Abstract
Background:
Peripheral artery disease (PAD) is associated with modifiable atherosclerotic risk factors (RF) like hypertension, diabetes, hyperlipidemia, and smoking. However, the effect of RF control on outcomes and disparities in achieving control are less well understood.
Methods:
All patients in an integrated, regional health system with PAD-related encounters, fee-for-service Medicare, and clinical RF control data were identified. Component RFs were dichotomized into controlled and uncontrolled categories (control defined as LDL <100 mg/dL, hemoglobin A1c <7.0%, SBP <140 mmHg, and current non-smoker) and composite categories (none, 1, 2 uncontrolled RFs) created. The primary outcome was major adverse vascular events (MAVE, a composite of all-cause mortality, myocardial infarction, stroke, and lower extremity revascularization and amputation).
Results:
The cohort included 781 patients with PAD, average age 72.5±9.8 years, of whom 30.1% were Black, and 19.1% were Medicaid dual-enrolled. In this cohort, 260 (33.3%) had no uncontrolled RFs and 200 (25.6%) had 2 uncontrolled RFs. Patients with the poorest RF control were more likely to be Black (p<0.001), Medicaid dual-enrolled (p<0.001), and have chronic limb threatening ischemia (p=0.009). Significant differences in MAVE by degree of RF control were observed at 30 days (none uncontrolled: 5.8%, 1 uncontrolled: 11.5%, 2 uncontrolled: 13.6%, p=0.01) but not at 1 year (p=0.08). RF control was not associated with outcomes at 1 year after adjustment for patient and PAD-specific characteristics.
Conclusions:
RF control is poor among patients with PAD, with significant disparities in achieving optimal control a potential target for reducing inequities in outcomes.
Keywords: peripheral artery disease, risk factor control
Background:
Peripheral artery disease (PAD) is characterized by atherosclerotic narrowing of lower extremity arteries and affects more than 10 million Americans and 230 million people globally.1,2 Its presentation ranges from asymptomatic to disabling, and limb complications are associated with impaired quality of life, high healthcare costs, and significant morbidity and mortality.3–6 Disparities in outcomes by sex, race, rurality, and socioeconomic status have been noted, however the complex interplay of demographic factors and PAD severity and treatment remains unclear.1,4,7,8 Improvements in PAD care and outcomes, including a reduction in inequities, are important targets of study for this growing health problem.
Several risk factors (RF) have been implicated in the development and progression of PAD, similar to atherosclerotic disease in other vascular beds. Hypertension (HTN), diabetes mellitus (DM), hyperlipidemia (HLD), and smoking are modifiable RFs that contribute the majority of risk associated with developing PAD.1 However, most previous analyses focus on the role of present comorbidities in PAD risk and outcomes rather than degree of control of these RFs according to clinical guidelines.4,6,9–11
We aimed to describe demographic and disease-specific factors in achieving optimal RF control. We used clinical and laboratory data from electronic health records (EHR) coupled with Centers for Medicare and Medicaid Services (CMS) claims data to explore the role of RF control on longitudinal cardiovascular outcomes in a real-world cohort of patients with confirmed PAD. We hypothesized that optimal RF control is associated with fewer adverse cardiovascular and limb events in patients with PAD but is unequally achieved across our integrated health system.
Methods:
Study population
This retrospective cohort study was approved by the Duke Health Institutional Review Board. Patients were selected from Duke University Health System (DUHS), comprising three hospitals and over 140 primary and specialty clinics in a network dispersed across North Carolina.
Patients with PAD were identified from a data warehouse query of the DUHS EHR. Eligible patients had one or more clinical encounters with PAD-related International Classification of Disease (ICD)-9 or ICD-10 diagnosis codes documented in the EHR between January 1, 2015 and March 31, 2016 (list of diagnosis codes available in Supplemental Table 1). The index encounter date was defined as the discharge or departure date of the earliest PAD encounter in the study period, meaning that the majority of patients had prevalent PAD rather than incident PAD. PAD was clinically adjudicated through a multi-step algorithmic reduction process using a method that has previously been described.8,12 PAD was defined as having one of four clinical criteria: (1) an abnormal ankle brachial index (ABI, abnormal defined as 0.9 or >1.4), (2) imaging (computed tomography angiography, invasive peripheral angiography, magnetic resonance angiography or lower extremity ultrasound) that demonstrated significant arterial stenosis (defined as 50% stenosis), (3) prior lower extremity revascularization, or (4) prior non-traumatic lower extremity amputation for symptomatic PAD.
In order to assess outcomes longitudinally, we included patients who were enrolled in fee-for-service Medicare during the index encounter as well as continuously enrolled in the year prior and also had documented clinical and laboratory data in the two years prior to the index encounter to determine control of RFs of interest. Patients missing clinical or laboratory data for any of the studied RFs were excluded (Figure 1). Eligible patients had at least one outpatient systolic blood pressure (SBP) and at least one low density lipoprotein (LDL) value. Additionally, patients were eligible for the study if (1) there was at least one documented hemoglobin A1c (HbA1c) value regardless of history of DM or (2) no documented HbA1c value and no history of DM. Patients with a history of DM but no documented HbA1c in the two years prior to the index encounter were excluded.13 Eligible patients also had documentation of tobacco use (none, former, current).
Figure 1.
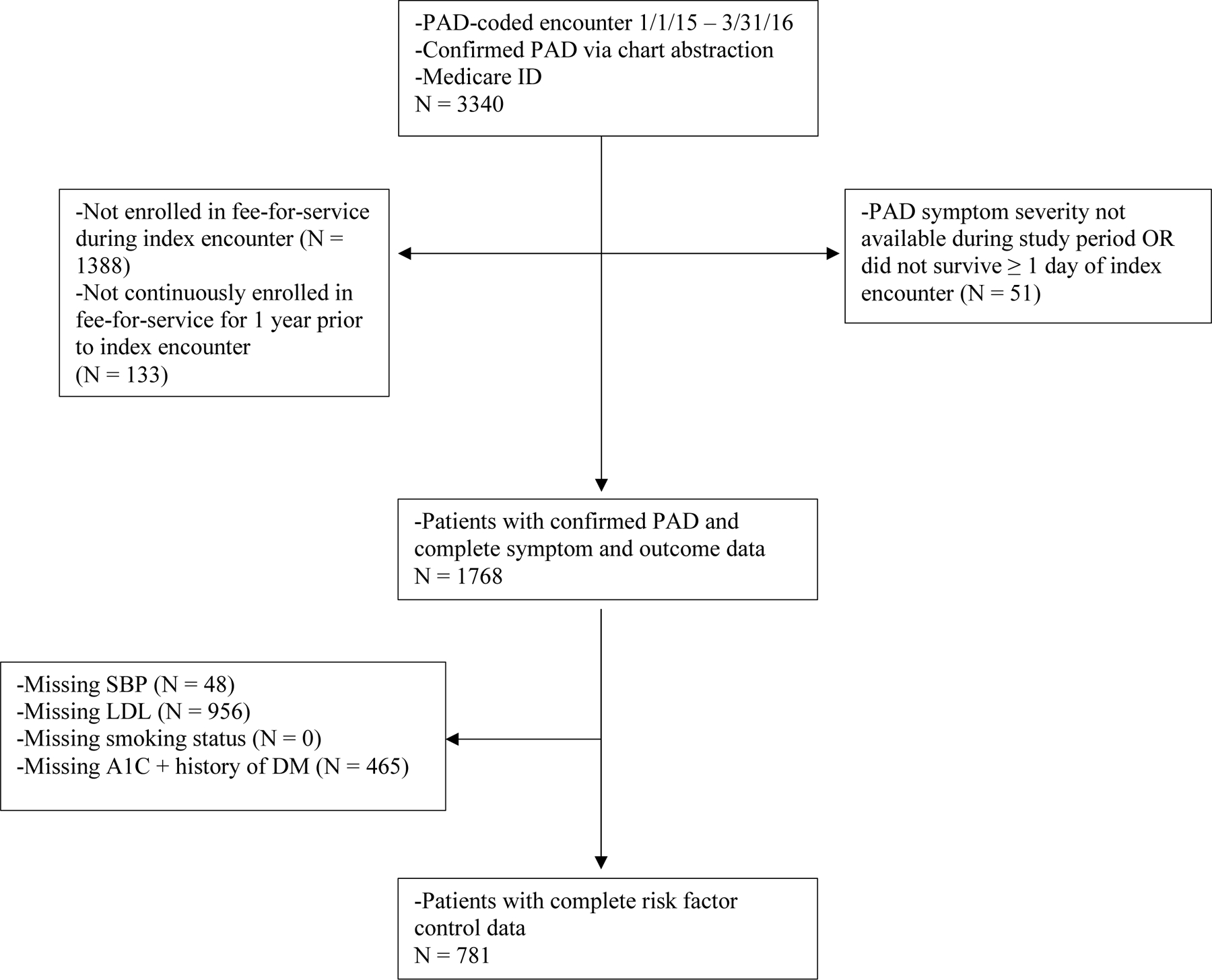
Ascertainment of Study Population
Study variables
Smoking status was manually abstracted from the EHR, while SBP and lab values (HbA1c, LDL) were extracted using an extraction tool from the Duke Enterprise Data Unified Content Explorer (DEDUCE) data warehouse.14 A review of patients with no lab values extracted by DEDUCE was performed to manually abstract missing data. PAD-specific characteristics were manually abstracted during the clinical adjudication process. These included the most severe PAD symptom (asymptomatic, intermittent claudication [IC], or chronic limb threatening ischemia [CLTI]) during the study period.
Other study variables included demographic characteristics of patients (age, sex, race), geographic region (rurality), socioeconomic status (census-tract or county level median household income), and insurance coverage (Medicaid dual enrollment), which were collected from Medicare files, EHR data, and American Community Survey tables as available. In accordance with the CMS cell size suppression policy that requires removal of cell counts <11, values of 1–10 were removed and other cells were represented as ranges to prevent the derivation of suppressed cells.15 Statin use at any time prior to the index encounter, as documented in the EHR, was reported as a way to give a general sense of guideline-based medication use. Use of other medications was not reported due to concerns about incomplete EHR documentation, fragmentation of patient care across multiple health systems, and lack of adherence and persistence data. Comorbid conditions were obtained from inpatient, outpatient, and carrier claims from the year prior to the index encounter using validated algorithms for cancer, cerebrovascular disease (CBVD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, DM, HTN, ischemic heart disease (IHD), renal disease, and the Charlson Comorbidity Index.13,16,17
Exposure definition
Degree of control for each RF in the period two years prior to the index encounter was dichotomized into controlled or uncontrolled categories, and patients were grouped by the number of uncontrolled RFs (none, 1, 2 uncontrolled RFs). RF control was dichotomized using clinical practice guideline recommendations instead of using RF values as continuous variables. Control was defined as LDL <100 mg/dL (<2.59 mmol/L), HbA1c <7.0%, SBP <140 mmHg, and tobacco use history of current non-smoker (never or former). Control thresholds were selected based on reasonable clinical targets outlined by contemporary guidelines during the lookback period.11 Only SBP values from outpatient encounters were obtained, while HbA1c and LDL values were obtained from both inpatient and outpatient encounters. SBP values >300 mmHg (none), LDL values >500 mg/dL (35), and HbA1c values >15% (1) were excluded as physiologically implausible. For continuous RF variables with multiple measurements per patient, control was determined by whether the majority of values fell into the controlled or uncontrolled category. In cases of a tie, the oldest value was dropped and the control category was reassessed. Patients without a documented HbA1c value and no history of DM were imputed to controlled HbA1c.
Outcome definitions and ascertainment
The primary outcome was major adverse vascular events (MAVE), a composite of all-cause mortality, myocardial infarction (MI), stroke, lower extremity revascularization, and lower extremity amputation. Secondary outcomes included major adverse cardiovascular events (MACE, a composite of all-cause mortality, MI, and stroke) and major adverse limb events (MALE, a composite of lower extremity revascularization and amputation). Secondary outcomes also included individual components for each composite plus all-cause hospitalization. Composite event dates were determined by the earliest occurring component outcome. Outcome events were ascertained by searching inpatient, outpatient, Master Beneficiary Summary File, and carrier files in the 100% Medicare claims data from 2014 through 2017. Outcomes were assessed through 1 year following the index encounter.
Statistical analysis
Baseline characteristics of the study cohort were described overall and stratified by number of uncontrolled RFs (none, 1, or 2) using proportions for categorical variables and means with standard deviations or medians with quartiles for continuous variables. Group differences were evaluated using chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Cumulative incidence for mortality at 30 days and 1 year from the index encounter date were calculated using Kaplan-Meier estimates. Group differences by number of uncontrolled RFs (none, 1, or 2) were calculated using the log-rank test. For all other outcomes (MAVE, MACE, MALE, component events, and all-cause hospitalization), the cumulative incidence function was used to account for the competing risk of death and group differences were evaluated using Gray’s test.
Cox proportional hazards regression models were used to evaluate the association of RF control and baseline characterisitcs with composite outocomes and all-cause hospitalization by one year. These models were adjusted using patient characteristic covariates. Two models were constructed for each outcome: the first model adjusted for control of individual component RFs and the second model adjusted for a single overall RF control variable (none, 1, or 2 RF uncontrolled). The two models explored the effects of individual component RF control and composite control, respectively. For all results, cell values <11 were suppressed in accordance with CMS cell suppression policy. All analyses were completed using SAS version 9.4.
Results:
Patient characteristics
A total of 3,340 patients with confirmed PAD and Medicare were identified during the study period, of which 1,768 had complete symptom and outcome data. Of these, 781 patients with complete data to determine RF control during the two years prior to the index date were included in the cohort (Figure 1). Patients with incomplete RF control data (N = 987) differed significantly from those with complete RF data across several demographic, geographic, socioeconomic, PAD symptom, and medical history baseline characteristics (Table 1).
Table 1:
Baseline characteristics of PAD patients included and excluded from cohort
| Variable | Included | Not included | p-value |
|---|---|---|---|
| N | 781 | 987 | |
| Symptom Severity | < .001 | ||
| Asymptomatic | 271 (34.7%) | 288 (29.2%) | |
| IC | 339 (43.4%) | 391 (39.6%) | |
| CLTI | 171 (21.9%) | 308 (31.2%) | |
| Demographics | |||
| Age (years), Mean (SD) | 72.51 (9.84) | 71.61 (9.71) | .02 |
| Age Category | .08 | ||
| < 65 | 144 (18.4%) | 204 (20.7%) | |
| 65 - < 75 | 293 (37.5%) | 408 (41.3%) | |
| 75 - < 85 | 257 (32.9%) | 282 (28.6%) | |
| ≥ 85 | 87 (11.1%) | 93 (9.4%) | |
| Sex, Male | 468 (59.9%) | 568 (57.5%) | .31 |
| Race (RTI) | < .001 | ||
| White | 522 (66.8%) | 606 (61.4%) | |
| Black | 235 (30.1%) | 296 (30.0%) | |
| Other/Unknown | 24 (3.1%) | 85 (8.6%) | |
| Medicaid Dual Enrollment | 149 (19.1%) | 273 (27.7%) | < .001 |
| Smoking History | .003 | ||
| Never | 145 (18.6%) | 180 (18.2%) | |
| Current | 120 (15.4%) | 213 (21.6%) | |
| Former | 516 (66.1%) | 594 (60.2%) | |
| Geographic-based | |||
| Non-metropolitan area (vs. metro) | 152 (19.5%) | 398 (40.3%) | < .001 |
| Household Income, Median $ (Q1, Q3) | 55,022 (41,184, 72,616) | 43,603 (32,407, 56,393) | < .001 |
| Medical History | |||
| Prior Statins, yes | 658 (84.3%) | 583 (59.1%) | < .001 |
| Comorbidities | |||
| Cancer | 146 (18.7%) | 176 (17.8%) | .64 |
| Cerebrovascular disease | 270 (34.6%) | 336 (34.0%) | .82 |
| Chronic obstructive pulmonary disease | 292 (37.4%) | 429 (43.5%) | .01 |
| Congestive heart failure | 232 (29.7%) | 287 (29.1%) | .77 |
| Dementia | 17 (2.2%) | 35 (3.5%) | .09 |
| Diabetes mellitus | 422 (54.0%) | 549 (55.6%) | .50 |
| Hypertension | 738 (94.5%) | 928 (94.0%) | .67 |
| Ischemic heart disease | 526 (67.3%) | 613 (62.1%) | .02 |
| Renal disease | 297 (38.0%) | 376 (38.1%) | .98 |
| Charlson score, Mean (SD) | 4.85 (2.97) | 4.95 (2.89) | .32 |
IC: intermittent claudication; CLTI: chronic limb-threatening ischemia;
A total of 260 (33.3%) patients had no uncontrolled RFs, 321 (41.1%) had 1 uncontrolled RF, and 200 (25.6%) had 2 uncontrolled RFs (Table 2). HTN was the RF most likely to be uncontrolled (33.6% of patients with 1 uncontrolled RF and 68.5% with 2 uncontrolled RF) while tobacco use was the least likely to be uncontrolled (11.5% and 41.5% of patients in 1 and 2 uncontrolled RF groups were currently smoking, respectively).
Table 2:
Baseline characteristics of patients with PAD, by number of uncontrolled risk factors
| Variable | None | 1 | 2 or more | p-value |
|---|---|---|---|---|
| N | 260 | 321 | 200 | |
| Risk Factor Control | ||||
| LDL ≥ 100 | 87 (27.1%) | 117 (58.5%) | < .001 | |
| HbA1c ≥ 7 | 89 (27.7%) | 102 (51.0%) | < .001 | |
| SBP ≥ 140 | 108 (33.6%) | 137 (68.5%) | < .001 | |
| Current Smoker | 37 (11.5%) | 83 (41.5%) | < .001 | |
| Symptom Severity | .009 | |||
| Asymptomatic | 103 (39.6%) | 114 (35.5%) | 54 (27.0%) | |
| IC | 116 (44.6%) | 129 (40.2%) | 94 (47.0%) | |
| CLTI | 41 (15.8%) | 78 (24.3%) | 52 (26.0%) | |
| Demographics | ||||
| Age (years), Mean (SD) | 74.15 (8.59) | 72.69 (10.84) | 70.07 (9.20) | < .001 |
| Age Category | < .001 | |||
| < 65 | 27 (10.4%) | 66 (20.6%) | 51 (25.5%) | |
| 65 - < 75 | 106 (40.8%) | 106 (33.0%) | 81 (40.5%) | |
| 75 - < 85 | 97 (37.3%) | 103 (32.1%) | 57 (28.5%) | |
| ≥ 85 | 30 (11.5%) | 46 (14.3%) | 11 (5.5%) | |
| Sex, Male | 166 (63.8%) | 189 (58.9%) | 113 (56.5%) | .25 |
| Race (RTI) | < .001 | |||
| White | 206 (79.2%) | 202 (62.9%) | 114 (57.0%) | |
| Black | 40–50 (15%−19%) | 105–115 (33%−36%) | 75–85 (38%−43%) | |
| Other/Unknown | * | * | * | |
| Medicaid Dual Enrollment | 30 (11.5%) | 72 (22.4%) | 47 (23.5%) | < .001 |
| Geographic-based | ||||
| Non-metropolitan area (vs. metro) | 48 (18.5%) | 64 (19.9%) | 40 (20.0%) | .88 |
| Household Income $, Median (Q1, Q3) | 56,100 (44,274, 75,971) | 55,204 (40,962, 72,616) | 53,750 (39,913, 64,483) | .02 |
| Medical History | ||||
| Prior Statins, yes | 229 (88.1%) | 265 (82.6%) | 164 (82.0%) | .11 |
| Comorbidities | ||||
| Cancer | 52 (20.0%) | 51 (15.9%) | 43 (21.5%) | .22 |
| Cerebrovascular disease | 103 (39.6%) | 94 (29.3%) | 73 (36.5%) | .03 |
| Chronic obstructive pulmonary disease | 98 (37.7%) | 120 (37.4%) | 74 (37.0%) | .99 |
| Congestive heart failure | 78 (30.0%) | 97 (30.2%) | 57 (28.5%) | .91 |
| Dementia | * | * | * | .13 |
| Diabetes mellitus | 112 (43.1%) | 177 (55.1%) | 133 (66.5%) | < .001 |
| Hypertension | 242 (93.1%) | 305 (95.0%) | 191 (95.5%) | .46 |
| Ischemic heart disease | 187 (71.9%) | 208 (64.8%) | 131 (65.5%) | .15 |
| Renal disease | 86 (33.1%) | 131 (40.8%) | 80 (40.0%) | .13 |
| Charlson score, Mean (SD) | 4.42 (2.80) | 5.01 (3.12) | 5.18 (2.89) | .01 |
Cell size of less than 11 removed in accordance with CMS cell suppression policy
HbA1c: Hemoglobin A1c; CLTI: chronic limb-threatening ischemia; IC: intermittent claudication; LDL: low density lipoprotein; SBP: systolic blood pressure
The average age overall was 72.5±9.8 years but differed by degree of RF control, with patients with complete RF control being older, on average, than patients with 2 uncontrolled RFs (p<0.001). Patients with the poorest RF control were more likely to be Black (38–43%, p<0.001) and dual Medicaid enrolled (23.5%, p<0.001) than were patients with optimal control. Overall, 19.5% of patients lived in non-metropolitan areas, which did not differ by degree of risk factor control (p=0.88). Median census-tract income per group ranged from $56,100 (IQR $44,274, $75,971; none uncontrolled) to $53,650 (IQR $39,913, $64,483; 2 uncontrolled, p=0.02).
PAD symptom severity significantly differed between groups (Table 2, p=0.009). Patients with 2 uncontrolled RFs were more likely to have CLTI and less likely to have asymptomatic disease compared to patients with optimal control (CLTI 26.0% ( 2 uncontrolled) vs. 15.8% (none uncontrolled); asymptomatic 27.0% ( 2 uncontrolled) vs. 39.6% (none uncontrolled)).
Clinical Outcomes
The cumulative incidence of outcomes are shown in Table 3 and Figure 2. Patients with 2 uncontrolled RFs were more likely to experience MAVE by 30 days (none uncontrolled: 5.8%, 1 uncontrolled: 11.5%, 2 uncontrolled: 13.6%, p=0.01). A high rate of MAVE was observed by 1 year, with numerically higher MAVE rates with less RF control (none uncontrolled: 31.4%, 1 uncontrolled: 34.2%, 2 uncontrolled: 40.5%, p=0.08).
Table 3:
Observed cumulative incidence of each composite event type at 30 days and 1 year, by number of uncontrolled risk factors.
| Variable | None | 1 | 2 or more | p-value |
|---|---|---|---|---|
| N | 260 | 321 | 200 | |
| Mortality | ||||
| 30 days | * | * | * | .43 |
| 1 year | 40 (15.5%) | 44 (14.2%) | 20 (10.2%) | .23 |
| All-cause hospitalization | ||||
| 30 days | 34 (13.1%) | 39 (12.2%) | 31 (15.6%) | .53 |
| 1 year | 119 (46.0%) | 145 (46.3%) | 94 (47.6%) | .96 |
| MAVE: Major Adverse Cardiac or Limb Events | ||||
| 30 days | 15 (5.8%) | 37 (11.5%) | 27 (13.6%) | .01 |
| 1 year | 81 (31.4%) | 107 (34.2%) | 80 (40.5%) | .08 |
| MACE: Major Adverse Cardiac Events | ||||
| 30 days | * | * | * | .51 |
| 1 year | 30 (11.6%) | 32 (10.3%) | 33 (16.8%) | .09 |
| Myocardial Infarction | ||||
| 1 year | * | 13 (4.2%) | 14 (7.1%) | .08 |
| Stroke | ||||
| 1 year | * | 20 (6.4%) | 21 (10.7%) | .21 |
| MALE: Major Adverse Limb Events | ||||
| 30 days | * | 26 (8.1%) | 19 (9.5%) | .006 |
| 1 year | 36 (13.9%) | 58 (18.4%) | 50 (25.2%) | .007 |
| Revascularization | ||||
| 1 year | 24 (9.3%) | 44 (13.9%) | 38 (19.2%) | .008 |
| Amputation | ||||
| 1 year | 18 (7.0%) | 24 (7.6%) | 17 (8.6%) | .79 |
Cell size of less than 11 removed in accordance with CMS cell suppression policy
MACE: major adverse cardiac events (mortality + myocardial infarction + stroke); MALE: major adverse limb events (revascularization + amputation); MAVE: major adverse vascular events (mortality + myocardial infarction + stroke + revascularization + amputation)
Figure 2.
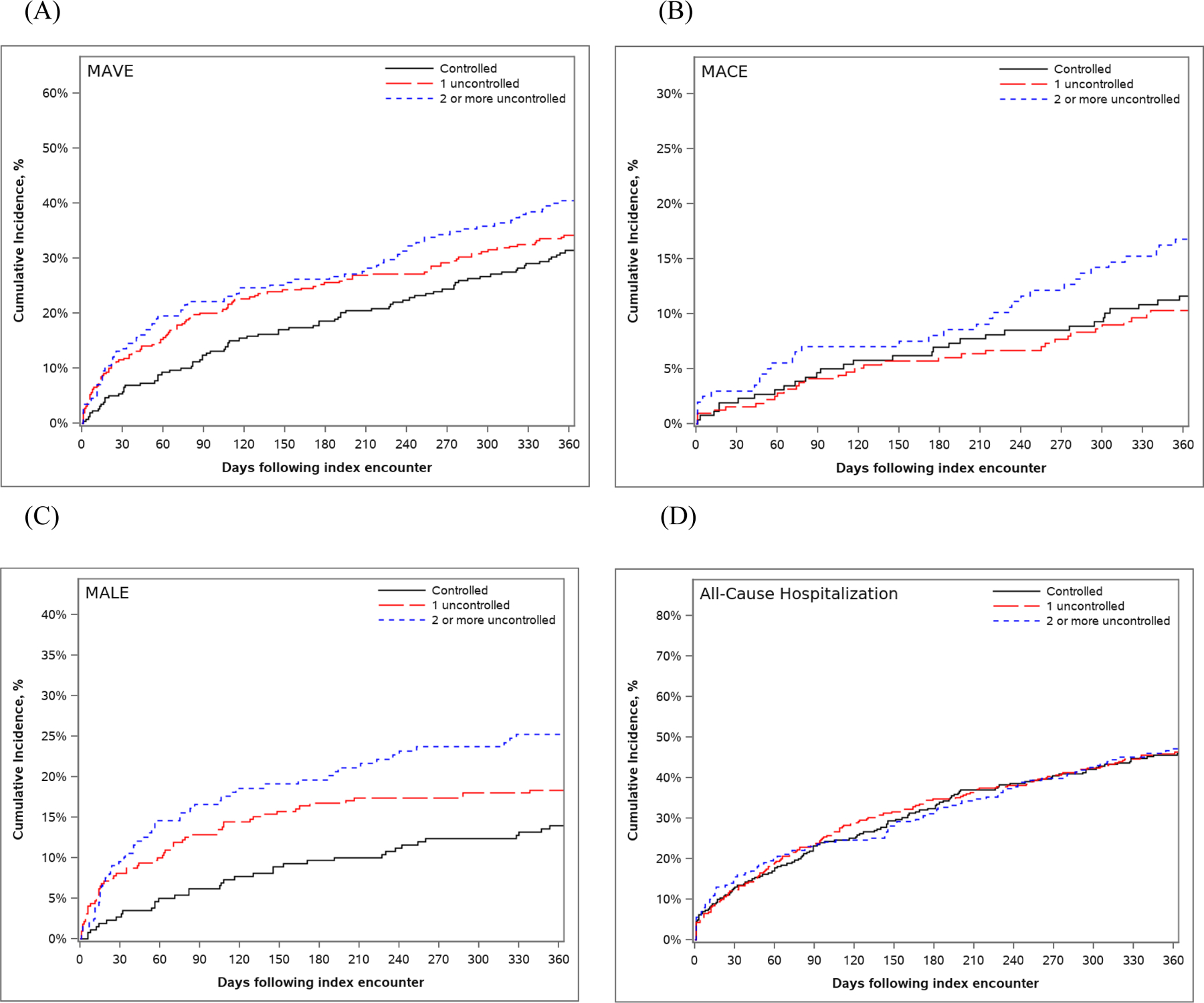
Cumulative incidence curves for (A) MAVE, (B) MACE, (C) MALE, and (D) all-cause hospitalization
Significant differences in MALE were observed between groups by both 30 days (none uncontrolled: suppressed (due to CMS policy), 1 uncontrolled: 8.1%, 2 uncontrolled: 9.5%, p=0.006) and 1 year (none uncontrolled: 13.9%, 1 uncontrolled: 18.4%, 2 uncontrolled: 25.2%, p=0.007). This was driven by rates of lower extremity revascularization at 1 year (none uncontrolled: 9.3%, 1 uncontrolled: 13.9%, 2 uncontrolled: 19.2%, p=0.008). No significant differences in rates of lower extremity amputation were observed between groups at 1 year. There were also no statistically significant differences in the mortality rate, MACE, or all-cause hospitalization between groups at either 30 days or 1 year, although there was a numerically lower rate of all-cause death in the 2 uncontrolled RFs group than among other groups.
Relationship between risk factor control and outcomes by one year
Patients with uncontrolled HbA1c were at heightened unadjusted risk of MAVE (HR 1.54, 95%CI 1.19–2.00, Supplementary Table 2) and MALE (HR 1.93, 95% CI 1.37–2.70, Supplementary Table 3), but not MACE (Supplementary Table 4). After adjustment for patient demographics, PAD symptom severity, and comorbidities, uncontrolled HbA1c was no longer associated with elevated risk of any outcome. Patients with uncontrolled SBP were at higher unadjusted risk of MACE (HR 1.53, 95% CI 1.01–2.29), but this association did not persist after adjustment for patient covariates. Current smoking and uncontrolled LDL were not associated with outcomes in unadjusted or adjusted analyses. None of the uncontrolled RF measures were associated with all-cause hospitalization in either unadjusted or adjusted analyses (Supplementary Table 5).
Patients with 2 uncontrolled RFs were at heightened unadjusted risk of MAVE (HR 1.42, 95% CI 1.05–1.93, Supplementary Table 6) and MALE (HR 1.93, 95% CI 1.26–2.94, Supplementary Table 7), but not MACE (Supplementary Table 8) or all-cause hospitalization (Supplementary Table 9). These higher risks were no longer significant following adjustment.
Relationship between patient characteristics and outcomes by one year
Patients who were Medicaid dual-enrolled were at higher unadjusted risk of MAVE (HR 1.53, 95% CI 1.17–2.00) and all-cause hospitalization (HR 1.55, 95% CI 1.23–1.95). The heightened risk of MAVE did not remain statistically significant following adjustment for other patient characteristics and control of individual RFs, however the elevated risk of all-cause hospitalization remained (HRadj 1.36, 95% CI 1.05–1.76). Patients who resided in rural areas were at a significantly lower risk of all-cause hospitalization when adjusting for covariates and control of individual RFs (HRadj 0.73, 95% CI 0.56–0.96). Conversely, patients in rural areas were at higher unadjusted risk of MALE (HR 1.55, 95% CI 1.07–2.25), but this elevated risk did not remain significant following multivariable adjustment. Patients who were Black were at higher unadjusted risk of MAVE (HR 1.65, 95% CI 1.28–2.12), MALE (HR 1.90, 95% CI 1.37–2.60), and all-cause hospitalization (HR 1.31, 95% CI 1.05–1.63), yet these risks were no longer significant after adjustment for confounding factors.
Discussion:
In this paper, we evaluated the relationship between RF control and longitudinal clinical outcomes in a large group of patients with PAD within an integrated health system, and the analysis yielded three key findings. First, control of RFs in patients with PAD is suboptimal and RF control varied significantly across patient demographics and clinical factors. Second, worse RF control was associated with a significantly higher incidence of major adverse events, but these increased risks did not persist after adjustment for patient characteristics. Finally, adjustment for RF control and patient characteristics attenuated but did not negate the relationships between social determinants of health and cardiovascular outcomes.
Guidelines recommend management of HTN, HLD, and DM as well as smoking cessation in patients with PAD through lifestyle modifications and/or medical therapy, with the goal of improved cardiovascular morbidity and mortality as well as quality of life.18,19 This study demonstrates the poor management of RFs in patients with PAD, with only one third of patients in this cohort achieving control of all modifiable RFs and one quarter lacking control of two or more. Poor RF management among patients with PAD has been previously documented, especially compared to conditions necessitating similar control such as ischemic heart disease and cerebrovascular disease.5,20–23 While insurance status and under recognition of PAD can be reasonably expected to influence access to and quality of care, this cohort of patients with insurance coverage and diagnosed PAD still demonstrated suboptimal control of RFs, pointing to the role of other provider- and patient-level factors in achieving ideal management. Identifying and addressing these factors will be crucial to ensuring better risk optimization for this patient population.
A higher incidence of cardiovascular events with poorer RF control is in keeping with analyses of larger databases. Analyses of the COMPASS trial and REACH registry have demonstrated higher rates of ischemic events at one year with worse individual as well as cumulative RF control for patients with PAD.5,24 Endpoints in this cohort were driven by limb events, particularly revascularization, as well as late cardiac events. A high rate of limb events was observed in all patients with available outcome data (i.e., including patients without RF control data) and has previously been noted in patients with both isolated PAD as well as polyvascular disease.5,8 This potentially suggests a greater burden of leg disease and symptoms despite the systemic atherosclerosis associated with PAD. The lack of significant association between RF control and outcomes in multivariable analysis in this cohort may have been due to limited statistical power due to the exclusion of patients with incomplete RF control data. This bears particular consideration given that patients who were excluded due to incomplete RF control data had characterisitics similar to included patients with poor RF control, suggesting that the excluded patients may have disproportionately had poor RF control and more ischemic events.
Patients in this cohort with the poorest degree of control of RFs ( 2 uncontrolled) had more severe PAD symptoms (i.e., more likely to have IC and CLTI) and were younger. Previous studies have demonstrated the independent and cumulative association of HTN, DM, HLD, and tobacco use with the risk of PAD and severity.9,10,25 Our findings suggest that the degree of control of RFs, not just their presence or absence, may be related to symptom severity. However, the relationship between RF control and symptom severity remains unclear and may be bidirectional – while worse RF control may accelerate PAD progression and worsen symptoms, more severe symptoms could limit mobility and exercise capability, for instance, thus impeding RF control. Large studies have demonstrated that older patients are more likely to have clinical RFs for PAD,25 but are also more likely to have better control of these RFs compared to younger patients.5 There are several possible reasons that older patients had better RF control in our study. Older patients may have more established PAD and cardiovascular care, resulting in more consistent management of RFs. It is also conceivable that patients with less RF control may develop PAD earlier due to a heightened risk profile. The relationship between degree of RF control and age of PAD incidence as well as symptom severity merits further exploration.
Several aspects of RF control in relation to race, ethnicity, and socioeconomic status were notable in this study. Patients with the poorest control of RFs were significantly more likely to be Black (38–43% vs. 15–19%), dual Medicaid enrolled (23.5% vs. 11.5%), and live in areas with lower annual median income ($53,750 vs. $56,100) when compared with patients with optimal control. Additionally, non-white, rural, and poorer (i.e., lower median income and greater Medicaid dual enrolment) patients were significantly more likely to be excluded from this cohort due to incomplete available RF control data. Incomplete data may be the result of less access to care, fragmented care or lower quality care, potentially pointing towards underservice of these patients. Importantly, among included patients, adjusting for degree of RF control and other patient characteristics attenuated but did not negate the elevated risk of many cardiovascular outcomes in historically underserved groups, suggesting multiple and potentially modifiable contributors to disparities in outcomes. However, an increased risk for all-cause hospitalization among dual Medicaid enrolled patients as well as those residing in metropolitan areas persisted after adjustment. Increased risk of hospitalization among Medicaid patients has previously been noted in post-MI care,26 as have worse in-hospital and overall outcomes for PAD patients with Medicaid.27,28 A greater burden of comorbidities, more advanced disease, less access to care, and other social determinants related to poverty may all contribute to this elevated risk of hospitalization.
Our findings are in line with a growing understanding of the relationship between social drivers of health, such as race and socioeconomic status, and cardiovascular RFs and outcomes.6,10,18,22,26 Disparities in RF control that mirror inequities in cardiovascular outcomes in patients with PAD suggest RF control is a potential intermediate step on the causal pathway of poorer outcomes in underserved groups. These social determinants may influence unmeasured drivers of differences in RF control. These factors may include access to care, ability to adhere to medications and lifestyle modifications, patient-provider communication and trust, racism or bias, patient attitudes towards disease and treatment, health literacy, access to nutritious foods, and ease of physical activity, among others.7,29–34Addressing disparities in RF control through equitable implementation of guideline-directed medical therapy, targeted implementation programs, culturally appropriate patient education in underserved communities, and patient and community empowerment is an important step in reducing inequalities in cardiovascular outcomes.
This study has several limitations. This observational study is from a single large referral center, therefore geographic variability in both RF control and PAD outcomes is likely not represented. The cohort only includes fee-for-service Medicare patients to facilitate robust outcome ascertainment, limiting generalizability. The cohort was further limited to patients with available EHR data necessary to determine risk factor control, excluding patients with more fragmented care. Patients with providers in multiple health systems (e.g., a local primary care physician but vascular specialist in a distant city) may have a different RF profile and outcomes due to fragmented care. Control of component RFs was dichotomized based on reasonable clinical targets, however person-time spent above and below control thresholds may contribute to outcomes and were not easily analyzed retrospectively. The study’s retrospective design makes some temporal and causal relationships impossible to discern (e.g., RF control and symptom severity), but determination of RF control during a two-year lookback period preserved the temporal relationship to measured outcomes.
Nevertheless, our analysis suggests further investigation into RF control and better implementation in underserved communities is warranted. Replicating this analysis among broader patient populations, including wider geographic areas with variable insurance status, can further evaluate the role of RF control in PAD outcomes and disparities in implementing guideline-directed care. A prospective design could assess the role of RF control in both PAD incidence, progression, and severity, including studies of primary and secondary prevention with guideline-directed medical therapy and lifestyle interventions. Trials or large dataset analyses testing different control thresholds can provide more evidence for and improve clinical treatment goals. These future investigations can refine the understanding of the relationship between RF control and PAD, optimize control definitions, and improve implementation.
Conclusions:
Patients with PAD have poor RF control, with significant differences in degree of control mirroring known racial and socioeconomic disparities in outcomes. Observed differences in cardiovascular outcomes by degree of RF control were mitigated by adjustment for other patient characteristics. Patients with dual Medicaid enrollment and metropolitan residence remained at elevated risk for all-cause hospitalization after adjustment for RF control and patient characteristics. Addressing disparities in RF control is an actionable step in reducing inequities in cardiovascular outcomes for patients with PAD. Further exploration of the role of RF control on PAD incidence, severity, and progression as well as contributors to disparities in control is desperately needed.
Supplementary Material
Acknowledgements:
None
Sources of Funding:
Doris Duke Charitable Foundation provided a Clinical Scientist Development Grant to Dr. Jones to complete this work. Dr. Weissler is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number F32HL151181. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures:
FWP: none
CBF: none
EHW: none
MMS: none
NCH: none
DIN: none
SJL: none
MAG: none
CL: none
JAR: none
JAG: Consulting: Janssen Pharmaceuticals and Amgen Inc.
MRP: Research Grants: Janssen, Bayer, NHLBI, Heartflow, Phillips, Advisory Board/Consulting: Bayer, Janssen, Heartflow
WSJ: Research support – Boehringer Ingelheim, Doris Duke Charitable Foundation, National Institute of Health, Patient-Centered Outcomes Research Institute; Advisory Board – Bayer, Bristol-Myers Squibb, Janssen Pharmaceuticals
References:
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. The Lancet Global Health 2019;7(8):e1020–e1030. [DOI] [PubMed] [Google Scholar]
- 3.Parvar SL, Fitridge R, Dawson J, Nicholls SJ. Medical and lifestyle management of peripheral arterial disease. Journal of Vascular Surgery 2018;68(5):1595–1606. [DOI] [PubMed] [Google Scholar]
- 4.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet 2013;382(9901):1329–1340. [DOI] [PubMed] [Google Scholar]
- 5.Cacoub PP, Abola MTB, Baumgartner I, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis 2009;204(2):e86–e92. [DOI] [PubMed] [Google Scholar]
- 6.Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. Journal of Vascular Surgery 2013;58(3):673–681.e671. [DOI] [PubMed] [Google Scholar]
- 7.Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998–2006. Journal of vascular surgery 2010;51(4):S21–S26. [DOI] [PubMed] [Google Scholar]
- 8.Narcisse DI, Ford CB, Weissler EH, et al. The Association of Healthcare Disparities and Patient-Specific Factors on Clinical Outcomes in Peripheral Artery Disease. Am Heart J 2021. [DOI] [PubMed] [Google Scholar]
- 9.Wyss TR, Adam L, Haynes AG, et al. Impact of cardiovascular risk factors on severity of peripheral artery disease. Atherosclerosis 2015;242(1):97–101. [DOI] [PubMed] [Google Scholar]
- 10.Eraso LH, Fukaya E, Mohler ER, III, Xie D, Sha D, Berger JS. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. European Journal of Preventive Cardiology 2014;21(6):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Journal of Vascular and Interventional Radiology 2006;17(9):1383–1398. [DOI] [PubMed] [Google Scholar]
- 12.Weissler EH, Lippmann SJ, Smerek MM, et al. Model-Based Algorithms for Detecting Peripheral Artery Disease Using Administrative Data From an Electronic Health Record Data System: Algorithm Development Study. JMIR Med Inform 2020;8(8):e18542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005:480–485. [DOI] [PubMed] [Google Scholar]
- 14.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. Journal of biomedical informatics 2011;44(2):266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ResDAC: Research Data Assistance Center. CMS cell size suppression policy. https://resdac.org/articles/cms-cell-size-suppression-policy. Accessed February 10, 2022. [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005:1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. European Journal of Vascular and Endovascular Surgery 2019;58(1):S1–S109. e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboyans V, Ricco J-B, Bartelink M-LEL, et al. Editor’s Choice – 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2018;55(3):305–368. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Hirsch AT. Contemporary risk factor control and walking dysfunction in individuals with peripheral arterial disease: NHANES 1999–2004. Atherosclerosis 2008;201(2):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehring TF, Sandhoff BG, Stolcpart RS, Merenich JA, Hollis HW Jr. Atherosclerotic risk factor control in patients with peripheral arterial disease. Journal of vascular surgery 2005;41(5):816–822. [DOI] [PubMed] [Google Scholar]
- 22.Nelson KM, Reiber G, Kohler T, Boyko EJ. Peripheral arterial disease in a multiethnic national sample: the role of conventional risk factors and allostatic load. Ethnicity and Disease 2007;17(4):669. [PubMed] [Google Scholar]
- 23.Berger JS, Katona B, Mulder H, et al. Risk Factor Control and Secondary Prevention of Cardiovascular Events in Peripheral Artery Disease: Insights From the Euclid Trial. Circulation 2019;140(Suppl_1):A13433–A13433. [Google Scholar]
- 24.Vanassche T, Verhamme P, Anand SS, et al. Risk factors and clinical outcomes in chronic coronary and peripheral artery disease: An analysis of the randomized, double-blind COMPASS trial. European Journal of Preventive Cardiology 2020;27(3):296–307. [DOI] [PubMed] [Google Scholar]
- 25.Joosten MM, Pai JK, Bertoia ML, et al. Associations Between Conventional Cardiovascular Risk Factors and Risk of Peripheral Artery Disease in Men. JAMA 2012;308(16):1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doll JA, Hellkamp AS, Goyal A, Sutton NR, Peterson ED, Wang TY. Treatment, Outcomes, and Adherence to Medication Regimens Among Dual Medicare-Medicaid–Eligible Adults With Myocardial Infarction. JAMA Cardiology 2016;1(7):787–794. [DOI] [PubMed] [Google Scholar]
- 27.Kim LK, Swaminathan RV, Minutello RM, et al. Trends in hospital treatments for peripheral arterial disease in the United States and association between payer status and quality of care/outcomes, 2007–2011. Catheter Cardiovasc Interv 2015;86(5):864–872. [DOI] [PubMed] [Google Scholar]
- 28.Hong MS, Beck AW, Nelson PR. Emerging national trends in the management and outcomes of lower extremity peripheral arterial disease. Ann Vasc Surg 2011;25(1):44–54. [DOI] [PubMed] [Google Scholar]
- 29.Lafata JE, Karter AJ, O’Connor PJ, et al. Medication adherence does not explain black-white differences in cardiometabolic risk factor control among insured patients with diabetes. Journal of general internal medicine 2016;31(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okunrintemi V, Spatz ES, Di Capua P, et al. Patient–provider communication and health outcomes among individuals with atherosclerotic cardiovascular disease in the United States: medical expenditure panel survey 2010 to 2013. Circulation: Cardiovascular Quality and Outcomes 2017;10(4):e003635. [DOI] [PubMed] [Google Scholar]
- 31.Nanna MG, Navar AM, Zakroysky P, et al. Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: insights from the patient and provider assessment of lipid management registry. JAMA cardiology 2018;3(8):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. Health & place 2010;16(5):876–884. [DOI] [PubMed] [Google Scholar]
- 33.Hawes AM, Smith GS, McGinty E, et al. Disentangling race, poverty, and place in disparities in physical activity. Int J Environ Res Public Health 2019;16(7):1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breathett K, Spatz ES, Kramer DB, et al. The groundwater of racial and ethnic disparities research: a statement From Circulation: Cardiovascular Quality and Outcomes. In: Am Heart Assoc; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


