Key Points
Question
Is preemptive foot and ankle care provided by podiatrists associated with a reduced risk of limb loss and/or mortality in patients with kidney failure receiving dialysis who are at risk of developing diabetic foot ulcers?
Findings
In this cohort study including 14 935 patients aged 40 years or older with kidney failure, foot and ankle care by podiatrists prior to the occurrence of a diabetic foot ulcer was associated with lower rates of amputation and/or death. The rate of major amputation (above-knee, below-knee) was also decreased.
Meaning
The findings of this study suggest that, for individuals with kidney failure receiving dialysis and susceptible to diabetes-related limb loss, receiving foot and ankle care by podiatrists before the onset of diabetic foot ulcers may be associated with improved outcomes.
Abstract
Importance
Patients with kidney failure have an increased risk of diabetes-related foot complications. The benefit of regular foot and ankle care in this at-risk population is unknown.
Objective
To investigate foot and ankle care by podiatrists and the outcomes of diabetic foot ulcers (DFUs) in patients with kidney failure.
Design, Setting, and Participants
This retrospective cohort study included Medicare beneficiaries with type 2 diabetes receiving dialysis who had a new DFU diagnosis. The analysis of the calendar year 2016 to 2019 data from the United States Renal Data System was performed on June 15, 2023, with subsequent updates on December 11, 2023.
Exposures
Foot and ankle care by podiatrists during 3 months prior to DFU diagnosis.
Main Outcomes and Measures
The outcomes were a composite of death and/or major amputation, as well as major amputation alone. Kaplan-Meier analysis was used to estimate 2 to 3 years of amputation-free survival. Foot and ankle care by podiatrists and the composite outcome was examined using inverse probability-weighted Cox regression, while competing risk regression models were used for the analysis of amputation alone.
Results
Among the 14 935 adult patients with kidney failure and a new DFU (mean [SD] age, 59.3 [12.7] years; 35.4% aged ≥65 years; 8284 men [55.4%]; Asian, 2.7%; Black/African American, 35.0%; Hispanic, 17.7%; White, 58.5%), 18.4% (n = 2736) received care by podiatrists in the 3 months before index DFU diagnosis. These patients were older, more likely to be male, and have more comorbidities than those without prior podiatrist visits. Over a mean (SD) 13.5 (12.0)-month follow-up, 70% of those with podiatric care experienced death and/or major amputation, compared with 74% in the nonpodiatric group. Survival probabilities at 36 months were 26.3% vs 22.8% (P < .001, unadjusted Kaplan-Meier survival analysis). In multivariate regression analysis, foot and ankle care was associated with an 11% lower likelihood of death and/or amputation (hazard ratio [HR], 0.89 95% CI, 0.84-0.93) and a 9% lower likelihood of major amputation (above or below knee) (HR, 0.91; 95% CI, 0.84-0.99) than those who did not.
Conclusions and Relevance
The findings of this study suggest that patients with kidney failure at risk for DFUs who receive foot and ankle care from podiatrists may be associated with a reduced likelihood of diabetes-related amputations.
This cohort study examines the occurrence of limb loss and mortality in patients with diabetic foot ulcers who are undergoing dialysis who have vs have not received preventive care by podiatrists.
Introduction
Foot ulceration is one of the most serious and costly complications of diabetes worldwide, including the US.1 Up to 34% of adults with diabetes develop diabetic foot ulcers (DFUs), and two-thirds of DFUs recur, even after healing.1,2 These foot ulcers precede 80% of lower extremity amputations and are associated with diminished physical function, reduced quality of life, and increased mortality risk.3,4,5 The co-occurrence of kidney insufficiency among individuals with DFUs increases the risk of peripheral artery disease (PAD), lower extremity amputation, and mortality.6,7 In one study, patients with kidney failure accounted for nearly one-third of amputations within the overall diabetes population.8
Among the population of patients with kidney failure, approximately 10% to 32% of those receiving hemodialysis have active DFUs, while 22% to 41% have had a history of such ulcers.9,10 The severity of kidney disease corresponds to DFU outcomes.11,12 Individuals undergoing kidney replacement therapy face a 5-fold increased risk of DFUs compared with those with chronic kidney disease (stage 4 or 5) not receiving dialysis.13 Moreover, a study by Game et al14 suggested the onset of dialysis therapy itself seems to amplify the risk of DFUs among individuals with diabetes. In addition, the impact of diabetic foot complications extends to health care use, with DFUs the leading cause of hospitalizations among patients with kidney failure.8,11 Collectively, the cost of annual cost of DFU care to Medicare is estimated to be $9 billion, and the cost to private insurers is $1.3 billion.15
Recognizing the heightened risk of DFUs and their adverse outcomes among patients with diabetes and kidney failure, the 2023 International Working Group on Diabetic Foot practice guidelines categorize these patients as high risk (category 3), regardless of a history of DFU or amputation.16 This classification underscores the necessity of regular foot care and screening. Podiatrists play an instrumental role in treating diabetic foot complications in the US.17,18,19 While previous research has reported the importance of foot and ankle care by podiatrists in preventing amputations and hospitalizations in those with DFUs, the specific impact of such preemptive care in patients with kidney failure and diabetes at high risk for DFUs remains unexplored.17,20
Using a comprehensive and nationally representative cohort of US patients undergoing dialysis, this study sought to elucidate whether the outcomes of DFUs differ between patients with kidney failure who had preemptive foot and ankle care by podiatrists within 3 months before the index DFU diagnosis and those who did not.
Methods
Data Sources
We queried the US Renal Data System claims data from 2016 to 2017 to identify Medicare beneficiaries with type 2 diabetes receiving dialysis with a new diagnosis of DFU in 2017.21 The US Renal Data System, a national repository of information on patients with kidney failure, was used for analysis (eMethods in Supplement 1).22 The University of Arizona Institutional Review Board exempted this study from review and the requirement for informed consent due to the use of deidentified data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.23
Study Populations
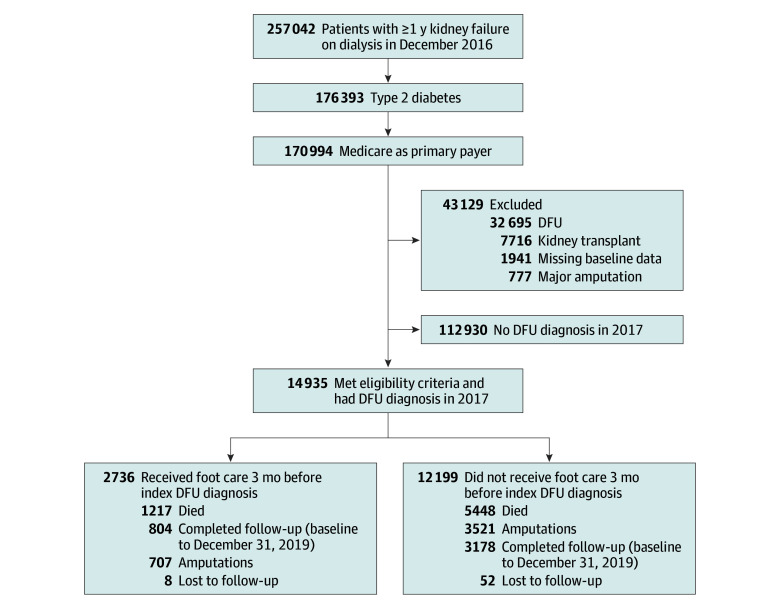
Figure 1 illustrates the study cohort selection criteria. From an initial pool of 176 393 patients with type 2 diabetes and kidney failure undergoing dialysis in December 2016, we focused on Medicare beneficiaries to ensure sufficient claims history (n = 170 994). The index date was the first DFU diagnosis in 2017 (study baseline). We excluded patients with a prior diagnosis of DFU in 2016 (n = 32 695), history of major amputation (above or below the knee) (n = 777), kidney transplant (n = 7716), and incomplete baseline information (n = 1941). The final study cohort (n = 14 935) was followed up until December 31, 2019, for major amputation, death, or loss to follow-up (defined as missing Medicare claims in 60 consecutive days). Median follow-up time was 12 (IQR, 2-26) months, and mean (SD) follow-up time was 13.5 (12.0) months. A full list of related International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Clinical Modification (ICD-10-CM) and Healthcare Common Procedure Coding System (HCPCS) procedures are provided in eTable 1 in Supplement 1.
Figure 1. Flowchart of Study Cohort Selection.
DFU indicates diabetic foot ulcer.
Outcomes
We assessed 2 outcomes: amputation-free survival, defined as the time (in months) to death and/or major amputation, and time to major amputation (above-knee or below-knee) identified by Current Procedure Terminology (CPT) codes (eTable 1 in Supplement 1). For amputation analysis, death was a competing event.
Exposures
The exposure of interest was foot and ankle care provided by a podiatrist (physician specialty code 48) within 3 months before study baseline (ie, index DFU diagnosis). It was identified through HCPCS codes (2028F, G0245, G02460, and G0127) and CPT codes (11055-57, 11102, 11103, 11305-08, 11420-24, 11426, 11719-21, and 11750) (eTable 1 in Supplement 1).
Covariates
Covariates included age at kidney failure onset; race; Hispanic ethnicity; sex; underlying cause of kidney disease; functional status, including amputation, inability to ambulate or transfer, need of assistance with daily activities, and being institutionalized; pre-kidney failure nephrology care; and body mass index. Race and ethnicity were considered in the analyses to assess whether there were differences in the use of foot and ankle care by podiatrists before DFU diagnosis. These variables were extracted from the Medical Evidence form at the index diagnosis of kidney failure. Major comorbid conditions evaluated were hypertension, coronary artery disease, congestive heart failure, PAD, cerebrovascular disease, stroke, chronic obstructive pulmonary disease, and other cardiac conditions. Patients were considered to have a comorbidity of interest if they had received at least 1 recorded diagnosis for the condition of interest at either an outpatient or hospital visit. Information on comorbid conditions was ascertained in the 3 months preceding the study baseline. The Charlson Comorbidity Index was used to measure the severity and range of patient comorbid conditions. Dialysis modality (hemodialysis or peritoneal dialysis), duration, and total hospital days during 3 months before the study baseline were also considered. A detailed description of variables, including their definitions and related data sources, is provided in the eMethods and eTable 2 in Supplement 1.
Statistical Analysis
We calculated bivariate frequencies and percentages to compare baseline characteristics of the podiatry care and nonpodiatry care groups using Pearson χ2 tests for categorical variables and t tests for continuous variables. Bivariate and adjusted multivariate logistic regression models were used to identify factors associated with receiving foot and ankle care by podiatrists. Odd ratios (ORs) and 95% CIs are reported. The Kaplan-Meier method was used to estimate amputation-free survival probability at 3 years.
To account for the nonrandomized distribution of the 2 groups, inverse probability of treatment weighting (IPTW)–adjusted analyses were used to control for differences in baseline characteristics. Propensity score weighting was used for adjusted analyses, and balance was assessed before and after weighting using absolute standardized mean difference. A value of less than 0.1 was considered balanced (eFigure in Supplement 1). Using IPTW Cox proportional hazards regression, hazard ratios (HRs) for a composite of death and major amputation were estimated for the podiatry care group compared with the no podiatry care group. We observed no departure from the proportional hazards assumption, as indicated by the absence of a significant interaction between the exposure group and time. The presence of competing events is an important consideration in the kidney failure population because of the high mortality risk. Mortality was treated as a competing risk in examining amputations. Specifically, the cumulative incidence curve of major amputation was estimated while accounting for early death as a competing risk. In IPTW multivariate analysis, we adopted a subdistribution hazard model to account for the possible nonindependence of censoring on death as a competing event.
Multiple sensitivity analyses were performed to ensure result robustness. First, a negative control outcome analysis assessed residual confounding, exploring the association between podiatric care and an unrelated outcome—hospitalization rates for pneumonia—as a potential surrogate marker for overall patient care quality. Second, to address lead time bias, we examined time intervals (in days) from study entry (January 1, 2017) to the index DFU diagnosis based on podiatry care receipt. Osteomyelitis or gangrene presence at the index DFU diagnosis, as indicators of ulcer severity, were also compared among study groups. Third, analyses were reiterated using propensity score as an adjustment variable and propensity score matching for 55% of the sample. Fourth, exposure measurement (ankle and foot care by podiatrists) was extended to 12 months before the index DFU diagnosis. All analyses were 2-sided, and P < .05 was considered statistically significant. Statistical analyses were completed on June 15, 2023, and an updated analysis was performed on December 11, 2023, using SAS software, version 9.4 (SAS Institute LLC).
Results
The study cohort included 14 935 adults with kidney failure and newly diagnosed DFUs; 6651 patients (44.5%) were female and 8284 were male (55.4%). Mean (SD) age was 59.3 (12.7) years, and 5291 patients (35.4%) were aged 65 years or older at the initiation of kidney replacement therapy. The mean (SD) duration of dialysis was 5.5 (3.3) years. Among the cohort, 414 (2.7%) were Asian, 5235 (35.0%) were Black/African American, 2652 (17.7%) were Hispanic, and 8745 (58.5%) were White. Other baseline characteristics at dialysis initiation are listed in Table 1.
Table 1. Characteristics of Dialysis Patients by Recipient of Foot and Ankle Care by Podiatrists in the 3 Months Before the Index DFU.
| Characteristica | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| Total (N = 14 935) | With podiatric care (n = 2736) | Without podiatric care (n = 12 199) | |||
| Demographic characteristics | |||||
| Sex | |||||
| Male | 8284 (55.4) | 1547 (56.5) | 6737 (55.2) | ||
| Female | 6651 (44.5) | 1189 (43.5) | 5462 (44.8) | ||
| Age, y | |||||
| Mean (SD), y | 59.3 (12.7) | 62.8 (12.1) | 58.5 (12.7) | ||
| <50 | 3253 (21.7) | 381 (13.9) | 2872 (23.5) | ||
| 50 to <65 | 6391 (42.7) | 1084 (39.6) | 5307 (43.5) | ||
| ≥65 | 5291 (35.4) | 1271 (46.5) | 4020 (33.0) | ||
| Race and ethnicityb | |||||
| Asian | 414 (2.7) | 77 (2.8) | 337 (2.8) | ||
| Black or African American | 5235 (35.0) | 951 (34.8) | 4284 (35.1) | ||
| Hispanic | 2652 (17.7) | 439 (16) | 2213 (18.1) | ||
| White | 8745 (58.5) | 1664 (60.8) | 7081 (58) | ||
| Other | 541 (3.6) | 44 (1.6) | 497 (4.1) | ||
| Dual eligibility of Medicare and Medicaid | 6467 (43.4) | 1121 (41) | 5346 (43.8) | ||
| Clinical characteristics at diagnosis of kidney failure | |||||
| BMI | |||||
| Mean (SD) | 32.1 (8.3) | 32.0 (8.1) | 32.1 (8.4) | ||
| 0 to <25 | 3011 (20.1) | 555 (20.3) | 2456 (20.1) | ||
| 25 to <30 | 3866 (25.8) | 708 (25.9) | 3158 (25.9) | ||
| 30 to <35 | 3389 (22.6) | 615 (22.5) | 2774 (22.7) | ||
| ≥35 | 4669 (31.2) | 858 (31.4) | 3811 (31.2) | ||
| Primary cause of kidney failure | |||||
| Diabetes | 10 897 (72.9) | 2011 (73.5) | 8886 (72.8) | ||
| Hypertension | 2732 (18.2) | 500 (18.3) | 2232 (18.3) | ||
| Glomerulonephritis | 441 (2.9) | 65 (2.4) | 376 (3.1) | ||
| Other/unknown | 865 (5.7) | 160 (5.8) | 705 (5.8) | ||
| Nephrology care before initiation of dialysis | |||||
| Yes | 9202 (61.6) | 1762 (64.4) | 7440 (61) | ||
| No | 3277 (21.9) | 491 (17.9) | 2786 (22.8) | ||
| Unknown | 2456 (16.4) | 483 (17.7) | 1973 (16.2) | ||
| Insulin-dependent diabetes at initiation of dialysis | 8916 (59.6) | 1660 (60.7) | 7256 (59.5) | ||
| Alcohol/drug use | 2179 (14.5) | 36 (1.3) | 200 (1.6) | ||
| Need assistance with activities of daily living | 1472 (9.8) | 303 (11.1) | 1169 (9.6) | ||
| Institutionalized | 757 (5.1) | 228 (8.3) | 529 (4.34) | ||
| Dialysis-related characteristics | |||||
| Dialysis modality | |||||
| Hemodialysis | 13 816 (92.5) | 2567 (93.8) | 11249 (92.2) | ||
| Peritoneal dialysis | 1119 (7.4) | 169 (6.2) | 950 (7.8) | ||
| Duration of dialysis, y | |||||
| Mean (SD) | 5.5 (3.3) | 5.6 (3.3) | 5.5 (3.3) | ||
| <2.5 | 2676 (17.9) | 462 (16.9) | 2214 (18.1) | ||
| 2.5 to <4 | 3393 (22.7) | 574 (21) | 2819 (23.1) | ||
| 4 to <6.5 | 4331 (28.9) | 821 (30) | 3510 (28.8) | ||
| ≥6.5 | 4535 (30.3) | 879 (32.1) | 3656 (30) | ||
| Comorbidities and hospitalizationc,d | |||||
| Hypertension | 12 547 (84.0) | 2333 (85.3) | 10 214 (83.7) | ||
| COPD | 2837 (18.9) | 553 (20.2) | 2284 (18.7) | ||
| CHF | 6464 (43.2) | 1174 (42.9) | 5290 (43.4) | ||
| CVA/TIA | 2789 (18.6) | 569 (20.8) | 2220 (18.2) | ||
| ASHD | 1614 (10.8) | 284 (10.4) | 1330 (10.9) | ||
| Peripheral arterial disease | 3429 (22.9) | 858 (31.4) | 2571 (21.1) | ||
| Coronary artery disease | 6840 (45.7) | 1320 (48.2) | 5520 (45.2) | ||
| Other cardiac conditions | 4913 (32.8) | 853 (31.2) | 4060 (33.3) | ||
| Charlson Comorbidity Index score | |||||
| Mean (SD) | 6.1 (2.3) | 6.4 (2.2) | 6.0 (2.3) | ||
| <6 | 6465 (43.2) | 1014 (37.1) | 5451 (44.7) | ||
| 6 to <8 | 5163 (34.5) | 1028 (37.6) | 4135 (33.9) | ||
| 8 to <9 | 1513 (10.1) | 335 (12.2) | 1178 (9.7) | ||
| ≥9 | 1794 (12.0) | 359 (13.1) | 1435 (11.8) | ||
| Duration of hospitalization, d | |||||
| Mean (SD) | 7.9 (13.2) | 5.5 (10.3) | 8.5 (13.7) | ||
| 0 | 7236 (48.4) | 1600 (58.5) | 5636 (46.2) | ||
| 1 to <10 | 3622 (24.2) | 603 (22) | 3019 (24.7) | ||
| ≥10 | 4077 (27.2) | 533 (19.5) | 3544 (29.1) | ||
Abbreviations: ASHD, atherosclerotic heart disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack.
All characteristics were found to be significantly different (P < .001) between the groups except for sex, BMI, primary cause of kidney failure, alcohol use, CHF, and COPD. eTable 2 in Supplement 1 provides detailed definition for each characteristic.
Identification of race and ethnicity was obtained from the Medical Evidence Form; ascertainment of race was made by self-report. The Other group includes American Indian, Alaska Native, Pacific Islander, or Native Hawaiian.
eTable 1 in Supplement 1 provides specific International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification codes used.
Both comorbid conditions and hospital days were measured during the 3 months prior to baseline.
Receipt of Foot and Ankle Care by Podiatrists in the 3 Months Before DFU Diagnosis
The study revealed substantial rates of mortality (44.6%) and major amputation (28.3%) in this high-risk population. Of the cohort, 18.4% (n = 2736) received foot and ankle care by podiatrists within 3 months before the index DFU diagnosis. Patients who received care by podiatrists were more likely to be male, to be older, of White race, receiving pre–kidney failure nephrology care, in need of assistance with daily activities, and institutionalized compared with those who did not. They also had higher Charlson Comorbidity Index scores, had a longer duration of dialysis dependency, a greater likelihood of receiving peritoneal dialysis, and extended hospital stay.
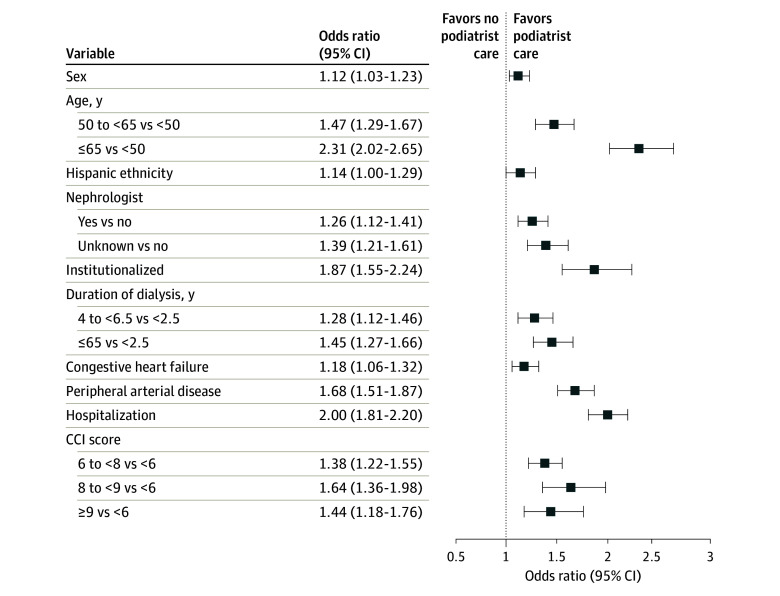
Adjusted logistic regression analysis (Figure 2) indicated that older age (≥65 vs <50 years: OR, 2.31; 95% CI, 2.02-2.65), institutionalization (OR, 1.87; 95% CI, 1.55-2.24), PAD (OR, 1.68; 95% CI, 1.51-1.87), hospitalization (OR, 2.00; 95% CI, 1.81-2.20), and higher Charlson Comorbidity Index score (8 to <9 vs <6: OR, 1.64; 95% CI, 1.36-1.98) were associated with the likelihood of receiving care by podiatrists. Patients with a longer dialysis duration (≥6.5 vs <2.5 years: OR, 1.45; 95% CI, 1.27-1.66) and receiving pre–kidney failure nephrologist care (OR, 1.26; 95% CI, 1.21-1.41) were also more likely to receive care by podiatrists. Complete logistic regression results can be found in eTable 3 in Supplement 1.
Figure 2. Adjusted Logistic Regression Analysis of Receipt of Foot and Ankle Care by Podiatrists in the 3 Months Before the Diabetic Foot Ulcer Diagnosis.
CCI indicates Charlson Comorbidity Index.
Associations Between Foot and Ankle Care by Podiatrists With Amputation-Free Survival and Major Amputation
At the end of the study follow-up, there were 1217 deaths (44.5%) among patients who received podiatry care (n = 2736) and 5448 deaths (44.7%) among patients who did not (n = 12 199); there were 707 (25.8%) major amputations for those with podiatry care and 3521 (28.9%) for those without podiatry care (Figure 1). Survival probabilities at 36 months were 26.3% vs 22.8% (P < .001, unadjusted Kaplan-Meier survival analysis) in the group who received care by podiatrists compared with those who did not (Figure 3, A). Consistently, the cumulative incidence of amputation alone at 36 months was 26.4% in the podiatry care and 29.8% in the no podiatry care groups (P = .001 by Gray test) (Figure 3, B).
Figure 3. Unadjusted Kaplan-Meier Survival Analysis.
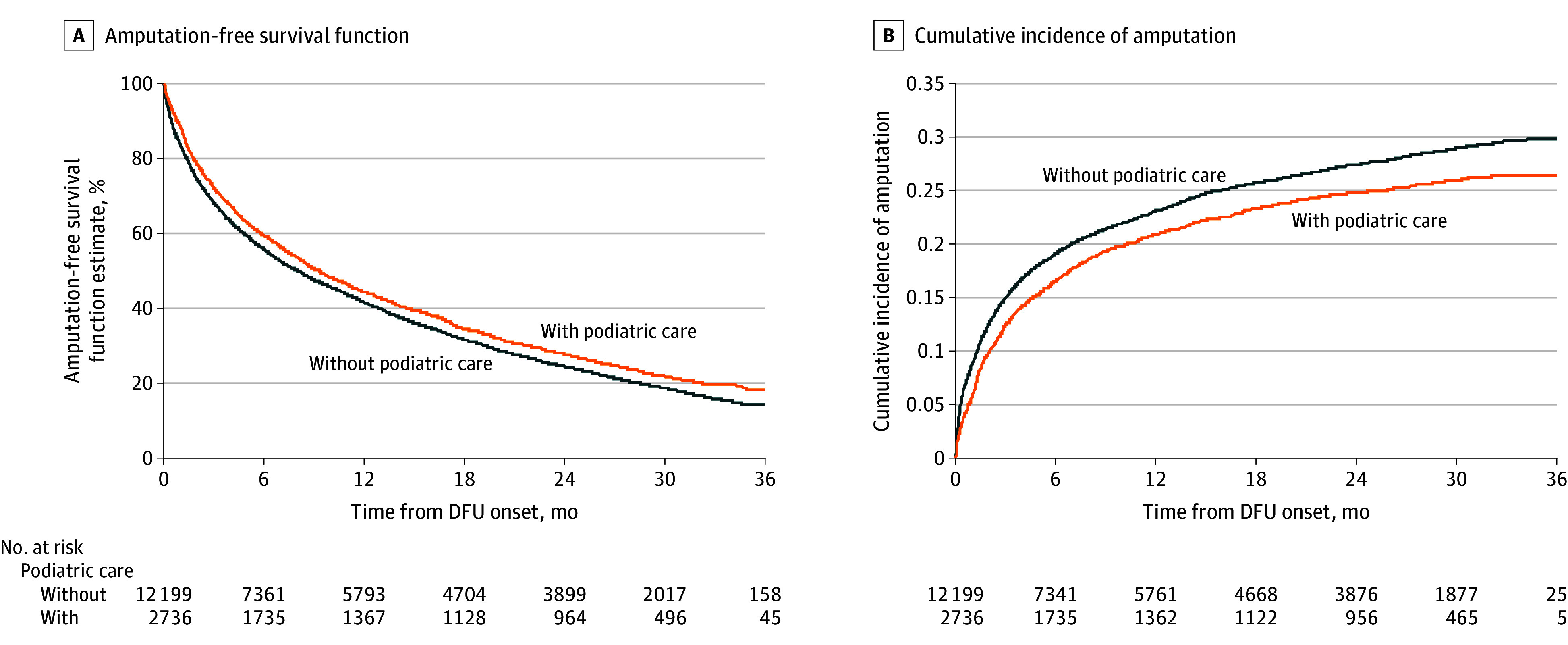
A, Higher amputation-free survival was observed at 36 months with vs without podiatry care (26.3% vs 22.8%; P < .001 by log-rank test). B, Cumulative incidence of amputation alone at 36 months was lower with vs without podiatry care (26.4% vs 29.8%; P = .001 by Gray test). DFU indicates diabetic foot ulcer.
In unadjusted analysis, receiving care from podiatrists appeared to have a 9% lower likelihood of death or major amputation (HR, 0.91; 95% CI, 0.86-0.95) and a 13% lower likelihood of major amputation alone (HR, 0.87; 95% CI, 0.80-0.93). After adjustment of baseline confounding, the multivariate IPTW model indicated that care by podiatrists was associated with an 11% lower likelihood of the composite of death or major amputation (HR, 0.89; 95% CI, 0.84-0.93; P < .001) and a 9% lower likelihood of major amputation alone (HR, 0.91; 95% CI, 0.84-0.99; P = .03) (Table 2). eTable 4 in Supplement 1 provides the full results of multivariate analyses.
Table 2. Association of Foot and Ankle Care by Podiatrists in the 3 Months Before the Index Diabetic Foot Ulcer Diagnosis With Outcomes.
| Outcome | No. of total events during follow-up | With vs without podiatric care | ||||||
|---|---|---|---|---|---|---|---|---|
| With podiatric care (n = 2735) | Without podiatric care (n = 12 199) | Unadjusted HR (95% CI) | IP-weighted HR (95% CI) | |||||
| Event, No. | % per Patient | % per Patient month | Event, No. | % per Patient | % per Patient month | |||
| Composite of death and amputation | 1924 | 70.3 | 5.9 | 8969 | 73.5 | 6.6 | 0.91 (0.86-0.95) | 0.89 (0.84-0.93) |
| Amputation alonea | 707 | 25.6 | 2.1 | 3521 | 28.9 | 2.6 | 0.87 (0.80-0.94) | 0.91 (0.84-0.99) |
Abbreviations: HR, hazard ratio; IP, inverse probability.
HRs were based on subdistribution hazard model.
Sensitivity Analysis
The sensitivity analysis results addressed concerns regarding residual confounding and lead-time bias. Specifically, pneumonia hospitalization rates were found to be comparable between patients with vs without podiatric care (1.2% vs 1.1%), with no significant difference in the IPTW model (HR, 0.98; 95% CI, 0.92-1.04). Time intervals to DFU diagnosis showed no association with podiatric care, averaging 164 days with care and 162 days without. The podiatric care group had a slightly lower prevalence of osteomyelitis (1.7% vs 1.9%) and gangrene (4.4% vs 4.6%). In the propensity score matching analysis, podiatrist care vs no care was associated with a 13% lower probability of death or amputation (HR, 0.87; 95% CI, 0.82-0.93). Similar results were observed in propensity score–adjusted analysis and when extending the exposure duration from 3 months to 12 months before the index DFU diagnosis.
Discussion
This claim-based study examined data from a cohort of 14 935 Medicare beneficiaries receiving kidney replacement therapy and newly diagnosed with DFUs to investigate the factors associated with receipt of foot and ankle care by podiatrists and its potential association with outcomes. The study revealed substantial rates of mortality (44.8%) and major amputation (28.3%) in this high-risk population. Only 18.4% of patients received care by podiatrists within the 3 months preceding the DFU diagnosis. Both Kaplan-Meier and adjusted analyses indicated that receipt of foot and ankle care by podiatrists was associated with improvement in 3-year amputation-free survival, a reduced risk of major amputation, and a lower risk of the composite outcome of death and/or major amputation. These findings suggest that preemptive foot and ankle care by podiatrists has the potential to offer benefits in reducing limb loss in high-risk patients with kidney failure and diabetes who are receiving dialysis.
Patients receiving dialysis have a disproportionate burden of peripheral neuropathy, PAD, and diabetes-related foot complications compared with individuals at earlier stages of chronic kidney disease.13 Diabetic peripheral neuropathy is prevalent in up to 90% of patients with advanced kidney disease,24 and the prevalence of PAD ranges from 23% to 46% in patients receiving dialysis, in contrast to 7% to 24% in those with chronic kidney disease.25 In addition, dialysis itself is an independent risk factor for limb loss in patients with both PAD and DFUs.12 Hospitalizations related to diabetic foot complications accounted for a higher proportion (33%) of amputation in patients with kidney failure and DFUs compared with nondialysis diabetic populations (14%).4 Furthermore, this cohort of 51 362 patients with kidney failure and DFUs exhibited a significantly higher mortality rate (71% over 3 years of follow-up)4 compared with usual cancer mortality and patients with kidney failure without diabetic foot complications.26
Given the substantial morbidity and mortality associated with DFUs, coupled with poor outcomes from revascularization in patients with kidney failure, care guidelines recommend regular foot screening and preventive care for individuals receiving dialysis.16,25 The 2023 International Working Group on Diabetic Foot guidelines categorize patients with kidney failure and peripheral neuropathy or PAD history as category 3 and recommend quarterly foot screenings.16 Implementing diabetes education and care management, including regular foot examinations, has been shown to improve glycemic control and reduce amputations in patients with kidney failure.27 In a study by Pernat et al,28 monthly foot examinations conducted by dialysis nurses at hemodialysis facilities resulted in a 17% reduction in major amputation rates.
Our study findings align with prior evidence underscoring the importance of care by podiatrists for patients with diabetes before and after the onset of DFUs. For example, Medicare beneficiaries with DFUs who received care from podiatrists and dedicated lower extremity complication specialists were less likely to undergo amputation compared with those treated by nondedicated specialists.29 A meta-analysis further highlighted that multidisciplinary care, including podiatrists, was associated with a 31% decreased risk of any amputation and a 55% lower risk of major amputation.17 Additionally, a study by Gibson et al18 reported that care provided by podiatrists in the year preceding a DFU diagnosis was associated with decreased risks of limb loss and hospitalizations. Taken together, these findings, including those from our study, underscore the potential of care by podiatrists to mitigate the heightened amputation risks faced by patients with diabetes who are receiving dialysis.
In our study, patients undergoing dialysis who received foot and ankle care by podiatrists exhibited distinct characteristics. Specifically, they tended to be older, presented with a higher burden of comorbidities, and needed assistance with activities of daily living, compared with those who did not receive such care. A substantial proportion of at-risk patients with kidney failure, accounting for more than 80% of the study cohort, did not receive such care within the 3 months leading up to their DFU diagnosis. This observation highlights a critical gap in the delivery of preventive measures for a population inherently susceptible to diabetes-related foot complications. Previous studies have highlighted wide variations in routine preventive podiatry care coverage across insurance types, states, and even within states depending on Medicaid Advantage–Managed Care programs.30,31 Addressing these challenges and the policy implications of expanded coverage are beyond this article’s scope, requiring further comprehensive studies. An understanding of the underlying patient-level and health care system–level factors that contribute to this gap can guide the development of targeted interventions to ensure more individuals at risk for DFUs receive appropriate preemptive foot and ankle care.
Limitations
This study has several important limitations. First, it relied on administrative claims data and the use of ICD-10-CM codes for identifying the study cohort, introducing inherent limitations associated with data accuracy and completeness. Second, due to data constraints, information regarding the frequency and content of podiatry visits, as well as foot and ankle care from nonpodiatry clinicians, was not captured. The absence of these details hampers the ability to examine the optimal nature and extent of foot and ankle care. Third, the study cohort focused on individuals who developed DFUs without a recent history of amputation or DFUs, which could potentially limit the generalizability of these findings to broader chronic kidney disease populations. Fourth, the relatively short duration of follow-up may limit our capability to draw definitive conclusions regarding the long-term efficacy of care provided by podiatrists within this population. We also did not examine how foot and ankle care by podiatrists could enhance the likelihood of kidney transplant in patients with kidney failure by reducing the presence of open wounds.
Conclusions
In this comprehensive analysis of a national cohort comprising Medicare beneficiaries with kidney failure and DFUs, the receipt of foot and ankle care provided by podiatrists within the 3 months preceding a DFU diagnosis was associated with reduction in the risks of major amputation and death among patients with kidney failure who were receiving dialysis. While future fully powered studies are needed to further support these findings, these results suggest positive potential benefits for preventive foot and ankle care to mitigate complications in this high-risk population.
eTable 1. Diagnosis and Procedure Codes
eMethods. Data Sources, Variable Constructions, and Missing Data
eTable 2. Independent Study Variables and Related Data Sources
eTable 3. Association Between Study Covariates and the Likelihood of Receiving Foot and Ankle Care by Podiatrists in the 3-Months Prior to Index DFU
eTable 4. Association for Receipt of Foot and Ankle Care by Podiatrists and Outcomes Using Inverse Probability of Treatment Weighting (IPTW) Cox Regression Analysis for Composite Outcomes of Death and/or Amputation and IPTW Competing Risk Analysis for Cumulative Incidence of Major Amputation
eFigure. Propensity Score Weighting for Adjusted Analyses
Data Sharing Statement
References
- 1.Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62-75. doi: 10.1001/jama.2023.10578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. doi: 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 3.Polikandrioti M, Vasilopoulos G, Koutelekos I, et al. Quality of life in diabetic foot ulcer: associated factors and the impact of anxiety/depression and adherence to self-care. Int J Low Extrem Wounds. 2020;19(2):165-179. doi: 10.1177/1534734619900415 [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. doi: 10.1186/s13047-020-00383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao SP, Houck JR, Waldman OV, Baumhauer JF, Oh I. Prediction of post-interventional physical function in diabetic foot ulcer patients using Patient Reported Outcome Measurement Information System (PROMIS). Foot Ankle Surg. 2021;27(2):224-230. doi: 10.1016/j.fas.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Qian H, Xu L, et al. Association between estimated glomerular filtration rate and outcomes in patients with diabetic foot ulcers: a 3-year follow-up study. Eur J Endocrinol. 2017;177(1):41-50. doi: 10.1530/EJE-17-0070 [DOI] [PubMed] [Google Scholar]
- 7.Petersen BJ, Linde-Zwirble WT, Tan TW, et al. Higher rates of all-cause mortality and resource utilization during episodes-of-care for diabetic foot ulceration. Diabetes Res Clin Pract. 2022;184:109182. doi: 10.1016/j.diabres.2021.109182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CW, Armstrong DG, Huang CH, et al. Diabetic foot disease in subjects with end-stage renal disease: a nationwide study over 14 years highlighting an emerging threat. Diabetes Res Clin Pract. 2022;193:110134. doi: 10.1016/j.diabres.2022.110134 [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MR, Raspovic A, McMahon LP, et al. Factors associated with foot ulceration and amputation in adults on dialysis: a cross-sectional observational study. BMC Nephrol. 2017;18(1):293. doi: 10.1186/s12882-017-0711-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brekelmans W, Borger van der Burg BLS, Vroom MA, Kreuger MJ, Schrander van der Meer AM, Hoencamp R. Prevalence of foot ulcers in dialysis-dependent patients. Wound Repair Regen. 2019;27(6):687-692. doi: 10.1111/wrr.12750 [DOI] [PubMed] [Google Scholar]
- 11.Lavery LA, Lavery DC, Hunt NA, La Fontaine J, Ndip A, Boulton AJ. Amputations and foot-related hospitalisations disproportionately affect dialysis patients. Int Wound J. 2015;12(5):523-526. doi: 10.1111/iwj.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care. 2008;31(7):1331-1336. doi: 10.2337/dc07-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndip A, Rutter MK, Vileikyte L, et al. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care. 2010;33(8):1811-1816. doi: 10.2337/dc10-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Game FL, Chipchase SY, Hubbard R, Burden RP, Jeffcoate WJ. Temporal association between the incidence of foot ulceration and the start of dialysis in diabetes mellitus. Nephrol Dial Transplant. 2006;21(11):3207-3210. doi: 10.1093/ndt/gfl427 [DOI] [PubMed] [Google Scholar]
- 15.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651-658. doi: 10.2337/dc13-2176 [DOI] [PubMed] [Google Scholar]
- 16.Bus SA, Sacco ICN, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. Published online June 11, 2023. doi: 10.1002/dmrr.3651 [DOI] [PubMed] [Google Scholar]
- 17.Blanchette V, Brousseau-Foley M, Cloutier L. Effect of contact with podiatry in a team approach context on diabetic foot ulcer and lower extremity amputation: systematic review and meta-analysis. J Foot Ankle Res. 2020;13(1):15. doi: 10.1186/s13047-020-0380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson TB, Driver VR, Wrobel JS, et al. Podiatrist care and outcomes for patients with diabetes and foot ulcer. Int Wound J. 2014;11(6):641-648. doi: 10.1111/iwj.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel N, Tan TW, Weinkauf C, et al. Economic value of podiatry service in limb salvage alliance. J Vasc Surg. 2022;75(1):296-300. doi: 10.1016/j.jvs.2021.07.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley CM, Perry IJ, Bradley CP, Kearney PM. Does contact with a podiatrist prevent the occurrence of a lower extremity amputation in people with diabetes? a systematic review and meta-analysis. BMJ Open. 2013;3(5):e002331. doi: 10.1136/bmjopen-2012-002331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Renal Data System . 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [Google Scholar]
- 22.National Institutes of Health . US Renal Data System. Accessed January 17, 2024. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 24.Jasti DB, Mallipeddi S, Apparao A, Vengamma B, Sivakumar V, Kolli S. A clinical and electrophysiological study of peripheral neuropathies in predialysis chronic kidney disease patients and relation of severity of peripheral neuropathy with degree of renal failure. J Neurosci Rural Pract. 2017;8(4):516-524. doi: 10.4103/jnrp.jnrp_186_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garimella PS, Hart PD, O’Hare A, DeLoach S, Herzog CA, Hirsch AT. Peripheral artery disease and CKD: a focus on peripheral artery disease as a critical component of CKD care. Am J Kidney Dis. 2012;60(4):641-654. doi: 10.1053/j.ajkd.2012.02.340 [DOI] [PubMed] [Google Scholar]
- 26.Ferreira ES, Moreira TR, da Silva RG, et al. Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: a 20-year cohort. BMC Nephrol. 2020;21(1):502. doi: 10.1186/s12882-020-02135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurray SD, Johnson G, Davis S, McDougall K. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis. 2002;40(3):566-575. doi: 10.1053/ajkd.2002.34915 [DOI] [PubMed] [Google Scholar]
- 28.Marn Pernat A, Peršič V, Usvyat L, et al. Implementation of routine foot check in patients with diabetes on hemodialysis: associations with outcomes. BMJ Open Diabetes Res Care. 2016;4(1):e000158. doi: 10.1136/bmjdrc-2015-000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan FA, Feinglos MN, Grossman DS. Receipt of care and reduction of lower extremity amputations in a nationally representative sample of US elderly. Health Serv Res. 2010;45(6, pt 1):1740-1762. doi: 10.1111/j.1475-6773.2010.01157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brewer TW, Bonnah GK, Cairns JS, Lanese BG, Waimberg J. Medicaid coverage for podiatric care: a national survey. Public Health Rep. 2023;138(2):273-280. doi: 10.1177/00333549221076552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labovitz JM, Kominski GF. Forecasting the value of podiatric medical care in newly insured diabetic patients during implementation of the Affordable Care Act in California. J Am Podiatr Med Assoc. 2016;106(3):163-171. doi: 10.7547/15-026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis and Procedure Codes
eMethods. Data Sources, Variable Constructions, and Missing Data
eTable 2. Independent Study Variables and Related Data Sources
eTable 3. Association Between Study Covariates and the Likelihood of Receiving Foot and Ankle Care by Podiatrists in the 3-Months Prior to Index DFU
eTable 4. Association for Receipt of Foot and Ankle Care by Podiatrists and Outcomes Using Inverse Probability of Treatment Weighting (IPTW) Cox Regression Analysis for Composite Outcomes of Death and/or Amputation and IPTW Competing Risk Analysis for Cumulative Incidence of Major Amputation
eFigure. Propensity Score Weighting for Adjusted Analyses
Data Sharing Statement