Abstract
Objective
In recent decades, there has been a notable increase in the morbidity and mortality rates linked to bacteremia and candidemia. This study aimed to investigate the clinical significance of inflammatory markers in assessing the disease severity in critically ill patients suffering from mixed-bloodstream infections (BSIs) due to Enterococcus spp. and Candida spp.
Methods
In this retrospective research, patients diagnosed with BSIs who were admitted to the intensive care unit (ICU) during the period of January 2019 to December 2022 were analyzed. The patients were divided into two groups: a mixed-pathogen BSI group with both Enterococcus spp. and Candida spp., and a single-pathogen BSI group with only Enterococcus spp. The study examined the differences in inflammatory marker levels and disease severity, including Acute Physiology and Chronic Health Evaluation (APACHE) II scores, duration of ICU stay, and 30-day mortality, between the two groups. Furthermore, we sought to scrutinize the potential associations among these aforementioned parameters.
Results
The neutrophil-to-lymphocyte ratios (NLRs) and levels of plasma C-reactive protein (CRP), interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) in the mixed-pathogen BSI group were higher than those in the single-pathogen BSI group. Spearman's rank correlation analysis showed that NLRs and plasma CRP and IL-6 levels were positively correlated with disease severity in the mixed-pathogen BSI group. Further, the levels of plasma IL-8 and TNF-α were also positively correlated with ICU stay duration and 30-day mortality. In multivariate analysis, plasma CRP and IL-6 levels were independently associated with 30-day mortality.
Conclusion
Mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp. may give rise to increased NLRs and plasma CRP, IL-6, IL-8, and TNF-α levels in comparison to BSI caused by Enterococcus spp. only, thus leading to elevated disease severity in critically ill patients.
Keywords: Bloodstream infection, Disease severity, 30-Day mortality, C-reactive protein, Pro-inflammatory cytokine
1. Introduction
Enterococcus spp. are a diverse group of lactic acid-fermenting cocci bacteria that are Gram-positive [1]. The classification of Enterococcus spp. is primarily based on the host and host environment. The most prevalent species, E. faecalis and E. faecium, are commonly found in the human intestinal tract, whereas E. mangii is typically found in natural environments [2]. Since the 1980s, there has been a rise in the prevalence of multi-drug-resistant strains of E. faecalis and E. faecium, leading to opportunistic infections in patients admitted to the intensive care unit (ICU) worldwide, posing a considerable threat to the health of hospitalized patients [3]. Based on the previous research findings [4], it has been established that over half of the clinical isolates of E. faecalis display resistance towards vancomycin, ampicillin, aminoglycosides, and macrolides. The occurrence of Enterococcus infections is typically observed in immunocompromised and critically ill patients who have potential risk factors such as advanced age, diabetes, malignant tumors, heart disease, transplantation, and surgery [5]. Enterococcus spp. can lead to various infections such as pelvic infection, neonatal infection, urinary tract infection, bloodstream infection (BSI), infectious endocarditis, and other diseases [6].
Fungi are widely distributed in nature, with approximately 400 species capable of causing various diseases in humans. However, it is worth noting that infections commonly arise from only 30 of these species [7]. The prevalence of these organisms has notably risen among ICU patients due to frequent invasive examinations and treatment with hormones, anti-tumor drugs, and immunosuppressants, leading to the occurrence of opportunistic or invasive infections [8]. A prior investigation revealed a notable disparity in the prevalence of fungal infection between the ICU and other hospital wards, with the mortality rate for invasive fungal infection ranging from 40 to 60% [9]. Candida spp., Aspergillus spp., Cryptococcus spp., and Mucor spp. are among the commonly encountered opportunistic fungal pathogens in clinical settings [10]. Enterococcus spp. and certain types of fungi are typically found in the human body as part of the normal flora. However, in patients with compromised immunity, these pathogens can cause opportunistic infections, often resulting in fatalities. The most severe condition among them is sepsis, with approximately 25%–30% of sepsis cases being caused by BSIs [11]. Sepsis is currently defined as a potentially life-threatening dysfunction of bodily organs in an individual, which is triggered by the person's abnormal response to an infection [12]. In China, E. faecalis and C. albicans are the most prevalent enterococcal and fungal pathogens, respectively, to cause co-infection in patients with sepsis [13]. Co-infections by these two pathogens can form mixed-species biofilms at infection sites, leading to increased resistance against antibacterial drugs and macrophages and posing significant challenges in the treatment [14]. Moreover, studies have demonstrated that the presence of biofilms shields lipoteichoic acid (derived from Enterococci), which in turn stimulates inflammation in host cells [15].
BSIs are associated with the highest mortality rate, especially among ICU patients [16]. A 4-year statistical study conducted in Romania from 2017 to 2020 found that the E. faecalis positivity rate in the blood cultures from the ICU was 6.8%, second only to Coagulase-negative Staphylococci, Klebsiella pneumoniae, Methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii [17]. Candida spp. is the most prevalent invasive fungus in critically ill patients, and the incidence of candidemia in the ICUs is approximately 10–20 times more than that in general wards [16]. An epidemiological study reported that Candida spp. was responsible for 22% of hospital-acquired BSIs, surpassing all other pathogens [18]. Notably, multiple studies have verified that BSIs caused by mixed pathogens (bacteria and Candida spp.) lead to higher morbidity and mortality compared to those caused by monomicrobial or polybacterial pathogens [19]. Inflammatory responses are common in critically ill patients with severe sepsis or septic shock, reflecting the host's unbalanced response to infection and its relation to morbidity and mortality [20]. This has also been observed in BSIs caused by a combination of bacterial and Candida spp. infection. Pathogenic bacteria in the bloodstream release toxins and metabolites, thus provoking a systemic inflammatory response [21]. On the other hand, candidemia is known for its highly invasive nature, which results in the massive secretion of inflammatory mediators and the recruitment of inflammatory neutrophils and monocytes [22]. From a broader perspective, it is particularly important to monitor the inflammatory response in mixed-pathogen BSIs caused by bacteria and Candida spp., paying particular attention to the link between inflammatory markers and the disease severity.
Bacterial or fungal infections cause an increase in the counts of neutrophils, and hence an increase in neutrophil-to-lymphocyte ratios (NLRs) may be observed in isolated cases. NLR is considered a reliable marker for the diagnosis of bacteremia and sepsis and has a potential value in assessing the severity of sepsis [23]. The diagnostic value of procalcitonin (PCT) and C-reactive protein (CRP) as inflammatory markers for the early detection of BSIs has been widely recognized. Nevertheless, the use of PCT and CRP for diagnosing fungemia remains controversial [24]. In addition, pro-inflammatory cytokines, including interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α), have been shown to predict the severity of sepsis in ICU patients [25,26]. These cytokines are also released by host cells in response to Candida albicans and play a crucial role in activating immune effector cells against invading microorganisms [27]. Patients with candidemia have been found to exhibit significantly elevated levels of these pro-inflammatory cytokines compared to healthy subjects [28]. Regrettably, there is a limited number of studies that have examined the correlation between levels of inflammatory markers and disease severity in critically ill patients with concurrent fungal and bacterial BSIs. Additionally, no specific strains have been investigated in these studies. Based on the aforementioned research, we proposed that inflammatory markers, including white blood cell (WBC) counts, NLRs, and plasma PCT, CRP, IL-6, IL-8, and TNF-α levels might be of clinical significance in assessing the disease severity in critically ill patients with mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp.
This study aimed to investigate the clinical significance of inflammatory markers in assessing disease severity in BSIs caused by mixed pathogens, specifically the simultaneous presence of Enterococcus spp. and Candida spp. We analyzed the association between levels of inflammatory markers and Acute Physiology and Chronic Health Evaluation (APACHE) II scores, ICU stay duration, and 30-day mortality. To the best of our knowledge, this is the first study to explore this aspect.
2. Materials and methods
2.1. Study population
The study enrolled critically ill patients admitted to the ICU with BSIs caused by Enterococci from January 2019 to December 2022. Critically ill patients were defined as those who exhibited unstable vital signs, rapidly changing conditions, and more than two organ systems functioning in an unstable state that could potentially endangers their lives at any time. BSI was determined by identifying microorganisms that were isolated from blood culture. Candidemia was defined based on criteria established by the Infectious Diseases Society of America [29]. The criteria for considering a blood culture positive were as follows: there must be at least one positive result from a blood culture, and subsequent blood cultures for the same patient should also yield positive results, indicating the presence of a single infection strain [30]. This study included patients who met the following inclusion criteria: (1) age ≥25 years and (2) Enterococcus BSI with or without concurrent Candida BSI as determined by blood culturing results. Further, the exclusion criteria were as follows: (1) the existence of other bacterial co-infections, (2) severely immunocompromised, and (3) receiving corticosteroid treatment. Severely immunocompromised patients were defined as individuals who met one or more of the following criteria: (1) a history of using antineoplastic drugs or immunosuppressants within 3 months prior to ICU admission, (2) the presence of hematologic malignancies, and (3) a diagnosis of acquired immunodeficiency syndrome. These exclusions were necessary in order to maintain the integrity and validity of the findings.
2.2. Classification
Depending on the presence of BSI caused by Candida spp, patients were categorized into two groups: (1) the single-pathogen BSI group, comprising patients without Candida BSI; and (2) the mixed-pathogen BSI group, encompassing patients with BSI caused by both Enterococcus spp. and Candida spp.
2.3. Data collection
Clinical data and laboratory results of all included patients in the study were retrospectively collected using the hospital information system (HIS). The integrated medical records during hospitalization can be queried at any time through HIS [31]; the records are regularly maintained by the staff of the hospital information department, thus avoiding the data to be missed. The main patient characteristics were age, sex, presence of major diseases, BSI classification, the foci of infection, invasive treatment received, antimicrobial treatment received, and blood culture time to positivity. The identification of the infected organ or tissue was established as the foci of infection when the clinical symptoms of bacteraemia appeared and a blood culture was obtained [32]. This study followed the convention of documenting the focus as uncertain if two or more potential foci were considered equally plausible [33]. The antibiotic treatment data obtained in this study mainly involve two aspects: the number of antibiotics used and inappropriate initial antimicrobial therapy. Inappropriate initial antimicrobial therapy was characterized as the antibiotic treatment administered within 72 h following the suspicion of BSI that was found to be ineffective against the particular pathogen determined via culture and in vitro susceptibility testing [34]. Prior to receiving the blood culture results, the administration of antibiotics as a form of empirical treatment did led to a percentage of patients receiving inappropriate initial antimicrobial therapy. Initiation of antimicrobial therapy was required if the patient had a temperature of >38.0 °C or <36.0 °C, experienced chills, or had a systolic blood pressure <90 mmHg [35]. The disease severity was defined based on APACHE II scores, ICU stay duration, and 30-day mortality. All patients underwent APACHE II score assessment within the first 24 h after admission to the ICU. BSIs were categorized as community-acquired or hospital-acquired infections. Community-acquired BSIs were identified from a positive blood culture collected within 48 h of hospital admission.
Blood samples for cultures were collected from patients presenting with a high fever and suspicion of BSI on at least 3 occasions. The samples for bacterial blood culture were divided into two bacterial culture bottles, each containing 8–10 mL of blood. The anaerobic bottle was inoculated first, followed by the aerobic bottle. For fungal blood culture, the samples were placed in fungal culture bottles, with each bottle containing 3–5 mL of blood. The time interval between the collection of samples for bacterial blood culture and fungal blood culture was controlled within 5 min. The BACT/ALERT-3D automatic blood culture system (BD Medical) was employed to conduct microbial culturing. Bacteraemia is characterized by the occurrence of bacteria in the bloodstream, as evident through the isolation of bacteria from blood cultures. Fungemia refers to the presence of fungi, specifically yeasts from the Candida spp., in the bloodstream [12]. The identification of Candida was accomplished through the utilization of colony coloration and the Vitek2 YST identification card (Biomerieux), whereas the ATB microbial identification and drug sensitivity analysis system (Biomerieux) was utilized for identifying bacterial species. The susceptibility test of Enterococcus spp. adhered to the guidelines stated in the 2017 version of the Clinical and Laboratory Standards Institute (CLSI) for the interpretation of susceptibility pattern [36]. The guidelines provided by CLSI were also followed for conducting the testing on Candida spp. The interpretation of susceptibility was based on the definitions outlined in the CLSI editions, specifically CLSI M27-S4 and CLSI M27-S3 [37].
At our hospital, which is a specialized center for hematology and oncology, the detection of inflammatory factors, including IL-6, IL-8, and TNF-α, is a routine practice in the clinical laboratory. Furthermore, in cases with infectious diseases, especially patients with sepsis, the levels of inflammatory markers are used as reference indicators for evaluating infection control in ICUs at our hospital. PCT, CRP, IL-6, IL-8, and TNF-α levels in blood samples that were collected at the same time as for culturing were analyzed using a SEBIA automatic enzyme-linked immunoassay detector. The upper reference ranges for plasma PCT, CRP, IL-6, IL-8, and TNF-α levels in healthy participants were 0.5 ng/mL, 5 mg/L, 5.9 pg/mL, 62 pg/mL, and 8.1 pg/mL, respectively. Normal reference intervals were acquired following the guidelines provided in the instruction manual of the Immulite 1000 automated chemiluminescence immunoassay analyzer (Siemens) kit. The establishment and verification of these intervals were conducted in adherence to the specifications outlined in the CLSI C28-A3 document [38]. Besides, WBC counts and NLRs were also analyzed as inflammatory markers. Following admission to the ICU, blood samples were collected on a daily basis and the complete blood cell counts were assessed using a Sysmex XT-2000i automatic five-category analyzer specialized in blood cell analysis. For the purpose of this study, the levels of inflammatory markers obtained on the day of collecting positive blood culture samples of Enterococcus spp. were utilized for analysis.
2.4. Statistical analysis
The Chi-squared test was applied to analyze categorical variables, and their presentation was expressed as numbers (percentages). The statistical analysis of parametric and non-parametric (natural log-transformed) continuous variables in this study was conducted using the two-sample t-test and the two-tailed Mann-Whitney test, respectively. All continuous variables are expressed as means (standard deviations). Survival curves were prepared to determine the dynamics of 30-day survival and compared using the log-rank (Mantel-Cox) test. Spearman's rank correlation analysis was employed to assess the associations between levels of inflammatory markers and disease severity. Additionally, in order to examine the association with 30-day mortality in detail, multivariate logistic regression analysis was conducted, incorporating age, number of major diseases, number of antibiotics used, inappropriate initial antimicrobial therapy received, the foci of infection, and all inflammatory markers included in this study as variables. p-values of less than 0.05 were deemed to be statistically significant, and odds ratios (OR) together with their corresponding 95% confidence intervals (CIs) were reported. Statistical analyses were conducted using GraphPad Prism 8.0.2 software.
3. Results
In the preliminary screening list, a total of 158 patients with initial and secondary infections caused by Enterococcus spp. were included. A total of 30 patients were excluded due to the presence of other bacterial co-infections, while 19 and 15 patients were excluded due to their immunocompromised status and the administration of corticosteroids during ICU admission, respectively. The final study cohort consisted of 94 patients, who were classified into two categories: individuals solely infected with Enterococcus spp. (n = 43; single-pathogen BSI group) and individuals concurrently infected with Enterococcus spp. and Candida spp. (n = 51; mixed-pathogen BSI group) (Fig. 1).
Fig. 1.
Flow chart showing the inclusion and exclusion criteria.
3.1. Demographic characteristics of included patients
Table 1 summarizes the demographic characteristics of included patients. Single- and mixed-pathogen BSI groups had 43 (45.7%; 30 men and 13 women; the mean age of 75.6 years) and 51 (54.3%; 35 men and 16 women; the mean age of 77.2 years) patients, respectively. Major diseases in patients of these two groups were cardiovascular (41.9% vs. 47.0%), digestive (16.3% vs. 19.6%), urinary system (16.3% vs. 23.5%) and neurological (34.9% vs. 37.2%) diseases, and diabetes mellitus (53.5% vs. 56.8%). The majority of patients had hospital-acquired BSIs in both the single and mixed pathogen BSI groups (53.5% vs. 58.8%). Regarding the foci of infection, in the single-pathogen BSI group, 10 (23.2%) patients had lower respiratory tract infections, 3 (6.9%) had urinary infections, and 14 (32.5%) had gastrointestinal tract infections. Additionally, 16 (37.2%) patients had no certain foci of infection. On the other hand, in the mixed-pathogen BSI group, 13 (25.4%) patients had lower respiratory tract infections, 5 (9.8%) had urinary infections, and 15 (29.4%) had gastrointestinal tract infections. Moreover, 18 (35.3%) patients had no certain foci of infection. All patients in the two groups had a central venous catheter and an indwelling urethral catheter. Further, 31 (72.1%), 5 (11.6%), and 2 (4.6%) patients in the single-pathogen BSI group and 43 (84.3%), 8 (15.7%), and 6 (11.8%) patients in the mixed-pathogen BSI group had indwelling nasogastric, indwelling thoracic drainage, and indwelling peritoneal drainage tubes, respectively. In the single-pathogen BSI group, 8 patients (18.6%) received inappropriate initial antimicrobial therapy, while in the mixed-pathogen BSI group, 15 patients (29.4%) received inappropriate initial antimicrobial therapy. There were no significant differences observed in age, sex, major diseases, BSI classification, primary infected foci, invasive treatment received, and inappropriate initial antimicrobial therapy received between the two groups (all p>0.05). However, the proportion of patients using 3 or more antibiotics in the single-pathogen BSI group was found to be significantly lower than that in the mixed-pathogen BSI group (65.1% vs. 88.2%, respectively; p = 0.012). Additionally, the blood culture time (in hours) to positivity for Enterococcus spp. in the single-pathogen BSI group was significantly shorter than in the mixed-pathogen BSI group [22.8 (7.6) vs. 27.6 (8.4), respectively; p = 0.005]. Furthermore, Candida spp. exhibited a longer blood culture time to positivity compared to Enterococcus spp.
Table 1.
Patient demographics of the final study cohort.
| Variables | Single-pathogen BSI group (n = 43) | Mixed-pathogen BSI group (n = 51) | p-value |
|---|---|---|---|
| Age, years, mean (SD) | 75.6 (10.6) | 77.2 (11.3) | 0.483 |
| Male sex, n (%) | 30 (69.8) | 35 (72.5) | 0.821 |
| Major diseases, n (%) | |||
| Cardiovascular disease | 18 (41.9) | 24 (47.0) | 0.679 |
| Digestive disease | 7 (16.3) | 10 (19.6) | 0.790 |
| Urinary system diseases | 7 (16.3) | 12 (23.5) | 0.446 |
| Neurological disease | 15 (34.9) | 19 (37.2) | 0.833 |
| Diabetes mellitus | 23 (53.5) | 29 (56.8) | 0.836 |
| BSI classification, n (%) | |||
| Community-acquired | 20 (46.5) | 21 (41.2) | 0.678 |
| Hospital-acquired | 23 (53.5) | 30 (58.8) | 0.678 |
| The foci of infection, n (%) | |||
| Lower respiratory tract | 10 (23.2) | 13 (25.4) | >0.999 |
| Urinary tract | 3 (6.9) | 5 (9.8) | 0.723 |
| Gastrointestinal tract | 14 (32.5) | 15 (29.4) | 0.824 |
| Uncertain | 16 (37.2) | 18 (35.3) | >0.999 |
| Invasive treatments, n (%) | |||
| Central venous catheterization | 43 (100) | 51 (100) | >0.999 |
| Indwelling urethral catheter | 43 (100) | 51 (100) | >0.999 |
| Indwelling nasogastric tube | 31 (72.1) | 43 (84.3) | 0.206 |
| Indwelling thoracic drainage tube | 5 (11.6) | 8 (15.7) | 0.766 |
| Indwelling peritoneal drainage tube | 2 (4.6) | 6 (11.8) | 0.282 |
| Antimicrobial treatment, n (%) | |||
| Antibiotics ≥ 3 | 28 (65.1) | 45 (88.2) | 0.012 |
| Inappropriate initial antimicrobial therapy | 8 (18.6) | 15 (29.4) | 0.241 |
| Blood culture time to positivity, hours, mean (SD) | |||
| Enterococcus spp. | 22.8 (7.6) | 27.6 (8.4) | 0.005 |
| Candida spp. | – | 40.6 (10.8) | – |
| Disease severity, mean (SD) or n (%) | |||
| APACHE II scores, mean (SD) | 19.9 (4.0) | 22.1 (3.7) | 0.003 |
| ICU stay duration, days, mean (SD) | 20.3 (5.2) | 23.6 (4.7) | 0.009 |
| 30-day mortality, n (%) | 10 (23.3) | 23 (45.1) | 0.032 |
Data are presented as mean (SD) or n (%). APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; ICU, intensive care unit; n, number; SD, standard deviation. For the analysis, the ICU hospitalization days of patients who died within 30 days were also considered as part of the ICU stay duration. p-value < 0.05 means statistically significant. BSIs were community-acquired or hospital-acquired.
3.2. Distribution of pathogens
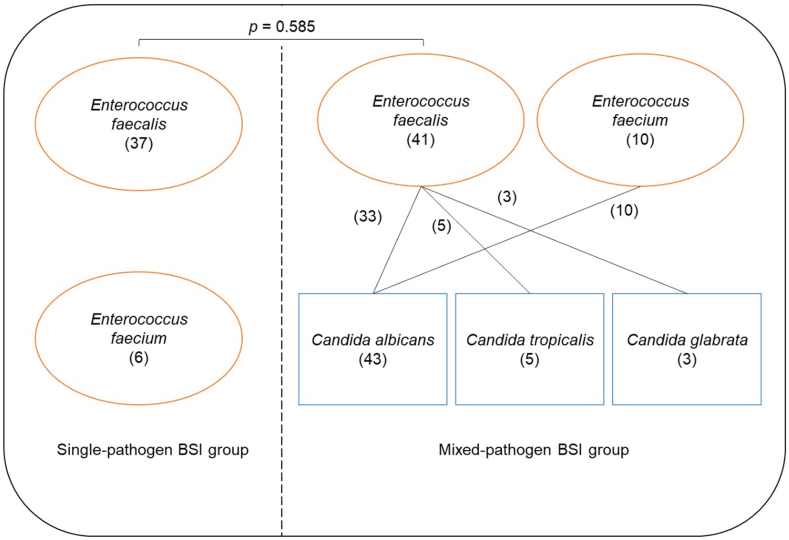
As depicted in Fig. 2, the included patients exhibit a varying distribution of pathogens. In the single-pathogen BSI group, it was found that 37 patients were infected with E. faecalis, while 6 patients were infected with E. faecium. On the other hand, in the mixed-pathogen BSI group, 41 patients were found to be infected with E. faecalis, with an additional 10 patients infected with E. faecium. Among these cases, 33 patients displayed co-infections of E. faecalis with C. albicans, 5 patients with C. tropicalis, and E. faecalis, 3 patients with C. glabrata. Furthermore, all of the remaining 10 patients were all co-infected with E. faecium and C. albicans. Notably, no significant differences were observed in the distribution of Enterococcus spp. between the two groups (p = 0.585).
Fig. 2.
Distribution of pathogens in the two groups. The numbers in parentheses represent the number of cases.
3.3. Disease severity
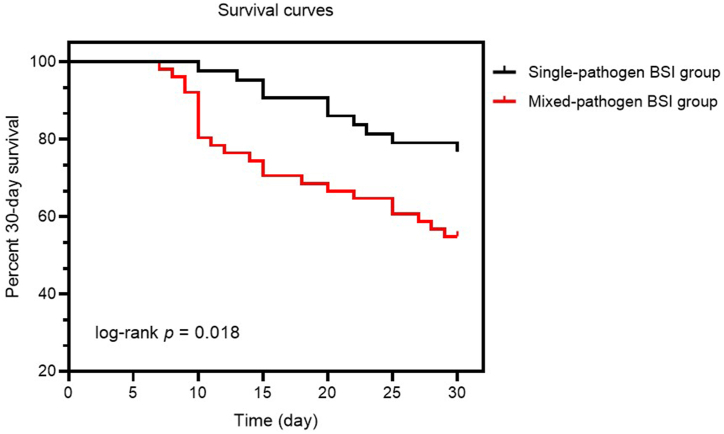
In Table 1, the disease severity in both groups is also presented. Within 24 h of ICU admission, APACHE II scores were determined for all 94 patients. Comparing the two groups, the mixed-pathogen BSI group had significantly higher APACHE II scores compared to the single-pathogen BSI group [22.1 (3.7) vs. 19.9 (4.0), respectively; p = 0.002]. In addition, ICU stay duration (in days) was significantly longer for the mixed-pathogen BSI group compared to the single-pathogen BSI group [23.6 (4.7) vs. 20.3 (5.2), respectively; p = 0.005]. The 30-day mortality in the mixed-pathogen BSI group was found to be significantly higher compared to the single-pathogen BSI group (45.1% vs. 23.3%, respectively; p = 0.032). The survival curves of the two groups indicated that deaths in the mixed-pathogen BSI group peaked at day 10, while in the single-pathogen BSI group, deaths were evenly distributed and occurred later. The percent 30-day survival also differed significantly between the two groups (log-rank p = 0.018) (Fig. 3).
Fig. 3.
Thirty-day survival curves for patients in two groups. Black and red lines represent 30-day survival curves for the single- and mixed-pathogen BSI groups, respectively. p < 0.05 indicates statistical significance.
3.4. Inflammatory markers
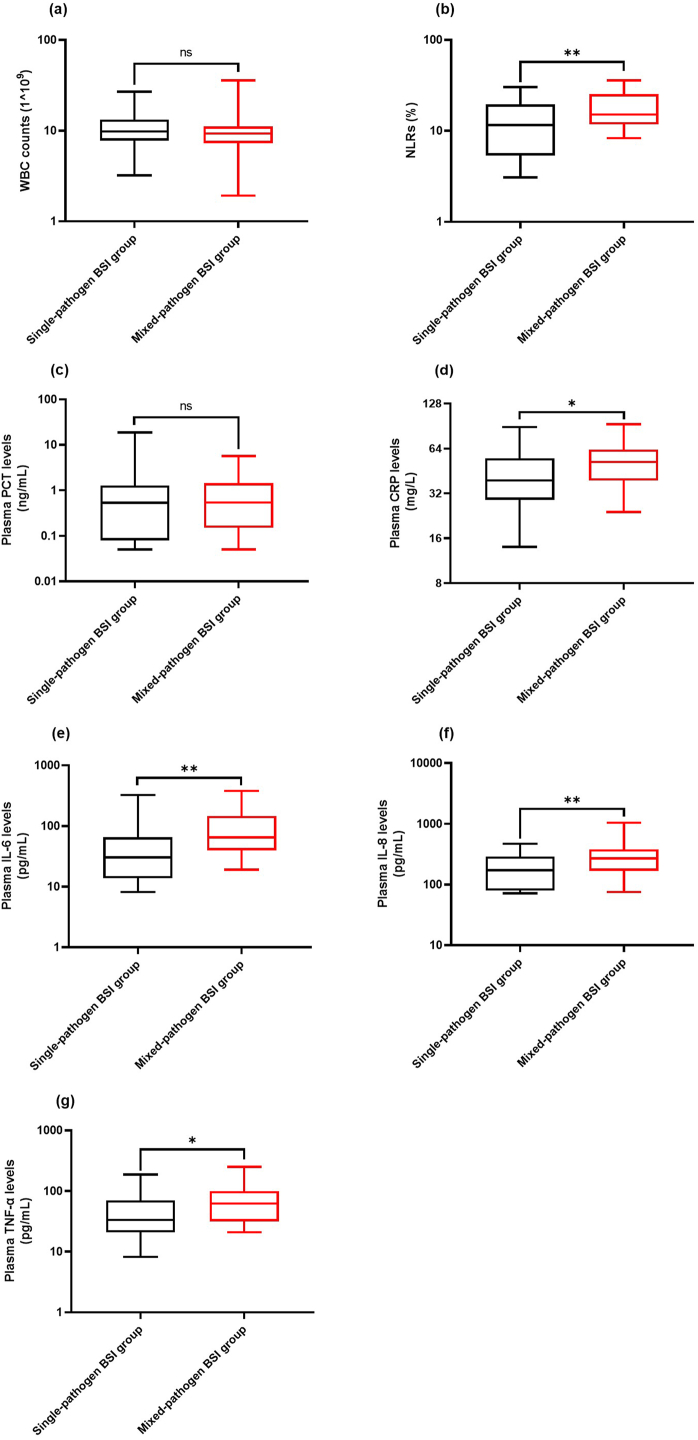
Fig. 4 illustrates the comparisons made between the two groups in terms of WBC counts, NLRs, and plasma PCT, CRP, IL-6, IL-8, and TNF-α levels. The NLRs of the mixed-pathogen BSI group were found to be significantly higher than those of the single-pathogen BSI group (p = 0.009) (Fig. 4b). Additionally, the mixed-pathogen BSI group exhibited significantly higher levels of plasma CRP (Fig. 4d), IL-6 (Fig. 4e), IL-8 (Fig. 4f), and TNF-α (Fig. 4g) compared to the single-pathogen BSI group (p = 0.012, p = 0.003, p = 0.006, and p = 0.026, respectively). However, no statistically significant differences were observed between the two groups in terms of WBC (Fig. 4a) counts and plasma PCT (Fig. 4c) levels (all p>0.05).
Fig. 4.
Comparison of inflammatory marker levels between the two groups. Inflammatory markers include WBC counts (a), NLRs (b), plasma PCT levels (c), plasma CRP levels (d), plasma IL-6 levels (e), plasma IL-8 levels (f), and plasma TNF-α levels (g). * and ** represent p < 0.05 and p < 0.01, respectively.
3.5. Association of inflammatory markers with disease severity
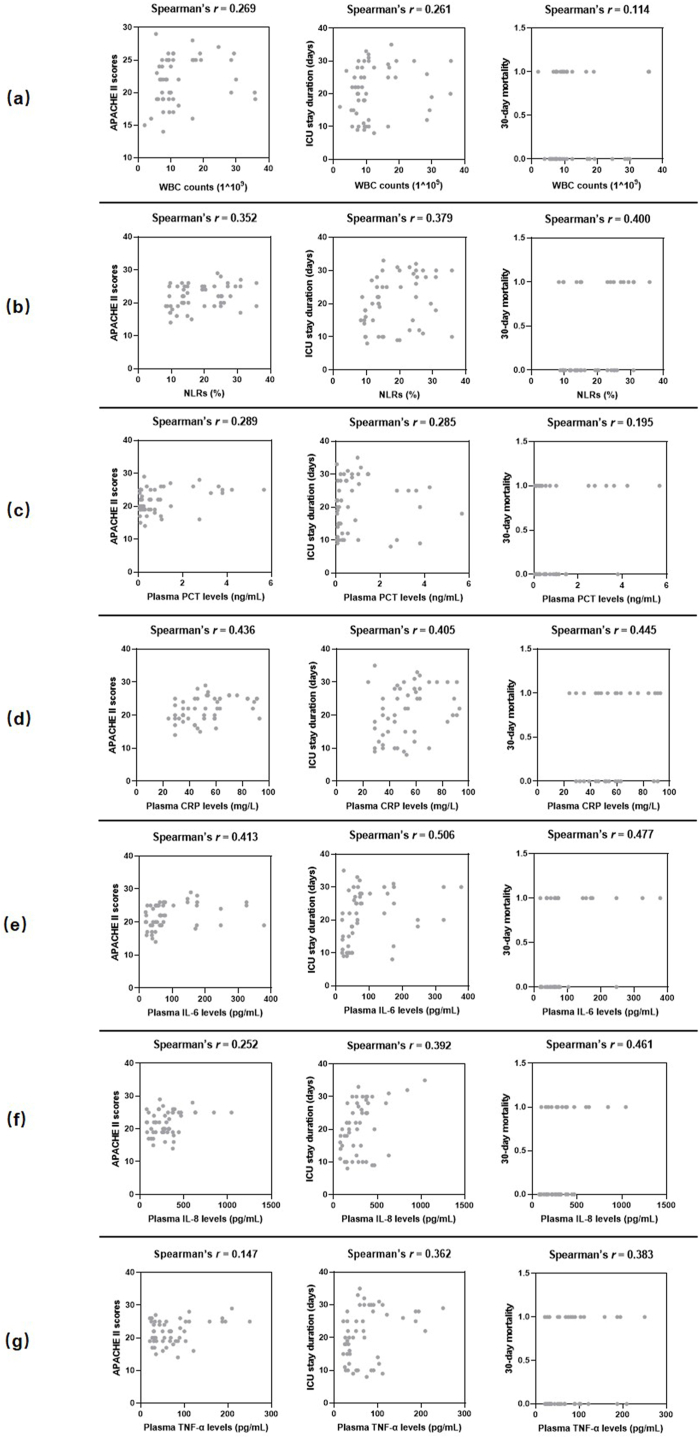
Fig. 5 presents the association between inflammatory markers and disease severity in the mixed-pathogen BSI group. NLRs (Fig. 5b) and plasma CRP (Fig. 5d) and IL-6 (Fig. 5e) levels were positively correlated with APACHE II scores [(r = 0.352 and p = 0.038), (r = 0.436 and p = 0.014), and (r = 0.413 and p = 0.019), respectively], ICU stay duration [(r = 0.379 and P = 0.025), (r = 0.405 and p = 0.024), and (r = 0.506 and p = 0.003), respectively], and 30-day mortality [(r = 0.400 and p = 0.017), (r = 0.445 and p = 0.012), and (r = 0.477 and p = 0.004), respectively]. Further, plasma IL-8 (Fig. 5f) and TNF-α (Fig. 5g) levels also showed a positive correlation with ICU stay duration [(r = 0.392 and p = 0.027) and (r = 0.362 and p = 0.041), respectively] and 30-day mortality [(r = 0.461 and p = 0.013) and [(r = 0.383 and p = 0.025), respectively]. Nevertheless, WBC counts (Fig. 5a) and plasma PCT levels (Fig. 5c) exhibited no significant correlation with APACHE II scores, ICU stay duration, and 30-day mortality (all p>0.05).
Fig. 5.
Scatterplot shows the correlations between inflammatory markers and disease severity. Inflammatory markers include WBC counts (a), NLRs (b), plasma PCT levels (c), plasma CRP levels (d), plasma IL-6 levels (e), plasma IL-8 levels (f), and plasma TNF-α levels (g). Disease severity was presented as APACHE II scores, ICU stay duration and 30-day mortality. r: Spearman's correlation conefficient. For the analysis, the ICU hospitalization days of patients who died within 30 days were also considered as part of the ICU stay duration.
In multivariate analyses, any confounding association between inflammatory markers and 30-day mortality were excluded using logistic regression analysis, considering patients' background variables, primarily age, number of major diseases, number of antibiotics used, and the foci of infection. Plasma CRP (OR = 1.130, 95% CI = 1.039–1.319, p = 0.027) and IL-6 (OR = 1.045, 95% CI = 1.017–1.100, p = 0.018) levels were independently associated with 30-day mortality (Table 2).
Table 2.
Multivariate logistic regression analysis of factors for 30-day mortality.
| Factors | odds ratios (95% confidence interval) | p-value |
|---|---|---|
| Age | 1.187 (1.021–1.621) | 0.095 |
| Number of major diseases | 5.261 (1.018–58.14) | 0.088 |
| Number of antibiotics used | 2.603 (0.496–22.23) | 0.296 |
| Inappropriate initial antimicrobial therapy | 1.812 (0.761–4.847) | 0.198 |
| The foci of infection | 0.315 (0.030–1.725) | 0.229 |
| WBC counts | 1.163 (0.971–1.889) | 0.291 |
| NLRs | 1.396 (1.072–2.895) | 0.094 |
| Plasma PCT levels | 0.432 (0.095–1.328) | 0.176 |
| Plasma CRP levels | 1.130 (1.039–1.319) | 0.027 |
| Plasma IL-6 levels | 1.045 (1.017–1.100) | 0.018 |
| Plasma IL-8 levels | 1.051 (1.018–1.156) | 0.054 |
| Plasma TNF-α levels | 1.028 (0.997–1.082) | 0.143 |
CRP, C-reactive protein; IL-6, interleukin 6; IL-8, interleukin 8; n, number; NLR, neutrophil to lymphocyte ratio; PCT, procalcitonin; TNF-α, tumor necrosis factor alpha; WBC, white blood cell. p-value <0.05 means statistically significant.
4. Discussion
In the past decade, BSIs have been frequently seen in patients admitted to the ICU, in particular, with bacteremia and candidemia being linked to significant levels of morbidity and mortality [39]. Enterococcus species are a significant group of bacterial pathogens that lead to hospital-acquired BSIs in ICU patients, with E. faecalis and E. faecium being the most prevalent pathogens responsible for the majority of infections [40]. The prevalence of E. faecalis-induced BSIs is estimated to be around 4.5 infections per 100,000 individuals each year. Additionally, the case fatality rate linked to these infections ranges from 10% to 20% [41]. Besides bacterial BSIs, fungal infections, specifically those caused by Candida species, have demonstrated an increase, especially among individuals undergoing significant abdominal surgical procedures [42]. A previous study conducted by MacBrayne et al. [43] indicated that the median blood culture time to positivity in Gram-negative pathogens was 14.33 h, while in fungi it was 32.5 h, which aligns with the findings of current study. This study also identified that co-infection with Candida spp. prolonged the blood culture time to positivity of Enterococcus spp. Further, co-infections that involve both C. albicans and E. faecalis are on the rise in hospital-acquired BSIs [44]. Hospital-acquired BSIs are frequently linked to multidrug-resistant strains, which have a strong association with antibiotic usage in healthcare settings [45]. In the present study, it was found that BSIs caused by mixed cultures were the most prevalent, with a majority of these cases being hospital-acquired. These results indicate a potentially elevated risk of developing BSIs from mixed cultures within healthcare facilities. Among them, a significant number of patients received three or more antibiotics. In addition, Zeise et al. found that a considerable proportion of BSIs caused by E. faecalis and C. albicans were linked to central venous catheters [44]. Similarly, all patients with BSIs included in this study received central venous catheterization after ICU admission. BSI refers to a patient displaying signs of systemic infection with a positive blood culture. The infection may originate from a known source or an unidentified source [46]. Evans et al. [47] conducted a previous prospective cohort study on BSIs and reported that around 30% of patients had no identified foci of infection, which aligns with our findings. A prior prospective cohort study conducted in Taiwan [48] revealed that polymicrobial BSIs were linked to higher risk of poor outcomes in comparison to mono-microbial BSIs. Similarly, the present study also observed that patients with the mixed-pathogen BSIs exhibited a higher APACHE II score, longer ICU stay, and increased 30-day mortality compared to patients with the single-pathogen BSIs, further highlighting a significant increase in disease severity when multiple pathogens (both Candida spp. and Enterococcus spp.) at present. Inappropriate initial antimicrobial therapy is a crucial factor affecting the prognosis of patients with severe infections [35]. Several previous studies have highlighted that inappropriate initial antimicrobial therapy leads to an increase in both hospital mortality rate and length of stay among critically ill patients with Gram-negative BSIs [34]. In this study, it was observed that the proportion of patients in the mixed-pathogen BSI group who received inappropriate initial antimicrobial therapy was higher compared to the single-pathogen BSI group. However, it is important to note that this difference was not statistically significant. In a previous study conducted by Hamada et al. [49], it was found that prior use of antibiotics for uncertain foci was significantly associated with a 28-day mortality in patients with ampicillin-resistant enterococcal bacteremia. This factor may also contribute to the higher disease severity and poor prognosis in patients with mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp. However, further studies with a larger sample size are needed to confirm these findings.
Systemic inflammation plays a crucial role in the advancement of diseases in BSIs and potentially sepsis, leading to heightened mortality risks [50]. Elevated NLRs are often characterized by increased neutrophils and decreased lymphocytes, reflecting an imbalance between acute inflammation and adaptive immunity [51]. A higher NLR, particularly >10, indicates a worse prognosis in patients with sepsis [23]. Salciccioli et al. found that higher NLRs in patients with sepsis were associated with higher disease severity characterized by a longer ICU stay and higher APACHE II score [52]. In the present study, the mixed-pathogen BSIs caused by Enterococcus spp. and Candida spp. resulted in higher NLRs than those of the single-pathogen BSIs by Enterococcus spp., and NLRs were also positively correlated with disease severity. The WBC counts, along with plasma PCT and CRP levels, are commonly used markers for identifying bacterial infection [53]. Yet, the predictive ability of WBC counts for BSIs is limited as a considerable number of individuals with BSI exhibit normal WBC counts [54]. Furthermore, studies have indicated that WBC counts are unable to distinguish between patients with fungal BSIs and those with negative blood cultures [55]. This explains why there were no significant differences in WBC counts between the two groups in the current study. Consistent with previous reports [56,57], WBC counts did not correlate with disease severity in ICU patients with BSIs, supporting that NLRs are a more valuable predictor of disease severity in the mixed-pathogen BSI caused by Enterococcus spp. and Candida spp.
PCT is recognized as a crucial marker of inflammation in clinical assessments of bacterial infections [58]. CRP is a valuable acute-phase protein that operates as a sensitive systemic marker for both inflammation and tissue injury [59]. Studies have shown a significant increase in plasma levels of both PCT and CRP in individuals with bacterial sepsis [58]. However, rare reports exist regarding the clinical significance of plasma PCT and CRP levels in cases of candidemia. Pieralli et al. found that critically ill patients in the ICU with sepsis and candidemia exhibited notably lower plasma PCT levels in comparison to those with bacteremia [60]. Li et al. [61] similarly reported that there was no significant difference in plasma PCT levels between patients with candidemia and bacteremia, but did find significantly higher plasma CRP levels in patients with candidemia. Our study also revealed that patients with mixed-pathogen BSIs involving Enterococcus spp. and Candida spp. had higher plasma CRP levels in comparison to PCT levels when compared to patients with single-pathogen BSIs.
In patients with bacteria-driven sepsis, the plasma PCT and CRP levels found to be linked to disease severity [62]. To determine if the same correlation applies to mixed-pathogen BSIs involving Enterococcus spp. and Candida spp., correlation analyses were conducted between inflammatory markers and disease severity. Contrary to previous findings [62], the present study found that plasma PCT levels did not correlate with disease severity in cases of mixed-BSIs caused by Enterococcus spp. and Candida spp. Interestingly, there was a positive correlation observed between plasma CRP levels and APACHE II scores, ICU stay duration, and 30-day mortality. This indicates that plasma CRP levels could serve as a more dependable marker for assessing disease severity and predicting outcomes in such infections. Several previous studies have consistently found that levels of PCT are higher in Gram-negative bacterial infections compared to Gram-positive bacterial and fungal infections. Additionally, PCT levels serve as a more sensitive marker for bacterial BSIs compared to fungal BSIs [63]. This discrepancy may elucidate the lack of a positive correlation between PCT levels and disease severity in the present study. Coexistence of C. albicans and bacteria like E. faecalis and Staphylococcus aureus has been shown to exacerbate bacterial infection, resulting in a heightened inflammatory response and ultimately a worse prognosis [64]. Based on our findings, we determined that higher disease severity in patients with mixed-pathogen BSIs was attributed to higher plasma CRP levels, which could be due to the exacerbation of bacterial infection in the presence of a fungal pathogen.
Pro-inflammatory cytokines IL-6, IL-8, and TNF-α are commonly activated during severe infections. In our previous study, plasma IL-6, IL-8, and TNF-α levels were observed to be higher during sepsis due to co-infections by Klebsiella pneumoniae and Acinetobacter baumannii in comparison to sepsis due to infection by only one of these species; further, the levels of these markers were correlated with disease severity [26]. Wang et al. [65] found that the levels of IL-6, IL-8, and TNF-α were significantly elevated in candidemia compared with Gram-positive bacteremia, indicating that the elevated levels of these pro-inflammatory cytokines may serve as valuable diagnostic markers for differentiating between candidemia and bacteremia. Co-infection by Candida albicans and Staphylococcus aureus has been proven to shift the balance between pro-inflammatory and anti-inflammatory cytokine production. Altered host responses may be due to direct host cell-microbe interactions, increased microbial transmission, or increased production of pro-inflammatory cytokines in host cell responses to co-infection [66]. In the present study, plasma IL-6, IL-8, and TNF-α levels in the mixed-pathogen BSI group were significantly higher than those in the single-pathogen BSI group, further supporting the previous findings. Additionally, we found that plasma IL-6, IL-8, and TNF-α levels were positively correlated with disease severity by Spearman's rank correlation analysis. These results indicated that IL-6 and CRP are of potential clinical significance in predicting the disease severity in critically ill patients with mixed-pathogen BSI caused by Enterococcus spp. and Candida spp. Furthermore, our findings again indicated that Candida spp. enhanced enterococcal infection and manifested as higher plasma levels of IL-6, IL-8, and TNF-α and associated disease severity.
While these findings indicated that NLRs, the plasma levels of CRP, IL-6, IL-8, and TNF-α were associated with disease severity, the strength of these associations was found to be weak. Additionally, it was noted that age, number of major diseases, number of antibiotics used, the foci of infection could not be disregarded when determining the impact on the mortality of patients with mixed-pathogen BSIs. Consequently, considering the aforementioned clinical context of the patients, we conducted a multivariate analysis taking multiple factors into account. The results revealed plasma CRP and IL-6 levels were independently associated with 30-day mortality, thereby reaffirming the significance of inflammatory markers in assessing disease severity.
This study had a few limitations. Firstly, it was conducted at a single-center ICU, which may limit the generalizability of the results. Secondly, patients with other bacterial co-infections, immunosuppression, and previous or ongoing corticosteroid treatments were excluded from the study, resulting in a further reduction in the sample size. Thirdly, despite the retrospective observational design of the study, the potential presence of confounding factors was not taken into account. Lastly, the study did not consider the potential impact of antibiotic prophylaxis based on full coverage of pathogenic microorganisms.
5. Conclusion
In summary, our study exposed that mixed-pathogen (Enterococcus spp. and Candida spp.) BSIs in the ICU were predominantly hospital-acquired, with E. faecalis and C. albicans as the main pathogens. The majority of this patient cohort consisted mainly of elderly individuals with cardiovascular disease, neurological disease, diabetes, and other underlying conditions. Moreover, the patients underwent multiple antimicrobial therapies, including an initial antimicrobial therapy that may have been inappropriate to some extent. Additionally, they received invasive interventions such as central venous catheterization and urethral catheterization as part of their necessary treatment. By analyzing our findings, it was observed that patients with mixed-pathogen BSIs exhibited higher NLRs and plasma CRP, IL-6, IL-8, and TNF-α levels as well as higher disease severity when compared to those with single-pathogen (Enterococcus spp.) BSIs. This result could be due to the enhancement of enterococcal infection by Candida spp.; however, further studies are required to confirm this effect. Additionally, this study revealed that NLRs as well as plasma CRP, IL-6, IL-8, and TNF-α levels were positively correlated with disease severity in patients with the mixed-pathogen BSIs caused by both Enterococcus spp. and Candida spp. Among these markers, plasma CRP and IL-6 levels exhibited independent associations with 30-day mortality. In addition, multivariate logistic regression analysis revealed that there were correlations between NLRs and plasma IL-8 levels, as well as age and the number of major diseases, with 30-day mortality. However, these correlations were not statistically significant, possibly due to limitations in sample size. Hence, these inflammatory markers may be of great clinical significance as predictors of the disease severity in this specific patient population. Furthermore, our study may provide insights for future larger multicenter prospective studies.
Funding statement
This work was supported by National Major Science and Technology Projects of China (No. 2018ZX10713-002-002-004). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability statement
Data will be made available on request.
Ethical statement
This study was approved by the Medical Ethics Committee of The Fifth Medical Center of Chinese PLA General Hospital [ky-2020-8-18]. Informed consent was not required as the study was retrospective in nature, and all data sources were anonymized.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Xin Wang: Writing – original draft, Investigation, Data curation. Ming Li: Writing – original draft, Data curation. Yang Yang: Writing – original draft, Software, Data curation. Xueyi Shang: Software, Data curation. Yonggang Wang: Writing – review & editing, Conceptualization. Yan Li: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge Editideas (www.editideas.cn) for English language editing.
Contributor Information
Xin Wang, Email: wangxinlming@163.com.
Ming Li, Email: zijun0621@163.com.
Yang Yang, Email: young62253@163.com.
Xueyi Shang, Email: sundayxster@163.com.
Yonggang Wang, Email: yonggwang@126.com.
Yan Li, Email: liyanmdhuxi@163.com.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- BSI
bloodstream infection
- CI
confidence interval
- CRP
C-reactive protein
- HIS
hospital information system
- ICU
intensive care unit
- IL-6
interleukin-6
- IL-8
interleukin-8
- NLR
neutrophil-to-lymphocyte ratios
- OR
odds ratio
- PCT
procalcitonin
- TNF-α:
tumor necrosis factor-α;
- WBC
white blood count
References
- 1.Ike Y. [Pathogenicity of enterococci] Nihon Saikingaku Zasshi. 2017;72(2):189–211. doi: 10.3412/jsb.72.189. Japanese. [DOI] [PubMed] [Google Scholar]
- 2.Fiore E., Van Tyne D., Gilmore M.S. Pathogenicity of enterococci. Microbiol. Spectr. 2019;7(4) doi: 10.1128/microbiolspec.GPP3-0053-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang J., Sickbert-Bennett E.E., Brown V.M., Weber D.J., Rutala W.A. Relative frequency of health care-associated pathogens by infection site at a university hospital from 1980 to 2008. Am. J. Infect. Control. 2012;40(5):416–420. doi: 10.1016/j.ajic.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 4.García-Solache M., Rice L.B. The Enterococcus: a model of adaptability to its environment. Clin. Microbiol. Rev. 2019;32(2) doi: 10.1128/CMR.00058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sava I.G., Heikens E., Huebner J. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 2010;16(6):533–540. doi: 10.1111/j.1469-0691.2010.03213.x. [DOI] [PubMed] [Google Scholar]
- 6.Raza T., Ullah S.R., Mehmood K., Andleeb S. Vancomycin resistant Enterococci: a brief review. J. Pakistan Med. Assoc. 2018;68(5):768–772. [PubMed] [Google Scholar]
- 7.Köhler J.R., Casadevall A., Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med. 2014;5(1):a019273. doi: 10.1101/cshperspect.a019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Lilienfeld-Toal M., Wagener J., Einsele H., Cornely O.A., Kurzai O. Invasive fungal infection. Dtsch Arztebl Int. 2019;116(16):271–278. doi: 10.3238/arztebl.2019.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frange P., Bougnoux M.E., Lanternier F., Neven B., Moshous D., Angebault C., et al. An update on pediatric invasive aspergillosis. Med. Maladies Infect. 2015;45(6):189–198. doi: 10.1016/j.medmal.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Pathakumari B., Liang G., Liu W. Immune defence to invasive fungal infections: a comprehensive review. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110550. [DOI] [PubMed] [Google Scholar]
- 11.Huerta L.E., Rice T.W. Pathologic difference between sepsis and bloodstream infections. J Appl Lab Med. 2019;3(4):654–663. doi: 10.1373/jalm.2018.026245. [DOI] [PubMed] [Google Scholar]
- 12.Guna Serrano M.R., Larrosa Escartín N., Marín Arriaza M., Rodríguez Díaz J.C. Microbiological diagnosis of bacteraemia and fungaemia: blood cultures and molecular methods. Enferm. Infecc. Microbiol. Clín. 2019;37(5):335–340. doi: 10.1016/j.eimc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y., Wang W., Zeng Q., Wang T., Qian W. Antibiofilm efficacy of luteolin against single and dual species of Candida albicans and Enterococcus faecalis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.715156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao Y., Shang W., Yang Y., Zhou R., Rao X. Fighting mixed-species microbial biofilms with cold atmospheric plasma. Front. Microbiol. 2020;11:1000. doi: 10.3389/fmicb.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theilacker C., Sanchez-Carballo P., Toma I., Fabretti F., Sava I., Kropec A., et al. Glycolipids are involved in biofilm accumulation and prolonged bacteraemia in Enterococcus faecalis. Mol. Microbiol. 2009;71(4):1055–1069. doi: 10.1111/j.1365-2958.2008.06587.x. [DOI] [PubMed] [Google Scholar]
- 16.Epelbaum O., Chasan R. Candidemia in the intensive care unit. Clin. Chest Med. 2017;38(3):493–509. doi: 10.1016/j.ccm.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Golli A.L., Cristea O.M., Zlatian O., et al. Prevalence of multidrug-resistant pathogens causing bloodstream infections in an intensive care unit. Infect. Drug Resist. 2022;15:5981–5992. doi: 10.2147/IDR.S383285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magill S.S., Edwards J.R., Bamberg W., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y.M., Huang P.Y., Cheng Y.C., Lee C.H., Hsu M.C., Lu J.J., et al. Enhanced virulence of Candida albicans by Staphylococcus aureus: evidence in clinical bloodstream infections and infected zebrafish embryos. J Fungi (Basel). 2021;7(12):1099. doi: 10.3390/jof7121099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molano Franco D., Arevalo-Rodriguez I., Roqué I Figuls M., Montero Oleas N.G., Nuvials X., Zamora J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst. Rev. 2019;4(4):CD011811. doi: 10.1002/14651858.CD011811.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huerta L.E., Rice T.W. Pathologic difference between sepsis and bloodstream infections. J Appl Lab Med. 2019;3(4):654–663. doi: 10.1373/jalm.2018.026245. [DOI] [PubMed] [Google Scholar]
- 22.Abe M., Kinjo Y., Ueno K., et al. Differences in ocular complications between Candida albicans and non-albicans Candida infection analyzed by epidemiology and a mouse ocular candidiasis model. Front. Microbiol. 2018;9:2477. doi: 10.3389/fmicb.2018.02477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drăgoescu A.N., Pădureanu V., Stănculescu A.D., Chiuțu L.C., Tomescu P., Geormăneanu C., et al. Neutrophil to lymphocyte ratio (NLR)-A useful tool for the prognosis of sepsis in the ICU. Biomedicines. 2021;10(1):75. doi: 10.3390/biomedicines10010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortegiani A., Misseri G., Ippolito M., et al. Procalcitonin levels in candidemia versus bacteremia: a systematic review. Crit. Care. 2019;23(1):190. doi: 10.1186/s13054-019-2481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao L., Ran X., Zhong Y., Le Y., Li S. A combined ratio change of inflammatory biomarkers at 72 h could predict the severity and prognosis of sepsis from pulmonary infections. Immunobiology. 2022;227(6) doi: 10.1016/j.imbio.2022.152290. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Zhang Q., Yan Y., Yang Y., Shang X., Li Y. Clinical significance of pro-inflammatory cytokines and their correlation with disease severity and blood coagulation in septic patients with bacterial Co-infection. Shock. 2021;56(3):396–402. doi: 10.1097/SHK.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 27.Dongari-Bagtzoglou A., Wen K., Lamster I.B. Candida albicans triggers interleukin-6 and interleukin-8 responses by oral fibroblasts in vitro. Oral Microbiol. Immunol. 1999;14(6):364–370. doi: 10.1034/j.1399-302x.1999.140606.x. [DOI] [PubMed] [Google Scholar]
- 28.Taj-Aldeen S.J., Mir F.A., Sivaraman S.K., AbdulWahab A. Serum cytokine profile in patients with candidemia versus bacteremia. Pathogens. 2021;10(10):1349. doi: 10.3390/pathogens10101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y.C., Chen J.S., Yin C.H., Shin-Jung Lee S., Chen W.C. Candidemia in hospitalized cirrhotic patients with bloodstream infection: a retrospective analysis and brief summary of published studies. J. Chin. Med. Assoc. 2022;85(3):295–303. doi: 10.1097/JCMA.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao H., Jia H., Yuan X., Zhou Q., Li W., Shan H., et al. The value of dynamic monitoring of procalcitonin in the early identification of pathogens and prognosis of bloodstream infections in the ICU. Ann. Palliat. Med. 2021;10(12):12208–12217. doi: 10.21037/apm-21-3232. [DOI] [PubMed] [Google Scholar]
- 31.Song H., Liu J., Cao Z., Luo W., Chen J.Y. Analysis of disease profile, and medical burden by lead exposure from hospital information systems in China. BMC Publ. Health. 2019;19(1):1170. doi: 10.1186/s12889-019-7515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuijpers M.L., Vos F.J., Bleeker-Rovers C.P., Krabbe P.F., Pickkers P., van Dijk A.P., et al. Complicating infectious foci in patients with Staphylococcus aureus or Streptococcus species bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26(2):105–113. doi: 10.1007/s10096-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 33.Larsen I.K., Pedersen G., Schønheyder H.C. Bacteraemia with an unknown focus: is the focus de facto absent or merely unreported? A one-year hospital-based cohort study. APMIS. 2011;119(4–5):275–279. doi: 10.1111/j.1600-0463.2011.02727.x. [DOI] [PubMed] [Google Scholar]
- 34.Zilberberg M.D., Shorr A.F., Micek S.T., Vazquez-Guillamet C., Kollef M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit. Care. 2014;18(6):596. doi: 10.1186/s13054-014-0596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y., Wu X., Cheng Q., Li X. Inappropriate initial antimicrobial therapy for hematological malignancies patients with Gram-negative bloodstream infections. Infection. 2020;48(1):109–116. doi: 10.1007/s15010-019-01370-x. [DOI] [PubMed] [Google Scholar]
- 36.Sengupta M., Sarkar S., SenGupta M., Ghosh S., Sarkar R., Banerjee P. Biofilm producing Enterococcus isolates from vaginal microbiota. Antibiotics (Basel). 2021;10(9):1082. doi: 10.3390/antibiotics10091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hii I.M., Liu C.E., Lee Y.L., Liu W.L., Wu P.F., Hsieh M.H., et al. Resistance rates of non-albicans Candida infections in taiwan after the revision of 2012 clinical and laboratory standards Institute breakpoints. Infect. Drug Resist. 2019;12:235–240. doi: 10.2147/IDR.S184884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem. Med. 2016;26(1):5–16. doi: 10.11613/BM.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laupland K.B. Incidence of bloodstream infection: a review of population-based studies. Clin. Microbiol. Infect. 2013;19(6):492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 40.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020;33(3) doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billington E.O., Phang S.H., Gregson D.B., Pitout J.D., Ross T., Church D.L., Laupland K.B., Parkins M.D. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int. J. Infect. Dis. 2014;26:76–82. doi: 10.1016/j.ijid.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Pongrácz J., Kristóf K. Candida bloodstream infection: a clinical microbiology laboratory perspective. Acta Microbiol. Immunol. Hung. 2014;61(3):389–398. doi: 10.1556/AMicr.61.2014.3.11. [DOI] [PubMed] [Google Scholar]
- 43.MacBrayne C.E., Williams M.C., Prinzi A., Pearce K., Lamb D., Parker S.K. Time to blood culture positivity by pathogen and primary service. Hosp. Pediatr. 2021;11(9):953–961. doi: 10.1542/hpeds.2021-005873. [DOI] [PubMed] [Google Scholar]
- 44.Zeise K.D., Woods R.J., Huffnagle G.B. Interplay between Candida albicans and lactic acid bacteria in the gastrointestinal tract: impact on colonization resistance, microbial carriage, opportunistic infection, and host immunity. Clin. Microbiol. Rev. 2021;34(4) doi: 10.1128/CMR.00323-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akova M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence. 2016;7(3):252–266. doi: 10.1080/21505594.2016.1159366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timsit J.F., Ruppé E., Barbier F., Tabah A., Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi: 10.1007/s00134-020-05950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans R.N., Pike K., Rogers C.A., Reynolds R., Stoddart M., Howe R., et al. Modifiable healthcare factors affecting 28-day survival in bloodstream infection: a prospective cohort study. BMC Infect. Dis. 2020;20(1):545. doi: 10.1186/s12879-020-05262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yo C.H., Hsein Y.C., Wu Y.L., Hsu W.T., Ma M.H., Tsai C.H., et al. Clinical predictors and outcome impact of community-onset polymicrobial bloodstream infection. Int. J. Antimicrob. Agents. 2019;54(6):716–722. doi: 10.1016/j.ijantimicag.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Hamada Y., Magarifuchi H., Oho M., Kusaba K., Nagasawa Z., Fukuoka M., et al. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: an eight-year retrospective comparison study. J. Infect. Chemother. 2015;21(7):527–530. doi: 10.1016/j.jiac.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Zheng C.F., Liu W.Y., Zeng F.F., Zheng M.H., Shi H.Y., Zhou Y., et al. Prognostic value of platelet-to-lymphocyte ratios among critically ill patients with acute kidney injury. Crit. Care. 2017;21(1):238. doi: 10.1186/s13054-017-1821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buonacera A., Stancanelli B., Colaci M., Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int. J. Mol. Sci. 2022;23(7):3636. doi: 10.3390/ijms23073636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salciccioli J.D., Marshall D.C., Pimentel M.A., et al. The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: an observational cohort study. Crit. Care. 2015;19(1):13. doi: 10.1186/s13054-014-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crouser E.D., Parrillo J.E., Seymour C., Angus D.C., Bicking K., Tejidor L., et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest. 2017;152(3):518–526. doi: 10.1016/j.chest.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marik P.E., Stephenson E. The ability of Procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. Analysis of a large database. J. Crit. Care. 2020;60:135–139. doi: 10.1016/j.jcrc.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q., Yang M., Wang C., Cui J., Li X., Wang C. Diagnostic efficacy of serum cytokines and chemokines in fungal bloodstream infection in febrile patients. J. Clin. Lab. Anal. 2020;34(4) doi: 10.1002/jcla.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray C.K., Hoffmaster R.M., Schmit D.R., Hospenthal D.R., Ward J.A., Cancio L.C., et al. Evaluation of white blood cell count, neutrophil percentage, and elevated temperature as predictors of bloodstream infection in burn patients. Arch. Surg. 2007;142(7):639–642. doi: 10.1001/archsurg.142.7.639. [DOI] [PubMed] [Google Scholar]
- 57.Djordjevic D., Rondovic G., Surbatovic M., Stanojevic I., Udovicic I., Andjelic T., et al. Neutrophil-to-Lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu L., Shi Q., Shi M., Liu R., Wang C. Diagnostic value of PCT and CRP for detecting serious bacterial infections in patients with fever of unknown origin: a systematic review and meta-analysis. Appl. Immunohistochem. Mol. Morphol. 2017;25(8):e61–e69. doi: 10.1097/PAI.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 59.Simon L., Gauvin F., Amre D.K., Saint-Louis P., Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 2004;39(2):206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 60.Perrella A., Giuliani A., De Palma M., Castriconi M., Molino C., Vennarecci G., et al. C-reactive protein but not procalcitonin may predict antibiotic response and outcome in infections following major abdominal surgery. Updates Surg. 2022;74(2):765–771. doi: 10.1007/s13304-021-01172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C., Cao J., Wang L., Jia X., He J., Zhang L. Up-regulation of chemokine CXCL13 in systemic candidiasis. Clin. Immunol. 2018;191:1–9. doi: 10.1016/j.clim.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Zhao L., Zang X., Chen W., Sheng B., Gu X., Zhang J. [Analysis of correlation between inflammatory parameters and severity of sepsis caused by bacterial bloodstream infection in septic patients] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27(6):448–453. doi: 10.3760/cma.j.issn.2095-4352.2015.06.007. Chinese. [DOI] [PubMed] [Google Scholar]
- 63.Wu H.N., Yuan E.Y., Li W.B., Peng M., Zhang Q.Y., Xie K.L. Microbiological and clinical characteristics of bloodstream infections in general intensive care unit: a retrospective study. Front. Med. 2022;9 doi: 10.3389/fmed.2022.876207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garsin D.A., Lorenz M.C. Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism? Gut Microb. 2013;4(5):409–415. doi: 10.4161/gmic.26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q., Wang C., Yang M., Li X., Cui J., Wang C. Diagnostic efficacy of serum cytokines and chemokines in patients with candidemia and bacteremia. Cytokine. 2020;130 doi: 10.1016/j.cyto.2020.155081. [DOI] [PubMed] [Google Scholar]
- 66.Allison D.L., Scheres N., Willems H.M.E., Bode C.S., Krom B.P., Shirtliff M.E. The host immune system facilitates disseminated Staphylococcus aureus disease due to phagocytic attraction to Candida albicans during coinfection: a case of bait and switch. Infect. Immun. 2019;87(11) doi: 10.1128/IAI.00137-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.