Highlights
-
•
The SBRT-SIB protocol used in our study, allowed us to treat the pancreatic cancer up to doses of 55 Gy/5 fractions/11 Gy per fraction.
-
•
Our treatment protocol, has been shown to be feasible and safe, in this particular patient́s cohort.
-
•
Although a larger sample size and an extended follow-up period are needed , we consider than the present findings are unquestionably promising.
Keywords: LAPC, BRPC, sbrt, SIB, Escalated dose, Feasibility, Safety
Abstract
Background
Pancreatic Stereotactic Body Radiotherapy (SBRT) allows for the administration of a higher biologically effective doses (BED), that would be essential to achieve durable tumor control. Escalating treatment doses need a very accurate tumor positioning and motion control during radiotherapy.
The aim of this study to assess the feasibility and safety of a Simultaneous Integrated Boost (SIB) dose-escalated protocol at 45 Gy, 50 Gy and 55 Gy in 5 consecutive daily fractions, in Border Line Resectable Pancreatic Cancer (BRCP) /Locally Advanced Pancreatic Cancer (LAPC) by means of a standard LINAC platform.
Methods
Patients diagnosed of BRPC/LAPC, candidates for neoadjuvant chemotherapy and SBRT, in four university hospitals of the province of Las Palmas (Canary Islands, Spain) were included in this prospective study. Radiotherapy was administered using standard technology (LINACS) with advanced positioning (Lipiodol® and metallic stent used as fiducial markers) and tumor motion control (4D, DBH, Calypso®). There were 3 planned dose-escalated SIB groups, 45 Gy/5f (9 patients) 50 Gy/5f (9 + 9 patients) and 55 Gy/5f (9 patients). The defined primary end points of the study were the safety and feasibility of the proposed treatment protocol. Secondary endpoints included radiological tumor response after SBRT, local control and survival.
Results
From June 2017 to December 2022, sixty-two patients were initially assessed for eligibility in the study in the four participating centers, and 49 were candidates for chemotherapy (CHT). Forty-one were referred to radiotherapy after CHT and 33 finally were treated by escalated-dose SIB, 45 Gy (9 patients) 50 Gy (16 patients), 55 Gy(8 patients). All patients completed the scheduled treatment and no acute or late severe (≥grade3) gastrointestinal toxicity was observed.
Local response was analyzed by CT/MRI two months after the end of SBRT. Ten patients (31,25 %) achieved objective response (2/9:45 Gy, 5/15:50 Gy, 3/8:55 Gy). Follow-up was closed as July 2023. Freedom from local progression at 1-2y were 89,3% (95 %CI:83,4–95,2%) and 66 % (95 %CI:54,6–77,4%) respectively. The 1-2y survival rates were 95,7% (95 %CI:91,4–100 % and 48,6% (95 %CI:37,7–59,5%) respectively.
Conclusion
These promising results should be confirmed by further studies with larger sample size and extended follow-up period.
Introduction
Pancreatic cancers are highly aggressive tumors that carry a poor prognosis [1]. Nevertheless, new radiation therapy delivery techniques allowing for radiation dose escalation may further improve local control (LC) and ultimately improve survival in patients with Border-line Resectable Pancreatic Cancer (BRPC) or locally advanced pancreatic cancer (LAPC) [2], [3].
In this context, stereotactic body radiation therapy (SBRT) has emerged as an effective component for the multimodal treatment of pancreatic cancer [4]. According to recent studies, SBRT after systemic therapy can increase survival in LAPC compared to either chemotherapy alone or conventionally fractionated radiotherapy CFRT [5], [6], [7], [8]. There is limited data to support a specific radiation therapy (RT) dosing for SBRT [9]. Reported SBRT doses include 3 fractions (total dose 30–45 Gy) or 5 fractions (total dose 25–50 Gy) [9]. While SBRT in the 25–33 Gy range may still be a reasonable option, the optimal SBRT schedule for this clinical situation has yet to be determined [9]. SBRT enables the administration of higher biologically effective doses (BED), which are crucial for achieving durable tumor control and impacting survival [10]. Furthermore, a reduced number of fractions increase patient compliance and facilitate a more seamless combination with systemic treatments [4].
However, limitations for high BED treatments, arise when tumors are close to critical organs at risk (OARs) such as the duodenum, stomach and small bowel [11], [12]. Therefore, researchers have designed different approaches to this challenging clinical situation [13]. Escalating treatment doses need a very accurate tumor positioning and motion during treatment, in order to reduce PTV margins, and therefore improving patient́s tolerance to treatment [14].
When standard LINAC technology is to be used for treatment administration, highly sophisticated planning (contrast CT/MRI) and tumor control motion systems (fiducials, Calypso® system, Deep Breath Holding (DBH) or 4D/CT ITV) are used [15], [16], [17], [18], [19].
Published experiences include high BED doses to the GTV including the use of a simultaneous integrated boost (SIB) approach [20], [21]. The advantage of a SIB approach, is that the total tumor volume may receive one dose, while a particular high risk area far away from OARs, may receive higher doses in the same number of fractions [21].
In recent years the use of magnetic resonance guided radiation therapy (MRgRT) has increased in this particular clinical situation [22], [23], [24], [25]. MRgRT provides superior soft tissue delineation, which is particularly important when the bowel/stomach (or other OARs) and tumor target are in close proximity. Furthermore the need for fiducial placement would be reduced [26]. Additionally, MRgRT platforms may allow advanced motion management and on-table, near real-time adaptive radiation capabilities [26]. Total doses up to 50 Gy or more, are routinely administered with a very safe toxicity profile and excellent preliminary clinical results [23], [24], [25]. Unfortunately, this technology is not widely available today in most European countries [16], [17]. Table 1
Table 1.
Dose escalation strategies in pancreatic cancer.
| Reference | Study design | Patients | Intervention | OS | FFLP | Toxicity |
|---|---|---|---|---|---|---|
| Krishnan et al.[11] | Retrospective | 47 | Dose escalated chemoradiation (BED > 70 Gy plus gemcitabine or capecitabine) | Median 17.8 months | Median 10.2 months | 2 % ≥grade 3 (≥G3) nausea |
| Parisi et al. [12] | Phase I/II | 8 | Induction chemotherapy followed by standard dose chemoradiation followed by SBRT boost to a median dose of 12 Gy in 1–3 fractions | Median 21.5 months | 73 % at 2 years | No G3 toxicities |
| T. Courtney [13] | Phase I | 30 | 40, 45, or 50 Gy SBRT in 5 fractions | Median 17.1 months | – | 6.7 % experienced G4 to G5 late toxicity, both of which occurred in the 45 Gy group |
| A. Tozzi et al. [12] | Prospective | 30 | 45 Gy in 6 daily fractions of 7.5 Gy | Median 11 months | 96 % at 1 year | No G3 toxicities |
| Comito et al. [15] | Retrospective | 31 | 45 Gy in 6 fractions | Median 18 months | 91 % at 1 year | No G3 toxicities |
| Mellon et al. [16] | Retrospective | 159 | 20–60 Gy in 3–5 fractions | Median 18,1 months | – | Acute and/or late toxicity grade 3 in 7 % (bleeding from de duodenum or stomach) |
| Shaib et al. [17] | Phase 1 | 13 | 45 Gy in 3 fractions | Median 11 months | – | No G3 toxicities |
| Rudra et al. [18] | Retrospective | 20 | High dose MRgRT (BED > 70 Gy, SBRT alone or hypofractionated RT plus concurrent gemcitabine, capecitabine, or gemcitabine-nab paclitaxel) vs standard‐dose groups (BED10 ≤ 70) | Median 20.8 months | 77 % at 2 years | 0 % ≥G3 GI toxicities |
| Hassanzadeh et al.[19] | Retrospective | 44 | MR guided SBRT (50 Gy in 5 fractions) with adaptive reoptimization | Median 15.7 months | 84 % at 1 year | 5 % ≥G3 ulcers |
| Chuong et al. [20] | Retrospective | 35 | MR guided SBRT (50 Gy in 5 fractions) with adaptive reoptimization | 59 % at 1 year | 88 % at 1 year | 6 % ≥G3 diarrhea and bile duct stenosis |
| SMART (Parikh et al.) [21] | Phase II | 136 | MR guided SBRT (50 Gy in 5 fractions) with adaptive reoptimization | 94 % at 1 year | 83 % at 1 year | No G3 toxicities |
Therefore, we aimed in this study to assess the feasibility and safety of dose escalation protocol using SIB dose escalated protocol at 45 Gy, 50 Gy and 55 Gy in 5 consecutive daily fractions, in BRCP/LAPC using a standard LINAC platform.
Materials and methods
Study design and patient selection
Patients diagnosed of BRPC and LAPC, elegible for neoadjuvant chemotherapy and local radiation treatment, were included in this prospective study. Patients were diagnosed and treated at four university hospitals of the province of Las Palmas (Canary Islands, Spain). Patient́s inclusion criteria were: age 18 years or older, histologically and radiologically proven borderline or unresectable locally advanced pancreatic adenocarcinoma, diagnosed in Las Palmas Province University Hospitals (Fuerteventura General, Lanzarote General, Insular-Materno Infantil y Gran Canaria Dr Negrín) and discussed in Multidisciplinary Tumor Boards (MTB) of each center. Exclusion criteria were: age less than 18 years, histological types other than adenocarcinoma, resectable adenocarcinoma, metastatic status or ECOG (Eastern Cooperative Oncology Group) > 3. Cancer staging was performed according to the 8th edition of the TNM classification system [34].
After being approved for inclusion in the study, patientś neoadjuvant chemotherapy was administered by the Medical Oncology Departments of these university hospitals, according to the standard of care of each institution. Once the systemic treatment was finished and response was evaluated [27], [28], the patient underwent a re-evaluation in the MTB to determine if they fulfill criteria for continuing in the trial and. If agreed upon, the patient proceed to receive SBRT at the Radiation Oncology Dept, University Hospital of Gran Canaria Dr Negrín.
This study was designed in a predefined escalating dose mode in sequential cohorts of 9 patients and stopping rules based on grade 3 severe acute toxicity. The first group of 9 patients was assigned to receive the first escalating dose. If no severe (Grade 3) acute side effects were observed, then the second dose level was started. The second dose level was planned to be administered to a study 9 patients cohort followed (if no stopping toxicity was observed) by a confirmatory 9 patients cohort at the same dose. Once the safety of the second dose level would be confirmed a third dose group of 9 patients would receive the third dose level.
The study was approved by the Ethical Committee of Hospital Dr. Negrín (Las Palmas) and registered by EudraCT Number: 2019–001715-23. Written inform consent for treatment was obtained in all the patients.
SBRT Protocol and planning
Patients were then assessed at the Radiation Oncology Dept, University Hospital of Gran Canaria Dr Negrín and enrolled in the simulation and treatment planning dedicated protocol.
Patients were immobilized in a supine position with their arms positioned over the head, on a flat table top, with knee and/ or foot support, to optimize setup reproducibility.
Abdominal compressor was also used to reduce the movement of abdominal organs and to better delimit the contours [36], [37].. Different techniques were used according to patient́s characteristics, to manage breathing induced tumor motion. According with the AAPM Task Group [29] recommendations, the respiratory motion options used (DBH, Calypso® gating system and 4D-CT) were tailored to accommodate patient́s tolerance, after prior evaluation and training, conducted by the physician and the technicians.
-
-
Deep breath hold (DBH) Gating. Patients underwent training to sustain a regular respiratory cycle, using the Real-time Position Management® system (RPM) (Varian Medical Systems, Palo Alto, CA) as patient́s visual guide.
-
-
The Calypso Gating System® (Varian Medical Systems, Palo Alto, CA, USA) was used incorporating a fiducial-based intra-fractional motion management system comprising an electromagnetic array and tumor-implanted fiducials (Beacon). The array, positioned above the patient during the treatment, detects the position as well as translational and rotational movements of the transponders. The Calypso® system offers continuous 3-dimensional intra-fractional motion management of all potential tumor movements, both intrinsic and those caused by the patient́s movements, in a real time. It operates at a frequency of 20 Hz and provides sub-millimetrical accuracy. The transponders are percutaneous implanted into, or adjacent to the lesion. Three anchored electromagnetic beacon transponders, were implanted under ultrasound guidance by an experienced radiologist [15].
-
-
The Motion management system 4D-CT generates an internal target volume (ITV) during free breathing (FB). An abdominal compressor was used to limit respiratory motion in these patients.
To enhance treatment́s precision for both tumor and organs at risk, a liquid iodinated contrast (Lipiodol®: ethyl esters of iodized fatty acids of poppy seed oil) was inserted by an experienced gastroenterologists into the second or third duodenal wall or in the antral stomach wall. The choice of location, depend on the tumoŕs position within the pancreas (head & neck of the pancreas). The procedure was conducted before the planning CT [30]. The Lipiodol®, has a retention rate in the wall throughout a 6-week to 7-week post-injection period and funtions as a radio-opaque fiducial marker [31].
A metallic endoscopically-placed stent, is routinely used in BRPC and LAPC in our hospitals. Reasons behind that standard institutional policy are the extended durability and reduced risk of obstruction inherent in metallic prostheses [32]. Furthermore, aside from its favorable impact on bilirubin levels and patient well-being, this metallic stent serves as a fiducial marker for precise treatment positioning [32]. No interference with radiation dose or increased side effects has been reported in the literature [33].
Planning CTs were subsequently conducted after 4 h fasting period. Following an initial unenhanced scan, a multi-phase contrast-enhanced simulation CT was performed [34]. In all cases, pretreatment diagnostic CT/MRI and the post chemotherapy re-evaluation CT/MRI, were fused with the images from the planning CT. Diagnostic and post-chemotherapy FDG-PET/CT, were also used in the planning and contouring process, when available. Additionally, information from the endoscopy reports of EUS (endoscopic ultrasound system) performed for lipiodol placement, was also taken into consideration.
Contouring
Only the pancreatic lesion was considered for SBRT. The gross tumor volume (GTV) was defined on the non-contrast CT simulation, fused with the contrast-enhanced CT in arterial, pancreatic, and portal-venous phases. The GTV was also defined from T1-weighted MRI images with and without contrast-enhancement [35].
The clinical target volume (CTV) was created by including the GTV plus the tumor–vessel interface volume (TVI). The TVI is the area nearby between major vessels and the GTV, and is included generally with an expansion of 5 mm [15].
The internal target volume (ITV) is generated only in case of 4D-CT scan simulation [36]. It was defined as the sum of the GTV in the different breathing phases (10 phases) acquired during the 4D-CT. The movement of biliary prosthesis could be measured and extrapolated for the expansion of the GTV to create an ITV in some patients [10], [35].
Another 3–5 mm isotropic expansion was generated from the ITV to obtain The Planning Target Volume (PTV) was generated by a 3–5 mm isotropic expansion from the ITV/CTV.
For critical organs at risk (OARs) such as the duodenum, stomach, and bowel, it was necessary to set up a planning organ at risk volume (PRVs). These volumes were generated from a 2 mm expansion over OARs [18].
Delineation of target volumes and organ at risk (OARs) it is shown in Fig. 1.
Fig. 1.

Delineation of target volumes and organ at risk (OARs). The delineation of various target volumes and organ at risk (OARs) is illustrated. The Gross Tumor Volume (GTV) is represented in orange. The Planning Target Volume (PTV) is PTV33, depicted in dark blue and Clinical Target Volumes (CTV) are CTV45 in light blue and CTV50-55 in red. The diagram also highlights the presence of specific OARs, as duodenum, marked in yellow and with Lipiodol® present within its wall, indicated as a radiopaque marker (white). Additionally, the stomach is denoted in dark green. For the treatment, the prescription dose consists of 50–55 Gy for CTV50-55, 45 Gy for CTV45, and 33 Gy for PTV33, all delivered in 5 daily fractions.
Definitive volumes to be treated based on 3 levels of dose escalation. Simultaneous integrated boost approach
The PTV33 is defined as either the ITV or CTV (in cases of 4D, DBH or Calypso® gating respectively) as previously described. This volume typically overlaps with the PRVs of the duodenum or stomach. The prescribed 33 Gy dose was administered in compliance with the constraints of theses OARs.
Escalated Simultaneous Integrated Boost (SIB) Dose Level 1
CTV45 is generated from de ITV or CTV, incorporating an internal margin of 5 mm. This inner volume ensures a safe distance between the 45 Gy dose and the critical OARs. The ultimate CTV45 avoids overlaps with PRV and critical OARs. The dose per fraction was 9 Gy, administered as a SIB every day. The predefined number of patients receiving this dose level was 9 cases.
Escalated Simultaneous Integrated Boost (SIB) Dose Level 2
CTV50 is created from CTV45, with an internal margin of 3 mm usually located.
in the most distant area of critical OARs duodenum, stomach, and gallbladder but inside the GTV, generally close to the great vessels. Dose per fraction was 10 Gy administered as a SIB every day. The predefined number of patients receiving this dose level was (9 + 9: 18 cases).
Escalated Simultaneous Integrated Boost (SIB) Dose Level 3
CTV 55 is derived from CTV45, incorporating an internal margin of 3 mm. It is typically situated in the farthest region from critical OARs such as the duodenum, stomach and gallbladder but, still within the GTV, usually in proximity to major vessels. The dose per fraction was 11 Gy administered as a SIB every day. The predefined number of patients receiving this dose level was 9 cases.
Dose prescription:
The prescribed target coverage aimed for D98% > 98 % for the CTV, with a minimum acceptable dose of D98% > 95 % and Dmax < 107 %. In regions of overlap between the PTV33 and OARs, the dose was constrained to 35 Gy. The PTV33 had a dose prescription of 33 Gy delivered in 5 consecutive fractions (EQD2 = 45.65 Gy, BED10 = 45.65 Gy.
SIB doses were prescribed according to the predefined treatment volumes.
(CTV45): 45 Gy in 5 consecutive fractions (EQD2 = 71.25 Gy, BED10 = 85.5 Gy).
(CTV50): 50 Gy in 5 consecutive fractions (EQD2 = 83.33 Gy, BED10 = 100 Gy). (CTV55): 55 Gy in 5 consecutive fractions (EQD2 = 96.25 Gy, BED10 = 115.5 Gy).
Treatment and IGRT
All SBRT plans were calculated for a TrueBeam® medical LINAC (Varian Medical System, Palo Alto, CA) equipped with a high definition multileaf collimator (HDMLC) and were created using Volumetric Modulated Arc Therapy (VMAT) technique. The plans involved 2––4 partial arcs, with 10MV photons, flattening filter free (FFF) beams at a dose rate of 2400 MU/min. Planning and contouring were performed through a yearly updated Eclipse® planner. The updated Timmermanńs constraints for 5 Fractions [37] were used to evaluate the limiting doses to the OARs.
The patients were prepared before every session with 4 h fasting period. Daily pre-treatment volumetric Imaging Guided Radiotherapy (IGRT) with cone-beam (CBCTs) was acquired to confirm the treatment reference position. Patient́s positioning was determined using the location of duodenum or stomach wall proximate to tumor (head or body/tail pancreas) in each fraction with the Lipiodol®. The metallic biliary prosthesis was also used as fiducial marker.
In patients treated with the Calypso® gating system, real-time monitoring of the target motion was conducted from the reference, in the three orthogonal directions. Treatment delivery was promptly interrupted if the beacons moved outside the 3 mm tracking predefined threshold.
For patients treated with DBH, the same DBH gating curve defined during the planning CT, was utilized in each radiotherapy session. Treatment delivery was promptly interrupted if curve moved outside the 3 mm predefined tracking thresholds. SBRT treatment sessions were administered daily.
Study end points
The defined primary end points of the study were the safety and feasibility of the proposed treatment protocol. Safety was assessed by the rate of grade 3 acute and late gastrointestinal toxicity observed in the patients treated with SBRT, according to the common toxicity criteria adverse effects (CTCAE 5.0) [38]. Patients were assessed for toxicity on a weekly basis during SBRT treatment, at the end of radiotherapy and one, three and six months thereafter. Patients were prospectively followed under the Spanish RD1566/1998 regulation for Radiation Therapy Quality Assurance. Follow-up visits were subsequently scheduled every 3 months during the first two years, and every six months, thereafter. Follow-up was conducted jointly by Medical and Radiation Oncology staff doctors, involving physical examination, general blood test and CT/MRI very 3 months for the first year and every 6 months thereafter or when there was clinical suspicion of relapse.
The treatment feasibility as evaluated through the number of patients that completed the scheduled treatment, the number of patients that suffered interruptions of the treatment, and treatment deviations from the expected treatment period (5 days) compared with the observed treatment period.
The defined secondary endpoints included: radiological tumor response after SBRT according to RECIST 1.1 criteria [39] and local control defined as the freedom from local pancreatic progression (FFLP) as the first cause of failure, and survival was assessed as the time to cancer-related death.
Statistical analysis
Statistical analyses were performed using IBM SPPSS Statistics, version 26 (IBM Corp., Armonk, NY). The Chi-square test was employed to analyze statistical differences in discrete variables. Survival figures were generated using the Kaplan-Meir tables. A p-value less than 0.05, was considered statistically significant.
Results
Patient and treatment characteristics
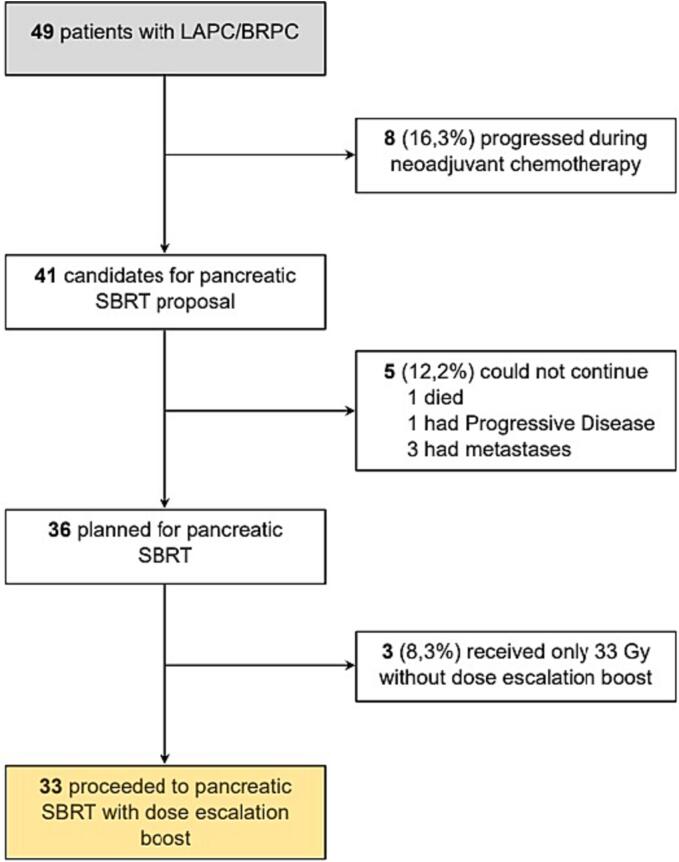
From June 2017 to December 2022, sixty-two patients were initially assessed for eligibility in the study across the four participating centers, and 49 identified as candidates for systemic treatment. Eight of them progressed during neoadjuvant chemotherapy and were consequently not elegible for SBRT. Forty-one patients were referred for SBRT consideration. Three of them were deemed ineligible, because of they were originally hepatic M1, although they achieved a complete response after neoadjuvant chemotherapy. One patient died of chemotherapy-induced toxicity prior to the planning CT and another patient exhibited disease progression in the planning CT/MRI. So, thirty-six patients met the criteria to be included in this study (Fig. 2). Unfortunately, three patients (two in the 50 Gy group and one in the 55 Gy group) did not receive the planned escalating dose SIB and were treated of a total dose of 33 Gy. Therefore 33 patients were ultimately included in this escalating SBRT/SIB trial.
Fig. 2.
Study and patients eligibility flowchart.
The mean age of the patients was 61.67 years (range 37–82). Patient́s characteristics are presented in Table 2 Briefly, the majority of them were females (57,6%), clinically staged as T3 (45,5%) and had negative lymph node involvement (75,8%). Tumors were predominantly localized in the head of the pancreas (45,5%) The majority of cases were unresectable LAPC (60,6%).
Table 2.
Baseline patient and tumor characteristics.
| Characteristic | Number of Patients (%) |
|---|---|
|
Sex Male Female |
14 (42,4%) 19 (57,6%) |
|
Histology Adenocarcinoma |
33 (100 %) |
|
Location of lesion Pancreatic head Pancreatic neck Body Tail Overlapping head/neck Overlapping head/body Overlapping body/tail |
15 (45,5%) 4 (12,1%) 4 (12,1%) 1 (3,0%) 3 (9,1%) 3 (9,1%) 3 (9,1%) |
|
Stage T T2 T3 T4 |
6 (18,2%) 15 (45,5%) 12 (36,3%) |
|
Stage N N0 N1 N2 |
25 (75,8%) 6 (18,1%) 2 (6,1%) |
|
Stage M M0 |
33 (100 %) |
|
Tumor classification Borderline (BRPC) Locally advanced pancreatic cancer (LAPC) |
13 (39,4%) 20 (60,6%) |
|
Adjacent organ invasion(duodenum) No |
33 (100 %) |
|
Neoadjuvant chemotherapy FOLFIRINOX Gemcitabine-nab-paclitaxel (Abraxane) |
17 (51,5 %) 16 (48,5%) |
Treatment administration
All patients had planning CTs as scheduled and all patients but one (97 %) had a metallic biliary stent in place. Pancreatic cancers located in the head and/or neck of the pancreas also had Lipiodol and was used as tumor marker in 24 patients (72,7%).
The Calypso® gating system was used for tumor motion control, in the majority of the patients (16 patients, 48.5 %), followed by 4D (12 patient,36.4 %) and few patients underwent breath holding (5 patients, 15.2 %) as part of their treatment.
All 33 patients received the standard 33 Gy/6,6Gy/5 days for the tumor PTV. Regarding the predefined escalating dose SIB, 3 patients (two in the 50 Gy group and other in the 55 Gy group) did not received the scheduled treatment due to uncompleted IGRT-assigned protocol (2 patients) and due to unnoticed lack of prescription of the treating physician (1 patient).
Therefore, nine predefined patients received a SIB of 45 Gy/9Gy/5 days, 16 patients received a SIB of 50 Gy/10 Gy/5 days and 8 patients received a SIB of 55 Gy/11 Gy/5 days.
The updated Timmermanńs constraints for 5 Fractions [37] were applied to evaluate the limiting doses to the OARs and were fulfilled by all patients. GTV coverage (33 Gy) was 93 ± 10,7% (median 97,50 %) and ultimate GTV45 (GTV minus the planning organ at risk volume (PRVs)) coverage was 90,07 %±9,57 (median 92,01), representing a 24 cc median volume. The 50 and 55 Gy had both a median volume of 12,50 cc.
Feasibility
All patients completed the SBRT schedule. One patient experienced a delay in the treatment schedule (10 days total treatment time) due to LINAC breakage. The total treatment time exceeded 5 days in 7 out of 33 (21,21 %) treated patients (6 days in 5 patients and 7 days in 2 patients) due to holydays and weekends. (mean treatment time 5,52+/-1.09 days, range: 5–10, median 5 days).
Safety
All patients were evaluable for toxicity. No patients presented acute or late severe (≥grade 3) gastrointestinal toxicity, according to the common toxicity criteria adverse effects (CTCAE 5.0) [38]. The most frequent acute grade 1–2 toxicities were diarrhea and abdominal pain in 4 patients (12,1%), cholangitis in one patient and one case of gastrointestinal bleeding (3 %). Regarding late toxicity, only 3 cases of grade II abdominal pain (9.1 %) and one case of duodenal bleeding (3 %) were reported. SIB dose escalation was not associated with higher acute (p = 0.368) or late toxicity (p = 0,374) (Table 3).
Table 3.
SIB dose and CTCAE 5.0 acute and late toxicity.
| SIB Dose (patients) |
Acute toxicity |
Late toxicity |
||||
|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G0 | G1 | G2 | |
| 45 Gy (9) | 5 15,15 % |
3 9,09 % |
1 3,03 % |
7 21,21 % |
0 0 % |
2 6,06 % |
| 50 Gy (16) | 12 36,36 % |
2 6,06 % |
2 6,06 % |
14 42,42 % |
0 0 % |
2 6,06 % |
| 55 Gy (8) | 6 18,18 % |
0 0 % |
2 6,06 % |
8 24,24 % |
0 0 % |
0 0 % |
| TOTAL | 23 (69,69 %) |
5 (15,15 %) |
5 (15,15 %) |
29 (87,87 %) |
0 (0 %) |
4 (12,12 %) |
Local response after neoadjuvant treatment
Local response after SIB-SBRT was analyzed by CT/MRI following the RECIST 1.1 criteria two months after the end of SBRT. One patient in the 50 Gy group underwent surgery in the referring hospital, without available radiological post-treatment evaluation. Three patients achieved a complete response and seven obtained a partial response. Therefore, 10 out of 32 patients (31,25 %) achieved objective response (OR). Other 20 cases showed stable disease. Only two patients showed local disease progression one month after SBRT. Two out of nine patients (22,22 %) in the 45 Gy group achieved an OR versus 5/15(33,33 %) in the 50 Gy group and 3/8 (37,5%) in the 55 Gy group. The radiological response after neoadjuvant treatment did not correlate with the escalated SIB dose (p = 0,816) Table 4
Table 4.
SIB dose and Clinical Response by RECIST 1.1.
| SIB Dose (patients) |
Clinical Response |
|||
|---|---|---|---|---|
| RC | RP | EE | PD | |
| 45 Gy (9) | 1 3,12 % |
1 3,12 % |
6 18,75 % |
1 3,12 % |
| 50 Gy (15) | 1 3,12 % |
4 12,5% |
10 31,25 % |
0 0 % |
| 55 Gy (8) | 1 3,12 % |
2 6,25 % |
4 12,5% |
1 3,12 % |
| TOTAL | 3 (9,36 %) | 7 (21,87 %) | 20 (62,5) | 2 (6,24 %) |
Follow-up was closed as July 2023. After a mean follow-up for surviving patients of 22,47 months (median 18,13 months and range 6,47–69,75 months), only 8 patients (24,2%) developed local progression. (Fig. 3). Freedom from local progression at 1-2y were 89,3% (95 %CI: 83,4–95,2%) and 66 % (95 %CI: 54,6–77,4%) respectively (Fig. 3). The median survival for freedom from local progression was not reached. No differences were found among the different dose groups in terms of local control.
Fig. 3.
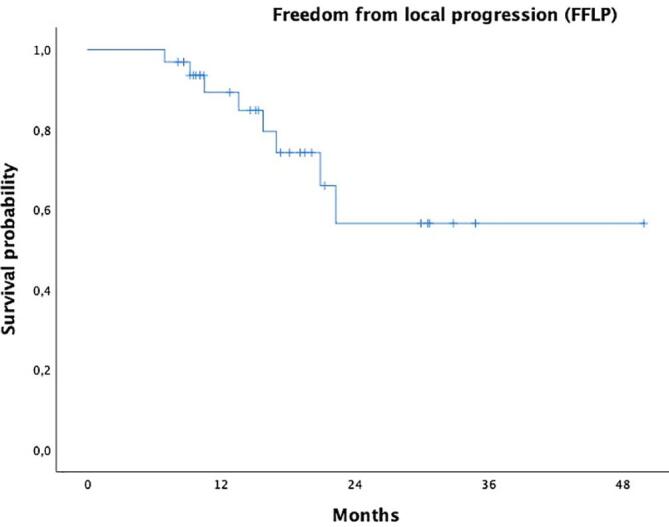
Kaplan-Meier curve for freedom from local progression (FFLP) in our patients.
The median survival from diagnosis was 22,5 months (95 % CI:6,907–38,168 months). The 1-2y survival rates were 95,7% (95 %CI: 91,4–100 % and 48,6% (95 %CI: 37,7–59,5%) respectively.
Discussion
The radiation dose for pancreatic cancer commonly employs non-ablative doses to avoid surpassing the radiation tolerance of gastrointestinal organs [40]. However, emerging evidence indicates the need for dose escalation beyond this range [10]. Nevertheless, substantial dose escalation in radiation, has been challenging due to technological constraints in effectively visualizing and sparing the mobile and radiosensitive stomach and small intestine during the actual treatment delivery [47].
Improvements in imaging [34],planning [41] and movement control [26], [42], allowed for dose escalation with SBRT in this particular setting [9]. Doses of 45 Gy/6 fractions [43], [44] or 50 Gy/5 fractions were safely administered using sophisticated technology [23], [24], [40]. Besides promising oncological results, the SBRT short treatment time (5 days) allows for an easy combination with systemic therapy [4], reduced hospital patient́s frequentation and improves the patient́s quality of life [45].
For these reasons, we aimed to carry out this dose escalation study in patients with unresectable BRPC and LAPC, using standard technology (LINACS) with advanced positioning (Lipiodol® and metallic stent used as fiducial markers) and tumor motion control (4D, DBH, Calypso®). Treatment administration was also fast (10 Mv photons, FFF at 2400MU/min) and precise (HDMLC).
Our treatment protocol including sophisticated treatment set-up and a SIB schedule for escalating dose, has been shown to be feasible and safe. All patients completed the treatment protocol and no severe grade 3 acute or late toxicity was observed. In fact, the toxicity profile of our escalating dose protocol is similar to or superior to, other published series with highly complex technological systems [23], [24], [25], [46]. Furthermore, 55 Gy/5 days is the highest dose published for SBRT pancreatic cancer and an excellent safety profile was observed for these patients.
The secondary endpoints of the study were related to local response, local freedom from progression and survival. Our figures were similar to the best results already published [23], [24], [25], [36], [44], [46], [47], although the possibility of a long-term local control of the disease was observed in our series of patients (median freedom from local progression survival was not reached).
Interestingly, we couldńt find any difference for those end points regarding the administered dose. In our opinion, the reduced number of cases by dose groups would limit the possibility of observing any differences. Anyhow, the observed 2/9 OR in 45 Gy group vs 8/23 OR in the 50–55 Gy groups without patient́s severe toxicity, would encourage us to use the higher doses levels.
Conclusion
This study emphasizes that implementing a dose escalation treatment using SBRT for patients with BRPC/LAPC is both feasible and safe, in this particular patient́s cohort. Although a larger sample size and an extended follow-up period are necessary to comprehensively grasp the long-term clinical implications, we consider than the present findings are unquestionably promising.
Limitations of the study include the relatively small sample size and the short follow-up.
Ethical approval
(Research involving human participants and/or animals) All human studies have been approved by the appropriate ethics committee and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All the persons gave their informed consent prior to their inclusion in the study and details that might disclose the identity of the subjects under study were omitted.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkoň P., Trna J., Slávik M., Němeček R., Kazda T., Pospíšil P., et al. Stereotactic Body Radiotherapy (SBRT) of Pancreatic Cancer—A Critical Review and Practical Consideration. Biomedicines. 2022;10(10) doi: 10.3390/biomedicines10102480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghaly M., Gogineni E., Herman J., Saif M.W. New Potential Options for SBRT in Pancreatic Cancer. Cancer Med J [internet] 2021;4(Suppl 3):41–50. http://www.ncbi.nlm.nih.gov/pubmed/34355218%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC8336074 Available from. [PMC free article] [PubMed] [Google Scholar]
- 4.Suker M, Nuyttens JJ, Eskens FALM, Haberkorn BCM, Coene PPLO, van der Harst E, et al. Efficacy and feasibility of stereotactic radiotherapy after folfirinox in patients with locally advanced pancreatic cancer (LAPC-1 trial). EClinicalMedicine [Internet]. 2019;17:100200. Available from: 10.1016/j.eclinm.2019.10.013. [DOI] [PMC free article] [PubMed]
- 5.Chen Y., Sun X.J., Jiang T.H., Mao A.W. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: A meta-analysis. World J Gastroenterol. 2013;19(42):7461–7471. doi: 10.3748/wjg.v19.i42.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi Y., Oh D.Y., Kim K., Chie E.K., Kim T.Y., Lee K.H., et al. Concurrent chemoradiotherapy versus chemotherapy alone for unresectable locally advanced pancreatic cancer: A retrospective cohort study. Cancer Res Treat. 2016;48(3):1045–1055. doi: 10.4143/crt.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rainer Fietkau, Michael Ghadimi, Robert Gr€utzmann, Uwe A Wittel, Lutz Jacobasch W, Uhl, Roland S. Croner, Wolf Otto Bechstein, Ulf Peter Neumann, Dirk Waldschmidt SH, Boeck, Nicolas Moosmann, Anke C. Reinacher-Schick, Henriette Golcher, Werner Adler S, Semrau, Annett Kallies, Markus Hecht, Andrea Tannapfel HO. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: First results of the CONKO-007 trial. Gastrointest CANCER—GASTROESOPHAGEAL, PANCREATIC, HEPATOBILIARY.
- 8.Comito T., Massaro M., Teriaca M.A., Franzese C., Franceschini D., Navarria P., et al. Can STEreotactic Body Radiation Therapy (SBRT) Improve the Prognosis of Unresectable Locally Advanced Pancreatic Cancer? Long-Term Clinical Outcomes, Toxicity and Prognostic Factors on 142 Patients (STEP Study) Curr Oncol. 2023;30(7):7073–7088. doi: 10.3390/curroncol30070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D., Morris J.S., Liu J., Hassan M.M., Day R.S., Bondy M.L., et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arcelli A., Guido A., Buwenge M., Simoni N., Mazzarotto R., Macchia G., et al. Higher biologically effective dose predicts survival in SBRT of pancreatic cancer: A multicentric analysis (PAULA-1) Anticancer Res. 2020;40(1):465–472. doi: 10.21873/anticanres.13975. [DOI] [PubMed] [Google Scholar]
- 11.Mazzarotto R., Simoni N., Guariglia S., Rossi G., Micera R., De Robertis R., et al. Dosimetric Feasibility Study of Dose Escalated Stereotactic Body Radiation Therapy (SBRT) in Locally Advanced Pancreatic Cancer (LAPC) Patients: It Is Time to Raise the Bar. Front Oncol. 2020;10(December):1–9. doi: 10.3389/fonc.2020.600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.1 NS, 1 RM, Paiella S, Guariglia S, Zivelonghi E, Malleo G, et al. Hypofractionated Stereotactic Body Radiation Therapy With Simultaneous Integrated Boost and Simultaneous Integrated Protection in Pancreatic Ductal Adenocarcinoma. Clin Oncol. 2020. [DOI] [PubMed]
- 13.Liu J., Lee P., McGee H.M., Chung V., Melstrom L., Singh G., et al. Advances in Radiation Oncology for Pancreatic Cancer: An Updated Review. Cancers (Basel) 2022;14(23):1–15. doi: 10.3390/cancers14235725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mary Feng, M.D.1, James M. Balter, Ph.D.1, Daniel Normolle, Ph.D.1 SA, M.D.2, Yue Cao, Ph.D.1, Thomas L. Chenevert, Ph.D.2, and Edgar Ben-Josef MD. Characterization of Pancreatic Tumor Motion Using Cine- MRI: Surrogates for Tumor Position Should be Used with Caution. Int J Radiat Oncol Biol Phys. 2009. [DOI] [PMC free article] [PubMed]
- 15.Kaučić H., Kosmina D., Schwarz D., Mack A., Šobat H., Čehobašić A., et al. Stereotactic Ablative Radiotherapy Using CALYPSO® Extracranial Tracking for Intrafractional Tumor Motion Management—A New Potential Local Treatment for Unresectable Locally Advanced Pancreatic Cancer? Results from a Retrospective Study. Cancers (Basel) 2022;14(11) doi: 10.3390/cancers14112688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishi T., Matsuo Yukinori, Nakamura Akira, Nakamoto Yuji, Itasaka Satoshi, Mizowaki Takashi, Kaori Togashi M.H. Comparative evaluation of respiratory-gated and ungated FDG-PET for target volume definition in radiotherapy treatment planning for pancreatic cancer. Radiother Oncol. 2016;120(2):217–221. doi: 10.1016/j.radonc.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Zeng C., Xiong W., Li X., Reyngold M., Gewanter R.M., Cuaron J.J., et al. Intrafraction tumor motion during deep inspiration breath hold pancreatic cancer treatment. J Appl Clin Med Phys. 2019;20(5):37–43. doi: 10.1002/acm2.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner T.B., Nestle U., Adebahr S., Gkika E., Wiehle R., Baltas D., et al. Simultan integrierte Protektion: Ein neues Konzept für die Hochpräzisionsbestrahlung. Strahlentherapie Und Onkol. 2016;192(12):886–894. doi: 10.1007/s00066-016-1057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gkika E., Kostyszyn D., Fechter T., Moustakis C., Ernst F., Boda-Heggemann J., et al. Interobserver agreement on definition of the target volume in stereotactic radiotherapy for pancreatic adenocarcinoma using different imaging modalities. Strahlentherapie Und Onkol. 2023;199(11):973–981. doi: 10.1007/s00066-023-02085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eugene J. Koay MD, PhD a 1, Alexander N. Hanania MD, MPH b 1, William A. Hall MD c, Cullen M. Taniguchi MD, PhD a, Neal Rebueno CMD a, Sten Myrehaug MD d, Katharine L. Aitken MD e, Laura A. Dawson MD f, Christopher H. Crane MD g, Joseph M. Herman MD a BEM c. Clinical Investigation Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract Radiat Oncol. 2020;10(6):e495–507. [DOI] [PMC free article] [PubMed]
- 21.Yang W, Reznik R, Fraass BA, Nissen N, Hendifar A, Wachsman A, et al. Dosimetric evaluation of simultaneous integrated boost during stereotactic body radiation therapy for pancreatic cancer. Med Dosim [Internet]. 2015;40(1):47–52. Available from: http://dx.doi.org/10.1016/j.meddos.2014.09.001. [DOI] [PubMed]
- 22.Rudra S., Jiang N., Rosenberg S.A., Olsen J.R., Roach M.C., Wan L., et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassanzadeh C, Rudra S, Bommireddy A, Hawkins WG, Wang-Gillam A, Fields RC, et al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv Radiat Oncol [Internet]. 2021;6(1):100506. Available from: 10.1016/j.adro.2020.06.010. [DOI] [PMC free article] [PubMed]
- 24.Chuong MD, Bryant J, Mittauer KE, Hall M, Kotecha R, Alvarez D, et al. Ablative 5-Fraction Stereotactic Magnetic Resonance–Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract Radiat Oncol [Internet]. 2021;11(2):134–47. Available from: 10.1016/j.prro.2020.09.005. [DOI] [PubMed]
- 25.Parikh PJ, Lee P, Low DA, Kim J, Mittauer KE, Bassetti MF, et al. A Multi-Institutional Phase 2 Trial of Ablative 5-Fraction Stereotactic Magnetic Resonance–Guided On-Table Adaptive Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Cancer. Int J Radiat Oncol [Internet]. 2023;000(00). Available from: 10.1016/j.ijrobp.2023.05.023. [DOI] [PubMed]
- 26.Ladbury C., Amini A., Schwer A., Liu A., Williams T., Lee P. Clinical Applications of Magnetic Resonance-Guided Radiotherapy: A Narrative Review. Cancers (basel) 2023;15(11):1–13. doi: 10.3390/cancers15112916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edge S., Byrd D.R., Compton C.C., Fritz A.G., Greene F., Trotti A.E. Springer; New York, NY: 2010. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. [Google Scholar]
- 28.Stephen B. Edge, David R. Byrd, Carolyn C. Compton, April G. Fritz, Frederick L. Greene AT. AJCC Cancer Staging Handbook From the AJCC Cancer Staging Manual. 2010.
- 29.Keall P.J., Mageras G.S., Balter J.M., Emery R.S., Forster K.M., Jiang S.B., et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33(10):3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 30.Be K.H., Khor R., Joon D.L., Starvaggi B., Chao M., Ng S.P., et al. Long-term clinical outcomes of lipiodol marking using standard gastroscopy for image-guided radiotherapy of upper gastrointestinal cancers. World J Gastroenterol. 2021;27(42):7387–7401. doi: 10.3748/wjg.v27.i42.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freilich J.M., Spiess P.E., Biagioli M.C., Fernandez D.C., Shi E.J., Hunt D.C., et al. Lipiodol as a fiducial marker for image-guided radiation therapy for bladder cancer. Int Braz J Urol. 2014;40(2):190–197. doi: 10.1590/S1677-5538.IBJU.2014.02.08. [DOI] [PubMed] [Google Scholar]
- 32.Lyu Y., Ye S., Wang B. Comparison of metal versus plastic stent for preoperative biliary drainage in patients with pancreatic cancer undergoing neoadjuvant therapy: a meta-analysis and systematic review. BMC Gastroenterol. 2023;23(1):1–8. doi: 10.1186/s12876-023-02874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eric Pepin PhD a b, Lindsey Olsen MS b, Shahed Badiyan MD b, Faris Murad MD b, Daniel Mullady MD b, Andrea Wang-Gillam MD b, David Linehan MD b, Parag Parikh MD b JOM. Comparison of implanted fiducial markers and self-expandable metallic stents for pancreatic image guided radiation therapy localization. Pract Radiat Oncol. 2015;5(3):e193–9. [DOI] [PubMed]
- 34.Lee E.S., Lee J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J Gastroenterol. 2014;20(24):7864–7877. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas B. Brunner a, Karin Haustermans b, Florence Huguet c d, Alessio G. Morganti e, Somnath Mukherjee f g, Claus Belka h, Robert Krempien j, Maria A. Hawkins k, Vincenzo Valentini m l FR i. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother Oncol. 2021;154:60–9. [DOI] [PubMed]
- 36.Parisi S., Ferini G., Cacciola A., Lillo S., Tamburella C., Santacaterina A., et al. A non-surgical COMBO-therapy approach for locally advanced unresectable pancreatic adenocarcinoma: preliminary results of a prospective study. Radiol Med. 2022;127:214–219. doi: 10.1007/s11547-021-01441-w. [DOI] [PubMed] [Google Scholar]
- 37.Timmerman R. A Story of Hypofractionation and the Table on the Wall. Int J Radiat Oncol Biol Phys [internet] 2022;112(1):4–21. doi: 10.1016/j.ijrobp.2021.09.027. Available from: 10.1016/j.ijrobp.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE).v.5.0. Cancer Ther Eval Progr [Internet]. 2017;155. Available from: http://upen.terengganu.gov.my/index.php/2017.
- 39.Jimenez V.J.M. Criterios de Evaluación en Tumores Sólidos 1.1 (RECIST 1.1) en pacientes con Cáncer Primario y/o Metastásico o Enfermedad Metastásica Recurrente. Rev Sci. 2018;April 2019(1):1–6. [Google Scholar]
- 40.Parikh PJ, Lee P, Low D, Kim J, Mittauer KE, Bassetti MF, et al. Stereotactic MR-Guided On-Table Adaptive Radiation Therapy (SMART) for Patients with Borderline or Locally Advanced Pancreatic Cancer: Primary Endpoint Outcomes of a Prospective Phase II Multi-Center International Trial. Radiat Oncol Biol. 2022;.
- 41.Stephen Chin, Cynthia L Eccles, Alan McWilliam, Robert Chuter, Emma Walker, Philip Whitehurst, Joseph Berresford, Marcel Van Herk, Peter J Hoskin AC. Magnetic resonance-guided radiation therapy: A review. J Med Imaging Radiat Oncol. 2019. [DOI] [PubMed]
- 42.Das I.J., Yadav P., Mittal B.B. Emergence of MR-Linac in Radiation Oncology: Successes and Challenges of Riding on the MRgRT Bandwagon. J Clin Med. 2022;11(17) doi: 10.3390/jcm11175136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tozzi A., Comito T., Alongi F., Navarria P., Iftode C., Mancosu P., et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8(1):1–8. doi: 10.1186/1748-717X-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Comito T., Cozzi L., Zerbi A., Franzese C., Clerici E., Tozzi A., et al. Clinical results of stereotactic body radiotherapy (SBRT) in the treatment of isolated local recurrence of pancreatic cancer after R0 surgery: A retrospective study. Eur J Surg Oncol [internet] 2017;43(4):735–742. doi: 10.1016/j.ejso.2016.12.012. Available from: [DOI] [PubMed] [Google Scholar]
- 45.Herman J.M., Chang D.T., Goodman K.A., Dholakia A.S., Raman S.P., Hacker-Prietz A., et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courtney P.T., Paravati A.J., Atwood T.F., Raja N., Zimmerman C.T., Fanta P.T., et al. Phase I Trial of Stereotactic Body Radiation Therapy Dose Escalation in Pancreatic Cancer. Int J Radiat Oncol Biol Phys [internet] 2021;110(4):1003–1012. doi: 10.1016/j.ijrobp.2021.02.008. Available from: 10.1016/j.ijrobp.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Mellon E.A., Hoffe S.E., Springett G.M., Frakes J.M., Strom T.J., Hodul P.J., et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol (madr) 2015;54(7):979–985. doi: 10.3109/0284186X.2015.1004367. [DOI] [PubMed] [Google Scholar]