Abstract
Objective
To investigate pain course over time and to identify baseline and 3-month predictors of unacceptable pain with or without low inflammation in early RA.
Methods
A cohort of 275 patients with early RA, recruited in 2012–2016, was investigated and followed for 2 years. Pain was assessed using a visual analogue scale (VAS; 0–100 mm). Unacceptable pain was defined as VAS pain >40, and low inflammation as CRP <10 mg/l. Baseline and 3-month predictors of unacceptable pain were evaluated using logistic regression analysis.
Results
After 2 years, 32% of patients reported unacceptable pain. Among those, 81% had low inflammation. Unacceptable pain, and unacceptable pain with low inflammation, at 1 and 2 years was significantly associated with several factors at 3 months, but not at baseline. Three-month predictors of these pain states at 1 and 2 years were higher scores for pain, patient global assessment, and the health assessment questionnaire, and more extensive joint tenderness compared with the number of swollen joints. No significant associations were found for objective inflammatory measures.
Conclusion
A substantial proportion of patients had unacceptable pain with low inflammation after 2 years. Three months after diagnosis seems to be a good time-point for assessing the risk of long-term pain. The associations between patient reported outcomes and pain, and the lack of association with objective inflammatory measures, supports the uncoupling between pain and inflammation in RA. Having many tender joints, but more limited synovitis, may be predictive of long-term pain despite low inflammation in early RA.
Keywords: rheumatoid arthritis, pain, patient reported outcomes, prediction, non-inflammatory pain, tender-swollen difference
Rheumatology key messages.
Nearly one-third of patients suffered from unacceptably high pain levels two years after rheumatoid arthritis (RA) diagnosis.
Three months after diagnosis was a better time-point for predicting long-term pain compared with baseline.
Patient-reported outcomes and extensive joint tenderness at 3-months follow-up predict long-term pain in RA.
Introduction
Rheumatoid arthritis (RA) is characterised by joint inflammation, resulting in stiffness and pain. Pain is a debilitating symptom of RA and has been reported as the most important predictor of psychosocial health and general health perception [1, 2]. Despite significant improvement in the treatment of RA over the last 20 years, pain remains a problem for a significant group of patients [3, 4]. Pain in RA is traditionally considered to be caused by inflammation, i.e. nociceptive pain. However, several studies indicate that uncoupling between pain and inflammation occurs, and that pain sometimes persists despite inflammation control [5, 6].
Nociplastic pain has been defined as ‘Pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain’ [7]. Such nociplastic pain could explain why a subgroup of patients with RA suffer from pain despite inflammation control. In line with this, previous studies have shown decreased pressure pain thresholds and increased temporal summation in patients with RA, which are signs of central sensitisation [8, 9]. Furthermore, the prevalence of fibromyalgia in RA has been reported to be up to 10 times higher compared with the general population [10].
Non-inflammatory pain could confound the interpretation of composite measures like DAS28, and may bias estimation of disease activity, e.g. in cases with extensive joint tenderness but limited synovitis. This could lead to patients receiving inadequate treatment that is not tailored to the patients’ needs. Measures like the ratio of tender to swollen joint counts (tender-swollen ratio) and the difference between tender and swollen joint counts (tender-swollen difference) have been associated with non-inflammatory pain mechanisms and may possibly help clinicians to better interpret DAS28 and differentiate between inflammatory disease activity and non-inflammatory pain [11].
Previously, we have investigated predictors of unacceptable pain, overall and with low inflammation, in an inception cohort of early RA patients with inclusion during 1995–2005 [12], before the implementation of a treat-to-target strategy [13], and before biologic DMARDs (bDMARDs) became standard treatment in severe disease. We found that low baseline swollen joint count and worse baseline patient reported outcomes (PROs) were risk factors for long-lasting pain in RA, and that anti-CCP negative patients were at greater risk of non-inflammatory pain. To improve pain management in RA there is need for new studies of predictors of persistent pain in the modern treatment era. It has previously been suggested that parameters of inflammation and disease activity are similar at diagnosis in patients with different pain phenotypes, and that differences are instead larger 3 months later [14]. Therefore, investigating predictors of long-term pain at a follow-up 3 months after diagnosis could be important to better predict future pain.
In this study we aimed to examine the course of pain and proportion of patients with unacceptable pain in early RA, and to identify those with increased risk of unacceptable pain, and unacceptable pain with low inflammation, up to 2 years after RA diagnosis using patient characteristics at diagnosis and after 3 months of follow-up.
Methods
Patients
An inception cohort of patients with early RA recruited in 2012–2016 (symptom duration ≤12 months) was investigated. All patients fulfilled the 2010 ACR/EULAR classification criteria for RA [15], or the 1987 ACR classification criteria [16] at inclusion, were diagnosed and followed-up at the rheumatology outpatient clinic of Skåne University Hospital and were included in the Swedish Rheumatology Quality register (SRQ). SRQ is a national clinical register of patients with chronic inflammatory joint diseases. The study was approved by the Regional Ethical Review Board for Southern Sweden (2018/878; 30 DEC 2018), with complementary approval for the use of comorbidities from a regional register (2022/01811/01; 20 APR 2022). All patients gave their informed consent before inclusion in the register. In accordance with Swedish law, written consent was not required for inclusion in the register, or for inclusion in the present study.
Clinical assessment
Clinical data was retrieved from the SRQ at inclusion and after 3, 6, 12 and 24 months. Acceptable time windows for every follow-up visit were defined in consistency with those used in the development of the LUNDEX method [17], i.e. month 6 visit: >5 and ≤8 months from inclusion; month 12 visit: >10 and ≤15 months from inclusion; month 24 visit: >22 and ≤27 months from inclusion. In addition, acceptable time windows for the month 3 visit were >2 and <5 months from inclusion. The visit closest to the selected follow-up time, with most complete data was used.
Pain, fatigue and the patients’ global assessment of disease activity (PGA) were assessed with a visual analogue scale (VAS; 0–100 mm) (Supplementary Table S1, available at Rheumatology online). For measuring disease activity, the DAS28 computed with CRP (DAS28-CRP) was used. This measure was chosen due to more missing data for ESR. Low disease activity (LDA) was defined as DAS28-CRP ≤3.2. Disability was evaluated with the Swedish validated version of the Stanford health assessment questionnaire (HAQ) [18]. These parameters, as well as dates of initiating and discontinuing anti-rheumatic treatment and treatment with glucocorticosteroids, were prospectively collected and registered in the SRQ by the physicians at every follow-up visit as part of standard clinical care. Tender-swollen difference was calculated as tender joint count out of 28 (TJC28) minus swollen joint count out of 28 (SJC28). Patients were defined as tender-dominated if the number of tender joints was >50% greater than the number of swollen joints (TJC28 >SJC28 × 1.5). Missing data in SRQ were retrieved by review of electronic medical records, where possible.
Laboratory parameters (ESR, CRP, RF and anti-CCP2 antibodies) were retrieved from clinical records and assessed using standard methods at the University Hospitals in Malmö and Lund.
Data on comorbidities were collected from linkage to a regional healthcare register, established in 1998, and were defined based on ICD-10 codes from inpatient and outpatient care, including primary care [19]. Comorbidities were divided into three groups as follows: pain-related comorbidities, psychiatric comorbidities and other relevant comorbidities (Supplementary Table S2, available at Rheumatology online). A comorbidity was considered prevalent if an ICD-10 code of the diagnosis was present in the register at any time before the inclusion date. In addition, the Charlson comorbidity index [20] was calculated for every patient.
Assessment of unacceptable pain and low inflammation
Unacceptable pain was defined as VAS pain >40 mm, based on the patient acceptable symptom state (PASS) [21] – a validated measure, captured from patient reports, indicating the cut-off level of acceptable pain. Low inflammation was defined as CRP <10 mg/l [5, 22], and a strict definition of low inflammation as CRP <10 mg/l and SJC28 ≤ 1 [5]. In addition, in a sensitivity analysis, low inflammation was defined as CRP <3 mg/l.
Statistics
Change in pain between visits was evaluated using the paired t test. Normality distribution of data was assessed using the Shapiro–Wilk test. Correlations between variables were assessed with Spearman’s rank test or Pearson’s test, as appropriate. Potential predictors (chosen based on subject matter knowledge), at baseline and at 3 months after inclusion, of the primary outcomes were evaluated using univariate and multivariate logistic regression analysis. The results were presented as odds ratios (OR) with 95% CI. Continuous variables were analysed per standard deviation. For the multivariate analyses, covariates were chosen based on the univariate analysis. Variables with a P-value ≤0.10 were potential candidates, and in case of collinearity (bivariate correlation between covariates with r > 0.3) only the covariate with the strongest association with the outcome variable was included in the multivariate regression model. In addition, the multivariate analysis was adjusted for the comorbidity group with greatest impact on the outcome variable. IBM SPSS statistics version 26 was used for all statistical analyses.
Results
Patients
A total of 275 patients with early RA (median symptom duration 5 months) were investigated in this study (Table 1). There were 148 and 117 patients with data on pain at the 1- and 2-year follow-up, respectively (Table 1). Most patients were treated with methotrexate, and 43% were treated with a bDMARD after 2 years. Nearly one-third of the patients had a diagnosis of psychiatric comorbidity at some point before inclusion and nearly half of the patients had a diagnosis of pain-related comorbidity (Supplementary Table S2).
Table 1.
Patient characteristics at inclusion and at follow-up visits.
| Characteristic | Inclusion | 3 months | 6 months | 12 months | 24 months |
|---|---|---|---|---|---|
| N | 269 | 191 | 169 | 185 | 157 |
| ACR/EULAR 2010a, n (%) | 260 (96.7) | 185 (96.9) | 162 (95.9) | 181 (97.8) | 152 (96.8) |
| ACR 1987a, n (%) | 197 (73.2) | 140 (73.3) | 122 (72.2) | 134 (72.4) | 118 (75.2) |
| Sex, female, n (%) | 198 (73.6) | 140 (73.3) | 125 (74.0) | 133 (71.9) | 114 (72.6) |
| Age, mean (SD), years | 58.9 (16.0) | 58.4 (15.7) | 58.0 (15.7) | 58.4 (15.2) | 59.4 (15.8) |
| Symptom duration at inclusion, months | 5.0 (3.0–8.0) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 5.0 (3.0–8.0) | 5.0 (3.0–7.0) |
| RF positive at inclusion, n/N (%) | 182/266 (68.4) | 129/188 (67.5) | 112/167 (67.1) | 126/183 (68.9) | 112/155 (72.3) |
| Anti-CCP positive at inclusion, n (%) | 182 (67.7) | 137 (71.7) | 119 (70.4) | 123 (66.5) | 114 (72.6) |
| Prednisolone, n (%) | 128 (47.6) | 88 (46.1) | 84 (49.7) | 63 (34.1) | 54 (34.4) |
| Methotrexate, n (%) | 177 (65.8) | 169 (88.5) | 143 (84.6) | 160 (86.5) | 122 (77.7) |
| >1 csDMARD, n (%) | 6 (2.3) | 8 (4.2) | 13 (7.7) | 11 (5.9) | 10 (6.4) |
| bDMARD, n (%) | 27 (10.3) | 45 (23.6) | 61 (36.1) | 79 (42.7) | 70 (44.6) |
| No csDMARD, n (%) | 79 (29.4) | 11 (5.8) | 17 (10.1) | 15 (8.1) | 25 (15.9) |
| VAS pain, mean (S.D.) | 50.7 (28.1) | 30.8 (26.8) | 28.5 (25.0) | 28.0 (24.9) | 26.9 (26.9) |
| SJC28 | 4.5 (2.0–9.0) | 1.0 (0–3.0) | 1.0 (0–3.0) | 1.0 (0–3.0) | 0 (0–2.0) |
| TJC28 | 6.0 (2.0–10.0) | 2.0 (0–6.0) | 1.0 (0–4.0) | 2.0 (0–5.0) | 1.0 (0–4.0) |
| HAQ | 1.0 (0.5–1.4) | 0.6 (0.1–1.0) | 0.5 (0.1–1.0) | 0.5 (0.1–1.1) | 0.5 (0.1–1.0) |
| CRP (mg/l) | 7.3 (2.8–24.0) | 3.7 (1.2–8.1) | 2.8 (1.1–6.3) | 2.2 (0.9–5.9) | 2.3 (0.8–5.8) |
| ESR (mm/h) | 26.0 (13.0–47.0) | 15.0 (9.0–26.0) | 14.0 (7.0–25.8) | 12.0 (7.0–23.0) | 16.0 (7.0–27.0) |
| VAS PGA, mean (S.D.) | 49.1 (28.2) | 32.8 (26.4) | 29.9 (26.2) | 29.5 (24.9) | 28.5 (26.7) |
| DAS28-CRP, mean (S.D.) | 4.4 (1.5) | 3.2 (1.3) | 2.9 (1.2) | 2.8 (1.1) | 2.7 (1.2) |
| Low disease activityb, n/N (%) | 47/213 (22.1) | 92/166 (55.4) | 87/132 (65.9) | 91/147 (61.9) | 77/115 (67.0) |
| Tender-swollen difference | 0 (0–3) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| Tender-dominatedc, n (%) | 73 (27.2) | 64 (33.5) | 57 (33.7) | 69 (37.3) | 54 (34.6) |
Values are median (interquartile range) unless otherwise indicated. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; DAS28-CRP: Disease Activity Score in 28 joints calculated with CRP; PGA: patient global assessment; SJC28: Swollen Joint Count in 28 joints; TJC28: Tender Joint Count in 28 joints; VAS: Visual Analogue Scale.
Data at inclusion: VAS pain (n = 216), VAS PGA (n = 217), HAQ (n = 215), DAS28-CRP (n = 213), CRP (n = 265), ESR (n = 243). Data at 3 months: VAS pain (n = 168), VAS PGA (n = 168); HAQ (n = 166), DAS28-CRP (n = 167), CRP (n = 190), ESR (n = 135). Data at 6 months: VAS pain (n = 133), VAS PGA (n = 134), HAQ (n = 133), DAS28-CRP (n = 132), CRP (n = 167), ESR (n = 136). Data at 12 months: VAS pain (n = 148), VAS PGA (n = 148), HAQ (n = 145), DAS28-CRP (n = 148), CRP (n = 183), ESR (n = 153).
Data at 24 months: VAS pain (n = 117), VAS PGA (n = 116), HAQ (n = 109), DAS28-CRP (n = 115), CRP (n = 155), ESR (n = 129).
Classification criteria fulfilled at inclusion.
DAS-28-CRP ≤3.2.
TJC28 > SJC28 × 1.5.
Pain over time
Mean VAS pain decreased significantly from inclusion to 3 months, and then remained largely unchanged during the rest of the follow-up period (Table 2). The mean decrease in pain from inclusion to 3 months was 23.5 (95% CI: 18.3–28.8). After 3 months there was no significant change in pain between the follow-up visits (Table 2).
Table 2.
VAS pain from inclusion to 2 years, and change in pain from the last follow-up.
| VAS pain | Δ VAS paina | |
|---|---|---|
| Inclusion | 50.7 (47.0, 54.4) | NA |
| 3 months | 30.8 (26.7, 34.9) | −23.5 (−28.8, −18.3) |
| 6 months | 28.5 (24.2, 32.8) | 0.1 (−4.3, 4.4) |
| 12 months | 28.0 (24.1, 31.9) | −1.4 (−6.4, 3.6) |
| 24 months | 26.9 (22.0, 31.8) | −4.0 (−10.1, 2.2) |
Means with 95% confidence intervals. NA: not applicable, VAS: visual analogue scale.
Paired samples t test.
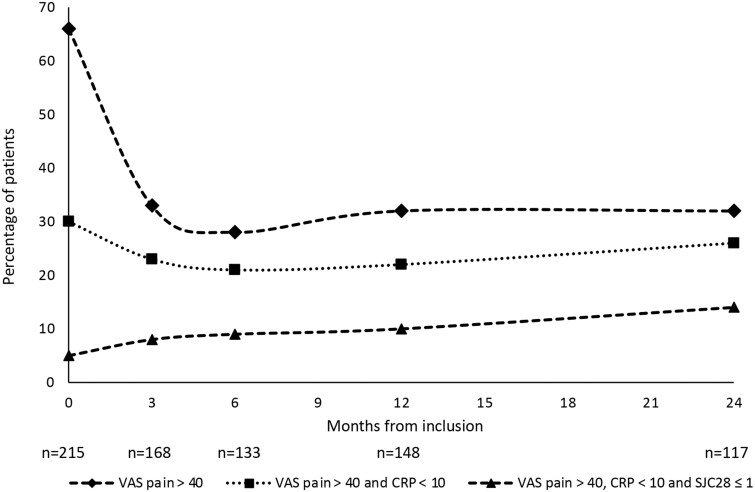
Sixty-six percent of the patients had unacceptable pain at inclusion, decreasing to 33% at 3 months. After 3 months the proportion of patients with unacceptable pain was essentially unchanged during the rest of the follow-up time (Fig. 1). Thirty percent of the patients had unacceptable pain with low inflammation at inclusion. This proportion decreased from inclusion to 6 months, and thereafter increased slightly during the rest of the follow-up period. The fraction of patients with unacceptable pain with the strict definition of low inflammation increased during the follow-up period, from 5% at inclusion to 14% at 2 years (Fig. 1).
Figure 1.
Percentage of patients with unacceptable pain over time, overall and in patients with low inflammation. Unacceptable pain: VAS pain >40. Low inflammation (standard): CRP <10. Low inflammation (strict): CRP <10 and SJC28 ≤ 1. SJC28: swollen joint count in 28 joints; VAS: visual analogue scale
Predictors of unacceptable pain
At baseline
There was no major difference in baseline treatment with methotrexate or bDMARDs in patients with and without unacceptable pain at 1 and 2 years (Supplementary Table S3, available at Rheumatology online). In univariate analysis, significant baseline predictors of unacceptable pain at 1 year were higher HAQ and female sex (Table 3). Fatigue was the only significant baseline predictor of unacceptable pain at 2 years. There was a borderline association between other relevant comorbidities before inclusion and unacceptable pain at 1 year, whereas psychiatric comorbidities were associated with unacceptable pain at 2 years in univariate analysis (Supplementary Table S4, available at Rheumatology online). When analysing the different psychiatric comorbidities separately, significant associations remained for anxiety-related conditions, but not for depression (Supplementary Table S5, available at Rheumatology online). Charlson comorbidity index was not associated with unacceptable pain at any time point (Supplementary Table S4). In multivariate logistic regression analysis, with further adjustment for other relevant comorbidities, there was a significant association for HAQ [OR 1.52 (95% CI 1.03–2.24)], but not for female sex [OR 1.90 (95% CI 0.72–5.03)] with unacceptable pain at 1 year.
Table 3.
Baseline predictors of unacceptable pain and unacceptable pain with low inflammation at follow-ups.
| Unacceptable pain |
Unacceptable pain with low inflammation |
|||
|---|---|---|---|---|
| 1 year | 2 years | 1 year | 2 years | |
| Variable | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Female | 2.59 (1.09, 6.13) | 0.90 (0.38, 2.12) | 3.72 (1.22, 11.34) | 1.13 (0.44, 2.87) |
| RF positive | 1.31 (0.60, 2.87) | 0.67 (0.28, 1.59) | 0.82 (0.36, 1.87) | 0.63 (0.25, 1.55) |
| Anti-CCP positive | 0.59 (0.29, 1.22) | 0.74 (0.32, 1.73) | 0.67 (0.30, 1.50) | 0.69 (0.28, 1.69) |
| Age | 1.07 (0.72, 1.60) | 1.11 (0.73, 1.68) | 1.26 (0.79, 2.01) | 0.90 (0.58, 1.39) |
| Symptom duration | 1.04 (0.73, 1.50) | 0.92 (0.60, 1.39) | 1.07 (0.72, 1.60) | 1.02 (0.66, 1.60) |
| VAS pain | 1.35 (0.91, 2.02) | 1.23 (0.76, 2.04) | 1.40 (0.90, 2.17) | 1.19 (0.70, 2.03) |
| SJC28 | 0.96 (0.67, 1.40) | 0.94 (0.65, 1.36) | 0.90 (0.60, 1.35) | 0.99 (0.67, 1.46) |
| TJC28 | 1.07 (0.76, 1.49) | 1.20 (0.83, 1.73) | 0.96 (0.66, 1.41) | 1.25 (0.85, 1.84) |
| HAQ | 1.58 (1.08, 2.30) | 1.33 (0.86, 2.07) | 1.50 (1.00, 2.25) | 1.14 (0.72, 1.82) |
| CRP | 0.99 (0.72, 1.35) | 0.72 (0.44, 1.17) | 0.72 (0.43, 1.19) | 0.61 (0.33, 1.15) |
| ESR | 1.01 (0.94, 1.46) | 0.88 (0.59, 1.30) | 0.83 (0.53, 1.30) | 0.77 (0.49, 1.21) |
| VAS PGA | 1.26 (0.83, 1.92) | 1.49 (0.91, 2.42) | 1.38 (0.87, 2.20) | 1.18 (0.71, 1.98) |
| Fatigue | 1.34 (0.83, 2.17) | 1.99 (1.03, 3.84) | 1.56 (0.93, 2.63) | 1.70 (0.84, 3.45) |
| DAS28-CRP | 1.08 (0.72, 1.63) | 1.07 (0.65, 1.74) | 1.05 (0.67, 1.65) | 1.03 (0.61, 1.74) |
| Tender-swollen difference | 1.16 (0.82, 1.64) | 1.29 (0.93, 1.79) | 1.07 (0.73, 1.57) | 1.29 (0.91, 1.81) |
| Tender-dominateda | 1.11 (0.52, 2.40) | 1.35 (0.59, 3.13) | 1.34 (0.58, 3.09) | 1.45 (0.60, 3.49) |
Univariate logistic regression analysis. Odds ratios are calculated per standard deviation for continuous variables. Values in bold indicate statistical significance with P-values <0.05. Unacceptable pain: VAS pain >40. Low inflammation: CRP <10.
DAS28-CRP: disease activity score in 28 joints calculated with CRP; OR: odds ratio; PGA: patient global assessment; SJC28: swollen joint count in 28 joints; TJC28: tender joint count in 28 joints; VAS: visual analogue scale.
TJC28 > SJC28 × 1.5.
At 3-month follow-up
A larger proportion of the patients without unacceptable pain at 1 year were treated with methotrexate at three months, compared with those with unacceptable pain at 1 year. However, treatment with methotrexate at 3 months was similar in patients with or without unacceptable pain at 2 years (Supplementary Table S6, available at Rheumatology online). In univariate analysis, significant 3-month predictors of unacceptable pain at both 1 and 2 years were higher VAS pain, higher HAQ, higher TJC28, higher VAS PGA and higher DAS28-CRP, while LDA was a negative predictor and the tender-swollen difference reached significance only for the 2-year outcome (Table 4). In multivariate analysis of 3-month predictors of unacceptable pain at 1 year, significant associations remained for HAQ, but not for female sex and tender-swollen difference (Table 5). In the corresponding multivariate analysis of predictors of unacceptable pain at 2 years, VAS PGA remained significantly predictive (Table 5). Adjustment for psychiatric comorbidities did not have a major impact on the results (Table 5). The selection of variables for the multivariate analyses is described in Supplementary Table S7, available at Rheumatology online.
Table 4.
Three-month predictors of unacceptable pain and unacceptable pain with low inflammation at follow-ups.
| Unacceptable pain |
Unacceptable pain with low inflammation |
|||
|---|---|---|---|---|
| 1 year | 2 years | 1 year | 2 years | |
| Variable | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| VAS pain | 2.08 (1.32, 3.27) | 2.73 (1.61, 4.60) | 2.28 (1.39, 3.74) | 2.84 (1.65, 4.87) |
| SJC28 | 1.26 (0.89, 1.78) | 1.19 (0.77, 1.83) | 1.35 (0.95, 1.92) | 1.25 (0.80, 1.93) |
| TJC28 | 1.68 (1.12, 2.52) | 1.73 (1.07, 2.81) | 1.75 (1.16, 2.64) | 1.81 (1.11, 2.97) |
| HAQ | 2.61 (1.63, 4.18) | 2.36 (1.45, 3.83) | 2.59 (1.57, 4.25) | 2.12 (1.32, 3.40) |
| CRP | 1.27 (0.88, 1.84) | 1.27 (0.88, 1.84) | 1.12 (0.78, 1.62) | 1.27 (0.88, 1.84) |
| ESR | 1.52 (0.95, 2.45) | 1.30 (0.80, 2.12) | 1.19 (0.74, 1.91) | 1.29 (0.78, 2.13) |
| VAS PGA | 2.25 (1.43, 3.56) | 3.32 (1.87, 5.88) | 2.69 (1.59, 4.54) | 3.05 (1.73, 5.38) |
| DAS28-CRP | 2.05 (1.34, 3.14) | 2.17 (1.33, 3.55) | 2.16 (1.36, 3.44) | 2.15 (1.31, 3.54) |
| Low disease activitya | 0.25 (0.10, 0.59) | 0.17 (0.06, 0.48) | 0.19 (0.07, 0.55) | 0.20 (0.07, 0.60) |
| Tender-swollen difference | 1.47 (0.97, 2.22) | 2.00 (1.14, 3.52) | 1.46 (0.97, 2.19) | 2.02 (1.15, 3.58) |
| Tender-dominatedb | 2.15 (0.97, 4.81) | 1.87 (0.75, 4.69) | 3.01 (1.24, 7.34) | 2.05 (0.79, 5.32) |
Univariate logistic regression analysis. Odds ratios are calculated per standard deviation for continuous variables. Bold text indicates statistical significance with P-values <0.05. Italic text indicates P-values ≥0.05 and <0.10. Bold plus italic text indicates P-values <0.001. Unacceptable pain: VAS pain >40. Low inflammation: CRP <10.
DAS28-CRP: disease activity score in 28 joints calculated with CRP; OR: odds ratio; PGA: patient global assessment; SJC28: swollen joint count in 28 joints; TJC28: tender joint count in 28 joints; VAS: visual analogue scale.
DAS28-CRP ≤3.2.
TJC28 > SJC28 × 1.5.
Table 5.
Three-month predictors of unacceptable pain and unacceptable pain with low inflammation, multivariate analysis.
| Variable | Basic model | Adj. for comorbidities |
|---|---|---|
| Unacceptable pain | ||
| 1 year after inclusion | ||
| Female sex | 1.58 (0.51, 4.86) | 1.64 (0.53, 5.14)a |
| HAQ | 2.44 (1.50, 3.95) | 2.40 (1.47, 3.92)a |
| Tender-swollen difference | 1.17 (0.78, 1.75) | 1.14 (0.75, 1.71)a |
| 2 years after inclusion | ||
| PGA | 3.02 (1.68, 5.43) | 2.79 (1.52, 5.12)b |
| Tender-swollen difference | 1.67 (0.87, 3.20) | 1.65 (0.86, 3.18)b |
| Unacceptable pain with low inflammation | ||
| 1 year after inclusion | ||
| Female sex | 5.57 (1.07, 28.83) | 5.14 (1.01, 26.17)b |
| PGA | 2.14 (1.16, 3.95) | 2.54 (1.44, 4.50)b |
| SJC28 | 1.56 (0.94, 2.58) | NIc |
| Tender-dominatedd | 6.18 (1.64, 23.27) | 3.58 (1.21, 10.62)b |
| 2 years after inclusion | ||
| PGA | 2.77 (1.54, 4.98) | 2.64 (1.44, 4.83)b |
| Tender-swollen difference | 1.64 (0.87, 3.11) | 1.63 (0.86, 3.07)b |
Both models include all variables in each sub-table. Results are presented as odds ratios (95% CI). Odds ratios are calculated per standard deviation for continuous variables. Unacceptable pain: VAS pain > 40. Low inflammation: CRP < 10.
NI: not included; PGA: patient global assessment; SJC28: swollen joint count in 28 joints.
Adjusted for other relevant comorbidities.
Adjusted for psychiatric comorbidities.
Excluded from this model due to limited power.
TJC28 > SJC28 × 1.5.
Predictors of unacceptable pain with low inflammation
At baseline
Treatment with methotrexate and with bDMARDs was similar at baseline in patients with or without unacceptable pain plus low inflammation at 1 and 2 years (Supplementary Table S8, available at Rheumatology online). In univariate analysis, female sex and higher HAQ were the only significant baseline predictors of unacceptable pain with low inflammation at 1 year, and there were no significant baseline predictors of this state at 2 years (Table 3). Among the comorbidity subsets, psychiatric comorbidities had the strongest association with unacceptable pain plus low inflammation at 1 and 2 years (Supplementary Table S4). In sensitivity analysis of CRP <3 mg/l as the definition of low inflammation, the results were largely similar to the main analysis, except that being tender-dominated was predictive of this outcome at 1 year (Supplementary Table S9, available at Rheumatology online). In multivariate analysis of baseline predictors of unacceptable pain with low inflammation at 1 year, including female sex and HAQ, with further adjustment for psychiatric comorbidities, there were no significant associations, although the same tendencies were seen as in the univariate analyses [adjusted ORs 1.41 (95% CI 0.93–2.13) per SD for HAQ and 2.39 (95% CI 0.74–7.71) for female sex].
At 3-month follow-up
A larger proportion of patients without unacceptable pain plus low inflammation at 1 year were treated with methotrexate at three months, compared with patients with unacceptable pain plus low inflammation (Supplementary Table S10, available at Rheumatology online). Three-month predictors of unacceptable pain with low inflammation at 1 and 2 years in univariate analysis were higher VAS pain, higher TJC28, higher HAQ, higher VAS PGA and higher DAS28-CRP, while LDA was a negative predictor (Table 4). Being tender-dominated was a significant predictor of this outcome at 1 year, and a higher tender-swollen difference for the 2-year outcome. In sensitivity analysis with CRP <3 mg/l as the definition of low inflammation, the results were similar to the main analysis (Supplementary Table S11, available at Rheumatology online). In multivariate analyses of 3-month predictors, there were significant associations for female sex, VAS PGA and being tender-dominated with unacceptable pain plus low inflammation at 1 year, whereas VAS PGA was predictive of this outcome at 2 years in adjusted analysis (Table 5). Further adjustment for psychiatric comorbidities did not have a major impact on the results (Table 5).
Discussion
We have investigated baseline and 3-month predictors of unacceptable pain with and without low inflammation 1 and 2 years after diagnosis in patients with RA. Nearly one-third of the patients had unacceptable pain at 1 and 2 years, and among those >70% had low inflammation. Mean VAS pain improved from inclusion to three months but was thereafter more or less unchanged during the rest of the follow-up period. Female sex and HAQ were the only significant baseline predictors of unacceptable pain both with and without low inflammation. However, at the three-month visit there were statistically significant associations for pain, PGA, HAQ, TJC28, DAS28-CRP and a higher tender-swollen difference with unacceptable pain with and without low inflammation at 1 and 2 years. In the multivariate analysis, significant associations remained for PGA with unacceptable pain at 2 years, and with unacceptable pain plus low inflammation at 1 and 2 years.
In this cohort nearly 80% of the patients with unacceptable pain at 2 years also had low inflammation. This likely explains why predictors of unacceptable pain and unacceptable pain with low inflammation were essentially the same. Most patients were quite well treated and did not have any substantial inflammatory activity. Pain without any clinically observed inflammation could possibly be due to central sensitisation, which has been supported by several studies [8, 11, 23]. It could also be due to subclinical disease activity. In one study, investigating subclinical synovitis with ultrasound [24], only 50% of the patients in clinical remission were also in ultrasound remission. The group of patients with unacceptable pain plus low inflammation could therefore include both patients with undetected, residual inflammation and patients with nociplastic pain not related to inflammation. Pain in RA without inflammation could also be due to comorbidities. However, in our cohort, adjustment for comorbidity groups that were associated with pain did not have a major impact on the results, suggesting that, in this case, associations between clinical parameters in early RA and pain at later follow-ups are not explained by comorbidities.
More significant associations with the pain outcomes were found for predictors at 3 months compared with baseline. At baseline the disease is not yet under inflammatory control and worse PROs could be due to high disease activity, but also other pain mechanisms like central sensitisation. After 3 months a substantial group of patients will probably have responded to treatment, resulting in improved PROs. Consequently, the group of patients with worse PROs at 3 months will be more homogeneous compared with baseline, which could explain why we found more significant 3-month predictors. In line with this, Lötsch et al. identified three different patient groups in early RA, based on persistence of pain (low, medium and high persistent pain). At baseline, measures like DAS28 and HAQ did not differ notably between the groups, but at 3 months there were significant differences [14].
We found that having higher PROs, i.e. PGA, pain and HAQ, at the three-month follow-up was associated with increased risk of unacceptable pain with or without low inflammation after 1 and 2 years. The more objective measures were not associated with future pain. DAS28-CRP includes both objective and subjective measures and was a significant predictor of unacceptable pain. However, looking at its subcomponents separately, only the more subjective measures, TJC28 and PGA, reached statistical significance, whereas SJC28 and CRP did not. Tender joint counts have previously been shown to more closely associate with pain compared with swollen joint counts [25], and the association between PROs and pain, as well as the lack of association between inflammatory markers and pain, suggests that long-term pain, in this cohort, is likely more nociplastic than nociceptive. Furthermore, we found that patients reaching LDA at 3 months were less likely to have future unacceptable pain, suggesting that patients not responding to treatment after 3 months are at increased risk of long-lasting pain. These patients could probably benefit from early pain interventions.
Tender-swollen ratio and tender-swollen difference have previously been suggested as measures indicative of non-inflammatory pain, and as predictors of response to tumor necrosis factor (TNF) inhibitors, where a high ratio would be associated with poor response [26]. Furthermore, Pollard et al. identified RA patients with fibromyalgia by using a cut-off point of tender-swollen difference ≥7 [27]. In our study, having more tender joints than swollen joints after 3 months follow-up was predictive of unacceptable pain and unacceptable pain with low inflammation at 1 and 2 years. As previously stated by McWilliams et al., pain might be more likely to persist where a non-inflammatory pain mechanism contributes to the pain spectrum [11], therefore patients would not respond to glucocorticoid or bDMARD treatment with reduced pain, and other interventions are probably needed to help these patients. It has been reported that Janus kinase inhibitors may have a direct impact on pain, and that factors beyond anti-inflammatory properties could contribute to the analgesic effects [28]. Potentially, this could be a better anti-rheumatic treatment option in patients with persistent high pain levels. However, further research is needed in this area.
To improve pain management in RA, patients with increased risk of persistent high pain levels and nociplastic pain features need to be identified and selected for evidence-based interventions, some of which may not be in the rheumatologist’s domain. For example, treatment with the serotonin-norepinephrine inhibitor, milnacipran [29], and moderate exercise may reduce pain in such RA patients [30]. Furthermore, comorbidities, in particular anxiety and depression, need to be taken into account, treating the whole patient.
Limitations of the study include the modest sample size, partly resulting from missing data, especially for pain, PGA and HAQ, leading to a risk of type II error. Furthermore, there might be a selection bias for patients with more severe disease in the SRQ, which could explain the higher proportion of patients with anti-CCP antibodies in this cohort compared with our previous study [12]. Additionally, there was no standardised protocol for joint assessments, and different physicians examined the patients during follow-up visits as standard clinical practice, which could lead to discrepancies in the assessment of tender and swollen joints. Finally, as this is an exploratory study, based on a retrospective sample, the results need to be tested in a prospective study.
The strengths of this study include the investigation of patients seen in daily clinical practice who have been treated and followed-up similar to patients seen in the clinic today. We have also adjusted our models for potential systemically captured comorbidities that could have an impact on long-term pain, strengthening the validity of our results.
Conclusion
In this cohort of patients with early RA we found that nearly one-third of the patients suffered from unacceptable pain up to 2 years after diagnosis. Out of these, >80% had low inflammation at 2 years, strengthening the concept of non-inflammatory pain as a long-term problem in RA. We found more significant associations for 3-month predictors, compared with baseline predictors, of long-term unacceptable pain, suggesting that 3 months after diagnosis is a good evaluation time-point for predicting long-term pain outcome. Our results indicate that patients with worse PROs at the 3-month follow-up have increased risk of long-lasting pain. Furthermore, extensive joint tenderness may also be useful for predicting long-term pain despite low inflammation. The association between patient reported outcomes and future pain, as well as the lack of association with objective inflammatory measures, are also compatible with an important role for a non-inflammatory pain spectrum in RA.
Supplementary Material
Contributor Information
Anna Eberhard, Rheumatology, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden; Helsingborg Hospital, Helsingborg, Sweden.
Stefan Bergman, Rheumatology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Spenshult Research and Development Centre, Halmstad, Sweden; Department of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Thomas Mandl, Rheumatology, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden.
Tor Olofsson, Rheumatology, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Rheumatology, Skåne University Hospital, Malmö, Sweden.
Ankita Sharma, Rheumatology, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden.
Carl Turesson, Rheumatology, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden; Department of Rheumatology, Skåne University Hospital, Malmö, Sweden.
Supplementary data
Supplementary data are available at Rheumatology online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
All co-authors have contributed significantly in accordance with authorship guidelines.
Funding
This work was supported by The Swedish Research Council (grant number 2015–02228), The Swedish Rheumatism Association (grant number R-664091) and Lund University (grant number ALFSKANE-446501).
Disclosure statement: C.T. has received consulting fees from Roche, speaking fees from Abbvie, Bristol Myers-Squibb, Nordic Drugs, Pfizer and Roche, and an unrestricted grant from Bristol Myers-Squibb. T.M. is an employee of the pharmaceutical company UCB, working as medical solution lead in rheumatology. T.O. has performed consulting tasks for Eli Lilly, and Merck Sharp & Dohme unrelated to the present work. A.E., A.S. and S.B. report no conflict of interests.
References
- 1. Courvoisier DS, Agoritsas T, Glauser J, et al. Pain as an important predictor of psychosocial health in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:190–6. [DOI] [PubMed] [Google Scholar]
- 2. Eurenius E, Brodin N, Lindblad S, Opava CH.. Predicting physical activity and general health perception among patients with rheumatoid arthritis. J Rheumatol 2007;34:10–5. [PubMed] [Google Scholar]
- 3. Ahlstrand I, Thyberg I, Falkmer T, Dahlström Ö, Björk M.. Pain and activity limitations in women and men with contemporary treated early RA compared to 10 years ago: the Swedish TIRA project. Scand J Rheumatol 2015;44:259–64. [DOI] [PubMed] [Google Scholar]
- 4. Altawil R, Saevarsdottir S, Wedren S. et al. Remaining pain in early rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res (Hoboken) 2016;68:1061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olofsson T, Wallman JK, Jöud A. et al. Pain over 2 years after start of biological versus conventional combination treatment in early rheumatoid arthritis: results from the randomised controlled SWEFOT trial. Arthritis Care Res (Hoboken) 2021;73:1312–21. [DOI] [PubMed] [Google Scholar]
- 6. McWilliams DF, Ferguson E, Young A, Kiely PD, Walsh DA.. Discordant inflammation and pain in early and established rheumatoid arthritis: latent Class Analysis of Early Rheumatoid Arthritis Network and British Society for Rheumatology Biologics Register data. Arthritis Res Ther 2016;18:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trouvin AP, Perrot S.. New concepts of pain. Best Pract Res Clin Rheumatol 2019;33:101415. [DOI] [PubMed] [Google Scholar]
- 8. Vladimirova N, Jespersen A, Bartels EM. et al. Pain sensitisation in women with active rheumatoid arthritis: a comparative cross-sectional study. Arthritis 2015;2015:434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friden C, Thoors U, Glenmark B. et al. Higher pain sensitivity and lower muscle strength in postmenonpausal women with early rheumatoid arthritis compared with age-matched healthy women–a pilot study. Disabil Rehabil 2013;35:1350–6. [DOI] [PubMed] [Google Scholar]
- 10. Atzeni F, Cazzola M, Benucci M. et al. Chronic widespread pain in the spectrum of rheumatological diseases. Best Pract Res Clin Rheumatol 2011;25:165–71. [DOI] [PubMed] [Google Scholar]
- 11. McWilliams DF, Kiely PDW, Young A. et al. Interpretation of DAS28 and its components in the assessment of inflammatory and non-inflammatory aspects of rheumatoid arthritis. BMC Rheumatol 2018;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eberhard A, Bergman S, Mandl T. et al. Predictors of unacceptable pain with and without low inflammation over 5 years in early rheumatoid arthritis-an inception cohort study. Arthritis Res Ther 2021;23:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolen JS, Breedveld FC, Burmester GR. et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lotsch J, Alfredsson L, Lampa J.. Machine-learning-based knowledge discovery in rheumatoid arthritis-related registry data to identify predictors of persistent pain. Pain 2020;161:114–26. [DOI] [PubMed] [Google Scholar]
- 15. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 16. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 17. Kristensen LE, Saxne T, Geborek P.. The LUNDEX, a new index of drug efficacy in clinical practice: results of a five-year observational study of treatment with infliximab and etanercept among rheumatoid arthritis patients in southern Sweden. Arthritis Rheum 2006;54:600–6. [DOI] [PubMed] [Google Scholar]
- 18. Ekdahl C, Eberhardt K, Andersson SI, Svensson B.. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol 1988;17:263–71. [DOI] [PubMed] [Google Scholar]
- 19. Lofvendahl S, Schelin MEC, Joud A.. The value of the Skane Health-care Register: prospectively collected individual-level data for population-based studies. Scand J Public Health 2020;48:56–63. [DOI] [PubMed] [Google Scholar]
- 20. Quan H, Sundararajan V, Halfon P. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 21. Tubach F, Ravaud P, Martin-Mola E. et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken) 2012;64:1699–707. [DOI] [PubMed] [Google Scholar]
- 22. Lourdudoss C, Di Giuseppe D, Wolk A. et al. Dietary intake of polyunsaturated fatty acids and pain in spite of inflammatory control among methotrexate-treated early rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2018;70:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christensen AW, Rifbjerg-Madsen S, Christensen R. et al. Non-nociceptive pain in rheumatoid arthritis is frequent and affects disease activity estimation: cross-sectional data from the FRAME study. Scand J Rheumatol 2016;45:461–9. [DOI] [PubMed] [Google Scholar]
- 24. Terslev L, Brahe CH, Ostergaard M. et al. Using a DAS28-CRP-steered treat-to-target strategy does not eliminate subclinical inflammation as assessed by ultrasonography in rheumatoid arthritis patients in longstanding clinical remission. Arthritis Res Ther 2021;23:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trovato GM, Pace P, Salerno S, Trovato FM, Catalano D.. Pain assessment in fibromyalgia and rheumatoid arthritis: influence of physical activity and illness perception. Clin Ter 2010;161:335–9. [PubMed] [Google Scholar]
- 26. Lee YC. Swollen to tender joint count ratio: a novel combination of routine measures to assess pain and treatment response in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:171–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollard LC, Kingsley GH, Choy EH, Scott DL.. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 2010;49:924–8. [DOI] [PubMed] [Google Scholar]
- 28. Taylor PC, Lee YC, Fleischmann R. et al. Achieving pain control in rheumatoid arthritis with baricitinib or adalimumab plus methotrexate: results from the RA-BEAM trial. J Clin Med 2019;8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee YC, Massarotti E, Edwards RR. et al. Effect of milnacipran on pain in patients with rheumatoid arthritis with widespread pain: a randomized blinded crossover trial. J Rheumatol 2016;43:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meeus M, Hermans L, Ickmans K. et al. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: a double-blind randomized controlled trial. Pain Pract 2015;15:98–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.