancreatic acinar cells against alcohol-induced endoplasmic reticulum stress and cellular injury. J Hepatobiliary Pancreat Sci, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chhetri G, et al. , Role of Mesencephalic Astrocyte-Derived Neurotrophic Factor in Alcohol-Induced Liver Injury. Oxid Med Cell Longev, 2020. 2020: p. 9034864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji C, Advances and New Concepts in Alcohol-Induced Organelle Stress, Unfolded Protein Responses and Organ Damage. Biomolecules, 2015. 5(2): p. 1099–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandol SJ, et al. , Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis, 2010. 28(6): p. 776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji C, New Insights into the Pathogenesis of Alcohol-Induced ER Stress and Liver Diseases. Int J Hepatol, 2014. 2014: p. 513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida H, et al. , Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem, 1998. 273(50): p. 33741–9. [DOI] [PubMed] [Google Scholar]
- 77.Wang D, et al. , XBP1 activation enhances MANF expression via binding to endoplasmic reticulum stress response elements within MANF promoter region in hepatitis B. Int J Biochem Cell Biol, 2018. 99: p. 140–146. [DOI] [PubMed] [Google Scholar]
- 78.Lee AH, Iwakoshi NN, and Glimcher LH, XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol, 2003. 23(21): p. 7448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang S, et al. , Age-dependent decrease in chaperone activity impairs MANF expression, leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron, 2014. 81(2): p. 349–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galli E, et al. , Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci Rep, 2016. 6: p. 29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galli E, et al. , Increased Serum Levels of Mesencephalic Astrocyte-Derived Neurotrophic Factor in Subjects With Parkinson’s Disease. Front Neurosci, 2019. 13: p. 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartley CL, et al. , Armet/Manf and Creld2 are components of a specialized ER stress response provoked by inappropriate formation of disulphide bonds: implications for genetic skeletal diseases. Hum Mol Genet, 2013. 22(25): p. 5262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henderson MJ, et al. , Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J Biol Chem, 2013. 288(6): p. 4209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glembotski CC, Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol, 2011. 51(4): p. 512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pelham HR, The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci, 1990. 15(12): p. 483–6. [DOI] [PubMed] [Google Scholar]
- 86.Oh-Hashi K, et al. , Intracellular trafficking and secretion of mouse mesencephalic astrocyte-derived neurotrophic factor. Mol Cell Biochem, 2012. 363(1–2): p. 35–41. [DOI] [PubMed] [Google Scholar]
- 87.Raykhel I, et al. , A molecular specificity code for the three mammalian KDEL receptors. J Cell Biol, 2007. 179(6): p. 1193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webb B, et al. , Ethanol and nerve growth factor effects on calcium homeostasis in cultured embryonic rat medial septal neurons before and during depolarization. Brain Res, 1995. 701(1–2): p. 61–74. [DOI] [PubMed] [Google Scholar]
- 89.Kouzoukas DE, et al. , Intracellular calcium plays a critical role in the alcohol-mediated death of cerebellar granule neurons. J Neurochem, 2013. 124(3): p. 323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.N’Gouemo P, Voltage-Sensitive Calcium Channels in the Brain: Relevance to Alcohol Intoxication and Withdrawal. Handb Exp Pharmacol, 2018. 248: p. 263–280. [DOI] [PubMed] [Google Scholar]
- 91.Chen G, et al. , Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res, 2008. 86(4): p. 937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matlik K, et al. , Poststroke delivery of MANF promotes functional recovery in rats. Sci Adv, 2018. 4(5): p. eaap8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y, et al. , MANF improves the MPP(+)/MPTP-induced Parkinson’s disease via improvement of mitochondrial function and inhibition of oxidative stress. Am J Transl Res, 2018. 10(5): p. 1284–1294. [PMC free article] [PubMed] [Google Scholar]
- 94.Neves J, et al. , MANF delivery improves retinal homeostasis and cell replacement therapies in ageing mice. Exp Gerontol, 2020. 134: p. 110893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hao F, et al. , Long-Term Protective Effects of AAV9-Mesencephalic Astrocyte-Derived Neurotrophic Factor Gene Transfer in Parkinsonian Rats. Exp Neurol, 2017. [DOI] [PubMed] [Google Scholar]
- 96.Yang W, et al. , Mesencephalic astrocyte-derived neurotrophic factor prevents neuron loss via inhibiting ischemia-induced apoptosis. J Neurol Sci, 2014. 344(1–2): p. 129–38. [DOI] [PubMed] [Google Scholar]
- 97.Huang J, et al. , Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells. Cell Biol Int, 2016. 40(7): p. 803–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


