Abstract
Objective
Patients with peripheral arterial disease (PAD) have a significant risk of myocardial infarction and death secondary to concomitant coronary artery disease (CAD). This is particularly true in patients with critical limb-threatening ischemia (CLTI) who exceed a 20% mortality rate at 6 months despite standard treatment with risk factor modification. Although systematic preoperative coronary testing is not recommended for patients with PAD without cardiac symptoms, the clinical manifestations of CAD are often muted in patients with CLTI due to poor mobility and activity intolerance. Thus, the true incidence and impact of “silent” CAD in a CLTI cohort is unknown. This study aims to determine the prevalence of ischemia-producing coronary artery stenosis in a CLTI cohort using coronary computed tomography angiography (cCTA) and computed tomography (CT)-derived fractional flow reserve (FFRCT), a noninvasive imaging modality that has shown significant correlation to cardiac catheterization in the detection of clinically relevant coronary ischemia.
Methods
Patients presenting with newly diagnosed CLTI at our institution from May 2020 to April 2021 were screened for underlying CAD. Included subjects had no known history of CAD, no cardiac symptoms, and no anginal equivalent complaints at presentation. Patients underwent cCTA and FFRCT evaluation and were classified by the anatomic location and severity of CAD. Significant coronary ischemia was defined as FFRCT ≤0.80 distal to a >30% coronary stenosis, and severe coronary ischemia was documented at FFRCT ≤0.75, consistent with established guidelines.
Results
A total of 170 patients with CLTI were screened; 65 patients (38.2%) had no coronary symptoms and met all inclusion/exclusion criteria. Twenty-four patients (31.2%) completed cCTA and FFRCT evaluation. Forty-one patients have yet to complete testing secondary to socioeconomic factors (insurance denial, transportation inaccessibility, testing availability, etc). The mean age of included subjects was 65.4 ± 7.0 years, and 15 (62.5%) were male. Patients presented with ischemic rest pain (n = 7; 29.1%), minor tissue loss (n = 14; 58.3%) or major tissue loss (n = 3; 12.5%). Significant (≥50%) coronary artery stenosis was noted on cCTA in 19 of 24 patients (79%). Significant left main coronary artery stenosis was identified in two patients (10%). When analyzed with FFRCT, 17 patients (71%) had hemodynamically significant coronary ischemia (FFRCT ≤0.8), and 54% (n = 13) had lesion-specific severe coronary ischemia (FFRCT ≤0.75). The mean FFRCT in patients with coronary ischemia was 0.70 ± 0.07. Multi-vessel disease pattern was present in 53% (n = 9) of patients with significant coronary stenosis.
Conclusions
The use of cCTA-derived fractional flow reserve demonstrates a significant percentage of patients with CLTI have silent (asymptomatic) coronary ischemia. More than one-half of these patients have lesion-specific severe ischemia, which may be associated with increased mortality when treated solely with risk factor modification. cCTA and FFRCT diagnosis of significant coronary ischemia has the potential to improve cardiac care, perioperative morbidity, and long-term survival curves of patients with CLTI. Systemic improvements in access to care will be needed to allow for broad application of these imaging assessments should they prove universally valuable. Additional study is required to determine the benefit of selective coronary revascularization in patients with CLTI.
Keywords: Coronary CTA, Critical limb ischemia, Critical limb-threatening ischemia, Fractional flow reserve, Peripheral arterial disease
Critical limb ischemia (CLI), or critical limb-threatening ischemia (CLTI), represents the most severe and aggressive form of atherosclerotic peripheral arterial disease (PAD), encompassing a constellation of symptoms that include ischemic rest pain, ischemic ulceration, and tissue gangrene. It is estimated that over 1.2 million patients in the United States are afflicted with CLTI,1 constituting a small fraction (1%-3%) of all patients with PAD.2,3 The initial treatment of patients with CLTI is directed at wound healing, pain relief, limb loss prevention, and improvement in quality of life. Equally important, albeit less urgent, is the critical attention devoted to risk factor modification (smoking cessation, structured exercise regimen, healthy diet) and guideline-directed medical therapy to address the underlying sources of ongoing peripheral vascular damage.4, 5, 6, 7, 8, 9
There is long-standing recognition that atherosclerosis is a systemic disease afflicting multiple vascular beds, and approximately 55% to 80% of patients with PAD have concomitant coronary artery disease (CAD).10, 11, 12 The combined diagnoses of lower extremity PAD and CAD carries an all-cause mortality of 4.6% per year, with nearly 75% of major adverse events being cardiac-related.13 The natural history of patients with symptomatic PAD is equally as dire, with a 5-year survival of 60% to 80% and 10-year survival of 20% to 60%.11,13 Moreover, the CASS registry14 demonstrated perioperative cardiac events occurred in 8.5% of patients with PAD with known CAD undergoing non-cardiac vascular surgery, and in another study, cardiac-related death (particularly fatal myocardial infarction [MI]) accounted for approximately 40% of all-cause perioperative mortality.15 In longer term follow-up after lower extremity vascular surgery, patients with overt CAD have an increased incidence of adverse cardiac events at 5 years compared with patients without CAD (28% vs 10%; P = .003), as well as decreased survival at 10 years (24% vs 51%; P < .001).16
Established recommendations do not advocate for CAD screening in patients with PAD, and current practice patterns generally defer preoperative cardiac revascularization in patients undergoing lower extremity vascular surgery in the absence of stable or unstable angina.5,6 However, significant restrictions in mobility often exist in the population of patients with CLTI, which may limit the detection of coronary ischemia symptoms via standard preoperative cardiac risk stratification.
A new noninvasive cardiac diagnostic modality, coronary computed tomography angiography (cCTA) derived fractional flow reserve (FFRCT) can identify patients with hemodynamically significant coronary stenoses who may benefit from coronary revascularization. FFRCT utilizes anatomical data provided by standard cCTA imaging with computational analysis of coronary blood flow to provide a three-dimensional color-coded map of FFR values throughout the coronary tree.17 Prospective clinical trials have shown that FFRCT accurately reflects invasively measured FFR18,19 and has a higher diagnostic accuracy than noninvasive myocardial perfusion tests.20 The clinical usefulness of FFRCT in patients with CAD is well documented,21, 22, 23 including patients with high coronary artery calcification.19 The 2021 American Heart Association/American College of Cardiology guideline for the evaluation and diagnosis of chest pain highlights the use of cCTA plus FFRCT as the frontline pathway for patients with known or suspected CAD to guide treatment decisions.24 This pathway has been applied to high-risk patients with PAD with no known CAD undergoing lower extremity vascular surgery, with early results showing improved survival with selective coronary revascularization.25,26
Therefore, the purpose of this study is to determine the presence, distribution, and severity of “silent” (asymptomatic) coronary ischemia in patients presenting with CLTI using cCTA and FFRCT. We hypothesize that a significant burden of coronary atherosclerosis is present in this CLTI patient population, which may have a consequential impact on long-term mortality.
Methods
For the period encompassing May 2020 through April 2021, all patients presenting with Rutherford Classification 4 - 6 were added to our active CLTI database. As part of the initial evaluation, patients were questioned about their cardiac history, namely any known diagnosis of CAD, prior MI, percutaneous coronary intervention, or coronary artery bypass graft surgery, and previous stress test of any type within 3 years. Patients were also screened for signs of coronary ischemia and anginal equivalents, including chest discomfort or tightness, dyspnea on exertion, shortness of breath, nausea, vomiting, lightheadedness, diaphoresis, fatigue, indigestion, or vague abdominal pain.27
In addition, patients were screened for appropriateness to undergo cCTA to optimize imaging quality and diagnostic integrity. Therefore, patients with body mass index >39 kg/m2, indwelling cardiac pacer or defibrillator, prosthetic cardiac valve, severe aortic stenosis (valve area <1.0 cm2), left ventricular ejection fraction <40%, heart transplant, or dysrhythmia were excluded from this study. The presence of chronic kidney disease (creatinine >1.8 mg/dL) or a contrast allergy were also basis for exclusion.
The cohort meeting the screening criteria were contacted, and a full disclosure of the study was provided, as approved by the institutional review board. Informed consent was not required specific to this study; however, all patients signed standard informed consent to proceed with cCTA. For those who agreed to participate, a prescription for metoprolol tartrate was provided if there were no contraindications, with instructions to administer 90 minutes prior to undergoing cCTA as per recommended protocol.28 A 50-mg dosage was prescribed for patients with a resting heart rate of 60 to 65 beats per minute (bpm), and 100 mg was prescribed for patients with a resting heart rate >65 bpm. Ivabradine 5 mg was prescribed for patients with a contraindication to metoprolol tartrate and was used in combination with metoprolol for patients with resting heart rate >80 bpm.
All cCTA scans were performed on a dual-source 96-row scanner with 196 slice acquisition. A 15- to 20-mL test bolus injection for was used to assist with timing of acquisition, and additional acquisition optimization was customized by patient calcium plaque burden, body habitus, electrocardiogram monitoring, and heart rate-dependent phase targeting. Interpretation of cCTA studies was completed by on-site certified reading cardiologists using TeraRecon Intuition software or GE Healthcare AW server-based software. Coronary artery stenosis was characterized by anatomic location, distribution (single-vessel or multi-vessel), and severity (significant if >50%). All cCTA image data was then sent via secure cloud-based server to HeartFlow for off-site computational analysis of FFRCT. Lesions were characterized by location, distribution, and severity; significant coronary ischemia were defined as FFRCT ≤0.80 distal to >30% coronary artery stenosis in a 2-mm diameter vessel, and severe coronary ischemia was defined as FFRCT ≤0.75 distal to >30% coronary artery stenosis in a 2-mm diameter vessel.
Continuous variables are expressed as mean ± standard deviation, and continuous variables are expressed as count (percentage) where appropriate. Significance between groups for continuous variable comparison was determined using the standard error of the mean with significance defined as P < .05.
Results
A total of 170 patients presenting to our institution with newly diagnosed CLTI were screened during the study period. Sixty-five patients (38.2%) had no coronary history or symptoms and met all inclusion/exclusion criteria. From this cohort, a total of 26 patients underwent cCTA; however, two patients did not complete cCTA or FFRCT analysis secondary to prohibitive coronary calcification. Patient demographics for the final cohort of 24 patients is illustrated in the Table. The mean age was 65.4 years, with a 62.5% male predominance (n = 15). Cardiovascular comorbidities include hypertension (n = 23; 95.8%), hyperlipidemia (n = 22; 91.6%), type 2 diabetes mellitus (n = 9; 37.5%), and active smoking (n = 19; 79.2%). CLTI presented as ischemic rest pain (Rutherford 4) (n = 7; 29.2%), minor tissue loss (Rutherford 5) (n = 14; 58.3%), and major tissue loss (Rutherford 6) (n = 3; 12.5%). Noninvasive vascular testing at the time of presentation revealed a mean ankle-brachial index (ABI) of 0.34 ± 0.19 and a mean toe pressure of 20.5 ± 15.4 mmHg.
Table.
Patient demographics
| Patient characteristics | n = 24 |
|---|---|
| Age, years | 65.4 ± 7.0 |
| Males | 15 (62.5) |
| Comorbidities | |
| Hypertension | 23 (95.8) |
| Hyperlipidemia | 22 (91.7) |
| Diabetes mellitus, type 2 | 9 (37.5) |
| Active or former smoker | 19 (79.2) |
| Rutherford classification | |
| 4 (ischemic rest pain) | 7 (29.2) |
| 5 (minor tissue loss) | 14 (58.3) |
| 6 (major tissue loss) | 3 (12.5) |
| Preoperative noninvasive testing | |
| Ankle-brachial index (n = 20) | 0.34 ± 0.19 |
| Noncompressible ankle-brachial index | 4 (16.7) |
| Toe pressure, in mmHg (n = 21) | 20.5 ± 15.4 |
Data are presented as number (%) or mean ± standard deviation.
Imaging results were reviewed separately between cCTA findings and FFRCT analysis. A significant stenosis (>50%) on cCTA was found in 19 of 24 patients (79.2%) based on the study interpretation. Two patients (8.3%) had a significant left main coronary artery stenosis on cCTA. All patients in the cohort completed FFRCT analysis. Two patients that were diagnosed with significant coronary stenosis on cCTA did not have significant findings on FFRCT (FFR ≥0.8). Therefore, a total of 17 of 24 patients with CLI (70.8%) were discovered to have hemodynamically significant coronary artery stenosis on FFRCT (FFR <0.8). Of the 17 patients with significant coronary stenosis, 13 patients (76.4%) had severe coronary ischemia (FFR ≤0.75), equating to 54.2% of the full cohort. The lowest calculated mean FFR for all patients with significant coronary ischemia (FFR <0.8; n = 17) was 0.71 ± 0.07 (range, 0.50-0.78). Nine of 17 patients (52.9%) had significant stenosis in a single coronary artery, and eight patients (47.1%) had multi-vessel stenosis (illustrated in Fig 1).
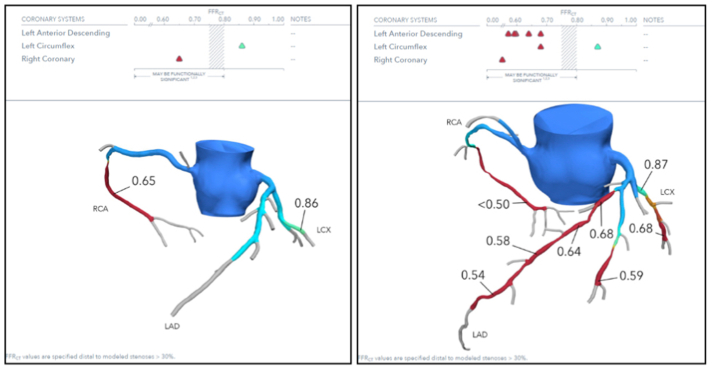
Fig 1.
Representative computed tomography-derived fractional flow reserve (FFRCT) report. Left, 72-year-old female with toe gangrene and single-vessel severe coronary ischemia. Right, 63-year-old male with toe gangrene and multi-vessel severe coronary ischemia. LAD, Left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
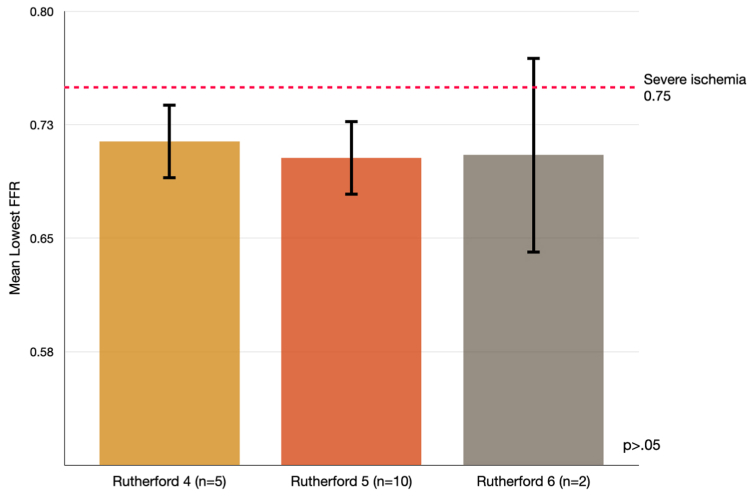
A secondary evaluation of patients that had significant coronary ischemia on FFRCT (n = 17) was then performed after grouping into presenting symptoms (ie, Rutherford 4, Rutherford 5, Rutherford 6). The severity of coronary ischemia was consistent across the three groups without a statistically significantly difference (Fig 2). The mean FFR was 0.71 ± 0.06 for patients presenting with Rutherford 4; 0.70 ± 0.08 for Rutherford 5; and 0.71 ± 0.09 for patients with Rutherford 6. There was no significant difference in the distribution of coronary stenosis between these groups: single vessel vs multi-vessel (P > .05).
Fig 2.
Mean lowest fractional flow reserve (FFR) based on Rutherford classification demonstrating nonsignificant differences between groups.
All patients in the cohort (n = 24) underwent lower extremity revascularization for CLI. Ten patients (41.7%) had surgical revascularization, and 14 patients (58.3%) had endovascular revascularization. Ten patients completed cCTA and FFRCT testing prior to surgical (n = 8) or endovascular (n = 2) revascularization. Preoperative cCTA identified significant coronary stenosis in eight of these 10 patients, and the mean FFRCT value was 0.76 ± 0.09. There were no cardiac deaths within 30 days of surgery. Two patients (8.3%) had troponin elevation above baseline without electrocardiogram changes during the perioperative period, both of whom had a positive cCTA and FFRCT values of 0.74 and 0.68.
Discussion
The findings of the current study demonstrate that patients presenting with CLTI who have no symptoms of coronary ischemia or other angina equivalents have a remarkable burden of significant and severe coronary artery stenosis. Approximately 70% of patients in this cohort were found to have a hemodynamically significant stenosis of at least one coronary artery (FFR <0.8), and nearly 55% of patients had severe lesion-specific coronary ischemia (FFR ≤0.75) in the coronary vasculature. These results illustrate the value of assessing patients with CLTI with FFRCT to visualize the anatomic and functional significance of coronary stenosis, thus allowing for more aggressive cardiac treatment on patients who may benefit most from coronary revascularization.
The 2016 American Heart Association/American College of Cardiology guidelines5 for treating patients with PAD focus on intensive atherosclerotic risk factor modification, including smoking cessation, a structured exercise program, and guideline-directed pharmacotherapy, to reduce cardiovascular ischemic events. The benefits of medical therapy with a regimen including an anti-platelet, statin, beta-blocker, and angiotensin-converting enzyme inhibitor are evident in symptomatic patients with PAD, resulting in significantly decreased cardiovascular death, MI, and stroke in multiple randomized controlled trials.7, 8, 9,29 However, there is not convincing evidence that these therapies have translated to a consequential improvement in 1- or 5-year survival rates for patients with CLTI in a review of population-based studies over the last 20 years.1
The reasons for unwavering long-term mortality rates in patients with CLTI despite these medical advancements is not clear. The high prevalence of cardiac-related death persists in patients with PAD and suggests that CAD disease remains a high-value target. Current recommendations5,6 do not support screening for CAD in patients with symptomatic PAD, citing “no evidence to demonstrate that screening all patients with PAD for asymptomatic atherosclerosis in other arterial beds improves clinical outcome.” Contemporary studies have challenged this assertion with advances in imaging technology and more aggressive interventional treatment algorithms for patients with CAD. For example, a recent meta-analysis reviewing 5460 patients undergoing FFRCT demonstrated that patients with FFR ≤0.8 were 2.3 times more likely to have a major adverse cardiac event, MI, or unplanned coronary revascularization at 1-year follow-up compared with patients with FFR >0.8.30 Given the reliable sensitivity between FFRCT and invasive coronary angiography with FFR,31,32 the potential for FFRCT to act as a screening tool and gatekeeper for coronary angiography appears to be fast approaching.33
Much of the basis for restricted screening in patients with PAD and hesitancy for preoperative cardiac revascularization in patients undergoing non-cardiac vascular surgery stems from the results of the Coronary Artery Revascularization Prophylaxis (CARP) trial.34 This trial found no difference in perioperative (30-day) or long-term (mean follow-up, 2.7 years) mortality between patients who were randomized to undergo cardiac revascularization (coronary artery bypass graft or percutaneous coronary intervention) prior to vascular surgery vs those that did not undergo preoperative cardiac revascularization. The limitations of the CARP study have been openly debated in other forums and do not require full rehashing here, but factors pertinent to the current study are worth reviewing. Importantly, initial screening protocols excluded 38% of 5859 patients by cardiology consultants, citing insufficient cardiac risk.35 It is uncertain if a lack of cardiac symptoms in the screened patient population of the CARP trial contributed to “insufficient cardiac risk” and thus exclusion from randomization, which would be inappropriate based on the severity of coronary ischemia discovered in the asymptomatic cohort of the current study. In addition, patients with left main stenosis were excluded from randomization in CARP, constituting 5% to 7% of patients with CAD in recently published trials,25,36 and is consistent with the 8% identified in the current data set. Patients with left main stenosis exhibit significantly better survival at 2.5 years following revascularization compared with those with no revascularization.37
Lastly, cardiac revascularization was considered based on ≥70% coronary artery stenosis interpreted from visual angiography, and subsequently, two-thirds of patients in the trial had incomplete coronary revascularization.35 The gold standard for coronary revascularization in current practice relies on invasive FFR measurements. Parikh et al demonstrated a significant mortality benefit at 1-year with FFR-directed revascularization over standard invasive coronary angiography.38 Outdated risk stratification models are also being confronted by modern research. Monaco et al39 recently published findings of a prospective randomized study that was undertaken to determine the effect of systematic coronary angiography in medium- to high-risk patients undergoing vascular surgery vs selective coronary angiography based on results of standard noninvasive tests. This resulted in increased revascularization rates in the systematic coronary angiography group, with improved long-term survival as well as freedom from major coronary events after a mean follow-up of 58 months.
The implications of the current study advocate for a reconsideration of the prevalent paradigm in addressing CAD in patients with CLI. Certainly, this data has shown that a lack of coronary symptoms is not a representative indicator of the severity of coronary atherosclerosis in a patient with CLI. These asymptomatic patients are largely excluded from routine screening and perioperative testing under current guidelines. It would seem prudent to consider the presence of CLI an angina equivalent, given that many patients with CLTI are unable to exert the level of activity required to elicit ischemic symptoms due to limitations in walking distance and mobility. Initial evidence supporting this algorithm for silently ischemic patients was recently presented by Krievins et al,26 showing their analysis of 231 patients with CLTI undergoing lower extremity surgical revascularization with preoperative FFRCT to guide selective postoperative coronary revascularization, compared with a standard preoperative risk stratification protocol and no postoperative revascularization. Of the 111 patients that underwent FFRCT evaluation with selective coronary revascularization, there was a significant reduction in MI (6.3% vs 17.5%; P = .009) and improved survival (91.9% vs 80.0%; P = .13) at 2-year follow-up. More investigation is required to confirm these findings before expanding this strategy to other patient populations.
It is worth noting the unexpected challenges we faced in completing this study, specifically surrounding the small number of patients that underwent scans. Although the intent of this research was to serve as a pilot investigation, the final cohort number was limited by socioeconomic factors, access to scanners, and insurance denials. Indeed, communication and coordination with our patients with CLTI were strained by a lack of reliable phone service to provide initial explanation of the study, prescription notification, scheduling issues, and confirmations. Delays resulted as we resorted to standard mail notifications for some patients. Furthermore, limited transportation options proved to be a significant barrier for patients who agreed to participate, and thus multiple no-shows resulted. Furthermore, appointment availability of CT machines with cCTA/FFRCT technology was initially thinly distributed, secondary to limited capacity. This limitation has been resolved at our institution but remains a concern for widespread adoption of this technology.
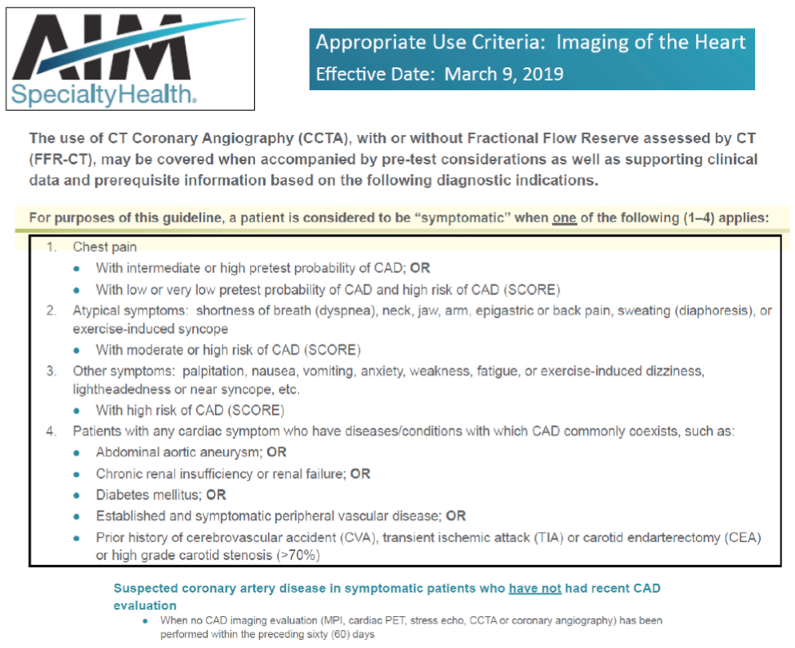
Lastly, the insurance payor approval rate for obtaining cCTA in an asymptomatic patient population was regrettably quite low. Approvals for cCTA in our region are largely based on current AIM Specialty Health guidelines, which require a patient to be symptomatic (chest pain or angina-equivalent) (Fig 3). This obstacle was expected given the lack of data supporting cCTA use in asymptomatic patients at the initiation of the study; however, we fared slightly better with a few private insurance companies. It is likely that payor approval will continue to be an impediment to obtaining scans until more data can be provided.
Fig 3.
AIM Specialty Health approved indications for coronary computed tomography angiography (CCTA) with or without computed tomography-derived fractional flow reserve (FFRCT). CAD, Coronary artery disease; MPI, myocardial perfusion imaging; PET, positron emission tomography.
This study has some important limitations. First, the small cohort size introduces the possibility of skewed means, Type I error, and may have inconsistent correlation to a larger sample of patients with CLTI. In addition, the patient cohort was an intentionally exclusive group for the purposes of this pilot investigation, selected specifically to optimize imaging quality and diagnostic precision. Thus, the results remain representative only to this group and may not be characteristic of a general PAD population. However, the findings of this study parallel the presence and severity of coronary ischemia reported by Krievins et al25,26 in a similar cohort of patients with CLTI in Latvia evaluated with cCTA and FFRCT. It is likely that future FFRCT research will be more inclusive of patients with previous coronary history or revascularization and with less restrictive comorbidities.
Lastly, the design of this study was intended to identify the severity and distribution of silent coronary ischemia in a CLTI cohort. The small patient cohort in this study does not permit an appropriate statistical comparison of preoperative cCTA/FFRCT results and adverse 30-day cardiac events. No claims can be made to the effectiveness of this imaging modality as a preoperative testing to predict perioperative coronary events from this data set. Nonetheless, the findings of this study and other previously mentioned publications strongly advocate for additional investigation with well-designed and controlled multi-center trials.
Conclusion
FFRCT identified a significant number of patients with CLTI with underlying silent (asymptomatic) coronary ischemia, including nearly 55% of patients who had lesion-specific severe ischemia. The use of FFRCT to diagnose and assist in management of otherwise asymptomatic coronary ischemia has the potential to improve perioperative complication rates and long-term survival curves of patients with CLTI. Current indications for CAD screening and justification for FFRCT may deserve reconsideration in the near future. Additional study is required to confirm the findings presented herein for wider applicability.
From the Southern Association for Vascular Surgery
Footnotes
Funding: This study was funded internally by Sanger Heart & Vascular Institute and received no outside funding source.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Discussion
Dr Claudie Sheahan (New Orleans, Louisiana). I appreciate the opportunity to have reviewed this manuscript. This was excellent, well-written, and submitted well ahead of time. I want to congratulate Dr Stanley and his colleagues for a well-designed pilot study in which they demonstrated a high prevalence of asymptomatic coronary artery disease (CAD) in their patients with critical limb ischemia. In the interest of time, I will not summarize these findings that were just presented.
I have one comment and two questions. My comment is, since patients with morbid obesity, congestive heart failure, and chronic kidney disease were excluded, it is likely that the actual prevalence of asymptomatic CAD is even higher than you have demonstrated.
My first question is, can you tell us how many of these patients had their actual treatment plan altered by the discovery of asymptomatic coronary disease? As you noted in the manuscript, all of them eventually underwent revascularization for their critical limb ischemia.
Second, you report that several barriers to care, as you just discussed, prevented many of these patients from undergoing the coronary imaging, including transportation, communication, and insurance obstacles. Can you give us an estimate of the relative frequency of these barriers? Obviously, overcoming these impediments to care should be a major focus of our specialty again in the future.
Again, congratulations on this excellent project, and I want to thank the Society for the opportunity to comment on this paper.
Gregory Stanley. Great, thank you, Dr Sheahan, for your comments and questions. To answer your first question, certainly I agree with you, the prevalence of significant CAD is likely higher in these patients that were not screened. The plan was not significantly altered in any of these patients, as they all did undergo vascular reconstruction. There were two patients in this cohort that had perioperative troponin leaks and ultimately underwent cardiac catheterization. One of those patients had an intervention at that time. The other did not; so, as actually has been shown in other trials, the perioperative outcomes haven't been shown to change much, and that's true in our patient population as well, so I think that much more of this focus will be on the long-term mortality and survival benefit in treating these patients with CAD.
To answer your second question, the barriers to care were significant. Less than 50% of all the patients that we screened ended up getting a scan, and the large majority of that was secondary to the socioeconomic issues. The insurance denials made up, I would say, about 20% to 25% of the patients that did not end up getting scanned, and that is not an insignificant number, and, in fact, one of the patients that was denied by insurance had a myocardial infarction 1 week after his insurance denial and ended up in the hospital for 14 days and underwent emergent coronary catheterization, etc, so it's certainly a relevant topic that needs to be overcome.
Dr Hernan Bazan (New Orleans). Thank you for presenting this pilot study. Just a couple of comments and a question. So Heartflow has a value now of $2.4 billion, and it's FDA-approved, and it seems like there's a search to try to find where to apply it in practice because it hasn't really been adopted by cardiologists very much, so one practical question for you is did any of those patients get percutaneous coronary intervention (PCI)? Can you tell us about that, in the ones that you found fractional flow reserve – computed tomography (FFR-CT) under 0.75?
Dr Stanley. Yes, so again, two of those patients did undergo cardiac catheterization during the perioperative period, and one did get a PCI. Less than 10 of the patients have undergone intervention since scanning, so again, there have been the same issues to access for those patients, sort of getting them to follow-up after the scan is complete, so less than 10 of them have undergone angiography, and a few have undergone PCI.
Dr Bazan. And any that didn't get PCI that had an FFR-CT under 0.75, did you follow them? Did they have any coronary events perioperatively?
Dr Stanley. So far, no, at this point. This study was done through the middle of last year, so our follow-up is still less than a year at this point.
Dr Bazan. The last question is so the mechanism of a myocardial infarction is an atherosclerotic plaque rupture and embolization, not necessarily low flow and ischemia. Can you comment on the imaging that's the vulnerable plaque vs ischemia?
Dr Stanley. Yes, thank you, and that certainly is one of the limitations of FFR-CT is that lesion-specific stability is not elucidated by FFR-CT as of yet, and that remains an area of active research.
Dr Chris Ramos (Atlanta, Georgia). What was the heart rate of your patients? As you know, the sensitivity of cardiac CT is more sensitive when patients are beta-blocked, heart rate 50 to 60. Were your patients pre-imaging beta-blocked to get better imaging?
Dr Stanley. Yes, great question. So, I don't have the exact number for their mean heart rate. I can tell you that 75% of the patients that underwent scan did get a preoperative beta blocker. Our goal was to have them down to a heart rate less than 70, and I was contacted by the technicians for several patients that had not met that criteria, and we added additional medications at the time of the scan to try to reach that goal, so 75% of the patients were pretreated.
Dr Sal Scali. Thank you, nice job.
Dr Stanley. Thank you.
References
- 1.Duff S., Mafilios M.S., Bhounsule P., Hasegawa J.T. The burden of critical limb ischemia: a review of the recent literature. Vasc Health Risk Manag. 2019;15:187–208. doi: 10.2147/VHRM.S209241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nehler M.R., Duval S., Diao L., et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 3.Baser O., Verpillat P., Gabriel S., Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vasc Dis Manag. 2013;10:26–36. [Google Scholar]
- 4.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G., TASC II Working Group Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. 2016 AHA/ACC Guideline of the management of patients with lower extremity peripheral artery disease. JACC (J Am Coll Cardiol) 2017;69:e71–e126. doi: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Aboyans V., Ricco J.B., Bartelink M., et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial disease, in collaboration with the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:305–368. doi: 10.1016/j.ejvs.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heart Protection Study Collaborative Group Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–654. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Aung P.P., Maxwell H.G., Jepson R.G., Price J.F., Leng G.C. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev. 2007;2007 doi: 10.1002/14651858.CD000123.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertzer N.R., Beven E.G., Young J.R., et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223–233. doi: 10.1097/00000658-198402000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welten G.M., Schouten O., Hoeks S.E., et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–1596. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 12.Hur D.J., Kizilgul M., Aung W.W., Roussillon K.C., Keeley E.C. Frequency of coronary artery disease in patients undergoing peripheral artery disease surgery. Am J Cardiol. 2012;110:736–740. doi: 10.1016/j.amjcard.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steg P.G., Bhatt D.L., Wilson P.W., et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 14.Eagle K.A., Rihal C.S., Mickel M.C., Holmes D.R., Foster E.D., Gersh B.J. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study. Circulation. 1997;96:1882–1887. doi: 10.1161/01.cir.96.6.1882. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson W.R., Janusz M.T., Miyagishima R.T., Gerein A.N. Influence of ischemic heart disease on early and late mortality after surgery for peripheral occlusive vascular disease. Circulation. 1982;66:I92–I97. [PubMed] [Google Scholar]
- 16.Farkouh M.E., Rihal C.S., Gersh B.J., et al. Influence of coronary heart disease on morbidity and mortality after lower extremity revascularization surgery: a population-based study in Olmsted County, Minnesota (1970–1987) J Am Coll Cardiol. 1994;24:1290–1296. doi: 10.1016/0735-1097(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 17.Taylor C.A., Fonte T.A., Min J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 18.Norgaard B.L., Leipsic J., Gaur S., et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomographic angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: next Steps) J Am Coll Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Norgaard B.L., Gaur S., Leipsic J., Ito H., Mijoshi T., Park S.J. Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease. A substudy of the NXT trial. J Am Coll Cardiol. 2015;8:1045–1055. doi: 10.1016/j.jcmg.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Driessen R.S., Danad I., Stuijfzand W.J., Raijmakers P.G., Schumaker S.P., van Diemen P.A. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. 2019;73:161–173. doi: 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 21.Norgaard B.L., Terkelsen C.J., Mathiassen O.N., et al. Coronary CT angiographic and flow reserve-guided management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2018;72:2123–2134. doi: 10.1016/j.jacc.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 22.Ihdayhid A.R., Norgaard B.L., Gaur S., et al. Prognostic value and risk continuum of noninvasive fractional flow reserve derived from coronary CT angiography. Radiology. 2019;292:343–351. doi: 10.1148/radiol.2019182264. [DOI] [PubMed] [Google Scholar]
- 23.Fairbairn T.A., Nieman K., Akasaka T., et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from ADVANCE registry. Eur Heart J. 2018;39:3701–3711. doi: 10.1093/eurheartj/ehy530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;78:e187–e3285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 25.Krievins D., Zellans E., Latkovskis G., et al. Pre-operative diagnosis of silent coronary ischaemia may reduce post-operative death and myocardial infarction and improve survival of patients undergoing lower extremity surgical revascularization. Eur J Vasc Endovasc Surg. 2020;60:411–420. doi: 10.1016/j.ejvs.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Krievins D., Zellans E., Latkovskis G., et al. Diagnosis of silent coronary ischemia with selective coronary revascularization might improve 2-year survival of patients with critical limb-threatening ischemia. J Vasc Surg. 2021;74:1261–1271. doi: 10.1016/j.jvs.2021.03.059. [DOI] [PubMed] [Google Scholar]
- 27.Writing Committee Members. Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2021;78:e187–e285. doi: 10.1016/j.jacc.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 28.Abbara S., Blanke P., Maroules C.D., et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Feringa H.H., van Waning V.H., Bax J.J., et al. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. J Am Coll Cardiol. 2006;47:1182–1187. doi: 10.1016/j.jacc.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 30.Nørgaard B.L., Gaur S., Fairbairn T.A., et al. Prognostic value of coronary computed tomography angiographic derived fractional flow reserve: a systematic review and meta-analysis. Heart. 2022;108:194–202. doi: 10.1136/heartjnl-2021-319773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakanishi R., Matsumoto S., Alani A., et al. Diagnostic performance of transluminal attenuation gradient and fractional flow reserve by coronary computed tomographic angiography (FFR(CT)) compared to invasive FFR: a sub-group analysis from the DISCOVER-FLOW and DeFACTO studies. Int J Cardiovasc Imaging. 2015;31:1251–1259. doi: 10.1007/s10554-015-0666-2. [DOI] [PubMed] [Google Scholar]
- 32.Baumann S., Renker M., Hetjens S., et al. Comparison of coronary computed tomography angiography-derived vs invasive fractional flow reserve assessment: meta-analysis with subgroup evaluation of intermediate stenosis. Acad Radiol. 2016;23:1402–1411. doi: 10.1016/j.acra.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Fukuoka R., Kawasaki T., Umeji K., Okonogi T., Koga N. The diagnostic performance of on-site workstation-based computed tomography-derived fractional flow reserve. Comparison with myocardium perfusion imaging. Heart Ves. 2022;37:22–30. doi: 10.1007/s00380-021-01897-w. [DOI] [PubMed] [Google Scholar]
- 34.McFalls E.O., Ward H.B., Moritz T.E., et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 35.Raghunathan A., Rapp J.H., Littooy F., et al. CARP Investigators. Postoperative outcomes for patients undergoing elective revascularization for critical limb ischemia and intermittent claudication: a subanalysis of the Coronary Artery Revascularization Prophylaxis (CARP) trial. J Vasc Surg. 2006;43:1175–1182. doi: 10.1016/j.jvs.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 36.Maron D.J., Hochman J.S., O’Brien S.M., et al. International study of comparative health effectiveness with medical and invasive approaches (ISCHEMIA) trial.: rationale and design. Am Heart J. 2018;201:124–135. doi: 10.1016/j.ahj.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia S., Moritz T.E., Ward H.B., et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol. 2008;102:809–813. doi: 10.1016/j.amjcard.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Parikh R.V., Liu G., Plomondon M.E., et al. Utilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J Am Coll Cardiol. 2020;75:409. doi: 10.1016/j.jacc.2019.10.060. [DOI] [PubMed] [Google Scholar]
- 39.Monaco M., Stassano P., Di Tommaso L., et al. Systematic strategy of prophylactic coronary angiography improves long-term outcome after major vascular surgery in medium- to high-risk patients: a prospective, randomized study. J Am Coll Cardiol. 2009;54:989–996. doi: 10.1016/j.jacc.2009.05.041. [DOI] [PubMed] [Google Scholar]