Abstract
The aims of this study were to explore the protective effect of Xiaochaihu decoction in mice with sepsis induced by intraperitoneal injection; to explore its anti-inflammatory effect on the TLR4-MyD88-NF-κB signalling pathway; and to explore the main material basis of the anti-inflammatory effect of Xiaochaihu decoction, with the aim of supplementing and expanding the associated research and providing a scientific foundation for the clinical use of the decoction. The effects of Xiaochaihu decoction on septic mice were analysed by measurements of white blood cells (WBC) and Platelets (PLT); Nitric Oxide (NO) level in serum; IL-6, IL-1β and TNF-α levels in serum; RT-PCR; Haematoxylin-Eosin (HE) immunohistochemistry; western blotting (WB). The results showed the excellent in vivo anti-inflammatory effects of Xiaochaihu decoction in LPS-induced septic mice, through down regulation of the gene and protein expression of TLR4, MYD88, TRAF6, IKK, IKBα and p65 and the subsequent reduction in the release of inflammatory mediators IL-6, IL-1β, TNF-α and NO. Moreover, significant anti-septic effect was observed from high and medium doses of Xiaochaihu decoction, but not from the low dose.
Keywords: Sepsis, Xiaochaihu decoction, TLR4, MyD88, NF-κB, LPS, Mice
1. Introduction
Sepsis, which is relatively common in the clinic, is mainly a systemic inflammatory reaction caused by pathogenic microbial infection [1]. It is an acute, life-threatening condition that can lead to organ dysfunction or circulatory dysfunction in severe cases [2]. The main symptoms of sepsis are chills and high fever. Patients can also have corresponding clinical symptoms at the site of infection [3]. However, in the face of acute and critical illness, such as sepsis, that endangers human life and health, even with today's highly developed medicine, the effects of treatment are poor. Therefore, there is an urgent need to examine the use of Chinese medicine as a means to assist in the treatment of sepsis.
Xiaochaihu decoction was first proposed by the medical sage Zhang Zhongjing in his Treatise on Febrile and Other Diseases. It is highly praised by famous doctors from all dynasties, and has gained the reputation of ‘typhoid fever especially wonderful little bupleurum’ [4]. The most consistent clinical indications of Xiaochaihu decoction is the concurrent cold and heat of Shaoyang disease, which is especially consistent with the main clinical symptoms of sepsis (chills and high fever), which is also one of the reasons why Xiaochaihu decoction was used to treat sepsis in this study. The indications of Xiaochaihu decoction are very wide. However, in most studies, Xiaochaihu decoction has be enlimited to the treatment of liver and bile diseases; there are few reports on the treatment of sepsis [5].
It has been reported that sepsis was related to the activation of TLR4 (6). The activation of TLR4 can initiate the downstream proinflammatory pathway of the MyD88/NF-κB pathway and then release a large number of inflammatory mediators, leading to sepsis [7]. MyD88 interacts with the IL-1R-associated kinase (IRAK) complex, which recruits tumor necrosis factor receptor-associated Factor 6 (TRAF6). TRAF6 can promote transcriptional activation of nuclear factor-KB (NF-kB) genes and pro-inflammatory factors tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) and other inflammatory factors such asinterleukin-1β (IL-1β) [8]. Phosphorylation of IkBa is a critical event after TRAF6 activation [9]. CHUK is an important component of the non-classical NF-kB signaling pathway [10]. Therefore,our aim of detection above indexes was to uncover the mechanism how do Xiaochaihu decoction work.
In this study, we observed the protective effect of Xiaochaihu decoction on mice with lipopolysaccharide (LPS)-induced sepsis, a common model of inflammation, and showed that the mechanism of the anti-sepsis effect. This will allow us to improve knowledge of the anti-sepsis effect of Xiaochaihu decoction and provide ascientific basis for its clinical application (Fig. 1).
Fig. 1.
Schematic of anti-sepsis effectiveness of Xiaochaihu decoction through the TLR4/MyD88/NF-κB signalling pathway.
2. Materials and methods section
2.1. Preparation of Xiaochaihu decoction
The Xiaochaihu decoction was prepared as per the original prescription in the Treatise on Febrile Diseases: Bupleurum chinense DC. (Chaihu) Half jin, Scutellaria baicalensis Georgi. (Huangqin) SAN liang, Panax ginseng C.A.Mey. (Renshen) SAN liang, Pinellia ternata (Thunb.) Half-litre, Glycyrrhiza uralensis Fisch (Zhigancao) SAN liang, Zingiber officinale Roscoe (Shengjiang) SAN liang and Ziziphus jujuba Mill. (Dazao) 12. For the high-dose group, the decoction administered was, according to the archaeology, 1 liang equal to approximately 15 g; for the medium-dose group, the decoction administered was, according to the clinical experience, 1 liang equal to approximately 5 g; and for the low-dose group, the decoction administered was, according to the teaching material, 1 liang equal to approximately 3 g. Namely, in the high-dose group (XCHD H), treatment comprised: Bupleurum 120 g, Scutellariae 45 g, Ginseng 45 g, Pinellia 45 g, Glycyrrhiza 45 g, Zingiber 45 g and Jujube 45 g (for a total daily dosage of 390 g per 60 kg adult). In the medium-dose group (XCHD M), treatment comprised: Bupleurum 40 g, Scutellariae 15 g, Ginseng 15 g, Pinellia 15 g, Glycyrrhiza 15 g, Zingiber 15 g and Jujube 15 g (for a total daily dosage of 130 g per 60 kg adult). In the low-dose group (XCHD L), treatment comprised: Bupleurum 24 g, Scutellariae 9 g, Ginseng 9 g, Pinellia 9 g, Glycyrrhiza 9 g, Zingiber 9 g and Jujube 9 g (for a total 60 kg adult daily dosage 78 g), the ingredients were presented in Table 1. Eight volumes of the medicinal materials were soaked in water for 30 min, heated and boiled, simmered for 60 min, and the residue was then filtered while hot. This process was repeated 3 times; The filtrate was combined 3 times and concentrated to the required concentration under the condition of 60 °C to obtain the brown viscous extract. High, medium and low doses of Xiaochaihu decoction were obtained and put in the refrigerator until use [[11], [12], [13], [14], [15], [16], [17], [18], [19]].
Table 1.
Treatment comprised of different dose groups.
| Dose of medication | XCHD H | XCHD M | XCHD L |
|---|---|---|---|
| Bupleurum Scutellariae |
120 g 45 g |
40 g 15 g |
24 g 9 g |
| Ginseng | 45 g | 15 g | 9 g |
| Pinellia | 45 g | 15 g | 9 g |
| Glycyrrhiza | 45 g | 15 g | 9 g |
| Zingiber | 45 g | 15 g | 9 g |
| Jujube | 45 g | 15 g | 9 g |
| Total | 390 g | 130 g | 78 g |
2.2. Design of animal experiment
Seventy-two healthy male KM mice (as the female mice had the effect of menstruation) were randomly divided according to body weight into each group. 12 mice were randomly allocated to the Normal group, 12 mice to the LPS group, 12 mice to the Dexamethasone (DXM, 5 mg/kg) group [20], 12 mice to the XCHD H (60.7 g/kg) group, 12 mice to the XCHD M (20.2 g/kg) group and 12 mice to the XCHD L (12.1 g/kg) group. The following formula was used: daily dose/mice = dose/kg (human body weight) × conversion factor 9.1 × body weight of each mice. The mice in each group were administered with 9.1 times the daily clinical crude drug dosage of Xiaochaihu decoction for a 60-kg adult, and received continuous intragastric administration for 7 days, twice a day, while the Normal group was given equal volume pure water. On day 6, 30min after the last dosing, sepsis modelling was performed. Earlier models of sepsis often involved direct administration of toxins such as lipopolysaccharide (LPS) into the blood, peritoneum, or lung. This induces a strong immediate inflammatory response that mimics activation of the innate immune system in human sepsis. The advantage of using this approach is its technical ease and reproducible response following injection of a quantifiable dose of toxin [21]. Therefore, all mice except those in the Normal group were injected intraperitoneally with LPS 0.1 mL/10 g at an injection dose of 15 mg/kg [22]; the Normal group was administered the same dose of normal saline [[23], [24], [25], [26], [27]].
The glucocorticoids often used for sepsis [28], so DXM was used in our experiment as a comparison group to measure the effects of experimental medicines.
These experiments has been approved by Laboratory Animal Ethics Committee, Shenzhen University Health Science Center. The ethical approval referenced number was 4403050195161. And we stated confirmation that these experiments were conducted according to established animal welfare guidelines.
2.3. Effects of Xiaochaihu decoction on organ index, whole blood, WBC, and PLT in septic mice
At 12 h after intraperitoneal injection of LPS, blood was collected from the orbit. All mice were sacrificed after collecting blood by neck mutilation and their organs were dissected immediately. Then, 20 μL of whole blood was taken to measure the white blood cell (WBC) count and platelet (PLT) count in the whole blood. The removed organs were weighed on a balance to calculate organ index, which was equal to organ weight divided by the mouse body weight [[29], [30], [31]].
2.4. Effect of Xiaochaihu decoction on serum NO in septic mice
The content of NO in serum was determined by Griess's method, and the content of NO in whole blood (centrifugation at 3500 rpm for 15min) and lung tissue was determined 12 h after modelling [[32], [33], [34]].
2.5. TNF-α, IL-6 and IL-1β in serum were determined by ELISA
Whole blood of mice was collected from the orbit at 12 h after intraperitoneal injection of LPS and centrifuged at 3000 rpm for 15 min to obtain serum. The serum levels of TNF-α, IL-6 and IL-1β were determined in accordance with the instructions of the ELISA kit. Finally, OD values were measured at 450 nm, and serum levels of TNF-α, IL-6 and IL-1β were calculated according to the established standard curve [[35], [36], [37], [38], [39]].
2.6. Preparation of HE pathological sections of lung tissue
Immediately after blood collection, each animal's lung was removed and immersed in a 10% neutral formalin buffer. The paraffin-embedded lung tissues were then cut into 5 μm thick slices, stained with haematoxylin–eosin (HE), and examined usinga digital trimograph microscope (BA400Digital, MacOdis Industrial Group Limited). To quantify the extent of histopathological changes, the number of neutrophils in the alveolar septum was calculated using Image-Pro Plus (version 6.0, Media Cybernetics) software. The infiltration of inflammatory cells was presentedas a change in the mean number of neutrophils in each of the six photographs taken at 200 × and 400 × magnifications to provide a more detailed view of the lung lesions [[40], [41], [42], [43], [44]]. Two observers utilize ImageJ software, for staining observations to ensure more objective data analysis. The dried sections were observed under a microscope and photographed [45].
2.7. RT-PCR
Real-time PCR was performed in accordance with the manufacturer's instructions for the total RNA miniprep kit (Axygen, USA), and total RNA was extracted from lung tissue using the Fast Quant RT kit (Tiangen, Beijing, China). The following conditions were used: 95 °C for 15 min, followed by 40 denaturation cycles of 95 °C for 10 s followed by extension at 64.1 °C for 30 s. Then, a 96-well reaction plate (Bio-Rad, Hercules, CA) with a reaction volume of 20 μL was applied directly for the PCR amplification reaction and the mRNA levels of TLR4, MYD88, TRAF6, IKK, IKKB-α, and p65 were detected.
2.8. The protein expression of TLR4, MYD88, TRAF6, IKK, IKKB-α and p65 in cell lysates were detected by western blotting
The total protein was extracted from the lungs with lysate buffer and the concentration was determined using a BCA protein assay kit. All samples were then adjusted to the same concentration. Proteins in lung tissue were separated on a gel containing 10% polyacrylamide by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. After transfer, the PVDF membranes were washed in TBST (Tris buffered saline containing 0.1% Tween 20) for 5 min, and then the antibodies were added in skim milk. An appropriate amount of sealing solution was added and left to oscillate slowly at 37 °C for 2 h. The membranes were then incubated overnight at 4 °C with primary antibody to TLR4, MYD88, TRAF6, IKK, IKKB-α, and p65 proteins were diluted in accordance with the manufacturer's instructions. After three washes with TBST, the HRP-labelled secondary antibody (1:1000) was incubated at 37 °C for 1.5 h, and then ECL western blotting reagent was used to detect the secondary antibody. The optical density was measured by the laborator imaging analysis system and the ratio of the optical density of the target protein to the internal reference protein band was used as the result.
The expression of TLR4, MYD88, TRAF6, IKK, IKKα, and p65 in lung tissues was determined by immunohistochemistry [46]
Lung tissue from each group was immobilised, dehydrated, and the clear sample was immersed in wax and cut into thin slices (approximately 4–5 μm in thickness). Next, the sections were routinely dewaxed and soaked in 3% hydrogen peroxide at room temperature for 10 min to inactivate endogenous peroxidase, and then washed three times in distilled water. The water bath was then heated for antigen recovery and sealed with goat serum at room temperature for 20 min. Primary antibodies to TLR4, MYD88, TRAF6, IKK, IKKB-α and p65 were incubated at 4 °C (diluted appropriately according to the manufacturer's instructions; Abcam) overnight. After three washes in PBS, biotinised goat anti-rabbit IgG was added dropwise and then incubated at 37 °C for 30 min. After three washes with PBS,the sections were incubated with horseradish peroxidase (HRP) at 37 °C for 30 min. Then, after four washes with PBS, diaminobenzidine (DAB) (OriGene, Beijing) reaction was performed. After washing with distilled water, haematoxylin was slightly redyed, dehydrated and sealed with neutral glue.
3. Results
3.1. Effects of Xiaochaihu decoction on whole blood WBC and PLT in mice
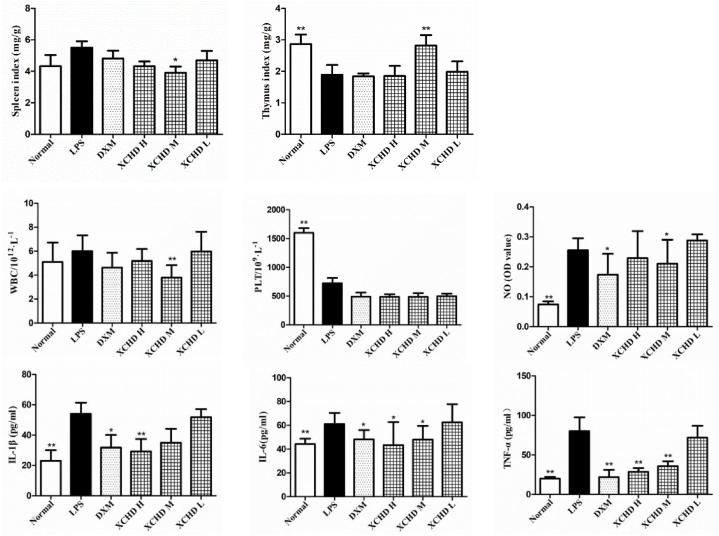
The results of WBC and PLT in whole blood are shown in Fig. 2. Compared with the LPS group, the spleen index and the thymus index were significantly decreased, and the whole blood white blood cell (WBC) count was significantly decreased in the medium-dose group of Xiaochaihu decoction, suggesting that the decoction could affect the immune defence function in the septicmodel mice and thereby relieve the inflammatory response.
Fig. 2.
Effects of Xiaochaihu decoction on the evolution of the spleen and thymus index and WBC, PLT, NO, IL-1β, IL-6, TNF-α in septic mice as determined by ELISA. Normal, blank group; LPS, model group; XCHD H, Xiaochaihu decoction high-dose group; XCHD M, Xiaochaihu decoction medium-dose group; and XCHD L, Xiaochaihu decoction low-dose group. Compared with the LPS group, p < 00.05, p < 00.01.
3.2. Effect of Xiaochaihu decoction on serum NO in mice
Compared with the Normal group, the serum NO content of mice in the LPS group was significantly increased (P < 0.01 or P < 0.05). Compared with LPS group, the Xiaochaihu decoction medium-dose group had significantly lower serum NO levels (P < 0.01) (Fig. 2).
3.3. Effects of Xiaochaihu decoction on serum levels of IL-1β, IL-6 and TNF-α in mice determined by ELISA
See Fig. 2 for the results. Compared with the Normal group, the serum contents of IL-1β, IL-6 and TNF-α were significantly higher in the LPS group (P < 0.01). Compared with the LPS group, the serum levels of IL-1β, IL-6 and TNF-α were significantly lower in the Xiaochaihu decoction high-dose group (P < 0.01 or P < 0.05).
3.4. RT-PCR
The RT-PCR results for the analysis of lung tissues for each group of mice are presented in Fig. 3. According to these results, the mRNA expression of IL-1β, IL-6, TNF-α, TLR4, MYD88, TRAF6, CHUK, IKB-α and NF-κB (p65) in lung tissue from LPS group was significantly higher than in the Normal group (P < 0.01 or P < 0.05). Compared with the LPS group, the mRNA expression of IL-1β, IL-6, TNF-α, TLR4, MYD88, TRAF6, CHUK, IKB-α and NF-κB (p65) in the lung tissue in the Xiaochaihu decoction high-dose and medium-dose groups was significantly reduced. However, the mRNA expression levels of the above genes in lung tissue was significantly decreased in the Xiaochaihu decoction low-dose group (P < 0.05), with a slight or no effect on individual indicators.
Fig. 3.
Effect of Xiaochaihu decoction on mRNA expression of IL-1β, IL-6, TNF-α, TLR4, MYD88, TRAF6, CHUK, IKB-α and p65 in lung tissue from septicmice. Normal, blank group; LPS, model group; XCHD H, Xiaochaihu decoction high-dose group; XCHD M, Xiaochaihu decoction medium-dose group; and XCHD L, Xiaochaihu decoction low-dose group. Compared with the LPS group, p < 00.05, p < 00.01.
3.5. HE staining of lung tissue sections
The HE staining of the sections revealed that, in the Normal group, the lung capsule was complete, the bronchial tubes and blood vessels at all levels were complete, the epithelium of the bronchial mucosa was arranged neatly, no cells were shed in the bronchial lumen, the vascular structure was complete, the epithelium cells were arranged regularly, the blood cell aggregation and plasma deposition were observed in the vascular lumen, the alveolar structure was normal and the alveolar epithelium cells were arranged regularly. Inflammatory cell infiltration into the lung tissue of mice with sepsis was alleviated in the Xiaochaihu decoction high-dose and medium-dose groups as shown in Fig. 4.
Fig. 4.
Effect of Xiaochaihu decoction HE staining of the lungs of septic mice in various conditions (400× magnification). Normal, blank group; LPS, model group; XCHD H, Xiaochaihu decoction high-dose group; XCHD M, Xiaochaihu decoction medium-dose group; and XCHD L, Xiaochaihu decoction low-dose group.
3.6. The expression of TLR4, MYD88, TRAF6, IKK, IKKα and p65 in lung tissues was determined by immunohistochemistry
As shown in Fig. 5, Immunohistochemical analysis showed that, compared with the Normal group, the positive staining of TLR4, MYD88, TRAF6, IKK, IKKα and p65 in the lung tissue of mice in the LPS group was significantly increased (P < 0.01). Compared with the LPS group, the Xiaochaihu decoction high-dose and medium-dose groups had lower positive staining of the above indicator proteins (P < 0.05).
Fig. 5.
Effects of Xiaochaihu decoction on TLR4, MYD88, TRAF6, IKK, IKKB-α and p65 protein levels in the lung tissues of septic mice determined by immunohistochemical staining (Magnification 400×). Normal, blank group; LPS, model group; XCHD H, Xiaochaihu decoction high-dose group; XCHD M, Xiaochaihu decoction medium-dose group; and XCHD L, Xiaochaihu decoction low-dose group. Data are expressed as the mean ± SEM of 10 mice per group, compared with the LPS group, p < 00.05, p < 00.01.
3.7. The protein expression of TLR4, MYD88, TRAF6, IKK, IKKB-α and p65 in cell lysate was detected by western blotting
As shown in Fig. 6, compared with the Normal group, the relative protein expression of TLR4, MYD88, TRAF6, IKK, IKKα and p65 in the lung tissue was significantly increased in mice with sepsis (the model group) (P < 0.05). Compared with the LPS group, the relative protein expression of TLR4, MYD88, TRAF6, IKK, IKKα and p65 in the Xiaochaihu decoction high-dose and medium-dose groups of was significantly decreased (P < 0.05). When the MYD88 inhibitor ST2825 was added to the model group (i.e., the LPS + ST2825 group), TLR4, TRAF6, IKK and IKKα in lung tissue of septicmice was obviously inhibited, whereas MYD88 and p65 were significantly inhibited.
Fig. 6.
Effects of Xiaochaihu decoction on the expression of TLR4, MYD88, TRAF6, IKK, IKKB-α and p65 in lung tissue of mice (refer to Fig. S1-Fig. S7). Normal, blank group; LPS, model group; XCHD H, Xiaochaihu decoction high-dose group; XCHD M, Xiaochaihu decoction medium-dose group; and XCHD L, Xiaochaihu decoction low-dose group. Comparison with LPS group, p < 00.05, p < 00.01.
4. Discussion
In this study, the clinical application of Xiaochaihu decoction to the treatment of sepsis was of great promise. In clinical practice, we found that Xiaochaihu decoction could reduce sepsis patients' hyperthermia. Eventually, we reviewed a lot of relevant papers and deduced that Xiaochaihu decoction played its anti-inflammatory and antipyretic effects through TLR4/MyD88/NF-κB signaling pathway. After the occurrence of sepsis, lung was an vulnerable organ that was the "first responders" to sepsis damage. Therefore, we deduced that Xiaochaihu decoction reduce inflammatory cell infiltration in lung tissue of septic mice [47]. The efficacy and mechanism of Xiaochaihu decoction against sepsis in mice were examined. The results showed that [1]: both high-dose and medium-dose Xiaochaihu decoction could significantly reduce hyperthermia in septic mice and ameliorates hivering symptoms [2]. The results of the organ index, whole blood WBC, and PLT count showed that the spleen index and thymus index were significantly decreased in the Xiaochaihu decoction medium-dose group, suggesting that it may have a certain effect on the body's defences and immune function, with a significant reduction in the count of WBC and alleviation of the inflammatory response in septic mice (P < 0.01) [3]. Compared with Normal group, the serum NO content in the LPS group was significantly increased (P < 0.01). Compared with the LPS group, the Xiaochaihu decoction medium-dose group had significantly lower serum NO levels (P < 0.05) [4]. ELISA showed that, compared with LPS group, the serum levels of IL-1β, TNF-α and IL-6 in the Xiaochaihu decoction high-dose and medium-dose groups were significantly decreased (P < 0.01 or P < 0.05) [5]. The histopathological results showed that Xiaochaihu decoction high-dose and medium-dose groups could both reduce inflammatory cell infiltration in lung tissue of septic mice [6]. Compared with the Normal group, the mRNA and protein expression of TLR4, MYD88, TRAF6, IKK, IKKα and p65 in the lung tissue of mice in the LPS group were significantly increased (P < 0.01 or P < 0.05). Compared with the LPS group, the Xiaochaihu decoction high-dose and medium-dose groups had significantly lower mRNA expression levels and protein expression levels in the lung tissue of septic mice (P < 0.01 or P < 0.05) [7]; Immunohistochemical staining revealed that compared with Normal group, the positive staining rates of TLR4, MYD88, TRAF6, IKK, IKKα and p65 proteins in the lung tissue of mice in the LPS group were significantly increased (P < 0.01). Compared with LPS group, the Xiaochaihu decoction high-dose and medium-dose groups had lower positive staining rates of the above indicator proteins (P < 0.05). All the evidence suggested that the mechanism and efficacy of the anti-sepsis effects of Xiaochaihu decoction high-dose and medium-dose was through the TLR4/MyD88/NF-κB signalling pathway. However, due to the complexity of Traditional Chinese Medicine (TCM) ingredients, we don't yet know exactly which ingredients are at work, that's where we're headed in the future.
5. Conclusion
In this study, Xiaochaihu decoction exerted a certain protective effect in septic mice, and this protective effect was notably better with the medium and high doses than with the low dose. Based on the above in vitro experiments, the mechanism and efficacy of the anti-sepsis effects of medicinal serum of medium-dose Xiaochaihu decoction were confirmed. In conclusion, Xiaochaihu decoction works through the TLR4/MyD88/NF-κB signalling pathway.
Data availability statement
The present study was guided by the basic principles of research integrity and research conduct with human participants, as stipulated in many documents. Participants were informed that their anonymity and the confidentiality of data pertaining to them will be retained. Hence, we were abided by this agreement with the participants not to share and deposit data for public use.
CRediT authorship contribution statement
Qingxin Yang: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Yulong Wang: Writing – review & editing, Writing – original draft, Validation, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Gefei Cao: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Formal analysis, Data curation, Conceptualization. Xiaoqing Li: Software, Methodology, Formal analysis, Data curation, Conceptualization. Tinghui Zhao: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation Fund of China (81502690).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26712.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yan J., Liao K., Wang T., Mai K., Xu W., Ai Q. Dietary lipid levels influence lipid deposition in the liver of large yellow croaker (Larimichthys crocea) by regulating lipo protein receptors, fatty acid uptake and triacylglycerol synthesis and catabolismat the transcriptional level. PLoS One. 2020;77(1):301–305. doi: 10.1371/journal.pone.0129937. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Z., Clouet P., Zheng W., Degrace P., Tian L., Liu Y. Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp(Ctenopharyngodon idella) fed high-fat diets. Br. J. Nutr. 2006;95:905–915. doi: 10.1079/bjn20061733. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 3.Gaylord T., Gatlin D. Dietary lipid level but not L-carnitine affects growth performance of hybrid striped bass (Morone chrysops female × M. saxatilis male) Aquaculture. 2000;190:237–246. [Google Scholar] [Google Scholar]
- 4.Spisni E., Tugnoli M., Ponticelli A., Mordenti T., Tomasi V. Hepatic steatosis inartificially fed marine teleosts. J. Fish. Dis. 1998;21:177–184. doi: 10.1046/j.1365-2761.1998.00089.x. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 5.Du Z., Liu Y., Tian L., Wang J., Wang Y., Liang G. Effect of dietary lipid level ongrowth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella) Aquacult. Nutr. 2005;11:139–146. [Google Scholar] [Google Scholar]
- 6.Zhao Fang, Wang Guangji, Huang Rui, et al. Astilbin protects from sepsis-induced cardiac injury through the NRF2/HO-1 and TLR4/NF-κB pathway. Phytother Res. 2023 doi: 10.1002/ptr.8093. undefined: undefined. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 7.Song Wenying, Huang Jing, Li Ying, et al. The role of TLR4/MyD88/NF-κB in the protective effect of ulinastatin on the intestinal mucosal barrier in mice with sepsis. BMC Anesthesiol. 2023;23:414. doi: 10.1186/s12871-023-02374-9. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selim Çomaklı, Küçükler Sefa. Değirmençay Şükrü et al. Quinacrine, a PLA2 inhibitor, alleviates LPS-induced acute kidney injury in rats: Involvement of TLR4/NF-κB/TNF α-mediated signaling. Int. Immunopharm. 2024;126 doi: 10.1016/j.intimp.2023.111264. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 9.Liu Meng, Guo Pengli, Zeng Mengnan, et al. Effects and mechanisms of frehmaglutin D and rehmaionoside C improve LPS-induced acute kidney injury through the estrogen receptor-mediated TLR4 pathway in vivo and in vitro. Phytomedicine. 2023;123 doi: 10.1016/j.phymed.2023.155218. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 10.Kim Minju, Chandra Chaudhary Shubhash, Kim Byeongkwon, et al. Acinetobacter baumanniiProtective effects of Rhamnetin in Carbapenem-Resistant -induced sepsis model and the Underlying mechanism. Int. J. Mol. Sci. 2023:24. doi: 10.3390/ijms242115603. undefined. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu K., Xu W., Li J., Li X., Huang G., Liu W. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013;79:661–671. [Google Scholar] [Google Scholar]
- 12.Zheng Q., Han C., Zhong Y., Wen R., Zhong M. Effects of dietary supplementation with green tea waste on growth, digestive enzyme and lipid metabolism of juvenilehybrid tilapia. Oreochromis niloticus × O. aureus. Fish Physiol. Biochem. 2017;43:361–371. doi: 10.1007/s10695-016-0292-5. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 13.Li D., Liu L. Introduction to diagnosis and treatment of fish fatty liver diseas. Prog.Veter. Med. 2016;37:114–117. [Google Scholar] [Google Scholar]
- 14.Xiao P., Ji H., Ye Y., Zhang B., Chen Y., Tian J., Liu P., Chen L., Du Z. Dietarysilymarin supplementation promotes growth performance and improves lipid metabolism and health status in grass carp (Ctenopharyngodon idellus) fed diets withelevated lipid levels. Fish Physiol. Biochem. 2017;43:245–263. doi: 10.1007/s10695-016-0283-6. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 15.Yuan B., Yang R., Ma Y., Zhou S., Zhang X., Liu Y. A systematic review of the activesaikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharmaceut. Biol. 2016;55:620–635. doi: 10.1080/13880209.2016.1262433. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao J.J. Study on the relationship between decoction method of Zhang Zhongjing's classical decoction and modern dosage. fujian journal of traditional chinese medicine. 2010;41(2):48. [Google Scholar] [Google Scholar]
- 17.Zhang W.A. Preliminary study on small dose decoction powder of traditional Chinese medicine. Journal of Chinese Materia Medica. 1992;15(18):38. [Google Scholar] [Google Scholar]
- 18.Zhang X.P. Comparison of decoction rate of high dose, medium dose and low dose of traditional Chinese medicine decoction. traditional medicine. 2007;16(12):54–55. [Google Scholar] [Google Scholar]
- 19.Tao H.H. Conversion and research of prescription volume. J. Shandong Univ. Tradit. Chin. Med. 1997;21(4):307. [Google Scholar] [Google Scholar]
- 20.Xu Baoshi, Yang Rongrong, Qiang Jingchao, et al. Gypenoside XLIX attenuates sepsis-induced splenic injury through inhibiting inflammation and oxidative stress. Int. Immunopharm. 2023;127 doi: 10.1016/j.intimp.2023.111420. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 21.Copeland S., Warren H.S., Lowry S.F., Calvano S.E., Remick D., Inflammation and the Host Response to Injury Investigators Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 2005;12(1):60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Yujiao, Yao Zhihua, Wang Hushan, et al. Severe inflammation in C57/BL6 mice leads to prolonged cognitive impairment by initiating the IL-1β/TRPM2 pathway. Int. Immunopharm. 2024;128 doi: 10.1016/j.intimp.2023.111380. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Wang J., Yuan L., Zhou L., Jia X., Tan X. Interaction of the main components from the traditional Chinese drug pair Chaihu-Shaoyao based on rat intestinal absorption. Molecules. 2011;16:9600–9610. doi: 10.3390/molecules16119600. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J., Cao L., Jia R., Yin G. Hepatoprotective and antioxidant effects of dietaryGlycyrrhiza polysaccharide against TCDD-induced hepatic injury and RT-PCRquantification of AHR2, ARNT2, CYP1A mRNA in Jian Carp (Cyprinus carpio var.Jian) J. Environ. Sci. 2017;51:181–190. doi: 10.1016/j.jes.2016.06.026. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 25.Jia R., Cao L., Xu P., Jeney G., Yin G. In vitro and in vivo hepatoprotective andantioxidant effects of Astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio) Fish Physiol. Biochem. 2012;38:871–881. doi: 10.1007/s10695-011-9575-z. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 26.Zhu Z., Zhao L., Liu X., Chen J., Zhang H., Zhang G., Chai Y. Comparative pharmacokinetics of baicalin and wogonoside by liquid chromatography-mass spectrometry after oral administration of Xiaochaihu Tang and Radix scutellariae extract torats. J. Chromatogr. B. 2010;878:2184–2190. doi: 10.1016/j.jchromb.2010.06.021. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 27.Taira Z., Yabe K., Hamaguchi Y. Effects of Sho-saiko-to extract and its components,Baicalin, baicalein, glycyrrhizin and glycyrrhetic acid, on pharmacokinetic behavior of salicylamide in carbon tetrachloride intoxicated rats. Food Chem. Toxicol. 2004;42:803–807. doi: 10.1016/j.fct.2003.12.017. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 28.Fang Yan, Xiao Chuang, Wang Lueli, et al. Synergistic Enhancement of Isoforskolin and Dexamethasone against sepsis and acute lung injury mouse models. J. Inflamm. Res. 2023;16:5989–6001. doi: 10.2147/JIR.S421232. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumino M., Saito Y., Ikegami F., Hirasaki Y., Namiki T. 2012. Extraction Efficiency ofShosaikoto (Xiaochaihu Tang) and Investigation of the Major Constituents in theResidual Crude Drugs. Evidence-Based Complementary and Alternative Medicine. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tajiri H., Kozaiwa K., Ozaki Y., Miki K., Shimizu K., Okada S. Effect of Sho-saiko-to(Xiao-chai-hu-tang) on HBeAg clearance in children with chronic hepatitis B virusinfection and with sustained liver disease. Am. J. Chin. Med. 1991;19:121–129. doi: 10.1142/S0192415X91000193. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 31.Woo K., Lim J., Suh S., Kwon Y., Shin S., Kim S. Differential inhibitory effects ofbaicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA binding activity. Immunobiology. 2006;211:359–368. doi: 10.1016/j.imbio.2006.02.002. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 32.Chao J., Su W., Liu H. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and surviving associated with oppositerole of p38 mitogen-activated protein kinase and AKT. Mol. Cancer Ther. 2006;7:3039–3048. doi: 10.1158/1535-7163.MCT-07-0281. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 33.Chang W., Shao Z., Yin J., Mehendale S., Wang C., Qin Y. Comparative effects offlavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur. J. Pharmacol. 2007;566:58–66. doi: 10.1016/j.ejphar.2007.03.037. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Othman A., Kawamura G., Senoo S., Ching F. Effects of different salinities ongrowth, feeding performance and plasma cortisol level in hybrid TGGG (Tigergrouper, Epinephelus fuscoguttatus × Giant grouper, Epinephelus lanceolatus) juveniles. Int. Res. J. Biol. Sci. 2015;4:15–20. [Google Scholar] [Google Scholar]
- 35.Triastuti J., Pursetyo K., Monica A., Lutfiyah L., Budi D. Abnormalities of hybridgrouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) in Situbondo. Asean-Fen Int. Fish. Symp. 2018;137 [Google Scholar] [Google Scholar]
- 36.Xu W., Liu W., Liu Z. Trichlorfon-induced apoptosis in hepatocyte primary cultures of Carassius auratus gibelio. Chemosphere. 2009;77:895–901. doi: 10.1016/j.chemosphere.2009.08.043. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 37.Holliday A.C., Moody M.N., Berlingeri-Ramos A. Methotrexate: role of treatment inskin disease. Skin Therapy Lett. 2013;18:4–9. [Google Scholar] [PubMed] [Google Scholar]
- 38.Bangert C.A., Costner M.I. Methotrexate in dermatology. Dermatol. Ther. 2007;20:216–228. doi: 10.1111/j.1529-8019.2007.00135.x. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 39.Bath R.K., Brar N.K., Forouhar F.A., Wu G.Y. A review of methotrexate-associatedhepatotoxicity. J. Dig. Dis. 2014;15:517–524. doi: 10.1111/1751-2980.12184. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 40.Marques P.E., Oliveira A.G., Pereira R.V., David B.A., Gomides L.F., Saraiva A.M., Pires D.A., Novaes J.T., Patricio D.O., Cisalpino D., Menezes-Garcia Z., et al. Hepatic DNA depositiondrives drug-induced liver injury and inflammation in mice. Hepatology. 2015;61:348–360. doi: 10.1002/hep.27216. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 41.Paludan S.R., Bowie A.G. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses toinjury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathinam V.A., Jiang Z., Waggoner S.N., Sharma S., Cole L.E., Waggoner L., Vanaja S.K., Monks B.G., Ganesan S., Latz E., Hornung V., Vogel S.N., Szomolanyi-Tsuda E., Fitzgerald K.A. The AIM2 inflammasome is essential for hostdefense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanneganti T.D., Lamkanfi M., Nunez G. Intracellular NOD-like receptors in hostdefense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 45.Wang J.L., Zou L., Chen S.P. To compare the inflammatory indexes and organ dysfunction indexes of septic rat models established by different modeling methods. Prog. Mod. Biomed. 2022;22(21):5. [Google Scholar] [Google Scholar]
- 46.Li Yang, Sun Zifa, Li Yuanyuan, et al. IL-1β-Stimulated Bone Mesenchymal Stem cell-Derived Exosomes Mitigate sepsis through Modulation of HMGB1/AKT pathway and M2 Macrophage Polarization. Curr. Mol. Med. 2024 doi: 10.2174/0115665240277763231206051401. undefined:undefined. [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 47.Zhou G., Xie D., Fan R., et al. Comparison of Pulmonary and Extrapulmonary models of sepsis-associated acute lung injury. Physiol. Res. 2023;72:741–752. doi: 10.33549/physiolres.935123. [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The present study was guided by the basic principles of research integrity and research conduct with human participants, as stipulated in many documents. Participants were informed that their anonymity and the confidentiality of data pertaining to them will be retained. Hence, we were abided by this agreement with the participants not to share and deposit data for public use.