Abstract
Water deficit is a critical obstacle that devastatingly impacts rice production, particularly in arid regions under current climatic fluctuations. Accordingly, it is decisive to reinforce the drought tolerance of rice by employing sustainable approaches to enhance global food security. The present study aimed at exploring the effect of exogenous application using different biostimulants on physiological, morphological, and yield attributes of diverse rice genotypes under water deficit and well-watered conditions in 2-year field trial. Three diverse rice genotypes (IRAT-112, Giza-178, and IR-64) were evaluated under well-watered (14400 m3/ha in total for the entire season) and water deficit (9170 m3/ha) conditions and were exogenously sprayed by nano-silicon, potassium sulfate, or proline. The results showed that drought stress substantially decreased all studied photosynthetic pigments, growth traits, and yield attributes compared to well-watered conditions. In contrast, antioxidant enzyme activities and osmoprotectants were considerably increased compared with those under well-watered conditions. However, the foliar application of nano-silicon, potassium sulfate, and proline substantially mitigated the deleterious effects of drought stress and markedly enhanced photosynthetic pigments, antioxidant enzyme activities, growth parameters, and yield contributing traits compared to untreated stressed control. Among the assessed treatments, foliar spray with nano-silicon or proline was more effective in promoting drought tolerance. The exogenous application of proline improved chlorophyll a, chlorophyll b, and carotenoids by 21.4, 19.6 and 21.0% followed by nano-silicon treatment, which enhanced chlorophyll a, chlorophyll b, and carotenoids by 21.1, 17.6 and 9.5% compared to untreated control. Besides, the application of proline demonstrated a superior improvement in the content of proline by 52.5% compared with the untreated control. Moreover, nano-silicon exhibited the maximum enhancement of catalase and peroxidase activity compared to the other treatments. The positive impacts of applied exogenously nano-silicon or proline significantly increased panicle length, number of panicles/plant, number of grains/panicle, fertility percentage, 1000-grain weight, panicle weight, and grain yield, compared to untreated plants under water deficit conditions. In addition, the physiological and agronomic performance of evaluated rice genotypes significantly contrasted under drought conditions. The genotype Giza-178 displayed the best performance under water deficit conditions compared with the other genotypes. Consequently, the integration of applied exogenously nano-silicon or proline with tolerant rice genotype as Giza-178 is an efficient approach to ameliorating drought tolerance and achieving agricultural sustainability under water-scarce conditions in arid environments.
Keywords: Antioxidant enzymes, Drought stress, Genotype, Genetics, Heatmap mediterranean environments, Principal component biplot
1. Introduction
Rice (Oryza sativa) is the staple food for almost half of the world's people [1]. It is grown on about 165 × 106 ha, yielding 787 × 106 tonnes of rice annually [2]. Rice production should be doubled by 2050 due to global population growth [3]. With an estimated production of 6.1 million tonnes per year and an area of over 0.5 million hectares, Egypt is the biggest rice producer in the Middle East [4]. However, this production is endangered by limited water supply and rising population. Drought is widely recognized as the greatest threat to global rice production and food security. Water scarcity causes yield losses of up to 50% worldwide [5,6]. Climate change will increase drought and damage water resources. Rice requires around 2500 L of water to produce 1 kg of grain during its life cycle [7]. Water scarcity disrupts plant biochemical and physiological processes, reducing growth and production. Water deficits during germination, seedling growth, tillering, flowering, and grain filling cause high yield losses. At the vegetative stage, drought causes senescence of leaves, lessened photosynthesis, limited leaf extension and tillering, stunted plant growth, and ultimately decreased grain yield [8,9]. Water shortages generate reactive oxygen species (ROS) which can destroy plant metabolism by denaturing enzyme activity [10,11]. ROS peroxides biological membranes disrupting transport [12]. Plants have adaptation mechanisms to cope with water scarcity, such as increased leaf water potential, improved root architecture, improved osmotic adjustment, increased proline accumulation, and increased leaf rolling and stomatal closure [13,14]. They also have effective enzymatic and non-enzymatic antioxidant defenses against ROS-induced oxidative damage [15,16]. Consequently, it is necessary to enhance the drought tolerance of field crops and alleviate the negative effects of water scarcity by utilizing innovative appropriate approaches. Various agricultural practices including applied exogenously bio-stimulative chemicals, growth regulators, or osmoprotectants, can protect plants from drought stress and enhance tolerance [[17], [18], [19], [20]].
The exogenous application of safe and effective substances is a crucial strategy for improving plant growth, development, production, and quality under drought stress [21]. Silicon application boosts rice root growth, physiological processes, shoot weight, and crop yield by 10–20% [22]. It also helps in maintaining mineral nutrition, membrane integrity, stress tolerance defense, and photosynthesis efficiency. Nano-materials have recently developed and become a popular approach to alleviate various environmental challenges in multiple fields [23]. Research has shifted toward green nanoparticle manufacturing and its use to alleviate abiotic and biotic stresses. Silicon nanoparticles (Si-NPs) improve silicon uptake and confer stress tolerance in crops against various abiotic and biotic stresses. Due to its small microscopic size, it possesses better physicochemical properties than bulk silicon [24,25]. It displays a higher surface area, increased surface solubility and reactivity, and many well-categorized surface characteristics [26]. Particle size affects absorption and transit into plant cells [27]. Additionally, nanoparticles interact with plant cells to transport chemicals that regulate plant metabolism and various physiological functions. Hence, the applied foliar Si-NP improves growth and yield for rice plants grown in water-deficit conditions [28]. It also boosts leaf-relative water content and antioxidant enzyme activity and decreases oxidative stress under drought stress [29]. However, there is still a lack of understanding of how Si-NP minimizes and mitigates drought stress damage in plants.
Proline as a compatible solute plays a dual role in maintaining osmotic balance and preserving the integrity of cellular components and organelles [30,31]. Proline scavenges free radicals, protecting plants from drought-induced ROS damage [32,33]. Subsequently, the exogenous application of proline effectively mitigates the detrimental effects of drought [34,35]. This is achieved by enhancing turgor potential, reducing oxidative stress caused by ROS, maintaining osmotic balance, and boosting the activity of antioxidant enzymes [36]. As a result, photosynthesis is improved, and the amount of oxidative damage is reduced [37].
Potassium (K) is a vital nutrient for plants, as it regulates numerous physiological processes that govern plant growth, yield, and quality attributes [38]. It regulates the function of stomata in transpiration and photosynthesis, including the maintenance of photophosphorylation, plant turgor, enzyme activation, and the transfer of photoassimilates. applied exogenously of K can attenuate the deleterious effects of drought on rice by improving various physiological processes including protein synthesis, enzyme activation, photosynthesis, water relations, and stomatal movement [39]. Moreover, it enhances water stress tolerance by increasing nutrient and water uptake and reducing transpiration water loss [38]. Consequently, K foliar spray enhances plant growth, crop productivity, and drought tolerance of rice plants grown under water-stress conditions [40,41]. Research focusing on the exogenous application of nano-silicon, potassium sulfate, and proline and their potential contributions to alleviating the detrimental effects of drought stress while enhancing rice productivity under water-deficit conditions, particularly in field settings, is currently limited. Drawing on insights from earlier studies, we posited that the exogenous application of nano-silicon, potassium sulfate, or proline could significantly enhance rice plant growth and productivity by improving the efficiency of physiological parameters and antioxidant enzyme activities. In light of this, our study aimed to explore the impact of exogenously applied nano-silicon, potassium sulfate, or proline on photosynthetic characteristics, antioxidants, growth, and yield parameters of diverse rice genotypes grown under water deficit conditions. This knowledge can help identify effective drought tolerance inducers to enhance crop performance and tolerance to drought stress.
2. Materials and methods
2.1. Experimental site and plant materials
The trial was performed during the two summer growing seasons of 2021 and 2022 at Sakha Agricultural Research Station, Egypt (31°6′N 30°56′E). The site is described by an arid and hot climate with no precipitation in the summer season. The maximum and minimum temperatures and relative humidity of both growing seasons at the experimental site were collected and displayed in table S1. Soil samples were collected before sowing (0–30 cm depth) and were analyzed (table S2). The soil analysis displayed that the soil was clay throughout the profile (56.0% clay, 12.0% silt, and 32.0% sand). Electrical conductivity, organic matter, and pH were 3.04 dS m−1, 1.35 g kg−1, and 8.15, in the same order. Three diverse rice genotypes; IRAT-112 (drought tolerant); Giza −178 (moderately tolerant) and IR-64 (drought sensitive) were utilized in the current study. The selected genotypes were chosen based on their drought tolerance from previous preliminary screening trials (unpublished data). The origin and pedigree of the evaluated genotypes are shown in table S3.
2.2. Experimental design and treatment
The field trial was applied in a strip-split-plot design with three replications. The vertical plots were designated for irrigation treatments, the horizontal plots were dedicated to foliar applications, and the rice genotypes as subplot factor. The vertical plots were represented by 72 units (720 m2) and the horizontal plots were represented by 36 experimental units (360 m2). The seeds of each genotype were sown in the nursery on May 20th. At 30 days from sowing, seedlings were pulled from the nursery and transplanted into plots (10 m2) in 20 × 20 cm spacing using one seedling per hill. The irrigation was applied according to the standard practice in the studied region using surface irrigation. The amount of irrigation water for rice cultivation is determined annually in various regions of Egypt based on soil type, climatic variables, and water requirements by the Department of Water Requirement and Field Irrigation belongs to the Egyptian Ministry of Agriculture and Land Reclamation. The recommended irrigation amount, set at 14400 m3/ha, was intentionally decreased by 35% to induce water stress, resulting in an application of 9170 m3/ha. The applied water amount for each irrigation treatment was measured employing a flow meter. The applied foliar treatments were potassium sulfate K2SO4 (at the rate of 2 ml/L); proline (at the rate of 2 g/L) and nano-silicon (at the rate of 0.4 g/L) as nano-silicon dioxide 20–30 nm particle size with a purity of 99.5 %, versus untreated control. The untreated control plants were applied with distilled water)and a spreading agent only. Treatments were sprayed twice, at mid-tillering and panicle initiation. was applied in the present study. Calcium superphosphate (15.5% P2O5) was applied in nursery land at the rate of 50 kg P2O5 ha−1 before plowing. After the last ploughing and directly before sowing Nitrogen was added at 165 kg N/ha using urea form (46.0% N). The field trial was fertilized before plowing with 50 kg P2O5 ha−1. Potassium fertilizer was applied in two equal doses after transplanting at 30 and 45 days by adding 60 kg K2O/ha using potassium sulfate form (48% K2O).
2.3. Studied traits
2.3.1. Physiological Measurements
Chlorophyll a and b and carotenoid (mg/g FW) contents were recorded at the heading stage following the method of Peng [42]. To begin, five fresh leaves were carefully washed to eliminate impurities. Subsequently, 2 g of leaf tissue was homogenized in 80% acetone using a mortar and pestle. After centrifugation, the resulting supernatants were utilized to measure absorbance at 663 nm, 645 nm, and 470 nm using a spectrophotometer. From these absorbance readings, the concentrations of chlorophyll a, chlorophyll b, and carotenoids were calculated (mg/g fresh weight).
Proline content was recorded at the panicle initiation stage as described in the method of Bates et al. [43]. Samples of 0.5 g of rice leaf were collected and homogenized in 4 ml of 3% sulfosalicylic acid using a mortar and pestle. After storing the homogenates at 5 °C for 24 h, they were centrifuged at 3000 rpm and room temperature for 5 min. The resulting supernatants were combined with 4 ml of acidic ninhydrin reagent, mixed, and heated in a water bath at 100 °C for 60 min. Following cooling, 4 ml of toluene was added. The upper toluene layer was separated, and its absorbance was measured at 520 nm using a spectrophotometer. Proline concentration was determined using a standard curve and expressed as μmol of proline per gram of fresh weight.
At the panicle initiation stage, the enzymatic antioxidant activity was assessed by freezing leaf samples in liquid nitrogen to prepare extracts. Catalase activity (unit/mg protein) was determined following the technique outlined by Aebi [44]. For the enzyme extract, 0.5 mL of 0.2 M H2O2 in 10 mM K-phosphate buffer (pH 7.0) was employed before the analysis. The catalase enzyme activity was measured using a spectrophotometer at 240 nm, tracking the consumed H2O2. Peroxidase activity (unit/mg protein) was recorded as described by Vetter et al. [45]. This involved combining 100 μL of enzyme extract with 2.9 mL of assay solution composed of 50 mM phosphate-citrate buffer (pH 6.5), 0.03% hydrogen peroxide, and 0.1% o-phenylenediamine. The reaction was initiated with the extract, and the increase in absorbance at 430 nm was monitored for 5 min. The change in absorbance at 430 nm was determined over a 5-min period.
2.3.2. Agronomic traits
Ten plants were randomly collected from each plot to record flag leaf area (cm2) according to Yoshida et al. [46] at the heading stage. Besides, plant height (PH) was recorded from the soil surface to the tip of the main panicle of each plant. Moreover, at harvesting, ten panicles were harvested at random from each plot to record panicle length, number of panicles/plant, number of grains/panicle, 1000-grain weight, panicle weight, and spikelet fertility. Spikelet fertility was calculated by dividing the filled spikelets from a panicle by the total spikelets. The grain yield was determined from a 6-m2 area in each experimental unit adjusted to 14% moisture content and converted to ton/ha.
2.4. Statistical analysis
The analysis of variance (ANOVA) was applied to explore the significant differences among applied treatments. R statistical software version 4.2.1 was utilized to analyze the obtained data. A strip-split-plot design with three replications, irrigation treatments as vertical strip, foliar applications as horizontal strip, and rice genotypes as the subplot factor. Tukey's HSD test was utilized for post-hoc analysis (p < 0.05). Moreover, the heatmap was applied with the package of RColorBrewer and biplot of the principal component with ggplot2 in R software to determine the relationship among studied traits and treatments.
3. Results
3.1. Physiological traits
Photosynthetic pigments were significantly (P ≤ 0.05) affected by irrigation treatments, foliar application, genotypic performance, and their interactions (Table 1). Chlorophyll a, chlorophyll b, and carotenoids levels significantly (P ≤ 0.05) reduced by 18.0, 29.5, and 14.3% under drought conditions, compared to regularly irrigated plants (Table 1). However, the foliar treatments using nano-silicon, potassium sulfate, or proline led to significant (P ≤ 0.05) increase in chlorophyll a, chlorophyll b, and carotenoids in comparison with untreated stressed plants. The best treatment was the foliage application of proline which improved chlorophyll a, chlorophyll b, and carotenoids by 21.4, 19.6 and 21.0% followed by nano-silicon treatment, which enhanced chlorophyll a, chlorophyll b, and carotenoids by 21.1, 17.6 and 9.5% compared with untreated control. The evaluated rice genotypes exhibited highly significant (P ≤ 0.01) variations in their responses to irrigation water treatments. The genotypes IRAT-112 and IR-64 displayed the highest chlorophyll a, chlorophyll b, and carotenoids under well-watered conditions, whereas Giza-178 possessed superior values under water deficit conditions (Fig. 1A–C). Likewise, the assessed genotypes exhibited contrasting responses to foliar-supplied treatments. The uppermost contents of chlorophyll a, chlorophyll b, and carotenoids were assigned for the genotype Giza-178 and IRAT-112 treated with foliar application of nano-silicon and proline, compared to their corresponding controls under water deficit conditions.
Table 1.
Impact of irrigation regimes and different exogenously applied substances on photosynthetic pigments of diverse rice genotypes over two summer seasons of 2021 and 2022.
| Studied Factor | Chlorophyll a (mg g−1 FW) |
Chlorophyll b (mg g−1 FW) |
Carotenoids (mg g−1 FW) |
|
|---|---|---|---|---|
| Irrigation | ||||
| Well-watered | 3.72 a | 2.61 a | 1.26 a | |
| Drought stress | 3.05 b | 1.84 b | 1.08 b | |
| Foliar application | ||||
| Untreated control | 2.94 c | 1.99 c | 1.05 c | |
| Nano-silicon | 3.56 a | 2.34 a | 1.15 b | |
| Potassium sulfate | 3.47 b | 2.20 b | 1.21 ab | |
| Proline | 3.57 a | 2.38 a | 1.27 a | |
| Genotypes | ||||
| IRRAT | 3.33 b | 2.36 a | 1.19 a | |
| G178 | 3.51 a | 2.28 ab | 1.21 a | |
| IR64 |
3.32 b |
2.04 b |
1.12 b |
|
| ANOVA | df | P value | ||
| Irrigation (IR) | 1 | 0.012 | <0.001 | <0.001 |
| Foliar application (FA) | 3 | 0.015 | <0.001 | <0.001 |
| Genotype (G) | 2 | <0.001 | <0.001 | <0.001 |
| Year (Y) | 1 | 0.342 | 0.092 | 0.072 |
| IR × FA | 3 | 0.007 | <0.001 | <0.001 |
| IR × G | 2 | <0.001 | <0.001 | <0.001 |
| IR × Y | 1 | 0.066 | 0.035 | 0.164 |
| FA × G | 6 | <0.001 | <0.001 | <0.001 |
| FA × Y | 3 | 0.153 | 0.083 | 0.048 |
| G × Y | 2 | 0.029 | 0.194 | 0.179 |
| IR × FA × G | 6 | <0.001 | <0.001 | <0.001 |
| IR × FA × G × Y | 3 | 0.337 | 0.694 | 0.952 |
Fig. 1.
Impact of different exogenously sprayed substances on chlorophyll a (A), chlorophyll b (B), and carotenoids (C) in diverse rice genotypes under well-watered and water deficit conditions. The bars on the top positioned above the columns of treatments correspond to Tukey's HSD (p ≤ 0.05). When the difference between two treatments extends beyond the HSD bar, it signifies their significant difference.
The irrigation treatments, exogenous application, genotypic performance, and their interactions exhibited highly significant (P ≤ 0.01) effects on the antioxidant enzymatic activity (peroxidase and catalase) and proline content (Table 2). Water deficit caused a substantial elevation in the activities of catalase, peroxidase, and proline content, by 42.08%, 33.23 %, and 140.20%, respectively, compared to well-watered treatment. The exogenous application of nano-silicon, potassium sulfate, and proline significantly (P ≤ 0.05) enhanced these parameters compared with untreated plants. The maximum enhancement of catalase and peroxidase activity was recorded by the foliar spray of nano-silicon (35.1 and 32.2 respectively). Otherwise, the application of proline demonstrated a superior improvement in the content of proline by 52.5% compared with the untreated control. The evaluated rice genotypes exhibited highly significant (P ≤ 0.01) variations in their responses to irrigation water treatments and foliar applications. It was noticed that the genotype Giza-178 showed the maximum values of catalase, peroxidase, and proline content. Moreover, both genotypes Giza-178 and IRAT-112 whether treated with nano-silicon or proline treatments achieved the uppermost values of catalase, peroxidase, and proline content under drought stress conditions (Fig. 2A–C)
Table 2.
Impact of irrigation regimes and different exogenously applied substances on the antioxidant enzymatic activity of diverse rice genotypes over two summer seasons of 2021 and 2022.
| Studied Factor | Catalase (Unit mg/protein) |
Peroxidase (Unit mg/protein) |
Proline content (μmol g/DW) |
|
|---|---|---|---|---|
| Irrigation | ||||
| Well-watered | 0.638 b | 1.58 b | 0.505 b | |
| Drought stress | 0.817 a | 1.93 a | 0.977 a | |
| Foliar application | ||||
| Untreated control | 0.627 d | 1.49 c | 0.552 c | |
| Nano-silicon | 0.847 a | 1.97 a | 0.799 ab | |
| Potassium sulfate | 0.685 c | 1.76 b | 0.772 b | |
| Proline | 0.752 b | 1.80 b | 0.842 a | |
| Genotypes | ||||
| IRRAT | 0.674 b | 1.70 b | 0.623 c | |
| G178 | 0.792 a | 1.85 a | 0.835 a | |
| IR64 |
0.717 ab |
1.71 b |
0.765 b |
|
| ANOVA | df | P value | ||
| Irrigation (IR) | 1 | <0.001 | <0.001 | <0.001 |
| Foliar application (FA) | 3 | <0.001 | <0.001 | <0.001 |
| Genotype (G) | 2 | <0.001 | <0.001 | <0.001 |
| Year (Y) | 1 | 0.052 | 0.402 | 0.059 |
| IR × FA | 3 | <0.001 | <0.001 | <0.001 |
| IR × G | 2 | <0.001 | <0.001 | <0.001 |
| IR × Y | 1 | 0.093 | 0.267 | 0.402 |
| FA × G | 6 | <0.001 | <0.001 | <0.001 |
| FA × Y | 3 | 0.059 | 0.046 | 0.267 |
| G × Y | 2 | 0.076 | 0.057 | 0.065 |
| IR × FA × G | 6 | <0.001 | <0.001 | <0.001 |
| IR × FA × G × Y | 3 | 0.771 | 0.608 | 0.608 |
Fig. 2.
Impact of different exogenously sprayed substances on catalase activity (A), peroxidase activity (B), and proline content (C) in diverse rice genotypes under well-watered and water deficit conditions. The bars on the top positioned above the columns of treatments correspond to Tukey's HSD (p ≤ 0.05). When the difference between two treatments extends beyond the HSD bar, it signifies their significant difference.
3.2. Agronomic traits
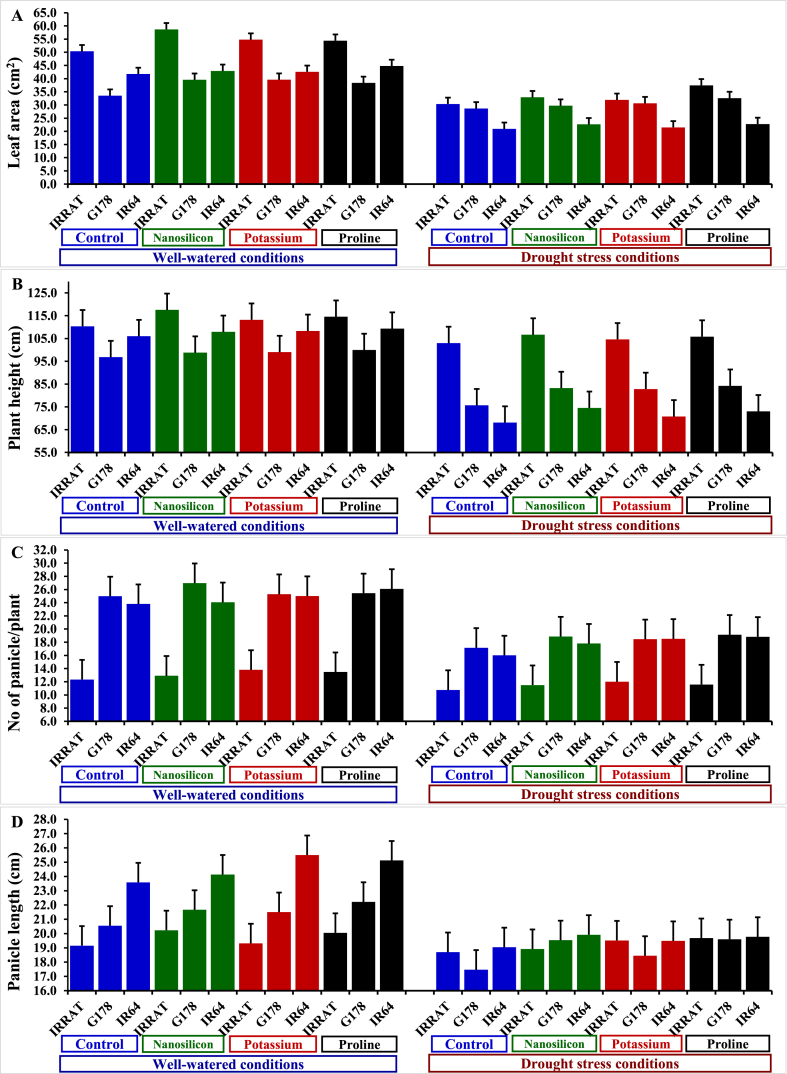
The evaluated agronomic traits were significantly (P ≤ 0.05) affected by irrigation treatment, exogenous application, genotypic performance, and their interactions (Table 3). Water deficit displayed an adverse impact on plant height and flag leaf area in comparison with well-watered conditions. There was a considerable reduction in plant height and flag leaf area by 19.4% and 36.8%, respectively, under water deficit conditions in comparison with those under complete irrigation conditions. Notwithstanding, the application of nano-silicon, potassium sulfate, and proline significantly increased flag leaf area by 10.1%, 7.5%, and 12.1%, respectively, and plant height by 5.1%, 3.4%, and 4.8%, in the same order, compared to untreated plants. The rice genotypes responded differently to irrigation treatments and foliar applications. The highest flag leaf area and plant height values belonged to IRAT-112 genotype, while IR-64 had the lowest values. Moreover, the genotype IRAT-112 treated with nano-silicon or proline treatments achieved superior flag leaf area and plant height under drought stress conditions (Fig. 3A–D).
Table 3.
Influence of irrigation regimes and different exogenously applied substances on some yield attributes of diverse rice genotypes over two summer seasons of 2021 and 2022.
| Studied Factor | Leaf area (cm2) |
Plant height (cm) |
No of panicles per plant |
Panicle length (cm) |
|
|---|---|---|---|---|---|
| Irrigation | |||||
| Well-watered | 45.11 a | 106.82 a | 21.18 a | 21.92 a | |
| Drought stress | 28.51 b | 86.05 b | 15.88 b | 19.17 b | |
| Foliar application | |||||
| Untreated control | 34.27 c | 93.33 c | 17.50 c | 19.75 c | |
| Nano-silicon | 37.74 a | 98.12 a | 18.68 b | 20.73 b | |
| Potassium sulfate | 36.84 b | 96.47 b | 18.84 ab | 20.63 b | |
| Proline | 38.40 a | 97.82 a | 19.09 a | 21.07 a | |
| Genotypes | |||||
| IRRAT | 43.86 a | 109.48 a | 12.29 b | 19.45 b | |
| G178 | 34.08 b | 90.07 b | 22.03 a | 20.12 b | |
| IR64 |
32.49 b |
89.75 b |
21.26 a |
22.07 a |
|
| ANOVA | df | P value | |||
| Irrigation (IR) | 1 | <0.001 | <0.001 | 0.011 | <0.001 |
| Foliar application (FA) | 3 | <0.001 | 0.032 | 0.006 | <0.001 |
| Genotype (G) | 2 | <0.001 | <0.001 | <0.001 | <0.001 |
| Year (Y) | 1 | 0.061 | 0.044 | 0.331 | 0.325 |
| IR × F | 3 | <0.001 | <0.001 | 0.022 | 0.008 |
| IR × G | 2 | <0.001 | <0.001 | <0.001 | <0.001 |
| IR × Y | 1 | 0.082 | 0.021 | 0.611 | 0.984 |
| FA × G | 6 | <0.001 | <0.001 | 0.379 | <0.001 |
| FA × Y | 3 | 0.051 | 0.855 | 0.498 | 0.021 |
| G × Y | 2 | 0.067 | 0.054 | 0.761 | 0.768 |
| IR × FA × G | 6 | <0.001 | <0.001 | 0.002 | <0.001 |
| IR × FA × G × Y | 3 | 0.225 | 0.065 | 0.527 | 0.093 |
Fig. 3.
Impact of different exogenously sprayed substances on leaf area (A), plant height (B), number of branches per plant (C), and panicle length (D) in diverse rice genotypes under well-watered and water deficit conditions. The bars on the top positioned above the columns of treatments correspond to Tukey's HSD (p ≤ 0.05). When the difference between two treatments extends beyond the HSD bar, it signifies their significant difference.
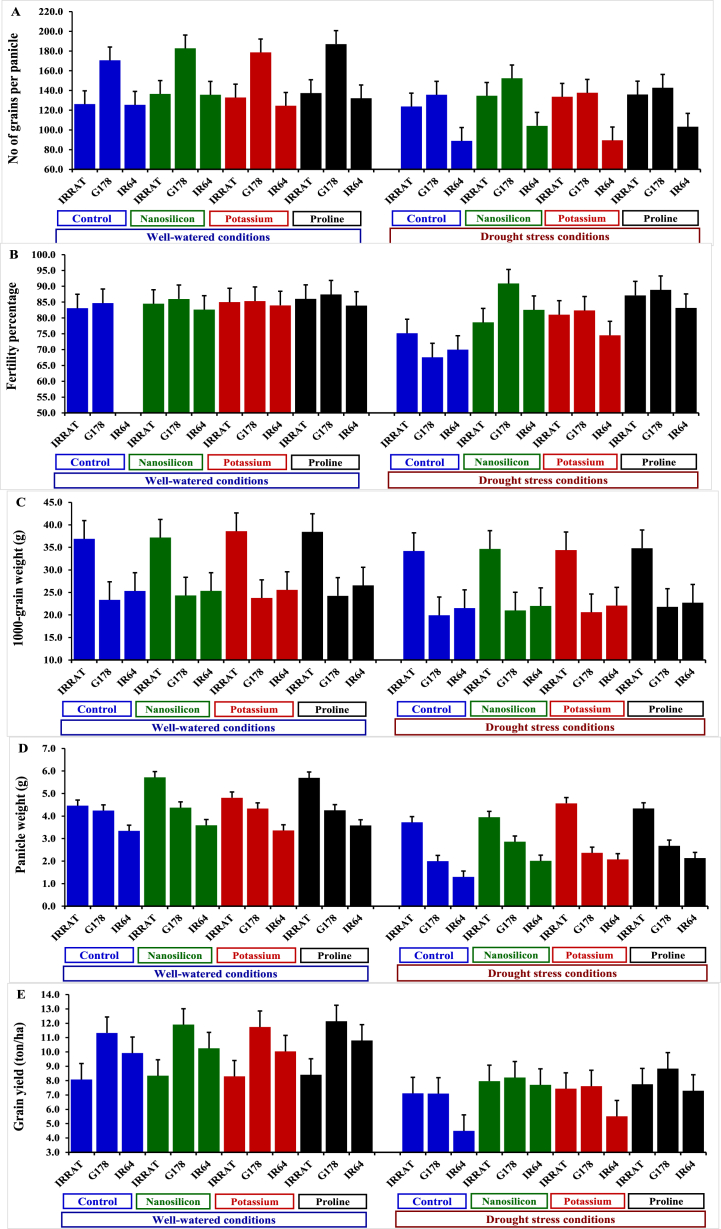
Deficient irrigation significantly decreased panicle length, number of panicles/plant, number of grains/panicle, fertility percentage, 1000-grain weight, panicle weight, and rice grain yield by 12.6%, 25.1%, 16.3%, 6.5%, 11.4%, 34.3%, and 28.2%, in the same order, in comparison with well-watered conditions (Table 4). However, the application of nano-silicon, potassium sulfate, and proline mitigated the devastating impacts of water deficit and considerably promoted all the aforementioned characteristics. Foliar treatment of proline displayed a superior boost of yield attributes followed by nano-silicon treatment. It significantly increased panicle length, number of panicles/plant, number of grains/panicle, fertility percentage, 1000-grain weight, panicle weight, and grain yield by 9.1, 6.7, 8.8, 13.1, 4.58, 18.9, and 14.9% compared to untreated plants. The evaluated genotypes displayed varied responses to irrigation treatments. The genotypes Giza-178 and IR-64 recorded superior yield traits under well-watered conditions, while Giza-178 and IRAT-112 possessed superior performance under drought stress conditions. Besides, the assessed genotypes displayed contrasting responses to the exogenous application. The highest yield traits were assigned for the genotypes Giza-178 and IRAT-112 treated with the foliar application of nano-silicon and proline, compared to their corresponding controls under water deficit conditions (Fig. 4A–E). Otherwise, the genotype IR64 exhibited the lowest agronomic performance under water deficit conditions under different foliar applications.
Table 4.
Influence of irrigation regimes and different exogenously applied substances on grain yield contributing traits of diverse rice genotypes over two summer seasons of 2021 and 2022.
| Studied Factor | No of grains per panicle |
Fertility (%) |
1000 grain Weight (g) |
Panicle weight (g) |
Grain yield (ton/ha) |
|
|---|---|---|---|---|---|---|
| Irrigation | ||||||
| Well-watered | 147.46 a | 84.77 a | 29.13 a | 4.31 a | 10.10 a | |
| Drought stress | 123.49 b | 80.15 b | 25.81 b | 2.83 b | 7.25 b | |
| Foliar application | ||||||
| Untreated control | 128.40 c | 76.09 d | 26.87 c | 3.18 c | 8.01 b | |
| Nano-silicon | 141.00 a | 84.19 b | 27.42 b | 3.75 a | 9.06 a | |
| Potassium sulfate | 132.78 b | 82.03 c | 27.50 b | 3.58 b | 8.44 b | |
| Proline | 139.72 a | 86.08 a | 28.10 a | 3.78 a | 9.20 a | |
| Genotypes | ||||||
| IRRAT | 132.58 b | 82.56 b | 36.15 a | 4.66 a | 7.92 b | |
| G178 | 160.94 a | 84.14 a | 22.38 b | 3.39 b | 9.86 a | |
| IR64 |
112.91 c |
80.09 c |
23.89 b |
2.67 b |
8.25 b |
|
| ANOVA | df | P value | ||||
| Irrigation (IR) | 1 | 0.018 | <0.001 | <0.001 | <0.001 | <0.001 |
| Foliar application (FA) | 3 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Genotype (G) | 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Year (Y) | 1 | 0.071 | 0.072 | 0.112 | 0.039 | 0.041 |
| IR × F | 3 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| IR × G | 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| IR × Y | 1 | 0.225 | 0.018 | 0.089 | 0.048 | 0.022 |
| FA × G | 6 | <0.001 | <0.001 | <0.001 | 0.051 | <0.001 |
| FA × Y | 3 | 0.083 | 0.061 | 0.087 | 0.064 | 0.073 |
| G × Y | 2 | 0.001 | 0.001 | 0.076 | 0.082 | 0.062 |
| IR × FA × G | 6 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| IR × FA × G × Y | 3 | 0.836 | 0.856 | 0.246 | 0.636 | 0.994 |
Fig. 4.
Impact of different exogenously sprayed substances on number of grains per panicle (A), fertility percentage (B), 1000-grain weight (C), panicle weight (D), and grain yield (E) in diverse rice genotypes under well-watered and water deficit conditions. The bars on the top positioned above the columns of treatments correspond to Tukey's HSD (p ≤ 0.05). When the difference between two treatments extends beyond the HSD bar, it signifies their significant difference.
3.3. Relationship among the evaluated treatments and traits
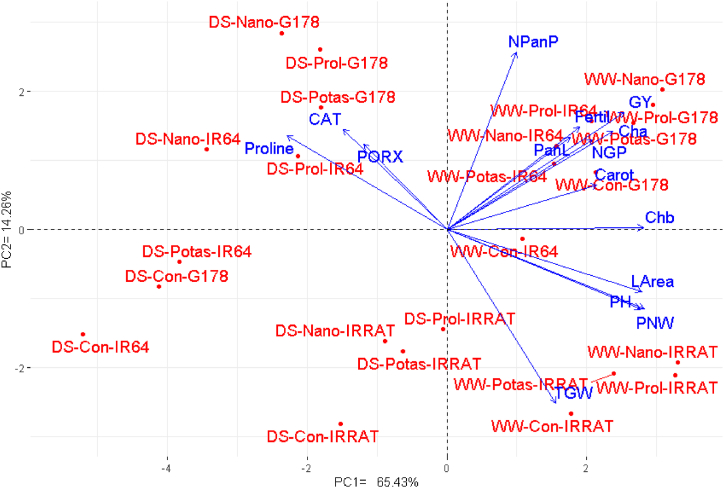
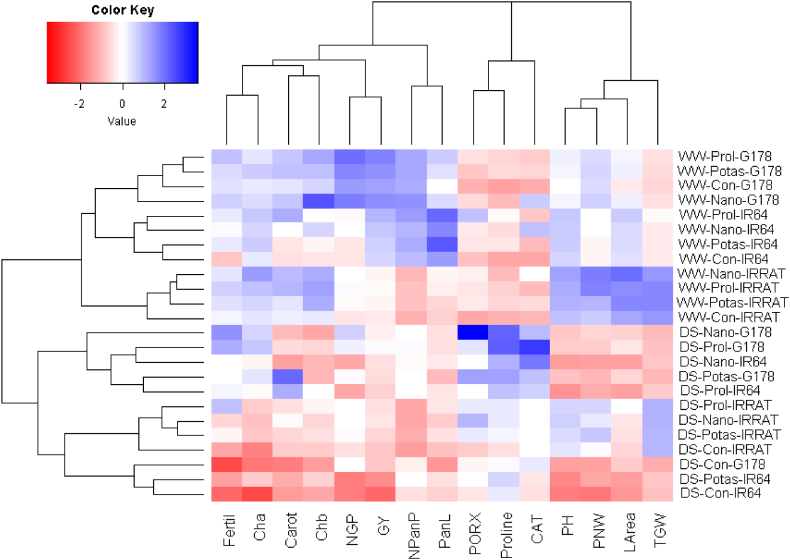
Principal component analysis (PC) was applied to study the association between the studied treatments and evaluated characteristics. The first two PCs displayed the most variability (79.69%) presenting 65.43% by PC1 and 14.26% by PC2 (Fig. 5). The PC1 was correlated with irrigation regimes, water deficit conditions were situated on the negative side while the well-watered conditions were positioned on the positive side. The PCA2 seems to correspond with rice genotypes from bottom to top as IRRAT-112, IR64, and Giza-178. Moreover, the genotype Giza-178 treated with nano-silicon and proline exhibited the highest values of most studied physiological and agronomic traits under well-watered and drought stress. Likewise, the heatmap based on the evaluated agronomic and physiological characters separated irrigation treatments, foliar applications, and rice genotypes into different clusters (Fig. 6). The irrigation treatment was the primary separating factor of the principal clusters. The genotype Giza-178 treated with proline and nano-silicon displayed the maximum values for most evaluated characteristics under drought stress (represented in blue). The evaluated characteristics presented by parallel vectors revealed a robust positive association, though those assigned almost opposite displayed a substantially negative relationship. The studied traits could be separated into two groups one contained photosynthetic pigments, growth, and agronomic traits, whereas the other contained enzymatic antioxidants and proline content. A robust positive relationship was identified among the traits within each group, whereas a negative relationship was perceived between the two groups.
Fig. 5.
Principal component biplot for the assessed rice genotypes; IRRAT, G178, and IR64 and exogenously applied substances untreated control (Con), nano-silicon (Nano), potassium sulfate (Potas), and proline (Prol) under water deficit (DS) and well-watered (WW) conditions. Cha: chlorophyll a, Chb: chlorophyll b, Carot: carotenoids, CAT: catalase, PORX: peroxidase, Proline: proline content, LArea: leaf area, PH: plant height, PanL: panicle length, NPanP: number of panicles/plant, NGP: number of grains/panicle, 1000-grain weight, Fertil: fertility percentage, PNW: panicle weight, GY: grain yield. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Heatmap and hierarchical clustering for the evaluated rice genotypes; IRRAT, G178, and IR64 and applied-foliar substances; untreated control (Con), nano-silicon (Nano), potassium sulfate (Potas), and proline (Prol) under water deficit (DS) and well-watered (WW) conditions. Cha: chlorophyll a, Chb: chlorophyll b, Carot: carotenoids, CAT: catalase, PORX: peroxidase, Proline: proline content, LArea: leaf area, PH: plant height, PanL: panicle length, NPanP: number of panicles/plant, NGP: number of grains/panicle, 1000-grain weight, Fertil: fertility percentage, PNW: panicle weight, GY: grain yield.
Red and blue colors were designated to high and low values for the studied trait, in the same order. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Drought is one of the main environmental stresses that pose great obstacles to rice production, especially in arid regions. Furthermore, it is projected to become more severe and frequent due to decreasing water supply and climate changes. Accordingly, it is decisive to recognize innovative attempts to boost drought tolerance, particularly under global climate fluctuations. In the present study, three safe and efficient substances, nano-silicon, potassium sulfate, and proline, were exogenously applied to explore and compare their influence on physio-biochemical, growth, and agronomic performance of three different rice genotypes grown under drought stress conditions in a 2-year field trial. Besides, to determine which tolerance inducer could be recommended for enhancing drought tolerance and boosting rice productivity under drought stress conditions in arid regions. The obtained results displayed that the studied irrigation treatments, foliar applications, assessed rice genotypes, and their interactions displayed substantial effects on most studied characteristics. In Egypt, the summer season is characterized by a hot and dry climate, with no occurrences of precipitation. Subsequently, the water deficit regime considerably reduced photosynthetic pigments, growth, and yield traits of all the evaluated rice genotypes. Otherwise, antioxidant enzymes and proline content were raised compared to non-stressed conditions. These results may be due to that proline and antioxidant enzymes are the most important osmoprotectants under stress conditions.
Photosynthetic pigments are important indicators for assessing drought tolerance in rice [47]. The obtained findings revealed that water deficit significantly decreased the contents of chlorophyll a and b as well as carotenoids of all tested rice genotypes compared with well-watered conditions. The reduction might be attributed to the detrimental effects of water deficit on ribulose-1,5-biphosphate activity, causing elevated chloroplast degradation and structural disarray. Consequently, this condition results in decreased chlorophyll content [48]. Likewise, growth traits such as flag leaf area and plant height considerably declined under water deficit conditions. This adverse effect could stem from reduced water uptake, resulting in decreased plant cell division and expansion [49]. Studies such as those conducted by Manickavelu et al. [50], Afroz and Akhtar [51], Yang et al. [52] elucidated the detrimental impact of water scarcity on rice, affecting photosynthetic pigments, flag leaf area, and plant height.
The exogenous application of nano-silicon, potassium sulfate, and proline significantly increased the content of chlorophyll a and b and carotenoids in the leaves of all tested genotypes under drought stress compared to untreated plants. These findings suggested an effective role for the applied substances in enhancing photosynthesis, plant growth, and productivity of rice under water deficit conditions. Similarly, previous studies of Elshayb et al. [28], Alharbi et al. [29], Hanif et al. [34], Ahmad et al. [38], Ali et al. [53] disclosed the positive impact of nano-silicon, potassium sulfate, and proline on promoting photosynthetic pigments while reducing oxidative damages, consequently improving plant performance under drought stress. Moreover, Farooq et al. [54], Mathur and Roy [55] deduced that these substances can aid in protecting and enhancing rice plants to counteract drought-induced oxidative damage. The uppermost enhancement was assigned for foliar-supplied with nano-silicon followed by proline. Rios et al. [56] demonstrated that foliar application of nano-silicon is more rapidly absorbed by plants compared to bulk silicon, resulting in more beneficial effects under drought stress. Esmaili et al. [57], Alharbi et al. [58] disclosed the application of nano-silicon promotes plant growth and development by expanding the leaf surface area for increased light absorption, regulating stomatal aperture, enhancing photosynthesis, and improving physiological processes like leaf water status, osmoregulation, and nutrient uptake. These beneficial effects significantly mitigated the deleterious effects of water deficit in rice. Likewise, the pivotal role of proline in increasing previous characteristics is attributed to its ability to protect the plasma membrane, and cytoplasmic enzymes, stabilize membranes, and substantially prevent chlorophyll degradation under water scarcity as demonstrated by Hosseinifard et al. [37], Bhaskara et al. [59].
The assessed rice genotypes displayed highly significant variations in their responses to irrigation water treatments and foliar applications. The evaluated genotypes accumulated catalase, peroxidase, and proline content in different trends under drought stress. The genotype Giza-178 exhibited the highest content of catalase, peroxidase, and proline under drought stress conditions. The accumulation of proline is a critical response of plant cells under water deficit to promote osmotic adjustment and antioxidant system [60]. Furthermore, proline has a decisive role in tolerance to drought stress due to its capability to detoxicate the generated detrimental free radical species [61]. Furthermore, the foliar applications of the three substances enhanced the proline under drought conditions compared with untreated plants. Exogenously sprayed proline exhibited the uppermost contents compared with the other applications. These results indicated that the increased accumulation of proline under water deficit conditions may be associated with the exogenous application of proline. Similarly, Abdelaal et al. [62], El-Bauome et al. [63] manifested that the exogenous foliar application of proline enhanced proline content and ameliorated tolerance to water deficit stress in rice. Besides, Farooq et al. [64], Ghaffari et al. [65] and AlKahtani et al. [36] proved an increase in levels of proline under drought stress following foliage proline treatment.
Water deficiency conditions considerably increased antioxidant enzymatic CAT and POX activities in all the evaluated genotypes compared with the well-watered conditions. Drought stress induces oxidative stress, as well as elevated levels of reactive oxygen species (ROS). To combat ROS toxicity, a highly effective antioxidant defense mechanism is necessary. It has been suggested that the increase in antioxidant activity exerts an ameliorative effect on drought stress [66]. The foliar application of nano-silicon, potassium sulfate, and proline significantly enhanced the activity of CAT and POX compared with stressed untreated plants. The enhanced activity of the antioxidant enzymes upon the application of these substances facilitated the conversion of H2O2 into non-toxic compounds (H2O and O2), thereby protecting the plants from the harmful impacts of drought stress [62]. These findings demonstrated the valuable impacts of the foliar application of these substances in ameliorating tolerance to water deficit by altering the antioxidant activities and detoxifying ROS. In agreement with our findings, similar results were noted in various crops under drought stress conditions [21,53,67]. Drought-stressed rice genotypes showed a decline in grain and its related traits compared to those of normal conditions. Elshayb et al. [28], Sakran et al. [68] depicted that the decrease in yield-contributing traits might resulted from reduced leaf area, decreased chlorophyll content, and disrupted carbohydrate metabolism, resulting in reduced assimilate transport and increased reproductive abortion. Additionally, this decline might be attributed to the adverse impact of water deficit on growth parameters, resulting from reduced water availability [69]. This reduction in nutrient uptake and organic carbon likely led to a decline in reproductive tillers, fertility percentage, 1000-grain weight, and ultimately, grain yield [52]. The exogenous application of nano-silicon, potassium sulfate, and proline significantly enhanced yield traits in treated plants under water deficit compared to untreated stressed plants. Notably, proline and nano-silicon exhibited greater effectiveness in improving the yield of the evaluated genotypes. These outcomes underscore the significant role of proline and nano-silicon in boosting grain yield under drought stress conditions. This enhancement was attributed to their impact on increasing chlorophyll content, antioxidant activity, proline content, number of panicles, plant growth, fertility percentage, and 1000-grain weight.
The evaluated genotypes showed significant alterations in all the measured traits under normal and water-deficit conditions. However, deficit irrigation treatment altered the performance of the genotypes more than normal watering. From this perspective Hassan et al. [70], Yadav et al. [6], Sedeek et al. [71], and Hussain et al. [72] elucidated highly significant variations among rice genotypes under normal and water-deficit conditions. The genotype Giza 178 displayed the uppermost physiological performance compared with other genotypes under drought stress conditions. Consequently, this genotype sustained to be tolerant under water deficit by stimulating photosynthetic efficiency, proline content, and enzymatic antioxidants. These improvements were exhibited in enhanced agronomic performance under drought stress conditions, in particular under the foliage application of nano-silicon, potassium sulfate, and proline. The interaction between irrigation level, foliar application, and assessed genotypes showed substantial impacts on most evaluated traits. Generally, under drought stress, Giza 178 genotype in combination with applied exogenously proline or nano-silicon exhibited superior enzymatic antioxidants, photosynthetic pigments, growth, and yield-related traits, compared to untreated treatment.
The PC-biplot, heatmap, and hierarchical clustering are helpful methods for exploring relationships among studied variables and parameters [[73], [74], [75]]. The obtained results of the heatmap and PCA biplot reinforced the positive impacts of exogenously applied substances on all evaluated parameters. The heatmap and PCA biplot exhibited that photosynthetic pigments, growth, and agronomic traits were positively related to foliar-applied proline and nano-silicon, in particular with the genotype Giza 178 under drought stress. These findings confirmed that the foliar application of both substances ameliorated the physiological characteristics, growth, and yield traits. Subsequently, the exogenously applied proline and nano-silicon could be a beneficial attempt to stimulate rice productivity, particularly under drought stress conditions. Besides, the PCA biplot showed that growth traits and most physiological parameters were positively associated with yield traits. Subsequently, selection for these traits is efficient for increasing rice productivity under water deficit conditions [[76], [77], [78]].
5. Conclusions
Water deficit significantly reduced photosynthetic pigment contents, growth parameters, and grain yield traits. Conversely, antioxidant enzyme activities and osmoprotectants showed significant increases under drought stress compared to well-watered conditions. However, foliar applications of nano-silicon, potassium sulfate, or proline significantly alleviated the adverse effects of water deficit and considerably enhanced all studied characteristics. Proline and nano-silicon emerged as the most effective treatments, further enhancing rice performance and drought stress tolerance. The assessed rice genotypes exhibited diverse responses to irrigation treatments and exogenously applied substances. The genotype Giza 178 when treated with foliar proline or nano-silicon demonstrated superior growth, photosynthetic pigments, enzymatic antioxidants, and yield traits compared to untreated plants under water deficit conditions. Thus, foliar application of proline or nano-silicon stands as a promising approach to enhance drought tolerance in high-yielding rice genotypes facing water scarcity.
Data availability statement
The data presented in this study are available upon request from the corresponding author.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R402), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Mohamed S. Abd-El-Aty: Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Mohamed M. Kamara: Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Walid H. Elgamal: Validation, Methodology, Investigation, Data curation. Mohamed I. Mesbah: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. ElSayed A. Abomarzoka: Visualization, Validation, Methodology, Investigation. Khairiah M. Alwutayd: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Formal analysis, Data curation. Elsayed Mansour: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Investigation, Formal analysis, Conceptualization. Imen Ben Abdelmalek: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Funding acquisition, Formal analysis. Said I. Behiry: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Formal analysis, Data curation. Ameina S. Almoshadak: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Funding acquisition, Formal analysis. Khaled Abdelaal: Writing – review & editing, Visualization, Validation, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R402), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26077.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Rezvi H.U.A., Tahjib Ui Arif M., Azim M.A., Tumpa T.A., Tipu M.M.H., Najnine F., Dawood M.F., Skalicky M., Brestič M. Rice and food security: climate change implications and the future prospects for nutritional security. Food Energy Secur. 2023;12:e430. [Google Scholar]
- 2.FAOSTAT . Statistical Database; 2023. Food and Agriculture Organization of the United Nations.http://www.fao.org/faostat/en/#data Availabe online: [Google Scholar]
- 3.Roy S.C., Shil P. Assessment of genetic heritability in rice breeding lines based on morphological traits and caryopsis ultrastructure. Sci. Rep. 2020;10:1–17. doi: 10.1038/s41598-020-63976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem K.F., Sallam A. Analysis of population structure and genetic diversity of Egyptian and exotic rice (Oryza sativa L.) genotypes. C. R. Biol. 2016;339:1–9. doi: 10.1016/j.crvi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 5.ElShamey E.A., Sakran R.M., ElSayed M.A., Aloufi S., Alharthi B., Alqurashi M., Mansour E., Abd El-Moneim D. Heterosis and combining ability for floral and yield characters in rice using cytoplasmic male sterility system. Saudi J. Biol. Sci. 2022;29:3727–3738. doi: 10.1016/j.sjbs.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav N., Sevanthi A.C., Pandey R., Chinnusamy V., Singh A.K., Singh N.K. Physiological response and agronomic performance of drought tolerance mutants of Aus rice cultivar Nagina 22 (Oryza sativa L) Field Crops Res. 2023;290 [Google Scholar]
- 7.Bañoc D.M., Yamauchi A., Kamoshita A., Wade L.J., Pardales J.R. Genotypic variations in response of lateral root development to fluctuating soil moisture in rice. Plant Prod. Sci. 2000;3:335–343. [Google Scholar]
- 8.Farooq M., Wahid A., Lee D.J., Ito O., Siddique K.H. Advances in drought resistance of rice. Crit. Rev. Plant Sci. 2009;28:199–217. [Google Scholar]
- 9.Rebolledo M., Luquet D., Courtois B., Henry A., Soulié J.-C., Rouan L., Dingkuhn M. Can early vigour occur in combination with drought tolerance and efficient water use in rice genotypes? Funct. Plant Biol. 2013;40:582–594. doi: 10.1071/FP12312. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. Handbook of plant and crop physiology. 2021:617–658. [Google Scholar]
- 11.Mansour E., El-Sobky E.-S.E., Abdul-Hamid M.I., Abdallah E., Zedan A.M., Serag A.M., Silvar C., El-Hendawy S., Desoky E.-S.M. Enhancing drought tolerance and water productivity of diverse maize hybrids (Zea mays) using exogenously applied biostimulants under varying irrigation levels. Agronomy. 2023;13:1320. [Google Scholar]
- 12.Nordberg J., Arnér E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y., Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015;72:673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desoky E.S.M., Alharbi K., Rady M.M., Elnahal A.S., Selem E., Arnaout S.M., Mansour E. Physiological, biochemical, anatomical, and agronomic responses of sesame to exogenously applied polyamines under different irrigation regimes. Agronomy. 2023;13:875. [Google Scholar]
- 15.Seleiman M.F., Al-Suhaibani N., Ali N., Akmal M., Alotaibi M., Refay Y., Dindaroglu T., Abdul-Wajid H.H., Battaglia M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021;10:259. doi: 10.3390/plants10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selem E., Hassan A.A., Awad M.F., Mansour E., Desoky E.-S.M. Impact of exogenously sprayed antioxidants on physio-biochemical, agronomic, and quality parameters of potato in salt-affected soil. Plants. 2022;11:210. doi: 10.3390/plants11020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafez E.M., Gowayed S.M., Nehela Y., Sakran R.M., Rady A.M., Awadalla A., Omara A.E.-D., Alowaiesh B.F. Incorporated biochar-based soil amendment and exogenous glycine betaine foliar application ameliorate rice (Oryza sativa L.) tolerance and resilience to osmotic stress. Plants. 2021;10:1930. doi: 10.3390/plants10091930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaya C., Ugurlar F., Ashraf M., Ahmad P. Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem. 2023;196:431–443. doi: 10.1016/j.plaphy.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Hussein H.A.A., Alshammari S.O., Abd El-Sadek M.E., Kenawy S.K., Badawy A.A. The promotive effect of putrescine on growth, biochemical constituents, and yield of wheat (Triticum aestivum L.) plants under water stress. Agriculture. 2023;13:587. [Google Scholar]
- 20.Sheikhalipour M., Gohari G., Esmaielpour B., Panahirad S., Milani M.H., Kulak M., Janda T. Melatonin and TiO2 NPs application-induced changes in growth, photosynthesis, antioxidant enzymes activities and secondary metabolites in stevia (Stevia rebaudiana bertoni) under drought stress conditions. J. Plant Growth Regul. 2023;42:2023–2040. [Google Scholar]
- 21.Desoky E.-S.M., Mansour E., El-Sobky E.-S.E., Abdul-Hamid M.I., Taha T.F., Elakkad H.A., Arnaout S.M., Eid R.S., El-Tarabily K.A., Yasin M.A. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.637783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizwan M., Ali S., Ibrahim M., Farid M., Adrees M., Bharwana S.A., Zia-ur-Rehman M., Qayyum M.F., Abbas F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ. Sci. Pollut. Res. 2015;22:15416–15431. doi: 10.1007/s11356-015-5305-x. [DOI] [PubMed] [Google Scholar]
- 23.Ansari S.A., Husain Q. Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol. Adv. 2012;30:512–523. doi: 10.1016/j.biotechadv.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Prasad T., Sudhakar P., Sreenivasulu Y., Latha P., Munaswamy V., Reddy K.R., Sreeprasad T., Sajanlal P., Pradeep T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012;35:905–927. [Google Scholar]
- 25.Rastogi A., Tripathi D.K., Yadav S., Chauhan D.K., Živčák M., Ghorbanpour M., El-Sheery N.I., Brestic M. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019;9:1–11. doi: 10.1007/s13205-019-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qados A.A., Moftah A.E. Influence of silicon and nano-silicon on germination, growth and yield of faba bean (Vicia faba L.) under salt stress conditions. Am. J. Exp. Agric. 2015;5:509–524. [Google Scholar]
- 27.Sarkar M.M., Mathur P., Mitsui T., Roy S. A review on functionalized silica nanoparticle amendment on plant growth and development under stress. Plant Growth Regul. 2022;98:421–437. [Google Scholar]
- 28.Elshayb O.M., Nada A.M., Ibrahim H.M., Amin H.E., Atta A.M. Application of silica nanoparticles for improving growth, yield, and enzymatic antioxidant for the hybrid rice ehr1 growing under water regime conditions. Materials. 2021;14:1150. doi: 10.3390/ma14051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alharbi K., Osman H.S., Rashwan E., Hafez E.M., Omara A.E.-D. Stimulating the growth, anabolism, antioxidants, and yield of rice plants grown under salt stress by combined application of bacterial inoculants and nano-silicon. Plants. 2022;11:3431. doi: 10.3390/plants11243431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Ervin E., Evanylo G., Haering K. Impact of biosolids on hormone metabolism in drought-stressed tall fescue. Crop Sci. 2009;49:1893–1901. [Google Scholar]
- 31.Habibullah M., Sarkar S., Islam M.M., Ahmed K.U., Rahman M.Z., Awad M.F., ElSayed A.I., Mansour E., Hossain M.S. Assessing the response of diverse sesame genotypes to waterlogging durations at different plant growth stages. Plants. 2021;10:2294. doi: 10.3390/plants10112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seki M., Umezawa T., Urano K., Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Kamara M.M., Rehan M., Mohamed A.M., El Mantawy R.F., Kheir A.M., Abd El-Moneim D., Safhi F.A., Alshamrani S.M., Hafez E.M., Behiry S.I. Genetic potential and inheritance patterns of physiological, agronomic and quality traits in bread wheat under normal and water deficit conditions. Plants. 2022;11:952. doi: 10.3390/plants11070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanif S., Saleem M.F., Sarwar M., Irshad M., Shakoor A., Wahid M.A., Khan H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J. Plant Growth Regul. 2021;40:305–312. [Google Scholar]
- 35.Zulfiqar F., Ashraf M. Proline alleviates abiotic stress induced oxidative stress in plants. J. Plant Growth Regul. 2022:1–23. [Google Scholar]
- 36.AlKahtani M.D., Hafez Y.M., Attia K., Rashwan E., Husnain L.A., AlGwaiz H.I., Abdelaal K.A. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants. 2021;10:398. doi: 10.3390/antiox10030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseinifard M., Stefaniak S., Ghorbani Javid M., Soltani E., Wojtyla Ł., Garnczarska M. Contribution of exogenous proline to abiotic stresses tolerance in plants: a review. Int. J. Mol. Sci. 2022;23:5186. doi: 10.3390/ijms23095186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad Z., Anjum S., Waraich E.A., Ayub M.A., Ahmad T., Tariq R.M.S., Ahmad R., Iqbal M.A. Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress–A review. J. Plant Nutr. 2018;41:1734–1743. [Google Scholar]
- 39.Zain N.A.M., Ismail M.R. Effects of potassium rates and types on growth, leaf gas exchange and biochemical changes in rice (Oryza sativa) planted under cyclic water stress. Agric. Water Manag. 2016;164:83–90. [Google Scholar]
- 40.Pervez H., Ashraf M., Makhdum M. Influence of potassium nutrition on gas exchange characteristics and water relations in cotton (Gossypium hirsutum L.) Photosynthetica. 2004;42:251–255. [Google Scholar]
- 41.Ullah H., Rahimi A.Z., Datta A. Growth and yield of lowland rice as influenced by potassium application and cultivation method under alternate wetting and drying water regime. J. Plant Nutr. 2019;42:1529–1542. [Google Scholar]
- 42.Peng Y., Liu E. A comparative study of methods of extracting chlorophyll. Acta Agric. Univ. Pekinensis. 1992;18:247–250. [Google Scholar]
- 43.Bates L., Waldren R.a., Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- 44.Aebi H. Methods in Enzymology. Elsevier; 1984. [13] Catalase in vitro; pp. 121–126. [Google Scholar]
- 45.Vetter J., Steinberg M., Nelson A. Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 1958;6:39–41. [Google Scholar]
- 46.Yoshida S., Forno D.A., Cock J.H. 1971. Laboratory Manual for Physiological Studies of Rice; pp. 69–72. Los Baños, Philippines. [Google Scholar]
- 47.Swapna S., Shylaraj K.S. Screening for osmotic stress responses in rice varieties under drought condition. Rice Sci. 2017;24:253–263. [Google Scholar]
- 48.Siddiqui M., Al-Khaishany M., Al-Qutami M., Al-Whaibi M., Grover A., Ali H., Al-Wahibi M., Bukhari N. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 2015;16:10214–10227. doi: 10.3390/ijms160510214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyer J.S. Cell enlargement and growth-induced water potentials. Physiol. Plant. 1988;73:311–316. [Google Scholar]
- 50.Manickavelu A., Nadarajan N., Ganesh S.K., Gnanamalar R.P., Chandra Babu R. Drought tolerance in rice: morphological and molecular genetic consideration. Plant Growth Regul. 2006;50:121–138. [Google Scholar]
- 51.Afroz R., Akhtar R. Climate Change and Rice Production: Adaptation Strategies and Capacity. Book Publisher International (a part of SCIENCEDOMAIN International); 2021. Climate change and rice production: adaptation strategies and capacity; pp. 1–2. [Google Scholar]
- 52.Yang X., Wang B., Chen L., Li P., Cao C. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-40161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali Q., Anwar F., Ashraf M., Saari N., Perveen R. Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int. J. Mol. Sci. 2013;14:818–835. doi: 10.3390/ijms14010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farooq M., Wahid A., Lee D.J., Cheema S.A., Aziz T. Drought stress: comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J. Agron. Crop Sci. 2010;196:336–345. [Google Scholar]
- 55.Mathur P., Roy S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020;157:114–127. doi: 10.1016/j.plaphy.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Rios J.J., Martínez-Ballesta M.C., Ruiz J.M., Blasco B., Carvajal M. Silicon-mediated improvement in plant salinity tolerance: the role of aquaporins. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esmaili S., Amiri V. Tavallali B. Nano-Silicon complexes enhance growth, yield, water relations and mineral composition in tanacetum parthenium under water deficit stress. Silicon. 2020;13:2493–2508. [Google Scholar]
- 58.Alharbi K., Rashwan E., Mohamed H.H., Awadalla A., Omara A.E.-D., Hafez E.M., Alshaal T. Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants. 2022;11:2026. doi: 10.3390/plants11152026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhaskara G.B., Yang T.-H., Verslues P.E. Dynamic proline metabolism: importance and regulation in water limited environments. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gholami Zali A., Ehsanzadeh P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crops Prod. 2018;111:133–140. [Google Scholar]
- 61.Mannan M.A., Tithi M.A., Islam M.R., Al Mamun M.A., Mia S., Rahman M.Z., Awad M.F., ElSayed A.I., Mansour E., Hossain M.S. Soil and foliar applications of zinc sulfate and iron sulfate alleviate the destructive impacts of drought stress in wheat. Cereal Res. Commun. 2022;50:1279–1289. [Google Scholar]
- 62.Abdelaal K.A.A., Attia K.A., Alamery S.F., El-Afry M.M., Ghazy A.I., Tantawy D.S., Al-Doss A.A., El-Shawy E.-S.E., Abu-Elsaoud A.M., Hafez Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability. 2020;12:1736. [Google Scholar]
- 63.El-Bauome H.A., Abdeldaym E.A., Abd El-Hady M.A.M., Darwish D.B.E., Alsubeie M.S., El-Mogy M.M., Basahi M.A., Al-Qahtani S.M., Al-Harbi N.A., Alzuaibr F.M., Alasmari A., Ismail I.A., Dessoky E.S., Doklega S.M.A. Exogenous proline, methionine, and melatonin stimulate growth, quality, and drought tolerance in cauliflower plants. Agriculture. 2022;12:1301. [Google Scholar]
- 64.Farooq M., Nawaz A., Chaudhry M.A.M., Indrasti R., Rehman A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J. Agron. Crop Sci. 2017;203:464–472. [Google Scholar]
- 65.Ghaffari H., Tadayon M.R., Nadeem M., Cheema M., Razmjoo J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019;41 [Google Scholar]
- 66.Zhang N., Zhao B., Zhang H.-J., Weeda S., Yang C., Yang Z.-C., Ren S., Guo Y.-D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.) J. Pineal Res. 2012;54:15–23. doi: 10.1111/j.1600-079X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 67.Wasaya A., Affan M., Ahmad Yasir T., Atique ur R., Mubeen K., Rehman H.u., Ali M., Nawaz F., Galal A., Iqbal M.A., Islam M.S., El-Sharnouby M., Rahman M.H.u., El Sabagh A. Foliar Potassium sulfate application improved photosynthetic characteristics, water relations and seedling growth of drought-stressed maize. Atmosphere. 2021;12:663. [Google Scholar]
- 68.Sakran R.M., Ghazy M.I., Rehan M., Alsohim A.S., Mansour E. Molecular genetic diversity and combining ability for some physiological and agronomic traits in rice under well-watered and water-deficit conditions. Plants. 2022;11:702. doi: 10.3390/plants11050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venuprasad R., Lafitte H.R., Atlin G.N. Response to direct selection for grain yield under drought stress in rice. Crop Sci. 2007;47:285–293. [Google Scholar]
- 70.Hassan H.M., Hadifa A.A., El-Leithy S.A., Batool M., Sherif A., Al-Ashkar I., Ueda A., Rahman M.A., Hossain M.A., Elsabagh A. Variable level of genetic dominance controls important agronomic traits in rice populations under water deficit condition. PeerJ. 2023;11 doi: 10.7717/peerj.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sedeek S., Mazal T.M., Osman M., Hefeina A., EL-Kallawy W., Bleih E.M. Genetic diversity among some of rice genotypes under water shortage. J. Plant Prod. Sci. 2022;13:929–936. [Google Scholar]
- 72.Hussain T., Hussain N., Tahir M., Raina A., Ikram S., Maqbool S., Fraz Ali M., Duangpan S. Impacts of drought stress on water use efficiency and grain productivity of rice and utilization of genotypic variability to combat climate change. Agronomy. 2022;12:2518. [Google Scholar]
- 73.Kasoma C., Shimelis H., Laing M.D., Shayanowako A.I., Mathew I. Revealing the genetic diversity of maize (Zea mays L.) populations by phenotypic traits and DArTseq markers for variable resistance to fall armyworm. Genet. Resour. Crop Evol. 2021;68:243–259. [Google Scholar]
- 74.Kocak B.A., Kilinc F., Bardak A., Güngör H., Dokuyucu T., Akkaya A., Dumlupinar Z. Association mapping of germination and some early seedling stage traits of a Turkish origin oat collection. Turk. J. Field Crops. 2022;27:41–50. [Google Scholar]
- 75.Mkhabela S.S., Shimelis H., Gerrano A.S., Mashilo J. Drought tolerance assessment of okra (Abelmoschus esculentus [l.] moench) accessions based on leaf gas exchange and chlorophyll fluorescence. Life. 2023;13:682. doi: 10.3390/life13030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gedam P.A., Thangasamy A., Shirsat D.V., Ghosh S., Bhagat K., Sogam O.A., Gupta A., Mahajan V., Soumia P., Salunkhe V.N. Screening of onion (Allium cepa L.) genotypes for drought tolerance using physiological and yield based indices through multivariate analysis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.600371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan M.A., Iqbal H.S.M.A., Akram M.W. Characterization of drought tolerance in bread wheat genotypes using physiological indices. Gesunde Pflanz. 2022;74:467–475. [Google Scholar]
- 78.Sun F., Chen Q., Chen Q., Jiang M., Qu Y. Yield-based drought tolerance index evaluates the drought tolerance of cotton germplasm lines in the interaction of genotype-by-environment. PeerJ. 2023;11 doi: 10.7717/peerj.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.