Abstract
Necrotizing pneumonia (NP) is characterized by destruction of pulmonary tissue, resulting in multiple thin-walled cavities. There are limited reports on NP and parapneumonic effusion cases in children associated with Pseudomonas aeruginosa. Currently, there is no consensus regarding the optimal timing for video-assisted thoracoscopic surgery (VATS) following failure of chest tube placement and antibiotic treatment. A healthy 20-month-old child was hospitalized with symptoms of community-acquired pneumonia, progressing to severe NP and parapneumonic effusion. Despite receiving broad-spectrum antibiotics and chest tube placement on the third day of treatment, the condition continued to deteriorate, prompting VATS intervention on the sixth day. The presence of a “split pleural sign“ and extensive lung necrosis on chest computed tomography contributed to initial treatment failure. Multidrug resistance P. aeruginosa was identified through nasal trachea aspiration specimens on the eighth day of treatment, leading to an adjustment in antibiotic therapy to high-dose meropenem and amikacin. Subsequently, the patient became afebrile, showed clinical improvement, and was discharged after 35 days of treatment. Through this case, we aim to emphasize an unusual pathogenic bacteria in the context of NP and the need for standardized surgical interventions in pediatric patients with NP.
Keywords: Necrotizing pneumonia, Pediatrics, VATS, Pseudomonas aeruginosa
Introduction
Necrotizing pneumonia (NP) is characterized by destruction of pulmonary tissue, resulting in multiple thin-walled cavities [1]. This condition predominantly affects children aged between 2 and 5 years and is an infrequent complication of community-acquired pneumonia (CAP), accounting for 3.7% of CAP cases [2]. The complication rate of pneumonia, including parapneumonic pleural effusion (PPE), empyema, NP, and lung abscess, is reported at 8.9% based on a retrospective study spanning nearly 2 decades by Masarweh et al. [3]. However, approximately 40% of pneumonia cases develop complications during hospitalization [3]. NP increases the risk of respiratory failure, PPE, empyema, pneumothorax, bronchopleural fistula, and septic shock. NP is also associated with increased mortality rates, prolonged febrile episodes, and extended hospitalization [2,4].
Antibiotics and surgical intervention are the 2 primary treatment modalities for cases of NP associated with PPE. A retrospective study involving 746 pediatric NP cases revealed 46.6% requirement for chest tube placement and 6.1% necessity for video-assisted thoracoscopic surgery (VATS) [2]. Although there is no consensus on the criteria for chest tube placement and VATS, studies indicated that the rate of surgical intervention for complicated CAP ranges from 38% to 77% [2,3,5]. The relatively high rate of surgical management, which includes chest tube placement, VATS, and lobular resection, emphasizes the necessity of early disease detection and prompt treatment.
There is a lack of large-sample studies on NP in pediatrics, as NP remains an uncommon complication of CAP. Many aspects of NP remain unclear, including risk factors, methods for early screening and diagnosis of NP in outpatients with CAP, the role of lung ultrasound, and the microbiological characteristics of NP-causing agents. Furthermore, there are no randomized controlled trials to inform guidelines on antibiotic therapy or the timing of surgical intervention following antibiotic treatment failure. We present a complex case of NP and PPE associated with the unusual pathogen P. aeruginosa in a healthy 20-month-old child. This case highlights the success of combined antibiotic therapy, chest tube placement, and VATS.
Case report
A 20-month-old female patient was admitted to Children's Hospital 2 due to fever and respiratory distress. The illness persisted for 7 days before hospitalization, initially presenting with upper respiratory infection symptoms such as coughing and rhinorrhea during the first 2 days. Subsequently, the patient developed a fever of 39°C and a worsening productive cough that did not respond to oral antibiotics (amoxicillin/clavulanic acid at 45 mg/kg/day, divided into 3 doses) over the next 3 days. The patient was diagnosed with pneumonia and treated with ceftriaxone (100 mg/kg/day, divided into 2 doses) at a lower-level hospital for 2 days. However, the symptoms did not improve, leading to the patient's transfer to Children's Hospital 2. No reported perinatal or psychomotor development disorders were reported, and no significant medical history was noted. The patient had received the complete series of the 5-in-one vaccine but had not received vaccinations against influenza and pneumococcus.
At Children's Hospital 2, the patient showed chest retractions, a heart rate of 150 beats per minute, a respiratory rate of 50 breaths per minute, weighing 11 kilograms, measuring 83 centimeters in height, and a SpO2 level of 95% with supplemental oxygen delivered via a cannula 3 liter/minute. Lung auscultation revealed diminished breath sounds in the left lung and right-sided crackles. Examination of other organs did not detect any abnormalities.
The clinical progression and antibiotic treatment are illustrated in Fig. 1, Fig. 2. During the first 3 days of antibiotic therapy, which included vancomycin, ceftriaxone, amikacin, and clarithromycin, the patient remained febrile, respiratory distress (requiring oxygen supplementation via nasal continuous positive airway pressure), and a chest X-ray revealed worsening pulmonary consolidation and pleural effusion (Fig. 3). The total vancomycin dose per day was modified based on the peak and trough levels of vancomycin (Table 1). The bedside lung ultrasound revealed consolidation and collapse of the left lung, with multiple hypoechoic lesions suggestive of NP. Additionally, there was a moderate amount of pleural effusion, with septations inside and pleural thickening (Fig. 4).
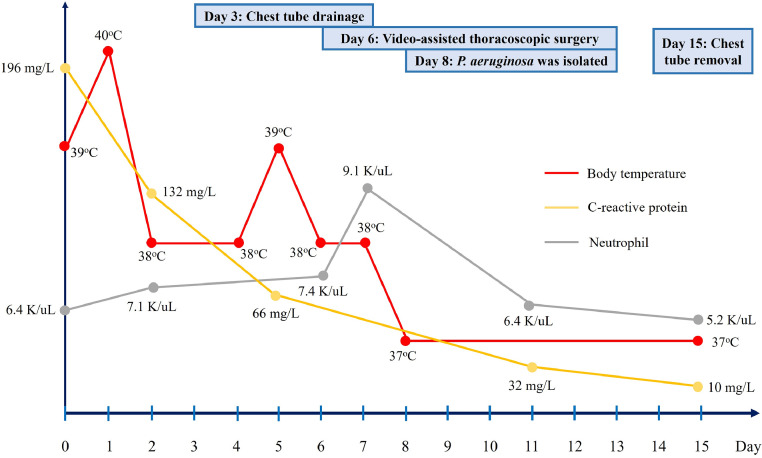
Fig. 1.
The progression of body temperature, C-reactive protein level, and neutrophil count from day 1 to day 15 after hospitalization.
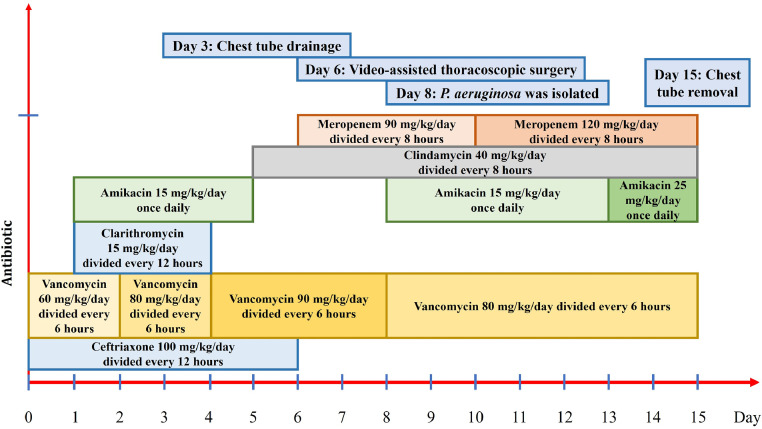
Fig. 2.
Summary of antibiotic types and duration of use by the patient.
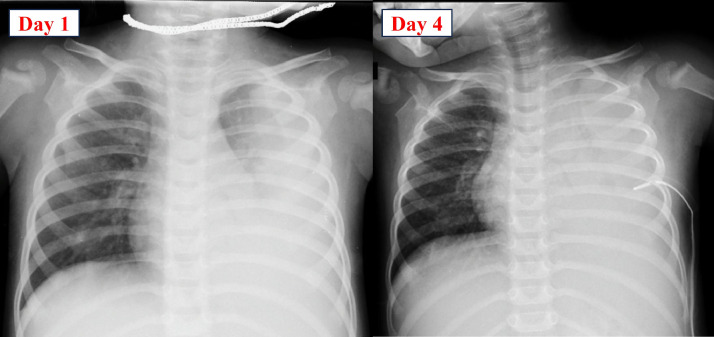
Fig. 3.
The chest X-ray from day 1 to day 4 post-hospitalization revealed progressing left lung consolidation and pleural effusion despite antibiotic treatment.
Table 1.
The patient's laboratory test results.
| Laboratory tests | Normal range | Value |
|---|---|---|
| Blood | ||
| Vancomycin trough level before fourth dose | µg/mL | 2.28 |
| Vancomycin peak level on the third day | µg/mL | 22.25 |
| Vancomycin trough level on the third day | µg/mL | 5.57 |
| AUC24 for vancomycin on the third day | µg/mL x hr | 360 |
| AST | <45 (U/L) | 135 |
| ALT | <40 (U/L) | 5 |
| Urea | 1.67-7.49 (mmol/L) | 2.2 |
| Creatinine | 20.33-88.4 (µmol/L) | 30 |
| Hemoglobin | 10.5-14 (g/dL) | 9.8 |
| Platelet | 150-400 (K/uL) | 383 |
| Chlamydia pneumoniae IgM | Negative | |
| Mycoplasma pneumoniae IgM | Negative | |
| Pleural fluid | ||
| Protein | g/L | 31.5 |
| Glucose | mmol/L | 2.5 |
| LDH | U/L | 3131 |
| ADA | U/L | 55 |
| Neutrophil cellular component | % | 80% |
| Mycobacterium tuberculosis PCR | Negative | |
| Culture for bacteria | Negative | |
| Nasotracheal aspiration specimens | ||
| Culture for bacteria | Multi-drug-resistant P. aeruginosa | |
| GeneXpert MTB/RIF | Negative | |
| Mycobacterium tuberculosis PCR | Negative | |
| AFB | Negative | |
ADA, adenosine deaminase; AFB, acid-fast bacilli; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC24, the area under the concentration-time curve from 0 to 24 hr; MTB, Mycobacterium tuberculosis; PCR, polymerase chain reaction; RIF, rifampicin. Abnormal values are highlighted in bold.
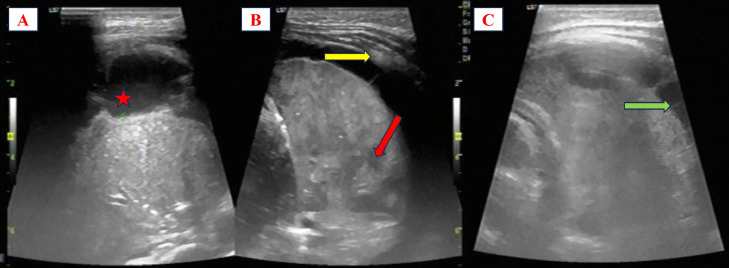
Fig. 4.
Bedside lung ultrasound on the third day. A shows echogenicity in the pleural fluid (red star). B shows multiple hypoechoic lesions within the left lung (red arrow) and pleural thickening (yellow arrow). C illustrates septation within the pleural cavity (green arrow).
On the third day, the patient underwent chest tube placement, leaking turbid yellow pleural fluid. The pleural fluid analysis is presented in Table 1, with elevated LDH levels (3131 U/L) and reduced glucose levels (2.5 mmol/L), consistent with complicated PPE. Other laboratory tests are presented in Table 1.
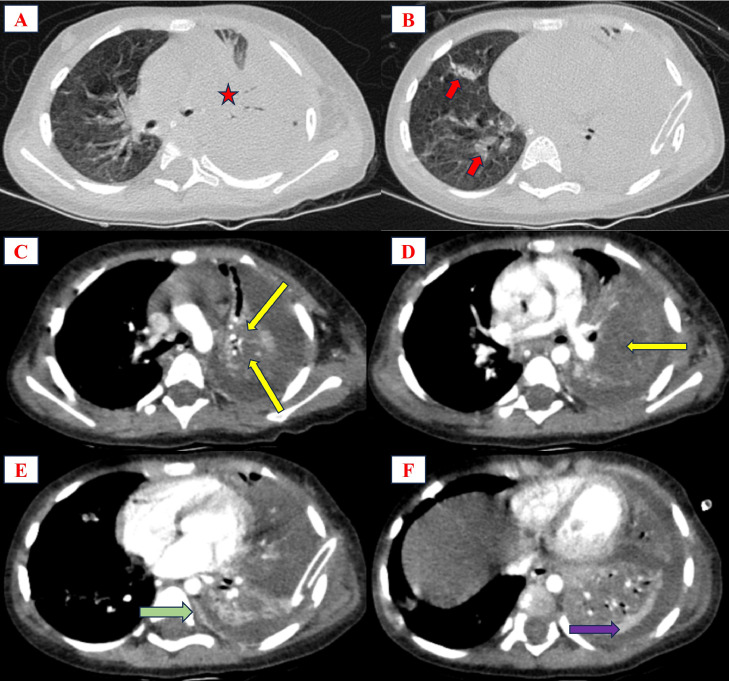
From day 3 to day 6 post-treatment (following the chest tube placement), the patient's fever persisted without improvement despite a reduction in CRP levels. Approximately 100 mL of turbid yellow pleural fluid through the chest tube per day was collected. The patient received additional intravenous clindamycin and an increased dose of vancomycin guided by AUC24. Blood cultures yielded negative results for bacterial growth. Transthoracic doppler echocardiography revealed no abnormalities. The contrast-enhanced chest computed tomography (CT) showed NP, PPE, and “split pleural sign” (Fig. 5).
Fig. 5.
Chest CT on the fourth day. A and B showed consolidation in the left lung with an air bronchogram sign (red star) and scattered consolidations in the right lung (red arrow). C and D revealed low-attenuated areas inside the consolidation, suggesting NP (yellow arrow). E and F showed thickened parietal (green arrow) and visceral (pink arrow) pleura, referred to as “split pleural sign”.
The patient received meropenem supplementation based on the multiplex PCR results of nasal tracheal aspiration (NTA) presented in Table 3, indicating a high concentration of Stenotrophomonas maltophilia, P. aeruginosa, and Streptococcus pneumonia. During the VATS procedure on day sixth, nearly complete necrosis of the left upper lobe parenchyma with numerous pseudomembranes was observed. The pleural membranes of both upper and lower lobes were decorticated, pseudomembranes were removed, and adherent lung parenchyma was dissected. Multidrug-resistant P. aeruginosa (Table 2) was isolated from nasotracheal aspiration specimens (grade of Bartlett's criteria was 2 points), which were collected on the third day.
Table 3.
The multiplex polymerase chain reaction results of nasal tracheal aspiration specimens.
| Organism | CT | Result | Organism | CT | Result |
|---|---|---|---|---|---|
| Community-acquired bacteria | Fungal | ||||
| Streptococcus pneumoniae | 28.14 | 7.47 × 105 | Pan Aspergillus | (-) | - |
| Haemophilus influenzae | 33.26 | 2.14 × 104 | Aspergillus fumigatus | (-) | - |
| Haemophilus influenzae type B | (-) | - | Aspergillus flavus | (-) | - |
| Moraxella catarrhalis | (-) | - | Aspergillus niger | (-) | - |
| Streptococcus pyogenes (GAS) | (-) | - | Aspergillus terrus | (-) | - |
| Streptococcus agalactiae (GBS) | (-) | - | Candida albicans | 33.34 | 2.03×104 |
| Streptococcus suis | (-) | - | Candida kefyr | (-) | - |
| Nosocomial bacteria | Candida tropicalis | (-) | - | ||
| Staphylococcus aureus (MRSA) | (-) | - | Candida krusei | (-) | - |
| Staphylococcus aureus (MSSA) | (-) | - | Candida glabrata | (-) | - |
| Staphylococcus epidermidis (MRSE) | (-) | - | Cryptococcus neoformans | (-) | - |
| Staphylococcus epidermidis (MSSE) | (-) | - | Pneumocystis jirovecii | (-) | - |
| Coagulase-negative staphylococcus | 32.11 | 4.76×104 | Penicillium marneffei | (-) | - |
| Panton Valentine Leukocidin (PVL) | (-) | - | Histoplasma capsulatum | (-) | - |
| Enterococcus faecalis | (-) | - | Fusarium oxysporum | (-) | - |
| Enterococcus faecium | (-) | - | Fusarium verticillioides | (-) | - |
| Escherichia coli | (-) | - | Coccidioides immitis/ posadasii | (-) | - |
| Enterobacter cloaceae | (-) | - | Sporothrix globosa | (-) | - |
| Enterobacter aerogenes | (-) | - | Sporothrix schenckii/ brasiliensis | (-) | - |
| Klebsiella pneumoniae | (-) | - | Mucormycosis (Rhizopus oryzae) | (-) | - |
| KPC | (-) | - | Fusarium solani | (-) | - |
| NDM-1 | (-) | - | Virus | ||
| Pseudomonas aeruginosa | 27.78 | 9.59 × 105 | Influenzavirus A | (-) | - |
| Burkhoideria cepacia | (-) | - | Influenzavirus B | (-) | - |
| Burkholderia pseudomallei | (-) | - | Influenzavirus C | (-) | - |
| Acinetobacter baumannii | 36.44 | 2.36 × 103 | Parainfluenzavirus 1 | (-) | - |
| Stenotrophomonas maltophilia | 25.65 | 4.20 × 106 | Parainfluenzavirus 2 | (-) | - |
| Morganella morganii | (-) | - | Parainfluenzavirus 3 | (-) | - |
| Providencia sp. | (-) | - | Rhinovirus | (-) | - |
| Proteus mirabilis | (-) | - | Respiratory syncytial virus (RSV) | 19.14 | 3.84×108 |
| Citrobacter freundii | (-) | - | Human metapneumovirus | (-) | - |
| Elizabethkingia meningoseptica | (-) | - | Measles virus | (-) | - |
| Fusobacterium nucleatum | 30.13 | 1.88 × 105 | Adenovirus | (-) | - |
| Atypical bacteria | Epstein-Barr Virus (EBV) | 26.81 | 1.88 × 106 | ||
| Mycoplasma | (-) | - | Cytomegalovirus (CMV) | 33.2 | 2.23 × 104 |
| Mycoplasma pneumoniae | (-) | - | Bocavirus | (-) | - |
| Chlamydia pneumoniae | (-) | - | Varicella-Zoster Virus (VZV) | (-) | - |
| Chlamydia trachomatis | (-) | - | Common-cold virus | (-) | - |
| Chlamydia psittaci | (-) | - | Rubella virus | (-) | - |
| Legionella pneumophila | (-) | - | SARS-CoV-2 | (-) | - |
| Bordetella pertussis | (-) | - | Mycobacterium | ||
| Bordetella parapertussis | (-) | - | Mycobacterium tuberculosis | (-) | - |
| Nocardia asteroides | (-) | - | |||
CT, cycle threshold.
Table 2.
Antibiotic susceptibility of Pseudomonas aeruginosa.
| Antibiotic | Result | MIC |
|---|---|---|
| Piperacillin-Tazobactam | Susceptible | 16/4 µg/mL |
| Ceftazidime | Resistant | > 16 µg/mL |
| Cefepime | Susceptible | 8 µg/mL |
| Imipenem | Resistant | > 8 µg/mL |
| Meropenem | Resistant | > 16 µg/mL |
| Gentamicin | Resistant | > 8 µg/mL |
| Amikacin | Susceptible | ≤ 8 µg/mL |
| Ciprofloxacin | Intermediate | 1 µg/mL |
| Ceftazidime-Avibactam | Resistant | > 8/4 µg/mL |
| Colistin | Intermediate | ≤ 1 µg/mL |
MIC, minimum inhibitory concentration.
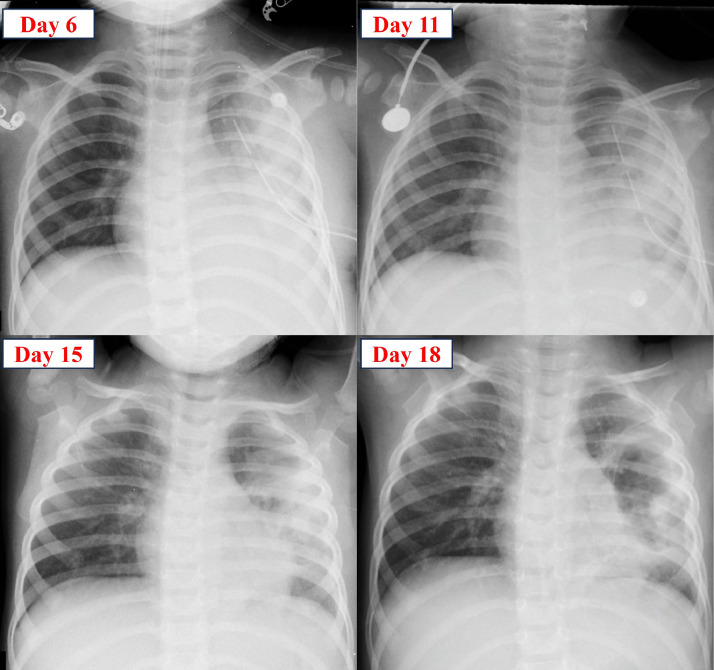
From the eighth day of treatment, the patient received additional amikacin, continued meropenem, vancomycin, and clindamycin. The patient's condition improved with the resolution of fever, reduced CRP levels, and decreased neutrophil count. Chest X-rays showed improvement from the sixth day to the 18th day of treatment (Fig. 6). The patient was discharged on the 35th day of treatment.
Fig. 6.
Progression of the patient's chest X-ray from the sixth day to the 18th day of treatment.
Discussion
Our case describes a complex scenario of NP and PPE associated with an uncommon pathogen, P. aeruginosa, in a healthy 20-month-old child. The patient underwent successful treatment involving a combination of broad-spectrum antibiotics, chest tube placement, and VATS. Through this case, we intend to highlight the complexities of managing NP and PPE associated with P. aeruginosa in children.
Epidemiological studies illustrate inconsistent findings regarding risk factors for NP in children with CAP. Hsieh et al. conducted a retrospective study involving 71 children with NP due to S. pneumoniae in Taiwan, revealing that NP often progresses rapidly in previously healthy children. Identified risk factors for NP included an elevated immature neutrophil count, C-reactive protein levels exceeding 12 mg/dL, and the absence of pre-existing medical conditions [6]. Conversely, another study involving 746 children with NP indicated that complex chronic conditions increased the risk of NP and mortality [2]. Other potential risk factors, such as asthma and prior NSAIDS usage, have yet to be confirmed [1]. Moreover, lower SaO2 levels, higher fever, and elevated CRP levels in the complicated CAP group may predict the likelihood of requiring surgical intervention [3]. Although NP is a rare complication in children with CAP, clinical practitioners must remain alert to the potential rapid progression from CAP to NP in previously healthy pediatric patients.
P. aeruginosa is mainly recognized as a nosocomial infection and frequently causes diseases in immunocompromised, burned, or pediatric patients with cystic fibrosis [7]. However, P. aeruginosa is rarely reported as a pathogen in cases of CAP with NP in children. Common causative pathogens of pediatric NP include S. pneumoniae, S. aureus, M. pneumoniae [4], Haemophilus influenzae, and Acinetobacter baumannii [8]. A review of 197 bacterial or fungal isolates in children with NP revealed that 82% of NP cases were due to S. pneumoniae and S. aureus, with only 3 cases isolating P. aeruginosa (1.5%) [1]. The frequency of NP caused by P. aeruginosa is rare, as reported by Yonghan, accounting for only 1 out of 282 cases [8]. However, in children with complex chronic conditions, the rate of P. aeruginosa infection can increase up to 12.8%, compared to 4% in those without pre-existing medical conditions [2]. In adults, P. aeruginosa is recognized as a severe but rare causative agent of CAP, NP, or cavitation lung disease, often associated with a high mortality rate [9]. In our case, P. aeruginosa, resistant to ceftazidime and carbapenem, was isolated from NTA specimens. We cannot definitively confirm P. aeruginosa as the sole pathogen causing the disease; moreover, based on the multiplex PCR results, S. pneumoniae is also a potential coinfecting agent. We hypothesize that P. aeruginosa is the causative pathogen, given the higher copies per mL of P. aeruginosa compared to S. pneumoniae in the multiplex PCR results. Furthermore, the clinical progression lacks a distinct resolution phase, minimizing the suspicion of P. aeruginosa superinfection in the hospital. However, determining the actual causative agent remains challenging; hence, antibiotic therapy should still encompass common pathogens to address this uncertainty. NP associated with P. aeruginosa in a previously healthy child adds complexity to the treatment, as it is an unusual pathogen in this population. This poses a significant challenge in NP management since empirical antibiotics covering P. aeruginosa are not routinely used in NP cases.
Complicated CAP should be considered if symptoms do not respond to appropriate treatment within 48 to 72 hours [10]. Patients should undergo screening for PPE, NP, lung abscess, sepsis, or metastasis infection. Chest X-rays show lower sensitivity in diagnosing NP than CT scans [1]. Donnelly et al. highlighted that 50% of cases with fluid-filled cavities may be undetected on chest X-rays [11]. In the early stages of NP, the liquefied lung parenchyma not connected to the airway may be challenging to notice on X-rays. In cases of suspected NP, a chest CT scan should be performed. Several studies have used lung ultrasonography as a potential alternative to CT scans in diagnosing NP [12,13]. Lung ultrasonography offers the advantage of being noninvasive and avoiding radiation exposure, especially in the pediatric population. Moreover, impaired perfusion and hypoechoic lesions observed on ultrasonography can predict pneumatocele formation and correlate with necrosis in CT scans [12]. Our case encountered a delay in diagnosing NP, despite the potential for earlier diagnosis through lung ultrasonography. The lung ultrasonography images from the case correlated with findings on CT scans, including pleural thickening, NP, and PPE.
The optimal timing for VATS intervention in cases of treatment failure with antibiotics and chest tube placement has yet to be confirmed. In our case, despite receiving broad-spectrum antibiotics and chest tube placement, the patient's condition did not improve. This could be attributed to the extensive pleural thickening and widespread lung parenchymal necrosis contributing to treatment failure. VATS may aid in removing fibrous walls within the pleural cavity, removing pleural peel to allow lung re-expansion, and eliminating pus from the pleural space under direct visualization. VATS is the first-line intervention when antibiotic treatment and chest tube placement fail, rather than an open thoracostomy [14]. Some authors define complicated NP as extensive necrosis or cavitation larger than 50% of the involved lung lobe [15,16]. Cases of complicated NP tend to require wedge resections or lobectomies, and postoperative complications, pneumothorax, and more extended hospital stays are frequently observed. The recommended timeframe for surgical intervention in cases of NP and PPE unresponsive to appropriate treatment is 7 days [14]. Other situations requiring surgical intervention include complex empyema with significant lung pathology (extensive pleural thickening, trapped lung), bronchopleural fistula, or secondary empyema [14]. Early VATS (e.g., within 24-48 hours of hospital admission) has been proposed by some authors due to its benefits in reducing hospital stay, complication rates, and the number of days with chest tube placement [17,18]. However, as VATS depends mainly on each center's and surgeon's experience, there is currently no consensus on the optimal timing for VATS procedures. Several studies have been conducted to predict extensive necrosis in NP using indicators such as CRP, serum albumin, IgM [19], lung ultrasonography [12], or IFN-γ [20]; however, there are still limited data and predictive factors for the likelihood of requiring surgery during hospitalization.
Conclusion
Our clinical case highlights the complexities of managing NP in children. Multidrug-resistant P. aeruginosa can contribute to severe community-acquired NP in a healthy pediatric patient. Further research is needed to predict the likelihood of P. aeruginosa infection in NP, as empiric antibiotic therapy does not initially cover P. aeruginosa. Pediatric NP inpatients require close monitoring of clinical progression, as there is no consensus on the optimal timing or method of surgical intervention. Successful treatment relies on broad-spectrum antibiotics, chest tube placement, and timely surgical intervention.
Patient consent
Written informed consent was obtained for the publication of this case report.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Masters IB, Isles AF, Grimwood K. Necrotizing pneumonia: an emerging problem in children? Pneumonia. 2017;9:11. doi: 10.1186/s41479-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochinwala NM, Kobaitri K, Totapally BR. Characteristics and outcomes of children with necrotizing pneumonia. Pediatr Crit Care Med. 2021;22(12):e640–e643. doi: 10.1097/PCC.0000000000002793. [DOI] [PubMed] [Google Scholar]
- 3.Masarweh K, Gur M, Toukan Y, Bar-Yoseph R, Kassis I, Gut G, et al. Factors associated with complicated pneumonia in children. Pediatr Pulmonol. 2021;56(8):2700–2706. doi: 10.1002/ppul.25468. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Li L, Wang C, Zhang Y, Zhou Y, et al. Necrotizing pneumonia in children: early recognition and management. J Clin Med. 2023;12(6):2256. doi: 10.3390/jcm12062256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frybova B, Koucky V, Pohunek P, Cejnarova K, Coufal S, Kokesova A, et al. Lung resection in children with necrotizing pneumonia: outcome and follow-up. Eur J Pediatr Surg. 2021;32(3):280–286. doi: 10.1055/s-0041-1725188. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh YC, Hsueh PR, Lu CY, Lee PI, Lee CY, Huang LM. Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by Streptococcus pneumoniae in children in Taiwan. Clin Infect Dis. 2004;38(6):830–835. doi: 10.1086/381974. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Infectious Diseases, American Academy of Pediatrics, David WK, Elizabeth DB, Ruth L, Mark HS. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. American Academy of Pediatrics; 2021. Pseudomonas aeruginosa Infections; pp. 614–616. [Google Scholar]
- 8.Luo Y, Wang Y. Clinical characteristics of necrotizing pneumonia caused by different pathogens. Infect Drug Resist. 2023;16:3777–3786. doi: 10.2147/IDR.S419294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maharaj S, Isache C, Seegobin K, Chang S, Nelson G. Necrotizing pseudomonas aeruginosa community-acquired pneumonia: a case report and review of the literature. Case Rep Infect Dis. 2017;2017:1717492. doi: 10.1155/2017/1717492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly LF, Klosterman LA. Cavitary necrosis complicating pneumonia in children: sequential findings on chest radiography. AJR Am J Roentgenol. 1998;171(1):253–256. doi: 10.2214/ajr.171.1.9648799. [DOI] [PubMed] [Google Scholar]
- 12.Lai SH, Wong KS, Liao SL. Value of lung ultrasonography in the diagnosis and outcome prediction of pediatric community-acquired pneumonia with necrotizing change. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrard J, Bacher S, Rochat-Guignard I, Knebel JF, Alamo L, Meuwly JY, et al. Necrotizing pneumonia in children: chest computed tomography vs. lung ultrasound. Front Pediatr. 2022;10:898402. doi: 10.3389/fped.2022.898402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balfour-Lynn IM, Abrahamson E, Cohen G, Hartley J, King S, Parikh D, et al. BTS guidelines for the management of pleural infection in children. Thorax. 2005;60(Suppl 1):i1–i21. doi: 10.1136/thx.2004.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai J-Y, Yang W, Ming Y-C. Surgical management of complicated necrotizing pneumonia in children. Pediatr Neonatol. 2017;58(4):321–327. doi: 10.1016/j.pedneo.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Reimel BA, Krishnadasen B, Cuschieri J, Klein MB, Gross J, Karmy-Jones R. Surgical management of acute necrotizing lung infections. Can Respir J. 2006;13(7):369–373. doi: 10.1155/2006/760390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurt BA, Winterhalter KM, Connors RH, Betz BW, Winters JW. Therapy of parapneumonic effusions in children: video-assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics. 2006;118(3):e547–e553. doi: 10.1542/peds.2005-2719. [DOI] [PubMed] [Google Scholar]
- 18.Padman R, King KA, Iqbal S, Wolfson PJ. Parapneumonic effusion and empyema in children: retrospective review of the duPont experience. Clin Pediatr. 2007;46(6):518–522. doi: 10.1177/0009922806299096. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Zhang X, Chen B, Ji Y, Chen W, Cai S, et al. Early predictors of lung necrosis severity in children with community-acquired necrotizing pneumonia. Pediatr Pulmonol. 2022;57(9):2172–2179. doi: 10.1002/ppul.26020. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Hu M, Ye B, Chen Z, Zhang Y. Early prediction of necrotizing pneumonia from mycoplasma pneumoniae pneumonia with large pulmonary lesions in children. Sci Rep. 2020;10(1):19061. doi: 10.1038/s41598-020-76083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]