Abstract
Background
Fungal keratitis is a fungal infection of the cornea. It is common in lower income countries, particularly in agricultural areas but relatively uncommon in higher income countries. Although there are medications available, their effectiveness is unclear.
Objectives
To assess the effects of different antifungal drugs in the management of fungal keratitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 2), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2015), EMBASE (January 1980 to March 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 16 March 2015.
Selection criteria
We included randomised controlled trials of medical therapy for fungal keratitis.
Data collection and analysis
Two review authors selected studies for inclusion in the review, assessed trials for risk of bias and extracted data. The primary outcome was clinical cure at two to three months. Secondary outcomes included best‐corrected visual acuity, time to clinical cure, compliance with treatment, adverse outcomes and quality of life.
Main results
We included 12 trials in this review; 10 trials were conducted in India, one in Bangladesh and one in Egypt. Seven of these trials were at high risk of bias in one or more domains, two of these studies were at low risk of bias in all domains. Participants were randomised to the following comparisons: topical 5% natamycin compared to topical 1% voriconazole; topical 5% natamycin compared to topical 2% econazole; topical 5% natamycin compared to topical chlorhexidine gluconate (0.05%, 0.1% and 0.2%); topical 1% voriconazole compared to intrastromal voriconazole 50 g/0.1 mL (both treatments combined with topical 5% natamycin); topical 1% voriconazole combined with oral voriconazole compared to both oral voriconazole and oral itraconazole (both combined with topical 5% natamycin); topical 1% itraconazole compared to topical 1% itraconazole combined with oral itraconazole; topical amphotericin B compared to topical amphotericin B combined with subconjunctival injection of fluconazole; intracameral injection of amphotericin B with conventional treatment compared to conventional treatment alone (severe fungal ulcers); topical 0.5% and 1% silver sulphadiazine compared to topical 1% miconazole. Overall the results were inconclusive because for most comparisons only one small trial was available. The exception was the comparison of topical natamycin and topical voriconazole for which three trials were available. In one of these trials clinical cure (healed ulcer) was reported in all 15 people allocated to natamycin and in 14/15 people allocated to voriconazole (risk ratio (RR) 1.07; 95% confidence interval (CI) 0.89 to 1.28, low quality evidence). In one trial people randomised to natamycin were more likely to have a microbiological cure at six days (RR 1.64; 95% CI 1.38 to 1.94, 299 participants). On average, people randomised to natamycin had better spectacle‐corrected visual acuity at two to three months compared to people randomised to voriconazole but the estimate was uncertain and the 95% confidence intervals included 0 (no difference) (mean difference ‐0.12 logMAR, 95% CI ‐0.31 to 0.06, 434 participants; 3 studies, low quality evidence) and a decreased risk of corneal perforation or therapeutic penetrating keratoplasty, or both (RR 0.61; 95% CI 0.40 to 0.94, 434 participants, high quality evidence). There was inconclusive evidence on time to clinical cure. Compliance with treatment and quality of life were not reported. One trial comparing natamycin and voriconazole found the effect of treatment greater in Fusarium species, but this subgroup analysis was not prespecified by this review.
Authors' conclusions
The trials included in this review were of variable quality and were generally underpowered. There is evidence that natamycin is more effective than voriconazole in the treatment of fungal ulcers. Future research should evaluate treatment effects according to fungus species.
Keywords: Humans; Antifungal Agents; Antifungal Agents/therapeutic use; Eye Infections, Fungal; Eye Infections, Fungal/drug therapy; Keratitis; Keratitis/drug therapy; Keratitis/microbiology; Natamycin; Natamycin/therapeutic use; Randomized Controlled Trials as Topic; Voriconazole; Voriconazole/therapeutic use
Plain language summary
Medical treatments for fungal infection of the cornea (clear front part of the eye)
Background and review question Fungal infection of the cornea occurs rarely in higher income countries but is relatively common in lower income countries. If left untreated the cornea may develop a hole and this may lead to blindness. Although there are a number of medications available, it is not clear which is the most effective and cost‐effective. Our review question was: which is the best treatment for fungal infection of the cornea (fungal keratitis)?
Study characteristics We identified 12 randomised controlled trials that included 981 people; the evidence is current up to March 2015. The trials were mainly conducted in India.
Key results and quality of the evidence The studies were small and many of them were at risk of bias. They also looked at different treatments. This meant that for most treatments we could not draw any conclusions as to which was better. There was one exception. Three trials (434 participants) compared topical natamycin and topical voriconazole. In these trials there was low quality evidence that people receiving topical natamycin were more likely to be cured and were more likely to have better vision three months after treatment started. There was high quality evidence that people receiving natamycin were less likely to develop a hole in the cornea and need a transplant. We did not find any evidence on quality of life. One trial found evidence that natamycin was particularly good when treating a particular type of fungal infection (Fusarium species).
Summary of findings
for the main comparison.
| Topical 5% natamycin compared with topical 1% voriconazole for fungal keratitis | ||||||
|
Patient or population: people with fungal ulcers Settings: hospital or community Intervention: topical 1% voriconazole Comparison: topical 5% natamycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Voriconazole | Natamycin | |||||
| Clnical cure at 2 to 3 months | 900 per 1000 | 963 (801 to 1000) | RR 1.07 (0.89 to 1.28) | 30 (1) | ⊕⊕⊝⊝1 low | MUTT 2010 reported on microbiological cure at 6 days. 132/155 (85.2%) people in the natamycin group were culture negative compared to 75/144 (52.1%) in the voriconazole group (RR 1.64, 95% CI 1.38 to 1.94) |
|
Best corrected visual acuity at 2 to 3 months (measured using logMAR scale. A score of 0 = good vision, higher score is worse vision) |
The mean visual acuity ranged across control groups from 0.39 to 1.37 logMAR units | The mean visual acuity in the intervention groups was 0.12 logMAR better, (0.06 worse to 0.31 better) | 434 (3) | ⊕⊕⊝⊝2 low | ||
| Time to clinical cure | Arora 2011 reported that the average time of complete resolution of corneal infiltrate in 15 patients allocated to natamycin was 24.3 days and in 14 patients (with healed ulcer) allocated to voriconazole was 27.4 days. MUTT 2010 reported a hazard ratio for re‐epithelialisation that was higher with natamycin but confidence intervals compatible with no difference (hazard ratio 1.25, 95% CI 0.95 to 1.65). Prajna 2010 reported time to re‐epithelialisation with a hazard ratio 0.95 (95% CI 0.88 to 1.15). | |||||
| Compliance with treatment | Not reported | |||||
| Corneal perforation or penetrating keratoplasty, or both, at 2 to 3 months | 200 per 1000 | 122 per 1000 (80 to 188) | RR 0.61 (0.40 to 0.94) | 434 (3) | ⊕⊕⊕⊕ high | |
| Quality of life | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for risk of bias (‐1) and imprecision (‐1)
2 Downgraded for imprecision (‐1) and inconsistency (‐1)
Background
Description of the condition
Fungal infections can involve different parts of the eye and periocular tissues including the lacrimal apparatus, conjunctiva, eyelids and bony orbit. The most common sites for fungal infections of the eye involve the cornea and the retina or vitreous (O' Brien 1997). In the past few decades there have been increased reports of fungal infections of the eye (O' Day 1996). These can be mainly attributed to increased clinical awareness and improved laboratory techniques but may also have been caused by widespread use of corticosteroids, antibiotics, immunosuppressants, chemotherapeutic drugs and ocular prosthetic devices (O' Brien 1997).
Epidemiology
Fungal keratitis or keratomycosis is relatively uncommon in developed countries. There have been no high quality published reports on the incidence rates of the disease. In the United States, it has been reported that the total number of fungal keratitis cases annually is approximately 1500 (O' Day 1996). It is, however, more common in agricultural and tropical countries. In South Florida, a nine year survey from 1968 to 1977 revealed that 133 out of 633 cases of corneal ulcers were fungal in origin (Liesegang 1980). In the Philippines, a 25 year survey on central microbial keratitis revealed a total of 430 cases (Valenton 2000). The most common aetiologic agents are Fusarium, Aspergillus fumigatus and Aspergillus flavus. In Hyderabad, India, a 10 year study on fungal keratitis showed 1352 culture proven cases; the most common aetiologic agents included Fusarium, Aspergillus, and Curvularia spp (Gopinathan 2002).
The most common predisposing factor in fungal keratitis is trauma associated with plant material. Other risk factors include long‐term corticosteroid use and immunocompromised patients (O' Day 1996).
Presentation and diagnosis
Fungal infections almost always present in an insidious manner. The infection may be recognised within days or weeks and it is not uncommon for the traumatised epithelium to heal completely before signs of infection appear. During this latent period the patient may be asymptomatic. However, within a few days or weeks the patient might complain of discomfort, photophobia and discharge.
During this period, a persistent infiltrate at the site of previous superficial trauma is present which may increase in size and density in time. The epithelium tends to heal over this inflammatory focus, although there may be recurrent episodes of epithelial breakdown. The cornea becomes slightly thickened and 'satellite' lesions may develop peripheral to the focal area of infiltration.
If not treated, the inflammatory signs gradually progress causing permanent breakdown of the epithelium, stromal ulceration, or formation of descemetocoele (corneal thinning). The cornea may eventually perforate. Neovascularisation may occur as a result of inflammation, which may lead to severe scarring of the cornea. Associated signs indicating the severity of inflammation include the presence of hypopyon (pus in the anterior chamber) and ciliary injection. Fungi can invade the deep stroma with great rapidity and may gain access to the anterior chamber.
It is important to determine the aetiologic agent of the corneal ulcer. Combined infections with bacteria and fungi or even with multiple fungi might occur. Diagnosis is usually achieved by scraping material from the base of the ulcer. Some of this material is stained for fungi and bacteria, the rest is cultured on solid and liquid media. In severe cases where diagnosis is unclear it may be necessary to take a larger corneal biopsy.
Description of the intervention
Management of fungal keratitis is mainly by antifungal agents. Keratoplasty or corneal transplant is usually reserved for acute management of corneal perforation and for visual rehabilitation following corneal scarring.
The number of antifungal agents available for therapy is few compared with the number of pathogens capable of infecting the eye (O' Brien 1997). Current antifungal agents are divided into four groups: polyenes, imidazoles, triazoles and fluorinated pyrimidines. These drugs can be administered topically, intravenously or orally. Topical antifungals can cause toxicity such as punctate keratitis, chemosis recurrent corneal epithelial erosions and conjunctival injection. Subconjunctival injections are quite painful and ulceration and necrosis of the conjunctival epithelium may occur.
Current practice in the treatment of fungal keratitis involves the use of topical antifungal drops such as natamycin and topical amphotericin B. Newly discovered triazoles such as voriconazole and posaconazole are also being studied as treatment for fungal keratitis (Galarreta 2007; Tu 2007). In developing countries, where the incidence of fungal keratitis is higher, the costs and availability of these polyene drops may be an issue. Hence, various studies have been performed to validate the effectiveness of chlorhexidine drops as an inexpensive alternative to the treatment of fungal keratitis (Martin 1996). Combination therapy using several antifungal drugs has been studied. The concomitant use of corticosteroids and antifungal agents remains controversial (O' Brien 1997).
In India, due to unavailability and high price of antifungal drugs, different antiseptic agents were studied in vitro and revealed a good dose response for chlorhexidine gluconate while povidone iodine showed a good response in all concentrations (Martin 1996). This initial study was then followed by a randomised controlled trial (RCT) to further determine the clinical effectiveness of chlorhexidine in confirmed fungal keratitis patients (Rahman 1997).
How the intervention might work
Antifungal medications such as the polyenes work by binding to the ergosterol in the cell membrane of the fungal organism. Likewise, imidazoles affect the plasma membrane formation by affecting the ergosterol through microsomal P‐450 enzyme. Pyrimidines are transformed to fluorouracil in the cell, therefore blocking thymidine synthesis (Mabon 1998).
Why it is important to do this review
The gold standard for treatment of fungal keratitis has not been identified. Due to the low incidence of the disease it is difficult to perform large trials, especially in developed countries. A systematic review of available trials will, therefore, contribute to the evidence base.
Objectives
To assess the effects of different antifungal drugs in the management of fungal keratitis.
Methods
Criteria for considering studies for this review
Types of studies
We considered only RCTs in this review.
Types of participants
We included trials where the participants had fungal keratitis diagnosed clinically or microbiologically. We also included trials which included both people with or without corneal perforation, if separate data were available for those without perforation. We excluded studies of participants with mixed bacterial and fungal infections.
Types of interventions
We considered studies using any antifungal drug in the management of fungal keratitis. This included placebo controlled trials or trials comparing one antifungal agent against another. We also considered trials comparing antifungal drugs with superficial keratectomy.
Types of outcome measures
Primary outcomes
Clinical cure: as defined by study investigators at two to three months.
Secondary outcomes
Best‐corrected visual acuity at two to three months.
Time to clinical cure.
Compliance with treatment.
Adverse outcomes, including: corneal thinning or descemetocoele formation, corneal perforation, endophthalmitis, chemosis, punctate keratopathy, recurrent epithelial erosions, conjunctival injections, ulceration and necrosis of conjunctiva, hepatotoxicity and renal toxicity.
Quality of life.
Follow‐up
We included trials with at least two months follow‐up.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 2), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to March 2015), EMBASE (January 1980 to March 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 16 March 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of identified trial reports to find additional trials. We contacted investigators and pharmaceutical companies to identify additional published, unpublished and ongoing studies. We searched conference abstracts for additional studies but journals were not handsearched.
Data collection and analysis
Selection of studies
Titles and abstracts resulting from the searches were assessed independently by both review authors against the inclusion criteria for the review. We obtained full copies of the studies that definitely or possibly met the inclusion criteria for further assessment on whether the paper should be excluded or included. We contacted trialists for further information as needed in order to determine the relevance of the study.
Data extraction and management
Both review authors extracted details about the methods, participants, interventions, outcomes measured and other details of the included studies and transferred them to the 'Characteristics of included studies' table in Review Manager (RevMan) (RevMan 2014). One review author extracted data using the form developed by the Cochrane Eyes and Vision Group. A second author compared the extraction to the original reports. If data were missing or difficult to determine from a paper, the trialists were approached for clarification and verification. Data were entered into RevMan by one review author, and the second author checked for errors.
Assessment of risk of bias in included studies
Assessment of the risk of bias of studies was undertaken in accordance with the methods given in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). Both review authors independently assessed the studies and any disagreements were resolved by discussion. The following bias domains were considered: selection bias, performance bias, detection bias, attrition bias, selective outcome reporting. Assessment was based on the following:
Selection bias (random sequence generation and allocation concealment): was the sequence of allocation of participants to groups randomly generated and concealed until after treatments were allocated?
Performance bias (masking of participants and researchers): were the recipients of care unaware of their assigned treatment? Were persons providing care unaware of the assigned treatment?
Detection bias: were persons assessing outcome unaware of the assigned treatment?
Attrition bias: were rates of follow up similar in the comparison groups? Was the analysis 'intention‐to‐treat' (were all participants analysed as randomised)?
Selective outcome reporting: were all outcomes reported?
We assessed each parameter as 'low risk of bias', 'high risk of bias' or 'unclear'. We contacted trialists for clarification of any parameter graded as unclear.
Measures of treatment effect
We calculated the risk ratio for dichotomous outcomes and mean difference for continuous outcomes
Unit of analysis issues
All the included studies were parallel group trials. People were randomised to treatment. In most studies the number of eyes included in the study was not clearly described but often fungal keratitis is unilateral and it is likely that one eye per person was included.
Dealing with missing data
Where possible, we did an intention‐to‐treat (ITT) analysis, using imputed data if computed by the trial investigators using an appropriate method. We did not impute missing data ourselves.
For most studies, ITT data were not available and we did an available case analysis. This assumes that data are missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow up and reasons for loss to follow up by treatment group, if reported.
Assessment of heterogeneity
We examined the overall characteristics of the studies, in particular the type of participants and types of interventions, to assess the extent to which the studies were similar enough to make pooling study results sensible.
We looked at the forest plots of study results to see how consistent the results of the studies are, in particular looking at the size and direction of effects.
We calculated I2 which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2003). We considered I2 values over 50% to indicate substantial inconsistency but also considered Chi2 P value. As this may have low power when the number of studies are few we considered P < 0.1 to indicate statistical significance of the Chi2 test.
Assessment of reporting biases
In future updates, if there are 10 trials or more included in a meta‐analysis, we will construct funnel plots and consider tests for asymmetry for assessment of publication bias.
Data synthesis
We pooled data using a fixed‐effect model where we had three or less trials and there was no evidence of substantial heterogeneity. For one analysis (Analysis 1.1) we had three trials but there was inconsistency in the results of these trials. We present both fixed‐ and random‐effects models for this analysis and report the random‐effects model in the abstract and summary of findings table. In future updates, if we have more than three trials contributing to an analysis we will use a random‐effects model.
1.1. Analysis.
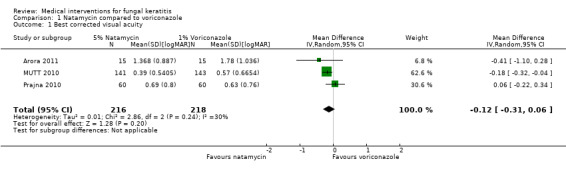
Comparison 1 Natamycin compared to voriconazole, Outcome 1 Best corrected visual acuity.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses. One trial included in this review noted a difference in effect according to species of fungal infection. In future updates of this review, we will conduct subgroup analyses according to type of fungal infection, if possible.
Sensitivity analysis
We did not conduct sensitivity analysis as we had few trials contributing to each meta‐analysis. If possible we will do so for future updates so that we can assess how robust the review results are to key decisions and assumptions that were made during the review. Analysis of data will be repeated with the following adjustments:
exclusion of studies at high risk of bias in one or more domains.
exclusion of unpublished studies
Summary of findings table
We prepared a summary of findings table presenting relative and absolute risks. One author (JE) graded the overall quality of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/). The other author checked the grading. We included the following outcomes in the summary of findings table but note that these were not specified a priori because the summary of findings table was included in the current (2015) update only.
Clinical cure at 2 to 3 months
Time to clinical cure
Best corrected visual acuity at 2 to 3 months
Corneal perforation or penetrating keratoplasty, or both, at 2 to 3 months
Compliance with treatment
Quality of life
The protocol for this review was originally published in 2003 (FlorCruz 2003). The methods have been updated at each update ‐ see Differences between protocol and review for details.
Results
Description of studies
Results of the search
The electronic searches resulted in 471 reports of possible medical interventions for fungal keratitis. Twenty three abstracts were retrieved in full for further assessment. Six RCTs were identified for inclusion (Agarwal 2001; Mohan 1987; Mohan 1988; Prajna 2003; Rahman 1997; Rahman 1998).
Contact with first authors of identified trials and searching the reference lists of these studies failed to identify any additional trials. We also approached pharmaceutical companies producing antifungal agents but there was no information on additional trials.
Update searches were done in January 2007 and Februrary 2010. The searches yielded a total of 206 and 23 references, respectively. The Trials Search Co‐ordinator (TSC) scanned the search results for both updates and removed any references which were not relevant to the scope of the review. These searches did not identify any references which met the inclusion criteria for the review.
A further update search was done in August 2011. After deduplication the search identified a total of 50 references. The TSC scanned the search results and removed 41 references which were not relevant to the scope of the review. We reviewed the remaining nine references of which five were published reports of studies and four were reports of ongoing studies. We assessed the five published reports of studies for potential inclusion in the review. We obtained full‐text copies of three studies and have included them in the review (Arora 2011; Mahdy 2010; Prajna 2010). The remaining two reports did not meet the inclusion criteria. Of the four reports of ongoing studies, trial NCT00557362 is the initial report of the published paper by Prajna 2010. The three other reports of ongoing studies are relevant to the review and have been added to the studies awaiting assessment section. The results of these will be included in the review when the studies have been completed (NCT00996736; MUTT II; NCT00516399).
An update search in March 2015 yielded a total of 249 references (Figure 1). The Trials Search Co‐ordinator scanned the search results, removed 70 duplicates and then removed 107 references which were not relevant to the scope of the review. We screened the remaining 72 reports and discarded 57 reports as not relevant. We obtained 15 full‐text reports for potential inclusion in the review, we included eight reports of four studies (Basak 2004; MUTT 2010; Parchand 2012; Sharma 2013) and excluded five studies (Chen 2013; Gupta 2006; Li 2011; Oude Lashof 2011; Shuai 2012). A study by Qu 2013 requires translation and will be assessed for inclusion when we have translated the report.
1.
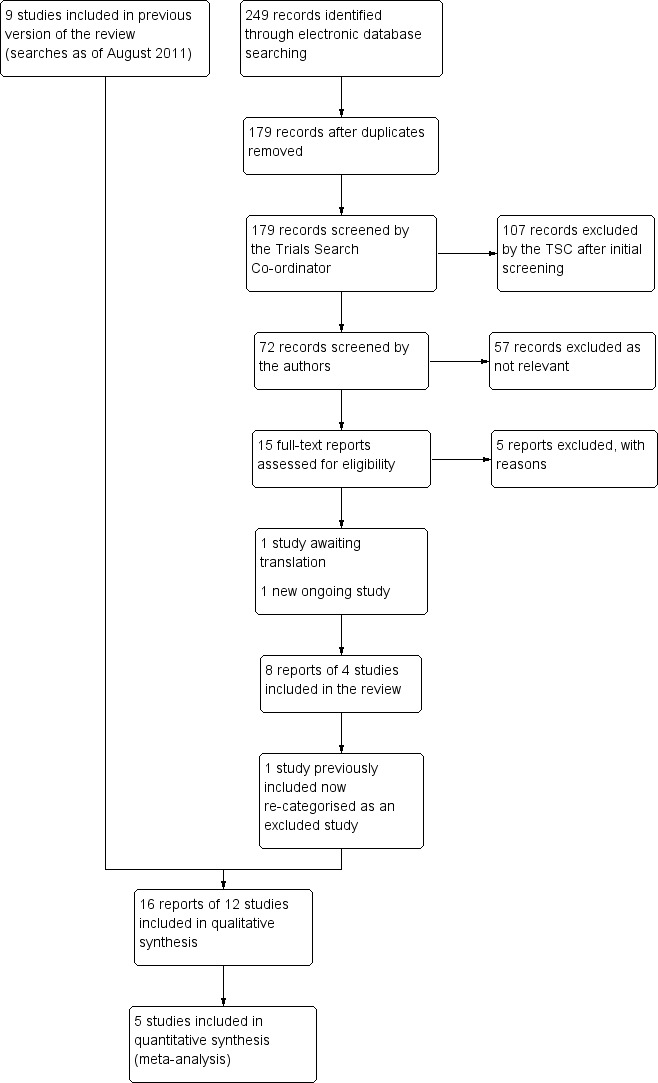
Results of searching for studies for inclusion in the review
The previously included study by Mohan 1988 has been reassessed during this update and has been deemed as not meeting the inclusion criteria so has now been re‐categorized as an excluded study. We have identified one new ongoing trial CTR 2011 091 000107 and will assess it when data become available. In the previous version of this review we had identified three potentially relevant ongoing studies, one is still awaiting data (MUTT II), one has been excluded NCT00516399 and one has been included (MUTT 2010).
Included studies
See the 'Characteristics of included studies' table for additional details for included studies.
Size of studies
The 12 included trials randomised a total of 981 participants: Agarwal 2001 (54 participants); Arora 2011 (30); Basak 2004 (45); Mahdy 2010 (48); Mohan 1987 (30); MUTT 2010 (323); Prajna 2003 (116); Prajna 2010 (120); Rahman 1997 (60); Rahman 1998 (70); Sharma 2013 (40).
Types of participants
Ten of the trials were conducted in India with one trial conducted in Bangladesh (Rahman 1998) and one trial in Egypt (Mahdy 2010). Trials included people with a wide range of ages, from seven to 84 years of age, although in general the patient populations were younger rather than older, with average ages between 33 and 47 years. The majority of the participants were male; the percentage male ranged from 57% to 77% in the included trials (median 69%).
The majority of the trials included participants with microbiological evidence of fungal keratitis. Two trials (Agarwal 2001; Mahdy 2010) included participants based on a clinical definition only.
Types of interventions
Table 2 summarises the antifungals studied. The trials were heterogeneous in terms of types of antifungals studied. Nine antifungal drugs in different preparations and routes of administration were used. Agarwal 2001 compared topical and systemic itraconazole versus topical itraconazole. Mohan 1987 compared 0.5% and 1% silver sulphadiazine in ointment form to 1% miconazole ointment. Prajna 2003 compared 2% econazole and 5% natamycin in topical preparations. Rahman 1997 compared different concentrations of chlorhexidine gluconate versus 5% natamycin while Rahman 1998 compared 0.2% chlorhexidine gluconate versus 2.5% natamycin. Three trials (Arora 2011; MUTT 2010; Prajna 2010) compared topical voriconazole 1% with natamycin 5%. Parchand 2012 compared oral and topical voriconazole, oral voriconazole and topical natamycin and oral itraconazole and topical natamycin. Mahdy 2010 compared amphotericin B combined with subconjunctival injection of fluconazole with amphotericin B alone. Basak 2004 compared amphotericin B injection plus conventional medication with conventional medication alone. Sharma 2013 compared 1% topical voriconazole with 50 μg/0.1 mL intrastromal voriconazole pretreated with recalcitrant to 5% topical natamycin.
1. Anti‐fungal agents studied in the included trials.
| Study | Intervention | Dose | Treatment duration | Intervention | Dose | Treatment duration | |||
| Agarwal 2001 | Topical itraconazole | 1% every hour | For 6 weeks after keratitis resolved | Oral itraconazole Topical itraconazole |
100 mg twice daily 1% every hour |
3 weeks For 6 weeks after keratitis resolved |
|||
| Arora 2011 | Topical natamycin | 5% every hour | Two weeks "Further dosage titrated according to the patient's response" |
Topical voriconazole | 1% every hour | Two weeks "Further dosage titrated according to the patient's response" |
|||
| Basak 2004 | Intracameral amphotericin B combined with conventional medication as given to control group | 5 to 15 μg | Depending upon the size of the ulcer and amount of hypopyon the injection was repeated after 7 days as indicated Complications were treated medically, surgically, or both |
Conventional medication: (1) oral fluconazole (2) topical natamycin (3) topical amphotericin B (4) broad‐spectrum topical antibiotic (5) topical antiglaucoma medication (6) topical cycloplegics |
(1) 150 to 200 mg (2) 5% every hour (3) 0.15% every hour (4) every 2 hours |
(1) twice a day for 3 weeks | |||
| Mahdy 2010 | Topical amphotericin B Subconjunctival injection of fluconazole |
0.05% every 2 hours 0.5 mL of 2 mg/mL daily |
N/A 20 injections, first 10 every day, second 10 every 2 days |
Topical amphotericin B | 0.05% every 2 hours | N/A | |||
| Mohan 1987 | Topical silver sulphadiazine | 2 doses studied: 0.5% and 1%, applied 5 times a day | N/A | Topical miconazole | 1% applied 5 times a day | N/A | |||
| MUTT 2010 | Topical natamycin 5% | 1 drop was applied to the affected eye every hour, while awake, for 1 week, then every 2 hours while awake until 3 weeks from enrolment | 3 weeks | Topical voriconazole 1% | 1 drop was applied to the affected eye every hour, while awake, for 1 week, then every 2 hours while awake until 3 weeks from enrolment | 3 weeks | |||
| Parchand 2012 | Oral and topical voriconazole 1% | Oral voriconazole was given in tablet form 400 mg twice a day on day 1 followed by 200 mg twice a day and continued until the resolution of the infiltrates. Topical voriconazole was given every hour, while awake, for 1 week, then every 2 hours while awake until healing of the epithelial defect and then gradual tapering off. | Until healed | Oral voriconazole and topical natamycin 5% | Oral voriconazole was given in tablet form 400 mg twice a day on day 1 followed by 200 mg twice a day and continued until the resolution of the infiltrates. Topical natamycin was given every hour, while awake, for 1 week, then every 2 hours while awake until healing of the epithelial defect and then gradual tapering off. | Until healed | |||
| Prajna 2003 | Topical natamycin | 5% every hour between 7am and 9pm | 4 weeks | Topical econazole | 2% every hour between 7am and 9pm | 4 weeks | |||
| Prajna 2010* | Topical natamycin | 5% every hour while awake | Every hour for 1 week followed by every 2 hours for 2 weeks, further continuation at discretion of physician | Topical voriconazole | 1% every hour while awake | Every hour for 1 week followed by every 2 hours for 2 weeks, further continuation at discretion of physician | |||
| Rahman 1997 | Topical natamycin | 5% | Day 1: Half‐hourly for 3 hours, hourly during waking hours for rest of day. Days 2 to 5: 2‐hourly, then 3‐hourly for a further 2 weeks. If no improvement at 5 days swapped to another treatment | Topical chlorhexidine gluconate | Three doses studied: 0.05%, 0.1% and 0.2% | Day 1: Half‐hourly for 3 hours, hourly during waking hours for rest of day. Days 2 to 5: 2‐hourly, then 3‐hourly for a further 2 weeks. If no improvement at 5 days swapped to another treatment | |||
| Rahman 1998 | Topical natamycin | 2.5% | Half‐hourly for first 3 hours, then 1‐hourly for 2 days, 2‐hourly for 5 days, and 3‐hourly for 3 weeks. If no improvement at 5 days treatment changed | Topical chlorhexidine gluconate | 0.2% | Half‐hourly for first 3 hours, then 1‐hourly for 2 days, 2‐hourly for 5 days, and 3‐hourly for 3 weeks. If no improvement at 5 days treatment changed | |||
| Sharma 2013 | Topical voriconazole as an adjunct to natamycin | 1% | Hourly for the initial 48 hours, then were tapered to every 2 hours while awake for 72 hours and thereafter the dosage was every 4 hours. Further tapering of the drug depended on the response of the infection to treatment and as per the clinician’s judgment. |
Instrastromal voriconazole as an adjunct to natamycin | 0.5 mg/mL voriconazole was injected obliquely into the cornea. 5 divided doses were given around the ulcer to form a deposit of the drug around the circumference of the lesion. At least 3 injections were given 72 hours apart. |
Both groups received topical 5% natamycin eye drops every 4 hours, 0.3% ciprofloxacin hydrochloride eye drops 4 times daily, and 2% homatropine eye drops 3 times daily. |
* Participants were also randomised to "scraping of the corneal epithelium"
Types of outcome measures
The majority of trials considered healing of ulcer, or time taken for ulcer to heal, as the primary outcome. MUTT 2010; Prajna 2010 and Sharma 2013 specified visual acuity as the primary outcome. Follow‐up varied: Rahman 1997 and Rahman 1998 considered healing of ulcer at three weeks; Mohan 1987 and Prajna 2003 considered healing at four weeks; Sharma 2013 did not specify a cut‐off time but noted healing of ulcers within two to four weeks; Agarwal 2001 considered healing of ulcer at six weeks as primary outcome; Arora 2011 followed up for a minimum of 10 weeks, or until the ulcer healed; Mahdy 2010; MUTT 2010; Parchand 2012 and Prajna 2010 followed up at three months. Parchand 2012 recorded time to disappearance of the hypopyon, resolution of the infiltrate and closure of the epithelial defect in days, as well as final logMAR visual acuity and adverse effects such as cataract, perforation, glaucoma, endophthalmitis and phthisis bulbi.
Excluded studies
See the 'Characteristics of excluded studies' table for details.
Risk of bias in included studies
2.
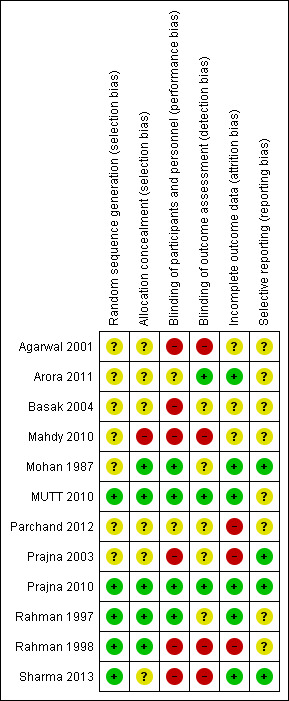
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
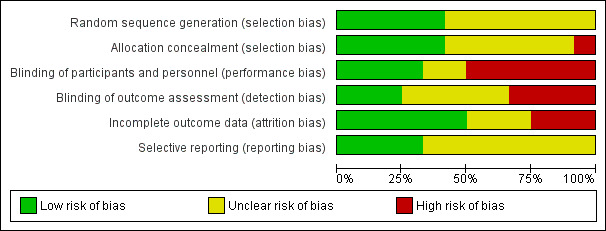
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Four trials reported adequate methods of sequence generation and allocation concealment (MUTT 2010; Prajna 2010; Rahman 1997; Rahman 1998). Sharma 2013 reported adequate sequence generation but did not elaborate on the allocation concealment.
Blinding
Masking of participants was not always possible. Only MUTT 2010 and Prajna 2010 reported adequate masking of participants, personnel and outcome assessment.
Incomplete outcome data
Arora 2011; MUTT 2010; Mohan 1987; Prajna 2010; Rahman 1997 and Sharma 2013 had reasonably complete data. In the other studies, attrition bias was considered to be possible.
Selective reporting
Selective reporting was not considered to be a major problem in the included trials but it was not always possible to assess this adequately.
Other potential sources of bias
Agarwal 2001 stated it was a cross‐over trial but it was not clear from the report that it actually was; Mohan 1987 randomly allocated participants to another treatment if they had not responded by one week.
Effects of interventions
See: Table 1
Natamycin
1. Topical 5% natamycin versus topical 1% voriconazole
Clinical cure
Arora 2011 reported on clinical cure at eight weeks. In the natamycin group clinical cure (healed ulcer) was reported in all 15 people allocated to natamycin and in 14/15 people allocated to voriconazole (RR 1.07; 95% CI 0.89 to 1.28). MUTT 2010 did not report on clinical cure but did report on microbiological cure at six days. In participants randomised to natamycin 132/155 (85.2%) were culture negative compared to 75/144 (52.1%) people in the voriconazole group (RR 1.64; 95% CI 1.38 to 1.94).
Time to clinical cure
Arora 2011 reported that the average time of complete resolution of corneal infiltrate in 15 participants allocated to natamycin was 24.3 days, and in 14 participants (with healed ulcer) allocated to voriconazole was 27.4 days. MUTT 2010 reported a hazard ratio for re‐epithelialisation that was higher with natamycin (hazard ratio 1.25, 95% CI, 0.95 to 1.65) but confidence intervals compatible with no difference. Prajna 2010 reported time to re‐epithelialisation with a hazard ratio 0.95 (95% CI, 0.88 to 1.15; P = 0.61).
Best‐corrected visual acuity
In Arora 2011 the best‐corrected (logMAR) visual acuity at last follow‐up was 1.37 (SD 0.88) in the natamycin group (N = 15) and 1.78 (SD 1.04) in the voriconazole group (N = 15) (MD ‐0.41; 95% CI ‐1.10 to 0.28 in favour of natamycin). MUTT 2010 reported best spectacle‐corrected visual acuity at three months. Participants treated with natamycin had a mean logMAR acuity of 0.39 (SD 0.53, 141 participants) compared to a mean of 0.57 logMAR (SD 0.66, 143 participants) in the voriconazole group (mean difference (MD) ‐0.18; 95% CI ‐0.32 to ‐0.04 in favour of natamycin). Prajna 2010 found that in people treated with natamycin the mean best spectacle‐corrected logMAR acuity at three months was 0.69 (SD 0.80) (N = 60) and for the voriconazole group the mean logMAR acuity was 0.63 (SD 0.76) (N = 60) (MD 0.06; 95% CI ‐0.22 to 0.34, in favour of voriconazole).
Using a fixed‐effect model, the pooled estimate of effect was in favour of natamycin (MD ‐0.14 logMAR, 95% CI ‐0.26 to ‐0.02; participants = 434; studies = 3; I2 = 30%). In our protocol for this review (FlorCruz 2003) we planned to use a fixed‐effect model "..if the total number of trials in the comparison is three or less provided that heterogeneity has not been detected either statistically or by review.". For this reason we report preferentially the random‐effects model, which is more conservative, as the estimates of effect were in different directions. (MD ‐0.12 logMAR, 95% CI ‐0.31 to 0.06). (Analysis 1.1, Figure 4).
4.
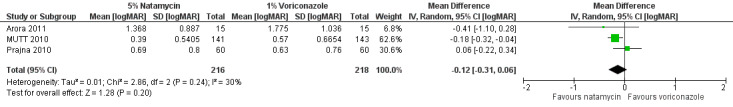
Forest plot of comparison: 1 Topical natamycin compared to topical voriconazole, outcome: 1.1 Best corrected visual acuity [logMAR].
Compliance with treatment
Not reported.
Adverse outcomes
MUTT 2010 found 18/141 (12.8%) people randomised to natamycin had corneal perforations or therapeutic penetrating keratoplasty, or both, compared to 34/143 (23.8%) in people given voriconazole (RR 0.54; 95% CI 0.32 to 0.90). One (out of 15) participants in the voriconazole group in Arora 2011 experienced a perforation and required therapeutic penetrating keratoplasty. None of the 15 participants in the natamycin groups required keratoplasty. In Prajna 2010 there were nine corneal perforations in the natamycin group and 10 in the voriconazole group (RR 0.90; 95% CI 0.39 to 2.06). The results of these studies were homogeneous (I2 = 0%) and the pooled risk ratio suggested a 39% relative risk reduction in favour of natamycin (RR 0.61; 95% CI 0.40 to 0.94) (Analysis 1.2, Figure 5).
1.2. Analysis.
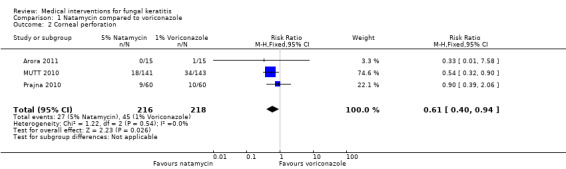
Comparison 1 Natamycin compared to voriconazole, Outcome 2 Corneal perforation.
5.
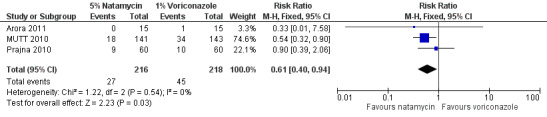
Forest plot of comparison: 1 Topical 5% natamycin versus topical 1% voriconazole, outcome: 1.2 Corneal perforation.
Two participants in Arora 2011 developed cataract but it was not clear which group these participants were in.
No systematic adverse effects were recorded in Prajna 2010.
No adverse reactions to study medications were noted in Arora 2011.
Quality of life
Not reported.
Subgroup analysis
MUTT 2010 did a subgroup analysis on the basis of type of fungal infection. The effect of natamycin versus voriconazole was different in the people infected withFusarium species compared to those infected with non‐Fusarium species. This subgroup analysis was not prespecified in this review and it was not clear if it was prespecified in the MUTT trial.
| Outcome measures | Natamycin versus voriconazole in people infected with Fusarium species | Natamycin versus voriconazole in people infected with non‐Fusarium species. |
| Effect estimate (95% CI) | Effect estimate (95% CI) | |
| Microbiological cure at 6 days | RR: 2.29 (1.67 to 3.15) | RR 1.33 (1.10 to 1.63) |
| Time to re‐epithelialization | HR: 1.89 (1.21 to 2.93) | HR: 1.00 (0.70 to 1.42) |
| Best spectacle‐corrected visual acuity | RC: 0.41 logMAR (0.61 to 0.20) | RC: 0.02 logMAR (‐0.17 to 0.13) |
| Perforation | OR: 0.06 (0.01 to 0.28) | OR 1.08 (0.48 to 2.43) |
RR: Risk ratio; HR: Hazard ratio; RC: Regression coefficient; OR: Odds ratio.
2. Topical 5% natamycin versus topical 2% econazole
Clinical cure
Prajna 2003 found that similar proportions of people comparing natamycin versus topical econazole had clinical cure (RR 1.05; 95% CI 0.81 to 1.35). Follow‐up was at four weeks.
There was no significant difference (log rank 0.52, P = 0.47) between the two arms for success which was defined as a healed or healing ulcer at four weeks.
Time to clinical cure
Data were not reported in a form that enabled extraction. The following quote is from the paper"There was no significant difference in the time to heal based on baseline size of epithelial defects (log rank 0.82, p=0.37), size of infiltrate (log rank 0.86, p=0.35) or depth of infiltrate (log rank 0.74, p=0.39) between the two arms of the study. There was no difference in the time to subside for signs including lid oedema (log rank 1.05, p=0.31), congestion of the conjunctiva (log rank 0.51, p=0.47) or hypopyon (log rank 0.23, p=0.63) between the two arms."
Best‐corrected visual acuity
Not reported.
Compliance with treatment
Not reported.
Adverse outcomes
Prajna 2003"Exit criteria from the study were determined as a clinical worsening of the ulcer—if the size and depth of the infiltrate had increased by at least 20% with respect to the previous visit or perforation—or adverse reactions to the drops." In the natamycin group 34/61 (55.7%) exited the study compared to 30/55 (54.5%) of the econazole group (RR 1.02; 95% CI 0.74 to 1.42).
Prajna 2003 did not elaborate on the ocular and systemic adverse reactions due to natamycin or econazole.
Quality of life
Not reported.
3. Topical 5% natamycin versus topical chlorhexidine gluconate (0.05%, 0.1% and 0.2%)
Clinical cure
In two trials by the same investigators (Rahman 1997; Rahman 1998) fewer cases of clinical cure at 21 days were observed in people treated with natamycin compared to chlorhexidine gluconate at various concentrations. However, the overall estimate of effect was uncertain (RR 0.70, 95% CI 0.45 to 1.09; participants = 110; studies = 2; I2 = 0%) (Analysis 2.1, Figure 6).
2.1. Analysis.
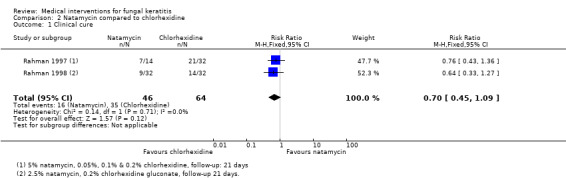
Comparison 2 Natamycin compared to chlorhexidine, Outcome 1 Clinical cure.
6.
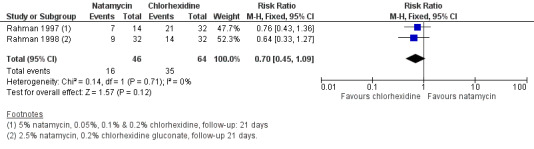
Forest plot of comparison: 2 Natamycin versus chlorhexidine, outcome: 2.1 Clinical cure.
Time to clinical cure
Not reported.
Best‐corrected visual acuity
Not reported.
Compliance with treatment
Not reported.
Adverse outcomes
There was no report of significant systemic or ocular adverse reactions from chlorhexidine gluconate or natamycin. A case of temporary punctate epitheliopathy was observed in one participant receiving chlorhexidine gluconate. This was attributed to increased frequency of application of the drops. No early cataract formation was observed at six months to one year after treatment for participants exposed to chlorhexidine gluconate or natamycin.
In Rahman 1998 1/36 (2.8%) participants allocated to natamycin had an enucleation compared to 3/35 (8.6%) participants allocated to chlorhexidine (RR 0.32; 95% CI 0.04 to 2.97). Six of 36 (16.7%) participants allocated to natamycin had a perforation or therapeutic penetrating keratoplasty, or both, compared to 0/35 participants allocated to chlorhexidine (RR 12.65; 95% CI 0.74 to 216.4). However, 3/36 (8.3%) participants in the natamycin were lost to follow‐up compared to 13/35 (37.1%) in the chlorhexidine group.
Quality of life
Not reported.
Voriconazole
See comparison with natamycin above (comparison 1).
4. Topical 1% voriconazole versus intrastromal voriconazole 50 g/0.1 mL (both treatments combined with topical 5% natamycin)
Clinical cure
In Sharma 2013 treatment was successful in 19/20 (95%) people receiving topical voriconazole compared to 16/20 (80%) people receiving intrastromal voriconazole (RR 1.19; 95% CI 0.93 to 1.51).
Time to clinical cure
The mean duration for healing in the 20 participants allocated to the topical group was 28.9 (SD 19.1) days compared to 36.1 (SD 20.2) days in the 20 participants in the intrastromal group (MD ‐7.20; 95% CI ‐19.38 to 4.98).
Best‐corrected visual acuity
Visual acuity at three months was improved in 15/20 (75%) of the topical group compared to 10/20 (50%) of the intrastromal group (RR 1.50; 95% CI 0.90 to 2.49). The mean visual acuity after treatment was 1.295 (SD 0.50) logMAR units in the topical group and 1.692 (SD 0.29) logMAR units in the intrastromal group (MD ‐0.40; 95% CI ‐0.65 to ‐0.14).
Compliance with treatment
Not reported.
Adverse outcomes
Corneal perforation was observed in 1/20 people in the topical group compared to 4/20 people in the intrastromal group (RR 0.25; 95% CI 0.03 to 2.05).
Quality of life
Not reported.
5. Oral and topical 1% voriconazole versus oral voriconazole and topical 5% natamycin versus oral itraconazole and topical 5% natamycin
Clinical cure
In Parchand 2012 at three months, treatment success was observed in 10/15 (66.7%) participants allocated to topical and oral voriconazole compared to 11/15 (73.3%) people receiving oral voriconazole and topical natamycin and 10/15 (66.7%) in the itraconazole and natamycin group.
Time to clinical cure
The mean time for disappearance of the hypopyon was 9.8 (SD 1.7), 12.3 (SD 3.6), and 16.0 (SD 10.5) days in the three groups (P = 0.231). The mean time of resolution of infiltrates was 36.8 (SD 10.66), 38.81 (SD 8.94), and 36.7 (SD 10.42) days (P = 0.860). The mean time of closure of epithelial defect was 31.1 (SD 11.4), 29.18 (SD 8.25), and 31.8 (SD 11.4) days (P = 0.837).
Best‐corrected visual acuity
Final logMAR visual acuity was 1.7 (SD 0.9) in participants in the topical and oral voriconazole group, 1.5 (SD 0.8) in the oral voriconazole and topical natamycin group and 1.2 (SD 0.6) in the itraconazole and natamycin group.
Compliance with treatment
Not reported.
Adverse outcomes
| Outcomes | Topical and oral voriconazole (N = 15) | Voriconazole and natamycin (N = 15) | Itraconazole and natamycin (N = 15) |
| Cataract | 2 | 2 | 1 |
| Perforation | 5 | 4 | 5 |
| Glaucoma | 1 | 0 | 1 |
| Endophthalmitis | 0 | 1 | 0 |
| Phthisis bulbi | 0 | 1 | 0 |
| Corneal opacity | 9 | 11 | 9 |
Quality of life
Not reported.
Itraconazole
See comparison with voriconazole and natamycin above (comparison 5).
6. Topical itraconazole versus topical and oral itraconazole
Clinical cure
Topical itraconazole was compared to topical and oral itraconazole (Agarwal 2001). Overall, 42/54 (78%) of the participants in the study "responded favourably" to treatment but the comparison between topical and topical and oral groups was not clearly presented making it difficult to draw conclusions as to comparative efficacy.
Time to clinical cure
Not reported.
Best‐corrected visual acuity
Not reported.
Compliance with treatment
Not reported.
Adverse outcomes
Mild adverse effects were noted in topical itraconazole, which included: corneal oedema in two cases; increased intraocular pressure in two cases; and prolonged congestion in four cases. No significant adverse effects were reported in participants with oral itraconazole.
Quality of life
Not reported.
Amphotericin B
7. Topical amphotericin B versus topical amphotericin B and subconjunctival injection of fluconazole
Clinical cure
Mahdy 2010 found a higher proportion of ulcers healed with combination treatment (amphotericin B and fluconazole) 20/24 (83%) compared to amphotericin alone 16/24 (67%). However, as the study was small there remains uncertainty as to the relative effect of these two interventions (RR 1.25; 95% CI 0.89 to 1.75).
Time to clinical cure
Mean duration of healing was 31 (SD 3) days in the combination group compared to 37 days (SD 2) in the monotherapy groups.
Best‐corrected visual acuity
Mean best‐corrected visual acuity was 0.23 in the combination group compared to 0.25 in the monotherapy group. This is presumably a decimal visual acuity and was reported for the healed cases only.
Compliance with treatment
Not reported.
Adverse outcomes
| Outcomes | Amphotericin B and fluconazole (N = 24) | Amphotericin B (N = 24) |
| Corneal perforation | 2 | 2 |
| Endophthalmitis | 1 | 0 |
| Penetrating keratoplasty | 1 | 0 |
| Conjunctival necrosis | 0 | 0 |
| Subconjunctival haemorrhage | 0 | 0 |
Quality of life
Not reported.
8. Intracameral injection of amphotericin B with conventional treatment (combination treatment) versus conventional treatment for severe fungal ulcers
Clinical cure
Basak 2004 reported that the ulcers healed in 18/23 (78.3%) people in the combination treatment group compared to 12/22 (54.5%) people in conventional treatment group at eight weeks (RR 1.43; 95% CI 0.93 to 2.22).
Time to clinical cure
Not reported.
Best‐corrected visual acuity
Nine of 23 (39%) of the combination treatment group achieved visual acuity of 6/18 or better after healing compared to 2/22 (9.1%) of the conventional treatment group (RR 4.30; 95% CI 1.04 to 17.74).
Compliance with treatment
Not reported.
Adverse outcomes
There was little evidence of any difference in adverse outcomes, although the study was underpowered to look at these.
| Complication | Intracameral amphotericin B plus conventional medication | Conventional medication |
| Perforation | 2/23 | 3/22 |
| Anterior staphyloma | 1/23 | 0/22 |
| Phthsis bulbi | 0/23 | 1/22 |
| Panophthalmitis | 0/23 | 1/22 |
Quality of life
Not reported.
Silver sulphadiazine
9. Topical 0.5% and 1% silver sulphadiazine versus topical 1% miconazole
Clinical cure
People given silver sulphadiazine (0.5% or 1%) were more likely to have a healed ulcer at two to four weeks (Mohan 1987). In the silver sulphadiazine group 15/20 (75%) people had a healed ulcer at two to four weeks compared to 6/10 (60%) of the 1% miconazole group. The overall effect was uncertain (RR 1.25; 95% CI 0.71 to 2.20).
Time to clinical cure
The average duration of healing ranged from two to four weeks in each group.
Best‐corrected visual acuity
Not reported.
Compliance with treatment
Data on compliance was collected but not reported.
Adverse outcomes
Mohan 1987 reported that "All the drugs were tolerated well and no significant ocular or systemic side effects were observed".
Quality of life
Not reported.
Discussion
This systematic review aimed to provide a critical, quantitative overview of previous clinical research, and to yield, where possible, summary effect measures with increased statistical power by combining multiple small clinical trials. The current review includes 12 trials comparing different antifungal drugs in topical drops, ointment and oral preparations for the treatment of fungal keratitis. All trials were done in lower income countries (mainly India) since the incidence is greater there compared to higher income countries such as the United States. There are still no large multicentre randomised trials on the treatment of fungal keratitis.
Eight antifungal agents, namely: voriconazole, econazole, itraconazole, miconazole, natamycin, amphotericin B, chlorhexidine gluconate and silver sulphadiazine were studied. The latter two are not part of the conventional drugs which act on the hyphal cell membranes. The use of alternative drugs such as chlorhexidine gluconate and silver sulphadiazine may indicate that conventional drugs are not always available, are expensive and ineffective. Since fungal keratitis is more common in lower income countries the use of inexpensive alternative drugs is promising. In addition, pharmaceutical companies have less financial incentive to invest in the development of ocular antifungal agents. The only commercially available antifungal drug in the United States in ophthalmic form is natamycin (Natacyn 5% by Alcon Laboratories). In Asia and Africa, Natacyn is given as a service drug but with limited availability. In India, topical natamycin is manufactured by a local pharmaceutical company, however, no clinical trials have been done on this drug.
Summary of main results
The trials included in this review were of variable quality and were generally underpowered so there is little good evidence for most comparisons reported in this review. The exception is the comparison between natamycin and voriconazole (Table 1). There is evidence that natamycin is more effective than voriconazole in the treatment of fungal ulcers and some evidence that this effect particularly applies toFusarium species.
Overall completeness and applicability of evidence
The evidence supporting the treatment of fungal keratitis appears to be weak. Only 12 trials of variable quality were identified. The trials considered different preparations and comparisons and so for most comparisons it was either not possible or not useful to pool the data.
Most participants included in this review belonged to studies that compared natamycin and voriconazole. There was no study related to the medical treatment of yeast infection.
The most important study (MUTT 2010) did not provide any information on clinical cure or time to clinical cure. The hazard ratio of re‐epithelialization may not give a true picture since re‐epithelization can still take place in the presence of underlying stromal infiltrate.
Quality of the evidence
In general the quality of the evidence included in this review was low: trials were at risk of bias and were underpowered; only two of the comparisons had more than one trial and no comparison had more than three trials contributing data. The exception is for the comparison of natamycin and voriconazole, specifically for the outcome corneal perforation, where there was high quality evidence from three trials that natamycin achieved better outcomes than voriconazole (Table 1).
Potential biases in the review process
The original protocol for this review was first published in 2003 (FlorCruz 2003). Since then recommended Cochrane review methods have changed considerably. The methods for this review have therefore been updated, in particular to include assessment of risk of bias and summary of findings tables but also refinement of the outcomes and methods for addressing heterogeneity and unit of analysis issues. However, the criteria for inclusion of studies and methods for data extraction have not changed. As there are few trials included for each comparison, and therefore key decisions have not been affected by these changes, we believe the evolution in methods in this review over time will not have biased the overall conclusions. See Differences between protocol and review for details.
For one analysis (Analysis 1.1) fixed‐ and random‐effects models provide different results in terms of statistical significance (but similar results in terms of size of the effect). We have chosen to report the more conservative random effects model in the abstract and summary of findings table.
Agreements and disagreements with other studies or reviews
Most of the trials on management of fungal keratitis gathered during the literature search are case series. We only included RCTs in this review.
Authors' conclusions
Implications for practice.
There are a variety of antifungal agents available for the treatment of fungal keratitis but the studies comparing them are of variable quality and generally underpowered. The results of these studies do not show significant clinical differences among the heterogeneous interventions, with the exception of the comparison between natamycin and voriconazole. People given natamycin had a lower risk of corneal perforation but there was less evidence to support an effect on the primary outcome of this review ‐ clinical cure at two to three months.
Implications for research.
There is a need for future multicentre RCTs of the interventions considered in this review that recruit enough numbers of participants to measure effects with appropriate precision. Future trials could consider subgroup analyses by type of fungal infection. The main outcome measures to be addressed should include clinical cure, visual acuity, serious adverse effects such as corneal perforation and patient reported outcome measures such as quality of life. Since the price of these drugs is likely to be prohibitive to patients in developing nations, cost‐effectiveness should also be examined. The search for a cheaper and more effective treatment alternative to what has already been proposed still continues.
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2015 | New citation required and conclusions have changed | Issue 4, 2015: Four new trials included in the update (Basak 2004; MUTT 2010; Parchand 2012; Sharma 2013) |
| 19 March 2015 | New search has been performed | Issue 4, 2015: Electronic searches were updated, plain language summary updated, Summary of findings table included |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 15 December 2011 | New search has been performed | Issue 2, 2012: Electronic searches were updated, risk of bias tables have been completed for all included trials and text modified. A new author joined the review team to help with updating the review. |
| 15 December 2011 | New citation required but conclusions have not changed | Issue 2, 2012: Three new trials were included in the update (Arora 2011; Mahdy 2010; Prajna 2010). |
| 22 October 2008 | Amended | Converted to new review format. |
| 13 November 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The Cochrane Eyes and Vision Group executed the electronic searches. We would like to thank Anupa Shah, Katherine Henshaw, Sally Green, Steve McDonald, Liam Smeeth, Ruben Lim Bon Siong, Leo Cubillan, Alejandro De Leon, Anna Lisa Yu, Johann Michael Reyes, Jaime FlorCruz, Guo Baoqi, Maoling Wei of the Chinese Cochrane Center and Richard Wormald. We would also like to thank the peer reviewers especially Catey Bunce for comments on the review and Mark Wilkins for comments on the protocol.
Richard Wormald (Co‐ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Eye Infections, Fungal #2 MeSH descriptor Keratitis #3 fung* near keratit* #4 fung* near infect* near eye* #5 fung* near infect* near ocular #6 keratomycosis #7 keratomicosis #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Antifungal Agents #10 MeSH descriptor Natamycin #11 natamycin* #12 MeSH descriptor Chlorhexidine #13 chlorhexidine* #14 MeSH descriptor Econazole #15 econazole* #16 MeSH descriptor Itraconazole #17 itraconazole* #18 MeSH descriptor Miconazole #19 miconazole* #20 anti fung* #21 antifung* #22 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 (#8 AND #22)
Appendix 2. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. 2 (randomized or randomised).ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 exp animals/ 10 exp humans/ 11 9 not (9 and 10) 12 8 not 11 13 exp eye infections, fungal/ 14 exp keratitis/ 15 (fung$ adj2 keratit$).tw. 16 (fung$ adj3 infect$ adj3 eye$).tw. 17 (fung$ adj3 infect$ adj3 ocular).tw. 18 keratom?cosis.tw. 19 or/13‐18 20 exp antifungal agents/ 21 exp natamycin/ 22 natamycin$.tw. 23 exp chlorhexidine/ 24 chlorhexidine$.tw. 25 exp econazole/ 26 econazole$.tw. 27 exp itraconazole/ 28 itraconazole$.tw. 29 exp miconazole/ 30 miconazole$.tw. 31 antifung$.tw. 32 anti fung$.tw. 33 or/20‐32 34 19 and 33 35 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1 exp randomized controlled trial/ 2 exp randomization/ 3 exp double blind procedure/ 4 exp single blind procedure/ 5 random$.tw. 6 or/1‐5 7 (animal or animal experiment).sh. 8 human.sh. 9 7 and 8 10 7 not 9 11 6 not 10 12 exp clinical trial/ 13 (clin$ adj3 trial$).tw. 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15 exp placebo/ 16 placebo$.tw. 17 random$.tw. 18 exp experimental design/ 19 exp crossover procedure/ 20 exp control group/ 21 exp latin square design/ 22 or/12‐21 23 22 not 10 24 23 not 11 25 exp comparative study/ 26 exp evaluation/ 27 exp prospective study/ 28 (control$ or prospectiv$ or volunteer$).tw. 29 or/25‐28 30 29 not 10 (930488) 31 30 not (11 or 23) 32 11 or 24 or 31 33 exp keratomycosis/ 34 exp keratitis/ 35 (fung$ adj2 keratit$).tw. 36 (fung$ adj3 infect$ adj3 eye$).tw. 37 (fung$ adj3 infect$ adj3 ocular).tw. 38 keratom?cosis.tw. 39 or/33‐38 40 exp antifungal agent/ 41 exp natamycin/ 42 natamycin$.tw. 43 exp chlorhexidine/ 44 chlorhexidine$.tw. 45 exp econazole/ 46 econazole$.tw. 47 exp itraconazole/ 48 itraconazole$.tw. 49 exp miconazole/ 50 miconazole$.tw. 51 antifung$.tw. 52 anti fung$.tw. 53 or/40‐52 54 39 and 53 55 32 and 54
Appendix 4. LILACS search strategy
eye$ or ocular and fungal keratitis or keratomycosis
Appendix 5. ISRCTN search strategy
"fungal keratitis" OR keratomycosis
Appendix 6. ClinicalTrials. gov search strategy
fungal keratitis OR keratomycosis
Appendix 7. ICTRP search strategy
fungal keratitis OR keratomycosis
Data and analyses
Comparison 1. Natamycin compared to voriconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Best corrected visual acuity | 3 | 434 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.31, 0.06] |
| 2 Corneal perforation | 3 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.94] |
Comparison 2. Natamycin compared to chlorhexidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2001.
| Methods | Cross‐over randomised controlled trial People enrolled and randomly allocated, number of eyes not reported Date conducted: June 1999 to September 2000 |
|
| Participants | Setting: Calcutta, India Participants: 54 (37 men, 17 women), average age 35 years (estimated from Table 1) Inclusion criteria: "Clinically suspected cases of fungal corneal ulcers" Exclusion criteria: not specified Participants were divided into 2 groups. Group I comprised new patients and Group II comprised patients who had been previously treated with agents. |
|
| Interventions |
Topical itraconazole was prepared by mixing 100 mg of itraconazole powder with 100 mL of artificial tear solution under sterile conditions. Oral itraconazole 100 mg was given twice daily for 3 weeks along with topical itraconazole every hour. The topical itraconazole was applied for 6 weeks after the ulcer healed. Cycloplegics were used in all cases. Antiglaucoma therapy was given in cases suspected to have raised intraocular pressure. Antibacterials (topical ciprofloxacin) were applied in all cases at the beginning of treatment but stopped once fungal aetiology confirmed. |
|
| Outcomes |
Follow‐up: 6 months |
|
| Notes | Funding: not reported Conflict of interest: not reported Although trial report states this was a cross‐over trial it was not clear from the study report that it actually was. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were divided into two groups" on the basis of new and untreated patients but no other information is given |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but treatments different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported but treatments different |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not possible to assess |
| Selective reporting (reporting bias) | Unclear risk | Not possible to assess |
Arora 2011.
| Methods | Parallel group randomised controlled trial People enrolled and randomly allocated, number of eyes not reported. Date conducted: September 2007 to March 2009 |
|
| Participants | Setting: tertiary care hospital in India Participants: 30 (21 men, 9 women), average age 43 years Inclusion criteria: fungal keratitis with corneal scrapings positive for fungal hyphae on 10% potassium hydroxide wet mount/Gram's staining, negative for bacteria Exclusion criteria: any prior usage of antifungal drugs, history of herpetic keratitis or previous corneal scars, impending perforation, no light perception |
|
| Interventions |
A commercial preparation of topical natamycin was used. Topical voriconazole drops were prepared by reconstituting lyophilised powder available as 200 mg vials with sterile deionised water to make 1% (10 mg/mL) solution which was stored in a refrigerator for 48 hours. The drug was reconstituted every 48 to 72 hours. For both preparations, 1 drop was applied every hour for 2 weeks. Further doses depended on patient response. The additional standard treatment protocol included topical ofloxacin hydrochloride 0.3% four times a day, homatropine bromide 2% four times a day, and timolol maleate 0.5% twice a day if needed. |
|
| Outcomes |
Follow‐up: 10 weeks or until complete resolution of the ulcer |
|
| Notes | Funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “This study was randomized, double‐masked, interventional, pilot study of patients with fungal keratitis”. Methods, first paragraph “They were randomly divided into two groups of 15 patients using the lottery methods”. Methods, first paragraph |
| Allocation concealment (selection bias) | Unclear risk | “Double masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment as natamycin is delivered via suspension, whereas VRC is in solution”. Methods, first paragraph |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Double masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment as natamycin is delivered via suspension, whereas VRC is in solution”. Methods, first paragraph |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Double masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment as natamycin is delivered via suspension, whereas VRC is in solution”. Methods, first paragraph |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no reported drop outs in both treatment and control groups. Follow‐up ranged from 10 days to 60 days |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome was defined as the “time taken for the complete resolution of the ulcer”. Methods, last paragraph Various other outcomes reported e.g. visual acuity and mean size of the ulcer |
Basak 2004.
| Methods | Parallel group randomised controlled trial Cases enrolled and randomly allocated, number of people/eyes not reported. Date conducted: not reported |
|
| Participants | Setting: community‐based tertiary care hospital in India Participants: 45 (31 men, 14 women), average age 33 years Inclusion criteria: deep keratomycosis with endothelial plaque; non‐mobile cheesy hypopyon of various height; all cases were smear positive for fungus on potassium hydroxide or Gram stain, or both; smear (Gram stain) was negative for bacteria in all cases. Exclusion criteria: keratomycosis without hypopyon; mixed ulcer on microscopic examination of the smear; ulcer with impending or frank perforation; after 48 hours if any bacterial culture report became positive. |
|
| Interventions |
Conventional medication was: oral fluconazole 150 mg to 200 mg twice a day for 3 weeks; topical natamycin 5% every hour; topical amphotericin B 0.15% every hour; broad‐spectrum topical antibiotic every 2 hours; topical antiglaucoma medication; topical cycloplegics. Intracameral injection of amphotericin B was given in a dose between 5 μg and 15 μg depending upon the size of the ulcer and amount of hypopyon. Injection was repeated after 7 days as indicated. Complications were treated medically or surgically, or both. |
|
| Outcomes |
Follow‐up: day 1, 3, 7 and then weekly until ulcer healed. |
|
| Notes | Funding: not reported Conflict of interest: "The Authors do not have any proprietary interest in the method or subject matter mentioned in this article." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but interventions quite different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Mahdy 2010.
| Methods | Parallel group randomised controlled trial 1 eye per patient, unclear how eye selected Date conducted: March 2008 to December 2009 |
|
| Participants | Setting: hospital in Egypt Participants: 48 (31 male, 17 female), average age 44 years Inclusion criteria: clinical signs of fungal keratitis Exclusion criteria: not specified |
|
| Interventions |
Topical amphotericin B (Fungizone, Squib) eye drops were prepared from the commercially available 50 mg vial with 5% dextrose dilution to get the 0.05% concentration required. These were used every 2 hours for both groups. In addition to this, one group also received a 1 mL subconjunctival injection prepared directly from the commercially available intravenous infusion form of fluconazole solution (Diflucan, Pfizer), which was injected daily for the first 10 injections and every 48 hours for a further 10 injections. For both groups, in addition to the use of antifungal agents, topical atropine sulphate 1% drops were given 3 times daily and gatifloxacin 0.3% eye drops 5 times daily in cases of negative bacterial results, using specific antibacterial drops according to the sensitivity reaction of bacterial culture. The ulcers were also regularly debrided using a sharp corneal keratome (every 48 hours). |
|
| Outcomes |
Follow‐up 3 months |
|
| Notes | Funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The study is a prospective, randomized one,..” Page 282 “Eyes with similar clinical and laboratory findings were classified into 2 groups of treatment.” Page 282 |
| Allocation concealment (selection bias) | High risk | No description on method of allocation concealment however the study groups were exactly matched for fungal species (table 2) which is unlikely on this number of patients if the allocation was truly random |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not masked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not masked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Difficult to judge from report |
Mohan 1987.
| Methods | Parallel group randomised controlled trial (after 1 week, patients who had not responded were randomly allocated to another treatment) Eyes enrolled, unclear if 1 eye per person Date conducted: not reported |
|
| Participants | Setting: New Delhi, India
Participants: 30, age and sex not reported Inclusion criteria: clinical diagnosis of fungal keratitis with positive for potassium hydroxide or Grams smear, or both Exclusion criteria: not specified |
|
| Interventions |
All three drugs were prepared in an ointment base and were applied 5 times a day. Cycloplegics (atropine or homatropine), antiglaucoma medication (acetazolamide, glycerol) and vitamins (A, B complex and C) were given where indicated. |
|
| Outcomes |
|
|
| Notes | Funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The cases were divided into 3 treatment groups […] on a random basis” Page 573 |
| Allocation concealment (selection bias) | Low risk | “The drugs […were] coded by the Ocular Pharmacology Laboratory” Page 573 “At the end of the trial, the code was broken and the result analyzed” Page 573 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Each patient was given a coded antifungal ointment tube of 5g to be applied 5 times a day and the entire study was conducted in a double blind manner” Page 573 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “The drugs […were] coded by the Ocular Pharmacology Laboratory” Page 573 “Each patient was given a coded antifungal ointment tube of 5g to be applied 5 times a day and the entire study was conducted in a double blind manner” Page 573 “At the end of the trial, the code was broken and the result analyzed” Page 573 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “There was no fallout from this study on account of poor patient compliance” Page 573 |
| Selective reporting (reporting bias) | Low risk | Probably not a problem as they reported ulcers responding to treatment |
MUTT 2010.
| Methods | Parallel group randomised controlled trial (multicentre) 1 eye per patient, unclear how eye selected Date conducted: April 2010 to December 2011 |
|
| Participants | Setting: 3 hospitals in India Participants: 323 (183 men, 140 women), average age 47 years Inclusion criteria: smear‐positive fungal corneal ulcer and baseline visual acuity of 20/40 (0.3 logMAR) to 20/400 (1.3 logMAR) Exclusion criteria: impending perforation, evidence of bacterial, Acanthamoeba, or herpetic keratitis, being younger than 16 years, and bilateral ulcers or visual acuity worse than 20/200 (1.0 logMAR) in the non affected eye. |
|
| Interventions |
In both groups, 1 drop was applied to the affected eye every hour while awake for 1 week, then every two 2 hours while awake until 3 weeks from enrolment. |
|
| Outcomes |
“The visual acuity measurement protocol was adapted from the Age‐Related Eye Disease Study using Early Treatment Diabetic Retinopathy Study tumbling “E” charts (charts 2305 and 2305A; PrecisionVision) at 4 m, using a protocol identical to that used in the Steroids for Corneal Ulcers Trial, with low‐vision testing at 0.5 m.” Follow‐up: 3 months |
|
| Notes | Funding: “This work was supported by grants U10 EY018573 (Dr Lietman) and K23 EY017897 (Dr Acharya) from the National Eye Institute and grants from That Man May See, the Harper/Inglis Trust, the South Asia Research Foundation, and Research to Prevent Blindness (Drs Lietman and Acharya). Natamycin and voriconazole were donated by Alcon and Pfizer, respectively” Conflict of interest: reported “nil” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A random allocation sequence was generated (T.C.P.and K.J.R.) for patients by center in random block sizes of 4, 6, and 8” Page 424 |
| Allocation concealment (selection bias) | Low risk | “Masked assignment to the treatment intervention was performed after determination of eligibility and consent to participate.” Page 423 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Double‐masking was achieved through Aurolab packaging both the natamycin suspension and the voriconazole solution in identical opaque containers (3 mL/container) and ophthalmic assistants carefully irrigating each patient’s eye prior to examination.” Page 423 “Patients, physicians, and investigators were all masked to treatment until the conclusion of the trial” page 423 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Double‐masking was achieved through Aurolab packaging both the natamycin suspension and the voriconazole solution in identical opaque containers (3 mL/container) and ophthalmic assistants carefully irrigating each patient’s eye prior to examination.” Page 423 “Patients, physicians, and investigators were all masked to treatment until the conclusion of the trial” page 423 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 143/161 (88.8%) in voriconazole group (114 and 28 LOCF) 141/162 (87.0%) in natamycin group (128 and 13 LOCF) |
| Selective reporting (reporting bias) | Unclear risk | Some differences with trial registration information on ClinicalTrials.gov |
Parchand 2012.
| Methods | Parallel group randomised controlled trial 1 eye per patient, unclear how eye selected Date conducted: not reported |
|
| Participants | Setting: Chandigarh, India Participants: 45, age and sex not reported Inclusion criteria: ulcer with epithelial defect more than 5 mm in the greatest dimension, infiltrates involving more than two thirds depth of corneal thickness, proven fungal corneal ulcer either on 10% potassium hydroxide west mount/Calcoflour white stain or growth of fungi on Sabouraud's dextrose agar, older than 18 years, willingness to be an inpatient and take part in follow‐up Exclusion criteria: perforated cornea or impending perforation, sclera involvement, total corneal involvement, endophthalmitis, acanthamoeba keratitis, evidence of bacterial infection or herpetic keratitis, bilateral ulcers, previous ocular surgery, pregnancy or breast‐feeding, known allergy to medication, no light perception, failed to attend for follow‐up at 3 months. |
|
| Interventions |
Oral voriconazole was given in tablet form 400 mg twice a day on day 1 followed by 200 mg twice a day and continued until the resolution of the infiltrates. Topical voriconazole and natamycin were given every hour while awake for 1 week, then every 2 hours while awake until healing of the epithelial defect and then gradual tapering off. |
|
| Outcomes |
Follow‐up: 3 months |
|
| Notes | Funding sources: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | People who did not attend at 3 months were excluded from the study |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Prajna 2003.
| Methods | Parallel group randomised controlled trial 1 eye per patient, unclear how eye selected Date conducted: March 2002 to October 2002 |
|
| Participants | Setting: Aravind, India
Participants: 116 (72 men, 44 women), average age 37 years Inclusion criteria: smear and culture positive for fungal infection; ulcer at least 2 mm2 and not more than 60 mm2 Exclusion criteria: did not consent to study |
|
| Interventions |
Participants were admitted to the hospital for a week. Interventions were applied every hour between 7am and 9pm. 1% atropine sulphate ointment was applied 3 times per day in the affected eye at least 15 minutes after application of the antifungal eye drops. |
|
| Outcomes |
Follow‐up: 4 weeks |
|
| Notes | Funding: Aravind Medica Research Foundation Conflict of interest: reported "none" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “…subjects were randomized to receive either…” Page 1235 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Since natamycin is available as a suspension, and precipitates in the corneal tissue, it was not possible to mask the investigator to the drugs used on subsequent visits.” Page 1235 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Since natamycin is available as a suspension, and precipitates in the corneal tissue, it was not possible to mask the investigator to the drugs used on subsequent visits.” Page 1235 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Four of the 116 patients randomized at baseline did not return for further follow‐up (Fig 1) and were dropped from the study.” Page 1236 However, this contradicts figure 1 where 5 people were lost to follow‐up by week 4. Also large numbers of people “exited” the study due to clinical worsening or reaction to drops. By week 4, 25/61 in the econazole group and 22/55 of natamycin group remained in the study |
| Selective reporting (reporting bias) | Low risk | Reported “time to cure” and no indication of any unreported variables |
Prajna 2010.
| Methods | Parallel group randomised controlled trial (multicentre) 1 eye per patient, only 1 eye enrolled in trial Date conducted: November 2007 to May 2008 |
|
| Participants | Setting: corneal clinics in Madurai and Pondicherry, India Participants: 120 (79 male, 41 female) average age 47 years Inclusion criteria: presence of a corneal ulcer, smear positive for filamentous fungi on potassium hydroxide wet mount, Giemsa or Gram stain, able to understand the purpose of the study and consent Exclusion criteria: overlying epithelial defect, impending perforation, evidence of acanthamoeba, evidence of herpetic keratitis, corneal scar, < 16 years, bilateral ulcers, previous penetrating keratoplasty, pregnancy, outside 200 km radius of hospital, best spectacle‐corrected visual acuity worse than 6/60 in the fellow eye, no light perception in the affected eye, not willing to return for follow‐up visits |
|
| Interventions |
The interventions were applied every hour while awake for 1 week, and then every 2 hours while awake until 3 weeks after enrolment. Further continuation was at the discretion of the physician. Patients were also randomly allocated to repeat scraping of the cornea. |
|
| Outcomes |
Follow‐up: 3 months |
|
| Notes | Funding: That Man May See and the South Asia Research Fund; core grant EY02162 from the National Eye Institute (Department of Ophthalmology at University of California, San Francisco); grant K23EY017897 from the National Eye Institute (Dr Acharya); a Research to Prevent Blindness Career Development Award (Drs Acharya and Lietman); grant U10‐EY015114 from the National Eye Institute (Dr Lietman); That Man May See Foundation at University of California, San Francisco (Dr Porco); Alcon Inc; and Pfizer Inc. Conflict of interest: reported "none". Role of the Sponsors: "Alcon Inc. donated natamycin and Pfizer Inc. donated voriconazole for the study. The sponsors did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “This study was a randomized, double‐masked, clinical trial of patients with fungal corneal ulcers.” Page 673 "Patients were block randomized in groups of 4 (using the statistical package R; http: //www.r‐project.org) by T.P." Page 673 |
| Allocation concealment (selection bias) | Low risk | "Double‐masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment. In addition, patients were no longer receiving treatment at 3 months, the time that the primary outcome of final visual acuity was measured.Only the biostatisticians responsible for the randomization coding and the study pharmacist were unmasked." Page 673 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment. In addition, patients were no longer receiving treatment at 3 months, the time that the primary outcome of final visual acuity was measured.Only the biostatisticians responsible for the randomization coding and the study pharmacist were unmasked." Page 673 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment. In addition, patients were no longer receiving treatment at 3 months, the time that the primary outcome of final visual acuity was measured.Only the biostatisticians responsible for the randomization coding and the study pharmacist were unmasked." Page 673 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Efficacy endpoints were analyzed on an intent‐to‐treat basis for all randomized patients enrolled in the study. The primary analysis included the actual 3‐month data when available and last observation carried forward for missing values." Page 674 "Sensitivity analyses were also performed in which we separately (1) assigned surgical patients the value 1.7 instead of 1.9, (2) assigned patients with perforation (but no surgery) the value 1.7 or 1.9 (instead of using last observation carried forward), (3) analyzed only patients with complete follow‐up, or (4) used multiple imputation (recursive random partitioning‐based hot deck method)" Page 674 11/120 lost to follow‐up but evenly distributed across study groups 2/2/4/3 |
| Selective reporting (reporting bias) | Low risk | "The primary efficacy endpoint was BSCVA at 3 months in the study eye, using a linear regression model with 3‐month logMAR BSCVA as the outcome variable and treatment arm (voriconazole vs natamycin) and enrollment logMAR BSCVA and scraping (yes or no) as covariates." Page 674 "Other prespecified endpoints included BSCVA at 3 weeks, adjusting for enrollment BSCVA, and infiltrate/scar size at 3 weeks and 3 months, adjusting for enrollment infiltrate/scar size." Page 674 |
Rahman 1997.
| Methods | Parallel group randomised controlled trial People enrolled and randomly allocated, number of eyes not reported Date conducted: not reported |
|
| Participants | Setting: Aravind Eye Hospital in Madurai, India Participants: 60 (46 men, 14 women estimated from data on subgroup) average age not reported Inclusion criteria: suppurative corneal ulcer with fungal elements demonstrated in a potassium hydroxide preparation and culture, agree to stay in hospital at 7 days and return at 21 days Exclusion criteria: only 1 eye, children under 1 year, diabetics, perforated corneal ulcer, mixed bacterial and fungal infections Male (76%), aged 50 years and above (33%) |
|
| Interventions |
One drop was applied every half hour for 3 hours, then once every hour during waking hours. From the second day, the drop was applied every 2 hours for 5 days, and then every 3 hours for a further 2 weeks. If there was no improvement by 5 days the code was broken and an alternative treatment used. People in the chlorhexidine groups were given natamycin, people in the natamycin group were given econazole 1%. |
|
| Outcomes |
Follow‐up: 3 weeks |
|
| Notes | Funding: British Council for the Prevention of Blindness Conflict of interest: not reported 12 patients with severe ulcers were excluded in the analysis of outcome at 21 days since only 1 (from chlorhexidine gluconate 0.05%) had favourable response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was computer generated by statisticians at Aravind, using the one‐sample run test.” Page 143 |
| Allocation concealment (selection bias) | Low risk | “… 60 consecutive patients were randomly allocated in a double‐masked fashion..” Page 142 “The bottles were prepared and labelled only with the randomized numbers by the Aravind executive staff” Page 143 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “… 60 consecutive patients were randomly allocated in a double‐masked fashion..” Page 142 “The bottles were prepared and labelled only with the randomized numbers by the Aravind executive staff” Page 143 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “… 60 consecutive patients were randomly allocated in a double‐masked fashion..” Page 142 “The bottles were prepared and labelled only with the randomized numbers by the Aravind executive staff” Page 143 But for “treatment failures” the code was broken on day 5 so presumably all assessments after that date were unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients were lost to follow‐up, so that 58 patients were left in the study" Page 144 |
| Selective reporting (reporting bias) | Unclear risk | A number of different outcome measures reported and no indication as to whether these were all outcomes on which data collected |
Rahman 1998.
| Methods | Parallel group randomised controlled trial People enrolled and randomly allocated, number of eyes not reported. Date conducted: not reported |
|
| Participants | Setting: Chittagong, Bangladesh
Participants: 70 (52 men, 18 women) average age 43 years (estimated from table 1) Inclusion criteria: suppurative keratitis, fungal hyphal elements observed on a wet mount in 10% potassium hydroxide and as a heat fixed mount with Gram stain Exclusion criteria: only 1 eye, diabetes, polymicrobial infections, unwilling to participate fully or attend for follow‐up, children under 1 year of age, ulcer had already perforated |
|
| Interventions |
Chlorhexidine gluconate 20% solution was supplied by Moorfields Eye Hospital, London, Small volumes of this solution were diluted with distilled, deionised, pyrogen‐free water (Glaxo Wellcome, Bangladesh). Natamycin 2.5% suspension consisted of natamycin 27.5 g, sodium hydroxide 1.2% solution 150 mL, hydrochloric acid 5% solution added to adjust pH to 6.0 to 7.0, benzalkonium chloride 1% 5.5 mL, distilled water to 1000 mL. One drop was applied every half hour for 3 hours, then every hour for 2 days, every 2 hours for 5 days, and every 3 hours for 2 weeks. Drops were applied during waking hours. If no response by 5 days the code was broken and alternative treatment given (econazole 1% or natamycin 5% or clotrimazole 1%). |
|
| Outcomes |
Follow‐up: 6 months to 1 year |
|
| Notes | Funding: British Council for the Prevention of Blindness Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization of individuals was computer generated in London....” Page 920 |
| Allocation concealment (selection bias) | Low risk | “... and the codes for the alternative treatments sealed in serially numbered opaque envelopes, which were opened in sequence by the research ophthalmologist as the trial progressed.” Page 920 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “It was not possible to mask the ophthalmologist or nurses to the medications because of their different appearances” Page 920 Blinding of participants not stated directly but can be inferred that they were masked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “It was not possible to mask the ophthalmologist or nurses to the medications because of their different appearances” Page 920 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/35 of chlorhexidine 0.2% group dropped out of the study by 21 days compared to 3/36 of the natamycin 2.5% group. Page 921, figure 1 |
| Selective reporting (reporting bias) | Unclear risk | Main outcome was healing at 21 days of treatment but other follow‐up periods also available and not clear that this outcome was prespecified or not |
Sharma 2013.
| Methods | Parallel group randomised controlled trial 1 eye per patient, only people with 1 affected eye enrolled Date conducted: December 2008 to June 2010 |
|
| Participants | Setting: Tertiary eye care hospital in India Participants: 40 (30 men, 10 women, estimated) average age 44 years Inclusion criteria: positive smear results (potassium hydroxide wet mount or gram stain) or positive culture results for fungal ulcers larger than 2 mm involving up to two thirds of the stromal thickness and not showing any signs of clinical improvement after 2 weeks of topical natamycin therapy, willingness to be treated on an inpatient basis and to return for follow up and medications Exclusion criteria: mixed infection on smear or culture analysis, evidence of herpetic keratitis, impending perforation, bilateral ulcers, vision worse than 6/60 in the fellow eye, < 18 years |
|
| Interventions |
Potential participants were treated with topical natamycin every hour round the clock for 2 days and every 2 hours thereafter along with ciprofloxacin hydrochloride 0.3% every 6 hours and cycloplegics. People with ulcers with an increase in size of epithelial defect, a decrease of less than 20% of stromal infiltrate or scar complex, or increasing hypopyon were enrolled. Both groups were treated with 5% Natamycin drops four times per day, 0.3% Ciprofloxacin hydrochloride drops 4 times per day and 2% homatropine drops 3 times per day. Topical voriconazole 1% was applied every hour for the first 48 hours. Topical voriconazole 1% eye drops were prepared in the Department of Ocular Pharmacology by reconstituting injection voriconazole 200 mg powder (VORAZE; Sun Pharma, Mumbai, India) in 19 mL Ringer lactate. The drops were tapered to every 2 hours while awake for 72 hours and then the dose was applied every 4 hours. Further tapering of the drug depended on the response of the infection to treatment and the clinician’s judgment. Instrastromal injections 50 g/0.1 mL intrastromal voriconazole. VORAZE 200 mg powder (Sun Pharma, Mumbai, India) was reconstituted with 19 mL Ringer lactate. 1 mL of this solution was diluted further with 20 mL Ringer lactate. The resulting 0.5 mg/mL (50 g/0.1 mL) solution was used for the intrastromal injection. All injections were given in an operating room under aseptic precautions after administering peribulbar anaesthesia. After loading the drug into a 1 mL tuberculin syringe fitted with a 26‐gauge needle, it was inserted obliquely into the cornea from the uninvolved, clear area to reach just flush to the ulcer at the midstromal level in each case. 5 divided doses were given around the ulcer to form a deposit of the drug around the circumference of the lesion. This was done in such a manner that the injected drug appeared to encompass the ulcer along each meridian. At least 3 injections were given 72 hours apart. Participants in both groups received topical therapy with 5% natamycin every 4 hours, cycloplegics, and 0.3% ciprofloxacin hydrochloride every 6 hours. |
|
| Outcomes |
Follow‐up: 3 months |
|
| Notes | Funding: Dr. Rajendra Prasad Centre for Ophthalmic Sciences, New Delhi, India Conflict of interest: reported "none" Trial id number: ISRCTN57259399 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was carried out using computer‐generated random numbers according to the variable block size.” Page 678 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported and interventions different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported and interventions different |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up not reported but assumed that all enrolled were reported |
| Selective reporting (reporting bias) | Low risk | No evidence for selective outcome reporting |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2013 | Treatment allocation depended on disease status ‐ not an RCT |
| Gupta 2006 | No response to request for information |
| ISRCTN84613089 | Not an RCT |
| Jones 1975 | This is a lecture on the principles in the management of keratomycosis |
| Kalavathy 2002 | The article is a commentary to Agarwal 2001 |
| Kalavathy 2005 | This is not a RCT. The first 50 consecutive patients received natamycin while the next 50 patients were given itraconazole |
| Lavingia 1986 | This is an in vitro study on antifungal properties of amphotericin B |
| Li 2011 | Allocation by administration number |
| Mabon 1998 | The article is not a RCT but an overview on fungal keratitis |
| Mahashabde 1987 | This is a case series |
| Maichuk 1990 | This is a case series using antifungal agents for different ocular fungal infections |
| Maichuk 1991 | This is a case series using antifungal agents for different ocular fungal infections |
| Maichuk 1994 | This is a case series using antifungal agents for different ocular fungal infections |
| Maichuk 1995 | This is a case series |
| Martin 1996 | The article is an in vitro study |
| Mitsui 1987 | This is a case series |
| Mohan 1988 | Quasi‐randomised trial: allocation by alternation |
| NCT00516399 | Study terminated |
| Oude Lashof 2011 | RCT but not fungal keratitis |
| Panda 1996 | It is not a RCT; 6 consecutive eyes were treated with topical fluconazole |
| Rao 1997 | It is a commentary to another article |
| Ray 2002 | The article is a another commentary to Agarwal 2001 |
| Reddy 1982 | Allocation was not random |
| Shuai 2012 | Treatment allocation depended on disease status ‐ not an RCT |
| Sun 1996 | There was attempt at randomisation but no mention of centralised randomisation. Masking of participants was impossible due to different forms of the medication given. Masking of care givers and outcome assessors was not reported although difficult to perform because the treatments were in different forms (suspension and oil mixture). There was also no report on drop‐out rates |
| Xie 2001 | This is a retrospective study on severe fungal ulcers which needed penetrating keratoplasty |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Qu 2013.
| Methods | TBC |
| Participants | TBC |
| Interventions | TBC |
| Outcomes | TBC |
| Notes | TBC |
TBC ‐ to be completed
Characteristics of ongoing studies [ordered by study ID]
CTR 2011 091 000107.
| Trial name or title | N/A |
| Methods | Parallel group RCT |
| Participants | Country: India 30 people with smear‐positive deep fungal keratitis with hypopyon who did not respond to topical natamycin and topical amphotericin B |
| Interventions |
Topical natamycin hourly during day and 2‐hourly during night. Topical amphotericin B every 2 hours. |
| Outcomes | Time to epithelialisation and resolution of the ulcer |
| Starting date | 2008 |
| Contact information | N/A |
| Notes | Currently submitted for publication (personal communication from PI) Trial registry website: http://apps.who.int/trialsearch/trial.aspx?trialid=CTRI/2011/091/000107, accessed 10th September 2013 |
MUTT II.
| Trial name or title | Mycotic Ulcer Treatment Trial II (MUTT II) |
| Methods | Parallel group RCT |
| Participants | People aged 16 years or older with fungal corneal ulcer |
| Interventions | Topical voriconazole 1% combined with oral voriconazole compared to topical voriconazole 1% alone |
| Outcomes | Following text from entry on ClinicalTrials.gov: Primary Outcome Measures: Rate of perforation [ Time Frame: 3 months from enrollment ] [ Designated as safety issue: No ] Comparison of rate of perforation between the treatment groups (topical voriconazole with oral voriconazole vs. topical voriconazole with oral placebo) Secondary Outcome Measures: Best spectacle‐corrected logMAR visual acuity [ Time Frame: 3 weeks after enrollment ] [ Designated as safety issue: No ] Best spectacle‐corrected logMAR visual acuity at 3 weeks after enrollment, adjusting for enrollment BSCVA and treatment arm in a multiple linear Best spectacle‐corrected logMAR visual acuity only in Indian sites [ Time Frame: 3 weeks and 3 months after enrollment ] [ Designated as safety issue: No ] Best spectacle‐corrected logMAR visual acuity only in Indian sites, 3 weeks and 3 months after enrollment, adjusting for enrollment BSCVA and treatment arm in a multiple linear regression model Best spectacle‐corrected logMAR visual acuity [ Time Frame: 3 months after enrollment ] [ Designated as safety issue: No ] Best spectacle‐corrected logMAR visual acuity 3 months after enrollment, adjusting for enrollment BSCVA and treatment arm in a multiple linear Hard contact‐lens corrected visual acuity measured in logMAR [ Time Frame: 3 months after enrollment ] [ Designated as safety issue: No ] Hard contact‐lens corrected visual acuity measured in logMAR 3 months after enrollment Size of infiltrate/scar [ Time Frame: 3 weeks and 3 months after enrollment ] [ Designated as safety issue: No ] Size of infiltrate/scar at 3 weeks and 3 months after enrollment, using enrollment infiltrate scar/size as a covariate Time to resolution of epithelial defect [ Time Frame: At the time of resolution of epithelial defect ] [ Designated as safety issue: No ] Number of adverse events [ Time Frame: At the time of adverse event ] [ Designated as safety issue: No ] Minimum inhibitory concentration of isolates [ Time Frame: 3 months after enrollment ] [ Designated as safety issue: No ] Microbiological cure at 7 days [ Time Frame: 7 days after enrollment ] [ Designated as safety issue: No ] |
| Starting date | May 2010. Estimated date of completion: August 2016 |
| Contact information | Nisha Acharya, MD, MS nisha.acharya@ucsf.edu |
| Notes | http://clinicaltrials.gov/ct2/show/NCT00997035 |
N/A = not available; RCT = randomised controlled trial
Differences between protocol and review
The methods have been updated since the protocol was originally published in line with developments in Cochrane methods.
The objectives and inclusion criteria (types of studies, types of participants and types of interventions) have stayed the same as specified in the orignal published protocol (FlorCruz 2003).
For the current update we simplified and reduced the number of outcomes to make the review clearer and more relevant. We also specified the list of outcomes for the summary of findings table at this update. We did not consider the data available when making this selection but we were aware of the results of the published trials.
The new Cochrane risk of bias tool has been introduced since the protocol was written and this has been implemented in this review. We have added in some more detail on unit of analysis issues, dealing with missing data and assessment of reporting biases that were not considered at the protocol stage. Plans for data synthesis remain the same as the protocol. We have included one subgroup analysis for future updates (type of fungal infection) and removed one sensitivity analysis ("changing inclusion criteria such as lowering methodological cut‐off points") because we felt that on reflection this was taken care of by the sensitivity analysis of risk of bias.
Contributions of authors
NVF conceived the review question, co‐ordinated the review, organised retrieval of full text copies, wrote to authors of papers for additional information, provided additional data about papers, obtained and screened data on unpublished studies, analysed and interpreted data, performed previous work that was the foundation of the review and wrote the review. NVF and IP screened initial search results, screened retrieved papers against inclusion criteria, extracted and entered data in to RevMan.
Updates, 2012 and 2015 NVF and JE screened search results, appraised quality of papers, extracted and entered data in to RevMan and wrote the update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London including Jennifer Evans who has assisted in updating this review in 2012 and 2015.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agarwal 2001 {published data only}
- Agarwal PK, Roy P, Das A, Banerjee A, Maity PK, Banerjee AR. Efficacy of topical and systemic itraconazole as a broad‐spectrum antifungal agent in mycotic corneal ulcer. A preliminary study. Indian Journal of Ophthalmology 2001;49(3):173‐6. [PubMed] [Google Scholar]
Arora 2011 {published data only}
- Arora R, Gupta D. Voriconazole versus natamycin as primary treatment in fungal ulcers. Clinical and Experimental Ophthalmology 2011;39(5):434‐40. [DOI] [PubMed] [Google Scholar]
Basak 2004 {published data only}
- Basak SK, Mahanta A, Bhowmick A, Bhattacharya D. Intracameral amphotericin B in deep keratomycosis with hypopyon: a randomized controlled clinical trial. American Academy of Ophthalmology 2004:176.
Mahdy 2010 {published data only}
- Mahdy RA, Nada WM, Wageh MM. Topical amphotericin B and subconjunctival injection of fluconazole (combination therapy) versus topical amphotericin B (monotherapy) in treatment of keratomycosis. Journal of Ocular Pharmacology and Therapeutics 2010;26(3):281‐5. [DOI] [PubMed] [Google Scholar]
Mohan 1987 {published data only}
- Mohan M, Gupta SK, Kalra VK, Vajpayee RB, Sachdev MS. Silver sulphadiazine in the treatment of mycotic keratitis. Indian Journal of Medical Research 1987;85:572‐5. [PubMed] [Google Scholar]
MUTT 2010 {published data only}
- Lalitha P, Sun CQ, Prajna NV, Karpagam R, Geetha M, O'Brien KS, et al. In vitro susceptibility of filamentous fungal isolates from a corneal ulcer clinical trial. American Journal of Ophthalmology 2014;157(2):318‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmology 2013;131(4):422‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Srinivasan M, Lalitha P, Krishnan T, Rajaraman R, Ravindran M, et al. Differences in clinical outcomes in keratitis due to fungus and bacteria. JAMA Ophthalmology 2013;131(8):1088‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CQ, Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Srinivasan M, et al. Expert prior elicitation and Bayesian analysis of the Mycotic Ulcer Treatment Trial I. Investigative Ophthalmology and Visual Science 20103;54(6):4167‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parchand 2012 {published data only}
- Parchand S, Gupta A, Ram J, Gupta N, Chakrabarty A. Voriconazole for fungal corneal ulcers. Ophthalmology 2012;119(5):1083. [DOI] [PubMed] [Google Scholar]
Prajna 2003 {published data only}
- Prajna NV, John RK, Nirmalan PK, Lalitha P, Srinivasan M. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. British Journal of Ophthalmology 2003;87(10):1235‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prajna 2010 {published data only}
- Krishnan T, Prajna NV, Gronert K, Oldenburg CE, Ray KJ, Keenan JD, et al. Gender differences in re‐epithelialisation time in fungal corneal ulcers. British Journal of Ophthalmology 2012;96(1):137‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha P, Prajna NV, Oldenburg CE, Srinivasan M, Krishnan T, Mascarenhas J, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea 2012;31(6):662‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Krishnan T, Mascarenhas J, Srinivasan M, Oldenburg CE, Toutain‐Kidd CM, et al. Predictors of outcome in fungal keratitis. Eye 2012;26(9):1226‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Lalitha PS, Mascarenhas J, Krishnan T, Srinivasan M, Vaitilingam CM, et al. Natamycin and voriconazole in Fusarium and Aspergillus keratitis: subgroup analysis of a randomised controlled trial. British Journal of Ophthalmology 2012;96(11):1440‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajna NV, Mascarenhas J, Krishnan T, Reddy PR, Prajna L, Srinivasan M, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Archives of Ophthalmology 2010;128(6):672‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 1997 {published data only}
- Rahman MR, Minassian DC, Srinivasan M, Martin MJ, Johnson GJ. Trial of chlorhexidine gluconate for fungal corneal ulcers. Ophthalmic Epidemiology 1997;4(3):141‐9. [DOI] [PubMed] [Google Scholar]
Rahman 1998 {published data only}
- Rahman MR, Johnson GJ, Husain R, Howlader SA, Minassian DC. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. British Journal of Ophthalmology 1998;82(8):919‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma N, Chacko J, Velpandian T, Titiyal JS, Sinha R, Satpathy G, et al. Comparative evaluation of topical versus intrastromal voriconazole as an adjunct to natamycin in recalcitrant fungal keratitis. Ophthalmology 2013;120(4):677‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2013 {published data only}
- Chen TH, Li SW, Niu XX, Ning JH, Lu FQ, Guo ZF. Clinical study of domestic natamycin medication for the treatment of fungal corneal ulcer. International Eye Science 2013;13(2):356‐8. [Google Scholar]
Gupta 2006 {published data only}
- Gupta A, Ram J, Brar GS, Pandav SS. Early vs. late intracameral amphotericin B in nonresponding severe keratomycosis. American Academy of Ophthalmology 2006:220.
ISRCTN84613089 {published data only}
- ISRCTN84613089. Intracameral voriconazole injection in the treatment of fungal endophthalmitis developed from keratitis. www.isrctn.com/ISRCTN84613089 (accessed 30 April 2013).
Jones 1975 {published data only}
- Jones BR. Principles in the management of oculomycosis. XXXI Edward Jackson memorial lecture. American Journal of Ophthalmology 1975;79(5):719‐51. [DOI] [PubMed] [Google Scholar]
Kalavathy 2002 {published data only}
- Kalavathy CM, Thomas PA. Efficacy of topical and systemic itraconazole as a broad‐spectrum antifungal agent in mycotic corneal ulcer. A preliminary study. Indian Journal of Ophthalmology 2002;50(1):71‐2. [PubMed] [Google Scholar]
Kalavathy 2005 {published data only}
- Kalavathy CM, Parmar P, Kaliamurthy J, Philip VR, Ramalingam MD, Jesudasan CA, et al. Comparison of topical itraconazole 1% with topical natamycin 5% for the treatment of filamentous fungal keratitis. Cornea 2005;24(4):449‐52. [DOI] [PubMed] [Google Scholar]
Lavingia 1986 {published data only}
- Lavingia B, Dave S. Comparative study of amphotericin‐B pimaricin and gentian violet on ocular fungi. Indian Journal of Ophthalmology 1986;34:73‐7. [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li QT. Clinical curative effect of irrigating the anterior chamber with solution of amphotericin B to treat the fungal keratitis. International Journal of Ophthalmology 2011;11(7):1194‐6. [Google Scholar]
Mabon 1998 {published data only}
- Mabon M. Fungal keratitis. International Ophthalmology Clinics 1998;38(4):115‐23. [DOI] [PubMed] [Google Scholar]
Mahashabde 1987 {published data only}
- Mahashabde S, Nahata MC, Shrivastava U. A comparative study of anti‐fungal drugs in mycotic corneal ulcer. Indian Journal of Ophthalmology 1987;35(5‐6):149‐52. [PubMed] [Google Scholar]
Maichuk 1990 {published data only}
- Maichuk I, Karimov MK, Lapshina NA. Ketoconazole in the treatment of ocular mycoses [Ketokonazol v lechenii mikozov glaza]. Vestnik Oftalmologii 1990;106(1):44‐6. [PubMed] [Google Scholar]
Maichuk 1991 {published data only}
- Maichuk I, Lapshina NA, Diadina UV. Midazoles in the treatment of ocular mycoses [Imidazoly v lechenii mikozov glaza]. Antibiotiki i Khimioterapiia 1991;36(1):45‐6. [PubMed] [Google Scholar]
Maichuk 1994 {published data only}
- Maichuk I, Diadina UV. Itraconazole in the treatment of ophthalmomycoses [Itrakonazol v lechenii oftal'momikozov]. Antibiotiki i Khimioterapiia 1994;39(7):54‐6. [PubMed] [Google Scholar]
Maichuk 1995 {published data only}
- Maichuk I, Diadina UV. Metamphocin in the treatment of ocular mycoses [Metamfotsin v lechenii mikozov glaza]. Antibiotiki i Khimioterapiia 1995;40(11‐12):55‐6. [PubMed] [Google Scholar]
Martin 1996 {published data only}
- Martin MJ, Rahman MR, Johnson GJ, Srinivasan M, Clayton YM. Mycotic keratitis: susceptibility to antiseptic agents. International Ophthalmology 1996;19(5):299‐302. [DOI] [PubMed] [Google Scholar]
Mitsui 1987 {published data only}
- Mitsui Y, Kitano S, Uchida Y, Tanaka N, Kobayashi S, Tokuda H, et al. Effect of 1% pimaricin ophthalmic ointment in the treatment of keratomycosis. Nippon Ganka Gakkai Zasshi 1987;91(2):304‐11. [PubMed] [Google Scholar]
Mohan 1988 {published data only}
- Mohan M, Gupta SK, Kalra VK, Vajpayee RB, Sachdev MS. Topical silver sulphadiazine ‐ a new drug for ocular keratomycosis. British Journal of Ophthalmology 1988;72(3):192‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00516399 {published data only}
- NCT00516399. A clinical trial of the treatment of fungal corneal ulcers with povidone‐iodine. ClinicalTrials.gov/show/NCT00516399 (accessed 20 Sept 2011).
Oude Lashof 2011 {published data only}
- Oude Lashof AM, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, et al. Ocular manifestations of candidemia. Clinical Infectious Diseases 2011;53(3):262‐8. [DOI] [PubMed] [Google Scholar]
Panda 1996 {published data only}
- Panda A, Sharma N, Angra SK. Topical fluconazole therapy of Candida keratitis. Cornea 1996;15(4):373‐5. [DOI] [PubMed] [Google Scholar]
Rao 1997 {published data only}
- Rao SK, Madhavan HN, Rao G, Padmanabhan P. Fluconazole in filamentous fungal keratitis. Cornea 1997;16(6):700. [PubMed] [Google Scholar]
Ray 2002 {published data only}
- Ray A, Rao SK, Fogla R, Padmanabhan P, Kalavathy CM, Thomas PA, et al. Efficacy of topical and systemic itraconazole as a broad‐spectrum antifungal agent in mycotic corneal ulcer. A preliminary study. Indian Journal of Ophthalmology 2002;50(1):70‐2. [PubMed] [Google Scholar]
Reddy 1982 {published data only}
- Reddy PR, Reddy PS, Reddy AR, Saboo NK. A comparative evaluation of nystatin, amphotericin‐b and miconazole in keratomycosis. Indian Journal of Ophthalmology 1982;30(4):249‐50. [PubMed] [Google Scholar]
Shuai 2012 {published data only}
- Shuai SS, Ning HZ, Chen F, He XY, Yu QS. Clinical observation of povidone iodine combined with carbolic acid in the treatment of fungal corneal ulcer. International Eye Science 2012;12(12):2329‐30. [Google Scholar]
Sun 1996 {published data only}
- Sun B, He Y, Wang Y. Comparison of various types of imidazole derivatives for treatment of filamentous fungal keratitis. Chung Hua Yen Ko Tsa Chih 1996;32(4):260‐3. [PubMed] [Google Scholar]
Xie 2001 {published data only}
- Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. British Journal of Ophthalmology 2001;85(9):1070‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Qu 2013 {published data only}
- Qu XL, Zhao GQ, Gao A, Che CY, Lin J, Hu LT, et al. Efficacy observation of the fungal keratitis treated by the Natamycin and recombinant bovine basic fibroblast growth factor ophthalmic gel. International Eye Science 2013;13(7):1322‐5. [Google Scholar]
References to ongoing studies
CTR 2011 091 000107 {published data only}
- CTRI/2011/091/000107. Evaluation of intracameral amphotericin B in the management of deep fungal keratitis:randomized controlled trial. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2625 (accessed 18 March 2015).
MUTT II {published data only}
- NCT00997035. Mycotic Ulcer Treatment Trial II. ClinicalTrials.gov/show/NCT00997035 (accessed 18 March 2015).
Additional references
Galarreta 2007
- Galarreta DJ, Tuft SJ, Ramsay A, Dart JK. Fungal keratitis in London: microbiological and clinical evaluation. Cornea 2007;26(9):1082‐6. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gopinathan 2002
- Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10 year review at a referral eye care center in South India. Cornea 2002;21(6):555‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins J P, Thompson S G, Deeks J J, Altman D G. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liesegang 1980
- Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. American Journal of Ophthalmology 1980;90(1):38‐47. [DOI] [PubMed] [Google Scholar]
O' Brien 1997
- O' Brien TP, Rhee P. Pharmacotherapy of fungus infections of the eye. In: Zimmerman TJ, Koonere KS, Fecthner RD, Sharir M editor(s). Textbook of ocular pharmacology. Hagerstown: Lipincott‐Raven, 1997:587‐607. [Google Scholar]
O' Day 1996
- O' Day D. Fungal keratitis. In: Pepose JS editor(s). Ocular infection and immunity. St Louis: Moseby, 1996:1048‐61. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Tu 2007
- Tu EY, Park AJ. Recalcitrant Beauveria bassiana keratitis: confocal microscopy findings and treatment with posaconazole (Noxafil). Cornea 2007;26(8):1008‐10. [DOI] [PubMed] [Google Scholar]
Valenton 2000
- Valenton M. Central microbial keratitis. Philippine Journal of Ophthalmology 2000;25(1):10‐21. [Google Scholar]
References to other published versions of this review
FlorCruz 2003
- FlorCruz NV, Peczon Jr, I. Medical interventions for fungal keratitis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004241] [DOI] [PubMed] [Google Scholar]
FlorCruz 2008
- FlorCruz NV, Peczon IV. Medical interventions for fungal keratitis. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004241.pub2] [DOI] [PubMed] [Google Scholar]
FlorCruz 2012
- FlorCruz NV, Peczon IV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004241.pub3] [DOI] [PubMed] [Google Scholar]


