Abstract
Transcriptional activation of heat shock genes is a reversible and multistep process involving conversion of inactive heat shock factor 1 (HSF1) monomers into heat shock element (HSE)-binding homotrimers, hyperphosphorylation, and further modifications that induce full transcriptional competence. HSF1 is controlled by multiple regulatory mechanisms, including suppression by additional cellular factors, physical interactions with HSP70, and integration into different cellular signaling cascades. However, the signaling mechanisms by which cells respond to stress and control the HSF1 activation-deactivation pathway are not known. Here we demonstrate that HSP90, a cellular chaperone known to regulate several signal transduction molecules and transcription factors, functions in the regulation of HSF1. The existence of HSF1-HSP90 heterocomplexes was shown by coimmunoprecipitation of HSP90 with HSF1 from unshocked and heat-shocked nuclear extracts, recognition of HSF1-HSE complexes in vitro by using HSP90 antibodies (Abs), and recognition of HSF1 in vivo by HSP90 Abs microinjected directly into oocyte nuclei. The functional impact of HSP90-HSF1 interactions was analyzed by using two strategies: direct nuclear injection of HSP90 Abs and treatment of cells with geldanamycin (GA), an agent that specifically blocks the chaperoning activity of HSP90. Both HSP90 Abs and GA delayed the disassembly of HSF1 trimers during recovery from heat shock and specifically inhibited heat-induced transcription from a chloramphenicol acetyltransferase reporter construct under control of the hsp70 promoter. HSP90 Abs activated HSE binding in the absence of heat shock, an effect that could be reversed by subsequent injection of purified HSP90. GA did not activate HSE binding under nonshock conditions but increased the quantity of HSE binding induced by heat shock. On the basis of these findings and the known properties of HSP90, we propose a new regulatory model in which HSP90 participates in modulating HSF1 at different points along the activation-deactivation pathway, influencing the interconversion between monomeric and trimeric conformations as well as transcriptional activation. We also put forth the hypothesis that HSP90 links HSF1 to cellular signaling molecules coordinating the stress response.
Cells respond to heat and other forms of stress by upregulating expression of a family of highly conserved heat shock proteins (HSPs) which function under both normal and stressful conditions as molecular chaperones mediating the folding, assembly, translocation, and degradation of proteins (reviewed in references 25 and 34). In eukaryotes, stress-induced expression of HSPs is regulated primarily by heat shock transcription factor HSF1, which acts through heat shock regulatory elements (HSEs) found in the promoters of HSP genes (33, 73). Under nonstress conditions, HSF1 exists as a repressed non-DNA-binding monomer (16, 53, 75), and the activation-deactivation pathway of HSF1 in metazoan cells involves several independently regulated steps (reviewed in references 40 and 73). Different forms of cellular stress trigger the rapid conversion of HSF1 from inert monomers to homotrimers, and this first step is accompanied by increased HSE-binding affinity (4, 53, 55, 66, 70). Detailed mutagenic analyses have suggested that HSF1 monomers are stabilized by intramolecular interactions between several hydrophobic heptad repeats and that activation during stress involves the disruption of these intramolecular interactions, leading to formation of intermolecular coiled coils with other HSF1 monomers (16, 55, 66, 75). Thus, the suppression of HSE-binding activity under normal conditions is regulated at least in part by hydrophobic sequences within HSF1, although other regions of the molecule have also been implicated in this regulation (48). Once it has trimerized, HSF1 undergoes additional modifications to upregulate transcriptional activity which appear to be regulated independently of trimerization and DNA binding (21, 59, 76). The final step of HSF1 regulation is deactivation or attenuation. Upon removal of stress, HSF1 dissociates into monomers and ceases to activate transcription (11, 44, 52).
It has become clear that HSF1 is subject to complex regulation under both normal and stress conditions. First, a simple model in which HSF1 is regulated by temperature alone is contradicted by observations that hsp genes are switched on by multiple unrelated stresses and that HSF1 molecules expressed in heterologous systems are reprogrammed according to the appropriate physiological temperatures of the host cells (8, 11, 30, 53). It therefore appears that HSF1 is under negative regulation by unknown cellular factors. Second, HSF1 has been shown to be phosphorylated by several different signal transduction molecules; for example, heat-induced activation of HSF1 was enhanced by phorbol ester treatment of human erythroleukemia cells, and thus HSF1 is apparently targeted by protein kinase C (26). HSF1 has also been shown to be phosphorylated by ERK1, linking HSF1 to the Ras signaling pathway (29). The constitutive phosphorylation on serine and threonine residues and inducible hyperphosphorylation in response to stress also suggested that HSF1 is modulated by cellular kinases and/or phosphatases (4, 30, 55). Although definitive correlations between different phosphorylation states and specific HSF1 activities have not been established (12, 31, 46, 72), recent evidence suggests that hyperphosphorylation could function both to increase transactivation potential and to delay the dissociation of trimers (74).
In addition to phosphorylation and suppression by cellular factors, it has been hypothesized that HSPs themselves, particularly HSP70, could function as part of an autoregulatory mechanism by which cells detect stress and regulate HSF1 (39). This idea is substantiated by a number of experimental observations with various cell types: activated expression of other HSPs after mutation of the hsp70 gene in yeast (9), continued transcription of hsp70 genes during recovery in cells blocked for HSP accumulation by treatment with translational inhibitors (13), and activation of hsp genes in response to artificially increased nuclear concentrations of denatured proteins (2, 37). It has been shown that physical interactions between HSP70 and HSF1 occur both in vitro and in vivo, but these appear to be unstable, and thus the stoichiometric relationships of these complexes have not been determined (1, 3, 5, 39, 47, 53, 70). In addition, different studies have shown that direct overexpression of HSP70 in cultured cells either reduced the level of HSF1 activation during induction (5, 41) or increased the rate of HSF1 deactivation (41, 54), and so the precise influence of HSP70 on oligomerization steps of the HSF1 activation-deactivation have not been fully elucidated. More recently, HSP70 has been shown to repress HSF1 through direct interaction with the transactivation domain (61); therefore, the primary autoregulatory role of HSP70 appears to be downregulation of HSF1-mediated transcription.
Another well-characterized HSP, HSP90, functions as the core of several chaperone heterocomplexes that contain different assortments of noncovalently linked proteins, including HSP70 (18). Numerous studies have established that HSP90 is required for higher-order folding of certain signal transduction molecules and transcription factors (reviewed in references 7 and 28) and that HSP90 functions in the later stages of protein folding after the formation of extensive secondary structure (35, 62, 63). HSP90 is thought not to be a general chaperone but rather to interact with and modulate the activity of various specific substrates, including hypoxia-inducible factor α (20), MyoD (58), steroid hormone receptors (50), the pp60v-src kinase (10), casein kinase II (15), and eIF-2α kinase (69).
Together, the known chaperone function and substrate specificity of HSP90, the conformational changes associated with HSF1 oligomerization, apparent integration of HSF1 into different signal transduction cascades, and a previous report describing an interaction between immobilized HSP90 and HSF from HeLa cell nuclear extracts (42) prompted us to consider HSP90 as a potential modulator of HSF1 activity. Further, HSF1 has recently been shown to assemble HSP90 and other chaperones common to the progesterone receptor assembly pathway in vitro (43). Here we investigated the potential autoregulatory role of HSP90 by using Xenopus oocytes. Results of coimmunoprecipitation and antibody recognition experiments provide clear evidence of a physical association in vivo between HSP90 and HSF1. Impairment of HSP90 function either by microinjected antibodies or by the specific binding agent geldanamycin (GA) resulted in a significant delay in HSF1 trimer disassembly during recovery and a marked reduction in heat-induced transcription from the hsp70 promoter. In addition, microinjected HSP90 antibodies (Abs) caused the activation of HSE-binding activity in the absence of heat shock, an effect which could be reversed by subsequent injection of exogenous HSP90. Together these observations support a new regulatory model in which formation of a heterocomplex between HSP90 and HSF1 plays a key role in modulating different steps of the HSF1 activation-deactivation pathway.
MATERIALS AND METHODS
Oocyte manipulations, stress treatments, and microinjection.
Xenopus laevis frogs were purchased from Xenopus I (Ann Arbor, Mich.). Ovary portions were surgically removed from adult female frogs, and follicle cells were removed from oocytes by treatment in calcium-free OR2 (45) buffer (82.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1 mM NaH2PO4, 5 mM HEPES [pH 7.8], 10 mg of streptomycin sulfate per liter, 10 mg of benzyl penicillin per liter) containing 2 mg of collagenase (type II; Sigma) per ml for 2 to 3 h at 18°C. Oocytes were washed extensively and allowed to recover overnight in OR2 containing 1 mM CaCl2 at 18°C, and only stage VI oocytes were selected for experiments. Nuclei were obtained under OR2 by scoring animal hemispheres with a needle and gently squeezing the equatorial region with watchmaker’s forceps. In some experiments, oocytes were incubated either in OR2 containing 10 μg of GA (a gift of the Developmental Therapeutics Program, National Cancer Institute, Bethesda, Md.) per ml dissolved in dimethyl sulfoxide (DMSO) or in OR2 with 1:250 (vol/vol) DMSO for 2 h at 18°C, rinsed, and then exposed to heat shock treatments. In all experiments, a minimum of 20 oocytes was used for each sample.
Plasmid constructs used for microinjection experiments were the human cytomegalovirus (CMV)-chloramphenicol acetyltransferase (CAT) and the Xenopus hsp70-CAT clones (kindly provided by Alan Wolffe, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.), which were previously described (32). Following defolliculation, oocytes were incubated for several hours at 18°C, after which healthy oocytes were selected and injected into nuclei with 20 nl of a solution containing 2 ng of either CMV-CAT or hsp70-CAT plasmid per μl (40 pg) by using a Narashige IM 300 microinjector. After incubation overnight at 18°C, oocytes were subjected to heat shock or GA treatments and then incubated for an additional 12 h at 18°C, washed in OR2, and assayed for CAT activity.
For Ab microinjection experiments, Abs were diluted (1:1) in sterile H2O immediately prior to microinjection. In experiments in which the effects of HSP90 Abs on induced transcription were measured, Ab solutions (15 nl) were injected directly into the nuclei of oocytes containing reporter constructs (injected 12 h previously). Oocytes were then incubated for 30 min at 18°C, heat shocked, and then incubated for a further 12 h to allow for CAT expression. In experiments to measure the effects of HSP90, HSF1, or YY1 Abs on HSF1 binding, Abs were injected into (previously uninjected) oocyte nuclei. Injected oocytes were allowed to recover for 30 min at 18°C and then incubated at a control temperature (18°C) or heat shocked at 33°C. Protein extracts were then prepared for gel mobility shift analyses and immunoblotting. HSP90 polyclonal Abs (PAbs) and purified bovine HSP90 were gifts of R. Matts, Oklahoma State University, Stillwater; anti-HSP90 monoclonal Ab (MAb) (SPA 830) was purchased from StressGen, Victoria, British Columbia, Canada; HSF1 PAbs (55) were a gift of K. Sarge, University of Kentucky, Lexington; and anti-Ying Yang 1 (anti-YY1) transcription factor PAbs were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif.
Protein extracts and gel mobility shift assays.
Protein extracts were prepared by homogenizing oocytes in buffer C (50 mM Tris-Cl [pH 7.9], 20% glycerol, 50 mM KCl, 0.1 mM EDTA, 2 mM dithiothreitol, 10 μg of aprotinin per ml, and 10 μg of leupeptin per ml) (14) in a Dounce homogenizer (10 μl/oocyte). Homogenates were transferred to Eppendorf tubes and spun for 5 min at 15,000 × g (4°C). The resultant supernatants were placed in a fresh tube and then immediately frozen in liquid nitrogen and stored at −80°C.
DNA mobility shift assays were performed by using HSE oligonucleotide probes as previously described (19). DNA-binding reaction mixtures contained 10 μl of extract (one oocyte equivalent by volume, or 20 μg of soluble protein). The relative amounts of protein in all samples were confirmed by Bradford assays, and extract volumes were adjusted so that equal protein concentrations were added to each binding reaction mixture. Binding reactions were performed with 1 μg of poly(dI-dC)–10 mM Tris (pH 7.8)–50 mM NaCl–1 mM EDTA–0.5 mM dithiothreitol–5% glycerol in a final volume of 20 μl. Reaction mixtures were incubated on ice for 20 min and immediately loaded onto 5% nondenaturing polyacrylamide gels containing 6.7 mM Tris-Cl (pH 7.5), 1 mM EDTA, and 3.3 mM sodium acetate. Gels were electrophoresed for 2.5 h at 150 V, dried, and exposed to autoradiography with X-ray film (XAR; Kodak). In vitro Ab recognition experiments were performed by adding Abs directly in DNA-binding reaction mixtures or by mixing Abs with oocyte extracts for 20 min on ice prior to DNA-binding reactions.
Immunoblotting and immunoprecipitations.
Protein extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide) and electroblotted onto polyvinylidene difluoride membranes, and the blots were blocked (for 2 h at room temperature) in TBST (20 mM Tris-Cl [pH 7.6], 137 mM NaCl, 0.1% [vol/vol] Tween 20) containing 5% milk powder. Abs were diluted in TBST with 2.5% milk (1:5,000 for anti-HSP90 PAb), and blots were incubated in primary antibody for 2 h at room temperature. Blots were washed in TBST and incubated with secondary Ab (horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G; Bio-Rad) diluted 1:10,000 in TBST and 2.5% milk for 2 h at room temperature. Blots were washed, and proteins were visualized by chemiluminescence (Renaissance system; Dupont NEN) and autoradiography with X-ray film (XAR; Kodak).
Immunoprecipitations were performed by the method described by Firestone and Winguth (17). Nuclei from nonshocked and heat-shocked (30 min, 33°C) oocytes were isolated as described above, and in each assay eight nuclei were subjected to immunoprecipitation with anti-mouse HSF1 PAb (55) or anti-YY1 transcription factor PAb (Santa Cruz Biotechnology).
CAT assays.
CAT assays were performed with one oocyte equivalent of whole-cell extract from uninjected or microinjected oocytes as previously described (49). A pool of at least 20 microinjected oocytes was used for each experimental treatment. The acetylated products were separated by thin-layer chromatography and visualized by autoradiography.
RESULTS
Detection of HSP90 in immune complexes with HSF1.
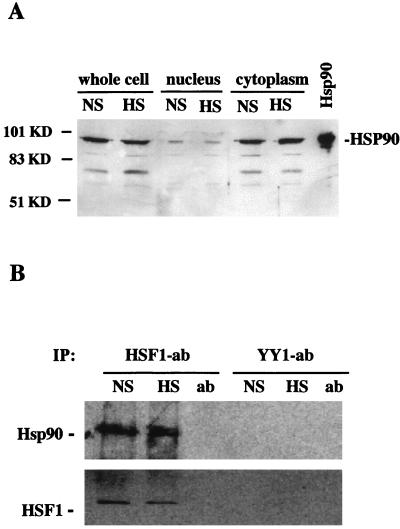
In this study we used the Xenopus oocyte model system to examine the potential role of the HSP90 chaperone in modulating the activities of HSF1. We have previously shown that endogenous HSF1 is a nuclear protein in Xenopus oocytes both before and after heat shock (36). Accordingly, we reasoned that endogenous HSP90 must be present in oocyte nuclei in order for HSP90-HSF1 interactions to occur in vivo. To test this, oocytes were manually enucleated and the subcellular distribution of HSP90 was determined by immunoblotting (Fig. 1A). In this assay, total protein from intact oocytes was compared directly to protein in a single large oocyte nucleus and in the cytoplasm of the same cell. Although the majority of HSP90 appeared to reside in the cytoplasm, HSP90 was clearly detectable in the nuclear compartments of both unshocked and heat shocked oocytes. Relatively equal quantities of HSP90 were detected in nuclei isolated from oocytes before and after heat shock, demonstrating that the nucleocytoplasmic distribution of HSP90 was not significantly affected by the heat stress conditions used in this experiment (33°C for 2 h). Heat shock did not result in a detectable increase in the total cellular levels of HSP90. This is in agreement with previous observations demonstrating that HSP synthesis is not significantly induced by heat shock in the single-cell Xenopus oocyte (27).
FIG. 1.
Identification of HSP90-HSF1 heterocomplexes in Xenopus oocyte nuclei. (A) Immunoblot showing subcellular distribution of HSP90. Oocytes were incubated at a nonshock (NS) temperature (18°C) or heat shocked (HS) at 33°C for 2 h, and proteins from single intact oocytes or from the nuclear and cytoplasmic fractions were separated by SDS-PAGE along with purified bovine HSP90 as a control. HSP90 was detected with an HSP90 PAb. The positions of molecular mass standards are shown on the left. (B) Coimmunoprecipitation of HSF1 and HSP90 from oocyte nuclei. HSF1 and YY1 were immunoprecipitated with rabbit anti-mouse HSF1 PAb (55) or YY1 PAb (Santa Cruz). Nuclear extracts were isolated from nonshocked or heat-shocked (33°C for 1 h) oocytes. Immunoprecipitated material was subjected to SDS-PAGE and transferred to nitrocellulose, and the blot was probed with HSP90 and HSF1 Abs as indicated.
In order to determine if in vivo HSP90-HSF1 interactions could be detected in vivo, HSF1 was immunoprecipitated from unshocked or heat-shocked oocyte nuclei by using HSF1 PAbs (anti-mouse HSF1 antiserum) (55), and the immunoprecipitated material was examined for the presence of HSP90 by immunoblotting (Fig. 1B). HSP90 was present in the HSF1-immunoprecipitated material from both nonshocked and heat-shocked nuclei, suggesting a physical association between HSP90 and HSF1. Identical quantities of HSP90 were detected before and after heat shock, indicating that HSP90 associates equally well with inactive HSF1 monomers or stress-activated HSF1 trimers. Control experiments show that the HSF1 PAbs used in these experiments do not cross-react with endogenous Xenopus or purified bovine HSP90 on immunoblots (data not shown). Also, as shown in Fig. 1B, HSP90 was not detected in control immunoprecipitations with PAbs against YY1 (60), which is a relatively abundant transcription factor in Xenopus oocytes (36a).
HSP90 Abs recognize heat-activated HSF1 in vivo and in vitro.
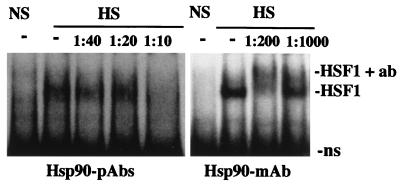
While coimmunoprecipitation of HSP90 and HSF1 suggested a physical interaction between these proteins, additional evidence was required to substantiate the existence of HSP90-HSF1 heterocomplexes. In the following series of experiments, we tested the ability of HSP90 Abs to recognize activated DNA-binding HSF1 trimers. HSP90 Abs were mixed with aliquots of unshocked or heat-shocked oocyte extracts prior to DNA-binding reactions with radiolabeled HSE probes, and the effect on the migration of HSF1-HSE complexes was analyzed in gel mobility supershift assays (Fig. 2). Heat-induced HSF1 complexes were clearly supershifted by an HSP90 MAb and were disrupted by HSP90 PAbs, although in comparison to the HSP90 MAb, a relatively large amount of antiserum was required for this effect. The results of these experiments were identical for each antibody when HSP90 MAbs or PAbs were mixed directly into DNA-binding reaction mixtures without preincubation (data not shown). We attributed the retardation or disruption of heat-induced HSE bandshifts observed in these assays to specific antibody recognition of HSP90 in direct association with HSF1 trimers. This interpretation is consistent with the coimmunoprecipitation of HSP90 and HSF1 (Fig. 1). The disruption of HSE binding by HSP90 PAbs, while differing somewhat from the clear supershift by the MAb, was similar to the results of supershift experiments with HSF1 PAbs (see Fig. 4B) (19). It is likely that recognition of multiple epitopes by PAbs present in HSP90 antiserum interfered with HSP90-dependent DNA-binding activities of HSF1.
FIG. 2.
Recognition of the heat shock-activated DNA-binding form of HSF1 in gel mobility shift assays by HSP90 Abs. Aliquots of nonshocked (NS) or heat-shocked (HS) oocyte extracts were mixed with HSP90 MAb or PAbs (antiserum) and incubated for 30 min prior to DNA-binding reactions with a 32P-labeled HSE probe, and the migration of HSF1-HSE complexes was analyzed in gel mobility shift assays. Lanes in which extracts were not mixed with antibodies are indicated (−), and the final antibody dilutions in the extract incubations are indicated above the panel. Positions of the nonspecific binding activity (ns) and heat-induced (HSF1) and supershifted (HSF1 + ab) complexes are indicated.
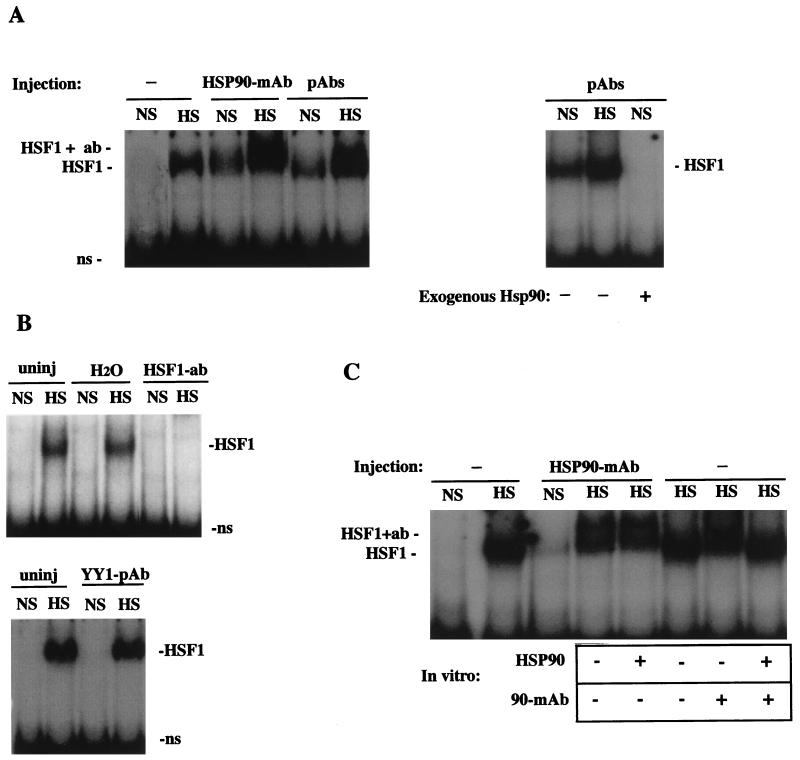
FIG. 4.
HSP90 Abs activate the HSE-binding activity of HSF1 under nonstress conditions. (A) Left, gel mobility shift assay of uninjected oocytes (−) and HSP90 Ab-injected oocytes (MAb or PAb) that were incubated at nonshock temperatures (NS) or heat shocked for 1 h (HS). The positions of heat-activated or Ab-activated HSF1-HSE complexes (HSF1) and HSP90 Ab-supershifted complexes (HSF1 + ab) are indicated. Right, gel mobility shift assay showing the effects of coinjected bovine HSP90 on the formation of HSF1-HSE complexes. In the third lane (+HSP90), 50 ng of purified bovine HSP90 was injected into oocytes 2 h after injection of HSP90 PAbs, and extracts were made following incubation at a nonshock temperature for a further 1 h. ns, nonspecific binding. (B) Bottom, gel mobility shift assay of oocytes injected with PAbs against YY1 (Santa Cruz; see Materials and Methods). Top, comparison of the activation of HSF1 in uninjected (uninj) and sham-injected (H2O) or HSF1 antiserum-injected (HSF1 PAb) (55) oocytes. (C) Recognition of HSF1 by microinjected HSP90 Abs occurred in vivo. A gel mobility shift assay was performed with uninjected (−) or HSP90 MAb-injected oocytes. In some lanes, HSP90 MAb or purified bovine HSP90 was added to extracts as indicated below the panel. HSP90 (1 μg) was added to Ab-injected oocytes at the time of homogenization (fifth lane from left), and HSP90 MAb or protein (1 μg) was added to uninjected heat-shocked oocyte extracts just prior to DNA-binding reactions (seventh and eighth lanes from left).
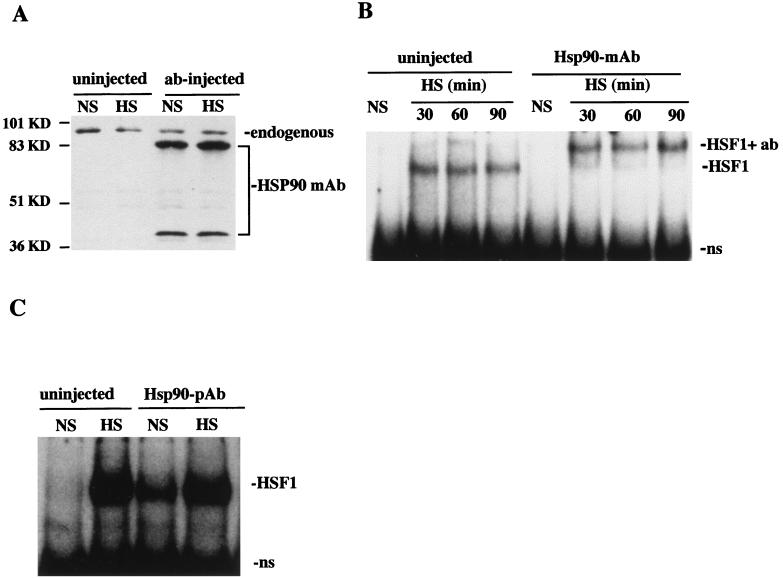
We next determined if the physical association between HSP90 and HSF1 implied by in vitro supershift experiments could be detected in vivo. A series of experiments in which HSP90 was targeted by direct introduction of HSP90 Abs into oocyte nuclei was performed. It was important, however, to first evaluate the efficacy of the Ab injection technique. Initial experiments were aimed at determining the stability of injected Abs during heat shock, as well as any quantitative effects that Ab injection might have on endogenous HSP90 levels. Following nuclear microinjection of HSP90 MAb, oocytes were allowed to recover for 1 h at 18°C and then were further incubated under nonshock or heat shock conditions (18 or 33°C for 2 h). Cell homogenates were then examined for the presence of anti-HSP90 MAbs by immunoblotting; in this experiment injected Abs were recognized by the secondary Ab used to detect endogenous HSP90 (Fig. 3A). The results show that both the quantity and apparent molecular sizes of microinjected MAbs were similar before and after heat shock and also that HSP90 levels were not affected by the presence of MAbs. Thus, it appeared that this technique could effectively be used to target or sequester HSP90 into immune complexes in vivo without affecting total HSP90 concentrations.
FIG. 3.
HSP90 interacts with HSF1 in vivo. (A) Immunoblot of nonshocked (NS) and heat-shocked (33°C, 2 h) oocytes that were either uninjected or microinjected with HSP90 MAb. HSP90 was detected with HSP90 primary Ab, and injected HSP90 Ab was detected with the secondary antibody (as described in Materials and Methods). Endogenous oocyte HSP90 and injected HSP90 MAbs present in extracts are indicated on the right. The positions of molecular mass standards are indicated on the left. (B) The migration of HSF1-HSE complexes was analyzed by gel mobility shift assay of uninjected or HSP90 MAb-injected oocytes that were not shocked (NS) or heat shocked (HS) at 33°C for the times indicated. The heat-activated HSF-HSE (HSF1) and HSP90 Ab-supershifted (HSF1+ab) complexes are indicated on the right. ns, nonspecific binding activity. (C) An experiment similar to that for panel B was performed with HSP90 antiserum (pAb).
The effect of microinjected HSP90 Abs on the DNA-binding activity of HSF1 was analyzed both before and after heat shock. HSP90 MAbs significantly retarded the mobility of heat shock-induced HSF1-HSE complexes (Fig. 3B). The relative amounts of induced HSF1 were similar in both uninjected and HSP90 MAb-injected oocytes, indicating that these antibodies did not suppress trimerization or the DNA-binding activity of HSF1. Supershift of heat shock-induced HSF1-HSE complexes was not observed after injection of HSP90 PAbs (Fig. 3C), but this was not surprising, since the maximum volume of antiserum that could be injected into oocyte nuclei was much less than the amount required to disrupt HSF1-HSE bandshifts in vitro (Fig. 2). Despite the differences between the effects of HSP90 MAb and PAbs seen in these experiments, the overall results of Ab injections are consistent with our interpretations of immunoprecipitation and in vitro gel supershift experiments and provide further evidence that HSP90 associates with HSF1 in vivo.
HSP90 associates with HSF1 in vivo.
In control experiments we also tested the effects of microinjected HSF1 Abs on HSF1 in vivo. We have previously shown that anti-mouse HSF1 PAbs (55) supershift or obliterate stress-activated HSF1 complexes in oocyte extracts in vitro (19). Similar to the results of in vitro gel supershifts, direct nuclear injection of HSF1 antiserum appeared to completely inhibit the formation of HSF1-HSE complexes in response to heat shock (Fig. 4B). We could not, however, distinguish between the possibility that HSF1 Abs actually bound to and inhibited the trimerization of inactive HSF1 monomers prior to heat shock and the possibility that HSF1 Ab-supershifted complexes were simply not detectable in these gel shift assays. The results of control experiments also showed that heat shock-induced HSF1-HSE complexes were not affected by sham nuclear injection of H2O or nuclear injection of Abs against the transcription factor YY1 (Fig. 4B), indicating that the supershift of heat-induced HSF1-HSE complexes by microinjected HSP90 Abs was attributable to specific effects on HSP90 in vivo.
HSP90 Abs induce HSE binding in the absence of heat shock.
In the mobility gel shift assay with the results shown in Fig. 3B, HSF1-HSE complexes were not observed in unshocked oocytes after nuclear injection of an HSP90 MAb; however, in repeated experiments HSF1 appeared to be induced by the same MAb in the absence of heat shock (Fig. 4A). Similar heat shock-independent activation was also observed in experiments with microinjected PAbs in HSP90 antiserum (Fig. 3C and 4A). Thus, it appeared that injection of HSP90 Abs mimicked the effects of heat shock in eliciting the DNA-binding activity of HSF1. The mobility of HSF1-HSE complexes induced by injected HSP90 Abs in unshocked oocytes did not appear to be supershifted but was similar to that of the heat-induced complexes in uninjected cells (Fig. 3C and 4A). This suggested that Ab-induced activation may have been the result of Ab interaction with HSP90 not in direct contact with HSF1.
Several control experiments were performed to determine if the activation of HSF1 under nonshock conditions by microinjected HSP90 Abs was attributable to specific effects on HSP90 in vivo. First, induction of HSE binding by HSP90 Abs could be reversed by subsequent injection of 50 ng of purified HSP90 (Fig. 4A). Second, sham injection or the nuclear presence of YY1 or HSF1 Abs did not induce HSE binding in the absence of heat shock (Fig. 4B). Thus, HSF1 activation by HSP90 Abs was not due to stress caused by the injection procedure and/or the presence of nonspecific antibodies alone but rather was a result of specific Ab interactions with HSP90 in vivo. Since injected HSP90 Abs probably inhibit or disrupt HSP90 function in vivo, these results indicate that HSP90 participates in repression of trimerization, or maintaining the monomeric state of HSF1, under nonshock conditions. We also found that gel supershifts of activated HSF1-HSE complexes by HSP90 MAb added in vitro could be quenched or inhibited by the addition of excess purified HSP90 (1 μg/oocyte) to DNA-binding reaction mixtures (Fig. 4C). Addition of the same quantity of excess HSP90 to heat-shocked oocytes immediately upon homogenization did not suppress the supershift of HSF1-HSE complexes by injected HSP90 MAb (Fig. 4C). We therefore conclude that the effects on HSF1-HSE complexes observed after microinjection of HSP90 MAbs were the result of Ab interactions occurring in vivo.
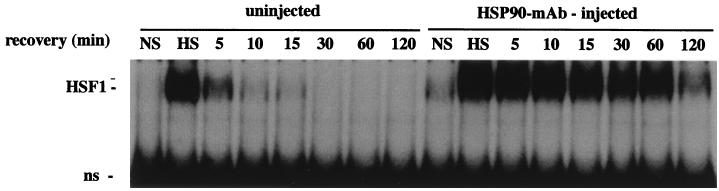
Nuclear injection of HSP90 Abs delays attenuation of HSF1-binding activity.
Taken together, the established role of HSP90 as a protein-folding chaperone targeting specific cellular substrates, the known structural changes associated with HSF1 oligomerization, and the results here of immunoprecipitation and antibody recognition experiments demonstrating association between HSP90 and HSF1 and activation of HSF1 by microinjected HSP90 Abs suggested that HSP90 could function as a modulator of HSF1 activity. To examine this further, we analyzed the effects of microinjected HSP90 Abs on the DNA-binding properties of HSF1 during the deactivation phase of the heat shock response. Since HSF1-HSE complexes persist indefinitely throughout prolonged heat shock treatments of oocytes (19), we could not monitor HSF1 deactivation during continuous stress. Instead, we compared attenuation of HSE binding in uninjected and HSP90 Ab-injected oocytes recovering at 18°C from a 33°C heat shock. As shown in Fig. 5, microinjected HSP90 MAbs caused a significant delay in the attenuation of HSE-binding activity relative to that for uninjected controls. The result of this experiment suggested that disruption of the chaperoning ability of HSP90 in vivo decreased the rate of HSF1 trimer disassembly. Thus, it appears that, in addition to suppressing trimerization, HSP90 also plays a role in the conformational changes required for the reverse step of HSF1 trimerization.
FIG. 5.
HSP90 Abs delay the deactivation of HSF1 in vivo. Uninjected or HSP90 MAb-injected oocytes were subjected to a heat shock at 33°C for 1 h (HS) and then allowed to recover at 18°C for the periods of time indicated, and the HSE-binding activity of HSF1 was analyzed by gel mobility shift assay. Both heat-induced and supershifted complexes are indicated as HSF1 at the left. ns, nonspecific binding activity.
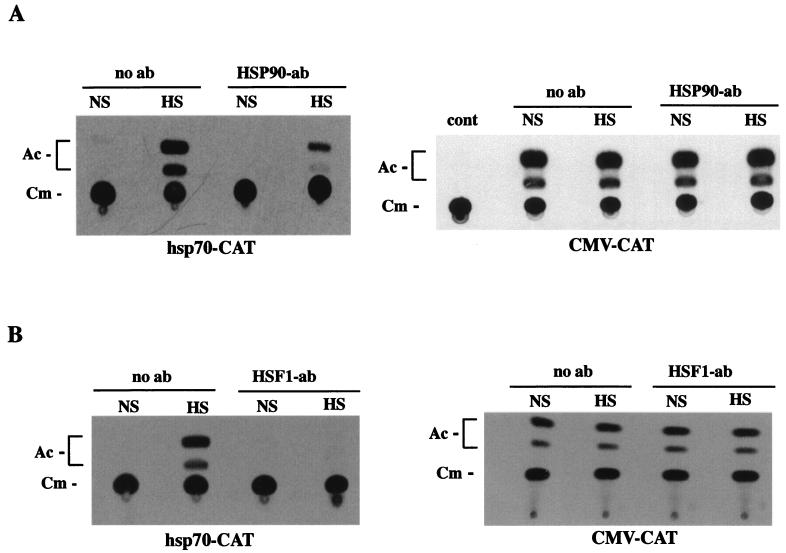
HSP90 Abs inhibit HSF1-mediated transcription from hsp70 promoter.
The transcriptional activation domain of HSF1 is regulated independently of trimerization, and it has been hypothesized that full induction requires additional conformational changes (21, 59, 76). We postulated that HSP90-HSF1 interactions might also have a functional impact on heat-inducible transcription. To investigate this possibility, we measured the effect of HSP90 Abs on heat-inducible expression from hsp70-CAT reporter constructs. Nuclear injection of HSP90 Abs resulted in a significant reduction of heat-induced CAT activity (Fig. 6A). The negative effect on HSF1-mediated CAT expression implied that binding of Abs to HSP90 in vivo could disrupt transactivation by HSF1 under heat shock conditions. In order to rule out the possibility that injected HSP90 Abs had a general inhibitory effect on transcription and/or CAT, parallel experiments were performed with CMV-CAT reporter constructs (Fig. 6A). The results show that expression from the CMV promoter was not affected by anti-HSP90. In additional control experiments, we assessed the effects of HSF1 Abs on heat shock-induced expression of hsp70-CAT. The results show that CAT activity was downregulated by HSF1 antiserum (Fig. 6B), and, as was the case for HSP90 MAbs, expression from the CMV promoter was not affected by HSF1 antiserum. Thus, the negative effects of both HSP90 and HSF1 Abs appeared to be specific to HSF1. On the basis of these expression assays, we suggest that HSP90 Ab interactions with HSF1-HSP90 heterocomplexes in vivo hindered or inhibited physical changes to HSF1 required for the acquisition of full transcriptional competence in response to heat shock. We have not ruled out the possibility that the inhibitory effect of injected Abs was exerted through interaction with uncomplexed HSP90 molecules. It is also possible that HSP90 must dissociate from HSF1 trimers before transcriptional activation, and therefore Abs may have sterically inhibited conformational changes to the HSF1 activation domain.
FIG. 6.
Microinjected HSP90 Abs inhibit the heat-induced transcriptional activity of HSF1. (A) CAT assay of oocytes microinjected with hsp70-CAT (left) or CMV-CAT (right). Oocytes were injected with HSP90 MAb 1 h before heat shock (33°C for 1 h) treatment (HS), and the expression from each promoter was compared directly to that in similarly treated oocytes without injected Ab. Each experiment was repeated at least five times with different batches of oocytes, yielding similar results. The positions of unacetylated (Cm) and acetylated (Ac) forms of [14C,]chloramphenicol are indicated on the left of each panel. Background CAT activity of non-plasmid-injected oocytes is shown in the right panel (cont). NS, no heat shock. (B) CAT assay of oocytes microinjected with reporters as described above. HSF1 PAbs were injected into oocyte nuclei essentially as described above for HSP90 Ab.
Effect of GA on the DNA-binding and transcriptional activities of HSF1.
In order to rule out potential artifacts of microinjected Abs, we examined the role of HSP90 in regulating HSF1 activities by an alternative method involving treatment of cells with GA. GA is a fungal benzoquinoid ansamycin known to block HSP90 function (65, 71). GA binds specifically to HSP90 at the ATP-binding domain (22, 51, 67) and prevents HSP90-mediated renaturation of proteins (24, 56, 68). GA has been shown to inhibit the HSP90-dependent activities of numerous proteins, including the glucocorticoid receptor and pp60v-src (71), the Src family kinase p56lck (23), Raf kinase (57), and progesterone receptor (65).
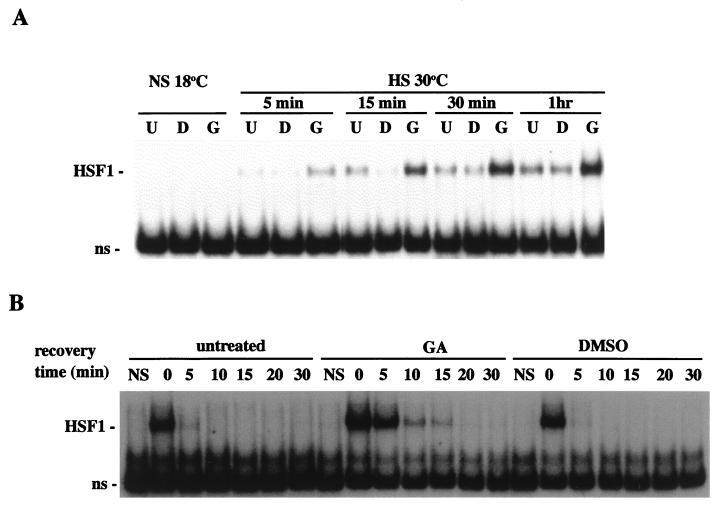
Oocytes were incubated in medium supplemented with GA (10 μg/ml) for 2 h, and the DNA-binding activity of HSF1 was analyzed by gel shift assay with labeled HSE. As shown in Fig. 7A, GA treatment alone did not activate HSF1. We did not observe HSF1 induction by GA at nonshock temperatures even after extended exposure of up to several hours or after acute exposure to GA concentrations as high as 100 μg/ml (data not shown). The failure of GA to induce HSE binding in the absence of heat shock indicated that GA inhibition of HSP90 in vivo was not sufficient to bring about HSF1 trimerization. In heat shock experiments, however, GA treatment (10 μg/ml) for 2 h prior to a heat shock of 30°C for 30 min caused a significant increase in the induced levels of HSF1-HSE complexes relative to those for identically stressed controls (Fig. 7A). Elevated levels of HSE-binding activity were observed at several different points during the induction phase, from between 5 min and 1 h of heat shock.
FIG. 7.
(GA) enhances the heat-inducible HSE-binding activity of HSF1 and delays deactivation during recovery. (A) Oocytes were incubated for 2 h in the presence of 10 μg of GA (G) per ml or in DMSO (D) or left untreated (U) prior to heat shock at 30°C for the times indicated. The HSE-binding activities of HSF1 were then compared by gel mobility shift assay. (B) Gel mobility shift assay of oocytes that were treated with GA or DMSO as described above, heat shocked at 33°C for 1 h and then allowed to recover at 18°C for the periods of time indicated. The positions of activated HSF1 and nonspecific (ns) bands are indicated at the left of each panel.
Enhanced activation of HSF1 in GA-treated oocytes could have been attributed to a synergistic effect of heat shock in combination with GA or to a shift in the oligomerization equilibrium towards formation of HSF1 trimers caused by GA inhibition of HSP90-mediated dissociation. In an attempt to distinguish between these possibilities, we examined the effect of GA on the DNA-binding properties of HSF1 during the deactivation phase of the heat shock response. The results of recovery experiments consistently showed that deactivation of HSF1 was delayed in GA-treated oocytes relative to that in untreated controls (Fig. 7B). It is important to note that even after severe heat treatments, HSE binding of endogenous HSF1 in oocytes returns to control levels within 5 to 15 min of recovery (19). Thus, GA disruption of the chaperoning activity of HSP90 in vivo appears to decrease the rate of HSF1 trimer disassembly. The results of recovery experiments with GA were similar to those of HSP90 Ab injections, and together these data imply that HSP90 functions in controlling the conformational changes associated with conversion of HSF1 from trimers to monomers. From these experiments, however, we cannot be certain whether GA and HSP90 Abs exerted their effects directly by inhibiting the function of HSP90 molecules in complex with HSF1 or indirectly by inhibiting the function of HSP90 molecules not stably complexed with HSF1.
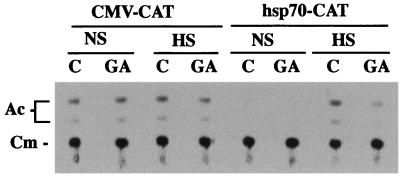
We next used GA to examine the functional significance of HSP90-HSF1 interactions for heat-inducible transcription. Expression from microinjected hsp70-CAT reporter constructs in untreated and GA-treated oocytes under nonshock and heat shock conditions was compared (Fig. 8). The results show that heat-induced CAT activity was significantly reduced in GA-treated oocytes relative to that in untreated controls. Thus, inhibition of HSF1-mediated CAT expression by GA mimicked the results with microinjected HSP90 Abs. Expression was unaffected by GA in parallel experiments with microinjected CMV-CAT reporter constructs (Fig. 8), thus ruling out the possibility that GA had a general inhibitory effect on transcription and/or CAT activity in oocytes. The results of expression experiments using both microinjected Abs and GA strongly suggest that HSP90 functions in regulating conformational changes to the transcriptional activation domain of HSF1.
FIG. 8.
GA inhibits the heat-induced transcriptional activity of HSF1. CAT activities of CMV-CAT- or hsp70-CAT-injected oocytes that were either untreated or treated with GA (10 μg/ml, 2 h) prior to incubation at 18°C (NS) or heat shock (HS) (33°C for 2 h) were compared. Each experiment was repeated at least five times with different batches of oocytes, yielding similar results. The positions of unacetylated (Cm) and acetylated (Ac) forms of [14C]chloramphenicol are indicated on the left.
DISCUSSION
HSF1 is one of the most intensely studied eukaryotic transcription factors. Numerous reports have described the functional domains and intrinsic properties of HSF1 molecules from different organisms, the changes in oligomerization and phosphorylation states, interactions with HSP70, and HSF1 activation by disparate stress signals. Despite knowledge of these events, however, the precise mechanisms by which cells regulate HSF1 are yet to be defined. This study is an important step towards understanding HSF1 regulation. It had previously been postulated, on the basis of HSP90-HSF1 heterocomplex formation in vitro, that HSP90 could modulate HSF1 activity (42, 43), an idea that has not been confirmed before this work. The results of this analysis, using novel experimental approaches made possible by the Xenopus oocyte model system, clearly establish a physical association between HSP90 and endogenous HSF1 in vivo and provide evidence that HSP90 functions to modulate oligomerization and possibly the inducible transcriptional activity of HSF1.
The existence of HSP90-HSF1 heterocomplexes is corroborated by several independent lines of evidence, including the detection of HSP90 in HSF1 Ab-immunoprecipitated material from both heat-shocked and control cells, HSP90 Ab-directed induction of HSE-binding activity, HSP90 Ab-directed supershifts of heat-activated HSF1 both in vitro and in vivo, and the delayed deactivation of HSF1-binding activity after disruption of HSP90 with GA or microinjected Abs. Results showing HSP90 Ab induction of HSF1 in the absence of heat shock and delayed deactivation of HSF1 after targeting of HSP90 in vivo suggest that HSP90 contributes to the conformational changes required for suppression of trimerization as well as disassembly of HSF1 trimers. Furthermore, independent experiments with microinjected HSP90 Abs and cell treatments with GA show specific effects on heat-induced transcriptional activity of HSF1. Although we have not directly confirmed the universality of these findings, we assume that HSP90-HSF1 interactions and the apparent regulatory role of HSP90 are not unique to Xenopus oocytes; in fact, the likelihood that HSP90-HSF complexes must exist in other cells is strengthened by the evolutionary conservation of these molecules and previous reports of HSP90-HSF interactions in human cell extracts or reticulocyte lysates (42, 43). Also of note is the recent observation by Farkas et al. (16) of a 94-kDa protein species copurifying with bacterially expressed HSF1; our results lead us to suggest that this could have been HSP90.
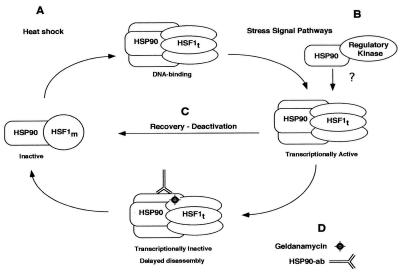
Based on the evidence presented here and the previously reported properties of both HSF1 and HSP90 molecules, we propose a regulatory model in which HSP90 functions as an HSF1-specific chaperone at different points along the activation-deactivation pathway: maintenance of the monomeric conformation under nonstress conditions, induction of transactivation potential, and disassembly of trimers to a non-DNA-binding state following stress (Fig. 9). The model predicts that HSP90 is associated with HSF1 at different points along the activation-deactivation pathway and exerts its regulatory effects directly through interactions with HSF1. The model also predicts that, in addition to coordinating the dynamic changes in HSF1 oligomerization, HSP90 could function in the unmasking of the transcriptional activation domain. Previous studies have shown that the carboxy-terminal transcriptional activator domain is regulated independently of oligomerization (21, 31, 46, 59, 76). The effects on HSF1-dependent transcription of GA and microinjected HSP90 Abs suggest that this step could be regulated at least in part by complexed HSP90. It must be emphasized that the current model does not exclude the involvement of HSP70 in controlling HSF1 activities. Rather, it is more likely that HSP70 and HSP90 act coordinately in this regard. The idea of cooperative and/or coordinate regulation of HSF1 by both HSPs is congruent with the previous identification of HSP70 and HSP90 in large chaperone complexes (63, 64), with the ability of HSF1 to assemble HSP90 along with HSP70 and several components of the cytoplasmic molecular chaperone machinery, and with the recent demonstration that HSP70 binds to the activation domain and negatively regulates transcription (61).
FIG. 9.
Model of HSF1 regulation by HSP90. Our data indicate that HSP90 interacts with the inactive monomeric and active trimeric forms of HSF1 and could be involved in regulating the interconversion between these forms (A and C). Both HSP90 Abs and GA delay trimer disassembly and inhibit heat-induced transcription of HSF1 (D). These observations suggest a role for HSP90 in modulation of the transcriptional activation domain (B) or that HSP90 dissociation from trimers is required for transcriptional competence. We also hypothesize that HSP90 could link HSF1 to cellular signaling molecules (B). Details of HSF1 structure are not shown, although the shape change from a circle to an ellipse is intended to indicate conformational changes associated with trimerization. No data are presented for the one-to-one stoichiometric relationship between HSP90 and HSF1, which is shown only for simplicity. Although HSP70 is not represented here, we do not imply its absence of involvement in any of these processes.
Apart from providing a mechanistic framework in which the HSF1-regulatory function of HSP90 could be explained, the model shown in Fig. 9 describes a hypothetical mechanism by which HSF1 could be linked to disparate signaling pathways. We put forth the hypothesis that HSP90 functions as an integral part of the cellular stress detection machinery, tethering HSF1 to cell signaling molecules. We suggest that this could occur through transient or reiterative interactions between HSP90-HSF1 heterocomplexes and other HSP90-associated cellular kinases or other regulatory molecules. Alternatively, HSF1 could assemble with HSP90 and other molecules into large heteromeric complexes; however, to our knowledge these have not been detected in vivo through biochemical approaches. Our proposal of interactions between HSP90 molecules simultaneously in complex with HSF1 and cellular signaling molecules provides a plausible mechanism by which signaling molecules that participate in regulating the cellular stress activation-deactivation pathway could be specifically presented to HSF1. The formation of HSP90 homodimers (38) is consistent with this idea.
Specific recognition of HSF1-HSE complexes by using HSP90 Abs was demonstrated both in vivo (Abs injected into oocyte nuclei) and in vitro (Abs added to DNA-binding reactions mixtures). Also, both active and inactive forms of HSF1 coimmunoprecipitated with HSP90 from heat-shocked and unshocked nuclear extracts. These results are consistent with the previous findings showing an interaction between immobilized HSP90 and HSF in nuclear extracts of unshocked HeLa cells (42) and between HSP90 and bacterially expressed HSF1 in a cell-free reticulocyte lysate assembly system (43), but they are in contrast to results of other studies in which HSF1-HSP90 interactions were not detected by immunoprecipitation (53) or supershift analyses (5); it is possible that such discrepancies could be accounted for by the use of different Abs. The simplest interpretation of our results is that a subset of nuclear HSP90 molecules exist in heterocomplexes with inactive HSF1 monomers and remain associated with trimers. Coimmunoprecipitation of equal quantities of HSP90 under both nonshock and heat shock conditions implies that HSP90-HSF1 heterocomplex formation is independent of the oligomeric state of HSF1. What remains to be determined is the relative affinity between HSP90 and HSF1 monomers and trimers and the stoichiometric relationship between these molecules. Also of interest is whether HSP90 remains associated with HSF1 throughout activation and deactivation or whether HSP90 interacts reiteratively and transiently with HSF1 at each step in the pathway.
Induction of the HSE-binding activities of HSF1 under nonshock conditions after nuclear injection of HSP90 Abs, although not after GA treatment, leads us to hypothesize that HSP90 participates as a molecular chaperone in the folding of HSF1 monomers, either to establish the formation of intramolecular hydrophobic interactions of inactive HSF1 monomers, or to maintain the monomeric conformation. Interestingly, the HSP90 Ab-induced HSE-binding activity in unshocked oocytes did not appear to be supershifted, suggesting that this heat shock-independent activation could have been the result of Ab interaction with uncomplexed HSP90. Why did HSP90 Abs but not GA induce HSF1 in unstressed cells? Presumably, the binding of Abs to certain epitopes was sufficient to inhibit the apparent HSP90-mediated repression of HSF1, either directly through interactions with molecules in HSF1-HSP90 heterocomplexes or indirectly through interactions with HSP90 not complexed with HSF1. The fact that GA treatments failed to activate HSF1 does not necessarily contradict a potential role for HSP90 in the folding or repression of HSF1 monomers. GA binds the ATP-binding domain of HSP90 and is hypothesized to inhibit substrate release or lead to substrate degradation (22, 24, 51, 56, 67, 68), effects that would not inexorably lead to trimerization.
Although the functional implications of an interaction between HSP90 and inactive monomers is still unclear, recovery experiments indicated a clear relationship between HSP90 and the disassembly of HSF1 trimers. Both GA treatments and nuclear injection of HSP90 Abs significantly delayed deactivation of HSF1 during recovery from heat shock. A number of potential mechanisms by which HSP90 might modulate reversion of HSF1 oligomerization could be considered. One is that HSP90 acts to stabilize the trimeric conformation of HSF1 and must be released prior to conversion to the monomeric form and deactivation of HSE binding. Alternatively, HSP90 could remain complexed with HSF1 throughout the disassembly process and act to mediate the conformational and folding changes associated with conversion of homotrimers to monomers. In either case, the delay in HSF1 deactivation by GA is probably due to inhibition of the ATPase activity of HSP90, but the mechanism by which HSP90 Abs inhibited HSP90 function in vivo is not known. It was interesting that HSP90 Ab-induced HSF1-HSE complexes detected in gel mobility shift assays were not supershifted. Consequently, we have not ruled out the possibility that GA or HSP90 Abs could have exerted their effects indirectly by affecting the chaperone activity of HSP90 molecules not in complex with HSF1.
Another key finding of this study is the repression of heat-inducible transcription after targeting HSP90 in vivo. It is very unlikely that this transcriptional repression of HSF1 was artifactual, since it was reproduced by two independent methods, microinjection of HSP90 Abs and cell treatments with GA. The results imply that physical binding of HSP90 by injected Abs or disruption of its ATPase domain by GA somehow blocked the conformational changes to the transactivation domain of HSF1. Therefore, we suggest, but have yet not confirmed, that the chaperoning function of HSP90 may be directly involved in this step of the activation pathway. This function would be entirely consistent with the known chaperoning role of HSP90 with other cellular proteins and transcription factors such as MyoD (58). Similar to the case for the delay of deactivation by HSP90 antibodies or GA, we have not yet determined whether these agents inhibited transcription directly through perturbing the function of HSP90 molecules complexed with HSF1 or indirectly through interactions with the uncomplexed pool of HSP90. Furthermore, additional factors such as HSP70 (61) or various signal transduction molecules could be involved in regulating the transcriptional activation domain.
There were two apparent contradictions in the results of expression experiments: (i) under heat shock conditions, GA treatment enhanced the HSE-binding activity of HSF1 while reducing transcriptional activity, and (ii) under nonshock conditions, HSP90 Ab injection induced HSE binding but not a corresponding increase in HSF1-mediated transcription from the HSP70 promoter. It could be argued that these results reflect the independent regulation of (i) trimerization and DNA binding and (ii) transactivation (21, 59, 76). In fact, the DNA-binding and transcriptional activities of HSF1 are also uncoupled in Xenopus oocytes (6).
Rather than a direct role for HSP90 in regulating transcriptional competence of HSF1, the observations of repression by GA and HSP90 Abs could indicate that dissociation of HSP90 from trimers is a necessary precondition for transcriptional activation. In this case, reduced transcriptional activity caused by HSP90 Abs or GA could have been the result of inhibition of HSP90 release and steric blocking of modifications to the transcriptional activation domain. It is possible that in our experiments with oocytes, these agents acted to stabilize a transcriptionally incompetent intermediate in the activation pathway. Regardless of the interpretations of these experiments, however, it is clear that HSP90 is involved in the transcriptional activation of HSF1. The precise details of this role will probably require more knowledge of the stoichiometric relationships between these two proteins throughout the activation-deactivation pathway.
In conclusion, the data presented here provide the first clear evidence in support of a role for HSP90 in the regulation of HSF1. This study raises a number of key questions. For example, what is the molecular basis for HSP90-HSF1 interactions, and what are the dynamics of HSP90-HSF1 interactions throughout the stress response? Do ATP hydrolysis and HSF1 hyperphosphorylation affect the physical association between these molecules, or does HSP90 actually coordinate HSF1 phosphorylation through interactions with other factors? Do HSP70 and HSP90 cooperate in modulating HSF1 activities, and are other molecules involved? It will be interesting to unravel the precise roles of HSP90 in coordinating the physical changes associated with induction of HSF1 by stress and to determine if HSP90 plays a role in the signaling pathways used to sense stress and coordinate the appropriate changes in cellular activities.
ACKNOWLEDGMENTS
This work was supported by a research grant from the Medical Research Council of Canada to N.O., a postdoctoral fellowship from the Health Services Utilization Research Council of Saskatchewan to A.A., and a PGSA scholarship from the Natural Sciences and Engineering Research Council of Canada to S.B.
We thank S. Hartson and R. Matts for purified HSP90 protein and antiserum; A. Wolffe for hsp70-CAT and CMV-CAT constructs; K. Sarge and R. Morimoto for HSF1 antiserum; A. Hnatov, J. Davies, and J. Xavier for technical assistance; and P. Mercier for critical reading of the manuscript.
A. Ali and S. Bharadwaj contributed equally to this paper.
REFERENCES
- 1.Abravaya K A, Myers M, Murphy S P, Morimoto R I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992;6:1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 2.Ananthan J, Golberg A L, Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–525. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 3.Baler R, Welch W J, Voellmy R. Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J Cell Biol. 1992;117:1151–1159. doi: 10.1083/jcb.117.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baler R, Dahl G, Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baler R, Zou J, Voellmy R. Evidence for a role of hsp70 in the regulation of the heat shock response of mammalian cells. Cell Stress Chaperones. 1996;1:33–39. doi: 10.1379/1466-1268(1996)001<0033:efaroh>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharadwaj S, Hnatov A, Ali A, Ovsenek N. Regulation of the DNA-binding and transcriptional activities of heat shock factor 1 is uncoupled in Xenopus oocytes. Biochim Biophys Acta. 1998;1402:79–85. doi: 10.1016/s0167-4889(97)00146-8. [DOI] [PubMed] [Google Scholar]
- 7.Bohen S P, Yamamoto K R. Modulation of steroid-receptor signal transduction by heat shock proteins. In: Mormoto R I, Tissiers A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 313–334. [Google Scholar]
- 8.Bonner J J, Heyward S, Fackenthal D L. Temperature-dependent regulation of a heterologous transcriptional activation domain fused to yeast heat shock transcription factor. Mol Cell Biol. 1992;12:1021–1030. doi: 10.1128/mcb.12.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boorstein W R, Craig E A. Transcriptional regulation of SSA3, an hsp70 gene from Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:3262–3267. doi: 10.1128/mcb.10.6.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugge J S, Erikson E, Erikson R L. The specific interaction of the Rous sarcoma virus transforming protein pp60src, with two cellular proteins. Cell. 1981;25:363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- 11.Clos J, Westwood J T, Becker P B, Wilson S, Lambert K, Wu C. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell. 1990;63:1085–1097. doi: 10.1016/0092-8674(90)90511-c. [DOI] [PubMed] [Google Scholar]
- 12.Cotto J J, Kline M, Morimoto R I. Activation of heat shock factor 1 DNA binding preceds stress-induced serine phosphorylation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- 13.DiDomenico B J, Bugaisky G E, Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982;31:593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- 14.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty J J, Rabideau D A, Iannoti A M, Sullivan W P, Toft D O. Identification of the 90 kDa substrate of rat liver type II casein kinase with the heat shock protein which binds steroid receptors. Biochim Biophys Acta. 1987;927:74–80. doi: 10.1016/0167-4889(87)90067-x. [DOI] [PubMed] [Google Scholar]
- 16.Farkas T, Kutskova Y A, Zimarino V. Intramolecular repression of mouse heat shock factor 1. Mol Cell Biol. 1998;18:906–918. doi: 10.1128/mcb.18.2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firestone G L, Winguth S D. Immunoprecipitation of proteins. Methods Enzymol. 1990;182:688–700. doi: 10.1016/0076-6879(90)82054-6. [DOI] [PubMed] [Google Scholar]
- 18.Georgopoulos C. The emergence of chaperone machines. Trends Biochem Sci. 1992;17:295–299. doi: 10.1016/0968-0004(92)90439-g. [DOI] [PubMed] [Google Scholar]
- 19.Gordon S, Bharadwaj S, Hnatov A, Ali A, Ovsenek N. Distinct stress-inducible and developmentally regulated heat shock transcription factors in Xenopus oocytes. Dev Biol. 1997;181:47–63. doi: 10.1006/dbio.1996.8441. [DOI] [PubMed] [Google Scholar]
- 20.Gradin K, McGuire J, Wenger R H, Kvietikova I, Whitelaw M L, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green M, Schuetz T J, Sullivan E K, Kingston R E. A heat shock-responsive domain of human HSF1 that regulates transcription activation domain function. Mol Cell Biol. 1995;15:3354–3362. doi: 10.1128/mcb.15.6.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grenert J P, Sullivan W P, Fadden P, Haystead T A J, Clark J, Mimnaugh E, Krutzsch H, Ochel H J, Schulte T W, Sausville E, Neckers L M, Toft D O. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 23.Hartson S D, Ottinger E A, Huang W, Barany G, Burn P, Matts R L. Modular folding and evidence for phosphorylation-induced stabilization of an Hsp90-dependent kinase. J Biol Chem. 1998;273:8475–8482. doi: 10.1074/jbc.273.14.8475. [DOI] [PubMed] [Google Scholar]
- 24.Hartson S D, Barrett D J, Burn P, Matts R L. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry. 1996;35:13451–13459. doi: 10.1021/bi961332c. [DOI] [PubMed] [Google Scholar]
- 25.Hendrick J P, Hartl F U. Molecular chaperone functions of heat shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 26.Holmberg C I, Leppa S, Eriksson J E, Sistonen L. The phorbol ester 12-O-tetradecanoylphorbol 13-acetate enhances the heat-induced stress response. J Biol Chem. 1997;272:6792–6798. doi: 10.1074/jbc.272.10.6792. [DOI] [PubMed] [Google Scholar]
- 27.Horrell A, Shuttleworth J, Coleman A. Transcript levels and translational control of hsp70 synthesis in Xenopus oocytes. Genes Dev. 1987;1:433–444. doi: 10.1101/gad.1.5.433. [DOI] [PubMed] [Google Scholar]
- 28.Jakob U, Buchner J. Assisting spontaneity: the role of hsp90 and small hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Nueda A, Meng Y H, Dynan W S, Michevi N F. Analysis of phosphorylation of human heat shock transcription factor-1 by MAPK family members. J Cell Biochem. 1997;67:43–54. doi: 10.1002/(sici)1097-4644(19971001)67:1<43::aid-jcb5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Kline M P, Morimoto R I. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol. 1997;17:2107–2115. doi: 10.1128/mcb.17.4.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knauf U, Newton E M, Kryiakis J, Kingston R E. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10:2782–2793. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- 32.Landsberger N, Ranjan M, Almouzni G, Stump D, Wolffe A P. The heat shock response in Xenopus oocytes, embryos, and somatic cells: a regulatory role for chromatin. Dev Biol. 1995;170:62–74. doi: 10.1006/dbio.1995.1195. [DOI] [PubMed] [Google Scholar]
- 33.Lis J, Wu C. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell. 1993;74:1–4. doi: 10.1016/0092-8674(93)90286-y. [DOI] [PubMed] [Google Scholar]
- 34.Mager W H, DeKruijff A J J. Stress-induced transcriptional activation. Microbiol Rev. 1995;59:506–531. doi: 10.1128/mr.59.3.506-531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melnick J, Aviel S, Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J Biol Chem. 1992;267:21303–21306. [PubMed] [Google Scholar]
- 36.Mercier P A, Foksa J, Ovsenek N, Westwood J T. Xenopus heat shock factor 1 is a nuclear protein before heat stress. J Biol Chem. 1997;272:14147–14151. doi: 10.1074/jbc.272.22.14147. [DOI] [PubMed] [Google Scholar]
- 36a.Meyer, D., and N. Ovsenek. Unpublished results.
- 37.Mifflin L C, Cohen R E. hsc70 moderates the heat shock (stress) response in Xenopus laevis oocytes and binds to denatured protein inducers. J Biol Chem. 1994;269:15718–15723. [PubMed] [Google Scholar]
- 38.Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I. Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J Biol Chem. 1991;266:10099–10103. [PubMed] [Google Scholar]
- 39.Morimoto R I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 40.Morimoto R I, Tissiers A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 41.Mosser D D, Duchaine J, Massie B. The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol Cell Biol. 1993;13:5427–5438. doi: 10.1128/mcb.13.9.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadeau K, Das A, Walsh C T. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J Biol Chem. 1993;268:1479–1487. [PubMed] [Google Scholar]
- 43.Nair S C, Toran E J, Rimerman R A, Hjermstad S, Smithgall T E, Smith D F. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor HSF1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakai A, Morimoto R I. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol Cell Biol. 1993;13:1983–1997. doi: 10.1128/mcb.13.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemoto T, Ohara-Nemoto Y, Ota M, Takagi T, Yokoyama K. Mechanism of dimer formation of the 90-kDa heat-shock protein. Eur J Biochem. 1995;233:1–8. doi: 10.1111/j.1432-1033.1995.001_1.x. [DOI] [PubMed] [Google Scholar]
- 46.Newton E M, Knauf U, Green M, Kinston R E. The regulatory domain of human heat shock factor 1 is sufficient to sense heat stress. Mol Cell Biol. 1996;16:839–846. doi: 10.1128/mcb.16.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunes S L, Calderwood S K. Heat shock factor 1 and the heat shock cognate 70 protein associate in high molecular weight complexes in the cytoplasm of NIH-3T3 cells. Biochem Biophys Res Commun. 1995;213:1–6. doi: 10.1006/bbrc.1995.2090. [DOI] [PubMed] [Google Scholar]
- 48.Orosz A, Wisniewski J, Wu C. Regulation of Drosophila heat shock factor trimerization: global sequence requirements and independence of nuclear localization. Mol Cell Biol. 1996;16:7018–7030. doi: 10.1128/mcb.16.12.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ovsenek N, Heikkila J J. DNA sequence specific binding activity of the Xenopus heat shock transcription factor is heat inducible before the midblastula transition. Development. 1990;110:427–433. doi: 10.1242/dev.110.2.427. [DOI] [PubMed] [Google Scholar]
- 50.Pratt W B, Welsh M J. Chaperone functions of the heat shock proteins associated with steroid receptors. Semin Cell Biol. 1994;5:83–93. doi: 10.1006/scel.1994.1012. [DOI] [PubMed] [Google Scholar]
- 51.Prodromou C, Roe S M, O’Brien R, Ladbury J E, Piper P W, Pearl L H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 52.Rabindran S K, Giorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci USA. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabindran S K, Haroun R I, Clos J, Wisniewski J, Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993;259:230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- 54.Rabindran S K, Wisniewski J, Li L, Li G C, Wu C. Interaction between heat shock factor and hsp70 is insufficient to supress induction of DNA-binding activity in vivo. Mol Cell Biol. 1994;14:6552–6520. doi: 10.1128/mcb.14.10.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarge K D, Murphy S P, Morimoto R I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider C, Sepp-Lorenzio L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen R, Hartl F U. Pharmacologic shifting of a balance between protein refolding and degradation mediated by hsp90. Proc Natl Acad Sci USA. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulte T W, Blagosklonny M V, Ingui C, Neckers L. Disruption of the Raf-1-hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 58.Shaknovich R, Shue G, Kohtz D S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84) Mol Cell Biol. 1992;12:5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, Kroeger P E, Morimoto R I. The carboxyl-terminal domain of heat shock factor 1 is negatively regulated and stress responsive. Mol Cell Biol. 1995;15:4309–4318. doi: 10.1128/mcb.15.8.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Y, Lee J S, Gavin K M. Everything you have ever wanted to know about Yin Yang 1. Biochim Biophys Acta. 1997;1332:49–66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 61.Shi Y, Mosser D D, Morimoto R I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shue G, Kohtz D S. Structural and functional aspects of basic helix-loop-helix protein folding by heat-shock protein 90. J Biol Chem. 1994;269:2707–2711. [PubMed] [Google Scholar]
- 63.Smith D F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- 64.Smith D F, Albers M W, Schreiber S L, Leach K L, Deibel M R. FKBP54, a novel FK506-binding protein in avian progesterone receptor complexes and HeLa extracts. J Biol Chem. 1993;268:24270–24273. [PubMed] [Google Scholar]
- 65.Smith D F, Whitesell L, Nair S, Chen S, Prapapanich V, Rimerman R A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorger P K. Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell. 1990;62:793–805. doi: 10.1016/0092-8674(90)90123-v. [DOI] [PubMed] [Google Scholar]
- 67.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl F U, Pavletich N P. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;18:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 68.Thalasiraman V, Matts R L. Effect of geldanamycin on the kinetics of chaperone-mediated renaturation of firefly luciferase in rabbit reticulocyte lysate. Biochemistry. 1996;35:13443–13450. doi: 10.1021/bi9615396. [DOI] [PubMed] [Google Scholar]
- 69.Uma S, Hartson S D, Chen J J, Matts R L. Hsp90 is obligatory for the heme-regulated eIF-2a kinase to acquire and maintain an activatable conformation. J Biol Chem. 1997;272:11648–11656. doi: 10.1074/jbc.272.17.11648. [DOI] [PubMed] [Google Scholar]
- 70.Westwood J T, Wu C. Activation of Drosophila heat shock factor: conformational change associated with a monomer-to-trimer transition. Mol Cell Biol. 1993;13:3481–3486. doi: 10.1128/mcb.13.6.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitesell L, Mimnaugh E G, DeCosta B, Myers C, Neckers L M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by bezoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winegarden N A, Wong K S, Sopta M, Westwood J T. Sodium salicylate decreases intracellular ATP, induces both heat shock factor binding and chromosomal puffing, but does not induce hsp70 gene transcription in Drosophila. J Biol Chem. 1996;271:26971–26980. doi: 10.1074/jbc.271.43.26971. [DOI] [PubMed] [Google Scholar]
- 73.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 74.Xia W, Voellmy R. Hyperphosphorylation of heat shock transcription factor 1 is correlated with transcriptional competence and slow dissociation of active factor trimers. J Biol Chem. 1997;272:4094–4102. doi: 10.1074/jbc.272.7.4094. [DOI] [PubMed] [Google Scholar]
- 75.Zou J, Baler R, Dahl G, Voellmy R. Activation of the DNA-binding ability of human heat shock factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled-coil structure. Mol Cell Biol. 1994;14:7557–7568. doi: 10.1128/mcb.14.11.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou J, Rungger D, Voellmy R. Multiple levels of regulation of human heat shock transcription factor 1. Mol Cell Biol. 1995;15:4319–4330. doi: 10.1128/mcb.15.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]