Abstract
Introduction
Outcomes for patients with multiple myeloma has significantly improved through the years. This is mainly related to the use of novel agents.
Methods
This is a retrospective study that reviewed presentation and outcome of 139 patients with multiple myeloma at the Windsor Essex Regional Cancer Centre from Jan. 1, 2015 to Dec. 31, 2019. Median age was 71 years and most patients had higher risk disease (65.5% either R ISS stage II or III). 30% had high risk FISH for myeloma including del.17P, t (4:14), t (14:16) and Gain (1q21). In terms of presentation, 38.8% had anemia (hemoglobin <100g/L), 18.7% had hypercalcemia, 74.1% had skeletal lytic lesions, 38.8% had pathologic fracture and 17.3% had plasmacytoma.
Results
Almost all (92%) of the patients were treated using at least one novel agent (proteasome inhibitor or immunomodulators [ImiDs]). Cyclophosphamide, bortezomib, and dexamethasone (CyBorD) was the most used treatment regimen (48.9%) followed by bortezomib, melphalan and prednisone (BMP) at 28.8% and lenalidomide, dexamethasone (LenDex) at 14.4%. With respect to response to therapy, 51.8% had at least Very good partial response (VGPR), while 9.4% had progressive disease. 33% had autologous stem cell transplant. After a median follow up of 2.4 years, median overall survival was 3.7 years. 2 years overall survival and relapse-free survival were 70% and 83%, respectively.
Discussion
Our study showed comparable outcome for patients with multiple myeloma despite older age and higher risk disease. Outcome is expected to improve with the introduction of more novel agents.
Keywords: multiple myeloma, proteasome inhibitors, immunomodulators
Introduction
Multiple myeloma was responsible for 1600 deaths in Canada in 2020 with an annual incidence of 3400 cases (https://www.cancer.ca/statistics). Although there are significant advances in its treatment, multiple myeloma is still an incurable disease. The discovery of novel therapeutic agents including proteasome inhibitors and immunomodulators made a huge improvement in the outcome of patients with multiple myeloma.1
The newly utilized combination of monoclonal antibodies and dual novel agents has resulted in a dramatic improvement in response rate, minimal residual disease (MRD) negativity and survival.2,3 However, access to newer anti-myeloma drugs is variable among different countries as well as provinces. This is likely in part responsible for the differences in patient outcomes among different institutions.
In this retrospective chart review, data on demographics, presentation and outcome of patients with multiple myeloma treated at the Windsor Essex Regional Cancer Centre are described. Outcome metrics including median overall survival and relapse-free survival were compared with the outcomes from local as well as international institutions.
Methods
The study is a retrospective chart review on 139 patients diagnosed and treated for multiple myeloma from Jan. 1, 2015 to Dec. 31, 2019 at the Windsor Essex Regional Cancer Centre. Information on demographics, presentation, treatment and outcome was collected.
The Revised International Staging system (R ISS) was included whenever information was available using the IMWG definition. Patients with high-risk FISH were defined as having one of the following: del.17p, t (4; 14), t (14; 16) and Gain (1q21). Response rates were classified as complete remission (CR), Very good partial response (VGPR), stable disease and progressive disease based on the IMWG criteria.4,5
Continuous variables are presented as median (interquartile range [IQR]) and categorical variables are reported as frequency (percentage). Time to all-cause mortality and relapse were assessed graphically using Kaplan-Meier curves. Median survival time was determined using the time where the Kaplan-Meier survival curve crossed the 50% threshold. Characteristics associated with death and relapse were assessed using hazard ratios obtained from univariable Cox proportional hazards models. Two-sided p-values <0.05 were considered statistically significant. All analyses were run using R version 4.0.2.
Results
A total of 139 patients with multiple myeloma were treated at the Windsor Essex Regional Cancer Centre from Jan. 1, 2015 to Dec. 31, 2019.
Median age was 71 years, ranging from 63–78 years, with 52% male. Most patients had higher risk disease (65.5% either R ISS stage II or III). 30% had high risk FISH for myeloma including del.17P, t (4:14), t (14:16) and Gain (1q21). In terms of presentation, close to half (47%) had moderate to severe renal impairment (eGFR < 60mL/min/1.73m2), 38.8% had anemia (hemoglobin <100g/L), 18.7% had hypercalcemia, 74.1% had skeletal lytic lesions, 38.8% had pathologic fracture and 17.3% had concurrent plasmacytoma (Table 1).
Table 1.
Baseline Characteristics of Patients with Multiple Myeloma
| Overall | |
|---|---|
| (N=139) | |
| Age (Years) | |
| Median (IQR) | 71 (63, 78) |
| Sex | |
| Male | 72 (51.8%) |
| Female | 67 (48.2%) |
| Revised International Staging System (R-ISS) | |
| I | 8 (5.8%) |
| II | 66 (47.5%) |
| III | 25 (18.0%) |
| N/A | 40 (28.8%) |
| Glomerular filtration rate (mL/min/1.73m²) | |
| >90 | 26 (18.7%) |
| 60–90 | 46 (33.1%) |
| 30–59 | 38 (27.3%) |
| 15–29 | 14 (10.1%) |
| <15 | 13 (9.4%) |
| Missing | 2 (1.4%) |
| Ionized calcium (mmol/L) | |
| Normal (<=1.29) | 107 (77.0%) |
| Elevated (>1.29) | 26 (18.7%) |
| Missing | 6 (4.3%) |
| Hemoglobin (g/L) | |
| ≥100 | 85 (61.2%) |
| <100 | 54 (38.8%) |
| Lactate dehydrogenase (LDH) (U/L) | |
| Normal (<234) | 110 (79.1%) |
| Elevated (≥234) | 25 (18.0%) |
| Missing | 4 (2.9%) |
| Skeletal lytic lesions | |
| Yes | 103 (74.1%) |
| No | 35 (25.2%) |
| Missing | 1 (0.7%) |
| Pathologic fracture | |
| Yes | 54 (38.8%) |
| No | 84 (60.4%) |
| Missing | 1 (0.7%) |
| Plasmacytoma | |
| Yes | 24 (17.3%) |
| No | 114 (82.0%) |
| Missing | 1 (0.7%) |
| Myeloma Fluorescence In Situ Hybridization | |
| Negative | 45 (32.4%) |
| High Risk | 43 (30.9%) |
| Standard Risk | 3 (2.2%) |
| N/A | 48 (34.5%) |
Almost all (92%) of the patients were treated using at least one novel agent (proteasome inhibitor or ImiDs). Cyclophosphamide, bortezomib, and dexamethasone (CyBorD) was the most commonly used treatment regimen (48.9%) followed by bortezomib, melphalan and prednisone (BMP) at 28.8% and lenalidomide, dexamethasone (LenDex) at 14.4%. Only five patients received melphalan-dexamethasone. 33% had autologous stem cell transplant and 31% were on maintenance therapy for a median of 10 months (Table 2).
Table 2.
Treatment and Outcome of Patients with Multiple Myeloma
| Overall | |
|---|---|
| (N=139) | |
| Initial chemotherapy regimen | |
| Lenalidomide-Dexamethasone | 20 (14.4%) |
| Bortezomib-Melphalan-Prednisone | 40 (28.8%) |
| Cyclophosphamide-Bortezomib-Dexamethasone | 68 (48.9%) |
| Melphalan-Dexamethasone | 5 (3.6%) |
| Missing | 6 (4.3%) |
| Number of chemotherapy cycles | |
| Median (IQR) | 5 (4, 7) |
| Missing | 1 (0.7%) |
| Best Response | |
| Complete response | 17 (12.2%) |
| Very good partial response | 55 (39.8%) |
| Partial response | 31 (22.3%) |
| Stable disease | 12 (8.6%) |
| Progressive disease | 13 (9.4%) |
| Missing | 11 (7.9%) |
| Toxicity requiring dose reduction/stop | |
| Yes | 28 (20.1%) |
| No | 94 (67.6%) |
| N/A | 10 (7.2%) |
| Missing | 7 (5.0%) |
| Toxicity type | |
| Peripheral neuropathy | 3 (2.2%) |
| Diarrhea | 3 (2.2%) |
| Fatigue | 1 (0.7%) |
| Cytopenia | 4 (2.9%) |
| Other | 15 (10.8%) |
| Multiple types | 3 (2.2%) |
| N/A | 94 (67.6%) |
| Missing | 16 (11.5%) |
| Palliative radiation | |
| Yes | 64 (46.0%) |
| No | 73 (52.5%) |
| Missing | 2 (1.4%) |
| First autologous stem cell transplant | |
| Yes | 46 (33.1%) |
| No | 90 (64.7%) |
| Missing | 3 (2.2%) |
| Tandem stem cell transplant | |
| Yes | 3 (2.2%) |
| No | 134 (96.4%) |
| Missing | 2 (1.4%) |
| Maintenance therapy | |
| Yes | 44 (31.7%) |
| No | 92 (66.2%) |
| Missing | 3 (2.2%) |
| Maintenance chemotherapy regimen | |
| Lenalidomide | 35 (25.2%) |
| Bortezomib | 3 (2.2%) |
| Ixazomib | 1 (0.7%) |
| Lenalidomide-Ixazomib | 4 (2.9%) |
| Lenalidomide-Dexamethasone | 1 (0.7%) |
| N/A | 95 (68.3%) |
| Duration of maintenance therapy (months) | |
| Median (IQR) | 10 (5, 20) |
| Missing | 95 (68.3%) |
| Palliative care referral | |
| Yes | 41 (29.5%) |
| No | 97 (69.8%) |
| Missing | 1 (0.7%) |
With respect to response to therapy, 51.8% had at least Very good partial response (VGPR) of which 12.2% had Complete response (CR). 9.4% had progressive disease. The rest include partial response, stable disease and missing (Table 2).
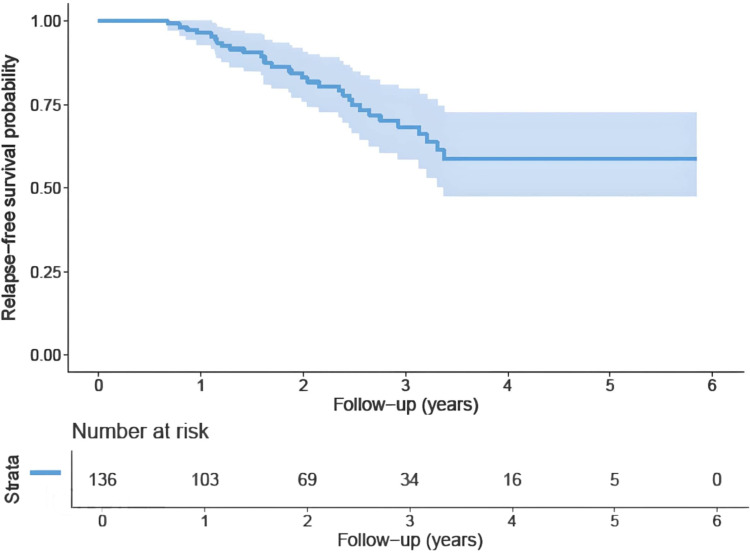
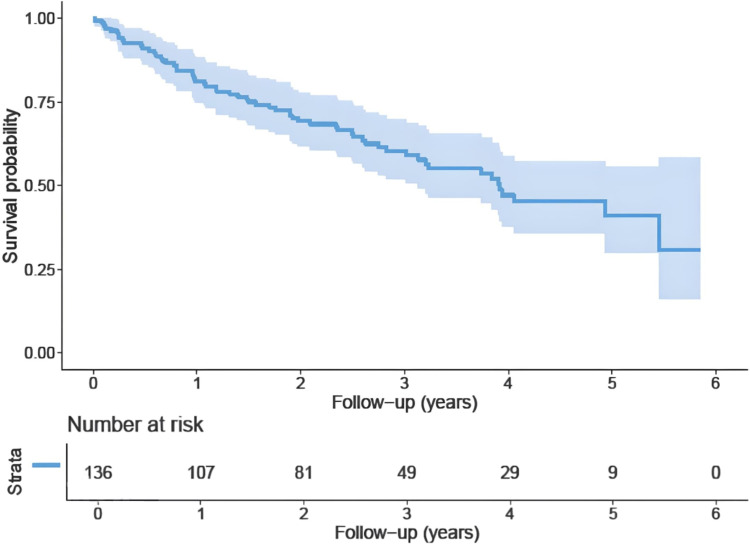
Patients were followed for a median of 2.4 years with 61 deaths and 31 relapse events. Median overall survival was 3.7 years. Two years overall survival and relapse-free survival were 70% and 83%, respectively. One year survival probability was 80% (Figures 1 and 2).
Figure 1.
Shows The Kaplan-Meier curve for patients’ relapse-free survival in years.
Figure 2.
Shows The Kaplan-Meier curve for patients’ overall survival in years.
Cox regression showed hypercalcemia as the only variable significantly associated with increased risk of relapse (HR 2.76, 95% CI 1.28–5.97, P value 0.009). On the other hand, older age (HR 1.03 per year, 95% CI 1.05–1.05, P value 0.02), response inferior to VGPR (stable disease/partial response) (HR 2.87, 95% CI 1.57–5.27, P<0.0001) and progressive disease (HR 10.25, 95% CI 4.99–21.06, P<0.0001) were significantly associated with risk of death. In addition, having autologous stem cell transplant (HR 0.18, 95% CI 0.08–0.38, P value 0.0001) and being on maintenance therapy (HR 0.25, 95% CI 0.12–0.51, P < 0.0001) were significantly associated with reduced risk of dying (Tables 3 and 4).
Table 3.
Hazard Ratios for Baseline Characteristics and the Outcome of Relapse
| Variable | Value | Unadjusted | |||
|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P-value | |||
| Age, per year | 0.99 | 0.96 | 1.02 | 0.48 | |
| Sex | |||||
| Female | 0.73 | 0.36 | 1.49 | 0.39 | |
| Male | 1.00 | (referent) | |||
| R-ISS | |||||
| I or II | 1.00 | (referent) | |||
| III | 2.20 | 0.88 | 5.45 | 0.09 | |
| Ionized calcium | |||||
| Normal (<=1.29) | 1.00 | (referent) | |||
| Elevated (>1.29) | 2.76 | 1.28 | 5.97 | 0.0099 | |
| Glomerular filtration rate | |||||
| ≥60 | 1.00 | (referent) | |||
| <60 | 1.62 | 0.78 | 3.33 | 0.19 | |
| Pathologic fracture | |||||
| No | 1.00 | (referent) | |||
| Yes | 1.50 | 0.74 | 3.06 | 0.26 | |
| Hemoglobin | |||||
| <100 | 1.37 | 0.66 | 2.82 | 0.40 | |
| ≥100 | 1.00 | (referent) | |||
| Best response | |||||
| Complete or very good partial response | 1.00 | (referent) | 0.90 | ||
| Partial response or stable disease | 0.94 | 0.42 | 2.13 | ||
| Progressive disease | 1.72 | 0.40 | 7.39 | ||
| Autologous stem cell transplant | |||||
| No | 1.00 | (referent) | |||
| Yes | 0.71 | 0.34 | 1.46 | 0.35 | |
| Maintenance therapy | |||||
| No | 1.00 | (referent) | |||
| Yes | 0.80 | 0.38 | 1.66 | 0.54 | |
Table 4.
Hazard Ratios for Baseline Characteristics and the Outcome of All-Cause Mortality
| Variable | Value | Unadjusted | |||
|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P-value | |||
| Age, per year | 1.03 | 1.01 | 1.05 | 0.02 | |
| Sex | |||||
| Female | 0.89 | 0.54 | 1.48 | 0.65 | |
| Male | 1.00 | (referent) | |||
| R-ISS | |||||
| I or II | 1.00 | (referent) | |||
| III | 0.98 | 0.43 | 2.27 | 0.97 | |
| Ionized calcium | |||||
| Normal (<=1.29) | 1.00 | (referent) | |||
| Elevated (>1.29) | 1.53 | 0.85 | 2.78 | 0.16 | |
| Glomerular filtration rate | |||||
| ≥60 | 1.00 | (referent) | |||
| <60 | 1.41 | 0.84 | 2.37 | 0.19 | |
| Pathologic fracture | |||||
| No | 1.00 | (referent) | |||
| Yes | 0.71 | 0.42 | 1.22 | 0.22 | |
| Hemoglobin | |||||
| <100 | 1.33 | 0.80 | 2.22 | 0.27 | |
| ≥100 | 1.00 | (referent) | |||
| Best response | |||||
| Complete or very good partial response | 1.00 | (referent) | <0.0001 | ||
| Partial response or stable disease | 2.87 | 1.57 | 5.27 | ||
| Progressive disease | 10.25 | 4.99 | 21.06 | ||
| Autologous stem cell transplant | |||||
| No | 1.00 | (referent) | |||
| Yes | 0.18 | 0.08 | 0.38 | <0.0001 | |
| Maintenance therapy | |||||
| No | 1.00 | (referent) | |||
| Yes | 0.25 | 0.12 | 0.51 | <0.0001 | |
| Relapse (time-varying covariate) | |||||
| No | 1.00 | (referent) | |||
| Yes | 2.03 | 0.96 | 4.29 | 0.06 | |
Discussion
Despite its limitations including small sample size and retrospective design with missing variables, the study revealed important information on the presentation, treatment and outcome of patients with multiple myeloma at the Windsor Essex Regional Cancer Centre. Outcome of patients in this study is comparable with other centres in Canada as well as patients internationally treated with similar regimen. However, the outcome is inferior to those centres that used dual novel agents with or without monoclonal antibody.6,7
A retrospective study from Alberta multiple myeloma registry on 147 patients with median age of 73 years showed median overall survival of 3.3 years for those on CyBorD regimen, which is comparable to our study (ASH poster 2020). Another study from the Swedish myeloma registry on 4904 patients from 2008 to 2015 showed median overall survival of 3.4 years for those patients older than 65 years of age which is again comparable with our outcome. However median overall survival was much higher at 7.7 years for those patients 65 years or younger suggesting the strong impact of age on survival.6 Another retrospective study on 1000 patients from the Emory myeloma database from January 2007 to August 2016 showed much higher median overall survival of 10.5 years. However, these patients were treated with a regimen that has dual novel agents [RVD: Revlimid (ImiD) and velcade (proteasome inhibitor)]. In the same study response of VGPR or better was also much higher at 89% compared with 51% in our study.7 This underscores the importance of using multiple novel agents to get a deeper response in the initial treatment of multiple myeloma. With the discovery of more and more novel therapies for the treatment of multiple myeloma, access to these drugs will be critical.
This is clearly demonstrated in the dramatic response seen with the combination of monoclonal antibody, daratumumab, and dual novel agents (ImiDs or proteasome inhibitors) in the frontline treatment of patients with multiple myeloma with a complete response rate of up to 79% and minimal residual disease (MRD) negativity of 14–24%.2,3
The most widely used, currently funded, first-line treatment regimen for transplant eligible patients with multiple myeloma in Windsor Essex is CyBorD. Whereas BMP and recently LenDex are funded and commonly used as first-line therapy for those who are ineligible for autologous stem cell transplant. (Systemic Treatment - Quality Based Procedure, Cancer Care Ontario; https://www.cancercareontario.ca/en/cancer-treatments/chemotherapy/funding-reimbursement/systemic-treatment-quality-based-procedure).
Although access to combination of novel agents is slowly improving, the data are compelling to incorporate multiple novel agents and monoclonal antibodies in the front-line setting. This will result in deeper response and higher rate of MRD negativity which in turn will have positive impacts on survival.2,3 Regulatory agents will need to consider this emerging scientific evidence and cost-benefit implications for society in deciding drug access, particularly in multiple myeloma.
Hypercalcemia was the only variable significantly associated with higher risk of relapse (HR 2.76, 95% CI 1.28–5.97, P 0.009) but not death in our study. Other studies on patients with symptomatic MM showed inferior survival in those patients with hypercalcemia.8
In our study, older age and poor response to therapy, particularly progressive disease (HR 10.2, 95% CI 4.99–21, P< 0.0001) were significantly associated with higher risk of death. On the other hand, those with autologous stem cell transplant had significantly lower risk of dying (HR 0.18, 95% CI 0.08–0.38, P < 0.0001). This could be associated with selection bias as patients who had autologous stem cell transplant are generally younger and healthier. Better survival for those on maintenance therapy can also be subjected to similar bias as patients who live longer are more likely to be on maintenance. Nevertheless, survival benefit for autologous stem cell transplant as well as maintenance therapy was demonstrated in several other studies.4,9–11
The impact of relapse on survival as a time-dependent variable was close to statistical significance but not quite, unlike other studies which showed early relapse as a risk for poor survival.12
Finally, despite the continuous effort of the scientific community in finding new and novel treatment options for multiple myeloma, studies still suggest that the outcome of patients with 17p deletion and t (4; 14) has not improved dramatically.13,14 This underscores the need for continued bench to bedside collaboration between scientists and clinicians for continued breakthrough in the fight against myeloma.
In conclusion, our study showed comparable outcomes for patients with multiple myeloma treated with similar treatment regimen in other centres. However, improved patient outcomes were observed with newer regimens that incorporate monoclonal antibodies and dual novel agents. As more and more novel drugs are introduced to the armament against the disease, it is expected that access to these drugs will affect outcomes. We recommend a prospective myeloma registry to acquire more comprehensive information as well as improved access to combination of novel agents and monoclonal antibodies to improve the outcome of our patients with multiple myeloma.
Ethical/Copyright
The study was cleared by the Windsor Regional Hospital (WRH) Ethics Review Board and supported by the Schulich-Windsor Opportunities for Research Excellence Program (SWORP). The study was exempt from informed consent as it was judged to not involve more than minimal risk as approved by the ethics review board of WRH. Maximum precaution was used to protect patient confidential data. The study was conducted in compliance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateos MV, Sonneveld P, Hungria V, et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: three-year Follow-up of CASTOR. Clin Lymphoma Myeloma Leuk. 2020;20(8):509–518. doi: 10.1016/j.clml.2019.09.623 [DOI] [PubMed] [Google Scholar]
- 3.Avet-Loiseau H, San-Miguel J, Casneuf T, et al. Evaluation of sustained minimal residual disease negativity with daratumumab-combination regimens in relapsed and/or refractory multiple myeloma: analysis of POLLUX and CASTOR. J Clin Oncol. 2021;39(10):1139–1149. doi: 10.1200/JCO.20.01814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. doi: 10.1056/NEJMoa1402888 [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e46. doi: 10.1016/S1470-2045(16)30206-6 [DOI] [PubMed] [Google Scholar]
- 6.Blimark CH, Turesson I, Genell A, et al. Outcome and survival of myeloma patients diagnosed 2008–2015. Real-world data on 4904 patients from the Swedish myeloma registry. Haematologica. 2018;103(3):506–513. doi: 10.3324/haematol.2017.178103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38(17):1928–1937. doi: 10.1200/JCO.19.02515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zagouri F, Kastritis E, Zomas A, et al. Hypercalcemia remains an adverse prognostic factor for newly diagnosed multiple myeloma patients in the era of novel antimyeloma therapies. Eur J Haematol. 2017;99(5):409–414. doi: 10.1111/ejh.12923 [DOI] [PubMed] [Google Scholar]
- 9.McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279–3289. doi: 10.1200/JCO.2017.72.6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parrondo RD, Ailawadhi S, Sher T, Chanan-Khan AA, Roy V. Autologous stem-cell transplantation for multiple myeloma in the era of novel therapies. JCO Oncol Pract. 2020;16(2):56–66. doi: 10.1200/JOP.19.00335 [DOI] [PubMed] [Google Scholar]
- 11.Syed YY. Lenalidomide: a review in newly diagnosed multiple myeloma as maintenance therapy after ASCT. Drugs. 2017;77(13):1473–1480. doi: 10.1007/s40265-017-0795-0 [DOI] [PubMed] [Google Scholar]
- 12.Majithia N, Rajkumar SV, Lacy MQ, et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia. 2016;30(11):2208–2213. doi: 10.1038/leu.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalopin T, Vallet N, Theisen O, et al. No survival improvement in patients with high-risk multiple myeloma harbouring del(17p) and/or t(4;14) over the two past decades. Br J Haematol. 2021;194:635–638. doi: 10.1111/bjh.17488 [DOI] [PubMed] [Google Scholar]
- 14.Corre J, Perrot A, Caillot D, et al. del(17p) without TP53 mutation confers a poor prognosis in intensively treated newly diagnosed patients with multiple myeloma. Blood. 2021;137(9):1192–1195. doi: 10.1182/blood.2020008346 [DOI] [PMC free article] [PubMed] [Google Scholar]