Abstract
Progress in the development of effective chemotherapy is producing a growing population of patients with acute and chronic painful chemotherapy-induced peripheral neuropathy (CIPN), a serious treatment-limiting side effect for which there is currently no US Food and Drug Administration-approved treatment.
CIPNs induced by diverse classes of chemotherapy drugs have remarkably similar clinical presentations, leading to the suggestion they share underlying mechanisms. Sensory neurons share with immune cells the ability to detect damage associated molecular patterns (DAMPs), molecules produced by diverse cell types in response to cellular stress and injury, including by chemotherapy drugs. DAMPs, in turn, are ligands for pattern recognition receptors (PRRs), several of which are found on sensory neurons, as well as satellite cells, and cells of the immune system. In the present experiments, we evaluated the role of two PRRs, TLR4 and RAGE, present in dorsal root ganglion (DRG), in CIPN.
Antisense (AS)-oligodeoxynucleotides (ODN) against TLR4 and RAGE mRNA were administered intrathecally before (‘prevention protocol’) or 3 days after (‘reversal protocol’) the last administration of each of three chemotherapy drugs that treat cancer by different mechanisms (oxaliplatin, paclitaxel and bortezomib). TLR4 and RAGE AS-ODN prevented the development of CIPN induced by all three chemotherapy drugs. In the reversal protocol, however, while TLR4 AS-ODN completely reversed oxaliplatin- and paclitaxel-induced CIPN, in rats with bortezomib-induced CIPN it only produced a temporary attenuation. RAGE AS-ODN, in contrast, reversed CIPN induced by all three chemotherapy drugs.
When a TLR4 antagonist was administered intradermally to the peripheral nociceptor terminal, it did not affect CIPN induced by any of the chemotherapy drugs. However, when administered intrathecally, to the central terminal, it attenuated hyperalgesia induced by all three chemotherapy drugs, compatible with a role of TLR4 in neurotransmission at the central terminal but not sensory transduction at the peripheral terminal.
Finally, since it has been established that cultured DRG neurons can be used to study direct effects of chemotherapy on nociceptors, we also evaluated the role of TLR4 in CIPN at the cellular level, using patch-clamp electrophysiology in DRG neurons cultured from control and chemotherapy-treated rats. We found that increased excitability of small-diameter DRG neurons induced by in vivo and in vitro exposure to oxaliplatin is TLR4-dependent. Our findings suggest that in addition to the established contribution of PRR-dependent neuroimmune mechanisms, PRRs in DRG cells also have an important role in CIPN.
Keywords: oxaliplatin, paclitaxel, bortezomib, chemotherapy-induced peripheral neuropathy (CIPN), Toll-like receptor 4 (TLR4), receptor for advanced glycation endproducts (RAGE)
Neuropathic pain is a common side-effect of chemotherapy and can have a profound impact on quality of life. Araldi et al. show in rats that transiently reducing the expression of two pattern recognition receptors in dorsal root ganglion cells prevents and reverses this debilitating condition.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN), a predominantly sensory neurotoxicity presenting in a distal symmetric stocking-glove distribution,1,2 is a common, debilitating, painful and treatment-limiting condition,3 for which there is currently no US Food and Drug Administration (FDA) approved treatment. CIPN symptoms may range from pain and allodynia to hypoaesthesia, and in some patients may be accompanied by motor and autonomic neuropathy,1,4 resulting in decreased quality of life.5,6 Symptoms generally improve over time following the discontinuation of chemotherapy, although complete recovery is frequently not attained.1,7,8 It is estimated that 50–90% of patients who receive chemotherapy develop CIPN in the acute setting, which persists in 30–40% as chronic CIPN.1,8 The frequency and severity of CIPN is dependent on the class of chemotherapy drug, its cumulative dose and duration of exposure.1,8,9 How diverse chemotherapy agents produce a phenotypically similar, distal, symmetric, small-fibre, painful, peripheral neuropathy,8,10-14 however, remains a critically important question.
One of the most extensively studied mechanisms underlying CIPN pain, induced by diverse chemotherapy drugs involves neuroimmune mechanisms, interactions between cells of the immune system, and nociceptive sensory neurons.15,16 Cells of the immune system respond to damage associated molecular patterns (DAMPs) produced in response to chemotherapy drugs,17-21 which act at pattern recognition receptors (PRRs) on immune cells that in turn release proinflammatory cytokines, which can sensitize nociceptors.22 Importantly, sensory neurons also express PRRs,23-30 including TLR4 and RAGE, which can further exacerbate cell stress and injury.18,31 In rat, TLR4 and RAGE are expressed by nociceptors,32-37 and it has been shown that paclitaxel increases nociceptor TLR4 expression,35 associated with damage to the peripheral nervous system38 and the development of mechanical allodynia.34,35 And, while it has been shown that oxaliplatin- and paclitaxel-induced hyperalgesia (CIPN) in rats and mice is attenuated by administration of TLR4 and RAGE antagonists,39 the location of the PRRs involved remains to be established.
We propose that the PRRs, TLR4 and RAGE, in DRG cells injured by exposure to neurotoxic chemotherapy drugs contribute to CIPN. To test this hypothesis, we explored the role of TLR4 and RAGE in CIPN induced by three commonly used neurotoxic chemotherapy drugs that are thought to produce CIPN by different mechanisms: oxaliplatin,40-42 paclitaxel43,44 and bortezomib.45-47 To directly evaluate the role of nociceptor PRRs in the enhanced excitability induced by chemotherapy drugs, we used patch-clamp electrophysiology to evaluate the effect of oxaliplatin on cultured dorsal root ganglia (DRG) neurons also treated in vitro with a PRR antagonist or in vivo with PRR oligodeoxynucleotide antisense.
Material and methods
For complete details, see the Supplementary material.
Animals
Experiments were performed on 260–460 g adult, male and female Sprague–Dawley rats (Charles River Laboratories). Animals were housed three per cage, under a 12-h light/dark cycle, in a temperature- and humidity-controlled animal care facility at the University of California, San Francisco. Food and water were available ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California at San Francisco and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Effort was made to minimize the number of animals used and their suffering.
Nociceptive threshold testing
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter® (Randall–Selitto paw-withdrawal device, Stoelting), which applies a linearly increasing mechanical force to the dorsum of a rat's hind paw, as previously described.48-54 To minimize restraint stress, rats were placed in cylindrical acrylic restrainers designed to provide ventilation and allow hind leg extension from lateral ports during the assessment of mechanical nociceptive threshold. To acclimatize rats to the testing procedure, they were placed in restrainers for 1 h prior to starting training sessions for 3 days consecutively, daily, and for 40 min prior to experimental manipulations. Nociceptive threshold was defined as the force in grams at which a rat withdrew its paw. Baseline nociceptive threshold was defined as the mean of the three readings taken before test agents were injected. Only one paw was used in an experiment, and each experiment was performed on a separate group of rats. To minimize experimenter bias, individuals conducting the behavioural experiments (D.A. and I.J.M.B) were blinded to experimental interventions.
Drugs
The following compounds were used in this study: oxaliplatin and paclitaxel (Sigma-Aldrich); bortezomib (LC Laboratories); and the TLR4 antagonist LPS-RS Ultrapure (lipopolysaccharide from Rhodobacter sphaeroides, InvivoGen).
Preclinical models of chemotherapy-induced peripheral neuropathy
Oxaliplatin-induced neuropathic pain
Oxaliplatin (2 mg/kg) was dissolved in saline and a single dose injected intravenously. This is a well-described and reproducible preclinical model in which distinct early and late phases of CIPN are present.55,56
Paclitaxel-induced neuropathic pain
Paclitaxel was prepared in a mixture of absolute ethanol and polyethoxylated castor oil (Cremophor EL; 1:1; Sigma-Aldrich), and further diluted in saline to a final concentration of 1 mg/ml, immediately before use. Paclitaxel (1 mg/kg) was injected intraperitoneally (i.p.) every other day for a total of four doses.55,57
Bortezomib-induced neuropathic pain
Bortezomib (0.2 mg/kg)58,59 was dissolved in 3% dimethyl sulphoxide (DMSO, Sigma-Aldrich) and 97% saline. Bortezomib was administered intravenously (i.v.)58,59 every other day for a total of four doses. The volume of drug solution injected was 1 ml/kg.
Oligodeoxynucleotides antisense to TLR4 and RAGE mRNA
To investigate the role of TLR4 and RAGE in the hyperalgesia induced by oxaliplatin, paclitaxel and bortezomib, validated oligodeoxynucleotides (ODNs) antisense (AS) for TLR448 and RAGE60 were employed.
AS-ODN sequences, directed against unique regions of the rat mRNA for TLR4 and RAGE, were as follows: TLR4 AS-ODN: 5′-AGGAAGTGAGAGTGCCAACC-3′ (GenBank accession No. AF057025.2); and RAGE AS-ODN: 5′-AGCTACTGTCCCCGTTGG-3′ (GenBank accession No. L33413).
As a control, the following mismatch (MM) and sense (SE) ODN sequences were used: TLR4 MM-ODN: 5′-ACGATCGAGAGAGTCACCG-3′; and RAGE SE-ODN: 5′- CCAACGGGGACAGTAGCT-3′. ODNs were synthesized by Thermo Fisher Scientific. More details about ODN dilution and in vivo administration are included in the Supplementary material.
SDS-PAGE and western blotting
The effect of the antisense treatment on TLR4 expression in rat DRG was analysed 24 h and 9 days following the last intrathecal (i.t.) injection. Rats were euthanized by exsanguination, while under isoflurane anaesthesia, and L4 and L5 DRG surgically removed and stored at −80°C until further use. Results were analysed using computer-assisted densitometry and levels of TLR4 immunoreactivity normalized with respect to the β-actin control levels in each sample. The percentage decrease in TLR4 expression was calculated as: (normalized density for AS / normalized density for MM × 100) − 100.61,62 More details are included in the Supplementary material.
Dorsal root ganglia neuron culture and in vitro patch-clamp electrophysiology
Primary neuronal cultures were made from dissociated DRG harvested from adult male Sprague-Dawley rats (300–400 g) as described previously.63,64
DRG neurons were used in electrophysiology experiments 24–96 h after plating. While small, medium and large sized DRG neurons were routinely observed in the same preparation, this study focused on cells with soma diameter less than 30 μm, representing predominantly C-type nociceptors.65-69 All experiments were performed at room temperature (20–22°C).
Whole-cell patch-clamp recordings, in ‘current clamp’ mode, were made to assess changes in the excitability of cultured DRG neurons. Holding current was adjusted to maintain membrane potential at −70 mV. Rheobase, the minimum magnitude of a current step needed to elicit an action potential (AP), was determined using a protocol in which increasing square wave (40–80 ms) current pulses were applied every 2 s with step adjusted for 5–10% precision.63,70,71
AP threshold potential was determined from an approximation of the initial part of the response to a square wave pulse of rheobase magnitude with the sum of decaying and rising exponents, representing membrane capacitance recharge and initial rising phase of AP development.70,72,73 AP threshold was defined as a potential on the original recording where the difference from the decaying component of the fit raised above the arbitrary selected value of 2 mV, representing sensitivity of the definition.64 More details are included in the Supplementary material.
Data analysis
All in vivo data are presented as mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 9.0 statistical software (GraphPad Software, San Diego, CA, USA). A P-value < 0.05 was considered statistically significant. In the behavioural experiments, the dependent variable was change in mechanical paw-withdrawal threshold, expressed as percentage change from baseline. As specified in the figure legends, Student's t-test or two-way repeated-measures ANOVA followed by Bonferroni's post hoc test was performed to compare the magnitude of hyperalgesia induced by chemotherapeutic agents.
In in vitro electrophysiology experiments, values of AP threshold in the three groups were analysed for differences between their means with one-way ANOVA followed by Dunnett's or Holm–Šídák's post hoc test.
Results
Oxaliplatin, paclitaxel and bortezomib produce distal symmetric CIPN
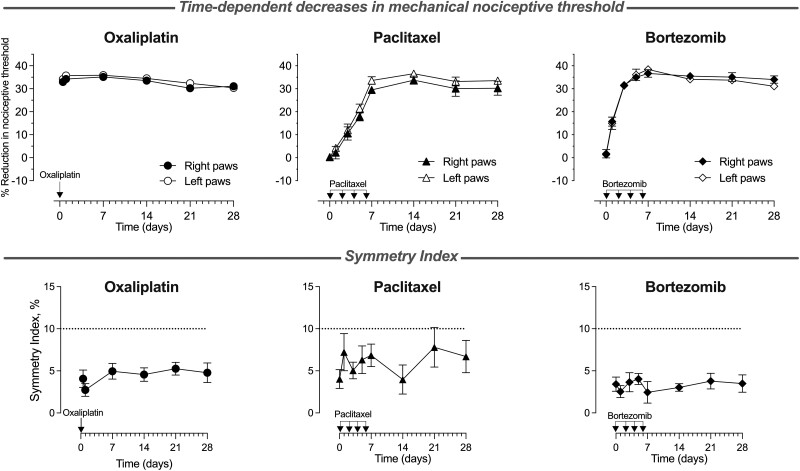
To assess for the presence of symmetry in CIPN pain induced by oxaliplatin, paclitaxel and bortezomib, we measured changes in mechanical nociceptive threshold over time in both hind paws of the same rat, following the systemic administration of each chemotherapy drug. Rats receiving oxaliplatin (2 mg/kg, i.v., single dose), paclitaxel (1 mg/kg, i.p., on Days 0, 2, 4 and 6) or bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6) demonstrated a time-dependent symmetric decrease in mechanical nociceptive threshold from Days 0 to 28 (Fig. 1; mean values: oxaliplatin 4.4%, paclitaxel 6.0% and bortezomib 3.3%; n = 12 for oxaliplatin- and paclitaxel-, and n = 8 for bortezomib-treated rats).
Figure 1.
Time-dependent symmetric decrease in mechanical nociceptive threshold produced by oxaliplatin, paclitaxel and bortezomib. Oxaliplatin [2 mg/kg, intravenously (i.v.), on Day 0], paclitaxel [1 mg/kg, intraperitoneally (i.p.), on Days 0, 2, 4 and 6] or bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6) were administered to groups of male rats, and the magnitude of hyperalgesia was measured over 28 days. Top: Oxaliplatin decreased the nociceptive threshold from the first time point (30 min), and it remained undiminished over the 28-day testing period. For paclitaxel and bortezomib, the time-dependent decrease in the nociceptive threshold reached a maximum on Day 7 and remained undiminished for the rest of the 28-day testing period. Bottom: A symmetry index was calculated at every time point, as the absolute (unsigned) value of the difference between the reduction in nociceptive threshold in the right minus left paws, for each rat, expressed as a percentage of the baseline nociceptive threshold. There was no time dependence of the symmetry index for any of the three agents tested, and the average levels remained very low at all time points (mean values: oxaliplatin 4.4%, paclitaxel 6.0% and bortezomib 3.3%), supporting the presence of symmetry in hind paw mechanical hyperalgesia throughout the time course of the study (Days 0–28) for all three chemotherapy drugs. Values are presented as mean ± SEM; n = 12 for oxaliplatin- and paclitaxel-, and n = 8 for bortezomib-treated rats.
Effect of attenuating TLR4 in dorsal root ganglia on prevention and reversal of CIPN
To evaluate if CIPN produced by diverse chemotherapy drugs share underlying PPR-dependent mechanisms, we evaluated the role of TLR4, present in DRG, in the onset and maintenance of CIPN induced by oxaliplatin, paclitaxel and bortezomib using: (i) intrathecal administration of ODN antisense to mRNA for TLR4; and (ii) TLR4 antagonist (LPS-RS Ultrapure), administered at central (intrathecally) or peripheral (intradermally) nociceptor terminals.
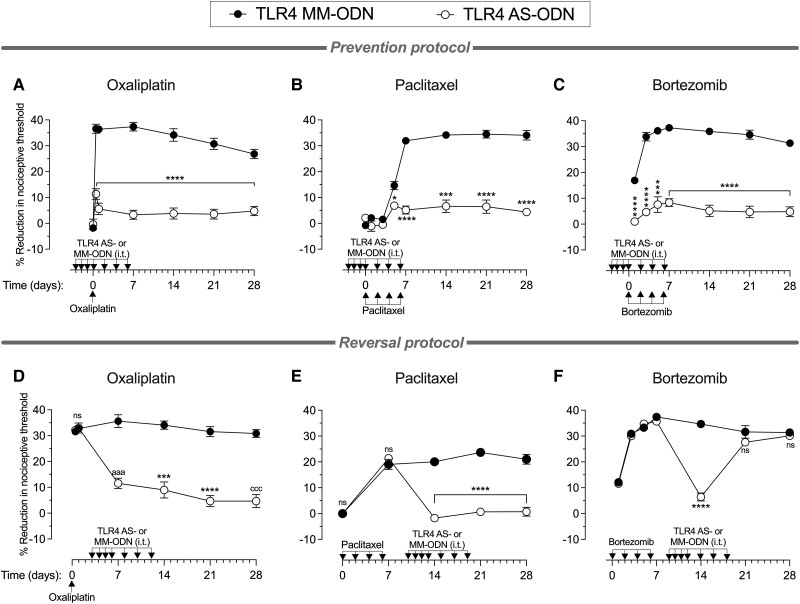
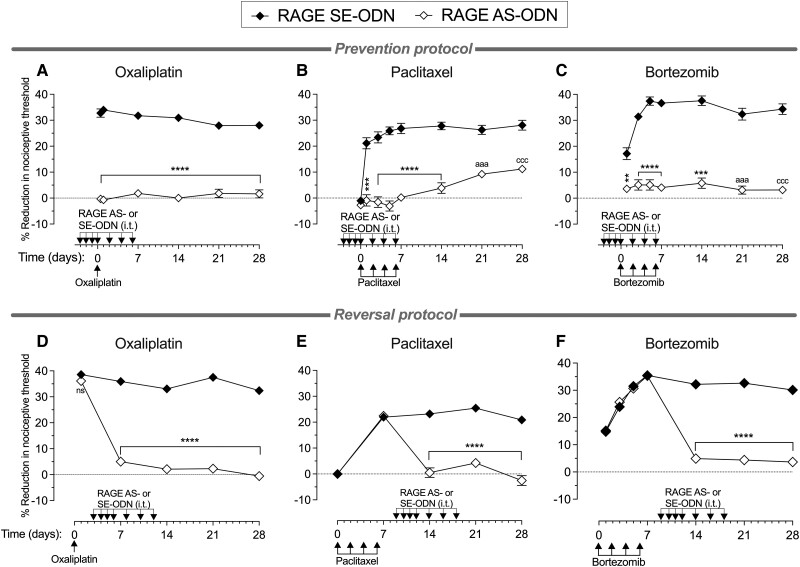
To determine whether TLR4 plays a role in the onset of hyperalgesia induced by oxaliplatin, paclitaxel or bortezomib, male rats received intrathecal AS-ODN or MM-ODN to TLR4 mRNA (120 μg/20 μl) daily for 4 days consecutively, and then three more doses, one every other day (a total of seven doses). Approximately 20 h after the third intrathecal administration of ODN, oxaliplatin (2 mg/kg, i.v.), paclitaxel (1 mg/kg, i.p.) or bortezomib (0.2 mg/kg, i.v.) were administered to induce CIPN. Oxaliplatin was administered as a single dose on Day 0; paclitaxel and bortezomib were administered on Days 0, 2, 4 and 6. The mechanical nociceptive threshold was measured from Days −3 (before the first intrathecal administration of ODN) to 28. In the groups treated with TLR4 AS-ODN, compared with the TLR4 MM-ODN-treated groups, oxaliplatin, paclitaxel and bortezomib were not able to induce hyperalgesia (Fig. 2A–C, respectively), indicating that the knock down of TLR4 in DRG cells ‘prevented’ the development of CIPN induced by diverse chemotherapy drugs.
Figure 2.
Effect of TLR4 antisense on preventing and reversing oxaliplatin, paclitaxel and bortezomib CIPN. Top: Groups of male rats were treated intrathecally (i.t.) with antisense (AS)-oligodeoxynucleotides (ODN) or mismatch (MM)-ODN (both 120 μg in 20 μl/day) against TLR4 mRNA, once a day starting 3 days before (Day −3) each chemotherapy drug, for seven doses, over 10 days. Rats received (A) oxaliplatin [2 mg/kg, intravenously (i.v.), on Day 0]; (B) paclitaxel [1 mg/kg, intraperitoneally (i.p.), on Days 0, 2, 4 and 6]; or (C) bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6). The mechanical nociceptive threshold was evaluated before the first intrathecal administration of ODN (Day −3) and then from Days 0 to 28. In the TLR4 antisense-treated rats, CIPN hyperalgesia was markedly inhibited in the oxaliplatin-, paclitaxel- and bortezomib-treated rats (A, B and C, respectively), and this attenuation was undiminished over the 28-day testing period (all data are mean ± SEM). Statistical analyses for (A) oxaliplatin: two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(6,60) = 27.22, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 210.6, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (TLR4 MM-ODN versus TLR4 AS-ODN); (B) paclitaxel: two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(7,70) = 39.54, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 333.2, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001, ***P = 0.0001, *P = 0.0299 (TLR4 MM-ODN versus TLR4 AS-ODN); and (C) ortezomib; two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(6,60) = 7.817, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 280.8, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001, ***P = 0.0004 (TLR4 MM-ODN versus TLR4 AS-ODN). n = 6 paws for each group. Bottom: Separate groups of male rats received: (D) oxaliplatin (2 mg/kg, i.v., on Day 0); (E) paclitaxel (1 mg/kg, i.p., on Days 0, 2, 4 and 6); or (F) bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6). Each group was then treated intrathecally with AS-ODN or MM-ODN (both 120 μg in 20 μl/day) against TLR4 mRNA once a day starting 3 days after the last dose of chemotherapy agent, for seven doses, over 10 days. The mechanical nociceptive threshold was evaluated from Days 0 to 28. In the TLR4 AS-ODN-treated group, CIPN hyperalgesia was markedly attenuated in oxaliplatin- and paclitaxel-treated rats, and this attenuation was undiminished for the 28-day testing period. Bortezomib-induced hyperalgesia was reversed on Day 14, but hyperalgesia returned, after ending antisense administration, on Days 21 and 28. Statistical analyses for (D) oxaliplatin: two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(5,50) = 41.94, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 67.68, P < 0.0001; Bonferroni's multiple post hoc comparisons test, aaaP = 0.0002, ***P = 0.0008, ****P < 0.0001, cccP = 0.0001 (MM-ODN versus AS-ODN); (E) paclitaxel: two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(4,40) = 75.11, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 100.6, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (MM-ODN versus AS-ODN); and (F) bortezomib: two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(6,60) = 44.08, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 23.6, P = 0.0007; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (MM-ODN versus TLR4 AS-ODN at Day 14 after first bortezomib administration). All data are mean ± SEM. n = 6 paws for each group. CIPN = chemotherapy-induced peripheral neuropathy.
To determine if maintenance of CIPN induced by oxaliplatin, paclitaxel and bortezomib is also PRR dependent, we next evaluated whether CIPN could be ‘reversed’ by intrathecal administration of TLR4 AS-ODN and if such reversal would again outlast the duration the TLR4 antisense action. Male rats received oxaliplatin (2 mg/kg, i.v., on Day 0), paclitaxel (1 mg/kg, i.p., on Days 0, 2, 4 and 6) or bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6), and 3 days after the last administration of each chemotherapy drug, when hyperalgesia was already fully established, TLR4 AS-ODN or MM-ODN was administered (120 μg/20 μl, i.t.) for 4 days consecutively; then three more doses were administered, one every other day (a total of seven doses). While TLR4 AS-ODN completely reversed the hyperalgesia induced by oxaliplatin and paclitaxel (Fig. 2D and E, respectively), it produced only a transient reversal of bortezomib-induced hyperalgesia (Fig. 2F), compatible with the time course of action of intrathecal antisense. The reversal of oxaliplatin and paclitaxel CIPN hyperalgesia produced by TLR4 AS-ODN persisted long after antisense was discontinued.
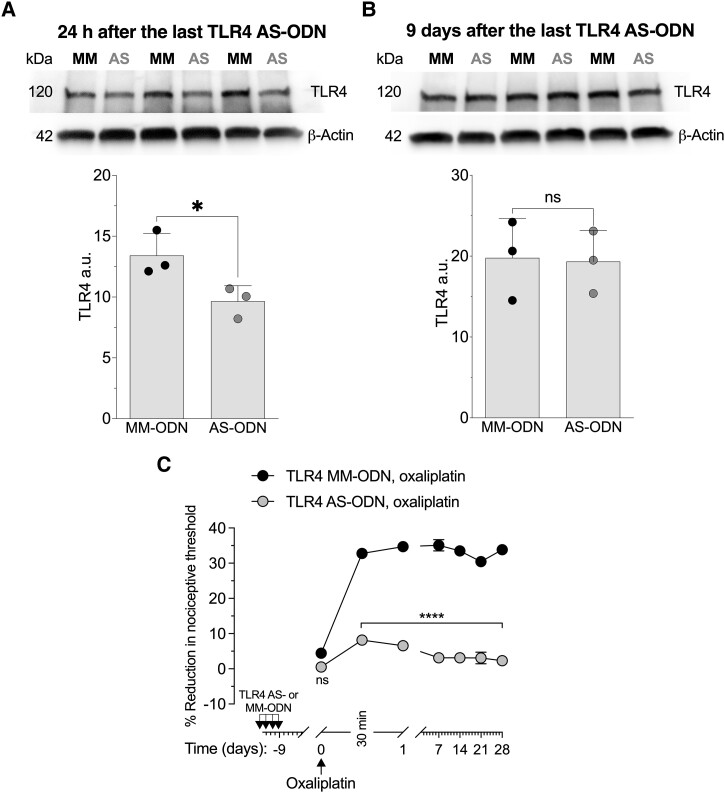
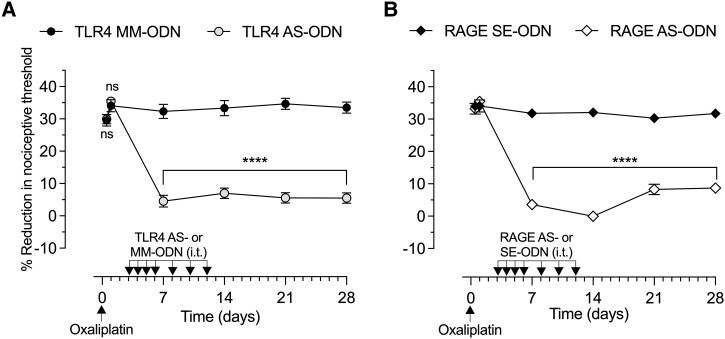
Since the effect of TLR4 AS-ODN on CIPN hyperalgesia persisted long after antisense was discontinued, we determined the level of TLR4 present in DRG from rats that received four consecutive doses of intrathecal TLR4 AS-ODN and had DRG collected 24 h (Fig. 3A) or 9 days after the last injection of TLR4 AS-ODN (Fig. 3B). Evidence for AS-induced reduction of TLR4 expression in DRG can be seen in western blots (pooled L4 and L5 DRG) from TLR4 AS-ODN-treated rats (Fig. 3A), in which we observed a 28.02 ± 1.29% (in arbitrary units normalized to the reference protein, unpaired Student's t-test, n = 3, P < 0.05) decrease in the expression of TLR4 relative to the extracts derived from TLR4 MM-ODN-treated rats. However, western blot analysis of DRG extracts from rats injected with TLR4 AS-ODN or MM-ODN for 4 days consecutively, but the DRGs collected 9 days after the last intrathecal injection of ODNs, showed no significant decrease in their anti-TLR4 immunoreactivity (Fig. 3B; −2.32 ± 3.6%, unpaired Student's t-test, n = 3, P > 0.05). Additionally, we treated male rats with TLR4 AS-ODN or MM-ODN for 4 days consecutively, and then 9 days later, when the levels of TLR4 in DRG had recovered; oxaliplatin (2 mg/kg, i.v.) was administered and the mechanical nociceptive threshold evaluated 30 min and 1, 7, 14, 21 and 28 days later. At a time when TLR4 levels in DRG had recovered, the rats that received the last administration of TLR4 AS-ODN 9 days prior to oxaliplatin did not develop hyperalgesia (Fig. 3C).
Figure 3.
TLR4 antisense attenuates TLR4 protein expression in L4 and L5 dorsal root ganglia and oxaliplatin-induced CIPN. Western blot analysis of dorsal root ganglia (DRG) extracts from male rats treated intrathecally with antisense-oligodeoxynucleotides (AS-ODN) against TLR4 mRNA, once a day for 4 days (120 µg in 20 µl/day). (A) TLR4 AS-ODN-treatment significantly decreased anti-TLR4 immunoreactivity 24 h after the last treatment (−28.06 ± 1.29%, unpaired Student's t-test, n = 3, *P < 0.05). Of note, the magnitude of the attenuation of TLR4 in DRG neurons is probably an underestimate, as TLR4 levels in other cells in the DRG are not affected by intrathecal antisense but are measured on the western blots. (B) Nine days after the last administration of TLR4 AS-ODN, anti-TLR4 immunoreactivity was not significantly different from the levels in DRG from TLR4 mismatch (MM)-ODN-treated rats (−2.32 ± 3.6%, unpaired Student's t-test, n = 3, P > 0.05). The calculated molecular weight of TLR4 is 96 kDa (according to UniProtKB database entry Q9QX05). The difference between the calculated and apparent molecular weights may be due to the glycosylation of TLR4. β-Actin, which was used as a loading control, has a calculated molecular weight of ∼42 kDa (according to UniProtKB database entry P60771). The full-length gels and blots are included in Supplementary Fig. 3. (C) Male rats were treated intrathecally with AS-ODN or MM-ODN (both 120 μg in 20 μl/day) against TLR4 mRNA once a day for 4 days consecutively. On Day 0 (9 days after the last intrathecal ODN injection), rats received oxaliplatin [2 mg/kg, intravenously (i.v.)], and the mechanical nociceptive threshold was evaluated before the first intrathecal administration of ODN (Day −12) and then from Days 0 to 28. In the TLR4 AS-ODN-treated group, oxaliplatin did not develop hyperalgesia until Day 28. Two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(6,60) = 35.66, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 881.1, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (TLR4 MM-ODN versus TLR4 AS-ODN). n = 6 paws for each group. All data are mean ± SEM. CIPN = chemotherapy-induced peripheral neuropathy.
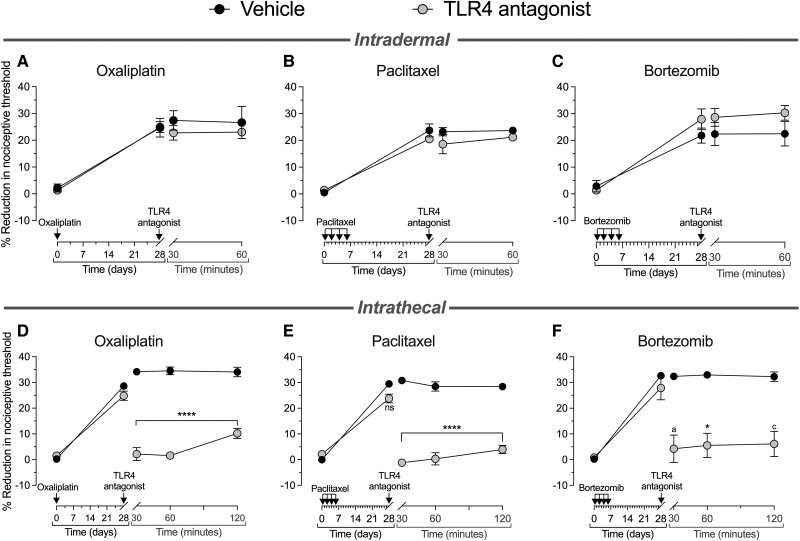
To evaluate the role of TLR4 in the peripheral and central terminals of DRG neurons, we administered LPS-RS Ultrapure, a selective TLR4 antagonist, intradermally (Fig. 4A–C) or intrathecally (Fig. 4D–F). Male rats pretreated with oxaliplatin, paclitaxel or bortezomib received the TLR4 antagonist intradermally (3 μg/5 μl), and the mechanical nociceptive threshold was measured 30 and 60 min later. Intradermal administration of LPS-RS Ultrapure did not affect oxaliplatin-, paclitaxel- or bortezomib-induced hyperalgesia (Fig. 4A–C, respectively). However, when administered intrathecally (10 μg/20 μl), this TLR4 antagonist markedly inhibited oxaliplatin-, paclitaxel- and bortezomib-induced hyperalgesia (Fig. 4D–F, respectively), as measured 30, 60 and 120 min after TLR4 antagonist administration. Twenty-four hours after intrathecal administration of the TLR4 antagonist, the hyperalgesia recovered to pre-TLR4 antagonist levels in all chemotherapy-treated rats (data not shown). These data support the suggestion that agonist stimulation of TLR4 in the central but not peripheral terminal of the nociceptor plays a role in the expression of CIPN induced by all three chemotherapy drugs.
Figure 4.
Intrathecal but not intradermal administration of a TLR4 selective antagonist reverses oxaliplatin, paclitaxel and bortezomib CIPN. Top: Groups of male rats received (A and D) oxaliplatin [2 mg/kg, intravenously (i.v.), on Day 0]; (B and E) paclitaxel [1 mg/kg, intraperitoneally (i.p.), on Days 0, 2, 4 and 6]; or (C and F) bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6). The mechanical nociceptive threshold was evaluated on Day 0 before the chemotherapy drug was administered and then at Day 28 after their administration. On Day 28 after chemotherapy drugs, rats received intradermal vehicle (saline, 5 μl) or TLR4 antagonist (LPS-RS Ultrapure, 3 μg/5 μl), and the mechanical nociceptive threshold was evaluated 30 and 60 min later. Intradermal TLR4 antagonist did not attenuate CIPN hyperalgesia in the oxaliplatin-, paclitaxel- and bortezomib-treated rats (A, B and C, respectively). Statistical analyses for intradermal TLR4 antagonist (A) oxaliplatin: two-way repeated-measures ANOVA, Time × TLR4 antagonist interaction, F(3,12) = 0.749, P = 0.5434; TLR4 antagonist treatment, F(1,10) = 0.347, P = 0.5876; Bonferroni's multiple post hoc comparisons test, not significant (ns; vehicle versus TLR4 antagonist); (B) paclitaxel: two-way repeated-measures ANOVA, Time × TLR4 antagonist interaction, F(3,12) = 1.136, P = 0.3738; TLR4 antagonist treatment, F(1,10) = 2.009, P = 0.3393; Bonferroni's multiple post hoc comparisons test, ns (vehicle versus TLR4 antagonist); and (C) bortezomib: two-way repeated-measures ANOVA, Time × TLR4 antagonist interaction, F(3,12) = 2.157, P = 0.1462; TLR4 antagonist treatment, F(1,10) = 1.382, P = 0.3050; Bonferroni's multiple post hoc comparisons test, ns (vehicle versus TLR4 antagonist). n = 6 paws for each group. Bottom: Separate groups of male rats, treated 28 days prior with (D) oxaliplatin (2 mg/kg, i.v.), (E) paclitaxel (1 mg/kg, i.p.) or (F) bortezomib (0.2 mg/kg, i.v.), received intrathecally vehicle (saline, 20 μl) or TLR4 antagonist (LPS-RS Ultrapure, 10 μg/20 μl), and the mechanical nociceptive threshold was evaluated 30, 60 and 120 min later. In the group treated with intrathecal TLR4 antagonist, oxaliplatin-, paclitaxel- and bortezomib-induced hyperalgesia was markedly attenuated (D, E and F, respectively), and this inhibition was undiminished for the 120-min testing period. Statistical analyses for intrathecal TLR4 antagonist (D) oxaliplatin: two-way repeated-measures ANOVA, Time × TLR4 antagonist interaction, F(4,40) = 74.54, P < 0.0001; TLR4 antagonist treatment, F(1,10) = 168.3, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (vehicle versus TLR4 antagonist); (E) paclitaxel; two-way repeated-measures ANOVA, Time × TLR4 antagonist interaction, F(4,40) = 73.01, P < 0.0001; TLR4 antagonist treatment, F(1,10) = 216.8, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (vehicle versus TLR4 antagonist); and (F) bortezomib: two-way repeated-measures ANOVA, Time × TLR4 antagonist interaction, F(4,32) = 32.66, P < 0.0001; TLR4 antagonist treatment, F(1,10) = 30.99, P = 0.0005; Bonferroni's multiple post hoc comparisons test, aP = 0.0341, *P = 0.0248, cP = 0.0252 (vehicle versus TLR4 antagonist). n = 6 paws for each group. All data are mean ± SEM. LPS-RS Ultrapure = lipopolysaccharide from Rhodobacter sphaeroides. CIPN = chemotherapy-induced peripheral neuropathy.
Since TLR4 AS-ODN prevented oxaliplatin-induced hyperalgesia, we evaluated if a second administration of oxaliplatin (14 days after its first administration) could produce hyperalgesia in these TLR4 AS-ODN treated rats. Male rats were treated intrathecally with TLR4 AS-ODN or MM-ODN (120 μg/20 μl) for 4 days consecutively, and then three more doses were administered, one every other day (a total of seven doses). When oxaliplatin was injected intravenously (2 mg/kg) on Day 0, the rats treated with TLR4 AS-ODN, compared with those treated with TLR4 MM-ODN, did not develop hyperalgesia. TLR4 AS-ODN was injected intrathecally until Day 6 after the first oxaliplatin administration, and then on Day 14 (8 days after the last intrathecal injection of TLR4 AS-ODN) oxaliplatin (2 mg/kg, i.v.) was injected again and the mechanical nociceptive threshold was evaluated 30 min and 24 h later. The second dose of oxaliplatin was also not able to produce hyperalgesia in rats that had received their last intrathecal administration of TLR4 AS-ODN 8 days earlier (Supplementary Fig. 4), at which time the levels of TLR4 in DRG had recovered, indicating that the effect of the transient attenuation of TLR4 in DRG neurons on CIPN persists long after antisense is discontinued. That knockdown of TLR4 reversed, while intrathecal TLR4 antagonist (LPS-RS Ultrapure) only transiently attenuated CIPN hyperalgesia, supports the suggestion TLR4 crosstalk with other molecules in nociceptors may be needed to maintain CIPN.
TLR4 attenuation prevents and reverses oxaliplatin-induced sensitization of small-diameter DRG neurons
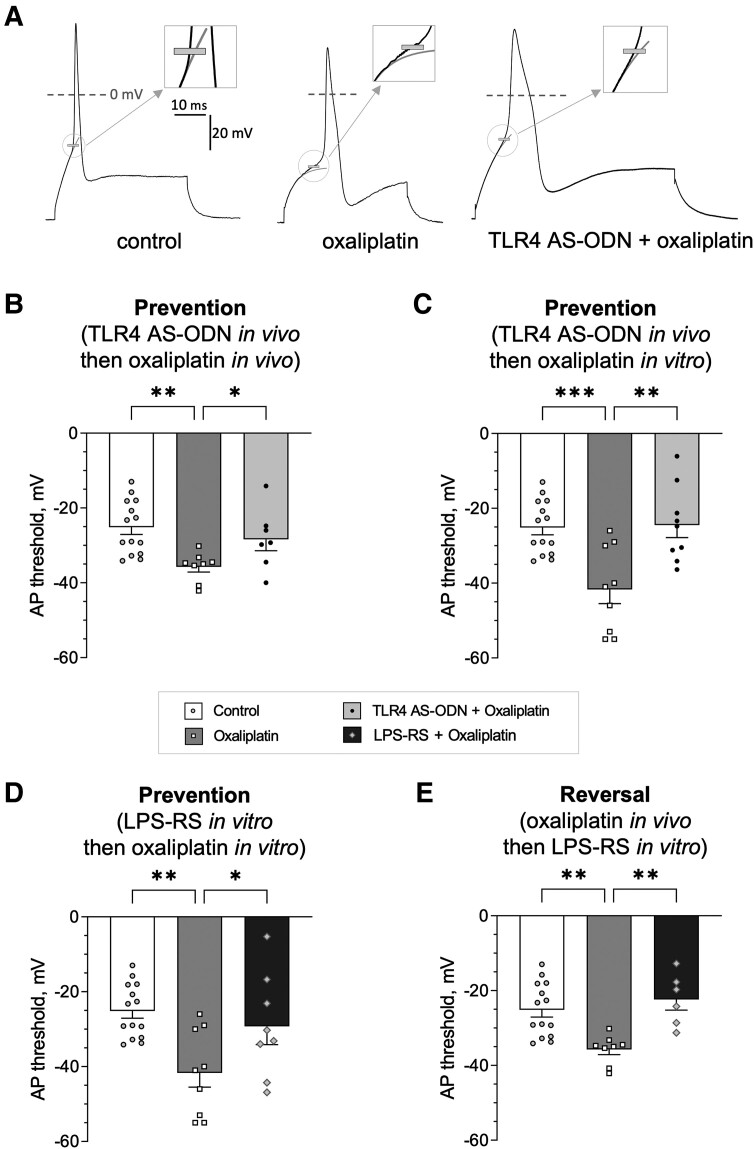
The results of our behavioural experiments provide support for the suggestion that oxaliplatin-induced hyperalgesia is TLR4-dependent in both the ‘induction’ and ‘maintenance’ of long-lasting CIPN hyperalgesia. To more directly elucidate the role of nociceptor TLR4, in vitro experiments were used to address the following questions: (i) does oxaliplatin-induced hyperalgesia implicate long-lasting nociceptor sensitization; (ii) can such sensitization be a direct effect of oxaliplatin on the nociceptor; and (iii) is TLR4 involved in the effect of oxaliplatin at the level of the nociceptor? To address these questions, we assessed oxaliplatin-induced nociceptor sensitization in electrophysiological experiments on cultured DRG neurons, examining electrical excitability in putative nociceptors (DRG neurons with soma diameter <30 μm, which majorly constitute the population of C-fibre nociceptors). We selected an AP threshold, the membrane potential at which an AP starts to develop as fast and significant depolarization (see the ‘Materials and methods’ section for details of calculation and Fig. 5A for the illustration), a robust measure of electrical excitability. To parallel in vivo behavioural experiments, in vitro experiments were also performed using both ‘prevention’ (Fig. 5B–D) and ‘reversal’ protocols (Fig. 5E).
Figure 5.
Oxaliplatin-induced nociceptor sensitization, in vitro, is TLR4 dependent. (A) Example traces for the lower (more negative) action potential (AP) threshold in a small dorsal root ganglia (DRG) neuron of an oxaliplatin-treated rat (middle) compared with naïve rats (left), and the lack of such oxaliplatin-induced lowering of the AP threshold after in vivo pretreatment with TLR4 antisense-oligodeoxynucleotides (AS-ODN) in the prevention protocol (right). Traces show APs generated in response to a current step with height equal to rheobase (minimum current required to induce an AP) in three different neurons. The scale is the same for all panels. AP threshold is indicated by short grey tick. Rectangle inset in the upper right corner of each panel shows magnified region of recordings near the position of the AP threshold. Thin light grey line shows fit of the initial phase of the depolarization with a single exponent corresponding to passive charging of the neuron's capacitance. Deviation of the actual neuronal depolarization of 2 mV above the fit was defined as the AP threshold (see the ‘Materials and methods’ section for details). (B) Effect of oxaliplatin administered in vivo on AP threshold, and the attenuation of this effect by TLR4 AS-ODN administered in vivo, in the prevention protocol. Oxaliplatin induced a reduction in the AP threshold compared with the control group [untreated naïve animals; one-way ANOVA: F(2,26) = 6.3, P = 0.006; Holm–Šídák's post hoc test: t(26) = 3.6, **adjusted P = 0.003]. TLR4 AS-ODN administered before oxaliplatin produced a significant shift in the AP threshold back towards the control value [t(26) = 2.1, #adjusted P = 0.04 compared with in vivo oxaliplatin]. (C) Effect of in vitro administration of oxaliplatin on the AP threshold, and its attenuation by TLR4 AS-ODN administered in vivo, in the prevention protocol. Oxaliplatin (50 μM) induced a significant reduction in the AP threshold (after preincubation for 3 h before recording) compared with the control group [one-way ANOVA: F(2,29) = 10.5, P = 0.0004; Dunnett's post hoc test: q(29) = 4.1, ***adjusted P = 0.0005]. TLR4 AS-ODN administered in vivo, before oxaliplatin, produced a significant shift in the AP threshold back towards the control value [Dunnett's post hoc test: q(29) = 3.9, ##adjusted P = 0.0010 compared with in vitro oxaliplatin]. (D) Effect of in vitro administration of LPS-RS Ultrapure, a selective inhibitor of TLR4, on oxaliplatin-induced reduction in AP threshold (‘all in vitro’ prevention). Preincubation of cultured neurons with LPS-RS Ultrapure (10 μg/ml for 24 h) before adding oxaliplatin (for 3 h before and during the recording) significantly shifted the AP threshold back towards the control value compared with the significantly more negative AP threshold observed in in vitro oxaliplatin-treated neurons [one-way ANOVA: F(2,28) = 7.0, P = 0.004; Dunnett's post hoc test: q(25) = 3.7, **adjusted P = 0.0018 for in vitro oxaliplatin, when compared with control; q(28) = 2.4, #adjusted P = 0.04 for LPS-RS Ultrapure, when compared with in vitro oxaliplatin]. (E) Effect of LPS-RS Ultrapure administered in vitro on AP threshold in neurons derived from animals treated with oxaliplatin in vivo (in vitro reversal after in vivo induction of nociceptor sensitization). Incubation of such neurons with LPS-RS Ultrapure (10 μg/ml for 24 h) produced a significant shift in the AP threshold back towards the control value when compared with the significantly more negative AP threshold observed in neurons from oxaliplatin-treated animals [one-way ANOVA: F(2,25) = 9.5, P = 0.0009; Dunnett's post hoc test: q(25) = 3.7, **adjusted P = 0.0019 for in vivo oxaliplatin, when compared with control; q(25) = 3.9, ##adjusted P = 0.0014 for LPS-RS Ultrapure, when compared with in vivo oxaliplatin]. Number of cells: B, C, D and E share the same data set for the control group (left bars), n = 14; B and E share the same data set for the in vivo oxaliplatin group (middle bars), n = 8; C and D share the same dataset for the in vitro oxaliplatin group (middle bars), n = 9. Number of cells in prevention or reversal groups (right bars): n = 7 in B, n = 9 in C, n = 8 in D, n = 6 in E. LPS-RS Ultrapure = lipopolysaccharide from Rhodobacter sphaeroides.
To determine if oxaliplatin-induced hyperalgesia is associated with long-lasting nociceptor sensitization, we compared the AP threshold in neurons from naïve animals (control group) to neurons derived from animals 3 weeks after in vivo oxaliplatin administration, at which time robust mechanical hyperalgesia was still present (Fig. 2A). In neurons from oxaliplatin-treated rats, the AP threshold was significantly lower than in neurons from the control group of rats [Fig. 5B; F(2,26) = 6.3; t(26) = 3.6, adjusted P = 0.003], revealing enhanced electrical excitability, an indication of nociceptor sensitization. The presence of sensitization in cultured neurons isolated from their complex cellular in vivo environment supports the suggestion that in vivo exposure of nociceptors to oxaliplatin produces long-lasting neuroplasticity. Submitting animals to the same prevention protocol used for behavioural experiments, pretreatment with TLR4 AS-ODN before administering oxaliplatin, which prevents oxaliplatin-induced hyperalgesia in vivo (Fig. 2A), and then preparing neuronal cultures at the same 3 week time point, revealed a significantly higher AP threshold compared with the oxaliplatin-treated group [Fig. 5B; t(26) = 2.1, adjusted P = 0.04]. This finding supports the suggestion that transient attenuation of TLR4 in vivo attenuates oxaliplatin-induced nociceptor sensitization, thus implicating a role of TLR4 signalling in the induction of such nociceptor sensitization.
Although in vivo administration of oxaliplatin induces nociceptor sensitization, this does not rule out the possibility that indirect signalling to the nociceptor (e.g. by neuroimmune interactions) is responsible for its sensitization. Therefore, we next examined if exposure to oxaliplatin in vitro could directly sensitize nociceptors, reducing the AP threshold, and whether this effect was attenuated by an in vivo prevention protocol. Indeed, oxaliplatin (50 μM, preincubation for 3 h) induced a significant reduction in the AP threshold compared with the control group [Fig. 5C; F(2,29) = 10.5, P = 0.0004; q(29) = 4.1, adjusted P = 0.0005], while in a group of nociceptors that received TLR4 AS-ODN in vivo and then in vitro oxaliplatin, the AP threshold remained significantly higher [q(29) = 3.9, adjusted P = 0.001], similar to the value in control nociceptors. These findings support the suggestion that direct action of oxaliplatin on nociceptors, which is TLR4 dependent, contributes to oxaliplatin-induced nociceptor sensitization.
To test the hypothesis that nociceptor TLR4 is involved in the sensitizing effect of oxaliplatin, we modelled prevention in vitro by incubating cultured neurons with a selective TLR4 antagonist, LPS-RS Ultrapure (10 μg/ml), for 24 h before exposure to oxaliplatin in vitro. In the TLR4 antagonist-treated group of nociceptors, the AP threshold was significantly higher compared with the oxaliplatin-treated group of nociceptors [Fig. 5D; F(2,28) = 7.0, P = 0.004; q(28) = 2.4, adjusted P = 0.04]. This finding supports the suggestion that nociceptor TLR4 is involved in the sensitization induced by oxaliplatin.
Finally, to examine if nociceptor TLR4 is involved in the maintenance of oxaliplatin-induced nociceptor sensitization, we modelled in vivo reversal, in vitro, by incubating cultured neurons, derived from animals that received oxaliplatin in vivo 3 weeks prior, with LPS-RS Ultrapure (10 μg/ml) for 24 h before measuring the AP threshold in vitro. In this ‘reversal’ group of nociceptors, the AP threshold was also significantly higher compared with an oxaliplatin-treated group [Fig. 5E; F(2,25) = 9.5, P = 0.0009; q(25) = 3.9, adjusted P = 0.0014]. This finding supports the suggestion that nociceptor TLR4 plays an important role in the long-lasting oxaliplatin-induced nociceptor neuroplasticity.
We also evaluated the resting membrane potential and Rheobase of cultured DRG neurons with and without oxaliplatin (Supplementary Fig. 5). Additionally, we assessed several other electrophysiological parameters of the examined neurons, including input resistance at the resting membrane potential, input resistance at baseline, series resistance, AP overshoot, AP amplitude from baseline, AP amplitude from AP threshold, baseline membrane potential and the holding current required to achieve the baseline membrane potential (Supplementary Table 1). No statistically significant differences were observed between the groups in any of these parameters.
Taken together, our in vitro findings support the hypothesis that nociceptor sensitization, and, in turn, hyperalgesia induced by oxaliplatin are mediated by a direct action of oxaliplatin on small-diameter DRG neurons. The accompanying long-lasting nociceptor neuroplasticity and its maintenance are TLR4 dependent.
AS-ODN for RAGE prevents and reverses bortezomib as well as oxaliplatin and paclitaxel CIPN
TLR4 and RAGE both act as PRRs for DAMPs, molecules produced in response to cell stress and injury.74 Since TLR4 antisense only transiently reversed bortezomib-induced CIPN (Fig. 2F), we evaluated whether the transient attenuation of another PRR found in DRG cells, RAGE, also plays a role in CIPN. Male rats received RAGE AS-ODN or MM-ODN (120 μg/20 μl, i.t.) for 4 days consecutively, and then three more doses were administered, one every other day (a total of seven doses). Approximately 20 h after the third administration of ODN, oxaliplatin (2 mg/kg, i.v., on Day 0), paclitaxel (1 mg/kg, i.p., on Days 0, 2, 4 and 6) or bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6) was administered, and the mechanical nociceptive threshold evaluated from Days −3 (before the first ODN injection) to 28. RAGE AS-ODN prevented hyperalgesia induced by oxaliplatin, paclitaxel and bortezomib (Fig. 6A–C, respectively). These data indicate that RAGE in DRG cells, like TLR4, is essential for the induction of CIPN triggered by diverse chemotherapy drugs.
Figure 6.
RAGE antisense prevents and reverses oxaliplatin, paclitaxel and bortezomib CIPN. Top: Separate groups of male rats were treated intrathecally (i.t.) with antisense (AS)- or sense (SE)-oligodeoxynucleotides (ODN) (both 120 μg in 20 μl/day) against RAGE mRNA, once a day, starting 3 days before the chemotherapy agents, for seven doses, over 10 days. Rats received: (A) oxaliplatin [2 mg/kg, intravenously (i.v.), on Day 0]; (B) paclitaxel [1 mg/kg, intraperitoneally (i.p.), on Days 0, 2, 4 and 6]; or (C) bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6). The mechanical nociceptive threshold was evaluated before the first intrathecal administration of ODN (Day −3) and then from Days 0 to 28. In the RAGE AS-ODN-treated groups, CIPN hyperalgesia was markedly inhibited in the oxaliplatin-, paclitaxel- and bortezomib-treated rats (A, B and C, respectively), and this attenuation was undiminished for the 28-day testing period. Statistical analyses for (A) oxaliplatin: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(5,50) = 6.772, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 637.3, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (RAGE SE-ODN versus AS-ODN); (B) paclitaxel: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(7,70) = 21.52, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 151.6, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ***P = 0.0002, ****P < 0.0001, aaaP = 0.0006, cccP = 0.0004 (RAGE SE-ODN versus AS-ODN); and (C) bortezomib: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(6,60) = 19.11, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 238.3, P < 0.0001; Bonferroni's multiple post hoc comparisons test, **P = 0.0071, ****P < 0.0001, ***P = 0.0002, aaaP = 0.0007, cccP = 0.0006 (RAGE SE-ODN versus AS-ODN). Bottom: Separate groups of male rats received: (D) oxaliplatin (2 mg/kg, i.v., on Day 0); (E) paclitaxel (1 mg/kg, i.p., on Days 0, 2, 4 and 6); or (F) bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6). Each group was then treated intrathecally with an AS-ODN or SE-ODN (both 120 μg in 20 μl/day) against RAGE mRNA once a day starting 3 days after the last dose of chemotherapy drug, for seven doses, over 10 days. The mechanical nociceptive threshold was evaluated from Days 0 to 28. In the RAGE AS-ODN-treated groups, CIPN hyperalgesia was markedly attenuated in oxaliplatin-, paclitaxel- and bortezomib-treated rats, and this attenuation was undiminished for the 28-day testing period. Statistical analyses for (D) oxaliplatin: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(4,40) = 94.86, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 862.4, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (RAGE SE-ODN versus AS-ODN); (E) paclitaxel: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(4,40) = 91.51, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 69.15, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (RAGE SE-ODN versus AS-ODN); and (F) bortezomib: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(6,60) = 78.44, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 246.5, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (RAGE SE-ODN versus AS-ODN). n = 6 paws for each group. All data are mean ± SEM. CIPN = chemotherapy-induced peripheral neuropathy.
We also evaluated whether RAGE antisense can reverse CIPN induced by oxaliplatin, paclitaxel and bortezomib. Male rats received oxaliplatin (2 mg/kg, i.v., on Day 0), paclitaxel (1 mg/kg, i.p., on Days 0, 2, 4 and 6) or bortezomib (0.2 mg/kg, i.v., on Days 0, 2, 4 and 6), and the mechanical nociceptive threshold was evaluated from Days 0 to 28. RAGE AS-ODN or SE-ODN was administered intrathecally (120 μg/20 μl) for 4 days consecutively, and then three more doses were administered, one every other day (a total of seven doses). Intrathecal treatment with ODNs started 3 days after administration of oxaliplatin and 3 days after the fourth administration of paclitaxel and bortezomib. In RAGE AS-ODN-treated rats, neither oxaliplatin (Fig. 6D), paclitaxel (Fig. 6E) nor bortezomib (Fig. 6F) were able to develop hyperalgesia at any time point evaluated, when compared with their respective RAGE SE-ODN-treated groups. The reversal of oxaliplatin, paclitaxel and bortezomib CIPN by RAGE AS-ODN persisted at time points when their levels in DRG would have been expected to have recovered, supporting the suggestion that RAGE-dependent activity in DRG is necessary for the maintenance of CIPN pain.
To determine if bortezomib-induced hyperalgesia is associated with long-lasting nociceptor sensitization, we compared the AP threshold in neurons from naïve animals (control group) to that in neurons derived from animals 3 weeks after in vivo bortezomib administration, at which time robust mechanical hyperalgesia was still present (Fig. 2C). In neurons from bortezomib-treated rats, the AP threshold was significantly lower, compared with neurons from a control group of rats [Supplementary Fig. 6; F(2,28) = 4.4; t(28) = 2.5, adjusted P = 0.02], revealing enhanced electrical excitability, an indication of nociceptor sensitization. Submitting animals to the same reversal protocol used for behavioural experiments, treatment with RAGE AS-ODN 3 days after the last administration of bortezomib, which reverses bortezomib-induced hyperalgesia in vivo (Fig. 6C), and then preparing neuronal cultures at the same 3 week time point, revealed a significantly higher AP threshold compared with the bortezomib-treated group [Supplementary Fig. 6; t(28) = 2.7, adjusted P = 0.02]. This finding supports the suggestion that transient attenuation of RAGE in vivo attenuates bortezomib-induced nociceptor sensitization, thus implicating a role of RAGE signalling in the reversal of such nociceptor sensitization.
Attenuating TLR4 and RAGE reverses CIPN induced by oxaliplatin in female rats
Given that studies have reported sexual dimorphism in chronic pain mechanisms,50,75-78 including for CIPN,55-57,79 we evaluated whether TLR4 (Fig. 7A) and RAGE (Fig. 7B) AS-ODN also reversed oxaliplatin-induced CIPN in female rats. Oxaliplatin was injected intravenously (2 mg/kg) on Day 0, and the mechanical nociceptive threshold was measured 30 min and 24 h after its administration. All female rats developed hyperalgesia (Fig. 7A and B). On the fourth day after administration of oxaliplatin, AS-ODN or MM/SE-ODN (120 μg/20 μl) for TLR4 or RAGE were administered intrathecally for 4 days consecutively, and then three more doses were administered, one every other day (a total of seven doses). In the female TLR4 (Fig. 7B) and RAGE (Fig. 7B) AS-ODN-treated groups, oxaliplatin-induced hyperalgesia was completely reversed, an effect that persisted even at time points when TLR4 and RAGE levels in DRG had recovered. These data support the suggestion that CIPN dependence on TLR4 and RAGE is not sexually dimorphic.
Figure 7.
TLR4 and RAGE antisense reverses oxaliplatin CIPN in female rats. Female rats received oxaliplatin [2 mg/kg, intravenously (i.v.)] on Day 0, and 4 days later, they were treated with intrathecal antisense-oligodeoxynucleotides (AS-ODN) or mismatch (MM)/sense (SE)-ODN (both 120 μg in 20 μl/day) against (A) TLR4 or (B) RAGE mRNA, once a day for 4 days consecutively, and then three more doses, one every other day, for a total of seven doses, over 10 days. The mechanical nociceptive threshold was evaluated from Days 0 to 28. Oxaliplatin-induced hyperalgesia was markedly reversed in the (A) TLR4 and (B) RAGE AS-ODN-treated groups of female rats, and this reversal was undiminished over the 28-day testing period. Statistical analyses for A: two-way repeated-measures ANOVA, Time × TLR4 AS-ODN interaction, F(5,50) = 64.68, P < 0.0001; TLR4 AS-ODN treatment, F(1,10) = 95.41, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (MM-ODN versus AS-ODN); and B: two-way repeated-measures ANOVA, Time × RAGE AS-ODN interaction, F(5,50) = 77.11, P < 0.0001; RAGE AS-ODN treatment, F(1,10) = 368.2, P < 0.0001; Bonferroni's multiple post hoc comparisons test, ****P < 0.0001 (SE-ODN versus AS-ODN). n = 6 paws for each group. All data are mean ± SEM. CIPN = chemotherapy-induced peripheral neuropathy.
Discussion
While small-fibre, painful peripheral neuropathy is a common, debilitating side-effect of diverse classes of cancer chemotherapy drugs,8,13,16,80,81 including taxanes,44,82 vinca alkaloids,83-85 platinum-based compounds,86-89 proteasome inhibitors (e.g. bortezomib),90-92 immunomodulators (e.g. thalidomide)93,94 and epothilones (e.g. ixabepilone),95,96 there are still no FDA approved treatments, and only duloxetine is recommended by the American Society of Clinical Oncology (ASCO) to treat CIPN pain. Although the primary cellular targets of these diverse classes of neurotoxic chemotherapy drugs, in tumour cells, differ markedly (e.g. while taxanes hyper-stabilize microtubules, platinum-based chemotherapies form DNA adducts and bortezomib is a potent selective proteasome inhibitor), the vast majority of chemotherapy drugs produce a phenotypically similar, distal, symmetric, small-fibre, painful, sensory neuropathy, which is, where studied, responsive to serotonin-noradrenaline reuptake inhibitors (SNRIs).8,10-14,97-112 In the present study, we first demonstrated a symmetric, time-dependent decrease in the mechanical nociceptive threshold in the hind paws of rats during the onset and maintenance phases of oxaliplatin-, paclitaxel- and bortezomib-induced CIPN.
While most classes of chemotherapy drugs are neurotoxic, damaging DRG neurons and peripheral nerves,113-115 how they produce clinically similar painful CIPN remains a critically important question. Moreover, even though the primary mechanisms by which diverse classes of chemotherapy drugs injure neurons differ, animals treated with chemotherapeutics as diverse as paclitaxel, oxaliplatin and vincristine exhibit remarkably similar painful peripheral neuropathies.113-117 This has led to the suggestion that the neuropathies produced by diverse chemotherapy drugs share underlying mechanisms, which may be different from those mediating their antineoplastic effects.114 While a substantial literature developed over the past two decades provides compelling evidence for a contribution of neuroimmune mechanisms in CIPN induced by diverse chemotherapy drugs,35,43,114,118-125 we tested the hypothesis that there is an important contribution of PRRs, TLR4 and RAGE, in nociceptors (and non-neuronal cells in the DRG) that have been stressed or injured by exposure to representatives of three different classes of chemotherapy drugs: platinum-based, taxanes and proteasome inhibitors.
Importantly, sensory neurons share with cells of the immune system the presence of PRRs and the ability to detect cell generated danger signals, DAMPs, a large family of molecules generated by diverse cell types13,114,118-121,123,125,126 in response to cell stress and injury,17,19-21,23,127 that, in turn, act as ligands at PRRs, such as TLR4 and RAGE, through which they can further exacerbate cellular stress and injury. In rats treated with AS-ODN to TLR4 or RAGE mRNA, CIPN produced by oxaliplatin, paclitaxel and bortezomib was prevented, even at time points when the effect of AS-ODN on PRR levels was no longer present. Thus, while we demonstrated, by western blot, a reduction of TLR4 expression in DRG from rats treated for 4 days consecutively with TLR4 AS-ODN and had DRG collected 24 h after the last intrathecal administration of TLR4 AS-ODN, when the DRGs were collected 9 days after the last TLR4 AS-ODN administration, no decrease in anti-TLR4 immunoreactivity between the TLR4 AS- and MM-ODN-treated groups was detected. That is, TLR4 protein levels in DRG neurons had returned to baseline when measured 9 days after the last TLR4 AS-ODN administration, in accordance with previous studies demonstrating that at 7 days after intrathecal AS-ODN cessation, the target protein levels in DRG neurons had returned to pre-AS-ODN levels.128,129 These data support the suggestion that, following knockdown of TLR4 in DRG cells, chemotherapy drugs are not capable of activating the signalling pathways responsible for the development of CIPN; also, this inhibition seems to be permanent, indicating that even when the level of TLR4 in DRG neurons returns to baseline, oxaliplatin does not induce CIPN. We have previously shown a contribution of hyperalgesic priming mechanisms to CIPN;130 interventions that reverse hyperalgesic priming also markedly attenuate CIPN.130 Interventions that permanently reverse priming also permanently reverse CIPN.130 Importantly, we have recently shown a role of TLR4 in hyperalgesic priming;48 transient attenuation of TLR4 also permanently reverses priming.48 How transient attenuation of priming mechanisms, or TLR4 and CIPN, permanently reverses priming is an active area of investigation.
We also demonstrated that while oxaliplatin and paclitaxel CIPN were reversed by treatment with TLR4 AS-ODN, in the bortezomib-treated group TLR4 AS-ODN only transiently attenuated hyperalgesia, by roughly the duration of action of AS-ODN on nociceptors (7 days after the last TLR4 AS-ODN administration, bortezomib-induced hyperalgesia had returned to the control group level). Why CIPN induced by bortezomib, but not oxaliplatin or paclitaxel, recovers post TLR4 AS-ODN treatment remains to be explained. To test the hypothesis that for persistence of bortezomib CIPN, other PRRs may be more important than TLR4, we tested the ability of AS-ODN for RAGE to reverse CIPN. While both TLR4 and RAGE are found in nociceptors32-37 and have substantial crosstalk,131-133 they have differential regulation of expression and independent signalling mechanisms and effects on cell function.134-138 For example, siRNA depletion of RAGE, but not TLR4, suppressed HMGB1 (high mobility group box 1)-induced p38 MAPK (mitogen-activated protein kinase) activation139; and RAGE, but not TLR4, signal via CaMKK-β (calmodulin-dependent protein kinase kinase β) and ERK1/2 (extracellular signal-regulated kinase 1/2),140 second messengers known to be involved in nociceptor neuroplasticity.141-143 Furthermore, bortezomib increases the expression of RAGE,144,145 activating STAT3 (signal transducer and activator of transcription 3),144,145 which has been implicated in pain mechanisms.146,147 How these diverse mechanisms could contribute to the differential role of TLR4 and RAGE in bortezomib, but not oxaliplatin and paclitaxel CIPN, remains to be established.
Nociceptors have two terminal fields: peripheral, involved in sensory transduction; and central, involved in neurotransmission. Thus, our finding that intrathecal, but not intradermal TLR4 antagonist markedly attenuated oxaliplatin-, paclitaxel- and bortezomib-induced hyperalgesia provides support for the novel suggestion that increased neurotransmission at the central terminal, rather than enhanced sensory transduction at the peripheral terminal, is enhanced in CIPN. The fact that TLR4 AS-ODN permanently reversed CIPN while a TLR4 antagonist, LPS-RS Ultrapure, only transiently attenuated CIPN indicates that there may be crosstalk between TLR4 and other molecules in nociceptors that play a critical role in maintenance of CIPN. In another study, where rats received paclitaxel (2 mg/kg, i.p.) every other day for a total of four injections, and were treated every 12 h with intrathecal LPS-RS Ultrapure (20 μg/20 μl) or PBS (vehicle), beginning 2 days before the first dose and continuing to 2 days after the last dose of paclitaxel or vehicle,35 paclitaxel-LPS-RS Ultrapure-treated rats showed an attenuated development of mechanical hypersensitivity.35 Our results and similar ones by others raise several interesting questions that will form the basis for follow-up investigations. For example, the results with the TLR4 antagonist suggest a tonic stimulation at TLR4. One candidate is reactive oxygen species, which have been implicated in the pathophysiology of CIPN148,149 and which are TLR4 agonists.150 Chemotherapeutics elicit the release of other DAMPs, including HMGB1,151 which stimulate TLR4 in neurons151 and promote neuropathic pain.152
Since TLR4 plays an important role in the ‘induction’ and ‘maintenance’ of CIPN in vivo, we next directly evaluated the cellular effects of a chemotherapy drug, oxaliplatin, on nociceptor excitability using in vitro patch-clamp electrophysiology. Consistent with our in vivo findings, we observed a significantly lower AP threshold in small-diameter DRG neurons cultured from rats treated in vivo with oxaliplatin, when compared with the naïve (control) group, confirming that oxaliplatin-induced sensitization can be observed in vitro. We next demonstrated, in DRG neurons cultured from rats that received TLR4 AS-ODN followed by oxaliplatin, both in vivo, an AP threshold not significantly different from that in nociceptors from the naïve group, indicating that treatment with TLR4 AS-ODN in vivo was able to prevent the reduction in AP threshold in cultured DRG neurons induced by oxaliplatin in vivo. The AP threshold was also reduced when oxaliplatin was applied in vitro to DRG neurons cultured from naïve control rats. This reduction in AP threshold induced by in vitro oxaliplatin was prevented both in vivo by treatment with TLR4 AS-ODN and in vitro by a specific TLR4 antagonist (LPS-RS Ultrapure). Using a reversal protocol, in vivo oxaliplatin-induced reduction in the AP threshold in DRG neurons was reversed by the in vitro application of a selective TLR4 antagonist. The induction of membrane depolarization and reduction in rheobase induced by in vitro oxaliplatin have previously been reported.153,154 Using nociceptors harvested from rats treated in vivo with TLR4 AS-ODN, our data further support the suggestion that PRR signalling in nociceptors, which maintains CIPN pain in vivo, also maintains oxaliplatin-induced nociceptor sensitization in vitro.
Since several studies have reported sexually dimorphic mechanisms in CIPN pain,55-57,79 we evaluated if oxaliplatin CIPN in female rats is also TLR4 and/or RAGE dependent. As observed in male rats, oxaliplatin-induced hyperalgesia was reversed by TLR4 and RAGE AS-ODN in females, indicating that at this point, the signalling pathway activated by oxaliplatin, downstream of TLR4 and RAGE, is not sexually dimorphic.
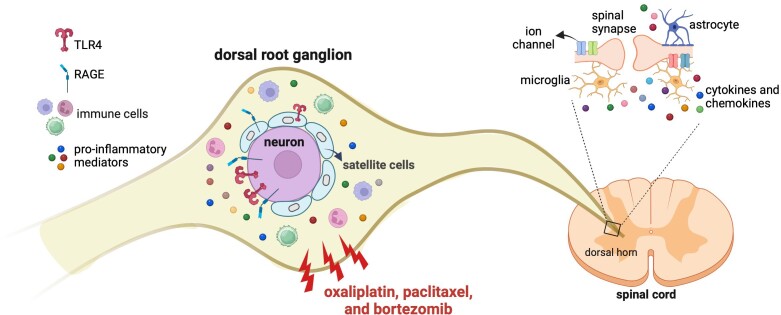
CIPN pain, induced by diverse chemotherapy drugs, involves neuroimmune mechanisms, interactions between cells of the immune system and nociceptive sensory neurons.15,16 Recent studies have highlighted the role of immune mechanisms and the release of pro-inflammatory mediators in CIPN pain, while neurons and satellite cells may contribute to the maintenance of the neuroinflammatory process in the DRG.120 Neuroinflammation has also been observed in the spinal cord, with microglia and astrocytes playing significant roles in CIPN development.155-157 However, since the intrathecal administration of TLR4 and RAGE antisense may affect other cell types in the DRG and spinal cord, as well as different subtypes of sensory neurons, it cannot be concluded that the knockdown of PRRs exclusively in nociceptors is the sole factor responsible for the prevention and reversal of CIPN. A summary of our current findings, as well as those from other studies, is provided in Fig. 8.
Figure 8.
Schematic summary of the effects of chemotherapeutic agents on pattern recognition receptors in dorsal root ganglia. Oxaliplatin, paclitaxel and bortezomib, administered systemically, act on pattern recognition receptors (PRRs; TLR4 and RAGE), present on cells in dorsal root ganglia (DRG) to produce chemotherapy-induced peripheral neuropathy (CIPN) hyperalgesia. Once TLR4 and RAGE are knocked down, in DRG cells, chemotherapeutic drugs are not able to produce CIPN hyperalgesia. It has previously been demonstrated that immune cells infiltrate DRG after administration of chemotherapeutic drugs158-160 and release potent pro-inflammatory mediators such as tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), nerve growth factor (NGF), nitric oxide (NO) and prostanoids.161 Additionally, chemotherapeutic drugs activate microglia in the spinal dorsal horn,157 releasing chemokines and cytokines, which can activate astrocytes.155,156 Chemotherapy also alters the expression and function of ion channels in the spinal dorsal horn.155,162 The schematic summary was designed using BioRender.com.
In conclusion, our in vivo and in vitro data support the hypothesis that PRRs in DRG cells, injured by exposure to diverse classes of chemotherapy drugs, contribute to the maintenance as well as the induction of CIPN pain. Our study provides evidence that the transient attenuation of TLR4 and RAGE may be useful for treating CIPN pain.
Supplementary Material
Acknowledgements
The authors thank Niloufar Mansooralavi for excellent technical assistance.
Contributor Information
Dionéia Araldi, Department of Oral and Maxillofacial Surgery, UCSF Pain and Addiction Research Center, University of California at San Francisco, San Francisco, CA 94143, USA.
Eugen V Khomula, Department of Oral and Maxillofacial Surgery, UCSF Pain and Addiction Research Center, University of California at San Francisco, San Francisco, CA 94143, USA.
Ivan J M Bonet, Department of Oral and Maxillofacial Surgery, UCSF Pain and Addiction Research Center, University of California at San Francisco, San Francisco, CA 94143, USA.
Oliver Bogen, Department of Oral and Maxillofacial Surgery, UCSF Pain and Addiction Research Center, University of California at San Francisco, San Francisco, CA 94143, USA.
Paul G Green, Department of Oral and Maxillofacial Surgery, UCSF Pain and Addiction Research Center, University of California at San Francisco, San Francisco, CA 94143, USA; Department of Preventative and Restorative Dental Sciences, Division of Neuroscience, University of California at San Francisco, San Francisco, CA 94143, USA.
Jon D Levine, Department of Oral and Maxillofacial Surgery, UCSF Pain and Addiction Research Center, University of California at San Francisco, San Francisco, CA 94143, USA; Department of Medicine, Division of Neuroscience, University of California at San Francisco, San Francisco, CA 94143, USA.
Data availability
All the data generated during the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by National Institutes of Health, National Cancer Institute Grant CA250017.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Maihofner C, Diel I, Tesch H, Quandel T, Baron R. Chemotherapy-induced peripheral neuropathy (CIPN): Current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer. 2021;29:4223–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miaskowski C, Mastick J, Paul SM, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. 2017;54:204–218.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salat K. Chemotherapy-induced peripheral neuropathy: Part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep. 2020;72:486–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Souza RS, Her YF, Jin MY, Morsi M, Abd-Elsayed A. Neuromodulation therapy for chemotherapy-induced peripheral neuropathy: A systematic review. Biomedicines. 2022;10:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolb NA, Smith AG, Singleton JR, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016;73:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer. 2014;22:2261–2269. [DOI] [PubMed] [Google Scholar]
- 7. Beijers A, Mols F, Dercksen W, Driessen C, Vreugdenhil G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. 2014;12:401–406. [DOI] [PubMed] [Google Scholar]
- 8. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. [DOI] [PubMed] [Google Scholar]
- 9. Briani C, Argyriou AA, Izquierdo C, et al. Long-term course of oxaliplatin-induced polyneuropathy: A prospective 2-year follow-up study. J Peripher Nerv Syst. 2014;19:299–306. [DOI] [PubMed] [Google Scholar]
- 10. Colvin LA. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain. 2019;160(Suppl 1):S1–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derman BA, Davis AM. Recommendations for prevention and management of chemotherapy-induced peripheral neuropathy. JAMA. 2021;326:1058–1059. [DOI] [PubMed] [Google Scholar]
- 12. Kachrani R, Santana A, Rogala B, Pawasauskas J. Chemotherapy-induced peripheral neuropathy: Causative agents, preventative strategies, and treatment approaches. J Pain Palliat Care Pharmacother. 2020;34:141–152. [DOI] [PubMed] [Google Scholar]
- 13. Selvy M, Kerckhove N, Pereira B, et al. Prevalence of chemotherapy-induced peripheral neuropathy in multiple myeloma patients and its impact on quality of life: A single center cross-sectional study. Front Pharmacol. 2021;12:637593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017;81:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46:927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene. 2016;35:5931–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Land WG. Emerging role of innate immunity in organ transplantation part II: Potential of damage-associated molecular patterns to generate immunostimulatory dendritic cells. Transplant Rev (Orlando). 2012;26:73–87. [DOI] [PubMed] [Google Scholar]
- 20. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and Damage-Associated Molecular Patterns (DAMPs). Am J Transplant. 2016;16:3338–3361. [DOI] [PubMed] [Google Scholar]
- 21. Rubartelli A, Lotze MT. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. [DOI] [PubMed] [Google Scholar]
- 22. Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation. 2015;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donnelly CR, Chen O, Ji RR. How do sensory neurons sense danger signals? Trends Neurosci. 2020;43:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goethals S, Ydens E, Timmerman V, Janssens S. Toll-like receptor expression in the peripheral nerve. Glia. 2010;58:1701–1709. [DOI] [PubMed] [Google Scholar]
- 25. Liu T, Xu Z-Z, Park C-K, Berta T, Ji R-R. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. [DOI] [PubMed] [Google Scholar]
- 27. Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: A mechanism for pain due to infection. J Dent Res. 2006;85:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Z-Z, Kim YH, Bang S, et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med. 2015;21:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeisel A, Hochgerner H, Lonnerberg P, et al. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng Y, Liu P, Bai L, Trimmer JS, Bean BP, Ginty DD. Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron. 2019;103:598–616.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou J, Chen X, Gilvary DL, et al. HMGB1 induction of clustering creates a chemoresistant niche in human prostate tumor cells. Sci Rep. 2015;5:15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allette YM, Due MR, Wilson SM, et al. Identification of a functional interaction of HMGB1 with receptor for advanced glycation end-products in a model of neuropathic pain. Brain Behav Immun. 2014;42:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bestall SM, Hulse RP, Blackley Z, et al. Sensory neuronal sensitisation occurs through HMGB-1-RAGE and TRPV1 in high-glucose conditions. J Cell Sci. 2018;131:jcs215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kato J, Agalave NM, Svensson CI. Pattern recognition receptors in chronic pain: Mechanisms and therapeutic implications. Eur J Pharmacol. 2016;788:261–273. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saleh A, Smith DR, Tessler L, et al. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol. 2013;249:149–159. [DOI] [PubMed] [Google Scholar]
- 37. Vincent AM, Perrone L, Sullivan KA, et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148:548–558. [DOI] [PubMed] [Google Scholar]
- 38. Ustinova EE, Shurin GV, Gutkin DW, Shurin MR. The role of TLR4 in the paclitaxel effects on neuronal growth in vitro. PLoS One. 2013;8:e56886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsubota M, Fukuda R, Hayashi Y, et al. Role of non-macrophage cell-derived HMGB1 in oxaliplatin-induced peripheral neuropathy and its prevention by the thrombin/thrombomodulin system in rodents: Negative impact of anticoagulants. J Neuroinflammation. 2019;16:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: A prospective study in 170 patients with colorectal cancer. Cancer. 2013;119:438–444. [DOI] [PubMed] [Google Scholar]
- 41. Sakurai M, Egashira N, Kawashiri T, Yano T, Ikesue H, Oishi R. Oxaliplatin-induced neuropathy in the rat: Involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain. 2009;147:165–174. [DOI] [PubMed] [Google Scholar]
- 42. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sekiguchi F, Domoto R, Nakashima K, et al. Paclitaxel-induced HMGB1 release from macrophages and its implication for peripheral neuropathy in mice: Evidence for a neuroimmune crosstalk. Neuropharmacology. 2018;141:201–213. [DOI] [PubMed] [Google Scholar]
- 44. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meregalli C, Canta A, Carozzi VA, et al. Bortezomib-induced painful neuropathy in rats: A behavioral, neurophysiological and pathological study in rats. Eur J Pain. 2010;14:343–350. [DOI] [PubMed] [Google Scholar]
- 46. Tsubota M, Miyazaki T, Ikeda Y, et al. Caspase-dependent HMGB1 release from macrophages participates in peripheral neuropathy caused by bortezomib, a proteasome-inhibiting chemotherapeutic agent, in mice. Cells. 2021;10:2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamamoto S, Egashira N. Pathological mechanisms of bortezomib-induced peripheral neuropathy. Int J Mol Sci. 2021;22:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Araldi D, Bogen O, Green PG, Levine JD. Role of nociceptor toll-like receptor 4 (TLR4) in opioid-induced hyperalgesia and hyperalgesic priming. J Neurosci. 2019;39:6414–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Araldi D, Ferrari LF, Levine JD. Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci. 2015;35:12502–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Araldi D, Ferrari LF, Levine JD. Hyperalgesic priming (type II) induced by repeated opioid exposure: Maintenance mechanisms. Pain. 2017;158:1204–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Araldi D, Ferrari LF, Levine JD. Mu-opioid receptor (MOR) biased agonists induce biphasic dose-dependent hyperalgesia and analgesia, and hyperalgesic priming in the rat. Neuroscience. 2018;394:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Araldi D, Khomula EV, Ferrari LF, Levine JD. Fentanyl induces rapid onset hyperalgesic priming: Type I at peripheral and type II at central nociceptor terminals. J Neurosci. 2018;38:2226–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. [DOI] [PubMed] [Google Scholar]
- 54. Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989;492:397–399. [DOI] [PubMed] [Google Scholar]
- 55. Staurengo-Ferrari L, Bonet IJM, Araldi D, Green PG, Levine JD. Neuroendocrine stress axis-dependence of duloxetine analgesia (anti-hyperalgesia) in chemotherapy-induced peripheral neuropathy. J Neurosci. 2022;42:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Staurengo-Ferrari L, Green PG, Araldi D, Ferrari LF, Miaskowski C, Levine JD. Sexual dimorphism in the contribution of neuroendocrine stress axes to oxaliplatin-induced painful peripheral neuropathy. Pain. 2021;162:907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferrari LF, Araldi D, Green PG, Levine JD. Marked sexual dimorphism in neuroendocrine mechanisms for the exacerbation of paclitaxel-induced painful peripheral neuropathy by stress. Pain. 2020;161:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hemeryck A, Geerts R, Monbaliu J, et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14 C-bortezomib. Cancer Chemother Pharmacol. 2007;60:777–787. [DOI] [PubMed] [Google Scholar]
- 59. Meregalli C, Maricich Y, Cavaletti G, et al. Reversal of bortezomib-induced neurotoxicity by suvecaltamide, a selective T-type Ca-channel modulator, in preclinical models. Cancers (Basel). 2021;13:5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu H-L, Vetri F, Lee H-K, et al. Estrogen replacement therapy in diabetic ovariectomized female rats potentiates postischemic leukocyte adhesion in cerebral venules via a RAGE-related process. Am J Physiol Heart Circ Physiol. 2009;297:H2059–H2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alvarez P, Bogen O, Green PG, Levine JD. Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. Pain. 2017;158:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. [DOI] [PubMed] [Google Scholar]
- 63. Khomula EV, Araldi D, Bonet IJM, Levine JD. Opioid-Induced hyperalgesic priming in single nociceptors. J Neurosci. 2021;41:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bonet IJM, Khomula EV, Araldi D, Green PG, Levine JD. PI3Kγ/AKT signaling in high molecular weight hyaluronan (HMWH)-induced anti-hyperalgesia and reversal of nociceptor sensitization. J Neurosci. 2021;41:8414–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. [DOI] [PubMed] [Google Scholar]
- 66. Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neuroscience. 2002;115:15–30. [DOI] [PubMed] [Google Scholar]
- 68. Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: Histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000;84:2365–2379. [DOI] [PubMed] [Google Scholar]
- 69. Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. [DOI] [PubMed] [Google Scholar]
- 70. Ferrari LF, Khomula EV, Araldi D, Levine JD. CD44 signaling mediates high molecular weight hyaluronan-induced antihyperalgesia. J Neurosci. 2018;38:308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khomula EV, Araldi D, Levine JD. In vitro nociceptor neuroplasticity associated with in vivo opioid-induced hyperalgesia. J Neurosci. 2019;39:7061–7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duzhyy DE, Viatchenko-Karpinski VY, Khomula EV, Voitenko NV, Belan PV. Upregulation of T-type Ca2+ channels in long-term diabetes determines increased excitability of a specific type of capsaicin-insensitive DRG neurons. Mol Pain. 2015;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Viatchenko-Karpinski V, Gu JG. Mechanical sensitivity and electrophysiological properties of acutely dissociated dorsal root ganglion neurons of rats. Neurosci Lett. 2016;634:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brown AS, Levine JD, Green PG. Sexual dimorphism in the effect of sound stress on neutrophil function. J Neuroimmunol. 2008;205:25–31. [DOI] [PubMed] [Google Scholar]
- 76. Ferrari LF, Khomula EV, Araldi D, Levine JD. Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep. 2016;6:31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Joseph EK, Levine JD. Sexual dimorphism for protein kinase c epsilon signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;119:831–838. [DOI] [PubMed] [Google Scholar]
- 78. Khomula EV, Ferrari LF, Araldi D, Levine JD. Sexual dimorphism in a reciprocal interaction of ryanodine and IP(3) receptors in the induction of hyperalgesic priming. J Neurosci. 2017;37:2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luo X, Huh Y, Bang S, et al. Macrophage toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J Neurosci. 2019;39:6848–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Addington J, Freimer M. Chemotherapy-induced peripheral neuropathy: An update on the current understanding. F1000Res. 2016;5:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jordan B, Jahn F, Sauer S, Jordan K. Prevention and management of chemotherapy-induced polyneuropathy. Breast Care (Basel). 2019;14:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. De Iuliis F, Taglieri L, Salerno G, Lanza R, Scarpa S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit Rev Oncol Hematol. 2015;96:34–45. [DOI] [PubMed] [Google Scholar]
- 83. Casey EB, Jellife AM, Le Quesne PM, Millett YL. Vincristine neuropathy. Clinical and electrophysiological observations. Brain. 1973;96:69–86. [DOI] [PubMed] [Google Scholar]
- 84. Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993;15:23–27. [DOI] [PubMed] [Google Scholar]
- 85. Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000;424:563–576. [PubMed] [Google Scholar]
- 86. Argyriou AA, Zolota V, Kyriakopoulou O, Kalofonos HP. Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. J BUON. 2010;15:435–446. [PubMed] [Google Scholar]
- 87. Arkenau H-K. Capecitabine combined with oxaliplatin (CAPOX) in clinical practice: How significant is peripheral neuropathy? Ann Oncol. 2011;22:239–240. [DOI] [PubMed] [Google Scholar]
- 88. Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Long-term neuropathy after oxaliplatin treatment: Challenging the dictum of reversibility. Oncologist. 2011;16:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tofthagen C, McAllister RD, McMillan SC. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin J Oncol Nurs. 2011;15:182–188. [DOI] [PubMed] [Google Scholar]
- 90. Cavaletti G, Gilardini A, Canta A, et al. Bortezomib-induced peripheral neurotoxicity: A neurophysiological and pathological study in the rat. Exp Neurol. 2007;204:317–325. [DOI] [PubMed] [Google Scholar]
- 91. Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care. 2011;5:1–7. [DOI] [PubMed] [Google Scholar]
- 92. Rampen AJJ, Jongen JLM, van Heuvel I, Scheltens-de Boer M, Sonneveld P, van den Bent MJ. Bortezomib-induced polyneuropathy. Neth J Med. 2013;71:128–133. [PubMed] [Google Scholar]
- 93. Cavaletti G, Beronio A, Reni L, et al. Thalidomide sensory neurotoxicity: A clinical and neurophysiologic study. Neurology. 2004;62:2291–2293. [DOI] [PubMed] [Google Scholar]
- 94. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15:3081–3094. [DOI] [PubMed] [Google Scholar]
- 95. Argyriou AA, Marmiroli P, Cavaletti G, Kalofonos HP. Epothilone-induced peripheral neuropathy: A review of current knowledge. J Pain Symptom Manage. 2011;42:931–940. [DOI] [PubMed] [Google Scholar]
- 96. Vahdat LT, Thomas ES, Roche HH, et al. Ixabepilone-associated peripheral neuropathy: Data from across the phase II and III clinical trials. Support Care Cancer. 2012;20:2661–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38:3325–3348. [DOI] [PubMed] [Google Scholar]
- 98. Rokhsareh S, Haghighi S, Tavakoli-Ardakani M. Evaluating the effects of duloxetine on prophylaxis of oxaliplatin-induced peripheral neuropathy in patients with gastrointestinal cancer: A randomized double-blind placebo controlled clinical trial. J Oncol Pharm Pract. 2023;29:60–65. [DOI] [PubMed] [Google Scholar]
- 99. Yang Y, Zhao B, Gao X, et al. Targeting strategies for oxaliplatin-induced peripheral neuropathy: Clinical syndrome, molecular basis, and drug development. J Exp Clin Cancer Res. 2021;40:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin Oncol. 2021;48:193–207. [DOI] [PubMed] [Google Scholar]
- 101. Anand P, Elsafa E, Privitera R, et al. Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8% patch: From pain relief towards disease modification. J Pain Res. 2019;12:2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Farshchian N, Alavi A, Heydarheydari S, Moradian N. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol. 2018;82:787–793. [DOI] [PubMed] [Google Scholar]
- 103. Gewandter JS, Gibbons CH, Campagnolo M, et al. Clinician-rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology. 2019;93:346–360. [DOI] [PubMed] [Google Scholar]
- 104. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: A pilot randomized trial. Int J Clin Oncol. 2015;20:866–871. [DOI] [PubMed] [Google Scholar]
- 105. Kanbayashi Y, Inagaki M, Ueno H, Hosokawa T. Predictors of the usefulness of duloxetine for chemotherapy-induced peripheral neuropathy. Med Oncol. 2017;34:137. [DOI] [PubMed] [Google Scholar]
- 106. Matsuoka H, Iwase S, Miyaji T, et al. Additive duloxetine for cancer-related neuropathic pain nonresponsive or intolerant to opioid-pregabalin therapy: A randomized controlled trial (JORTC-PAL08). J Pain Symptom Manage. 2019;58:645–653. [DOI] [PubMed] [Google Scholar]
- 107. Ruscher V, Lieber S, Kuhl JS, et al. Long-term small-fiber neuropathy and pain sensitization in survivors of pediatric acute lymphoblastic leukemia after stem cell transplantation. J Cancer Res Clin Oncol. 2020;146:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA. 2013;309:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Song SY, Ko YB, Kim H, et al. Effect of serotonin-norepinephrine reuptake inhibitors for patients with chemotherapy-induced painful peripheral neuropathy: A meta-analysis. Medicine (Baltimore). 2020;99:e18653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tamburin S, Park SB, Alberti P, Demichelis C, Schenone A, Argyriou AA. Taxane and epothilone-induced peripheral neurotoxicity: From pathogenesis to treatment. J Peripher Nerv Syst. 2019;24(Suppl 2):S40–S51. [DOI] [PubMed] [Google Scholar]
- 111. Timmins HC, Li T, Kiernan MC, Horvath LG, Goldstein D, Park SB. Quantification of small fiber neuropathy in chemotherapy-treated patients. J Pain. 2020;21:44–58. [DOI] [PubMed] [Google Scholar]
- 112. Uhelski ML, Li Y, Fonseca MM, Romero-Snadoval EA, Dougherty PM. Role of innate immunity in chemotherapy-induced peripheral neuropathy. Neurosci Lett. 2021;755:135941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang X-M, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Weimer LH. Medication-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2003;3:86–92. [DOI] [PubMed] [Google Scholar]
- 116. Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. [DOI] [PubMed] [Google Scholar]
- 118. Agalave NM, Mody PH, Szabo-Pardi TA, Jeong HS, Burton MD. Neuroimmune consequences of eIF4E phosphorylation on chemotherapy-induced peripheral neuropathy. Front Immunol. 2021;12:642420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bennett GJ. A neuroimmune interaction in painful peripheral neuropathy. Clin J Pain. 2000;16(3 Suppl):S139–S143. [DOI] [PubMed] [Google Scholar]
- 120. Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol. 2020;11:626687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hore ZL, Villa-Hernandez S, Denk F. Probing the peripheral immune response in mouse models of oxaliplatin-induced peripheral neuropathy highlights their limited translatability. Wellcome Open Res. 2021;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Illias AM, Yu K-J, Hwang S-H, et al. Dorsal root ganglion toll-like receptor 4 signaling contributes to oxaliplatin-induced peripheral neuropathy. Pain. 2022;163:923–935. [DOI] [PubMed] [Google Scholar]
- 123. Janes K, Wahlman C, Little JW, et al. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun. 2015;44:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lees JG, Makker PG, Tonkin RS, et al. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer. 2017;73:22–29. [DOI] [PubMed] [Google Scholar]
- 125. Lowy DB, Makker PGS, Moalem-Taylor G. Cutaneous neuroimmune interactions in peripheral neuropathic pain states. Front Immunol. 2021;12:660203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Blitzer BL, Terzakis C, Scott KA. Hydroxyl/bile acid exchange. A new mechanism for the uphill transport of cholate by basolateral liver plasma membrane vesicles. J Biol Chem. 1986;261:12042–12046. [PubMed] [Google Scholar]
- 127. Talbot S, Foster SL, Woolf CJ. Neuroimmunity: Physiology and pathology. Annu Rev Immunol. 2016;34:421–447. [DOI] [PubMed] [Google Scholar]
- 128. Aley KO, Levine JD. Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in rat. J Neurosci. 1997;17:8018–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. [DOI] [PubMed] [Google Scholar]
- 130. Staurengo-Ferrari L, Araldi D, Green PG, Levine JD. Neuroendocrine mechanisms in oxaliplatin-induced hyperalgesic priming. Pain. 2023;164:1375–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Prantner D, Nallar S, Vogel SN. The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways. FASEB J. 2020;34:15659–15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: Relatives, friends or neighbours? Mol Immunol. 2013;56:739–744. [DOI] [PubMed] [Google Scholar]
- 133. Zhong H, Li X, Zhou S, et al. Interplay between RAGE and TLR4 regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J Immunol. 2020;205:767–775. [DOI] [PubMed] [Google Scholar]
- 134. Cohen MJ, Carles M, Brohi K, et al. Early release of soluble receptor for advanced glycation endproducts after severe trauma in humans. J Trauma. 2010;68:1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Medeiros MC, Frasnelli SC, Bastos Ade S, Orrico SR, Rossa C Jr. Modulation of cell proliferation, survival and gene expression by RAGE and TLR signaling in cells of the innate and adaptive immune response: Role of p38 MAPK and NF-KB. J Appl Oral Sci. 2014;22:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. [DOI] [PubMed] [Google Scholar]
- 137. Tafani M, Schito L, Pellegrini L, et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-kappaB. Carcinogenesis. 2011;32:1167–1175. [DOI] [PubMed] [Google Scholar]
- 138. Xia C, Braunstein Z, Toomey AC, Zhong J, Rao X. S100 proteins as an important regulator of macrophage inflammation. Front Immunol. 2017;8:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yang WS, Kim JJ, Lee MJ, Lee EK, Park S-K. Ectodomain shedding of RAGE and TLR4 as a negative feedback regulation in high-mobility group box 1-activated aortic endothelial cells. Cell Physiol Biochem. 2018;51:1632–1644. [DOI] [PubMed] [Google Scholar]
- 140. Paudel YN, Angelopoulou E, Piperi C, Othman I, Aamir K, Shaikh MF. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): From risk factors to therapeutic targeting. Cells. 2020;9:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chakraborty S, Elvezio V, Kaczocha M, Rebecchi M, Puopolo M. Presynaptic inhibition of transient receptor potential vanilloid type 1 (TRPV1) receptors by noradrenaline in nociceptive neurons. J Physiol. 2017;595:2639–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hucho T, Suckow V, Joseph EK, et al. Ca++/CaMKII switches nociceptor-sensitizing stimuli into desensitizing stimuli. J Neurochem. 2012;123:589–601. [DOI] [PubMed] [Google Scholar]
- 143. Ferrari LF, Bogen O, Levine JD. Second messengers mediating the expression of neuroplasticity in a model of chronic pain in the rat. J Pain. 2014;15:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kang R, Tang D, Lotze MT, Zeh HJ 3rd. AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6–pSTAT3 pathway. Autophagy. 2012;8:989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wei J-Y, Liu C-C, Ouyang H-D, et al. Activation of RAGE/STAT3 pathway by methylglyoxal contributes to spinal central sensitization and persistent pain induced by bortezomib. Exp Neurol. 2017;296:74–82. [DOI] [PubMed] [Google Scholar]
- 146. Liu X, Tian Y, Lu N, Gin T, Cheng CHK, Chan MTV. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS One. 2013;8:e75804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pang H, Ren Y, Li H, Chen C, Zheng X. LncRNAs linc00311 and AK141205 are identified as new regulators in STAT3-mediated neuropathic pain in bCCI rats. Eur J Pharmacol. 2020;868:172880. [DOI] [PubMed] [Google Scholar]
- 148. Doyle T, Chen Z, Muscoli C, et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci. 2012;32:6149–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fidanboylu M, Griffiths LA, Flatters SJ. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One. 2011;6:e25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. [DOI] [PubMed] [Google Scholar]
- 151. Liu L, Yang M, Kang R, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25:23–31. [DOI] [PubMed] [Google Scholar]
- 152. Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cerles O, Goncalves TC, Chouzenoux S, et al. Preventive action of benztropine on platinum-induced peripheral neuropathies and tumor growth. Acta Neuropathol Commun. 2019;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhang Y, Zhang Y, Wang Z, et al. Suppression of delayed rectifier K(+) channels by gentamicin induces membrane hyperexcitability through JNK and PKA signaling pathways in vestibular ganglion neurons. Biomed Pharmacother. 2021;135:111185. [DOI] [PubMed] [Google Scholar]
- 155. Brandolini L, d'Angelo M, Antonosante A, Allegretti M, Cimini A. Chemokine signaling in chemotherapy-induced neuropathic pain. Int J Mol Sci. 2019;20:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tay N, Laakso EL, Schweitzer D, Endersby R, Vetter I, Starobova H. Chemotherapy-induced peripheral neuropathy in children and adolescent cancer patients. Front Mol Biosci. 2022;9:1015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. [DOI] [PubMed] [Google Scholar]
- 158. Zhang H, Li Y, de Carvalho-Barbosa M, et al. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain. 2016;17:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. [DOI] [PubMed] [Google Scholar]
- 160. Huang Z-Z, Li D, Liu C-C, et al. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun. 2014;40:155–165. [DOI] [PubMed] [Google Scholar]
- 161. Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. [DOI] [PubMed] [Google Scholar]
- 162. Aromolaran KA, Goldstein PA. Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy; cause and effect? Mol Pain. 2017;13:1744806917714693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated during the current study are available from the corresponding author upon reasonable request.