Abstract
Investigations on acute carbon monoxide (CO) poisoning struggle to highlight a relevant discriminant criterion related to CO poisoning severity for predicting complications, such as delayed neurological syndromes. In this context, it remains difficult to demonstrate the superiority of one method of oxygen (O2) administration over others or to identify the optimal duration of normobaric 100% oxygen (NBO) treatment. Myoglobin, as hemoglobin, are a potential binding site for CO, which could be a source of extravascular CO storage that impacts the severity of CO poisoning. It is not possible in routine clinical practice to estimate this potential extravascular CO storage. Indirect means of doing so that are available in the first few hours of poisoning could include, for example, the carboxyhemoglobin half-life (COHbt1/2), which seems to be influenced itself by the level and duration of CO exposure affecting this store of CO within the body. However, before the elimination of CO can be assessed, the COHbt1/2 toxicokinetic model must be confirmed: research still debates whether this model mono- or bi-compartmental. The second indirect mean could be the assessment of a potential COHb rebound after COHb has returned to 5% and NBO treatment has stopped. Moreover, a COHb rebound could be considered to justify the duration of NBO treatment. On an experimental swine model exposed to moderate CO poisoning (940 ppm for ±118 min until COHb reached 30%), we first confirm that the COHb half-life follows a bi-compartmental model. Secondly, we observe for the first time a slight COHb rebound when COHb returns to 5% and oxygen therapy is stopped. On the basis of these two toxicokinetic characteristics in favor of extravascular CO storage, we recommend that COHbt1/2 is considered using the bi-compartmental model in future clinical studies that compare treatment effectiveness as a potential severity criterion to homogenize cohorts of the same severity. Moreover, from a general toxicokinetic point of view, we confirm that a treatment lasting less than 6 hours appears to be insufficient for treating moderate CO poisoning.
Keywords: Carbon monoxide, Swine, Toxicokinetics, Carboxyhemoglobin (COHb), Carboxyhemoglobin half-life (COHbt1/2), Carbon monoxide poisoning management
Graphical Abstract
Highlights
-
•
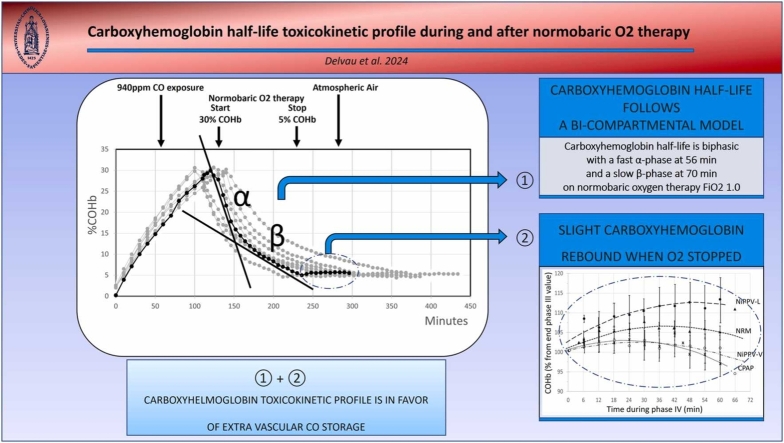
Carboxyhemoglobin half-life is biphasic and thus follows a bi-compartmental model.
-
•
A slight COHb rebound occurred when COHb returns to 5% and oxygen therapy is stopped.
-
•
Carboxyhemoglobin toxicokinetic profiles are in favor of extra vascular CO storage.
-
•
Carboxyhemoglobin half-life could be a potential CO poisoning severity criterion.
1. Background
Carbon monoxide (CO) is estimated to be responsible for approximately 40,000 deaths and significantly affects more than one million people per year worldwide[1], [2]. CO poisoning results in delayed neurological sequelae (DNS) at 6 weeks in 37% of cases[3]. It has been a major unresolved medical and public health problem for over 120 years[2]. Accidental CO poisoning in the US alone bears a cost to society of over $1.3 billion a year as a direct result of medical costs and lost earnings[4].
It is therefore essential that patients at risk of DNS are identified promptly so that effective early treatment can be proposed to avoid that risk. Nevertheless, the American College of Emergency Physicians (ACEP) has concluded that no clinical variables seemed to accurately identify severely poisoned patients for whom hyperbaric oxygen (HBO) therapy would be most likely to provide this benefit[5]. Furthermore, the exact role of HBO therapy and of its protocol adapted for the severity of the poisoning are still controversial[2]. Indeed, in the absence of reliable severity criteria that might otherwise allow us to compare homogeneous cohorts, it is difficult to demonstrate the superiority of one treatment over another[6], [7]. In this context, normobaric oxygen (NBO) therapy is considered as a therapeutic option to treat moderate CO poisoning, leading to an outcome that is comparable with that of HBO therapy[2], [6], [8], [9].
An individual's carboxyhemoglobin (COHb) level is easily measured in clinical practice but, unfortunately, publications confirmed poor correlations between an isolated COHb level and the severity of CO poisoning [10]. By contrast, in guidelines, a COHb level >25% has been retained as a potential severity criterion; but regardless of when the blood is drawn once CO exposure has stopped, what post-exposure treatment is applied, or of the CO exposure duration estimated, which makes it an unreliable criterion[7], [11], [12].
The assessment of a COHb level alone cannot be relevant because it does not take into account the complexity of CO pathophysiology[2], [13]. Approximatively 80% of the CO is bonded to hemoglobin (Hb) to form COHb. The remaining is bonded to other metalloprotein as myoglobin (Mb) for ≈15% to form carboxymyoglobin (COMb) and less than 5% to other compound stores as neuroglobin and cytochromes[14], [15], [16], [17]. This is considered as CO extravascular storage and could be correlated to CO poisoning severity, but it is not possible to technically evaluate this CO storage in clinical or experimental practice. Thus, the objective but not specific clinical selection criteria that tend to be used to judge severe CO poisoning include transient or prolonged unconsciousness, abnormal neurological signs, cardiovascular dysfunction, severe acidosis, or having been exposed to CO for >24 hours. These criteria, however, have not yet demonstrated their effectiveness as truly discriminating criteria[18]. It is therefore necessary to reexamine early severe criteria of CO poisoning[19].
Whereas an isolated level of COHb is of limited value, to date, the relevance of COHb toxicokinetics, i.e., the COHb half-life (COHbt1/2) estimation, as a specific early means of identifying CO storage and/or poisoning severity has not been accurately assessed by studies that compare treatments.
In the light of results obtained using a swine model, in the following we discuss two potential indices that could provide an indirect estimation of this CO storage that potentially reflect the severity of CO poisoning: first, the COHbt1/2 profile; and, secondly, a COHb rebound after COHb returns to 5% when treatment is stopped.
First, before evaluating the role of COHbt1/2 as a severity criterion in future prospective clinical studies comparing different treatments, it is necessary to confirm the right toxicokinetic model that corresponds to the accurate COHb elimination profile. Yet there is still active debate surrounding whether the COHbt1/2 toxicokinetic model is mono- or bi-compartmental[19], [20], [21], [22]. Only a few studies have mentioned biphasic COHbt1/2 elimination and these tend to do so only in atmospheric air-treated (AA) studies: on dogs (Godin, 1972), humans (Myhre, 1974), dogs again (Wagner, 1975), sheep (Shimazu, 1990 and confirmed in 2000), and human smokers (Croenenberg, 2007)[23], [24], [25]. To date, only two studies of NBO therapy have been published (both on dogs), and they contradict one another: Schwerma (1948) favors of a biphasic profile, while Sasaki (1975) favors a mono-compartmental profile[25], [26]. Additionally, based on a retrospective clinical study, Weaver's research published in 2001 favors a model of mono-compartmental elimination [21], [22]. More recently, Bruce (2006) published a mathematical model using data from an AA-treated human cohort modeled as ‘NBO-treated’ and demonstrated a bi-compartmental COHbt1/2 elimination[17].
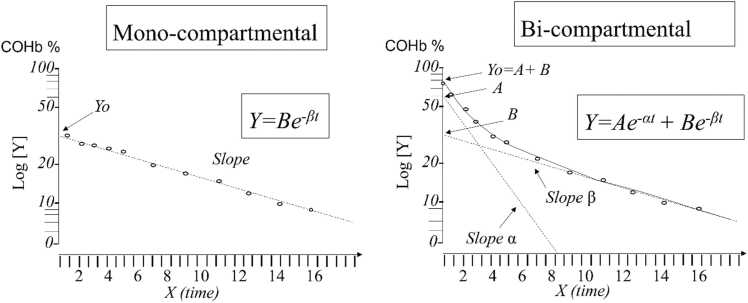
Theoretically, after cessation of CO exposure, a mono-exponential washout would be consistent with the hypothesis that CO is being removed from either a single vascular compartment or a vascular compartment in rapid equilibrium with extravascular tissues[27].
Then again, a bi-compartmental toxicokinetic model shows a biphasic COHb elimination profile and is consistent with the myoglobin CO affinity leading to the extravascular storage of CO. The initial rapid decrease (α-phase) with a steep slope (α-cst) is correlated to CO that is removed from COHb in blood and is exhaled by the lungs and is associated with a CO distribution to extravascular tissues as myoglobin (MbCO). This is followed by a slower phase (β-phase) with a softer slope (β-cst) that correlate with CO exhalation from intra- and extravascular compartments characterized by a slow transfer of CO between Mb and Hb [17], [20], [28].Fig. 1
Fig. 1.
COHb blood concentration vs time profiles during CO elimination on semi-logarithmic scale for CO which would exhibit a mono- or bi-compartmental model. Inspired by [19], [29], where ‘α’ is the distribution constant, ‘β’ is the terminal elimination constant. ‘A’ is the y-axis intercept for the α-phase and ‘B’ the y-axis intercept for the β-phase; ‘t’ is the time after the end of intoxication. COHbt1/2-α=0.693/α and COHbt1/2-β=0.693/β[19].
In concrete terms, breathing a high concentration of CO for a short period results in a high percentage of COHb but a short COHbt1/2 due to the low extravascular distribution of CO during this brief period of exposure [20]. On the opposite end of the spectrum, exposure to a lower CO concentration for a longer period will result in a lower COHb level, but will lead to a higher tissue CO burden with a longer COHbt1/2[17]. In guidelines, a CO exposure duration estimated to be at least 24 hours at any COHb level is considered a severity criterion and is a current selection criterion for HBO[7].
It seems that the duration and level of CO exposure (difficult to estimate in practice and rarely reported in clinical studies), CO body storage (not measurable in clinical practice) and the severity of CO intoxication (without reliable objective criteria for early identification) could be correlated. We hypothesize that the COHbt1/2, which is available in clinical practice, could be an indirect reflection of these three factors[11], [17], [19], [20], [21], [22], [28], [30].
Secondly, in addition to the demonstration of a bi-compartmental COHbt1/2 model, another objective argument in favor of CO storage would be to observe a significant COHb rebound after cessation of treatment once the COHb returns to <5%. This could in fact signify a delayed CO release from extravascular storage. To date, the behavior of COHb it returns to <5% has only rarely been explored in humans[31]. Only Anderson in 1978 suggested that if exposure was long enough to reach equilibrium, COMb could account for COHb rebounding to significant levels several hours after therapy, but no data from that study were published[32]. And, Dolan, citing Anderson, noted only that in patients who had received HBO therapy for severe CO poisoning until COHb was no longer detectable, COHb could be detected again several hours later, possibly as a result of the slow release of CO from tissular compartments. He therefore claimed that patients with severe CO poisoning could benefit from having a new COHb sample taken several hours following HBO[32], [33].
Furthermore, no systematic review, randomized control trials or cohort studies indicate the optimal duration of NBO 100% O2 treatment after moderate CO poisoning[34]. Even O2 itself has not been rigorously evaluated as a therapy for decreasing the side effects of CO poisoning and may not be devoid of O2-induced side effects[35], [36], [37], [38], [39]. The duration of NBO treatment should therefore be adapted as accurately as possible and be correlated to the severity of the poisoning without over-treating the patient in order to avoid DNS. Objective arguments to balance NBO dose (level and duration) are needed. In clinical practice, to date, in NBO, the usual therapeutic approach for CO poisoning is empirical and recommends the use of continuous O2 therapy for at least 6–12 hours or until COHb levels fall to <5% or even ≤3%, considered as a safe level[7], [9], [11], [21], [34], [40], [41], [42]. Following this, further evaluation of COHb levels is described as unnecessary, but this strategy has not been investigated in detail[7].
If COHb rebound occurs after treatment has ceased, the clinical implication should be continuous 100% NBO beyond the time just needed to return to 5% COHb, with an objective argument of opting for a treatment time of up to 6 or 12 hours in order to remove potential CO extravascular storage that is clinically unmeasurable. This treatment duration might have an impact not only on the patient’s outcome, but also on the length of their hospital stay and the costs incurred in and by emergency departments. Optimizing the duration of NBO therapy is also supported by recent studies indicating that CO, once stored in the brain, is more difficult to eliminate from here than it is from other tissues [43].
Using a swine model, therefore, our goal was first to reach a conclusion regarding whether our COHbt1/2 toxicokinetic model is mono- or bi-compartmental. The correlation between an accurately calculated COHbt1/2 and the severity of CO poisoning could then be explored in future prospective studies. Our second goal was to study the COHb profile one hour after the cessation of NBO treatment in order to explore a potential late release of CO after a return to a COHb level of <5%.
2. Materials & methods
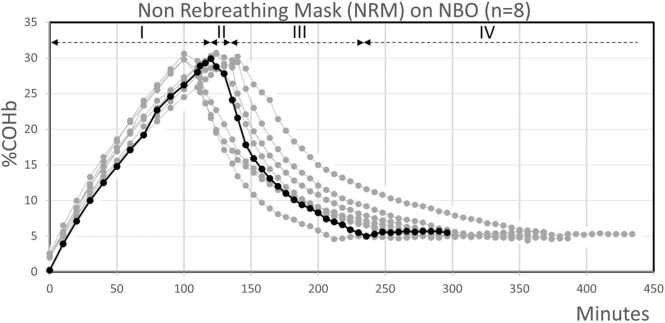
Based on a previously published study investigating the half-life COHb elimination (COHbt1/2) using a model of swine treated testing several normobaric oxygenation (NBO) methods, we aimed first to investigate whether the COHb toxicokinetic model is to be understood as mono- or bi- compartmental, and, secondly, to assess the COHb late terminal behavior when COHb has dropped below 5% and treatment has been stopped. The experimental conditions are outlined in a previous paper[44]. The study was approved by the Université Catholique de Louvain’s ethics committee for animal experimentation (UCL, Brussels, Belgium) (ref 2013/UCL/MD/001 and ref 2015/UCL/MD/28). Briefly, eight pigs (25 experimentations) were acutely exposed to CO at 0.094% or 940 ppm until the COHb level reached 30%. During the treatment phase, their COHbt1/2 were then investigated on atmospheric air, or using NBO therapies with different devices (non-rebreathing mask (NRM), continuous positive airway pressure (CPAP), non-invasive positive airway pressure (NiPPV) on two different devices (NiPPV-V and NiPPV-L) delivering %FiO2 at 72 [59–83]; 54 [49–73]; 51 [51–53] and 92 [76–93], respectively). Our main objective was to assess the COHbt1/2 until COHb level reached 5%. This toxicokinetic analysis was focused on phase III of the experiment as shown in Fig. 2., which, as an illustration of the protocol, includes only the eight procedures using NRM.
Fig. 2.
COHb samples during CO exposure and the NRM treatment arm on eight swine[44]. For the example, the curve for one of the eight experiments on NRM (in bold) is split into the four phases of the protocol (dashed black horizontal lines). Phase I: Median CO exposure time to reach COHb at 30% being 118 min [±10.4]; Phase II: no treatment (AA) for 10 min; Phase III (treatment): Median time needed to reach COHb at 5% on NRM being 163.2 min [±15.3]. This time varied on other oxygenation/ventilation modalities (not included in Fig. 2; see Table 1); Phase IV: last hour only on AA to verify occurrence of a percentage COHb rebound. From[44].
Firstly, we assessed the toxicokinetic COHbt1/2 model using the experimental COHb values obtained during washout under NBO in phases III (n=21). We plotted COHb values as percentages (%) on a log scale vs. time on a linear scale, using the linear regression method with the least square method[17]. To decide on the best model, we compared the models using the Bayesian information criterion (BIC).
Secondly, we also collected data during the final phase (phase IV) on AA (Fig. 2). When any therapy was stopped because the COHb level target had reached 5%, blood was sampled every 6 min for 60 min[44]. Our analysis focused on potential COHb rebound during phase IV. In the case of no COHb decay, COHbt1/2 would be impossible to assess. We therefore tested whether data corresponded to a quadratic model that is used to assess the importance of a potential rebound. In order to assess this rebound, we did not consider swine that were immediately on AA after CO exposure without NBO treatment (the control group; n=4).
All analyses were performed using JMP statistical software version Pro 16.0.0 (SAS Institute Inc., Cary, NC, USA). To gain functional understanding of the COHb response following the different treatments stopped, polynomial regression models using the least square method were fitted to the data. Three mathematical expressions (i.e., linear, quadratic and exponential) were tested to mathematically shape the evolution during phase IV. Corrected-Akaike criterion (AICc) and r² values were assessed to compare and evaluate the adequacy of the models tested. In addition, we performed a visual inspection of the residuals to exclude any potential bias. The best fit was selected based on a 2 AICc improvement (lower scores) when comparing 2 models. In any case, p<0.05 was considered to be statistically significant.
3. Results
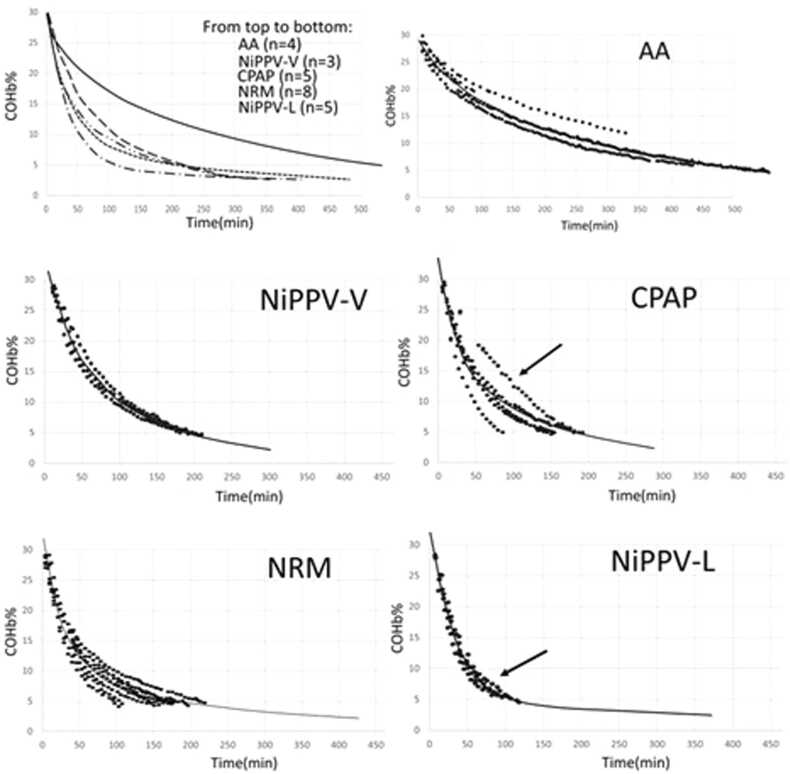
With regard to our first question, concerning whether we observed a mono- or bi-compartmental toxicokinetic model on this swine model, overall, for all treatments, a bi-compartmental model provided the best fit for our data (▲BIC compared to mono-compartment=−302.8) (Fig. 3; Table 1). When analyzing the data for each pig individually, the bi-compartmental model was the most appropriate, except in the case of two experiments on two different pigs (Fig. 3).
Fig. 3.
Bi-compartmental COHbt1/2 elimination observed during ‘treatment phase III’ with all treatments; AA; NiPPV-V; CPAP; NRM; NiPPV-L. COHb elimination curves for each treatment suggest individually that the distribution and elimination of CO from the blood are best explained by a bi-compartmental model, with the exception of the two experiments marked with arrows.
Table 1.
Comparison between different toxicokinetic approaches (as seen in Fig. 1; right) on different O2 therapy modalities compared to median COHbt1/2 (min)* using mono-compartmental analysis in the previous study which, despite a lack of statistical power(p=0.18), favored NiPPV-L (58 min, n=5) as opposed to NRM (85 min, n=8) [44].
| NRM | CPAP | NiPPV-V | NiPPV-L | AA | All | |
|---|---|---|---|---|---|---|
| COHbt1/2from (*) | 85 [46–116] | 82 [40–94] | 93 [92–113] | 58 [52–79] | 251 [130−273] | |
| COHbt1/2-α | 56.2 ±16.2 | 34.03 ±13.5 | 26.9 ±5.0 | 23.6 ±3.9 | 41.6 ±5.7 | 39.4 ±6.2 |
| COHbt1/2-β | 70.0 ±22.1 |
99.6 ±17.3 |
120.6 ±10.0 |
174.7 ±63.8 |
310.5 ±23.5 |
141.8 ±22.4 |
| α-cst | 0.022 ±0.0055 |
0.0032 ±0.0084 |
0.028 ±0.0047 |
0.0032 ±0.0042 |
0.018 ±0.0032 |
0.026 ±0.0027 |
| β-cst | 0.026 ±0.0079 |
0.0081 ±0.0021 |
0.0058 ±0.00053 |
0.0053 ±0.0013 |
0.0023 ±0.00015 |
0.012 ±0.0034 |
| A | 15.7 ±0.8 | 16.0 ±3.4 | 12.3 ±1.8 | 21.6 ±2.0 | 6.6 ±0.4 | 15.1 ±1.2 |
| B | 12.7 ±1.0 | 15.1 ±1.4 | 17.5 ±1.7 | 8.7 ±1.2 | 21.2 ±1.1 | 14.5 ±1.0 |
| A/B ratio | 1.33 ±0.18 | 0.90 ±0.20 | 0.73 ±0.15 | 2.56 ±0.64 | 0.32 ±0.036 | 1.21 ±0.19 |
| AUC0-∞ | 2614.8 ±231.2 |
2716.3 ±368.0 |
3505.0 ±173.0 |
2263.6 ±340.4 |
9946.5 ±1032.6 |
3844.8 ±579.1 |
| AUC0−t | 1849.1 ±169.3 |
2041.9 ±312.6 |
2661.2 ±144.5 |
1292.7 ±42.0 |
6574.5 ±525.4 |
2629.9 ±376.0 |
| Time to reach 5% | 163.2 ±15.3 | 168.7 ±22.4 | 217.1 ±5.9 | 110.15 ±4.1 | 647.9 ±64.0 | 237.7 ±38.7 |
In answer to our second question regarding COHb rebound, on more than a thousand COHb assessments during all treatments over the phase III period (COHb decay from 30% to 5%), absolutely no rebound was observed at any moment, not even 0.1% COHb (Fig. 2. Phase III)[44]. However, over the first hour after termination of the different NBO methods (n=19), once COHb had returned to 5%, a steady state of COHb level, or even a slight rebound, was observed. The profile curves methodologically did not allow for a calculation of COHbt1/2 value (Fig. 4).
Fig. 4.
After treatment phase III on NRM, CPAP, NiPPV-V or NiPPV-L: %COHb evolution during phase IV, the additional hour without any treatment with blood samples taken every 6 min (11 samples for each) (n=19).
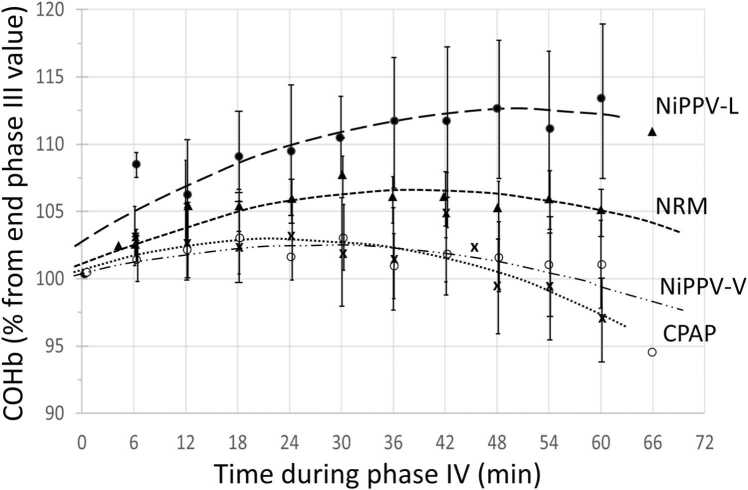
The quadratic model fitted the data the best (ΔAICc=−10.04 compared to the linear model, r²=0.08, p=0.0003) and the plot of the residuals did not reveal any particular bias. This observation suggests that a parabola is a better fit for our dataset than a linear or exponential model. The fit was improved when the treatment was introduced in the quadratic model as an independent factor (ΔAICc=−43.03 compared to the model without covariates, r²=0.33, p<0.0001), reflecting that the shape of the curve depends on the treatment previously received. Compared to other treatments, the strongest rebound was observed in NiPPV-L (p<0.0001; Figs. 4 and 5).
Fig. 5.
Phase IV. COHb expressed in percentage from last measured value of Phase III (range 4.5–5.2% considered individually as 100%) according to time post-phase III (min). This phase IV is the last hour without any intervention (on AA). Data are expressed as means ± SD. The lines represent the quadratic regression model according to each treatment. This allows us, firstly, to homogenize the starting values, to compare curves and to better visualize the evolution of %COHb if it continues to fall as in treatment during phase III or if it stabilizes or even rises in phase IV.
4. Discussion
While analyzing the COHbt1/2 profiles of swine exposed to CO and treated on NBO, the COHbt1/2 is biphasic following a bi-compartmental toxicokinetic model. This confirms Shimazu's findings from studying sheep treated using atmospheric air and contrasts with Weaver’s conclusions drawn in a retrospective clinical study of human patients. This bi-compartmental CO distribution suggests an extravascular CO storage.
Moreover, while NBO treatment for CO poisoning (≈118 min) at COHb 30% brought COHb down to 5% and was stopped, during one hour on atmospheric air, %COHb failed to decrease. On the contrary, we observed a slight rebound of COHb, which also accords with potential extravascular CO storage, meaning that further consideration of the duration of NBO treatment is necessary.
4.1. On the bi-compartmental model
In a previous study, we considered a non-compartmental assessment to avoid ambiguity regarding mono- vs bi-compartmental models and to compare the data with clinical studies suggesting the mono-compartmental COHbt1/2 toxicokinetic model[21]. In this much more detailed data analysis, which includes a large number of samples, the bi-compartmental model was confirmed for all experiments, except for two; in these two experiments, a mono-compartmental model was applied because it provided the best fit for the data (arrows in Fig. 3). Indeed, and in a very interesting way, one of the pigs treated with NiPPV (NiPPV-L-4) showed such a fast early COHbt1/2 that the second β-phase did not appear. Conversely, for one of the swine treated with CPAP (CPAP-1), due to a technical problem, CO values from 6 to 48 min were not collected and the α-phase could not be modeled. This is consistent with Weaver’s clinical study where only few late samples were made[21]. As already developed by Shimazu (bi-compartmental model observed on AA-treated sheep) and Weaver (mono-compartmental model observed in NBO-treated humans) and supported later by Bruce (bi-compartmental model observed in a mathematical model developed from data on AA-treated humans), the argument between mono- vs bi-compartmental COHb elimination is a methodological one[17], [20], [22]. An early rapid fall with shorter COHbt1/2 (α-phase) is probably not observed in Weaver’s retrospective clinical study, in which early COHb measurements are missing, and is quickly followed by a second longer COHbt1/2 (β-phase) that is more significant[20]. Early sampling is most often lacking in clinical practice during the α-phase, which is concomitant with the removal of the patient from the toxic environment, transport by ambulance, and/or admission to the emergency department. Usually, only two or three samples are taken from a patient with CO poisoning. As a result, we cannot observe a precise COHbt1/2 and are even less able to observe a biphasic character[3].
Further factors could generate confusion. Mono-compartmental elimination could be observed in the case of bi-compartmental distribution if the intravascular compartment is in rapid equilibrium with the extravascular tissues[27]. In the context of CO, MbCO levels fall more slowly during washout than the COHb, even if CO links Mb with a lower relative affinity ‘M’ constant than for Hb (39 compared to 218, respectively). The slow rate of exchange of CO between blood and muscle tissues, compared to that of O2, results from both the blood-tissue conductance for CO and the pressure difference driving this flow[28]. Therefore, the mono-compartmental model is theoretically not expected[45], [46], [47]. Conversely, a bi-compartmental elimination could be observed in spite of a mono-compartmental elimination if some peripheral organs are poorly perfused[20]. In this swine model, blood pressure, heart frequency and lactate were continuously monitored and physiological values remained steady. Thus, these results supported a bi-compartmental COHbt1/2 toxicokinetic model.
In retrospective clinical studies, COHbt1/2 is calculated on the basis of only two COHb blood samples collected at random times (t1 and t2) where COHbt1/2 = (t2-t1) x ln[2] ÷ ln(COHbt1 - COHbt2). This ‘two point method’ assumes that COHb follows a mono-exponential elimination[3], [17], [48]. Bruce warned against this method. He demonstrated on AA-treated humans (n=12) significant COHbt1/2 discrepancies depending on the time of sampling. COHbt1/2 was assessed at 208±78 min; 275±75 min or 358±73 min if the two blood samples considered were collected at five and 67 min (i.e., pre-hospital transport time estimated to be the rapid fall α-phase); 67 and 131 min (i.e., moment of admission to emergency department) or 131 and 259 min (i.e., during potential waiting before entry in HBO chamber estimated to be the slow fall β-phase), respectively. Nevertheless, using a bi-compartmental model including all samples and thus reflecting real COHb toxicokinetics, a biphasic COHbt1/2 was assessed at 236.2 ±34.4 min for the first α-phase and 302.3±38.9 for the β-phase[17]. In this swine model, we compared COHbt1/2 assessed on mono- and bi-compartmental models highlighting the same discrepancies (Table 1). Different analyses are shown in this table to illustrate the variety of comparative toxicokinetic analyses possible between the different treatments studied. Moreover, the results for the α-phase may well have been underestimated because, in order to mimic ‘real’ life, the protocol included an initial 10 minute post-exposure period without any treatment (AA) between the end of CO poisoning and the start of treatment (Fig. 2); this was intended to replicate the victim’s removal from the toxic environment and the arrival of emergency services [44].
In addition, the impact of the specific characteristics of the absorption phase (CO exposure level and duration) have been poorly studied [49]. While the reference studies such as Peterson's (human experimental study, 1970) reported a COHbt1/2 at 320 min on AA or Weaver’s study on NBO (human clinical study, 2001) published a COHbt1/2 at 74±25 min (mean±SD), both present a very wide range from 128 to 409 min (n=39) and from 26 to 148 min (n=93), respectively [21], [50]. For Peterson, variations in COHbt1/2 did not appear to be related to the duration of exposure or to the concentration inhaled but no assessment was reported[50]. Weaver accepted that COHbt1/2 may be affected by CO exposure duration and dose but provided no exploration of the matter [2], [22]. Bruce used modelling data to estimate COHbt1/2-α and -β to be 20 and 15% longer, respectively, for a long exposure to low CO concentrations compared to a shorter exposure to higher CO concentrations [17].
These wide COHbt1/2 ranges might be explained: (a) by variations in ventilation, although ventilation is not impacted during a moderate CO poisoning; (b) exclusively by an inter-individual variability, but this is not suggested in Benignus's study [17], [51]; or (c) by the uncertain FiO2 level administered which, moreover, is seldom reported in clinical studies. Indeed, the adequate installation of the NRM exercises a significant influence by varying the delivered FiO2 and sometimes there is an interval between the end of CO exposure and O2 treatment initiation (1.2±2.5 hours) [3]. Further explanations for the wide COHbt1/2 ranges include (d) the random sampling times or the lack of samples inherent in retrospective studies; but, in the end, we hypothesize that (e) COHbt1/2 discrepancies could also correlate to the absorption phase due to heterogeneity of duration and level of CO exposure resulting in different severities of CO poisoning and therefore potentially requiring different treatments[17]. To test the COHbt1/2 relevance as a severity criterion in future clinical studies, however, it was necessary to confirm the correct bi-compartmental toxicokinetic model to apply [52].
This biphasic nature of the COHbt1/2 curve does not seem altered by the various factors that might affect the COHbt1/2 value, such as peak COHb level, CO exposure concentration, CO duration or FiO2 level supply [17], [19], [53]. This last is in accordance with this study where results confirm that the biphasic profile is not influenced either by the different devices used or the FiO2 supply. Unfortunately, the number of animals included in the study made it impossible to test and to confirm the constancy of the biphasic profile after different COHb levels had been reached or under different CO exposure protocols (CO exposure level x duration). In this swine protocol, CO the exposure time target did not last the four to five hours necessary to reach the COHb plateau phase corresponding to the equilibrium between CO absorption and elimination[44]. However, this biphasic elimination observed after 118 min SD±10.4 CO exposure, considered as an intermediate time exposure, was also observed after short exposure (<6 min) as shown in Wagner, Godin or Shimazu in experimental dog or sheep studies. By contrast, in cases of longer CO exposure, Wagner puts forward that the distribution phase could not be observed since the CO distribution may have been almost complete in the muscle tissue[52]. However, COHb elimination after long CO exposure to moderate CO levels (±500 ppm), as seen in the studies on human smokers or sheep exposed for 5 or even 10 hours, also confirmed a biphasic elimination, even if this was more discreet[17], [19], [53].
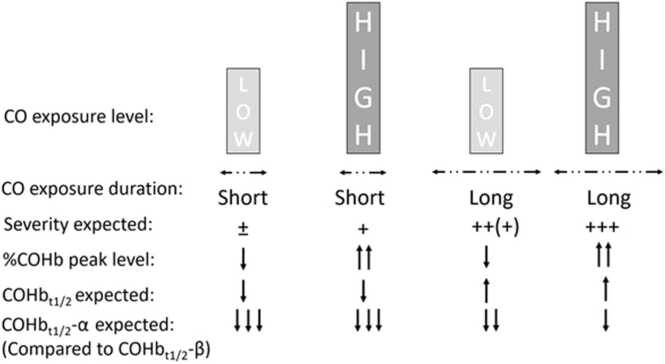
Globally, a longer COHbt1/2 would be observed in cases of longer CO exposure allowing higher CO tissue storage regardless of high or low CO exposure concentrations and should be considered as more severe poisoning[49]. It will be necessary to establish an average threshold of what would be considered a long or short COHbt1/2. The current COHbt1/2 reference is an average of 74 minutes but is assessed on the mono-compartmental model[21]. And, at this stage, no COHbt1/2-α or -β, references for human on NBO are known except for estimations using mathematical models with COHbt1/2-α at 86.9 ±10.5 min and COHbt1/2-β at 160.7±11.9 min[17]. It would seem that COHbt1/2 discrepancies issues arising from CO exposure characteristics are more pronounced for the α-phase than for the β-phase[21], [49], [54]. Moreover, COHbt1/2-α seems to be lower as the %COHb peak level increases, regardless of whether this higher %COHb level was due to a longer CO exposure or a higher inhaled CO concentration[17]. Fig. 6 provides an overview of the relationships between exposure conditions, perceived CO poisoning severity and COHb elimination profile.
Fig. 6.
Global overview of the impact of CO exposure criteria (duration x level) on the peak %COHb and COHbt1/2 expected. This could approximate severity CO poisoning in relation to potential CO storage. Pending future studies, the two reference thresholds that could be considered as severity criteria are COHb peak >25% and COHbt1/2 > 74 min[12], [21], [22].
In addition, to illustrate the importance of bicompartmental analysis, the different toxicokinetic approaches described in Fig. 1 can be detailed (Table 1). In this study design used a similar protocol for all experiments (940 ppm x 118 min [±10.4]) and no significant difference was observed for COHbt1/2-α suggesting that distribution of CO occurs with similar intensity whatever the treatment (Table 1). By contrast, the associated A-constant reflecting the y-axis intercept for the α-phase was significantly higher for NiPPV-L, CPAP and NRM when compared to AA with values of 23.6%, 16.0%, 15.7% and 6.6%, respectively (p=0.0004, 0.03 and 0.02 respectively). And, B-associated constant was significantly lower in the NiPPV-L group when compared to AA (p<0.0001), NiPPV-V (p=0.003) and CPAP (p=0.02) and not significant when compared to the NRM treated group (p=0.13). This observation reflects a smaller contribution of the β-phase when compared to the α-phase for %COHb decrease in NiPPV-L (8.7% versus 21.6%, p=0.02). For other groups of treatment, the differences between A-constant and B-constant are not significant except for AA-treated pigs for which B-constant (21.2%) is significantly higher than α-constant (6.6%), (p=0.002). To demonstrate this relative importance of both elimination phases, it’s interesting to compute the ratio of A- and B- constants. NiPPV-L treated pigs had a significantly higher ratio A/B than every other group, corroborating the observation that the α-phase contributes more to the elimination of COHb in NiPPV-L treated pigs than in every other groups. This benefit in favour of NiPPV-L is also supported by the fact that the time to reach 5% COHb was the lowest in NiPPV-L treated pigs (110.1 min), despite not reaching significant difference with other treatment groups (p=0.15 with NIPPV-V [217.1 min], p=0.55 with CPAP [168.7 min], and p=0.55 with NRM [163.2 min]. Supporting these observations, similar differences are also observed when comparing AUC0−t values which reflect the total exposure to COHb till time to reach 5%. This is why it might be interesting to investigate in future clinical studies, not only the two elimination phases separately, COHbt1/2-α and COHbt1/2-β, but also their A/B ratio and AUC0−t.
Assessment of the COHb biphasic elimination could thus allow us to open up the field for further investigations. We encourage a systematic evaluation of this COHbt1/2 from several venous COHb samples taken during treatment, including very early samples gained during the patient's transport in an ambulance. COHbt1/2 might accurately reflect the CO burden and, therefore, the real severity of CO intoxication. Nevertheless, whatever the reason for having an extended COHbt1/2, the treatment modalities or duration could be adapted to consider a higher COHbt1/2 as a criterion of CO poisoning severity. This COHbt1/2 should be considered as a potential criterion for inclusion and comparison in future clinical studies, in which it is essential to homogenize the cohorts compared under different treatments[2].
4.2. On COHb rebound
In our previous study, visually and without statistical analysis regarding this secondary point, we prematurely concluded that there was no COHb rebound. But, by focusing statistical analysis accurately on this point, a rebound up to 1 h after the treatment had ended was demonstrated as significant[44]. As discussed above and according to biphasic CO elimination, this rebound could result from significant extravascular CO storage[47], [55], [56]. In practice, these extravascular bindings could then delay the complete elimination of CO but, unfortunately, the estimation of this CO burden level is not accessible in clinical investigations[17], [20], [47], [50]. Thus, a rebound and its level of importance could reflect the severity of CO poisoning and suggests the use of ongoing treatment when COHb returns to 5% and for a minimum of 6 hours following exposure.
In this protocol, not having reached the five hours of CO exposure time necessary to reach a %COHb equilibrium state between absorption and elimination[19], [57], at the end of treatment, a %COHb remaining at 5% for one hour or even a modest COHb rebound might not be expected. Nevertheless, rebound is systematically observed albeit subtly. The CO body burden therefore seems to occur even as a result of a moderate duration of CO exposure. This rebound could be more significant in cases of longer exposure, even if this extravascular CO only accounts for up to 15% of the global CO body burden[15]. Nevertheless, we are not able to estimate the clinical impact of this CO burden on other metalloprotein-binding CO such as cytochromes or neuroglobin which could be significant, even in small amounts.
Another perspective could be that the fall of arterial pO2 at the end of treatment (switching to AA after phase III) could allow more free CO in the blood to bind to Hb and thus cause %COHb to increase. Bruce observed this phenomenon in his mathematical model when switching from AA to O2 therapy in the early phase of treatment[17], [28]. Nevertheless, the Haldane constant (M-value) at 218 means that Hb has 218-fold more affinity for CO than O2[46]. But, more accurately, the overall affinity for a gas is its equilibrium constant (KEq) corresponding to the ratio between its association and dissociation constant being, respectively, Ka and Kd (KEq=Ka/Kd). M-value is the ratio KEq CO/ KEq O2. In the case of CO, what explains the high global affinity of Hb for CO is not a fast linking of CO to Hb compared with O2, but a very slow COHb dissociation (kd≈0.008 s−1), i.e., 2500 times slower than HbO2 (kd≈20 s−1)[58], [59]. Thus, ventilation, which did not change between the switch from O2 to AA therapy, should have enough time to eliminate the free CO already in the blood and the CO slowly released from COHb on AA when treatment stopped. For this reason, this COHb rebound presents an argument in favor of a delayed CO release from extravascular CO storage and not from a new uptake of CO previously released by Hb during O2 therapy.
Moreover, as briefly suggested by Wazawa on AA-treated rabbits, we could hypothesize that this slight rebound may arise as a result of the endogenous CO production resulting from the physiological hemoglobin catabolism, which contributes to the physiological basal COHb rate from 0.4% to 0.8%[20], [60]. Nevertheless, this production is continuously present and in the order of 6.1 µl/h/kg or 0.007 ml/min in humans of similar weight to the swine used in this study (±80 kg). Hemoglobin catabolism should therefore produce only ±0.5 ml CO during the hour of observation. So, it does not seem consistent that the observed CO rebound corresponds exclusively to endogenous CO production. Furthermore, rebound from a tissular storage is also observed in cases of lithium intoxication once hemodialysis has removed circulating lithium. In this context, serum concentrations often rebound, so repeated or prolonged treatment may be required[61]. In our opinion, whatever the reason for this rise or stagnation of COHb, it might be wise to continue NBO beyond the time necessary to reach a %COHb level of 5% as long as COHbt1/2 does not commence its normal decrease under AA.
This therapeutic point of view is in accordance with the theoretical complete clearance (±97%) of COHb expected after five-fold COHbt1/2 (5 ×74 ±25 min [26−148]). The theoretical total O2 treatment time could then be estimated at 370 min (±6 h), but 12 hours if we consider the extreme range at 148 min[62]. Despite this, in clinical practice, the decision to stop O2 therapy is taken when COHb is estimated to be below 5% and the patient is asymptomatic[34]. The duration of 100% normobaric O2 therapy seems to vary depending on empiric decisions or residual subjective symptoms. To illustrate this point, recent prospective clinical studies examined the influence of CPAP or NRM on COHbt1/2 for CO poisoned patients with a NBO treatment duration targeting only a COHb return close to 5%. It was reported that patients were discharged from ED after 127 minutes in the CPAP group and 201 minutes in the NRM group[63]. It is also interesting to note that in half of the significant studies comparing NBO to HBO selected in meta-analyses assessing HBO efficiency, NBO was only administered for less than or equal to 6 hours[6], [42]. This could be a significant bias in these comparative studies and highlights the heterogeneity of treatment strategies. Moreover, among these few studies selected by meta-analyses which provided an NBO of >6 h, none identified a benefit of HBO[42], [62], [64]. Ideally, for severe CO poisoning, a homogenous NBO duration, probably 12 hours, should be achieved and compared with HBO.
Based on [1] this COHbt1/2 bi-compartmental toxicokinetic evidence, [2] a rebound objectively observed for the first time, [3] the mathematical model suggesting an extravascular CO store and [4] waiting for future clinical impact assessments of CO body burden, we confirm the suggested strategy of providing a minimum of 6 hours NBO for all cases of CO poisoning. If the decision to use NBO rather than HBO is made, conditions the for stopping NBO after 6 hours could be: particular attention to delivered FiO2 close to 1.0 (optimal NRM placement); a CO exposure duration estimated at less than five hours; COHbt1/2-α very low compared to COHbt1/2-β; an estimated COHbt1/2-β of less than or close to 74 minutes; and an asymptomatic patient. Otherwise, there are arguments for a higher CO body burden level and to continuing treatment for up to 12 hours.
5. Conclusion
This experimental model that subjected swine to moderate CO poisoning confirms that the COHb half-life is biphasic and thus follows a bi-compartmental model. It also provides the first observation of a slight COHb rebound when COHb returns to 5% and oxygen therapy is stopped. On the basis of these two toxicokinetic profiles in favor of extra vascular CO storage, it suggests that future studies might consider the COHbt1/2 from multiple samples and, using a bi-compartmental model, assess it as a potential criterion for discerning the severity of CO poisoning. Nevertheless, this relationship between the COHb elimination profile and/or the COHbt1/2 value as a reflection of CO storage and/or the potential use of the COHbt1/2 value as a criterion for estimating the severity of CO poisoning remain to be confirmed. The significant goal is to homogenize cohorts of the same severity in further large multicenter randomized clinical trials regarding the management of CO poisoning. Furthermore, the extent of COHb rebound needs to be assessed on other CO exposure protocols in order for it to be confirmed as well as its correlation with CO storage. While waiting for further studies to emerge, our findings suggest that a treatment lasting less than 6 hours does not seem to be adapted for any case of moderate CO poisoning.
CRediT authorship contribution statement
Nicolas Delvau: Conceptualization,Investigation, Methodology, Writing - original draft, Writing - review &editing. Laure Elens: Formal analysis, Software. Andrea Penaloza: Writing -original draft. Giuseppe Liistro: Writing - review &editing. Frederic Thys: Writing - review &editing, Resources. Pierre-Marie Roy: Writing - review &editing, Resources. Pierre Gianello: Resources. Philippe Hantson: Writing -review &editing, Supervision. All authors gave final approval of the present manuscriptprior to submission.
Funding
This study was supported by the Saint-Luc Foundation and the Experimental Surgery and Transplantation unit (CHEX, Université Catholique de Louvain,UCL, Brussels, Belgium).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Delvau Nicolas reports financial support was provided by Vygon. Delvau Nicolas reports financial support was provided by Saint-Luc Foundation.
Acknowledgments
The authors wish to thank Sabine Jaumotte (Radiometer©, Medical Aps, Denmark) for her essential technical support and the nurses from the emergency unit (Cliniques universitaires Saint-Luc, UCL, Brussels, Belgium) for their assistance and invaluable help. We also want to thank Professor Eugene N. Bruce for his feedback on the interpretation of the COHb rebound, which was highly appreciated.
Handling Editor: Lawrence Lash
Data Availability
Data will be made available on request.
References
- 1.Mattiuzzi C., Lippi G. Worldwide epidemiology of carbon monoxide poisoning. Hum. Exp. Toxicol. 2020;39(4):387–392. doi: 10.1177/0960327119891214. [DOI] [PubMed] [Google Scholar]
- 2.Juurlink D. Hyperbaric oxygen should not be used routinely for carbon monoxide poisoning: CON. Authorea Preprints. 2022. [DOI] [PubMed]
- 3.Weaver L.K., Hopkins R.O., Chan K.J., Churchill S., Elliott C.G., Clemmer T.P., et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N. Engl. J. Med. 2002;347(14):1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 4.Hampson N.B. Cost of accidental carbon monoxide poisoning: A preventable expense. Prev. Med Rep. 2016;3:21–24. doi: 10.1016/j.pmedr.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf S.J., Maloney G.E., Shih R.D., Shy B.D., Brown M.D. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute carbon monoxide poisoning. Ann. Emerg. Med. 2017;69(1):98–107. doi: 10.1016/j.annemergmed.2016.11.003. e6. [DOI] [PubMed] [Google Scholar]
- 6.Buckley N.A., Juurlink D.N., Isbister G., Bennett M.H., Lavonas E.J. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst. Rev. 2011;2011(4):Cd002041. doi: 10.1002/14651858.CD002041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampson N.B., Piantadosi C.A., Thom S.R., Weaver L.K. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am. J. Respir. Crit. Care Med. 2012;186(11):1095–1101. doi: 10.1164/rccm.201207-1284CI. [DOI] [PubMed] [Google Scholar]
- 8.Casillas S., Galindo A., Camarillo-Reyes L.A., Varon J., Surani S.R. Effectiveness of hyperbaric oxygenation versus normobaric oxygenation therapy in carbon monoxide poisoning: a systematic review. Cureus. 2019;11(10) doi: 10.7759/cureus.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logue C.J. An inconvenient truth? Ann. Emerg. Med. 2008;51(3):339–340. doi: 10.1016/j.annemergmed.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Hampson N.B., Hauff N.M. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am. J. Emerg. Med. 2008;26(6):665–669. doi: 10.1016/j.ajem.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Weaver L.K. Clinical practice. Carbon monoxide poisoning. N. Engl. J. Med. 2009;360(12):1217–1225. doi: 10.1056/NEJMcp0808891. [DOI] [PubMed] [Google Scholar]
- 12.Weaver L.K., Valentine K.J., Hopkins R.O. Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am. J. Respir. Crit. care Med. 2007;176(5):491–497. doi: 10.1164/rccm.200701-026OC. [DOI] [PubMed] [Google Scholar]
- 13.Goldbaum L., Orellano T., Dergal E. Mechanism of the toxic action of carbon monoxide. Ann. Clin. Lab. Sci. 1976;6(4):372–376. [PubMed] [Google Scholar]
- 14.Stewart R.D. The effect of carbon monoxide on humans. Annu. Rev. Pharmacol. 1975;15(1):409–423. doi: 10.1146/annurev.pa.15.040175.002205. [DOI] [PubMed] [Google Scholar]
- 15.Coburn R.F. The carbon monoxide body stores. Ann. N. Y Acad. Sci. 1970;174(1):11–22. doi: 10.1111/j.1749-6632.1970.tb49768.x. [DOI] [PubMed] [Google Scholar]
- 16.Penney D.G. CRC Press; 2000. Carbon monoxide toxicity. [Google Scholar]
- 17.Bruce M.C., Bruce E.N. Analysis of factors that influence rates of carbon monoxide uptake, distribution, and washout from blood and extravascular tissues using a multicompartment model. J. Appl. Physiol. 2006;100(4):1171–1180. doi: 10.1152/japplphysiol.00512.2005. [DOI] [PubMed] [Google Scholar]
- 18.Rose J.J., Nouraie M., Gauthier M.C., Pizon A.F., Saul M.I., Donahoe M.P., et al. Clinical outcomes and mortality impact of hyperbaric oxygen therapy in patients with carbon monoxide poisoning. Crit. care Med. 2018;46(7) doi: 10.1097/CCM.0000000000003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazu T., Ikeuchi H., Sugimoto H., Goodwin C.W., Mason A.D., Jr., Pruitt B.A., Jr. Half-life of blood carboxyhemoglobin after short-term and long-term exposure to carbon monoxide. J. Trauma. 2000;49(1):126–131. doi: 10.1097/00005373-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Shimazu T. Half-life of blood carboxyhemoglobin. Chest. 2001;119(2):661–663. doi: 10.1378/chest.119.2.661. [DOI] [PubMed] [Google Scholar]
- 21.Weaver L.K., Howe S., Hopkins R., Chan K.J. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117(3):801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 22.Weaver L.K., Howe S., Hopkins R., Chan K.J. Half-life of blood carboxyhemoglobin. Chest. 2001;119(2):662–663. doi: 10.1378/chest.119.2.661. [DOI] [PubMed] [Google Scholar]
- 23.Shimazu T., Ikeuchi H., Hubbard G.B., Langlinais P.C., Mason A.D. Smoke inhalation injury and the effect of carbon monoxide in the sheep model. Army Inst Surgical Res. Fort Samhouston. 1990 doi: 10.1097/00005373-199002000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Godin G., Shephard R. On the course of carbon monoxide uptake and release. Respiration. 1972;29(4):317–329. doi: 10.1159/000192905. [DOI] [PubMed] [Google Scholar]
- 25.Schwerma H., Wolman W., Sidwell Jr A., Ivy A. Elimination of carbon monoxide from the blood of acutely poisoned dogs. J. Appl. Physiol. 1948;1(5):350–363. doi: 10.1152/jappl.1948.1.5.350. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T., Igarashi A., Okonogi K., Ohiwa H. Study on Half-Clearance Time of Carbon Monoxide Hemoglobin in Blood at Hyperbaric Oxygen Therapy (OHP) Nippon Eiseigaku Zasshi (Jpn. J. Hyg. ) 1975;30(3):387–396. doi: 10.1265/jjh.30.387. [DOI] [PubMed] [Google Scholar]
- 27.Bruce M.C., Bruce E.N. Analysis of factors that influence rates of carbon monoxide uptake, distribution, and washout from blood and extravascular tissues using a multicompartment model. J. Appl. Physiol. (1985) 2006;100(4):1171–1180. doi: 10.1152/japplphysiol.00512.2005. [DOI] [PubMed] [Google Scholar]
- 28.Bruce E.N., Bruce M.C., Erupaka K. Prediction of the rate of uptake of carbon monoxide from blood by extravascular tissues. Respir. Physiol. Neurobiol. 2008;161(2):142–159. doi: 10.1016/j.resp.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan J., de Lannoy I.A.M. Pharmacokinetics. Biochem. Pharmacol. 2014;87(1):93–120. doi: 10.1016/j.bcp.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Hampson N.B. CHRN SLD. Symptoms of carbon monoxide poisoning do not correlate with the initial carboxyhemoglobin level. Undersea Hyperb. Med. 2012;39(2):657. [PubMed] [Google Scholar]
- 31.Fukuda S., Niimi Y., Andersen C.R., Manyeza E.R., Rojas J.D., Prough D.S., et al. Blood carboxyhemoglobin elimination curve, half-lifetime, and arterial-venous differences in acute phase of carbon monoxide poisoning in ovine smoke inhalation injury model. Biochem. Biophys. Res. Commun. 2020;526(1):141–146. doi: 10.1016/j.bbrc.2020.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson G.K. Treatment of carbon monoxide poisoning with hyperbaric oxygen. Mil. Med. 1978;143(8):538–541. [PubMed] [Google Scholar]
- 33.Dolan M.C. Carbon monoxide poisoning. Cmaj. 1985;133(5):392–399. [PMC free article] [PubMed] [Google Scholar]
- 34.Smollin C., Olson K. Carbon monoxide poisoning (acute) BMJ Clin. Evid. 2010:2010. [PMC free article] [PubMed] [Google Scholar]
- 35.Brent J. What does the present state of knowledge tell us about the potential role of hyperbaric oxygen therapy for the treatment of carbon monoxide poisoning? Toxicol. Rev. 2005;24:145–147. doi: 10.2165/00139709-200524030-00001. [DOI] [PubMed] [Google Scholar]
- 36.Chu D.K., Kim L.H., Young P.J., Zamiri N., Almenawer S.A., Jaeschke R., et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 37.Piantadosi C., Zhang J., Demchenko I. Production of hydroxyl radical in the hippocampus after CO hypoxia or hypoxic hypoxia in the rat. Free Radic. Biol. Med. 1997;22(4):725–732. doi: 10.1016/s0891-5849(96)00423-6. [DOI] [PubMed] [Google Scholar]
- 38.Annane D., Chadda K., Gajdos P., Jars-Guincestre M.-C., Chevret S., Raphael J.-C. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: two randomized controlled trials. Intensive care Med. 2011;37(3):486–492. doi: 10.1007/s00134-010-2093-0. [DOI] [PubMed] [Google Scholar]
- 39.Rucker J., Tesler J., Fedorko L., Takeuchi A., Mascia L., Vesely A., et al. Normocapnia improves cerebral oxygen delivery during conventional oxygen therapy in carbon monoxide–exposed research subjects. Ann. Emerg. Med. 2002;40(6):611–618. doi: 10.1067/mem.2002.129723. [DOI] [PubMed] [Google Scholar]
- 40.Ilano A.L., Raffin T.A. Management of carbon monoxide poisoning. Chest. 1990;97(1):165–169. doi: 10.1378/chest.97.1.165. [DOI] [PubMed] [Google Scholar]
- 41.Scheinkestel C.D., Jones K., Myles P.S., Cooper D.J., Millar I.L., Tuxen D.V. Where to now with carbon monoxide poisoning? Emerg. Med Austral. 2004;16(2):151–154. doi: 10.1111/j.1742-6723.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 42.Rose J.J., Wang L., Xu Q., McTiernan C.F., Shiva S., Tejero J., et al. Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am. J. Respir. Crit. Care Med. 2017;195(5):596–606. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao Q., Kawaguchi A.T., Mizobata S., Motterlini R., Foresti R., Kitagishi H. Sensitive quantification of carbon monoxide in vivo reveals a protective role of circulating hemoglobin in CO intoxication. Commun. Biol. 2021;4(1):425. doi: 10.1038/s42003-021-01880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delvau N., Penaloza A., Liistro G., Thys F., Delattre I.K., Hantson P., et al. Effect of pressure support ventilation on carboxyhemoglobin toxicokinetic after acute carbon monoxide intoxication: a swine model. J. Med Toxicol. 2018;14(2):128–133. doi: 10.1007/s13181-018-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi-Fanelli A., Antonini E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch. Biochem Biophys. 1958;77(2):478–492. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- 46.Delvau N., Penaloza A., Liistro G., Thys F., Megarbane B., Hantson P., et al. Relative affinity M constant of adult and fetal hemoglobin for carbon monoxide in humans: a systematic review and meta-analysis. Hum. Physiol. 2020;46:191–199. [Google Scholar]
- 47.Bruce E.N., Bruce M.C. A multicompartment model of carboxyhemoglobin and carboxymyoglobin responses to inhalation of carbon monoxide. J. Appl. Physiol. (1985) 2003;95(3):1235–1247. doi: 10.1152/japplphysiol.00217.2003. [DOI] [PubMed] [Google Scholar]
- 48.Weaver L.K. Carbon monoxide poisoning. Undersea Hyperb. Med. 2020;47(1):151–169. [PubMed] [Google Scholar]
- 49.Pan K.T., Leonardi G.S., Croxford B. Factors contributing to CO uptake and elimination in the body: a critical review. Int J. Environ. Res Public Health. 2020;17(2) doi: 10.3390/ijerph17020528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson J.E., Stewart R.D. Absorption and elimination of carbon monoxide by inactive young men. Arch. Environ. Health. 1970;21(2):165–171. doi: 10.1080/00039896.1970.10667215. [DOI] [PubMed] [Google Scholar]
- 51.Benignus V.A., Hazucha M.J., Smith M.V., Bromberg P.A. Prediction of carboxyhemoglobin formation due to transient exposure to carbon monoxide. J. Appl. Physiol. (1985) 1994;76(4):1739–1745. doi: 10.1152/jappl.1994.76.4.1739. [DOI] [PubMed] [Google Scholar]
- 52.Wagner J.A., Horvath S.M., Dahms T.E. Carbon monoxide elimination. Respir. Physiol. 1975;23(1):41–47. doi: 10.1016/0034-5687(75)90070-5. [DOI] [PubMed] [Google Scholar]
- 53.Cronenberger C., Mould D.R., Roethig H.J., Sarkar M. Population pharmacokinetic analysis of carboxyhaemoglobin concentrations in adult cigarette smokers. Br. J. Clin. Pharmacol. 2008;65(1):30–39. doi: 10.1111/j.1365-2125.2007.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norman J., Ledingham I.M. Vol. 24. Elsevier; 1967. Carbon monoxide poisoning: investigations and treatment; pp. 101–122. (Progress in brain research). [DOI] [PubMed] [Google Scholar]
- 55.Möller P., Sylvén C. Myoglobin in human skeletal muscle. Scand. J. Clin. Lab Invest. 1981;41(5):479–482. doi: 10.3109/00365518109090486. [DOI] [PubMed] [Google Scholar]
- 56.Sokal J.A., Majka J., Palus J. The content of carbon monoxide in the tissues of rats intoxicated with carbon monoxide in various conditions of acute exposure. Arch. Toxicol. 1984;56(2):106–108. doi: 10.1007/BF00349080. [DOI] [PubMed] [Google Scholar]
- 57.Peterson J.E., Stewart R.D. Predicting the carboxyhemoglobin levels resulting from carbon monoxide exposures. J. Appl. Physiol. 1975;39(4):633–638. doi: 10.1152/jappl.1975.39.4.633. [DOI] [PubMed] [Google Scholar]
- 58.Roughton F.J.W. The kinetics of hœmoglobin VI-The competition of carbon monoxide and oxygen for hœmoglobin. Proc. R. Soc. Lond. Ser. B, Contain. Pap. a Biol. Character. 1934;115(795):473–495. [Google Scholar]
- 59.Killick E.M. Carbon monoxide anoxemia. Physiol. Rev. 1940;20(3):313–344. [Google Scholar]
- 60.Wazawa H., Yamamoto K., Yamamoto Y., Matsumoto H., Fukui Y. Elimination of CO from the body: an experimental study on the rabbit. Nihon Hoigaku Zasshi. 1996;50(4):258–262. [PubMed] [Google Scholar]
- 61.Waring W.S. Management of lithium toxicity. Toxicol. Rev. 2006;25(4):221–230. doi: 10.2165/00139709-200625040-00003. [DOI] [PubMed] [Google Scholar]
- 62.Scheinkestel C.D., Bailey M., Myles P.S., Jones K., Cooper D.J., Millar I.L., et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial. Med J. Aust. 1999;170(5):203–210. doi: 10.5694/j.1326-5377.1999.tb140318.x. [DOI] [PubMed] [Google Scholar]
- 63.Turgut K., Yavuz E. Comparison of non-invasive CPAP with mask use in carbon monoxide poisoning. Am. J. Emerg. Med. 2020;38(7):1454–1457. doi: 10.1016/j.ajem.2020.04.050. [DOI] [PubMed] [Google Scholar]
- 64.Mathieu D., Wattel F., Mathieu-Nolf M., Durak C., Tempe J., Bouachour G., et al. Randomized prospective study comparing the effect of HBO versus 12 h NBO in non comatose CO poisoned patients: results of the interim analysis. 1996.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.