Abstract
The c-Cbl protein is tyrosine phosphorylated and forms complexes with a wide range of signalling partners in response to various growth factors. How c-Cbl interacts with proteins, such as Grb2, phosphatidylinositol 3-kinase, and phosphorylated receptors, is well understood, but its role in these complexes is unclear. Recently, the Caenorhabditis elegans Cbl homolog, Sli-1, was shown to act as a negative regulator of epidermal growth factor receptor signalling. This finding forced a reassessment of the role of Cbl proteins and highlighted the desirability of testing genetically whether c-Cbl acts as a negative regulator of mammalian signalling. Here we investigate the role of c-Cbl in development and homeostasis in mice by targeted disruption of the c-Cbl locus. c-Cbl-deficient mice were viable, fertile, and outwardly normal in appearance. Bone development and remodelling also appeared normal in c-Cbl mutants, despite a previously reported requirement for c-Cbl in osteoclast function. However, consistent with a high level of expression of c-Cbl in the hemopoietic compartment, c-Cbl-deficient mice displayed marked changes in their hemopoietic profiles, including altered T-cell receptor expression, lymphoid hyperplasia, and primary splenic extramedullary hemopoiesis. The mammary fat pads of mutant female mice also showed increased ductal density and branching compared to those of their wild-type littermates, indicating an unanticipated role for c-Cbl in regulating mammary growth. Collectively, the hyperplastic histological changes seen in c-Cbl mutant mice are indicative of a normal role for c-Cbl in negatively regulating signalling events that control cell growth. Consistent with this view, we observed greatly increased intracellular protein tyrosine phosphorylation in thymocytes following CD3ε cross-linking. In particular, phosphorylation of ZAP-70 kinase in thymocytes was uncoupled from a requirement for CD4-mediated Lck activation. This study provides the first biochemical characterization of any organism that is deficient in a member of this unique protein family. Our findings demonstrate critical roles for c-Cbl in hemopoiesis and in controlling cellular proliferation and signalling by the Syk/ZAP-70 family of protein kinases.
The c-Cbl protooncogene is the cellular homolog of the acutely transforming v-Cbl oncogene, which was originally identified in the murine Cas NS-1 retrovirus (27). v-Cbl induces pre-B-cell and myeloid tumors in mice, transforms rodent fibroblast cell lines, and encodes a protein of 357 amino acids which encompasses the amino-terminal region of c-Cbl (2). Recently the 913-amino-acid product of c-Cbl has been identified as a ubiquitous substrate of protein tyrosine kinases that is rapidly phosphorylated following stimulation of growth factor, antigen, and integrin receptors (4, 11, 13, 17, 25, 33, 36–38, 40, 52, 60). c-Cbl protein has no known catalytic function but contains a novel phosphotyrosine binding (PTB) domain, a RING finger domain, an extensive proline-rich region, and a carboxy-terminal leucine zipper. To date four Cbl proteins have been described: c-Cbl and Cbl-b in mammals, Drosophila Cbl, and Caenorhabditis elegans Cbl (known as Sli-1) (2, 19, 21, 34, 64). The partial sequence of a third human Cbl homolog has recently been lodged in the National Institutes of Health expressed sequence tag (EST) database (AA112513). All Cbl proteins are highly conserved in their amino-terminal PTB and RING finger domains but are divergent at their carboxy termini. The carboxy-terminal half of c-Cbl contains proline-rich SH3-binding domains, three major sites of tyrosine phosphorylation for SH2 domain interactions, and the binding site for interactions with 14-3-3 proteins (30).
While the multiple complexes that c-Cbl is found in suggest diverse roles in signal transduction, a definitive function for c-Cbl has not emerged. The most revealing clue about the function of Cbl proteins came originally from genetic studies in C. elegans, where Sli-1 was identified as a negative regulator of the epidermal growth factor (EGF) receptor tyrosine kinase (Let-23) (20). These experiments demonstrated that Sli-1 acts at the level of Let-23 and the C. elegans Grb2 homolog (Sem5), consistent with mammalian studies that place Cbl at an early point in tyrosine kinase-mediated signal transduction (64). Recent studies have provided initial evidence that mammalian c-Cbl may also negatively regulate the activity of cytoplasmic protein tyrosine kinases. Overexpression of c-Cbl in mast cells suppresses Syk kinase activity (39) and reduces Ras-dependent AP1 activation following T-cell ligation in Jurkat cells (43). In addition, treatment of cells with antisense c-Cbl can enhance the activation of the JAK-STAT pathway (56). It is unclear whether these effects on kinase activity are direct. Interestingly, c-Cbl has been shown to be transiently ubiquitinated in colony-stimulating factor 1 (CSF-1) stimulated macrophages, leading to the suggestion that c-Cbl may negatively regulate signalling in these cells by participating in ubiquitin-mediated degradation of the CSF-1 receptor (60).
Studies of oncogenic forms of c-Cbl have also provided clues to Cbl function. c-Cbl can be rendered oncogenic either by a large carboxy-terminal truncation (v-Cbl) or by a small internal deletion immediately amino terminal to the RING finger (70Z-Cbl) (1, 2, 27). Studies of v-Cbl demonstrated that the PTB domain forms a direct association with the EGF receptor and the ZAP-70 tyrosine kinase (4, 17, 31, 53). These findings indicate that v-Cbl may complete with c-Cbl for binding sites on activated receptor complexes, thereby blocking regulation by c-Cbl and resulting in transformation. In contrast, transformation by 70Z-Cbl appears to involve a positive signalling mechanism. Expression of 70Z-Cbl causes hyperphosphorylation of a range of substrates by enhancing the kinase activity of the platelet-derived growth factor and EGF receptors (3, 54). These studies indicate that 70Z-Cbl has lost its ability to function as a negative regulator but still provides docking sites for complexes that mediate growth-stimulatory signals. This notion is supported by the finding that 70Z-Cbl, but not c-Cbl or v-Cbl, induces transcriptional activation of the nuclear factor of activated T cells (NFAT) (29). 70Z-Cbl-mediated NFAT activation was markedly enhanced by stimulation with calcium ionophore and abrogated by the expression of a dominant-negative Ras, implicating 70Z-Cbl in the Ras signalling pathway. Reduced activation of Erk2 and decreased stimulation of a Ras-sensitive AP1 reporter in c-Cbl-overexpressing Jurkat cells (43) is also consistent with a role for c-Cbl in T-cell receptor (TCR)-mediated Ras activation.
These studies have led to the realization that c-Cbl may function as a negative regulator of protein tyrosine kinases and also to provide docking sites for a multitude of signalling proteins. To study these proposed roles in detail, we have generated mice that are deficient in c-Cbl. The results obtained with these animals provide compelling evidence that mammalian c-Cbl is involved in the control of tissue cellularity and that it functions as a negative regulator of protein tyrosine kinases.
MATERIALS AND METHODS
c-Cbl targeting vector and isolation of targeted ES cells and mice.
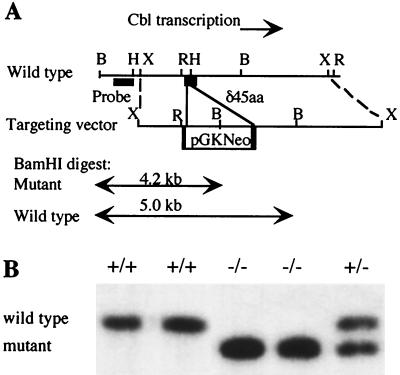
Murine c-Cbl genomic clones were isolated from a λUNI-ZAP 129Sv library (Stratagene) by hybridization with a 32P-labelled murine c-Cbl cDNA, and the positions of the coding exons were determined by standard procedures (44). A 7-kb XbaI fragment was subcloned into the XbaI site of pBSII (Stratagene), and from this an EcoRI-HindIII fragment that contained a c-Cbl coding exon, beginning at amino acid codon 146, was subcloned into pALTER (Promega). A ClaI site was introduced at amino acid codon 153 by site-directed mutagenesis, converting the sequence CAAATTATCCCTGATCTT to CAAATTATCGAGGATCTT. This fragment was returned to the 7-kb XbaI fragment, and a 1.8-kb pGKNeo-selectable marker was inserted between the ClaI and HindIII sites, removing amino acid codons 153 to 190 and creating an in-frame stop codon within the pGK promoter. The construct was linearized with XhoI and electroporated into W9.5 embryonic stem (ES) cells (26). Targeted ES cells were selected in G418 at 200 μg/ml. DNA was obtained for Southern analysis of these clones by using proteinase K and sodium dodecyl sulfate (SDS) and was restricted with BamHI. To identify targeted clones, filters were probed with a 32P-labelled genomic fragment outside the targeting construct (Fig. 1A). As predicted, targeted clones gave a new band of 4.2 kb. Fifteen clones were obtained at a frequency of 1 clone per 27 Neor ES cell clones.
FIG. 1.
Targeted disruption of the c-Cbl locus. (A) Mutation strategy in which c-Cbl coding sequences were replaced by a neomycin resistance cassette (pGKneo). Predicted sizes are shown for the wild-type and mutant alleles following BamHI digestion of genomic DNA and probing with the indicated fragment outside the targeting construct. B, BamHI; H, HindIII; X, XbaI; R, EcoRI. (B) Southern blot analysis of tail DNA from progeny of an intercross of heterozygous mice, restricted with BamHI and probed with the fragment indicated in A. Mice bearing the mutant allele showed the predicted restriction fragment.
Four G418-resistant clones that had only single integration events, as evidenced by probing a Southern blot with neomycin-specific probe, were used to generate chimeric mice by microinjection of embryonic day 3.5 C57BL/6J blastocysts. Male chimeras were mated to C57BL/6J females, and germ line transmission of the ES cell genome was detected by coat color. Southern blot analysis of the tail DNA of pups was performed by standard procedures (44), and approximately half of the agouti pups were heterozygous for the c-Cbl mutation. C57BL/6J and 129Sv mice were obtained from the Animal Resource Center, Murdoch, Western Australia. Western blot analysis of tissues from homozygous mutant mice was performed essentially as described previously (59) but with an antiserum directed to a C-terminal c-Cbl peptide, C15 (Santa Cruz).
Thymocyte stimulation by antibody cross-linking.
Single-cell suspensions of thymocytes were prepared at 5 × 107/ml in RPMI 1640 supplemented with 5% fetal calf serum (FCS) (RPMI–5% FCS). Hamster anti-CD3ε (500A2) or biotinylated anti-CD3ε (500A2) and anti-CD4 (L3T4) antibodies were added to the cells at 10 μg/ml, and the cells were incubated on ice for 10 min and washed once in RPMI–5% FCS. Cross-linking was carried out by addition of 12.5 μg of anti-hamster immunoglobulin G (IgG) antibodies/ml in RPMI–5% FCS or 40 μg of streptavidin/ml in RPMI. The cells were incubated on ice for 10 min before stimulation at 37°C for 5 min or various times as indicated. All antibodies were obtained from PharMingen.
Immunoprecipitations and immunoblotting.
Following stimulation, the thymocytes were washed once in ice-cold phosphate-buffered saline and then lysed at 3 × 107 to 5 × 107 cells/ml in ice-cold Nonidet P-40 (NP-40) lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1 mM sodium orthovanadate, 1% NP-40) supplemented with 10 μg of aprotinin/ml, 10 mM NaF, and 1 μg (each) of chymostatin, leupeptin, and pepstatin/ml. After being incubated for 10 min on ice, the lysates were cleared by centrifugation at 14,000 × g for 8 min. The cleared lysates (0.5-ml aliquots) were then analyzed by immunoprecipitation and immunoblotting as previously described (4). Anti-Lck antibodies were purchased from Zymed, anti-SLP-76 and anti-Fyn antibodies were purchased from Santa Cruz, anti-ZAP-70 antibodies were purchased from Transduction Laboratories and L. Samelson, and anti-phosphotyrosine (4G10) antibodies were purchased from B. Druker.
Immune complex kinase assays.
Anti-Lck immunoprecipitates from unstimulated or anti-CD3- and anti-CD3-CD4-stimulated thymocytes were washed three times in NP-40 lysis buffer and then washed once in kinase buffer (20 mM MOPS [morpholinepropanesulfonic acid] buffer [pH 7.0], 5 mM MgCl2, 5 mM MnCl2). Immunoprecipitates were then incubated with 25 μl of kinase buffer containing 12.5 μCi of [γ-32P]ATP (4,000 Ci/mmol; Bresatec) for 10 min at room temperature with occasional mixing. The kinase reaction was stopped by addition of 1 ml of ice-cold modified RIPA buffer (20 mM MOPS [pH 7.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS), the mixture was centrifuged briefly, and the supernatant containing unincorporated radioisotope was discarded. Immunoprecipitates were washed a further three times in RIPA buffer, by which time minimal radioactivity was detected in the discarded supernatant. Samples were resuspended in 30 μl of 1× Laemmli sample buffer, incubated at room temperature for 10 min, and then boiled for 3 min, and the supernatant was transferred to a fresh tube. The samples were separated by electrophoresis through a SDS–10% polyacrylamide gel, dried at 80°C under vacuum for 30 min, and analyzed by autoradiography after a 30-min exposure.
Thymocyte proliferation assay.
Thymocytes (4 × 105) from normal or c-Cbl-deficient mice were cultured in 200 μl of RPMI–10% FCS with plate-bound anti-TCRαβ (H57-597) antibodies for up to 3 days in round-bottom 96-well culture dishes (Nunc). Eight hours before being harvested, the cells were pulsed with 0.6 μCi of [3H]thymidine and the radioactivity of individual filters was measured by liquid scintillation counting. All cultures were in triplicate, and the results are presented as mean counts per minute ± standard error of the mean from thymuses of 10 wild-type and 10 c-Cbl−/− mice.
Assay of myeloid progenitors.
Bone marrow and spleen cell suspensions (2,500 and 10,000 cells per dish, respectively) were assayed for the presence of macrophage lineage progenitor cells in a double-layer nutrient agar culture system exactly as previously described (5). A predetermined optimal concentration of partially purified pregnant mouse uterus extract was used as a source of macrophage CSF (6). Colonies of at least 50 cells were scored after 14 days of incubation. The values are means ± standard errors of the mean for mice 7 to 9 weeks of age except where indicated. The statistical significance was determined by the Student t test, using the SigmaStat statistical package (version 2.0; Jandel Corp., St. Rafael, Calif.).
Histological preparations.
Standard histology was performed as described previously (59). Tartrate-resistant acid phosphatase stains for osteoclasts were performed with reagents from Sigma, according to the manufacturer’s protocols. Whole-mount stains of mammary fat pads were performed as described previously (58).
Flow cytometric analysis.
Immunophenotyping was performed with a FACStarPlus flow cytometer. List files of at least 10,000 events were collected for each cell population and were analyzed with Lysis II software. CD4 and CD8 cell subsets and B220-positive cells expressing IgM or IgD were analyzed by two-color fluorescence (fluorescein isothiocyanate or phycoerythrin) following fluorescence compensation. Monoclonal antibodies directed against murine CD3ε (145-2C11), CD4 (GK1.5), CD8 (53-6.7), TCRβ (H57), IgM (R6-60.2), and IgD were purchased from PharMingen. The monoclonal antibody directed against B220 (RA36B2) (9) was purified from rat hybridoma conditioned medium. Streptavidin-phycoerythrin was purchased from Caltag Laboratories.
RESULTS
Targeting of the c-Cbl locus.
To generate c-Cbl-deficient mice, we first sought to disrupt one allele of the gene in ES cells by homologous recombination of a targeting construct into the c-Cbl locus (Fig. 1A). Following electroporation of the targeting construct, multiple ES cell clones were obtained in which the c-Cbl gene was precisely disrupted. Four such clones were used to generate mice with a germ line mutation. Homozygous c-Cbl−/− mice, detected by Southern blot analysis (Fig. 1B), were produced in expected numbers from an intercross of heterozygotes (Table 1). c-Cbl is widely expressed in adult mouse tissues but is most abundant in the testes and thymus (28). Despite this, homozygous c-Cbl−/− male and female animals were fertile, and the litter sizes of c-Cbl−/− intercrosses were comparable to those of wild-type controls (Table 2). Homozygous mutant animals had normal growth rates and longevity (data not shown) and were externally indistinguishable from their wild-type littermates.
TABLE 1.
Numbers of offspring of c-Cbl−/− mice and control animalsa
| Genotype | Total no. | Ratio
|
|
|---|---|---|---|
| Expected | Actual | ||
| +/+ | 63 | 1 | 1.05 |
| +/− | 121 | 2 | 2.01 |
| −/− | 56 | 1 | 0.94 |
From heterozygous intercross.
TABLE 2.
Litter numbers and sizes of c-Cbl−/− mice and control animals
| Genotype of parents | Avg litter size | No. of litters |
|---|---|---|
| +/+ | 7 | 11 |
| +/− | 9 | 13 |
| −/− | 6 | 11 |
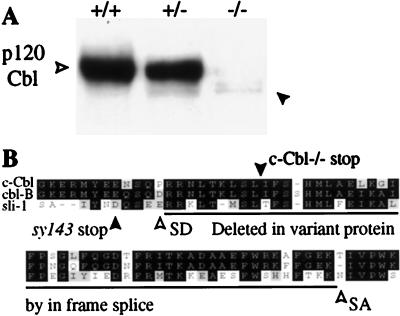
To further confirm that the gene had been functionally disrupted, Western blot analysis was performed on tissue lysates with an antiserum directed to the C terminus of c-Cbl which does not cross-react with the related Cbl-b protein. There was a complete absence of full-length protein in all tissues tested in homozygous mutant mice. Trace amounts of a smaller protein were apparent, however, in the thymus, where c-Cbl is especially abundant and easy to detect (Fig. 2A). A survey of other tissues, including those of the spleen, lymph node, breast, and brain, also showed very weak expression of the smaller protein in proportion to the normal levels of the protein in wild-type tissue (data not shown). The residual protein was seen only in c-Cbl+/− and c-Cbl−/− mice and therefore appeared to be a product of the mutant allele rather than a normal splice variant. Consistent with this notion, the transcript corresponding to this protein was cloned only from mutant mice when reverse transcription and PCR amplification were performed. DNA sequence analysis demonstrated that the smaller protein was encoded by an in-frame splicing event that deleted the targeted exon (Fig. 2B). The predicted protein lacked highly conserved residues in the region of the PTB domain of c-Cbl (Fig. 2B) (31, 53). Thus, the c-Cbl mice lack any wild-type c-Cbl protein and produce trace amounts of an abnormal protein that lacks highly conserved residues within the PTB domain, indicating that these mice are likely to be null for c-Cbl function.
FIG. 2.
Western blot analysis of c-Cbl mutant mice. (A) Protein levels of c-Cbl were greatly reduced in homozygote mutant thymocytes (open arrowhead), although a trace amount of a slightly smaller protein remained (solid arrowhead). Note that the smaller protein is only apparent in homozygous and heterozygous mutant mice. (B) Alignment showing the positions of splice donor (SD) and splice acceptor (SA) sites adjacent to the targeting site (c-Cbl−/− stop) and the sequences deleted in the smaller protein (panel A, solid arrowhead). The majority of the c-Cbl protein was truncated by insertion of a Neor cassette several residues downstream of a site corresponding to a null allele in Sli-1 (syl143 stop). Cloning of the mRNA for the residual mutant protein demonstrated that it was produced by an atypical, in-frame splicing event that was generated only from the mutant allele. Black boxes, identical amino acids; grey boxes, conservative amino acid differences; white boxes, nonconservative amino acid differences.
Hemopoietic and mammary hyperplasia.
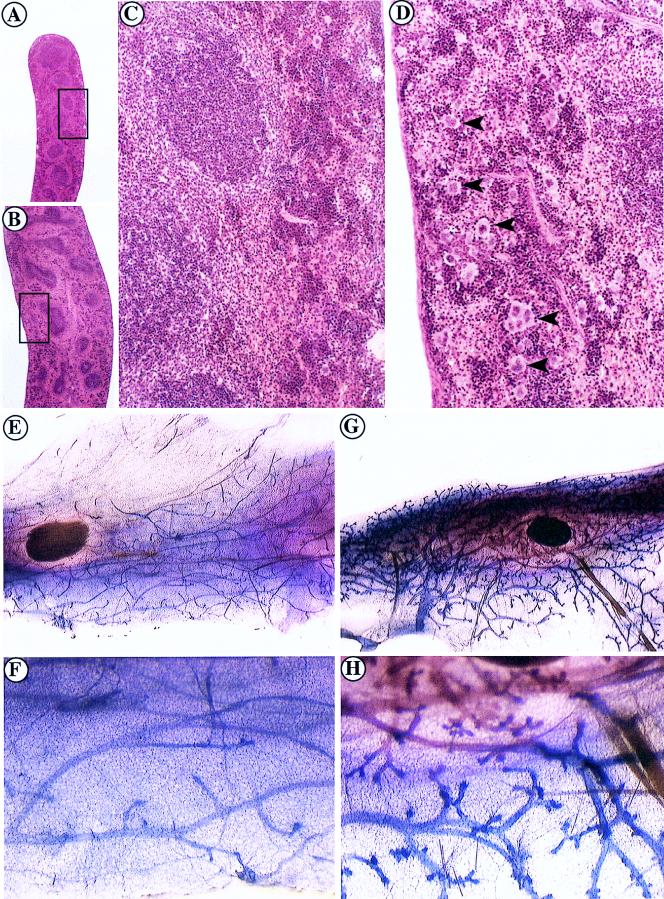
We performed a histological survey of neonatal and adult mice to identify tissue abnormalities in c-Cbl-deficient mice. A prominent feature of c-Cbl−/− mice was a severe splenic disorder characterized by splenomegaly (Table 3 and Fig. 3), fibrosis, and extensive extramedullary hemopoiesis (EMH), in which large numbers of megakaryocytes and normoblasts were apparent in the spleen parenchyma. Increased EMH in the spleens and livers of mutants was apparent from birth (data not shown). EMH can occur as a secondary response to failure of bone marrow hemopoiesis or due to anemia, for example, following hemorrhage. However, erythrocyte numbers and other blood parameters examined were comparable in mutant and wild-type animals, except for an increase in circulating platelets in c-Cbl−/− mice (Table 3). The thrombocytosis seen in c-Cbl−/− mice may reflect the large numbers of splenic megakaryocytes seen in these animals (Fig. 3D). Bone marrow cellularity and the incidence of bone marrow progenitor colony-forming cells (CFC) were not significantly different in wild-type and c-Cbl−/− mice (Table 3). There was an increase in the number of CFC in some c-Cbl−/− mice, but due to a large variability between animals, the changes noted were not significantly different from those of the controls. Lymph nodes were significantly enlarged in c-Cbl−/− mice and were comprised of increased numbers of both T and B cells (Table 3 and data not shown). Interestingly, mice aged between 5 and 5.5 weeks had significantly increased numbers of thymocytes, but by 7 to 9 weeks of age, thymic cellularity in c-Cbl−/− mice was comparable to that in their wild-type littermates (Table 3).
TABLE 3.
Hemopoietic parameters of wild-type and Cbl−/− mice
| Parameter | Value (± SD) for genotype:
|
P value | |
|---|---|---|---|
| +/+ | −/− | ||
| Blood | |||
| Leukocytes (106/ml) | 8.5 ± 1.2 | 10.9 ± 1.3 | NSb |
| Erythrocytes (109/ml) | 9.3 ± 0.5 | 9.3 ± 0.6 | NS |
| Platelets (109/ml) | 1,091 ± 39 | 1,755 ± 87 | <0.001 |
| Reticulocytes (109/ml) | 0.39 ± 0.03 | 0.40 ± 0.06 | NS |
| Packed cell vol (%) | 46.9 ± 1.2 | 46.3 ± 0.9 | NS |
| Spleen | |||
| Wt (mg) | 79.6 ± 5.8 | 136.6 ± 9.7 | <0.001 |
| CFC/104 cells | 30.5 ± 8.6 | 65.8 ± 17.8 | NSc |
| Bone marrow | |||
| Cells/femur (107) | 2.3 ± 0.3 | 2.0 ± 0.2 | NS |
| CFC/femur (106)a | 5.55 ± 1.54 | 6.02 ± 1.38 | NS |
| Thymus cellularity (107) | |||
| 5–5.5 wk old | 16.3 ± 2.0 | 24.8 ± 1.3 | <0.005 |
| 7–9 wk old | 16.1 ± 1.6 | 14.9 ± 1.5 | NS |
| Lymph node cellularity (106) | 16.3 ± 2.2 | 31.7 ± 3.7 | 0.004 |
CFC responsive to CSF-1 alone were assayed as an index of the committed progenitor cell compartment (5).
NS, not significant.
The incidence of spleen CFC appeared elevated in Cbl−/− mice but did not reach statistical significance (P = 0.1).
FIG. 3.
Primary splenomegaly, EMH, and mammary duct hyperplasia in c-Cbl−/− mice. (A to D) Hematoxylin and eosin staining of splenic sections from wild-type (A and C) and c-Cbl−/− (B and D) mice. (A and B) Low-power magnification demonstrating splenomegaly and disruption of the architecture of the spleen of a c-Cbl−/− mutant. (C and D) High-power magnification of the boxed regions in panels A and B showing EMH with large numbers of megakaryocytes (arrowheads) in the spleen of a c-Cbl−/− mutant (D) compared with that of its normal littermate (C). (E to H) Whole-mount stains of the abdominal mammary fat pads of virgin 13-week-old female mice. Excessive ductal branching was seen in c-Cbl−/− mice (G and H) compared to that in age-matched wild-type mice (E and F).
We observed that the mammary fat pads of virgin adult female mice were slightly thickened compared with those of controls. When stained as whole-mount preparations to reveal the ductal development, a striking increase in duct density and branching was observed in mature virgin c-Cbl−/− mice compared with that in controls (Fig. 3E to H). Examination of other branching and secretory tissues, including the glands associated with the male reproductive system and the salivary glands, did not reveal any morphological difference between wild-type and c-Cbl−/− mice (data not shown). c-Cbl expression was readily detectable in wild-type animals by Western blot analysis in all tissues affected by the c-Cbl mutation (data not shown).
The splenic, lymphoid, and mammary changes we observed appeared to uniformly affect each type of tissue and were not suggestive of focal clonal expansion(s). Whether these abnormalities represent low-grade neoplastic changes is unclear; however, we have not observed the development of progressive, malignant tumors in c-Cbl−/− mice maintained up to 12 months of age.
Bone development and remodelling.
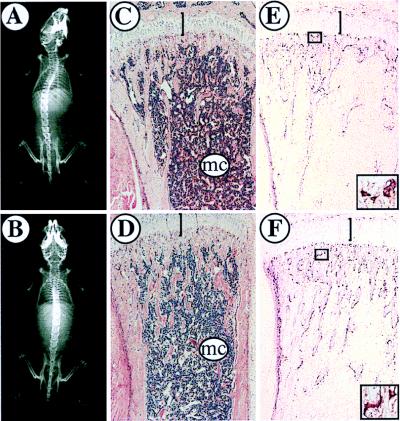
c-Cbl has been reported to be required for osteoclast activity in an in vitro bone resorption assay (51). To investigate whether there were any skeletal abnormalities in mutant animals, mice were examined by high-resolution X ray and histologically to reveal general morphology and osteoclasts. X-ray analysis showed distinct marrow cavities in the long bones of mutant animals and demonstrated that their general skeletal architecture was normal (Fig. 4A and B). This was confirmed by hematoxylin and eosin staining of decalcified femurs and tibias from juvenile and adult mice and serial sections through neonatal mice (Fig. 4C and D and data not shown). Finally, bone sections stained for tartrate-resistant acid phosphatase as a marker of osteoclasts revealed normal numbers and morphology of osteoclasts in mutant animals compared with those in their littermates (Fig. 4E and F). A more detailed in vitro analysis of osteoclast activity and a histomorphometric analysis of bones from mutant mice may be required to reveal a subtle requirement for c-Cbl in bone remodelling.
FIG. 4.
Bone morphology in c-Cbl−/− and wild-type control mice. (A and B) X-ray analysis of 7-week-old wild-type (A) and c-Cbl−/− (B) mice showing comparable skeletal morphology. (C and D) Hematoxylin and eosin staining of the femoral heads of a 6-week-old wild-type mouse (C) and its c-Cbl−/− littermate (B). The morphologies of the growth plate (bar) and marrow cavity (mc) were comparable. (E and F) Sections comparable to those in panels C and D stained for tartrate-resistant acid phosphatase to reveal osteoclasts. The numbers and morphologies (high-power insets of boxed regions) of osteoclasts were comparable in wild-type (E) and c-Cbl−/− (F) mice.
Abnormalities in T-cell profiles.
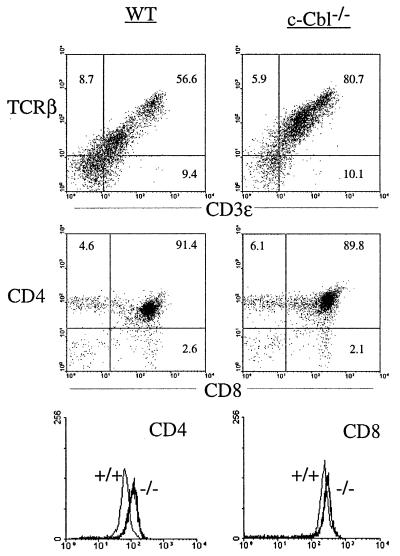
c-Cbl is highly expressed in the thymus and has been implicated in TCR and B-cell receptor signalling (11, 13, 22). To investigate its role in T- and B-cell development, and to further characterize the hemopoietic abnormalities in the c-Cbl−/− mouse, we performed flow cytometric analyses on 7- to 9-week-old mice. Although c-Cbl−/− mice had normal numbers of thymic cells (Table 3), there was a pronounced alteration in their phenotype. There was a large increase in the number of TCRβ- and CD3ε-expressing thymocytes in the c-Cbl−/− mice compared with those in their wild-type littermates (Fig. 5). The percentage of CD4-CD8 double-positive and CD4 and CD8 single-positive thymic cells was largely normal in c-Cbl−/− mice, except for a tendency for fewer CD8 single-positive cells. The slight reduction in CD8 single-positive cells was not statistically significant when data from a large number of animals was compared and may therefore reflect a chance occurrence or a phenotype that occurs at low penetrance in the c-Cbl−/− mice (data not shown). Although CD4/CD8 ratios were largely unaffected in c-Cbl−/− mice, there was a consistent increase in the intensity of CD4 and CD8 staining in the double-positive thymocytes, but not in single-positive cells (Fig. 5). Similar changes in the TCRβ-CDε and CD4-CD8 profiles were observed in the spleens of c-Cbl−/− mice, but they were less pronounced and a proportion of animals appeared largely normal (data not shown).
FIG. 5.
Altered T-cell parameters in c-Cbl−/− mice. Flow cytometry profiles from wild-type (WT) and c-Cbl−/− cells as indicated. The flow cytometry profiles were consistent in 12 or more mice of each genotype.
Whereas the thymic-T-cell changes seen in c-Cbl−/− mice were highly reproducible, more variable changes were observed in B cells. The mesenteric lymph nodes of c-Cbl−/− mice demonstrated absolute increases in both B and T cells (Table 3). The distribution of immature and mature B cells in the spleen, lymph nodes, and bone marrow, assessed by IgM, IgD, and B220 staining, was generally normal (data not shown). However, some c-Cbl−/− mice had a larger proportion of IgM-positive, IgD-negative cells in the bone marrow and spleen. Antibody stains for cells in the granulocyte and macrophage/monocyte lineages did not reveal any abnormalities in mutant hemopoietic tissues (data not shown). Depletion of c-Cbl therefore had its most apparent impact on cells in the lymphoid lineage, most notably in the T-cell compartment.
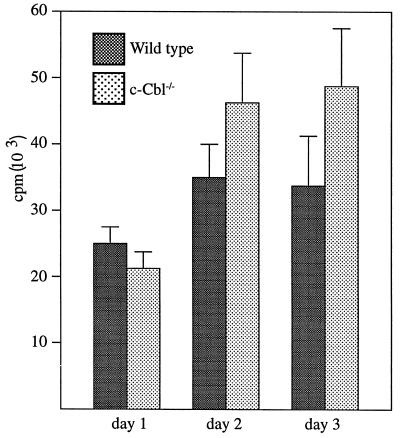
Given the changes observed by flow cytometric analysis, we investigated the functional integrity of the immune system by measuring antibody responses to antigenic challenge. Mice were immunized with the hapten nitrophenol coupled either to lipopolysaccharide or keyhole limpet hemocyanin, in order to measure T-cell-independent and T-cell-dependent antigens, respectively. The immunized mice were then assayed for their isotypic responses over a 3-week period. The magnitude and timing of the responses to these antigens were comparable in mutant and wild-type animals (data not shown). In vitro proliferation of thymocytes and peripheral T cells in response to TCR cross-linking was also investigated. While there was a trend towards a higher proliferative response in c-Cbl−/− thymocytes, this was not statistically significant (Fig. 6). Further evaluation of response to limiting amounts of cross-linking antibodies or to ovalbumin with T cells from antigen-primed animals failed to detect significant proliferative differences between wild-type and c-Cbl−/− cells (data not shown).
FIG. 6.
Analysis of thymocyte proliferation in response to anti-TCR stimulation. Thymocytes from wild-type and c-Cbl−/− mice were cultured with plate-bound anti-TCRαβ (H57-597) antibodies for the indicated times and pulsed with 0.6 mCi of [3H]thymidine 8 h before being harvested. All cultures were in triplicate, and the results represent mean radioactive values ± standard errors of the mean from cells harvested from the thymuses of 10 wild-type and 10 c-Cbl−/− mice. No response was observed in the absence of antibody stimulation.
CD3-mediated activation of ZAP-70 in c-Cbl-deficient thymocytes.
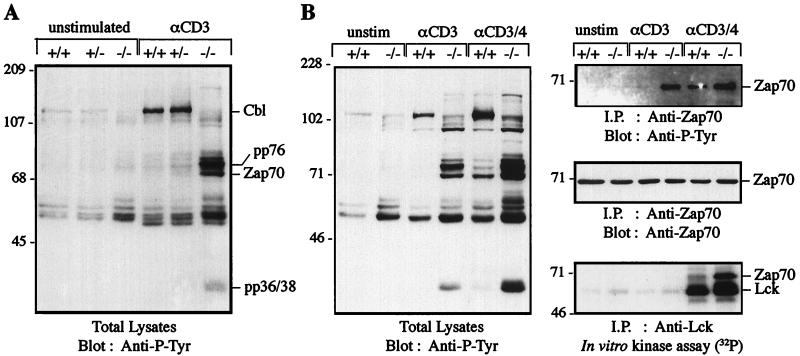
c-Cbl is a prominent target of the TCR-regulated Fyn kinase (55). Overexpression of c-Cbl reduces Fcε Receptor I-mediated phosphorylation of the Syk tyrosine kinase in mast cells (39) and decreases TCR-mediated, Ras-dependent activation of AP1 (43). To investigate the intracellular consequences of loss of c-Cbl, we examined signalling events in thymocytes before and after TCR activation by cross-linking with anti-CD3 antibodies. Immunoblotting of lysates with anti-phosphotyrosine antibodies revealed that unstimulated c-Cbl−/− thymocytes had higher basal levels of tyrosine-phosphorylated proteins in the 50- to 60-kDa molecular mass range compared with normal cells (Fig. 7A). Anti-CD3 cross-linking of thymocytes from normal mice resulted in a large induction of c-Cbl tyrosine phosphorylation, consistent with Fyn kinase activation, and an increase in the tyrosine phosphorylation of proteins in the 50- to 60-kDa molecular mass range. In contrast, stimulation of c-Cbl−/− thymocytes through the TCR-CD3 complex revealed the tyrosine phosphorylation of additional substrates not evident in normal cells. Most striking was the massive induction in tyrosine phosphorylation of three polypeptides with molecular masses of 70, 75, and 80 kDa and the hyperphosphorylation of a protein of a mobility equivalent to that of pp36-38 (48) (Fig. 7A).
FIG. 7.
(A) Anti-CD3 stimulation of thymocytes from c-Cbl-deficient mice induces the tyrosine phosphorylation of proteins that are not detectably phosphorylated in normal thymocytes. Thymocytes from c-Cbl+/+, c-Cbl+/−, and c-Cbl−/− mice were left unstimulated or stimulated with anti-CD3 (αCD3) antibodies for 5 min at 37°C before lysis and immunoblotting with anti-phosphotyrosine (Anti-P-Tyr) antibodies. The 76-kDa phosphoprotein from the stimulated c-Cbl−/− thymocytes has a mobility equivalent to that of SLP-76. (B) Anti-CD3-induced phosphorylation of ZAP-70 in c-Cbl-deficient thymocytes is not dependent on Lck activation. Thymocytes from c-Cbl+/+ or c-Cbl−/− mice were left unstimulated (unstim) or stimulated with anti-CD3 or anti-CD3-CD4 antibodies for 5 min at 37°C. Cell lysates were either analyzed by immunoblotting with anti-phosphotyrosine antibodies or immunoprecipitated (i.p.) with anti-Lck or anti-ZAP-70 antibodies. The anti-Lck immunoprecipitates were analyzed in an immune complex kinase assay for Lck kinase activity, and the anti-ZAP-70 immunoprecipitates were analyzed by anti-phosphotyrosine immunoblotting.
The electrophoretic mobility of these proteins raised the possibility that anti-CD3 stimulation of Cbl-deficient thymocytes had inappropriately phosphorylated and activated the ZAP-70 protein tyrosine kinase, which is known to phosphorylate the substrates SLP-76 and pp36-38 (44a, 62). ZAP-70 in normal thymocytes is activated via the CD4-coupled tyrosine kinase Lck (63). We compared the effects of anti-CD3 and anti-CD3-CD4 stimulation of thymocytes from normal and c-Cbl-deficient mice (Fig. 7B). Again anti-CD3 stimulation of normal thymocytes resulted in the phosphorylation of a limited number of substrates, with c-Cbl being most prominent, whereas in c-Cbl-deficient thymocytes tyrosine phosphorylation of the 70- to 80-kDa substrates predominated. Immunoprecipitation of ZAP-70 and immunoblotting with anti-phosphotyrosine antibodies confirmed the lack of activated ZAP-70 in anti-CD3-stimulated normal thymocytes, whereas in c-Cbl-deficient thymocytes ZAP-70 is inappropriately activated (Fig. 7B). Following costimulation of normal thymocytes with anti-CD3-CD4, there was an induction of a hyperphosphorylated 70-kDa protein and, to a lesser extent, proteins of approximately 75 and 80 kDa. Anti-ZAP-70 immunoprecipitation of costimulated normal thymocytes confirmed the identity of this protein as tyrosine-phosphorylated ZAP-70. The immunoprecipitation also showed that ZAP-70 tyrosine phosphorylation was slightly enhanced in the c-Cbl-deficient thymocytes, but there was a disproportionately large increase in the phosphorylation of the 75-, 80, and 36- to 38-kDa proteins compared to that in normal thymocytes (Fig. 7B).
These findings suggested that c-Cbl was functioning as a negative regulator of ZAP-70. To test whether this regulation is a direct effect or is mediated indirectly by c-Cbl’s effect on Lck, we analyzed Lck in an immune complex kinase assay. Lck is an upstream activator of ZAP-70 that phosphorylates tyrosine 493 in the kinase domain of ZAP-70, resulting in catalytic activation of ZAP-70 (8, 61). The results of the immune complex kinase assay shown in Fig. 7B demonstrated a lack of activated Lck in both normal and c-Cbl-deficient thymocytes stimulated by anti-CD3 antibodies, consistent with the requirement for CD4 cross-linking for Lck activation (63). Thus, it appears that the inappropriate activation of ZAP-70 via CD3 in the c-Cbl-deficient thymocytes was occurring without the overt activation of Lck. In contrast costimulation by anti-CD3-CD4 antibodies results in a large induction of Lck kinase activity in both normal and c-Cbl-deficient thymocytes. Lck induction and ZAP-70 phosphorylation were slightly higher in the c-Cbl-deficient thymocytes, but there was a disproportionately increased association of Lck and ZAP-70. Furthermore, there is a very large increase in the level of phosphorylation of the 75- to 80-kDa proteins in c-Cbl−/− cells relative to that in wild-type control cells, which was disproportionate to the level of ZAP-70 phosphorylation. Taken together these findings suggest that the depletion of c-Cbl greatly enhances ZAP-70 activity via a mechanism that independent of its immediate upstream activator.
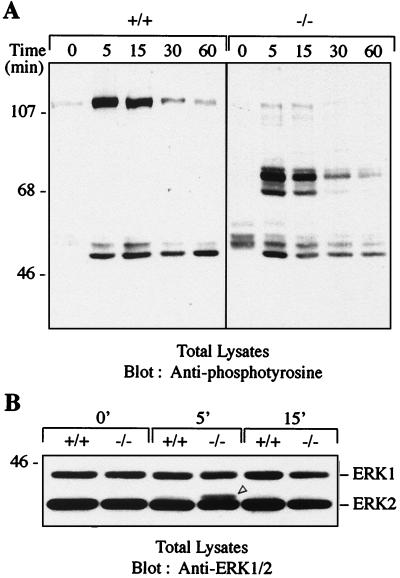
To test the possibility that this enhanced phosphorylation could be a consequence of decreased tyrosine phosphatase activity rather than a deregulation of ZAP-70 activity, we compared a time course of tyrosine phosphorylation of proteins in anti-CD3ε-stimulated thymocytes. Dephosphorylation of prominent substrates occurred at a comparable rate in both wild-type and c-Cbl−/− thymocytes (Fig. 8A), indicating that increased kinase activity is responsible for the increased level of phosphoproteins seen in c-Cbl−/− thymocytes. Furthermore, there appears to be some degree of specificity, as the enhancement in tyrosine phosphorylation due to c-Cbl depletion appears to be restricted to about four substrates, which is in contrast to the array of hyperphosphorylated proteins in pervanadate-treated thymocytes (data not shown).
FIG. 8.
(A) Time course analysis comparing the kinetics of tyrosine phosphorylation and dephosphorylation of protein substrates in c-Cbl+/+ and c-Cbl−/− thymocytes following anti-CD3 stimulation. Thymocytes were stimulated for the times indicated, lysed, and immunoblotted with anti-phosphotyrosine antibodies. (B) Enhanced activation of MAP kinase Erk2 in CD3-stimulated thymocytes from c-Cbl−/− mice. c-Cbl+/+ and c-Cbl−/− thymocytes were left unstimulated (0′) or stimulated with anti-CD3 antibodies for 5 (5′) or 15 (15′) min. MAP kinase activation was assessed by SDS-polyacrylamide gel electrophoresis and immunoblotting of lysates with an antibody that recognizes both p42 (Erk2) and p44 (Erk1) to detect electrophoretic mobility shifts. The mobility shift representing an increase in Erk2 phosphorylation in c-Cbl−/− thymocytes is indicated by an open arrowhead.
Consistent with the increased phosphorylation seen in c-Cbl−/− thymocytes we also found an increased level of activated Erk2 following anti-CD3 stimulation (Fig. 8B). This finding is consistent with the suppression of Erk2 activity by c-Cbl overexpression in Jurkat T cells (43) and the ability of 70Z-Cbl to induce NFAT activation via the Ras pathway (29). Collectively these findings indicate that c-Cbl functions to directly regulate the activity of ZAP-70 in stimulated thymocytes and that the absence of c-Cbl results in enhanced phosphorylation of downstream targets of ZAP-70. Furthermore, these findings support the model originally proposed for Cbl regulating the events leading to Ras activation in C. elegans (64).
DISCUSSION
Current understanding of Cbl protein function comes from the convergence of two streams of work: biochemical studies of mammalian cells, which identify c-Cbl as a major phosphoprotein that forms diverse protein complexes, and more limited genetic studies that implicate Cbl proteins as negative regulators of signalling (39, 43, 64). The work described here unifies these experimental studies by describing the effects of germ line loss of c-Cbl in mice and provides a coherent model for c-Cbl function.
Mutation of the c-Cbl locus.
While our disruption of the c-Cbl gene resulted in a complete absence of wild-type protein, the mice unexpectedly produced a very low level of a variant protein at less than 5% of wild-type levels. This protein was the result of an in-frame transcriptional splice around the targeted exon and was specific to the mutant allele. Whether the residual protein retains any biological activity is unknown. The atypically spliced exon removes 37 amino acids from a region of c-Cbl that is very highly conserved among the known Cbl proteins (19). A nearby mutation, at residue 316 in C. elegans Sli-1, results in a severe loss-of-function phenotype (64). Both the C. elegans mutation and the c-Cbl−/− mutation described here occur within a large block of homology that is within a region of c-Cbl that encodes a novel PTB domain, the boundaries of which have not yet been mapped precisely (53). This domain is functionally distinct from a conserved, more C-terminal peptide sequence that is deleted in the oncogenic 70Z-Cbl protein (1). Importantly, we have seen no evidence that the variant protein produced in c-Cbl−/− mice results in a gain-of-function phenotype. Indeed deletions that impinge on the region affected in the c-Cbl mutant mice destabilize the full-length protein and render the v-Cbl protein inactive (reference 53 and unpublished results). Residual protein expression cannot be excluded whenever a targeting event preserves any part of the coding region of the gene of interest, as detection of residual expression is limited by the sensitivity of the antisera used. The large size of the c-Cbl gene (3a) will hamper complete deletion of the locus.
Tissue hyperplasia in c-Cbl mutant mice.
Loss of c-Cbl was characterized by hyperplastic changes in lymphoid and mammary tissues. The abundant EMH observed in the spleen appeared to be a direct consequence of the loss of c-Cbl, as there was no evidence of stimuli, such as anemia or bone marrow hypoplasia, that could have triggered this response. In the absence of such an underlying cytopenia in the bone marrow, and the presence of thrombocythemia with normal numbers of circulating erythrocytes and granulocytes, the EMH should be regarded as a pathological rather than a physiological response (47). The accumulation of the large number of megakaryocytes in the spleens of c-Cbl mutant mice may arise from dysregulated paracrine or autocrine signalling loops. Interestingly, c-Cbl is a prominent phosphoprotein in thrombopoietin-stimulated cells (7, 35, 45) and sustained activation of the ERK-mitogen-activated protein (MAP) kinase pathway promotes autocrine secretion of megakaryocyte differentiation factors from myeloid cells in vitro (42).
Enhanced growth factor responsiveness may also account for the altered mammary morphology seen in c-Cbl mutant mice. Members of the EGF ligand and receptor families are interesting candidates for perturbed signalling in this tissue, given their prominent role in mammary branching and development (10, 12, 15, 49, 57), Sli-1’s proposed role in attenuating EGF receptor signalling in C. elegans (64), and c-Cbl’s interaction with the EGF receptor in murine cell lines (4, 17).
Although chromosomal aberrations occur in the region of the c-Cbl locus in a small number of leukemias, it is unclear whether loss of c-Cbl is directly involved in transformation (46). While we did see hyperplastic changes involving lymphoid and myeloid lineage cells that may represent premalignant lesions, we did not see the development of frank tumors in the c-Cbl mutant mice over a period of 8 to 12 months. Loss of c-Cbl does not appear to have phenocopied the effects of overexpression of v-Cbl in vivo (27). If indeed v-Cbl acts as a dominant negative for c-Cbl (53), our findings indicate that v-Cbl probably also blocks the activity of other Cbl proteins in exerting its transforming effects.
T-cell and bone development in c-Cbl-deficient mice.
Thymocytes represent one of the most abundant sites of c-Cbl expression, and c-Cbl has been strongly implicated in TCR signalling. It was therefore of considerable interest to investigate the consequences of c-Cbl depletion for T-cell development. Development of a functional T-cell repertoire occurred normally in c-Cbl−/− mice as evidenced by a correct ratio of CD4 and CD8 single- and double-positive cells and normal in vivo responses to antigen. That T-cell development was not severely affected in c-Cbl−/− mice is surprising for several reasons: the surface expression of CD4, CD8, and TCR was increased in c-Cbl−/− thymocytes; there was hyperphosphorylation of intracellular proteins in response to receptor cross-linking; and there was a marked qualitative difference between wild-type and c-Cbl−/− thymocytes in that the latter responded acutely to a TCR-mediated signal in the absence of the normal requirement for coreceptor aggregation (63). Perhaps c-Cbl occupies an unusual niche as a negative regulator that finely tunes T-cell development rather than being one of the critical positive components of T-cell signalling for which a severe loss-of-function phenotype is normally seen (reviewed in reference 41). Alternatively, redundancy with Cbl-b may allow cells to accommodate the loss of c-Cbl, as is seen with Lck and Fyn in mice that are mutant for these genes (18).
Increased expression of the TCR and its coreceptors in c-Cbl mutant mice is of interest given the proposed role of c-Cbl in mediating degradation of the CSF-1 receptor (60). The increased expression of CD4 and CD8 in the double-positive thymocytes closely resembles that seen in mice that are deficient in a specific splice variant of CD45 (23). The altered CD4-CD8 expression seen in CD45 mutant mice may be a consequence of the profound block in thymocyte development that occurs at the transition from immature CD4-CD8 double-positive cells to mature, single-positive cells. However, it is notable that c-Cbl−/− mice do not display a similar block in their development. Whether the cell surface changes observed in the c-Cbl−/− mice reflects a direct effect of loss of c-Cbl, such as increased stability of these proteins, or an altered T-cell developmental sequence requires further investigation.
c-Cbl phosphorylation is reduced in osteoclast-like cells from c-Src mutant mice, indicating that c-Cbl may be involved in signalling by Src (51), which in turn is required for osteoclast activity and bone remodelling (50). Consistent with this notion, in vitro bone resorption by osteoclasts was inhibited with antisense oligonucleotides to c-Cbl. Despite these findings we did not observe any bone defects in c-Cbl mutant mice. Although Cbl-b expression was not assessed in the antisense experiments, the oligonucleotides used were directed to sequences that were nonhomologous between the Cbl-b and c-Cbl genes. In addition, c-Cbl expression was more profoundly reduced in this study than in the experiments by Tanaka et al. (51). Thus, a difference in the degree or scope of attenuation of Cbl protein expression does not readily explain the discrepancy between the two studies. Rather this difference more likely reflects the assays used, one in vitro and the other measuring steady-state bone morphology in vivo. Detailed bone morphometry and in vitro analysis of c-Cbl−/− osteoclast activity is currently in progress to further characterize the role of c-Cbl in these cells.
c-Cbl as a negative regulator of ZAP-70.
Our analysis of signalling via the TCR-CD3 complex and the CD4 receptor on thymocytes has provided compelling evidence that c-Cbl acts as a negative regulator of the ZAP-70 protein tyrosine kinase. The initial analysis of CD3-mediated signalling revealed a marked induction in ZAP-70 tyrosine phosphorylation (Fig. 7). Three other proteins in c-Cbl-deficient thymocytes also revealed a massive induction in tyrosine phosphorylation following CD3 cross-linking. Based on their relative mobilities, two of these proteins may represent the ZAP-70 substrates SLP-76 and pp36-38 (or LAT) (44a, 62). Importantly, costimulation of normal thymocytes with anti-CD3-CD4 antibodies induced a level of phosphorylation of ZAP-70 that was only slightly lower than that seen in c-Cbl-deficient thymocytes (Fig. 7B). Despite this, phosphoproteins other than ZAP-70 showed a massive increase in c-Cbl-deficient thymocytes costimulated with anti-CD3-CD4. Thus, the activating signal appears to be enhanced by c-Cbl depletion beyond that accounted for by an activation of ZAP-70 alone.
A striking aspect of the response of c-Cbl−/− thymocytes to receptor cross-linking was that loss of c-Cbl abrogated the normal requirement for CD4-TCR costimulation for ZAP-70 activation in thymocytes (63). TCR signalling in these cells was not completely deregulated, however, as a signal through CD3 was still necessary for ZAP-70 activation. In normal CD4+ CD8+ thymocytes ZAP-70 forms an inactive complex with the TCRζ chain and cannot be activated by CD3 cross-linking alone because CD4-Ia interactions sequester available Lck (63). We considered it possible that loss of c-Cbl increased the availability of Lck or that Lck was being activated by CD3 alone in c-Cbl−/− thymocytes. We were also concerned that a trivial explanation for these findings was that they simply reflected the increased number of TCR-expressing thymocytes in c-Cbl−/− mice. This does not appear to be the case, however, as Lck immune complex kinase assays revealed no obvious difference in Lck activity between normal and c-Cbl-deficient thymocytes following CD3 stimulation (Fig. 7B). We would expect that if the altered signalling observed in c-Cbl−/− thymocytes reflected an expanded TCR-expressing pool, then Lck activation would parallel the other intracellular changes we observed. The profound CD3-mediated intracellular phosphorylation that occurred in c-Cbl−/− thymocytes in the absence Lck activation indicated that signalling through ZAP-70 had become uncoupled from Lck activation in these cells. This implied that a kinase other than Lck may be responsible for inappropriate activation of ZAP-70 in CD3-cross-linked c-Cbl−/− thymocytes. Several reports have described an interaction between c-Cbl and the CD3ε chain-associated Src family kinase, Fyn (14, 16, 55). Although it is possible that the loss of c-Cbl increases the availability of Fyn to phosphorylate ZAP-70 following CD3ε cross-linking, we have found no evidence of this (data not shown). Alternatively there may be a direct loss of ZAP-70 regulation so that a normally weak signal via CD3 is sufficient to trigger its activation. Consistent with the notion that loss of c-Cbl may impact directly on ZAP-70 regulation is the recent finding that the c-Cbl PTB domain binds selectively to Tyr-292 of ZAP-70 (32). Phosphorylated Tyr-292 is thought to negatively regulate ZAP-70 through the binding of regulatory molecules (24). We aim to further test this hypothesis by generating thymocyte cell lines from c-Cbl−/− mice into which wild-type and mutant alleles of the gene can be introduced.
Experiments first performed in C. elegans, where the weak signal from a reduction-of-function Let-23 receptor mutant is rescued by the depletion of Sli-1, provided the first indication that Cbl proteins may negatively regulate tyrosine kinases (64). Our findings, together with studies revealing the suppressive effect of c-Cbl overexpression on Syk kinase activity, provides compelling evidence for c-Cbl to be classified as a negative regulator of the Syk/ZAP-70 family of tyrosine kinases. Peptides closely matching the Tyr-292 negative regulatory sequence are represented in several other proteins to which c-Cbl binds, including the EGF receptor, Syk, and platelet-derived growth factor alpha (32), suggesting a potential general mechanism for c-Cbl regulation of protein tyrosine kinases. While our observations support this general mechanism for c-Cbl action, the moderate phenotype of the c-Cbl−/− mice suggests that substantial functional redundancy may exist with other Cbl family members. Further elucidation of the mechanism by which this unique family of proteins regulates intracellular signalling is likely to be of considerable importance, given the central role that tyrosine kinases play in controlling cell survival, growth, and differentiation.
ACKNOWLEDGMENTS
This work was supported by grants from Howard Hughes Medical Institute and Wellcome Trust and NH&MRC, Australia. D.D.L.B. is a Howard Hughes International Research Scholar.
We are indebted to Frank Kontgen for assistance in gene targeting and to Fiona Christensen for excellent assistance with animals and microinjection. We very much appreciate extensive histological processing performed by Lynn Trute, additional processing by Keith Cole, and assistance with fluorescence-activated cell sorter analyses by Ralph Rossi, Gillian Bradford, Brenda Williams, and Leslie Barber. We thank David Tarlington, Andreas Strasser, Ken Shortman, Wu Li, Kirsten Puls, Brian Drucker, Larry Samelson, and Sandy Morse for reagents and valuable advice and members of the Peter Mac for comments on the manuscript.
REFERENCES
- 1.Andoniou C E, Thien C, Langdon W Y. Tumour induction by activated Abl involves tyrosine phosphorylation of the product of the Cbl oncogene. EMBO J. 1994;13:4515–4523. doi: 10.1002/j.1460-2075.1994.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake T J, Shapiro M, Morse H D, Langdon W Y. The sequences of the human and mouse c-Cbl proto-oncogenes show v-Cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene. 1991;6:653–657. [PubMed] [Google Scholar]
- 3.Bonita D P, Miyake S, Lupher M R, Langdon W Y, Band H. Phosphotyrosine binding domain-dependent upregulation of PDGF receptor signaling cascade by transforming mutants of Cbl: implications for Cbl’s function and oncogenicity. Mol Cell Biol. 1997;17:4597–4610. doi: 10.1128/mcb.17.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Bowtell, D. Unpublished data.
- 4.Bowtell D, Langdon W Y. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene. 1995;11:1561–1567. [PubMed] [Google Scholar]
- 5.Bradley T R, Hodgson G S, Rosendaal M. The effect of oxygen tension on haemopoietic and fibroblast cell proliferation in vitro. J Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 6.Bradley T R, Stanley E R, Sumner M A. Factors from mouse tissues stimulating colony growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1971;49:595–603. doi: 10.1038/icb.1971.65. [DOI] [PubMed] [Google Scholar]
- 7.Brizzi M F, Dentelli P, Lanfrancone L, Rosso A, Pelicci P G, Pegoraro L. Discrete protein interactions with the Grb2/c-Cbl complex in Scf- and Tpo-mediated myeloid cell proliferation. Oncogene. 1996;13:2067–2076. [PubMed] [Google Scholar]
- 8.Chan A C, Dalton M, Johnson R, Kong G H, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman R L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 10.Coleman S, Silberstein G B, Daniel C W. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- 11.Cory G, Lovering R C, Hinshelwood S, MacCarthy-Morrogh L, Levinsky R J, Kinnon C. The protein product of the c-Cbl protooncogene is phosphorylated after B cell receptor stimulation and binds the SH3 domain of brutons tyrosine kinase. J Exp Med. 1995;182:611–615. doi: 10.1084/jem.182.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis S W, Washburn T, Sewall C, Diaugustine R, Lindzey J, Couse J F, Korach K S. Physiological coupling of growth factor and steroid receptor signaling pathways—estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93:12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donovan J A, Wange R L, Langdon W Y, Samelson L E. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 14.Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase zap-70 with the protooncogene product p120(c-cbl) in T lymphocytes. J Exp Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler K J, et al. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukazawa T, Reedquist K A, Trub T, Soltoff S, Panchamoorthy G, Druker B, Cantley L, Shoelson S E, Band H. The SH3 domain-binding T cell tyrosyl phosphoprotein p120—demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2, and phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:19141–19150. doi: 10.1074/jbc.270.32.19141. [DOI] [PubMed] [Google Scholar]
- 17.Galisteo M L, Dikic I, Batzer A G, Langdon W Y, Schlessinger J. Tyrosine phosphorylation of the c-Cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem. 1995;270:20242–20245. doi: 10.1074/jbc.270.35.20242. [DOI] [PubMed] [Google Scholar]
- 18.Groves T, Smiley P, Cooke M P, Forbush K, Perlmutter R M, Guidos C J. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 19.Hime G R, Dhungat M P, Ng A, Bowtell D. D-cbl, the Drosophila homologue of the c-cbl proto-oncogene, interacts with the Drosophila EGF receptor in vivo, despite lacking c-terminal adaptor binding sites. Oncogene. 1997;14:2709–2719. doi: 10.1038/sj.onc.1201223. [DOI] [PubMed] [Google Scholar]
- 20.Jongeward G D, Clandinin T R, Sternberg P W. Sli-1, a negative regulator of let-23-mediated signaling in C. elegans. Genetics. 1995;139:1553–1566. doi: 10.1093/genetics/139.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keane M M, Riverolezcano O M, Mitchell J A, Robbins K C, Lipkowitz S. Cloning and characterization of Cbl-b—a SH3 binding protein with homology to the c-Cbl proto-oncogene. Oncogene. 1995;10:2367–2377. [PubMed] [Google Scholar]
- 22.Kim T J, Kim Y T, Pillai S. Association of activated phosphatidylinositol 3-kinase with p120(cbl) in antigen receptor-ligated B cells. J Biol Chem. 1995;270:27504–27509. doi: 10.1074/jbc.270.46.27504. [DOI] [PubMed] [Google Scholar]
- 23.Kishihara K, et al. Normal B-lymphocyte development but impaired T-cell maturation in cd45-exon6 protein tyrosine phosphatase deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 24.Kong G H, Dalton M, Wardenburg J B, Straus D, Kurosaki T, Chan A C. Distinct tyrosine phosphorylation sites in Zap-70 mediate activation and negative regulation of antigen receptor function. Mol Cell Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kontani K, Kukimoto I, Nishina H, Hoshino S, Hazeki O, Kanaho Y, Katada T. Tyrosine phosphorylation of the c-cbl proto-oncogene product mediated by cell surface antigen cd38 in hl-60 cells. J Biol Chem. 1996;271:1534–1537. doi: 10.1074/jbc.271.3.1534. [DOI] [PubMed] [Google Scholar]
- 26.Kontgen F, Grumont R J, Strasser A, Metcalf D, Li R L, Tarlinton D, Gerondakis S. Mice lacking the c-Rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 27.Langdon W Y, Hartley J W, Klinken S P, Ruscetti S K, Morse H C. v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci USA. 1989;86:1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langdon W Y, Hyland C D, Grumont R J, Morse H D. The c-cbl proto-oncogene is preferentially expressed in thymus and testis tissue and encodes a nuclear protein. J Virol. 1989;63:5420–5424. doi: 10.1128/jvi.63.12.5420-5424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y C, Elly C, Langdon W Y, Altman A. Ras-dependent Ca2+-stimulated activation of nuclear factor of activated T cells by a constitutively active Cbl mutant in T cells. J Biol Chem. 1997;272:168–173. doi: 10.1074/jbc.272.1.168. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y C, Liu Y H, Elly C, Yoshida H, Lipkowitz S, Altman A. Serine phosphorylation of cbl induced by phorbol ester enhances its association with 14-3-3 proteins in T cells via a novel serine-rich 14-3-3-binding motif. J Biol Chem. 1997;272:9979–9985. doi: 10.1074/jbc.272.15.9979. [DOI] [PubMed] [Google Scholar]
- 31.Lupher M L, Reedquist K A, Miyake S, Langdon W Y, Band H. A novel phosphotyrosine-binding domain in the N-terminal transforming region of cbl interacts directly and selectively with Zap-70 in T cells. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- 32.Lupher M L, Songyan Z, Shoeson S E, Cantley L C, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the TyrP292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- 33.Marcilla A, Riverolezcano O M, Agarwal A, Robbins K C. Identification of the major tyrosine kinase substrate in signaling complexes formed after engagement of Fc gamma receptors. J Biol Chem. 1995;270:9115–9120. doi: 10.1074/jbc.270.16.9115. [DOI] [PubMed] [Google Scholar]
- 34.Meisner H, Daga A, Buxton J, Fernández B, Chawla A, Banerjee U, Czech M P. Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol Cell Biol. 1997;17:2217–2225. doi: 10.1128/mcb.17.4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oda A, Ozaki K, Druker B J, Miyakawa Y, Miyazaki H, Handa M, Morita H, Ohashi H, Ikeda Y. P120(c-Cbl) is present in human blood platelets and is differentially involved in signaling by thrombopoietin and thrombin. Blood. 1996;88:1330–1338. [PubMed] [Google Scholar]
- 36.Odai H, Sasaki K, Hanazono Y, Ueno H, Tanaka T, Miyagawa K, Mitani K, Yazaki Y, Hirai H. c-Cbl is inducibly tyrosine-phosphorylated by epidermal growth factor stimulation in fibroblasts, and constitutively tyrosine-phosphorylated and associated with v-Src in v-Src-transformed fibroblasts. Jpn J Cancer Res. 1995;86:1119–1126. doi: 10.1111/j.1349-7006.1995.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ojaniemi M, Martin S S, Dolfi F, Olefsky J M, Vuori K. The proto-oncogene product p120(Cbl) links c-Src and phosphatidylinositol 3′-kinase to the integrin signaling pathway. J Biol Chem. 1997;272:3780–3787. doi: 10.1074/jbc.272.6.3780. [DOI] [PubMed] [Google Scholar]
- 38.Ota Y, Beitz L O, Scharenberg A M, Donovan J A, Kinet J P, Samelson L E. Characterization of cbl tyrosine phosphorylation and a Cbl-Syk complex in rbl-2h3 cells. J Exp Med. 1996;184:1713–1723. doi: 10.1084/jem.184.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ota Y, Samelson L E. The product of the proto-oncogene c-Cbl—a negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- 40.Panchamoorthy G, Fukazawa T, Miyake S, Soltoff S, Reedquist K, Druker B, Shoelson S, Cantley L, Band H. P120(Cbl) is a major substrate of tyrosine phosphorylation upon B cell antigen receptor stimulation and interacts in vivo with Fyn and Syk tyrosine kinases, Grb2 and Shc adaptors, and the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:3187–3194. doi: 10.1074/jbc.271.6.3187. [DOI] [PubMed] [Google Scholar]
- 41.Qian D, Weiss A. T cell antigen receptor signal transduction. Curr Opin Cell Biol. 1997;9:205–212. doi: 10.1016/s0955-0674(97)80064-6. [DOI] [PubMed] [Google Scholar]
- 42.Racke F K, Lewandowska K, Goueli S, Goldfarb A N. Sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway is required for megakaryocyte differentiation in K562 cells. J Biol Chem. 1997;272:23366–23370. doi: 10.1074/jbc.272.37.23366. [DOI] [PubMed] [Google Scholar]
- 43.Rellahan B L, Graham L J, Stocia B, DeBell K E, Bonvini E. Cbl-mediated regulation of T cell receptor-induced AP1 activation. J Biol Chem. 1997;272:30806–30811. doi: 10.1074/jbc.272.49.30806. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44a.Samelson, L. Personal communication.
- 45.Sasaki K, et al. Tpo/c-Mpl ligand induces tyrosine phosphorylation of multiple cellular proteins including proto-oncogene products, vav and c-Cbl, and Ras signaling molecules. Biochem Biophys Res Commun. 1995;216:338–347. doi: 10.1006/bbrc.1995.2629. [DOI] [PubMed] [Google Scholar]
- 46.Savage P D, Shapiro M, Langdon W Y, Geurts van Kessel A D, Seuanez H N, Akao Y, Croce C, Morse H D, Kersey J H. Relationship of the human protooncogene CBL2 on 11q23 to the t(4;11), t(11;22), and t(11;14) breakpoints. Cytogenet Cell Genet. 1991;56:112–115. doi: 10.1159/000133062. [DOI] [PubMed] [Google Scholar]
- 47.Schafer A I. Essential (primary) thrombocythemia. In: Beutler E, Lichtman M A, Coller B S, Kipps T J, editors. Williams hematology. New York, N.Y: McGraw-Hill; 1995. pp. 340–345. [Google Scholar]
- 48.Sieh M, Batzer A, Schlessinger J, Weiss A. GRB2 and phospholipase C-γ1 associate with a 36- to 38-kilodalton phosphotyrosine protein after T-cell receptor stimulation. Mol Cell Biol. 1994;14:4435–4442. doi: 10.1128/mcb.14.7.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snedeker S M, Brown C F, DiAugustine R P. Expression and functional properties of transforming growth factor alpha and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc Natl Acad Sci USA. 1991;88:276–280. doi: 10.1073/pnas.88.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-Src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy J B, Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka S, Neff L, Baron R, Levy J B. Tyrosine phosphorylation and translocation of the c-Cbl protein after activation of tyrosine kinase signaling pathways. J Biol Chem. 1995;270:14347–14351. doi: 10.1074/jbc.270.24.14347. [DOI] [PubMed] [Google Scholar]
- 53.Thien C, Langdon W Y. EGF receptor binding and transformation by v-cbl is ablated by the introduction of a loss-of-function mutation from the Caenorhabditis elegans Sli-1 gene. Oncogene. 1997;14:2239–2249. doi: 10.1038/sj.onc.1201193. [DOI] [PubMed] [Google Scholar]
- 54.Thien C B F, Langdon W Y. Tyrosine kinase activity of the EGF receptor is enhanced by the expression of oncogenic 70Z-Cbl. Oncogene. 1997;15:2909–2920. doi: 10.1038/sj.onc.1201468. [DOI] [PubMed] [Google Scholar]
- 55.Tsygankov A Y, Mahajan S, Fincke J E, Bolen J B. Specific association of tyrosine-phosphorylated c-Cbl with Fyn tyrosine kinase in T cells. J Biol Chem. 1996;271:27130–27137. doi: 10.1074/jbc.271.43.27130. [DOI] [PubMed] [Google Scholar]
- 56.Ueno H, Sasaki K, Miyagawa K, Honda H, Mitani K, Yazaki Y, Hirai H. Antisense repression of proto-oncogene c-Cbl enhances activation of the Jak-Stat pathway but not the Ras pathway in epidermal growth factor receptor signaling. J Biol Chem. 1997;272:8739–8743. doi: 10.1074/jbc.272.13.8739. [DOI] [PubMed] [Google Scholar]
- 57.Vonderhaar B K. Local effects of EGF, alpha-TGF, and EGF-like growth factors on lobuloalveolar development of the mouse mammary gland in vivo. J Cell Physiol. 1987;132:581–584. doi: 10.1002/jcp.1041320324. [DOI] [PubMed] [Google Scholar]
- 58.Vonderhaar B K, Greco A E. Lobulo-alveolar development of mouse mammary glands is regulated by thyroid hormones. Endocrinology. 1979;104:409–418. doi: 10.1210/endo-104-2-409. [DOI] [PubMed] [Google Scholar]
- 59.Wang D Z-M, Hammond V E, Abud H E, Bertoncello I, McAvoy J W, Bowtell D D L. Mutation in Sos1 dominantly enhances a weak allele of the EGFR, demonstrating a requirement for Sos1 in EGFR signaling and development. Genes Dev. 1997;11:309–320. doi: 10.1101/gad.11.3.309. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Yeung Y G, Langdon W Y, Stanley E R. c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following Csf-1 stimulation of macrophages. J Biol Chem. 1996;271:17–20. doi: 10.1074/jbc.271.1.17. [DOI] [PubMed] [Google Scholar]
- 61.Wange R L, Guitian R, Isakov N, Watts J D, Aebersold R, Samelson L E. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of Zap-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 62.Wardenburg J B, et al. Phosphorylation of Slp-76 by the Zap-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 63.Wiest D L, Ashe J M, Abe R, Bolen J B, Singer A. TCR activation of Zap70 is impaired in cd4(+)cd8(+) thymocytes as a consequence of intrathymic interactions that diminish available p56(lck) Immunity. 1996;4:495–504. doi: 10.1016/s1074-7613(00)80415-x. [DOI] [PubMed] [Google Scholar]
- 64.Yoon C H, Lee J H, Jongeward G D, Sternberg P W. Similarity of Sli-1, a regulator of vulval development in C. elegans, to the mammalian proto-oncogene c-Cbl. Science. 1995;269:1102–1105. doi: 10.1126/science.7652556. [DOI] [PubMed] [Google Scholar]