Summary
Fat infiltration in skeletal muscle (also known as myosteatosis) is now recognized as a distinct disease from sarcopenia and is directly related to declining muscle capacity. Hence, understanding the origins and regulatory mechanisms of fat infiltration is vital for maintaining skeletal muscle development and improving human health. In this article, we summarized the triggering factors such as aging, metabolic diseases and metabolic syndromes, nonmetabolic diseases, and muscle injury that all induce fat infiltration in skeletal muscle. We discussed recent advances on the cellular origins of fat infiltration and found several cell types including myogenic cells and non-myogenic cells that contribute to myosteatosis. Furthermore, we reviewed the molecular regulatory mechanism, detection methods, and intervention strategies of fat infiltration in skeletal muscle. Based on the current findings, our review will provide new insight into regulating function and lipid metabolism of skeletal muscle and treating muscle-related diseases.
Subject areas: Health sciences, Physiology, Human metabolism
Graphical abstract

Health sciences; Physiology; Human metabolism
Introduction
Skeletal muscle is among one of the largest organs in the human body accounting for approximately 40% of total body weight and playing an indispensable role for locomotion and glucose and lipid homeostasis. Many multinucleated fast and slow myofibers constitute skeletal muscle through the activation of resident myogenic precursor satellite cells (SCs). Activated SCs undergo proliferation and differentiation of the myoblast, fusion of myocytes, and finally maturation of myofibers, during the biological process of myogenesis.1 Many myogenic transcription factors such as paired box 7 (Pax7), myogenic differentiation (MyoD), myogenic factor 5 (Myf5), and myogenin regulate myogenesis.2 Besides, adipose tissue can secrete several adipokines and lipokines that regulate skeletal muscle development and homeostasis.3 Various adipokines (e.g., adiponectin, leptin, chemerin, and resistin) and lipokines (e.g., oxylipins, fatty acid-hydroxy-fatty acids, lysophosphatidic acid, and palmitoleate) play important roles in mediating the crosstalk between adipose tissue and skeletal muscle. After muscle injury, skeletal muscle has a mighty regenerative capacity. The damaged muscle elicits the activation of quiescent SCs and their niche for repair. A number of signaling molecules take part in this process to maintain muscle stem cells homeostasis.4 However, muscle fiber is replaced by ectopic tissues including fat and fibrous tissue during progressing muscle regeneration. Muscle regeneration ultimately leads to the loss of many muscle functions, this process results from a decreased SCs number.5 The decline in skeletal muscle mass, strength, and other functions is directly related to human myopathies, including muscular dystrophy, cachexia, and sarcopenia. Hence, how does regulating skeletal muscle development, function, and regeneration benefits human health?
Fat infiltration (also known as fat deposition/accumulation), often exists in non-adipose tissue, such as skeletal muscle, bone, and liver. Myosteatosis, defined as the pathologic fat accumulation in skeletal muscle with poor metabolic and musculoskeletal health, is now considered as a distinct disease from sarcopenia, which is also recognized as a common feature of aging and is related to the decline of muscle strength, muscle architecture, muscle contraction, and muscle capacity.6 Fat deposition in skeletal muscle has two patterns: (1) fat deposition between skeletal muscle fibers (muscle cells), including the intermuscular adipose tissue (IMAT) between the epimysium of skeletal muscle and intramuscular fat (IMF) in the endomysium and perimysium and (2) fat deposition (lipid droplets) in skeletal muscle fibers, called intramyocellular (IMCL).7 In this article, IMF content refers to IMF in a broad sense, including IMAT, IMF, and IMCL. However, intramuscular triacylglycerol or lipid droplets also serve as energy storage organelles and the primary goal of lipid droplet biogenesis is to alleviate cellular lipotoxic stress, as well as stresses associated with disturbed endoplasmic reticulum homeostasis, oxidation, and starvation.8 Recently, Yue et al. have shown lipid droplet dynamics could regulate stem cell fate determination.9 Hence, lipid droplets play different roles in different diseases and might even have opposite functions during different stages of the same disease.8 In parallel, animal production research has provided extensive insight into the role of IMF in meat quality. Several muscle disorders and physiological stresses always accompany with fat infiltration in skeletal muscle, which is directly related to insulin resistance and muscular dysfunction, such as aging, sarcopenia, diabetes, and obesity.10,11 Previous studies found that many triggering factors, including aging, diseases, and muscle injury, can contribute to fat infiltration in skeletal muscle and fat infiltration is modulated by many regulators, genes, and signaling pathways.12,13 Thus, understanding the origins of IMF deposition and the regulatory mechanism of fat infiltration in skeletal muscle are vital for maintaining the development, function and homeostasis of skeletal muscle, and the treatment of muscle-related disease.
In this review, we mainly discuss the triggering factors contributing to fat infiltration, including aging, metabolic diseases, non-metabolic diseases, and muscle injury as well as the cellular origin of fat infiltration. Additionally, recent advances and current discoveries of regulatory mechanisms, detection methods, and intervention strategies (exercise, diet, secreted factors, and gut microbiota) on fat infiltration in skeletal muscle are also discussed.
Influential triggers of fat infiltration in skeletal muscle
Several triggering factors have been reported to induce fat formation and accumulation in skeletal muscle, including aging, metabolic diseases and metabolic syndromes, non-metabolic diseases, muscle injury, and other factors (Figure 1).
Figure 1.
Influential triggers on fat infiltration in skeletal muscle
Several triggering factors induce IMF deposition in skeletal muscle, including aging, diseases, muscle injury, and others. COPD, chronic obstructive pulmonary disease; DMD, duchenne muscular dystrophy; HIV, Human immunodeficiency virus; NO, nitric oxide; T2D, type 2 diabetes.
Aging
Aging relates to physiological, metabolic, and functional dysfunction, in part through age-related alterations in body composition. Aging is always accompanied by a decreased mass and strength of skeletal muscle, also referred to as “sarcopenia,” which is a significant contributor to the emergence of age-related metabolic dysfunction, comorbidities, and premature death.14 Fat infiltration and IMF deposition often occur in skeletal muscles of elderly people as well as aged animals. In human, a previous study found an age-related remodeling of body composition with a reduction in skeletal muscle and IMAT in older African American women, which suggested that aging-induced fat infiltration in skeletal muscle is always correlated with sarcopenia.10 Molecularly, based on the quantitative proteomic analysis in skeletal muscle of human,15 we performed a correlation analysis and found the levels of adipogenesis-related protein (ADIPOQ, FABP4, and PLIN1) were positive with age (Figure 1). Additionally, fat infiltration in skeletal muscle is often correlated with the emergence of some diseases for the elderly. In Caucasian aged men, skeletal muscle fat infiltration is positively associated with all-cause and cardiovascular mortality.16 There is a clear linear positive correlation between the greater content of IMF and increasing age in individuals across disease states, including cancer and chronic stroke survivors, total knee arthroplasty, and anterior cruciate deficiency.17 In older adults, lower muscle mass (also called smaller thigh muscle area) and more IMF content in the muscle are related to the risk of mobility losses.18 Recently, Perez et al. identified the source of IMF cell types including mesenchymal stem cells (MSCs), fibro/adipogenic progenitors (FAPs), and endothelial cells (ECs) in vastus lateralis of old people by using single-nucleus RNA-seq (snRNA-seq).19 Similarly, in animals, aging is also related to IMAT and IMF deposition in skeletal muscle. Jing et al. identified adipocytes clusters and other pre-adipocytes clusters (MSCs, FAPs, ECs, and pericytes) in aged muscle of monkeys through snRNA-seq.20 In brief, aging is always accompanied with fat infiltration in skeletal muscle which might be a significant trigger for muscular dysfunction. However, whether the cell types in IMF of aged skeletal muscle are similar with adipocytes needs to be further explored.
Metabolic diseases and metabolic syndromes
Metabolic diseases are diseases caused by metabolic disorders and metabolic exuberance. Diabetes mellitus (DM) is a common metabolic disease in elderly people. Recent statistics showed that DM is estimated to affect approximately 578 million by 2030 and 700 million by 2045.21 Importantly, type 2 diabetes (T2D) is the most common form of DM and accounts for about 90% of cases diagnosed. Recently, more and more studies have found IMF content is proportionately higher in men and women with T2D and the metabolic syndrome than in people without these conditions.22 Greater ectopic fat deposition and lower skeletal muscle mass are associated with T2D and is a heritable trait.23 Particularly, therefore, lipid accumulation in skeletal muscle in Africans is more prevalent than in Caucasian men.24 T2D is always accompanied with fat infiltration in skeletal muscle. For example, obese patients with T2D had the highest IMAT and it was strongly associated with insulin resistance.25 Failure of fat cell proliferation, impairment of fat oxidation, and increased fat intake all lead to ectopic IMF deposition, insulin resistance, and T2D.26 Myosteatosis contributed to the development of T2D in African men27 and the degree of myosteatosis was significantly and positively correlated with IMAT content, which can alter skeletal muscle quality and relate to sarcopenia and skeletal muscle aging.28 A group of complex metabolic disorder syndrome and a risk factor leading to DM, cardiovascular and cerebrovascular diseases, and obesity is one of its main common symptoms. Importantly, obesity is always accompanied by increasing fat deposition between skeletal muscle fibers or surrounding skeletal muscle.29 Based on the RNA-seq data of primary differentiated human myotubes, we used heatmap and found the expression of adipogenic marker gene (Pparγ and C/ebpα) was significantly upregulated in obese group30 (Figure 1). This meant obesity is related to fat deposition in skeletal muscle in vitro. Overall, metabolic diseases and metabolic syndromes are always accompanied with IMF deposition but the regulatory mechanism of these processes is still unknown.
Nonmetabolic diseases
Disease is a major risk factor for skeletal muscle mass and strength loss. Emerging evidences implicate that some diseases may be major causes of fat infiltration in skeletal muscle.
Muscle-related diseases
Duchenne muscular dystrophy (DMD) is a degenerative muscle disorder in which muscle cells are damaged and replaced by fibrofatty tissue. Compared with normal muscle, boys suffering from DMD had increased fat infiltration.31 Specifically, gluteus maximus had the greatest degree of fatty infiltration in boys with DMD.32 Additionally, in expiratory muscle, Barnard et al. also found fatty infiltration in individuals with DMD.33 Muscle degeneration in low back pain is characterized by a decrease in cross-sectional area and an increase in fat infiltration in the lumbar paraspinal muscles.34 These results indicate muscle-related diseases are influential triggers of fat infiltration in skeletal muscle.
Viral diseases
Recently, the COVID-19 pandemic caused an extraordinary global emergency and was threatening human health. Current evidence suggests that skeletal muscle was affected by the COVID-19-related malfunction.35 COVID-19 leads to the decrease of skeletal muscle strength and mass36 and is associated with skeletal muscle fat index.37 Therefore, COVID-19 may impair skeletal muscle function but the special relationship between it and fat deposition in muscle needs further study. Besides, men infected with HIV had lower thigh muscle density and more lipid accumulation in skeletal muscle during aging.38 Based on these research, viral diseases contribute to fat infiltration in skeletal muscle.
Inflammatory diseases
In patients with rheumatoid arthritis, IMF accumulation has a special association with physical function and physical activity.39 Similarly, in thigh muscles of people with knee osteoarthritis, increased infiltration of fat was shown both between and within the muscles.40 Besides, muscle fat content is strongly associated with non-alcoholic steatohepatitis.41 Kaibori et al. also observed that after partial hepatectomy surgery, patients with hepatocellular carcinoma had even less overall survival and greater IMF accumulation.42 These data suggest that inflammatory diseases have a powerful role in IMF deposition.
Other diseases
Additionally, other diseases are also correlated with fat deposition in skeletal muscle. Computed tomography images showed that fat accumulation in thigh and calf muscle is increased in patients with chronic obstructive pulmonary disease43 and it is related to relevant clinical outcomes and comorbidities.44 Specifically, in patients with lumbar diseases, increased fat infiltration was shown in the multifidus muscle in those patients with lumbar radiculopathy or lumbar degenerative kyphosis.45 Recently, Avesani et al. reviewed the correlation between muscle fat infiltration and chronic kidney disease, which mainly showed fat infiltration has been associated with a decrease in muscle strength and impaired muscle quality as well as with metabolic abnormalities, cardiovascular disease, and increased mortality.46 Also, myosteatosis is common in patients with cirrhosis and is associated with higher mortality.47
In short, many non-metabolic diseases are also important triggering factors for fat deposition in skeletal muscle accompanying with skeletal muscle mass and strength loss (Figure 1). However, the specific regulatory mechanism is still not clear.
Muscle injury
After various types of injuries and diseases, skeletal muscle has a striking regenerative capacity. Several studies demonstrated that muscle injury is always accompanied with degenerative changes such as fat infiltration in recent years.48 A recent study found that 1-week post motor vehicle collision, muscle attenuation values were significantly related to IMF content in magnetic resonance imaging.49 Compared with individuals with healthy controls and mild/moderate symptoms, patients with persistent whiplash injury-associated disorders (WAD) had increased muscle fat infiltration within the cervical multifidus muscle after 2 weeks and 3 months.50,51 Within the severe WAD group and the healthy group, there was a relevance observed between local and distal fat infiltration in skeletal muscle but not in the mild/moderate group.52 Similarly, people with persistent pain following whiplash injury always have varying levels of fat infiltration in their cervical extensor muscle.53 However, a meta-analysis suggests that there is still deficient evidence to confirm whether fat infiltration in muscle increases right after whiplash injury.54 Hence, the crosstalk between WAD and fat infiltration is still controversial. Besides, after chronic spinal cord injury, patients always suffer from IMF infiltration leading to metabolic disease and related mortality.55
In murine models, injecting glycerol in skeletal muscle has been used as a new animal model system for inducing muscle damage and regeneration. Our previous data showed the mRNA levels of adipogenesis-related genes (Fabp4, Adipoq, Plin1, Pparγ, and C/ebpα) were significantly increased in muscle of glycerol-injured mice56 (Figure 1). This result indicates fat deposition always accompanies with muscle injury. In detail, Joe et al. showed that muscle damage could activate resident FAPs that facilitate myogenesis.57 Additionally, impaired skeletal muscle regeneration is always accompanied with significant fat deposition in diabetic mice,58 suggesting that diabetes may increase sarcopenia in obesity through enhancing the anomalous differentiation of FAPs. In brief, there is a close correlation between muscle injury and fat infiltration in skeletal muscle and the glycerol-induced injury model could provide a new and feasible mouse model to study fat deposition and muscle regeneration.
Disuse and inactivity
Pagano et al. found that even after a short period of inactivity, intermuscular adipose tissue content is increased in adult men.59 In the lumbar multifidus and erector spinae muscles in subjects with sway-back posture, disuse can predispose these muscles to atrophy, which is characterized by a reduced cross-sectional area and fat infiltration.60 Also, fat infiltration in the gluteus minimus muscle is related to disuse following aging in embalmed elder cadavers.61 These results emphasize that inactivity and disuse is associated with IMF development in muscle.
Other influential factors
Apart from the aforementioned factors, there are other triggering factors that have been reported to induce fat infiltration. In mdx mice, nitric oxide treatment reduces the number of PDGFRα+ cells and IMF deposition.62 In sarcopenic mice, vibration and β-hydroxy-β-methylbutyrate treatment promote the transdifferentiation of adipocytes and thus could inhibit intramuscular lipid accumulation.63 Recently, our studies have discovered cold exposure could increase fat infiltration and alter lipid metabolism in skeletal muscle of mice64 and also increased IMF content in longissimus dorsi muscle of pigs.65 Taken together, fat filtration in skeletal muscle was induced by many influential triggering factors, which is related to skeletal muscle quality and mass.
The cellular origin of fat infiltration in skeletal muscle
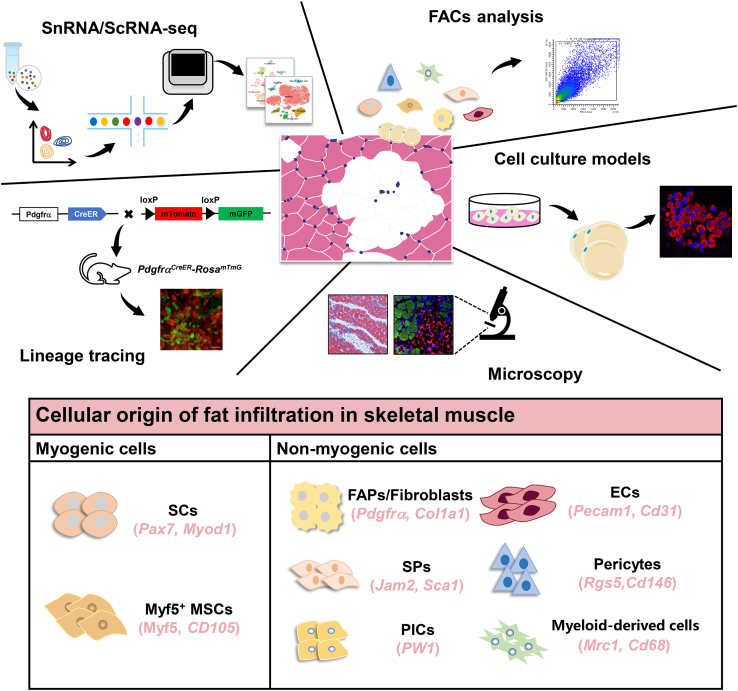
Recently, multiple studies have investigated the cellular origin of fat infiltration and discovered several cell types leading to the formation of ectopic IMF through multiple technologies including single-cell RNA sequencing, snRNA-seq, genetic lineage tracing, in vitro cell culture models, and microscopy5,56 (Figure 2).
Figure 2.
Fat infiltration in skeletal muscle derived from cell types with myogenic or non-myogenic origins
ScRNA-seq, snRNA-seq, genetic lineage tracing, in vitro cell culture models, and microscopy represent classical tools for studying the formation of fat infiltration in skeletal muscle and have found myogenic cells and non-myogenic cells types lead to fat infiltration. ECs, endothelial cells; FAPs, fibro/adipogenic progenitors; MSCs, mesenchymal stem cells; PICs, PW1-expressing cells; SCs, satellite cells; scRNA-seq, single-cell RNA sequencing; snRNA-seq, single-nucleus RNA sequencing; SPs, side population cells. Reprinted from56 Copyright 2020, Wiley Online Library66; Copyright 2023, Springer nature67; Copyright 2023, Wiley Online Library; under the Creative Commons CC BY License.
Myogenic cells
Early studies demonstrated that some primary muscle SCs could differentiate into adipocytes in vitro under different conditions.68 However, recent studies suggest that the primary myoblast isolation method used in the early stage may be contaminated by other cells; lineage tracing studies used by the Cre-loxP recombinant system found that IMF was derived from Myod− and Pax3- cells rather than the muscle SCs spectrum.69,70 However, it was also found that the primary muscle SCs achieved by fluorescence-activated cell sorting had lipid droplet deposition under certain culture conditions, but did not activate the adipogenesis process, and did not express the terminal differentiation marker genes of mature adipocytes. Fat accumulation in muscle SCs increased under some special conditions such as increased insulin resistance, decreased oxygen supply, and changed local metabolic environment.71 Additionally, studies have reported the existence of brown adipose tissue (BAT) derived from Myf5+ MSCs in skeletal muscle, and Pax7-CreER linage tracing studies have found that muscle SCs in vivo can differentiate into BAT regulated by Prdm16 (a transcription factor of BAT differentiation).72 In addition, MSCs are pluripotent stem cells; studies have reported that MSCs isolated from skeletal muscle of adult and mouse can proliferate and differentiate into mesenchymal tissue and non-mesenchymal tissue in vitro and in vivo.73
Non-myogenic cells
FAPs
Different from previous studies on the potential of adipogenic differentiation in vitro, Uezumi et al.74,75 verified in vivo transplantation of different cell populations in skeletal muscle and found that a subpopulation of non-myogenic MSCs is the main source of intramuscular adipocytes during muscle regeneration in mice and humans which is different from muscle SCs. This subpopulation of progenitor cells is PDGFRα+ or CD140α+ and has the biological potential of differentiating into lipid-rich adipocytes and expressing collagen I fibroblasts, which are defined as FAPs.74 Unlike muscle SCs, FAPs are located in the space between muscle fibers and muscle bundles in mice and humans, which is similar to IMF.74,75 FAPs play an important role in homeostasis and disease of skeletal muscle.76 Furthermore, interstitial muscle connective tissue cells expressing the transcription factor Osr1 which is found in mouse embryos skeletal muscle are embryo-like FAPs that can differentiate into fibroblasts and adipocytes in vivo and in vitro. Part of FAPs in adult skeletal muscle are derived from Osr1+ cells,77 and Osr1 has a low expression level of silent FAPs in skeletal muscle after birth.78 Uezumi et al. believed the proliferation of FAPs in adult skeletal muscle mainly came from PDGFRα+ cells rather than PDGFRα− cells or stem cells in the circulatory system.79 Historically, FAPs are the main source of IMF57,74 and recent study have found that MME+ FAPs are highly adipogenic and are reduced in fatty infiltrated human muscle.80 In our previous study, we found FAPs could differentiate into adipocytes in 2D and 3D cultured conditions; specially, FAPs could differentiate into PDE4D+/PDE7B+ adipocytes.66 In conclusion, FAPs are the main sources of IMF.
Fibroblasts
Fibroblasts can directly differentiate into adipocytes, such as 3T3 cell line. Fibroblasts that activate and produce fibers expressing vimentin and α-SMA are also known as myofibroblasts. Due to the fact that myoblasts also express fibroblast marker proteins vimentin and α-SMA, it is difficult to identify fibroblasts in skeletal muscle; studies on the fate and function of fibroblasts in skeletal muscle are very limited. Recently, TE-7 has been found to be a fibroblast-specific protein in humans; TE-7+/CD56- fibroblasts isolated from human skeletal muscle can be induced into adipocytes in vitro.81
ECs
ECs in the human fetal placenta have adipogenic differentiation potential, and CD34+/CD31+ ECs from human omental and subcutaneous adipose tissue can be induced into adipocytes.82,83 Based on our previous cell-to-cell communication, we found ECs may also have potential capacity to differentiate into adipocytes.66
Side population cells and pericytes
In early studies, the heterogeneous cells that express Sca-1 and c-Kit, are located in the muscle fiber space (outside the basement membrane) in skeletal muscle, are referred to as side population cells (SPs). Some of these CD34+/SCA-1+/CD45- SPs are also named myoendothelial progenitors that could differentiate into adipocytes in vitro.84 Besides, some CD31-/CD45- SPs also have the potential of adipogenic differentiation in vitro.85 Pericytes expressing NG2, α-SMA, CD146, and PDGFRβ surrounding ECs in capillaries and microvessels can express peroxisome proliferator-activated receptors γ2 (PPARγ2) and form into lipid droplets when cultured in adipogenic induction medium.86 The mesodermal tissue-derived pluripotent progenitors (mesoangioblasts) expressing Sca-1 and CD34 in skeletal muscle blood vessels may be a subpopulation of pericytes that can also differentiate into adipocytes under appropriate culture conditions.87
PW1+/Pax7- interstitial cells
Mitchell et al. found a subpopulation of MSCs which expressed PW1 but not Pax7 in the muscle stroma in the early postnatal period of mice, called PW1+/Pax7- interstitial cell (PIC).88 PIC cells express different level of PW1 and Sca-1 and have different differentiation potential, among which PW1+ cells expressing medium level of Sca-1 had adipogenic potential. Some of PIC cells express PDGFRα, so some PDGFRα+/PW1+ cells may overlap with some FAPs. All the PIC cells expressed NG2, suggesting that these cells may also overlap with muscle pericytes.
Others
In our previous study, we revealed that myeloid-derived cells may be involved in regulating fat infiltration in skeletal muscle by single-cell RNA sequencing.56 Heterogeneous myeloid-derived cells in muscle tissue were divided into 10 subclusters; some of them express abundant adipose-related genes (DLK1, CD38, ZFP423, and CD34) and adipogenesis-regulated genes (C/EBPα and PPARγ).56 However, whether there are other sources of IMF needs to be further investigated.
Taken together, numerous kinds of cell types contribute to fat infiltration because skeletal muscle tissues are highly heterogeneous. Specifically, skeletal muscle contains several cell types including myogenic cells (SCs and Myf5+ MSCs) and non-myogenic cells (FAPs, fibroblasts, ECs, pericytes, SPs, PICs, and myeloid-derived cells) (Figure 2). However, FAPs are the main source of IMF, but the mechanism of FAPs committed differentiate into IMF and whether there are other sources of IMF needs to be further investigated.
Fat infiltration regulatory mechanism in skeletal muscle
The regulatory mechanism of fat infiltration in skeletal muscle is intricate and involves the regulation of signaling pathways. Genes and regulators regulate fat filtration in skeletal muscle through related signaling pathways in the animal organism (Figure 3).
Figure 3.
Molecular mechanism of fat infiltration in skeletal muscle
Regulation of IMF deposition involves in many genes and signaling pathways including cAMP-PKA, hedgehog, Wnt/β-catenin, AMPK, MAPK, miRNAs, and lncRNAs. ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; aP2, adipocyte fatty acid-binding protein; ATGL, adipose triglyceride lipase; cAMP-PKA, cAMP-protein kinase A; CASR, calcium-sensing receptor; CREB, cAMP response element-binding protein; CRTCs, CREB-regulated transcription coactivators; CTRP6, C1q/tumor necrosis factor-related protein 6; DGAT1, diacylglycerol acyltransferase 1; DNJ, 1-deoxynojirimycin; ERK, e extracellular signal-regulated kinases; FABP4, fatty acid-binding protein 4; GADD45A, growth arrest and DNA damage-inducible alpha; IMF, intramuscular fat; LKB1, liver kinase B1; MAPK, mitogen-activated protein kinase; miRNAs, microRNAs; PPARγ, peroxisome proliferator-activated receptors γ; PRMT5, protein arginine methyl transferase 5; SREBP1a, sterol regulatory element-binding transcription factor 1a; TAZ, transcriptional co-activator with PDZ-binding motif; YAP, Yes-associated protein.
Lkb1-AMPK
AMP-activated protein kinase (AMPK) exerts a significant effect on regulating metabolism and energy balance in the animals’ body. Adiposity is associated with the AMPK signaling pathway in aged skeletal muscle.89 AMPK regulated the expression of precursor of adipocyte differentiation and maturity-related genes (Pparγ, C/ebpα, and Fas).90 These studies suggested that AMPK might regulate MSCs and intramuscular preadipocyte differentiation. In skeletal muscle, AMPK promoted lipid oxidation and reduced triglyceride synthesis via acetyl-CoA carboxylase 2 phosphorylation.91 Liver kinase B1 (Lkb1) is a serine/threonine protein kinase which can be phosphorylated to activate AMPK activity. Recent studies found Lkb1 affected SCs proliferation and differentiation through the AMPK signaling pathway and regulated lipid metabolism and ectopic fat deposition in muscle progenitor cells and mature muscles.92 Overall, these observations suggest that the Lkb1-AMPK signaling pathway inhibits intramuscular preadipocyte differentiation in skeletal muscle.
MAPK
Mitogen-activated protein kinase (MAPK) signaling pathway participates in physiological process such as cell proliferation and differentiation and including extracellular signal-regulated kinases 1 and 2 (ERK1/2), ERK5, p38, and C-Jun amino-terminal kinase, this pathway regulates adipogenesis from stem cells to adipocytes. After rotator cuff repair, inhibition of p38 resulted in a reduction of muscle fat infiltration.93 In porcine intramuscular adipocytes, C1q/tumor necrosis factor-related protein 6 inhibited intramuscular adipocytes proliferation and promoted differentiation by the AdipoR1/MAPK signaling pathway94 and 1-deoxynojirimycin inhibited lipid accumulation by repressing of the ERK/PPARγ signaling pathway95 (Figure 3). These results indicate that MAPK regulates adipogenesis from stem cells to intramuscular adipocytes in animal models.
Wnt/β-catenin
The Wnt/β-catenin signaling pathway exerts a vital function on regulating adipocyte differentiation and lipid metabolism. Reggio et al. found the WNT5a/GSK3/β-catenin axis could affect adipogenesis of skeletal muscle FAPs.96 After muscle injury, inhibition of Wnt10b signaling could activate the adipogenic potential during muscle regeneration.97 Recently, Fu et al. found WNT7A inhibited adipogenesis of skeletal muscle FAPs and fat infiltration through Wnt-Rho-Yes-associated protein/transcriptional co-activator with PDZ-binding motif signaling axis.98 Besides, in sarcopenic mice, vibration and β-hydroxy-β-methylbutyrate treatment inhibited IMF accumulation and adipogenic differentiation through the Wnt/β-catenin pathway.63 These findings demonstrate that Wnt/β-catenin pathway occupies an important position in inhibiting fat deposition in skeletal muscle.
cAMP/PKA
The cAMP-protein kinase A (cAMP-PKA) signaling pathway acts downstream the cAMP response element-binding protein and its transcriptional coactivators (CRTCs) and cAMP-PKA signaling pathway affects lipolysis and lipid metabolism.99 Overexpression of CRTC2 could increase IMF content and the cross-sectional area of muscle fibers in skeletal muscle.99 CRTC3 is a member of the CRTC family and significantly affects energy metabolism. Overexpression of CRTC3 increases triglyceride deposition in skeletal muscle by upregulating diacylglycerol acyltransferase 1100 (Figure 3). Our previous study showed that CRTC3 regulates lipid and energy homeostasis and the adipogenic differentiation of porcine intramuscular adipocytes through activating the calcium pathway.101 These data suggest that cAMP-PKA signaling pathway could enhance IMF content in skeletal muscle.
Hedgehog pathway
The hedgehog signaling pathway regulates the differentiation fates of precursor cells and influences adipocyte development. Activation of hedgehog downregulated the expression of adipogenic marker genes (C/ebpα and Pparγ) and mature adipocyte marker proteins (leptin and adipocyte fatty acid-binding protein [aP2]), following inhibited adipogenic differentiation of preadipocyte as well as suppressed adipocytes development.102 During skeletal muscle regeneration, hedgehog inhibited the transformation of FAPs to adipocytes and suppressed adipogenesis in skeletal muscle.103 Overall, hedgehog pathway inhibits adipogenesis and the transformation of FAPs to adipocytes in muscle.
miRNAs
MicroRNAs (miRNAs) are important post-transcriptional regulators of gene expression in skeletal muscle and are associated with aging.104 Deeply, miRNAs affect lipid accumulation through regulating the expression of adipogenesis-related genes in many animal models. In mice, Michael et al. found miR-206 mimicry in vivo limits adipogenic conversion of skeletal muscle MSCs.13 MiR-34a enhanced fat deposition by regulating PDGFRα to promote the adipogenic differentiation of porcine intramuscular preadipocytes via the ERK signaling pathway.105 In the aged skeletal muscle, FGF-2 signaling increased miR-29a expression and enhanced miR-29a levels stimulated differentiation of FAPs into adipocytes via the reduction of SPARC, promoting IMAT formation.106 Taken together, miRNAs could affect IMF deposition in many animal models but different miRNAs have different function. These differences may be due to the cell lines and the dosage used being different.
lncRNAs
In addition to common signaling pathways, more and more long-chain non-coding RNAs (lncRNAs) have been considered to be involved in the regulation of adipogenesis.107 lncRNAs are a class of non-coding RNAs with a length of over 200 nucleotides and recent studies suggested that lncRNAs function on regulating fat accumulation in skeletal muscle. Zhang et al. discovered an lncRNA, which they named IMF-associated long non-coding RNA, could promote the differentiation of intramuscular adipocyte by sponging miR-128-3p and miR-27b-3p.108 Recent study has found lncRNAs contribute to fat infiltration by regulating the expression of nuclear receptor subfamily 4 group A member 3.109 These findings indicate that lncRNAs plays a crucial role in regulating adipogenesis in skeletal muscle through sponging miRNAs.
Others
Previous study has found that adipose triglyceride lipase (ATGL) is associated with lipid droplets biogenesis in skeletal muscle and muscle SCs.9 Recently, Kim et al. discovered protein arginine methyl transferase 5 methylated and stabilized sterol regulatory element-binding transcription factor 1a to promote lipid droplets biogenesis in myofibers; it also increased the repressive H4R3Me2s modification to suppress ATGL gene expression and lipolysis.110
Overall, the molecular and regulatory mechanism of fat infiltration is manifold, complicated, and still challenging; a further studying of interventions combined with these signaling pathways will provide a basis for discovering novel potentially effective therapeutic approaches.
Detection methods of fat infiltration in skeletal muscle
There are many imaging approaches to detect myosteatosis including computed tomography (CT), peripheral quantitative computed tomography (pQCT), MRI, and quantitative ultrasound (QUS).111 CT is one of the most extensively employed imaging modalities for indirect myosteatosis assessment; it could differentiate SAT and IMAT and allows 3D reconstruction. However, its major disadvantages include high cost and limited access, and it cannot directly measure the location of fat storage or lipid droplets within the muscle. Compared with CT, pQCT has the advantage of lower cost and significantly reduced ionizing radiation emission but it cannot distinguish between IMAT and IMF.111,112 MRI can be utilized to detect myosteatosis, presenting a distinct advantage over CT devices due to the absence of ionizing radiation; however, a quantitative assessment of muscle density is currently unavailable and it also has high cost and limited access limitations.111 Besides, QUS is increasingly recognized as an affordable, convenient, and practical device for detecting myosteatosis; it can yield trustworthy measurements of muscle thickness and tissue echogenicity in both appendicular and axial skeletal muscles;113 however, it also cannot distinguish between IMAT and IMF. IMAT and IMF can be identified using CT and MRI, whereas IMCL necessitates specialized imaging techniques for visualization and quantification, such as magnetic resonance spectroscopy or muscle biopsies. Consequently, an increasing number of potential imaging methods are accessible for detecting fat infiltration in skeletal muscle, yet current challenges persist.
Interventions of fat infiltration in skeletal muscle
In recent years, many researchers have focused on intervention strategies of fat infiltration in skeletal muscle via randomized controlled trials and animal models.
Exercise
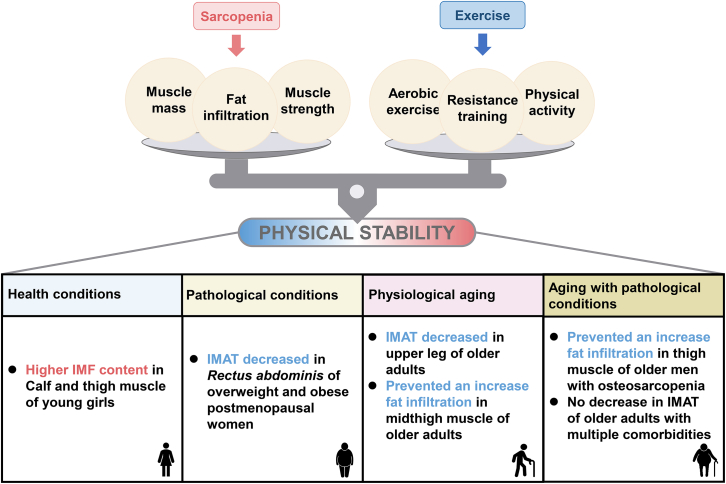
Regular exercise and physical activity can help people to maintain health-promoting physical fitness. For humans, exercise is the key intervention to counteract sarcopenia. The recent study concluded that persistent exercise can prevent muscle fat infiltration and can also improve muscle mass and strength among the elderly.114 A randomized controlled trial showed that IMAT was significantly reduced in older adults after a 12-week, low-intensity, short-interval, slow-jogging.115 Similarly, Ryan et al. found that older women could have significantly decreased lipid storages in the spinal and abdominal muscles of the trunk region after an aerobic exercise training.116 In elderly men with osteosarcopenia, long-term high-intensity resistance training prevented a further increase of muscle fat infiltration of the thigh muscle.117 Apart from the elderly, exercise also affects fat infiltration in young people. For young girls, a lower physical activity leads to higher IMF content of the calf and thigh.118 Additionally, more and more studies have been testing whether persistent exercise could decrease fat infiltration in people suffering from different conditions and diseases. Goodpaster et al. found that in older adults having moderate functional impairments, the age-related loss of skeletal muscle strength can be prevented by regular physical activity and increased IMF deposition.119 Marcus et al. concluded that persistent exercise decreases IMAT contents in skeletal muscle in older patients with a variety of comorbidities.120 However, Jacobs et al. demonstrated that there is no reduction in thigh IMAT following a three-month exercise intervention in older adults when considering the risk for falling.121 Hence, the function of exercise on fat deposition in muscle depends on methods of exercise and physical conditions (Figure 4) and whether exercise alone can reduce fat infiltration in patients suffering from different diseases remains to be further explored.
Figure 4.
The balance between sarcopenia and exercise under different conditions
The effects of exercise on regulating fat infiltration in skeletal muscle under health, pathological, physiological aging, and aging with pathological conditions.
Nutritional interventions
Apart from exercise, in recent years, nutritional interventions are the important strategies to regulate fat deposition in muscle based on clinic trials and many animal models. The main nutrients to regulate IMF deposition are vitamin D, vitamin A and retinoic acid, conjugated linoleic acid (CLA), linseed, amino acid, betaine, and so on (Table 1).
Table 1.
Nutritional interventions of fat infiltration in skeletal muscle
| Meansa | Subjects/Animals | Measures | Duration | Effects | Muscle | Reference |
|---|---|---|---|---|---|---|
| Vitamin D | Sarcopenic elderly | Whey protein, fish oil, vitamin D | 12 weeks | Fat mass was lower | / | Li et al. 122 |
| Subacute post-stroke rehabilitation patients | Whey protein and vitamin D | 16 weeks | Fat infiltration was lower | Thigh muscle | Honaga et al. 123 | |
| Vitamin A | Iberian pigs | Vitamin A restriction | At early growing and at finishing | Increased the preadipocyte number | Longissimus thoracis muscle | Ayuso et al. 124 |
| CLA | Chinese adults | 3.2 g/d CLA supplementation | 12 weeks | Increased muscle mass | Trunk muscle | Chang et al. 125 |
| Student athletes | 0.9 g/day intake | 14 days | Increased muscle mass | / | Terasawa et al.126 | |
| Female and male participants | 5 g/d CLA | 7 weeks | Increased in lean tissue mass and greater losses of fat mass | Lean tissue | Pinkoski et al. 127 | |
| Older adults | 5 g/d creatine monohydrate + 6 g/d CLA | 6 mouths | Improved muscular endurance and lower fat mass | / | Tarnopolsky et al.128 | |
| Sedentary older adults | 4000 mg/d CLA | 8 weeks | No significant muscle anabolic effects | Vastus lateralis | van Vliet et al.129 | |
| Mice | 0.7 g/kg or 0.5% CLA with endurance training | 6 weeks | induced a fiber-type-specific hypertrophy | Plantaris muscle | Barone et al. 130 | |
| Pigs | 1% CLA | 35 days | Increased IMF content | LDM | Wang et al. 131 | |
| Linseed | Patients with non-alcoholic fatty liver disease | 20 g/d flaxseed oil | 12 weeks | reduced fat mass | / | Rezaei et al. 132 |
| Dystrophic hamsters | 30% flaxseed | / | Promote regeneration of injured skeletal muscle | Biceps femoris muscles | Carotenuto et al.133 | |
| Barrows | 10% linseed | 90 days | IMF content increased | Longissimus muscle | Luo et al. 134 | |
| Herbal extract ALS-L1023 | Obese rats | 0.4% or 0.8% (w/w) of ALS-L1023 | 4 weeks | Reduced fat deposition | Gastrocnemius muscle | Shin et al.135 |
| Chronic sugar | C57BL/6J male mice | 15% fructose or 15% glucose | 7 months | Intramyocellular lipids accumulated fed with fructose | Gastrocnemius muscle | De Stefanis et al.136 |
| Betaine | In vitro (C2C12 cells) | 10 mM betaine | 24 and 48 h | Elevated lipid accumulation | / | Wu et al.137 |
| EGCG | Mice | 100 mg/kg EGCG | 14 days | Decreased IMF deposition | Tibialis anterior | You et al.67 |
| Gut microbiota | Patients with metabolic syndrome and C57BL/6J mice | Porphyromonas gingivalis | 6 weeks | Increased fat infiltration | Lumbar muscle | Watanabe et al.138 |
| SMAP8 mice | Lactobacillus casei Shirota | 12 weeks | Delayed age-related muscle mass deposition | Gastrocnemius muscle | Chen et al.139 | |
| SPF C57BL/6J mice | bacterial consortium | 4 weeks | Increased IMF content | Quadricep muscle | Xie et al.140 |
CLA, conjugated linoleic acid; EGCG, epigallocatechin-3-gallate; FAPs, fibro/adipogenic progenitors; HFD, high-fat diet; IMAT, intermuscular adipose tissue; IMCL, intramyocellular lipid; IMF, intramuscular fat; LDM, longissimus dorsi muscle.
Vitamin D
Vitamin D is a pro-steroid hormone which has been reported as the earliest hormone to arise on earth. The expression of the vitamin D receptor in human skeletal muscle declines with age. Low vitamin D levels are associated with the emergence of many diseases especially myopathies such as sarcopenia, cachexia, type 2 diabetes mellitus, osteoporosis, and cancer, and it also plays a vital role in the immune system that has become a global public health issue.141 Clinical trials and animal dietary intervention studies have demonstrated that vitamin D treatments exert positive function on muscle mass and the deletion of vitamin D receptor leads to impaired muscle function and sarcopenia.142,143 However, recent study showed that vitamin D may have adverse effects on muscle health in humans (except athletes)144; this systematic review and meta-analysis showed that vitamin D was associated with a significantly longer time spent performing the time up and go test and a significant reduction in maximum knee flexion strength. Many studies have found that vitamin D levels were associated with lipid deposition in skeletal muscle for young/older and healthy/unhealthy people. Gilsanz et al. also showed that vitamin D insufficiency was significantly associated with increased muscle fat infiltration in healthy post pubertal females.145 In older adults, vitamin D status could affect gastrocnemius intramyocellular lipid content independently of body mass and physical activity146 and in older adults with sarcopenia, fat mass was significantly lower in nutrition supplementation (whey protein, fish oil, and vitamin D) group.122 In post-stroke convalescent rehabilitation patients, vitamin D supplement decreases lipid accumulation into the thighs muscle.123 Additionally, reduced limb muscle strength is correlated to increased IMF levels and vitamin D deficiency in some patients with metabolic diseases, such as T2D and impaired glucose tolerance.147 Hence, the aforementioned findings confirmed that vitamin D can influence IMF deposition in human but the results need to be elaborated further and the regulatory mechanism also needs to be investigated further.
Vitamin A and retinoic acid
Vitamin A (also known as retinol), an essential micronutrient for human health, whose dietary supply is always in the form of retinyl esters, originates from animal sources. Vitamin A has important influences on regulating the metabolism and homeostasis of glucose and lipid in skeletal muscle. An increasing number of studies have shown that vitamin A inhibits adipocyte differentiation and restrictive vitamin A intake could increase IMF deposition in skeletal muscle in many animal models.124 Retinoic acid, a metabolite of vitamin A, regulates the roles of vitamin A that are required in growth and development. Retinoic acid plays increasingly important roles in adipogenesis and terminal maturation of adipocytes. In skeletal muscle, retinoic acid can restrict myoblast development and regulate the immature state of human skeletal muscle progenitor cells in vitro.148 In conclusion, vitamin A and retinoic acid play major roles in regulating muscle fat infiltration. However, the functions of vitamin A on skeletal muscle development and regeneration especially the effects of retinoic acid on IMF content in human are rarely reported.
CLA
CLA is a class of positional and geometric isomers of linoleic acid with a conjugated double bond which is generally found in ruminant animals and dairy products. CLA has anti-adipogenic, anti-cancer, anti-diabetic, and anti-hypertension effects. For Chinese adults with elevated body fat percentage, 3.2 g/day CLA supplementation significantly increased trunk muscle mass.125 For student athletes, after administering 0.9 g/day CLA intake, body weight variation significantly increased, amount of body fat percentage variation tended to decrease, and muscle mass increased.126 Pinkoski et al. found 5 g/day CLA combined with resistance training could increase lean tissue mass and decrease fat mass in participants.127 Liquid chromatography-mass spectrometry metabolomics showed CLA altered 57 metabolites enriched in lipids/lipid-like molecules including glycerophospholipids, fatty acyls, and sphingolipids in plasma.149 For old adults, 5 g/d creatine monohydrate + 6 g/d CLA for 6 months following resistance exercise training improved muscular endurance, isokinetic knee extension strength, and had lower fat mass.128 However, van Vliet S et al. discovered CLA supplementation does not have muscle anabolic effects in sedentary older adults.129 Hence, the specific effects of CLA on muscle function are still controversial. In mice, CLA has been identified to prevent sarcopenia by maintaining redox balance during aging, positively regulate mitochondrial adaptation, improve muscle metabolism, and induce hypertrophy in type IIx muscle fibers after endurance exercise.130,150 Besides, in porcine models, our previous studies have demonstrated that adding CLA into the diet could improve IMF contents in longissimus dorsi muscle of lean pig breeds and Chinese indigenous pig breeds.131,151 However, CLA supplementation positively affects IMF content in porcine models, but the effects on fat infiltration and deposition in human skeletal muscle as well as in rodents need to be further explored.
Linseed
Linseed is the ripe seed of flax which is rich in n-3 PUFAs and has many physiological functions including anti-adipogenic, anti-inflammatory, and anti-cancerous while regulating the metabolism and homeostasis of glucose and lipid metabolism. Previous studies have found that patients with non-alcoholic fatty liver disease after 20 g/d flaxseed oil had significantly reduced fat mass, after 20 g/d sunflower oil had significantly reduced muscle mass.132 However, Babajafari et al. found that there is no significant difference in triglyceride and cholesterol content in serum between patients who received isolated soy protein with or without flaxseed oil.152 Like CLA, the function of flaxseed oil on fat deposition in muscle is still unknown and the effects of fat infiltration in human skeletal muscle also need to be studied in the future. In animal models, linseed could promote regeneration of injured skeletal muscle in vivo and in vitro in dystrophic hamsters.133 In porcine models, linseed, flax, or flaxseed oil affect fatty acid composition, gene expression in skeletal muscle, and sensory quality through affecting the expression of adipogenesis-related genes (PPARγ and aP2).134,153 In a word, based on these studies, linseed and linseed oil are considered to affect IMF content but their specific function needs to be further elucidated.
Plant extract
After treatment with the herbal extract ALS-L1023, which was isolated from Melissa officinalis, the reduction of fat deposition was observed in the skeletal muscle of obese rats.135 In addition, Wu et al. described that 10 mM betaine treatment promoted lipid accumulation and regulated lipid metabolism in C2C12 cells through activating the ERK/PPAR signaling pathway.137 Recently, You et al. found dietary factor epigallocatechin-3-gallate protects against fat infiltration via repressing GADD45A expression in muscle of mice.67 These data suggest that plant extract could regulate fat deposition in skeletal muscle in mice and in vitro.
Muscle-adipose crosstalk
Skeletal muscle and adipose tissue both can serve as secretory organs that act in an endocrine, paracrine, or autocrine manner to release myokines and adipokines to facilitate tissue-to-tissue communication, work together to improve overall metabolic health, and regulate IMF deposition in muscles.154,155
Myokines
Myostatin is one of the best-characterized myokines that negatively regulates skeletal muscle growth and development.156 In animal models, myostatin mutation leads to higher lean mass, reduced body fat content, and lower IMF in M. longissimus and M. quadriceps.157 Leukemia inhibitory factor transplantation could reduce the number of FAPs and inhibit fibrogenesis of muscle cells, further diminishing pathology through transforming growth factor β signaling. Besides, fibroblast growth factor 21 significantly inhibits the differentiation of porcine intramuscular preadipocytes and goat intramuscular preadipocytes.158,159 Myonectin has shown beneficial effects on systemic lipid homeostasis;160 however, Petro et al. have found serum levels of myonectin are lower in adults with metabolic syndrome and serum myonectin was negatively correlated with the android/gynoid fat mass ratio but not with the IMF content.161
Adipokines and lipokines
Apart from lipid storage and metabolism, adipose tissue serves as an endocrine organ and secretes regulatory factors such as adipokines and lipokines, which can regulate lipid metabolism in skeletal muscle.3 In goat intramuscular preadipocytes, knockdown of adiponectin promoted goat IMF deposition162 and FGF10 promoted the adipogenesis during adipogenic differentiation of intramuscular preadipocyte.163 Another adipokine, chemerin, could promote lipolysis and induce adipogenesis during preadipocyte differentiation in bovine intramuscular adipocytes.164 Lipokines are described as important fatty acid-derived products that emanate from adipose tissue. Palmitoleic acid is an adipose tissue-derived lipokine and is catalyzed by stearoyl-CoA desaturase-1,165 which is reported to reduce intramuscular lipid and restores insulin sensitivity in obese sheep.166
Overall, these data confirmed the association between adipose tissue and skeletal muscle through myokines and adipokines, which also regulate fat infiltration in skeletal muscle, but the underlying mechanisms remain to be elucidated.
Gut microbiota
Gut microbiota play important roles in skeletal muscle metabolism and function. Gut microbiota are associated with muscle mass, muscle function, physical performance, and muscle fat infiltration in animal models and humans.167 In patients with metabolic syndrome, the anti-Porphyromonas gingivalis (Pg) IgG antibody titers positively correlated with intramuscular adipose tissue content and Pg administration increased fat infiltration in murine skeletal muscle.138 In the SAMP8 mice, Lactobacillus casei Shirota delayed the appearance of senescence and age-related muscle mass deposition.139 Compared with control mice, inoculating the bacterial consortium into mice increased IMF content in SPF C57BL/6J mice.140 Deeply, alteration of gut microbiota may lead to skeletal muscle atrophy via a bile acid-farnesoid X receptor pathway.168 Furthermore, PPARs may regulate fat deposition in skeletal muscle along the gut-muscle axis in both health and disease.169 Besides, a systematic review has shown that potential mechanisms of microbiome modulating muscle mainly include lipid and glucose metabolism and mitochondrial function.170 Short-chain fatty acids (SCFAs) have been effective in enhancing muscle mass and host function,171 and increase fatty acid uptake and oxidation while preventing lipid accumulation in skeletal muscle.172 In children, total body fat content mediated the associations of gut microbiota and SCFAs with skeletal muscle quality.173 These evidences showed gut microbiota are tightly related to lipid metabolism in skeletal muscle and could regulate the lipid deposition in the muscle of pigs and mice. However, little studies have targeted the human microbiome associated with fat infiltration in skeletal muscle.
Concluding remarks
Summarizing the previously presented data, we concluded the triggering factors such as aging, diseases, muscle injury, etc. and we detailed the cellular origin of muscle fat infiltration is at the beginning of being understood. We also discussed regulatory mechanisms, detection methods, and interventions related to fat infiltration in skeletal muscle, including exercise, several nutritional interventions, muscle-adipose crosstalk, and gut microbiota. Full understanding of affected factors and the cellular origins of fat infiltration may prove useful for the building up of skeletal muscle, for regeneration of injured muscle, and for ultimately improving human health. Regulating genes in farmed animals, persistent exercise, and several nutritional interventions may be a great and safe way to alleviate fat infiltration in skeletal muscle and to treat muscle-related diseases. Besides, we could apply these strategies to increase the IMF content in skeletal muscle and to improve meat quality in animal production. However, there are still some questions that need to be further addressed: (1) In addition to the aforementioned cell types, we need to find out whether other cell types are involved in fat infiltration and how the cellular origins of fat infiltration could be better investigated and understood. (2) Influential triggering factors affect fat infiltration in human and animal models, but how these factors are interconnected and interact through molecular mechanisms need to be further studied. (3) The interactions between key genes, regulators, and signaling pathways regulating fat infiltration in skeletal muscle need to be further explored. (4) The effects of some important nutrients, such as CLA, linseed, and vitamin A, on fat infiltration have been mainly studied in animal models or in vitro studies so far, but their effects on fat infiltration in skeletal muscle in humans or specifically in individuals with skeletal muscle-related diseases are largely unknown. (5) How to apply exercise and nutrients safely and efficiently to alleviate fat infiltration in skeletal muscle, and treat muscle-related diseases in humans need to be further studied. In a word, our current contribution summarized and discussed cellular origins, influential triggering factors, regulatory mechanisms, detection methods, and intervention strategies relating to fat infiltration in skeletal muscle and we provided useful information for regulating function and lipid metabolism of skeletal muscle, further treating muscle-related diseases by using nutritional interventions.
Acknowledgments
This work was partially supported by the Natural Science Foundation of Zhejiang Province (LZ22C170003) and the “Hundred Talents Program” funding from Zhejiang University to T.S. and we thank the members of the Shan laboratory for their comments.
Author contributions
L.W.: conceptualization, methodology, investigation, and writing – Ooiginal draft preparation. T.G.V.: writing – reviewing and editing. T.S.: conceptualization, funding acquisition, supervision, validation, and writing – reviewing and editing.
Declaration of interests
The authors declare no conflict of interest.
References
- 1.Bentzinger C.F., Wang Y.X., Rudnicki M.A. Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zammit P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Gu X., Wang L., Liu S., Shan T. Adipose tissue adipokines and lipokines: Functions and regulatory mechanism in skeletal muscle development and homeostasis. Metabolism. 2023;139 doi: 10.1016/j.metabol.2022.155379. [DOI] [PubMed] [Google Scholar]
- 4.Kuang S., Gillespie M.A., Rudnicki M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Sciorati C., Clementi E., Manfredi A.A., Rovere-Querini P. Fat deposition and accumulation in the damaged and inflamed skeletal muscle: cellular and molecular players. Cell. Mol. Life Sci. 2015;72:2135–2156. doi: 10.1007/s00018-015-1857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn H., Kim D.W., Ko Y., Ha J., Shin Y.B., Lee J., Sung Y.S., Kim K.W. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res. Rev. 2021;70 doi: 10.1016/j.arr.2021.101398. [DOI] [PubMed] [Google Scholar]
- 7.Al Saedi A., Debruin D.A., Hayes A., Hamrick M. Lipid metabolism in sarcopenia. Bone. 2022;164 doi: 10.1016/j.bone.2022.116539. [DOI] [PubMed] [Google Scholar]
- 8.Zadoorian A., Du X., Yang H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023;19:443–459. doi: 10.1038/s41574-023-00845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yue F., Oprescu S.N., Qiu J., Gu L., Zhang L., Chen J., Narayanan N., Deng M., Kuang S. Lipid droplet dynamics regulate adult muscle stem cell fate. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2021.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M.Y., Ruts E., Kim J., Janumala I., Heymsfield S., Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am. J. Clin. Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 11.Li C.W., Yu K., Shyh-Chang N., Jiang Z., Liu T., Ma S., Luo L., Guang L., Liang K., Ma W., et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J. Cachexia Sarcopenia Muscle. 2022;13:781–794. doi: 10.1002/jcsm.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biferali B., Bianconi V., Perez D.F., Kronawitter S.P., Marullo F., Maggio R., Santini T., Polverino F., Biagioni S., Summa V., et al. Prdm16-mediated H3K9 methylation controls fibro-adipogenic progenitors identity during skeletal muscle repair. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wosczyna M.N., Perez Carbajal E.E., Wagner M.W., Paredes S., Konishi C.T., Liu L., Wang T.T., Walsh R.A., Gan Q., Morrissey C.S., Rando T.A. Targeting microRNA-mediated gene repression limits adipogenic conversion of skeletal muscle mesenchymal stromal cells. Cell Stem Cell. 2021;28:1323–1334.e8. doi: 10.1016/j.stem.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tieland M., Trouwborst I., Clark B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ubaida-Mohien C., Lyashkov A., Gonzalez-Freire M., Tharakan R., Shardell M., Moaddel R., Semba R.D., Chia C.W., Gorospe M., Sen R., Ferrucci L. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife. 2019;8 doi: 10.7554/eLife.49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miljkovic I., Kuipers A.L., Cauley J.A., Prasad T., Lee C.G., Ensrud K.E., Cawthon P.M., Hoffman A.R., Dam T.T., Gordon C.L., et al. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1133–1140. doi: 10.1093/gerona/glv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus R.L., Addison O., Kidde J.P., Dibble L.E., Lastayo P.C. Skeletal muscle fat infiltration: Impact of age, inactivity, and exercise. J. Nutr. Health Aging. 2010;14:362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser M., Goodpaster B.H., Kritchevsky S.B., Newman A.B., Nevitt M., Rubin S.M., Simonsick E.M., Harris T.B. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 19.Perez K., Ciotlos S., McGirr J., Limbad C., Doi R., Nederveen J.P., Nilsson M.I., Winer D.A., Evans W., Tarnopolsky M., et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging (Albany N. Y.) 2022;14:9393–9422. doi: 10.18632/aging.204435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing Y., Zuo Y., Yu Y., Sun L., Yu Z., Ma S., Zhao Q., Sun G., Hu H., Li J., et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell. 2023;14:497–512. doi: 10.1093/procel/pwac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster B.H., Bergman B.C., Brennan A.M., Sparks L.M. Intermuscular adipose tissue in metabolic disease. Nat. Rev. Endocrinol. 2023;19:285–298. doi: 10.1038/s41574-022-00784-2. [DOI] [PubMed] [Google Scholar]
- 23.Waddell T., Bagur A., Cunha D., Thomaides-Brears H., Banerjee R., Cuthbertson D.J., Brown E., Cusi K., Després J.P., Brady M. Greater ectopic fat deposition and liver fibroinflammation and lower skeletal muscle mass in people with type 2 diabetes. Obesity. 2022;30:1231–1238. doi: 10.1002/oby.23425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miljkovic I., Cauley J.A., Petit M.A., Ensrud K.E., Strotmeyer E., Sheu Y., Gordon C.L., Goodpaster B.H., Bunker C.H., Patrick A.L., et al. Greater Adipose Tissue Infiltration in Skeletal Muscle among Older Men of African Ancestry. J. Clin. Endocrinol. Metab. 2009;94:2735–2742. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodpaster B.H., Thaete F.L., Kelley D.E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. J. Clin. Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 26.Ravussin E., Smith S.R. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 27.Miljkovic I., Kuipers A.L., Cvejkus R., Bunker C.H., Patrick A.L., Gordon C.L., Zmuda J.M. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity. 2016;24:476–482. doi: 10.1002/oby.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoico E., Corzato F., Bambace C., Rossi A.P., Micciolo R., Cinti S., Harris T.B., Zamboni M. Myosteatosis and myofibrosis: Relationship with aging, inflammation and insulin resistance. Arch. Gerontol. Geriatr. 2013;57:411–416. doi: 10.1016/j.archger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H., Ballantyne C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Invest. 2017;127:43–54. doi: 10.1172/JCI88880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Väremo L., Henriksen T.I., Scheele C., Broholm C., Pedersen M., Uhlén M., Pedersen B.K., Nielsen J. Type 2 diabetes and obesity induce similar transcriptional reprogramming in human myocytes. Genome Med. 2017;9 doi: 10.1186/s13073-017-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrood P., Hollingsworth K.G., Eagle M., Aribisala B.S., Birchall D., Bushby K., Straub V. MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J. Magn. Reson. Imaging. 2009;30:1130–1138. doi: 10.1002/jmri.21941. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.K., Merrow A.C., Shiraj S., Wong B.L., Horn P.S., Laor T. Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr. Radiol. 2013;43:1327–1335. doi: 10.1007/s00247-013-2696-z. [DOI] [PubMed] [Google Scholar]
- 33.Barnard A.M., Lott D.J., Batra A., Triplett W.T., Willcocks R.J., Forbes S.C., Rooney W.D., Daniels M.J., Smith B.K., Vandenborne K., Walter G.A. Characterizing Expiratory Respiratory Muscle Degeneration in Duchenne Muscular Dystrophy Using MRI. Chest. 2022;161:753–763. doi: 10.1016/j.chest.2021.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goubert D., Van Oosterwijck J., Meeus M., Danneels L. Structural Changes of Lumbar Muscles in Non-Specific Low Back Pain. Pain Physician. 2016;19:E985–E999. [PubMed] [Google Scholar]
- 35.Casey P., Ang Y., Sultan J. COVID-19-induced sarcopenia and physical deconditioning may require reassessment of surgical risk for patients with cancer. World J. Surg. Oncol. 2021;19:8. doi: 10.1186/s12957-020-02117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osuna-Padilla I.A., Rodríguez-Moguel N.C., Rodríguez-Llamazares S., Orsso C.E., Prado C.M., Ríos-Ayala M.A., Villanueva-Camacho O., Aguilar-Vargas A., Pensado-Piedra L.E., Juárez-Hernández F., Hernández-Cárdenas C.M. Low muscle mass in COVID-19 critically-ill patients: Prognostic significance and surrogate markers for assessment. Clin. Nutr. 2022;41:2910–2917. doi: 10.1016/j.clnu.2022.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi X., Liu H., Zhu L., Wang D., Xie F., Shi L., Mei J., Jiang X., Zeng Q., Hu P., et al. Myosteatosis predicting risk of transition to severe COVID-19 infection. Clin. Nutr. 2022;41:3007–3015. doi: 10.1016/j.clnu.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natsag J., Erlandson K.M., Sellmeyer D.E., Haberlen S.A., Margolick J., Jacobson L.P., Palella F.J., Koletar S.L., Lake J.E., Post W.S., Brown T.T. HIV Infection Is Associated with Increased Fatty Infiltration of the Thigh Muscle with Aging Independent of Fat Distribution. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoja S.S., Moore C.G., Goodpaster B.H., Delitto A., Piva S.R. Skeletal Muscle Fat and Its Association With Physical Function in Rheumatoid Arthritis. Arthritis Care Res. 2018;70:333–342. doi: 10.1002/acr.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedroso M.G., de Almeida A.C., Aily J.B., de Noronha M., Mattiello S.M. Fatty infiltration in the thigh muscles in knee osteoarthritis: a systematic review and meta-analysis. Rheumatol. Int. 2019;39:627–635. doi: 10.1007/s00296-019-04271-2. [DOI] [PubMed] [Google Scholar]
- 41.Nachit M., Kwanten W.J., Thissen J.P., Op De Beeck B., Van Gaal L., Vonghia L., Verrijken A., Driessen A., Horsmans Y., Francque S., Leclercq I.A. Muscle fat content is strongly associated with NASH: A longitudinal study in patients with morbid obesity. J. Hepatol. 2021;75:292–301. doi: 10.1016/j.jhep.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 42.Kaibori M., Ishizaki M., Iida H., Matsui K., Sakaguchi T., Inoue K., Mizuta T., Ide Y., Iwasaka J., Kimura Y., et al. Effect of Intramuscular Adipose Tissue Content on Prognosis in Patients Undergoing Hepatocellular Carcinoma Resection. J. Gastrointest. Surg. 2015;19:1315–1323. doi: 10.1007/s11605-015-2838-8. [DOI] [PubMed] [Google Scholar]
- 43.Robles P.G., Sussman M.S., Naraghi A., Brooks D., Goldstein R.S., White L.M., Mathur S. Intramuscular Fat Infiltration Contributes to Impaired Muscle Function in COPD. Med. Sci. Sports Exerc. 2015;47:1334–1341. doi: 10.1249/MSS.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 44.Martin M., Almeras N., Després J.P., Coxson H.O., Washko G.R., Vivodtzev I., Wouters E.F., Rutten E., Williams M.C., Murchison J.T., et al. Ectopic fat accumulation in patients with COPD: an ECLIPSE substudy. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:451–460. doi: 10.2147/COPD.S124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M.S., Moon S.H., Kim T.H., Oh J., Lee S.J., Chang H.G., Shin J.H. Paraspinal Muscles of Patients with Lumbar Diseases. J Neurol Surg Part A. 2018;79:323–329. doi: 10.1055/s-0038-1639332. [DOI] [PubMed] [Google Scholar]
- 46.Avesani C.M., de Abreu A.M., Ribeiro H.S., Brismar T.B., Stenvinkel P., Sabatino A., Lindholm B. Muscle fat infiltration in chronic kidney disease: a marker related to muscle quality, muscle strength and sarcopenia. J. Nephrol. 2023;36:895–910. doi: 10.1007/s40620-022-01553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebadi M., Tsien C., Bhanji R.A., Dunichand-Hoedl A.R., Rider E., Motamedrad M., Mazurak V.C., Baracos V., Montano-Loza A.J. Skeletal Muscle Pathological Fat Infiltration (Myosteatosis) Is Associated with Higher Mortality in Patients with Cirrhosis. Cells. 2022;11 doi: 10.3390/cells11081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sastourné-Arrey Q., Mathieu M., Contreras X., Monferran S., Bourlier V., Gil-Ortega M., Murphy E., Laurens C., Varin A., Guissard C., et al. Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury. Nat. Commun. 2023;14 doi: 10.1038/s41467-022-35524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elliott J.M., Smith A.C., Hoggarth M.A., Albin S.R., Weber K.A., Haager M., Fundaun J., Wasielewski M., Courtney D.M., Parrish T.B. Muscle fat infiltration following whiplash: A computed tomography and magnetic resonance imaging comparison. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott R., Peolsson A., West J., Elliott J.M., Åslund U., Karlsson A., Leinhard O.D. The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J. 2018;18:717–725. doi: 10.1016/j.spinee.2017.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karlsson A., Leinhard O.D., Åslund U., West J., Romu T., Smedby Ö., Zsigmond P., Peolsson A., Peolsson A. An Investigation of Fat Infiltration of the Multifidus Muscle in Patients With Severe Neck Symptoms Associated With Chronic Whiplash-Associated Disorder. J. Orthop. Sports Phys. Ther. 2016;46:886–893. doi: 10.2519/jospt.2016.6553. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson A., Peolsson A., Elliott J., Romu T., Ljunggren H., Borga M., Dahlqvist Leinhard O. The relation between local and distal muscle fat infiltration in chronic whiplash using magnetic resonance imaging. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elliott J., Sterling M., Noteboom J.T., Treleaven J., Galloway G., Jull G. The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates in the cervical extensor musculature: a preliminary investigation. Eur. Spine J. 2009;18:1371–1378. doi: 10.1007/s00586-009-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owers D.S., Perriman D.M., Smith P.N., Neeman T., Webb A.L. Evidence for cervical muscle morphometric changes on magnetic resonance images after whiplash: A systematic review and meta-analysis. Injury. 2018;49:165–176. doi: 10.1016/j.injury.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Moore C.D., Craven B.C., Thabane L., Laing A.C., Frank-Wilson A.W., Kontulainen S.A., Papaioannou A., Adachi J.D., Giangregorio L.M. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal Interact. 2015;15:32–41. [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Z., You W., Chen W., Zhou Y., Nong Q., Valencak T.G., Wang Y., Shan T. Single-cell RNA sequencing and lipidomics reveal cell and lipid dynamics of fat infiltration in skeletal muscle. J. Cachexia Sarcopenia Muscle. 2021;12:109–129. doi: 10.1002/jcsm.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mogi M., Kohara K., Nakaoka H., Kan-No H., Tsukuda K., Wang X.L., Chisaka T., Bai H.Y., Shan B.S., Kukida M., et al. Diabetic mice exhibited a peculiar alteration in body composition with exaggerated ectopic fat deposition after muscle injury due to anomalous cell differentiation. J. Cachexia Sarcopenia Muscle. 2016;7:213–224. doi: 10.1002/jcsm.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pagano A.F., Brioche T., Arc-Chagnaud C., Demangel R., Chopard A., Py G. Short-term disuse promotes fatty acid infiltration into skeletal muscle. J. Cachexia Sarcopenia Muscle. 2018;9:335–347. doi: 10.1002/jcsm.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pezolato A., de Vasconcelos E.E., Defino H.L.A., Nogueira-Barbosa M.H. Fat infiltration in the lumbar multifidus and erector spinae muscles in subjects with sway-back posture. Eur. Spine J. 2012;21:2158–2164. doi: 10.1007/s00586-012-2286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takano Y., Kobayashi H., Yuri T., Yoshida S., Naito A., Kiyoshige Y. Fat infiltration in the gluteus minimus muscle in older adults. Clin. Interv. Aging. 2018;13:1011–1017. doi: 10.2147/Cia.S157402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cordani N., Pisa V., Pozzi L., Sciorati C., Clementi E. Nitric Oxide Controls Fat Deposition in Dystrophic Skeletal Muscle by Regulating Fibro-Adipogenic Precursor Differentiation. Stem Cell. 2014;32:874–885. doi: 10.1002/stem.1587. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Cui C., Chim Y.N., Yao H., Shi L., Xu J., Wang J., Wong R.M.Y., Leung K.S., Chow S.K.H., Cheung W.H. Vibration and beta-hydroxy-beta-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J. Cachexia Sarcopenia Muscle. 2020;11:564–577. doi: 10.1002/jcsm.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W., Xu Z., You W., Zhou Y., Wang L., Huang Y., Shan T. Cold exposure alters lipid metabolism of skeletal muscle through HIF-1alpha-induced mitophagy. BMC Biol. 2023;21:27. doi: 10.1186/s12915-023-01514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Z., Chen W., Wang L., Zhou Y., Nong Q., Valencak T.G., Wang Y., Xie J., Shan T. Cold Exposure Affects Lipid Metabolism, Fatty Acids Composition and Transcription in Pig Skeletal Muscle. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.748801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L., Zhao X., Liu S., You W., Huang Y., Zhou Y., Chen W., Zhang S., Wang J., Zheng Q., et al. Single-nucleus and bulk RNA sequencing reveal cellular and transcriptional mechanisms underlying lipid dynamics in high marbled pork. NPJ Sci. Food. 2023;7:23. doi: 10.1038/s41538-023-00203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.You W., Liu S., Ji J., Ling D., Tu Y., Zhou Y., Chen W., Valencak T.G., Wang Y., Shan T. Growth arrest and DNA damage-inducible alpha regulates muscle repair and fat infiltration through ATP synthase F1 subunit alpha. J Cachexia Sarcopeni. 2023;14:326–341. doi: 10.1002/jcsm.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L., Shan T. Factors inducing transdifferentiation of myoblasts into adipocytes. J. Cell. Physiol. 2021;236:2276–2289. doi: 10.1002/jcp.30074. [DOI] [PubMed] [Google Scholar]
- 69.Chen J.C.J., Mortimer J., Marley J., Goldhamer D.J. MyoD-cre transgenic mice: a model for conditional mutagenesis and lineage tracing of skeletal muscle. Genesis. 2005;41:116–121. doi: 10.1002/gene.20104. [DOI] [PubMed] [Google Scholar]
- 70.Liu W., Liu Y., Lai X., Kuang S. Intramuscular adipose is derived from a non-Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev. Biol. 2012;361:27–38. doi: 10.1016/j.ydbio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vettor R., Milan G., Franzin C., Sanna M., De Coppi P., Rizzuto R., Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Endocrinol. Metab. 2009;297:E987–E998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 72.Yin H., Pasut A., Soleimani V.D., Bentzinger C.F., Antoun G., Thorn S., Seale P., Fernando P., van Ijcken W., Grosveld F., et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.da Silva Meirelles L., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 74.Uezumi A., Fukada S.i., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 75.Uezumi A., Fukada S., Yamamoto N., Ikemoto-Uezumi M., Nakatani M., Morita M., Yamaguchi A., Yamada H., Nishino I., Hamada Y., Tsuchida K. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen W., You W., Valencak T.G., Shan T. Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Res. Rev. 2022;80 doi: 10.1016/j.arr.2022.101682. [DOI] [PubMed] [Google Scholar]
- 77.Vallecillo-Garcia P., Orgeur M., Vom Hofe-Schneider S., Stumm J., Kappert V., Ibrahim D.M., Borno S.T., Hayashi S., Relaix F., Hildebrandt K., et al. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nat. Commun. 2017;8:1218. doi: 10.1038/s41467-017-01120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stumm J., Vallecillo-García P., Vom Hofe-Schneider S., Ollitrault D., Schrewe H., Economides A.N., Marazzi G., Sassoon D.A., Stricker S. Odd skipped-related 1 (Osr1) identifies muscle-interstitial fibro-adipogenic progenitors (FAPs) activated by acute injury. Stem Cell Res. 2018;32:8–16. doi: 10.1016/j.scr.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., Yamaguchi M., Ogawa R., Matev M.M., Miyagoe-Suzuki Y., et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 80.Fitzgerald G., Turiel G., Gorski T., Soro-Arnaiz I., Zhang J., Casartelli N.C., Masschelein E., Maffiuletti N.A., Sutter R., Leunig M., et al. MME(+) fibro-adipogenic progenitors are the dominant adipogenic population during fatty infiltration in human skeletal muscle. Commun. Biol. 2023;6:111. doi: 10.1038/s42003-023-04504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agley C.C., Rowlerson A.M., Velloso C.P., Lazarus N.R., Harridge S.D.R. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J. Cell Sci. 2013;126:5610–5625. doi: 10.1242/jcs.132563. [DOI] [PubMed] [Google Scholar]
- 82.Lang I., Schweizer A., Hiden U., Ghaffari-Tabrizi N., Hagendorfer G., Bilban M., Pabst M.A., Korgun E.T., Dohr G., Desoye G. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation. 2008;76:1031–1043. doi: 10.1111/j.1432-0436.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- 83.Haynes B.A., Huyck R.W., James A.J., Carter M.E., Gaafar O.U., Day M., Pinto A., Dobrian A.D. Isolation, Expansion, and Adipogenic Induction of CD34+CD31+ Endothelial Cells from Human Omental and Subcutaneous Adipose Tissue. J. Vis. Exp. 2018:57804. doi: 10.3791/57804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tamaki T., Akatsuka A., Ando K., Nakamura Y., Matsuzawa H., Hotta T., Roy R.R., Edgerton V.R. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uezumi A., Ojima K., Fukada S.i., Ikemoto M., Masuda S., Miyagoe-Suzuki Y., Takeda S. Functional heterogeneity of side population cells in skeletal muscle. Biochem. Biophys. Res. Commun. 2006;341:864–873. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 86.Farrington-Rock C., Crofts N.J., Doherty M.J., Ashton B.A., Griffin-Jones C., Canfield A.E. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 87.Roobrouck V.D., Clavel C., Jacobs S.A., Ulloa-Montoya F., Crippa S., Sohni A., Roberts S.J., Luyten F.P., Van Gool S.W., Sampaolesi M., et al. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cell. 2011;29:871–882. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- 88.Mitchell K.J., Pannérec A., Cadot B., Parlakian A., Besson V., Gomes E.R., Marazzi G., Sassoon D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 89.Burton M.A., Antoun E., Garratt E.S., Westbury L., Baczynska A., Dennison E.M., Harvey N.C., Cooper C., Patel H.P., Godfrey K.M., et al. Adiposity is associated with widespread transcriptional changes and downregulation of longevity pathways in aged skeletal muscle. J Cachexia Sarcopeni. 2023;14:1762–1774. doi: 10.1002/jcsm.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bijland S., Mancini S.J., Salt I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013;124:491–507. doi: 10.1042/Cs20120536. [DOI] [PubMed] [Google Scholar]
- 91.Zong H., Ren J.M., Young L.H., Pypaert M., Mu J., Birnbaum M.J., Shulman G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shan T., Zhang P., Bi P., Kuang S. Lkb1 deletion promotes ectopic lipid accumulation in muscle progenitor cells and mature muscles. J. Cell. Physiol. 2015;230:1033–1041. doi: 10.1002/jcp.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilde J.M., Gumucio J.P., Grekin J.A., Sarver D.C., Noah A.C., Ruehlmann D.G., Davis M.E., Bedi A., Mendias C.L. Inhibition of p38 mitogen-activated protein kinase signaling reduces fibrosis and lipid accumulation after rotator cuff repair. J. Shoulder Elbow Surg. 2016;25:1501–1508. doi: 10.1016/j.jse.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu W., Zhang J., Zhao C., Sun Y., Pang W., Yang G. CTRP6 Regulates Porcine Adipocyte Proliferation and Differentiation by the AdipoR1/MAPK Signaling Pathway. J. Agric. Food Chem. 2017;65:5512–5522. doi: 10.1021/acs.jafc.7b00594. [DOI] [PubMed] [Google Scholar]
- 95.Wang G.Q., Zhu L., Ma M.L., Chen X.C., Gao Y., Yu T.Y., Yang G.S., Pang W.J. Mulberry 1-Deoxynojirimycin Inhibits Adipogenesis by Repression of the ERK/PPAR gamma Signaling Pathway in Porcine Intramuscular Adipocytes. J. Agric. Food Chem. 2015;63:6212–6220. doi: 10.1021/acs.jafc.5b01680. [DOI] [PubMed] [Google Scholar]
- 96.Reggio A., Rosina M., Palma A., Cerquone Perpetuini A., Petrilli L.L., Gargioli C., Fuoco C., Micarelli E., Giuliani G., Cerretani M., et al. Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/beta-catenin axis. Cell Death Differ. 2020;27:2921–2941. doi: 10.1038/s41418-020-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wagatsuma A. Adipogenic potential can be activated during muscle regeneration. Mol. Cell. Biochem. 2007;304:25–33. doi: 10.1007/s11010-007-9482-x. [DOI] [PubMed] [Google Scholar]
- 98.Fu C., Chin-Young B., Park G., Guzmán-Seda M., Laudier D., Han W.M. WNT7A suppresses adipogenesis of skeletal muscle mesenchymal stem cells and fatty infiltration through the alternative Wnt-Rho-YAP/TAZ signaling axis. Stem Cell Rep. 2023;18:999–1014. doi: 10.1016/j.stemcr.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruno N.E., Kelly K.A., Hawkins R., Bramah-Lawani M., Amelio A.L., Nwachukwu J.C., Nettles K.W., Conkright M.D. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. EMBO J. 2014;33:1027–1043. doi: 10.1002/embj.201386145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ho J.N., Kim O.K., Nam D.E., Jun W., Lee J. Pycnogenol supplementation promotes lipolysis via activation of cAMP-dependent PKA in ob/ob mice and primary-cultured adipocytes. J. Nutr. Sci. Vitaminol. 2014;60:429–435. doi: 10.3177/jnsv.60.429. [DOI] [PubMed] [Google Scholar]
- 101.Liu J., Wang L., Chen W., Li J., Shan T. CRTC3 Regulates the Lipid Metabolism and Adipogenic Differentiation of Porcine Intramuscular and Subcutaneous Adipocytes by Activating the Calcium Pathway. J. Agric. Food Chem. 2021;69:7243–7255. doi: 10.1021/acs.jafc.1c02021. [DOI] [PubMed] [Google Scholar]
- 102.Suh J.M., Gao X., McKay J., McKay R., Salo Z., Graff J.M. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3:25–34. doi: 10.1016/j.cmet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 103.Kopinke D., Roberson E.C., Reiter J.F. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell. 2017;170:340–351.e12. doi: 10.1016/j.cell.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGregor R.A., Poppitt S.D., Cameron-Smith D. Role of microRNAs in the age-related changes in skeletal muscle and diet or exercise interventions to promote healthy aging in humans. Ageing Res. Rev. 2014;17:25–33. doi: 10.1016/j.arr.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Sun Y.M., Qin J., Liu S.G., Cai R., Chen X.C., Wang X.M., Pang W.J. PDGFR alpha Regulated by miR-34a and FoxO1 Promotes Adipogenesis in Porcine Intramuscular Preadipocytes through Erk Signaling Pathway. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18112424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mathes S., Fahrner A., Ghoshdastider U., Rüdiger H.A., Leunig M., Wolfrum C., Krützfeldt J. FGF-2-dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2021013118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Squillaro T., Peluso G., Galderisi U., Di Bernardo G. Long non-coding RNAs in regulation of adipogenesis and adipose tissue function. Elife. 2020;9 doi: 10.7554/eLife.59053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang M., Li F., Sun J.W., Li D.H., Li W.T., Jiang R.R., Li Z.J., Liu X.J., Han R.L., Li G.X., et al. LncRNA IMFNCR Promotes Intramuscular Adipocyte Differentiation by Sponging miR-128-3p and miR-27b-3p. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L., Zhou Z.Y., Zhang T., Zhang L., Hou X., Yan H., Wang L. IRLnc: a novel functional noncoding RNA contributes to intramuscular fat deposition. BMC Genom. 2021;22:95. doi: 10.1186/s12864-020-07349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim K.H., Jia Z., Snyder M., Chen J., Qiu J., Oprescu S.N., Chen X., Syed S.A., Yue F., Roseguini B.T., et al. PRMT5 links lipid metabolism to contractile function of skeletal muscles. EMBO Rep. 2023;24 doi: 10.15252/embr.202357306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Correa-de-Araujo R., Addison O., Miljkovic I., Goodpaster B.H., Bergman B.C., Clark R.V., Elena J.W., Esser K.A., Ferrucci L., Harris-Love M.O., et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020;11:963. doi: 10.3389/fphys.2020.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erlandson M.C., Lorbergs A.L., Mathur S., Cheung A.M. Muscle analysis using pQCT, DXA and MRI. Eur. J. Radiol. 2016;85:1505–1511. doi: 10.1016/j.ejrad.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Nijholt W., Scafoglieri A., Jager-Wittenaar H., Hobbelen J.S.M., van der Schans C.P. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J. Cachexia Sarcopenia Muscle. 2017;8:702–712. doi: 10.1002/jcsm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamrick M.W., McGee-Lawrence M.E., Frechette D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016;7 doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ikenaga M., Yamada Y., Kose Y., Morimura K., Higaki Y., Kiyonaga A., Tanaka H., Nakagawa Study Group Effects of a 12-week, short-interval, intermittent, low-intensity, slow-jogging program on skeletal muscle, fat infiltration, and fitness in older adults: randomized controlled trial. Eur. J. Appl. Physiol. 2017;117:7–15. doi: 10.1007/s00421-016-3493-9. [DOI] [PubMed] [Google Scholar]
- 116.Ryan A.S., Harduarsingh-Permaul A.S. Effects of weight loss and exercise on trunk muscle composition in older women. Clin. Interv. Aging. 2014;9:395–402. doi: 10.2147/CIA.S56662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ghasemikaram M., Chaudry O., Nagel A.M., Uder M., Jakob F., Kemmler W., Kohl M., Engelke K. Effects of 16 months of high intensity resistance training on thigh muscle fat infiltration in elderly men with osteosarcopenia. Geroscience. 2021;43:607–617. doi: 10.1007/s11357-020-00316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farr J.N., Van Loan M.D., Lohman T.G., Going S.B. Lower Physical Activity is Associated with Fat Infiltration within Skeletal Muscle in Young Girls. Med. Sci. Sports Exerc. 2011;43:443. doi: 10.1249/MSS.0b013e31824749b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goodpaster B.H., Chomentowski P., Ward B.K., Rossi A., Glynn N.W., Delmonico M.J., Kritchevsky S.B., Pahor M., Newman A.B. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J. Appl. Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marcus R.L., Addison O., Kidde J.P., Dibble L.E., Lastayo P.C. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J. Nutr. Health Aging. 2010;14:362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jacobs J.L., Marcus R.L., Morrell G., LaStayo P. Resistance exercise with older fallers: its impact on intermuscular adipose tissue. Biomed Res. Int. 2014. 2014;2014 doi: 10.1155/2014/398960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Z., Cui M., Yu K., Zhang X.W., Li C.W., Nie X.D., Wang F. Effects of nutrition supplementation and physical exercise on muscle mass, muscle strength and fat mass among sarcopenic elderly: a randomized controlled trial. Appl Physiol Nutr Me. 2021;46:494–500. doi: 10.1139/apnm-2020-0643. [DOI] [PubMed] [Google Scholar]
- 123.Honaga K., Mori N., Akimoto T., Tsujikawa M., Kawakami M., Okamoto T., Sakata Y., Hamano H., Takeda Y., Kondo K. Investigation of the Effect of Nutritional Supplementation with Whey Protein and Vitamin D on Muscle Mass and Muscle Quality in Subacute Post-Stroke Rehabilitation Patients: A Randomized, Single-Blinded, Placebo-Controlled Trial. Nutrients. 2022;14 doi: 10.3390/nu14030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ayuso M., Óvilo C., Rodríguez-Bertos A., Rey A.I., Daza A., Fenández A., González-Bulnes A., López-Bote C.J., Isabel B. Dietary vitamin A restriction affects adipocyte differentiation and fatty acid composition of intramuscular fat in Iberian pigs. Meat Sci. 2015;108:9–16. doi: 10.1016/j.meatsci.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 125.Chang H., Gan W., Liao X., Wei J., Lu M., Chen H., Wang S., Ma Y., Wu Q., Yu Y., Liu X. Conjugated linoleic acid supplements preserve muscle in high-body-fat adults: A double-blind, randomized, placebo trial. Nutr Metab Cardiovas. 2020;30:1777–1784. doi: 10.1016/j.numecd.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 126.Terasawa N., Okamoto K., Nakada K., Masuda K. Effect of Conjugated Linoleic Acid Intake on Endurance Exercise Performance and Anti-fatigue in Student Athletes. J. Oleo Sci. 2017;66:723–733. doi: 10.5650/jos.ess17053. [DOI] [PubMed] [Google Scholar]
- 127.Pinkoski C., Chilibeck P.D., Candow D.G., Esliger D., Ewaschuk J.B., Facci M., Farthing J.P., Zello G.A. The effects of conjugated linoleic acid supplementation during resistance training. Med. Sci. Sports Exerc. 2006;38:339–348. doi: 10.1249/01.mss.0000183860.42853.15. [DOI] [PubMed] [Google Scholar]
- 128.Tarnopolsky M., Zimmer A., Paikin J., Safdar A., Aboud A., Pearce E., Roy B., Doherty T. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007;2:e991. doi: 10.1371/journal.pone.0000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Vliet S., Fappi A., Reeds D.N., Mittendorfer B. No independent or combined effects of vitamin D and conjugated linoleic acids on muscle protein synthesis in older adults: a randomized, double-blind, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2020;112:1382–1389. doi: 10.1093/ajcn/nqaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barone R., Sangiorgi C., Marino Gammazza A., D'Amico D., Salerno M., Cappello F., Pomara C., Zummo G., Farina F., Di Felice V., Macaluso F. Effects of Conjugated Linoleic Acid Associated With Endurance Exercise on Muscle Fibres and Peroxisome Proliferator-Activated Receptor gamma Coactivator 1 alpha Isoforms. J. Cell. Physiol. 2017;232:1086–1094. doi: 10.1002/jcp.25511. [DOI] [PubMed] [Google Scholar]
- 131.Wang L., Zhang S., Huang Y., You W., Zhou Y., Chen W., Sun Y., Yi W., Sun H., Xie J., et al. CLA improves the lipo-nutritional quality of pork and regulates the gut microbiota in Heigai pigs. Food Funct. 2022;13:12093–12104. doi: 10.1039/d2fo02549c. [DOI] [PubMed] [Google Scholar]
- 132.Rezaei S., Sasani M.R., Akhlaghi M., Kohanmoo A. Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: a randomised double-blind controlled trial. Br. J. Nutr. 2020;123:994–1002. doi: 10.1017/S0007114520000318. [DOI] [PubMed] [Google Scholar]
- 133.Carotenuto F., Costa A., Albertini M.C., Rocchi M.B.L., Rudov A., Coletti D., Minieri M., Di Nardo P., Teodori L. Dietary Flaxseed Mitigates Impaired Skeletal Muscle Regeneration: in Vivo, in Vitro and in Silico Studies. Int. J. Med. Sci. 2016;13:206–219. doi: 10.7150/ijms.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo H.F., Wei H.K., Huang F.R., Zhou Z., Jiang S.W., Peng J. The Effect of Linseed on Intramuscular Fat Content and Adipogenesis Related Genes in Skeletal Muscle of Pigs. Lipids. 2009;44:999–1010. doi: 10.1007/s11745-009-3346-y. [DOI] [PubMed] [Google Scholar]
- 135.Shin Y., Lee D., Ahn J., Lee M., Shin S.S., Yoon M. The herbal extract ALS-L1023 from Melissa officinalis reduces weight gain, elevated glucose levels and beta-cell loss in Otsuka Long-Evans Tokushima fatty rats. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113360. [DOI] [PubMed] [Google Scholar]
- 136.De Stefanis D., Mastrocola R., Nigro D., Costelli P., Aragno M. Effects of chronic sugar consumption on lipid accumulation and autophagy in the skeletal muscle. Eur. J. Nutr. 2017;56:363–373. doi: 10.1007/s00394-015-1086-8. [DOI] [PubMed] [Google Scholar]
- 137.Wu W., Wang S., Xu Z., Wang X., Feng J., Shan T., Wang Y. Betaine promotes lipid accumulation in adipogenic-differentiated skeletal muscle cells through ERK/PPAR signalling pathway. Mol. Cell. Biochem. 2018;447:137–149. doi: 10.1007/s11010-018-3299-7. [DOI] [PubMed] [Google Scholar]
- 138.Watanabe K., Katagiri S., Takahashi H., Sasaki N., Maekawa S., Komazaki R., Hatasa M., Kitajima Y., Maruyama Y., Shiba T., et al. Porphyromonas gingivalis impairs glucose uptake in skeletal muscle associated with altering gut microbiota. FASEB J. 2021;35:e21171. doi: 10.1096/fj.202001158R. [DOI] [PubMed] [Google Scholar]
- 139.Chen L.H., Chang S.S., Chang H.Y., Wu C.H., Pan C.H., Chang C.C., Chan C.H., Huang H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle. 2022;13:515–531. doi: 10.1002/jcsm.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xie C., Teng J., Wang X., Xu B., Niu Y., Ma L., Yan X. Multi-omics analysis reveals gut microbiota-induced intramuscular fat deposition via regulating expression of lipogenesis-associated genes. Anim. Nutr. 2022;9:84–99. doi: 10.1016/j.aninu.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garcia M., Seelaender M., Sotiropoulos A., Coletti D., Lancha A.H., Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019;60:66–69. doi: 10.1016/j.nut.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 142.Montenegro K.R., Pufal M.A., Newsholme P. Vitamin D Supplementation and Impact on Skeletal Muscle Function in Cell and Animal Models and an Aging Population: What Do We Know So Far? Nutrients. 2021;13 doi: 10.3390/nu13041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Girgis C.M., Cha K.M., So B., Tsang M., Chen J., Houweling P.J., Schindeler A., Stokes R., Swarbrick M.M., Evesson F.J., et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle. 2019;10:1228–1240. doi: 10.1002/jcsm.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bislev L.S., Grove-Laugesen D., Rejnmark L. Vitamin D and Muscle Health: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials. J. Bone Miner. Res. 2021;36:1651–1660. doi: 10.1002/jbmr.4412. [DOI] [PubMed] [Google Scholar]
- 145.Gilsanz V., Kremer A., Mo A.O., Wren T.A.L., Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J. Clin. Endocrinol. Metab. 2010;95:1595–1601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Redzic M., Powell D.K., Thomas D.T. Vitamin D status is related to intramyocellular lipid in older adults. Endocrine. 2014;47:854–861. doi: 10.1007/s12020-014-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Almurdhi M.M., Reeves N.D., Bowling F.L., Boulton A.J.M., Jeziorska M., Malik R.A. Distal lower limb strength is reduced in subjects with impaired glucose tolerance and is related to elevated intramuscular fat level and vitamin D deficiency. Diabet. Med. 2017;34:356–363. doi: 10.1111/dme.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.El Haddad M., Notarnicola C., Evano B., El Khatib N., Blaquière M., Bonnieu A., Tajbakhsh S., Hugon G., Vernus B., Mercier J., Carnac G. Retinoic acid maintains human skeletal muscle progenitor cells in an immature state. Cell. Mol. Life Sci. 2017;74:1923–1936. doi: 10.1007/s00018-016-2445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He Y., Xu K., Li Y., Chang H., Liao X., Yu H., Tian T., Li C., Shen Y., Wu Q., et al. Metabolomic Changes Upon Conjugated Linoleic Acid Supplementation and Predictions of Body Composition Responsiveness. J. Clin. Endocrinol. Metab. 2022;107:2606–2615. doi: 10.1210/clinem/dgac367. [DOI] [PubMed] [Google Scholar]
- 150.Chen P.B., Yang J.S., Park Y. Adaptations of Skeletal Muscle Mitochondria to Obesity, Exercise, and Polyunsaturated Fatty Acids. Lipids. 2018;53:271–278. doi: 10.1002/lipd.12037. [DOI] [PubMed] [Google Scholar]
- 151.Wang L., Huang Y., Wang Y., Shan T. Effects of Polyunsaturated Fatty Acids Supplementation on the Meat Quality of Pigs: A Meta-Analysis. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.746765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Babajafari S., Hojhabrimanesh A., Sohrabi Z., Ayaz M., Noorafshan A., Akrami A. Comparing isolated soy protein with flaxseed oil vs isolated soy protein with corn oil and wheat flour with corn oil consumption on muscle catabolism, liver function, blood lipid, and sugar in burn patients: a randomized clinical trial. Trials. 2018;19:308. doi: 10.1186/s13063-018-2693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wei H., Zhou Y., Jiang S., Huang F., Peng J., Jiang S. Transcriptional response of porcine skeletal muscle to feeding a linseed-enriched diet to growing pigs. J. Anim. Sci. Biotechno. 2016;7 doi: 10.1186/s40104-016-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen W., Wang L., You W., Shan T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2021;236:2393–2412. doi: 10.1002/jcp.30033. [DOI] [PubMed] [Google Scholar]
- 155.Scheele C., Wolfrum C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020;41:53–65. doi: 10.1210/endrev/bnz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou X., Wang J.L., Lu J., Song Y., Kwak K.S., Jiao Q., Rosenfeld R., Chen Q., Boone T., Simonet W.S., et al. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 157.Kärst S., Strucken E.M., Schmitt A.O., Weyrich A., de Villena F.P.M., Yang H., Brockmann G.A. Effect of the myostatin locus on muscle mass and intramuscular fat content in a cross between mouse lines selected for hypermuscularity. BMC Genom. 2013;14:16. doi: 10.1186/1471-2164-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y., Liu X., Hou L., Wu W., Zhao S., Xiong Y. Fibroblast Growth Factor 21 Suppresses Adipogenesis in Pig Intramuscular Fat Cells. Int. J. Mol. Sci. 2015;17 doi: 10.3390/ijms17010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Q., Lin S., Li Q., Lin Y., Xiong Y., Zhu J., Wang Y. Fibroblast growth factor 21 regulates lipid accumulation and adipogenesis in goat intramuscular adipocyte. Anim. Biotechnol. 2021;32:318–326. doi: 10.1080/10495398.2019.1691010. [DOI] [PubMed] [Google Scholar]
- 160.Seldin M.M., Peterson J.M., Byerly M.S., Wei Z., Wong G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012;287:11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petro J.L., Fragozo-Ramos M.C., Milán A.F., Aristizabal J.C., Gallo-Villegas J.A., Calderón J.C. Serum Levels of Myonectin Are Lower in Adults with Metabolic Syndrome and Are Negatively Correlated with Android Fat Mass. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24086874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xie G., Wang Y., Xu Q., Hu M., Zhu J., Bai W., Lin Y. Knockdown of adiponectin promotes the adipogenesis of goat intramuscular preadipocytes. Anim. Biotechnol. 2022;33:408–416. doi: 10.1080/10495398.2020.1800484. [DOI] [PubMed] [Google Scholar]
- 163.Xu Q., Lin S., Wang Y., Zhu J., Lin Y. Fibroblast growth factor 10 (FGF10) promotes the adipogenesis of intramuscular preadipocytes in goat. Mol. Biol. Rep. 2018;45:1881–1888. doi: 10.1007/s11033-018-4334-1. [DOI] [PubMed] [Google Scholar]
- 164.Fu Y.Y., Chen K.L., Li H.X., Zhou G.H. The adipokine Chemerin induces lipolysis and adipogenesis in bovine intramuscular adipocytes. Mol. Cell. Biochem. 2016;418:39–48. doi: 10.1007/s11010-016-2731-0. [DOI] [PubMed] [Google Scholar]
- 165.Foryst-Ludwig A., Kreissl M.C., Benz V., Brix S., Smeir E., Ban Z., Januszewicz E., Salatzki J., Grune J., Schwanstecher A.K., et al. Adipose Tissue Lipolysis Promotes Exercise-induced Cardiac Hypertrophy Involving the Lipokine C16:1n7-Palmitoleate. J. Biol. Chem. 2015;290:23603–23615. doi: 10.1074/jbc.M115.645341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duckett S.K., Volpi-Lagreca G., Alende M., Long N.M. Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep. Diabetes Metab. Syndr. Obes. 2014;7:553–563. doi: 10.2147/DMSO.S72695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ticinesi A., Nouvenne A., Cerundolo N., Catania P., Prati B., Tana C., Meschi T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients. 2019;11 doi: 10.3390/nu11071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mancin L., Wu G.D., Paoli A. Gut microbiota-bile acid-skeletal muscle axis (vol 31, pg 254, 2023) Trends Microbiol. 2023;31:322. doi: 10.1016/j.tim.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 169.Manickam R., Duszka K., Wahli W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020;21:8056. doi: 10.3390/ijms21218056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu C., Cheung W.H., Li J., Chow S.K.H., Yu J., Wong S.H., Ip M., Sung J.J.Y., Wong R.M.Y. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopeni. 2021;12:1393–1407. doi: 10.1002/jcsm.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li G., Jin B., Fan Z. Mechanisms Involved in Gut Microbiota Regulation of Skeletal Muscle. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/2151191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Frampton J., Murphy K.G., Frost G., Chambers E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020;2:840–848. doi: 10.1038/s42255-020-0188-7. [DOI] [PubMed] [Google Scholar]
- 173.Chen F., Li Q., Chen Y., Wei Y., Liang J., Song Y., Shi L., Wang J., Mao L., Zhang B., Zhang Z. Association of the gut microbiota and fecal short-chain fatty acids with skeletal muscle mass and strength in children. FASEB J. 2022;36 doi: 10.1096/fj.202002697RRR. [DOI] [PubMed] [Google Scholar]