Abstract
Local and systemic low-grade inflammation, mainly involving the innate immune system, plays an important role in the development of OA. A receptor playing a key role in initiation of this inflammation is the pattern-recognition receptor Toll-like receptor 4 (TLR4). In the joint, various ligands for TLR4, many of which are damage-associated molecular patterns (DAMPs), are present that can activate TLR4 signalling. This leads to the production of pro-inflammatory and catabolic mediators that cause joint damage. In this narrative review, we will first discuss the involvement of TLR4 ligands and signalling in OA. Furthermore, we will provide an overview of methods for inhibit, TLR4 signalling by RNA interference, neutralizing anti-TLR4 antibodies, small molecules and inhibitors targeting the TLR4 co-receptor MD2. Finally, we will focus on possible applications and challenges of these strategies in the dampening of inflammation in OA.
Keywords: osteoarthritis, toll-like receptor 4, inflammation
Rheumatology key messages.
Activation of Toll-like receptor 4 (TLR4) signalling is important in the induction/amplification of inflammation during osteoarthritis.
Multiple approaches have been developed for inhibiting TLR4 signalling.
To minimize the side-effects of TLR4 inhibition, proper patient selection and local delivery methods are essential.
Introduction
OA is the most common degenerative joint disease. The >240 million patients worldwide suffer from pain, stiffness, and impaired mobility, seriously affecting quality of life. OA is considered a multifactorial complex disease of the joint as an organ in which all articular and peri-articular tissues are involved [1]. A relatively low-grade chronic inflammation of both local and systemic origin, mainly involving cells of the innate immune system, is observed in the majority of knee OA patients and is considered an active player in disease development (reviewed in [2]). However, inhibition of classical cytokines such as IL-1β and TNFα has been largely disappointing in clinical trials [3].
An extended group of mediators released upon tissue damage and involved in the inflammatory process in OA are the damage-associated molecular patterns (DAMPs). These molecules activate cells by binding to pattern-recognition receptors (PRRs), such as the receptor for advanced glycation end products (RAGE) [4] and Toll-like receptors (TLRs) [5] that are present on many cell types in the joint. TLR4 is of particular interest, as a plethora of TLR4-binding ligands are present in the OA environment. This narrative review aims to provide an overview of the involvement of TLR4 signalling in OA and to summarize methods that can be deployed for inhibiting this signalling pathway.
The TLR4 pathway
The TLR4/NF-κB signalling pathway is of central importance for host defence and during many inflammatory diseases. As the pathway has been extensively reviewed [6–9], we here only provide a short overview of those factors that are needed for the interpretation of this review.
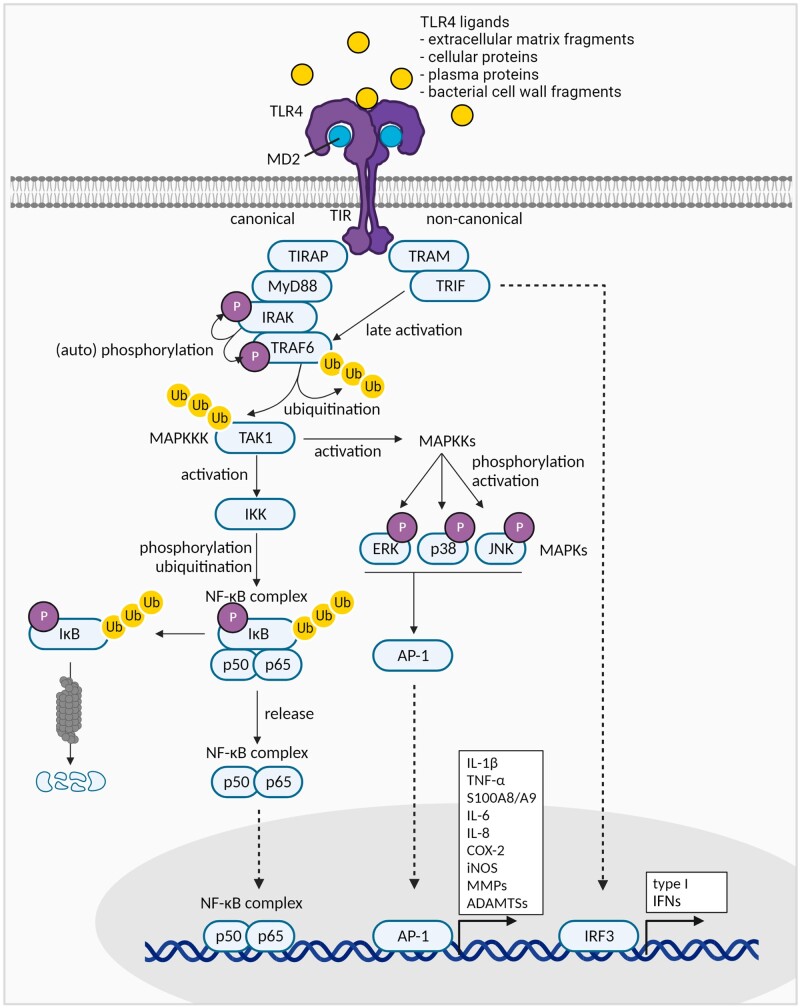
TLR4 ligands, like pathogen-associated molecular patterns (PAMPs) and DAMPs, are recognized by TLR4, which consists of an ectodomain, a transmembrane domain, and a cytoplasmic Toll/IL-1R (TIR) domain. Ligand binding induces TLR4 dimerization and cooperation with the co-receptors CD14 and MD2 to induce signalling [10–15]. This induces conformational changes and recruitment of downstream signalling mediators, which can lead to activation of two downstream pathways: the canonical (MyD88-dependent) pathway resulting in activation of the transcription factors NF-κB and AP-1, and the non-canonical (MyD88-independent) pathway, resulting in activation of the transcription factor IFN regulatory factor 3 (IRF3) (Fig. 1) [16, 17].
Figure 1.
Schematic overview of TLR4 signalling in OA. Various TLR4 ligands present in the OA environment can induce TLR4 signalling. Receptor activation can lead to canonical and non-canonical signalling, ultimately leading to activation of the transcription factors NF-κB, and AP-1 and IRF3, respectively. This results in the transcription of various relevant pro-inflammatory cytokines, matrix-degrading enzymes, and type I IFNs. Figures were created with BioRender.com
The canonical TLR4 pathway shares many of its intracellular signalling components with IL-1R and all other TLR signalling routes. Upon TLR4 activation, TIR-domain–containing adaptor proteins (TIRAPs) recruit MyD88, which subsequently interacts with IL-1-receptor–associated kinases (IRAKs). These IRAKs autophosphorylate and activate TNF-receptor–associated factor 6 (TRAF6), which ubiquitinates itself and TGF-β-activated kinase 1 (TAK1), which leads to activation of two types of transcription factors: NF-κB and AP-1. The NF-κB transcription factor family consists of five subunits that can form homo- or hetero-dimer complexes: RelA (p65), RelB, cRel, NF-κB1 (p50 or p105), and NF-κB2 (p52 or p100). In its inactive state, the NF-κB dimers are restricted to the cytoplasm by inhibitor of nuclear factor kappa B (IκB). TAK1 activates IκB kinases (IKKs) that phosphorylate, ubiquitinate and subsequently degrade IκB. Released NF-κB dimers then translocate to the nucleus, where they can bind DNA κB sites and trigger the expression of many pro-inflammatory mediators (e.g. IL-1β, TNF-α, IL-6, COX-2), cartilage-degrading enzymes like MMPs, and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs), angiogenic factors, and apoptosis-related molecules [6–9, 18–22]. The AP-1 transcription factors are activated via the mitogen-activated protein kinase (MAPK) activation cascade starting from TAK. AP-1 consists of homo- and hetero-dimers formed by proteins from the Jun, Fos, Maf and ATF families. Like NF-κB, AP-1 binding to DNA induces the production of inflammatory and catabolic factors [8, 23, 24].
The non-canonical pathway, only employed by TLR3 and TLR4 receptors [8], is activated by the TRIF-related adaptor molecule (TRAM) that recruits the TIR-domain–containing adaptor-inducing IFN-β (TRIF), which when combined stimulate signalling proteins, including TRAF6. This converges in the induction of type I IFNs, but also late NF-κB activation [8].
Based on its pro-inflammatory and catabolic downstream effects, it is mainly the MyD88-dependent TLR4 signalling pathway that is considered important during OA. This is underlined by a study showing that the induction of catabolic responses in chondrocytes upon stimulation with low-molecular-weight HA and HMGB1 was reduced in MyD88-deficient chondrocytes [25].
TLR4 signalling in OA
Increased TLR4 levels have been found in OA cartilage, synovium, subchondral bone, and chondrocytes, and are positively associated with disease severity [26–30]. In addition, increased levels of soluble TLR4, shed from the cell membrane during inflammation, are associated with disease progression in OA patients in some, but not all studies [31–33]. Moreover, soluble CD14 is positively associated with OA characteristics, including macrophage activation, joint-space narrowing, osteophytes, and pain [33–35].
During OA, many DAMPs are released into the joint environment: extracellular matrix fragments (tenascin-C, low-molecular-weight hyaluronan, biglycan, decorin, lumican, heparan sulphf, fibronectin), cellular proteins (S100 family members, HMGB1, HSPs, advanced glycation end products) and plasma proteins (fibrinogen, serum amyloid A, oxidized low-density lipoprotein). Activation of TLR4 by DAMPs leads to expression of pro-inflammatory mediators (including cytokines, iNOS and COX-2) and matrix-degrading enzymes, but can also lead to chondrocyte hypertrophy or apoptosis [4, 5, 21, 36, 37].
Interestingly, although the inflammation in OA is generally considered to be sterile, increased systemic levels of the classical TLR4 ligand lipopolysaccharide (LPS) have recently been associated with OA, likely being the result of the ‘leaky gut syndrome’ [32, 38–43]. Together, these data point to a relationship between TLR4 signalling and OA disease, indicating it might be worth investigating TLR4 as a therapeutic target for OA.
S100A8/A9 in OA
A DAMP that might be of particular importance in OA is S100A8/A9 (calprotectin). A first study showed an early increase, but later decrease, of S100A8 and S100A9 after induction of the destabilization of the medial meniscus (DMM) model. The authors therefore suggested that S100A8/A9 can have an effect in early but probably not late cartilage degradation [44]. In agreement, our lab found that S100A8/A9 levels were increased only for a short time after induction of DMM, whereas prolonged increased expression was observed in the more inflammatory collagenase-induced OA (CiOA) model [45, 46]. Furthermore, a recent study showed that S100A8/A9 levels were lower in more progressed OA stages [47]. Moreover, cartilage destruction [46] and osteophyte size [48] were reduced in CiOA-induced S100a9–/–- mice, which also do not express S100A8 protein, whereas no significant differences were measured in the DMM model [46].
Interestingly, Ruan et al. showed that serum S100A8/A9 levels are associated with total WOMAC, and WOMAC weight-bearing pain and physical dysfunction scores, and showed a positive association with cartilage degeneration [49]. Furthermore, OA patients had increased S100A8 and S100A9 mRNA levels in their synovium and serum S100A8/A9 levels [47] compared with controls; in addition, among OA patients, serum S100A8/A9 levels were higher in patients who showed progression of cartilage damage and osteophyte formation compared with non-progressors [46, 48]. Another study, however, showed no association between S100A8/A9 serum level and pain, stiffness or function, and even a negative association with osteophytes, which might be explained by the relatively advanced OA stage of the patients involved [50].
Underlining these data, in vitro studies showed increased expression of pro-inflammatory and catabolic factors upon stimulation of human OA cartilage explants and chondrocytes [29] and OA synovium and macrophages [51] with S100A8 and/or S100A9.
Inhibition of TLR4 signalling
Because of its involvement in a multitude of diseases, the development of inhibitors of the TLR4/NF-κB axis has been of great interest. Here, we will provide an overview of various strategies for targeting TLR4 signalling (Fig. 2). First, we describe two well-studied miRNAs in OA, which both inhibit multiple proteins in the canonical TLR4/NF-κB signalling pathway. Next, we discuss inhibition of TLR4 itself using neutralizing antibodies and the small molecule TAK-242. Finally, we give an overview of molecules inhibiting the co-receptor MD2, including lipid A mimetics and small molecules. Whereas neutralizing antibodies against the co-receptor CD14 and inhibitors of downstream kinases definitely belong to the armamentarium for inhibiting TLR4 signalling, these have been extensively reviewed recently [52–55].
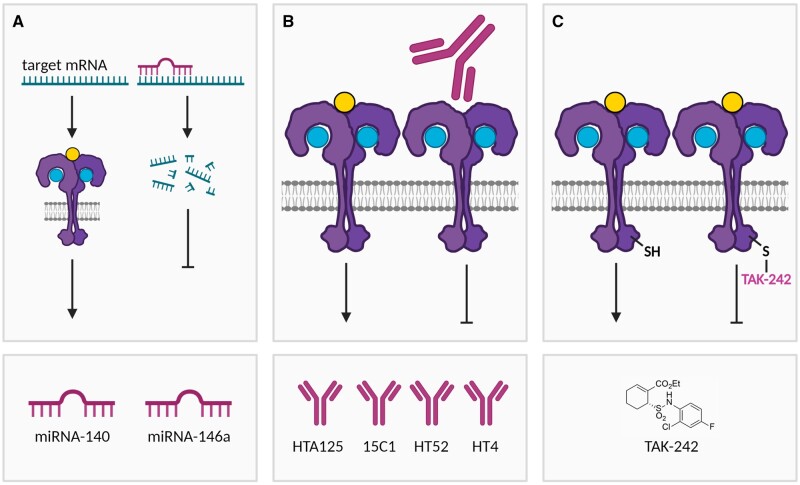
Figure 2.
Overview of various strategies for inhibiting TLR4. (A) miRNA-140 has been reported to directly target the mRNA of the TLR4 gene, thereby leading to mRNA degradation. In addition, both miRNA-140 and miRNA-146a target the mRNA of various genes involved in TLR4 signal transduction, thereby collectively reducing TLR4 signalling. (B) Neutralizing anti-TLR4 antibodies (HTA125, 15C1, HT52, HT4) prevent ligand-induced TLR4 signalling. (C) TAK-242 binds to Cys747 in the intracellular TIR domain of TLR4, thereby inhibiting ligand-induced signalling. Figures were created with BioRender.com
Inhibition of the TLR4 receptor
miRNAs inhibiting TLR4 signalling in OA
miRNAs are small non-coding RNAs that bind to 3′-untranslated regions of target mRNAs, resulting in target gene silencing. Synthetic miRNAs (miRNA mimics or agomirs) mimic the function of endogenous miRNAs, leading to target mRNA degradation. In contrast, antagomirs or anti-miRs inhibit the action of endogenous miRNAs [56]. Various miRNAs have been implicated in OA pathophysiology, and this has recently been reviewed elsewhere [57, 58]. Here, we will discuss the two most well-studied miRNAs in OA: miRNA-140 and miRNA-146a.
miRNA-140
The first miRNA that has been extensively described in OA is miRNA-140, which is specifically expressed in cartilage and has been implicated in cartilage homeostasis [59, 60]. Reporter cell experiments showed that miRNA-140 targets include TLR4 [61–64], HMGB1 [65] and ADAMTS5 [66].
miRNA-140 levels were lower in OA cartilage and SF compared with controls [65–67] and negatively associated with OA disease [64, 67] and HMGB1 levels [65]. Furthermore, miRNA-140 knock-out mice developed more severe age-related and experimentally induced OA pathologies [66]. In agreement, IA injection of a miRNA-140 mimic after induction of a preclinical OA model in rats reduced OA characteristics [67].
As expected, transfection with miRNA-140 reduced TLR4 [61–63], but also NF-κB and MyD88 expression [63]. Furthermore, LPS-induced IκBα and p65 phosphorylation was reduced by miRNA-140 overexpression [64], and miRNA-140 mimics reduced pro-inflammatory cytokines and cartilage-degrading enzymes [65, 66]. Interestingly, the effects of miRNA-140 treatment were higher in chondrocytes derived from early- and middle-stage OA patients, the most promising groups for treatment, rather than patients with late-stage disease [67].
In agreement, downregulation of miRNA-140 increased TLR4 levels [61, 62]. Together, these studies prove that miRNA-140 can dampen TLR4-mediated inflammatory responses; thus, miRNA-140 might be a promising treatment for OA patients, and it might simultaneously inhibit TLR4 [61–64], its ligand HMGB1 [65] and its downstream aggrecanase ADAMTS5 [66].
miRNA-146a
A second miRNA associated with OA development is miRNA-146a, which has often been reported as targeting TRAF6 and IRAK1 as intracellular mediators of canonical NF-κB signalling. Luciferase reporter assays have demonstrated direct binding to TRAF6 and IRAK1 [68–70], as well as to Notch1 [71] and SMAD4 [72]. Furthermore, previous studies have shown that miRNA-146a expression is lower in lesioned as compared with preserved cartilage [71], and that its expression is reversely associated with disease grade [73, 74], indicating the pro-homeostatic function of miRNA-146a. Moreover, several animal studies have indicated a beneficial role for miRNA-146a. Lower miRNA-146a levels were found in the dorsal root ganglia of rats with OA knee joint pain [75]. Furthermore, mice deficient in miRNA-146a had normal cartilage at birth, but showed more OA characteristics upon ageing [71]. Also, they showed higher OA scores after induction of experimental OA. In agreement, miRNA-146a transgenic mice presented with decreased age-induced OA development [71].
In contrast, other studies showed sex-dependent regulation of miRNA-146a in human OA patients, with increased expression in females and decreased expression in males; however, the entire OA group was not significantly different from controls [76]. Moreover, this same study found a positive correlation between miRNA-146a expression and OA severity. Other studies showed increased miRNA-146a expression in cartilage [68, 77], peripheral blood mononuclear cells (PBMCs) [73] and fibroblast-like synoviocytes [70] from OA patients as compared with controls. Lastly, two studies showed that induction of experimental OA resulted in upregulated miRNA-146a expression [72, 78].
On a functional level, miRNA-146a transfection decreased the expression of pro-inflammatory factors and matrix-degrading enzymes, whereas anabolic factors were induced by miRNA-146a [71, 75]. Furthermore, miRNA-146 reduced pain-related target genes, including NOS2, PTGS2 and TRPV1, which might also be beneficial for patients [75]. In agreement, miRNA-146a–deficient chondrocytes had increased MMP13 and collagen X, whereas aggrecan was decreased [71]. Interestingly, the promotor region of miRNA-146a contains NF-κB binding sites, which leads to negative feedback upon TLR4 stimulation [69]. Indeed, LPS and IL-1β induced miRNA-146a in THP-1 cells, chondrocytes, and fibroblast-like synoviocytes [68–70, 72, 74].
Interestingly, miRNA-146a and miRNA-146b have a high sequence similarity, although it remains unclear whether both have the same function [79]. Like miRNA-146a, miRNA-146b targets not only IRAK1 and TRAF6, but also MyD88 and TLR4 [79]. Although it has not been investigated whether miRNA-146a directly targets TLR4 or MyD88, its transfection functionally mimics the reduction of TLR4, MyD88, IRAK1 and NF-κB [71, 79, 80].
Although the association between OA severity and miRNA-146a levels remains inconclusive and might be dependent on disease stage and sex, based on the anti-inflammatory and anti-catabolic effects, it is tempting to speculate that treatment with miRNA-146a mimics might be a good strategy for ameliorating OA.
TLR4-blocking antibodies
Another method for inhibiting the TLR4 receptor is by using antibodies. The mAb HTA125, which is one of the first and most-studied anti-TLR4 antibodies, inhibited LPS-induced NF-κB activation [13, 81–83]. The subsequently developed 15C1 clone inhibited LPS-induced IL-8 production in TLR4/MD2-expressing HEK cells and IL-6 (MyD88-dependent) and IP-10 (MyD88-independent) production in whole blood of healthy donors [84] and proved more potent than HTA125. These effects were conserved in the humanized antibody variant, called NI-0101 or Hu15C1 [84, 85]. In a first clinical trial using anti-TLR4 antibodies, NI-0101 proved safe and protected against LPS-induced pro-inflammatory cytokine production [86]. Two other TLR4 antibodies, HT52 and HT4, showed higher potency than HTA125 for inhibiting LPS-mediated NF-κB activation and production of pro-inflammatory cytokines. However, little is known about these antibodies to date [87].
Unfortunately, no studies with TLR4-neutralizing antibodies in OA models have been published, but the favourable safety profiles and pharmacokinetic characteristics suggest great potential.
TAK-242
Identified in a library screened to inhibit LPS-stimulation of macrophages, TAK-242 (also called resatorvid or CLI-095) is a very potent and the most studied small molecule TLR4 inhibitor to date. Structure–activity optimization of a lead that inhibited LPS-induced NO, TNFα and IL-6 resulted in the small-molecule TAK-242, which inhibits both human and mouse TLR4 signalling [88, 89]. TAK-242 is selective for TLR4 over other TLRs [89, 90] and inhibits signalling [89] by binding Cys747 of the intracellular TLR4 domain [91], thereby disrupting the interaction of TLR4 with its MyD88-dependent (TIRAP) and MyD88-independent (TRAM) adaptor molecules [90], independent of the TLR4 ligand used [91]. TAK-242 had IC50 values in the low nanomolar range for NO, TNF-α and Il-6 production in vitro, and protected mice from a lethal LPS challenge by suppressing the cytokine storm [92]. A phase III clinical trial of patients with severe sepsis-induced shock or respiratory failure was terminated because the compound failed to suppress IL-6, IL-8 or TNF-α levels. It is speculated that the lack of cytokine reduction was due to redundancies in the inflammatory signalling system. Overall the treatment was without harm and well tolerated [93].
Multiple studies have investigated the potential of TAK-242 to dampen the inflammation in OA. TAK-242 inhibited NF-κB activation and subsequent production of pro-inflammatory and matrix-degrading molecules induced by several TLR4 ligands in cells obtained from OA patients [94–97]. Furthermore, TAK-242 prevented biglycan-induced TLR4 expression in OA cartilage explants [95]. TAK-242 treatment in various preclinical OA models reduced inflammation and resulted in decreased bone and cartilage damage [28, 98–100]. Moreover, TAK-242 treatment decreased signs of low back pain and disc degeneration in a pre-clinical model [101]. Altogether, these studies indicate that TAK-242 could be a potential treatment option for inhibiting TLR4-mediated induction of inflammation and joint destruction in OA.
Inhibition of the MD2 co-receptor
Other than by targeting TLR4 itself, a potential way to inhibit TLR4 signalling is to inhibit the MD2 co-receptor. Inhibition of MD2 has been shown to inhibit TLR4 signalling, and some MD2 inhibitors have already progressed into clinical trials. Here we discuss various strategies employed for inhibiting MD2 (Fig. 3).
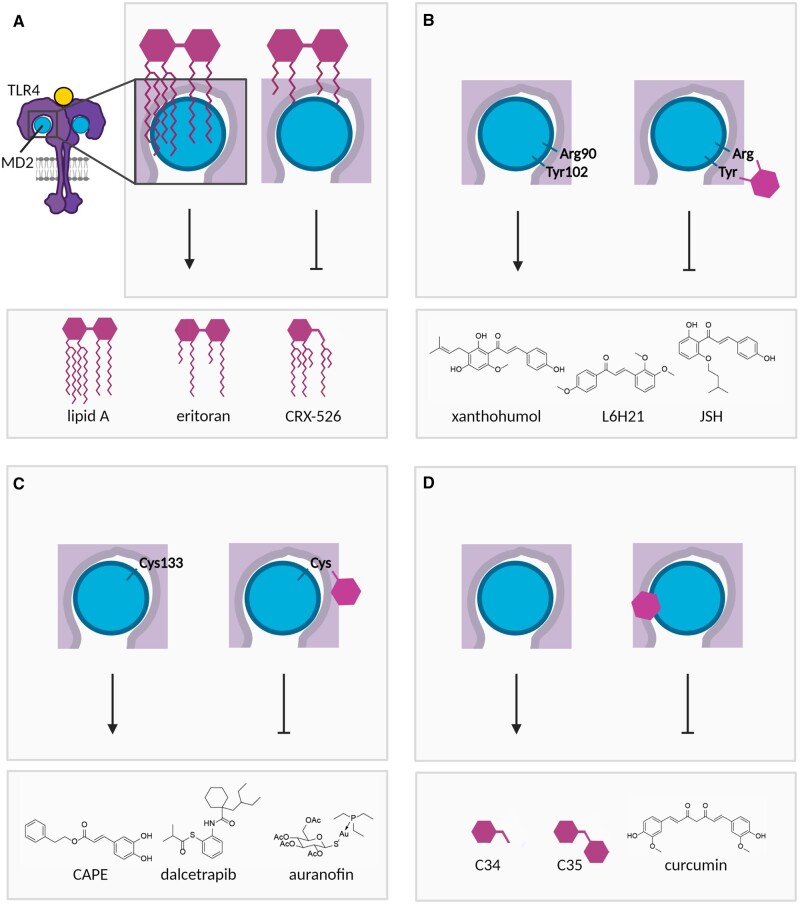
Figure 3.
Schematic overview of strategies for inhibiting the TLR4 co-receptor MD2. (A) The lipid A part of lipopolysaccharide (LPS) binds to MD2 and TLR4, thereby inducing downstream signalling. Non-signalling lipid A derivatives with modified lipid tails (eritoran, CRX-526) can bind MD2, preventing activation by TLR4 ligands. (B) The chalcones xanthohumol, L6H21 and JSH bind to Arg90 and Tyr102 in MD2, thereby inhibiting ligand-induced TLR4 signalling. (C) Caffeic acid phenethyl ester (CAPE), dalcetrapib and auranofin inhibit TLR4 signalling by binding to Cys133 in MD2. (D) C34, C35 and curcumin bind to the hydrophobic pocket of MD2, thereby preventing TLR4 signalling. Figures were created with BioRender.com
Non-signalling lipid A mimetics
Upon binding of LPS to MD2, five out of six lipid chains of the LPS lipid A component are buried inside the hydrophobic pocket in MD2, whereas the sixth interacts with TLR4. This binding of LPS causes the dimerization of two TLR4/MD2 complexes and subsequent activation of downstream signalling [17]. Based on the LPS lipid A structure, several inhibitors have been developed that competitively bind MD2 but do not induce downstream signalling (Fig. 3A). Eritoran (E5564, eritoran tetrasodium) is a structural analogue of the lipid A part of Rhodobacter sphaeroides. The four acyl chains of eritoran bind to the hydrophobic pocket in MD2, but eritoran does not directly interact with TLR4 and cannot induce formation of active TLR4/MD2 complex dimers [102]. Whereas eritoran reduced LPS-induced cytokine expression in multiple phase I trials, this could not be confirmed in phase II trials [103, 104]. Furthermore, a phase III study in sepsis patients showed no significantly different effect between patients treated with placebo or eritoran [105]. Since the inflammation observed in OA is different from the septic response, eritoran might still be beneficial for OA patients. A reported downside is that eritoran rapidly becomes inactive in whole blood and serum [103], but this might be circumvented by IA administration.
In silico similarity searches based on eritoran led to the identification of C34 and C35 (Fig. 3D), which share their backbone structure with eritoran, but lack the lipid chains, thereby overcoming the inactivation in blood. They bind to the hydrophobic pocket in MD2 and inhibit LPS-induced NF-κB-signalling [106, 107].
The length of the lipid chains of lipid A mimetics appeared crucial for TLR4 activation, leading to the discovery of CRX-526: a lipid A mimetic that is itself unable to induce inflammatory signalling in vitro and in vivo [108]. CRX-526 reduced pro-inflammatory effects in in vitro and in vivo studies in disease models of IBD [109], diabetic nephropathy [110], and gram-negative sepsis [111], but has not progressed into clinical trials.
Chalcones and curcumin
Chalcones (Fig. 3B) are compounds with a wide range of biological activities, including anti-oxidation and anti-inflammation, present in natural products. Their function depends on their characteristic αβ-unsaturated bond that can react with free cysteines that are abundantly present in cells [112]. Among their effects is the modulation of NF-κB signalling via binding MD2. Xanthohumol and L6H21 are chalcones reported to decrease LPS-induced production of pro-inflammatory factors. Although MD2 contains a free cysteine in its hydrophobic pocket (Cys133), which can be expected to bind to the αβ-unsaturated bond of the described chalcones, the action of xanthohumol is dependent on its binding in the hydrophobic pocket and interaction with Tyr102 and Arg90 of MD2 [113–117] (Fig. 3B). Interestingly, L6H21 exerted beneficial effects in in vitro and in vivo models of a plethora of inflammatory diseases [118–122].
Another compound described in this context is curcumin, which binds a variety of targets [123], including the hydrophobic pocket in MD2. Although curcumin possesses an αβ-unsaturated bond, it does not bind Cys133 of MD2 [124]. However, caution is warranted, since curcumin has many targets, and non-specific effects on lipid bilayer properties and membrane protein function have been described.
MD2 Cys133-binding inhibitors
Caffeic acid phenethyl ester (CAPE) is not a chalcone but does possess an αβ-unsaturated bond and inhibited LPS-induced production of inflammatory factors, which was shown to be partially mediated via Cys133 binding in the hydrophobic pocket of MD2 (Fig. 3C). However, it also inhibited NF-κB activity in cells overexpressing the MD2 Cys133Ser mutation [125]. In an anterior cruciate ligament transection (ACLT) rabbit model, IA administration of CAPE reduced cartilage destruction and proteoglycan loss, but synovial inflammation did not differ from that of control animals [126]. Other Cys133-binding molecules are dalcetrapib and auranofin [127], although they do not have an αβ-unsaturated bond. Dalcetrapib (also called JTT-705, R1658 and RO4607381), first identified as cholesteryl ester transfer protein (CETP) inhibitor [128], additionally inhibits NF-κB [127]. Clinical trials in dyslipidemic patients showed a favourable safety profile [129]. Auranofin is a gold salt used as therapy for inflammatory arthritis. Interestingly, auranofin is reported to target IKKβ [130], and NF-κB itself, in addition to MD2 [131] and was found to attenuate the progression of DMM-induced OAs [132].
Challenges and possibilities for targeting TLR4 signalling in OA
Possible side effects of TLR4 inhibition
TLR4 is a central player in host defence by recognizing PAMPs. Therefore, its inhibition may result in increased susceptibility for infections. Indeed, mutations in TLR4 increased the chance of gram-negative infection [133, 134] and showed a trend towards increased mortality in systemic inflammatory response syndrome (SIRS) patients [135]. Nevertheless, trials with the anti-TLR4 antibody NI-0101 showed no increased susceptibility to gram-negative infections [86, 136]. Furthermore, a mouse sepsis model treated with TAK-242 did not increase bacterial blood counts, suggesting that the susceptibility to infection is not problematically increased upon TLR4 inhibition [91]. However, it remains to be investigated whether long-term treatment, which is likely needed in the case of OA, in contrast with more acute treatment regimens for sepsis, increases the chance of infections or other adverse events.
Targeting MD2 instead of TLR4 itself could offer a level of selectivity for the ligands for which signalling is inhibited. However, most TLR4 ligands, including HMGB1 and LPS, require MD2 to induce TLR4 signalling [12, 13, 15]. Concerning S100A8/A9, one study showed that S100A9 does not interact with MD2 [137], whereas a more recent study using molecular docking indicated interactions of S100A9 with both TLR4 and MD2 [138]. This is supported by the finding that the TLR4/MD2 binding sites of S100A8 and LPS might be close together, since the effect on both could be inhibited by the HTA123 anti-TLR4 antibody [139].
Delivery methods
Since OA is often a local disease, adverse effects could potentially be reduced by treating locally. Furthermore, IA injection can improve drug delivery to the avascular cartilage, which has been reviewed previously [140–142]. Unfortunately, drug retention in the joint is low, especially for small molecules, in which the range can be as little as hours; this suggests a challenge, since IA injections cannot be used too frequently. In addition, IA treatment of multiple-joint and small-joint OA (e.g. hand OA) is not feasible. To improve joint residence time, delivery systems are being developed. Liposomes can be used to deliver hydrophobic small molecules, such as the lipid A mimetics, with slow solubilization and sustained release. More polar compounds like miRNAs can be delivered using hydrogels, or micro- or nano-particles [140]. HA and synthetic hydrogels have been employed as drug carriers for IA delivery, but the hydrogels themselves are also rapidly cleared and are not yet able to control long-term small-molecule drug release [141, 143]. Micro- or nano-particles might, therefore, be more effective. Indeed, a microparticle-based formulation of triamcinolone alleviated knee OA pain for a longer period than conventional triamcinolone [144]. Nanoparticles have a phagocytosable size, and can be taken up by macrophages, leading to higher clearance [145]. This downside, however, might be beneficial in OA, in which macrophages play a key role in the inflammation.
Patient phenotyping
Another difficulty with developing OA therapies, is the disease heterogeneity. Deep phenotyping using multi-omics approaches will allow for much more detailed endotyping and information on druggable pathways at the population level. Moreover, it could additionally allow selection of patients who likely would benefit from a specific therapy, e.g. patients with clear involvement of inflammation, which might benefit from inhibition of TLR4 signalling [146]. In this light, it is interesting that higher NF-κB expression was observed in patients with early compared with more advanced OA [147], and expression of the TLR4 ligands HMGB1 and S100A8/A9 was higher in knee OA compared with hip OA [148].
Conclusions
TLR4 signalling plays a pivotal role in the chronic low-grade inflammation that is present during OA. Inhibition of this inflammatory pathway can be achieved by directly targeting the TLR4 receptor itself, its co-receptor MD2, or more downstream mediators of TLR4 signalling, using multiple approaches. However, it remains to be investigated whether long-term inhibition of TLR4 signalling leads to adverse effects. Moreover, deep phenotyping approaches are necessary to identify patients who are likely to benefit from TLR4 inhibition.
Acknowledgements
The figures were created with BioRender.com.
Contributor Information
Yvonne L Bartels, Experimental Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Peter L E M van Lent, Experimental Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Peter M van der Kraan, Experimental Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Arjen B Blom, Experimental Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Kimberly M Bonger, Synthetic Organic Chemistry, Institute for Molecules and Materials, Radboud University, Nijmegen, The Netherlands.
Martijn H J van den Bosch, Experimental Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Funding
Y.B. was funded by a grant from the Dutch Arthritis Society (grant number 16–2-401) and the ZonMW/Enabling Technology Hotels research program (project number 435004005). K.B. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement number 802940). MvdB received funding from the ZonMW/VENI research program (project number 09150161810015), which is financed by the Dutch Research Council (NWO). The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Loeser RF, Goldring SR, Scanzello CR, Goldring MB.. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Bosch MHJ, van Lent P, van der Kraan PM.. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthritis Cartilage 2020;28:532–43. [DOI] [PubMed] [Google Scholar]
- 3. Chevalier X, Eymard F, Richette P.. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol 2013;9:400–10. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg JH, Rai V, Dilisio MF, Agrawal DK.. Damage-associated molecular patterns in the pathogenesis of osteoarthritis: potentially novel therapeutic targets. Mol Cell Biochem 2017;434:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep 2013;15:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akira S, Takeda K.. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 7. Akira S, Uematsu S, Takeuchi O.. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 8. Kawasaki T, Kawai T.. Toll-like receptor signaling pathways. Front Immunol 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satoh T, Akira S.. Toll-like receptor signaling and its inducible proteins. Microbiol Spectr 2016;4. [DOI] [PubMed] [Google Scholar]
- 10. He Z, Riva M, Bjork P. et al. CD14 is a co-receptor for TLR4 in the S100A9-induced pro-inflammatory response in monocytes. PLoS One 2016;11:e0156377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S, Kim SY, Pribis JP. et al. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med 2013;19:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagai Y, Akashi S, Nagafuku M. et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 2002;3:667–72. [DOI] [PubMed] [Google Scholar]
- 13. Shimazu R, Akashi S, Ogata H. et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 1999;189:1777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC.. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–3. [DOI] [PubMed] [Google Scholar]
- 15. Yang H, Wang H, Ju Z. et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med 2015;212:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park BS, Lee JO.. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 2013;45:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park BS, Song DH, Kim HM. et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009;458:1191–5. [DOI] [PubMed] [Google Scholar]
- 18. Nefla M, Holzinger D, Berenbaum F, Jacques C.. The danger from within: alarmins in arthritis. Nat Rev Rheumatol 2016;12:669–83. [DOI] [PubMed] [Google Scholar]
- 19. Scanzello CR, Goldring SR.. The role of synovitis in osteoarthritis pathogenesis. Bone 2012;51:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sokolove J, Lepus CM.. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013;5:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gómez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G.. TLR4 signalling in osteoarthritis–finding targets for candidate DMOADs. Nat Rev Rheumatol 2015;11:159–70. [DOI] [PubMed] [Google Scholar]
- 22. Nabel GJ, Verma IM.. Proposed NF-kappa B/I kappa B family nomenclature. Genes Dev 1993;7:2063. [DOI] [PubMed] [Google Scholar]
- 23. Atsaves V, Leventaki V, Rassidakis GZ, Claret FX.. AP-1 transcription factors as regulators of immune responses in cancer. Cancers (Basel) 2019;11:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaulian E, Karin M.. AP-1 as a regulator of cell life and death. Nat Cell Biol 2002;4:E131–E136. [DOI] [PubMed] [Google Scholar]
- 25. Liu-Bryan R, Terkeltaub R.. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum 2010;62:2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim HA, Cho ML, Choi HY. et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum 2006;54:2152–63. [DOI] [PubMed] [Google Scholar]
- 27. Kuroki K, Stoker AM, Sims HJ, Cook JL.. Expression of Toll-like receptors 2 and 4 in stifle joint synovial tissues of dogs with or without osteoarthritis. Am J Vet Res 2010;71:750–4. [DOI] [PubMed] [Google Scholar]
- 28. Liu X, Cai HX, Cao PY. et al. TLR4 contributes to the damage of cartilage and subchondral bone in discectomy-induced TMJOA mice. J Cell Mol Med 2020;24:11489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schelbergen RF, Blom AB, van den Bosch MH. et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum 2012;64:1477–87. [DOI] [PubMed] [Google Scholar]
- 30. Sillat T, Barreto G, Clarijs P. et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop 2013;84:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han W, Chen X, Wang X. et al. TLR-4, TLR-5 and IRF4 are diagnostic markers of knee osteoarthritis in the middle-aged and elderly patients and related to disease activity and inflammatory factors. Exp Ther Med 2020;20:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang ZY, Perry E, Huebner JL. et al. Biomarkers of inflammation - LBP and TLR- predict progression of knee osteoarthritis in the DOXY clinical trial. Osteoarthritis Cartilage 2018;26:1658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajandran SN, Ma CA, Tan JR. et al. Exploring the association of innate immunity biomarkers with MRI features in both early and late stages osteoarthritis. Front Med (Lausanne) 2020;7:554669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daghestani HN, Pieper CF, Kraus VB.. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol 2015;67:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nair A, Kanda V, Bush-Joseph C. et al. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum 2012;64:2268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barreto G, Manninen M, K KE.. Osteoarthritis and toll-like receptors: when innate immunity meets chondrocyte apoptosis. Biology (Basel) 2020;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rigoglou S, Papavassiliou AG.. The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 2013;45:2580–4. [DOI] [PubMed] [Google Scholar]
- 38. Collins KH, Paul HA, Reimer RA. et al. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage 2015;23:1989–98. [DOI] [PubMed] [Google Scholar]
- 39. Li K, Liu A, Zong W. et al. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J Biol Sci 2021;28:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watanabe K. [PGF synthetase]. Seikagaku 1986;58:23–8. [PubMed] [Google Scholar]
- 41. Huang ZY, Stabler T, Pei FX, Kraus VB.. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage 2016;24:1769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hao X, Shang X, Liu J. et al. T. The gut microbiota in osteoarthritis: where do we stand and what can we do? Arthritis Res Ther 2021;23:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramires LC, Santos GS, Ramires RP. et al. The association between gut microbiota and osteoarthritis: does the disease begin in the gut? Int J Mol Sci 2022;23:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zreiqat H, Belluoccio D, Smith MM. et al. S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res Ther 2010;12:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van den Bosch MH, Blom AB, Schelbergen RF. et al. Induction of canonical wnt signaling by the alarmins S100A8/A9 in murine knee joints: implications for osteoarthritis. Arthritis Rheumatol 2016;68:152–63. [DOI] [PubMed] [Google Scholar]
- 46. van Lent PL, Blom AB, Schelbergen RF. et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 2012;64:1466–76. [DOI] [PubMed] [Google Scholar]
- 47. Safa A, Bagherifard A, Hadi Al-Baseesee H. et al. Serum calprotectin as a blood-based biomarker for monitoring knee osteoarthritis at early but not late stages. Cartilage 2021;13(Suppl 1):1566S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schelbergen RF, de Munter W, van den Bosch MH. et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann Rheum Dis 2016;75:218–25. [DOI] [PubMed] [Google Scholar]
- 49. Ruan G, Xu J, Wang K. et al. Associations between serum S100A8/S100A9 and knee symptoms, joint structures and cartilage enzymes in patients with knee osteoarthritis. Osteoarthritis Cartilage 2019;27:99–105. [DOI] [PubMed] [Google Scholar]
- 50. Mahler EA, Zweers MC, van Lent PL. et al. Association between serum levels of the proinflammatory protein S100A8/A9 and clinical and structural characteristics of patients with established knee, hip, and hand osteoarthritis. Scand J Rheumatol 2015;44:56–60. [DOI] [PubMed] [Google Scholar]
- 51. van den Bosch MH, Blom AB, Schelbergen RF. et al. Alarmin S100A9 induces proinflammatory and catabolic effects predominantly in the M1 macrophages of human osteoarthritic synovium. J Rheumatol 2016;43:1874–84. [DOI] [PubMed] [Google Scholar]
- 52. Kabanov DS, Grachev SV, Prokhorenko IR.. Monoclonal antibody to CD14, TLR4, or CD11b: impact of epitope and isotype specificity on ROS generation by human granulocytes and monocytes. Oxid Med Cell Longev 2020;2020:5708692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loeser RF, Erickson EA, Long DL.. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr Opin Rheumatol 2008;20:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patterson H, Nibbs R, McInnes I, Siebert S.. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol 2014;176:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zarrin AA, Bao K, Lupardus P, Vucic D.. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discov 2021;20:39–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lam JK, Chow MY, Zhang Y, Leung SW.. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids 2015;4:e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tavallaee G, Rockel JS, Lively S, Kapoor M.. MicroRNAs in synovial pathology associated with osteoarthritis. Front Med (Lausanne) 2020;7:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li YP, Wei XC, Li PC. et al. The role of miRNAs in cartilage homeostasis. Curr Genomics 2015;16:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oliviero A, Della Porta G, Peretti GM, Maffulli N.. MicroRNA in osteoarthritis: physiopathology, diagnosis and therapeutic challenge. Br Med Bull 2019;130:137–47. [DOI] [PubMed] [Google Scholar]
- 60. Wienholds E, Kloosterman WP, Miska E. et al. MicroRNA expression in zebrafish embryonic development. Science 2005;309:310–1. [DOI] [PubMed] [Google Scholar]
- 61. Li H, Guan SB, Lu Y, Wang F.. MiR-140-5p inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4. Biomed Pharmacother 2017;96:208–14. [DOI] [PubMed] [Google Scholar]
- 62. Liu H, Mao Z, Zhu J, Shen M, Chen F.. MiR-140-5p inhibits oxidized low-density lipoprotein-induced oxidative stress and cell apoptosis via targeting toll-like receptor 4. Gene Ther 2021;28:413–21. [DOI] [PubMed] [Google Scholar]
- 63. Yang Y, Liu D, Xi Y. et al. Upregulation of miRNA-140-5p inhibits inflammatory cytokines in acute lung injury through the MyD88/NF-kappaB signaling pathway by targeting TLR4. Exp Ther Med 2018;16:3913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Q, Weng Y, Jiang Y. et al. Overexpression of miR-140-5p inhibits lipopolysaccharide-induced human intervertebral disc inflammation and degeneration by downregulating toll-like receptor 4. Oncol Rep 2018;40:793–802. [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Shen S, Li Z, Li W, Weng X.. MIR-140-5p affects chondrocyte proliferation, apoptosis, and inflammation by targeting HMGB1 in osteoarthritis. Inflamm Res 2020;69:63–73. [DOI] [PubMed] [Google Scholar]
- 66. Miyaki S, Sato T, Inoue A. et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev 2010;24:1173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Si HB, Zeng Y, Liu SY. et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage 2017;25:1698–707. [DOI] [PubMed] [Google Scholar]
- 68. Shao J, Ding Z, Peng J. et al. MiR-146a-5p promotes IL-1beta-induced chondrocyte apoptosis through the TRAF6-mediated NF-kB pathway. Inflamm Res 2020;69:619–30. [DOI] [PubMed] [Google Scholar]
- 69. Taganov KD, Boldin MP, Chang KJ, Baltimore D.. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006;103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang JH, Shih KS, Wu YW, Wang AW, Yang CR.. Histone deacetylase inhibitors increase microRNA-146a expression and enhance negative regulation of interleukin-1beta signaling in osteoarthritis fibroblast-like synoviocytes. Osteoarthritis Cartilage 2013;21:1987–96. [DOI] [PubMed] [Google Scholar]
- 71. Guan YJ, Li J, Yang X. et al. Evidence that miR-146a attenuates aging- and trauma-induced osteoarthritis by inhibiting Notch1, IL-6, and IL-1 mediated catabolism. Aging Cell 2018;17:e12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li J, Huang J, Dai L. et al. miR-146a, an IL-1beta responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res Ther 2012;14:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Okuhara A, Nakasa T, Shibuya H. et al. Changes in microRNA expression in peripheral mononuclear cells according to the progression of osteoarthritis. Mod Rheumatol 2012;22:446–57. [DOI] [PubMed] [Google Scholar]
- 74. Yamasaki K, Nakasa T, Miyaki S. et al. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum 2009;60:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li X, Gibson G, Kim JS. et al. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene 2011;480:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Soyocak A, Kurt H, Ozgen M. et al. miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene 2017;627:207–11. [DOI] [PubMed] [Google Scholar]
- 77. Kopańska M, Szala D, Czech J. et al. MiRNA expression in the cartilage of patients with osteoarthritis. J Orthop Surg Res 2017;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Castanheira C, Anderson JR, Fang Y. et al. Mouse microRNA signatures in joint ageing and post-traumatic osteoarthritis. Osteoarthr Cartil Open 2021;3:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Curtale G, Mirolo M, Renzi TA. et al. Negative regulation of toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci U S A 2013;110:11499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tan Y, Yu L, Zhang C. et al. miRNA-146a attenuates inflammation in an in vitro spinal cord injury model via inhibition of TLR4 signaling. Exp Ther Med 2018;16:3703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paik YH, Schwabe RF, Bataller R. et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003;37:1043–55. [DOI] [PubMed] [Google Scholar]
- 82. Wang JE, Warris A, Ellingsen EA. et al. Involvement of CD14 and toll-like receptors in activation of human monocytes by Aspergillus fumigatus hyphae. Infect Immun 2001;69:2402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yonekawa K, Neidhart M, Altwegg LA. et al. Myeloid related proteins activate Toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis 2011;218:486–92. [DOI] [PubMed] [Google Scholar]
- 84. Dunn-Siegrist I, Leger O, Daubeuf B. et al. Pivotal involvement of Fcgamma receptor IIA in the neutralization of lipopolysaccharide signaling via a potent novel anti-TLR4 monoclonal antibody 15C1. J Biol Chem 2007;282:34817–27. [DOI] [PubMed] [Google Scholar]
- 85. Shang L, Daubeuf B, Triantafilou M. et al. Selective antibody intervention of Toll-like receptor 4 activation through Fc gamma receptor tethering. J Biol Chem 2014;289:15309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Monnet E, Lapeyre G, Poelgeest EV. et al. Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin Pharmacol Ther 2017;101:200–8. [DOI] [PubMed] [Google Scholar]
- 87. Tsukamoto H, Fukudome K, Takao S. et al. Multiple potential regulatory sites of TLR4 activation induced by LPS as revealed by novel inhibitory human TLR4 mAbs. Int Immunol 2012;24:495–506. [DOI] [PubMed] [Google Scholar]
- 88. Ii M, Matsunaga N, Hazeki K. et al. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol 2006;69:1288–95. [DOI] [PubMed] [Google Scholar]
- 89. Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H.. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol 2008;584:40–8. [DOI] [PubMed] [Google Scholar]
- 90. Matsunaga N, Tsuchimori N, Matsumoto T, Ii M.. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol 2011;79:34–41. [DOI] [PubMed] [Google Scholar]
- 91. Takashima K, Matsunaga N, Yoshimatsu M. et al. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol 2009;157:1250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yamada M, Ichikawa T, Ii M. et al. Discovery of novel and potent small-molecule inhibitors of NO and cytokine production as antisepsis agents: synthesis and biological activity of alkyl 6-(N-substituted sulfamoyl)cyclohex-1-ene-1-carboxylate. J Med Chem 2005;48:7457–67. [DOI] [PubMed] [Google Scholar]
- 93. Rice TW, Wheeler AP, Bernard GR. et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med 2010;38:1685–94. [DOI] [PubMed] [Google Scholar]
- 94. Barreto G, Senturk B, Colombo L. et al. Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization. Osteoarthritis Cartilage 2020;28:92–101. [DOI] [PubMed] [Google Scholar]
- 95. Barreto G, Soininen A, Ylinen P. et al. Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res Ther 2015;17:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. de Seny D, Cobraiville G, Charlier E. et al. Acute-phase serum amyloid a in osteoarthritis: regulatory mechanism and proinflammatory properties. PLoS One 2013;8:e66769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lambert C, Borderie D, Dubuc JE, Rannou F, Henrotin Y.. Type II collagen peptide Coll2-1 is an actor of synovitis. Osteoarthritis Cartilage 2019;27:1680–91. [DOI] [PubMed] [Google Scholar]
- 98. Lin X, Kong J, Wu Q, Yang Y, Ji P.. Effect of TLR4/MyD88 signaling pathway on expression of IL-1beta and TNF-alpha in synovial fibroblasts from temporomandibular joint exposed to lipopolysaccharide. Mediators Inflamm 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lin X, Xie J, Sun S. et al. Toll-like receptor 4 (TLR4) stimulates synovial injury of temporomandibular joint in rats through the activation of p38 mitogen-activated protein kinase (MAPK) signaling pathway. Med Sci Monit 2018;24:4405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kong J, Yang Y, Sun S. et al. Effect of toll-like receptor 4 on synovial injury of temporomandibular joint in rats caused by occlusal interference. Mediators Inflamm 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Krock E, Millecamps M, Currie JB, Stone LS, Haglund L.. Low back pain and disc degeneration are decreased following chronic toll-like receptor 4 inhibition in a mouse model. Osteoarthritis Cartilage 2018;26:1236–46. [DOI] [PubMed] [Google Scholar]
- 102. Kim HM, Park BS, Kim JI. et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007;130:906–17. [DOI] [PubMed] [Google Scholar]
- 103. Barochia A, Solomon S, Cui X, Natanson C, Eichacker PQ.. Eritoran tetrasodium (E5564) treatment for sepsis: review of preclinical and clinical studies. Expert Opin Drug Metab Toxicol 2011;7:479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bennett-Guerrero E, Grocott HP, Levy JH. et al. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg 2007;104:378–83. [DOI] [PubMed] [Google Scholar]
- 105. Opal SM, Laterre PF, Francois B. et al. ; ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 2013;309:1154–62. [DOI] [PubMed] [Google Scholar]
- 106. Neal MD, Jia H, Eyer B. et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS One 2013;8:e65779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wipf P, Eyer BR, Yamaguchi Y. et al. Synthesis of anti-inflammatory alpha-and beta-linked acetamidopyranosides as inhibitors of toll-like receptor 4 (TLR4). Tetrahedron Lett 2015;56:3097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stover AG, Da Silva Correia J, Evans JT. et al. Structure-activity relationship of synthetic toll-like receptor 4 agonists. J Biol Chem 2004;279:4440–9. [DOI] [PubMed] [Google Scholar]
- 109. Fort MM, Mozaffarian A, Stöver AG. et al. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol 2005;174:6416–23. [DOI] [PubMed] [Google Scholar]
- 110. Lin M, Yiu WH, Li RX. et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int 2013;83:887–900. [DOI] [PubMed] [Google Scholar]
- 111. Zhou J, Soltow M, Zimmermann K. et al. Experimental TLR4 inhibition improves intestinal microcirculation in endotoxemic rats. Microvasc Res 2015;101:33–7. [DOI] [PubMed] [Google Scholar]
- 112. Arshad L, Jantan I, Bukhari SN, Haque MA.. Immunosuppressive effects of natural alpha, beta-unsaturated carbonyl-based compounds, and their analogs and derivatives, on immune cells: a review. Front Pharmacol 2017;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Peluso MR, Miranda CL, Hobbs DJ, Proteau RR, Stevens JF.. Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: structure-activity relationships and in silico binding to myeloid differentiation protein-2 (MD-2). Planta Med 2010;76:1536–43. [DOI] [PubMed] [Google Scholar]
- 114. Roh E, Lee HS, Kwak JA. et al. MD-2 as the target of nonlipid chalcone in the inhibition of endotoxin LPS-induced TLR4 activity. J Infect Dis 2011;203:1012–20. [DOI] [PubMed] [Google Scholar]
- 115. Fu W, Chen L, Wang Z. et al. Determination of the binding mode for anti-inflammatory natural product xanthohumol with myeloid differentiation protein 2. Drug Des Devel Ther 2016;10:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang Y, Shan X, Chen G. et al. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br J Pharmacol 2015;172:4391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu J, Li J, Cai Y. et al. Evaluation and discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J Med Chem 2011;54:8110–23. [DOI] [PubMed] [Google Scholar]
- 118. Fang Q, Zhao L, Wang Y. et al. A novel chalcone derivative attenuates the diabetes-induced renal injury via inhibition of high glucose-mediated inflammatory response and macrophage infiltration. Toxicol Appl Pharmacol 2015;282:129–38. [DOI] [PubMed] [Google Scholar]
- 119. Chen X, Yu W, Li W. et al. An anti-inflammatory chalcone derivative prevents heart and kidney from hyperlipidemia-induced injuries by attenuating inflammation. Toxicol Appl Pharmacol 2018;338:43–53. [DOI] [PubMed] [Google Scholar]
- 120. Fang Q, Wang L, Yang D. et al. Blockade of myeloid differentiation protein 2 prevents obesity-induced inflammation and nephropathy. J Cell Mol Med 2017;21:3776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Han J, Zou C, Mei L. et al. MD2 mediates angiotensin II-induced cardiac inflammation and remodeling via directly binding to Ang II and activating TLR4/NF-kappaB signaling pathway. Basic Res Cardiol 2017;112:9. [DOI] [PubMed] [Google Scholar]
- 122. Fang Q, Wang J, Zhang Y. et al. Inhibition of myeloid differentiation factor-2 attenuates obesity-induced cardiomyopathy and fibrosis. Biochim Biophys Acta Mol Basis Dis 2018;1864:252–62. [DOI] [PubMed] [Google Scholar]
- 123. Zhou H, Beevers CS, Huang S.. The targets of curcumin. Curr Drug Targets 2011;12:332–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gradisar H, Keber MM, Pristovsek P, Jerala R.. MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol 2007;82:968–74. [DOI] [PubMed] [Google Scholar]
- 125. Kim SY, Koo JE, Seo YJ. et al. Suppression of Toll-like receptor 4 activation by caffeic acid phenethyl ester is mediated by interference of LPS binding to MD2. Br J Pharmacol 2013;168:1933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Elmali N, Ayan I, Turkoz Y. et al. Effect of caffeic acid phenethyl ester on cartilage in experimental osteoarthritis. Rheumatol Int 2002;22:222–6. [DOI] [PubMed] [Google Scholar]
- 127. Mancek-Keber M, Gradisar H, Iñigo Pestaña M, Martinez de Tejada G, Jerala R.. Free thiol group of MD-2 as the target for inhibition of the lipopolysaccharide-induced cell activation. J Biol Chem 2009;284:19493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Okamoto H, Yonemori F, Wakitani K. et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 2000;406:203–7. [DOI] [PubMed] [Google Scholar]
- 129. Dalcetrapib: JTT 705; JTT-705; R 1658; R1658; RG1658; RO 4607381; RO4607381. Drugs R D 2010;10:33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jeon KI, Byun MS, Jue DM.. Gold compound auranofin inhibits IkappaB kinase (IKK) by modifying Cys-179 of IKKbeta subunit. Exp Mol Med 2003;35:61–6. [DOI] [PubMed] [Google Scholar]
- 131. Yang JP, Merin JP, Nakano T. et al. Inhibition of the DNA-binding activity of NF-kappa B by gold compounds in vitro. FEBS Lett 1995;361:89–96. [DOI] [PubMed] [Google Scholar]
- 132. Liang J, Wang S, Hu J. et al. Targeted inhibition of TXNRD1 prevents cartilage extracellular matrix degeneration by activating Nrf2 pathway in osteoarthritis. Biochem Biophys Res Commun 2022;635:267–76. [DOI] [PubMed] [Google Scholar]
- 133. Agnese DM, Calvano JE, Hahm SJ. et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis 2002;186:1522–5. [DOI] [PubMed] [Google Scholar]
- 134. Lorenz E, Mira JP, Frees KL, Schwartz DA.. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med 2002;162:1028–32. [DOI] [PubMed] [Google Scholar]
- 135. Child NJ, Yang IA, Pulletz MC. et al. Polymorphisms in Toll-like receptor 4 and the systemic inflammatory response syndrome. Biochem Soc Trans 2003;31:652–3. [DOI] [PubMed] [Google Scholar]
- 136. Monnet E, Choy EH, McInnes I. et al. Efficacy and safety of NI-0101, an anti-toll-like receptor 4 monoclonal antibody, in patients with rheumatoid arthritis after inadequate response to methotrexate: a phase II study. Ann Rheum Dis 2020;79:316–23. [DOI] [PubMed] [Google Scholar]
- 137. Bjork P, Bjork A, Vogl T. et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 2009;7:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vogl T, Stratis A, Wixler V. et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J Clin Invest 2018;128:1852–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vogl T, Tenbrock K, Ludwig S. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007;13:1042–9. [DOI] [PubMed] [Google Scholar]
- 140. Evans CH, Kraus VB, Setton LA.. Progress in intra-articular therapy. Nat Rev Rheumatol 2014;10:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Maudens P, Jordan O, Allemann E.. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today 2018;23:1761–75. [DOI] [PubMed] [Google Scholar]
- 142. Rai MF, Pham CT.. Intra-articular drug delivery systems for joint diseases. Curr Opin Pharmacol 2018;40:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mancipe Castro LM, Garcia AJ, Guldberg RE.. Biomaterial strategies for improved intra-articular drug delivery. J Biomed Mater Res A 2021;109:426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Latourte A, Kloppenburg M, Richette P.. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol 2020;16:673–88. [DOI] [PubMed] [Google Scholar]
- 145. Yameen B, Choi WI, Vilos C. et al. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release 2014;190:485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Mobasheri A, Kapoor M, Ali SA, Lang A, Madry H.. The future of deep phenotyping in osteoarthritis: how can high throughput omics technologies advance our understanding of the cellular and molecular taxonomy of the disease? Osteoarthritis and Cartilage Open 2021;3:100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ostojic M, Soljic V, Vukojevic K, Dapic T.. Immunohistochemical characterization of early and advanced knee osteoarthritis by NF-kappaB and iNOS expression. J Orthop Res 2017;35:1990–7. [DOI] [PubMed] [Google Scholar]
- 148. Rosenberg JH, Rai V, Dilisio MF, Sekundiak TD, Agrawal DK.. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol Cell Biochem 2017;436:59–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.