Abstract
Background
Tetrahydrobiopterin (BH4) deficiency is a rare cause of hyperphenylalaninemia (HPA). The incidence of this condition varies based on region and ethnicity. In the early stages, patients typically do not exhibit any symptoms, and HPA is identified only through newborn screening for diseases. It is important to distinguish BH4 deficiency from phenylketonuria (PKU, MIM # 261600). Timely diagnosis and treatment of BH4 deficiency are crucial for the prognosis of patients.
Case presentation
We present two rare cases of Chinese Tibetan children with BH4D, diagnosed through biochemical tests and genetic sequencing. Case 1 is a male infant, 2 months old, with a newborn screening (NBS) Phe level of 1212 μmol/L (reference range <120 μmol). The biopterin(B) level was 0.19 mmol/molCr (reference range: 0.42–1.92 mmol/molCr), with a B% of 5.67% (reference range: 19.8%–50.3%). Gene sequencing revealed a homozygous missense variant [NM_000317.3 (PTS): c.259C > T (p.Pro87Ser), rs104894276, ClinVar variation ID: 480]. The patient was treated with a Phe-reduced diet and oral sapropterin, madopar and is currently 3 years and 4 months old, showing mild global developmental delay. Case 2 is a 40-day-old female infant with a Phe level of 2442.11 μmol/L and dihydropteridine reductase (DHPR) activity of 0.84 nmol/(min. 5 mm disc) (reference range: 1.02–3.35 nmol/min.5 mm disc. Gene sequencing revealed a compound heterozygous genotype [NM_000320.3(QDPR): c.68G > A (p.Gly23Asp), rs104893863, ClinVar Variation ID: 490] and [NM_000320.3(QDPR) c.419C > A (p. Ala140Asp), ClinVar ID: 2444501]. The patient was treated with a Phe-reduced diet and oral madopar, 5-hydroxytryptophan. At the age of 1 year, she exhibited severe global developmental delay with seizures.
Conclusion
We identified and treated two cases of BH4D in Tibetan populations in China, marking the first confirmed instances. Our report emphasizes the significance of conducting differential diagnosis tests for BH4D.
Keywords: Tetrahydrobiopterin (BH4) -deficient, PTS, QDPR, Tibetan, Case report
1. Introduction
Dysfunction in phenylalanine (Phe) metabolism can result in the accumulation of Phe in the blood and cerebrospinal fluid, leading to hyperphenylalaninemia (HPA). This condition can cause irreversible neurological damage and psychiatric symptoms in severe cases. A common cause of HPA is the functional deficiency of phenylalanine hydroxylase due to variations in the PAH gene, also known as phenylketonuria (PKU, MIM # 261600). Another rare cause is tetrahydrobiopterin (BH4) deficiency, referred to as BH4D (MIM # 261640). BH4 plays a crucial role not only as a cofactor of PAH but also in the metabolism of tyrosine and two tryptophan hydroxylases. This explains why BH4D patients not only experience HPA similar to PKU patients but also exhibit symptoms such as central hypotonia [1]. The incidence of BH4D varies among different populations, ethnicities, and regions. Early differential diagnosis and effective treatment of PKU and BH4D deficiency can help prevent progressive neurological damage in affected patients. In the mainland China, screening for neonatal diseases started in 1981, and BH4 differential screening has been available since 2003. The overall screening rate in newborn screening (NBS) has been increasing annually [2]. According to statistics from the National Center for Clinical Laboratories in 2008, the prevalence of HPA in China was estimated to be approximately 1 in 11,763. Among HPA cases, the proportion of BH4D was found to be approximately 8.55%. Furthermore, it was observed that the incidence of HPA varies between the southern and northern regions of China [[2], [3], [4]]. Based on the data collected by the Chinese Newborn Screening Information System (CNBSIS) between 2013 and 2019, the prevalence of BH4D in mainland China was found to be 3.8 per 1,000,000 live births. Interestingly, during the screening of 20,658 newborns in Tibet, no cases of BH4D were identified [5]. Tibetan people, who primarily inhabit the Plateau on the Qinghai-Tibet (with a mean elevation of over 4,000 m), constitute 8.17% of the permanent residents in mainland China. The Han nationality comprises 88% of the population, making Tibetan the eighth largest ethnic group in the country. In November 2015, the Tibet Region initiated screening for neonatal diseases. However, due to factors such as language, culture, customs, education, and economies specific to Tibet, the overall screening rate by the National Bureau of Statistics (NBS) is lower than that in other coastal regions in China. Notably, no cases of HPA or BH4D have been reported among the Tibetan population in this region. In our recent study, we presented two cases of Tibetan BH4 deficiency and provided a detailed introduction to the patients' condition.
1.1. Case series presentation
Case 1
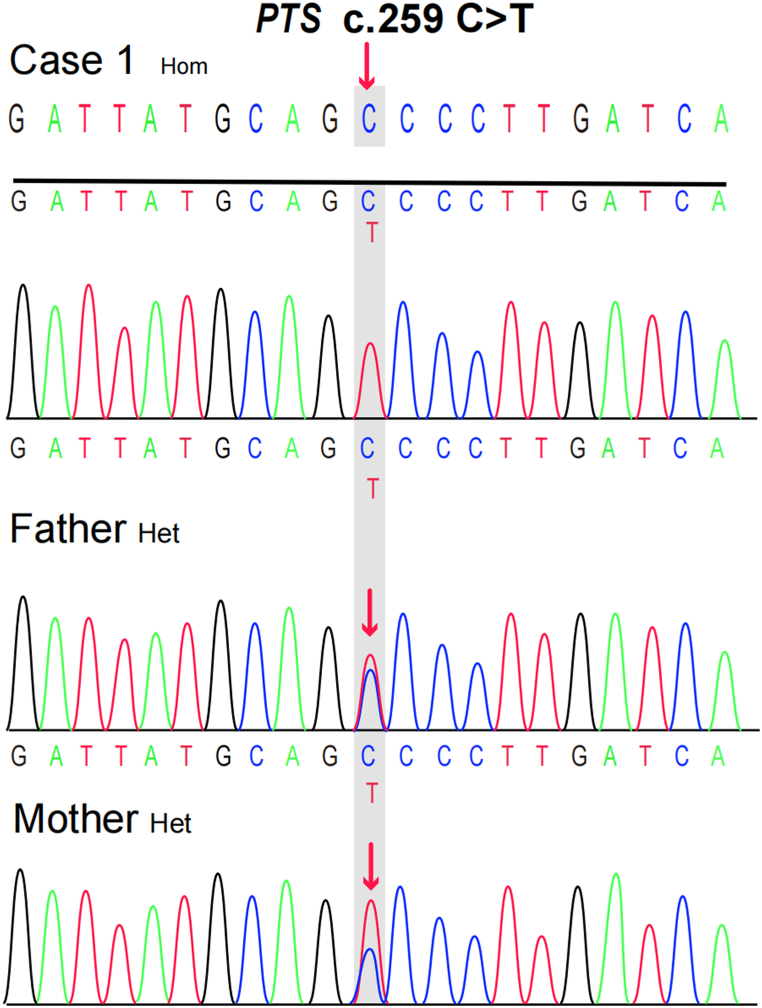
A 2-month-old Tibetan male was delivered at term with a birth weight of 3.0 kg, a length of 50 cm, and a head circumference of 34 cm. He was born into a healthy Tibetan family, and the parents are non-consanguineous. Three days after birth, the level of NBS Phe was measured at 1212 μmol/L (reference range <120 μmol). The child was fed a phenylalanine-free formula for 1 week, and the retested Phe level was 504 μmol/L. A preliminary diagnosis of HPA was made, as the child did not present with any other unusual symptoms. However, the parents refused the HPA-related biochemical differential test. Despite this, the child was taken home and continued to breastfeed until he was 2 months old. At that time, NBS staff contacted the parents to remind them of the follow-up appointment, and the Phe level was measured at 723 μmol/L. To clarify the etiology of the disease and formulate a plan for subsequent treatment, we obtained the family's informed consent and performed differential diagnosis of biochemical tests and genetic tests. Biochemical tests revealed a neopterin (N) concentration of 3.08 mmol/molCr (reference range: 1.21–2.92 mmol/molCr), a biopterin (B) concentration of 0.19 mmol/molCr (reference range: 0.42–1.92 mmol/molCr), a B% of 5.67% (reference range: 19.8%–50.3%), and a DHPR activity of 2.84 nmol/(min.5 mm disc) (reference range: 1.02–3.35 nmol/min.5 mm disc), which is 94.61% of the control activity. These results suggest the possibility of 6-pyruvoyltetrahydropterin synthase deficiency (PTPSD, MIM # 261640). The patient underwent treatment with oral madopar, sapropterin dihydrochloride. Target sequencing was performed on all exons and flanking regions (20 bp average) of HPA-associated genes (PAH, PTS, GCH1, QDPR, PCBD1, and GFRP/DNAJC12) in the Human Gene Mutation Database (HGMD). A homozygous missense variant [NM_000317.3(PTS): c.259C > T (p.Pro87Ser), rs104894276, ClinVar variation ID: 480] was identified, which was inherited from both the father and the mother (Fig. 1).
According to the American College of Medical Genetics and Genomics (ACMG) guidelines, variant c.259C > T is considered pathogenic (PS1+PM2+PP3+PP4) [6]. The results of gene sequencing were consistent with the biochemical tests, confirming the diagnosis of PTPSD. The child continued to require medication, and their Phe level was monitored every 3 months, ranging from 140.01 to 206.77 μmol/L. At the age of 2, the patient experienced a febrile seizure, specifically a generalized tonic-clonic seizure (GTCS). The patient successfully acquired walking ability at the age of 1 and began speaking words at the age of 2. Currently, the patient is three years and four months old and exhibits mild global developmental delay.
Case 2
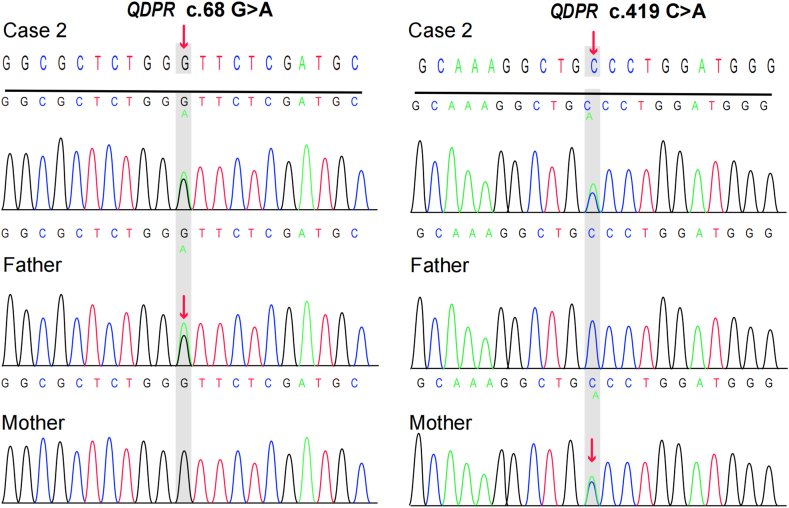
A 40-day-old Tibetan female who was breastfed after birth had non-consanguineous parents. The initial screening showed a Phe level of 358 μmol/L. We contacted the parents by phone to inform them about the abnormal test results. However, for various reasons, the child was not retested until one month after birth, and the Phe level was found to be 2442.11 μmol/L. As a result, the child was given a phenylalanine-free formula. Biochemical tests and genetic sequencing were immediately conducted to determine the cause of the abnormal results. The biochemical results showed a neotrexin (N) level of 2.12 mmol/mol Cr, urinary pterin (B) level of 0.93 mmol/mol Cr, B% of 30.61%, and DHPR activity of 0.84 nmol/(min. 5 mm disc), which was 21.91% of the control activity (The dried blood spots of the patient were sent to institute of Xin Hua Hospital of Shanghai for determination of DHPR activity). Based on these findings, the child was initially diagnosed with dihydropteridine reductase disease (DHPRD, MIM # 261630). The treatment involved a Phe-reduced diet along with supplementation with -madopar, 5-hydroxytryptophan, and calcium folinic acid. Further genetic analysis revealed that the child had a compound heterozygous genotype [NM_000320.3(QDPR): c.68G > A (p.Gly23Asp), rs104893863, ClinVar variation ID: 490] and [NM_000320.3(QDPR) c.419C > A (p.Ala140Asp), ClinVar ID: 2444501] (Fig. 2).
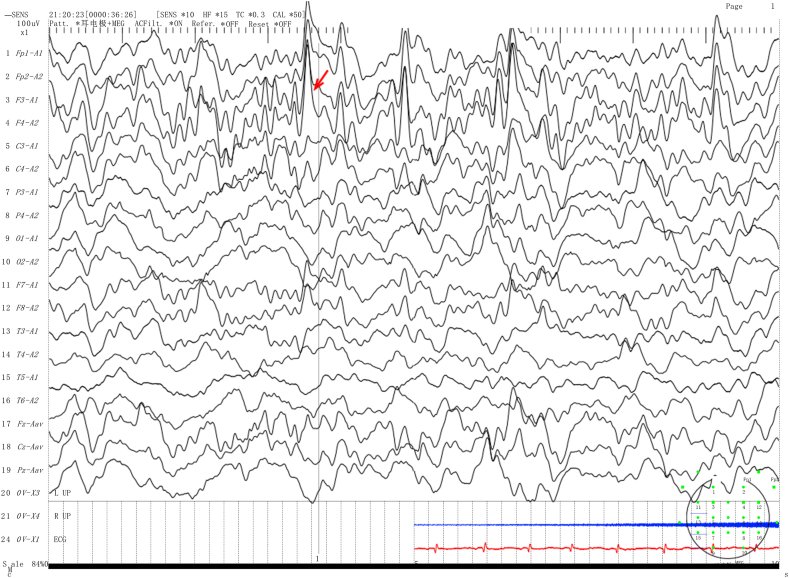
The Sanger sequencing results demonstrated that the patient inherited the QDPR variants c.68G > A and c.419C > A from their parents. The c.68G > A variant was not found in the gnomAD, 1000 Genomes, or ESP databases, but it has been reported in the HGMD and CinVar databases. Similarly, the c.419C > A variant was not found in the HGMD or CinVar databases. The mutation test software predicted both variants to be deleterious. According to the ACMG guidelines, the c.68G > A and c.419C > A variants were judged to be possibly pathogenic based on the criteria PM2, PM3_Strong, PP3, and PP4 for the former and PM2, PM3(Trans), PP3, and PP4 for the latter. The patient was diagnosed with DHPRD. The treatment plan involves a Phe-reduced diet and drugs such as madopar, 5-hydroxytryptophan, and calcium folinic acid. Phe levels were monitored every 4–6 months and fluctuated between 180.29 and 839.07 μmol/L. After 6 months of age, the patient's diet includes low phenylalanine rice and noodles, and animal foods such as eggs and lean meat are gradually introduced to ensure the intake of all necessary nutrients. This patient exhibited a severe global developmental delay. We recommended the addition of sapropterin to the therapeutic regimen, but it was not implemented due to economic hardship and difficulties in purchasing the medicine. Although the patient's Phe-reduced diet remained irregular, we promptly advised the family against feeding the child Zanba (a mixture containing ghee and barley, which is not Phe-reduced) at 1–2 months of age. At 1 year and 2 months of age, the child presented with seizures, focal myoclonus, and an abnormal electroencephalogram (EEG) showing spike/spike waves in the top central frontal area. (Fig. 3).
Fig. 1.
Sanger sequencing chromatograms were used to analyze the PTS case 1 family. The presence of a red arrow confirmed that the parents of Case 1 were heterozygous carriers of the variant. Het: heterozygous; Hom: homozygous. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Sanger sequencing chromatograms were used to analyze the QDPR case 2 family. The presence of a red arrow confirmed that the parents of Case 2 were heterozygous carriers of both variants. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
The EEG results for Case 2 show spike/spike waves in the top central frontal area, as indicated by the red arrow. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Discussion
We reported two cases of Tibetan children with BH4D. In Table 1.
Table 1.
The genotype and phenotype of 6 patients with PTS c.259C > T or QDPR c.68G > A reported in the literature.
| Patient no. | Gender | Newborn screening Phe (μmol/L) |
Gene | Sequence variation | Allelic status | Outcomes | References |
|---|---|---|---|---|---|---|---|
| P1 | Male | 1949.14 | PTS | c.259C > T (p.Pro87Ser) | Hom | NA | [6] |
| P2 | Male | 375.78 | PTS | c.155A > G (p.Asn52Ser); c.259C > T (p.Pro87Ser) |
Het | NA | [6] |
| P3 | Male | 879.39 | PTS | c.155A > G (p.Asn52Ser) c.259C > T (p.Pro87Ser) |
Het | NA | [6] |
| P4 | Female | 1320 | QDPR | c.68G > A (p.Gly23Asp) | Hom | Bad | [7] |
| P5 | Male | 399 | QDPR | c.68G > A (p.Gly23Asp) | Hom | Died | [7] |
| P6 | NA | NA | QDPR | c.68G > A (p.Gly23 Asp) | Hom | NA | [8] |
Het: heterozygous; Hom: homozygous; NA: Not applicable.
We compared these cases with previously reported cases that had the same pathogenic variants. The PTS c.259C > T variant is frequent in Southeast Asian individuals, with an allele frequency of 0.0001033 according to the gnomAD database [9]. In a study by Lin et al. [6], a case of PTS c.259C > T was reported as a homozygous variant and two cases of individuals with heterozygous variants were reported. Biochemistry analysis showed that the Phe value of the homozygous variant was higher than that of the heterozygous variants during the newborn screening period. It was speculated that the enzyme activity center of p.Pro87, which is close to p.Asp89, is related to the binding of 2-valent metal ions, and this might affect the integrity of secondary structure elements that contribute important residues to the active site [10]. Although it is not yet possible to determine whether our case 1 febrile sexual convulsions were associated with
PTPSD, it is worth noting that the incidence of epilepsy in patients with PTPSD is approximately 25%-50%. Therefore, long-term monitoring of convulsive seizures in children is still needed.
Glycine (Gly) located at codon 23 of the QDPR gene is the third glycine in the Gly-X-Gly-X-X-Gly repeat sequence. This repeat sequence allows for the tight binding of the β-fold and α-helix, and the pathogenic variant may disrupt the structural domain of NADH formed by protein folding [11]. The variant p.Gly23Asp is frequently observed in Mediterranean populations and was identified in 272 randomly selected Maltese neonatal DNA samples. The allele frequency of this variant was determined to be 0.016 [8]. The QDPR gene mutation has been observed in 75% of the Turkish population deficient in BH4. In Tables 1 and it has been reported that the p.Gly23Asp variant was found in two individuals (P4/P5) who are homozygous variants [7]. There was a significant difference in Phe levels between the two patients, which we hypothesized was due to the older age of diagnosis of P5 (15 months). We observed that our Case 2 had a monitoring level of 2442.11 μmol/L on day 30 of neonatal disease screening after birth, and the DHPR activity was decreased to approximately 21% of normal levels. She experienced irregular treatment, which led to the development of seizures, severe global growth retardation, and microcephaly. All three p.G23D children showed a poor response to treatment. Overall, due to the low prevalence of BH4D and geographical variations, no genotype-phenotype-biochemical association has been established.
BH4D patients in the neonatal period do not exhibit abnormalities. However, as time progresses, untreated patients may develop classical symptoms such as hypotonia, cognitive impairment, dyskinesia, seizures, and microcephaly at approximately 3 months of age. These symptoms place a significant burden on the patient, their family, and society as a whole. Previous studies, along with our two cases, suggest that early diagnosis and regular treatment of BH4D can improve prognosis and potentially reduce the cost of future treatments. However, achieving early diagnosis and treatment in less economically developed areas remains challenging. The establishment of a newborn disease screening laboratory in such regions requires comprehensive support from the government, including economic and political assistance. Factors such as birth population, test sample volume, sample management, transportation, and training of medical staff need to be considered [12].
The clinical symptoms of BH4D vary and differ, and there are no accurate descriptions of the phenotype-genotype in existing studies. Therefore, these differences in clinical symptoms cannot be used to differentiate between the various subspecies of BH4D. Clinicians still require a simple classification of each type of BH4D through the analysis of blood/urine metabolites. The results of this analysis will ultimately affect the treatment options for the disease and are closely related to the prognosis of the patient. The measurement of urine/dried blood spot (DBS) biopterin or percentage of biopterin is a crucial indicator of PTPSD and can distinguish it from GTPCH (MIM # 233910). It is worth noting that urine tests are more sensitive than dried blood spot tests [13]. The BH4 loading test is commonly used to distinguish between different types of BH4-responsive PKU. However, this test has some limitations. For example, the results may be affected by the degradation of urine due to light and heat, as well as improper handling of the specimen, which can impact the accuracy of the results. Additionally, the BH4 loading test is expensive and not widely available, and there is no standardized method for the test across countries. As a result, genetic testing has become increasingly important in conjunction with biochemical testing for the diagnosis and differential diagnosis of BH4D in cases where it is difficult to determine and presents with atypical clinical symptoms. The guidelines recommend a Phe-reduced diet as the initial treatment for all patients with HPA, regardless of whether they have PKU or BH4D, when blood Phe levels exceed 360 mmol/L. Individualized treatment protocols should be established based on factors such as diet habits, enzyme activity, and age [13].
For patients with BH4D who have developed neurological symptoms, Phe-reduced diet therapy and drugs such as dopamine agonists are recommended, depending on the enzyme deficiency. The use of sapropterin in the treatment of DHPRD is controversial due to the possibility of high doses leading to a buildup of 7,8 dihydrobiopterin (BH2), but the evidence from cellular experiments is insufficient to support these impairments. However, sapropterin has been found to be effective in improving neurotransmitter metabolites in the cerebrospinal fluid and treating DHPRD [14]. In our study, Case 2 was not treated with sapropterin drugs due to their high cost and the challenges associated with acquiring them, which is unfortunate.
Sichuan Province, located in the interior of Southwest China, is the second largest Tibetan region in the country. The Tibetan population is primarily concentrated in the western plateau of Sichuan. Approximately 95% of Tibetans speak Tibetan and Kajang, while approximately 40% are bilingual in Chinese and Tibetan, despite significant cultural differences. Due to variations in geography, economy, and customs, the rate of out-of-hospital births is higher among Tibetans than among Han Chinese, and there is generally a lower screening rate for newborn diseases. Since 2002, the Sichuan Maternal and Child Health Centre has been responsible for screening newborns from ethnic minorities across the entire plateau region of Sichuan Province. The center has also trained specialists in newborn screening in the region. However, there are still some challenges that need to be addressed. Collection of unqualified samples leads to a higher rate of false positives, thereby increasing the cost of screening. Additionally, the nomadic lifestyle and inconvenient transportation often result in delayed diagnosis as some samples take more than a week to be tested. The region lacks regular guidance and follow-up visits by genetic counseling professionals.
3. Conclusion
In our 20-year screening for neonatal diseases, we identified only 2 cases of Tibetan BH4D. This is the first reported case of BH4D in Tibetans in China. Although this number is small and not representative of the entire ethnic group, we have developed an individualized Phe-reduced diet in conjunction with Tibetan lifestyle practices. This approach serves as a basis and provides ideas for the prevention and early diagnosis of rare diseases in ethnic minorities. Additionally, we identified one case of a high-frequency pathogenic variant in the PTS gene (c.259C > T homozygous variant) in Southeast Asian populations, and we also discovered a new pathogenic variant in the QDPR gene. These findings contribute to the enrichment of the human gene mutation database and offer data support for future studies.
Funding
This research was funded by Chengdu Bureau of Science and Technology Project (2021-YF05-01658-SN).
Data availability statement
Suggested Data Availability Statements are available in section ClinVar bank at SCV003840210, SCV003840224 and SCV003840211.
Ethics statement
All methods were carried out in accordance with relevant guidelines and regulations by the 1975 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the Sichuan Provincial Maternity and Child Health Care Hospital (20230331-024), and all patients signed informed consent forms.
CRediT authorship contribution statement
Shuyao Zhu: Conceptualization, Investigation, Resources, Writing – original draft, Writing – review & editing. Qi Hu: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. Yunxia Yang: Data curation, Methodology, Supervision, Writing – review & editing. Hui Zhu: Conceptualization, Investigation, Supervision, Writing – original draft. Jin Wang: Data curation, Formal analysis, Project administration, Software, Writing – review & editing. Zemin Luo: Conceptualization, Formal analysis, Resources, Visualization, Writing – review & editing. Mincai Ou: Data curation, Methodology, Software, Visualization, Writing – review & editing. Ai Chen: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. Yu Huang: Data curation, Investigation, Resources, Supervision, Writing – review & editing. Fu Xiong: Data curation, Project administration, Resources, Visualization, Writing – review & editing. Jiaji Zhou: Conceptualization, Formal analysis, Investigation, Project administration, Visualization, Writing – review & editing. Jinglin Liu: Data curation, Methodology, Supervision, Writing – review & editing. Xunming Lei: Formal analysis, Resources, Validation, Writing – review & editing. Lan Zeng: Conceptualization, Formal analysis, Software, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:AI CHEN reports financial support was provided by Chengdu Bureau of Science and Technology Project. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to Dr. Heping Fang for reading and reviewing this article, thanks to my family and my favorite singer Alan Tam.
References
- 1.Longo N. Disorders of biopterin metabolism. J. Inherit. Metab. Dis. 2009;32(3):333–342. doi: 10.1007/s10545-009-1067-2. [DOI] [PubMed] [Google Scholar]
- 2.Gu X., W Z., Ye J., Han L., Qiu W. Newborn screening in China: phenylketonuria, congenital hypothyroidism and expanded screening. Ann. Acad. Med. Singapore. 2008:107–110. [PubMed] [Google Scholar]
- 3.Liu N., et al. Spectrum of PAH gene variants among a population of Han Chinese patients with phenylketonuria from northern China. BMC Med. Genet. 2017;18(1):108. doi: 10.1186/s12881-017-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie K., et al. Mutation spectrum of PTS gene in patients with tetrahydrobiopterin deficiency from jiangxi province. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1077729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan X., et al. Birth prevalence of tetrahydrobiopterin deficiency in China: data from the national newborn screening program, 2013-2019. J. Pediatr. Endocrinol. Metab. 2021;34(7):835–841. doi: 10.1515/jpem-2021-0077. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y., et al. Newborn screening and genetic features of patients with hyperphenylalaninemia in a southern Chinese population. Clin. Chim. Acta. 2022;535:13–18. doi: 10.1016/j.cca.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Romstad A., et al. Molecular analysis of 16 Turkish families with DHPR deficiency using denaturing gradient gel electrophoresis (DGGE) Hum. Genet. 2000;107(6):546–553. doi: 10.1007/s004390000407. [DOI] [PubMed] [Google Scholar]
- 8.Farrugia R., et al. Molecular genetics of tetrahydrobiopterin (BH4) deficiency in the Maltese population. Mol. Genet. Metabol. 2007;90(3):277–283. doi: 10.1016/j.ymgme.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Ye J., et al. Demographics, diagnosis and treatment of 256 patients with tetrahydrobiopterin deficiency in mainland China: results of a retrospective, multicentre study. J. Inherit. Metab. Dis. 2013;36(5):893–901. doi: 10.1007/s10545-012-9550-6. [DOI] [PubMed] [Google Scholar]
- 10.Muniz J.R.C., et al. Role of protein structure in variant annotation: structural insight of mutations causing 6-pyruvoyl-tetrahydropterin synthase deficiency. Pathology. 2019;51(3):274–280. doi: 10.1016/j.pathol.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Dianzani I., et al. Two new mutations in the dihydropteridine reductase gene in patients with tetrahydrobiopterin deficiency. J. Med. Genet. 1993;30(6):465–469. doi: 10.1136/jmg.30.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therrell B.L., Jr., Padilla C.D. Newborn screening in the developing countries. Curr. Opin. Pediatr. 2018;30(6):734–739. doi: 10.1097/MOP.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 13.Opladen T., et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH(4)) deficiencies. Orphanet J. Rare Dis. 2020;15(1):126. doi: 10.1186/s13023-020-01379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coughlin C.R., 2nd, et al. Dihydropteridine reductase deficiency and treatment with tetrahydrobiopterin: a case report. JIMD Rep. 2013;10:53–56. doi: 10.1007/8904_2012_202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Suggested Data Availability Statements are available in section ClinVar bank at SCV003840210, SCV003840224 and SCV003840211.