Abstract
Immune checkpoint inhibitors (ICIs) have revolutionary effects on therapeutic strategies for multiple malignancies. Their efficacy depends on their ability to reactivate the host immune system to fight cancer cells. However, adverse reactions to ICIs are common and involve several organs, limiting their use in clinical practice. Although the incidence of cardiovascular toxicity is relatively low, it is associated with serious consequences and high mortality rates. The primary cardiovascular toxicities include myocarditis, pericarditis, Takotsubo syndrome, arrhythmia, vasculitis, acute coronary syndrome, and venous thromboembolism. Currently, the mechanism underlying ICI-associated cardiovascular toxicity remains unclear and underexplored. The diagnosis and monitoring of ICI-associated cardiovascular toxicities mainly include the following indicators: symptoms, signs, laboratory examination, electrocardiography, imaging, and pathology. Treatments are based on the grade of cardiovascular toxicity and mainly include drug withdrawal, corticosteroid therapy, immunosuppressants, and conventional cardiac treatment. This review focuses on the incidence, underlying mechanisms, clinical manifestations, diagnoses, and treatment strategies.
Keywords: Immune checkpoint inhibitors, Immunotherapy, Cardiovascular toxicities, Myocarditis, Multidisciplinary treatments
1. Introduction
Immune checkpoint inhibitors (ICIs), which are often combined with other treatments such as chemotherapy, radiotherapy, and targeted therapy, have become the standard treatment modalities for some malignancies, including melanoma, lung cancer, renal cell carcinoma, urothelial cancer, and endometrial cancer [1]. ICIs are monoclonal antibodies that block immune checkpoints and invigorate T cell activity, which can activate the immune system to produce an antitumour response [2,3]. The targets of currently available ICIs include cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), PD-1 ligand 1 (PD-L1), and lymphocyte activating gene 3 (LAG-3) [[4], [5], [6], [7]]. The US Food and Drug Administration (FDA) has approved nine agents, including one CTLA-4 blocking antibody (ipilimumab); four PD-1 blocking antibodies (nivolumab, pembrolizumab, cemiplimab, and dostarlimab); three PD-L1 blocking antibodies (atezolizumab, avelumab, and durvalumab); and one LAG-3 blocking antibody (relatlimab) (Table 1). Multiple clinical studies have confirmed that ICIs can improve patients’ survival rates and quality of life [8].
Table 1.
[26] Summary of the FDA-approved immune checkpoint inhibitors.
Abbreviations; FDA, US Food and Drug Administration, CTLA-4, cytotoxic T lymphocyte antigen-4, dMMR, mismatch repair deficient, MSI-H, microsatellite instability-high, PD-1, programmed death-1, PD-L1, programmed death ligand-1, LAG-3, lymphocyte activation gene-3.
| Target | Drug name | Company | Initial approval year | FDA-approved indications | Cardiovascular toxicities |
|---|---|---|---|---|---|
| CTLA-4 | Ipilimumab (Yervoy)[8] | Bristol-Myers Squibb | 2011 | melanoma | arrhythmia |
| renal cell carcinoma | angiopathy | ||||
| MSI-H or dMMR metastatic colorectal cancer | myocarditis | ||||
| hepatocellular carcinoma | pericarditis | ||||
| non-small cell lung cancer (PD-L1≥1) | temporal arteritis | ||||
| malignant pleural mesothelioma | vasculitis | ||||
| esophageal cancer | hypertension | ||||
| hypotension | |||||
| PD-1 | Nivolumab (Opdivo)[9] | Bristol-Myers Squibb | 2014 | melanoma | arrhythmia |
| non-small cell lung cancer | myocarditis | ||||
| malignant pleural mesothelioma | pericarditis | ||||
| renal cell carcinoma | vasculitis | ||||
| classical Hodgkin's lymphoma | hypertension | ||||
| head and neck squamous cell carcinoma | hypotension | ||||
| urothelial carcinoma | cardiovascular failure | ||||
| MSI-H or dMMR metastatic colorectal cancer | myocardial infarction | ||||
| hepatocellular carcinoma | |||||
| esophageal cancer | |||||
| gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma | |||||
| PD-1 | Pembrolizumab (Keytruda)[10] | Merck | 2014 | melanoma | myocarditis |
| non-small cell lung cancer | pericarditis | ||||
| head and neck squamous cell carcinoma | vasculitis | ||||
| classic Hodgkin's lymphoma | cardiac failure | ||||
| primary mediastinal large B-cell lymphoma | cardiac tamponade | ||||
| urothelial carcinoma | cardiac ischemia | ||||
| MSI-H or dMMR cancer | myocardial infarction | ||||
| MSI-H or dMMR metastatic colorectal cancer | pericardial effusion | ||||
| gastric cancer | pericarditis | ||||
| esophageal cancer | arrhythmia | ||||
| cervical cancer | cardiac arrest | ||||
| hepatocellular carcinoma | hypertension | ||||
| merkel cell carcinoma | |||||
| renal cell carcinoma | |||||
| endometrial carcinoma | |||||
| TMB-high cancer | |||||
| cutaneous squamous cell carcinoma | |||||
| triple-negative breast cancer | |||||
| PD-1 | Cemiplimab (Libtayo)[11] | Regeneron | 2018 | cutaneous squamous cell carcinoma | myocarditis |
| basal cell carcinoma | pericarditis | ||||
| non-small cell lung cancer | vasculitis | ||||
| hypertension | |||||
| PD-1 | Dostarlimab (Jemperli)[12] | GSK | 2021 | dMMR endometrial cancer | myocarditis |
| dMMR solid tumour | pericarditis | ||||
| vasculitis | |||||
| PD-L1 | Atezolizumab (Tecentriq)[13] | Genentech | 2016 | non-small cell lung cancer | myocarditis |
| small cell lung cancer | pericarditis | ||||
| hepatocellular carcinoma | vasculitis | ||||
| melanoma | arrhythmia | ||||
| alveloar soft part sarcoma | acute cardiac failure | ||||
| acute myocardial infarction | |||||
| cardiac arrest | |||||
| myocardial ischemia | |||||
| myocardial infarction | |||||
| ejection fraction decreased | |||||
| electrocardiogram QT prolonged | |||||
| pericardial effusion | |||||
| cardiac tamponade | |||||
| hypertension | |||||
| PD-L1 | Avelumab (Bavencio)[14] | EMD Serono | 2017 | Merkel cell carcinoma | myocardial infarction |
| urothelial carcinoma | congestive heart failure | ||||
| renal cell carcinoma | myocarditis | ||||
| pericarditis | |||||
| vasculitis | |||||
| myocardial infarction | |||||
| hypertension | |||||
| sudden cardiac death | |||||
| PD-L1 | Durvalumab (Imfinzi)[15] | AstraZeneca | 2017 | non-small cell lung cancer | myocarditis |
| small cell lung cancer | pericarditis | ||||
| biliary tract cancer | vasculitis | ||||
| hepatocellular carcinoma | cardiac arrest | ||||
| PD-1+LAG-3 | Relatlimab + Nivolumab (Opdualag)[16] | Bristol-Myers Squibb | 2022 | melanoma | myocarditis |
| pericarditis | |||||
| vasculitis | |||||
| acute myocardial infarction |
Despite their anticancer efficacy, immune-related adverse events (irAEs) associated with ICIs are common and involve multiple organ systems, such as the colon, lung, liver, skin, pituitary, thyroid, and heart [9]. According to reports, 2/3 of drug users develop irAEs, and 13–23% have grade 3 to 4 irAEs, resulting in 40% of recipients needing drug withdrawal. Consequently, its curative effect has also been affected to varying degrees [[10], [11], [12]]. The incidence of cardiotoxicity is lower than those of irAEs in other organs; however, the mortality rate is higher [13]. Therefore, ICI-induced cardiovascular toxicities must be discussed separately.
This review provides an overview of the mechanisms of ICI-associated cardiovascular toxicities, focuses on the epidemiology, pathophysiological mechanisms, diagnosis, and management of ICI-associated cardiovascular toxicities, provides perspectives on potential strategies, and summarises ongoing research developments to prevent and mitigate their occurrence.
2. Overview of ICI-associated cardiovascular toxicities
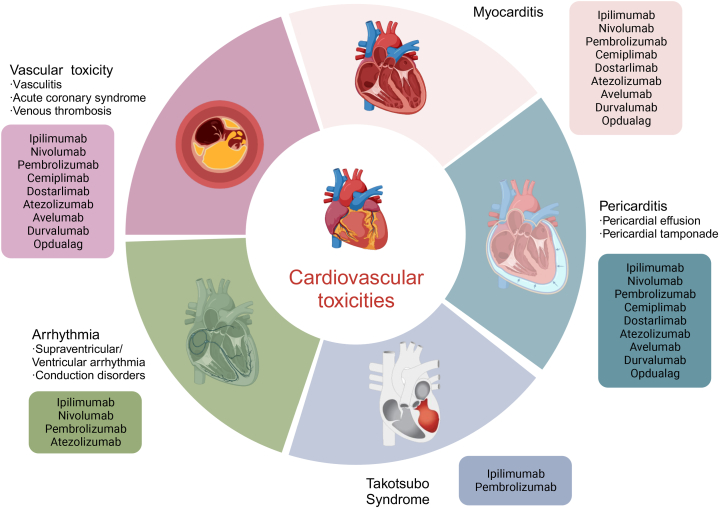
Cardiovascular toxicity is emerging as a serious concern with the extensive use of ICIs, particularly with the application of two or a single ICI in combination with another cardiovascular toxic anticancer therapy. The median time to onset of cardiovascular toxicities is 6 weeks but can range from 2 to 54 weeks [8]. Myocarditis, pericarditis, Takotsubo syndrome, arrhythmia, vasculitis, acute coronary syndrome, and venous thromboembolism are the cardiovascular toxicities of concern. In an observational, retrospective study, compared with the full database, ICIs were associated with higher reporting of myocarditis (reporting odds ratio [ROR] 11.21 [95% CI 9.36–13.43]), pericardial diseases (3.80 [3.08–4.62]), and vasculitis (1.56 [1.25–1.94]) [4]. According to a meta-analysis including 4751 patients, 1.3% of persons presented cardiovascular irAEs, and the overall incidence of cardiovascular irAEs was 3.1% (95% CI 0.73–7.06) with ICI monotherapy and 5.8% (95% CI 3.86–15.53) with dual immunotherapy [14]. Another meta-analysis of 25 studies with 20,244 patients reported an increased risk of cardiac arrhythmia with dual immunotherapy compared to monotherapy (OR 3.90, 95% CI: 1.08–14.06) [15]. Moreover, the World Health Organization (WHO) database reported that mortality from ICI-associated myocarditis increased almost two-fold with combination ICIs (67% vs 36%; P = 0·008) compared with monotherapy [16]. Moreover, the combination of ICI with other antitumour treatments that may cause cardiovascular toxicity may further aggravate cardiovascular toxicities, such as targeted agents (e.g. sorafenib, sunitinib, and trastuzumab), chemotherapy (e.g. anthracyclines and 5-fluorouracil), and radiotherapy [[17], [18], [19], [20], [21]]. For example, VEGF receptor tyrosine kinase inhibitors (VEGFR TKIs) are associated with hypertension and heart failure, and ICIs combined with VEGFR TKIs can significantly increase the incidence of hypertension [18]. Likewise, the presence of cardiovascular risk factors, including diabetes mellitus, obesity, preexisting cardiac pathology or peripheral arterial disease, history of smoking, and dyslipidaemia, is postulated as a possible risk factor for ICI-related cardiovascular toxicity [[22], [23], [24]].
The diagnosis of immune-related cardiovascular toxicities includes symptoms, signs, laboratory tests, imaging data, and tissue biopsy. Tissue biopsy is the gold standard diagnostic method [8]. After its occurrence, different management approaches are based on the grade of cardiovascular toxicities, which often require multidisciplinary discussions, especially with the cooperation of oncologists and cardiologists [25]. Currently, the most common treatment methods are drug withdrawal and corticosteroid therapy, mainly through the sequestration of CD4+ T cells in the reticuloendothelial system, suppression of lymphocyte activity, and inhibition of cytokine production [26]. If the effect of corticosteroids is poor, other immunosuppressants can be used, including mycophenolate, anti-calcineurin, anti-thymocyte globulin, or intravenous immunoglobulin [25,27]. Immunomodulators targeting heart muscle that can be administered prophylactically or in combination with ICIs may be further developed to avoid cardiovascular irAEs. The immunomodulators described in case reports and small case series can effectively reverse near-fatal ICI-associated myocarditis. These drugs mainly include tocilizumab (IL-6R antibody), alemtuzumab (anti-CD52), and abatacept (CTLA-4 agonist) [[28], [29], [30]]. Moreover, in contrast to the current targets, the focus has shifted to the research and development of new mechanisms of action of ICIs. The targets of these novel agents are not shared by the myocardium and tumours, which may limit the inflammatory response in myocardial cells [31]. The new drugs under study include anti-T-cell immunoglobulin and mucin 3 (anti-TIM-3), anti-V-domain Ig suppressor of T-cell activation (anti-VISTA), anti-T-cell immunoglobulin and ITIM domain (anti-TIGIT), and anti-B- and T-cell lymphocyte attenuators (anti-BTLA) [[32], [33], [34], [35]].
3. Mechanisms of ICI-associated cardiovascular toxicities
The mechanisms of ICI-associated cardiovascular toxicities are complex and incompletely understood, which has led to increased interest in the fundamental role that immune checkpoints play in cardiovascular homeostasis, as well as their global effects at the intersection of the immune and cardiovascular systems [17]. Based on a large number of preclinical and clinical studies, the mechanisms mainly involve the following aspects: T cell activation, the mechanism and function of ICIs, and the cause and process of immune-related cardiotoxicity [4,36,37]. To better understand ICI-associated cardiovascular toxicities, we discuss the basic biology of immune checkpoints and their roles in regulating cardiovascular function.
3.1. T cell activation
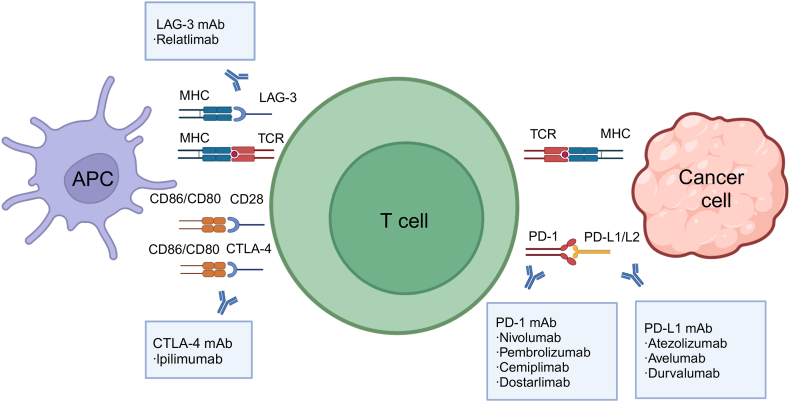
The adaptive immune system is a key contributor to antitumour responses triggered by cancer immunotherapy [38]. T lymphocytes play an important role in the adaptive immune response, each of which bears a unique T cell receptor (TCR) generated by random somatic recombination to endow each T cell with specificity for its cognate antigen [3]. In T-cell-mediated immunity, new antigens released by dead cancer cells are captured by antigen-presenting cells (APCs), such as dendritic cells, and present on major histocompatibility complex (MHC) molecules (Fig. 1) [39]. CD8+T cells act as cytotoxic effectors that kill target cells by triggering cell death; CD4+T cells that classically recognise antigens processed from the extracellular environment recognise new antigen-MHC I and - MHC II complexes, respectively, become activated, and migrate to the tumour bed, where they recognise cancer cells through the interaction between the new antigen-MHC complex and TCR on T cells [40]. In short, T-cell activation requires TCR to recognise the specific peptide presented by MHC on APC. The activation of T cells requires a second signal, the co-stimulatory signal provided by molecules on the surface of T cells, such as CD28 [41]. T cell activation can also be restricted by co-inhibitory molecules such as CTLA-4, PD-1, and LAG-3 [42].
Fig. 1.
Schematic diagram of immune checkpoint inhibitors blocking T cell activation. Abbreviations: APC, antigen-presenting cell; CTLA-4, cytotoxic T lymphocyte antigen-4; ICI, immune checkpoint inhibitor; MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, programmed death ligand-1; TCR, T cell receptor; LAG-3, lymphocyte activation gene-3. Figure created with BioRender.com.
The adaptive immune system utilised by the host to attack tumours may cause autoimmune reactions. Hosts contain many intrinsic steps to ensure that T lymphocytes do not attack themselves while simultaneously being activated and attacking foreign substances [43]. Both central and peripheral tolerances affect T cell activation and regulation [17]. Peripheral tolerance is one of the most important mechanisms: blockade of co-stimulatory signals between T cells and APCs in the target tissue [44,45]. Based on this contradictory relationship, the delicate regulation of the adaptive immune system may play a vital role in cardiovascular irAEs [46].
3.2. Immune checkpoint inhibitors
ICIs block immune inhibitory signals to enhance immune responses against tumours. This is mainly achieved by blocking signalling through the CTLA-4, PD-1/PD-L1, and LAG-3 receptors. Although ICIs activate T cells to produce antitumour effects, they can also enhance autoimmune responses and exert adverse effects on multiple organs, such as the lungs, heart, thyroid, and skin. Among these, cardiovascular toxicities are rare, but often severe [7,47].
3.2.1. CTLA-4
CTLA-4 is an inhibitory coreceptor that is expressed almost exclusively in T cells. It plays a critical role in the control of T cell activation and tolerance by antagonising CD28-mediated costimulation in combination with CD80 and/or CD86 [48]. Benefited by this kind of combination, CTLA-4 can mediate the immunosuppressive capacity of regulatory T cells [49]. What's more, the expression of CTLA-4 on activated resting T cells provides a negative feedback signal to balance the strength of the adaptive immune response through regulating the production of cytokines (e.g. IFN-γ), differentiation and expansion of T cells, and cell contact and migration [50]. One study reported that CTLA-4 deficient mice rapidly develop lymphoproliferative diseases with multiple organ lymphocyte infiltration and tissue destruction, especially severe myocarditis and pancreatitis, and eventually died at 3–4 weeks. This study indicated that CTLA-4 may have a detrimental effect on T cell activation. The absence of CTLA-4 in these mice results in a severe phenotype, emphasising the crucial role of this molecule in regulating T cell activation and preserving the immune balance [51].
The anti-CTLA-4 antibody blocks the binding between CTLA-4 and its ligands CD80/CD86 and causes the co-stimulatory surface protein CD28 to bind with these ligands, thus leading to T cell activation. Downstream stimulation signalling leads to an increase in T cell proliferation, differentiation, and cytokine production, thus mediating antitumour activity [52]. Ipilimumab is currently the only FDA-approved CTLA-4 inhibitor. Owing to its efficacy, it has been approved for use in seven types of tumours, including melanoma, renal cell carcinoma, and colorectal cancer. For example, the 5-year survival rate of melanoma patients with the use of ipilimumab was significantly improved from 10% to 20–26% [53].
3.2.2. PD-1/PD-L1 axis
PD-1 is a co-inhibitory member of the CD80 or CD86/CD28 molecular superfamily and is expressed on the surface of activated CD4+ and CD8+ T cells, B cells, monocytes, natural killer cells, and dendritic cells. It interacts with PD-L1 and PD-L2 to transmit inhibitory signals for T cell activation [54]. PD-1 is thought to inhibit T cells in the late stages of the immune response [55]. A study showed that in the model of CD8+ T cell-mediated adoptive transfer, compared to the effects of PD-1 (+/+) CD8 (+) T cells, PD-1 (−/−) CD8 (+) T cells led to the aggravation of the disease and increased inflammatory infiltration, especially rich in neutrophils. Moreover, Tarrio ML reported that PD-1 deficient T lymphocytes enhance the proliferation and cytotoxicity of cardiac endothelial cells in vitro [56]. In another study, mice lacking PD-1 showed limited and variable model-dependent autoimmunity, including arthritis and cardiomyopathy [57].
Anti-PD-1 and anti-PD-L1 antibodies block the interaction between PD-1 and PD-L1, thus releasing the immunosuppressive and cancer immune escape effects of PD-1/PD-L1 binding and subsequently restoring the activity of exhausted CD8 effectors by activating the PI3K/AKT/mTOR and RAS/MEK/ERK pathways [58,59]. Currently, PD-1/PD-L1 inhibitors are considered the most effective immune checkpoint inhibitors. Four PD-1 and three PD-L1 inhibitors have been approved by the FDA [17]. Pembrolizumab, a PD-1 inhibitor, has been approved for the treatment of 16 types of tumours, including melanoma, non-small cell lung cancer, and head and neck squamous cell carcinoma. Furthermore, it has been approved for use in cases where the tumour type is not specified as long as it exhibits MSI-H, dMMR, or TMB-high characteristics [60].
3.2.3. LAG-3
LAG-3, a type I transmembrane protein, is an inhibitory receptor of T cells that is often upregulated on the surface of regulatory T cells, contributing to the depletion of T cells in the tumour microenvironment (TME) and limiting the antitumour T cell response [61,62]. LAG-3 is overexpressed in many tumour types including melanoma, lung cancer, and breast cancer. However, the mechanism through which LAG-3 induces immunosuppression remains unclear. It is generally associated with the participation of APCs in TME and MHC II expressed on some tumour cells, as well as other reported ligands such as Galectin-3, LSECtin, and fibrinogen-like protein-1 [63,64], which lead to reduced production of IL-2 and IFN-γ [65]. Mice lacking both PD-1 and LAG-3 developed spontaneous T-cell-mediated myocarditis [66,67]. LAG-3 deficiency alone did not induce myocarditis, but led to a slight increase in the number of lymphocytes in BALB/c and NOD mice [68].
The anti-LAG-3 antibody can bind to LAG-3 and restore the effector function of exhausted T cells. Another study confirmed that the anti-LAG-3 treatment restored NK and T cell-mediated responses and promoted the secretion of TNF-α, IFN-γ and IL-2 [69]. Opdualag, a combination of relatlimab (LAG-3 antibody) and nivolumab (PD-1 antibody), was approved by the FDA in 2022 for melanoma based on the RELATIVITY-047 trial, which demonstrated superior efficacy compared with single-agent nivolumab [36].
3.3. ICI-associated cardiovascular toxicities
The exact mechanism of immune-related cardiotoxicity is not completely understood but is believed to be related to the role of immune checkpoints in maintaining immune homeostasis [70]. The heart has a complex and dynamic immune system. The T cells in the heart are relatively deficient under physiological conditions [71]. The presence of resident tissue macrophages and dendritic cells in the heart suggests that T-cell responses could be triggered. T-lymphocyte accumulation in the heart may lead to immune-mediated myocardial diseases, mainly myocarditis, dilated cardiomyopathy, and ischaemic cardiomyopathy [[72], [73], [74]]. Fortunately, when the heart encounters inflammatory insults, the expression of immune checkpoints (e.g. CTLA-4, PD-1, PD-L1, and LAG-3) in T cells increases [44]. However, the use of immunotherapy in cancer treatment disrupts this balance and may make cardiac cells more vulnerable to damage, leading to serious toxicity [75].
In animal models, these immune checkpoints protect the heart from immune-mediated damage following stress [63,76]. The mouse gene knockout experiment also proved that checkpoints play a key role in regulating cardiac autoimmune responses [56,77]. Because of the different mechanisms of immune checkpoints in T cell activation, it is worth noting that although combining ICIs can improve therapeutic outcomes, it also leads to an increased risk of cardiovascular toxicity [2,78,79]. Three mechanisms have been identified to explain the occurrence of ICI-associated cardiovascular toxicities: (1) ICIs interfere with CTLA-4, PD-L1, PD-1, and LAG-3 signalling in the heart, causing a breakdown of peripheral immune tolerance by lowering the threshold for T cell activation; and (2) the presence of shared antigens between tumours and skeletal or cardiac muscles can result in activated T cell infiltration, macrophages, and monocytes into normal muscles. This cross-reactivity can lead to various muscular disorders (3) Elevation of inflammatory factors (e.g., IL-1a, IL-2a, IFN-α, and IL-17); induced by ICI therapy further aggravates the injury [80].
4. Detailed description of ICI-associated cardiovascular toxicities (Fig. 2)
Fig. 2.
The spectrum of cardiovascular toxicities associated with immune checkpoint inhibitors. Figure created with BioRender.com.
4.1. Myocarditis
4.1.1. Epidemiology
Myocarditis is a common cardiovascular toxicity associated with ICIs. The incidence of myocarditis in patients receiving ICIs ranges from 0.04% to 1.14%, with the median onset time being around 34 days after treatment initiation [22,81]. Although its incidence is low, the mortality rate can reach 46% [4]. Males have a slightly higher incidence, comprising 67% of all cases [4]. The combination of anti-PD-1 and anti-CTLA4 antibodies results in a higher occurrence and fatality rate of myocarditis than a single immune drug alone [[81], [82], [83]]. According to a study published in the New England Journal of Medicine, myocarditis occurred in 1.7% of patients who received a combination of anti-LAG-3 and anti-PD-1 agents [36]. Additionally, ICIs combined with anticancer therapies involving other mechanisms may increase the risk of myocarditis. In a trial of 55 patients receiving avelumab and axitinib (a VEGFR TKI), 1 patient (2%) developed fatal myocarditis [84]. Moreover, myocarditis is more common in patients with melanoma [4]..
4.1.2. Aetiology and pathology
Similarly, the mechanism underlying ICI-induced myocarditis caused by ICIs is not fully understood. Two main mechanisms are primarily involved in this process. One is the breakdown of immune tolerance to the heart mediated by the CTLA-4, PD-1, and LAG-3 pathways, and the other involves the expansion of T cells targeting a common antigen shared by the tumour and the heart [2,80]. The main features of myocarditis include myocardial inflammation and immune cell infiltration into the cardiac tissue, cardiac sinus, and atrioventricular nodes [22,85]. Histopathological analysis of the heart have revealed that the infiltrating cells within the myocardium are positive for the T cell marker CD3 or the macrophage marker CD68. Moreover, tissue infiltrations are characterized by a high proportion of CD4+ and CD8+ T cells [81,86].
4.1.3. Clinical manifestation
ICI-related myocarditis lacks specific clinical manifestations such as shortness of breath, chest pain, palpitations, fatigue, hypotension due to cardiogenic shock, and sudden cardiac death [83]. Notably, special care may be required when using ICIs in patients with systemic autoimmune diseases because they may develop subclinical myocarditis without apparent signs and symptoms [87]. Besides, ICIs can lead to the simultaneous development of myocarditis, myositis, and myasthenia gravis, indicating poor patient prognoses [4,16,46]. Among patients with ICI-related myocarditis, 23–33% have myositis, while 11% are diagnosed with myasthenia gravis [4,81]. Another analysis of 180 patients with ICI-associated myositis revealed that at least 16% of the patients were diagnosed with myocarditis [88]. As mentioned above, this phenomenon may be due to the cardiac and skeletal muscles sharing antigens targeted by T cells [81].
4.1.4. Medical inspection and examination
In addition to the symptoms, myocarditis can present with abnormalities on laboratory and imaging examinations. Knowledge of these indicators can assist in the identification and diagnosis of the condition.
First, the diagnosis of myocarditis can be aided by elevated levels of myocardial markers, such as creatine kinase, creatine kinase-MB, troponin T (TNT), B-type natriuretic peptide (BNP), and N-terminal pro-BNP (NTpro-BNP). A multicentre case-control study found that 94% and 66% of patients with ICI-associated myocarditis had elevated TNT and BNP levels, respectively [22]. Further, some inflammatory factors, including C-reactive protein, IL-6, and IL-10, are used to assist in diagnosis [89].
Second, up to 89% of patients have an abnormal ECG, which seems to be one of the most sensitive signs of ICI-related myocarditis but lacks specificity [90]. ECG abnormalities mainly include tachycardia, bradycardia, atrioventricular, intraventricular conduction block, and ST-T changes [91,92]. A clinical study showed that ICIs could prolong the QRS duration, increasing the occurrence of major adverse cardiac events (MACEs) [93]. However, myocarditis cannot be excluded from a normal ECG cannot exclude the diagnosis of myocarditis [22]. If an abnormality is detected on the ECG, emergency cardiovascular imaging (e.g. coronary angiography) should be conducted promptly to exclude other causes of myocardial injury, especially acute coronary syndrome and acute infectious myocarditis [94].
Echocardiography is an important imaging modality for patients with suspected ICI-related myocarditis. However, unlike other forms of myocarditis, at least half of patients with ICI-associated myocarditis maintain a normal ejection fraction on presentation [22,90]. The global longitudinal strain (GLS) is an important echocardiographic indicator that decreases during the development of ICI-related myocarditis. Lower GLS counts are strongly associated with MACEs in ICI-associated myocarditis presenting with either a preserved or reduced ejection fraction [95].
Cardiovascular magnetic resonance imaging (CMR) is the preferred imaging modality for diagnosing myocarditis, allowing tissue characterisation techniques to be used as surrogates for myocardial injury. CMR can identify the characteristics of fibrosis and inflammatory tissues in the early stages of the disease because of smaller operator supervisory factors and technologies more advanced than echocardiography. Hence, abnormal values in T1 and T2 mapping of CMR may provide significant diagnostic value [96]. In 2009, the ‘Lake Louise Criteria’ for the diagnosis of myocarditis using CMR were published. These criteria propose three common features of myocardial tissue: oedema, hyperaemia, and necrosis or scarring. The corresponding imaging features included T2-weighted CMR images, myocardial early gadolinium enhancement, and late gadolinium enhancement. According to these criteria, meeting two of the three aforementioned criteria indicates a relatively high likelihood of acute myocarditis [97]. Although echocardiography and MRI provide new technologies for obtaining sufficient information, the diagnostic sensitivity for ICI-associated myocarditis remains low. Additionally, cardiac 18F-fluorodeoxyglucose position emission tomography can reveal active myocardial inflammatory changes and help diagnose myocarditis [81].
Lastly, myocardial biopsy is the gold standard for diagnosis. Pathological examination usually reveals T-lymphocyte and macrophage infiltration as well as the death of cardiomyocytes [98]. Immunohistochemistry following myocardial biopsy revealed an exuberant immune response by CD8+ cytotoxic T cells and uniformly high CD68+ cells representing histiocytes (macrophages), with mild CD4+ T cell infiltration [99]. Patients with possible ICI-associated myocarditis should actively undergo myocardial biopsy, which can not only confirm the diagnosis but also facilitate the treatment and improve the prognosis [100].
4.1.5. Diagnosis and treatment
Diagnosis can be made based on medication history, symptoms, and the tests mentioned above. Grading the disease severity aids the diagnosis and treatment. Although no apparent risk stratification has been established for ICI-associated myocarditis, the noticeable increase in biological standards, myocardial inflammatory cell infiltration, decreased myocardial muscle strength, and arrhythmia are apparent high-risk indicators [22,81,86]. The Common Terminology Criteria for Adverse Events (CTCAE) was devised to provide a uniform approach for classifying adverse events resulting from antineoplastic therapies in clinical trials. In general, myocarditis severity can be classified into five grades (G1–G5) according to Version 5 criteria (2017). G1 did not exhibit any apparent symptoms. In G2, mild symptoms and abnormal screening test results were observed. G3 signified moderately abnormal testing results or symptoms with mild activity that may necessitate intervention. G4 is considered life-threatening and typically requires medical treatment. G5 refers: death [101].
Drug withdrawal and corticosteroid therapy are the most important treatments [25,27]. Corticosteroids are the first line of treatment. The initial corticosteroid dose and treatment strategies varied significantly across different guidelines. This retrospective study provides insights into this perplexing issue. A higher initial dose (e.g. methylprednisolone 1000 mg/day) and earlier use of corticosteroids (within 24 h) can improve the prognosis of ICI-related myocarditis [102]. Some guidelines recommend that ICI therapy should be discontinued and intravenous methylprednisolone 500–1000 mg once daily for 3–5 days as soon as possible after diagnosis, followed by oral prednisone at 1–2 mg/kg/d for 4–6 weeks to reduce the incidence of MACEs in cases of non-fluctuating and fulminant ICI-associated myocarditis [102,103]. If corticosteroid therapy is ineffective, other immunosuppressants, such as mycophenolate mofetil, anticalcineurin, anti-thymocyte globulin, or intravenous immunoglobulin, are administered [8,22]. New drugs are under development for the treatment of immunomyocarditis. For example, abatacept, which targets CD80/CD86 on APCs to impede the interaction between T cells and APCs, thereby inhibiting T cell activation, is currently under clinical study for the treatment of patients with ICI-associated myocarditis [17]. Patients who develop heart failure or arrhythmia can be treated according to relevant guidelines [[104], [105], [106]]. Once the abnormality is resolved, a multidisciplinary treatment (MDT) discussion is recommended to determine whether to permanently discontinue or resume the ICI [100].
4.2. Pericarditis
4.2.1. Epidemiology
Pericarditis, occurring either in isolation or concomitantly with myocarditis, is a common ICI-associated cardiovascular toxicity that manifests as pericardial effusion or cardiac tamponade. The WHO database identified 95 cases of pericardial disease in over 30,000 patients who experienced side effects of treatment with ICIs. Lung cancer patients who received ICIs had the highest incidence of pericardial effusion [4]. A retrospective study involving 2842 patients who had received ICIs and 2699 patients who did not found that the incidence of pericarditis or pericardial effusion was more than four times higher in patients who received ICIs than in those who did not (HR 4.37, P < 0.001) [107]. The risk was also higher for single agents containing anti-PD-1 or anti-PD-L1 antibodies than for anti-CTLA-4 drugs (0.36% vs 0.16%, respectively). Surprisingly, the combination of two ICIs does not increase the risk of pericarditis compared with the use of only one ICI (0.33% vs 0.3%) [4]. A retrospective study reported that the median onset time of ICI-related pericarditis is approximately 6 months [108].
4.2.2. Aetiology and pathology
No relevant preclinical or clinical studies have investigated the mechanisms underlying ICI-related pericardial diseases. Several case reports on ICI-associated pericarditis have found that inflammatory cell infiltrates are almost always present in the pericardium, including large numbers of CD4+ and CD8+ T cells, CD68+ macrophages, and scattered CD20+ B cells [99].
4.2.3. Clinical manifestation and examination
The main clinical manifestations of pericarditis are chest pain and dyspnoea [109]. A significant amount of pericardial effusion or tamponade may lead to hypotension, cardiogenic shock, or cardiac arrest. When a patient exhibits symptoms of pericardial disease, physical examination, cardiac biomarkers, electrocardiography, radiography, computed tomography (CT), and echocardiography can be performed to evaluate the condition [8]. Cardiac magnetic resonance (CMR) is an important option for evaluating myocarditis. Pericardial biopsy is the gold standard for its diagnosis and is currently used relatively infrequently in clinical practice [25].
4.2.4. Treatment
The prognosis of ICI-related pericardial diseases was poor, especially in cases with concomitant myocarditis [108,110]. According to current guidelines, the top recommended treatment for patients with moderate-to-severe pericardial effusion is still the interruption of ICIs [25,27]. Prednisolone should be firstly recommended for patients with ICI-associated pericarditis. Nonsteroidal anti-inflammatory drugs and colchicine can relieve the symptoms of pericarditis and reduce pericardial effusion [111]. Mycophenolate mofetil, infliximab, or anti-thymocyte globulin can be used as a second-line treatment for steroid-refractory pericarditis, but no systematic evidence exists [8,112]. If a patient presents with massive pericardial effusion or cardiac tamponade, the preferred first-aid measures include pericardiocentesis or surgical pericardiotomy [108]. Once pericarditis or pericardial effusion is resolved, the decision to restart ICI treatment under close monitoring can be discussed using the MDT.
4.3. Takotsubo syndrome
4.3.1. Epidemiology
Takotsubo syndrome (TTS), a form of acute left ventricular dysfunction, is a relatively rare form of cardiovascular toxicity associated with ICIs [113]. One study reported that TTS accounted for 14% (4/29) of the total ICI-related cardiotoxicities [90]. No specific information regarding the onset time has been reported, owing to limited data on ICI-associated TTS. However, the incidence rate is inevitably higher in the initial three months of medication [90].
4.3.2. Aetiology and pathology
The mechanisms underlying the ICI-associated TTS remain unknown. It remains unclear whether the TTS associated with ICI reflects a direct effect of ICI on the myocardial or coronary vascular system or an indirect effect via a sudden release of large amounts of adrenaline from the adrenal glands or norepinephrine from postganglionic sympathetic nerves [8,114]. Unlike ICI-related myocarditis, the underlying pathological mechanism of ICI-related TTS is non-inflammatory [114].
4.3.3. Clinical manifestation and examination
The main clinical symptoms include acute chest pain, breathlessness, and palpitations caused by arrhythmias. In severe cases, patients may present with pre-syncope or syncope resulting from ventricular tachyarrhythmias, severe left ventricular outflow tract obstruction (LVOTO), or cardiogenic shock [113].
Abnormalities can be detected using ECG, biochemical tests, and imaging. ECG displays ST-T changes, widespread T-wave inversion, and QT prolongation [115]. Elevation of myocardial enzymes is frequently observed along with elevated levels of BNP or NT-pro-BNP. However, as in myocarditis, these markers lack specificity. Cardiac catheterisation revealed no significant coronary artery stenosis. Echocardiography is an invaluable tool for diagnosing and monitoring TTS as it can identify possible complications such as LVOTO, acute mitral regurgitation, right ventricular involvement, or apical thrombus. In typical TTS, abnormal circumferential wall motion can be detected in the short-axis view of the mid-left ventricular [116]. CMR has also been used to evaluate concurrent myocarditis [117]. Invasive coronary angiography can help to exclude acute myocardial infarction in most patients. For patients with advanced malignancy or severe thrombocytopenia, invasive coronary angiography is contraindicated and coronary CT angiography is recommended [27]. Therefore, ventriculography is a reliable diagnostic tool for the treatment of TTS. Once coronary occlusion is ruled out, ventriculography should be performed unless contraindicated. This is crucial because wall motion anomalies may recover within hours and may be missed if imaging is delayed [114].
4.3.4. Treatment
According to these guidelines, it should be discontinued ICIs in patients with TTS [118]. It is uncertain whether immunosuppressive drugs are useful in such patients. However, one recommendation proposes the use of methylprednisolone because of the similarity between ICI-induced TTS and myocarditis [27]. Furthermore, high-dose corticosteroid therapy (methylprednisolone, 1000 mg/day) has proven effective in some reported cases [117]. However, QT-prolonging drugs should be avoided [114]. If a patient recovers from acute-phase TTS, MDT should be performed to determine whether to resume ICI therapy [119].
4.4. Arrhythmia
4.4.1. Epidemiology
Arrhythmia is also the most common cardiovascular toxicity but often coexists with other ICI-associated cardiovascular toxicities (e.g. thyroiditis with thyrotoxicosis, myocarditis, pericarditis, or severe systemic inflammatory syndromes) [6]. Several types of therapy have been linked to ICI arrhythmias, including supraventricular arrhythmias, ventricular arrhythmias, and conduction disturbances. Data from 5518 cancer patients who received at least one cycle of ICIs extracted from a vast network of healthcare organisations indicated that by the 12th month, 12.5% of patients developed cardiotoxicity, the most common cardiotoxicity being arrhythmia (9.3%) [129]. In a descriptive observational analysis of patients, cardiotoxicity, atrial fibrillation, ventricular arrhythmia, and conduction disorders were observed in 30%, 27%, and 17% of patients, respectively [99]. ICI-associated conduction abnormalities in the absence of generalised myocarditis are emerging as common and potentially serious causes of ICI-mediated sudden death [99].
4.4.2. Aetiology and pathology
The pathophysiology of arrhythmogenesis varies for different cancer therapies and may be attributed to direct cellular effects, electrolyte abnormalities, or toxicities related to cancer therapy, such as heart failure, ischemia, myocarditis, or combined ICI-associated thyroiditis [120]. The mechanism of arrhythmias associated with ICIs is largely unknown, although it has been associated with direct T cell-mediated cytotoxicity. Some studies have shown that there is immune cell infiltration in the sinoatrial node, the atrioventricular node, and the His-Purkinje conduction system [22,85]. Transgenic mouse strains harbouring loss-of-function alleles of Ctla4 (encoding CTLA-4) and Pdcd1 (encoding PD-1) recapitulate some characteristics of conduction abnormalities related to ICI treatment, although further research is required [37]. A review published in the Lancet suggested that arrhythmia may be related to the following mechanisms: (1) triggered activity or re-entry mechanism due to fibrosis and inflammation of myocardium; (2) inflammation or direct immune interaction involving the cardiac conduction; (3) effect of systemic inflammatory state on atrial and ventricular arrhythmias; (4) effect of left ventricular impairment due to non-inflammatory functional cardiotoxicity on atrial and ventricular arrhythmias; or (5) other causes of arrhythmias such as electrolyte disorder, pulmonary embolism, other drugs, or pre-existing cardiovascular disease [8].
4.4.3. Clinical manifestation and examination
The main symptoms of patients with ICI-associated arrhythmia are palpitations, fatigue, presyncope, syncope, and sudden cardiac death. ECG has high diagnostic value, and Holter electrocardiogram is feasible when necessary [121]. Despite the relatively high incidence of arrhythmia, its actual occurrence is frequently underestimated as routine cardiac monitoring is either not performed or involves only non-continuous 12-lead ECG. ECG changes are often nonspecific, including supraventricular arrhythmia, ventricular arrhythmias, conduction abnormalities (e.g. conduction block, QRS prolongation, and Q-T prolongation), and ST-T abnormalities [122]. Echocardiography and cardiac MRI can help determine whether it is accompanied by myocarditis or pericarditis [25,27].
4.4.4. Treatment
Currently, no dedicated protocol is available for the management of ICI-associated arrhythmias. According to the guidelines for non-specific arrhythmias, antiarrhythmic drugs, catheter ablation, and device therapy (e.g., pacemakers) are potential treatment options [106,123,124]. If combined with ICI-related myocarditis, the aforementioned treatment methods for myocarditis can be followed.
4.5. Vascular toxicity
4.5.1. Vasculitis
4.5.1.1. Epidemiology
Vasculitis is a rare form of cardiovascular toxicity associated with ICIs. Currently, no reliable statistical data are available on the incidence rate. In an observational retrospective pharmacovigilance study including 31321 adverse events reported in patients who received ICIs, 82 had vasculitis, including 18 with temporal arteritis and 16 with polymyalgia rheumatica [4]. The incidence of ICI-associated vasculitis is not related to age but appears to be sex-specific, with a higher incidence in men. The median time to onset of toxicity was 55 days (IQR: 21–98) [4,8].
4.5.1.2. Aetiology and pathology
The mechanism of ICI-associated vasculitis is unclear and may involve a defective checkpoint pathway in the arteries that makes these arterial walls vulnerable to autoimmune attack, producing a broad spectrum of inflammatory cytokines (IFN-γ, IL-17, and IL-21) and playing a direct role in promoting intimal hyperplasia and intramural neovascularization [125]. ICI-associated vasculitis can affect vessels of any size, but is most commonly reported in large vessels, such as temporal arteritis (giant cell), aortitis, and primary angiitis of the nervous system [4,126]. A recent systematic literature review examined 20 cases of vasculitis in patients who had received ICIs including ipilimumab (n = 8), pembrolizumab (n = 6), nivolumab (n = 5), and a combination of anti-PD-L1 and targeted therapy with a BRAF inhibitor (n = 1). The histological diagnosis confirmed vasculitis involving vessels of different sizes [126]. A study analysing 15 kidney biopsies of antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis in correlation with glomerular and tubulointerstitial lesions found that the predominant tubulointerstitial expression of PD-1 decreased in ANCA-associated renal vasculitis. Moreover, the loss of tubulointerstitial PD-1 strongly correlates with active ANCA-associated renal vasculitis [127]. A case of myocardial vasculitis due to pembrolizumab was reported and confirmed by endomyocardial biopsy [128]. Giant cell arteritis (GCA) is a large vessel vasculitis that can be caused by PD-L1 and CTLA-4 blockade and may be associated with symptoms of rheumatic polymyalgia [129].
4.5.1.3. Clinical manifestation and examination
The clinical symptoms of ICI-associated vasculitis vary, depending on the affected vessel. For example, vascular inflammation in GCA patients is accompanied by systemic inflammation, which manifests as fever, failure to thrive, polymyalgia rheumatica, and exuberant production of acute-phase reactants [130]. Temporal arteritis (giant cells) presents with headache, jaw claudication, transient monocular visual loss, and diplopia. Clinical consequences are mainly associated with aortic wall injury, aortic arch syndrome, and ocular and posterior cerebral ischemic complications [131].
Complete blood count, comprehensive metabolic panel (CMP), erythrocyte sedimentation rate, C-reactive protein, ANCA, serum complement (C3/C4), serology for viral hepatitis, serum cryoglobulins, urinalysis, and blood cultures (to rule out endocarditis) were helpful in diagnosis vasculitis [132]. The evaluation for vasculitis of grade ≥2 should include a biopsy of the affected organs, or imaging through CT or MRI if a biopsy is not possible [103].
4.5.1.4. Treatment
No well-established guidelines are available for the treatment of ICI-associated vasculitis. Vasculitis can initially resolve following the withdrawal of ICIs and corticosteroid therapy. A systematic review included 53 cases of large-vessel vasculitis, and central and peripheral nervous system vasculitis. All cases were resolved by either holding the ICIs and/or administering corticosteroid therapy, and none happened to death [126]. In addition to corticosteroids, other immune drugs such as cyclophosphamide can also be considered [133].
4.5.2. Acute coronary syndromes (ACS)
4.5.2.1. Epidemiology
ACS is a rare but severe cardiovascular complication associated with ICI treatment. Multiple cases of ACS have a cause-and-effect relationship with ICI use [24,134]. In a matched cohort study including 2842 patients treated with ICI and 2842 matched controls, the risk of cardiovascular events (including myocardial infarction, coronary revascularization, and ischaemic stroke) increased 3-fold after the initiation of ICI (HR 3.3 [95% CI, 2.0–5.5]; P < 0.001). Moreover, the rate of atherosclerotic plaque progression (based on total aortic plaque volume) was more significant than 3-fold higher in the ICI-treated group than in the control group [135]. A meta-analysis of 22 clinical trials on ICI treatment for lung cancer patients reported that the incidence of ICI-related myocardial infarction was 1% [136].
4.5.2.2. Aetiology and pathology
Currently, the pathophysiological mechanism of ICI-related ACS is not precise and is thought to be related to several factors. First, ICIs can activate inflammation in preexisting atherosclerotic coronary plaques and cause fibre cap rupture, acute coronary thrombosis, and myocardial infarction. The second explanation is the direct activation of T-cell-mediated coronary vasculitis without atherosclerosis [8,137]. A third reason is coronary vasospasm; one case of transient ST-segment elevation accompanying pembrolizumab treatment for bronchial adenocarcinoma has been reported [138].
Furthermore, preclinical and clinical trials have assessed the impact of immune checkpoint signalling via CTLA-4 and PD-1 on atherosclerosis. In the atherosclerotic mouse model using apolipoprotein E knockout (Apoe −/−) mice, the overexpression of CTLA-4 improved the formation of atherosclerotic lesions by down-regulating the activity of CD4+T cells and inhibiting the migration of macrophages to atherosclerotic plaques [139]. Moreover, CTLA-4 is constitutively expressed in Treg cells and that the amount of CTLA-4 negatively correlates with human plaque size and vulnerability [140].
Anti-PD-1 treatment is considered to hasten the progression of atherosclerosis, as evidenced by the increased formation of atherosclerotic lesions and elevated infiltration of macrophages and T cells in PD-1 deficient low-density lipoprotein receptor knockout (Ldlr −/−) mice [141]. Further, plaques from ICI-treated patients contain T cell subsets with cell depletion markers (high levels of PD-1 and transcriptional signatures of PDCD1 and LAG-3) and macrophages with an activated phenotype associated with plaque vulnerability [142]. The presence of PD-1 expressing depleted T cells within atherosclerotic plaques indicates that PD-1 inhibitors may activate these T cells and worsen atherosclerosis [143].
4.5.2.3. Brief clinical process
ACS is characterised by acute chest pain, elevated cardiac enzyme levels, new ischaemic changes on ECG, and regional wall motion abnormalities on echocardiography [133]. Coronary angiography can not only confirm the diagnosis but also clarify the degree of coronary stenosis [144]. The first-aid measures include percutaneous coronary intervention, coronary artery bypass grafting, and the use of related drugs [94,144]. Temporary interruption of ICIs therapy is recommended for patients with ICI-associated ACS [27]. Corticosteroids are used to treat these patients [24,134]. The decision to resume ICI therapy should only be made after MDT.
4.5.3. Venous thromboembolism (VTE)
4.5.3.1. Epidemiology
VTE is a common complication of ICI-associated vascular injury. Attributing thromboembolic events exclusively to ICI treatment may be difficult because these events can be caused by cancer [145]. Most retrospective studies have found an elevated incidence of VTE after treatment with ICIs, which ranges from 8% to-30%. A retrospective study suggested that the overall incidence of thromboembolic events in ICI-treated patients is approximately 8%; however, the study did not distinguish between events induced by ICI therapy and those caused by the malignancy itself [146]. A study involving 672 patients who underwent ICI treatment reported 47 VTE events, with a cumulative incidence of 12.9%. Another single-centre study of 228 patients with melanoma treated with ICI identified VTE events in 16.2% of the participants [147,148]. A retrospective study including 2854 patients who had received ICIs at a single academic centre suggested that the incidence of VTE increased by over four times after the initiation of ICI (HR 4.98, 95% CI 3.65–8.59, P < 0.001) [149]. A systematic review and meta-analysis of 59 trials, 25 of 5578 patients with melanoma, and 34 of 6543 patients with non-small cell lung cancer (NSCLC) reported that the incidence of VTE was significantly higher in patients receiving ICIs. Among these, the incidence of dual immunotherapy is higher than that of monotherapy in NSCLC patients [150]. Furthermore, a study showed that ICIs combined with targeted therapy and chemotherapy resulted in the highest cumulative incidence of deep vein thrombosis [151].
4.5.3.2. Aetiology and pathology
Currently, the pathophysiology of ICI leading to venous thrombotic events remains under investigation. Some studies have suggested that systemic proinflammatory states induced by ICIs may enhance the prothrombotic state by activating coagulation and platelets and impairing fibrinolysis [152,153]. Moreover, activated T cells can induce the synthesis of tissue factors in monocytes and macrophages, which may be a mechanism that promotes a hypercoagulable state [154]. Additionally, ICIs have the ability to cause a rise in inflammatory molecules like IL-1β, IL-6, and TNF, which are strongly associated with the development of VET [155]. The pre-existing proinflammatory condition could be worsened by the inhibition of PD-1 and/or CTLA-4, causing the activation of platelets and coagulation, as well as the impairment of fibrinolysis [153].
4.5.3.3. Brief clinical process
The symptoms associated with VTE include pain, swelling of the extremities, increased visibility of the skin veins or purpuric rash, erythema, and cyanosis [156]. One study showed that the risk stratification of thromboembolic events using the Khorana score and guiding anticoagulation therapy in patients treated with ICIs can benefit these patients [157]. Vascular colour Doppler ultrasonography and angiography are valuable tools for diagnosing VTE [25]. The main treatment options for cancer-related thrombosis include direct oral anticoagulants and low-molecular-weight heparin [27]. Recent prospective clinical trials have shown that prophylactic anticoagulation prevents VTE in high-risk cancer patients (defined as Khorana scores ≥2) receiving conventional chemotherapy, with a reduction in VTE of up to 6% [158]. Even without chemotherapy, the prophylactic use of anticoagulants may be beneficial for patients at high risk of thrombosis. Patients who experience thromboembolic events or are deemed at risk should not receive corticosteroids [159]. When the patient's condition is stable, ICIs are continued in the absence of other irAEs [25].
5. Conclusion
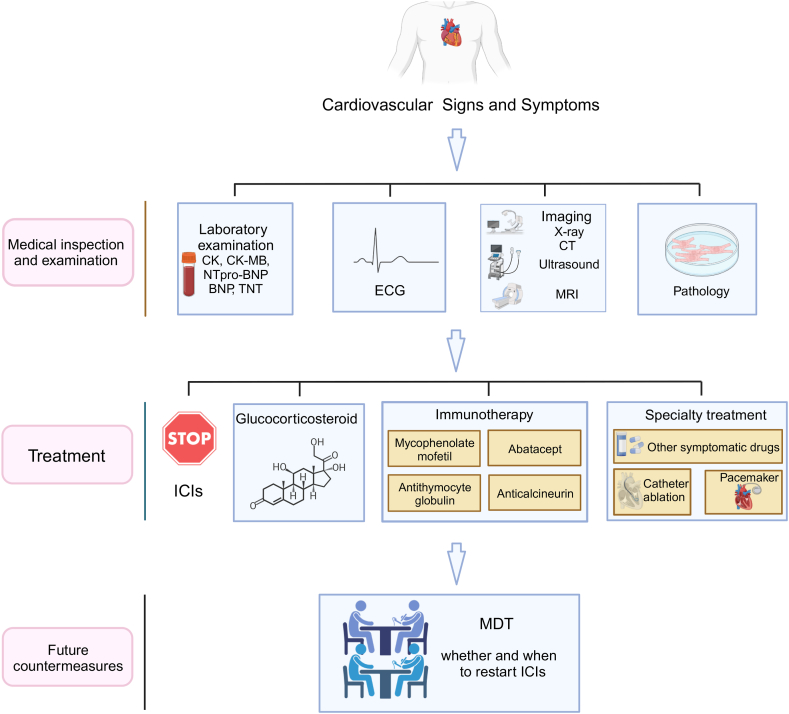
ICIs have revolutionised the field of oncology treatment. With the widespread use of ICIs, adverse reactions, particularly cardiovascular toxicity, have attracted increasing attention. Among these, myocarditis requires greater attention because of its high morbidity and mortality. The pathological mechanisms of ICI-related cardiovascular toxicities remain unclear and require further confirmation through preclinical and clinical studies. The diagnosis depends on a comprehensive assessment of symptoms, laboratory tests, imaging, and pathology. Effective management of toxicity often necessitates interdisciplinary collaboration, particularly between oncology and cardiology, and the specific course of treatment may vary based on the severity of toxicity. Currently, the primary treatments include drug withdrawal and corticosteroid therapy. Typically, the diagnostic and treatment processes for cardiovascular irAEs involve the following steps: (1) identifying and evaluating the type and severity of cardiovascular toxicities; (2) determining whether to discontinue ICI therapy; (3) considering whether to use corticosteroid therapy and immunotherapies; (4) specialised treatment for cardiovascular irAEs; and (5) assessing whether and when to restart ICIs [31] (Fig. 3). More targeted drugs to prevent cardiovascular toxicities and new agents against ICI-related cardiovascular toxicities are currently being tested in preclinical and clinical trials. ICI-related cardiovascular toxicities should be prevented and treated effectively.
Fig. 3.
Overview of diagnosis and treatments for ICI-associated cardiovascular toxicities. Abbreviations: ICI, Immune checkpoint inhibitor; CK, creatine kinase; CK-MB, creatine kinase-MB; BNP, B-type natriuretic peptide; NTpro-BNP, N-terminal pro-BNP; TNT, troponin T; ECG, electrocardiogram; CT, computed tomography; MRI, magnetic resonance imaging; MDT, multi-disciplinary treatment. Figure created with BioRender.com.
Funding statement
This study was financially supported by the National Natural Science Foundation of China (grant no.81201788).
Data availability statement
No data were used for the research described in this article.
CRediT authorship contribution statement
Guihong Liu: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Formal analysis, Data curation. Tao Chen: Writing – review & editing, Writing – original draft, Resources, Data curation. Xin Zhang: Resources, Investigation, Data curation, Conceptualization. Binbin Hu: Writing – original draft, Resources, Data curation. Huashan Shi: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Data curation.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Not applicable.
References
- 1.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 2.Baik A.H., Oluwole O.O., Johnson D.B., et al. Mechanisms of cardiovascular toxicities associated with immunotherapies. Circ Res. 2021;128:1780–1801. doi: 10.1161/CIRCRESAHA.120.315894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baik A.H., Tsai K.K., Oh D.Y., et al. Mechanisms and clinical manifestations of cardiovascular toxicities associated with immune checkpoint inhibitors. Clin Sci (Lond) 2021;135:703–724. doi: 10.1042/CS20200331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolladille C., Ederhy S., Sassier M., et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Souza M., Nielsen D., Svane I.M., et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2021;42:1621–1631. doi: 10.1093/eurheartj/ehaa884. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyon A.R., Yousaf N., Battisti N.M.L., et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 9.Manuel Ramos-Casals J.R.B., Margaret K Callahan, Flores-Chávez Alejandra, Keegan Niamh, Khamashta Munther A., Lambotte Olivier, Mariette Xavier, Prat Aleix, Suárez-Alma Maria E. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):39. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D.B., Chandra S., Sosman J.A. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 12.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 13.Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio-Infante N., Ramirez-Flores Y.A., Castillo E.C., et al. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail. 2021;23:1739–1747. doi: 10.1002/ejhf.2289. [DOI] [PubMed] [Google Scholar]
- 15.Hu J., Tian R., Ma Y., et al. Risk of cardiac adverse events in patients treated with immune checkpoint inhibitor regimens: a systematic review and meta-analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.645245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moslehi J.J., Salem J.E., Sosman J.A., et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waliany S., Lee D., Witteles R.M., et al. Immune checkpoint inhibitor cardiotoxicity: understanding basic mechanisms and clinical characteristics and finding a cure. Annu Rev Pharmacol Toxicol. 2021;61:113–134. doi: 10.1146/annurev-pharmtox-010919-023451. [DOI] [PubMed] [Google Scholar]
- 18.Waliany S., Sainani K.L., Park L.S., et al. Increase in blood pressure associated with tyrosine kinase inhibitors targeting vascular endothelial growth factor. JACC CardioOncol. 2019;1:24–36. doi: 10.1016/j.jaccao.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers C.J., Lu B. Decreased survival after combining thoracic irradiation and an anti-PD-1 antibody correlated with increased T-cell infiltration into cardiac and lung tissues. Int J Radiat Oncol Biol Phys. 2017;99:1129–1136. doi: 10.1016/j.ijrobp.2017.06.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahyon H.A., Al-Harbi S.A. Chemoprotective role of an extract of the heart of the Phoenix dactylifera tree on adriamycin-induced cardiotoxicity and nephrotoxicity by regulating apoptosis, oxidative stress and PD-1 suppression. Food Chem Toxicol. 2020;135 doi: 10.1016/j.fct.2019.111045. [DOI] [PubMed] [Google Scholar]
- 21.Porter C., Azam T.U., Mohananey D., et al. Permissive cardiotoxicity: the clinical crucible of cardio-oncology. JACC CardioOncol. 2022;4:302–312. doi: 10.1016/j.jaccao.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norwood T.G., Westbrook B.C., Johnson D.B., et al. Smoldering myocarditis following immune checkpoint blockade. Journal for ImmunoTherapy of Cancer. 2017;5 doi: 10.1186/s40425-017-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita Y.S.D., Kakiuchi Y., Saeki S., Saruwatari K., Sakata S., Jodai T., Migiyama Y., Akaike K., Hirosako S., et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann Oncol. 2017;28:2893–2895. doi: 10.1093/annonc/mdx326. [DOI] [PubMed] [Google Scholar]
- 25.Bryan J., Schneider Mjn M.D., Santomasso Bianca D., PhD M.D., Lacchetti Christina, Mhsc, Adkins Sherry, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy- ASCO guideline Update.pdf. Journal of Clinical Oncology. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 26.Jonathan D. Ashwell FWML, Vacchio aMS: glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 27.Lyon A.R., Lopez-Fernandez T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS) Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 28.Doms J., Prior J.O., Peters S., et al. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann Oncol. 2020;31:1273–1275. doi: 10.1016/j.annonc.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esfahani K., Buhlaiga N., Thebault P., et al. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 30.Joe-Elie Salem Y.A. Abatacept for severe immune checkpoint inhibitor–associated Myocarditis.pdf. The New England Journal of Medicine. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 31.Zito C., Manganaro R., Ciappina G., et al. Cardiotoxicity induced by immune checkpoint inhibitors: what a cardio-oncology team should know and do. Cancers (Basel) 2022;14(21):5403. doi: 10.3390/cancers14215403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monney L.S.C., Gaglia J.L., Ryu A., Waldner H., Chernova T., et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 33.Lines J.L., Pantazi E., Mak J., et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X., Harden K., Gonzalez L.C., et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 35.Ceeraz S., Nowak E.C., Noelle R.J. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tawbi H.A., Schadendorf D., Lipson E.J., et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34. doi: 10.1056/NEJMoa2109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei S.C., Meijers W.C., Axelrod M.L., et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 2021;11:614–625. doi: 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lian X., Yang K., Li R., et al. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol Cancer. 2022;21(1):27. doi: 10.1186/s12943-021-01486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halle S., Halle O., Forster R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 2017;38:432–443. doi: 10.1016/j.it.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Godfrey D.I., Le Nours J., Andrews D.M., et al. Unconventional T cell targets for cancer immunotherapy. Immunity. 2018;48:453–473. doi: 10.1016/j.immuni.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucca L.E., Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat Rev Immunol. 2020;20:680–693. doi: 10.1038/s41577-020-0296-3. [DOI] [PubMed] [Google Scholar]
- 43.Finn O.J. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):6–9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grabie N., Lichtman A.H., Padera R. T cell checkpoint regulators in the heart. Cardiovasc Res. 2019;115:869–877. doi: 10.1093/cvr/cvz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Borght K., Scott C.L., Nindl V., et al. Myocardial infarction primes autoreactive T cells through activation of dendritic cells. Cell Reports. 2017;18:3005–3017. doi: 10.1016/j.celrep.2017.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu J.R., Florido R., Lipson E.J., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–868. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephen Hodi Md F., Steven J. O'Day M.D., David F. McDermott M.D., Robert W., Weber M.D., Jeffrey A., Sosman M.D., John B., Haanen M.D., Rene Gonzalez M.D., Caroline Robert M.D.PhD., Dirk Schadendorf M.D., Jessica C., Hassel M.D., Wallace Akerley M.D., Alfons J.M., van den Eertwegh M.D.PhD., Jose Lutzky M.D., Paul Lorigan M.D., Julia M., Vaubel M.D., Gerald P., Linette M.D., PhD, David Hogg M.D., Christian H., Ottensmeier M.D.PhD., Celeste Lebbé M.D., Christian Peschel M.D., Ian Quirt M.D., Joseph I., Clark M.D., Jedd D., Wolchok M.D.PhD., Jeffrey S., Weber M.D.PhD., Jason Tian PhD., Michael J., Yellin M.D., Geoffrey M., Nichol M.B.ChB., Axel Hoos M.D.PhD., Walter J., Urba M.D. PhD. Improved survival with ipilimumab in patients with metastatic melanoma. The new england journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krummel M.F.A.J. CTLA-4 engagement inhibits 11.+-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao J., Shi L.Z., Zhao H., et al. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167:397–404. doi: 10.1016/j.cell.2016.08.069. e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tivol E.A.B.F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 52.Maurer M.F., Lewis K.E., Kuijper J.L., et al. The engineered CD80 variant fusion therapeutic davoceticept combines checkpoint antagonism with conditional CD28 costimulation for anti-tumor immunity. Nat Commun. 2022;13(1):1790. doi: 10.1038/s41467-022-29286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schadendorf D., Hodi F.S., Robert C., et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keir M.E., Butte M.J., Freeman G.J., et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boussiotis V.A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarrio M.L., Grabie N., Bu D.X., et al. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188:4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimura H.N.M., Hiai H., Minato N., Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 58.Yi M., Zheng X., Niu M., et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaikwad S., Agrawal M.Y., Kaushik I., et al. Immune checkpoint proteins: signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol. 2022;86:137–150. doi: 10.1016/j.semcancer.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 60.Malmberg R., Zietse M., Dumoulin D.W., et al. Alternative dosing strategies for immune checkpoint inhibitors to improve cost-effectiveness: a special focus on nivolumab and pembrolizumab. Lancet Oncol. 2022;23(12):e552–e561. doi: 10.1016/S1470-2045(22)00554-X. [DOI] [PubMed] [Google Scholar]
- 61.Blackburn S.D., Shin H., Haining W.N., et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gandhi M.K., Lambley E., Duraiswamy J., et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108:2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Sanmamed M.F., Datar I., et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:334–347. doi: 10.1016/j.cell.2018.11.010. e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao X., Ou M.T., Karuppagounder S.S., et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353(6307):aah3374. doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sauer N., Szlasa W., Jonderko L., et al. LAG-3 as a potent target for novel anticancer therapies of a wide range of tumors. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23179958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okazaki T., Okazaki I.M., Wang J., et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woo S.R., Turnis M.E., Goldberg M.V., et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.WorkmanCJ C., KimI J., Blackman M.A., Woodland D.L., Vignali D.A.A. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172(9):5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 69.Sordo-Bahamonde C., Lorenzo-Herrero S., Gonzalez-Rodriguez A.P., et al. LAG-3 blockade with relatlimab (BMS-986016) restores anti-leukemic responses in chronic lymphocytic leukemia. Cancers (Basel) 2021;13(9):2112. doi: 10.3390/cancers13092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alissafi T., Hatzioannou A., Legaki A.I., et al. Balancing cancer immunotherapy and immune-related adverse events: the emerging role of regulatory T cells. J Autoimmun. 2019;104 doi: 10.1016/j.jaut.2019.102310. [DOI] [PubMed] [Google Scholar]
- 71.Swirski F.K., Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 72.Tang T.T., Zhu Y.C., Dong N.G., et al. Pathologic T-cell response in ischaemic failing hearts elucidated by T-cell receptor sequencing and phenotypic characterization. Eur Heart J. 2019;40:3924–3933. doi: 10.1093/eurheartj/ehz516. [DOI] [PubMed] [Google Scholar]
- 73.Stephenson E., Savvatis K., Mohiddin S.A., et al. T-cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation. Br J Pharmacol. 2017;174:3914–3925. doi: 10.1111/bph.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwimmbeck Cb P.L., Rohn G., Schulze K., Schultheiss H.P. The role of sensitized T-cells in myocarditis and dilated cardiomyopathy. Int J Cardiol. 1996;54(2):117–125. doi: 10.1016/0167-5273(96)02588-0. [DOI] [PubMed] [Google Scholar]
- 75.Frodermann V., Nahrendorf M. Macrophages and cardiovascular Health. Physiol Rev. 2018;98:2523–2569. doi: 10.1152/physrev.00068.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okazaki T., Tanaka Y., Nishio R., et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 77.Grabie N., Gotsman I., DaCosta R., et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 78.Gestermann N., Saugy D., Martignier C., et al. LAG-3 and PD-1+LAG-3 inhibition promote anti-tumor immune responses in human autologous melanoma/T cell co-cultures. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1736792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zelba H., Bedke J., Hennenlotter J., et al. PD-1 and LAG-3 dominate checkpoint receptor-mediated T-cell inhibition in renal cell carcinoma. Cancer Immunol Res. 2019;7:1891–1899. doi: 10.1158/2326-6066.CIR-19-0146. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J.C., Chen W.D., Alvarez J.B., et al. Cancer immune checkpoint blockade therapy and its associated autoimmune cardiotoxicity. Acta Pharmacol Sin. 2018;39:1693–1698. doi: 10.1038/s41401-018-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. New England Journal of Medicine. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zamami Y., Niimura T., Okada N., et al. Factors associated with immune checkpoint inhibitor–related myocarditis. JAMA Oncology. 2019;5 doi: 10.1001/jamaoncol.2019.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moslehi J., Lichtman A.H., Sharpe A.H., et al. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021:131. doi: 10.1172/JCI145186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choueiri T.K., Larkin J., Oya M., et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19:451–460. doi: 10.1016/S1470-2045(18)30107-4. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L., Awadalla M., Mahmood S.S., et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–1743. doi: 10.1093/eurheartj/ehaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lehmann L.H., Cautela J., Palaskas N., et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. JAMA Cardiol. 2021;6:1329–1337. doi: 10.1001/jamacardio.2021.2241. [DOI] [PubMed] [Google Scholar]
- 87.Varricchi G., Galdiero M.R., Marone G., et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anquetil C., Salem J.E., Lebrun-Vignes B., et al. Immune checkpoint inhibitor-associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation. 2018;138:743–745. doi: 10.1161/CIRCULATIONAHA.118.035898. [DOI] [PubMed] [Google Scholar]
- 89.Kaya Z., Leib C., Werfel S., et al. Comparison of IL-10 and MCP-1-7ND gene transfer with AAV9 vectors for protection from murine autoimmune myocarditis. Cardiovasc Res. 2011;91:116–123. doi: 10.1093/cvr/cvr063. [DOI] [PubMed] [Google Scholar]
- 90.Escudier M., Cautela J., Malissen N., et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 91.Delombaerde D., Vervloet D., Franssen C., et al. Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Power J.R., Alexandre J., Choudhary A., et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. 2021;144:1521–1523. doi: 10.1161/CIRCULATIONAHA.121.055816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zlotoff D.A., Hassan M.Z.O., Zafar A., et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barbato E., Mehilli J., Sibbing D., et al. Questions and answers on antithrombotic therapy and revascularization strategies in non-ST-elevation acute coronary syndrome (NSTE-ACS): a companion document of the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1368–1378. doi: 10.1093/eurheartj/ehaa601. [DOI] [PubMed] [Google Scholar]
- 95.Awadalla M., Mahmood S.S., Groarke J.D., et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75:467–478. doi: 10.1016/j.jacc.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thavendiranathan P., Zhang L., Zafar A., et al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol. 2021;77:1503–1516. doi: 10.1016/j.jacc.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Friedrich M.G., Sechtem U., Schulz-Menger J., et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caforio A.L., Pankuweit S., Arbustini E., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 99.Altan M., Toki M.I., Gettinger S.N., et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14:1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frey N., Meder B., Katus H.A. Left ventricular biopsy in the diagnosis of myocardial diseases. Circulation. 2018;137:993–995. doi: 10.1161/CIRCULATIONAHA.117.030834. [DOI] [PubMed] [Google Scholar]
- 101.Brahmer Jrl C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., Ernstoff M.S., Gardner J.M., Ginex P., et al. National comprehensive cancer network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang L., Zlotoff D.A., Awadalla M., et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141:2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brahmer J.R., Abu-Sbeih H., Ascierto P.A., et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 105.Brugada J., Katritsis D.G., Arbelo E., et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:655–720. doi: 10.1093/eurheartj/ehz467. [DOI] [PubMed] [Google Scholar]
- 106.Zeppenfeld K., Tfelt-Hansen J., de Riva M., et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 107.Gong F.F., Cascino G.J., Murtagh G., et al. Circulating biomarkers for cardiotoxicity risk prediction. Curr Treat Options Oncol. 2021;22:46. doi: 10.1007/s11864-021-00845-0. [DOI] [PubMed] [Google Scholar]
- 108.Gong J., Drobni Z.D., Zafar A., et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Troughton R.W., Asher C.R., Klein A.L. Pericarditis. Lancet. 2004;363:717–727. doi: 10.1016/S0140-6736(04)15648-1. [DOI] [PubMed] [Google Scholar]
- 110.Adler Y., Charron P., Imazio M., et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European society of cardiology (ESC)endorsed by: the European association for cardio-thoracic surgery (EACTS) Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S.R., Kim E.K., Cho J., et al. Effect of anti-inflammatory drugs on clinical outcomes in patients with malignant pericardial effusion. J Am Coll Cardiol. 2020;76:1551–1561. doi: 10.1016/j.jacc.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 112.Thibault C., Vano Y., Soulat G., et al. Immune checkpoint inhibitors myocarditis: not all cases are clinically patent. Eur Heart J. 2018;39:3553. doi: 10.1093/eurheartj/ehy485. [DOI] [PubMed] [Google Scholar]
- 113.Kastaun S., Gerriets T., Tschernatsch M., et al. Psychosocial and psychoneuroendocrinal aspects of Takotsubo syndrome. Nat Rev Cardiol. 2016;13:688–694. doi: 10.1038/nrcardio.2016.108. [DOI] [PubMed] [Google Scholar]
- 114.Lyon A.R., Bossone E., Schneider B., et al. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 115.Kato K., Lyon A.R., Ghadri J.R., et al. Takotsubo syndrome: aetiology, presentation and treatment. Heart. 2017;103:1461–1469. doi: 10.1136/heartjnl-2016-309783. [DOI] [PubMed] [Google Scholar]
- 116.Citro R., Rigo F., Ciampi Q., et al. Echocardiographic assessment of regional left ventricular wall motion abnormalities in patients with tako-tsubo cardiomyopathy: comparison with anterior myocardial infarction. Eur J Echocardiogr. 2011;12:542–549. doi: 10.1093/ejechocard/jer059. [DOI] [PubMed] [Google Scholar]
- 117.Ederhy S., Cautela J., Ancedy Y., et al. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC: Cardiovascular Imaging. 2018;11:1187–1190. doi: 10.1016/j.jcmg.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 118.Ghadri J.R., Wittstein I.S., Prasad A., et al. International expert consensus document on Takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thompson J.A., Schneider B.J., Brahmer J., et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. 2020;18:230–241. doi: 10.6004/jnccn.2020.0012. [DOI] [PubMed] [Google Scholar]
- 120.Alomar M., Fradley M.G. Electrophysiology translational considerations in cardio-oncology: QT and beyond. J Cardiovasc Transl Res. 2020;13:390–401. doi: 10.1007/s12265-019-09924-y. [DOI] [PubMed] [Google Scholar]
- 121.Tisdale J.E., Chung M.K., Campbell K.B., et al. Drug-induced arrhythmias: a scientific statement from the American heart association. Circulation. 2020;142(15):e214–e233. doi: 10.1161/CIR.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 122.Elhaj F.A., Salim N., Harris A.R., et al. Arrhythmia recognition and classification using combined linear and nonlinear features of ECG signals. Comput Methods Programs Biomed. 2016;127:52–63. doi: 10.1016/j.cmpb.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 123.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 124.Glikson M., Nielsen J.C., Kronborg M.B., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 125.Boland P., Heath J., Sandigursky S. Immune checkpoint inhibitors and vasculitis. Curr Opin Rheumatol. 2020;32:53–56. doi: 10.1097/BOR.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 126.Daxini A., Cronin K., Sreih A.G. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37:2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 127.Hakroush S., Tampe B. Association between loss of immune checkpoint programmed cell death protein 1 and active ANCA-associated renal vasculitis. Int J Mol Sci. 2023:24. doi: 10.3390/ijms24032975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oishi H., Morimoto R., Shimoyama Y., et al. Myocardial vasculitis associated with the immune checkpoint inhibitor pembrolizumab. JACC Case Rep. 2020;2:1937–1941. doi: 10.1016/j.jaccas.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watanabe R., Zhang H., Berry G., et al. Immune checkpoint dysfunction in large and medium vessel vasculitis. Am J Physiol Heart Circ Physiol. 2017;312:H1052–H1059. doi: 10.1152/ajpheart.00024.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lazarewicz K., Watson P. Giant cell arteritis. BMJ. 2019;365:l1964. doi: 10.1136/bmj.l1964. [DOI] [PubMed] [Google Scholar]
- 131.Hoffman G.S. Giant cell arteritis. Ann Intern Med. 2016;165:ITC65–ITC80. doi: 10.7326/AITC201611010. [DOI] [PubMed] [Google Scholar]
- 132.Saadoun D., Vautier M., Cacoub P. Medium- and large-vessel vasculitis. Circulation. 2021;143:267–282. doi: 10.1161/CIRCULATIONAHA.120.046657. [DOI] [PubMed] [Google Scholar]
- 133.Crout T.M., Lennep D.S., Kishore S., et al. Systemic vasculitis associated with immune check point inhibition: analysis and review. Curr Rheumatol Rep. 2019;21:28. doi: 10.1007/s11926-019-0828-7. [DOI] [PubMed] [Google Scholar]
- 134.Cautela J., Rouby F., Salem J.E., et al. Acute coronary syndrome with immune checkpoint inhibitors: a proof-of-concept case and pharmacovigilance analysis of a life-threatening adverse event. Can J Cardiol. 2020;36:476–481. doi: 10.1016/j.cjca.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 135.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu Y.B., Zhang Q., Li H.J., et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6(Suppl 1):S8–S20. doi: 10.21037/tlcr.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kusters P.J.H., Lutgens E., Seijkens T.T.P. Exploring immune checkpoints as potential therapeutic targets in atherosclerosis. Cardiovasc Res. 2018;114:368–377. doi: 10.1093/cvr/cvx248. [DOI] [PubMed] [Google Scholar]
- 138.Nykl R., Fischer O., Vykoupil K., et al. A unique reason for coronary spasm causing temporary ST elevation myocardial infarction (inferior STEMI) - systemic inflammatory response syndrome after use of pembrolizumab. Arch Med Sci Atheroscler Dis. 2017;2:e100–e102. doi: 10.5114/amsad.2017.72531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsumoto T., Sasaki N., Yamashita T., et al. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016;36:1141–1151. doi: 10.1161/ATVBAHA.115.306848. [DOI] [PubMed] [Google Scholar]
- 140.Dietel B., Cicha I., Voskens C.J., et al. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis. 2013;230:92–99. doi: 10.1016/j.atherosclerosis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 141.Bu D.X., Tarrio M., Maganto-Garcia E., et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernandez D.M., Rahman A.H., Fernandez N.F., et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baitsch L., Baumgaertner P., Devevre E., et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Collet J.P., Thiele H., Barbato E., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 145.Allouchery M., Beuvon C., Perault-Pochat M.C., et al. Immune checkpoint inhibitors and venous thromboembolism: an analysis of the WHO pharmacovigilance database. Clin Pharmacol Ther. 2022;112:164–170. doi: 10.1002/cpt.2615. [DOI] [PubMed] [Google Scholar]
- 146.Gutiérrez Sainz L., Martínez-Marin V., Viñal Lozano D., et al. Incidence of vascular thromboembolism events in cancer patients receiving immunotherapy: a single institution experience. Annals of Oncology. 2019;30 doi: 10.1007/s12094-020-02515-3. [DOI] [PubMed] [Google Scholar]
- 147.Sussman T.A., Li H., Hobbs B., et al. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer. 2021:9. doi: 10.1136/jitc-2020-001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Florian Moik W.-S.E.C., Wiedemann Sarah. etc.: incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy.pdf. blood. 2021;137(12):1669–1678. doi: 10.1182/blood.2020007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong J., Drobni Z.D., Alvi R.M., et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer. 2021;158:99–110. doi: 10.1016/j.ejca.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Giustozzi M., Becattini C., Roila F., et al. Vascular events with immune checkpoint inhibitors in melanoma or non-small cell lung cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2021;100 doi: 10.1016/j.ctrv.2021.102280. [DOI] [PubMed] [Google Scholar]
- 151.Hill H., Robinson M., Lu L., et al. Venous thromboembolism incidence and risk factors in non-small cell lung cancer patients receiving first-line systemic therapy. Thromb Res. 2021;208:71–78. doi: 10.1016/j.thromres.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 152.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 153.Franco A.T., Corken A., Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Horio Y., Takamatsu K., Tamanoi D., et al. Trousseau's syndrome triggered by an immune checkpoint blockade in a non-small cell lung cancer patient. Eur J Immunol. 2018;48:1764–1767. doi: 10.1002/eji.201847645. [DOI] [PubMed] [Google Scholar]
- 155.Sato R., Imamura K., Sakata S., et al. Disorder of coagulation-fibrinolysis system: an emerging toxicity of anti-PD-1/PD-L1 monoclonal antibodies. J Clin Med. 2019;8 doi: 10.3390/jcm8060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Young A., Chapman O., Connor C., et al. Thrombosis and cancer. Nat Rev Clin Oncol. 2012;9:437–449. doi: 10.1038/nrclinonc.2012.106. [DOI] [PubMed] [Google Scholar]
- 157.Overvad T.F., Skjoth F., Piazza G., et al. The Khorana score and venous and arterial thrombosis in patients with cancer treated with immune checkpoint inhibitors: a Danish cohort study. J Thromb Haemost. 2022;20:2921–2929. doi: 10.1111/jth.15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khorana A.A., Soff G.A., Kakkar A.K., et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 159.Li A., Garcia D.A., Lyman G.H., et al. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res. 2019;173:158–163. doi: 10.1016/j.thromres.2018.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in this article.