Abstract
Background
Diabetes is associated with left ventricular remodeling. Myocardial wall stress is a measurable factor connected to the ventricular breadth and force and is related to myocardial thickness; it can be measured by echocardiography. The present study aimed to assess the link between heart failure (HF) and echocardiography-derived myocardial wall stress in diabetic patients with ST elevation myocardial infarction (STEMI) who were managed with revascularization.
Methods
This study was a comparative prospective study that took place between February 2022 and February 2023. It included 100 diabetic patients presented with STEMI and managed by percutaneous coronary intervention (PCI). Patients were selected from the cardiology departments at Al-Azhar University Hospital, Damietta, Egypt. During the hospital stay, patients were checked for HF symptoms and signs. They were also observed for 3 months after discharge for detection of HF. Those who did not develop HF were assigned to group I, and those with HF were assigned to group II.
Results
The mean value of end-systolic wall stress (ESWS) was 77.09 ± 12.22 and 97 ± 13.44, and the mean value of end-diastolic wall stress (EDWS) was 12.61 ± 2.76 and 15.87 ± 2.86 in groups I and II respectively, with significant differences between the 2 groups. The cutoff point to detect HF was 88 KPa for ESWS and 13.5 KPa for EDWS, with a sensitivity of 70% and 79% and a specificity of 80% and 61% for ESWS and EDWS, respectively.
Conclusion
Elevated left ventricle (LV) myocardial stress is related to increased HF in diabetic patients whose HF was managed by PCI after STEMI. LV wall stress is a potentially helpful risk stratification tool using routine echocardiography to determine the treatment plane according to the risk status.
Keywords: Heart Failure, Myocardial Wall Stress, Acute Myocardial Infarction, Revascularization
↑What is “already known” in this topic:
Cardiac remodeling with diabetes is a significant risk for heart failure. Methods to measure this remodeling directly or indirectly are limited. Furthermore, the process of risk classification following an ST-elevation myocardial infarction (STEMI) is critical to the planning and monitoring of medical interventions.
→What this article adds:
The significance of measuring the left ventricle myocardial stress (systolic and diastolic) using echocardiography as a marker of cardiac remodeling was highlighted by this study. It is a non-invasive technique with a respectable level of sensitivity and specificity. The findings need to be handled carefully and submitted for additional validation in subsequent research.
Introduction
Diabetes is considered an essential cause of left ventricular remodeling (1, 2); in another words, dia betes was defined in the section on cardiomyopathy related to diabetes, as no other cause can lead to left ventricular remodeling aside from diabetes (3). Myocardial remodeling caused by acute myocardial infarction (MI) has been suggested in about two-thirds of the 5 million yearly patients of heart failure (HF) (4).
Left ventricular adverse remodeling is a process of maladjustment caused by cardiac lesions distinguished by morphological changes in left ventricular construction and appearance, with the following impairment of the cardiac function (4).
Myocardial wall stress is a measurable factor connected to the ventricular breadth and force and is contrary to myocardial thickness. The current echo machine has no direct method to assess myocardial wall stress, nonetheless, it can be measured by Laplace's low using a related formula (5).
Risk stratification is an essential element of protecting cases with acute MI. Prognostic data are important for the ideal method and material assignment to provide MI cases with the perfect intensity and location of care (6).
Major adverse cardiac events are expected after ST elevation myocardial infarction (STEMI); thus, identifying patients who are at risk for future cardiac events is important for optimization of treatment and improving prognosis(7). Nowadays, the imaging data that can be used for risk stratification after ST-elevation myocardial for the clinical outcome are limited (8).
Hence, there is a critical requirement for development in echo machines to discover dispassionately variables that can be measured by echocardiography for risk stratification (9).
The present study aimed to assess the link between HF and echocardiography derived myocardial wall stress in diabetic cases with ST-elevation MI managed with revascularization.
Methods
The present study was a comparative prospective study that took place between February 2022 and February 2023 and included 100 diabetic patients presented with ST elevation anterior MI that was managed with primary revascularization. Patients were admitted to cardiology departments in Al-Azhar University Hospital, Damietta, Egypt. Patients were monitored for signs and symptoms of HF while they were in the hospital. In order to diagnose HF, patients were also monitored for 3 months following their discharge.
Inclusion Criteria
Adult diabetic patients with first ST elevation anterior MI that was managed with percutaneous revascularization in the first 12 hours after MI met the inclusion criteria.
Exclusion Criteria
History of previous ischemic heart disease (IHD), history of MI or cardiac surgery, nondiabetic patients, aortic stenosis patients, and poor echo window were the exclusion criteria.
Patients were divided into 2 divisions according to their 3-month follow-up data following primary percutaneous coronary intervention (PCI): group I: nonheart failure group, and group II: HF group.
Applied Methods
Patients underwent assessment using the following schemes:
• Full history taking, including personal history (eg, age, sex, and social status); medical history (eg, patient complaint, history of diabetes mellitus [DM], hypertension [HTN], and smoking); and history of coronary artery disease and arrhythmia. In addition, general and local cardiac examinations were fulfilled.
• Standard 12 leads electrocardiogram (ECG), besides laboratory investigations (eg, complete blood count, liver and renal function tests, and lipid profile.
• Cardiac imaging by echocardiography was performed within 72 hours after PCI and before discharge. Left ventricular studies by M-Mode and Doppler were carried out in the standard echo views. It was performed in the left lateral position. Philips iE 33 Matrix machine was used (Philips IE 33 Ultrasound) and a 4S-RS (3.5-Mhz) probe. Two cardiologists performed the investigations. They were experts in the field and were blinded to categorization of patients. Images were recorded from the parasternal long axis, apical 4 chambers, apical 5 chambers, and apical 2-chamber view. All examinations were done in agreement with the American Society of Echocardiography guidelines.
LV Myocardial Stress
The left ventricular wall stress in systole and diastole was computed using the Laplace law and depended on the equation adopted by Mirskey (10–12).
Left Ventricular Wall Stress(kpa)
Where LV = left ventricular, P = Diastolic or systolic pressure, V lum = Diastolic or systolic volume in the left ventricle, V myo = Myocardial volume.
End Systolic Pressure and End Diastolic Pressure Measurement
The value of systolic wall stress equals the value of systolic blood pressure that measured during echocardiogram; pulmonary capillary wedge pressure measurement was used instead of end diastolic pressure for assessment of end diastolic wall stress using the Nagueh's formula (13).
PCWP=1.24*( )+1.9
Where, E = Early mitral inflow velocity; E’ = tissue Doppler of the mitral annulus.
Measurement of Left Ventricular Volume
The Simpson's biplane method, which was recommended by the American Society of echocardiography was used to measure the left ventricular luminal volume (14), and myocardial volume was measured by the left ventricular mass and specific gravity of the myocardial wall, which was previously reported to be 1.05g/cc.
Yocardial Volume=
Measurement of LV Mass
LV mass was measured using the following equation as recommended by the American Society of echocardiography (15):
Left ventricular Mass (g) =0.8{1.04(((LVEDD +IVSd +PWd) 3−LVEDD3))} +0.6
Where LVEDD = left ventricular end-diastolic diameter in cm; IVSd = interventricular septal thickness at the end of diastole; PWd = Posterior wall diameter at the end of diastole.
Patients of the study were followed up during their hospital stay and three months after PCI for the detection of HF.
Ethical Considerations
The ethical and research committee of Damietta Faculty of Medicine, Al-Azhar University approved the thesis protocol. Before the study started and after its objectives and techniques were explained to each participant, they all signed an informed consent form. The rights of every patient were respected. The Helsinki Declaration's ethical code for conducting research was followed in the completion of the study (2013).
Statistical Analysis
SPSS software Version 16 was used after the computerization of patient data to run different statistical analyses. The results were presented in other formats—eg, text, tables, and figures of graphic representation. For qualitative data, the relative frequency and percentages are calculated and used as the primary representation of this type of variable. The χ2 test and its suitable substitutes or equivalents were used to estimate the association between groups. However, mean, standard deviation, minimal, and maximal values represent quantitative data. The independent-sample Student t test was calculated as a comparison test between the 2 groups. The receiver operating characteristic (ROC) curve was used to assess some values for HF prediction using the cutoff point of that value. The normality of data was tested by the Kolmogorov–Smirnov test, and P < 0.05 was considered statistically significant.
Results
Clinical and Demographic Characteristics
We included 100 patients in the present study. There were 76 in group I (nonheart failure) and 24 in group II (HF). Out of them, 50% were men and 50% were female, without significant differences. The mean age was 57.56 ± 11.67 years and 68.58 ± 7.73 years in groups I and II, respectively, with significant differences between groups. However, hypertension was significantly higher in group II than in group I (75% vs 36.8%). The remaining patients did not significantly differ across groups; 50 had obesity, 45 smoked, 62 had dyslipidemia, and 7 had a positive family history of IHD. History of COVID-19 was reported among 8 patients, with a significant increase in group II than group I (25% vs 2.6%, respectively) (Table 1).
Table 1. Clinical and Demographic Characteristics of all Participants, Including Nonheart Failure Group Versus Heart Failure Group.
| Variable | All Patients(n=100) | Non-HF Group I (n=76) | HF Group II (n =24) | P Value |
|---|---|---|---|---|
| Age | 60.21±11.80 | 57.56±11.67 | 68.58±7.73 | <0.001* |
| Male sex | 50 (50%) | 39 (51.4%) | 11 (45.8%) | 0.815 |
| Female sex | 50 (50%) | 37 (48.6%) | 13 (54.1%) | 0.830 |
| HTN | 46 (46%) | 28 (36.8%) | 18 (75%) | 0.002* |
| Obese | 50 (50%) | 37 (48.6%) | 13 (54.1%) | 0.815 |
| Smoking | 45 (45%) | 31 (40.7%) | 14 (58.3%) | 0.161 |
| Dyslipidemia | 62 (62%) | 51 (67.1%) | 11 (45.8%) | 0.090 |
| FH ofIHD | 26 (26%) | 3 (3.9%) | 4 (16.6%) | 0.055 |
| COVID | 8 (8%) | 2 (2.6%) | 6 (25%) | 0.002* |
HTN: hypertension, FH: family history; IHD: ischemic heart disease; COVID: coronavirus disease, * Indicate statistical significance.
The echocardiographic data revealed that the left ventricle (LV) ejection fraction was significantly lower in group II than in group I (42.83 ± 5.76 vs 52.56 ± 4.63, respectively). The end-systolic wall stress (ESWS) was considerably higher in group II than in group I (97 ± 13.44 vs 77.09 ± 12.22, respectively). Similarly, the end-diastolic wall stress (EDWS) was significantly higher in group II than group I (Table 2).
Table 2. Echocardiographic Characteristics of all Participants, Including Heart Failure and Heart Failure Groups.
| Variable | All Patients (n = 100) | Non-HF Group I (n=76) | HF Group II (n =24) | P Value |
|---|---|---|---|---|
| LVEF | 50.33±6.47 | 52.69±4.63 | 42.83±5.76 | <0.001* |
| ESWS | 81.87±15.10 | 77.09±12.22 | 97.00±13.44 | <0.001* |
| EDWS | 13.40±3.10 | 12.61±2.76 | 15.87±2.86 | <0.001* |
The percutaneous coronary angiography results revealed a significant increase in LAD disease among group II when compared with group I (87.5% vs 48.6%). Similarly, LCX and RCA diseases were significantly higher in group II than in group I (66.6%, 83.3% vs 35.5%, 26.3% successively). The single coronary vessel disease was reported among 68 patients (64 in group I and 4 in group II). However, the 2-coronary vessel disease was reported among 21 patients (8 in groups I and 13 in group II), with significant differences. Furthermore, there was a significant increase in multivessel coronary disease in group II than in group I (29.1% vs 5.2%, respectively) (Table 3).
Table 3. Coronary Angiography Characteristics of all Participants, Including Nonheart failure and Heart Failure Groups.
| Variable | All Patients (n=100) | Non-HF Group I (n=76) | HF Group II (n = 24) | P Value |
|---|---|---|---|---|
| LAD | 58(58%) | 37(48.6%) | 21(87.5%) | 0.001* |
| LCX | 43(43%) | 27(35.5%) | 16(66.6%) | 0.009* |
| RCA | 40(40%) | 20(26.3%) | 20(83.3%) | <0.001* |
| One vessel | 68(68%) | 64(84.2%) | 4(16.6%) | <0.001* |
| Two vessels | 21(21%) | 8(10.5%) | 13(54.1%) | <0.001* |
| Three vessels | 11(11%) | 4(5.2%) | 7(29.1%) | <0.001* |
LAD: Left anterior descending; LCX: Left circumflex; RCA: right coronary artery, * Indicates statistical significance.
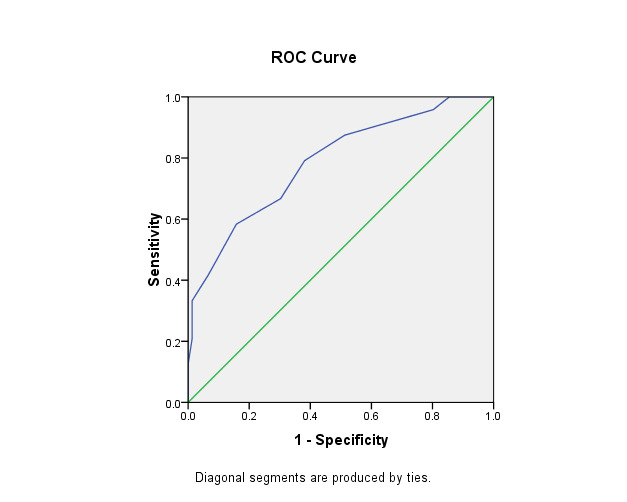
The ROC curve was built for ESWS and EDWS to test the potentiality of both measurements to predict HF. For ESWS, the finding raveled an area under the curve (AUC) of 0.849, with a cutoff point of 88 Kpa, with a sensitivity of 70% and a specificity of 80%. EDWS showed an AUC of the curve of 0.787, with a cutoff point of 13.5, sensitivity of 79%, and specificity of 61% (Table 4, Figure 1 and Figure 2).
Table 4. Optimal cut-off Point of End Systolic and Diastolic Wall Stress to Predict Heart Failure.
| Variable | AUC | Std. Error | P | Asymptotic 95% CI | Cut off Point | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| LB | UB | |||||||
| ESWS | 0.849 | 0.09 | <0.001 | 0.754 | 0.944 | 88 KPa | 70% | 80 % |
| EDWS | 0.787 | 0.005 | <0.001 | 0.680 | 0.895 | 13.5 KPa | 79% | 61% |
ESWS: End systolic wall stress; EDWS: End diastolic wall stress; Kpa: Kilopascal; CI: Confidence interval; LB: Lower Bound, UB: Upper Bound.
Figure 1.
ROC curve of the ESWS that differentiate patients with and without heart failure.
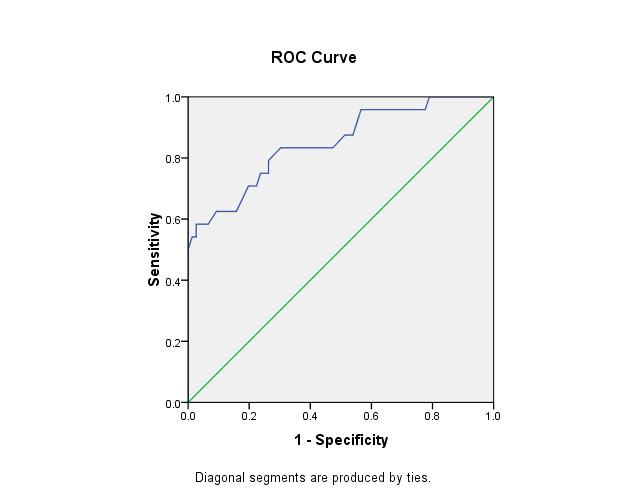
Figure 2.
ROC curve of the EDWS that differentiate patients with and without heart failure.
Correlation Between Ejection Fraction and Wall Stress in Systole and Diastole
In the present study, there was an inverse, statistically nonsignificant correlation between EF and ESWS. Similarly, there was a negative, statistically nonsignificant correlation between EF and EWDS (Table 5).
Table 5. Correlation between Ejection Fraction, End-Systolic Wall Stress, and End-Diastolic Wall Stress.
| Variable | Ejection Fraction | |
|---|---|---|
| Spearman's correlation coefficient | P Value | |
| ESWS | -0.201 | 0.345 |
| EDWS | -0.038 | 0.860 |
Discussion
One of the most important prognostic factors for acute or subacute ST-elevation MI is HF (16). After the development of a new modality in the treatment of acute MI, including primary intervention, the prognosis of HF after STEMI is improved, but despite all of the major advanced cardiac events after STEMI, it is still high (17,18). Hemodynamic changes of the LV (immediate or chronic) can occur after ST-elevation MI (19).
Eccentric LV dilatation can occur as an adaptive mechanism after a chronic increase of the left ventricular load. Myocardial geometry change and impairment of cardiac function are considered a confirmed result after elevated myocardial wall stress (20). The risk of HF, ischemic events, and death is substantially increased by diabetes, and the risk of cardiovascular death is elevated if the patient is diseased with both diabetes and HF (21).
The aim of the present study was to assess the link between HF and echocardiography myocardia stress in diabetic patients with ST elevation myocardial infarction managed by revascularization. The results of the present study showed the development of HF in older patients. This agrees with the study done by Liang et al (22), who showed advanced age was of statistical significance in HF patients after STEMI. These results also agree with the study done by Vicent et al (23).
Another important finding in the present study is the significant increase of hypertension and history of COVID-19 in patients who developed HF. These results align with the study done by Wellings et al (15). They found that many risk factors, such as hypertension, diabetes, and organic diseases (eg, kidney and lung disease) were linked to increasing risk of HF morbidity. Our results are comparable to those reported by Dogan et al (24), who studied the incidence of major adverse cardiovascular events (MACE) during COVID-19 infection in acute MI, and they reported higher MACE incidence with COVID-19 infection.
Our study showed significant differences between the 2 groups regarding the LV systolic function, which agrees with Shuichi et al (25).They reported that lower ejection fraction was linked to HF in a patients with ST-elevation myocardial infarction. In addition, our results revealed significant differences between the 2 groups regarding IV systolic myocardial stress and left ventricular end-diastolic wall stress. These results are in line with previous studies. For example, Kattel et al (8) reported that increased systolic myocardial stress was related to increased mortality in patients with acute MI managed by revascularization. Another study by Clerfond et al (26) calculated the systolic wall stress in STEMI and reported a significant increase in patients complicated by HF. Our results were agreeable with those of Mosleh et al (19), and they concluded that end-diastolic myocardial stress can be considered a prognostic factor for risk stratification of patients with acute MI. It is a predictive of MACE independent of left ventricular ejection fraction (LVEF).
Regarding the predictive power, our results agreed with Kattel et al (8), who found that an elevated ESWS of ˃62.5 KPa is associated with 8 folds higher odds of mortality. Also, Mosleh et al (19)reported that an elevated EDWS of ˃13.5 KPa had a sensitivity of 67% and a specificity of 68%. EDWS was associated with increased major advanced cardiac events. They also reported that systolic myocardial stress was negatively correlated with LVEF, as in the present study. However, their correlation was significant, but ours was not. This could be attributed to different sample sizes.
Conclusion
Elevated LV myocardial stress is related to increased HF in diabetic cases treated by percutaneous revascularization after acute MI. LV wall stress is a potentially useful risk stratification tool using routine echo to determine the treatment plane according to the risk status. However, these results must be treated cautiously because of the limitations of the study. The LV has a half-ellipsoid shape; therefore, measuring its mass and volume is technically limited. In addition, defining the borders of the LV is a manual procedure that depends on the echo window and the patient's experience. Furthermore, wall stress was evaluated as the LV underwent remodeling, and this observed wall stress could alter over time.
Ethical Approval
DFM-IRB 00012367-22-01-009.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as : Abdul khalek A, Abdel-Khalek El-Bahnasy H, Alshahat Omar M, Ibrahim Elraghy M, Ahmed Ahmed Dabash T, Berengy MS, Abozid E, Saad Reihan M. Heart Failure and Echocardiography Derived Myocardial Wall Stress Link in Diabetic Cases with Acute Myocardial Infarction Managed by Revascularization. Med J Islam Repub Iran. 2024 (15 Jan);38:3. https://doi.org/10.47176/mjiri.38.3
References
- 1.Jankauskas SS, Kansakar U, Varzideh F, Wilson S, Mone P, Lombardi A. et al. Heart failure in diabetes. Metabolism. 2021 Dec;125:154910. doi: 10.1016/j.metabol.2021.154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Tan K, Xia H, Gao Y. Left ventricular metabolic remodeling and accompanied dysfunction in type 2 diabetic patients: A 3D speckle tracking analysis. Echocardiography. 2019 Mar;36(3):486–494. doi: 10.1111/echo.14248. [DOI] [PubMed] [Google Scholar]
- 3.Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015 Jul 14;36(27):1718. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- 4.Jenca D, Melenovsky V, Stehlik J, Staněk V, Kettner J, Kautzner J. et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021 Feb;8(1):222–237. doi: 10.1002/ehf2.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchiyama N, Yuasa T, Miyata M, Horizoe Y, Chaen H, Kubota K. et al. Correlation of Right Ventricular Wall Stress With Plasma B-Type Natriuretic Peptide Levels in Patients With Pulmonary Hypertension. Circ J. 2019 May 24;83(6):1278–1285. doi: 10.1253/circj.CJ-18-1155. [DOI] [PubMed] [Google Scholar]
- 6.Smulders KRR, Demandt JPA, Vlaar PJ. Early risk assessment in patients with suspected NSTE-ACS; a retrospective cohort study. Am J Emerg Med. 2022 Oct;60:106–115. doi: 10.1016/j.ajem.2022.07.053. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim IM, Taha M, Abdelaziz M, Alawadi MI, Elzayat A. A Novel Angiographic Index Can Independently Predict 1-Year Cardiovascular Outcomes after Anterior ST-Elevation Myocardial Infarction. Med J Cairo Univ. 2021;89(3):1221–1228. [Google Scholar]
- 8.Kattel S, Bhatt H, Gurung S, Karthikeyan B, Sharma UC. Elevated myocardial wall stress after percutaneous coronary intervention in acute ST elevation myocardial infraction is associated with increased mortality. Echocardiography. 2021;38(8):1263–1271. doi: 10.1111/echo.15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alter P, Koczulla AR, Nell C, Figiel JH, Vogelmeier CF, Rominger MB. Wall stress determines systolic and diastolic function--Characteristics of heart failure. Int J Cardiol. 2016 Jan 1;202:685. doi: 10.1016/j.ijcard.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Mirsky I. Left ventricular stresses in the intact human heart. Biophys J. 1969 Feb;9(2):189–208. doi: 10.1016/S0006-3495(69)86379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res. 1973 Aug;33(2):233. doi: 10.1161/01.res.33.2.233. [DOI] [PubMed] [Google Scholar]
- 12.Alter P, Rupp H, Stoll F, Adams P, Figiel JH, Klose KJ. et al. Increased end diastolic wall stress precedes left ventricular hypertrophy in dilative heart failure--use of the volume-based wall stress index. Int J Cardiol. 2012 May 31;157(2):233. doi: 10.1016/j.ijcard.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 13.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997 Nov 15;30(6):1527. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015 Jan;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Wellings J, Kostis JB, Sargsyan D, Cabrera J, Kostis WJ;. Risk Factors and Trends in Incidence of Heart Failure Following Acute Myocardial Infarction. Am J Cardiol. 2018 Jul 1;122(1):1–5. doi: 10.1016/j.amjcard.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Desta L, Jernberg T, Lofman I, Hofman-Bang C, Hagerman I, Spaak J. et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. 2015;3(3):234. doi: 10.1016/j.jchf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal M, Agrawal S, Garg L, Mohananey D, Garg A, Bhatia N. et al. National Trends in the Incidence, Management, and Outcomes of Heart Failure Complications in Patients Hospitalized for ST-Segment Elevation Myocardial Infarction. Mayo Clin Proc Innov Qual Outcomes. 2017 Jun 8;1(1):26–36. doi: 10.1016/j.mayocpiqo.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA. et al. ; China PEACE Collaborative Group. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385(9966):441. doi: 10.1016/S0140-6736(14)60921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosleh W, Elango K, Shah T, Chaudhari M, Gandhi S, Kattel S. et al. Elevated end-diastolic wall stress after acute myocardial infarction predicts adverse cardiovascular outcomes and longer hospital length of stay. Echocardiography. 2018 Nov;35(11):1721–1728. doi: 10.1111/echo.14136. [DOI] [PubMed] [Google Scholar]
- 20.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000 Jun 27;101(25):2981. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 21.Cavender MA, Steg PG, Smith SC, Eagle K, Ohman EM. et al. ; REACH Registry Investigators. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132(10):923. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 22.Liang J, Zhang Z. Predictors of in-hospital heart failure in patients with acute anterior wall ST-segment elevation myocardial infarction. Int J Cardiol. 2023 Mar 15;375:104–109. doi: 10.1016/j.ijcard.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Vicent L, Velásquez-Rodríguez J, Valero-Masa MJ, Valero-Masa MJ, Díez-Delhoyo F, González-Saldívar H. et al. Predictors of high Killip class after ST segment elevation myocardial infarction in the era of primary reperfusion. Int J Cardiol. 2017 Dec 1;248:46–50. doi: 10.1016/j.ijcard.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Dogan Z, Erden I, Bektasoglu G, Karabulut A. Association Between History of Polymerase Chain Reaction-verified COVID-19 Infection and Outcomes of Subsequent ST-Elevation Myocardial Infarction. Angiology. 2022 Nov 18;75(2):131–138. doi: 10.1177/00033197221139918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuichi T, Satoru S, Takeshi B, Hiroshi T, Naohiko A, Yoshio Y. et al. Predictors of left ventricular remodeling in patients with acute myocardial infarction participating in cardiac rehabilitation. Circ J. 2004 Mar;68(3):214. doi: 10.1253/circj.68.214. [DOI] [PubMed] [Google Scholar]
- 26.Clerfond G, Bière L, Mateus V, Grall S, Willoteaux S, Prunier F. et al. End-systolic wall stress predicts post-discharge heart failure after acute myocardial infarction. Arch Cardiovasc Dis. 2015 May;108(5):310. doi: 10.1016/j.acvd.2015.01.008. [DOI] [PubMed] [Google Scholar]


