Abstract
Background
The 1-min sit-to-stand test (1STST) is a practical tool to evaluate physical capacity. The aim of this study was to assess the impact of tezacaftor and ivacaftor on functional exercise capacity, muscle strength and symptoms in people with cystic fibrosis (PwCF).
Methods
The assessments were performed during the first year of tezacaftor and ivacaftor using the 1STST, 6-min walk test (6MWT), MicroFET2 dynamometer®, CF Questionnaire-Revised (CFQ-R), Leicester Cough Questionnaire (LCQ). Forced expiratory volume in 1 s (FEV1), body mass index (BMI), pancreatic sufficiency status, genotype and microbiologic data were also collected.
Results
Fifty-four PwCF participated to the study and took at least one dose of tezacaftor-ivacaftor. Mean age was 26y±10 (±SD), median BMI 20.9 kg/m2 (interquartile range) (19.4; 23.5) and mean FEV1 82 percent of predicted values (%PV) ± 21. Significant correlations were found at baseline between the 1STST and the 6MWT (r = 0.617, p < 0.0001), the quadriceps strength (r = 0.6556, p < 0.0001) and the FEV1 (r = 0.29, p = 0.03). After one year of treatment, the 1STST increased significantly in terms of number of repetitions (n) (median 50 versus 58.5, p < 0.0001), %PV (101.1 versus 115.2%PV, p = 0.0003) and n times weight in kg (2885 versus 3389nxkg, p < 0.0001). The 6MWT distance and quadriceps strength were not modified after treatment but during the 6MWT, oxygen desaturation decreased significantly. FEV1, BMI, CFQ-R, LCQ improved as previously demonstrated.
Conclusion
After one year of tezacaftor and ivacaftor, the 1STST improves, suggesting that the 1STST seems more responsive than the 6MWT and the MicroFET2 dynamometer® to assess the effects of CFTR modulators.
Keywords: Cystic fibrosis, Quadriceps strength, Functional exercise capacity, One-minute sit-to-stand test, CFTR modulators
Highlights
-
•
Muscular strength is easily evaluated by the 1STST in patients with CF.
-
•
The 1STST at baseline and after one year of CFTR modulator therapy is correlated to 6MWT, MVCQ, CFQ-R and FEV1.
-
•
Tezacaftor and ivacaftor improve the 1STST and reduce oxygen desaturation during 6MWT in patients with CF.
1. Introduction
Cystic fibrosis (CF) is an autosomal recessive, multisystemic disease caused by defects in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Although the prognosis is mainly linked to the respiratory status, due to chronic bronchial and lung infections along with excessive inflammation and local immune dysfunction [1], skeletal muscle weakness is also one of the best markers of prognosis and survival in CF [2]. In addition, it is also associated with reduced aerobic capacity and impaired quality of life [3].
Previously, Troosters et al. found that quadriceps force was reduced in 56% of people with CF (PwCF) and that it correlated to the 6-min walk distance [4]. The 1-min sit-to-stand test (1STST), which is easier to perform in clinical practice, is a valuable tool to assess muscle strength and functional exercise capacity and was previously considered as a valid alternative to the 6-min walk test (6MWT) [5]. Muscular weakness in CF was also confirmed using the 1STST and it was furtherly correlated to quadriceps force [6], to the maximal oxygen consumption (VO2) during cardiopulmonary exercise testing (CPET) [7,8] and recently to the 6MWT distance in children with CF [9].
With the emergence of CFTR modulators, the perspectives related to the muscles change. These modulators enhance or even restore the function of the CFTR protein which is expressed in bronchial smooth muscle [10], in cardiac muscle [11] and in skeletal muscle [12]. One case report on 2 patients taking ivacaftor showed conflicting results on CPET [13] and one randomized placebo-controlled trial showed no effect on peak VO2 after 28 days of ivacaftor in G551D mutated patients even if the duration of exercise was enhanced [14]. In a case series, after 6 months of tezacaftor and ivacaftor, all 8 patients showed an improvement of peak VO2 [15]. The same results were shown recently in a study following 3 patients after 6 weeks of elexacaftor, tezacaftor and ivacaftor [16].
Given these observations, our hypothesis was that CFTR modulators can improve the physical capacity of PwCF. Thus, the primary aim of this study was to measure the impact of tezacaftor and ivacaftor on functional exercise capacity by measuring the 1STST, and the 6MWT, as well as the quadriceps muscle strength using a dynamometer during one year of treatment. The secondary aims were to assess the impact of CFTR modulators on the quality of life (through the revised CF Questionnaire (CFQ-R)) and cough (Leicester Cough Questionnaire (LCQ)), as well as the correlations between the functional exercise capacity and clinical parameters.
2. Methods
2.1. Study population
The PwCF were prospectively recruited in the CF Reference Centre of the Cliniques universitaires Saint-Luc between April 2021 and January 2022. The study was approved by the local ethic committee (2021/21JUI/282) and registered (B4032021000078). We respected the Helsinki declaration and good clinical practice. All participants signed a written informed consent. The study followed the STROBE guidelines.
Inclusion criteria consisted of a confirmed diagnosis of CF (following Farrell definition [17]) and an eligibility to tezacaftor and ivacaftor in Belgium. Exclusion criteria consisted of pregnancy, disturbed musculoskeletal conditions (interfering with the evaluation of the muscle strength and/or motility), or a medical history of lung transplant. Age, body mass index (BMI), microbiology, forced expiratory volume in 1 s (FEV1), genotype and pancreatic sufficiency status of participants were collected.
2.2. Outcomes
To assess the functional exercise capacity and the muscular strength, the 1STST, the 6MWT, and the MicroFET2 dynamometer® (Hoggan, Salt Lake City, USA) were used. Measurements were made during a routine visit before the starting of the CFTR modulator treatment as well as after 1, 4, 7 and 12 months of therapy.
2.2.1. 1STST
The 1STST was performed using a chair of 46 cm in height, without armrests, following a recent standard procedure [18]. Briefly, the operator demonstrated it and all participants performed a training test to reduce the learning effect. Patients were required to fully stand up with straight legs and then sit down with the buttocks on the chair, the knees at 90° flexion, with arms crossed on chest, as many times as possible during 1 min. The encouragements were standardised and when necessary, a stop time was authorised. The results of the 1STST were expressed in number of repetitions per minute, percent of predicted values (%PV) using reference values from Haile et al. and Strassman et al. [19,20] as well as in the number of repetitions as the product of the bodyweight (nxkg). In addition, heart rate, pulsed oxygen saturation, dyspnea (visual analog scale (VAS) scored from 0 to 10) and muscular fatigue (VAS) were recorded before and after the test. At 2 min rest, heart rate and pulsed oxygen saturation were collected again to evaluate recovery.
2.2.2. 6MWT
The 6MWT was realised following the ATS statements [21]. Briefly, the patient was asked to walk the longest distance possible back and forth in a 30 m hallway during 6 min. Heart rate, pulsed oxygen saturation, dyspnea VAS scored from 0 to 10) and muscular fatigue (VAS) were recorded before and after the test. At 2 min rest, heart rate and pulsed oxygen saturation were collected again to evaluate recovery. The results were then expressed as absolute and relative values [22].
2.2.3. Maximal voluntary contraction of the quadriceps (MVCQ)
The MVCQ of both legs were measured with the MicroFET2 dynamometer® stabilised with a hand-belt, during at least 3 consecutive efforts with less than 10% of variability [23,24]. Standardised encouragements were given. The MVCQ was expressed in Newton-meter (N-m) by means of the lever arm measurement and in %PV [25].
2.2.4. Symptoms
Quality of life and cough were assessed using the CF Questionnaire-Revised (CFQ-R) and the Leicester Cough Questionnaire (LCQ), respectively.
2.3. Statistical analysis
The sample size was determined assuming 5 repetitions to be clinically relevant [7] and a drop off of 10%. The study required a sample size of 48 pairs to achieve a power of 80% and a level of significance of 5% (two sided), to detect a mean of the differences of 5 between pairs, assuming the standard deviation of the differences to be 12. The Gaussian distribution of the data was evaluated with Kolmogorov and Shapiro-Wilk tests. Descriptive statistics were shown by means and standard deviation (SD) for parametric data or medians and interquartile range (IQR) for non-parametric data. Missing values are specified in each figure. A Wilcoxon test was performed for the comparison between 2 groups of paired non-parametric data, and a paired student t-test for the comparison of 2 groups of paired parametric data (minimum n = 20). Mixed-effects models were used for multiple comparisons. Correlation coefficients were determined by Pearson test if parametric and by Spearman test if non-parametric. A p value less than 0.05 was considered as statistically significant. Statistical analyses were performed using IBM SPSS Statistics (version 27 for Windows, Chicago, USA) and figures were made using GraphPad Prism (version 9.00 for Windows; GraphPad Software, San Diego, USA; www.graphpad.com).
3. Results
3.1. Study population at baseline
We recruited 54 stable patients with CF at a routine visit. All patients took at least one dose of the medication and only one patient stopped the treatment during the study due to headaches and one other shifted to elexacaftor-tezacaftor-ivacaftor. Full characteristics of the patients are summarised in Table 1.
Table 1.
Characteristics of study population at baseline.
| Subjects, n | 54 |
|---|---|
| Sex (F/M) | 24/30 |
| Age, yrs | 26 ± 10 |
| Children (<18 yrs)/Adults | 12/42 |
| F508del/F508del (yes/no) | 49/5 |
| Pancreatic sufficiency (yes/no) | 4/50 |
| Pseudomonas aeruginosa infection (yes/no) | 19/35 |
| Days of intravenous antibiotics the year before tezacaftor/ivacaftor | 15 (0–36.25) |
| Smokers (yes/no) | 0/54 |
| BMI, kg/m2 | 20.9 (19.4; 23.5) |
| FEV1, %PV | 82 ± 21 |
| 1STST, n | 49.2 ± 12.9 |
| 1STST, %PV | 101.1 ± 27.6 |
| 1STST, nxkg | 2885 ± 916.1 |
| 6MWT distance, m | 620.7 ± 59.4 |
| 6MWT %PV | 87.02 (79.37; 94.49) |
| MVCQ, N-m | 67.16 ± 21.11 |
| MVCQ, %PV | 48.43 (38.41; 54.65) |
Data are mean ± SD if parametric or median (interquartile ranges) if non-parametric (Kolmogorov and Shapiro-Wilk tests). Definition of abbreviations: n, number; F, female; M, male; yrs, years; BMI, body mass index; kg/m2, kilogram divided by the square meter; FEV1, forced expiratory volume in 1 s; PV, predicted values; 1STST, the 1-min sit-to-stand test; nxkg, number of repetitions as a product of bodyweight expressed in kilogram; 6MWT, 6-min walk test; m, meter; MVCQ, maximal isometric voluntary contraction of the quadriceps; N-m, Newton-meter.
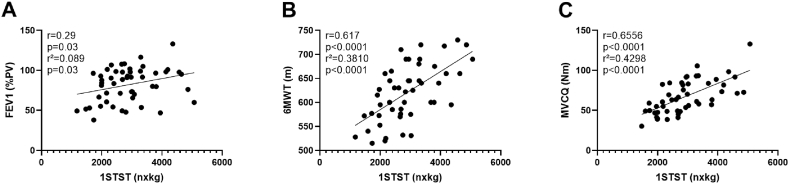
Most of the 54 patients were adults (78%), F508del homozygous (91%) and pancreatic insufficient (93%). Nineteen were infected by Pseudomonas aeruginosa. The mean FEV1 was 82%PV and the median BMI was 20.9 kg/m2. Mean 1STST results were not reduced at baseline in this cohort while the median MVCQ was strongly decreased compared to the general population. The 6MWT distance was below the lower limit of the norm in 13 patients. We confirmed a correlation between the 1STST and the FEV1 (Fig. 1A), the 6MWT distance (Fig. 1B), MVCQ (Fig. 1C) and CFQ-R (r = 0.4059, p = 0.004). At baseline, the 6MWT distance in meter and %PV was correlated with CFQ-R (r = 0.404, p = 0.006 and r = 0.41, p = 0.005 respectively). No correlations between MVCQ and clinical parameters were found.
Fig. 1.
Correlations at baseline between 1STST and lung function n = 51, Spearman correlation and simple linear regression (A), 6MWT distance n = 49, Pearson correlation and simple linear regression (B) and MVCQ n = 49, Pearson correlation and simple linear regression (C). FEV1, forced expiratory volume in 1 s; PV, predicted values; 1STST, the 1-min sit-to-stand test; nxkg, number of repetitions as a product of bodyweight expressed in kilogram; 6MWT, 6-min walk test; m, meter; MVCQ, maximal isometric voluntary contraction of the quadriceps; N-m, Newton-meter.
3.2. Increased 1STST results after one year of tezacaftor and ivacaftor
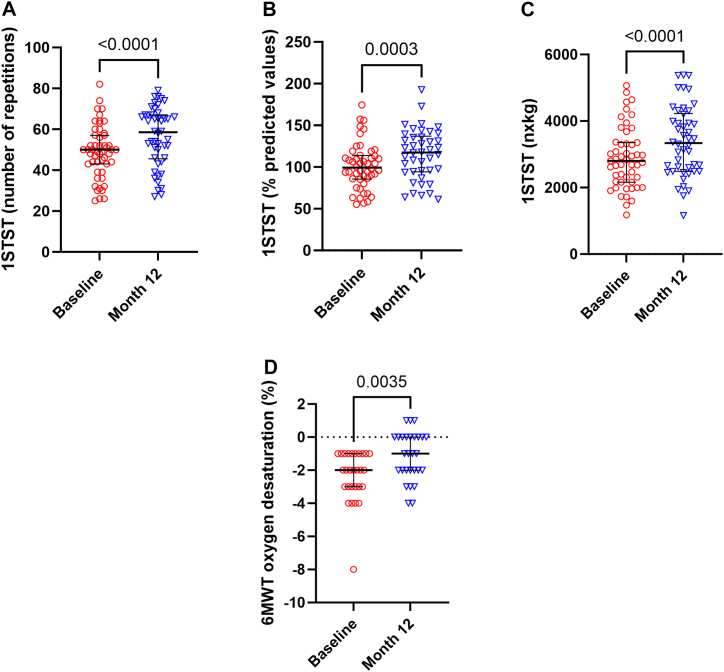
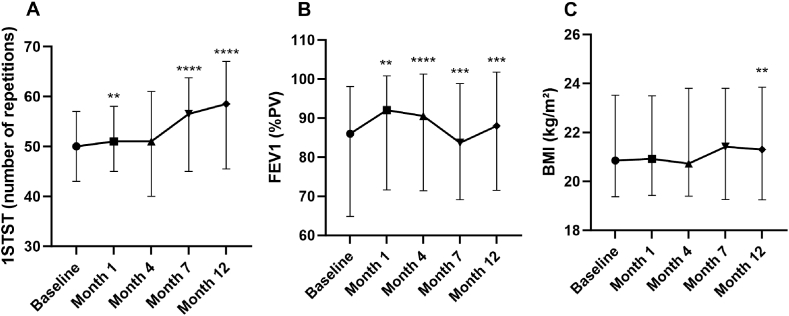
We observed a significant increase of the number of repetitions during 1STST: 50 (43–57) (median (IQR)) before versus 58.5 (45.5–67) (p < 0.0001) after treatment (Fig. 2A), as well as 101.1%PV (±27.6) (mean ± SD) versus 115.2%PV (±30.5) (p = 0.0003) (Figs. 2B) and 2885nxkg (±916.1) versus 3389nxkg (±1058) (p < 0.0001) (Fig. 2C). The mean absolute increase of repetition was 7.26 and 32 patients improved their score by 5 or more repetitions, resulting in clinical relevance. After 1 and 4 months, we observed a slight increase (although not significant at 4 months) while at 7 months the observed increase in repetitions became significant (Fig. 3A).
Fig. 2.
1STST before and after 12 months of tezacaftor and ivacaftor expressed in number of repetitions n = 51 and 46, Wilcoxon test (A), % predicted values n = 48 and 42, paired t-test (B) and nxkg n = 51 and 46, paired t-test (C). 6MWT oxygen desaturation in people with CF having oxygen desaturation during the test n = 28 and 28, Wilcoxon test (D). 1STST, 1-min sit-to-stand test; nxkg, number of repetitions as a product of bodyweight expressed in kilogram; 6MWT, 6-min walk test.
Fig. 3.
Evolution of 1STST expressed in number of repetitions n = 51, n = 51, n = 47, n = 40, n = 46 (A), FEV1 n = 54, n = 53, n = 50, n = 45 and n = 50 (B) and BMI n = 54, n = 53, n = 51, n = 46 and n = 50 (B) after 1, 4, 7 and 12 months of treatment. 1STST, the 1-min sit-to-stand test; FEV1, forced expiratory volume in 1 s; PV, predicted values; BMI, body mass index; kg/m2, kilogram divided by the square meter. Mixed-effects model, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to baseline.
This improved physical condition was not seen with the 6MWT distance (620.7 m–87.4%PV versus 614.1 m–87.3%PV; mean absolute difference −4.04 m, p = 0.632 and p = 0.736) or the MCVQ (67.16N-m – 48.33%PV versus 67.95N-m – 47.78%PV, p = 0.496 and p = 0.806) at 12 months nor at the other time points. Even though the walked distance did not increase, the 28 patients showing an oxygen desaturation at the end of the 6MWT at baseline, presented less desaturation after 12 months of treatment (Fig. 2D). At 12 months, we still found a significant correlation between 1STST and the FEV1 (Fig. S1A), the 6MWT distance (Fig. S1B), the MVCQ (Fig. S1C) and the CFQ-R (r = 0.3301, p = 0.02).
3.3. Increased FEV1, BMI, CFQ-R and LCQ scores after one year of tezacaftor and ivacaftor
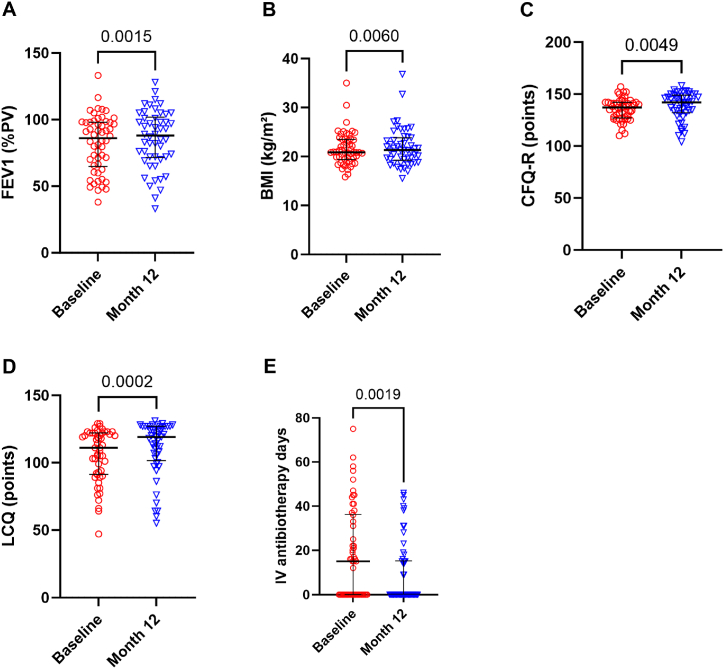
In real conditions, tezacaftor and ivacaftor improved clinical conditions of the PwCF slightly. Thirty-three patients improved their FEV1 after treatment. Mean FEV1 increased significantly from 82.1 to 85.2%PV (p = 0.0015) (Fig. 4A), mean BMI from 21.6 to 21.9 kg/m2 (p = 0.006) (Fig. 4B), mean CFQ-R from 135.4 to 138.5 points (p = 0.0049) (Fig. 4C) and mean LCQ from 105.5 versus 111 points (p = 0.0002) (Fig. 4D) at 12 months. The positive effect on lung function was more rapid, already significant at 1 month, while the effect was slower to become apparent on the BMI (Fig. 3B and C). The mean number of days of intravenous antibiotics significantly decreases after treatment from 18.4 to 9.7 days (p = 0.019) (Fig. 4E). Pseudomonas aeruginosa infection rate was not modified after treatment. No correlations between the changes in clinical data (FEV1/weight/BMI/CFQ-R/LCQ scores/days of intravenous antibiotics) versus the changes of exercise capacity (6MWT distance in meter or %PV/MVCQ in N-m and %PV/1STST in number of repetitions, %PV and nxkg) were highlighted.
Fig. 4.
Effects of one year of tezacaftor and ivacaftor on FEV1 n = 54 and n = 50 (A), BMI n = 54 and n = 50 (B), CFQ-R n = 49 and n = 47 (C), LCQ n = 52 and n = 49 (D), intravenous antibiotherapy days the year before and after tezacaftor and ivacaftor n = 54 and n = 54 (E). FEV1, forced expiratory volume in 1 s; PV, predicted values; BMI, body mass index; kg/m2, kilogram divided by the square meter; CFQ-R, cystic fibrosis questionnaire-revised; LCQ, Leicester cough questionnaire; IV, intravenous. Wilcoxon test.
4. Discussion
In our study, after one year of treatment with tezacaftor and ivacaftor, we observed an improvement of the performance in the 1STST but not in the 6MWT nor the MVCQ in a cohort of 54 PwCF. Nevertheless, less oxygen desaturation occurred during the 6MWT. Our cohort displayed at baseline a usual level of exercise capacity measured by 1STST and 6MWT compared to previous studies in PwCF [6,8,26]. In contrast, our cohort presented a very low MVCQ compared to the general population and to other CF studies [4]. The latest guidelines stated that the 1STST is a good tool to use to follow the effects of a treatment [18] and indeed, an improvement has been shown after intravenous antibiotics course [6]. Furthermore, as expected, the FEV1, BMI and the quality of life improved and we also showed that cough decreased, evaluated by LCQ after treatment. The increase of repetitions in the 1STST and the increase of the BMI were shown after a few months while the improvement of lung function occurred more rapidly as also observed in phase 3 studies [27,28]. In healthy adults, it is expected that the number of repetitions during the 1STST decreases with age, and that it is lower in women [20], while in children, it remains stable until 13 years old after which it decreases [19]. In PwCF not taking CFTR modulators, functional exercise capacity measured by 1STST and 6MWT is lower than the general population [6] and decreases with time and advanced disease [29].
Data on the effects of CFTR modulation on functional exercise capacity are scarce and conflicting. Ivacaftor was the first modulator to be available and one case-report on 2 patients showed conflicting results concerning peak VO2 after 12 weeks of treatment [13]. In a prospective study involving 20 PwCF, 4 weeks of ivacaftor increased exercise time but did not improve peak VO2 [14]. More recently, Salvatore et al. published the long-term effects of this treatment (12 months) and showed a mean absolute increase of 55.7 m in the walked distance during the 6MWT [30]. Concerning lumacaftor and ivacaftor, a small study conducted on 7 patients did not observe a change in endurance time after one month [31], while two other studies demonstrated an improved walked distance after 3 months on 11 PwCF (+75 m) [32] and after 12 months on 10 patients (+118 m) [33]. Concerning peak VO2 following 6 months of lumacaftor and ivacaftor, one case series (3 patients) did not notice a clear improvement [34]. Data are more infrequent on the association of tezacaftor and ivacaftor. One poster described a positive effect of 6–8 months of tezacaftor and ivacaftor based on peak VO2 in 8 patients [15].
Knowing this, we decided to evaluate the effect during one year of CFTR modulators as short-term effects were not conclusive. To do this, we used classical exercise tests (6MWT and MVCQ) as well as the promising and practical 1STST. We previously used it to evaluate the impact of intravenous antibiotics and it correlated, like in this study, with lung function and quadriceps strength. It was also more sensitive than the MVCQ to show the improvement in exercise capacity after intravenous antibiotics [6]. The 1STST seems more responsive, accurate and sensitive to exercise capacity changes as in our cohort, we did not find any differences in the walked distance nor in the quadriceps strength. The 1STST is reliable in PwCF and induced fewer cardio-respiratory responses than the 6MWT [35]. Both tests probably measure different components of functional exercise capacity and the 1STST could thus be a good alternative to 6MWT in CF as recently suggested by the ECFS exercise working group [9,18].
As limitations, our cohort of people with CF was not compared to a prospective control arm but rather to reference values of the general population. Some learning effects has also been previously described with exercise tests [26], but to reduce these, all our patients performed a training test beforehand [18].
Looking at the future, the highly efficient CFTR modulators (elexacaftor, tezacaftor and ivacaftor) are treatments with hopeful outcomes. First studies showed consistent results in terms of 6MWT after 3 months (9 patients) [36] and 12 months (9 patients) [37] or in terms of CPET after 6 weeks (3 patients) [16]. Regarding rare mutations, three PwCF with complex allele enhanced their 6MWT distance after 4 weeks of CFTR modulators [38].
In conclusion, this is the largest study to date available on exercise capacity assessing the long-term effects of tezacaftor and ivacaftor in PwCF and the 1STST seems to be the most responsive and easier-to-use tool to assess exercise in routine clinical follow-up.
Ethics statement
The study was reviewed and approved by the local ethic committee of the Cliniques universitaires Saint-Luc with the approval number: 2021/21JUI/282 and registered code (B4032021000078). All participants/patients (or their proxies/legal guardians) provided informed consent to participate in the study.
Funding
SG is a researcher clinician of the Fonds National de la Recherche Scientifique (FNRS; grant 1R01618).
Data availability statements
Data will be available on request.
CRediT authorship contribution statement
Aubriot Anne-Sophie: Writing – original draft, Data curation, Conceptualization. Morgane Penelle: Writing – original draft, Formal analysis, Data curation. Gonçalvès Clémence: Data curation. Silvia Berardis: Writing – review & editing. Christophe Goubau: Writing – review & editing. Gregory Reychler: Writing – review & editing. Sophie Gohy: Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank Miss Alessia Qiu for the revision of the English language and Dr Celine Bugli for the statistical revision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26729.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Gohy S., Moeremans A., Pilette C., Collin A. Immunoglobulin A mucosal immunity and altered respiratory epithelium in cystic fibrosis. Cells. 2021;10(12) doi: 10.3390/cells10123603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nixon P.A., Orenstein D.M., Kelsey S.F., Doershuk C.F. The prognostic value of exercise testing in patients with cystic fibrosis. N. Engl. J. Med. 1992;327(25):1785–1788. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 3.Gruet M., Troosters T., Verges S. Peripheral muscle abnormalities in cystic fibrosis: etiology, clinical implications and response to therapeutic interventions. J. Cyst. Fibros. 2017;16(5):538–552. doi: 10.1016/j.jcf.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Troosters T., Langer D., Vrijsen B., Segers J., Wouters K., Janssens W., Gosselink R., Decramer M., Dupont L. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. Eur. Respir. J. 2009;33(1):99–106. doi: 10.1183/09031936.00091607. [DOI] [PubMed] [Google Scholar]
- 5.Kohlbrenner D., Benden C., Radtke T. The 1-minute sit-to-stand test in lung transplant candidates: an alternative to the 6-minute walk test. Respir. Care. 2020;65(4):437–443. doi: 10.4187/respcare.07124. [DOI] [PubMed] [Google Scholar]
- 6.Hardy S., Berardis S., Aubriot A.S., Reychler G., Gohy S. One-minute sit-to-stand test is practical to assess and follow the muscle weakness in cystic fibrosis. Respir. Res. 2022;23(1):266. doi: 10.1186/s12931-022-02176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke T., Hebestreit H., Puhan M.A., Kriemler S. The 1-min sit-to-stand test in cystic fibrosis - insights into cardiorespiratory responses. J. Cyst. Fibros. 2017;16(6):744–751. doi: 10.1016/j.jcf.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Gruet M., Peyre-Tartaruga L.A., Mely L., Vallier J.M. The 1-minute sit-to-stand test in adults with cystic fibrosis: correlations with cardiopulmonary exercise test, 6-minute walk test, and quadriceps strength. Respir. Care. 2016;61(12):1620–1628. doi: 10.4187/respcare.04821. [DOI] [PubMed] [Google Scholar]
- 9.Combret Y., Boujibar F., Gennari C., Medrinal C., Sicinski S., Bonnevie T., Gravier F.E., Laurans M., Marguet C., Le Roux P., Lamia B., Prieur G., Reychler G. Measurement properties of the one-minute sit-to-stand test in children and adolescents with cystic fibrosis: a multicenter randomized cross-over trial. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michoud M.C., Robert R., Hassan M., Moynihan B., Haston C., Govindaraju V., Ferraro P., Hanrahan J.W., Martin J.G. Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2009;40(2):217–222. doi: 10.1165/rcmb.2006-0444OC. [DOI] [PubMed] [Google Scholar]
- 11.Du X.Y., Finley J., Sorota S. Paucity of CFTR current but modest CFTR immunoreactivity in non-diseased human ventricle. Pflugers Arch. 2000;440(1):61–67. doi: 10.1007/s004240000254. [DOI] [PubMed] [Google Scholar]
- 12.Lamhonwah A.M., Bear C.E., Huan L.J., Kim Chiaw P., Ackerley C.A., Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle: dysfunction causes abnormal metabolic recovery in exercise. Ann. Neurol. 2010;67(6):802–808. doi: 10.1002/ana.21982. [DOI] [PubMed] [Google Scholar]
- 13.Saynor Z.L., Barker A.R., Oades P.J., Williams C.A. The effect of ivacaftor in adolescents with cystic fibrosis (G551D mutation): an exercise physiology perspective. Pediatr. Phys. Ther. 2014;26(4):454–461. doi: 10.1097/PEP.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 14.Edgeworth D., Keating D., Ellis M., Button B., Williams E., Clark D., Tierney A., Heritier S., Kotsimbos T., Wilson J. Improvement in exercise duration, lung function and well-being in G551D-cystic fibrosis patients: a double-blind, placebo-controlled, randomized, cross-over study with ivacaftor treatment. Clin. Sci. (Lond.) 2017;131(15):2037–2045. doi: 10.1042/CS20170995. [DOI] [PubMed] [Google Scholar]
- 15.Molla Imaduddin Ahmed N.D., Joe Madge, Gaillard Erol. The impact of Symkevi (Tezacaftor/Ivacaftor) on exercise capacity in adolescents with CF. Eur. Respir. J. 2021;58:PA2101. doi: 10.1183/13993003.congress-2021.PA2101. [DOI] [Google Scholar]
- 16.Causer A.J., Shute J.K., Cummings M.H., Shepherd A.I., Wallbanks S.R., Pulsford R.M., Bright V., Connett G., Saynor Z.L. Elexacaftor-Tezacaftor-Ivacaftor improves exercise capacity in adolescents with cystic fibrosis. Pediatr. Pulmonol. 2022;57(11):2652–2658. doi: 10.1002/ppul.26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell P.M. The prevalence of cystic fibrosis in the European Union. J. Cyst. Fibros. 2008;7(5):450–453. doi: 10.1016/j.jcf.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Saynor Z.L., Gruet M., McNarry M.A., Button B., Morrison L., Wagner M., Sawyer A., Hebestreit H., Radtke T., Urquhart D.S. G. European Cystic Fibrosis Society Exercise Working, Guidance and standard operating procedures for functional exercise testing in cystic fibrosis. Eur. Respir. Rev. : an official journal of the European Respiratory Society. 2023;32(169) doi: 10.1183/16000617.0029-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haile S.R., Fuhner T., Granacher U., Stocker J., Radtke T., Kriemler S. Reference values and validation of the 1-minute sit-to-stand test in healthy 5-16-year-old youth: a cross-sectional study. BMJ Open. 2021;11(5) doi: 10.1136/bmjopen-2021-049143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strassmann A., Steurer-Stey C., Lana K.D., Zoller M., Turk A.J., Suter P., Puhan M.A. Population-based reference values for the 1-min sit-to-stand test. Int. J. Publ. Health. 2013;58(6):949–953. doi: 10.1007/s00038-013-0504-z. [DOI] [PubMed] [Google Scholar]
- 21.Laboratories ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 23.Bohannon R.W., Kindig J., Sabo G., Duni A.E., Cram P. Isometric knee extension force measured using a handheld dynamometer with and without belt-stabilization. Physiother. Theory Pract. 2012;28(7):562–568. doi: 10.3109/09593985.2011.640385. [DOI] [PubMed] [Google Scholar]
- 24.Bachasson D., Villiot-Danger E., Verges S., Hayot M., Perez T., Chambellan A., Wuyam B. [Maximal isometric voluntary quadriceps strength assessment in COPD] Rev. Mal. Respir. 2014;31(8):765–770. doi: 10.1016/j.rmr.2013.10.645. [DOI] [PubMed] [Google Scholar]
- 25.Hogrel J.Y., Payan C.A., Ollivier G., Tanant V., Attarian S., Couillandre A., Dupeyron A., Lacomblez L., Doppler V., Meininger V., Tranchant C., Pouget J., Desnuelle C. Development of a French isometric strength normative database for adults using quantitative muscle testing. Arch. Phys. Med. Rehabil. 2007;88(10):1289–1297. doi: 10.1016/j.apmr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Radtke T., Puhan M.A., Hebestreit H., Kriemler S. The 1-min sit-to-stand test--A simple functional capacity test in cystic fibrosis? J. Cyst. Fibros. 2016;15(2):223–226. doi: 10.1016/j.jcf.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Middleton P.G., Mall M.A., Drevinek P., Lands L.C., McKone E.F., Polineni D., Ramsey B.W., Taylor-Cousar J.L., Tullis E., Vermeulen F., Marigowda G., McKee C.M., Moskowitz S.M., Nair N., Savage J., Simard C., Tian S., Waltz D., Xuan F., Rowe S.M., Jain R., Group V.X.S. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., Mall M.A., Welter J.J., Ramsey B.W., McKee C.M., Marigowda G., Moskowitz S.M., Waltz D., Sosnay P.R., Simard C., Ahluwalia N., Xuan F., Zhang Y., Taylor-Cousar J.L., McCoy K.S., McCoy K., Donaldson S., Walker S., Chmiel J., Rubenstein R., Froh D.K., Neuringer I., Jain M., Moffett K., Taylor-Cousar J.L., Barnett B., Mueller G., Flume P., Livingston F., Mehdi N., Teneback C., Welter J., Jain R., Kissner D., Patel K., Calimano F.J., Johannes J., Daines C., Keens T., Scher H., Chittivelu S., Reddivalam S., Klingsberg R.C., Johnson L.G., Verhulst S., Macedo P., Downey D., Connett G., Nash E., Withers N., Lee T., Bakker M., Heijerman H., Vermeulen F., Van Braeckel E., Knoop C., De Wachter E., van der Meer R., Merkus P., Majoor C. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/s0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapnadak S.G., Dimango E., Hadjiliadis D., Hempstead S.E., Tallarico E., Pilewski J.M., Faro A., Albright J., Benden C., Blair S., Dellon E.P., Gochenour D., Michelson P., Moshiree B., Neuringer I., Riedy C., Schindler T., Singer L.G., Young D., Vignola L., Zukosky J., Simon R.H. Cystic Fibrosis Foundation consensus guidelines for the care of individuals with advanced cystic fibrosis lung disease. J. Cyst. Fibros. 2020;19(3):344–354. doi: 10.1016/j.jcf.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Salvatore D., Terlizzi V., Francalanci M., Taccetti G., Messore B., Biglia C., Pisi G., Calderazzo M.A., Caloiero M., Pizzamiglio G., Majo F., Cresta F., Leonetti G., De Venuto D. Ivacaftor improves lung disease in patients with advanced CF carrying CFTR mutations that confer residual function. Respir. Med. 2020;171 doi: 10.1016/j.rmed.2020.106073. [DOI] [PubMed] [Google Scholar]
- 31.Quon B.S., Ramsook A.H., Dhillon S.S., Mitchell R.A., Boyle K.G., Wilcox P.G., Guenette J.A. Short-term effects of Lumacaftor/Ivacaftor (Orkambi) on exertional symptoms, exercise performance, and ventilatory responses in adults with cystic fibrosis. Respir. Res. 2020;21(1):135. doi: 10.1186/s12931-020-01406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauwers E., Belmans D., Mignot B., Ides K., Van Hoorenbeeck K., Snoeckx A., Van Holsbeke C., Nowe V., Van Braeckel E., De Backer W., De Backer J., Verhulst S. The short-term effects of ORKAMBI (lumacaftor/ivacaftor) on regional and distal lung structures using functional respiratory imaging. Ther. Adv. Respir. Dis. 2021;15 doi: 10.1177/17534666211046774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wark P.A.B., Cookson K., Thiruchelvam T., Brannan J., Dorahy D.J. Lumacaftor/Ivacaftor improves exercise tolerance in patients with Cystic Fibrosis and severe airflow obstruction. BMC Pulm. Med. 2019;19(1):106. doi: 10.1186/s12890-019-0866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savi D., Gramegna A., Vicenzi M., Di Paolo M., Messore B., Palange P., Blasi F. Changes in exercise endurance and inspiratory capacity after lumacaftor/ivacaftor therapy in cystic fibrosis. Pulmonology. 2023;29(4):338–341. doi: 10.1016/j.pulmoe.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Combret Y., Prieur G., Medrinal C. Validity and reliability of the one-minute sit-to-stand test for the measurement of cardio-respiratory responses in children with cystic fibrosis. Authors' reply, Pulmonology. 2023;29(2):174–175. doi: 10.1016/j.pulmoe.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Gur M., Bar-Yoseph R., Hanna M., Abboud D., Keidar Z., Palchan T., Toukan Y., Masarweh K., Alisha I., Zuckerman-Levin N., Bentur L. Effect of Trikafta on bone density, body composition and exercise capacity in CF: a pilot study. Pediatr. Pulmonol. 2023;58(2):577–584. doi: 10.1002/ppul.26243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giallongo A., Parisi G.F., Papale M., Manti S., Mule E., Aloisio D., Terlizzi V., Rotolo N., Leonardi S. Effects of elexacaftor/tezacaftor/ivacaftor on cardiorespiratory polygraphy parameters and respiratory muscle strength in cystic fibrosis patients with severe lung disease. Genes. 2023;14(2) doi: 10.3390/genes14020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terlizzi V., Centrone C., Ferrari B., Castellani C., Gunawardena T.N.A., Taccetti G., Laselva O. Modulator Therapy in Cystic Fibrosis Patients with cis Variants in F508del Complex Allele: a Short-Term Observational Case Series. J Pers Med. 2022;12(9) doi: 10.3390/jpm12091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available on request.