Pollutant and allergen exposures encountered at the workplace can cause or exacerbate asthma, and these potential pollutants are numerous, varying, and often under-recognized. Commonly, these causative pollutants are associated with a specific immunologic response, referred to as occupational sensitizers, and are broadly classified as high-molecular-weight (HMW) and low-molecular-weight (LMW) agents.1 HMW agents include vertebrate and invertebrate animal, plant, bacteria, and fungi exposures commonly encountered in farming, laboratories, office buildings, healthcare, and processing/harvesting environments. LMW agents such as diisocyanates, wood dusts, chemicals, biocides, acid anhydrides are usually encountered in painting, industrial, health care, cleaning, and hairdressing work. Pollutants can also induce asthma in the absence of a specific immunologic response that can be observed after high-level exposure, referred to as irritant-induced occupational asthma.1 The collapse of the World Trade Center that created exposure to very high concentrations of highly irritating dust, gases, fumes, is an example of irritant-induced occupational asthma experienced by emergency first-responders.1 In this brief review, the current perspective and paradigm shifts of workplace pollutants focused on latex and farm sensitizers with occupational asthma are examined (Figure 1, overview schematic).
Figure 1.
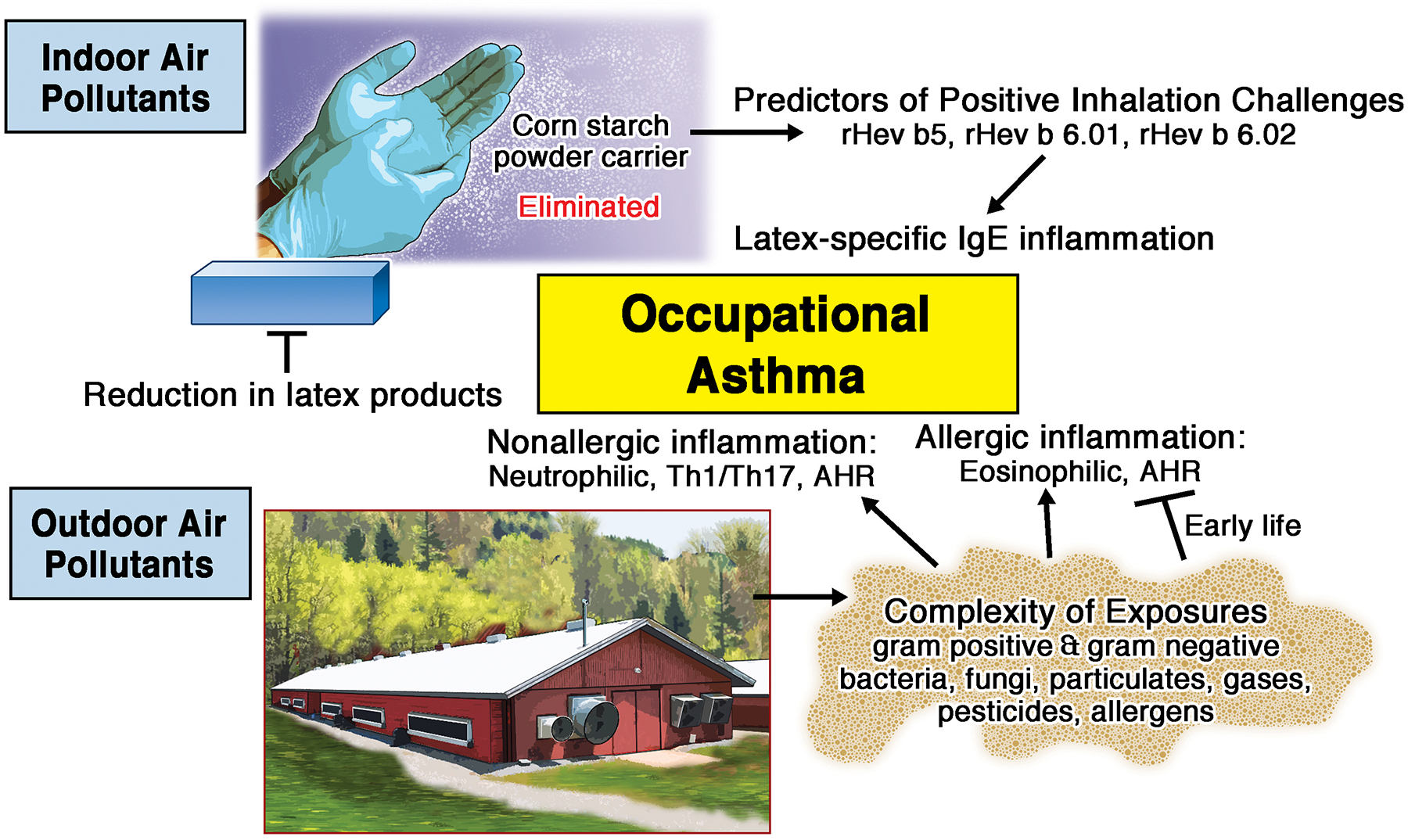
Indoor and Outdoor Air Pollutants Impact Occupational Asthma. Latex, an indoor air pollutant, is a high molecular weight allergen mediating IgE sensitization that is potentiated through corn starch carrier with recent advances in component diagnostic testing to demonstrate specificity to occupational asthma. Agriculture exposures are a complex, outdoor air pollutant that can mediate occupational asthma through nonallergic and allergic mechanisms with early life exposure demonstrating protection against allergic asthma. AHR (airway hyper-responsiveness)
Indoor Air Pollutants: The Latex Allergy Example
Despite >400 occupational sensitizers identified to induce allergy or asthma in workers, specific predictive testing has been less than satisfactory with false negative and positive results. Advances in the molecular diagnosis of sensitization to occupational exposures shows promising results with latex allergy as one example of a successful approach.2 Diagnosis of OA is made by a combination of medical history, physical exam, positive methacholine challenge or bronchodilator responsiveness, determination of IgE mediated sensitization to HMW allergens, and possibly basophil activation testing to LMW chemicals and HMW allergens. Diagnostic testing is complicated by the numerous HMW proteins recognized in various indoor air pollutant environments. In the example of latex allergy, over 250 peptides have been identified, yet only15 allergens from Hevea brasiliensis Latex (Hev b 1–15) are included in the official allergen list of the World Health Organization and International Union of Immunological Societies Allergen Nomenclature database. Unfortunately, current serologic testing for Latex specific IgE demonstrate sensitivity of 35% - 76%,3 but with high specificity.
Promising alternatives to latex challenge testing is the utilization of component resolved diagnostic reagents and the cloning of recombinant latex allergens. The “spiking” of rHev b 5 into the ImmunoCap (Uppsala, Sweden) greatly improved the diagnostic capacity of the ImmunoCap test and serves as a model for improved testing in diagnosing other pollutant induced asthma.2 For example, patterns of reactivity to rHev b 1 and rHev b 3 identify up to 86% of latex allergic spina bifida patients. Similarly, reactivity of specific IgE to rHev b 5, rHev b 6.01, and rHev b 6.02 are the most accurate predictors of a positive response in an inhalation challenge test of latex allergen.4 This pattern of reactivity to allergen components in latex-mediated disease demonstrates superior diagnostic capacity compared to ImmunoCap. The global success of reduction of latex allergy in health care workers by reducing environmental contamination of latex allergens carried on cornstarch powder has been nothing short of a remarkable achievement.4
Outdoor Air Pollutants: Agriculture Exposures
In the past 20 years, the protective role for early life farming exposures in reducing the development of asthma has been established, and animal modeling studies demonstrate that exposure to these dust extracts prior to and during allergic sensitization and challenge reduces airway hyper-reactivity and eosinophilia.5 However, this paradigm of early life farm exposures as protective against asthma development does not explain the observations in adults that occupational agriculture exposure can cause or exacerbate asthma. Among farm operators with farm work-related asthma, 33% had asthmatic exacerbations while doing farm work, suggesting that farm exposure is a risk factor for worsening asthma disease.6 The “agriculture exposure” is complex and characterized by its abundance and wide-variety of gram positive and gram negative bacteria, fungi, particulates, gases, pesticides, and allergens. All of these complex factors, which can be either HMW or LMW, can sensitize or irritate the airway response towards allergic or nonallergic asthmatic disease. The timing and magnitude of the exposure plays a significant role as higher endotoxin levels, which serves a surrogate measure of bacterial burden, has been associated with lower lung function in asthma cases of adult farmers.7 Interestingly, this relationship is modified by early-life farm exposures as those not born on a farm had higher rates of asthma as compared to adult farmers who were born and raised on a farm.7 Similarly, exposure to swine and dairy confinements are risk factors for new onset non-allergic asthma and worsening lung function over time, while being born and raised on a farm reduces this risk.7 Finally, the use of various types of pesticides including, but not limited to organophosphorus pesticides,8 should be highlighted because pesticides are LMW agents that can be important, independent risk factors to explain workplace exacerbated asthma among exposed adults.
The mechanisms to explain occupational asthma and workplace exacerbated asthma in farming occupations are not well-defined. Non-allergic asthma tends to prevail over allergic asthma, but atopy can be certainly found in these asthmatics. Microbial components within agricultural dust extracts activate pathogen recognition receptor signaling pathways to elicit strong inflammatory responses marked by neutrophil and Th1/Th17 lymphocyte influx as well as trigger airway hyper-responsiveness.7 Neutrophilic inflammation has also been associated with airway dehydration in asthmatics, which is hypothesized to contribute to bronchoconstriction. Endotoxins can also enhance histamine release caused by allergens and potentiate eosinophilic inflammation.9 Large, prospective longitudinal studies of adult agriculture workers are warranted to better understand the pathogenesis of asthma associated with agriculture work. Comprehensive exposure assessments incorporating molecular technique tools to move the field forward to the ultimate goal of understanding the occupational “exposome”10 will be necessary. It will also be important to identify how the changing demographics of this industry with racially/ethnically diverse and potential expansion of female workers might impact asthma outcomes. Finally, the non-uniform use of respirators within agricultural work contributes to workplace asthma, and education for respiratory use should clearly be considered by health care providers.
Funding:
No funding source supported this manuscript.
Footnotes
Disclosure of interest: All others authors confirm that they have no conflicts of interest to disclose. JAP is funded by the NIH (ES-019325) and NIOSH (U54OH010162).
References
- 1.Tarlo SM, Lemiere C. Occupational asthma. N Engl J Med. 2014. Feb 13;370(7):640–9. [DOI] [PubMed] [Google Scholar]
- 2.Kelly KJ, Sussman G. Latex allergy: Where are we now and how did we get there? J Allergy Clin Immunol Pract. 2017. Sep – Oct;5(5):1212–6. [DOI] [PubMed] [Google Scholar]
- 3.Accetta Pedersen DJ, Klancnik M, Elms N, Wang ML, Hoffmann RG, Kurup VP, et al. Analysis of available diagnostic tests for latex sensitization in an at-risk population. Ann Allergy Asthma Immunol. 2012. Feb;108(2):94–7. [DOI] [PubMed] [Google Scholar]
- 4.Vandenplas O, Froidure A, Meurer U, Rihs HP, Rifflart C, Soetaert S, et al. The role of allergen components for the diagnosis of latex-induced occupational asthma. Allergy. 2016. Jun;71(6):840–9. [DOI] [PubMed] [Google Scholar]
- 5.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016. Aug 4;375(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazurek JM, White GE, Rodman C, Schleiff PL. Farm work-related asthma among US primary farm operators. J Agromedicine. 2015;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wunschel J, Poole JA. Occupational agriculture organic dust exposure and its relationship to asthma and airway inflammation in adults. J Asthma. 2016. Jun;53(5):471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaffo FC, Grodzki AC, Fryer AD, Lein PJ. Mechanisms of organophosphorus pesticide toxicity in the context of airway hyperreactivity and asthma. Am J Physiol Lung Cell Mol Physiol. 2018. Oct 1;315(4):L485–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger M, de Boer JD, Bresser P, van der Poll T, Lutter R, Sterk PJ, et al. Lipopolysaccharide amplifies eosinophilic inflammation after segmental challenge with house dust mite in asthmatics. Allergy. 2015. Mar;70(3):257–64. [DOI] [PubMed] [Google Scholar]
- 10.Agache I, Miller R, Gern JE, Hellings PW, Jutel M, Muraro A, et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: A practall document. Allergy. 2019. Mar;74(3):449–63. [DOI] [PubMed] [Google Scholar]


