Abstract
Tenofovir, as nucleotide reverse transcriptase inhibitors (NRTIs), is used to prevent and cure HIV/AIDS. Ample evidence confirmed that the nephrotoxicity of tenofovir has been linked to mitochondrial dysfunction. It seems that transplantation with healthy mitochondria instead of damaged mitochondria may be a beneficial approach to therapy. Therefore, it decided to investigate the impact of mitotherapy on tenofovir against renal proximal tubular cells (RPTCs) toxicity by measurement of oxidative stress and cytotoxicity biomarkers and restoring of mitochondrial function on isolated mitochondria. EC50 of tenofovir was achieved at 40 μM following 2 h incubation in Earle's solution (pH = 7.4; 37 °C). Freshly isolated mitochondria (80 μg/ml) were added to damage RPTCs affected by tenofovir in treated groups. One Way ANOVA analysis showed that healthy mitochondrial transplantation decreased oxidative stress biomarkers following tenofovir toxicity in RPTCs. Our data revealed that mitotherapy makes cell survival possible in RPTCs affected by tenofovir. In addition, it supposed that a novel and ideal strategy for the treatment of chemicals-induced nephrotoxicity.
Keywords: Tenofovir, Oxidative stress, Nephrotoxicity, Mitochondrial transplantation, Renal proximal tubular cells (RPTCs)
Highlights
-
•
Correlation of tenofovir nephrotoxicity and mitochondrial dysfunction.
-
•
Mitotherapy as a novel and potential therapeutic strategy in tenofovir-induced nephrotoxicity.
-
•
Healthy mitochondrial transplantation decreased oxidative stress biomarkers following tenofovir toxicity in RPTCs.
1. Introduction
Tenofovir, a nucleotide analog reverse transcriptase inhibitor, has been approved by the FDA for the treatment of CHB as monotherapy since 2008 and for the treatment of HIV infection since 2001 [1]. Tenofovir enters cells where it is phosphorylated by the cellular adenylate kinase nucleotide kinase to the intermediate monophosphate form, which is then quickly transformed into the active diphosphate form by nucleoside diphosphate kinase [2]. Unfortunately, the clinical evidence revealed that tenofovir caused renal impairment, including Fanconi syndrome and acute renal failure. Also, monitoring of renal function and creatinine clearance should be determined in all patients before beginning tenofovir medication [3]. It seems that increased tenofovir plasma levels may directly result in its greater accumulation in the renal tubular cells, which more or less certainly causes nephrotoxicity specifically in renal proximal tubular such as cytoplasmic vacuolization and hydropic damage [4]. Therefore, by prevention of mitochondrial DNA polymerase, tenofovir, and other acyclic nucleotides reduce the amount of mtDNA, which is linked to mitochondrial dysfunction and cell death signaling in the kidney. It is supposed that mitochondrial energy-producing ability and mitochondrial membrane can be impaired because of the deteriorative impacts of nephrotoxicant agents [5]. Therefore, mitochondrial therapy (mitotherapy) transfers functional exogenous mitochondria into cells with impaired mitochondria for cell viability recovery and consequently, inhibition of the disease toxicity and progress [6].
In recent years, mitochondrial transplantation has gained popularity as a new technique for treating mitochondrial illnesses by augmentation and replacement of mitochondria. Freshly isolated mitochondria are able to enter mammalian cells through incubation and protect them against cytotoxic effects due to mitochondrial injury because of high performance and efficiency in toxic conditions [[7], [8], [9], [10], [11], [12]]. Another study showed mitochondrial transference as a new treatment based on transplanting normal mitochondria to cells in cadmium-induced nephrotoxicity following uptake and cellular functional integration of the transplanted mitochondria appears to kidney cells [13].
Due to limited research on mitochondrial transplantation, we examined the protective possible role of mitotherapy against tenofovir-induced nephrotoxicity via transferring functioning exogenous mitochondria into mitochondrion-deficient cells by measurements of oxidative stress biomarkers.
2. Material and methods
2.1. Animals
Male Wistar rats (250–300 g) were provided by the Pasteur Institute, Iran, and maintained in separate cages at controlled room temperature (20–25 °C), humidity (70–80%), and exposure to daylight 12 h a day (Standard laboratory condition). The Animal Ethics Committee of Shahid Beheshti University of Medical Sciences conducted all experiments (IR.SBMU.PHARMACY.REC.1400.171) in accordance with the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86–23, revised 1985). The researchers tried to minimize the suffering of the animals during the experiments.
2.2. Chemicals
All chemicals were provided in the highest commercial quality from Merck Company (Darmstadt, Germany).
2.3. Preparation and isolation of RPTCs
Isolation of rat RPTCs was done by modified enzymatic procedures adapted from Boom et al., 1992 [14,15]. Xylazine (10 mg/kg) and Ketamine (40 mg/kg) were applied to anesthetize animals and following kidney harvesting, cervical dislocation was applied to scarify the rats. Ca2+-free Hank's balanced salt solution (HBSS) plus 0.5 mM EGTA perfused the kidneys. Then, the digestion of the specimens was done in a HBSS solution with 4 mM CaCl2, 0.05% collagenase type II, and 1% streptomycin/penicillin. The cortical segments of the kidneys were subjected to decapsulation and dissection to achieve proximal cell tubules mechanically isolated by consecutive filtration at respectively 120 and 60 μm mesh, and sections with a thickness of 0.5–1 mm. Pellets were obtained from the extracted and washed RPTCs. Then, the obtained cells were subjected to re-suspension in Earl's solution (pH = 7.4) at 1 × 106 cells per milliliter, followed by placement in round-bottomed containers, circulating in a water bath (37 °C), and adding to 28 mM HEPES with 10% O2, 5% CO2, and 85% N2. Cell viability was determined by measuring the exclusion of trypan blue (0.2% w/v). RPTCs were pre-incubated for 30 min prior to addition of tenofovir (5–80 μM). At least 80–90% of control cells were still viable after this 2 h incubation period.
2.4. Preparation of freshly isolated mitochondria from kidney
Using differential centrifugation procedures, mitochondria were prepared from the animals’ kidneys [16,17]. Following removing of kidneys and chopping with scissors, a cold isolation solution including sucrose (75 mM), EDTA (0.2 mM) and D-mannitol (0.225 M) was used. Kidneys were homogenized with a glass homogenizer and centrifuged at 1000 g for 10 min at 4 °C. Then, supernatant was treated with 250 μL of BSA solution, which was then continuously filtered through 40 and 5 μm meshes, respectively. The supernatant was centrifuged at 10,000 g for 10 min. A lower layer of centrifuge is mitochondrial fraction. To purity of isolated mitochondria, heavy mitochondrial sediments was re-suspended in the homogenization buffer including Tris–HCl (0.05 M), MgCl2 (2.0 mM) KCl (20 mM), sucrose (0.25 M), and Na2HPO4 (1.0 mM) at pH = 7.4 at 4 °C and centrifuged twice for 10 min at 10,000 g, again. Finally, mitochondrial sediments were suspended in the desired solution buffer before each assay.
The protein concentrations of the isolated mitochondria were done by bradford assay based on the Coomassie blue protein-binding method using bovine serum albumin as the standard to keep the uniformity of experimental conditions [18]. The normalization procedure for each sample was done based on 80 μg mitochondrial protein per ml of sample.
2.5. Incubation of RPTCs with freshly isolated mitochondria
Rat RPTCs (106 cells/ml) were subjected to suspension in Earle's solution (pH = 7.4; 37 °C) for 2 h after adding of different concentration of tenofovir (5–80 μM). Also, isolation of the mitochondria for transplantation was done from the kidney at 4 °C. The optimum dose of isolated mitochondria and optimal time of incubation were chosen based on pilot and previous research [19,20]. Mitochondrial supplemented media (37 °C) was applied for the replacement of the media on RPTCs and maintained on the cells for 1,2 and 3 h. Mitochondrial succinate dehydrogenase activity was measured by the reduction of MTT to formazan crystals after dissolving in 100 μL DMSO and reading the absorbance at 570 nm by an ELISA reader (Tecan, Rainbow Thermo, Austria) to check mitochondrial functionality [21].
2.6. Cytotoxicity biomarker in RPTCs
2.6.1. Viability assay
Lactate dehydrogenase (LDH) as a stable enzyme is present in all cell types which is rapidly released from cells into the medium upon damage of the plasma membrane. It was measured by LDH kit from Millipore Sigma (St. Louis, Missouri) by spectrophotometry at 340 nm and was reported as μM substrate/min/l.
2.6.2. ROS measurement in the RPTCs
It was measured by spectrofluorometric assay with emission and excitation wavelengths of 520 and 500 nm, respectively as described by previous study [22]. DCFH DA (1.6 μM) as a reagent of ROS formation was applied in the RPTCs following mitochondrial transplantation (mitotherapy). The findings were expressed as fluorescence intensity units per 106 cells.
2.6.3. Mitochondrial membrane potential assay in the RPTCs
The uptake of Rhodamine123 (Rh123) was utilized as a reagent for determining the mitochondrial membrane potential (MMP). Briefly, following separating 0.5 ml of the RPTCs by centrifuge, the pellet was re-suspended in 2 ml of Rh123 (1.5 μM) with medium and subjected to incubation for 10 min at 37 °C. A spectrofluorometric method was used with emission and excitation wavelengths of 520 and 490 nm, respectively. The difference in Rh123 fluorescence between the treatment and control groups was applied to determine the mitochondrial capacity in absorbing Rh123 [22,23].
2.6.4. GSH and GSSG level in the RPTCs
Measurement of GSSG and GSH, o-phthalaldehyde (OPT) was employed as a fluorescent reagent, which can react specifically with GSSG at pH 12 and GSH at pH 8 respectively. The fluorescence intensity was assessed by a spectrofluorimetric method at the emission and excitation wavelength of respectively 420 and 350 nm [19,24].
2.6.5. ATP content in the RPTCs
Measurement of mitochondrial uptake process was evaluated following pre-incubation of 106 cells per ml of tenofovir treated RPT cells by 5-(N-Ethyl-N-isopr opyl) amiloride (EIPA) (100 μM), cytochalasin D (10 μM) and methyl-β-cyclodextrin (1 mM) and pre-incubation was done within distinct flasks for 30min. Addition of isolated mitochondria to each flask and consequent co-incubation was then carried out at 37 °C using 5% CO2 for 4 h. Eventually, mitochondria administration inhibitory impacts on ATP levels were assessed by luciferase enzyme [25].
2.7. Statistical analysis
Results were reported as mean ± SD. One-way or two-way analysis of variance (ANOVA) followed by proper post hoc tests were applied to analyze the data. The Shapiro-Wilk test checked the normality and a p < 0.05 was considered significant. We used GraphPad Prism 9 for graphic design and data analysis.
3. Results
3.1. Function assessment of isolated mitochondria in mitotherapy
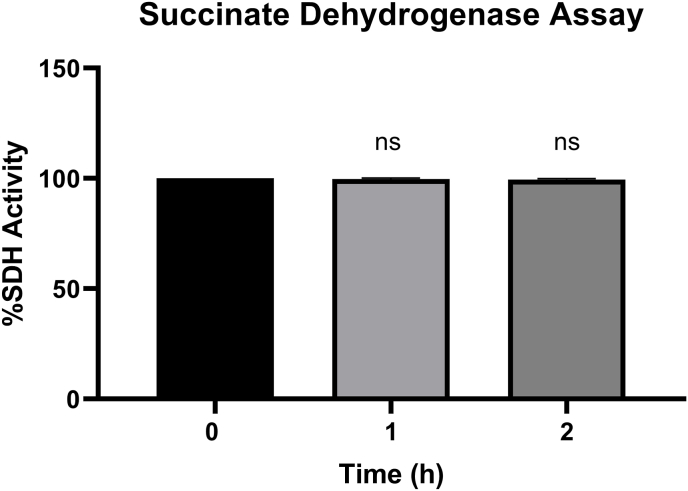
The succinate dehydrogenase activity was assessed by measuring the MTT reduction to formazan in the mitochondrial fraction at 570 nm. The results of Fig. 1 revealed that there is no significant difference regarding succinate dehydrogenase activity after 1 and 2 h of incubation of the mitochondria suspension than zero time (p > 0.05).
Fig. 1.
Effect of time on functionality of isolated mitochondria. Values are expressed as the mean of three separate experiments (±SD). NS. No significant difference compared to zero time as the control group (p > 0.05).
3.2. The impact of tenofovir and mitochondrial transplantation on viability
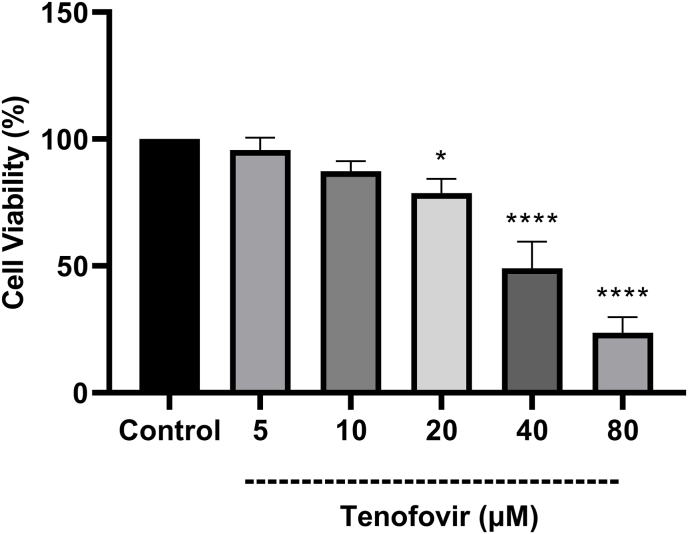
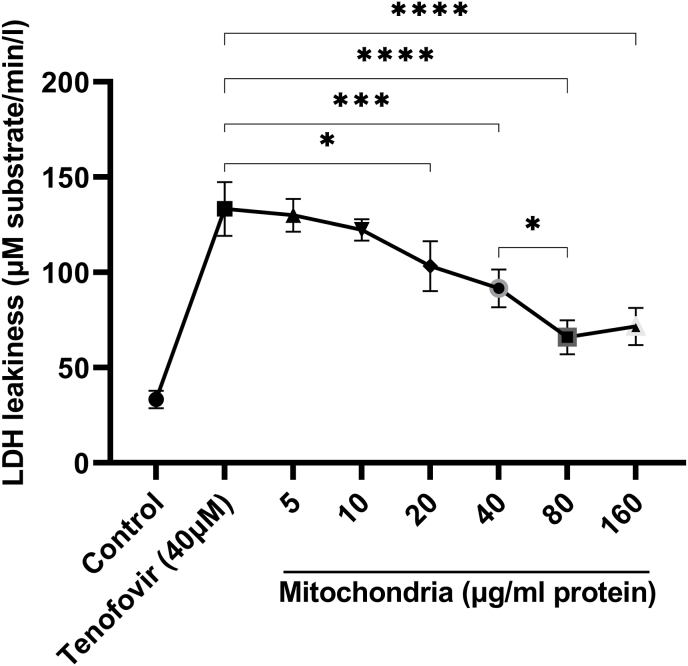
As shown in Fig. 2, the cytotoxic effect of Tenofovir on rat RPTCs following 2 h incubation was determined by trypan blue assay. Tenofovir (5, 10, 20, 40 and 80 μM) showed a dose-dependent cytotoxic manner on RPTCs by the reduction in cell viability at higher than 20 μM. (*p < 0.05 vs. control, ****p < 0.0001 vs. control). Our data revealed that the IC50 of Tenofovir in the RPTCs used in this study was reported 40 μM. Then, the results of cytotoxicity by LDH following exposure of different concentrations of isolated mitochondria (5–160 μg protein/ml) in RPTCs exposure with tenofovir (40 μM) revealed that pre-incubation of RPTCs with freshly isolated mitochondria (80 μg/mg protein) prevented RPTCs against tenofovir-induced cytotoxicity (p < 0.05) (Fig. 3). Although the different concentrations of the transferred mitochondria were able to reduce the LDH release caused by tenofovir, 80 μg protein/ml isolated mitochondria showed highest cytoprotection against RPTCs exposure with tenofovir (40 μM) revealed (p < 0.05) (Fig. 3).
Fig. 2.
Effect of Tenofovir concentrations on RPTCs viability. Values are expressed as the mean of three separate experiments (±SD). *p < 0.05 and ****p < 0.0001 compared with control groups.
Fig. 3.
Protective effect of transplantation of isolated mitochondria (5–160 μg/ml) against tenofovir (40 μM) induced cytotoxicity on RPTCs. Values are expressed as the mean of three separate experiments (±SD). *p < 0.05 and ****p < 0.0001 compared with control groups.
3.3. The impact of mitochondrial transplantation on ROS levels
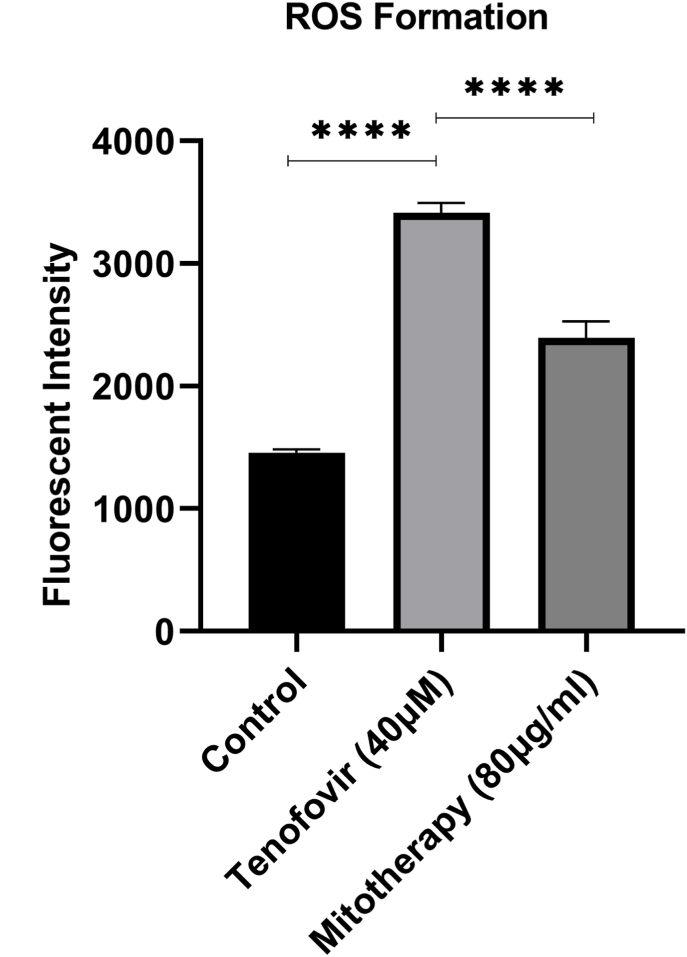
Data revealed a significant elevation in ROS levels after incubation of Tenofovir with RPTCs than control group (***p < 0.001; Fig. 4). Nonetheless, following mitochondrial transplantation (80 μg/mg protein), the ROS levels were significantly decreased in Tenofovir-treated RPTCs (***p < 0.001; Fig. 4). It seems that mitochondrial transplantation rescued the RPTCs from oxidative damage due to Tenofovir.
Fig. 4.
Effect of mitochondrial transplantation on RPTCs ROS level. It was measured by DCFH-DA (1.6 μM) following incubation of RPTCs. The fluorescence intensity was measured using a Shimadzu RF5000U fluorescence spectrophotometer with excitation and emission wavelengths 500 and 520 nm, respectively. The results were expressed as fluorescent intensity per 106 cells. Values are expressed as the mean of three separate experiments (±SD). *p < 0.05 and ****p < 0.0001 compared with control groups.
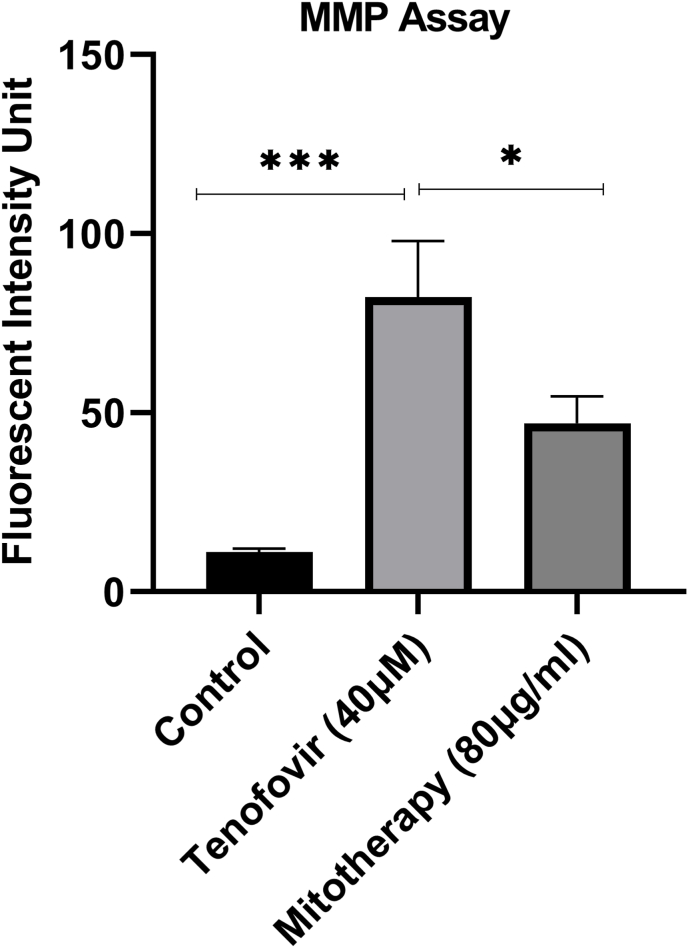
3.4. The impact of mitochondrial transplantation on MMP assay
As shown in Fig. 5, a rapid reduction was seen in the MMP in tenofovir-treated RPTCs in comparison with control groups. Also, mitochondrial transplantation could restore MMP decline, as a direct reason for Tenofovir-related mitochondrial damage.
Fig. 5.
Effect of mitochondrial transplantation on RPTCs mitochondrial membrane potential. RPTCs (106 cells/ml) were incubated for 2 h following the addition of Tenofovir (40 μM) and incubation with freshly isolated mitochondria. Mitochondrial membrane potential was determined as the difference in mitochondrial uptake of the rhodamine 123 between the control, treated cells, and mitotherapy (Freshly isolated mitochondria addition) group. Values are expressed as means of three separate experiments (±SD). *** Significant difference in comparison with control (p < 0.001). * Significant difference in comparison with the tenofovir group (p < 0.05).
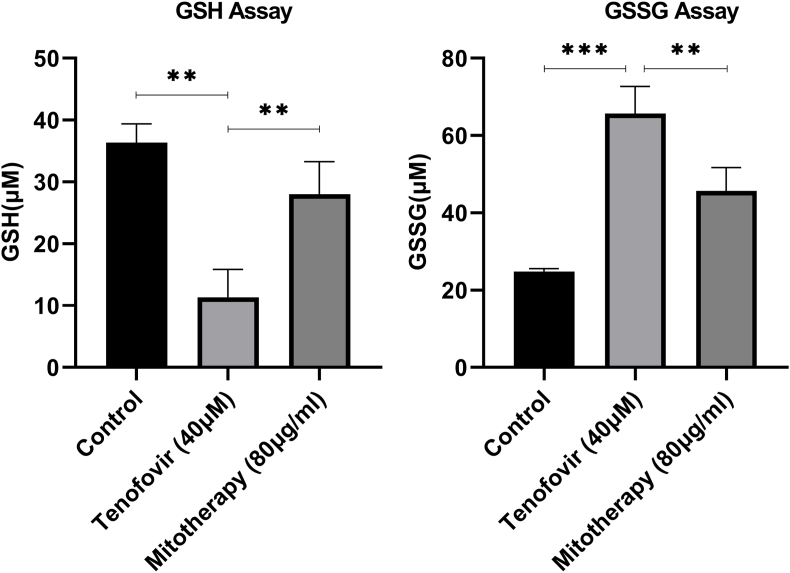
3.5. The impact of mitochondrial transplantation on GSH and GSSG levels
The exposure of the mitochondria with Tenofovir decreased the GSH concentrations (Fig. 6A) and increased the GSSG concentrations (Fig. 6B) in RPTCs. A significant increase was found in GSH levels in mitochondrial transplantation and Tenofovir treated groups (40 μM) than in the Tenofovir treated groups (p < 0.01). A decrease in GSSG concentrations was found in mitochondrial transplantation and Tenofovir-treated groups (40 μM) compared with the Tenofovir-treated groups (Fig. 6B; p < 0.01).
Fig. 6.
Effect of mitochondrial transplantation on RPTCs (A) GSH and (B) GSSG levels. Values are expressed as mean ± SD of separate experiments (n = 3). ** Significant difference in comparison with control group (P < 0.01). ** Significant difference in comparison with Tenofovir (P < 0.01).
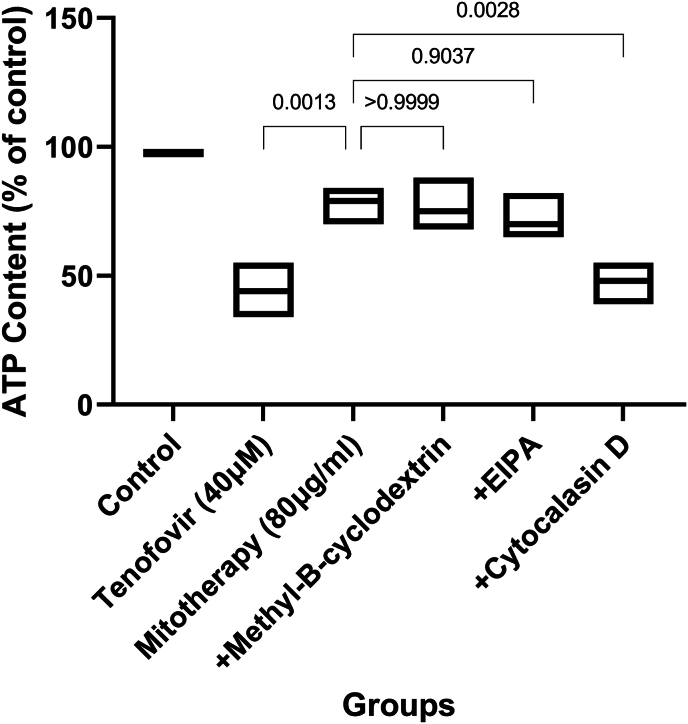
3.6. The impact of mitochondrial transplantation on ATP content
Evaluation of ATP level revealed that tenofovir (40 μM) caused a significant decrease in ATP content in RPT cells (Fig. 7). Also, we observed rise in ATP level following mitochondrial transplantation after 2 h tenofovir incubation in RPT cells. Besides, the protective effects in RPT cells and significantly reserve was seen in ATP amount following pre-incubation with 10 μM cytochalasin D which related to actin-dependent endocytosis plays a role in mitochondrial internalization (Fig. 7; p < 0.001).
Fig. 7.
Effect of mitochondrial transplantation on RPTCs ATP contents. Values are expressed as mean ± SD of separate experiments (n = 3). ** Significant difference in comparison with control group (P < 0.01). ** Significant difference in comparison with tenofovir (P < 0.01).
4. Discussion
Previous data showed that mitochondrial dysfunction has a key role in Tenofovir-induced nephrotoxicity and recovering mitochondrial dysfunction is the main aim in the reduction of this Tenofovir-induced nephrotoxicity [26]. In the present investigation, treatment with freshly isolated mitochondria or mitochondrial transplantation was evaluated and thus freshly isolated mitochondria for rescue of damaged RPTCs was added to tenofovir-affected cells. To observe the positive effect of mitotherapy on tenofovir-induced nephrotoxicity oxidative stress biomarkers including cell viability, ROS level, MMP, and reduced and oxidized glutathione level and ATP content we evaluated.
This technique in isolation of mitochondria permits mitochondria as intact and normal organelles to recover tenofovir-related nephrotoxicity in the RPTCs and introduces mitochondrial transplantation as a new therapeutic strategy [21,27,28].
It was proven that uptake of isolated mitochondria to culture media or RPTCs by endocytosis [29]. In our previous data, we checked the cellular uptake mechanism of isolated mitochondria in cisplatin-treated RPTCs by pre-incubation of our mitochondria-transplanted cells with 5-(N-Ethyl-N-isopropyl) amiloride (EIPA) as a pinocytosis inhibitor, cytochalasin D as an action-associated endocytosis inhibitor, and methyl-cyclodextrin as an endocytosis inhibitor associated with clathrin. Our data showed the mitochondrial transplantation protective effects in the RPTCs were significantly suppressed after pre-incubation using cytochalasin D, which confirms actin-dependent endocytosis and the internalization of the mitochondria into the RPTCs [19,20].
Tenofovir is a nucleoside reverse transcriptase inhibitor (NRTI) drug as a pyrimidine and purine analog, which is used for the treatment of HIV/AIDS following phosphorylation in cell and then quickly transformed to the active diphosphate form and competes with the natural substrate deoxyadenosine 5′-triphosphate for DNA inclusion to inhibits HIV reverse transcriptase [30]. The important adverse effect of tenofovir is limited to damage in proximal tubular dysfunction and renal dysfunction as primary clinical manifestations of Tenofovir nephrotoxicity [31].
Current evidence suggests that mitochondria and membrane transporters are the main targets of tenofovir nephrotoxicity of tenofovir. Also, the energy demand for this transport is provided by mitochondria in proximal tubular cells [32]. Our present study showed that tenofovir-induced nephrotoxicity is the result of ROS production and MMP collapse which is involved in reduction of energy (ATP) production level and increase in oxidized glutathione level which is supposed involvement of oxidative stress and mitochondrial dysfunction and start of cell death signaling [26,33,34].
In summary, mitochondrial dysfunction following exposure by tenofovir caused releasing of cytochrome C from mitochondria into the cytoplasm and consequently, activation of cell death signaling especially caspase-9&3&7. Our data suggested oxidative stress is considered as a mechanistic hypothesis for tenofovir-induced nephrotoxicity, which is derived from the imbalance of pro-oxidant (overproduction of ROS) and antioxidant level (depletion of cellular antioxidants). ROS also have considerable effects on MMP in cells as signaling molecules in inducing of apoptosis pathways. Oxidative stress is also responsible for transmitting mitochondrial derived ROS (H2O2) into lysosomes and initiating the lysosomal Fenton's reaction which leads to hydroxyl radical generation and lysosomal membrane damage. We observed that after the mitochondrial transplantation to RPTCs, all the toxicity parameters in tenofovir affected RPTCs returned to normal condition via rising in reduced glutathione and ATP levels. It supposed our hypothesis that transplantation of freshly isolated mitochondria to the tenofovir-affected RPTCs can cause cell functions improvement, cell viability restoring and Therefore, decrease of mitochondrial permeability which leads to decrease in cell injuries and decrease in activation of caspases cascade through cytochrome c leakage from mitochondria.
Funding
This work was supported by the deputy of research of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Project No: 30043).
Ethical approval
All experimental procedures were carried out by the ethical standards and protocols approved by the Animal Experimentation Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, with IR. SBMU.PHARMACY.REC.1400.171 ethical Committee code.
Consent for publication
All authors are agreed to publish this manuscript.
CRediT authorship contribution statement
Mir-Jamal Hosseini: Writing – review & editing, Writing – original draft, Software, Project administration, Investigation, Formal analysis. Aysan Hassanbeigloo: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation. Hamideh Abbasi: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation. Abdollah Arjmand: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation. Fatemeh Sherkat: Writing – review & editing, Writing – original draft, Supervision, Software, Project administration, Methodology, Investigation. Jalal Pourahmad: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Fatemeh Sherkat, Email: sherkatfatemeh75@gmail.com.
Jalal Pourahmad, Email: j.pourahmadjaktaji@utoronto.ca, j.pourahmadjaktaji@alumni.utoronto.ca.
Data availability
The authors do not have permission to share data.
References
- 1.De Clercq E. In search of a selective therapy of viral infections. Antivir. Res. 2010;85(1):19–24. doi: 10.1016/j.antiviral.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Dietz J.-P., et al. Di-tert-butyl phosphonate route to the antiviral drug tenofovir. Org. Process Res. Dev. 2021;25(4):789–798. doi: 10.1021/acs.oprd.0c00473. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann A.E., et al. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin. Infect. Dis. 2006;42(2):283–290. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 4.Lewis W., Copeland W.C., Day B.J. Mitochondrial DNA depletion, oxidative stress, and mutation: mechanisms 0f dysfunction from nucleoside reverse transcriptase inhibitors. Lab. Invest. 2001;81(6):777–790. doi: 10.1038/labinvest.3780288. [DOI] [PubMed] [Google Scholar]
- 5.Lewis W. Nucleoside reverse transcriptase inhibitors, mitochondrial DNA and AIDS therapy. Antivir. Ther. 2005;10(2_suppl):13–27. [PubMed] [Google Scholar]
- 6.Fu A. Mitotherapy as a novel therapeutic strategy for mitochondrial diseases. Curr. Mol. Pharmacol. 2020;13(1):41–49. doi: 10.2174/1874467212666190920144115. [DOI] [PubMed] [Google Scholar]
- 7.Nowak G. Protein kinase C-and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na transport, and cisplatin-induced apoptosis in renal cells. J. Biol. Chem. 2002;277:43377–43388. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuertes M., et al. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003;10(3):257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl D., Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur. J. Cancer. 1998;34(10):1522–1534. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 10.Weide L.G., et al. Detrimental effect of mitochondria on hybrid cell survival. Somat. Cell Genet. 1982;8(1):15–21. doi: 10.1007/BF01538647. [DOI] [PubMed] [Google Scholar]
- 11.Prockop D.J. Mitochondria to the rescue. Nat. Med. 2012;18(5):653–654. doi: 10.1038/nm.2769. [DOI] [PubMed] [Google Scholar]
- 12.Kesner E., Saada-Reich A., Lorberboum-Galski H. Characteristics of mitochondrial transformation into human cells. Sci. Rep. 2016;6(1) doi: 10.1038/srep26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Cruz E.Y., et al. Renal damage induced by cadmium and its possible therapy by mitochondrial transplantation. Chem. Biol. Interact. 2022;361 doi: 10.1016/j.cbi.2022.109961. [DOI] [PubMed] [Google Scholar]
- 14.Boom S.P., Gribnau F., Russel F. Organic cation transport and cationic drug interactions in freshly isolated proximal tubular cells of the rat. J. Pharmacol. Exp. Therapeut. 1992;263(2):445–450. [PubMed] [Google Scholar]
- 15.Schafer J., et al. A simplified method for isolation of large numbers of defined nephron segments. Am. J. Physiol. Ren. Physiol. 1997;273(4):F650–F657. doi: 10.1152/ajprenal.1997.273.4.F650. [DOI] [PubMed] [Google Scholar]
- 16.Preble J.M., et al. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. JoVE. 2014;(91) doi: 10.3791/51682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassani S., et al. Mechanistic view for toxic effects of arsenic on isolated rat kidney and brain mitochondria. Biologia. 2015;70(5):683–689. [Google Scholar]
- 18.Salimi A., et al. Selective anticancer activity of acacetin against chronic lymphocytic leukemia using both in vivo and in vitro methods: key role of oxidative stress and cancerous mitochondria. Nutr. Cancer. 2016;68(8):1404–1416. doi: 10.1080/01635581.2016.1235717. [DOI] [PubMed] [Google Scholar]
- 19.Arjmand A., et al. Mitochondrial transplantation therapy against ifosfamide induced toxicity on rat renal proximal tubular cells. Drug Research. 2023;73(2):113–120. doi: 10.1055/a-1967-2066. [DOI] [PubMed] [Google Scholar]
- 20.Arjmand A., et al. Mitochondrial transplantation against gentamicin-induced toxicity on rat renal proximal tubular cells: the higher activity of female rat mitochondria. In Vitro Cell. Dev. Biol. Anim. 2023;59(1):31–40. doi: 10.1007/s11626-022-00743-1. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini M.-J., et al. Toxicity of cigarette smoke on isolated lung, heart, and brain mitochondria: induction of oxidative stress and cytochrome c release. Toxicol. Environ. Chem. 2013;95(9):1624–1637. [Google Scholar]
- 22.Pourahmad J., et al. Mitochondrial/lysosomal toxic cross-talk plays a key role in cisplatin nephrotoxicity. Xenobiotica. 2010;40(11):763–771. doi: 10.3109/00498254.2010.512093. [DOI] [PubMed] [Google Scholar]
- 23.Pourahmad J., et al. Involvement of mitochondrial/lysosomal toxic cross-talk in ecstasy induced liver toxicity under hyperthermic condition. Eur. J. Pharmacol. 2010;643(2–3):162–169. doi: 10.1016/j.ejphar.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 25.Lundin A. 2000. Use of Firefly Luciferase in ATP-Related Assays of Biomass, Enzymes, and Metabolites. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Fernandez B., et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. doi: 10.1155/2011/354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulger O., Kubat G.B. Therapeutic applications of mitochondrial transplantation. Biochimie. 2022;195:1–15. doi: 10.1016/j.biochi.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Hosseini M.-J., et al. Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem. Biophys. 2014;70(1):367–381. doi: 10.1007/s12013-014-9922-7. [DOI] [PubMed] [Google Scholar]
- 29.Rieder H., Decker K. 1984. Phagocytosis of Hepatocyte Mitochondria by Rat Kupffer Cells in Vitro. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J., et al. Fast and ultrasensitive electrochemical detection for antiviral drug tenofovir disoproxil fumarate in biological matrices. Biosensors. 2022;12(12):1123. doi: 10.3390/bios12121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herlitz L.C., et al. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78(11):1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 32.Sons J. John Wiley & Sons; Hoboken, NJ, USA: 2008. Mitochondrial Dysfunction in Drug-Induced Toxicity. [Google Scholar]
- 33.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham P., Ramamoorthy H., Isaac B. Depletion of the cellular antioxidant system contributes to tenofovir disoproxil fumarate-induced mitochondrial damage and increased oxido-nitrosative stress in the kidney. J. Biomed. Sci. 2013;20(1):1–15. doi: 10.1186/1423-0127-20-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.