Abstract
Sleep, circadian rhythms, and mental health are reciprocally interlinked. Disruption to the quality, continuity, and timing of sleep can precipitate or exacerbate psychiatric symptoms in susceptible individuals, while treatments that target sleep—circadian disturbances can alleviate psychopathology. Conversely, psychiatric symptoms can reciprocally exacerbate poor sleep and disrupt clock-controlled processes. Despite progress in elucidating underlying mechanisms, a cohesive approach that integrates the dynamic interactions between psychiatric disorder with both sleep and circadian processes is lacking. This review synthesizes recent evidence for sleep—circadian dysfunction as a transdiagnostic contributor to a range of psychiatric disorders, with an emphasis on biological mechanisms. We highlight observations from adolescent and young adults, who are at greatest risk of developing mental disorders, and for whom early detection and intervention promise the greatest benefit. In particular, we aim to a) integrate sleep and circadian factors implicated in the pathophysiology and treatment of mood, anxiety, and psychosis spectrum disorders, with a transdiagnostic perspective; b) highlight the need to reframe existing knowledge and adopt an integrated approach which recognizes the interaction between sleep and circadian factors; and c) identify important gaps and opportunities for further research.
Keywords: mental disorder, mood disorder, psychosis, sleep, chronobiology
Mood, anxiety, and psychotic disorders rank among the most prevalent contributors to the burden of disability and disease worldwide (1), yet our understanding of their causes, and targets for therapeutic intervention, remains limited. In this perspective, we argue that a closer integration of psychiatry with sleep and circadian science will catalyze the improved understanding and treatment of mental disorders.
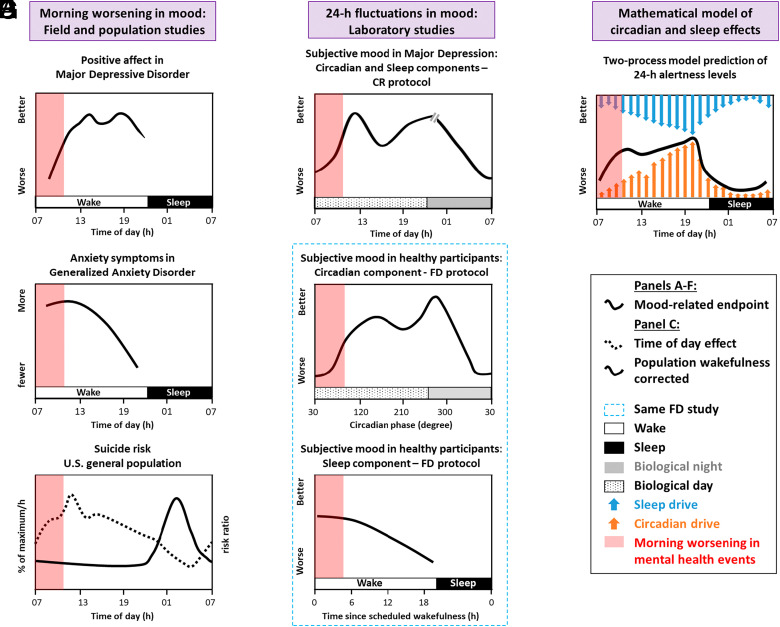
Sleep–circadian disturbances are the rule, rather than the exception, across every diagnostic category of psychiatric disorder (2). In addition to being a risk factor for the subsequent development of psychopathology (3), problems with sleep and its timing are one of the earliest signs of relapse (4). Challenges to the sleep–circadian system through nightshift work increases the risk of developing depression and anxiety disorder (5) while 8% of individuals with depression experience deterioration in mood over winter (6), implicating shorted day-length and changes in light exposure in mood disorders. Carefully controlled laboratory studies have elucidated that sleep homeostatic and circadian processes contribute to diurnal mood variation in healthy volunteers (7), and these processes may contribute to the observations from field-based studies that mood symptoms, including anxiety and risk of suicide, are greater in the mornings (Fig. 1).
Fig. 1.
Contribution of sleep and circadian factors to diurnal mood variation and greater probability of adverse psychiatric outcomes during the morning hours. Field studies in patient populations demonstrate (A) lower mood (8), (B) greater anxiety symptoms (9), and (C) elevated suicide risk, after adjusting for population sleep/wake timing (solid line) and greater proportion of suicides per hour (dotted line) (10) in the morning, indicating a diurnal rhythm in these variables when sampled under real-world conditions. Note that these time courses are likely to be underpinned by multiple additional factors (e.g., alcohol use and social isolation may contribute to elevated suicide risk) and show significant individual differences. Measures of outcomes such as suicide differ depending on the type of study and assumptions of the analysis. Stringently controlled laboratory protocols allow the contribution of sleep homeostasis and circadian factors on mood to be disentangled from environmental and behavioral influences. (D) Constant routine (CR) study illustrating the modulation of mood by sleep homeostatic and circadian processes across the 24-h cycle, in women with depression (11). (E and F) Forced desynchrony (FD) protocol revealing the relative contribution of the endogenous circadian and sleep homeostatic process on mood in healthy control participants (12). These are representative, and not definitive, time-courses. (G) the two-process model posits that sleep and alertness are regulated by the close interaction of two counterpoised processes—homeostatic sleep pressure and the circadian system. Although alertness, (black line) and not mood, is predicted by the mathematical model, note that the time-course for alertness is similar to that for the circadian component of mood in the forced desynchrony study.
Intervention studies have begun to reveal how targeting sleep-circadian disturbances can also lead to improvements in co-morbid psychiatric symptoms (13). Most such interventions are yet to enter widespread clinical practice, however, and when presenting together with psychiatric disorder, sleep problems are often overlooked. How do we harness the potential of sleep and chronobiology to foster innovation and improve the lives of those living with mental illness?
We address this critical question by integrating clinical and translational sleep–circadian findings in mood, anxiety, and psychosis spectrum disorders over the past two decades, and set out a roadmap of key barriers, gaps, and facilitators to progress. Foremost, although the interaction between circadian rhythmicity and sleep homeostasis has been well characterized in the classical two-process model of sleep regulation (14), studies in psychiatric disorders have largely examined sleep and circadian function as independent and parallel processes. We illustrate how both are implicated in a range of psychiatric disorders, and adopting a transdiagnostic approach that integrates the dynamic interaction between sleep and circadian processes with behavioral and environmental variables, promises to deliver a richer understanding of the mechanisms underpinning mental disorder, and in turn promote the development of blended interventions that address them. Sleep and circadian physiology evolve over the lifespan to play essential, age-specific roles in neuroplasticity and brain maturation (15). We therefore pay particular attention to adolescence—a time over which sleep–circadian disturbance is likely to play critical roles in the development of psychiatric disorders.
Transdiagnostic Associations Between Sleep–Circadian Disturbance and Mental Disorder
The notion that common sleep–circadian profiles are seen across mental disorders is compelling, as it suggests not only that shared sleep–circadian mechanisms play a role in the development and maintenance of psychiatric symptoms, but also that the same sleep–circadian interventions might be effective in treating both sleep–circadian and psychiatric symptoms, across a range of disorders (16).
The elevated prevalence of sleep disorders across mood, anxiety, and psychotic disorders, summarized in SI Appendix, Table S1 supports a transdiagnostic formulation. Insomnia occurs more commonly in mental disorders than in the general population (17), both during remission, and even more so during acute mood episodes (18) or in early psychosis (19, 20), when difficulty falling and staying asleep affects over half of individuals. Although data are limited in anxiety-related and bipolar disorders, nightmares are also significantly more common across psychiatric disorders, and associated with greater symptom severity and elevated risk of suicidality (21). Studies investigating circadian rhythm sleep–wake disorders (CRSWD) are scarce; however, those that are available suggest sleep-timing disorders, particularly delayed sleep–wake phase disorder, are present in 32% of bipolar (22) and 8% of early psychosis (20) patients. Another important yet under-recognized transdiagnostic phenotype is hypersomnia, characterized by an increased quantity of, or excessive need for, sleep (10 h of sleep needed during 24-h). In bipolar disorder, hypersomnia predicts relapse of mood episodes (23). Notably, hypersomnia co-exists with insomnia in around a quarter (18) to a third (24) of individuals with mood disorder, and similar proportions in psychosis (20, 25), suggesting that as well as experiencing disturbed sleep initiation and maintenance at night, patients are more sleepy during the day, spend longer in bed and/or have a longer overall sleep duration, in a vicious cycle. Indeed, co-morbidity within individuals is the norm (26): Of 121 adults receiving treatment in a community mental health center, over 85% met full or subdiagnostic criteria for two or more sleep–circadian problems (13). Similarly high rates of co-morbidity have been reported in psychosis (20), with both insomnia and nightmare disorder occurring together in 33% of patients.
Do objective measures support a transdiagnostic approach? Case–control actigraphy has been the most widely employed objective measure, permitting meta-analyses in depression (27), bipolar disorder (28, 29), early psychosis (30), and schizophrenia (29, 31). Here, data show notable consistency across diagnostic categories: parameters consistent with insomnia (reduced total sleep time, increased sleep latency and sleep fragmentation), hypersomnia (greater time in bed, reduced daytime activity), CRSWD (delayed sleep–wake timing), together with increased variability in sleep timing between days, have been reported across depression, euthymic and depressed phases of bipolar disorder, anxiety disorder, and psychosis (SI Appendix, Table S1). Similar patterns have been found in at-risk and early bipolar disorder (32), but findings were less consistent in at-risk and early psychosis (30).
A strength of actigraphy is that it allows the concurrent and longitudinal investigation of both sleep and wake dimensions, and the relative timing of each phase, under free-living conditions. In inter-episode bipolar disorder, patients show reduced daytime activity with shorter duration of the active phase, lower amplitude rest-activity rhythms, and also less organized and predictable patterns of motor behavior (33, 34), with the latter being identified as a heritable phenotype (34). In a study combining actigraphy with mobile symptom monitoring, unidirectional associations between motor activity and depressed mood, and bidirectional associations between motor activity and sleep, were found (35), suggesting that activation is a distinct dimension of bipolar disorder that is intimately connected with sleep and psychopathology. Other studies have reported unique motor signatures in schizophrenia and depression, using nonlinear analysis (36). The effects of antipsychotic medication on activation in schizophrenia, however, may be a significant confounding variable. These findings advocate for further research which examines the inter-relationships between activation, sleep, and symptomatology and integrates them with concepts of hypersomnia.
Findings from circadian and polysomnography studies have been less consistent, due in part to heterogenous patient groups, medication effects, methodological limitations such as masking, as well as notable areas where evidence is lacking (see SI Appendix for a summary of sleep–circadian principles, definitions, and measures). Circadian markers have been studied in mood disorder (37), discussed below; however, in anxiety and psychosis-spectrum disorders (38), findings are sparse and conflicting, making it difficult to reach firm conclusions. Of the meta-analyses of polysomnographic studies, indices of poorer sleep continuity and shorter sleep time have been reported in depression, anxiety disorders, and early and chronic stages of psychosis (2, 19, 39, 40), and increased REM pressure were found in depression, obsessive compulsive disorder (OCD), and chronic psychosis (2, 39). Within the anxiety-related disorders, reduction in sleep time and increased rapid eye movement (REM) pressure were particularly pronounced in OCD (40), suggesting a differential profile to the other anxiety disorders. In comparison to actigraphy, few significant differences in polysomnographic parameters were found in inter-episode bipolar disorder. In the following sections, we examine some of the putative mechanisms by which these perturbations may interact to play a causal role in the development and maintenance of psychiatric symptoms.
Mechanisms Underlying Sleep–Circadian Disturbance in Psychiatric Disorders
Longitudinal Sleep–Circadian Interactions and the Development of Mental Disorders.
Adolescence and early adulthood are a time of elevated risk of onset of mood, psychotic, and substance use disorders (41), with a recent US Task Force recommending screening for depression in adolescents aged 12 to 18 (42). This critical window coincides with widespread changes in the structure and timing of sleep and provides a prototypical example of the dynamic and inseparable interplay between sleep and circadian processes and their association with mental health.
During adolescence, developmental shifts in sleep physiology, including slow wave dynamics, sleep spindles, and sleep need, index maturational changes in gray and white matter (43, 44). These changes are accompanied by concurrent shifts in sleep timing, underpinned by the slower accumulation of homeostatic sleep pressure, and delay in the phase of the endogenous circadian rhythm (45). These variables interact with environmental and behavioral factors including exposure to evening light and greater autonomy in deciding bedtimes, thereby promoting a longer phase relationship between circadian timing and bedtime, pushing bedtimes even later (46). Later sleep onset, however, is often incompatible with social schedules including school start times, leading to curtailed sleep duration and the accumulation of sleep debt during school days. Adolescents may compensate for sleep debt by sleeping longer and later on free days, resulting in greater variability in the duration and timing of sleep over the week (47) and misalignment between circadian and sleep–wake rhythms so-called “social jet-lag” (48). In turn, the risk of sleep disturbances, and adverse health and mental health outcomes, are increased (47).
Interindividual variations in sleep–circadian physiology appear to confer vulnerability to developing psychiatric disorders. A preference for later bedtimes and rise times (late chronotype) and/or excessively delayed sleep timing during adolescence have been associated with a range of disorders, including depression (49), anxiety (50), bipolar disorders (51), psychosis (52), and suicidality (53). Conversely, Mendelian randomization approaches indicate that genes associated with an earlier, “morningness” chronotype are associated with a lower risk of developing depression, supporting a causal link between chronotype and mood disorder (54). A greater magnitude of sleep and social rhythm disturbance has been observed in young people with depressive or bipolar disorders compared with healthy controls (55), and a higher prevalence of delayed sleep phase syndrome has been found in the depressed phase of bipolar disorder, compared with unipolar depression (56). Similar associations have been reported in the few studies utilizing objective measures of sleep timing and circadian phase (57). Lower relative amplitude and more irregular diurnal rest–activity rhythms were observed in young people with emerging mood disorders, with less robust rhythms also being associated with greater symptom severity (58).
Though defined phenotypically, late chronotype is determined by a number of endogenous sleep–circadian variables, including a longer circadian period leading to a later phase of entrainment (59), lower light sensitivity (60), and differences in sleep-homeostasis dynamics. Together, these vulnerabilities may interact with age-dependent changes in physiology, environmental and social variables, to place some young people at increased risk of developing sleep–circadian disorder, and a range of psychiatric disorders (43).
Genetic Associations.
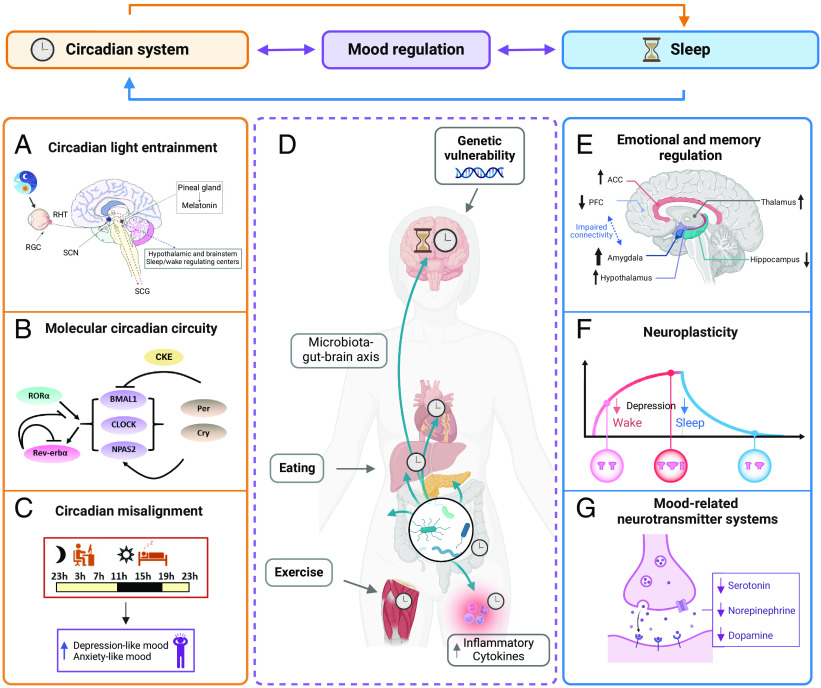
Our understanding of the molecular mechanisms linking sleep–circadian factors with psychiatric disorders is founded on animal studies focusing on the contribution of clock genes (Fig. 2). Targeted disruption of molecular or cellular rhythms in the mouse suprachiasmatic nucleus (SCN) increases depression and anxiety-related behaviors, suggesting that clock machinery contributes to the regulation of mood, anxiety, and reward (64, 65). Manipulation of core circadian genes within mood and reward-related circuits can also lead to changes in neuronal activity and behavior, though the results are more complex. For example, knock-down of Per1 and Per2 specifically in the mouse nucleus accumbens (NAc), a central hub for emotion and reward regulation, leads to increased anxiety-related behavior (66) while a knock-down of the Clock paralogue Npas2 in the NAc leads to decreased anxiety-related behavior (67).
Fig. 2.
Conceptual framework of circadian (Left) and sleep (Right) mechanisms in mental disorders. (A) Nonvisual light information is conveyed from the retina to areas involved in arousal, emotion, and cognition through an indirect pathway via the SCN, but also through direct pathways which bypass the SCN. Mis-timed or low-amplitude light exposure can therefore exert widespread effects on sleep, alertness, and mood. (B) Circadian rhythmicity is generated by the transcription of core clock genes and their suppression by their protein products. (C) Misalignment between the endogenous circadian timing system and behavioral or environmental cycles can negatively affect mood. (D) Physical activity and eating that occurs at a nonoptimal circadian phase, leading to misalignment between central and peripheral clocks, have been implicated in the pathogenesis of mood disorder. Preliminary findings in healthy humans also suggest that alterations in the gut microbiome influence mood via circadian mechanisms (5, 61) and increase depression and anxiety-like symptoms in healthy participants (62). (E–G) Sleep disturbances, particularly insomnia, are implicated in mood dysregulation through several pathways including maladaptive emotional processing, aberrant neuroplasticity and memory consolidation, and dysregulated neurotransmitter signaling (63). For instance, depression reduces both increase in synaptic density build up during wakefulness and increase synaptic pruning during sleep (black arrow, F). We emphasize, however, the many points of interaction and synergy between sleep and circadian systems. Abbreviations: RHT, retino hypothalamic tract; RGC, retinal ganglion cells; SCN, suprachiasmatic nucleus; SCG, superior cervical ganglion; ACC, anterior cingulate cortex; PFC, prefrontal cortex.
In humans, although associations between polymorphisms in circadian genes including CLOCK, PER, TIMELESS, and RORA have been implicated in mood disorders (37, 68), the genetic landscape for sleep–circadian interactions with psychiatric disorders has more recently built upon studies of heritability in rest-activity phenotypes using large-scale accelerometer and genome-wide association study (GWAS) datasets. These approaches have identified numerous independent genetic loci associated with chronotype/sleep timing (34, 69) and sleep quality/duration (70), including insomnia (71). Polygenic risk score for low relative amplitude in rest-activity profiles from UK Biobank participants was significantly associated with depression and also mood instability and neuroticism (72).
Both candidate-gene and GWAS approaches have yet to reveal consistent and robust associations between clock genes and psychiatric disorder (37, 68). However, recent human data implicate associations between a combination of variants in many genes and a range of heritable trait-like sleep–circadian endophenotypes, including later sleep timing, longer sleep duration, sleep fragmentation, and lower amplitude of rest-activity rhythm, that are not unique to any one disorder. These traits associate with phenotypes such as mood instability, neuroticism, and paranoia, thereby playing a role in the pathophysiology of psychiatric disorders. Novel analysis pipelines using state-of-the-art algorithms for classifying accelerometer data (73) promise to further extend the utility of such large-scale datasets, by identifying more nuanced digital biomarkers of sleep–circadian phenotypes.
Light.
Substantial evidence implicates the duration, timing, and intensity exposure to light with mental illness. Much of this interest has centered on mood disorder, following from the observation that depression can emerge or worsen over autumn and winter months, correlating with shortening of photoperiod (6), and that light therapy has antidepressant effects in seasonal, unipolar, and bipolar depression (74, 75). A recent analysis of a population-level survey, for example, found that greater self-reported time outdoors was associated with lower cross-sectional and longitudinal probability of mood disorder (76). Light in anxiety and psychotic disorders have received far less attention, an exception being a recent study reporting an association between greater variability in sleep timing in schizophrenia with reduced duration of exposure to bright light (77).
Mis-timed and low-amplitude light exposure can drive misalignment and diminished amplitude of the endogenous pacemaker through the pathway from the nonimage forming ipRGCs to the SCN, and in turn affect pathways downstream from the SCN (Fig. 2), including the sleep-promoting VLPO, other hypothalamic areas involved in appetite and homeostasis, and nuclei involved in mood regulation such as the dorsal raphe (78). Additionally, the SCN has dense serotonergic innervation, and one mechanism by which selective serotonin reuptake inhibitor antidepressants may exert therapeutic effects is by amplifying the nonvisual effects of light (79) and phase-advancing circadian rhythms (80). A 50-fold interindividual difference in sensitivity of the circadian system to light (81), arising from age, sex, ocular and genetic factors, and photic history (60), may explain why certain individuals are more susceptible to developing circadian dysfunction, and mood disorder, in response to aberrant light input.
Beyond its effects on the clock, however, understanding has more recently extended to include the effects of light on sleep, alertness, cognitive function, and mood, through pathways that are independent of the SCN and visual systems (78, 82). Light modulates sleep homeostasis and slow-wave sleep (83), possibly through a melanopsin-dependent direct pathway from ipRGCs to the VLPO (78). Evidence from rodent models suggests that light also affects alertness via direct pathways from the ipRGCs to monoaminergic and orexinergic centers and the VLPO, and mood via projections to the amygdala, nucleus accumbens, and perihabenular nucleus (82, 84). Serotonin, dopamine, and norepinephrine levels and activity of neurons expressing these neurotransmitters are also strongly influenced by photoperiod length (85), and positive correlations between serotonin synthesis and hours of sunlight have been reported (86).
Light is a particularly powerful explanatory variable because it provides a mechanistic and testable link between the behavioral and environmental context and sleep–circadian biology. Environmental factors in the home can lead to low quantity and quality of daylight (87), while symptoms such as low mood and motivation, anhedonia, negative symptoms, and paranoia may predispose to behaviors that reduce exposure to daylight, and increase exposure to light at night. Together, these factors may result in an attenuated amplitude and aberrant timing of the light signal. Thus far, these contextual factors have received little systematic investigation in mental disorders, however. Integrating these levels of explanation with a more detailed measurement of light exposure across psychiatric diagnoses, and their effects on both circadian and noncircadian pathways, is a key goal.
Circadian Misalignment.
“Circadian health” has been defined as the condition in which circadian clocks are allowed to stably entrain to zeitgebers, and thereby establish and appropriate, stable phase relationship in its cyclic environment (88). Displacement in timing between the central circadian pacemaker and daily environmental and behavioral rhythms, including the light–dark and sleep–wake cycles, is therefore hypothesized to negatively impact mood and well-being, and contributes to the increased risk of mood disorders among night workers (5) and psychiatric disorders more widely (Fig. 2). It was first postulated that the timing of the central circadian clock in depression is abnormally early relative to the timing of the sleep/wake cycle, such that a “critical phase” of the circadian rhythm that is normally aligned with wakefulness, is advanced into sleep (89, 90). More recent clinical studies have pointed toward delayed circadian phase relative to sleep (91, 92), with severity of misalignment correlating with the severity of depressive symptoms both in patients with depression (91) and seasonal affective disorder (SAD) (93).
In bipolar disorder, sleep–wake cycle abnormalities can precede mood fluctuations (94), with shortened, fragmented, and irregularly timed sleep during manic or depressive episodes. A 7 h phase advance in cortisol rhythm and PER1 and ARNTL1 (also known as BMAL1) expression has been reported in mania, and 4 to 5 h phase delay in the depressed phase (95). Misalignment subsequently normalized upon successful treatment of mood. Consistent with the hypothesis that circadian misalignment plays a causal role in mood disorder, the use of lithium—a mood stabilizer used primarily in the treatment and prophylaxis of mania—appears to have a direct effect on the molecular clock, perhaps through phosphorylation and inhibition of GSK3, resulting in a lengthening of cellular and behavioral period, with consequent phase delay and slowing of the clock (96). In schizophrenia, abnormalities in the circadian organization of sleep–wake cycles have been less well characterized, though delayed and advanced sleep phases, free running rest–activity patterns, or irregular sleep–wake patterns have been reported (97), which also predict clinical deterioration (98).
Given the far-reaching effects of circadian timing on the structure of sleep, circadian misalignment may exert some of its pathophysiological effects by virtue of its effects on sleep. Few clinical studies, however, have concurrently investigated sleep, using polysomnography or actigraphy, together with circadian parameters, and this area warrants greater attention.
Recent developments suggest a connection between the microbiome and mental health. Circadian misalignment may increase inflammatory microbiota taxa and decrease microbiota-tryptophan biosynthesis (99), and disrupted microbiome profile is associated with increased systemic inflammation and symptom severity in major depression (61, 100). Hence, sleep–circadian factors regulating the microbiome are a putative mechanism in the onset and maintenance of psychiatric disorders (Fig. 2).
Neuroplasticity.
A fundamental function of sleep is hypothesized to be the consolidation and active reorganization of information that has accrued during wakefulness through long-term synaptic potentiation (LTP) (101). The synaptic homeostasis hypothesis (102) is a candidate mechanism by which the efficiency and signal-to-noise ratio of neuronal networks are maintained within a dynamic working range, through selective down-scaling of synaptic strength during slow-wave sleep (Fig. 2). For example, the amount of slow-wave activity during sleep reflects net synaptic strength, and underlying slow oscillations may contribute directly to synaptic renormalization (102).
In depression, diminished LTP-like plasticity, and deficient accumulation and dissipation of homeostatic sleep pressure (103), have been demonstrated in preclinical and human studies (104). There is indirect human evidence in support of deficient LTP in depression: Using the paired associative stimulation paradigm, significantly lower motor evoked potentials were found in depression relative to controls, with patients who responded to sleep deprivation showing higher inducibility of associative LTP-like plasticity than nonresponders (104). In this context, it is notable that total and partial (first or second half of the sleep episode) sleep deprivation paradigms have shown comparable efficacy in the treatment of unipolar and bipolar depression, with one meta-analysis reporting a significant response in 50 to 60% of patients (105). The synaptic homeostasis hypothesis has been invoked to account for the efficacy of sleep deprivation, by boosting net synaptic strength and shifting the inducibility of associative plasticity into an optimal window, thereby reversing LTP impairments associated with depression (104, 106). Nonetheless, although response to sleep deprivation is rapid, benefits are not sustained, with deterioration of mood following recovery sleep occurring in 80% of patients. Wake therapy has therefore yet to be widely adopted in clinical practice, and prolonging its therapeutic effects remains an important challenge.
Circadian timing influences every aspect of physiology, including neuroplasticity. The initial encoding of experiences during the active phase, and their consolidation into hippocampal and cortical structures, are both energetically demanding processes which are hypothesized to be apportioned by clock-controlled processes into wakefulness and sleep, respectively (107). Centrally mediated mechanisms including 24-h rhythms of corticosteroid and melatonin secretion, fluctuations in brain temperature, and GABAergic inhibition together provide the appropriate conditions for circadian-dependent modulation of plasticity (107). The implications of sleep that is mistimed with respect to circadian phase on aberrant neuroplasticity underlying psychiatric disorders, however, has yet to be explored.
Insomnia, Cognition and Emotional Regulation.
Insomnia is a prevalent sleep disorder that is likely to play an important causal role in the development and maintenance of most psychiatric disorders, with which it is highly co-morbid (3, 108). Characterized by hyperarousal with consequent difficulty in falling and staying asleep, the increased vulnerability to depression and anxiety in insomnia has been hypothesized to arise from instability in REM sleep and impaired overnight emotional memory regulation, which in turn impedes adaptation to emotional distress (63). This results in greater hyperarousal and associated stress reactivity, rumination, and cognitive impairments during wakefulness, which interact with sleep and circadian physiology, and in turn amplifies these vulnerabilities in a bidirectional feedback loop (Fig. 2).
More broadly, animal studies also indicate that sleep is essential for the offline processing and encoding of episodic and emotional memory in limbic, striatal, and neocortical regions (109). These experiments have informed a model of how neural signatures of waking experience are reactivated during ensuing sleep, prioritizing consolidation of salient information into long-term memory (110). Disruption to these mechanisms may therefore contribute to inaccurate and unstable mental models of the world, the mis-association of different aspects of experience, and impairments in setting that experience in its correct emotional context (111). Animal models (112) and electroencephalography (EEG) or magnetoencephalography (MEG) studies in adults with schizophrenia (113) have demonstrated reduced sleep spindle density and dyscoordination of spindles and slow-waves across the cortex, potentially reflecting dysfunctional (cortico-)thalamo-cortical synchrony downstream of synaptic abnormalities in underlying circuits (19, 114).
However, the extent to which these EEG abnormalities coincide with symptoms such as insomnia or circadian misalignment remains unclear, since most patient EEG studies are relatively small and run over one or two nights in sleep laboratory settings. Setting studies of sleep-dependent information processing in circadian context—for example by quantifying memory consolidation relative to different circadian phases—remains a fundamental and clinically relevant next step. In addition, the term insomnia is often used imprecisely, as a symptom rather than as a diagnosis, and in some cases, the sleep disturbance may be more accurately explained by intermixed disorders including circadian rhythm sleep–wake disorder, nightmares, and night terrors, all of which are over-represented in mental disorders (20, 21).
Sleep and Circadian Interventions in Psychiatric Disorders
Targeting the diverse sleep and circadian risk factors and disease mechanisms discussed above, particularly over critical neurodevelopmental windows, offers an opportunity for developing novel preventative and therapeutic interventions for psychiatric disorders. These range from population-level considerations, such as the timing of the school and work day and the design of the built environment which optimizes light exposure from a sleep–circadian perspective; to personalized interventions including psychological therapies, neuromodulation, and medication and meal timing that are tailored to individual circadian parameters (SI Appendix, Fig. S1).
Cognitive Behavioral Therapy for Insomnia (CBT-I).
CBT-I is a multimodal psychological therapy that addresses cognitive factors and behaviors that perpetuate insomnia (115). International guidelines endorse CBT-I as first-line treatment (116) and meta-analysis of randomized trials finds improvement in both night and daytime symptoms (117, 118).
While investigation of treatment mechanisms is in its infancy, mediation analyses within randomized trials suggest CBT-I works, at least in part, through reduction of dysfunctional beliefs about sleep and cognitive arousal (119). To improve mechanistic precision, more recent work has systematically interrogated individual components of CBT-I, like sleep restriction therapy, showing reduced pre-sleep arousal, increased sleep consolidation, and potentiated EEG delta power and decreased beta power during NREM sleep (120, 121). CBT-I may also improve the regularity of the sleep–wake pattern and realign circadian phase angle, by delaying sleep opportunity (122).
Anxiety and depressive symptoms have been assessed as secondary outcomes in trials of CBT-I, and have been shown to reduce after treatment, relative to waiting-list or psychological placebo controls (118). Reductions in insomnia were also found to mediate depression improvement (123). A smaller number of trials have assessed the effect of CBT-I on symptoms of psychiatric disorders in specific patient groups. For example, a recent meta-analysis (108) found that CBT-I reduced depressive symptoms in patients with depression, and reduced trauma symptoms in patients with post-traumatic stress disorder (PTSD). However, the number of trials and sample sizes have been small to date, and there is uncertainty around whether treatment effects are maintained at follow-up. Another approach has been to treat insomnia in the absence of comorbidity and assess whether treatment leads to reduced incidence of future psychiatric disorder. A recent trial found that CBT-I reduced both the incidence and recurrence of major depression in elderly adults compared to control (124).
In contrast to depression, there has been limited evaluation of CBT-I in the context of anxiety disorders and psychosis. Intriguingly, in a head-to-head comparison of digital CBT-I versus digital cognitive behavioral therapy (CBT) for generalized anxiety, both treatments improved anxiety symptoms, yet CBT-I had larger initial effects on insomnia (125). There is also a need to examine the brain and behavioral mechanisms through which CBT-I leads to improvement in psychopathology, and to test whether combining sleep and circadian treatments (126), or personalizing sleep treatment based on presenting sleep and circadian phenotype (127) can enhance mental health outcomes.
Noninvasive Brain Stimulation.
Sleep is a highly active process, characterized by reactivation and reorganization of waking experience (110). Given the hypothesized contribution of dysfunctional nocturnal replay and neuroplasticity to psychiatric disorders, the use of noninvasive brain stimulation to selectively enhance or disrupt specific sleep stages, particularly slow-wave sleep, and impede maladaptive processing, has attracted interest.
Transcranial magnetic simulation (TMS) employs magnetic pulses designed to induce action potentials in localized cortical areas, whereas the transcranial electrical simulation (tES) paradigm uses weak electric currents to drive local shifts in neuronal excitability, that extends across the neural network (128). Auditory stimulation (AS) during sleep may activate arousal, or induce brief global reductions in cortical excitability, depending on the strength of stimulus (129). Phase-locking tES and AS to up- or down-phases of slow wave sleep has been shown to strengthen or suppress slow-oscillations and evoke K-complexes, while extended trains of stimulation can gradually entrain the frequency of neuronal activity to that of the stimulus (128).
The effects of slow wave modulation using noninvasive brain stimulation on sleep physiology and memory consolidation have been studied in healthy adults (128). With the exception of TMS, however, these approaches are currently experimental, and studies that examine its potential in treating psychiatric disorders remain scarce. In the context of theories of deficient neuoroplasticity in depression (106, 130), selective attenuation of slow waves using tES or AS may function as a more acceptable alternative compared with sleep deprivation. Signatures seen in other disorders, such as deficient sleep spindles in schizophrenia (131), may also be targeted by tES, which has been shown to augment spindle activity and procedural memory in healthy adults (132). Finally, brain stimulation has received little attention in REM sleep, and modulating REM sleep may be particularly relevant in light of theories of REM instability and aberrant emotional processing in insomnia, depression, anxiety, and PTSD (63).
Light Therapy.
With wide-ranging effects on sleep and circadian biology, emotion, and cognition through on both circadian and noncircadian pathways, light is one of the most studied nonpharmacological, biologically oriented treatment approaches in psychiatry. Therapeutic use of light arose from the hypothesis that seasonal depression results from a shortened photoperiod and lack of daylight over winter months, with most consistent effects of morning bright light therapy having been demonstrated in SAD (93). Efficacy of light therapy against placebo has subsequently been demonstrated in unipolar (74) and bipolar depression (75). Superiority of combination light therapy plus medication over pharmacotherapy alone has also been reported, and in unipolar depression, equivalent effect sizes to treatment with SSRIs (133). Recent findings suggest light is effective in treating perinatal depression (134); however, robust studies in psychosis and anxiety disorders are lacking (135). Combined light therapy with sleep deprivation and sleep phase-shifting may be more effective and durable than light therapy alone (136).
Light therapy is typically delivered on rising in the morning, which targets the phase advance portion of the phase–response curve, while also encouraging regular rise times ( 137). Bright light may also have antidepressant effects by directly modulating monoaminergic pathways that regulate mood (82), increasing alertness, and strengthening homeostatic sleep pressure (133). Appropriately timed light therapy also results in modest improvements in sleep timing and continuity in neuropsychiatric disorders (135); the extent to which improvements in sleep–circadian parameters mediate the effects on psychopathology is an important and as yet unexplored avenue of investigation.
Though recommended as a first-line treatment option of moderate to severe depression (75), the optimal parameters including the timing, duration, and intensity of dose, light spectra, and duration of treatment remain unclear, and larger and longer trials are needed. Consensus guidelines suggest the overall risk of switches in mood polarity from depression to mania in bipolar disorder is low (75); however, close monitoring and pre-treatment with a mood stabilizer are nonetheless recommended.
Chronopharmacology.
The administration of medications can be timed with respect to circadian phase, in order to optimize efficacy, minimize adverse effects, or modulate circadian rhythms. For example, when taken in the morning, the antipsychotic drug aripiprazole resulted in significantly favorable lipid and cholesterol measures compared to evening dosing (138), while evening dosing of risperidone was associated with greater weight gain and elevated glycosylated hemoglobin when compared to morning dosing (139). Melatonin taken in the evening promotes phase-advance of the circadian clock in delayed sleep–wake phase disorder and may have beneficial effects on psychopathology in other disorders where co-morbid sleep–circadian disturbance is suspected (140).
Certain medications used in the treatment of mood disorders have direct effects on the molecular clock which may underlie their therapeutic efficacy (141). Lithium lengthens the period of the clock and increases amplitude of the rhythm in vitro (142). Importantly, studies using patient-derived fibroblast (143) and neuronal precursor cells (144) found that cells with a longer period and lower amplitude have a weak circadian response to lithium, and these patients are more likely to be lithium nonresponders (145). In contrast, cells taken from lithium responders tended to have a short circadian period and be more responsive to the circadian effects of lithium (144, 145). SSRI antidepressants have also been shown to cause large phase advances in SCN neuronal activity (80) and increase the melatonin-suppressing effects of light (79). Thus, SSRIs and morning bright light therapy may modulate the circadian clock through a common circadian mechanism. There are also indications that rapid-acting antidepressants such as ketamine, which is acutely wake-promoting, change circadian gene expression, similar to total sleep restriction, and changes to sleep–wake profiles in response to ketamine can predict the durability of antidepressant effect (146).
Timed Meals and Exercise.
Although nutrition and exercise have long been associated with mental health (147), whether the timing of food intake or exercise influences mental health is a recent question. In humans, food timing can influence peripheral circadian rhythms, without impacting circadian rhythms driven by the central circadian clock (148). Conversely, timed exercise can shift the central circadian clock, including under dim-light conditions (149), but there are no human data testing whether physical activity can shift peripheral clocks. The circadian timing of meals and exercise have also been shown to differentially influence physiological functions, including glucose control, energy expenditure, and cardiovascular risk (150).
In shift-workers, a recent randomized controlled trial showed that while simulated night work with night eating increased depression-like and anxiety-like impairments, daytime eating prevented mood impairment during simulated night work (62). These beneficial effects of daytime eating correlated with the degree of synchrony between central and peripheral circadian rhythms, suggesting that meal timing is a promising countermeasure against the adverse mental health effects of night work. Regarding timed exercise in psychiatric disorders, future carefully controlled clinical trials are needed.
Conclusions and Future Directions
A central objective for the field is to develop treatment approaches that target sleep–circadian disturbances, and also address concomitant psychiatric symptoms, by virtue of the two sharing overlapping mechanisms. Though incomplete, current evidence suggests that there are many commonalities in subjective and objectively determined sleep–circadian profiles between psychiatric diagnoses, in the prodromal, early, and established phases of disorder. What is not yet clear is how common patterns of sleep–circadian dysfunction are differentially expressed and contribute to unique psychiatric phenotypes. Are the pathways through which circadian misalignment, insomnia, or sleep deprivation drive mania in bipolar disorder similar to those that might lead to the emergence of delusions and hallucinations in nonaffective psychosis? Although there is mounting evidence for overlapping genetic, cellular, and neurotransmitter mechanisms that underlie both sleep–circadian and mood symptoms in depression and bipolar disorder (151), similar links are lacking in psychosis-spectrum disorders, particularly from the circadian perspective. A further important area of future work will be to understand interindividual vulnerability with respect to sleep–circadian disturbance: Why are some individuals susceptible to developing mental disorder in the face sleep–circadian disruption, while others not?
Addressing these questions demands an integrated, co-ordinated research agenda (SI Appendix, Box 2). We advocate future studies which concurrently examine sleep and circadian parameters across multiple conditions and symptom clusters, in order to identify areas of diagnostic specificity, and convergence. Much research to date has been fragmented by its focus on specific age groups, and longitudinal approaches which capture changes across the lifespan are necessary. Promoting the adoption of common, operationalized standards for the collection of biomarkers of sleep, activity, and circadian rhythms (152–154) will aid harmonization across research groups.
A related question is how sleep–circadian disruption interacts with environmental and behavioral factors on the causal pathway to psychiatric disorder. Laboratory studies have afforded valuable insights into the role of homeostatic sleep and circadian processes in sleep–wake regulation (14, 155). They are limited however in their integration of variables such as light, social schedules, and psychopathology under real-world conditions and overlook the complex and dynamic interplay between vulnerabilities in sleep and circadian function, developmental trajectory, behavior and environment, and psychiatric disorder. Devices have been developed which allow investigation of the nonvisual effects of light, at the ocular level (156). Remote sleep-sensing technologies including wireless EEG, wearable sleep trackers, and mobile devices (157) may aid measurement of sleep, rest-activity patterns, and light exposure across developmental time-points in the home setting and reveal critical windows of vulnerability in which to focus preventative interventions. Together with improvements in automated sleep scoring algorithms, these technologies may facilitate real-time sleep analysis and closed-loop neurostimulation paradigms that recursively adapt to the effects of modulation on sleep physiology (128).
Studies examining circadian biomarkers in mental illness are scarce, even though accurate assessment of circadian phase is essential in understanding the contribution of circadian misalignment to psychopathology and for informing the correct timing of light or melatonin therapy. For example, up to 20% of individuals with a diagnosis of insomnia initiate sleep at too early a circadian phase (158), suggesting a circadian contribution to the disorder in a subset of individuals. Experimental approaches including multivariate-based biomarkers from blood samples (159), and the combination of physiologically informed mathematical models (45) with data from rest-activity profiles from wearable accelerometers (160), show promise in estimating circadian phase at scale, at least in those with fixed sleep schedules, and will help to uncover the contribution of circadian dysfunction to disease.
A further goal is to promote cost-effective and scalable interventions that are effective across different categories of psychiatric disorder. With the exception of CBT-I, and in more specialist settings melatonin and light therapy, pharmacological and behavioral sleep–circadian therapies are yet to enter widespread clinical practice. Aided by a closer theoretical and practical convergence of chronobiology with sleep science, the development of technologies which allow longitudinal measurement of sleep and circadian variables in the field, improved education in sleep medicine among clinicians and rigorous clinical trials, a key opportunity exists for overcoming barriers to clinical translation.
Current sleep and chronotherapeutic interventions are mostly supported by evidence from uncontrolled within-subject designs, or small randomized controlled trials which test a single intervention against the current standard of care or placebo. We have argued however that the contribution of sleep and circadian disturbances to psychopathology is multifactorial; examining each component in isolation can be inefficient and fails to account for the complexity of sleep–circadian problems experienced by people living with psychiatric disorder (26). Innovative multi-component interventions such as the Transdiagnostic Intervention for Sleep and Circadian dysfunction (26) adopt a sleep-health framework to combine modules that address sleep and circadian dimensions including sleep regularity, sleep phase, daytime dysfunction, nightmares, excessive time in bed, and the sleep environment, that are applicable to a range of disorders including depression, bipolar disorder, and psychosis. Such approaches acknowledge the co-morbidity of sleep–circadian pathology in psychiatric disorders, but also their associations with a spectrum of psychiatric phenotypes. Similar composite interventions could be tested and refined through efficient mulitarm, multistage adaptive trial designs, and adherence to treatments such as sleep restriction can potentially be enhanced by leveraging wearable and smartphone technologies. Future work should also untangle the complex mechanisms by which reduced activity and social behavior, psychotropic medications, and co-morbidities including nightmares, parasomnias, and sleep-disordered breathing, all of which are over-represented in mental disorder, contribute both directly and indirectly to psychopathology and disability via sleep and circadian pathways.
Collectively, research into mental health is poised to take advantage of the extraordinary advances that have been made in sleep and circadian science and translate these into improved understanding of the pathophysiology and treatment of psychiatric disorders.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
R.L. is supported by U.S. Department of Defense (W81XWH-16-1-0223); C.S. in part by ERC-Stg-GA757763 and FNRS-PDR-T.0220.20; S.D.K. by the National Institute of Health and Care Research (NIHR) Oxford Health Biomedical Research Centre, NIHR Efficacy and Mechanisms Evaluation Programme (Ref: 131789), NIHR Programme Grants for Applied Research (Ref: 203667) and the Wellcome Trust (226784/Z/22/Z and 227093/Z/23/Z); C.A.M. from NIH grants MH111601, MH106460, DA039865, DA046346, F.A.J.L.S. by NIH grants R01-HL140574 and R01-HL153969; M.W.J. from Wellcome grant 226709/Z/22/Z and MRC grant MR/X009726/1; S.L.C. was supported by the Alexander von Humboldt Stifung. Figures were created with permission from http://Biorender.com.
Author contributions
N.M., R.L., C.S., S.D.K., C.A.M., C.C., F.A.J.L.S., M.W.J., and S.L.C. contributed to the writing and editing; N.M., R.L., M.W.J., and S.L.C. developed the outline; and all authors contributing to the writing and editing of this paper.
Competing interests
N.M. has received speaker fees from Idorsia Pharmaceuticals. S.D.K. declares nonfinancial support from Big Health Ltd. in the form of no-cost use of Sleepio in clinical trial research. F.A.J.L.S. served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham and Morehouse School of Medicine. F.A.J.L.S. interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. F.A.J.L.S. consultancies are not related to the current work. M.W.J. has received speaker fees from Boehringer Ingelheim. All other authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Nicholas Meyer, Email: nicholas.meyer@kcl.ac.uk.
Sarah L. Chellappa, Email: S.L.Chellappa@soton.ac.uk.
Data, Materials, and Software Availability
There are no data underlying this work.
Supporting Information
References
- 1.Wittchen H. U., et al. , The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychophar. 21, 655–79 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Baglioni C., et al. , Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 142, 969–990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertenstein E., et al. , Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 43, 96–105 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Wehr T. A., Sack D. A., Rosenthal N. E., Sleep reduction as a final common pathway in the genesis of mania. Am. J. Psych. 144, 201–204 (1987). [DOI] [PubMed] [Google Scholar]
- 5.S. L. Chellappa, C. J. Morris, F. A. J. L. Scheer, Circadian misalignment increases mood vulnerability in simulated shift work. Sci. Rep. 10, 18614 (2020). [DOI] [PMC free article] [PubMed]
- 6.Wirz-Justice A., Ajdacic V., Rossler W., Steinhausen H. C., Angst J., Prevalence of seasonal depression in a prospective cohort study. Eur. Arch. Psychiatry Clin. Neurosci. 269, 833–839 (2019). [DOI] [PubMed] [Google Scholar]
- 7.F. A. J. L. Scheer, S. L. Chellappa, Endogenous circadian rhythms in mood and well-being. Sleep Heal. (2023). [DOI] [PubMed]
- 8.Peeters F., Berkhof J., Delespaul P., Rottenberg J., Nicolson N. A., Diurnal mood variation in major depressive disorder. Emotion 6, 383–391 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Geraci M. F., Uhde T. W., Diurnal rhythms and symptom severity in panic disorder. A preliminary study of 24-hour changes in panic attacks, generalised anxiety, and avoidance behaviour. Br. J. Psychiatry 161, 512–516 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Tubbs A. S., Fernandez F. X., Grandner M. A., Perlis M. L., Klerman E. B., The mind after midnight: Nocturnal wakefulness, behavioral dysregulation, and psychopathology. Front. Netw. Physiol. 1, 830228 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birchler-Pedross A., et al. , Subjective mood in young unmedicated depressed women under high and low sleep pressure conditions. Biol. (Basel) 5, 52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boivin D. B., et al. , Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch. Gen. Psychiatry 54, 145–152 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Sarfan L. D., et al. , Outcomes of the transdiagnostic intervention for sleep and circadian dysfunction (TranS-C) in a community setting: Unpacking comorbidity. Behav. Res. Therapy 145, 103948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borbely A. A., A two process model of sleep regulation. Hum. Neurobiol. 1, 195–204 (1982). [PubMed] [Google Scholar]
- 15.Logan R. W., McClung C. A., Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 20, 49–65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey A. G., Murray G., Chandler R. A., Soehner A., Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clin. Psychol. Rev. 31, 225–235 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford D. E., Kamerow D. B., Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA: J. Am. Med. Assoc. 262, 1479–1484 (1989). [DOI] [PubMed] [Google Scholar]
- 18.Soehner A. M., Kaplan K. A., Harvey A. G., Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J. Affect. Disord. 167, 93–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagautdinova J., et al. , Sleep abnormalities in different clinical stages of psychosis: A systematic review and meta-analysis. JAMA Psych. 80, 202–210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeve S., Sheaves B., Freeman D., Sleep disorders in early psychosis: Incidence, severity, and association with clinical symptoms. Schizophr. Bull. 45, 287–295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkaoui M. A., Lejoyeux M., D’ortho M. P., Geoffroy P. A., Nightmares in patients with major depressive disorder, bipolar disorder, and psychotic disorders: A systematic review. J. Clin. Med. 9, 1–26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaesu Y., et al. , Prevalence of circadian rhythm sleep-wake disorders and associated factors in euthymic patients with bipolar disorder. PLoS One 11, e0159578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan K. A., et al. , Hypersomnia subtypes, sleep and relapse in bipolar disorder. Psychol. Med. 45, 1751–1763 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geoffroy P. A., et al. , Insomnia and hypersomnia in major depressive episode: Prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J. Affect. Disord. 226, 132–141 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Sharma P., Excessive daytime sleepiness in schizophrenia: A naturalistic clinical study. J. Clin. Diagnost. Res. 10, 10–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey A. G., Treating sleep and circadian problems to promote mental health: Perspectives on comorbidity, implementation science and behavior change. Sleep 45, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tazawa Y., et al. , Actigraphy for evaluation of mood disorders: A systematic review and meta-analysis. J. Affect. Disord. 253, 257–269 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Geoffroy P. A., et al. , Sleep in patients with remitted bipolar disorders: A meta-analysis of actigraphy studies. Acta Psychiatr. Scand. 131, 89–99 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Meyer N., et al. , Sleep and circadian rhythm disturbance in remitted schizophrenia and bipolar disorder: A systematic review and meta-analysis. Schizophr. Bull. 46, 1126–43 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dondé C., et al. , Sleep disturbances in early clinical stages of psychotic and bipolar disorders: A meta-analysis. Aust. New Zealand J. Psy. 56, 1068–1079 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Wainberg M., et al. , Association of accelerometer-derived sleep measures with lifetime psychiatric diagnoses: A cross-sectional study of 89,205 participants from the UK biobank. PLOS Med. 18, e1003782 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott J., et al. , A systematic review and meta-analysis of sleep and circadian rhythms disturbances in individuals at high-risk of developing or with early onset of bipolar disorders. Neurosci. Biobehav. Rev. 135, 104585 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott J., et al. , Activation in bipolar disorders a systematic review. JAMA Psychiatry 74, 189–196 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Pagani L., et al. , Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc. Natl. Acad. Sci. U.S.A. 113, E754–E761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merikangas K. R., et al. , Real-time mobile monitoring of the dynamic associations among motor activity, energy, mood, and sleep in adults with bipolar disorder. JAMA Psych. 76, 190–198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauge E. R., Berle J. Ø., Oedegaard K. J., Holsten F., Fasmer O. B., Nonlinear analysis of motor activity shows differences between schizophrenia and depression: A study using Fourier analysis and sample entropy. PLoS One 6, e16291 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy M. J., et al. , Neurobiological and behavioral mechanisms of circadian rhythm disruption in bipolar disorder: A critical multi-disciplinary literature review and agenda for future research from the ISBD task force on chronobiology. Bipolar. Disord. 24, 232–263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delorme T. C., Srivastava L. K., Cermakian N., Are circadian disturbances a core pathophysiological component of schizophrenia? J. Biol. Rhy. 35, 325–339 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Benca R. M., Obermeyer W. H., Thisted R. A., Gillin J. C., Sleep and psychiatric disorders: A meta-analysis. Arch. Gen. Psych. 49, 651–668 (1992). [DOI] [PubMed] [Google Scholar]
- 40.Cox R. C., Olatunji B. O., Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Med. Rev. 51, 101282 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Solmi M., et al. , Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 27, 281–295 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.USPST Force et al., Screening for depression and suicide risk in children and adolescents: US preventive services task force recommendation statement. JAMA 328, 1534–1542 (2022). [DOI] [PubMed]
- 43.Anastasiades P. G., de Vivo L., Bellesi M., Jones M. W., Adolescent sleep and the foundations of prefrontal cortical development and dysfunction. Prog. Neurobiol. 218, 102338 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarokh L., Saletin J. M., Carskadon M. A., Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 70, 182–188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skeldon A. C., Derks G., Dijk D. J., Modelling changes in sleep timing and duration across the lifespan: Changes in circadian rhythmicity or sleep homeostasis? Sleep Med. Rev. 28, 96–107 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Crowley S. J., Wolfson A. R., Tarokh L., Carskadon M. A., An update on adolescent sleep: New evidence informing the perfect storm model. J. Adolesc. 67, 55–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bei B., Manber R., Allen N. B., Trinder J., Wiley J. F., Too long, too short, or too variable? SLeep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep 40, zsw067 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Foster R. G., et al. , Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog. Mol. Biol. Transl. Sci. 119, 325–346 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Saxvig I. W., Pallesen S., Wilhelmsen-Langeland A., Molde H., Bjorvatn B., Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. 13, 193–199 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Brown W. J., et al. , A review of sleep disturbance in children and adolescents with anxiety. J. Sleep Res. 27, e12635 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Vidafar P., Yocum A. K., Han P., McInnis M. G., Burgess H. J., Late chronotype predicts more depressive symptoms in bipolar disorder over a 5 year follow-up period. Int. J. Bipolar. Disord. 9, 28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linke M., Jankowski K. S., Chronotype in individuals with schizophrenia: A meta-analysis. Schizophr. Res. 235, 74–79 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Chan N. Y., et al. , The associations of insomnia symptoms and chronotype with daytime sleepiness, mood symptoms and suicide risk in adolescents. Sleep Med. 74, 124–131 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Daghlas I., Lane J. M., Saxena R., Vetter C., Genetically proxied diurnal preference, sleep timing, and risk of major depressive disorder. JAMA Psychiatry 78, 903–910 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mondin T. C., et al. , Mood disorders and biological rhythms in young adults: A large population-based study. J. Psychiatr. Res. 84, 98–104 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Robillard R., et al. , Delayed sleep phase in young people with unipolar or bipolar affective disorders. J. Affect. Disord. 145, 260–263 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Robillard R., et al. , Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl. Psychiatry 8, 213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robillard R., et al. , Ambulatory sleep-wake patterns and variability in young people with emerging mental disorders. J. Psychiatry Neurosci. 40, 28–37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gronfier C., Wright K. P., Kronauer R. E., Czeisler C. A., Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl. Acad. Sci. U.S.A. 104, 9081–9086 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chellappa S. L., Individual differences in light sensitivity affect sleep and circadian rhythms. Sleep 44, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teichman E. M., O’Riordan K. J., Gahan C. G., Dinan T. G., Cryan J. F., When rhythms meet the blues: Circadian interactions with the microbiota-gut-brain axis. Cell Metab. 31, 448–471 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Qian J., et al. , Daytime eating prevents mood vulnerability in night work. Proc. Natl. Acad. Sci. U.S.A. 119, e2206348119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Someren E. J. W. V., Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 101, 995–1046 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Landgraf D., et al. , Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol. Psychiatry 80, 827–835 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vadnie C. A., et al. , The suprachiasmatic nucleus regulates anxiety-like behavior in mice. Front Neurosci. 15, 765850 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spencer S., et al. , Circadian genes period 1 and period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur. J. Neurosci. 37, 242–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozburn A. R., et al. , NPAS2 regulation of anxiety-like behavior and GABAA receptors. Front. Mol. Neurosci. 10, 360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melhuish Beaupre L., Brown G. M., Kennedy J. L., Circadian genes in major depressive disorder. World J. Biol. Psychiatry 21, 80–90 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Jones S. E., et al. , Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 10, 343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dashti H. S., et al. , Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10, 1100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lane J. M., et al. , Biological and clinical insights from genetics of insomnia symptoms. Nat. Genet. 51, 387–393 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferguson A., et al. , Genome-wide association study of circadian rhythmicity in 71,500 UK biobank participants and polygenic association with mood instability. EBioMedicine 35, 279–287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katori M., Shi S., Ode K. L., Tomita Y., Ueda H. R., The 103,200-arm acceleration dataset in the UK biobank revealed a landscape of human sleep phenotypes. Proc. Natl. Acad. Sci. U.S.A. 119, e2116729119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Karawi D., Jubair L., Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J. Affect. Disord. 198, 64–71 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Gottlieb J. F., et al. , The chronotherapeutic treatment of bipolar disorders: A systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipol. Disord. 21, 741–773 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Burns A. C., et al. , Time spent in outdoor light is associated with mood, sleep, and circadian rhythm-related outcomes: A cross-sectional and longitudinal study in over 400,000 UK Biobank participants. J. Affect. Disord. 295, 347–352 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skeldon A. C., Dijk D. J., Meyer N., Wulff K., Extracting circadian and sleep parameters from longitudinal data in schizophrenia for the design of pragmatic light interventions. Schizophr. Bull. 48, 447–456 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maruani J., Geoffroy P. A., Multi-level processes and retina-brain pathways of photic regulation of mood. J. Clin. Med. 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGlashan E. M., et al. , The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacol. (Berl) 235, 3201–3209 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Sprouse J., Braselton J., Reynolds L., Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biol. Psychiatry 60, 896–899 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Phillips A. J., et al. , High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. U.S.A. 116, 12019–12024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LeGates T. A., Fernandez D. C., Hattar S., Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 15, 443–454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chellappa S. L., et al. , Acute exposure to evening blue-enriched light impacts on human sleep. J. Sleep Res. 22, 573–580 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Fernandez D. C., et al. , Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vadnie C. A., McClung C. A., Circadian rhythm disturbances in mood disorders: Insights into the role of the suprachiasmatic nucleus. Neural Plast. 2017, 1504507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambert G. W., Reid C., Kaye D. M., Jennings G. L., Esler M. D., Effect of sunlight and season on serotonin turnover in the brain. Lancet 360, 1840–1842 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Münch M., et al. , The role of daylight for humans: Gaps in current knowledge. Clocks Sleep 2, 61–85 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roenneberg T., Foster R. G., Klerman E. B., The circadian system, sleep, and the health/disease balance: A conceptual review. J. Sleep Res. 31, 1–14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kripke D. F., Mullaney D. J., Atkinson M., Wolf S., Circadian rhythm disorders in manic-depressives. Biol. Psychiatry 13, 335–351 (1978). [PubMed] [Google Scholar]
- 90.Borbély A. A., Wirz-Justice A., Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum. Neurobiol. 1, 205–210 (1982). [PubMed] [Google Scholar]
- 91.Emens J., Lewy A., Kinzie J. M., Arntz D., Rough J., Circadian misalignment in major depressive disorder. Psychiatry Res. 168, 259–261 (2009). [DOI] [PubMed] [Google Scholar]
- 92.Swanson L. M., et al. , Relationships between circadian measures, depression, and response to antidepressant treatment: A preliminary investigation. Psychiatry Res. 252, 262–269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewy A. J., Lefler B. J., Emens J. S., Bauer V. K., The circadian basis of winter depression. Proc. Natl. Acad. Sci. U.S.A. 103, 7414–7419 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bauer M., et al. , Temporal relation between sleep and mood in patients with bipolar disorder. Bipol. Disord. 8, 160–167 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Moon J. H., et al. , Advanced circadian phase in mania and delayed circadian phase in mixed mania and depression returned to normal after treatment of bipolar disorder. EBioMedicine 11, 285–295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moreira J., Geoffroy P. A., Lithium and bipolar disorder: Impacts from molecular to behavioural circadian rhythms. Chronobiol. Int. 33, 351–373 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Wulff K., Dijk D. J., Middleton B., Foster R. G., Joyce E. M., Sleep and circadian rhythm disruption in schizophrenia. Br. J. Psychiatry 200, 308–316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meyer N., et al. , The temporal dynamics of sleep disturbance and psychopathology in psychosis: A digital sampling study. Psychol. Med. 52, 2741–2750 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chellappa S. L., et al. , Proof-of-principle demonstration of endogenous circadian system and circadian misalignment effects on human oral microbiota. FASEB J. 36, e22043 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valles-Colomer M., et al. , The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Vyazovskiy V. V., Cirelli C., Pfister-Genskow M., Faraguna U., Tononi G., Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 11, 200–208 (2008). [DOI] [PubMed] [Google Scholar]
- 102.Tononi G., Cirelli C., Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Borbely A. A., The S-deficiency hypothesis of depression and the two-process model of sleep regulation. Pharmacopsychiatry 20, 23–9 (1987). [DOI] [PubMed] [Google Scholar]
- 104.Wolf E., et al. , Synaptic plasticity model of therapeutic sleep deprivation in major depression. Sleep Med. Rev. 30, 53–62 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Boland E. M., et al. , Meta-analysis of the antidepressant effects of acute sleep deprivation. J. Clin. Psychiatry 78, e1020–e1034 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Kuhn M., et al. , Indices of cortical plasticity after therapeutic sleep deprivation in patients with major depressive disorder. J. Affect. Disord. 277, 425–435 (2020). [DOI] [PubMed] [Google Scholar]
- 107.M. G. Frank, Clocking in: A circadian model of synaptic plasticity. Curr. Opin. Physiol. 15, 96–103 (2020).
- 108.Hertenstein E., et al. , Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 62, 101597 (2022). [DOI] [PubMed] [Google Scholar]
- 109.Girardeau G., Lopes-Dos-Santos V., Brain neural patterns and the memory function of sleep. Science 374, 560–564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klinzing J. G., Niethard N., Born J., Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Feld G. B., Born J., Neurochemical mechanisms for memory processing during sleep: Basic findings in humans and neuropsychiatric implications. Neuropsychopharmacology 45, 31–44 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phillips K. G., et al. , Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron 76, 526–33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nour M. M., Liu Y., Arumuham A., Kurth-Nelson Z., Dolan R. J., Impaired neural replay of inferred relationships in schizophrenia. Cell 184, 4315–4328 e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartsch U., et al. , Distributed slow-wave dynamics during sleep predict memory consolidation and its impairment in schizophrenia. NPJ Schizophr. 5, 18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morin C. M., Benca R., Chronic insomnia. Lancet 379, 1129–1141 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Riemann D., et al. , European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 26, 675–700 (2017). [DOI] [PubMed] [Google Scholar]
- 117.van Straten A., et al. , Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Med. Rev. 38, 3–16 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Benz F., et al. , The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: A systematic review and network meta-analysis. Clin. Psychol. Rev. 80, 101873 (2020). [DOI] [PubMed] [Google Scholar]
- 119.C. E. Parsons, R. Zachariae, C. Landberger, K. S. Young, How does cognitive behavioural therapy for insomnia work? A systematic review and meta-analysis of mediators of change. Clin. Psychol. Rev. 86, 102027 (2021). [DOI] [PubMed]
- 120.L. F. Maurer, C. A. Espie, S. D. Kyle, How does sleep restriction therapy for insomnia work? A systematic review of mechanistic evidence and the introduction of the Triple-R model. Sleep Med. Rev. 42, 127–138 (2018). [DOI] [PubMed]
- 121.Maurer L. F., et al. , Isolating the role of time in bed restriction in the treatment of insomnia: A randomized, controlled, dismantling trial comparing sleep restriction therapy with time in bed regularization. Sleep 43, zsaa096 (2020). [DOI] [PubMed] [Google Scholar]
- 122.Maurer L. F., Ftouni S., Espie C. A., Bisdounis L., Kyle S. D., The acute effects of sleep restriction therapy for insomnia on circadian timing and vigilance. J. Sleep Res. 30, e13260 (2021). [DOI] [PubMed] [Google Scholar]
- 123.Henry A. L., et al. , Insomnia as a mediating therapeutic target for depressive symptoms: A sub-analysis of participant data from two large randomized controlled trials of a digital sleep intervention. J. Sleep Res. 30, e13140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Irwin M. R., et al. , Prevention of incident and recurrent major depression in older adults with insomnia: A randomized clinical trial. JAMA Psychiatry 79, 33–41 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mason E. C., et al. , Co-occurring insomnia and anxiety: A randomized controlled trial of internet cognitive behavioral therapy for insomnia versus internet cognitive behavioral therapy for anxiety. Sleep 46, zsac205 (2023). [DOI] [PubMed] [Google Scholar]
- 126.Leerssen J., et al. , Treating insomnia with high risk of depression using therapist-guided digital cognitive, behavioral, and circadian rhythm support interventions to prevent worsening of depressive symptoms: A randomized controlled trial. Psychoth. Psychos. 91, 168–179 (2022). [DOI] [PubMed] [Google Scholar]
- 127.Harvey A. G., et al. , A randomized controlled trial of the Transdiagnostic Intervention for Sleep and Circadian Dysfunction (TranS-C) to improve serious mental illness outcomes in a community setting. J. Consul. Clin. Psychol. 89, 537–550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fehér K. D., et al. , Shaping the slow waves of sleep: A systematic and integrative review of sleep slow wave modulation in humans using non-invasive brain stimulation. Sleep Med. Rev. 58, 101438 (2021). [DOI] [PubMed] [Google Scholar]
- 129.Ngo H. V. V., Martinetz T., Born J., Mülle M., Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Wolf E., et al. , Synaptic plasticity model of therapeutic sleep deprivation in major depression. Sleep Med. Rev. 30, 53–62 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Manoach D. S., Stickgold R., Abnormal sleep spindles, memory consolidation, and schizophrenia. Annu. Rev. Clin. Psychol. 15, 451–479 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lustenberger C., et al. , Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 26, 2127–2136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Geoffroy P. A., Palagini L., Biological rhythms and chronotherapeutics in depression. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 106, 110158 (2021). [DOI] [PubMed] [Google Scholar]
- 134.Garbazza C., et al. , Sustained remission from perinatal depression after bright light therapy: A pilot randomised, placebo-controlled trial. Acta Psychiatr. Scand. 146, 350–356 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faulkner S. M., Bee P. E., Meyer N., Dijk D. J., Drake R. J., Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: A systematic review and meta-analysis. Sleep Med. Rev. 46, 108–123 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Humpston C., et al. , Chronotherapy for the rapid treatment of depression: A meta-analysis. J. Affect. Disord. 261, 91–102 (2020). [DOI] [PubMed] [Google Scholar]
- 137.S. B. S. Khalsa et al. , A phase response curve to single bright light pulses in human subjects, J. Physiol. 549, 945–952 (2003). [DOI] [PMC free article] [PubMed]
- 138.Chipchura D. A., Freyberg Z., Edwards C., Leckband S. G., McCarthy M. J., Does the time of drug administration alter the metabolic risk of aripiprazole? Front. Psychiatry 9, 494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zapata R. C., et al. , Antipsychotic-induced weight gain and metabolic effects show diurnal dependence and are reversible with time restricted feeding. Schizophrenia 8, 70 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palagini L., et al. , International expert opinions and recommendations on the use of melatonin in the treatment of insomnia and circadian sleep disturbances in adult neuropsychiatric disorders. Front. Psychiatry 12, 688890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cederroth C. R., et al. , Medicine in the fourth dimension. Cell Metabol. 30, 238–250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J., Lu W. Q., Beesley S., Loudon A. S., Meng Q. J., Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One 7, e33292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCarthy M. J., et al. , Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology 44, 620–628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mishra H. K., et al. , Circadian rhythms in bipolar disorder patient-derived neurons predict lithium response: Preliminary studies. Mol. Psychiatry 26, 3383–3394 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanghani H. R., et al. , Patient fibroblast circadian rhythms predict lithium sensitivity in bipolar disorder. Mol. Psychiatry 26, 5252–5265 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kohtala S., Alitalo O., Rosenholm M., Rozov S., Rantamaki T., Time is of the essence: Coupling sleep-wake and circadian neurobiology to the antidepressant effects of ketamine. Pharmacol. Ther. 221, 107741 (2021). [DOI] [PubMed] [Google Scholar]
- 147.Jacka F. N., et al. , A randomised controlled trial of dietary improvement for adults with major depression (the “smiles’’ trial). BMC Med. 15, 23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chellappa S. L., et al. , Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 7, eabg9910 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buxton O. M., Lee C. W., L’Hermite-Baleriaux M., Turek F. W., Van Cauter E., Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R714–R724 (2003). [DOI] [PubMed] [Google Scholar]
- 150.S. L. Chellappa, N. Vujovic, J. S. Williams, F. A. J. L. Scheer, Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 30, 767–779 (2019). [DOI] [PMC free article] [PubMed]
- 151.Carpenter J. S., et al. , Circadian depression: A mood disorder phenotype. Neurosci. Biobehav. Rev. 126, 79–101 (2021). [DOI] [PubMed] [Google Scholar]
- 152.Klerman E. B., et al. , Keeping an eye on circadian time in clinical research and medicine. Clin. Transl. Med. 12, e1131 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown T. M., et al. , Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 20, 1–24 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mazzotti D. R., et al. , Sleep and circadian informatics data harmonization: A workshop report from the Sleep Research Society and Sleep Research Network. Sleep 45, 1–10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dijk D. J., Czeisler C. A., Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 15, 3526–3538 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mohamed A., et al. , Wearable light spectral sensor optimized for measuring daily alpha-opic light exposure. Opt. Express 29, 27612–27627 (2021). [DOI] [PubMed] [Google Scholar]
- 157.Perez-Pozuelo I., et al. , The future of sleep health: A data-driven revolution in sleep science and medicine. NPJ Digital Med. 3, 1–15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Flynn-Evans E. E., et al. , Circadian phase and phase angle disorders in primary insomnia. Sleep 40, zsx163 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Dijk D. J., Duffy J. F., Novel approaches for assessing circadian rhythmicity in humans: A review. J. Biol. Rhy. 35, 421–438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheng P., et al. , Predicting circadian misalignment with wearable technology: Validation of wrist-worn actigraphy and photometry in night shift workers. Sleep 44, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
There are no data underlying this work.