Abstract
Background
Residual neuromuscular block is associated with serious postoperative complications. Some anaesthesiologists use neostigmine to reverse neuromuscular blockade for all paediatric surgical patients. However, the incidence of residual neuromuscular block may be lower in paediatric patients than in adults. The use of neostigmine has also caused complications, such as postoperative nausea, vomiting, excessive salivation and bradycardia. Therefore, whether neostigmine should be used routinely to reverse neuromuscular blockade in each paediatric patient is an important question for paediatric anaesthesiologists.
Objectives
To assess the necessity of routine usage of neostigmine in preventing residual neuromuscular blockade in paediatric patients following the use of muscle relaxants.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 8, part of The Cochrane Library; MEDLINE via Ovid (1946 to August 2013); EMBASE via Ovid SP (1974 to August 2013); ClinicalTrials.gov (18 August 2013) and Chinese Clinical Trial Registry (18 August 2013) with no language restrictions.
Selection criteria
We planned to include randomized controlled trials (RCTs) comparing neostigmine versus placebo in American Society of Anaesthesiologists (ASA) I or II paediatric surgical participants (younger than 12 years of age, including newborns) who had received non‐depolarizing muscle relaxants.
Data collection and analysis
Two review authors independently assessed the studies for inclusion.
Main results
We found no study that satisfied the inclusion criteria. We found one study awaiting classification.
Authors' conclusions
No RCTs were found that supported, or argued against, the routine use of neostigmine to reverse neuromuscular block in paediatric patients.
Keywords: Child, Humans, Neuromuscular Blockade, Neostigmine, Neostigmine/adverse effects, Neostigmine/therapeutic use, Neuromuscular Nondepolarizing Agents, Neuromuscular Nondepolarizing Agents/adverse effects, Neuromuscular Nondepolarizing Agents/therapeutic use
Plain language summary
Neostigmine for reversing muscle paralysis in children following surgery
Review question
We reviewed the evidence about whether neostigmine should be routinely used to prevent residual muscle paralysis in children who received muscle relaxants during surgery.
Background
Neuromuscular blocks are drugs that cause paralysis of the skeletal muscles. They are used to facilitate certain surgical procedures. Residual muscle paralysis after surgery is associated with serious complications in children such as low oxygen content (hypoxia). Neostigimine is a drug that is used to reverse the effects of neuromuscular blocks. Neostigime reduces the risk of paralysis, but it can also cause children to feel sick and vomit and to produce excessive saliva and have a slow heart rate (bradycardia). As risk of residual paralysis is lower in children than in adults after neuromuscular blocks, the use of neostigmine in all surgeries performed on children should be carefully considered.
Study characteristics
This evidence is current to August 2013. We found no study that satisfied the inclusion criteria.
Key results
We found only one study that is awaiting classification. No RCTs supported, or argued against, the routine use of neostigmine to reverse neuromuscular block in paediatric patients.
Quality of the evidence
We found no relevant evidence.
Background
Description of the condition
Residual neuromuscular block refers to symptoms and signs such as muscle paralysis, apnoea and hypoxia in the postoperative period that are related to the administration of muscle relaxants during a surgical procedure. It is important for patient safety to assess residual muscle paralysis in the correct manner. Traditional methods used to evaluate recovery of neuromuscular function include head lift, hand grip and respiratory function. Moreover, anaesthesiologists often use train‐of‐four (TOF) monitoring to assess residual muscle paralysis. Residual neuromuscular block has been defined as a TOF ratio < 0.7, but recently the value was changed to 0.9 (Murphy 2010). Although patients may have satisfactory recovery of muscle strength with a TOF ratio > 0.9 (Eriksson 1997; Kopman 1997), obvious muscle weakness may be present in some patients. Therefore, a residual neuromuscular block assessment requires not only TOF monitoring but also clinical observation (Murphy 2010).
The incidence of residual neuromuscular block in adults varies from 2% to 64% in different clinical trials owing to different muscle relaxants and different definitions (Murphy 2010). According to Bevan's study (Bevan 1996), which used mivacurium and defined residual neuromuscular block as TOF ratio < 0.7, no residual blockade was found in all children regardless of whether the blockade was reversed. This study showed that the incidence of residual neuromuscular blockade is different between adults and children, and that it may be associated with metabolic alterations. Therefore, it is unclear how frequently residual blockade occurs or whether a routine reversal is necessary in children.
Description of the intervention
Neostigmine is a reversible acetylcholinesterase inhibitor; its distribution and elimination half‐lives are 3.4 and 77 minutes, respectively (Miller 2009). The elimination half‐life in children is shorter than in adults (Fisher 1983). Neostigmine is usually used to reverse the effects of non‐depolarizing muscle relaxants at the end of an operation. It takes a shorter time to reverse the blockade in infants than in children or adolescents (Kirkegaard‐Nielsen 1995). Larger doses of neostigmine reverse the blockade more rapidly and completely than smaller doses. However, it has a maximum effective dosage, and administering additional neostigmine will not produce further reversal. The maximum effective dose is in the range of 0.06 to 0.08 mg/kg, and the recommended dose for blockade reversal in paediatric patients is 0.02 to 0.06 mg/kg when combined with 0.02 mg/kg atropine (Miller 2009).
Drugs such as quinidine and aminoglycoside may interfere with neuromuscular transmission and could decrease the efficacy of neostigmine. Increased acetylcholine resulting from neostigmine administration stimulates both nicotinic and muscarinic receptors. Adverse effects of neostigmine are associated with overstimulation of cholinergic nerves and include bradycardia, salivation, nausea and vomiting. In the worst cases, arrhythmia and bronchospasm can also occur (Hunter 2006).
How the intervention might work
Acetylcholinesterase contains two sites of action. One is an anionic site, which attracts acetylcholine (ACh). The other is an esteratic site, which combines with ACh and hydrolyzes it rapidly to deactivate it. Neostigmine occupies both sites of acetylcholinesterase and thus prevents it from binding to and hydrolyzing ACh (Caldwell 2009). Consequently, the amount of ACh in the synaptic cleft increases. The increased ACh is in competition with the non‐depolarizing agents to reverse the neuromuscular block. However, neostigmine has a ceiling effect. When neostigmine inhibits all the acetylcholinesterase, giving further neostigmine has no clinical effects on reversal. In addition, both the antagonism induced by neostigmine and the natural decrease in the muscle relaxant concentration contribute to recovery of neuromuscular function when neostigmine is administered.
Why it is important to do this review
Muscle relaxants are commonly used for endotracheal intubation or for maintenance of muscle paralysis during surgery in children (Meretoja 2010). Residual neuromuscular block may cause serious respiratory complications, such as hypoxia (low oxygen content), during the postoperative period. Residual blockade may have greater influence in children because of their limited respiratory reserve capacity. So it is important for anaesthesiologists to ensure that no residual paralysis is present in such children during the postoperative period. For infants and neonates, most of the traditional methods (such as head lift, hand grip and so on) used to assess residual neuromuscular blockade are impractical because co‐operation is lacking. One study (Meretoja 1990) showed that the average TOF ratio was only 0.5 at the time of extubation if recovery was assessed by clinical criteria. It seems that we should assess neuromuscular blockade in children by using the TOF monitor, but, unfortunately, most anaesthesiologists do not use a neuromuscular monitor in their daily work (Naguib 2010). Therefore, it has been recommended to use neostigmine with atropine to reverse neuromuscular blockade in all neonates and small infants, even if they have shown clinical signs of recovery (Miller 2009). However, some disagree because researchers have indicated that spontaneous and induced recovery from neuromuscular block may be faster in children than in adults (Goudsouzian 1984; Meakin 1983). Sometimes anaesthesiologists administer muscle relaxants just for the purpose of achieving endotracheal intubation. Therefore, it may be unnecessary to reverse neuromuscular blockade in every paediatric patient at the end of surgery. Furthermore, large doses of neostigmine may not reverse the blockade but may prolong muscle weakness (Bevan 1992), and salivation or bradycardia may be induced after neostigmine is used. Whether we should routinely use neostigmine in paediatric patients at the end of an operation to reverse neuromuscular blockade is an important question for paediatric anaesthesiologists.
Objectives
To assess the necessity of routine usage of neostigmine in preventing residual neuromuscular blockade in paediatric patients following the use of muscle relaxants.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs) that compared neostigmine versus placebo, irrespective of language and publication status.
Types of participants
We planned to include American Society of Anesthesiologists (ASA) I or II paediatric patients (younger than 12 years of age, including newborns) who had received non‐depolarizing muscle relaxants when undergoing any kind of operation.
We planned to exclude individuals with a known history of neuromuscular disease, cardiovascular disease, respiratory disease, hepatic disease or drug abuse and those who had recently taken medication affecting neuromuscular transmission. We also planned to exclude premature babies.
Types of interventions
Paediatric participants who were administered neostigmine at the end of surgery to reverse neuromuscular blockade constituted the intervention group.
Paediatric participants who were administered placebo at the end of surgery constituted the control group.
We intended to compare the intervention group versus the control group.
Types of outcome measures
Primary outcomes
Mortality of children in the postoperative period.
Risk of assisted ventilation (including mask ventilation, laryngeal mask airway and intubation) after extubation.
Secondary outcomes
Risk of drug‐related adverse effects: bradycardia, heart rate < 60 per minute; excessive salivation; emesis.
Risk of postoperative residual neuromuscular block: We defined residual neuromuscular block as TOF ratio < 0.9 using acceleromyography (mechanomyography or electromyography).
Time between neostigmine or placebo administration and TOF ratio recovery to 0.9.
Risk of hypoxia (partial pressure of arterial oxygen < 60 mmHg or oxygen saturation (SpO2) < 90%) after extubation.
Length of stay in the postanaesthesia care unit (PACU).
Search methods for identification of studies
Electronic searches
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 8, part of The Cochrane Library; MEDLINE via Ovid (1946 to August 2013); and EMBASE via Ovid SP (1974 to August 2013).
We combined free‐text terms and subject headings when searching CENTRAL (for our detailed search strategy, see Appendix 1).
We used the sensitive strategies described in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search for RCTs in MEDLINE and EMBASE via Ovid. We combined the exploded Medical Subject Headings (MeSH) terms (or EMTREE terms) and associated free‐text terms with the RCT sensitive search strategy (for detailed search strategies, see Appendix 2 and Appendix 3).
We applied no language restrictions to the search strategy.
Searching other resources
We searched for ongoing trials in the following databases: ClinicalTrials.gov (18 August 2013) and Chinese Clinical Trial Registry (18 August 2013).
Data collection and analysis
Selection of studies
Two review authors (LY and DY) independently screened all studies for eligibility based on their titles, abstracts and keywords (see Appendix 4 for a copy of the Study Selection Form). We resolved disagreements by consulting with a third review author (QL). We retrieved full‐text copies of all not to be excluded papers for further assessment. We contacted the first author of these papers to ask for non‐reported information.
We planned to list all eligible trials on a form (see Appendix 5 for a copy of the Eligible Trials Form).
Data extraction and management
Two review authors (LY and DY) independently extracted data onto a paper form (see Appendix 6 for a copy of the Data Extraction Form). We resolved disagreements by discussion. DY planned to enter data into a RevMan file, and LY planned to check the accuracy of the data entry.
Assessment of risk of bias in included studies
Two review authors (LY and DY) planned to independently assess the methodological quality of included papers. We planned to resolve disagreements by discussion with a third review author (QL).
We planned to perform risk of bias assessment using the 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A copy of the form that we planned to use to do this will be found in Appendix 7. We planned to assess each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other potential threats to validity.
We intended to consider a trial as having low risk of bias if all domains were assessed as low risk. We intended to consider a trial as having high risk of bias if one or more domains were assessed as high or unclear risk.
We planned to report the 'Risk of bias' table as part of the 'Table of characteristics of included studies' and to present a 'Risk of bias summary' figure, which would detail all judgements made for all included studies in the review.
Measures of treatment effect
We planned to present dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous data as mean differences (MD) with 95% CIs. In the case of outcomes with continuous data in different scales, we planned to use standardized mean differences (SMDs) with 95% CIs.
Unit of analysis issues
All comparisons were intended to be based on individual trial participants.
Dealing with missing data
We planned to contact the first author to request missing data. We planned to include in the review any relevant information obtained in this way.
We planned to perform both available case analysis and intention‐to‐treat analysis. We intended to compare these results in a sensitivity analysis.
Assessment of heterogeneity
We planned to assess clinical diversity (e.g. different types of muscle relaxants, length of surgery, age of children) and methodological diversity (risk of bias assessment). If they were similar enough, we intended to pool the data in a meta‐analysis. Then we intended to assess statistical significance with a P value < 0.1. We also intended to calculate the I2 statistic to assess the impact of heterogeneity on the meta‐analysis. We considered heterogeneity to be substantial if the value of the I2 statistic was greater than 50%. If substantial heterogeneity (I2 > 50%) was found in the results, we intended to explore possible sources of heterogeneity using the sensitivity and subgroup analyses described below.
Assessment of reporting biases
We planned to assess publication bias by using funnel plots if more than 10 studies were included in the meta‐analysis (Egger 1997).
Data synthesis
If heterogeneity was not substantial, we intended to combine all trial results. We planned to use RevMan 5.1 to perform the analysis along with a random‐effects model.
For dichotomous data, we planned to use Mantel‐Haenszel methods.
For continuous data, we planned to use MDs if all outcomes used the same scale; otherwise we planned to use SMDs.
If heterogeneity was considerable, we intended to refrain from combining any results. We planned to perform a descriptive analysis.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses for different interventions and participants.
Different definitions of residual neuromuscular block.
Types of muscle relaxants.
Length of surgery greater than one hour.
Different age groups (neonates younger than 28 days, younger than one year old, one to three years and older than three years).
Different races.
Neuromuscular monitoring (quantitative or qualitative means or not).
Sensitivity analysis
We planned to reanalyse the data using different statistical approaches (random‐effects model or fixed‐effect model). We planned to approach missing data using different imputation methods (best case, worst case; last observation carried forward).
Summary of findings
We planned to use the principles of the GRADE system in the review (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (mortality of children in postoperative period; risk of assisted ventilation after extubation; risk of drug‐related adverse effects (bradycardia, excessive salivation, vomiting)) and to construct the 'Summary of findings' (SoF) table using the GRADE software. The GRADE approach is used to appraise the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
We found 387 records using our search strategy and 12 records via other sources. A total of 343 records remained after we removed duplicates. We screened all titles, abstracts and keywords for eligibility and excluded all but three studies. We retrieved the three possible studies in full text for further assessment. Of those three studies, we excluded two (Baxter 1991; Neidhar 1996) and placed one study (Watcha 1995) in awaiting classification (see Figure 1). We tried to contact the lead author (Watcha 1995) via email, as we needed more information for the review. We also tried to contact the study author through the Cochrane Anaesthesia Review Group; however no response was received from the lead author.
1.
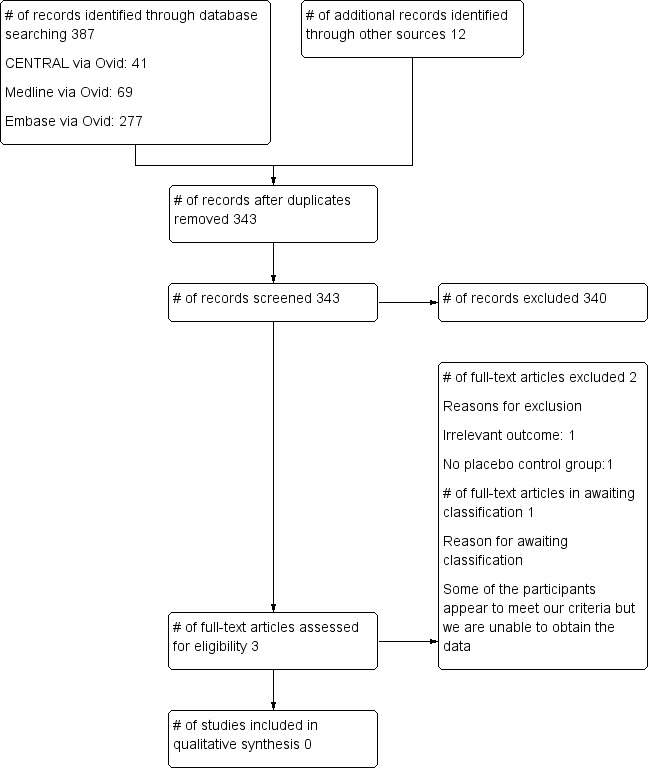
Flow diagram.
Included studies
No eligible studies were identified (see Characteristics of studies awaiting classification).
Excluded studies
We excluded two studies after reading the full papers (Baxter 1991; Neidhar 1996). The reasons for exclusion are documented in the Characteristics of excluded studies.
Risk of bias in included studies
We planned to report mortality and risk of assisted ventilation as primary outcomes, and risk of residual neuromuscular block, risk of adverse effects, time for recovery, risk of hypoxia and length of stay in the PACU as secondary outcomes. But unfortunately, no eligible studies were identified.
Allocation
No eligible studies were identified.
Blinding
No eligible studies were identified.
Incomplete outcome data
No eligible studies were identified.
Selective reporting
No eligible studies were identified.
Other potential sources of bias
No eligible studies were identified.
Effects of interventions
No eligible studies were identified.
Discussion
Summary of main results
We found no RCTs comparing neostigmine versus placebo in paediatric participants. So far, no RCT has reported benefit from the routine administration of neostigmine in each child at the end of surgery. One study, which is awaiting classification (Watcha 1995), reported that neostigmine increased the risk of emesis over placebo. However, some participants in that study were probably older than 12 years of age (our inclusion criterion was younger than 12 years of age). We tried to contact the lead author of that study to obtain the information needed but failed. Therefore, we have found no evidence that directly addresses our research question and that would enable us to determine the effects of routine usage of neostigmine in paediatric participants following the use of muscle relaxants.
Overall completeness and applicability of evidence
To better illuminate the influence of routine use of neostigmine on important clinical outcomes, we used patient‐relevant, non‐surrogate measurable outcomes as primary outcomes. But unfortunately, the participants included in most of the studies were not children. Therefore, even though we searched CENTRAL, MEDLINE, EMBASE, ClinicalTrials.gov and the Chinese Clinical Trial Registry without language restrictions, we found no relevant evidence.
Quality of the evidence
We found no relevant evidence.
Potential biases in the review process
We found no relevant evidence.
Agreements and disagreements with other studies or reviews
We found no relevant evidence.
Authors' conclusions
Implications for practice.
No RCTs were found to support, or argue against, the routine use of neostigmine to reverse neuromuscular block in paediatric patients.
Implications for research.
Randomized controlled trials are required to assess the necessity of routine usage of neostigmine in preventing residual neuromuscular blockade in paediatric patients following the use of muscle relaxants. So far, few studies have been concerned about the issue. In future clinical trials, the following need to be considered.
Risk of residual neuromuscular block in paediatric participants.
Adverse effects of neostigmine as antagonists to muscle relaxants in paediatric participants.
Time when administering neostigmine with or without neuromuscular monitor.
Types of muscle relaxants (such as long‐time and short‐time drugs).
Different ages of participants (such as neonates, infants and children).
Neuromuscular monitoring (quantitative or qualitative means or not).
Acknowledgements
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia Review Group (CARG)) and Karen Hovhannisyan (Trials Search Co‐ordinator, CARG) for their help. We would like to thank Huang Han (West China Second University Hospital) for advice provided. We would like to thank Patrick Brass for helping us identify and extract data from the German paper (Neidhar 1996).
We would like to thank Javier Eslava‐Schmalbach (content editor); Jeffrey K Lu, Dominic A Cave and Oliver Karam (peer reviewers); and Robert Wyllie (consumer referee) for help and editorial advice provided during the preparation of this systematic review.
We also would like to thank Wu Taixiang (Professor of Chinese Evidence‐Based Medcine Centre) for his help and guidance. We would like to thank Mathew Zacharias (content editor); Nathan Pace (statistical editor); and Dominic Cave, Oliver Karam, Santhanam Suresh, Jeffrey Lu and William Mcilvaine (peer reviewers) for help and editorial advice provided during the preparation of this protocol for the systematic review.
Appendices
Appendix 1. The Cochrane Library search strategy
#1 synstigmin or prostigmin or prostigmine or vagostigmin or polstigmine or proserine or prozerin or syntostigmine
#2 MeSH descriptor: [Neostigmine] explode all trees
#3 #1 or #2
#4 MeSH descriptor: [Muscle Relaxation] explode all trees
#5 paralysis or residual neuromuscular block
#6 #4 or #5
#7 #3 and #6
Appendix 2. MEDLINE (Ovid) search strategy
1. (synstigmin or prostigmin or prostigmine or vagostigmin or polstigmine or proserine or prozerin or syntostigmine).mp. or exp neostigmine 2. exp muscle relaxation/ or paralysis/ or paralysis.mp. or residual neuromuscular block.mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp neostigmine/ or synstigmin.mp. or prostigmin.mp. or prostigmine.mp. or vagostigmin.mp. or polstigmine.mp. or proserine.mp. or prozerin.mp. or syntostigmine.mp. 2. exp muscle relaxation/ or exp paralysis/ or paralysis.mp. or residual neuromuscular block.mp. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Study selection form
Study selection form
| First author | Journal/Conference proceedings etc | Year |
Study eligibility
| RCT | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No*/Unclear |
*Issue relates to selective reporting—when authors may have taken measurements for particular outcomes but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in 'Studies awaiting assessment' until clarified. If no clarification is received after three attempts, the study should then be excluded.
| Do not proceed if any of the above answers is 'No'. If study to be included in 'Excluded studies' section of the review, record below the information to be inserted into 'Table of excluded studies.' |
| Freehand space for comments on study design and treatment: |
Appendix 5. Eligible trials form
Eligible trials form
| Code each paper | Author(s) | Journal/Conference proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers | ||
Appendix 6. Data extraction form
Data extraction form
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers/%, etc) | |
| Other | |
| Trial characteristics | |
| Further details | |
| Single centre/Multi‐centre | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomly assigned? | |
| Number of participants in each intervention group/Control group | / |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Anaesthesia drugs | |
| Dose of neostigmine | |
| Anticholinergic agents/Dose | |
| Dose of placebo | |
| Trial design (e.g. parallel/Cross‐over*) | |
| Neuromuscular monitoring | Quantitative means/Qualitative means/Not |
| Other | |
Data extraction
| Outcomes | Reported in paper (circle) |
| Primary outcomes | |
| Outcome 1—Mortality of children in postoperative period | Yes/No |
| Outcome 2—Risk of assisted ventilation (including mask ventilation, laryngeal mask airway and intubation) after extubation | Yes/No |
| Outcome 3—Risk of drug‐related adverse effects | Bradycardia/Salivation/Vomiting/Others |
| Secondary outcomes | |
| Outcome 1—Risk of postoperative residual neuromuscular block: We defined residual neuromuscular block as TOF ratio < 0.9 in acceleromyography (mechanomyography or electromyography) | Yes/No |
| Outcome 2—Time between neostigmine or placebo administration and TOF ratio recovery to 0.9 | Yes/No |
| Outcome 3—Risk of hypoxia after extubation | Yes/No |
| Outcome 4—Length of stay in PACU | Yes/No |
| Subgroups | Reported in paper |
| Definition of residual neuromuscular block | |
| Muscle relaxant | |
| Length of surgery | |
| Age | Neonate/Younger than 3 years old/Older than 3 years old |
| Race | White/Black/Yellow |
| For continuous data | |||||||
| Code of paper | Outcomes | Unit of measurement | Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Time between neostigmine or placebo administration and TOF ratio recovery to 0.9 | |||||||
| Length of stay in PACU | |||||||
| For dichotomous data | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| Mortality of children in postoperative period | |||
| Risk of assisted ventilation after extubation | |||
| Risk of postoperative residual neuromuscular block | |||
| Risk of hypoxia after extubation | |||
| Risk of drug‐related adverse effects (list below, if any are reported) | |||
| Bradycardia | |||
| Salivation | |||
| Vomiting | |||
|
Other information that you feel is relevant to the results Indicate whether any data were obtained from the primary author; or whether results were estimated from graphs etc or were calculated by you using a formula (this should be stated and the formula given). In general if results not reported in the paper(s) are obtained, this should be made clear here to be cited in the review. |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, list contact names and details | ||
Appendix 7. Risk of bias table
Risk of bias table
| Domain | Judgement | Description |
| Random sequence generation | Low risk/High risk/Unclear | Quote: Comment: |
| Allocation concealment | Low risk/High risk/Unclear | Quote: Comment: |
| Blinding of participants and personnel | Low risk/High risk/Unclear | Quote: Comment: |
| Blinding of outcome assessment | Low risk/High risk/Unclear | Quote: Comment: |
| Incomplete outcome data | Low risk/High risk/Unclear | Quote: Comment: |
| Selective reporting | Low risk/High risk/Unclear | Quote: Comment: |
| Other sources of bias | Low risk/High risk/Unclear | Quote: Comment: |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baxter 1991 | No placebo control group |
| Neidhar 1996 | Irrelevant outcomes |
Characteristics of studies awaiting assessment [ordered by study ID]
Watcha 1995.
| Methods | Randomized, double‐blind, placebo‐controlled study |
| Participants | ASA I or II children scheduled for brief, elective peripheral procedures (not involving body cavities) under general endotracheal anaesthesia. 113 participants were randomly allocated to placebo (37 participants), neostigmine (38 participants) and edrophonium groups (38 participants) Placebo group (N = 37 participants): age (y): 8.6 ± 4.3, weight (kg): 35.7 ± 24.4, surgical time (min): 56 ± 38, anaesthetic time (min): 106 ± 48 Neostigmine group (N = 38 participants): age (y): 9.4 ± 4.8, weight (kg): 37.2 ± 23.6, surgical time (min): 69 ± 47, anaesthetic time (min): 117 ± 56 (Values are mean ± SD) |
| Interventions | Neostigmine: 70 μg/kg with glycopyrrolate 10 μg/kg in 10 ml Placebo: 10 ml saline At the end of surgical procedure, participants received one of the drugs |
| Outcomes | Lowest oxyhaemoglobin saturation by pulse oximetry (%): neostigmine 97.6 ± 1.8, placebo 98.5 ± 2.5 Risk of postoperative emesis in PACU (%): neostigmine 34, placebo 11 (P value < 0.05) Risk of postoperative emesis without analgesic therapy in PACU (%): neostigmine 36, placebo 10 (P value 0.04) Risk of overall 24‐hour emesis (%): neostigmine 54, placebo 44 (Values are mean ± SD) |
| Notes | We placed the study in 'awaiting classification' because some of the children in the study are older than 12 years (our inclusion criterion is younger than 12 years of age, including newborns). We wrote to the study author to request relevant information in February 2013, but as yet have not received a reply |
Abbreviations:
PACU: Postanaesthesia care unit.
Differences between protocol and review
We removed the age limit terms from the search strategy shown in the protocol (Yang 2012). The reason for this change is that the age limit can prevent collection of studies in which both adults and children are included. To consider the effect of monitoring devices, we added neuromuscular monitoring to the Subgroup analysis and investigation of heterogeneity.
Contributions of authors
Conceiving of the review: Lei Yang (LY).
Co‐ordinating the review: LY.
Undertaking manual searches: LY.
Screening search results: LY, Di Yang (DY).
Organizing retrieval of papers: LY.
Screening retrieved papers against inclusion criteria: LY, DY, Qian Li (QL).
Appraising quality of papers: LY, DY, QL.
Abstracting data from papers: LY, DY.
Writing to authors of papers for additional information: LY.
Providing additional data about papers: LY.
Obtaining and screening data on unpublished studies: LY.
Managing data for the review: LY, DY.
Entering data into Review Manager (RevMan 5.1): LY, DY.
Handling RevMan statistical data: LY, DongHao Lu (DL).
Performing other statistical analysis not using RevMan: LY, DL.
Performing double entry of data (data entered by person one: LY; data entered by person two: DY).
Interpreting data: LY, DY, QL, Yunxia Zuo (YZ).
Making statistical inferences: LY, DL.
Writing the review: LY, DY, YZ.
Securing funding for the review: DY, LY.
Performing previous work that served as the foundation of the present study: DY, LY.
Serving as guarantor for the review (one author): LY.
Taking responsibility for reading and checking the review before submission: DY.
LY and DY contributed equally to the review.
Sources of support
Internal sources
None, China.
External sources
None, China.
Declarations of interest
Qian Li and Di Yang took part in a clinical multi‐centre study on suggamadex in China, but they have not published any article about this and have received no material aid to attend any meetings on suggamadex.
Lei Yang: none known.
Yunxia Zuo: none known.
DongHao Lu: none known.
New
References
References to studies excluded from this review
Baxter 1991 {published data only}
- Baxter MR, Bevan JC, Samuel J, Donati F, Bevan DR. Postoperative neuromuscular function in pediatric day‐care patients. Anesthesia and Analgesia 1991;72:504‐8. [PUBMED: 1672489] [DOI] [PubMed] [Google Scholar]
Neidhar 1996 {published data only}
- Neidhart G, Pabelick C, Kuhn I, Leuwer M, Vettermann J. Influence of halothane, enflurane and isoflurane on the pharmacodynamics of mivacurium in children (German) [EinfluB von Halothan, Enfluran und Isofluran auf die Pharmakodynamik von Mivacurium bei Kindern]. Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 1996;31(5):293‐7. [PUBMED: 8767242] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Watcha 1995 {published data only}
Additional references
Bevan 1992
- Bevan DR, Donati F, Kopman AF. Reversal of neuromuscular blockade. Anesthesiology 1992;77(4):785‐805. [PUBMED: 1416176] [DOI] [PubMed] [Google Scholar]
Bevan 1996
- Bevan DR, Kahwaji R, Ansermino JM, Reimer E, Smith MF, O'Connor GA, et al. Residual block after mivacurium with or without edrophonium reversal in adults and children. Anesthesiology 1996;84(2):362‐7. [PUBMED: 8602667] [DOI] [PubMed] [Google Scholar]
Caldwell 2009
- Caldwell JE. Clinical limitations of acetylcholinesterase antagonists. Journal of Critical Care 2009;24(1):21‐8. [PUBMED: 19272535] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksson 1997
- Eriksson LI, Sundman E, Olsson R, Nilsson L, Witt H, Ekberg O, et al. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology 1997;87(5):1035‐43. [PUBMED: 9366453] [DOI] [PubMed] [Google Scholar]
Fisher 1983
- Fisher DM, Cronnelly R, Miller RD, Sharma M. The neuromuscular pharmacology of neostigmine in infants and children. Anesthesiology 1983;59(3):220‐5. [PUBMED: 6309036] [DOI] [PubMed] [Google Scholar]
Goudsouzian 1984
- Goudsouzian NG, Martyn JJ, Liu LM, Ali HH. The dose response effect of long‐acting nondepolarizing neuromuscular blocking agents in children. Canadian Anaesthetists' Society Journal 1984 May;31(3 Pt 1):246‐50. [PUBMED: 6722619] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hunter 2006
- Hunter JM, Flockton EA. The doughnut and the hole: a new pharmacological concept for anaesthetists. British Journal of Anaesthesia 2006 Aug;97(2):123‐6. [PUBMED: 16831877] [DOI] [PubMed] [Google Scholar]
Kirkegaard‐Nielsen 1995
- Kirkegaard‐Nielsen H, Meretoja OA, Wirtavuori K. Reversal of atracurium‐induced neuromuscular block in paediatric patients. Acta Anaesthesiologica Scandinavica 1995 Oct;39(7):906‐11. [PUBMED: 8848890] [DOI] [PubMed] [Google Scholar]
Kopman 1997
- Kopman AF, Yee PS, Neuman GG. Relationship of the train‐of‐four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology 1997;86(4):765‐71. [PUBMED: 9105219 ] [DOI] [PubMed] [Google Scholar]
Meakin 1983
- Meakin G, Sweet PT, Bevan JC, Bevan DR. Neostigmine and edrophonium as antagonists of pancuronium in infants and children. Anesthesiology 1983;59:316‐21. [PUBMED: 6311057] [DOI] [PubMed] [Google Scholar]
Meretoja 1990
- Meretoja OA, Gebert R. Postoperative neuromuscular block following atracurium or alcuronium in children. Canadian Journal of Anaesthesia 1990 Oct;37(7):743‐6. [PUBMED: 2171792] [DOI] [PubMed] [Google Scholar]
Meretoja 2010
- Meretoja OA. Neuromuscular block and current treatment strategies for its reversal in children. Paediatric Anaesthesia 2010;20(7):591‐604. [PUBMED: 20642658] [DOI] [PubMed] [Google Scholar]
Miller 2009
- Miller RD. Miller's Anesthesia. 7th Edition. Philadelphia: Elsevier Churchill Livingstone, 2009. [Google Scholar]
Murphy 2010
- Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesthesia and Analgesia 2010;111(1):120‐8. [PUBMED: 20442260] [DOI] [PubMed] [Google Scholar]
Naguib 2010
- Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesthesia and Analgesia 2010 Jul;111(1):110‐9. [PUBMED: 19910616 ] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
References to other published versions of this review
Yang 2012
- Yang L, Yang D, Li Q, Zuo Y, Lu D. Neostigmine for reversal of neuromuscular block in paediatric patients. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010110] [DOI] [PMC free article] [PubMed] [Google Scholar]


