Summary
Background
Tuberculosis (TB) remains a global public health challenge, causing substantial mortality and morbidity. While TB treatment has made significant progress, it often leaves survivors with post-TB sequelae, resulting in long-term health issues. Current healthcare systems and guidelines lack comprehensive strategies to address post-TB sequelae, primarily due to insufficient evidence. This systematic review and meta-analysis aimed to identify effective interventions for preventing post-TB sequelae.
Methods
A systematic search was conducted across four databases including PubMed, SCOPUS, Web of Science, and Cochrane Central Register of Controlled Trials from inception to September 22, 2023. Eligible studies reported interventions designed to prevent post-TB sequelae were included. A random effect meta-analysis was conducted where applicable, and heterogeneity between studies was evaluated visually using forest plots and quantitatively using an index of heterogeneity (I2). This study is registered with PROSPERO (CRD42023464392).
Findings
From the 2525 unique records screened, 25 studies involving 10,592 participants were included. Different interventions were evaluated for different outcomes. However, only a few interventions were effective in preventing post-TB sequelae. Rehabilitation programs significantly improved lung function (Hedges's g = 0.21; 95% confidence interval (CI): 0.03, 0.39) and prevented neurological sequelae (relative risk (RR) = 0.10; 95% CI: 0.02, 0.42). Comprehensive interventions and cognitive-behavioural therapy significantly reduced the risk of mental health disorders among TB survivors (Hedges's g = −1.89; 95% CI: −3.77, −0.01). In contrast, interventions targeting post-TB liver sequelae, such as vitamin A and vitamin D supplementation and hepatoprotective agents, did not show significant reductions in sequelae (RR = 0.90; 95% CI: 0.52, 1.57). Moreover, adjunctive therapies did not show a significant effect in preventing post-TB neurological sequelae (RR = 0.62, 95% CI: 0.31, 1.24).
Interpretation
Rehabilitation programs prevented post-TB lung, neurologic and mental health sequelae, while adjuvant therapies and other interventions require further investigation.
Funding
Healy Medical Research Raine Foundation, Curtin School of Population Health and the Australian National Health and Medical Research Council.
Keywords: Tuberculosis, Sequelae, Prevention, Intervention, Systematic review, Meta-analysis
Research in context.
Evidence before this study
There is a growing body of evidence indicating that tuberculosis (TB) survivors often experience a range of post-TB sequelae, resulting from the disease itself and the side effects of TB treatment. We searched PubMed, Cochrane Central, SCOPUS, and Web of Science databases from the inception of each database to 22 September 2023, for papers published in English, using terms related to TB, sequelae, and prevention. Multiple systematic reviews were found through our search, examining the prevalence and impact of post-TB sequelae and highlighting the substantial burden faced by TB survivors. Previous individual studies have also explored various interventions to mitigate these sequelae, including pulmonary rehabilitation programs, adjunctive therapies, nutritional supplementation, and psychological support. However, the evidence on the effectiveness of these interventions is limited, and there is a lack of comprehensive systematic reviews that synthesise the available evidence across different domains of post-TB sequelae.
Added value of this study
This comprehensive systematic review and meta-analysis identifies effective interventions for preventing a wide range of post-TB sequelae, including lung, liver, neurologic, and mental health sequelae. The results show the importance of timing for specific interventions in addressing different sequelae. Rehabilitation programs were effective when implemented during treatment in preventing post-TB lung and neurologic sequelae as well as mental health disorders, whereas the effectiveness of adjuvant therapies and other interventions requires further investigation. This comprehensive analysis fills a crucial knowledge gap and helps inform evidence-based strategies for post-TB care and management.
Implications of all the available evidence
Existing evidence shows the importance of considering the long-term health of TB survivors. Integrating pulmonary rehabilitation programs during TB treatment can substantially improve lung function and prevent other types of post-TB sequelae. However, further research and innovation are required, particularly in preventing post-TB liver sequelae arising from drug-induced liver injury (DILI), where existing interventions show limited efficacy. The findings of current evidence can also inform healthcare providers and policymakers about the importance of post-TB care and the potential benefits of specific interventions. Expanding the remit of current TB programs to include strategies for preventing post-TB sequelae, such as pulmonary rehabilitation programs, will help reduce the morbidity and suffering associated with this disease.
Introduction
Tuberculosis (TB) is a serious public health concern and the leading cause of death from an infectious disease, killing a total of 1.3 million people in 2022.1 An estimated 10.6 million people develop TB disease every year, with around 70% receiving treatment.1 While substantial progress has been achieved with an estimated 66 million lives saved through TB diagnosis and treatment over the past two decades,1 people who survive TB face a considerable and under-recognised burden of ongoing morbidity including physical impairment and psychosocial problems even after completion of treatment (i.e. collectively known as post-TB sequelae).2,3 While post-TB lung disease refers to consequences associated with pulmonary TB, post-TB sequelae are broader, encompassing sequelae that affect various organ systems and parts of the body beyond the pulmonary region.4 These post-TB sequelae mainly arise from the disease process or the side effects related to TB treatment and increase the risk of permanent disability, premature mortality, ongoing stigma, and poor quality of life.5 Previous systematic reviews on the global burden of TB-related disability showed that the prevalence of respiratory function impairment among TB survivors ranges from 15% to 60%.2,6 A modelling study has shown that nearly half of the global burden of morbidity and mortality related to TB is attributed to post-TB lung diseases.7
As the number of TB survivors is expected to rise in the future due to improved diagnosis and treatment, there is an urgent need to focus on post-treatment care. However, current healthcare systems and TB programs do not generally focus attention on people affected by TB after treatment completion. For example, the current World Health Organization (WHO) guidelines for TB management define ‘treatment success’ using microbiological outcomes and survival only, and measures of TB-associated morbidity remain solely focused on the period prior to and during treatment.8 The recent WHO policy brief on TB-associated disability recognizes post-TB sequelae as a substantial challenge in TB programs. However, it neglects post-TB care and lacks strategies for following up with TB survivors after completing their treatment.9 Moreover, the recent United Nations New York declaration on TB has neglected to address the issue of post-TB sequelae.10 The reason why current TB guidelines and policy documents omit consideration of post-TB sequelae is partly due to a lack of comprehensive evidence.
While several systematic reviews have been conducted to identify strategies for improving end-of-treatment outcomes, there remains a critical research gap in identifying preventive strategies for mitigating the post-TB treatment sequelae burden. The limited availability of observational studies or clinical trials has yielded inconclusive evidence. This systematic review and meta-analysis aims to provide comprehensive evidence on the effectiveness of existing interventions in preventing post-TB sequelae.
Methods
Search strategy
This systematic review and meta-analysis was conducted following the updated guideline for reporting systematic reviews (Table S1 and Table S2).11 The study protocol has been registered at the PROSPERO international registry for systematic reviews [CRD42023464392]. Systematic literature searches were conducted in PubMed, Cochrane Central, SCOPUS, and Web of Science databases from the inception of each database to 22 September 2023. The Medical Subject Headings (MeSH) term and a combination of keywords related to TB, sequelae, and prevention were used for the search. We systematically conducted searches for key terms across titles and abstracts. A complete search strategy for each database is available in the supplementary file (Table S3). The reference lists and citations of the retrieved articles were checked manually for additional studies. The authors of the papers were contacted through email when there was a need for additional information.
Eligibility criteria
The selection criteria to identify potential studies were based on the PICOS (Population, Intervention, Control, Outcome and Study type) criteria. Interventional and observational studies conducted on adults or children with TB were included in the systematic review if they reported relevant information about the effectiveness of interventions in preventing post-TB sequelae. The main outcome of interest was a reduction in post-TB sequelae, which was broadly defined as any clinical condition that TB survivors experience after and as a consequence of TB illness or TB medication.5 These include any long-term sequelae such as pulmonary, liver, neurologic, visual, hearing, and cardiac sequelae as well as mental health disorders that occur as a result of TB illness or side effects of TB medications.12
We excluded studies that reported temporary adverse events during treatment such as hepatotoxicity and renal toxicity that was resolved by a change of medication. We also excluded correspondence, reviews, editorials, and conference abstracts without sufficient information on the main outcome of interest. Studies conducted on animals and non-English language articles were also excluded. When multiple studies used the same data, we included the study with the most detailed clinical data, with the largest sample size or with the longest follow-up period to avoid duplication. Detailed information about the inclusion and exclusion criteria based on the PICOS principle is available in the supplementary information (Table S4).
Procedures
All citations identified through our search strategy were imported into EndNote library version X8 (Thompson Reuters, New York, NY, USA). After removing duplicates, articles were uploaded into Rayyan13 for screening. Two authors (KAA and BG) independently screened the titles and abstracts of studies and reviewed full-text articles to identify eligible studies, ensuring a blinded review process between the two reviewers. Any discrepancies were discussed and resolved by consensus.
Data were extracted from included articles using a piloted form by the same two researchers (KAA and BG). We collected information about the characteristics of patient cohorts, studies, and outcomes of interest, and recorded this information in an MS Excel spreadsheet. Comprehensive details on the extracted variables from included studies can be found in the supplementary information (Table S5).
The quality of the included studies and the risk of bias were assessed by the same two researchers (KAA and BG) using the revised Cochrane Risk of Bias Tool for interventional studies14 and Newcastle–Ottawa Quality Assessment for observational studies.15 The Cochrane methodology assessed each study with the following five domains: bias arising from (I) the randomisation process; (II) deviations from interventions; (III) missing the outcome data; (IV) measurement of outcome; and (V) selection of the reported result. Each domain had specific signalling questions and responses to these questions led to the judgment of “low risk of bias”, “some concerns” and “high risk of bias”. Therefore, a study with a low risk of bias for all domains and some concern for at least one domain was judged to have “low risk of bias” and “some concerns” respectively. A study with a high risk of bias for at least one domain or that was judged as having some concerns in multiple domains in a way that substantially affected the results was judged to have a “high risk of bias”. The modified Newcastle-Ottawa Quality Assessment Scale16 used to assess observational studies had scores ranging from zero to nine with scores 1 to 4, 5 to 7, and 8 to 9 representing low, medium and high-quality groupings, respectively.
Statistical analysis
Narrative syntheses were conducted using all included studies to describe the outcomes of the studies. Additionally, meta-analyses were performed separately for each outcome only when two or more studies were available on the outcome of interest. The effectiveness of interventions on the outcome of interest (in preventing post-TB sequelae) was assessed using pooled relative risk (RR) with 95% confidence intervals (CIs) for dichotomous outcomes (i.e. normal vs abnormal liver and neurologic function). A RR and corresponding 95% CI were computed using the total numbers of participants and events in each arm within each study. For continuous variables such as lung function tests and mental health disorder scores (i.e., anxiety and depression scores), Hedges's g with 95% CIs was utilized. Hedges's g was selected over standardized mean differences due to its appropriateness for handling limited sample sizes, incorporating a correction factor to account for potential overestimation of the effect size in studies with small numbers of participants. Heterogeneity between studies was assessed visually by forest plots and quantitatively by the index of heterogeneity squared (I2) statistics, with 95% CI. The I2 statistic measures the proportion of observed variance between studies that is not due to chance (rather due to real differences across study populations and interventions). An I2 value greater than 75% was interpreted as evidence of a substantial level of heterogeneity.17 Sensitivity analyses were conducted using fixed effects meta-analyses for endpoints with little evidence of heterogeneity. Sub-group analysis was conducted to explore the source of heterogeneity. The risk of publication bias was assessed by examining funnel plot symmetry and by conducting Egger's regression test where A significant P-value <0.05 indicates publication bias. Adjusted variables for the observational studies are available in Table S6. The data analysis was conducted by Stata Software version 17.
Ethics
The present study did not require informed consent or approval by the local Ethics Committees, due to the nature of the research.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
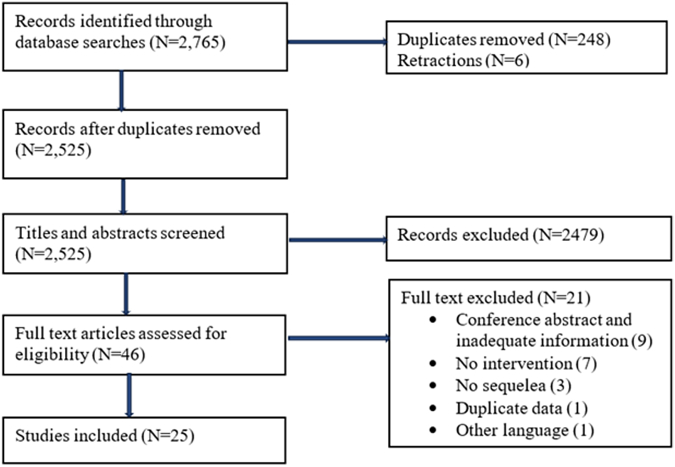
Our systematic search identified a total of 2765 articles. After removing 248 duplicates and 6 retracted papers, the remaining 2525 articles were screened by title and abstract, leaving 46 articles for full-text review. Finally, 25 articles fulfilling the eligibility criteria were included in this systematic review and meta-analysis, representing a total of 10,592 study participants (Fig. 1). The reasons for exclusion are provided in the supplementary files (Table S7).
Fig. 1.
Flow chart showing literature search and study selection.
Study characteristics
Table 1 provides detailed characteristics of the included studies. The studies were conducted between 1983 and 2023 in 12 different countries. The majority of studies were conducted in China (n = 7), South Africa (n = 5), and India (n = 4). The sample size of the included studies varied from 29 to 6743 (median 86). Most studies assessed possible interventions for preventing post-TB lung function sequelae (n = 9), liver function sequelae (n = 6), neurological sequelae (n = 5), and mental health disorders (n = 4). A few studies also assessed interventions for preventing hearing sequelae (n = 2), visual sequelae (n = 2), and cardiac sequelae (n = 1). Several possible interventions were investigated in the existing literature for their effectiveness in preventing post-TB sequela. These interventions include pulmonary rehabilitation programs, adjuvant therapies, comprehensive nursing care, micronutrient supplementation, hepatoprotective agents, cognitive behavioural therapy, and early medical interventions (Table 2).
Table 1.
Characteristics of studies included in the systematic review and meta-analyses.
| First author | Publication year | Country | Study design | Mean/median age (year) | Sample size | Intervention | Control | Length of intervention | Type of post-TB sequelae |
|---|---|---|---|---|---|---|---|---|---|
| Strange JIG | 1988 | South Africa | RCT | NA | 240 | Prednisolone | Placebo | 11 weeks | Cardiac function |
| Ando M | 2003 | Japan | RCT | 71 | 32 | Pulmonary rehabilitation | Before intervention | 9 weeks | Lung function |
| Nas K | 2004 | Turkey | Prospective cohort | 37.9 | 47 | Rehabilitation program | Before intervention | 6 months | Neurologic |
| Torok MS | 2011 | Vietnam | RCT | 35 | 545 | Dexamethasone | Placebo | 6–8 weeks | Neurologic |
| Grass DD | 2014 | South Africa | RCT | NA | 67 | Pulmonary rehabilitation | No intervention | 6 weeks | Lung function |
| Jones R | 2017 | Uganda | RCT | 45 | 34 | Pulmonary rehabilitation | Before intervention | 6 weeks | Lung function |
| Singh SM | 2018 | India | Prospective cohort | 48 | 29 | Pulmonary rehabilitation | Before intervention | 8 weeks | Lung function |
| Li X | 2019 | China | RCT | 72 | 183 | Comprehensive care | Routine care | 6 months | Anxiety & depression |
| Vashakidze SA | 2019 | USA | Cross-sectional | 31 | 54 | Surgical resection | No surgery | NA | Lung function |
| Shangase KK | 2019 | South Africa | Retrospective | 33.3 | 86 | Early medical intervention | No intervention | 6 months | Hearing |
| Xiong K | 2021 | China | RCT | 45 | 753 | Vitamins A & D | No supplement | 2 months | Liver function |
| Xu Z | 2021 | China | RCT | NA | 150 | Comprehensive intervention | Routine care | NA | Pulmonary depression & anxiety |
| Chen Q | 2022 | China | Retrospective cohort | 47.1 | 6743 | Silymarin/glycyrrhetinic acid | No intervention | 1–30 days | Liver function |
| Manesh A | 2023 | India | Retrospective cohort | NA | 90 | Infliximab | No infliximab | ≥1 dose | Neurological |
| Orooj M | 2023 | India | RCT | 31.1 | 90 | Pulmonary rehabilitation | No intervention | 4 weeks | Pulmonary & mental health |
| Ahmed S | 2020 | India | RCT | 25.5 | 62 | Pulmonary rehabilitation | No intervention | 12 weeks | Lung function |
| Visca D | 2019 | Italy | Retrospective cohort | 75 | 43 | Pulmonary rehabilitation | No intervention | 3 weeks | Lung function |
| Zuo X | 2022 | China | RCT | 41 | 45 | Cognitive behavioural therapy | Routine care | 2 months | Depression & Anxiety |
| Girgis NI | 1983 | Egypt | RCT | 14 | 55 | Dexamethasone | No steroids | 2 weeks | Vision |
| Gu J | 2015 | China | RCT | 36 | 568 | Silibinin | No Silibinin | 8 weeks | Liver function |
| Hakimizad R | 2021 | Iran | RCT | 53 | 87 | Acetyl-l-carnitine, alpha-lipoic acid & coenzyme Q10 | Placebo | 2 weeks | Liver function |
| Luangchosiri C | 2015 | Thailand | RCT | 54 | 55 | Silybum marinums | Placebo | 4 weeks | Liver function |
| Schoeman JF | 1997 | South Africa | RCT | 3 | 117 | Prednisone | No steroid | 1 month | Neurologic, hearing & vision |
| Schoeman JF | 2003 | South Africa | RCT | 52 | 47 | Thalidomide | Placebo | 1 month | Neurologic |
| Zhang S | 2015 | China | RCT | NA | 370 | Silybum marianum | Vitamin C tablet | 6 months | Liver function |
Table 2.
Number of studies reporting post-tuberculosis sequela and possible intervention strategies.
| Post-TB sequelae | Number of studies | Intervention strategies for preventing sequelae |
|---|---|---|
| Lung function | 9 |
|
| Liver function | 6 |
|
| Neurologic | 5 |
|
| Mental health disorder | 4 |
|
| Hearing | 2 |
|
| Vision | 2 |
|
| Cardiac function | 1 |
|
Quality assessment
Twenty-four studies were evaluated using the Cochrane risk of bias assessment tool, resulting in the classification of 17 studies as “low risk”, four as “some concerns”, and three as “high risk” of bias. Among the studies classified as “high risk”, all had a bias in the cohort selection and one study also had the potential for bias in the matching of exposed and unexposed cohorts. One study evaluated using the adapted Newcastle Ottawa scale was classified as medium quality. The assessment scores for each of the included articles are available in the supplementary file (Table S8 and Table S9).
Intervention to prevent post-TB lung sequelae
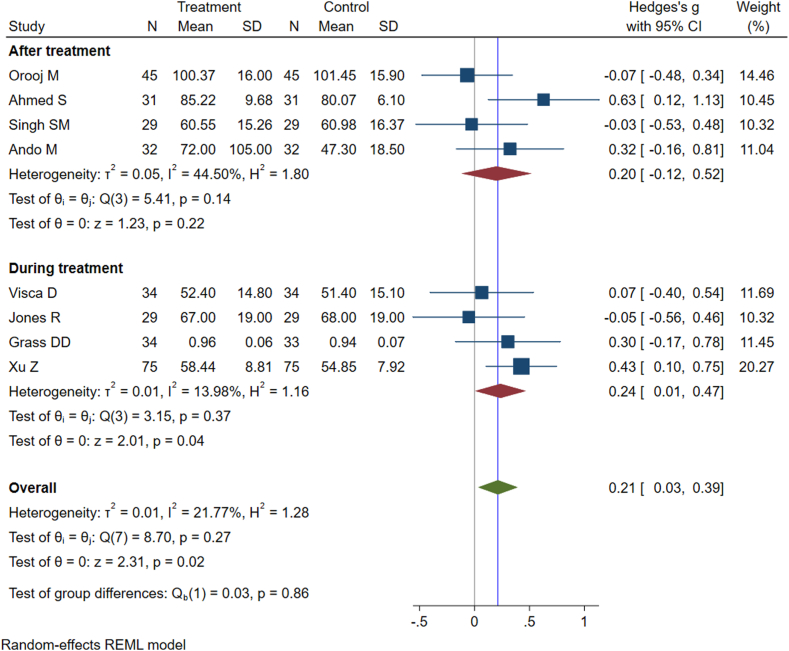
Table 3 summarises interventions and their effectiveness in preventing post-TB lung sequelae.18, 19, 20, 21, 22, 23, 24, 25, 26 Nine studies were conducted to examine the effectiveness of various intervention strategies in mitigating post-TB lung function sequelae. These interventions include pulmonary rehabilitation programs, comprehensive nursing care and adjunctive surgical resection. Post-TB lung function was assessed using various tools, including a 6-min walk test, a daily activity score, clinical dyspnea rating, and spirometry measurements such as forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and the ratio of FEV1 to FVC (FEV1/FVC). Notably, the FEV1/FVC ratio was commonly used across all studies (except one) to evaluate the impact of pulmonary rehabilitation programs on post-TB lung sequelae. We categorized interventions based on timing as those implemented during TB treatment and interventions implemented after the completion of TB treatment. Overall pooled effect estimates and subgroup analyses according to the timing of intervention are provided in Fig. 2. The pooled result shows that pulmonary rehabilitation programs can increase the grade of FEV1/FVC Ratio (Hedges's g = 0.21; 95% CI, 0.03–0.39; I2 = 21.7%; P = 0.02). Subgroup analyses by the timing of intervention showed that pulmonary rehabilitation programs provided during TB treatment significantly improved pulmonary function (Hedges's g = 0.24; 95% CI, 0.01–0.47; I2 = 13.98%; P = 0.04), whereas pulmonary rehabilitation programs provided after TB treatment did not significantly increase pulmonary function (Hedges's g = 0.20; 95% CI, −0.12 to 0.52; I2 = 44.5%; P = 0.22). The interaction test assessing the influence of pulmonary rehabilitation programs in relation to timing (during or after TB treatment) yielded non-significant results (Coefficient = 0.02, P = 0.8). This implies that the impact of the intervention is likely consistent across both time periods. Hower, it is important to interpret the findings cautiously, considering the small number of studies included in the meta-analysis and the lack of statistical significance does not necessarily imply the absence of a clinically meaningful difference. One study examining the effects of adjunctive surgical resection on pulmonary function showed that adjunctive surgical resection can improve pulmonary function. Results from sensitivity analyses, conducted using fixed-effects meta-analyses, were consistent with the values obtained from the random-effects meta-analysis (Figure S1). Analysis of funnel plots showed no obvious evidence of publication bias or that results of smaller trials were systematically different from those of larger trials (Figure S2). Main changes in lung function tests, pre- and post-intervention are available in Table S10.
Table 3.
Studies reporting possible interventions for preventing post-tuberculosis lung function impairment and their main findings.
| First author (year of publication), country, and study design | Category of intervention | Included interventions | Number of participants | Main findings |
|---|---|---|---|---|
| Ando M (2003), Japan, RCT | Pulmonary Rehabilitation | A 9-week pulmonary rehabilitation program | 32 | PRP appears to have merit when it is given to patients with post-TBC lung disorders |
| Grass DD (2014), South Africa, RCT | Pulmonary rehabilitation | A 6-week home-based pulmonary rehabilitation program that included cardiovascular exercises and walking. | 67 | PRP showed increasing trends towards lung function, but there was no statistical difference between the intervention and control groups |
| Jones R (2017), Uganda, RCT | Pulmonary rehabilitation | A 6-week pulmonary rehabilitation program consisted of exercise and education. | 34 | PRP was feasible and associated with clinically important improvements in respiratory outcomes, exercise capacity, and quality of life |
| Singh SM (2018), India, Prospective cohort | Pulmonary rehabilitation | An 8-week pulmonary rehabilitation program that included exercise and education. | 29 | Pulmonary function tests trended towards improvement with pulmonary rehabilitation but were not statistically significant |
| Vashakidze SA (2019), USA, Cross-sectional | Adjunctive surgical resection | Adjunctive lung surgery | 54 | Adjunctive surgery was significantly associated with improved lung function test |
| Xu Z (2021), China, RCT | Comprehensive nursing care | Comprehensive nursing intervention combined with respiratory functional exercises | 150 | Comprehensive nursing intervention combined with respiratory functional exercises can significantly improve pulmonary function |
| Orooj M (2023), India, RCT | pulmonary rehabilitation | A 4-week pulmonary rehabilitation program comprised of supervised exercise and education | 90 | Short-term PRP was insufficient to register changes in pulmonary function. |
| Ahmed S (2020), India, RCT | Pulmonary rehabilitation | 12-week pulmonary rehabilitation program consisted of exercise training, breath retraining, and education. | 62 | PRP administered along with treatment improved lung function capacity. |
| Visca D (2019), Italy, Retrospective cohort | Pulmonary rehabilitation | 3-week pulmonary rehabilitation programme including nurse training, exercises, relaxation, psychological support and nutritional counselling | 43 | PRP was effective in improving lung function capacity. |
Fig. 2.
Forest plot representing the effects of interventions during and after treatment on preventing post-tuberculosis lung impairment.
Interventions to prevent drug-induced liver injury
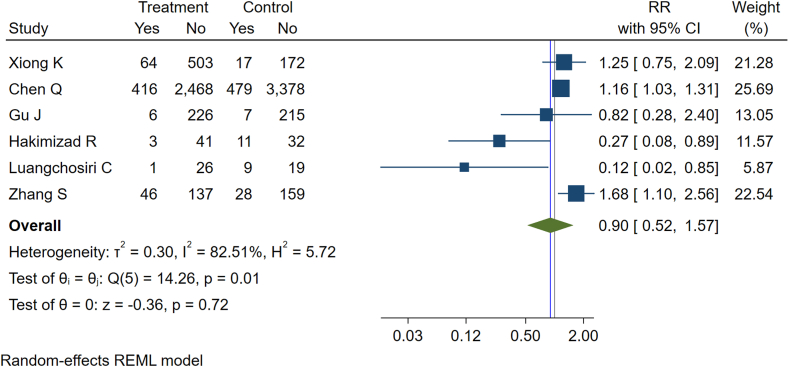
Six studies reported the effectiveness of various interventions in preventing DILI that commonly leads to liver function sequelae (Table 4).27, 28, 29, 30, 31, 32 The interventions investigated included micronutrient supplementation (e.g., vitamin A and vitamin D), administration of mitochondrial nutrients (e.g., acetyl-l-carnitine, alpha-lipoic acid, and coenzyme Q10), and the use of hepatoprotective agents (such as silymarin, glycyrrhetinic acid, silibinin, and silybum marianum). The overall incidence rate of DILI was 128 per 1000 TB survivors. When comparing the intervention groups to control groups across six studies included in the meta-analysis, the pooled relative risk (RR) was 0.90 (95% CI: 0.52–1.57, P = 0.72), indicating a lack of statistical significance and, therefore, a lack of evidence that existing interventions effectively reduced the incidence of DILI (Fig. 3). Subgroup analyses based on intervention characteristics were unfeasible due to the limited number of studies available. However, when examining individual studies, it was found that two trials involving silymarin and mitochondrial nutrients demonstrated a significant reduction in liver injury, while the remaining four studies did not show a significant decrease in liver injury (Table 4). Publication bias was not observed in the funnel plot of studies included in the prevention of liver function impairment (Figure S3).
Table 4.
Studies reporting possible interventions for preventing post-tuberculosis liver function impairment and their main findings.
| First author (year of publication), country, and study design | Category of intervention | Included interventions | Number of participants | Main findings |
|---|---|---|---|---|
| Xiong K (2021), China, RCT | Vitamin A and Vitamin D | Vitamin A (2000 IU/d), vitamin D (400 IU/d), and combination of vitamins A & D (2000 IU/d) supplementation | 753 | Vitamin A and D supplementation did not protect against TB-drug-induced liver injury. |
| Chen Q (2022), China, Retrospective cohort | Hepatoprotection prophylactic therapies | Chemoprotective agents including silymarin and/or glycyrrhetinic acid during for the first 30 days | 6743 | Prophylactic utilisation of hepatoprotective agents was not associated with a reduction in TB-DILI risks |
| Gu J (2015), China, RCT | Preventive hepatoprotective therapy | Silibinin capsules (oral administration of 70 mg/time, 3 times/day for 8 weeks) | 568 | There was no statistical difference in the incidences of liver injury between the two groups at different treatment periods |
| Hakimizad R (2021), Iran, RCT | Protective mitochondrial nutrients | Combination of acetyl-l-carnitine (250 mg), alpha-lipoic acid (250 mg), and coenzyme Q10 (200 mg) orally twice per day for 2 weeks as mitochondrial nutrients against anti-TB drug-induced liver injury | 87 | The incidence of DILI in the experimental group was significantly lower than that in the placebo group. |
| Luangchosiri C (2015), Thailand, RCT | Hepatoprotection | Silymarin (a traditional herbal drug extracted from milk thistle (Silybum marinums) seeds, has been used as a supplement remedy for hepatoprotection) | 55 | Silymarin reduced the incidence of antiTB-DILI. |
| Zhang S (2015), China, RCT | Hepatoprotective | Silybum marianum hepatoprotectants ((oral, 200 mg, twice a day)) to prevent anti-tuberculosis drug-induced liver injury (ATLI). | 370 | No significant preventive effect of silymarin was found for either lowering the risk of liver injury or boosting the positive outcomes. Worse, it has found a potential risk of liver damage caused by the hepatoprotectant. |
Fig. 3.
Forest plot showing the effect of intervention vs control in preventing post-tuberculosis liver function impairment.
Intervention to prevent neurological sequelae
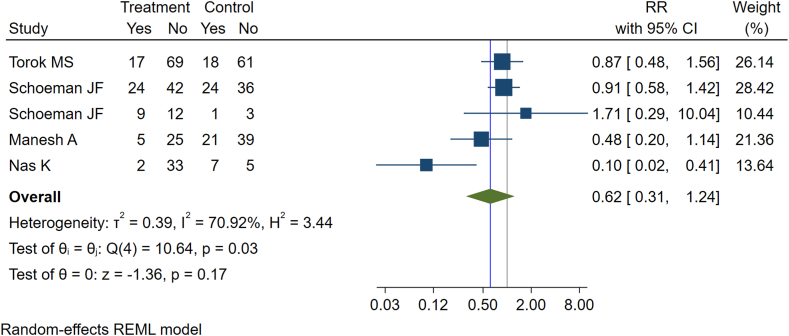
There were 5 studies representing 806 participants that evaluated the impacts of interventions in preventing post-TB neurological sequelae such as neuromotor disability, hemiparesis, quadriparesis, and neurological deficits (Table 5).33, 34, 35, 36, 37 The overall incidence rate of neurological sequelae was 282 per 1000 TB survivors (239 per 1000 TB survivors in the intervention group and 330 per 1000 TB survivors in the control group). We identified two main components of interventions delivered in the study: adjunctive therapy and rehabilitation programs. Adjunctive therapy includes dexamethasone, prednisone, thalidomide, and infliximab, which were reported as a component of TB treatment in four studies. The results of the meta-analysis for these adjunctive therapies showed that there was no statistically significant difference in the incidence of post-TB neurological sequelae between the intervention and control groups (RR = 0.62, 95% CI: 0.31–1.24) (Fig. 4). No significant heterogeneity was observed among the studies (I2 = 70.92%, P = 0.17%). The sensitivity analyses using fixed-effects meta-analyses yielded similar results (Figure S4). In one study implementing rehabilitation programs, a significant reduction in the incidence of neurological sequelae was observed within the intervention group when compared to the control group (Table 5). Inspection of the funnel plot did not indicate publication bias (Figure S5).
Table 5.
Studies reporting possible interventions for preventing post-tuberculosis neurological function impairment and their main findings.
| First author (year of publication), country, and study design | Type of neurological function impairment | Category of intervention | Included interventions | Number of participants | Main findings |
|---|---|---|---|---|---|
| Nas K (2004), Turkey, Prospective cohort | Motor impairment | Rehabilitation program | Muscle-strengthening exercises postoperative for 6 months | 47 | Rehabilitation programs on motor and functional improve patients motor and functional development of patients with spinal tuberculosis. |
| Torok MS (2011), Vietnam, RCT | Neurological disability | Adjunctive dexamethasone therapy | Intravenous dexamethasone (0.3–0.4 mg/kg day) at presentation and tapered over six to eight weeks | 545 | Adjunctive treatment with dexamethasone improves patient survival with TB meningitis but probably does not prevent severe disability |
| Schoeman JF (1997), South Africa, RCT | Hemiparesis and quadriparesis | Adjuvant steroids therapy | High-dose prednisone as adjuvant steroids (i.e., corticosteroids) therapy | 117 | No significant difference was found in the incidence of motor deficit between the steroid and nonsteroid groups after the completion of treatment |
| Schoeman JF (2003), South Africa, RCT | Hemiparesis | Adjunctive Thalidomide therapy | Thalidomide (24 mg/kg/day orally) adjunctive therapy for one month | 47 | The results do not support the use of adjunctive high-dose thalidomide therapy in the treatment of TB meningitis. The motor outcome was similar in the intervention and control groups |
| Manesh A (2023), India, Retrospective cohort | Neurological deficits (severe disability) | Adjunctive Infliximab Therapy | Adjunctive High-Dose Infliximab Therapy (Cohort A received at least 1 dose of infliximab after optimal anti-TB treatment and steroids) | 50 | Infliximab may be an effective and safe adjunctive strategy among severely disabled patients with CNS TB. |
Fig. 4.
Forest plot showing the effect of intervention vs control in preventing post-tuberculosis neurological impairment.
Intervention to prevent mental health disorders
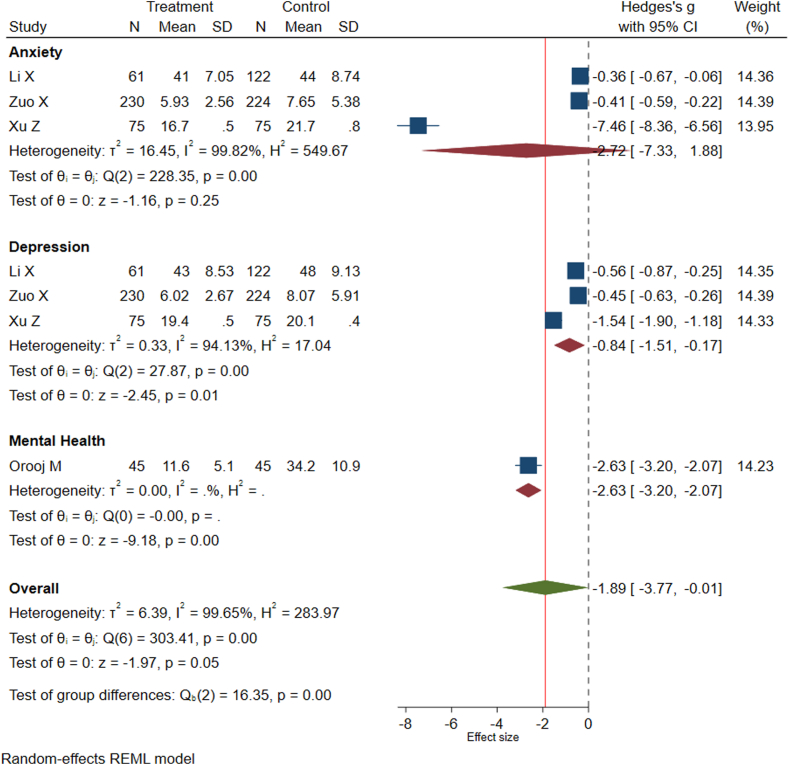
Four studies investigated the role of different interventions in preventing post-TB mental health disorders.25,26,38,39 Three of the studies assessed depression and anxiety as separate outcomes, while one study reported mental health as a general outcome (Table 6). We identified three different interventions reported in these studies for preventing the risk of post-TB mental health disorders, which included comprehensive interventions, pulmonary rehabilitation programs and cognitive-behavioural therapy (Table 6). These interventions significantly reduced the risk of mental health disorders among TB survivors (Hedges's g = −1.89; 95% CI: −3.77 to −0.01) (Fig. 5). However, there was publication bias (Figure S6) and the heterogeneity between studies was substantial (I2 = 99.65%, P < 0.001), with one study identified as an outlier among studies investigating the outcome of anxiety (Fig. 4). In contrast to the other studies that used standardised tools for anxiety measurement, the outlier study used a health-related quality of life questionnaire to assess anxiety. Sensitivity analyses were performed by excluding the outlier study, and the result showed a statistically significant reduction in anxiety within the intervention group compared to the control group (Hedges's g = −0.40; 95% CI: −0.56 to −0.24) (Figure S7).
Table 6.
Studies reporting possible interventions for preventing post-tuberculosis mental health disorders impairment and their main findings.
| First author (year of publication), country, and study design | Mental health disorder | Category of intervention | Included interventions | Number of participants | Main findings |
|---|---|---|---|---|---|
| Li X (2019), China, RCT | Depression and anxiety | Comprehensive interventions | Comprehensive interventions (health education, psychotherapy, home visits, peer support, and psycho-educational workshops) | 183 | Comprehensive interventions could alleviate the anxiety and depression of elderly tuberculosis patients sustainably and effectively. |
| Xu Z (2021), China, RCT | Depression and anxiety | Comprehensive interventions | Comprehensive nursing intervention combined with respiratory functional exercises | 150 | Comprehensive nursing intervention combined with respiratory functional exercises can significantly improve the pulmonary function, self-care ability, and quality of life of patients with pulmonary tuberculosis, with obvious clinical efficacy |
| Orooj M (2023), India, RCT | Mental health | Pulmonary rehabilitation program | Pulmonary rehabilitation program (comprised of supervised endurance and resistance training, breathing techniques, self-management strategies, and patient education) | 90 | A short-term pulmonary rehabilitation program enhances TB patient functional capacity, HRQoL, and dyspneal scores. |
| Zuo X (2022), China, RCT | Depression and anxiety | Cognitive-behavioral therapy | Cognitive-behavioral therapy for two months. The intervention group underwent CBT for 2 months, whereas the control group received routine follow-up | 45 | CBT can relieve anxiety, and depression symptoms and increase the quality of life in subjects with pulmonary tuberculosis. |
Fig. 5.
Forest plot representing the effect of intervention in preventing post-tuberculosis mental health disorders including anxiety and depression.
Intervention to prevent other types of post-TB sequelae
Table 7 presents summary information of four studies investigating interventions for post-TB cardiac function, hearing and vision sequelae.33,40, 41, 42 Adjuvant steroid therapy (i.e., prednisone) demonstrated a significant reduction in post-TB cardiac function sequelae while showing no preventive effect on hearing and visual sequelae.33,41 These results suggest that the effectiveness of prednisolone and other interventions may vary based on the specific type of sequelae and affected body sites. Additionally, one study reported the potential effects of early medical interventions including dose reduction, discontinuation of toxic medications, and regimen adjustments with safer drugs, in preventing post-TB hearing sequelae.42
Table 7.
Studies reporting possible interventions for preventing other post-tuberculosis sequelae and their main findings.
| First author (year of publication), country, and study design | Type of post-tuberculosis sequelae | Category of intervention | Included interventions | Number of participants | Main findings |
|---|---|---|---|---|---|
| Strange JIG (1988), South Africa, RCT | Abnormal cardiac function | Adjuvant steroids therapy | Adjuvant steroids therapy (i.e., prednisolone 5 mg tablets for first 11 weeks) | 240 | Prednisolone significantly reduced post-TB treatment sequala and corticosteroids can be prescribed in addition to anti-TB chemotherapy in the treatment of TB pericarditis. |
| Shangase KK (2019), South Africa, Retrospective | Hearing impairment | Early medical intervention | Early medical intervention to reduce the dose or stop treatment with ototoxic drugs, replaced by other drugs such as bedaquiline. | 86 | Early medical strategies implemented had a significant preventive impact. |
| Girgis NI (1983), Egypt, RCT | Vision impairment | Adjuvant steroids therapy | Adjunct therapy of dexamethasone was given (intramuscular 8–12 mg/day) for the first three weeks of therapy. | 55 | Dexamethasone does not affect the overall mortality from TBM but appears to prevent the development of severe ocular complications. |
| Schoeman JF (1997), South Africa, RCT | Vision and hearing impairment | Adjuvant steroids therapy | High-dose prednisone as adjuvant steroids (i.e., corticosteroids) therapy | 117 | No significant difference was found in the incidence of blindness or deafness between the steroid and nonsteroid groups. |
Discussion
This systematic review and meta-analysis quantifies the impacts of various interventions for preventing post-TB sequelae across different domains, including lung function impairment, liver function impairment, neurological impairment, and mental health disorders. These are the most common types of post-TB sequelae reported in previous systematic reviews and meta-analyses.2,6 In the current systematic review, several classes of interventions such as pulmonary rehabilitation programs, adjunctive surgical resection, comprehensive nursing care, micronutrient supplementation, adjunctive therapy, cognitive-behavioural therapy, hepatoprotective agents, and close patient follow-up and monitoring for timely medical intervention (e.g., dose reduction, treatment cessation, or switching to less toxic alternatives) were evaluated for their effectiveness in preventing different types of post-TB sequelae. The results demonstrated that only a few of these interventions have effectively prevented post-TB sequelae, providing important evidence for informed decision-making in managing post-TB sequelae. The scarcity of effective interventions to prevent and reduce post-TB sequelae may be attributed to the omission of post-TB sequelae from the TB program in the End TB Strategy. Furthermore, the recent UN New York declaration has also overlooked the emerging challenges of post-TB sequelae.
Our systematic review identified pulmonary rehabilitation programs as the most important intervention to prevent post-TB pulmonary sequelae among TB survivors. This finding aligns with previous systematic reviews that have also identified pulmonary rehabilitation programs as effective interventions in preventing sequelae in patients with chronic obstructive pulmonary disease (COPD).43,44 The studies included in our review presented diverse models of pulmonary rehabilitation programs, reflecting variations in healthcare contexts and access to such services. The rehabilitation programmes typically included physical exercises, strength training and education. Our study demonstrated that early initiation of the intervention may result in better lung function compared to late initiation; however, the evidence was not conclusive. This finding is clinically relevant and introduces a novel perspective on the significance of intervention timing in the context of TB care to optimise post-TB lung health. While integrating rehabilitation programs with TB treatment is important, further study is required to identify individuals most at risk for post-TB lung health complications. By targeting these high-risk groups, healthcare providers can ensure that pulmonary rehabilitation programs are delivered where they are needed most, thus maximising their potential impact to prevent post-TB sequelae. Here, it is also important to mention that early diagnosis and appropriate treatment of TB are crucial in preventing post-TB pulmonary sequelae by halting disease progression, minimizing lung tissue damage, preventing drug resistance, and reducing the risk of TB complications.4,45 A recent review showed that vaccination against respiratory tract infections including influenza, pneumococcal and COVID-19 potentially prevent post-TB lung sequelae.46 The Brazilian Thoracic Association has also recommended the use of these vaccinations to prevent post-TB lung sequelae.47
While the extent of liver function impairment among TB survivors may vary based on the treatment regimen, our systematic review examined potential interventions to prevent liver function impairment following TB treatment. Various interventions, including micronutrient supplementation, administration of mitochondrial nutrients, and the use of hepatoprotective agents, were evaluated in the existing literature to assess their effectiveness in preventing liver injury in patients with TB treatment. However, our meta-analysis did not reveal a statistically significant reduction in liver function impairment across the included studies, suggesting that there is a lack of evidence supporting their effectiveness. However, the low number of patients and methodological quality should be considered as a limitation when interpreting this finding. Previous studies also reported that while hepatoprotective agents were effective in preventing temporary and mild side effects of TB medications, they are not effective in preventing severe and long-term liver function impairment.48,49
Few studies have investigated the effects of rehabilitation programs and adjunctive therapy on preventing post-TB neurological impairment and findings are inconsistent.33, 34, 35, 36, 37 The pooled meta-analysis of adjunctive therapy revealed no statistically significant difference in neurological impairment incidence between the intervention and control groups, whereas a separate study on rehabilitation programs showed a significant reduction in neurological impairment within the intervention group compared to controls. While these findings suggest the potential benefit of rehabilitation programs in preventing neurological sequelae following TB treatment, further studies are needed to establish conclusive evidence.
Our systematic review identified four randomised controlled trials examining the effect of various interventions to prevent the occurrence of post-TB mental health disorders such as anxiety and depression.25,26,38,39 The interventions were based on comprehensive care, pulmonary rehabilitation programs, and cognitive-behavioural therapy. Meta-analysis of the included studies demonstrated that the interventions were considerably more effective than the control group (i.e., routine care or no intervention) in preventing post-TB mental health disorders. This has important implications for the longer-term management of depression and anxiety within TB programs. Interventions to prevent mental health disorders during TB illness have been studied extensively and the WHO guidelines have also recommended the integration of mental health programs during TB treatment.50 However, our findings showed that long-term integration of programs beyond the treatment period is also required.
The findings of this systematic review and meta-analysis need to be interpreted in the context of the following limitations. First, our results for most of the interventions are based on a limited number of studies. For example, while there were studies investigating the effectiveness of interventions for preventing post-TB cardiac impairment,41 hearing impairment,33,42 and vision impairments,33,40 conducting a meta-analysis was not possible due to the small number of studies. Second, as our systematic review and meta-analysis focused on identifying effective interventions for preventing long-term sequelae of TB or its medication, several studies evaluating the impacts of interventions in preventing temporary side effects of TB medication or disease complications were excluded. This conservative approach might miss some studies that did not separately report post-TB sequelae. Third, the comprehensive inclusion of diverse studies covering various topics, including pulmonary and extrapulmonary TB, and drug-sensitive and drug-resistant TB, in our systematic review and meta-analysis, poses a challenge in maintaining a cohesive narrative and may obscure the clarity of key findings. Fourth, the evidence in this systematic review is also not sufficiently robust to properly assess the effect of each intervention on the outcome of interest. This is primarily due to the small sample size and the very limited data in the existing literature, which is an important knowledge gap. Finally, our search was limited to English language studies, and this might have resulted in missing studies published in other languages.
In conclusion, this systematic review and meta-analysis identified various interventions for the prevention of post-TB sequelae. The results showed the importance of timing and specific interventions in addressing different sequelae. Rehabilitation programs show promise in preventing post-TB sequelae, while adjuvant therapies including hepato-protective drugs require further investigation. The varying quality of included studies and the presence of substantial heterogenicity suggest the need for adequately powered future studies using standardised endpoints. Clinicians and TB program leaders can use these findings to inform their strategies for preventing post-TB sequelae and improving the long-term health outcomes of TB patients.
Contributors
KAA conceived and designed the study, ran the analysis, and drafted the manuscript. LH, BG, ACAC, and MBM made substantial contributions in reviewing the draft manuscript. All authors contributed to the final approval of the version to be submitted.
All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The datasets generated during and/or analysed during the current study are available in the text and Supplementary material. Rough data supporting reported results are available from the corresponding author on reasonable request.
Declaration of interests
The authors have no conflicts of interest to declare.
Acknowledgements
This project is funded by the Healy Medical Research Raine Foundation and Curtin School of Population Health. KAA is funded by an Australian National Health and Medical Research Council Investigator Grant (APP1196549). The funders provided no input into the undertaking or reporting of the research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102511.
Contributor Information
Kefyalew Addis Alene, Email: kefyalew.alene@curtin.edu.au.
Lucas Hertzog, Email: lucas.hertzog@curtin.edu.au.
Beth Gilmour, Email: Beth.Gilmour@telethonkids.org.au.
Archie C.A. Clements, Email: archie.clements@plymouth.ac.uk.
Megan B. Murray, Email: megan_murray@hms.harvard.edu.
Appendix A. Supplementary data
References
- 1.WHO . World Health Organization; Geneva: 2023. Global tuberculosis report 2023. [Google Scholar]
- 2.Alene K.A., Wangdi K., Colquhoun S., et al. Tuberculosis related disability: a systematic review and meta-analysis. BMC Med. 2021;19(1):1–19. doi: 10.1186/s12916-021-02063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alene K.A., Clements A.C., McBryde E.S., et al. Mental health disorders, social stressors, and health-related quality of life in patients with multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Infect. 2018;77(5):357–367. doi: 10.1016/j.jinf.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Nightingale R., Carlin F., Meghji J., et al. Post-TB health and wellbeing. Int J Tubercul Lung Dis. 2023;27(4):248–283. doi: 10.5588/ijtld.22.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alene K.A., Clements A.C., McBryde E.S., et al. Sequelae of multidrug-resistant tuberculosis: protocol for a systematic review and meta-analysis. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-019593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akalu T.Y., Clements A.C., Wolde H.F., Alene K.A. Prevalence of long-term physical sequelae among patients treated with multi-drug and extensively drug-resistant tuberculosis: a systematic review and meta-analysis. eClinicalMedicine. 2023;57 doi: 10.1016/j.eclinm.2023.101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzies N.A., Quaife M., Allwood B.W., et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Global Health. 2021;9(12):e1679–e1687. doi: 10.1016/S2214-109X(21)00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; Geneva: 2022. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-susceptible tuberculosis treatment. [PubMed] [Google Scholar]
- 9.WHO. Policy brief on tuberculosis-associated disability. Policy brief on tuberculosis-associated disability; 2023. [Google Scholar]
- 10.UN . 2023. Comprehensive review of progress towards the achievement of global tuberculosis targets and implementation of the political declaration of the United Nations high-level meeting of the General Assembly on the fight against tuberculosis. New York. [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.Migliori G.B., Marx F., Ambrosino N., et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tubercul Lung Dis. 2021;25(10):797–813. doi: 10.5588/ijtld.21.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Syst Rev. 2016;5(210) doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:366. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa. Ottawa Hosp Res Inst. 2011;2(1):1–12. [Google Scholar]
- 16.Wells G., Shea B., O'Connell D., et al. 2014. Newcastle-Ottawa quality assessment scale cohort studies. [Google Scholar]
- 17.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 18.Ando M., Mori A., Esaki H., et al. The effect of pulmonary rehabilitation in patients with post-tuberculosis lung disorder. Chest. 2003;123(6):1988–1995. doi: 10.1378/chest.123.6.1988. [DOI] [PubMed] [Google Scholar]
- 19.de Grass D., Manie S., Amosun S.L. Effectiveness of a home-based pulmonary rehabilitation programme in pulmonary function and health related quality of life for patients with pulmonary tuberculosis: a pilot study. Afr Health Sci. 2014;14(4):866. doi: 10.4314/ahs.v14i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones R., Kirenga B.J., Katagira W., et al. A pre-post intervention study of pulmonary rehabilitation for adults with post-tuberculosis lung disease in Uganda. Int J Chronic Obstr Pulm Dis. 2017;12:3533–3539. doi: 10.2147/COPD.S146659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S.K., Naaraayan A., Acharya P., Menon B., Bansal V., Jesmajian S. Pulmonary rehabilitation in patients with chronic lung impairment from pulmonary tuberculosis. Cureus. 2018;10(11) doi: 10.7759/cureus.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vashakidze S.A., Kempker J.A., Jakobia N.A., et al. Pulmonary function and respiratory health after successful treatment of drug-resistant tuberculosis. Int J Infect Dis. 2019;82:66–72. doi: 10.1016/j.ijid.2019.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visca D., Zampogna E., Sotgiu G., et al. Pulmonary rehabilitation is effective in patients with tuberculosis pulmonary sequelae. Eur Respir J. 2019;53(3) doi: 10.1183/13993003.02184-2018. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed S., Sharma N., Patrikar S., Samiullah Efficacy of early structured pulmonary rehabilitation program in pulmonary function, exercise capacity, and health-related quality of life for patients with post-tubercular sequelae: a pilot study. Med J Armed Forces India. 2020;78(2):164–169. doi: 10.1016/j.mjafi.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z.J., Chen W.W., Li X.S. Effects of comprehensive nursing intervention combined with respiratory functional exercises on pulmonary function and self-care ability in patients with pulmonary tuberculosis: results of a randomized trial. Ann Palliat Med. 2021;10(7):7543–7550. doi: 10.21037/apm-21-1178. [DOI] [PubMed] [Google Scholar]
- 26.Orooj M., Pant A., Mujaddadi A., Jain M., Ahmed I. Short-term pulmonary rehabilitation among post-pulmonary tuberculosis patients during coronavirus disease 2019 pandemic. Thorac Res Pract. 2023;24(3) doi: 10.5152/ThoracResPract.2023.22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Gu S.-J.T., Shou-Yong T., Qi W., et al. An open-label, randomized and multi-center clinical trial to evaluate the efficacy of Silibinin in preventing drug-induced liver injury. Int J Clin Exp Med. 2015;8(3):4320–4327. [PMC free article] [PubMed] [Google Scholar]
- 28.Luangchosiri C., Thakkinstian A., Chitphuk S., Stitchantrakul W., Petraksa S., Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Compl Alternative Med. 2015;15:334. doi: 10.1186/s12906-015-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S., Pan H., Peng X., et al. Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: a randomized controlled trial. J Gastroenterol Hepatol. 2016;31(2):409–416. doi: 10.1111/jgh.13070. [DOI] [PubMed] [Google Scholar]
- 30.Hakimizad R., Soltani R., Khorvash F., Marjani M., Dastan F. The effect of acetyl-L-carnitine, alpha-lipoic acid, and coenzyme Q10 combination in preventing anti-tuberculosis drug-induced hepatotoxicity: a randomized, double-blind, placebo-controlled clinical trial. Iran J Pharm Res (IJPR) 2021;20(3):431–440. doi: 10.22037/ijpr.2021.114618.14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong K., Wang J.Y., Zhang B., Xu L., Hu Y.D., Ma A.G. Vitamins A and D fail to protect against tuberculosis-drug-induced liver injury: a post hoc analysis of a previous randomized controlled trial. Nutrition. 2021;86 doi: 10.1016/j.nut.2021.111155. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q., Hu A.R., Ma A.X., et al. Effectiveness of prophylactic use of hepatoprotectants for tuberculosis drug-induced liver injury: a population-based cohort analysis involving 6,743 Chinese patients. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.813682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johan F., Schoeman L.E.V.Z., Jacoba A L., Peter R D. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99(2):1997. doi: 10.1542/peds.99.2.226. [DOI] [PubMed] [Google Scholar]
- 34.Schoeman J.F., Springer P., van Rensburg A.J., et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J Child Neurol. 2004;19:250–257. doi: 10.1177/088307380401900402. [DOI] [PubMed] [Google Scholar]
- 35.Nas K., Kemaloglu M.S., Cevik R., et al. The results of rehabilitation on motor and functional improvement of the spinal tuberculosis. Joint Bone Spine. 2004;71(4):312–316. doi: 10.1016/S1297-319X(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 36.Török M.E., Nguyen D.B., Tran T.H., et al. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manesh A., Gautam P., Kumar D.S.S., et al. Effectiveness of adjunctive high-dose infliximab therapy to improve disability-free survival among patients with severe central nervous system tuberculosis: a matched retrospective cohort study. Clin Infect Dis. 2023;77:1460. doi: 10.1093/cid/ciad401. [DOI] [PubMed] [Google Scholar]
- 38.Li X.H., Wang B., Xu Y.H., et al. Comprehensive intervention for anxiety and depression among the community elderly with tuberculosis. Basic Appl Soc Psychol. 2019;41(3):179–187. [Google Scholar]
- 39.Zuo X., Dong Z., Zhang P., et al. Cognitive-behavioral therapy on psychological stress and quality of life in subjects with pulmonary tuberculosis: a community-based cluster randomized controlled trial. BMC Publ Health. 2022;22(1):2160. doi: 10.1186/s12889-022-14631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girgis Zf N.I., Hanna L.S., Yassin M.W., Wallace C.K. The use of dexamethasone in preventing ocular complications in tuberculous meningitis. Trans R Soc Trop Med Hyg. 1983;77(5):658–659. doi: 10.1016/0035-9203(83)90195-5. [DOI] [PubMed] [Google Scholar]
- 41.Strang J.I., Kakaza H.H., Gibson D.G., et al. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet. 1988;2(8614):759–764. doi: 10.1016/s0140-6736(88)92415-4. [DOI] [PubMed] [Google Scholar]
- 42.Khoza-Shangase K., Lecheko L., Ntlhakana L. The impact of medical interventions for reducing ototoxicity during treatment for multi-drug resistant tuberculosis. Acta Otorrinolaringol Esp. 2020;71(6):349–357. doi: 10.1016/j.otorri.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Jácome C., Marques A. Pulmonary rehabilitation for mild COPD: a systematic review. Respir Care. 2014;59(4):588–594. doi: 10.4187/respcare.02742. [DOI] [PubMed] [Google Scholar]
- 44.Habib G.M., Rabinovich R., Divgi K., et al. Systematic review of clinical effectiveness, components, and delivery of pulmonary rehabilitation in low-resource settings. NPJ Prim Care Respir Med. 2020;30(1):52. doi: 10.1038/s41533-020-00210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Kampen S.C., Wanner A., Edwards M., et al. International research and guidelines on post-tuberculosis chronic lung disorders: a systematic scoping review. BMJ Glob Health. 2018;3(4) doi: 10.1136/bmjgh-2018-000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasiri M., Silva D., Rommasi F., et al. Vaccination in post-tuberculosis lung disease management: a review of the evidence. Pulmonology. 2023;S2531-0437 doi: 10.1016/j.pulmoe.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Silva D.R., Santos A.P., Visca D., et al. Brazilian Thoracic Association recommendations for the management of post-tuberculosis lung disease. J Bras Pneumol. 2024;49 doi: 10.36416/1806-3756/e20230269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Floro C, Fausto A, Velasco M, Camenforte J, De Lusong M. Silymarin for prevention of anti-tuberculosis drug-induced liver injury: a meta-analysis.
- 49.Niu H., Sanabria-Cabrera J., Alvarez-Alvarez I., et al. Prevention and management of idiosyncratic drug-induced liver injury: systematic review and meta-analysis of randomised clinical trials. Pharmacol Res. 2021;164 doi: 10.1016/j.phrs.2020.105404. [DOI] [PubMed] [Google Scholar]
- 50.Ravimohan S., Kornfeld H., Weissman D., Bisson G.P. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27(147) doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.