Abstract
The burden of noncommunicable chronic diseases has relevant and negative consequences to persons, health care systems, and economies worldwide. Chronic diseases are the leading cause of disability and mortality and are responsible for 90% of health care expenditure. The most common chronic diseases are diabetes mellitus (DM), cardiovascular disease, and cerebrovascular disease (stroke and vascular cognitive impairment). Modifiable risk factors (MRFs) for these conditions include hypertension, hyperlipidemia, smoking, poor diet, and low-physical activity; with hypertension being the most prevalent MRF. Most MRFs can be successfully targeted through lifestyle medicine (LSM), which is a medical specialty that addresses the root causes of chronic diseases through its primary, secondary, and tertiary preventative approaches. Lifestyle medicine comprises 6 pillars (nutrition, physical activity, sleep health, stress reduction, social connections, and substance use) which through various behavioral approaches, focus on regular physical activity, healthy eating, good quality and quantity sleep, and meaningful social connections coupled with the reduction of stress and substance use. This paper will briefly review the evidence and promise of individual LSM pillars in addressing the underlying MRFs of DM, cardiovascular and cerebrovascular disease (specifically stroke and vascular cognitive impairment). Lifestyle medicine holds a great promise for comprehensive and much improved population health. However, the adoption of LSM at the societal scale requires a multifaceted approach and widespread integration would galvanize a paradigm shift to prevent, treat or reverse chronic diseases from the root causes and achieve health equity.
Article Highlights.
-
•
Noncommunicable chronic diseases (specifically diabetes mellitus, cardiovascular disease, and cerebrovascular disease- stroke and vascular cognitive impairment) have significant and negative consequences to persons, health care systems, and economies worldwide. Noncommunicable chronic diseases share common modifiable risk factors (MRFs), including hypertension, hyperlipidemia, smoking, poor diet, and low-physical activity; with hypertension being the most prevalent MRF.
-
•
The majority of MRFs can be successfully targeted through lifestyle medicine, which is a medical, evidence-based specialty that addresses the underlying root causes of chronic diseases through its primary, secondary, and tertiary preventative approach.
-
•
Lifestyle medicine comprises 6 pillars: nutrition, physical activity, sleep health, stress reduction, social connections, and substance use. Addressing lifestyle habits and factors through these pillars has the potential to address and reduce MRFs, which may moderate the global burden of disease.
-
•
It is imperative for clinicians to account for the unique circumstances and the inextricably interrelated aspects of lifestyle and social determinants of health at the micro, macro, and meso levels.
-
•
Lifestyle medicine has the potential to contribute to the restoration of health by modifying the behaviors that have contributed to the global burden of disease. The adoption of lifestyle medicine requires a multifaceted approach to support this paradigm shift and achieve health equity.
Global Burden of Chronic Disease
The epidemiologic burden of chronic diseases has important implications on persons, health care systems, and economies worldwide, making it a global challenge1 and its prevention and management is a global priority.2 Chronic diseases are the leading cause of disability and mortality, accounting for 74% of deaths worldwide (equivalent to 41 million deaths per year) and projections of this rising to 52 million by 2030.3 Chronic diseases are responsible for 90% of annual health care expenditure4 and by 2030, will account for an accumulated global economic loss of $47 trillion.3 The most common chronic diseases are diabetes mellitus (DM), cardiovascular diseases (CVDs), and cerebrovascular disease (stroke and vascular cognitive impairment (VCI)5; with one in 3 adults living with more than 1 chronic condition worldwide.6 In 2021, there were 529 million persons living with DM (96% of this being type 2 diabetes [T2D]), with projections of this doubling to >1.31 billion globally by 2050 (T2D accounting for 1.27 billion of this).7 All CVD (eg, coronary artery disease [CAD], peripheral arterial disease, and rheumatic heart disease), nearly doubled from 271 million in 1990 to 523 million in 2019.8 Ischemic heart disease (IHD) comprises most of CVD; with 197 million persons living with IHD in 2019 and global trends of mortality increasing (from 1990) to 9.14 million in 2019.8
In parallel to this, stroke and VCI are emerging as major global public health challenges; with stroke accounting for >40% of the global burden of all neurological diseases (∼101 million prevalent strokes), with 12.2 million new stroke cases per year; equivalent to 1 stroke every 3 seconds.9 This is in conjunction with a dramatic rise of stroke among younger adults; ∼40% of strokes occurring among the working and middle-aged population (<65 years of age).10,11 More importantly, ∼ 50% of persons with stroke develop some cognitive impairment and meet clinical and radiographic definitions of VCI (accounting for ∼30% of all dementia diagnoses).12 Presence of VCI affects at least 1 cognitive domain and comprises an entire spectrum of vascular brain pathologies (not only stroke) that contribute to any cognitive deficit-from mild cognitive impairment to dementia.12 As illustrated, the burden of all chronic diseases mentioned above is on a steep rise, likely owing to a current lifestyle in the modern society or the lack of access to care in the developing world.
The global burden of diseases (GBD), specifically for DM, CVD and cerebrovascular disease (stroke and VCI) are attributed to modifiable risk factors (MRFs).8,13 Approximately 90% of stroke,14 ∼70% of CVD or T2D, and ∼40% of dementia have been linked to shared MRFs.15 Common MRFs for all, include hypertension (HTN), hyperlipidemia, hyperglycemia, alcohol consumption, smoking, poor diet, and low-physical activity (PA); with HTN being the most significant of all (40%-60%).15 Currently, HTN as a MRF and an independent chronic disease affects 1 billion persons worldwide with 1 in 3 middle-aged adults living with HTN and worldwide projections of this increasing by ∼60% (1.56 billion) by 2025.16 As illustrated, the risks for DM, CVD, stroke, and VCI are shared through MRFs, many of which can be targeted through comparable lifestyle medicine (LSM) approaches.17
Lifestyle Medicine
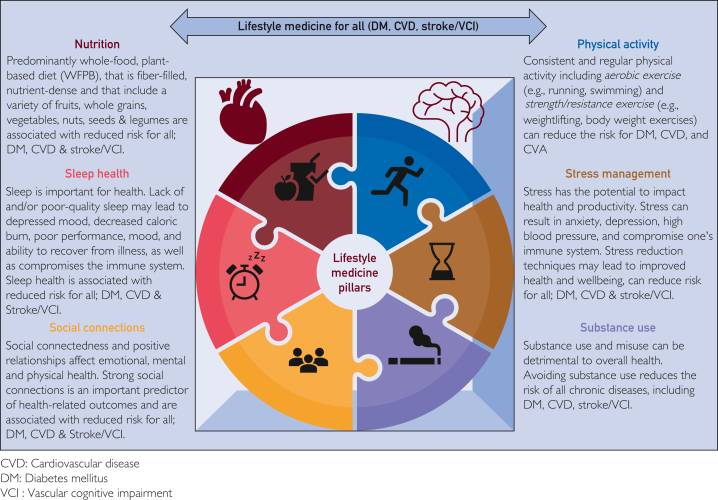
Lifestyle medicine, as defined by the American College of Lifestyle Medicine (ACLM), is a medical specialty that applies behavioral, motivational, environmental, and clinical principles to prevent, treat or manage chronic diseases.17 It addresses the underlying root causes of chronic diseases through its primary, secondary, and tertiary preventative approach; with direct and indirect multiscale effects on overall health, quality of life, well-being, and health care expenditure.18 It comprises 6 pillars: nutrition, PA, sleep health, stress reduction, social connections, and substance use. In the following sections, we will illustrate the importance and promises of individual LSM pillars in addressing the underlying MRFs of DM, cardiovascular and cerebrovascular disease (specifically stroke and VCI) (Figure 1).
Figure 1.
Lifestyle medicine pillars for all DM, CVD, Stroke/VCI.
Nutrition
Nutrition is a fundamental component of lifestyle habits that affect DM, CVD, stroke, and VCI. Suboptimal diet is estimated to be responsible for 1 in 5 premature deaths worldwide.19
Diabetes
One of the most essential components of T2D lifestyle therapy is supporting persons to adopt a healthful and wholesome diet (with adequate calorie restrictions). There is objective importance of >10%-15% body weight loss and implementing a weight-centric approach in treating T2D to reverse the underlying metabolic abnormalities, to improve glycemic control, and CVD risk factors,20 with the goal to achieve T2D remission.21 The T2D remission is defined as a return of hemoglobin A1C (HbA1c) to <6.5% (<48 mmoL/moL) spontaneously or after an intervention; lasting 3 months in the absence of usual glucose-lowering pharmacotherapy. In the event of A1c inaccuracy or unreliability, remission can be defined as fasting plasma glucose <126 mg/dL (<7.0 mmoL/L) or estimated HbA1c <6.5% calculated from continuous glucose monitor values.22 The landmark Diabetes Remission Clinical Trial22 examined the effect of a structured, intensive, weight management program in comparison to usual DM and obesity management delivered in primary care settings to achieve T2D remission. At the end of the 2 years, 36% of the participants (n=194) in the intervention arm stayed in remission, reported a 50% reduction in the use of T2D medications, and higher weight loss with higher rates of T2D remission; with 86% of those patients with T2D who lost ≥15 kg body weight going into remission (OR/kg weight loss 1.25).22 The Diabetes Remission Clinical Trial trial extension data also found that the proportion of participants in T2D remission after 5years were > 3 times to that of the control arm, and still in remission with an average weight loss of 8.9 kg (ie, 20 pounds) at 5 years.23 Lifestyle medicine for DM management emphasizes the incorporation of more nutrient and fiber dense food sources such as nonstarchy vegetables, whole grains, legumes, nuts, seeds and limiting calorie dense, hyperpalatable, nutrient-poor food groups such as ultraprocessed foods (UPF), and red and processed meats.24
CVD or Stroke
Based on the 2022 Global Burden of CVD and the NHLBI-Risks Collaboration study, IHD comprises most of CVD with poor dietary habits (eg, higher dietary sodium, low-whole grains, and fruits intake) accounting for the most associated disease risk.25, 26, 27 In the Framingham Cohort study, every additional serving of UPFs was associated with a 5% (95% CI, 1.02-1.08) and 9% (95% CI, 1.02-1.16) increase in overall CVD risk and CVD mortality, respectively.28 Among all MRFs, HTN is the most prevalent vascular risk for all CVD and all cerebrovascular disease.29 Healthy dietary patterns, such as the Dietary Approaches to Stopping Hypertension diet, the Mediterranean Diet (MeDi), and other plant-predominant diets, hold the most effective promise in reducing that risk, with several randomized control trials (RCTs) confirming the effectiveness of dietary interventions for reduction of blood pressure (BP) and HTN management. The general recommendations for these dietary patterns also include a higher intake of whole grains, legumes, fruits, vegetables, fish, vegetable (olive) oils, seeds, and nuts, and a lower intake of low-fat dairy products and poultry; all resulting in optimal content of fibers, but also macro and micronutrients (specifically low sodium per potassium ratio).30, 31, 32, 33, 34, 35, 36
In a meta-analysis of 30 RCTs, the DASH diet (low sodium, high potassium, predominantly whole food plant-based [WFPB]) considerably lowered BP with the net effect on both systolic (SBP) and diastolic blood pressure (DBP), 5.5 and 3.0 mm Hg, respectively,30 which was achieved by the second week of the diet introduction.31 When combined with additional sodium lowering (1500 mg/day vs 2300 mg [a teaspoon]), the DASH diet had a more powerful SBP lowering effect (∼20 mm Hg).32 Although the DASH study did not include CVD outcomes, other studies showed that lowering BP can dramatically reduce congestive heart disease (CHD) risk and stroke.33 Such findings are consistent with a large meta-analysis (147 RCTs) that also found BP reduction of only 10 mm Hg SBP and 5 mm Hg DBP associated with a 41% stroke reduction for all trials (46% in primary, 44% in secondary prevention, and by 35% in trials including participants with a history of CAD).34
Although the effect of low sodium per potassium ratio may explain some of the DASH diet effects on lowering BP, other studies explored these associations through consumption and measurements of different food items. In the Danish diet, cancer, and health study, after a 23-year follow-up of 53,150 individuals, the participants who consumed the highest intake of vegetable nitrate (141 mg/d) were associated with lower SBP and DBP, whereas even a modest intake of 60 mg/d (1 cup of green leafy vegetables) was associated with a 15% and 17% lower CVD and ischemic stroke risk, respectively.35 In the PREDIMED trial, the MeDi (predominantly WFPB, higher fish and low-meat intake) when supplemented with extra virgin olive oil or mixed nuts vs controls (low-fat diet) was associated with ∼40% of relative stroke risk reduction,36 whereas the provegetarian food pattern consumption when compared with diet, including animal, egg, dairy, or meat products, reported ∼ 40% all-cause mortality rate reduction.37
VCI
Although there are no specific studies around the effects of dietary patterns on VCI specifically, the most compelling evidence on the dietary effect on vascular mediation of dementia risk comes from studies related to increased consumption of food items rich in vitamin E (nuts), acting as an antioxidant, fish, polyunsaturated fats, B12 vitamin, and folates, as parts of the MeDi; showing dementia risk reduction of 20%-40% in higher quartiles of MeDi consumption.38 However, in a systematic review of 56 studies (population per RCT), higher adherence to all predominantly WFPB dietary patterns, such as the DASH, MeDi, and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, was associated with significantly lower dementia risk (∼50%), and even moderate adherence to the MIND diet reduced dementia risk by 35%.39
Key Message on LSM and Nutrition
Currently, a predominantly WFPB dietary pattern is supported by several clinical practice guidelines (the American Diabetes Association,40 the American heart association (AHA)/ACC, and the AHA/American stroke association)41,42 and a low-sodium intake (<2400 mg/d) for CVD and stroke prevention.42 However, minimizing UPF consumption globally is easier said than done without vigorous policy, public health, and cross-sectoral synergized efforts. Health promotion experts advocated for broader evidence-informed policies, making clearer dietary guidelines, unambiguous food labeling, taxation, restriction of fast-food outlets near schools, and reducing the size and appeal of food portions and packages, coupled with fresh food prescriptions for all.41 Simple and cost-effective lifestyle strategies with implementation of whole food, predominantly WFPB dietary patterns is the most effective tool in combating GBD, including DM, CVD, and stroke or VCI. Effective counseling with LSM specialists or nutritionists on dietary behavior change and WFPB dietary food items is crucial to address the root causes of lifestyle-related chronic diseases.
Physical Activity
Physical activity is critical for overall health and quality of life in conjunction with its substantial effect across multiple organ systems and on the risk of developing DM, CVD, and stroke or VCI. The direct and indirect effects of PA are broad, complex, and poorly understood at a molecular level.43 However, numerous exercise-related signaling pathways and molecules, called exerkines, have been identified. The immune, nervous, and cardiometabolic systems produce and are influenced by exerkines, contributing to the PA response.43 The biological effects of PA vary and include improved energy utilization, angiogenesis, neurogenesis, endothelial and immune function, increased skeletal muscle mass and mitochondria, and inflammation mediation.43 The benefits of PA on primary and secondary prevention of DM, CVD, stroke, and cognitive function are undeniable.42,44
DM and CVD
Worldwide, 7.2% of CVD deaths are attributed to physical inactivity.45 Physical inactivity also substantially affects the risk of developing CAD, stroke, HTN, and T2D, in both low-income and high-income countries (Table 1).46 The benefits of both aerobic and resistance exercise on lowering BP and improving lipids (Table 2).45,47 Exercise interventions are comparable with medication in mortality benefits in the secondary prevention of heart disease, rehabilitation after stroke, treatment of heart failure, and prevention of DM.33 These findings are consistent with a meta-epidemiologic study of 305 RCTs that found PA interventions to be more effective in reducing mortality than drug intervention among patients with stroke (odds ratio [OR], 0.09-0.10 exercise vs anticoagulants and antiplatelets, respectively),33 although such an effect was not significant for CHD or prediabetes. Shifting from medication to medical procedures, PA has been shown to be superior to percutaneous coronary intervention (PCI) in patients with stable CAD. A 12-month RCT (n=101) that compared an exercise training program (20 minutes of bicycle ergometry daily) to PCI among patients with stable CAD found a significantly higher event-free survival (88% vs 70% in the PCI group), increased maximal oxygen uptake, and lowered cost ($3429 vs $6956) among those in the exercise training program when compared with PCI.48
Table 1.
Population Attributable Risk of Physical Inactivity on Cardiovascular Disease and All-Cause Mortality in Low-Income and High-Income Countries46
| Adapted from Katzmarzyk, et al46 | Population Attributable Risk Low-Income Country |
Population Attributable Risk High-Income Country |
|---|---|---|
| Cardiovascular Mortality | 4.6% | 9.9% |
| Coronary Heart Disease | 3.0% | 6.5% |
| Stroke | 3.0% | 6.5% |
| Hypertension | 1.0% | 2.2% |
| Type 2 Diabetes | 2.7% | 5.9% |
| All-Cause Mortality | 4.3% | 9.3% |
Table 2.
Blood Pressure and Lipid Effects of Regular Aerobic and Resistance Exercisea
| Adapted from Eckel et al47 | Aerobic Exerciseb | Resistance Exercisec |
|---|---|---|
| Systolic blood pressure | −2-5 mm Hg | Small |
| Systolic blood pressure (patients with HTN) | −8.69 mm Hg | −7.23 mm Hg |
| Diastolic blood pressure | −1-4 mm Hg | Slight |
| HDL-C | No change | No change |
| LDL-C | −3-6 mg/dL | −6-9 mg/dL |
| Non-HDL-C | −6 mg/dL | −6-9 mg/dL |
| Triglycerides | No change | −6-9 mg/dL |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; Non-HDL-C, non-high- density lipoprotein cholesterol.
Effective aerobic BP and lipid lower interventions involve moderate-intensity to vigorous-intensity physical activity and are an average of 12-week duration, with 3-4 sessions per week, lasting 40 minutes per session.
Effective resistance training interventions for lipid lowering effects last for an average of 24 weeks, including 3 or more sessions per week, 9 exercises per session, for 3 sets of 11 repetitions at 70% of 1 repetition maximum.
Stroke or VCI
Engagement in any type of PA has been shown not only to reduce stroke risk but also the progression of cognitive decline and dementia, including VCI.46 Physical activity can enhance neuronal connections, maintain neuronal plasticity and improve the release of neurotrophic factors.49 Although the RCTs have not yet been performed, a meta-analysis of 18 cohort and 5 case-control studies, showed lower stroke incidence and mortality (∼30%) with moderate to high PA,50 whereas high levels of occupational PA reduced stroke risk by 43%, and high levels of leisure-time PA reduced stroke risk by 20%-25% when compared with inactivity.51 Similar findings were noted in a systematic review on PA and VCI,52 in which the overall effect of PA on VCI reduction was ∼30% (hazard ratio [HR], 0.68; 95% CI, 0.54-0.86; I2 6.8%); with higher levels of PA associated with lower VCI risk overtime; together supporting the AHA recommendations for prevention of stroke and VCI.45,53
Key Message on LSM and PA
Physical activity is a highly beneficial lifestyle approach in the long-term prevention, management or treatment of chronic diseases such as DM, CVD, and stroke or VCI. The dose (frequency, intensity, duration, and activity type) of PA to achieve specific health outcomes varies. Currently, the PA and clinical guidelines recommend adults with or without chronic disease, obtain ≥150-300 min/wk of moderate-intensity or 75-150 minutes of vigorous-intensity aerobic activity, coupled with ≥2 resistance training sessions per week. Older adults should also incorporate PA that includes a balance component.54,55 Physical activity prescription should be encouraged during each clinic visit through general counseling on PA guidelines with referral to rehabilitation or exercise specialists within the community, when appropriate.
Sleep Health
Sleep is an integral contributor to cardiometabolic and brain health. Sleep is also a foundation for overall improvement in other lifestyle pillars.56 The evidence on sleep health and DM, CVD, stroke, and VCI is growing, and most of existing research are from observational studies.57
DM, CVD, Stroke, or VCI
Poor sleep has been consistently identified as a risk factor for poor glycemic control in T2D. In a cross-sectional study,58 total sleep duration and subjective sleep quality (measured by Pittsburgh Sleep Quality Index with scores ≥6 indicating poor sleep) were significantly associated with higher HbA1c; explaining 10.3% of the HbA1c variance. A review of studies published between 2015 and 2020 also showed a consistent association of sleep variability with increased risk for adiposity, glucose dysregulation, and T2D.59 Sleep perturbations also has a negative effect on CVD and cardiometabolic health.60 In a systematic review and meta-analysis of 15 prospective studies60 among 474,684 participants, short sleep duration was associated with a greater risk of developing or dying of CHD (risk reduction [RR], 1.48; 95% CI, 1.22-1.80; P<.0001) and stroke (RR, 1.15; 95% CI, 1.00-1.31; P=.047). Similarly, long sleep duration was also associated with a greater risk of CHD (1.38, 95% CI, 1.15-1.66; P=.0005), stroke (1.65, 95% CI, 1.45-1.87; P<.0001), and total CVD (1.41, 95% CI, 1.19-1.68; P<.0001). The findings regarding the negative effect of sleep on VCI risk are also consistent with a population-based study, in which persons who slept <6 hours reported a higher risk for possible VCI61 and 30% increased risk of dementia later in life, respectively.62
Key Message on LSM and Sleep
Compromised sleep health has deleterious consequences on DM, CVD, and stroke or VCI; highlighting the importance of LSM interventions and preventative approaches (eg, sleep hygiene, sleep education, relaxation techniques, and cognitive behavior therapy) to promote healthy sleep, specifically sleep duration and quality to improve cardiovascular and neurocognitive outcomes. Sleep health should be assessed during clinical visits and a referral to LSM, or a sleep specialist should be encouraged if indicated.
Stress Reduction
Stress, which is considered to be the health epidemic of the 21st century,63 is undeniably linked with increased risk of DM, CVD, stroke, and cognitive impairment.64 Stress contributes to hormonal dysregulation, maladaptation of the neuroendocrine pathways, increased sheer stress, and plaque vulnerability, disrupts the immune system function, causes chronic inflammation,65 increases sugar and fat levels in the blood, and perpetuates unhealthy lifestyle habits such as overeating, smoking, and drinking.64
DM or CVD
It is well documented that environmental stress from social determinants of health (SDOH) (eg, socioeconomic status, education level, access to health care, living in food deserts, lack of safe outdoor space for exercise, race, ethnicity, gender, or other minority status, and adverse childhood experiences) contribute to higher T2D risk.20 Chronic stress and burnout, such as job strain, marital discord, important adverse life events, caregiving duties, and perceived discrimination have also been found to contribute to obesity, HTN, atrial fibrillation, strokes, myocardial infarction, and sudden death.66 Chronic stress and burnout also lead to poor coping mechanisms and adverse behaviors, such as smoking, increased alcohol intake, poor sleep habits, and unhealthy dietary choices (eg, eating higher sugar or fat foods); further increasing the rate of adverse cardiovascular and cerebrovascular outcomes and aggravating dysglycemia.
Various studies highlight the beneficial DM and CVD (including stroke) outcomes associated with stress reduction techniques (eg, meditation, yoga, tai-chi, visualizations, modified slow breathing, and progressive muscle relaxation techniques). For example, in a meta-analysis67 of 28 RCTs on mind-and-body based interventions (eg, meditation, yoga, qigong, mindfulness-based stress reduction) in conjunction with standard DM treatment for persons with T2D, an average HbA1c reduction of 0.84% (compared with standard treatment alone) was noted; an impressive finding particularly given that many of these studies were conducted in lower middle-income countries, such as India where there are numerous and additional SDOH-related stressors. Similarly, in a study of the Transcendental Meditation program, improvements in CVD risk factors, surrogate end points, and lower mortality among African Americans and other populations were noted. More specifically, the program was associated with a 48% RR in the composite of mortality, nonfatal MI, and nonfatal stroke in African American men and women with CHD, with a mean of 5.4 years follow-up.68 Comparable results of a RCT in which a 12-week slow breathing exercise training intervention significantly decreased (P<.05), heart rate, SBP, and DBP for participants compared with the control arm.69 Of note, the exact effect of many stress-reducing techniques on important CVD endpoints still requires further investigation, mostly because all stress and burnout studies rely on self-reporting and objective measures are lacking.
Stroke or VCI
There is limited research on the role of psychosocial risk factors, including stress, on the risk of stroke and VCI. It has been proposed that stress-related changes in hormones and immune system may lead to neural degeneration and the development of cognitive impairment.65 This was to some degree illustrated in a 35-year longitudinal population study70 that observed an association between psychological stress in middle-aged women and the development of dementia, specifically AD, later in life. In parallel, high-stress intensity doubled the risk of fatal stroke compared with persons who were stress-free (RR, 1.89; 95% CI,1.11-3.21), among participants in the 13-year Copenhagen City Heart Study. However, there are no specific interventions that have been studied for stress reduction in primary prevention of stroke or VCI other than those mentioned above.
Key Message on LSM and Stress Reduction
It is undeniable that stress has negative consequences on DM, CVD, and stroke or VCI; highlighting the importance for more studies on stress and burnout pathophysiology, but also on management techniques as one of the components of LSM to normalize stress management; hence, improve sleep habits, alcohol craving coupled with reducing CVD events, both acute and long-term, and improving cardiovascular and neurocognitive outcomes. An assessment of stress level, even in the most simplified forms during clinical visits is suggested, and appropriate referrals to LSM specialists or health coaches and when appropriate, psychologists are encouraged.
Social Connections
Our core fundamental existence as a human species is to be socially connected. Social connections are a web of relations that enable persons to experience a sense of belonging, being loved, cared for, esteemed, and part of mutual obligations. Social connections, which are important for physical, emotional, and mental health transform one’s whole health and well-being.71 This was articulated by the US Surgeon General: “What if there is something in our everyday lives that can transform our whole health and well-being? … That something is called Social Connection.”71 Unfortunately, in a recent study exploring human connections across diverse regions around the world, only 39% of adults in the United States, felt connected to others.72 This is in conjunction with a postpandemic look at the state of loneliness in America with alarming trends of ∼ 60% reporting being lonely; with the prevalence of loneliness among seniors being 44% and ∼ 80% among young adults.73 The complex interaction between these various components and their effect on health and disease are often underappreciated and yet, Medicare is spending an estimated $6.7 billons annually on persons with poor social connections.74
DM and CVD
There is strong evidence75, 76, 77, 78 in support of poor social connections and increased T2D and CVD risk. The risk of premature death from loneliness and social isolation was estimated to be similar to that of smoking 15 cigarettes per day.76 Specific to T2D, a large, 20-year longitudinal Norwegian population study found that persons who self-reported the loneliest score had a 2-fold higher risk of developing T2D relative to those who did not feel lonely (adjusted OR 2.19 [1.16-4.15]).75 Conversely, a systematic review of 17 studies (9 cross-sectional and 8 interventional) on social support (studied in 4 categories: emotional, tangible, informational, and companionship), noted improved DM-related clinical outcomes (HbA1c, BP, and lipids) with higher social support levels.77 More importantly, in 2 studies the relationship between social support and mortality was investigated; higher social support levels decreased mortality rates in patients with T2D.77 Specific to cardiovascular risk, for CAD, the data are mixed. In a systematic review and meta-analysis of longitudinal observational studies, it was noted that poor social relationships were associated with a 29% increased CHD risk (pooled RR, 1.29; 95% CI, 1.04-1.59) and a 32% increase in stroke risk (pooled RR, 1.32, 95% CI, 1.04-1.68).78 In parallel, a population-based cohort study (n=119,894; 20 countries per 5 continents) showed increased stroke risk (HR,1.23; 95% CI, 1.07-1.40) and CVD (HR,1.15; 95% CI, 1.05-1.25) with social isolation.79
Stroke or VCI
Empirical evidence on the association between social connections with stroke and VCI are sparse but emerging.12,80 Social isolation and loneliness have been found to be an independent risk factor for cardiovascular and brain health.80 In a meta-analysis of longitudinal observational studies, loneliness and social isolation were found to increase stroke risk incidences among persons by 32% after adjustments to sex, age, and socioeconomic status.78 Such findings are consistent with several prospective cohort studies in which persistent loneliness in midlife and older age, was significantly associated with a decrease in cognitive function,81 dementia and AD, whereas recovery from loneliness suggested resilience to dementia risk.82
Key Message on LSM and Social Connections
As detailed above, social connections have negative consequences on DM, CVD, and stroke or cognitive function. This highlights the importance of social connectedness (eg, connecting with family and friends, and participating in a peer or social network) as one of the components of LSM to improve cardiovascular and neurocognitive outcomes. During clinical visits, health care providers (HCPs) are highly encouraged to promote social connections as a health promotion tool to all patients.
Substance Use
Substance use (eg, smoking, vaping, illicit drug use, and alcohol drinking) has become prevalent in developed and developing countries, and all imposing considerable, and negative effects on T2D, CVD and stroke or cognitive impairment.
DM and CVD
Substance use (smoking, alcohol misuse, and illicit drug use) has a negative effect on T2D and is associated with early onset cardiac and cerebrovascular events.40, 41, 42 This is largely because of the effect of substances on the following: (a) glucose metabolism; (b) transient increase in BP (particularly with binge drinking); and (c) cells and endothelial cell function, arterial-vascular function, and hormone imbalance.83 Among young adults (>35year old), irrespective of gender, being ever smokers was associated with higher prevalence of DM.84 Chronic, heavy alcohol consumption (≥14 drinks/wk for man and ≥7drinks/wk for woman) were also found to increase the risk of HTN, DM complications, but also the risk of CVD and all stroke42 and VCI.53 Similarly, in a population-based study, young persons who used cannabis had a higher prevalence of cardiovascular risk factors, specifically HTN, CAD, dyslipidemia, DM, and obesity in conjunction with greater predilection for substance abuse (including tobacco, cocaine, and alcohol).85
Stroke or VCI
Substance use changes the brain chemistry and circuitry and affects or disrupts multiple regions of the brain (eg, brain stem, cerebral cortex, and limbic system).86 Chronic alcohol use may increase cytokines, prostaglandins, toll-like receptor activation, microglia activation, and inducible nitric oxide synthase; all of which lead to neurodegeneration and neuronal loss.49 The negative effect of substance use on the brain health was observed in the Honolulu-Asia Aging Study, that found middle-aged smoking resulted in higher risk of cognitive impairment later in life.87 Similarly, in a nationwide retrospective cohort study,88 alcohol use disorder was found to be the strongest MRF for dementia onset for all (P<.0001), highlighting the importance of cessation-related efforts through lifestyle. It is important to note that because of the heterogeneity of the definition of alcohol consumption, the findings on the effect of alcohol use on cognition are inconsistent.53
Key Message on LSM and Substance Use
As detailed above, substance use, which is by itself an MRF, has negative consequences on DM, CVD, and stroke or VCI. Using robust pillars of LSM, such as prevention and mitigation of stress and burnout, and responsible alcohol consumption or abstinence, can considerably improve cardiovascular, cerebrovascular, and neurocognitive outcomes. Evidence-based counseling around substance use, with a trauma-informed and nonjudgmental approach in conjunction with appropriate referrals to specialized programs, is encouraged during clinical visits for all patients to support overall health and prevention of chronic diseases.
Discussion
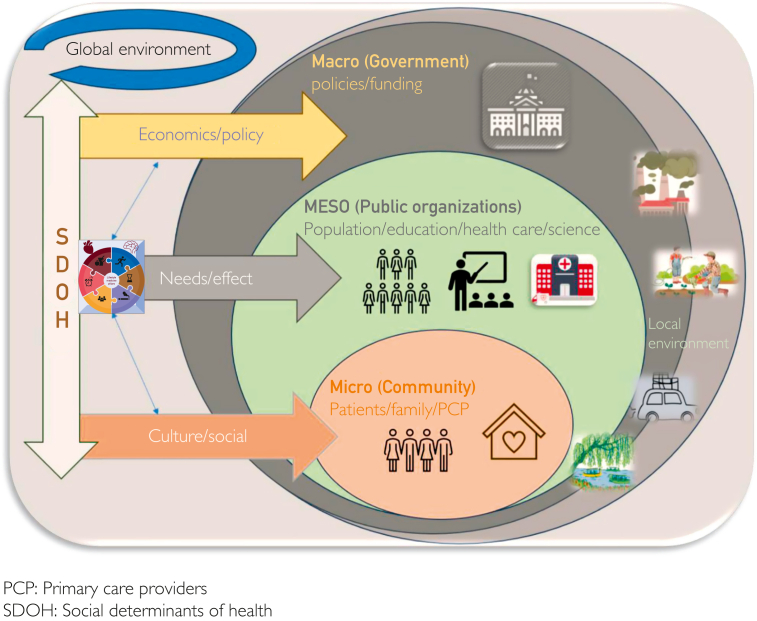
As illustrated, the wealth of clinical and observational evidence (Table 3) reports that addressing lifestyle factors—regular PA, healthy eating, good quality and quantity sleep, meaningful social connections coupled with the reduction of stress and substance use—through the 6 LSM pillars has the potential to address and reduce these MRFs and in turn, moderate the GBD (including but not limited to DM, CVD, and stroke or VCI). Lifestyle medicine, through its holistic, person-centered and root-cause approach, is also the most fundamental and promising approach to achieving health equity in conjunction with optimizing health and well-being.89 For the effective implementation of the 6 LSM pillars (Table 4), it is imperative for HCPs to account for the unique circumstances and the inextricably interrelated aspects of lifestyle and SDOH, at the micro, macro, and meso level.90 This is because recommending lifestyle changes (eg, a predominantly WFPB eating pattern and reducing stress) without understanding circumstances (eg, income and work status), access to necessary resources (eg, grocery store) and the SDOH that support or impede the lifestyle changes (eg, cultural, social, and economic constructs), poses a risk of unintentionally perpetuating health disparity for structurally marginalized persons and populations, without achieving desirable behavior changes and addressing the MRFs91 (Figure 2).
Table 3.
| Chronic Condition | Diabetes (ADA) | CVD (AHA/ACC) | Stroke (AHA/ASA) | VCI (ASA) |
|---|---|---|---|---|
| Lifestyle Pillar | ||||
| Nutrition | ||||
| Physical activity | ||||
| Sleep | ||||
| Stress | ||||
| Social connections | ||||
| Substance use | ||||
| Smoking | ||||
| Alcohol | ||||
Table 4.
General Recommendations Through LSM Pillars for Prevention/Management of MRFs, DM, CVD, Stroke/VCI
| LSM Pillar | Recommendations (Key messages) |
|---|---|
| Nutrition | • DASH Diet • MeDI Diet • MIND Diet General recommendations for these dietary patterns include the higher intake of whole grains, legumes, fruits, vegetables (green leafy), fish, vegetable (olive) oils, seeds, and nuts, and the lower intake of low-fat dairy products and poultry; all resulting in optimal content of fibers, but also macro and micronutrients (specifically low sodium/potassium ratio). |
| Physical Activity | • ≥150-300 min/week of moderate intensity and/or 75-150 min of vigorous intensity of aerobic activity • ≥ 2 resistance training sessions per week • For older adults, incorporate PA that includes a balance component |
| Sleep Health | • Healthy sleep-quality & sleep duration (7-9h/night) • Cognitive Behaviour Therapy • Sleep Hygiene |
| Stress Management | • Mind and-body interventions such as meditation, yoga, tai-chi, qigong, visualizations, modified slow breathing, and progressive muscle relaxation techniques |
| Social Connections | • Connections with friends and family • Part of a broader social network such as volunteering, group-activities, etc. |
| Substance Use | • Smoking cessation • Responsible alcohol consumption or abstinence |
DASH: Dietary Approaches to Stopping Hypertension
MeDI: Mediterranean Diet
MIND: Mediterranean-DASH Intervention for Neurodegenerative Delay
Figure 2.
Lifestyle medicine at the micro, macro, and meso level.
Building health equity is within the scope and mission of LSM and can be achieved through collaborative, organized, multilevel, person-centered, disease prevention and economically feasible innovations and efforts at local, national, and global levels.92 Innovations and efforts to address health inequity through LSM and in turn, include but are not limited to (a) Community-engaged Lifestyle Medicine (CELM); (b) clinicians and HCP’s education to improve health equity in practice; (c) innovating care delivery; (d) using community-based participatory research; and (e) advocating for public policy change (eg, food access and environmental health).
Community-Engaged Lifestyle Medicine
Community-engaged Lifestyle Medicine is an evidence-based framework for promoting health equity through multistakeholder, multilevel, collaborative, community-engaged, culturally responsive and intersectoral approaches for structurally marginalized populations.92 Through CELM, partnering with the community of interest supports the development of culturally appropriate, tailored, acceptable, and meaningful LSM interventions. In conjunction, intersectoral partnerships, through CELM, supports the coordination of services and resources from social, environmental, and political sectors to advance and sustain community-driven LSM interventions and initiatives.92
Clinician and HCP’s Education to Improve Health Equity in Practice
There is limited LSM curriculum and training in medical education.89,91 Addressing this limitation in medical and health care education is to enhance HCPs’ knowledge and understanding of LSM particularly as a recommended first-line of prevention and treatment based on many chronic disease guidelines. For example, the ACLM has various free resources for HCPs to enhance their knowledge, skills, and confidence in delivering LSM interventions. In addition, the ACLM offers online continued medical education courses in conjunction with in-person and virtual conferences to educate and guide HCPs in integrating LSM into their respective practice.89 In parallel with LSM-related education, educating HCPs about the history and current state of health disparities among structurally marginalized populations is integral to inform their LSM practice in a trauma-informed, meaningful, and person-centered manner.
Innovating Care Delivery
Shared medical appointments (SMAs), defined as multiple patients collectively seen in a group visit for the treatment or management of a shared medical condition, is an emergent model of care to offset the noted challenges of the current care delivery processes and structure for chronic disease.93 Delivering LSM through a group visit model (such as SMAs) has shown improvements in patient clinical outcomes (eg, BP and weight management), resource utilization, provider-patient interaction, and patient self-efficacy, satisfaction and trust.94 This is in conjunction with the SMA providing social support for persons that may encourage, motivate, and sustain lifestyle changes.89 Teaching kitchens are another innovative care delivery model in which communities, clinics, organizations and patients can learn about healthy foods and how to prepare the food through practical and hands-on learning; shifting away from the passive and abstract learning.89
Using Community-Based Participatory Research
The community-based participatory research uses research and social activist practices through the engagement and equal collaboration of community members, persons with lived experience, organizational representatives, and researchers throughout the research process.95 The community-based participatory research approach is driven by the 3 concepts—participation, action, and community95 with the goals of: (1) producing a meaningful social change to reduce health disparities and improve health; and (2) developing innovative, meaningful, and sustainable initiatives such as LSM interventions, based on the community’s unique circumstances, needs and social and health goals.95
Advocating for Public Policy Change
It is imperative to leverage public policy levers at local and national levels to advance and support the adoption of LSM. Policies ought to focus on (a) government subsidies to healthier foods; (b) supporting access to food and nutrition services (appropriate sourcing); (c) improving the physical and social conditions of underdeveloped and under-resourced neighborhoods and communities; (d) the provision of more reimbursement options for LSM activities (eg, gym and parks for PA); (e) integrating LSM in medical education (eg, all HCPs); and (f) integrating SDOH within guidelines and recommendations on lifestyle and behavior change.89,91
In summary, LSM has the potential to address most of MRFs for DM, CVD, stroke or VCI; contributing to the restoration of health by modifying the behaviors that have contributed to the GBD. Lifestyle medicine adoption requires a multifaceted approach and widespread integration would galvanize a paradigm shift to prevent, treat or reverse chronic diseases from the root causes, by focusing on the MRFs, and achieving health equity. Lifestyle medicine as a health promoting and evidence-based discipline is here to empower policymakers and stakeholders to actively work with HCPs and communities on developing economically sustainable, person-centered, and innovative health care models that are scalable to achieve health equity.
Potential Competing Interests
The authors have no conflict of interest.
Footnotes
Grant Support: Dr. Pikula receives support from the Jay and Sari Sonshine Chair in Stroke Prevention and Cerebrovascular Brain Health, University of Toronto, University Health Networks, Toronto.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) About chronic diseases. Centers for Disease Control and Prevention. https://www.cdc.gov/chronicdisease/about/index.htm [PubMed]
- 2.Hajat C., Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Rep. 2018;12:284–293. doi: 10.1016/j.pmedr.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Financial Burden of NCDs NCD Alliance. https://ncdalliance.org/why-ncds/the-financial-burden-of-ncds
- 4.Holman H.R. The relation of the chronic disease epidemic to the health care crisis. ACR Open Rheumatol. 2020;2(3):167–173. doi: 10.1002/acr2.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noncommunicable diseases. World Health Organization https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases#:∼:text=Noncommunicable%20diseases%20(NCDs)%20kill%2041,%2D%20and%20middle%2Dincome%20countries
- 6.Marengoni A., Angleman S., Melis R., et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 7.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):P203–P234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boot E., Ekker M.S., Putaala J., Kittner S., De Leeuw F.E., Tuladhar A.M. Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry. 2020;91(4):411–417. doi: 10.1136/jnnp-2019-322424. [DOI] [PubMed] [Google Scholar]
- 11.Singhal A.B., Biller J., Elkind M.S., et al. Recognition and management of stroke in young adults and adolescents. Neurology. 2013;81(12):1089–1097. doi: 10.1212/WNL.0b013e3182a4a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rundek T., Tolea M., Ariko T., Fagerli E.A., Camargo C.J. Vascular cognitive impairment (VCI) Neurotherapeutics. 2022;19(1):68–88. doi: 10.1007/s13311-021-01170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell M.J., Chin S.L., Rangarajan S., et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 15.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 17.Rippe J.M. Lifestyle Medicine: the health promoting power of daily habits and practices. Am J Lifestyle Med. 2018;12(6):499–512. doi: 10.1177/1559827618785554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edington D.W., Burton W.N., Schultz A.B. Health and economics of lifestyle medicine strategies. Am J Lifestyle Med. 2020;14(3):274–277. doi: 10.1177/1559827620905782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingvay I., Sumithran P., Cohen R.V., Le Roux C.W. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394–405. doi: 10.1016/S0140-6736(21)01919-X. [DOI] [PubMed] [Google Scholar]
- 21.Riddle M.C., Cefalu W.T., Evans P.H., et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438–2444. doi: 10.2337/dci21-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lean M.E., Leslie W.S., Barnes A.C., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 23.Weight loss puts type 2 diabetes into remission for five years. Newcastle University. https://www.ncl.ac.uk/press/articles/latest/2023/04/type2diabetesintoremissionfor5years/
- 24.Rosenfeld R.M., Kelly J.H., Agarwal M., et al. Dietary interventions to treat type 2 diabetes in adults with a goal of remission: an expert consensus statement from the American college of lifestyle medicine. Am J Lifestyle Med. 2022;16(3):342–362. doi: 10.1177/15598276221087624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 26.GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He F.J., Tan M., Ma Y., MacGregor G.A. Salt reduction to prevent hypertension and cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(6):632–647. doi: 10.1016/j.jacc.2019.11.055. [DOI] [PubMed] [Google Scholar]
- 28.Juul F., Vaidean G., Lin Y., Deierlein A.L., Parekh N. Ultra-processed foods and incident cardiovascular disease in the Framingham offspring study. J Am Coll Cardiol. 2021;77(12):1520–1531. doi: 10.1016/j.jacc.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 29.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filippou C.D., Tsioufis C.P., Thomopoulos C.G., et al. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(5):1150–1160. doi: 10.1093/advances/nmaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ettehad D., Emdin C.A., Kiran A., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 32.Juraschek S.P., Miller E.R., Weaver C.M., Appel L.J. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841–2848. doi: 10.1016/j.jacc.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naci H., Ioannidis J.P.A. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347(oct01 1) doi: 10.1136/bmj.f5577. f5577-f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaciong Z., Siński M., Lewandowski J. Blood pressure control and primary prevention of stroke: summary of the recent clinical trial data and meta-analyses. Curr Hypertens Rep. 2013;15(6):559–574. doi: 10.1007/s11906-013-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bondonno C.P., Dalgaard F., Blekkenhorst L.C., et al. Vegetable nitrate intake, blood pressure and incident cardiovascular disease: Danish diet, cancer, and health study. Eur J Epidemiol. 2021;36(8):813–825. doi: 10.1007/s10654-021-00747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees K., Takeda A., Martin N., et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019;3(3):CD009825. doi: 10.1002/14651858.CD009825.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martínez-González M.A., Sánchez-Tainta A., Corella D., et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(suppl 1):320S–328S. doi: 10.3945/ajcn.113.071431. [DOI] [PubMed] [Google Scholar]
- 38.Scarmeas N., Stern Y., Mayeux R., Manly J.J., Schupf N., Luchsinger J.A. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H., Dhana K., Huang Y., et al. Association of the mediterranean dietary approaches to stop hypertension intervention for neurodegenerative delay (MIND) diet with the risk of dementia. JAMA Psychiatry. 2023;80(6):630–638. doi: 10.1001/jamapsychiatry.2023.0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craig W., Mangels A.R. Position of the American Diabetes Association: vegetarian diets. J Am Diet Assoc. 2009;109(7):1266–1282. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Hess A., Passaretti M., Coolbaugh S. Fresh Food Farmacy. Am J Health Promot. 2019;33(5):830–832. doi: 10.1177/0890117119845711d. [DOI] [PubMed] [Google Scholar]
- 42.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College Of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e563–e595. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chow L.S., Gerszten R.E., Taylor J.M., et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ElSayed N.A., Aleppo G., Aroda V.R., et al. Addendum 3. Prevention or delay of diabetes and associated comorbidities: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S41–S48. doi: 10.2337/dc23-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckel R.H., Jakicic J.M., Ard J.D., et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25_suppl_2) doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [Google Scholar]
- 46.Katzmarzyk P.T., Friedenreich C., Shiroma E.J., Lee I.M. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br J Sports Med. 2022;56(2):101–106. doi: 10.1136/bjsports-2020-103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naci H., Salcher-Konrad M., Dias S., et al. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53(14):859–869. doi: 10.1136/bjsports-2018-099921. [DOI] [PubMed] [Google Scholar]
- 48.Hambrecht R., Walther C., Möbius-Winkler S., et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109(11):1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 49.Jaqua E., Biddy E., Moore C., Browne G. The impact of the six pillars of lifestyle medicine on brain health. Cureus. 2023;15(2) doi: 10.7759/cureus.34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee C.D., Folsom A.R., Blair S.N. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34(10):2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 51.Wendel-Vos G.C., Schuit A.J., Feskens E.J., et al. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33(4):787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 52.Vítor J., Melita C., Rodrigues M., et al. Physical activity in vascular cognitive impairment: systematic review with meta-analysis. J Stroke Cerebrovasc Dis. 2023;32(8) doi: 10.1016/j.jstrokecerebrovasdis.2023.107133. [DOI] [PubMed] [Google Scholar]
- 53.Gorelick P.B., Scuteri A., Black S.E., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Physical Activity Guidelines for Americans. 2nd ed. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
- 55.Colberg S.R., Sigal R.J., Yardley J.E., et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dedhia P., Maurer R. Sleep and health—a lifestyle medicine approach. J Fam Pract. 2022;71(suppl 1 Lifestyle):S30–S34. doi: 10.12788/jfp.0295. [DOI] [PubMed] [Google Scholar]
- 57.Koo D.L., Nam H., Thomas R.J., Yun C.H. Sleep disturbances as a risk factor for stroke. J Stroke. 2018;20(1):12–32. doi: 10.5853/jos.2017.02887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brouwer A., Van Raalte D.H., Rutters F., et al. Sleep and HbA1c in patients with type 2 diabetes: which sleep characteristics matter most? Diabetes Care. 2020;43(1):235–243. doi: 10.2337/dc19-0550. [DOI] [PubMed] [Google Scholar]
- 59.Zuraikat F.M., Makarem N., Redline S., Aggarwal B., Jelic S., St-Onge M.P. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diab Rep. 2020;20(8):38. doi: 10.1007/s11892-020-01324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cappuccio F.P., Cooper D., D’Elia L., Strazzullo P., Miller M.A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Mendoza J., He F., Calhoun S.L., Vgontzas A.N., Liao D., Bixler E.O. Objective short sleep duration increases the risk of all-cause mortality associated with possible vascular cognitive impairment. Sleep Health. 2020;6(1):71–78. doi: 10.1016/j.sleh.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabia S., Fayosse A., Dumurgier J., et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. doi: 10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stress: The Health Epidemic of the 21st Century. Published online 2016. http://scitechconnect.elsevier.com/stress-health-epidemic-21st-century/ SciTech Connect (elsevier.com)
- 64.Heart and Stroke: Stress Basics. https://www.heartandstroke.ca/healthy-living/reduce-stress/stress-basics#:∼:text=stroke%20and%20stress-,There%20are%20undeniable%20links%20between%20heart%20disease%2C%20stroke%20and%20stress,a%20heart%20attack%20or%20stroke
- 65.Leonard B.E. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13(5-6):268–276. doi: 10.1159/000104854. [DOI] [PubMed] [Google Scholar]
- 66.Rosengren A., Hawken S., Ôunpuu S., et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 67.Sanogo F., Xu K., Cortessis V.K., Weigensberg M.J., Watanabe R.M. Mind- and body-based interventions improve glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. J Integr Complement Med. 2023;29(2):69–79. doi: 10.1089/jicm.2022.0586. [DOI] [PubMed] [Google Scholar]
- 68.Rainforth M.V., Schneider R.H., Nidich S.I., Gaylord-King C., Salerno J.W., Anderson J.W. Stress reduction programs in patients with elevated blood pressure: a systematic review and meta-analysis. Curr Hypertens Rep. 2007;9(6):520–528. doi: 10.1007/s11906-007-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naik G.S., Gaur G.S., Pal G.K. Effect of modified slow breathing exercise on perceived stress and basal cardiovascular parameters. Int J Yoga. 2018;11(1):53–58. doi: 10.4103/ijoy.IJOY_41_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johansson L., Guo X., Waern M., et al. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(8):2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- 71.General U.S. Social connection–current priorities of the U.S. surgeon general. https://www.hhs.gov/surgeongeneral/priorities/connection/index.html
- 72.Survey G. Meta-Gallup State of Social Connections Study: Exploring human connection across diverse regions around the world. https://www.gallup.com/analytics/509675/state-of-social-connections.aspx Published online 2022.
- 73.Corporation C The Loneliness Epidemic Persists: A Post-Pandemic Look at the State of Loneliness among U.S. Adults. Cigna Newsroom (thecignagroup.com) https://newsroom.thecignagroup.com/loneliness-epidemic-persists-post-pandemic-look
- 74.Flowers L., Houser A., Noel-Miller C., et al. Medicare spends more on socially isolated older adults. Insight Issues. 2017;125:1119–1143. [Google Scholar]
- 75.Henriksen R.E., Nilsen R.M., Strandberg R.B. Loneliness increases the risk of type 2 diabetes: a 20 year follow-up – results from the HUNT study. Diabetologia. 2023;66(1):82–92. doi: 10.1007/s00125-022-05791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holt-Lunstad J., Robles T.F., Sbarra D.A. Advancing social connection as a public health priority in the United States. Am Psychol. 2017;72(6):517–530. doi: 10.1037/amp0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strom J.L., Egede L.E. The impact of social support on outcomes in adult patients with type 2 diabetes: a systematic review. Curr Diab Rep. 2012;12(6):769–781. doi: 10.1007/s11892-012-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valtorta N.K., Kanaan M., Gilbody S., Ronzi S., Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102(13):1009–1016. doi: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naito R., Leong D.P., Bangdiwala S.I., et al. Impact of social isolation on mortality and morbidity in 20 high-income, middle-income and low-income countries in five continents. BMJ Glob Health. 2021;6(3) doi: 10.1136/bmjgh-2020-004124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cené C.W., Beckie T.M., Sims M., et al. Effects of objective and perceived social isolation on cardiovascular and brain health: a scientific statement from the American Heart Association. J Am Heart Assoc. 2022;11(16) doi: 10.1161/JAHA.122.026493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lara E., Caballero F.F., Rico-Uribe L.A., et al. Are loneliness and social isolation associated with cognitive decline? Int J Geriatr Psychiatry. 2019;34(11):1613–1622. doi: 10.1002/gps.5174. [DOI] [PubMed] [Google Scholar]
- 82.Akhter-Khan S.C., Tao Q., Ang T.F.A., et al. Associations of loneliness with risk of Alzheimer’s disease dementia in the Framingham Heart Study. Alzheimers Dement. 2021;17(10):1619–1627. doi: 10.1002/alz.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piano M.R. Alcohol’s effects on the cardiovascular system. Alcohol Res. 2017;38(2):219–241. doi: 10.35946/arcr.v38.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jankowich M., Choudhary G., Taveira T.H., Wu W.C. Age-, race-, and gender-specific prevalence of diabetes among smokers. Diabetes Res Clin Pract. 2011;93(3):e101–e105. doi: 10.1016/j.diabres.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 85.Chami T., Kim C.H. Cannabis abuse and elevated risk of myocardial infarction in the young: a population-based study. Mayo Clin Proc. 2019;94(8):1647–1649. doi: 10.1016/j.mayocp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Drugs, brains and behaviour: the science of addition. drugs and the brain. National Institute on Drug Abuse. https://nida.nih.gov/publications/drugs-brains-behavior-science-addiction/drugs-brain Published n.d.
- 87.Galanis D.J., Petrovitch H., Launer L.J., Harris T.B., Foley D.J., White L.R. Smoking history in middle age and subsequent cognitive performance in elderly Japanese-American Men. The Honolulu-Asia Aging Study. Am J Epidemiol. 1997;145(6):507–515. doi: 10.1093/oxfordjournals.aje.a009138. [DOI] [PubMed] [Google Scholar]
- 88.Schwarzinger M., Pollock B.G., Hasan O.S.M., Dufouil C., Rehm J., QalyDays Study Group Contribution of alcohol use disorders to the burden of dementia in France 2008-13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3(3):e124–e132. doi: 10.1016/S2468-2667(18)30022-7. [DOI] [PubMed] [Google Scholar]
- 89.The potential of lifestyle medicine as a HighValue approach to address health equity. Inst Adv Health Value West Gov Univ. https://lifestylemedicine.org/wp-content/uploads/2022/09/institute_ib_lifestyle-medicine-aclm-0922.pdf
- 90.Shurney D., Webb D. Optimizing health and well-being: the interplay between lifestyle medicine and social determinants of health. J Fam Pract. 2022;71(suppl 1 Lifestyle):eS78–eS82. doi: 10.12788/jfp.0247. [DOI] [PubMed] [Google Scholar]
- 91.Cassoobhoy A., Sardana J.J., Benigas S., Tips J., Kees A. Building health equity: action steps from the American College of Lifestyle medicine’s health disparities solutions summit (HDSS) 2020. Am J Lifestyle Med. 2022;16(1):61–75. doi: 10.1177/15598276211052248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krishnaswami J., Sardana J., Daxini A. Community-engaged lifestyle medicine as a framework for health equity: principles for lifestyle medicine in low-resource settings. Am J Lifestyle Med. 2019;13(5):443–450. doi: 10.1177/1559827619838469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Edelman D., McDuffie J.R., Oddone E., et al. Department of Veterans Affairs (US); 2012. Shared medical appointments for chronic medical conditions: a systematic review.http://www.ncbi.nlm.nih.gov/books/NBK99785/ [PubMed] [Google Scholar]
- 94.Frates E.P., Morris E.C., Sannidhi D., Dysinger W.S. The art and science of group visits in lifestyle medicine. Am J Lifestyle Med. 2017;11(5):408–413. doi: 10.1177/1559827617698091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huffman T. In: The International Encyclopedia of Communication Research Methods. 1st ed. Matthes J., Davis C.S., Potter R.F., editors. Wiley; 2017. Participatory/action research/ CBPR; pp. 1–10. [DOI] [Google Scholar]