Abstract
Context
Patients with acromegaly are characterized by chronic exposure to high growth hormone (GH) and insulin-like growth factor-1 levels, known for their anabolic effect on skeletal muscle. Therefore, an increased skeletal muscle mass could be hypothesized in these individuals. Herein, we have performed a systematic revision of published evidence regarding skeletal muscle mass, quality, and performance in patients with acromegaly.
Evidence Acquisition
A systematic review of the literature in the PubMed database up to September 1, 2023, was conducted with the following query: acromegaly AND (“muscle mass” OR “skeletal muscle”). We excluded studies that did not compare different disease states or used nonradiological methods for the skeletal muscle analyses, except for bioelectrical impedance analysis.
Evidence Synthesis
Fifteen studies met the inclusion criteria. A total of 360 patients were evaluated for skeletal muscle mass, 122 for muscle fatty atrophy, and 192 for muscle performance. No clear evidence of increased skeletal muscle mass in patients with active disease compared to control or healthy individuals emerged. As for skeletal muscle quality, we observed a trend toward higher fatty infiltration among patients with acromegaly compared to healthy participants. Likewise, patients with active disease showed consistently worse physical performance compared to control or healthy individuals.
Conclusion
Skeletal muscle in acromegaly has lower quality and performance compared to that of healthy individuals. The small number of published studies and multiple confounding factors (eg, use of different radiological techniques) contributed to mixed results, especially regarding skeletal muscle mass. Well-designed prospective studies are needed to investigate skeletal muscle mass in patients with acromegaly.
Keywords: acromegaly, skeletal muscle, mass, quality, function, myopathy
Acromegaly is a chronic and systemic disease characterized by elevated levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1), caused in the vast majority of cases (>95%) by a GH-secreting pituitary tumor [1]. Long-term exposure to supraphysiological levels of GH and IGF-1 may lead to several comorbidities, such as cardiovascular, respiratory, osteoarticular, and metabolic diseases, among others [2].
Skeletal muscle is one of the main target tissues both of GH and IGF-1 [2].Under physiological conditions, the activation of the GH/IGF-1 axis inhibits proteolysis and exerts an anabolic action on muscles [2]. In patients with acromegaly, a shift of amino acid metabolism toward protein synthesis and free fatty acids toward lipolysis has been described, likely due to the direct activation of the GH receptor and the impairment of insulin signaling [3]. Therefore, differently from the general population in which insulin resistance is often associated with increased body fat [4], active acromegaly constitutes a unique and paradoxical metabolic scenario in which patients harbor insulin resistance despite a relative decrease in adipose tissue [5]. Overall, the skeletal muscle of active acromegaly patients is characterized by decreased protein breakdown and increased protein synthesis. An increase in skeletal muscle mass, and thus better physical performance, would be expected in these patients. However, some of the most prevalent and debilitating comorbidities of acromegaly include musculoskeletal pain and weakness, as well as osteoarticular diseases [6, 7]. Nevertheless, there is limited evidence regarding the effect of the disease and its biochemical control on skeletal muscle (including tissue mass, quality, and function).
Nowadays, skeletal muscle is considered more than a tissue responsible only for mechanical functions (such as mobility). A number of studies have already demonstrated its role as an endocrine organ exerting a variety of functions, including glucose and lipid metabolism regulation [8-16]. Therefore, it is relevant to investigate and properly describe skeletal muscle status in a complex endocrinological disease like acromegaly, which is tightly linked to metabolic comorbidities.
Patients and Methods
Search Strategy and Selection Criteria
We performed a systematic review of the literature in the PubMed database with the following query: acromegaly AND (“muscle mass” OR “skeletal muscle”) (search up to September 1, 2023). A total of 67 studies were identified. We included only English-written studies. We excluded studies that assessed or estimated entire lean mass without reporting skeletal muscle data. Then, we included only studies comparing different disease statuses (ie, active acromegaly vs controlled acromegaly) or studies that compared a specific disease status vs a control group (ie, active acromegaly vs control group or controlled acromegaly vs control group). Except for bioelectrical impedance analysis (BIA), studies that analyzed or estimated skeletal muscle condition by use of nonradiological methods were excluded. In detail, studies using magnetic resonance imaging (MRI) and ultrasound sonography (US) were included since they provide a direct measure of skeletal muscle mass; likewise, studies employing dual x-ray absorptiometry (DXA) and BIA were been included since they allow a reliable estimation of the same parameter. Regarding skeletal muscle quality, studies using MRI and proton magnetic resonance spectroscopy (HMRS) were included, since they provide a measurement of intermuscular adipose tissue (IMAT) and intramyocellular lipids (IMCLs), respectively. We then considered studies using physical tests such as gait speed, 30-second chair stand, Timed Up and Go, as well as hand grip, thigh flex, and extension to evaluate skeletal muscle performance. A single study using pennation angle (PA) evaluated by US has been included as a reliable estimation of muscle performance.
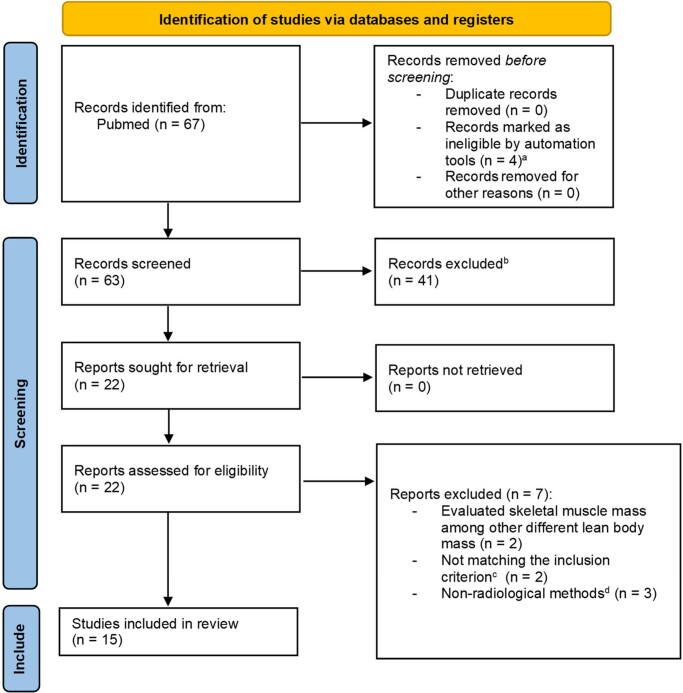
To enhance the quality of the systematic review, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was applied. Fig. 1 shows a flow diagram of the study identification, screening, and inclusion process. After applying the aforementioned criteria, 15 manuscripts were identified and then analyzed.
Figure 1.
PRISMA flowchart. aNot written in English. bExcluded by title. cOnly studies comparing different disease statuses (ie, active acromegaly vs controlled acromegaly) or a specific disease status vs control group (ie, active acromegaly vs control group or controlled acromegaly vs control group) have been included. dBioelectrical impedance analysis has been included.
Patients Stratification and Control Populations
The criteria to define controlled disease (CD) were not consistent among the identified manuscripts. Some authors defined patients as reaching biochemical control by presence of circulating IGF-1 levels below the upper limit of normal (IGF-1 ≤ 1 ×ULN), adjusted for age in most cases [17-19]. However, other authors preferred to consider GH levels—during fasting conditions, after an oral glucose tolerance test, or evaluated as a daily curve—to define the biochemical control of the disease [20-23].
In most studies, the control group (CTR) was selected among “healthy” or “nonacromegalic” individuals matched for several criteria such as age, sex, height, weight, and body mass index. In one manuscript, the control group was composed of participants affected by clinically nonfunctioning pituitary tumor [24].
Results
Skeletal Muscle Mass
Skeletal muscle mass was assessed in 9 studies [17-19, 22-27], most of them with a cross-sectional design only [17, 18, 22, 23, 25], except 4 manuscripts that adopted both a cross-sectional and a prospective design [19, 24, 26, 27] (Table 1). MRI was the most employed method (n = 5/9) [18, 19, 25-27], followed by DXA (n = 4/9) [17, 18, 23, 25], then BIA (n = 2/9) [23, 24], and US, this latter performed only once (n = 1/9) [22]. As mentioned earlier, MRI and US both can provide a direct measurement of skeletal muscle tissue based on the area or the volume of a specific region (or evaluation of the entire body). On the other hand, DXA and BIA can provide an estimate of skeletal muscle mass derived from the measurement of appendicular lean tissue or tissue impedance, respectively (see “Discussion.”).
Table 1.
Skeletal muscle mass evaluation
| Acromegaly patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Design | Technique | Body region | Total | AD | CD | CTR | Outcome |
| Eroğlu et al [18] | Cross-sectional | DXA | Appendicular | 33 | 16 | 17 | 19 | AD = CD |
| AD = CTR | ||||||||
| CD = CTR | ||||||||
| MRI | Abdominal | 33 | 16 | 17 | 19 | AD = CD | ||
| AD = CTR | ||||||||
| CD = CTR | ||||||||
| Lopes et al [23] | Cross-sectional | DXA | Appendicular | 28 | 13 | 15 | — | AD = CD |
| BIA | Total body | 28 | 13 | 15 | — | AD = CD | ||
| Kuker et al [26] | Cross-sectional/Prospectivea | MRI | Total body | 16 | 16 | — | n.a.b | AD < CTR |
| Ozturk Gokce et al [22] | Cross-sectional | US | Thigh and calfc | 39 | 22 | 17 | 39 | AD < CTR |
| AD = CD | ||||||||
| CD = CTR | ||||||||
| Guo et al [24] | Cross-sectional/Prospective | BIA | Total body | 36 | 36 | — | 37d | AD > CTR |
| — | 16e | 16e | — | AD < CD | ||||
| Bredella et al [19] | Cross-sectional/Prospective | MRI | Thigh | 20 | 20e | 16e | 20 | AD > CD |
| AD = CTR | ||||||||
| CD = CTR | ||||||||
| Reyes-Vidal et al [27] | Cross-sectional/Prospectivea | MRI | Total body | 23 | 23 | — | n.a.f | AD = CTR |
| Reid et al [17] | Cross-sectional | DXA | Total body | 138g | 77 | 61 | — | AD > CD |
| Freda et al [25] | Cross-sectional | MRI | Total body | 27 | 27 | — | n.a.h | AD = CTR |
| DXA | Total body | 25 | 25 | — | n.a.h | AD = CTR | ||
| 360 | 250 | 142 | ||||||
The total number of patients included is shown in the bottom row.
Abbreviations: AD, active disease; BIA, bioelectrical impedance analysis; CD, controlled disease; CTR, control group; DXA, dual-energy x-ray absorptiometry; MRI, magnetic resonance imaging; n.a., not available; US, ultrasound.
a Prospective data were not included since AD and CD patients were pooled together.
b A prediction equation was developed for skeletal muscle accounting for sex, age, height, weight, and race using generalized linear models from data obtained from 315 nonacromegalic patients.
c Vastus medial, vastus intermedius, vastus lateralis, and gastrocnemius medial head were evaluated.
d In this study, participants affected by a nonfunctioning pituitary adenoma were considered the control group.
e The AD and CD groups included the same acromegaly patients before and after disease control.
f The observed acromegaly values were compared with predicted values calculated using a previously derived prediction equation described by generalized linear models that account for age, weight, race, height, and sex [25].
g Of the 138 patients included, 22 did not undergo DXA assessment. However, it is not possible to determine how many of these individuals were active and how many were in the control group.
h The control group was selected from a larger group of 315 nonacromegalic patients in a 3-4:1 ratio to acromegalic patients and matched for sex, weight, and age.
Computed tomography (CT) was not employed in any of the studies included in our systematic review.
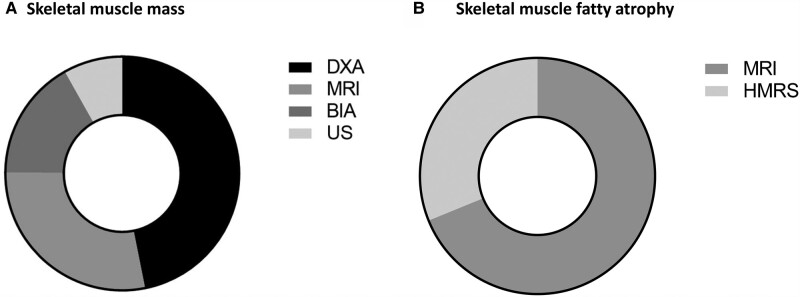
Of note, in 3 studies more than 1 technique was used (namely, 12 skeletal muscle mass analyses were performed in 9 studies) [18, 23, 25]. A total body assessment was performed in all but 4 studies: Eroğlu et al [18] employed DXA to estimate the appendicular skeletal mass and used MRI to evaluate the abdominal muscle area; Lopes et al [23] used DXA to estimate the appendicular skeletal mass; Ozturk Gokce et al [22] performed US on the thigh and calf; Bredella et al [19] employed MRI to evaluate the thigh region. A total of 360 patients with acromegaly underwent 478 skeletal muscle mass analyses: DXA was employed in 224 cases, followed by MRI (135 cases) and BIA (80 cases). Lastly, 39 cases were assessed by US (Fig. 2A).
Figure 2.
Techniques employed to assess A, skeletal muscle mass, and B, fatty atrophy. A technique was counted as one each time it was performed on a patient with acromegaly. If the same technique was used on the same patient with acromegaly at a different time and with a different disease status, it has been counted as two. BIA, bioelectrical impedance analysis; DXA, dual-energy x-ray absorptiometry; HMRS, proton magnetic resonance spectroscopy; MRI, magnetic resonance imaging; US: ultrasound.
The results of skeletal muscle mass analyses in patients with active disease (AD), CD, and CTR groups are shown in Fig. 3A. The AD group was compared to the CD group in 6 studies (including 8 analyses globally). In 2 analyses, the AD group showed higher skeletal muscle mass than the CD group [17, 19], whereas the opposite was reported in 1 analysis [24]. No differences between the 2 groups (AD vs CD) were identified in the remaining 5 evaluations [18, 22, 23].
Figure 3.
Analyses performed on A, skeletal muscle mass; B, fatty atrophy; and C, performance. Each thick line on the y-axis represents 1 analysis defined as a comparison of 2 different groups with a given technique. Some studies performed more than 1 analysis because they compared 2 groups with more than 1 technique or compared more than 2 groups with each other. AD, active disease group; CD, controlled disease group; CTR, control group.
When comparing the AD group with the CTR group, only 1 analysis found a greater muscle mass in AD vs CTR [24], whereas 2 analyses found the opposite (ie, participants in the CTR group had higher muscle mass than in the AD group) [22, 26]. Of note, most analyses (n = 6) did not find any significant difference in skeletal muscle mass between AD and CTR [18, 19, 25, 27].
Finally, the skeletal muscle mass of CD and CTR groups were compared in 4 analyses, showing no significant differences [18, 19, 22].
Skeletal Muscle Quality
To evaluate the quality of skeletal muscle, fatty atrophy was assessed (ie, the higher the fatty atrophy, the lower the quality of the muscle, and vice versa). A cross-sectional design was used in all 6 publications that evaluated skeletal muscle quality [5, 19-21, 26, 27], and 3 of them cited a prospective study design [19, 26, 27] (Table 2). The techniques employed were MRI and HMRS in 4 [5, 21, 26, 27] and 2 studies [19, 20], respectively. A total of 91 analyses involving 91 patients with acromegaly were performed by MRI (Fig. 2B). Likewise, HMRS was used for 43 analyses conducted on 27 patients with acromegaly (see Fig. 2B). Of note, HMRS was performed twice for 16 patients with acromegaly, before and after biochemical control was achieved. In 3 out of 4 studies that used MRI, IMAT was measured across all body regions to assess muscle quality [5, 26, 27], whereas in 1 study the fat fraction of thighs (ie, percentage of fatty infiltration in skeletal muscle) was assessed [21]. In the 2 studies that employed HMRS, IMCL was assessed in the soleus muscle in 1 case [19] and both in the soleus and tibialis anterior muscles in the other one [20].
Table 2.
Fatty atrophy of skeletal muscle
| Acromegaly patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Technique | Body region | Evaluation | Total | AD | CD | CTR | Outcome |
| Kuker et al [26] | Cross-sectional/Prospectivea | MRI | Total body | IMAT | 16 | 16 | — | n.a.b | AD > CTR |
| Martel-Duguech [21] | Cross-sectional | MRI | Thigh | Fat Fraction | 36 | — | 36 | 36 | CD > CTR |
| Bredella et al [19] | Cross-sectional/Prospective | HMRS | Soleus muscle | IMCLs | 20 | 20c | 16c | 20 | AD = CD |
| AD = CTR | |||||||||
| CD = CTR | |||||||||
| Reyes-Vidal et al [27] | Cross-sectional/Prospectivea | MRI | Total body | IMAT | 23 | 23 | — | n.a.d | AD > CTR |
| Szendroedi et al [20] | Cross-sectional | HMRS | Soleus and tibialis anterior muscles | IMCLs | 7 | — | 7 | 7 | CD = CTR |
| Freda et al [5] | Cross-sectional | MRI | Total body | IMAT | 16 | 16 | — | n.a.b | AD > CTR |
| 118 | 75 | 59 | |||||||
The total number of unique patients included is shown in the bottom row.
Abbreviations: AD, active disease; CD, controlled disease; CTR, control group; HMRS, proton magnetic resonance spectroscopy; IMAT, intermuscular adipose tissue; IMCLs, intramyocellular lipids; MRI, magnetic resonance imaging; n.a., not available.
a Prospective data were not included since AD and CD patients were pooled together.
b A prediction equation was developed for IMAT accounting for sex, age, height, weight, and race using generalized linear models from data obtained from 315 nonacromegalic patients.
c The AD and CD groups included the same acromegaly patients before and after disease control.
d The observed acromegaly values were compared with predicted values calculated using a previously derived prediction equation described by generalized linear models that account for age, weight, race, height, and sex [28].
Fig. 3B shows the results of fatty atrophy analyses among AD, CD, and CTR groups. In one analysis, skeletal muscle fatty atrophy in the AD group was compared with the CD group, revealing no significant difference [19].
Among the 4 studies that compared the AD group vs the CTR group, 3 analyses showed a higher degree of skeletal muscle fatty atrophy in AD patients [5, 26, 27], while in 1 analysis no significant difference was found [19].
Furthermore, no difference in skeletal muscle fatty atrophy was demonstrated between CD and CTR groups in 2 analyses [19, 20]. However, one analysis demonstrated more fatty atrophy in the CD group compared to the CTR [21].
Skeletal Muscle Performance
A total of 5 cross-sectional studies used functional exercises [18, 21], dynamometers [6, 18, 21, 29], and US [22] to evaluate or estimate the skeletal muscle performance in patients with acromegaly (Table 3). Among these, one study also used a prospective design [6]. Overall, 477 performance tests were conducted in 209 acromegalic patients; the results are shown in Table 3 and Fig. 3C.
Table 3.
Skeletal muscle performance
| Acromegaly patients | |||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Test | Total | AD | CD | CTR | Outcome |
| Eroğlu et al [18] | Cross-sectional | Hand grip | 33 | 16 | 17 | 19 | AD = CD |
| AD = CTR | |||||||
| CD = CTR | |||||||
| Thigh extension | 33 | 16 | 17 | 19 | AD = CD | ||
| AD = CTR | |||||||
| CD = CTR | |||||||
| Gait speed | 33 | 16 | 17 | 19 | AD = CD | ||
| AD = CTR | |||||||
| CD = CTR | |||||||
| Martel-Duguech et al [21] | Cross-sectional | Gait speed | 36 | 36 | — | 36 | CD < CTR |
| 30-s chair stand | 36 | 36 | — | 36 | CD < CTR | ||
| Timed Up and Go | 36 | 36 | — | 36 | CD < CTR | ||
| Hand grip | 36 | 36 | — | 36 | CD = CTR | ||
| Ozturk Gokce et al [22] | Cross-sectional | Pennation anglea | 39 | 22 | 17 | 39 | AD = CD |
| AD < CTR | |||||||
| CD = CTR | |||||||
| Füchtbauer et al [6] | Cross-sectional/Prospective | Thigh flex and extension | 48 | 48 | — | n.a.b | AD > CTR |
| — | — | 23 | n.a.b | CD = CTR | |||
| Hand grip | 48 | 48 | — | n.a.b | AD < CTR | ||
| — | 23c | 23c | — | AD < CD | |||
| — | — | 23 | n.a.b | CD = CTR | |||
| Lopes et al [29] | Cross-sectional | Thigh extension | 53 | 23 | 30 | — | AD < CD |
| 209 | 145 | 87 | |||||
The total number of unique patients included is shown in the bottom row. A detailed explanation of the different methods used to assess muscle performance is reported in the text of the related reference.
Abbreviations: AD, active disease; CD, controlled disease; CTR, control group; n.a., not available.
a Vastus medial, vastus intermedius, vastus lateralis, and gastrocnemius medial head were evaluated by ultrasonography.
b The control group was selected from a larger group of 144 nonacromegalic patients in a 6-27:1 ratio to acromegalic patients, matched for age and sex.
c The AD and CD groups included the same acromegaly patients before and after disease control.
In 3 analyses in which patients with AD were compared to the CD group, AD patients performed worse than those with CD [6, 29]. On the other hand, 4 performance analyses found no difference between AD and CD groups [18, 22].
When the AD group was compared to CTR, patients with AD performed worse than controls in 2 analyses [6, 22], whereas in 1 evaluation the opposite result was found (AD performed better than CTR) [6]. No difference between AD and CTR groups was reported in the remaining 3 analyses [18].
Finally, 10 analyses compared CD and CTR groups: In 3 evaluations the CD group had worse results [21], while in 7 analyses no difference was found (ie, CD and CTR groups performed similarly) [6, 18, 21, 22].
Discussion
Skeletal muscle is one of the main target tissues of GH and IGF-1 activity, and it is typically affected in acromegaly. Despite the first evaluation of skeletal muscle status in patients with acromegaly dating back to 1965 [30], the number of studies primarily focused on this issue is still relatively low.
Overall, performing a critical analysis of our systematic review on available literature data, the assumption that patients with acromegaly and AD could have more muscle mass than patients with CD (or healthy individuals) does not emerge clearly. Indeed, most analyses performed to compare patients with AD with both CD or healthy individuals did not find a significantly higher skeletal muscle mass in AD patients (14/17 evaluations). In 3 other analyses, the authors reported higher skeletal muscle mass in AD compared to CD or CTR individuals (Fig. 3A). Of note, 1 of these 3 analyses, carried out by Reid and colleagues [17], included the highest number of patients evaluated in a single study (n = 138; see Table 1).
On the other hand, a clear trend toward lower muscle quality in patients with acromegaly is apparent. Indeed, in all reported analyses, patients with acromegaly (either AD or CD) had equal or higher muscle fatty atrophy compared to healthy controls (see Fig. 3B).
A fair degree of consistency was found in the muscle performance assessment, as in all but one analysis the AD group performed worse than both the CD and the CTR groups (see Fig. 3C). In line with this finding, CD patients performed equal to (6 analyses) or worse than (3 analyses) healthy controls.
Therefore, while data on muscle mass are still conflicting, the evaluated studies are consistent in reporting higher fatty atrophy and lower muscle performance in patients with acromegaly compared to controls, with a further detrimental effect of AD. Patients with acromegaly presenting with high fatty atrophy and reduced muscle performance may show impaired mobility, walking ability, and higher fall risk [21]. Together with the well-known bone impairment and skeletal frailty reported in patients with acromegaly [31, 32], these factors may significantly affect patients’ quality of life and contribute to the increased fracture risk observed in this particular population [2]. Overall, musculoskeletal complications represent one of the most debilitating conditions associated with acromegaly [31].
However, it is crucial to examine the potential confounding factors of the included studies to correctly interpret the reported findings. First, the impairment of skeletal muscle function and the osteoarticular pain often observed in patients with acromegaly may contribute to a more sedentary lifestyle compared to age- and sex-matched populations. Nevertheless, physical activity has been included among the matching factors for healthy controls in only one study [21]. Additionally, comorbidities such as diabetes mellitus or impairment of other pituitary axes, such as the gonadal axis or, to a lesser extent, the corticotroph or thyrotroph axis, could affect skeletal muscle metabolism [33-36]. Unfortunately, detailed information on the complete hormonal status of evaluated patients is missing in most of the studies we have analyzed in the present manuscript. The potential effect of distinct imaging modalities and heterogeneous treatments (ie, medical therapy and surgical approach) on the evaluated clinical outcomes is described in the following sections.
Radiological Factors
Recent discoveries on the clinical implications of sarcopenia [8] led several groups to focus on skeletal muscle evaluation. In 2019, the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) indicated MRI and CT as the gold-standard techniques to evaluate skeletal muscle mass and quality due to their precision and reliability. However, the high cost of MRI and the use of ionizing radiation for CT make the use of both techniques not always suitable on a large scale in clinical practice. At the same time, the group defined various pitfalls of DXA (lack of consistency among different DXA instrument brands, the influence of subject hydration status) and BIA (lack of consistency among different age and ethnic groups, the influence of subject hydration status) [37]. Differently from MRI, CT, and US, neither DXA nor BIA can directly assess skeletal muscle mass. DXA can estimate the body lean mass, which includes organs and soft tissue in addition to muscle tissue [25]. Kim and colleagues [38] proposed an equation that estimates skeletal muscle mass in healthy individuals from DXA-estimated appendicular lean tissue, which strongly correlates with MRI-assessed skeletal muscle mass. Because of the well-established fluid-retentive effect of high IGF-1 and GH, patients with acromegaly represent a special population with a condition similar to an overhydration status. Consistent with this consideration, patients with acromegaly show an increase in lean mass [25], which may lead to an overestimation of skeletal muscle mass by DXA. However, the equation proposed by Kim and colleagues [38] was later validated in a group of acromegalic patients with AD [25].
As mentioned earlier, even BIA devices cannot directly measure skeletal muscle mass but rather estimate it through an equation that defines a positive correlation between the measured resistance expressed in ohms and the estimated skeletal muscle mass. While this equation has been shown to strongly correlate with skeletal muscle mass measured by MRI in healthy adults [39], there are currently no data validating its use in patients with acromegaly. Considering the high water conductivity and the fluid retention present in acromegalic patients, and the direct proportionality between BIA-estimated muscle mass and measured resistance, it can be inferred that resistance is undermeasured, leading to an underestimation in skeletal muscle mass in these patients. However, Lopes et al [23] reported a strong correlation in muscle mass estimation among acromegalic patients by comparing DXA with BIA in AD vs CD groups.
As previously mentioned, among the studies we have identified in our search, a total of 4 different techniques (MRI, BIA, DXA, US) have been employed to assess or estimate skeletal muscle mass in patients with acromegaly; while MRI and HMRS have been used for the evaluation of skeletal muscle quality. This heterogeneity in the applied techniques reflects the lack of a standardized method proposed to study muscle mass in individuals with acromegaly in a clinical setting.
Finally, there is also no agreement on how to evaluate muscular performance among patients with acromegaly. Indeed, 5 different physical tests and 1 sonographic technique have been performed in the studies we have reported. Again, there is a lack of consensus on how to estimate muscle performance in different populations with various health statuses.
Therapeutical Approaches
Nowadays, several medical therapies for acromegaly are available in clinical practice. First-generation somatostatin receptor ligands (fg-SRLs, octreotide and lanreotide) and the second-generation SRL, pasireotide, mainly act at the pituitary level reducing GH secretion and result in the lowering of circulating IGF-1 level [1]. On the other hand, pegvisomant is a recombinant GH analogue that acts on blocking GH signaling in the periphery—mainly at the liver—resulting in reduced IGF-1 production [1].
As concerns fg-SRLs, an early study showed that subcutaneous octreotide treatment leads to a reduction in lean body bass estimated by DXA; however, this finding has been attributed to the reduction in soft tissue fluid, more than a direct effect on skeletal muscle mass [40].
To our knowledge, there are no available clinical data on the effects of pasireotide on skeletal muscle; however, one in vitro study hypothesized a direct stimulation of this compound on protein synthesis in rat myoblast cells, although these data have not so far been confirmed by other studies [41].
As concerns pegvisomant, Kuker and colleagues [26] evaluated the effect of this drug on body composition in 21 patients with acromegaly, reporting no significant changes in skeletal muscle mass and quality after long-term treatment.
Furthermore, 2 studies investigated the effect of the surgical approach on skeletal muscle in patients with acromegaly. Guo and colleagues [24] reported a decrease in skeletal muscle mass in acromegaly patients 1 year after surgery compared to the presurgical evaluation. Similarly, Reyes-Vidal and colleagues [27] found a decrease in skeletal muscle mass 1 year after surgery only in male patients and a decrease in fatty atrophy only in female patients at the same time point.
To our knowledge, currently there are no studies directly comparing the various medical therapies with each other or vs the surgical approach. Therefore, further research is needed to investigate whether different therapeutic approaches may affect skeletal muscle characteristics in a different way.
Conclusions
To the best of our knowledge, this is the first systematic review focusing on skeletal muscle evaluation in patients with acromegaly. We identified a few studies, mostly with a cross-sectional design, that investigated this topic. The rarity of acromegaly, the high prevalence of confounding factors, and the heterogeneity of the methods used to assess skeletal muscle characteristics led to mixed results.
However, performing an analysis of the evaluated studies, we found a fair degree of consistency in reporting higher fatty atrophy and lower muscle performance in patients with acromegaly compared to healthy controls, with an additional detrimental effect observed in patients with AD. On the other hand, data on skeletal muscle mass are still conflicting, particularly as concerns the effect of disease activity.
It is therefore necessary to identify which method could be the most reliable, reproducible, and available on a large scale to properly investigate skeletal muscle mass, quality, and performance in a clinical setting. Then, larger studies with a longitudinal design should be conducted to better define the effect that GH, IGF-1, and the aforementioned confounding factors may have on skeletal muscle in this special population.
Abbreviations
- AD
active disease
- BIA
bioelectrical impedance analysis
- CD
controlled disease
- CT
computed tomography
- CTR
control group
- DXA
dual-energy x-ray absorptiometry
- fg-SRL
first-generation somatostatin receptor ligand
- GH
growth hormone
- HMRS
proton magnetic resonance spectroscopy
- IGF-1
insulin-like growth factor-1
- IMAT
intermuscular adipose tissue
- IMCLs
intramyocellular lipids
- MRI
magnetic resonance imaging
- PA
pennation angle
- US
ultrasound
Contributor Information
Angelo Milioto, Endocrinology Unit, Department of Internal Medicine and Medical Specialties (DIMI), University of Genoa, Genoa 16138, Italy.
Giuliana Corica, Endocrinology Unit, Department of Internal Medicine and Medical Specialties (DIMI), University of Genoa, Genoa 16138, Italy.
Federica Nista, Endocrinology Unit, Department of Internal Medicine and Medical Specialties (DIMI), University of Genoa, Genoa 16138, Italy.
Luiz Eduardo Armondi Wildemberg, Neuroendocrinology Research Center/Endocrinology Division, Medical School and Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro 21941-913, Brazil.
Federica Rossi, Department of Radiology, Ospedale Santa Corona, Pietra Ligure 17027, Italy.
Bianca Bignotti, Department of Radiology, IRCCS Ospedale Policlinico San Martino, Genoa 16139, Italy.
Mônica R Gadelha, Neuroendocrinology Research Center/Endocrinology Division, Medical School and Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro 21941-913, Brazil.
Diego Ferone, Endocrinology Unit, Department of Internal Medicine and Medical Specialties (DIMI), University of Genoa, Genoa 16138, Italy; Endocrinology Unit, IRCCS Ospedale Policlinico San Martino, Genoa 16139, Italy.
Alberto Stefano Tagliafico, Email: alberto.tagliafico@unige.it, Department of Health Sciences (DISSAL), University of Genoa, Genoa 16138, Italy; Department of Radiology, IRCCS Ospedale Policlinico San Martino, Genoa 16139, Italy.
Federico Gatto, Endocrinology Unit, IRCCS Ospedale Policlinico San Martino, Genoa 16139, Italy.
Funding
This work was partially supported by a research grant from the Italian Ministry of Health (Ricerca Corrente, project No. R743A to F.G.).
Disclosures
L.E.W. has received personal honoraria for lectures and advisory board from Ipsen and Crinetics. M.R.G. has received funding as principal investigator from Crinetics and Recordati in the last 3 years; she has received personal honoraria for lectures, consulting, and advisory boards from Crinetics and Recordati; and is associate editor of the Journal of Clinical Endocrinology & Metabolism. D.F. has received honoraria for lectures or advisory boards from Recordati, Ipsen, and Novartis-AAA, as well as research grants from Camurus and Pfizer; and he serves on the executive committee of the Italian Endocrine Society (SIE). A.S.T. discloses patents, royalties, or other intellectual property from Springer and Elsevier. F.G. has received personal honoraria for lectures, manuscript writing, educational events, and consultancy from Ipsen, Novartis, Pfizer, and Recordati; and he serves on the executive committee of the European Neuroendocrine Association (ENEA). The other authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Colao A, Grasso LFS, Giustina A, et al. Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. [DOI] [PubMed] [Google Scholar]
- 2. Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. 2019;40(1):268‐332. [DOI] [PubMed] [Google Scholar]
- 3. Arlien-Søborg MC, Dal J, Madsen MA, et al. Reversible insulin resistance in muscle and fat unrelated to the metabolic syndrome in patients with acromegaly. EBioMedicine. 2022;75:103763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5(6):2019‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freda PU, Shen W, Heymsfield SB, et al. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab. 2008;93(6):2334‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Füchtbauer L, Olsson DS, Bengtsson BA, Norrman LL, Sunnerhagen KS, Johannsson G. Muscle strength in patients with acromegaly at diagnosis and during long-term follow-up. Eur J Endocrinol. 2017;177(2):217‐226. [DOI] [PubMed] [Google Scholar]
- 7. Miller A, Doll H, David J, Wass J. Impact of musculoskeletal disease on quality of life in long-standing acromegaly. Eur J Endocrinol. 2008;158(5):587‐593. [DOI] [PubMed] [Google Scholar]
- 8. Tagliafico AS, Bignotti B, Torri L, Rossi F. Sarcopenia: how to measure, when and why. Radiol Med. 2022;127(3):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee SW, Youm Y, Lee WJ, et al. Appendicular skeletal muscle mass and insulin resistance in an elderly korean population: the korean social life, health and aging project-health examination cohort. Diabetes Metab J. 2015;39(1):37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shou J, Chen PJ, Xiao WH. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr. 2020;12(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang J. Enhanced skeletal muscle for effective glucose homeostasis. Prog Mol Biol Transl Sci. 2014;121:133‐163. [DOI] [PubMed] [Google Scholar]
- 12. Merz KE, Thurmond DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. 2020;10(3):785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perakakis N, Triantafyllou GA, Fernández-Real JM, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13(6):324‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baskin KK, Winders BR, Olson EN. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015;21(2):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012 8:8. 2012;8(8):457‐465. [DOI] [PubMed] [Google Scholar]
- 16. Vechetti IJ Jr, Valentino T, Mobley CB, McCarthy JJ. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J Physiol. 2021;599(3):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reid TJ, Jin Z, Shen W, et al. IGF-1 levels across the spectrum of normal to elevated in acromegaly: relationship to insulin sensitivity, markers of cardiovascular risk and body composition. Pituitary. 2015;18(6):808‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eroğlu I, İremli BG, Erkoç A, et al. Osteosarcopenia in acromegaly: reduced muscle quality and increased vertebral fat deposition. J Endocrinol Invest. 2023;46(12):2573‐2582. [DOI] [PubMed] [Google Scholar]
- 19. Bredella MA, Schorr M, Dichtel LE, et al. Body composition and ectopic lipid changes with biochemical control of acromegaly. J Clin Endocrinol Metab. 2017;102(11):4218‐4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szendroedi J, Zwettler E, Schmid AI, et al. Reduced basal ATP synthetic flux of skeletal muscle in patients with previous acromegaly. PLoS One. 2008;3(12):e3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martel-Duguech L, Alonso-Pérez J, Bascuñana H, et al. Intramuscular fatty infiltration and physical function in controlled acromegaly. Eur J Endocrinol. 2021;185(1):167‐177. [DOI] [PubMed] [Google Scholar]
- 22. Ozturk Gokce B, Gogus F, Bolayir B, et al. The evaluation of the tendon and muscle changes of lower extremity in patients with acromegaly. Pituitary. 2020;23(4):338‐346. [DOI] [PubMed] [Google Scholar]
- 23. Lopes AA, Albuquerque L, Fontes M, Rego D, Bandeira F. Body composition in acromegaly according to disease activity—performance of dual x-ray absorptiometry and multifrequency bioelectrical impedance analysis. Front Endocrinol (Lausanne). 2022;13:866099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo X, Gao L, Shi X, et al. Pre- and postoperative body composition and metabolic characteristics in patients with acromegaly: a prospective study. Int J Endocrinol. 2018;2018:4125013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freda PU, Shen W, Reyes-Vidal CM, et al. Skeletal muscle mass in acromegaly assessed by magnetic resonance imaging and dual-photon X-ray absorptiometry. J Clin Endocrinol Metab. 2009;94(8):2880‐2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuker AP, Shen W, Jin Z, et al. Body composition changes with long-term pegvisomant therapy of acromegaly. J Endocr Soc. 2021;5(3):bvab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reyes-Vidal CM, Mojahed H, Shen W, et al. Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J Clin Endocrinol Metab. 2015;100(8):2946‐2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopes AJ, Ferreira AS, Walchan EM, Soares MS, Bunn PS, Guimarães FS. Explanatory models of muscle performance in acromegaly patients evaluated by knee isokinetic dynamometry: implications for rehabilitation. Hum Mov Sci. 2016;49:160‐169. [DOI] [PubMed] [Google Scholar]
- 30. Palmieri G, Ikkos D. Water and electrolyte content of skeletal muscle in acromegaly. Acta Endocrinol (Copenh). 1965;48:469‐472. [DOI] [PubMed] [Google Scholar]
- 31. Fleseriu M, Langlois F, Lim DST, Varlamov EV, Melmed S. Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2022;10(11):804‐826. [DOI] [PubMed] [Google Scholar]
- 32. Nazzari E, Casabella A, Paolino S, et al. Trabecular bone score as a reliable measure of lumbar spine bone microarchitecture in acromegalic patients. J Clin Med. 2022;11(21):6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isidori AM, Balercia G, Calogero AE, et al. Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Invest. 2015;38(1):103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salvio G, Martino M, Balercia G, Arnaldi G. Acromegaly and male sexual health. Rev Endocr Metab Disord. 2022;23(3):671‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferraù F, Albani A, Ciresi A, Giordano C, Cannavò S. Diabetes secondary to acromegaly: physiopathology, clinical features and effects of treatment. Front Endocrinol (Lausanne). 2018;9:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93 Suppl 1:S52‐S59. [DOI] [PubMed] [Google Scholar]
- 37. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim J, Heshka S, Gallagher D, et al. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol. 2004;97(2):655‐660. [DOI] [PubMed] [Google Scholar]
- 39. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985). 2000;89(2):465‐471. [DOI] [PubMed] [Google Scholar]
- 40. Hansen TB, Gram J, Bjerre P, Hagen C, Bollerslev J. Body composition in active acromegaly during treatment with octreotide: a double-blind, placebo-controlled cross-over study. Clin Endocrinol (Oxf). 1994;41(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 41. Gatto F, Florio T. Does pasireotide directly modulate skeletal muscle metabolism? Endocrine. 2017; 57(1):6‐8. doi: 10.1007/s12020-017-1291-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.